Milk Fat Globule Membrane Proteome and Micronutrients in the Milk Lipid Fraction: Insights into Milk Bioactive Compounds
Abstract
:1. Introduction
2. MFGM Proteome and Comparative Proteomics
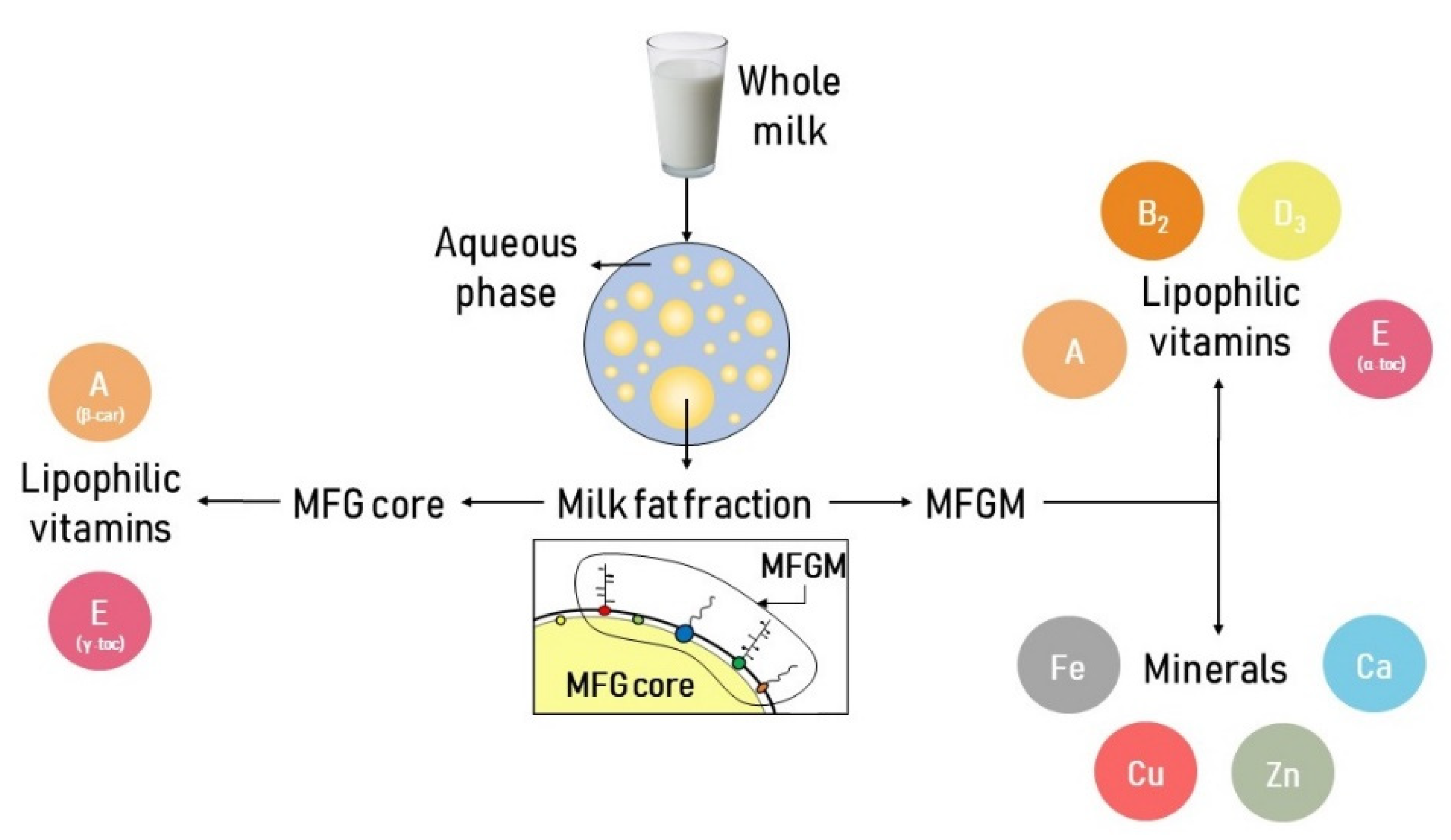
3. Milk Minerals and Vitamins
3.1. Minerals
3.2. Vitamins
4. Prospective Opportunities for the Future
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, Y.W. Overview of bioactive components in milk and dairy products. In Bioactive Components in Milk and Dairy Products, 1st ed.; Park, Y.W., Ed.; Wiley-Blackwell Publishers: Oxford, UK, 2009; pp. 3–14. [Google Scholar]
- Gaucheron, F. Milk and dairy products: A unique micronutrient combination. J. Am. Coll. Nutr. 2011, 30, 400S–409S. [Google Scholar] [CrossRef]
- Spitsberg, V.L. Invited review: Bovine milk fat globule membrane as a potential nutraceutical. J. Dairy Sci. 2005, 88, 2289–2294. [Google Scholar] [CrossRef]
- Arranz, E.; Corredig, M. Invited review: Milk phospholipid vesicles, their colloidal properties, and potential as delivery vehicles for bioactive molecules. J. Dairy Sci. 2017, 100, 4213–4222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, L.; Bonnet, M.; Delavaud, C.; Delosiere, M.; Ferlay, A.; Fougere, H.; Graulet, B. Milk Fat Globule in Ruminant: Major and Minor Compounds, Nutritional Regulation and Differences Among Species. Eur. J. Lipid Sci. Tech. 2018, 120, 1700039. [Google Scholar] [CrossRef]
- Lee, H.; Padhi, E.; Hasegawa, Y.; Larke, J.; Parenti, M.; Wang, A.; Hernell, O.; Lönnerdal, B.; Slupsky, C. Compositional Dynamics of the Milk Fat Globule and Its Role in Infant Development. Front. Pediatr. 2018, 6, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- German, J.B.; Dillard, C.J. Composition, structure and absorption of milk lipids: A source of energy, fat-soluble nutrients and bioactive molecules. Crit. Rev. Food Sci. Nutr. 2006, 46, 57–92. [Google Scholar] [CrossRef]
- Koletzko, B. Human Milk Lipids. Ann. Nutr. Metab. 2016, 69, 28–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldi, A.; Pinotti, L. Lipophilic microconstituents of milk. Adv. Exp. Med. Biol. 2008, 606, 109–125. [Google Scholar]
- Bernard, L.; Leroux, C.; Chilliard, Y. Nutritional regulation of mammary lipogenesis and milk fat in ruminant: Contribution to sustainable milk production. Rev. Colomb. Cienc. Pecu. 2013, 26, 292–302. [Google Scholar]
- Bionaz, M.; Loor, J.J. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genom. 2008, 9, 366. [Google Scholar] [CrossRef] [Green Version]
- Manoni, M.; Di Lorenzo, C.; Ottoboni, M.; Tretola, M.; Pinotti, L. Comparative Proteomics of Milk Fat Globule Membrane (MFGM) Proteome across Species and Lactation Stages and the Potentials of MFGM Fractions in Infant Formula Preparation. Foods 2020, 9, 1251. [Google Scholar] [CrossRef]
- Fox, P.F. Advanced Dairy Chemistry, 3rd ed.; Fox, P.F., McSweeney, P.L.H., Eds.; Chapman & Hall: London, UK; New York, NY, USA, 1992. [Google Scholar]
- Timby, N.; Domellöf, E.; Hernell, O.; Lönnerdal, B.; Domellöf, M. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 860–868. [Google Scholar] [CrossRef] [Green Version]
- Gallier, S.; Vocking, K.; Post, J.A.; Van De Heijning, B.; Acton, D.; van der Beek, E.M.; Van Baalen, T. A novel infant milk formula concept: Mimicking the human milk fat globule structure. Colloid Surf. B 2015, 136, 329–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davoodi, S.H.; Shahbazi, R.; Esmaeili, S.; Sohrabvandi, S.; Mortazavian, A.; Jazayeri, S.; Taslimi, A. Health-Related Aspects of Milk Proteins. Iran. J. Pharm. Res. 2016, 15, 573–591. [Google Scholar] [PubMed]
- Giromini, C.; Cheli, F.; Rebucci, R.; Baldi, A. Invited review: Dairy proteins and bioactive peptides: Modeling digestion and the intestinal barrier. J. Dairy Sci. 2019, 102, 929–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giromini, C.; Lovegrove, J.A.; Givens, D.I.; Rebucci, R.; Pinotti, L.; Maffioli, E.; Tedeschi, G.; Sundaram, T.S.; Baldi, A. In vitro-digested milk proteins: Evaluation of angiotensin-1-converting enzyme inhibitory and antioxidant activities, peptidomic profile, and mucin gene expression in HT29-MTX cells. J. Dairy Sci. 2019, 102, 10760–10771. [Google Scholar] [CrossRef]
- Ye, A.; Cui, J.; Singh, H. Proteolysis of milk fat globule membrane proteins during in vitro gastric digestion of milk. J. Dairy Sci. 2011, 94, 2762–2770. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; Van de Wiele, T.; Do, T.N.H.; Debyser, G.; Struijs, K.; Devreese, B.; Dewettinck, K.; Van Camp, J. Stability of milk fat globule membrane proteins toward human enzymatic gastrointestinal digestion. J. Dairy Sci. 2012, 95, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Thum, C.; Young, W.; Montoya, C.A.; Roy, N.C.; McNabb, W.C. In vitro Fermentation of Digested Milk Fat Globule Membrane from Ruminant Milk Modulates Piglet Ileal and Caecal Microbiota. Front. Nutr. 2020, 7, 91. [Google Scholar] [CrossRef]
- Mather, I.H. A review and proposed nomenclature for major proteins of the milk-fat globule membrane. J. Dairy Sci. 2000, 83, 203–247. [Google Scholar] [CrossRef]
- Brink, L.R.; Herren, A.W.; McMillen, S.; Fraser, K.; Agnew, M.; Roy, N.; Lönnerdal, B. Omics analysis reveals variations among commercial sources of bovine milk fat globule membrane. J. Dairy Sci. 2020, 103, 3002–3016. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, D.A.; Reith, W.; Trowsdale, J. Regulation of immunity by butyrophilins. Annu. Rev. Immunol. 2016, 34, 151–172. [Google Scholar] [CrossRef] [Green Version]
- Struijs, K.; Van de Wiele, T.; Le, T.T.; Debyser, G.; Dewettinck, K.; Devreese, B.; Van Camp, J. Milk fat globule membrane glycoproteins prevent adhesion of the colonic microbiota and result in increased bacterial butyrate production. Int. Dairy J. 2013, 32, 99–109. [Google Scholar] [CrossRef]
- Cavaletto, M.; Giuffrida, M.G.; Conti, A. The proteomic approach to analysis of human milk fat globule membrane. Clin. Chim. Acta. 2004, 347, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Hettinga, K.; van Valenberg, H.; de Vries, S.; Boeren, S.; van Hooijdonk, T.; van Arendonk, J.; Vervoort, J. The host defense proteome of human and bovine milk. PLoS ONE 2011, 6, e19433. [Google Scholar] [CrossRef]
- Pisanu, S.; Ghisaura, S.; Pagnozzi, D.; Biosa, G.; Tanca, A.; Roggio, T.; Uzzau, S.; Addis, M.F. The sheep milk fat globule membrane proteome. J. Proteom. 2011, 74, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, X.; Zhang, W.; Liu, L.; Pang, X.; Zhang, S.; Lv, J. Comparative proteomics of milk fat globule membrane in different species reveals variations in lactation and nutrition. Food Chem. 2016, 196, 665–672. [Google Scholar] [CrossRef]
- Ji, X.; Xu, W.; Cui, J.; Ma, Y.; Zhou, S. Goat and buffalo milk fat globule membranes exhibit better effects at inducing apoptosis and reduction the viability of HT-29 cells. Sci. Rep. 2019, 9, 2577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinhardt, T.A.; Lippolis, J.D. Developmental changes in the milk fat globule membrane proteome during the transition from colostrum to milk. J. Dairy Sci. 2008, 91, 2307–2318. [Google Scholar] [CrossRef] [Green Version]
- Fortunato, D.; Giuffrida, M.G.; Cavaletto, M.; Garoffo, L.P.; Dellavalle, G.; Napolitano, L.; Giunta, C.; Fabris, C.; Bertino, E.; Coscia, A.; et al. Structural proteome of human colostral fat globule membrane proteins. Proteomics 2003, 3, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Alvarado, R.; Phinney, B.; Lönnerdal, B. Proteomic characterization of human milk fat globule membrane proteins during a 12 month lactation period. J. Proteome Res. 2011, 10, 3530–3541. [Google Scholar] [CrossRef]
- Yang, M.; Deng, W.; Cao, X.; Wang, L.; Yu, N.; Zheng, Y.; Wu, J.; Wu, R.; Yue, X. Quantitative Phosphoproteomics of Milk Fat Globule Membrane in Human Colostrum and Mature Milk: New Insights into Changes in Protein Phosphorylation during Lactation. J. Agric. Food Chem. 2020, 68, 4546–4556. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, T.A.; Lippolis, J.D. Bovine milk fat globule membrane proteome. J. Dairy Res. 2006, 73, 406–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honan, M.C.; Fahey, M.J.; Fischer-Tlustos, A.J.; Steele, M.A.; Greenwood, S.L. Shifts in the Holstein dairy cow milk fat globule membrane proteome that occur during the first week of lactation are affected by parity. J. Anim. Sci. Biotechnol. 2020, 11, 81. [Google Scholar] [CrossRef]
- Scuderi, R.A.; Lam, Y.-W.; Ebenstein, D.B.; Tacoma, R.; Cersosimo, L.M.; Kraft, J.; Brito, A.F.; Greenwood, S.L. Comparative analysis of the skim milk and milk fat globule membrane proteomes produced by Jersey cows grazing pastures with different plant species diversity. J. Dairy Sci. 2020, 3, 7498–7508. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Cao, X.; Yang, M.; Han, H.; Kong, F.; Yue, X. Quantitative proteomic analysis of milk fat globule membrane (MFGM) proteins from donkey colostrum and mature milk. Food Funct. 2019, 10, 4256–4268. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Sun, X.; Jiang, S.; Guo, M. Characterization of the milk fat globule membrane proteome in colostrum and mature milk of Xinong Saanen goats. J. Dairy Sci. 2020, 103, 3017–3024. [Google Scholar] [CrossRef]
- Spertino, S.; Cipriani, V.; De Angelis, C.; Giuffrida, M.G.; Marsano, F.; Cavaletto, M. Proteome profile and biological activity of caprine, bovine and human milk fat globules. Mol. Biosyst. 2012, 8, 967–974. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, N.; Zhao, X.W.; Zhang, Y.D.; Han, R.W.; Ma, L.; Zhao, S.; Li, S.; Guo, T.; Wang, J. Proteomic characterization and comparison of mammalian milk fat globule proteomes by iTRAQ analysis. J. Proteom. 2015, 116, 34–43. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, N.; Wang, W.; Zhao, X.; Zhang, Y.; Han, R.; Ma, L.; Zhao, S.; Li, S.; Guo, T.; et al. N-glycosylation proteomic characterization and cross-species comparison of milk fat globule membrane proteins from mammals. Proteomics 2016, 16, 2792–2800. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Peng, X.; Wu, J.; Wu, R.; Liu, B.; Ye, W.; Xu, X.; Yue, X. Differential proteomic analysis of milk fat globule membrane proteins in human and bovine colostrum by iTRAQ-coupled LC-MS/MS. Eur. Food Res. Technol. 2017, 243, 901–912. [Google Scholar] [CrossRef]
- Juvarajah, T.; Wan-Ibrahim, W.I.; Ashrafzadeh, A.; Othman, S.; Hashim, O.H.; Fung, S.Y.; Abdul-Rahman, P.S. Human Milk Fat Globule Membrane Contains Hundreds of Abundantly Expressed and Nutritionally Beneficial Proteins That Are Generally Lacking in Caprine Milk. Breastfeed. Med. 2018, 13, 631–637. [Google Scholar] [CrossRef]
- Cao, X.; Zheng, Y.; Wu, S.; Yang, N.; Yue, X. Characterization and comparison of milk fat globule membrane N-glycoproteomes from human and bovine colostrum and mature milk. Food Funct. 2019, 10, 5046–5058. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Sun, X.; Guo, M. Comparative Proteomics of Whey and Milk Fat Globule Membrane Proteins of Guanzhong Goat and Holstein Cow Mature Milk. J. Food Sci. 2019, 84, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, L.; Chen, T. The effects of secretory IgA in the mucosal immune system. Biomed. Res. Int. 2020, 2020, 2032057. [Google Scholar] [CrossRef] [PubMed]
- Stelwagen, K.; Carpenter, E.; Haigh, B.; Hodgkinson, A.; Wheeler, T.T. Immune components of bovine colostrum and milk. J. Anim. Sci. 2009, 87, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Zou, Y.; Wu, Z.H.; Li, S.L.; Cao, Z.J. Colostrum quality affects immune system establishment and intestinal development of neonatal calves. J. Dairy Sci. 2015, 98, 7153–7163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallier, S.; Tolenaars, L.; Prosser, C. Whole Goat Milk as a Source of Fat and Milk Fat Globule Membrane in Infant Formula. Nutrients 2020, 12, 3486. [Google Scholar] [CrossRef]
- Zamberlin, Š.; Antunac, N.; Havranek, J.; Samaržija, D. Mineral elements in milk and dairy products. Mljekarstvo 2011, 62, 111–125. [Google Scholar]
- Melse-Boonstra, A. Bioavailability of Micronutrients from Nutrient-Dense Whole Foods: Zooming in on Dairy, Vegetables, and Fruits. Front. Nutr. 2020, 7, 101. [Google Scholar] [CrossRef]
- Graulet, B. Ruminant milk: A source of vitamins in human nutrition. Anim. Front. 2014, 4, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Górska-Warsewicz, H.; Rejman, K.; Laskowski, W.; Czeczotko, M. Milk and Dairy Products and Their Nutritional Contribution to the Average Polish Diet. Nutrients 2019, 11, 1771. [Google Scholar] [CrossRef] [Green Version]
- Pabón, M.L.; Lönnerdal, B. Effects of Type of Fat in the Diet on Iron Bioavailability Assessed in Suckling and Weanling Rats. J. Trace Elem. Med. Biol. 2001, 15, 18–23. [Google Scholar] [CrossRef]
- Hayes, K.C.; Pronczuk, A.; Perlman, D. Vitamin E in fortified cow milk uniquely enriches human plasma lipoproteins. Am. J. Clin. Nutr. 2001, 74, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Preveraud, D.; Desmarchelier, C. Bioavailability of vitamin E in humans: An update. Nutr. Rev. 2013, 71, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D. Milk minerals (including trace elements) and bone health. Int. Dairy J. 2006, 16, 1389–1398. [Google Scholar] [CrossRef]
- Fransson, G.B.; Lönnerdal, B. Iron, copper, zinc, calcium, and magnesium in human milk fat. Am. J. Clin. Nutr. 1984, 39, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Bingham, E.W.; Malin, E.L. Alkaline phosphatase in the lactating bovine mammary gland and the milk fat globule membrane. Release by phosphatidylinositol-specific phospholipase C. Comp. Biochem. Physiol. B 1992, 102, 213–218. [Google Scholar] [CrossRef]
- Lönnerdal, B. Effects of milk and milk components on calcium, magnesium, and trace element absorption during infancy. Physiol. Rev. 1997, 77, 643–669. [Google Scholar] [CrossRef] [PubMed]
- Mulder, H.; Walstra, P. The milk fat globule Emulsion Science as Applied to Milk Products and Comparable Foods. In Commonwealth Agricultural Bureau; Farnham Royal and Centre for Agricultural Publishing and Documentation: Wageningen, The Netherlands, 1974. [Google Scholar]
- Fransson, G.B.; Lonnerdal, B. Distribution of trace elements and minerals in human and cow’s milk. J. Pediatr. 1983, 17, 912–915. [Google Scholar] [CrossRef] [Green Version]
- Soliman, Z.A. Comparison of chemical and mineral content of milk from human, cow, buffalo, camel and goat in Egypt. Egypt J. Hosp. Med. 2005, 21, 116–130. [Google Scholar]
- Walton, J.; Flynn, A. Nutritional adequacy of diets containing growing up milks or unfortified cow’s milk in Irish children (aged 12–24 months). Food Nutr. Res. 2013, 57, 21836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chassaing, C.; Sibra, C.; Verbič, J.; Harstad, O.M.; Golecký, J.; Martin, B.; Ferlay, A.; Constant, I.; Delavaud, C.; Hurtaud, C.; et al. Mineral, vitamin A and fat composition of bulk milk related to European production conditions throughout the year. Dairy Sci. Technol. 2016, 96, 715–733. [Google Scholar] [CrossRef] [Green Version]
- Pietrzak-Fiećko, R.; Kamelska-Sadowska, A.M. The Comparison of Nutritional Value of Human Milk with Other Mammals’ Milk. Nutrients 2020, 12, 1404. [Google Scholar] [CrossRef]
- Couvreur, S.; Hurtaud, C. Relationships between milks differentiated on native milk fat globule characteristics and fat, protein and calcium compositions. Animal 2017, 11, 507–518. [Google Scholar] [CrossRef]
- Cazzola, R.; Della Porta, M.; Manoni, M.; Iotti, S.; Pinotti, L.; Maier, J.A. Going to the roots of reduced magnesium dietary intake: A tradeoff between climate changes and sources. Helyion 2020, 6, e05390. [Google Scholar] [CrossRef]
- Oh, H.E.; Deeth, H.C. Magnesium in milk. Int. Dairy J. 2017, 71, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Claeys, W.L.; Cardoen, S.; Daube, G.; De Block, J.; Dewettinck, K.; Dierick, K.; De Zutter, L.; Huyghebaert, A.; Imberechts, H.; Thiange, P.; et al. Raw or heated cow milk consumption: Review of risks and benefits. Food Control. 2013, 31, 251–262. [Google Scholar] [CrossRef]
- Assefa, B. The Effect of Different Heat Treatment on the Nutritional Value of Milk and Milk Products and Shelf-Life of Milk Products. A Review. Dairy Vet. Sci. J. 2019, 11, 555822. [Google Scholar]
- Corredig, M. Properties of the Milk Fat Globule Membrane Derived from Buttermilks from Different Sources. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, January 1998. [Google Scholar]
- Öste, R.; Jägerstad, M.; Andersson, I. Vitamins in Milk and Milk Products. In Advanced Dairy Chemistry Volume 3; Fox, P.F., Ed.; Springer: Boston, MA, USA, 1997. [Google Scholar]
- Park, Y.W. Impact of goat milk and milk products on human nutrition. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2007, 2, 1–19. [Google Scholar] [CrossRef]
- Fantuz, F.; Salimei, E.; Papademas, P. Macro- and micronutrients in non-cow milk and products and their impact on human health. In Non-Bovine Milk and Milk Products, 1st ed.; Tsakalidou, E., Papadimitriou, K., Eds.; Elsevier Academic Press: London, UK, 2016; pp. 209–261. [Google Scholar]
- Biadala, A.; Konieczny, P. Goat´s milk derived bioactive components—A review. Mljekarstvo 2018, 68, 239–251. [Google Scholar] [CrossRef]
- Jensen, S.K.; Nielsen, K.N. Tocopherols, retinol, b-carotene and fatty acids in fat globule membrane and fat globule core in cows’ milk. J. Dairy Res. 1996, 63, 565–574. [Google Scholar] [CrossRef]
- Zahar, M.; Smith, D.E. Vitamin A distribution among fat globule core, fat globule membrane, and serum fraction in milk. J. Dairy Sci. 1995, 78, 498–505. [Google Scholar] [CrossRef]
- Conway, V.; Gauthier, S.; Pouilot, Y. Buttermilk: Much more than a source of milk phospholipids. Anim. Front. 2014, 4, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Fuller, K.L.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Jiménez-Flores, R.; Donovan, S.M. Milk fat globule membrane isolated from buttermilk or whey cream and their lipid components inhibit infectivity of rotavirus in vitro. J. Dairy Sci. 2013, 96, 3488–3497. [Google Scholar] [CrossRef] [Green Version]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.A.; Biesalski, H.K. Beta-carotene is an important vitamin A source for humans. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar]
- Gallier, S.; Gordon, K.C.; Jiménez-Flores, R.; Everett, D.W. Composition of bovine milk fat globules by confocal Raman microscopy. Int. Dairy J. 2011, 21, 402–412. [Google Scholar] [CrossRef]
- Patton, S.; Kelly, J.J.; Keenan, T.W. Carotene in bovine milk fat globules: Observations on origin and high content in tissue mitochondria. Lipids 1980, 15, 33–38. [Google Scholar] [CrossRef]
- Wang, X.; Quinn, P.J. Vitamin E and its function in membranes. Prog. Lipid Res. 1999, 38, 309–336. [Google Scholar] [CrossRef]
- Lindmark-Månsson, H.; Akesson, B. Antioxidative factors in milk. Br. J. Nutr. 2000, 84, S103–S110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aiqian, Y.; Singh, H.; Taylor, M.; Anema, S. Interactions of whey proteins with milk fat globule membrane proteins during heat treatment of whole milk. Le Lait 2004, 84, 269–283. [Google Scholar]
- Bulgari, O.; Caroli, A.M.; Chessa, S.; Rizzi, R.; Gigliotti, C. Variation of Vitamin D in Cow’s Milk and Interaction with β-Lactoglobulin. Molecules 2013, 18, 10122–10131. [Google Scholar] [CrossRef] [Green Version]
- Kanno, C.; Kanehara, N.; Shirafuji, K.; Tanji, R.; Imai, T. Binding form of vitamin B2 in bovine milk: Its concentration, distribution and binding linkage. J. Nutr. Sci. Vitam. 1991, 37, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Sieber, R.; Eberhard, P.; Fuchs, D.; Gallmann, P.U.; Strahm, W. Effect of microwave heating on vitamins A, E, B1, B2 and B6 in milk. J. Dairy Res. 1996, 63, 169–172. [Google Scholar] [CrossRef] [Green Version]
- Peterson, J.A.; Patton, S.; Hamosh, M. Glycoproteins of the human milk fat globule in the protection of the breast-fed infant against infections. Biol. Neonate 1998, 74, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gao, J.; Du, M.; Mao, X. Milk fat globule membrane supplementation modulates the gut microbiota and attenuates metabolic endotoxemia in high-fat diet-fed mice. J. Funct. Foods 2018, 47, 56–65. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on the essential composition of infant and follow-on formulae. EFSA J. 2014, 12, 3760. [Google Scholar] [CrossRef] [Green Version]
- Corkins, K.G.; Shurley, T. What’s in the Bottle? A Review of Infant Formulas. Nutr. Clin. Pr. 2016, 31, 723–729. [Google Scholar] [CrossRef]
- Zavaleta, N.; Kvistgaard, A.S.; Graverholt, G.; Respicio, G.; Guija, H.; Valencia, N.; Lönnerdal, B. Efficacy of an MFGM-enriched complementary food in diarrhea, anemia, and micronutrient status in infants. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 561–568. [Google Scholar] [CrossRef]
- Billeaud, C.; Puccio, G.; Saliba, E.; Guillois, B.; Vaysse, C.; Pecquet, S.; Steenhout, P. Safety and tolerance evaluation of milk fat globule membrane-enriched infant formulas: A randomized controlled multicenter non-inferiority trial in healthy term infants. Clin. Med. Insights Pediatr. 2014, 8, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Holzmueller, W.; Kulozik, U. Isolation of milk fat globule membrane (MFGM) material by coagulation and diafiltration of buttermilk. Int. Dairy J. 2016, 63, 88–91. [Google Scholar] [CrossRef]
- Spitsberg, V.L.; Ivanov, L.; Shritz, V. Recovery of milk fat globule membrane (MFGM) from buttermilk: Effect of Ca-binding salts. J. Dairy Res. 2019, 86, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Jash, A.; Ubeyitogullari, A.; Rizvi, S. Synthesis of multivitamin-loaded heat stable liposomes from milk fat globule membrane phospholipids by using a supercritical-CO2 based system. Green Chem. 2020, 22, 5345–5356. [Google Scholar] [CrossRef]

| Samples | Objective | Main Findings | Ref. |
|---|---|---|---|
| Human | |||
| Human colostrum | Update the human colostrum MFGM proteome | 107 proteins identified; 46% were typical MFGM proteins (e.g., lactadherin and BTN) | [33] |
| Human milk from 1, 2, 3, 6, and 12 months of lactation | Examine the variations of human MFGM proteins during lactation | Human MFGM proteins: cell communication and signal transduction, immune function, metabolism and energy production; Variations during the course of lactation | [34] |
| Human colostrum and mature milk | Identify the MFGM phosphoproteome | 323 phosphorylation sites and 203 phosphoproteins; 48 MFGM phosphoproteins differentially expressed between colostrum and mature milk | [35] |
| Bovine | |||
| Holstein cow milk in mid-lactation | Update the proteome | Proteins identified (120) responsible for membrane/protein trafficking (23%) and cell signaling (23%) | [36] |
| Holstein cow colostrum and 7-days milk | Quantify protein changes in bovine MFGM from colostrum and 7-days milk. | 138 proteins identified; 26 proteins upregulated and 19 proteins downregulated in 7-days MFGM compared with colostrum MFGM; Upregulated proteins: FABP and MUC1; Early developmental shift in milk fat transport | [32] |
| Holstein dairy cow milk | Characterize variations in MFGM proteome of multiparous and primiparous dairy cows at four timepoints postpartum (M1, M2, M3, M4) | 104 shared proteins identified in each timepoint; M1 proteome enriched in immune-associated proteins and regulatory proteins; 70% of proteins affected by milking; 44% of proteins affected by parity; 33% of proteins affected by milking and parity | [37] |
| Jersey cow mature milk | Characterize the effect of pasture profile on the bovine MFGM proteome | 365 proteins identified in the MFGM fraction; Diet can alter the bovine MFGM proteome | [38] |
| Other species | |||
| Sarda dairy sheep in mid-lactation | Characterize the ovine MFGM proteome | 140 total proteins identified; Major MFGM proteins are the most abundant in ovine MFGM proteome | [28] |
| Donkey colostrum and mature milk | Characterize MFGM proteins in at two lactation periods | 902 and 913 MFGM proteins in donkey colostrum and mature milk, respectively | [39] |
| Colostrum and mature Xinong Saanen goat milk | Investigate the variations between goat colostrum and mature MFGM proteome | 543 and 858 proteins in colostrum and mature milk; 394 common proteins; Colostrum enriched in protein processing in the ER; Mature milk enriched in oxidative phosphorylation functions | [40] |
| Comparative analyses | |||
| Human and cow mature milk | Determine the differences in host defense proteomes | Proteins identified: human milk 268, bovine milk 269; 147 common proteins; More antimicrobial proteins in bovine milk; More Igs in human milk | [27] |
| Caprine, bovine and human milk | Elucidate the proteome profile and biological activity of different species | Major proteins shared among species | [41] |
| Holstein and Jersey cows, yak, buffalo, goat, camel, horse, and human mature milk | Characterize the MFGM proteome from different species | 520 proteins identified among all species, of which the most shared among all species | [42] |
| Human, cow, goat and yak milk | Investigate the MFGM proteins in human milk and three typically consumed milk in China | Major MFGM proteins conserved across species; Human MFGM proteome enriched in immunological factors and proteins involved in triglyceride and fatty acid catabolic process | [29] |
| Holstein and Jersey cows, buffalo, yak, goat, camel, horse, and human mature milk | Identify the N-glycoproteins of the MFGM fractions from different species | Extended number of known glycosylation sites in the milk proteins from dairy animal species: N-glycosylated proteins mostly related to the “response to stimulus” | [43] |
| Human and bovine colostrum | Identify and analyze the composition and expression changes of colostrum MFGM proteins in humans and bovines | 411 proteins identified from humans and bovines; 9 upregulated and 17 downregulated in humans compared to bovine; Upregulated proteins in human colostrum mainly involved in immune functions; Many of the differentially expressed proteins involved in immune function | [44] |
| Human and caprine milk | Identify differences in MFGM proteome of human and caprine milk to evaluate nutritional benefits of caprine milk for infant’s growth | 128 and 42 proteins in human and caprine MFGM proteome, respectively; Higher content of bioactive proteins in human than caprine MFGM proteome; Caprine milk could be a hypoallergenic alternative to newborns affected by CMPA and without any chance of breastfeeding | [45] |
| Human and bovine colostrum and mature milk | Compare MFGM N-glycoproteomes among species and lactation stages | 362 common proteins among human colostrum and mature milk; 155 common proteins among bovine colostrum and mature milk; Higher variations among species than lactation stages | [46] |
| Holstein cow and Guanzhong goat mature milk | Compare whey and MFGM proteins across species | Major MFGM proteins shared across species; 21% goat MFGM proteins: metabolic processes; 49% cow MFGM proteins: disease-associated pathways | [47] |
| Molecular Function | Human Proteins | Cow Proteins | Common Proteins |
|---|---|---|---|
| Cell wall/adhesion | 21 | 17 | 8 |
| Coagulation | 3 | 7 | 3 |
| Cytoskeleton | 12 | 8 | 7 |
| Enzymes | 70 | 50 | 25 |
| Host defense | 44 | 51 | 33 |
| Protease inhibitors | 12 | 15 | 8 |
| Protein synthesis/chaperone | 11 | 9 | 4 |
| Signaling | 15 | 19 | 7 |
| Transport | 48 | 64 | 39 |
| Others | 32 | 29 | 13 |
| Total proteins | 268 | 269 | 147 |
| Minerals | Ca | P | Na | K | Mg | Fe | Zn | Cu | I |
|---|---|---|---|---|---|---|---|---|---|
| Contribution (%) | 55 | 25 | 7.3 | 12 | 11 | 3.5 | 14 | 5.8 | 9.1 |
| Vitamins | B1 | B2 | B5 | B12 | C | A | D | E | K |
| Contribution (%) | 7–10 | 28–39 | 19 | 42 | 2–4 | 11 | 5 | 2 | 1 |
| Minerals | Whole Milk | Milk Lipid Fraction | ||
|---|---|---|---|---|
| Human | Cow | Human | Cow | |
| Calcium (mg/100 g) | 22–41 | 107–133 | 3.5–6.6 (16%) | 0.2–0.4 (1%) |
| Iron (µg/100 g) | 40–50 | 30–70 | <20 (40%) | <1 (14%) |
| Copper (µg/100 g) | 30–50 | 12–17 | <8 (15%) | <0.3 (2%) |
| Zinc (µg/100 g) | 145–165 | 350–400 | <30 (18%) | <4 (1%) |
| Magnesium (mg/100 g) | 3–3.5 | 9–16 | 0.06–0.07 (2%) | - |
| Phosphorus (mg/100 g) | 12–17 | 90–102 | - | - |
| Vitamins | Human Milk | Cow’s Milk |
|---|---|---|
| Vitamin A + all-trans-β-carotene (mg/100 g) | 0.05–0.06 | 0.04 |
| Vitamin D (µg/100 g) | 0.04–0.07 | 0.05–0.06 |
| Vitamin E (mg/100 g) | 0.24–0.28 | 0.10–0.13 |
| Vitamin K (µg/100 g) | 0.3–0.5 | 1.1 |
| Source of Vitamin A | Cream with Larger MFGs | Cream with Smaller MFGs |
|---|---|---|
| Buttermilk | 98.13 a ± 2.20 | 103.85 a ± 2.18 |
| Butter oil | 90.40 b ± 1.84 | 87.83 b ± 0.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manoni, M.; Cattaneo, D.; Mazzoleni, S.; Giromini, C.; Baldi, A.; Pinotti, L. Milk Fat Globule Membrane Proteome and Micronutrients in the Milk Lipid Fraction: Insights into Milk Bioactive Compounds. Dairy 2021, 2, 202-217. https://doi.org/10.3390/dairy2020018
Manoni M, Cattaneo D, Mazzoleni S, Giromini C, Baldi A, Pinotti L. Milk Fat Globule Membrane Proteome and Micronutrients in the Milk Lipid Fraction: Insights into Milk Bioactive Compounds. Dairy. 2021; 2(2):202-217. https://doi.org/10.3390/dairy2020018
Chicago/Turabian StyleManoni, Michele, Donata Cattaneo, Sharon Mazzoleni, Carlotta Giromini, Antonella Baldi, and Luciano Pinotti. 2021. "Milk Fat Globule Membrane Proteome and Micronutrients in the Milk Lipid Fraction: Insights into Milk Bioactive Compounds" Dairy 2, no. 2: 202-217. https://doi.org/10.3390/dairy2020018
APA StyleManoni, M., Cattaneo, D., Mazzoleni, S., Giromini, C., Baldi, A., & Pinotti, L. (2021). Milk Fat Globule Membrane Proteome and Micronutrients in the Milk Lipid Fraction: Insights into Milk Bioactive Compounds. Dairy, 2(2), 202-217. https://doi.org/10.3390/dairy2020018








