Lameness in Dairy Cow Herds: Disease Aetiology, Prevention and Management
Abstract
1. Introduction
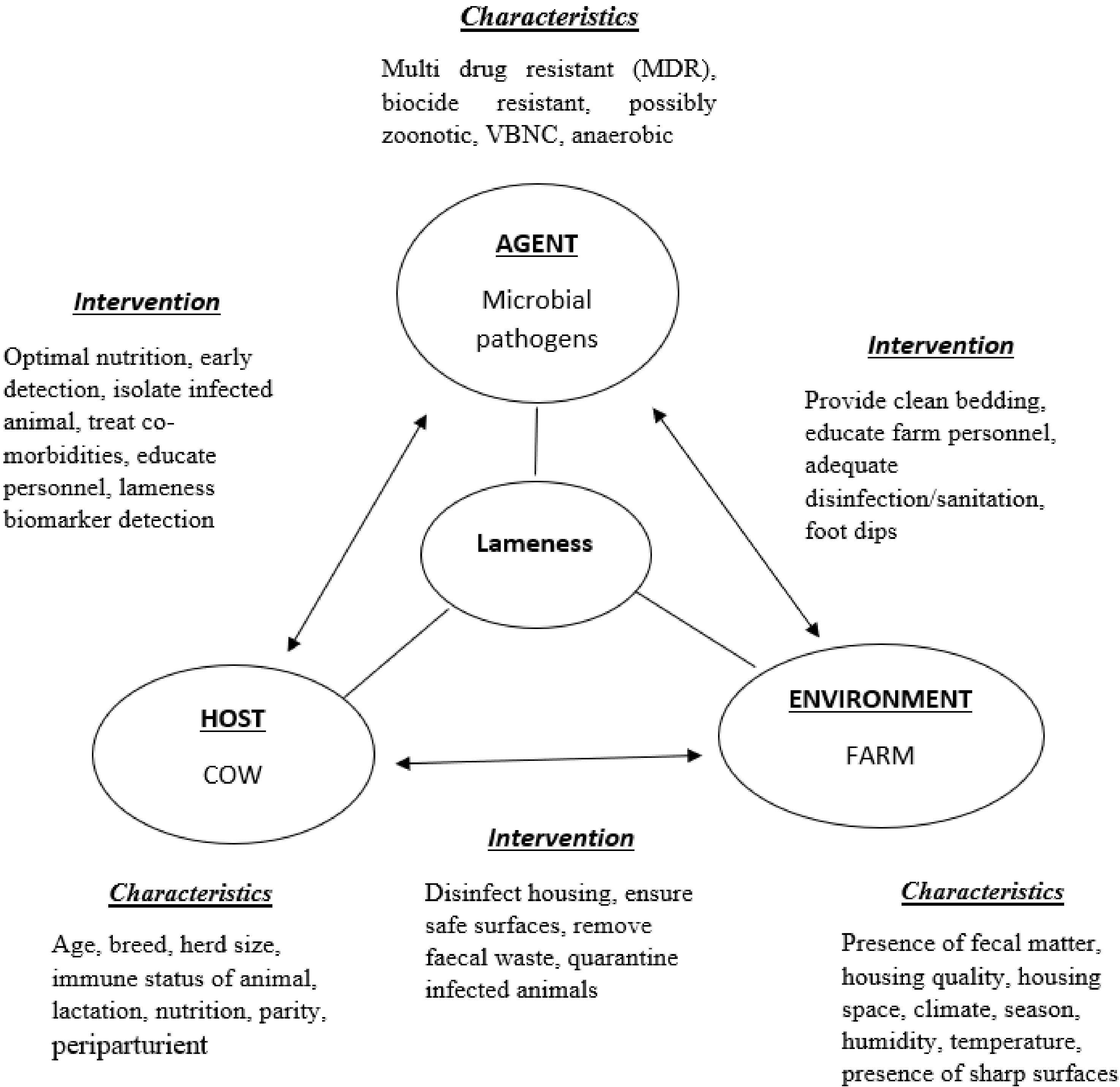
2. Disease Aetiology
Risk Factors
3. Lameness Impact
3.1. Impact on Milk Yield
3.2. Additional Impacts of Lameness
4. Disease Management
Controlling Lameness towards Sustainable Food Production Goals
5. Conclusions
Funding
Conflicts of Interest
References
- Archer, S.; Bell, N.; Huxley, J. Lameness in UK dairy cows: A review of the current status. Practice 2010, 32, 492–504. [Google Scholar] [CrossRef]
- Van Nuffel, A.; Zwertvaegher, I.; Pluym, L.; Van Weyenberg, S.; Thorup, V.M.; Pastell, M.; Sonck, B.; Saeys, W. Lameness Detection in Dairy Cows: Part 1. How to Distinguish between Non-Lame and Lame Cows Based on Differences in Locomotion or Behavior. Animals 2015, 5, 838–860. [Google Scholar] [CrossRef] [PubMed]
- Langova, L.; Novotna, I.; Nemcova, P.; Machacek, M.; Havlicek, Z.; Zemanova, M.; Chrast, V. Impact of Nutrients on the Hoof Health in Cattle. Animals 2020, 10, 1824. [Google Scholar] [CrossRef] [PubMed]
- Afonso, J.S.; Bruce, M.; Keating, P.; Raboisson, D.; Clough, H.; Oikonomou, G.; Rushton, J. Profiling Detection and Classification of Lameness Methods in British Dairy Cattle Research: A Systematic Review and Meta-Analysis. Front. Vet. Sci. 2020, 7, 542. [Google Scholar] [CrossRef] [PubMed]
- Ramanoon, S.Z.; Sadiq, M.B.; Mansor, R.; Syed-Hussain, S.S.; Mossadeq, W.M.S. The Impact of Lameness on Dairy Cattle Welfare: Growing Need for Objective Methods of Detecting Lame Cows and Assessment of Associated Pain. Anim. Welf. 2018. [Google Scholar] [CrossRef]
- Blackie, N.; Maclaurin, L. Influence of Lameness on the Lying Behaviour of Zero-Grazed Lactating Jersey Dairy Cattle Housed in Straw Yards. Animals 2019, 9, 829. [Google Scholar] [CrossRef]
- Palmer, M.; O’Connell, N. Digital Dermatitis in Dairy Cows: A Review of Risk Factors and Potential Sources of Between-Animal Variation in Susceptibility. Animals 2015, 5, 512–535. [Google Scholar] [CrossRef]
- Edwards, A.; Dymock, D.; Jenkinson, H. From tooth to hoof: Treponemes in tissue-destructive diseases. J. Appl. Microbiol. 2003, 94, 767–780. [Google Scholar] [CrossRef]
- Zinicola, M.; Lima, F.; Lima, S.; Machado, V.; Gomez, M.; Doepfer, D.; Guard, C.; Bicalho, R. Altered Microbiomes in Bovine Digital Dermatitis Lesions, and the Gut as a Pathogen Reservoir. PLoS ONE 2015, 10, e0120504. [Google Scholar] [CrossRef]
- Potterton, S.L.; Bell, N.J.; Whay, H.R.; Berry, E.A.; Atkinson, O.C.D.; Dean, R.S.; Huxley, J.N. A descriptive review of the peer and non-peer reviewed literature on the treatment and prevention of foot lameness in cattle published between 2000 and 2011. Vet. J. 2012, 193, 612–616. [Google Scholar] [CrossRef]
- Klitgaard, K.; Nielsen, M.W.; Ingerslev, H.C.; Boye, M.; Jensen, T.K. Discovery of Bovine Digital Dermatitis-Associated Treponema spp. in the Dairy Herd Environment by a Targeted Deep-Sequencing Approach. Appl. Environ. Microbiol. 2014, 80, 4427–4432. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.L.; Read, D.H.; Famula, T.R.; Mongini, A.; Döpfer, D. Long-term observations on the dynamics of bovine digital dermatitis lesions on a California dairy after topical treatment with lincomycin HCl. Vet. J. 2012, 193, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Döpfer, D.; Holzhauer, M.; van Boven, M. The dynamics of digital dermatitis in populations of dairy cattle: Model-based estimates of transition rates and implications for control. Vet. J. 2012, 193, 648–653. [Google Scholar] [CrossRef]
- Hussein, A.; Al-Jumaily, E.F. Extraction and Titration of Leukotoxins from Fusobacterium necrophorum Isolates Recovered from Bovine Liver Abscesses in Sulaimaniyah Region Suha. In In Proceedings of the Eleventh Veterinary Scientific Conference, Baghdad, Iraq; 2012; pp. 110–114. [Google Scholar]
- Hernandez, J.A.; Garbarino, E.J.; Shearer, J.K.; Risco, C.A.; Thatcher, W.W. Comparison of milk yield in dairy cows with different degrees of lameness. J. Am. Vet. Med. Assoc. 2005, 227, 1292–1296. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sadiq, M.B.; Ramanoon, S.Z.; Mansor, R.; Syed-Hussain, S.S.; Shaik Mossadeq, W.M. Claw Trimming as a Lameness Management Practice and the Association with Welfare and Production in Dairy Cows. Animals 2020, 10, 1515. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, K.; McCabe, M.; Earley, B. Leukocyte profile, gene expression, acute phase response, and metabolite status of cows with sole hemorrhages. J. Dairy Sci. 2017, 100, 9382–9391. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhang, H.; Guo, D.; Sun, A.; Wang, H. Shotgun Proteomic Analysis of Plasma from Dairy Cattle Suffering from Footrot: Characterization of Potential Disease-Associated Factors. PLoS ONE 2013, 8, e55973. [Google Scholar] [CrossRef]
- Reader, J.D.; Green, M.J.; Kaler, J.; Mason, S.A.; Green, L.E. Effect of mobility score on milk yield and activity in dairy cattle. J. Dairy Sci. 2011, 94, 5045–5052. [Google Scholar] [CrossRef]
- Somers, J.; Frankena, K.; Noordhuizen-Stassen, E.; Metz, J. Risk factors for digital dermatitis in dairy cows kept in cubicle houses in The Netherlands. Prev. Vet. Med. 2005, 71, 11–21. [Google Scholar] [CrossRef]
- Detilleux, J.C.; Koehler, K.J.; Freeman, A.; Kehrli, M.E.; Kelley, D.H. Immunological Parameters of Periparturient Holstein Cattle: Genetic Variation. J. Dairy Sci. 1994, 77, 2640–2650. [Google Scholar] [CrossRef]
- Calderon, D.F.; Cook, N.B. The effect of lameness on the resting behavior and metabolic status of dairy cattle during the transition period in a freestall-housed dairy herd. J. Dairy Sci. 2011, 94, 2883–2894. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Stamer, E.; Junge, W.; Thaller, G. Genetic parameters for lameness and claw and leg diseases in dairy cows. J. Dairy Sci. 2013, 96, 3310–3318. [Google Scholar] [CrossRef]
- Blowey, R.W. Cattle Lameness and Hoof Care, 3rd ed.; 5m Publishing: Sheffield, UK, 2015. [Google Scholar]
- Daros, R.R.; Eriksson, H.K.; Weazry, D.M.; VonKeyserlingk, M.A.G. Lameness during the dry period: Epidemiology and associated factors. J. Dairy Sci. 2019, 102, 11414–11427. [Google Scholar] [CrossRef] [PubMed]
- Somers, J.R.; Huxley, J.; Lorenz, I.; Doherty, M.L.; O’Grady, L. The effect of Lameness before and during the breeding season on fertility in 10 pasture-based Irish dairy herds. Ir. Vet. J. 2015, 68, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Westin, R.; Vaughan, A.; de Passillé, A.M.; DeVries, T.J.; Pajor, E.A.; Pellerin, D.; Rushen, J. Cow- and farm-level risk factors for lameness on dairy farms with automated milking systems. J. Dairy Sci. 2016, 99, 3732–3743. [Google Scholar] [CrossRef]
- Main, D.C.J.; Leach, K.A.; Barker, Z.E.; Sedgwick, A.K.; Maggs, C.M.; Bell, N.J.; Whay, H.R. Evaluating an intervention to reduce lameness in dairy cattle. J. Dairy Sci. 2012, 95, 2946–2954. [Google Scholar] [CrossRef] [PubMed]
- Barker, Z.E.; Leach, K.A.; Whay, H.R.; Bell, N.J.; Main, D.C.J. Assessment of lameness prevalence and associated risk factors in dairy herds in England and Wales. J. Dairy Sci. 2010, 93, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Warnick, L.D.; Janssen, D.; Guard, C.L.; Gröhn, Y.T. The Effect of Lameness on Milk Production in Dairy Cows. J. Dairy Sci. 2001, 84, 1988–1997. [Google Scholar] [CrossRef]
- Pavlenko, A.; Bergsten, C.; Ekesbo, I.; Kaart, T.; Aland, A.; Lidfors, L. Influence of digital dermatitis and sole ulcer on dairy cow behaviour and milk production. Animal 2011, 5, 1259–1269. [Google Scholar] [CrossRef]
- Charfeddine, N.; Perez-Cabal, M.A. Effect of claw disorders on milk production, fertility, and longevity, and their economic impact in Spanish holstein cows. J. Dairy Sci. 2017, 100, 653–665. [Google Scholar] [CrossRef]
- Green, L.E.; Hedges, V.J.; Schukken, Y.H.; Blowey, R.W.; Packington, A.J. The impact of clinical lameness on the milk yield of dairy cows. J. Dairy Sci. 2002, 85, 2250–2256. [Google Scholar] [CrossRef]
- Bach, A.; Dinarés, M.; Devant, M.; Carré, X. Associations between lameness and production, feeding and milking attendance of Holstein cows milked with an automatic milking system. J. Dairy Res. 2006, 74, 40. [Google Scholar] [CrossRef] [PubMed]
- King, M.T.M.; LeBlanc, S.J.; Pajor, E.A.; DeVries, T.J. Cow-level associations of lameness, behavior, and milk yield of cows milked in automated systems. J. Dairy Sci. 2017, 100, 4818–4828. [Google Scholar] [CrossRef] [PubMed]
- Bicalho, R.C.; Machado, V.S.; Caixeta, L.S. Lameness in dairy cattle: A debilitating disease or a disease of debilitated cattle? A cross-sectional study of lameness prevalence and thickness of the digital cushion. J. Dairy Sci. 2009, 92, 3175–3184. [Google Scholar] [CrossRef]
- Vermeersch, A.; Opsomer, G. Digital dermatitis in cattle Part I: Factors contributing to the development of digital dermatitis Digitale dermatitis bij rundvee Deel I: Factoren die bijdragen tot de ontwikkeling van digitale dermatitis. Vlaams Diergeneeskd. Tijdschr. 2019, 88, 247–257. [Google Scholar] [CrossRef]
- DeVries, T.J.; Dufour, S.; Scholl, D.T. Relationship between feeding strategy, lying behavior patterns, and incidence of intramammary infection in dairy cows. J. Dairy Sci. 2010, 93, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Antanaitis, R.; Juozaitiene, V.; Urbonavicius, G.; Malašauskiene, D.; Televicius, M.; Urbutis, M.; Baumgartner, W. Impact of Lameness on Attributes of Feeding Registered with Noseband Sensor in Fresh Dairy Cows. Agriculture 2021, 11, 851. [Google Scholar] [CrossRef]
- Booth, C.J.; Warnick, L.D.; Gröhn, Y.T.; Maizon, D.O.; Guard, C.L.; Janssen, D. Effect of Lameness on Culling in Dairy Cows. J. Dairy Sci. 2004, 87, 4115–4122. [Google Scholar] [CrossRef]
- Thomas, H.J.; Remnant, J.G.; Bollard, N.J.; Burrows, A.; Whay, H.R.; Bell, N.J.; Mason, C.; Huxley, J.N. Recovery of chronically lame dairy cows following treatment for claw horn lesions: A randomised controlled trial. Vet. Rec. 2016, 178, 116. [Google Scholar] [CrossRef]
- Machado, V.S.; Caixeta, L.S.; McArt, J.A.A.; Bicalho, R.C. The effect of claw horn disruption lesions and body condition score at dry-off on survivability, reproductive performance, and milk production in the subsequent lactation. J. Dairy Sci. 2010, 93, 4071–4078. [Google Scholar] [CrossRef]
- Van Hertem, T.; Parmet, Y.; Steensels, M.; Maltz, E.; Antler, A.; Schlageter-Tello, A.A.; Lokhorst, C.; Romanini, C.E.; Viazzi, S.; Bahr, C.; et al. The effect of routine hoof trimming on locomotion score, ruminating time, activity, and milk yield of dairy cows. J. Dairy Sci. 2014, 97, 4852–4863. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, P.T.; Sørensen, J.T.; Ersbøll, A.K. Evaluation of Three Commercial Hoof-Care Products Used in Footbaths in Danish Dairy Herds. J. Dairy Sci. 2008, 91, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.B.; Rieman, J.; Gomez, A.; Burgi, K. Observations on the design and use of footbaths for the control of infectious hoof disease in dairy cattle. Vet. J. 2012, 193, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Meade, E.; Slattery, M.A.; Garvey, M. Biocidal Resistance in Clinically Relevant Microbial Species: A Major Public Health Risk. Pathogens 2021, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Shillings, J.; Bennett, R.; Rose, D.C. Exploring the Potential of Precision Livestock Farming Technologies to Help Address Farm Animal Welfare. Front. Anim. Sci. 2021. [Google Scholar] [CrossRef]
- Silva, S.R.; Araujo, J.P.; Guedes, C.; Silva, F.; Almeida, M.; Cerqueira, J.L. Precision Technologies to Address Dairy Cattle Welfare: Focus on Lameness, Mastitis and Body Condition. Animals 2021, 11, 2253. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.C.; Chilvers, J. Agriculture 4.0: Broadening Responsible Innovation in an Era of Smart Farming. Front. Sust. Food Syst. 2018, 2, 87. [Google Scholar] [CrossRef]
- Berckmans, D. General introduction to precision livestock farming. Anim. Front. 2017, 7, 6–11. [Google Scholar] [CrossRef]
- Odintsov Vaintrub, M.; Levit, H.; Chincarini, M.; Fusaro, I.; Giammarco, M.; Vignola, G. Review: Precision livestock farming, automats and new technologies: Possible applications in extensive dairy sheep farming. Animal 2021, 15, 100143. [Google Scholar] [CrossRef]
- Meade, E.; Savage, M.; Garvey, M. Investigation of Alternative Biocidal Options against Foodborne Multidrug Resistant Pathogens. Eur. Exp. Biol. 2020, 10, 8. [Google Scholar] [CrossRef]
- Garvey, M. Bacteriophages and the One Health Approach to Combat Multidrug Resistance: Is This the Way? Antibiotics 2020, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Omazic, A.; Bylund, H.; Boqvist, S. Identifying climate-sensitive infectious diseases in animals and humans in Northern regions. Acta Vet. Scand. 2019, 61, 53. [Google Scholar] [CrossRef] [PubMed]
- Doane, M.; Sarenbo, S. Exposure of farm laborers and dairy cattle to formaldehyde from footbath use at a dairy farm in New York State. Sci. Total Environ. 2014, 487, 65–71. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. In NTP Report on Carcinogens, 12th ed.; Department of Health and Human Services, Public Health Services: Research Triangle Park, NC, USA, 2011.
- Downing, T.W.; Stiglbauer, K.; Gamroth, M.J.; Hart, J. CASE STUDY: Use of Copper Sulfate and Zinc Sulfate in Footbaths on Oregon Dairies. Prof. Anim. Sci. 2010, 26, 332–334. [Google Scholar] [CrossRef]
- Rohr, J.R.; Barrett, C.B.; Civitello, D.J.; Craft, M.E.; Delius, B.; Deleo, G.A.; Hudson, P.J.; Jouanard, N.; Nguyen, K.H.; Ostfeld, R.S.; et al. Emerging human infectious diseases and the links to global food production. Nat. Sustain. 2019, 2, 445–456. [Google Scholar] [CrossRef] [PubMed]
| Locomotion Score | Mobility Impact | Clinical Manifestations |
|---|---|---|
| 1 | Movement is normal, equal weight on all legs [2] | None—normal gait and walking—Normal |
| 2 | Movement not perfect, but animal willing to move and not hindered | Gait is affected [2], limbs do not share weight, and track is affected, level back standing—Mildly lame |
| 3 | Movement is possible but seriously compromised | Lack of tracking, limp visible, short strides [2], arched back standing and lying, reduced milk yield and milkings—Moderately lame |
| 4 | Movement is greatly reduced; animal is unwillingly to move [2] | Well-defined limp, loss of tracking, always arched back, unable to put weight on affected limb [19], lying time increased, reduced milk yield and milkings—Lame |
| 5 | Movement is severely restricted; animal is unable to move | Short stride [2], unable to put weight on limb [19] and may have additional limbs affected, lying time increased, reduced milk yield and milkings—Severely lame |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garvey, M. Lameness in Dairy Cow Herds: Disease Aetiology, Prevention and Management. Dairy 2022, 3, 199-210. https://doi.org/10.3390/dairy3010016
Garvey M. Lameness in Dairy Cow Herds: Disease Aetiology, Prevention and Management. Dairy. 2022; 3(1):199-210. https://doi.org/10.3390/dairy3010016
Chicago/Turabian StyleGarvey, Mary. 2022. "Lameness in Dairy Cow Herds: Disease Aetiology, Prevention and Management" Dairy 3, no. 1: 199-210. https://doi.org/10.3390/dairy3010016
APA StyleGarvey, M. (2022). Lameness in Dairy Cow Herds: Disease Aetiology, Prevention and Management. Dairy, 3(1), 199-210. https://doi.org/10.3390/dairy3010016






