Abstract
Subacute ruminal acidosis (SARA) represents one of the most important nutritional disorders in high-producing dairy farms. The determination of ruminal pH is a key factor for the diagnosis of SARA. However, measuring ruminal pH in the field is not practicable. Therefore, indicators that reflect the ruminal pH are in demand. The main objective of this study was to examine the relationship between the milk fat-to-protein ratio (FPR) and ruminal pH parameters (daily mean pH, daily time with pH < 5.8, and pH range) on a meta-analytical level including 47 studies with 189 treatment means. Besides the FPR, it was examined how a stepwise extension of further indicators (milk yield, rumination time, and dietary starch and structure effectiveness) can improve the prediction of ruminal pH parameters. Significant associations between milk FPR and ruminal pH parameters were found. The inclusion of further on-farm indicators improved the prediction of daily mean ruminal pH up to = 0.46 and time with pH < 5.8 up to . Still, a considerable part of variability was explained by the random factor study. Additional information (dietary PUFA content) may improve the models in further investigations.
1. Introduction
Subacute ruminal acidosis (SARA) represents a relevant problem in dairy cattle husbandry and nutrition. Reports on its frequency described its prevalence to be between 8% and 29% [1] and recent field studies classified 42% to 44% of tested dairy farms in Poland and Germany as SARA positive [2,3]. While it must be considered a complex syndrome [4,5], definitions of SARA largely rely on the pH of rumen fluid [6,7,8] using different specifications for pH thresholds. In a meta-analysis, Zebeli et al. [6] described that both a daily mean ruminal pH lower than 6.16 and a period of more than 5.24 h per day with a ruminal pH lower than 5.8 should be avoided to exclude the risk of SARA. These limits have been adopted as official feeding recommendations in Germany [9]. Besides such absolute pH thresholds, Villot et al. [10] suggested that relative pH indicators like pH range may be more reliable for SARA detection. In fact, besides a lower absolute pH, increased diurnal ranges from 0.9 to 1.1 pH units where observed when the offered daily amount of grain was raised from 50% to 70% [11].
All these approaches to SARA detection are based on a continuous measurement of ruminal pH, which severely limits their regular application in practical farming conditions. Although indwelling sensors represent a tool for such measurements with non-fistulated animals [12], their use in farms is very limited to date due to costs and life expectancy. In consequence, an indirect approach allowing reliable conclusions on ruminal pH is still strongly desirable.
Milk fat content is the first candidate for an indirect SARA indicator [6,13,14], representing an easily accessible data source in addition. Obviously, milk fat must not be considered as being constant during a lactation, irrespective of ruminal pH, e.g., body fat mobilization due to an energy deficit during early stages leads to significant increases in milk fat. Since protein contents are also increased at the onset of lactation and change to some degree in parallel with fat, to relate milk fat to protein (FPR) may have the advantage of correcting for such general changes, resulting in a more robust indicator. Already, Grieve et al. [15] concluded that FPR is a more reliable indicator than milk fat or milk protein alone.
The fat-protein ratio is generally accepted as an indicator for energy deficit [16] or subclinical ketosis [17] but is far less frequently used for low ruminal pH. However, individual trials provided evidence for some association [10,18] and a threshold has also been defined for SARA indication (FPR < 1.0; [19,20]). In a recent explorative meta-analysis, Mensching et al. [21] also identified milk FPR as an indicator for rumen pH parameters but without including it in the final prediction model. On the other hand, limitations of FPR in prediction of SARA have repeatedly been pointed to [22,23].
The objective of this study was to test in a meta-analysis how well ruminal pH may be estimated based on indicators available under practical farm conditions. A focus was on the relationship of ruminal pH parameters (daily mean pH, daily time with pH < 5.8, and daily pH range) and milk FPR. Further, it was tried to what extent the predictive power could be improved by the inclusion of additional indicators (milk yield, rumination time, dietary starch content, and dietary physically effective fiber).
2. Materials and Methods
2.1. Literature Research
Based on the study of Zebeli et al. [6] and using ScienceDirect, PubMed, and Google Scholar, a literature research was conducted in order to create the database for this study. The following keywords were used in different combinations: ruminal pH, fat-to-protein ratio, physically effective fiber, particle size, rumen fermentation, dairy, acidosis, lactation performance, and roughage.
A total of 54 studies met the following criteria: full manuscripts from peer-reviewed journals, research on lactating dairy cows, and information on pH parameters and milk parameters. Because measurement technology (rumenocentesis, stomach tube, continuous measurement in fistulated cows) and sample location (rumen or reticulum) as well as sampling time (continuous or several individual measurement points) have a large impact on pH parameters [4], it was continued only with studies that measured in the rumen, measuring continuously with Dascor data loggers or with a minimum of five measurement points in 24 h. The mean DIM (days in milk) of the cows could have only been considered per study but not per treatment. Furthermore, the trials in Latin square designs covered a large period. Therefore, DIM was not considered in the models. Number of lactations was not often reported and could not be taken into account, too. In the end, 50 studies representing 189 treatments fitted the above criteria and were used for further analysis. A summary of the studies used for this meta-analytical approach is listed in the Supplementary Materials.
2.2. Statistical Analysis
All statistical analysis was performed within the statistical software environment R [24]. As a preliminary examination, known relations between cow parameters, diet composition, and rumen parameters were checked in linear mixed models with study as a random effect. Then, in a first step, the pH parameters (daily mean pH, daily time with pH < 5.8, daily pH range) were predicted with FPR as a single indicator using linear mixed models. Afterwards, the complexity of the models was increased by including milk yield and rumination time as additional indicators as well as the diet characteristics starch and peNDF8mm (physically effective fiber retained on a >8-mm sieve). In the meta-regression analysis, linear mixed models were used, which can be described as:
where is the j-th observation hierarchically nested in the i-th study of one of the pH parameters, is the intercept, and correspond to the regression coefficients of the explanatory variables . It was assumed that the random effects of the study and the residuals are independent and identically distributed to a normal distribution with and . Table 1 shows which explanatory variables were used in the 5 models, respectively.

Table 1.
Explanatory variables (x1 to x5) used in the models.
Pseudo-R-squared for general mixed-effect models were estimated in two types [25]. The marginal R2 () represents the variance explained by the fixed effects, and is defined as:
The conditional R-GLMM2 () represents the variance explained by the entire model, including fixed (f) and random effects (a), and is calculated according to the equation:
where is the variance of the fixed effect components, is the variance of the random effects, and is the “observation-level” variance [26].
3. Results
Table 2 summarizes the descriptive statistics of the data. Not all studies reported the total set of variables, which is reflected in the varying number of observations. It was our intent to predict ruminal pH values and therefore SARA risk parameters (daily mean pH, daily time with pH < 5.8, and daily pH range) with indicators that are quantifiable on-farm (milk yield and composition, dietary composition, and chewing behavior).

Table 2.
Descriptive statistics of the database of the analysis.
3.1. Preliminary Examination
Before model development, some expected interrelations between diet parameters, rumen conditions, and milk composition were checked for plausibility. They were found to be present in the expected direction generally, as dietary starch content influenced daily mean ruminal pH negatively (p < 0.001), while time with pH < 5.8 (p < 0.001) and pH range (p = 0.01) were influenced positively. Contrarily, the amount of forage and physically effective fiber (peNDF8mm) had a positive influence on daily mean pH (p < 0.001), and a negative influence on time with pH < 5.8 (p < 0.001) as well as on pH range (p < 0.05). Higher dry matter intake (DMI) was associated with a lower daily mean pH. The acetate-to-propionate ratio (A:P) in the rumen was lower in cows with decreased mean pH and increased time with pH < 5.8 (p < 0.001). A higher concentration of ruminal acetate and lower concentration of ruminal propionate led to increased milk fat (p < 0.001) and milk FPR (p < 0.001). Milk yield was increased when daily mean pH decreased (p < 0.01). Rumination time was positively correlated to peNDF8mm (p < 0.001) and negatively to starch in the diet (p < 0.01). All observed pH values were correlated to rumination time, as cows with decreased mean pH and increased time with pH < 5.8 showed lower rumination.
3.2. Prediction of Ruminal pH Parameters
Daily mean ruminal pH (Table 3) was positively correlated to FPR while daily time with pH < 5.8 (Table 4) and daily pH range (Table 5) were negatively correlated to milk FPR (model 1). In total, the variance explained by the FPR () was 0.30 for daily mean pH, 0.32 for time with pH < 5.8, and 0.17 for daily pH range. The variance explained by FPR plus study as a random factor () was 0.79, 0.85, and 0.86, respectively. When looking at milk contents individually, SARA-risk cows (lower daily mean pH, higher time with pH < 5.8, and increased pH range) showed a decrease in milk fat (p < 0.001) while at the same time milk protein increased (p < 0.05). Model 2 included the milk yield in addition to FPR (Table 3, Table 4 and Table 5). Only for the daily mean pH was milk yield significant and improved the fit statistics slightly. In model 3, we included rumination time besides FPR and milk yield. Because less studies quantified rumination time, the number of observations changed. Although all pH values were correlated to the rumination time, only the model for daily mean pH was improved (: 0.42). In the models for daily time with ruminal pH < 5.8 and pH range, rumination time was not significant (Table 3, Table 4 and Table 5). Because of the known influence of dietary starch content on the ruminal pH, this variable was included in model 4. The number of observations changed for the model of daily mean pH. The variance explained by milk FPR, milk yield, rumination time, and dietary starch () was 0.46 for daily mean pH, 0.32 for daily time with pH < 5.8, and 0.20 for the pH range (Table 3, Table 4 and Table 5). Because not all farmers have the possibility to record the daily rumination time, rumination time was exchanged with the amount of physically effective fiber in the diet (peNDF8mm) in model 5. Again, the number of observations decreased. For daily mean pH, fit statistics were like in the previous models. An improvement could be seen for the daily time with pH < 5.8 (= 0.58). The prediction of the pH range was lower than in the former models ( = 0.07) (Table 3, Table 4 and Table 5).

Table 3.
Mixed-effects regression modelling of daily mean pH with variables based on milk, rumination, and diet.

Table 4.
Mixed-effects regression modelling of daily time with pH < 5.8 with variables based on milk, rumination, and diet.

Table 5.
Mixed-effects regression modelling of daily pH range with variables based on milk, rumination, and diet.
Finally, the models established above were used to cross-check relations between the pH parameter and FPR values regarded as critical (= threshold values as communicated in the literature). When using an FPR threshold of 1.0 for SARA indication, daily mean pH was 5.96 (Figure 1a), time with pH < 5.8 was 9.02 h (Figure 1b), and pH range was 1.34. Using the pH thresholds of daily ruminal mean pH < 6.16 and time with pH < 5.8 higher than 5.24 h to identify SARA-risk cows [6], the resulting FPR values were 1.22 (daily mean pH) and 1.18 (time with pH < 5.8).
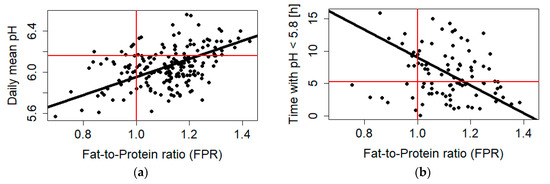
Figure 1.
Linear mixed model of daily mean ruminal pH (a) and time with pH < 5.8 (b) with milk fat-to-protein ratio (FPR) and literature thresholds for SARA (subacute ruminal acidosis) indication (in red: daily mean pH < 6.16; FPR < 1.0).
4. Discussion
In the endeavor to assess the SARA situation on a farm, FPR has been considered as a promising indicator [21,22,27]. First, it is based on the known decrease of its numerator (milk fat) as a consequence of low ruminal pH. Since, in the course of a lactation, the denominator (protein) is changing roughly in parallel with fat, e.g., due to milk yield-related dilution effects, use of their ratio should optimize its validity for the entire course of a lactation and therefore increase its robustness as an indicator. In fact, FPR is often considered in dairy practice and some studies support the view of FPR as a good SARA indicator (as an example, 8 Holstein Friesian cows with a milk yield of 25 kg under trial conditions [18]). However, others did not find a sufficiently close correlation of FPR and ruminal pH (as an example, 24 transition Holstein Friesian cows on a practical farm [23]; 6 Danish Holstein cows with 250 DIM in a trial setting [5]) and the shortcomings of FPR as an SARA indicator are discussed.
Despite the urgent demand for a sensible and non-invasive SARA indication on farm, hitherto, the meta-analytical approach has not been used to improve the indication of SARA via FPR. Besides the obvious advantage to be able to capitalize from the existing body of literature (in our case, 47 studies/189 individual treatments in the best and 11 studies/40 individual treatments in the worst case), it should be regarded as an opportunity that the diversity of conditions present on farms will be represented much more realistically in a summary of several experimental studies than in a single experiment, irrespective of its comprehensiveness. In fact, the random variable study explained considerable variability. Obviously, some shortcomings typical for meta-analyses were also present; besides the fact that the means of several individuals form the database, it should be stated once that when increasing the number of variables included in the models, the number and selection of studies necessarily changed at the same time due to missing values for variables, which should be considered in any interpretation.
It should be considered a first important result of this study that FPR reflected pH parameters to some extent. Changes in FPR were contingent on a reduction of milk fat but also on an increased milk protein content. As a result of too much highly fermentable carbohydrates and insufficient structure effectiveness in the diet, a shift of volatile fatty acids (VFAs) with increased propionate and decreased acetate in the rumen has long been recognized as the reason for milk fat depression. Sutton [28] explained up to 80% variation in milk fat by variations of molar proportions of VFAs in the rumen. In addition, a decrease of milk fat synthesis due to particular products of ruminal fat biohydrogenation is considered a comparably strong explanation today [29]. While the physiological concept behind the association of low ruminal pH and low milk fat is well established, this is less clear concerning milk protein. Any explanatory approach to this remains more speculative than for milk fat; while the decrease in protozoa often seen in SARA diets will increase the efficiency of bacterial growth via reduced predation, a low pH is associated with less efficient bacterial growth in general, which will have the opposite effect [30]. While the physiological background may not be completely clear, the presence of a negative association between FPR and ruminal pH was confirmed by Plaizier et al. [4], who also described an increased milk protein content in experimentally induced SARA, or by the meta-analysis of Mensching et al. [21].
While an association of ruminal pH and FPR was clearly present and confirmed their linkage, its predictive power was restricted to a maximal of 0.32 in the model 1 series. The considerable scatter of data becomes clearly visible in Figure 1, which also indicates some discrepancy between the SARA thresholds commonly used for FPR and pH parameters. Milk fat depression, as the major background of using FPR as a proxy for SARA, is a multifactorial metabolic syndrome with several conditions involved. Consequently, a strategy was followed to increase the predictive power by including additional variables available from the data set and linked to the fiber adequacy of the diet (peNDF; starch) and rumination. Further, these variables have in common that all can be quantified in a practical farm setting. In fact, the predictive power of individual models was increased to a level of coefficient of determination of ~0.46 for daily mean pH or even 0.58 for time with pH < 5.8.
Besides factors involved in this study like overall diet composition, further factors will have an influence. Among more detailed nutritional factors is, e.g., starch degradability, which has a direct effect on rumen pH and milk fat as a consequence [31]. As mentioned above, the presence of unsaturated fatty acids in the diet is also considered a nutritional factor of relevance. As a difference to many other nutritional factors, it will not take its influence via ruminal pH but via its negative association with milk fat [32]. However, dietary PUFA content is rarely analyzed in ruminant diets, which is why this factor could not be considered in this meta-analysis. An attempt was made to estimate PUFA contents of the diets of this study according to Feedipedia [33] and NRC database [34] using table values of ingredients of the dietary treatments. While this approach did slightly improve the prediction in a test analysis, the relation between PUFAs and milk fat was not in the expected direction: Increasing PUFA contents had an increasing rather than a decreasing effect on milk fat (p < 0.05). It can be speculated that especially the amounts of PUFAs in silages vary considerably. Furthermore, if forage is considered the major PUFA source in this data set, PUFAs and the structure effectiveness of diet will be correlated to some extent.
In addition, non-nutritional factors (like genetics, stage of lactation, and parity [35]) have been described to be of influence. While nutritional factors will often influence FPR via ruminal pH, many non-nutritional factors are not linked to FPR in this way. Obviously, all such factors will finally weaken the prediction of ruminal pH via milk fat/FPR. Therefore, it is felt that any attempt to further increase the predictive power of models should focus on an elucidation of these factors. As an example, breeding values for the milk composition might improve the predictiveness. It can be speculated that such factors may form the background of a considerable part of the variability covered by the random factor study.
Given the considerable difference between and , to cover such factors in future approaches shows some promise. Among the first additional factors to be considered are the dietary amount of PUFAs [28], cows’ lactational stage (days in milk), and parity (age) [16].
While the inclusion of further variables resulted in some improvement of the predictions for the pH parameters officially recommended for indication of SARA in Germany (daily mean pH and time with pH < 5.8), it was somehow surprising that the prediction of pH range could not be improved by diet and chewing parameters. Surprisingly, the inclusion of further variables did not result in a higher coefficient of determination for this pH parameter. In the study of Nocek et al. [11], higher amounts of grain in the diet led to a greater decrease in ruminal pH post feeding. They found an adaption with time that led to less variation. In our meta-analysis, we found no relation between pH range and DMI, but the amount of forage in the diet influenced pH range negatively. Mean ruminal pH decreased with increased DMI. It can be stated that our study gives no support of the idea of range as a pH parameter superior to the 24-h mean or time with pH < 5.8 in SARA detection.
5. Conclusions
In conclusion, it can be said that significant associations between milk FPR and ruminal pH parameters were found. Using FPR as a single indicator for daily mean ruminal pH, time with pH < 5.8, and pH range resulted in coefficients of determination of 0.30, 0.32, and 0.17, respectively. The inclusion of further on-farm indicators improved the prediction of daily mean ruminal pH (= 0.46) and time with pH < 5.8 (= 0.58). Still, a considerable part of variability was explained by the random factor. Additional information (dietary PUFA content, cows’ age, or lactation stage) may improve future models. The study gives no support to the idea of relative pH parameters like daily pH range being superior or more robust than the absolute pH parameters used in general.
Supplementary Materials
The following are available online at https://www.mdpi.com/2624-862X/1/3/17/s1.
Author Contributions
Conceptualization, M.Z. and J.H.; Data curation, M.Z.; Formal analysis, M.Z., A.M. and A.R.S.; Funding acquisition, J.H.; Investigation, M.Z.; Methodology, A.M. and A.R.S.; Project administration, J.H.; Supervision, J.H.; Validation, M.Z., A.M. and A.R.S.; Visualization, M.Z., A.M. and A.R.S.; Writing – original draft, M.Z.; Writing – review & editing, A.M., A.R.S. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was done within the project “Evaluation of Animal Welfare in Dairy Farming – Indicators for the Metabolism and Feeding” (IndiKuh, funding code: 2817905815). The project was supported by funds of the Federal Ministry of Food and Agriculture (BMEL) based on a decision of the parliament of the Federal Republic of Germany via the Federal Office for Agriculture and Food (BLE) in the course of the program for promotion of innovations.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Oetzel, G.R. Subacute ruminal acidosis in dairy herds: Physiology, pathophysiology, milk fat responses, and nutritional management. Dairy Herd Problem Investigation Strategies, Proceedings of the 40th Annual Conference, Vancouver, Canada, 17 September 2007. Available online: https://www.vetmed.wisc.edu/fapm/wp-content/uploads/2020/01/sara1aabp.pdf (accessed on 30 September 2020).
- Stefańska, B.; Nowak, W.; Komisarek, J.; Taciak, M.; Barszcz, M.; Skomiał, J. Prevalence and consequence of subacute ruminal acidosis in Polish dairy herds. J. Anim. Physiol. Anim. Nutr. 2017, 101, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Kleen, J.L.; Upgang, L.; Rehage, J. Prevalence and consequences of subacute ruminal acidosis in German dairy herds. Acta Vet. Scand. 2013, 55, 48. [Google Scholar] [CrossRef] [PubMed]
- Plaizier, J.C.; Krause, D.O.; Gozho, G.N.; McBride, B.W. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J. 2008, 176, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Danscher, A.M.; Li, S.; Andersen, P.H.; Khafipour, E.; Kristensen, N.B.; Plaizier, J.C. Indicators of induced subacute ruminal acidosis (SARA) in Danish Holstein cows. Acta Vet. Scand. 2015, 57, 1–14. [Google Scholar] [CrossRef]
- Zebeli, Q.; Dijkstra, J.; Tafaj, M.; Steingass, H.; Ametaj, B.N.; Drochner, W. Modeling the adequacy of dietary fiber in dairy cows based on the responses of ruminal pH and milk fat production to composition of the diet. J. Dairy Sci. 2008, 91, 2046–2066. [Google Scholar] [CrossRef]
- Kleen, J.L.; Hooijer, G.A.; Rehage, J.; Noordhuizen, J.P.T.M. Subacute ruminal acidosis (SARA): A review. J. Vet. Med. Ser. A 2003, 50, 406–414. [Google Scholar] [CrossRef]
- Garrett, E.; Pereira, M.N.; Nordlund, K.; Armentano, L.; Goodger, W.; Oetzel, G. Diagnostic methods for the detection of subacute ruminal acidosis in dairy cows. J. Dairy Sci. 1999, 82, 1170–1178. [Google Scholar] [CrossRef]
- GfE (Gesellschaft für Ernährungsphysiologie). Evaluation of structural effectiveness of mixed rations for dairy cows—Status and perspectives (Communications of the committee for requirement standards of the society of nutrition physiology). Proc. Soc. Nutr. Physiol. 2014, 23, 165–179. [Google Scholar]
- Villot, C.; Meunier, B.; Bodin, J.; Martin, C.; Silberberg, M. Relative reticulo-rumen pH indicators for subacute ruminal acidosis detection in dairy cows. Animal 2018, 12, 481–490. [Google Scholar] [CrossRef]
- Nocek, J.; Allman, J.; Kautz, W. Evaluation of an indwelling ruminal probe methodology and effect of grain level on diurnal pH variation in dairy cattle. J. Dairy Sci. 2002, 85, 422–428. [Google Scholar] [CrossRef]
- Mottram, T.; Lowe, J.; McGowan, M.; Phillips, N. Technical note: A wireless telemetric method of monitoring clinical acidosis in dairy cows. Comput. Electron. Agric. 2008, 64, 45–48. [Google Scholar] [CrossRef]
- De Brabander, D.L.; De Boever, J.L.; De Smet, A.M.; Vanacker, J.M.; Boucqué, C.V. Evaluation of the physical structure of fodder beets, potatoes, pressed beet pulp, brewers grains, and corn cob silage. J. Dairy Sci. 1999, 82, 110–121. [Google Scholar] [CrossRef]
- Glatz-Hoppe, J.; Losand, B.; Kampf, D.; Onken, F.; Spiekers, H. Nutzung von Milchkontrolldaten zur Fütterungs- und Gesundheitskontrolle bei Milchkühen. DLG Merkbl. 2020, 451, 15. [Google Scholar]
- Grieve, D.G.; Korver, S.; Rijpkema, Y.S.; Hof, G. Relationship between milk composition and some nutritional parameters in early lactation. Livest. Prod. Sci. 1986, 14, 239–254. [Google Scholar] [CrossRef]
- Buttchereit, N.; Stamer, E.; Junge, W.; Thaller, G. Evaluation of five lactation curve models fitted for fat:protein ratio of milk and daily energy balance. J. Dairy Sci. 2010, 93, 1702–1712. [Google Scholar] [CrossRef]
- Jenkins, N.T.; Peña, G.; Risco, C.; Barbosa, C.C.; Vieira-Neto, A.; Galvão, K.N. Utility of inline milk fat and protein ratio to diagnose subclinical ketosis and to assign propylene glycol treatment in lactating dairy cows. Can. Vet. J. Rev. Vet. Can. 2015, 56, 850–854. [Google Scholar]
- Li, S.; Gozho, G.N.; Gakhar, N.; Khafipour, E.; Krause, D.O.; Plaizier, J.C. Evaluation of diagnostic measures for subacute ruminal acidosis in dairy cows. Can. J. Anim. Sci. 2012, 92, 353–364. [Google Scholar] [CrossRef]
- Enemark, J.; Jorgensen, R.; Enemark, P. Rumen acidosis with special emphasis on diagnostic aspects of subclinical rumen acidosis: A review. Vet. Zootech. 2002, 20, 16–29. [Google Scholar]
- KTBL (Kuratorium für Technik und Bauwesen in der Landwirtschaft). Tierschutzindikatoren: Leitfaden für die Praxis—Rind; KTBL: Darmstadt, Germany, 2016; 60p. [Google Scholar]
- Mensching, A.; Hummel, J.; Sharifi, A.R. Statistical modeling of ruminal pH parameters from dairy cows based on a meta-analysis. J. Dairy Sci. 2020, 103, 750–767. [Google Scholar] [CrossRef]
- Humer, E.; Petri, R.M.; Aschenbach, J.R.; Bradford, B.J.; Penner, G.B.; Tafaj, M.; Südekum, K.-H.; Zebeli, Q. Practical feeding management recommendations to mitigate the risk of subacute ruminal acidosis in dairy cattle. J. Dairy Sci. 2017, 101, 872–888. [Google Scholar] [CrossRef]
- Stein, S.K. Determination of subclinical metabolic disorders in transition dairy cows. Ph.D. Dissertation, Degree at the Faculty of Organic Agricultural Sciences, University of Kassel, Kassel, Germany, 2016. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Nakagawa, S.; Johnson, P.C.D.; Schielzeth, H. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 2017, 14, 20170213. [Google Scholar] [CrossRef] [PubMed]
- Bartoń, K. MuMIn: Multi-Model Inference. R Package Version 1.43.6. Available online: https://cran.r-project.org/package=mumin (accessed on 30 September 2020).
- Enemark, J.M.D. The monitoring, prevention and treatment of sub-acute ruminal acidosis (SARA): A review. Vet. J. 2008, 176, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J.D. Altering milk composition by feeding. J. Dairy Sci. 1989, 72, 2801–2814. [Google Scholar] [CrossRef]
- Harvatine, K.J.; Boisclair, Y.R.; Bauman, D.E. Recent advances in the regulation of milk fat synthesis. Animal 2009, 3, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Strobel, H.J.; Russell, J.B. Effect of pH and energy spilling on bacterial protein synthesis by carbohydrate-limited cultures of mixed rumen bacteria. J. Dairy Sci. 1986, 69, 2941–2947. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Overton, T.R.; Mechor, G.D.; Bauman, D.E.; Jenkins, T.C.; Nydam, D.V. Field study to investigate the associations between herd-level risk factors for milk fat depression and bulk tank milk fat percent in dairy herds feeding monensin. J. Dairy Sci. 2018, 101, 3118–3125. [Google Scholar] [CrossRef]
- Bauman, D.E.; Griinari, J.M. Nutritional regulation of milk fat synthesis. Annu. Rev. Nutr. 2003, 23, 203–227. [Google Scholar] [CrossRef]
- Feedipedia—Animal Feed Resources Information System. Available online: https://www.feedipedia.org/ (accessed on 12 November 2018).
- National Research Council. Nutrient Requirements of Swine: 11th Revised Edition; The National Academies Press: Washington, DC, USA, 2012; p. 400. [Google Scholar]
- Le, K.; Gj, L. Milk fat depression: Etiology, theories, and soluble carbohydrate interactions. J. Anim. Res. Nutr. 2018, 3. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).