Effects of Capulin (C. xalapensis) on the Microbiological, Physicochemical and Sensory Properties of Yogurt
Abstract
1. Introduction
2. Materials and Methods
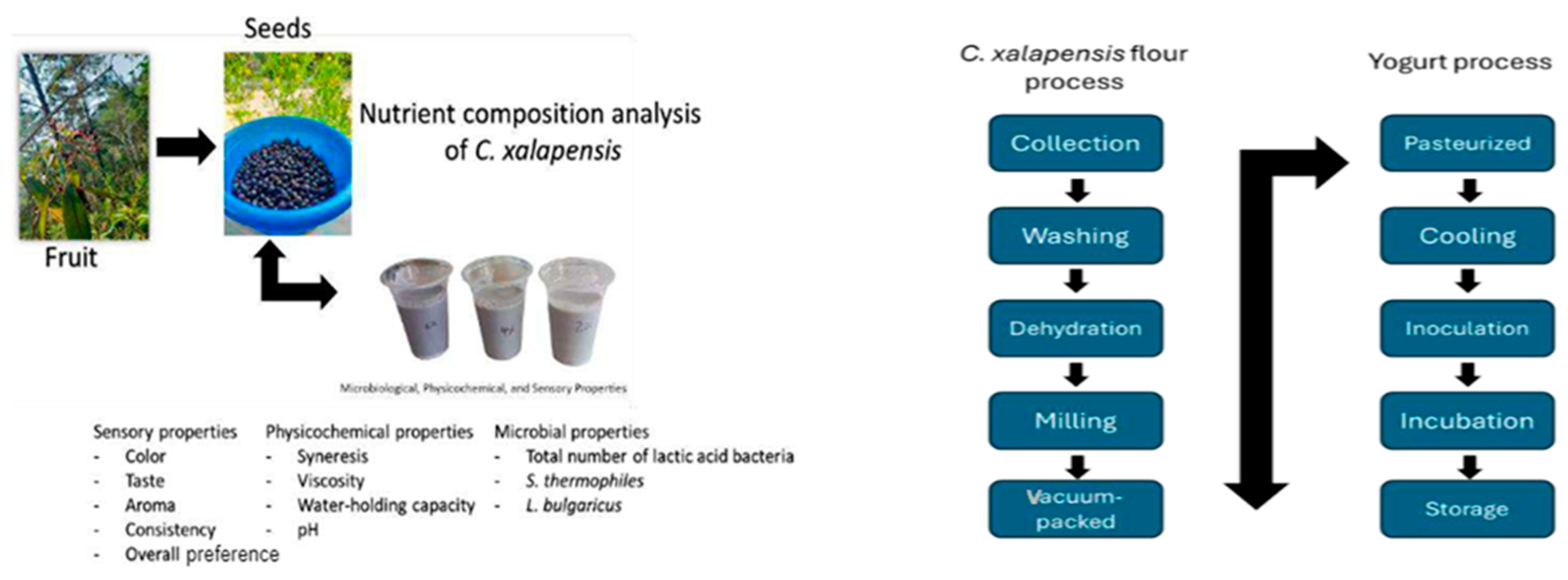
2.1. Preparation of C. xalapensis
2.2. Preparation of C. xalapensis Yogurt
2.3. Nutrient Composition Analysis of C. xalapensis Flour
2.4. Determination of the Physicochemical Properties of Yogurt
2.5. Determination of Lactic Acid Bacteria Counts
2.6. Sensory Evaluation of Yogurt
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Analysis of Capulin Flour and Yogurt
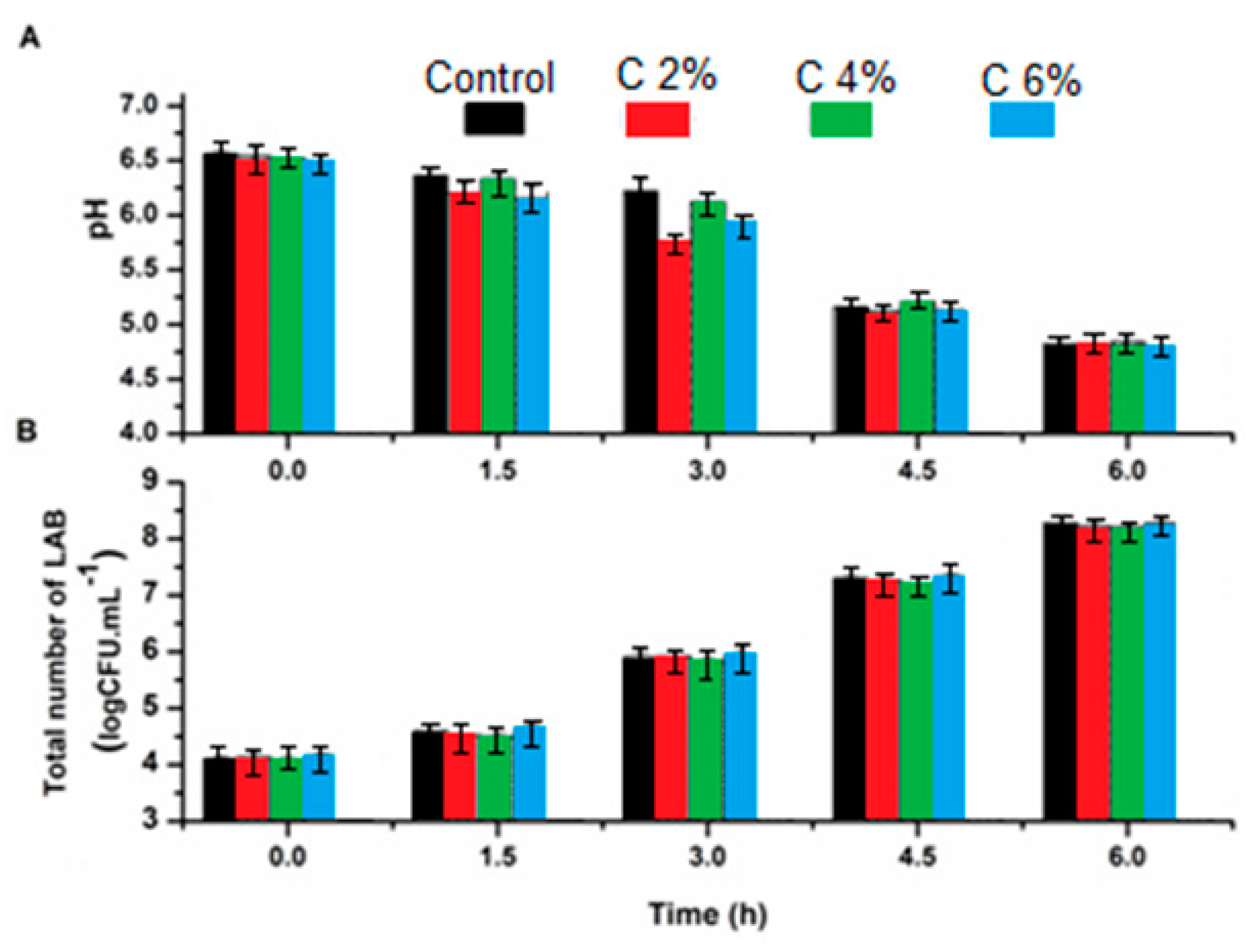
3.2. pH Changes and the Total Number of Lactic Acid Bacteria (LAB) in Yogurt during Fermentation
3.3. Effect of Capulin on the pH and Viscosity of Yogurts during Refrigeration
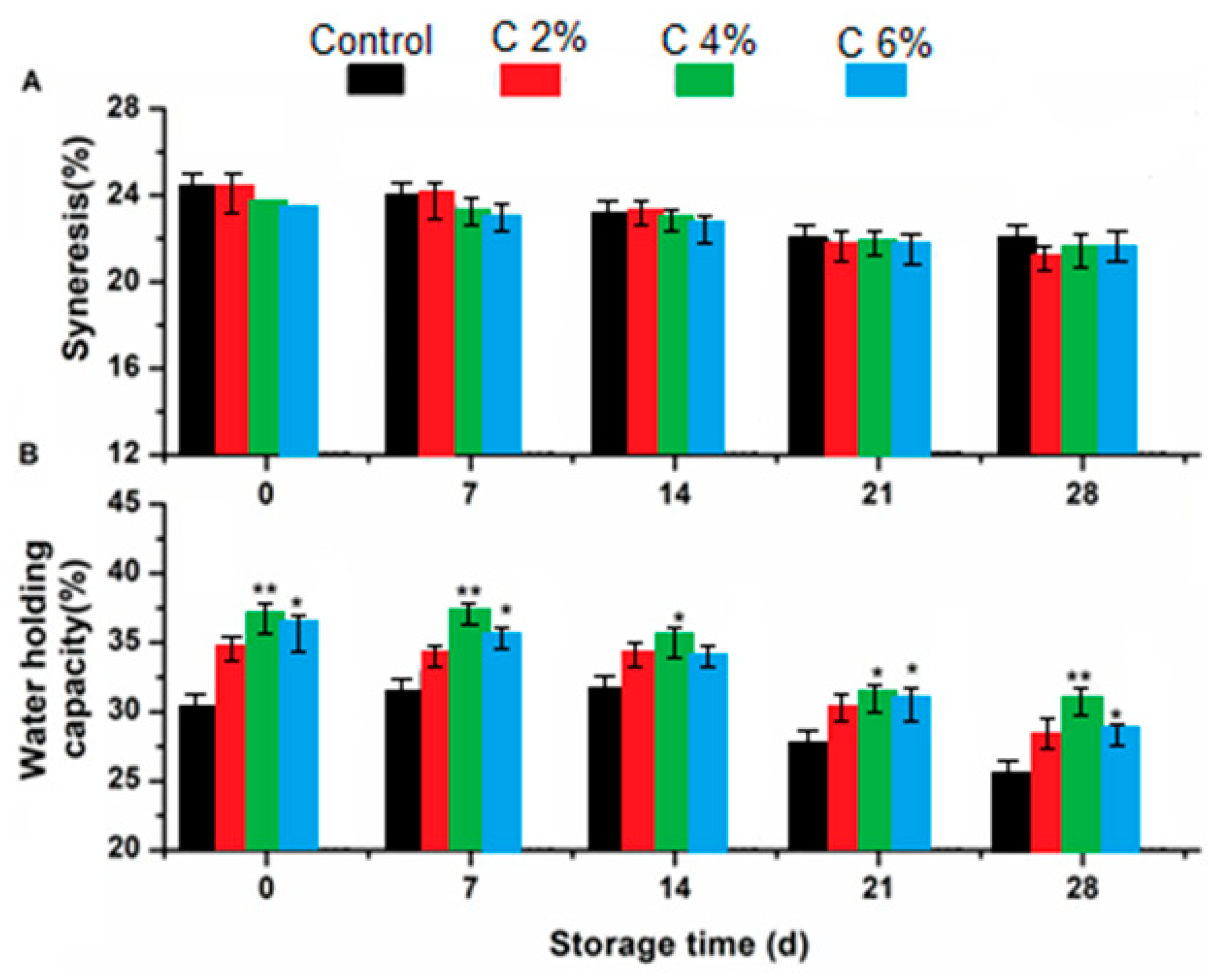
3.4. Effect of Capulin on Syneresis and Water Holding Capacity of Yogurts during Refrigeration
3.5. The Total Number and Primary LAB during Refrigeration
3.6. Effect of Capulin on the Sensory Quality of Yogurt
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuentes, J.A.M.; López-Salas, L.; Borrás-Linares, I.; Navarro-Alarcón, M.; Segura-Carretero, A.; Lozano-Sánchez, J. Development of an Innovative Pressurized Liquid Extraction Procedure by Response Surface Methodology to Recover Bioactive Compounds from Carao Tree Seeds. Foods 2021, 10, 398. [Google Scholar] [CrossRef]
- Maddela, N.R.; García, L.C. (Eds.) Innovations in Biotechnology for a Sustainable Future; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Ros, E. Health Benefits of Nut Consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef]
- Rusu, M.E.; Simedrea, R.; Gheldiu, A.-M.; Mocan, A.; Vlase, L.; Popa, D.-S.; Ferreira, I.C.F.R. Benefits of tree nut consumption on aging and age-related diseases: Mechanisms of actions. Trends Food Sci. Technol. 2019, 88, 104–120. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Bes-Rastrollo, M. Nut consumption, weight gain and obesity: Epidemiological evidence. Nutr. Metab. Cardiovasc. Dis. 2011, 21 (Suppl. S1), S40–S45. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, A.; Mc Auley, K.; Mann, J.; Williams, S.; Skeaff, M. Cholesterol lowering effects of nuts compared with a Canola oil enriched cereal of similar fat composition. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 284–292. [Google Scholar] [CrossRef]
- Hidalgo, P.; Vasquez, D. Pigmentos vegetales: Una alternativa frente a colorantes sintéticos. Revista de Divulg. Científica iBIO 2023, 5, CO113. [Google Scholar]
- Espinosa, E.B.L.; Martínez, C.J.; Dávila-Ortiz, G. Péptidos Bioactivos de Fuentes Vegetales: Un Nuevo Ingrediente para Alimentos Funcionales. In Tendencias de Innovación en la Ingeniería de Alimentos. OmniaScience. 2015, p. 37. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=8628654 (accessed on 20 July 2024).
- Santos, A.P.M.D.; Fracasso, C.M.; Santos, M.L.D.; Romero, R.; Sazima, M.; Oliveira, P.E. Reproductive biology and species geographical distribution in the Melastomataceae: A survey based on New World taxa. Ann. Bot. 2012, 110, 667–679. [Google Scholar]
- Jiao, B.; Wu, B.; Fu, W.; Guo, X.; Zhang, Y.; Yang, J.; Luo, X.; Dai, L.; Wang, Q. Effect of roasting and high-pressure homogenization on texture, rheology, and microstructure of walnut yogurt. Food Chem. X 2023, 20, 101017. [Google Scholar] [CrossRef]
- Yilmaz-Ersan, L.; Sahin, S.; Ozcan, T.; Akpinar-Bayizit, A.; Usta-Gorgun, B.; Ciniviz, M.; Keser, G. Interaction of probiotic activity, antioxidative capacity, and gamma-amino butyric acid (GABA) in chestnut milk-fortified yogurt. J. Food Process. Preserv. 2022, 46, e17266. [Google Scholar]
- Al-Bedrani, D.I.; ALKaisy, Q.H.; Rahi, A.K. Evaluation of milk source on physicochemical, texture, rheological and sensory properties of yogurts. J. Appl. Nat. Sci. 2023, 15, 128–136. [Google Scholar]
- Wairimu, N.; Elkana, O.E.; Koskei, K. Development and evaluation of goat milk yoghurt enriched with baobab fruit pulp. Eur. J. Agric. Food Sci. 2022, 4, 100–106. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 21st ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2019. [Google Scholar]
- Aleman, R.S.; Paz, D.; Cedillos, R.; Tabora, M.; Olson, D.W.; Aryana, K. Attributes of Culture Bacteria as Influenced by Ingredients That Help Treat Leaky Gut. Microorganisms 2023, 11, 893. [Google Scholar] [CrossRef] [PubMed]
- Gocer, E.M.C.; Koptagel, E. Production and evaluation of microbiological & rheological characteristics of kefir beverages made from nuts. Food Biosci. 2023, 52, 102367. [Google Scholar] [CrossRef]
- Marcia, J.; Aleman, R.S.; Montero-Fernández, I.; Martín-Vertedor, D.; Manrique-Fernández, V.; Moncada, M.; Kayanush, A. Attributes of Lactobacillus acidophilus as Effected by Carao (Cassia grandis) Pulp Powder. Fermentation 2023, 9, 408. [Google Scholar] [CrossRef]
- Medina, L.; Aleman, R.S.; Cedillos, R.; Aryana, K.; Olson, D.W.; Marcia, J.; Boeneke, C. Effects of carao (Cassia grandis L.) on physico-chemical, microbiological and rheological characteristics of yogurt. LWT 2023, 183, 114891. [Google Scholar] [CrossRef]
- Aleman, R.S.; Cedillos, R.; Page, R.; Olson, D.; Aryana, K. Physico-chemical, microbiological, and sensory characteristics of yogurt as affected by various ingredients. J. Dairy Sci. 2023, 106, 3868–3883. [Google Scholar] [CrossRef]
- Lim, L.T.; TANG, J.; He, J. Moisture sorption characteristics of freeze dried blueberries. J. Food Sci. 1995, 60, 810–814. [Google Scholar] [CrossRef]
- Coral, V. Determinación Proximal de los Principales Componentes Nutricionales de Siete Alimentos: Yuca, Zanahoria Amarilla, Zanahoria Blanca, Chocho, Avena Laminada, Harina de maíz y Harina de Trigo Integral. Master’s Thesis, Pontificia Universidad Católica del Ecuador, Quito, Ecuador, 2014. Available online: https://repositorio.puce.edu.ec/handle/123456789/20991 (accessed on 20 July 2024).
- Bardales, D.N.; Garay, S.G.M.; Tiburcio, J.E.V.; Portal, R.M.R.; Gómez, R.E.C.; Rosales, C.R.C.; Romaina, J.M.B.; Bedoya, J.R.B.; Aguilar, A.M. Caracterización fisicoquímica de cuatro variedades de papas nativas (Solanum tuberosum) con aptitud para fritura, cultivadas en dos zonas en huánuco. Revista de la Sociedad Química del Perú 2022, 88, 237–250. [Google Scholar] [CrossRef]
- MAPA. Almendra, Prunus Amygdalus (en línea). Frutos Secos. 2013, pp. 305–306. Available online: http://www.magrama.gob.es/es/ministerio/servicios/informacion/almendra_tcm7-315319.pdf%0Ahttp://www.fen.org.es/mercadoFen/pdfs/almendra.pdf (accessed on 20 July 2024).
- Urango, L. Componentes del maíz en la Nutrición Humana (en línea). Nutricionista Dietista, Magíster en Sc y Tecnología de Alimentos. Profesora de cátedra. Escuela de Nutrición y Dietética, Universidad de Antioquia. 2018, pp. 185–209. Available online: https://revistas.udea.edu.co/index.php/biogenesis/article/view/336229 (accessed on 20 July 2024).
- Ortega, I.L.; Montes, M.C. Composición Nutricional Y Calidad De La Miel Producida En El Territorio Patagonia Verde. Apic. En El Territ. Patagon. Verde Región De Los Lagos 2020, 6, 107–123. [Google Scholar]
- Udayarajan, C.T.; Mohan, K.; Nisha, P. Tree nuts: Treasure mine for prebiotic and probiotic dairy free vegan products. Trends Food Sci. Technol. 2022, 124, 208–218. [Google Scholar] [CrossRef]
- da Silva, A.P.R.; Longhi, D.A.; Dalcanton, F.; de Aragão, G.M.F. Modelling the growth of lactic acid bacteria at different temperatures. Braz. Arch. Biol. Technol. 2018, 61, e18160159. [Google Scholar] [CrossRef]
- Soukoulis, C.; Panagiotidis, P.; Koureli, R.; Tzia, C. Industrial yogurt manufacture: Monitoring of fermentation process and improvement of final product quality. J. Dairy Sci. 2007, 90, 2641–2654. [Google Scholar] [CrossRef] [PubMed]
- Erturk, M.Y.; Bonilla, J.C.; Kokini, J. Relationship of non-linear rheological properties and quantitative network analysis parameters as a function of increasingly large amplitude deformations in non-fat, low-fat and high-fat yogurt products. Food Hydrocoll. 2021, 111, 106194. [Google Scholar]
- Bensmira, M.; Jiang, B. Organic acids formation during the production of a novel peanut-milk kefir beverage. Br. J. Dairy Sci. 2011, 2, 18–22. [Google Scholar]
- Bensmira, M.; Jiang, B. Total phenolic compounds and antioxidant activity of a novel peanut based kefir. Food Sci. Biotechnol. 2015, 24, 1055–1060. [Google Scholar] [CrossRef]

| Proximal Analysis | g/100 g |
|---|---|
| Moisture | 9.05 ± 1.03 |
| Fat | 3.69 ± 0.45 |
| Proteín | 6.9 ± 0.69 |
| Ash | 4.24 ± 0.24 |
| Fiber | 13.1 ± 0.31 |
| Carbohydrates | 76.2 ± 2.19 |
| Lactic Acid Bacteria (log CFU·mL−1) | Day | Control | C2% | C4% | C6% |
|---|---|---|---|---|---|
| 1 | 8.07 ± 0.26 A | 8.05 ± 0.57 A | 7.94 ± 0.14 A | 8.03 ± 0.46 A | |
| 7 | 7.94 ± 0.51 A | 7.91 ± 0.42 A | 7.83 ± 0.32 A | 7.93 ± 0.31 A | |
| Total number of lactic acid bacteria | 14 | 7.89 ± 0.4 A | 7.81 ± 0.46 A | 7.78 ± 0.31 A | 7.86 ± 0.31 A |
| 21 | 7.81 ± 0.44 A | 7.78 ± 0.77 A | 7.71 ± 0.58 A | 7.79 ± 0.36 A | |
| 28 | 7.69 ± 0.67 B | 7.66 ± 0.63 B | 7.62 ± 0.45 A | 7.68 ± 0.88 A | |
| 1 | 7.18 ± 0.22 aA | 6.87 ± 0.19 bA | 6.84 ± 0.66 bA | 7.62 ± 0.73 acA | |
| 7 | 7.06 ± 0.39 aA | 6.75 ± 0.72 aA | 6.72 ± 0.65 aA | 7.56 ± 0.29 aA | |
| S. thermophilus | 14 | 6.90 ± 0.44 aA | 6.64 ± 0.7 aA | 6.57 ± 0.75 abA | 7.48 ± 0.24 adA |
| 21 | 6.79 ± 0.16 aB | 6.51 ± 0.4 aB | 6.49 ± 0.17 aA | 7.43 ± 0.84 cA | |
| 28 | 6.67 ± 0.22 aB | 6.42 ± 0.78 aB | 6.42 ± 0.85 aB | 7.16 ± 0.22 bB | |
| 1 | 6.84 ± 0.57 aA | 7.21 ± 0.81 bA | 6.98 ± 0.38 abA | 6.55 ± 0.86 acA | |
| 7 | 6.79 ± 0.32 aA | 7.10 ± 0.38 aA | 6.89 ± 0.88 aA | 6.46 ± 0.1 bA | |
| L. bulgaricus | 14 | 6.77 ± 0.21 aA | 7.00 ± 0.79 aA | 6.81 ± 0.6 aA | 6.39 ± 0.3 bA |
| 21 | 6.64 ± 0.67 aA | 6.93 ± 0.87 aA | 6.71 ± 0.52 aA | 6.36 ± 0.51 abA | |
| 28 | 6.55 ± 0.52 aB | 6.90 ± 0.13 bA | 6.56 ± 0.68 aB | 6.30 ± 0.88 aA |
| Treatment | Color | Taste | Aroma | Consistency | Preference |
|---|---|---|---|---|---|
| Control | 6.87 ± 1.18 a | 6.48 ± 1.54 c | 6.71 ± 1.11 c | 6.77 ± 1.21 c | 6.91 ± 1.29 c |
| C 2% | 6.88 ± 1.44 a | 7.00 ± 1.30 d | 6.64 ± 1.14 c | 6.80 ± 1.09 c | 6.97 ± 1.06 c |
| C 4% | 4.73 ± 1.17 b | 4.95 ± 1.08 b | 5.61 ± 1.40 b | 5.56 ± 1.10 b | 5.83 ± 1.26 b |
| C 6% | 3.92 ± 0.91 c | 4.23 ± 0.89 a | 4.23 ± 0.92 a | 4.36 ± 0.95 a | 4.43 ± 0.91 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, C.; García, S.K.C.; Marcía Fuentes, J.; Ore Areche, F.; Yadav, A.; Aleman, R.S. Effects of Capulin (C. xalapensis) on the Microbiological, Physicochemical and Sensory Properties of Yogurt. Dairy 2024, 5, 515-525. https://doi.org/10.3390/dairy5030039
Molina C, García SKC, Marcía Fuentes J, Ore Areche F, Yadav A, Aleman RS. Effects of Capulin (C. xalapensis) on the Microbiological, Physicochemical and Sensory Properties of Yogurt. Dairy. 2024; 5(3):515-525. https://doi.org/10.3390/dairy5030039
Chicago/Turabian StyleMolina, Cheyli, Sindy Karina Campos García, Jhunior Marcía Fuentes, Franklin Ore Areche, Ajitesh Yadav, and Ricardo S. Aleman. 2024. "Effects of Capulin (C. xalapensis) on the Microbiological, Physicochemical and Sensory Properties of Yogurt" Dairy 5, no. 3: 515-525. https://doi.org/10.3390/dairy5030039
APA StyleMolina, C., García, S. K. C., Marcía Fuentes, J., Ore Areche, F., Yadav, A., & Aleman, R. S. (2024). Effects of Capulin (C. xalapensis) on the Microbiological, Physicochemical and Sensory Properties of Yogurt. Dairy, 5(3), 515-525. https://doi.org/10.3390/dairy5030039






