Whey Proteins and Its Derivatives: Bioactivity, Functionality, and Current Applications
Abstract
1. Introduction
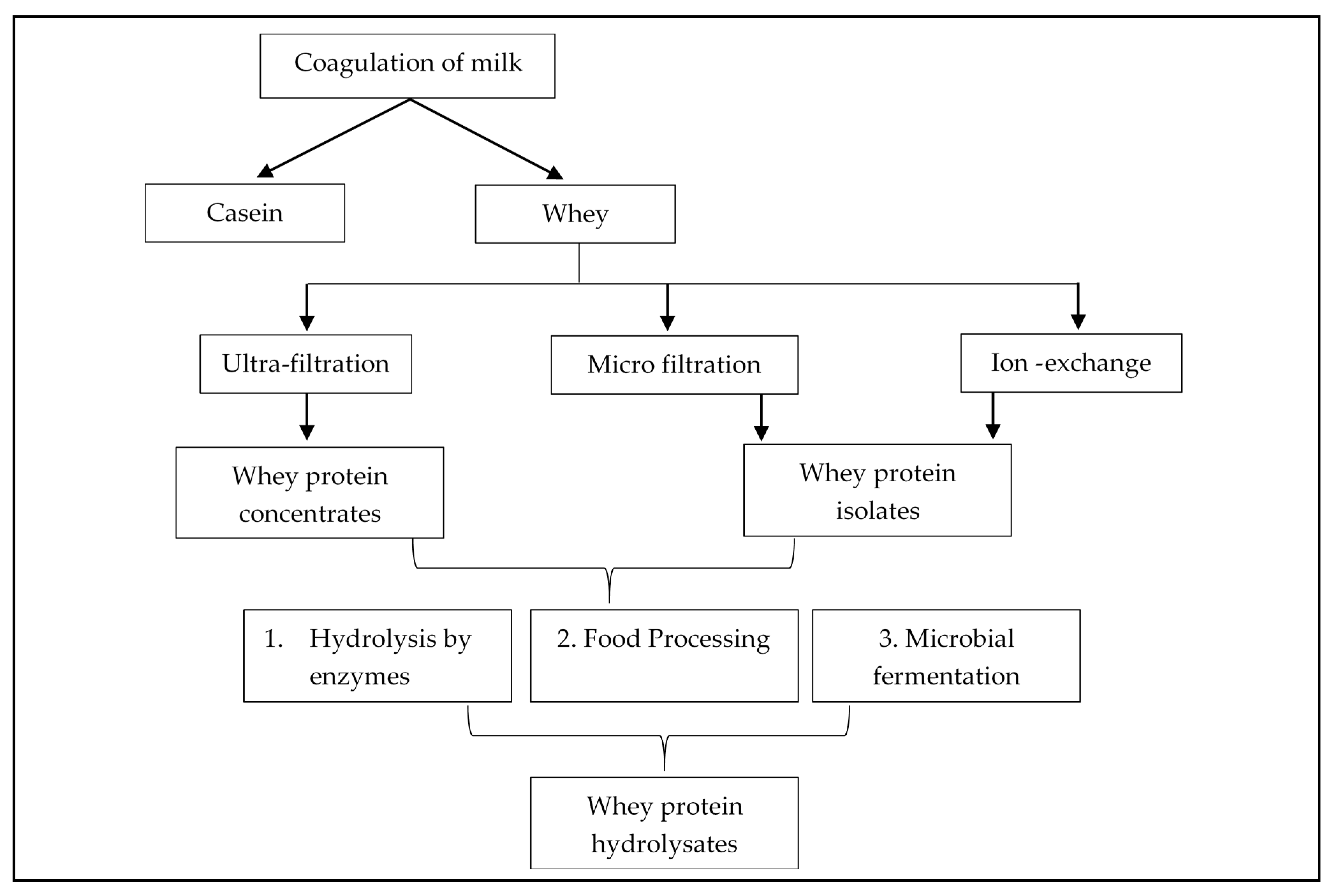
2. Whey Protein Derivatives: Concentrates, Isolates, and Hydrolysates
3. Biological Properties of Whey Proteins Associated with Bioactive Peptides
3.1. Whey Protein-Associated Bioactive Peptides
3.2. Manufacture of Bioactive Peptides from Whey Proteins
3.2.1. Enzymatic Hydrolysis of Whey Proteins
3.2.2. Microbial Fermentation and Food Processing of Whey Proteins
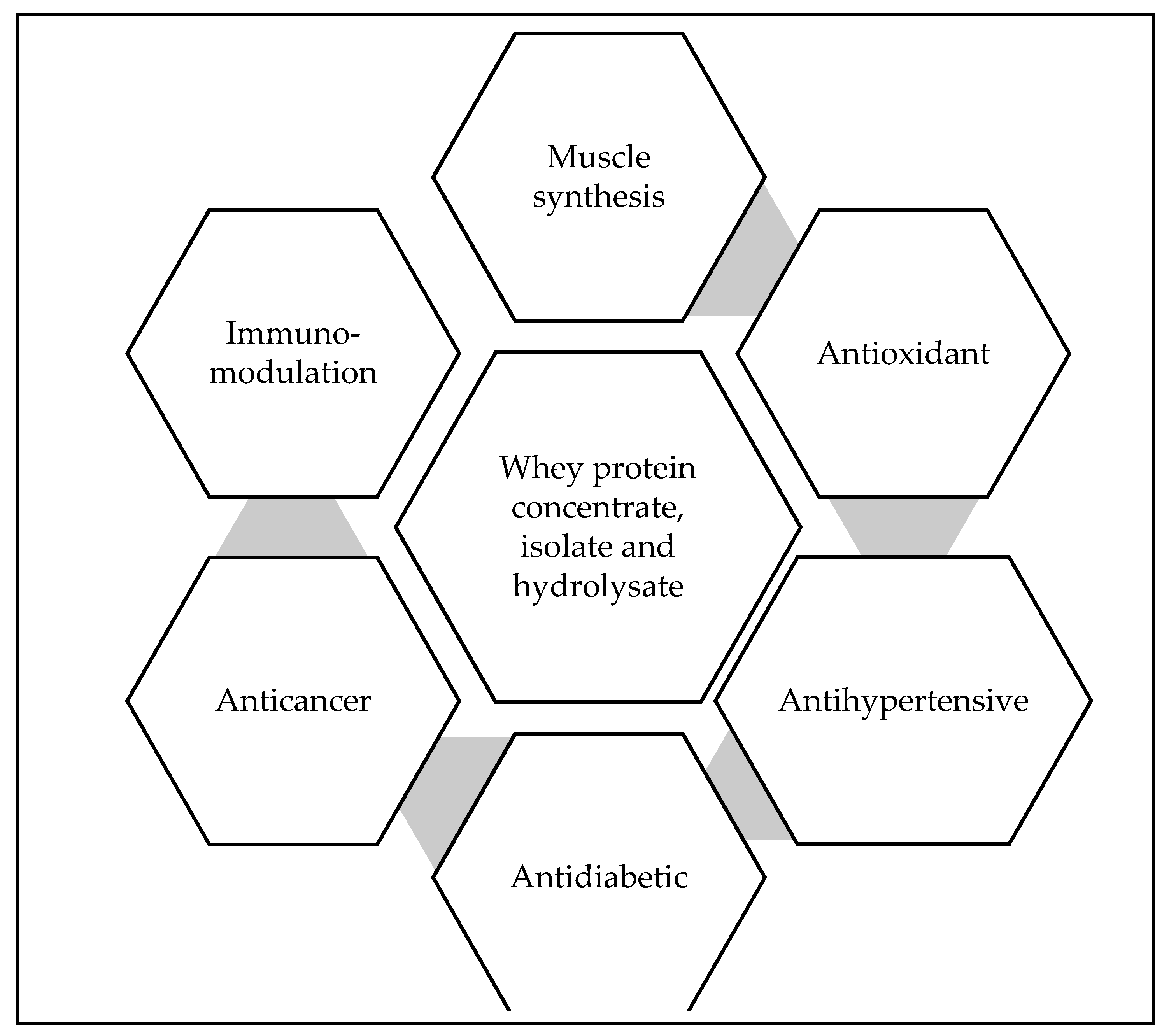
3.3. Bioactive Properties Associated with the Bioactive Peptides Isolated from Whey Proteins and Derivatives
3.3.1. Antioxidant Activity of the Bioactive Peptides
3.3.2. Antihypertensive Activity of the Bioactive Peptides
3.3.3. Opioid Activity of the Bioactive Peptides
3.3.4. Antidiabetic Property of the Bioactive Peptides
3.3.5. Anticancer Activity of the Bioactive Peptides
3.3.6. Immunomodulatory Activity of the Bioactive Peptides
3.3.7. Muscle Protein Synthesis by Bioactive Peptides
3.4. Identification of Bioactive Peptides Isolated from Whey Proteins and Derivatives
4. Functional Properties of Whey Proteins
4.1. Thermal Denaturation of Whey Proteins
4.2. Hydration and Solubility of Whey Proteins
4.3. Gelation Ability of Whey Proteins
4.4. Emulsification Property of Whey Proteins
4.5. Improvement in the Functionality through Conjugation
5. Current Applications of Whey Proteins and Its Derivatives
5.1. Role of Whey Proteins and Derivatives as Food Ingredients
5.2. Benefits of Combination of Whey Proteins and Derivatives with Other Supplements
5.3. Role of Whey Proteins and Derivatives as Encapsulating Agents and Coating Materials
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yalcin, A.S. Emerging therapeutic potential of whey proteins and peptides. Curr. Pharm. Des. 2006, 12, 1637–1643. [Google Scholar] [CrossRef]
- Mølgaard, C.; Larnkjær, A.; Arnberg, K.; Michaelsen, K.F. Milk and growth in children: Effects of whey and casein. In Milk and Milk Products in Human Nutrition; Karger Publishers: Basel, Switzerland, 2011; Volume 67, pp. 67–78. [Google Scholar]
- Madureira, A.R.; Pereira, C.I.; Gomes, A.M.; Pintado, M.E.; Malcata, F.X. Bovine whey proteins–Overview on their main biological properties. Food Res. Int. 2007, 40, 1197–1211. [Google Scholar] [CrossRef]
- Séverin, S.; Wenshui, X. Milk biologically active components as nutraceuticals. Crit. Rev. Food Sci. Nutr. 2005, 45, 645–656. [Google Scholar] [CrossRef]
- Chou, C.J.; Affolter, M.; Kussmann, M. A Nutrigenomics View of Protein Intake: Macronutrient, Bioactive Peptides, and Protein Turnover. In Progress in Molecular Biology and Translational Science; Elsevier BV: Amsterdam, The Netherlands, 2012; Volume 108, pp. 51–74. [Google Scholar]
- Bertenshaw, E.J.; Lluch, A.; Yeomans, M.R. Satiating effects of protein but not carbohydrate consumed in a between-meal beverage context. Physiol. Behav. 2008, 93, 427–436. [Google Scholar] [CrossRef]
- Gomes, S.P.; Nyengaard, J.R.; Misawa, R.; Girotti, P.A.; Castelucci, P.; Blazquez, F.H.J.; Ribeiro, A.A.C. Atrophy and neuron loss: Effects of a protein-deficient diet on sympathetic neurons. J. Neurosci. Res. 2009, 87, 3568–3575. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Jung, Y.C. Wetting, adhesion and friction of superhydrophobic and hydrophilic leaves and fabricated micro/nanopatterned surfaces. J. Phys. Condens. Matter 2008, 20, 225010. [Google Scholar] [CrossRef]
- Katayama, M.; Wilson, L.A. Utilization of okara, a byproduct from soymilk production, through the development of soy-based snack food. J. Food Sci. 2008, 73, S152–S157. [Google Scholar] [CrossRef]
- Pavlovich-Abril, A.; Rouzaud-Sández, O.; Carvajal-Millán, E.; Navarro, R.E.; Robles-Sánchez, R.M.; Barrón-Hoyos, J.M. Molecular characterization of water extractable arabinoxylans isolated from wheat fine bran and their effect on dough viscosity. LWT 2016, 74, 484–492. [Google Scholar] [CrossRef]
- Sousa, G.T.; Lira, F.S.; Rosa, J.C.; de Oliveira, E.P.; Oyama, L.M.; Santos, R.V.; Pimentel, G.D. Dietary whey protein lessens several risk factors for metabolic diseases: A review. Lipids Health Dis. 2012, 11, 67. [Google Scholar] [CrossRef]
- Codex Alimentarius. Milk and Milk Products, Codex Stan 243–2003, 2nd ed.; World Health Organization and Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Ahn, W.S.; Park, S.J.; Lee, S.Y. Production of poly (3-hydroxybutyrate) from whey by cell recycle fed-batch culture of recombinant Escherichia coli. Biotechnol. Lett. 2001, 23, 235–240. [Google Scholar] [CrossRef]
- Almeida, G.; Magalhães, R.; Carneiro, L.; Santos, I.; Silva, J.; Ferreira, V.; Teixeira, P. Foci of contamination of Listeria monocytogenes in different cheese processing plants. Int. J. Food Microbiol. 2013, 167, 303–309. [Google Scholar] [CrossRef]
- Park, Y.W.; Juárez, M.; Ramos, M.; Haenlein, G.F.W. Physico-chemical characteristics of goat and sheep milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef]
- Evans, E.W. Uses of milk proteins in formulated foods. In Developments in Food Proteins; Applied Science: London, UK, 1982. [Google Scholar]
- Pintado, M.E.; Macedo, A.C.; Malcata, F.X. Technology, chemistry and microbiology of whey cheeses. Food Sci. Technol. Int. 2001, 7, 105–116. [Google Scholar] [CrossRef]
- De Wit, J.N. Nutritional and functional characteristics of whey proteins in food products. J. Dairy Sci. 1998, 81, 597–608. [Google Scholar] [CrossRef]
- Eigel, W.N.; Butler, J.E.; Ernstrom, C.A.; Farrell, H.M., Jr.; Harwalkar, V.R.; Jenness, R.; Whitney, R.M. Nomenclature of proteins of cow’s milk: Fifth revision. J. Dairy Sci. 1984, 67, 1599–1631. [Google Scholar] [CrossRef]
- Brew, K.; Castellino, F.J.; Vanaman, T.C.; Hill, R.L. The complete amino acid sequence of bovine α-lactalbumin. J. Biol. Chem. 1970, 245, 4570–4582. [Google Scholar]
- Korhonen, H.J. Whey as raw material for development of new products for human nutrition and health: A review. In Milk in Nutrition: Effects of Production and Processing Factors: Proceedings of NJF/NMR-Seminar No. 252, Turku, Finland 13.-15.1. NJF-Report 102/Edited by: Säde Mantere-Alhonen and Kalle Maijala; Scandinavian Association of Agricultural Scientists: Helsinki, Finland, 1995; pp. 207–219. [Google Scholar]
- Westhoek and Colleagues. Available online: http://www.fao.org/fileadmin/user_upload/animalwelfare/Protein_Puzzle_web_1.pdf (accessed on 17 July 2017).
- Lemon, P.W. Do athletes need more dietary protein and amino acids? Int. J. Sport Nutr. Exerc. Metab. 1995, 5, S39–S61. [Google Scholar] [CrossRef] [PubMed]
- Smithers, G.W. Whey and whey proteins—From ‘gutter-to-gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Shang, N.; Chaplot, S.; Wu, J. Food Proteins for Health and Nutrition. In Proteins in Food Processing; Woodhead Publishing Series in Food Science; Technology and Nutrition: Duxford, UK, 2018; pp. 301–336. [Google Scholar]
- Suárez, E.; Lobo, A.; Alvarez, S.; Riera, F.A.; Álvarez, R. Demineralization of whey and milk ultrafiltration permeate by means of nanofiltration. Desalination 2009, 241, 272–280. [Google Scholar] [CrossRef]
- Wright, B.J.; Zevchak, S.E.; Wright, J.M.; Drake, M.A. The impact of agglomeration and storage on flavor and flavor stability of whey protein concentrate 80% and whey protein isolate. J. Food Sci. 2009, 74, S17–S29. [Google Scholar] [CrossRef]
- Morifuji, M.; Ishizaka, M.; Baba, S.; Fukuda, K.; Matsumoto, H.; Koga, J.; Higuchi, M. Comparison of different sources and degrees of hydrolysis of dietary protein: Effect on plasma amino acids, dipeptides, and insulin responses in human subjects. J. Agric. Food Chem. 2010, 58, 8788–8797. [Google Scholar] [CrossRef]
- Buckley, J.D.; Thomson, R.L.; Coates, A.M.; Howe, P.R.; DeNichilo, M.O.; Rowney, M.K. Supplementation with a whey protein hydrolysate enhances recovery of muscle force-generating capacity following eccentric exercise. J. Sci. Med. Sport 2010, 13, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K.; Lönnerdal, B.; Fernstrom, J.D. Applications for α-lactalbumin in human nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef]
- Ulfman, L.H.; Leusen, J.H.; Savelkoul, H.F.; Warner, J.O.; van Neerven, R.J. Effects of bovine immunoglobulins on immune function, allergy, and infection. Front. Nutr. 2018, 5, 52. [Google Scholar] [CrossRef]
- Walzem, R.L. Health enhancing properties of whey proteins and whey fractions. Blood 1999, 1, 1–6. [Google Scholar]
- Hulmi, J.J.; Lockwood, C.M.; Stout, J.R. Effect of protein/essential amino acids and resistance training on skeletal muscle hypertrophy: A case for whey protein. Nutr. Metab. 2010, 7, 51. [Google Scholar] [CrossRef]
- Chen, W.C.; Huang, W.C.; Chiu, C.C.; Chang, Y.K.; Huang, C.C. Whey protein improves exercise performance and biochemical profiles in trained mice. Med. Sci. Sports Exerc. 2014, 46, 1517. [Google Scholar] [CrossRef]
- Bell, S.J. Whey protein concentrates with and without immunoglobulins: A review. J. Med. Food 2000, 3, 1–13. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Valle, N.R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef]
- Sharma, R.; Rajput, Y.S.; Mann, B. Chemical and functional properties of glycomacropeptide (GMP) and its role in the detection of cheese whey adulteration in milk: A review. Dairy Sci. Technol. 2013, 93, 21–43. [Google Scholar]
- Barth, C.A.; Behnke, U. Nutritional physiology of whey and whey components. Die Nahr. 1997, 41, 2–12. [Google Scholar] [CrossRef]
- Markus, C.R.; Olivier, B.; de Haan, E.H. Whey protein rich in α-lactalbumin increases the ratio of plasma tryptophan to the sum of the other large neutral amino acids and improves cognitive performance in stress-vulnerable subjects. Am. J. Clin. Nutr. 2002, 75, 1051–1056. [Google Scholar] [CrossRef]
- Ganjam, L.S.; Thornton, W.H., Jr.; Marshall, R.T.; MacDonald, R.S. Antiproliferative effects of yogurt fractions obtained by membrane dialysis on cultured mammalian intestinal cells. J. Dairy Sci. 1997, 80, 2325–2329. [Google Scholar] [CrossRef]
- Puyol, P.; Perez, M.D.; Ena, J.M.; Calvo, M. Interaction of bovine β-lactoglobulin and other bovine and human whey proteins with retinol and fatty acids. Agric. Biol. Chem. 1991, 55, 2515–2520. [Google Scholar] [CrossRef]
- Wu, S.Y.; Pérez, M.D.; Puyol, P.; Sawyer, L. β-Lactoglobulin binds palmitate within its central cavity. J. Biol. Chem. 1999, 274, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Allen, J.C.; Swaisgood, H.E. Binding of vitamin D and cholesterol to β-lactoglobulin. J. Dairy Sci. 1997, 80, 1054–1059. [Google Scholar] [CrossRef]
- Perez, M.D.; Sanchez, L.; Aranda, P.; Ena, J.; Oria, R.; Calvo, M. Effect of β-lactoglobulin on the activity of pregastric lipase. A possible role for this protein in ruminant milk. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1992, 1123, 151–155. [Google Scholar] [CrossRef]
- Farrell, H.M., Jr.; Behe, M.J.; Enyeart, J.A. Binding of p-nitrophenyl phosphate and other aromatic compounds by β-lactoglobulin. J. Dairy Sci. 1987, 70, 252–258. [Google Scholar] [CrossRef]
- Warme, P.K.; Momany, F.A.; Rumball, S.V.; Tuttle, R.W.; Scheraga, H.A. Computation of structures of homologous proteins alpha-lactalbumin from lysozyme. Biochemistry 1974, 13, 768–782. [Google Scholar] [CrossRef]
- Walzem, R.L.; Dillard, C.J.; German, J.B. Whey components: Millennia of evolution create functionalities for mammalian nutrition: What we know and what we may be overlooking. Crit. Rev. Food Sci. Nutr. 2002, 42, 353–375. [Google Scholar] [CrossRef] [PubMed]
- Bosselaers, I.E.M.; Caessens, P.W.J.R.; Van Boekel, M.A.J.S.; Alink, G.M. Differential effects of milk proteins, BSA and soy protein on 4NQO-or MNNG-induced SCEs in V79 cells. Food Chem. Toxicol. 1994, 32, 905–909. [Google Scholar] [CrossRef]
- Laursen, I.; Briand, P.; Lykkesfeldt, A.E. Serum albumin as a modulator on growth of the human breast cancer cell line, MCF-7. Anticancer Res. 1990, 10, 343. [Google Scholar]
- Mitra, A.K.; Mahalanabis, D.; Ashraf, H.; Unicomb, L.; Eeckels, R.; Tzipori, S. Hyperimmune cow colostrum reduces diarrhoea due to rotavirus: A double-blind, controlled clinical trial. Acta Paediatr. 1995, 84, 996–1001. [Google Scholar] [CrossRef]
- Loimaranta, V.; Laine, M.; Soèderling, E.; Vasara, E.; Rokka, S.; Marnila, P.; Tenovuo, J. Effects of bovine immune and non-immune whey preparations on the composition and pH response of human dental plaque. Eur. J. Oral Sci. 1999, 107, 244–250. [Google Scholar] [CrossRef]
- Freedman, D.J.; Tacket, C.O.; Delehanty, A.; Maneval, D.R.; Nataro, J.; Crabb, J.H. Milk immunoglobulin with specific activity against purified colonization factor antigens can protect against oral challenge with enterotoxigenic Escherichia coli. J. Infect. Dis. 1998, 177, 662–667. [Google Scholar] [CrossRef]
- Okhuysen, P.C.; Chappell, C.L.; Crabb, J.; Valdez, L.M.; Douglass, E.T.; DuPont, H.L. Prophylactic effect of bovine anti-Cryptosporidium hyperimmune colostrum immunoglobulin in healthy volunteers challenged with Cryptosporidium parvum. Clin. Infect. Dis. 1998, 26, 1324–1329. [Google Scholar] [CrossRef]
- Sharpe, S.J.; Gamble, G.D.; Sharpe, D.N. Cholesterol-lowering and blood pressure effects of immune milk. Am. J. Clin. Nutr. 1994, 59, 929–934. [Google Scholar] [CrossRef]
- Mellander, O.L.O.F. The physiological importance of the casein phosphopeptide calcium salts. II. Peroral calcium dosage of infants. Some aspects of the pathogenesis of rickets. Acta Soc. Bot. Pol. 1950, 55, 247–257. [Google Scholar]
- Korhonen, H.; Pihlanto-Leppälä, A. Milk-Derived Bioactive Peptides: Formation and Prospects for Health Promotion. In Handbook of Functional Dairy Products, Functional Foods and Neutraceuticals Series 6.0; CRC Press: Boca Raton, FL, USA, 2004; pp. 109–124. [Google Scholar]
- Dziuba, J.; Nałęcz, D.; Minkiewicz, P.; Dziuba, B. Identification and determination of milk and soybean protein preparations using enzymatic hydrolysis followed by chromatography and chemometrical data analysis. Anal. Chim. Acta 2004, 521, 17–24. [Google Scholar] [CrossRef]
- Clare, D.A.; Catignani, G.L.; Swaisgood, H.E. Biodefense properties of milk: The role of antimicrobial proteins and peptides. Curr. Pharm. Des. 2003, 9, 1239–1255. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; Stepaniak, L.; De Angelis, M.; Corsetti, A.; Di Cagno, R. Latent bioactive peptides in milk proteins: Proteolytic activation and significance in dairy processing. Crit. Rev. Food Sci. Nutr. 2002, 42, 223–239. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. The scientific evidence for the role of milk protein-derived bioactive peptides in humans: A Review. J. Funct. Foods 2015, 17, 640–656. [Google Scholar] [CrossRef]
- Li-Chan, E.C. Bioactive peptides and protein hydrolysates: Research trends and challenges for application as nutraceuticals and functional food ingredients. Curr. Opin. Food Sci. 2015, 1, 28–37. [Google Scholar] [CrossRef]
- Fekete, A.A.; Givens, D.I.; Lovegrove, J.A. The impact of milk proteins and peptides on blood pressure and vascular function: A review of evidence from human intervention studies. Nutr. Res. Rev. 2013, 26, 177–190. [Google Scholar] [CrossRef]
- Shimizu, M. Food-derived peptides and intestinal functions. Biofactors 2004, 21, 43–47. [Google Scholar] [CrossRef]
- FitzGerald, R.J.; Meisel, H. Milk protein hydrolysates and bioactive peptides. In Advanced Dairy Chemistry—1 Proteins; Springer: New York, NY, USA, 2003; pp. 675–698. [Google Scholar]
- Panchaud, A.; Affolter, M.; Kussmann, M. Mass spectrometry for nutritional peptidomics: How to analyze food bioactives and their health effects. J. Proteom. 2012, 75, 3546–3559. [Google Scholar] [CrossRef] [PubMed]
- Agyei, D.; Danquah, M.K. Industrial-scale manufacturing of pharmaceutical-grade bioactive peptides. Biotechnol. Adv. 2011, 29, 272–277. [Google Scholar] [CrossRef]
- Clemente, A. Enzymatic protein hydrolysates in human nutrition. Trends Food Sci. Technol. 2000, 11, 254–262. [Google Scholar] [CrossRef]
- Byun, H.G.; Kim, S.K. Purification and characterization of angiotensin I converting enzyme (ACE) inhibitory peptides from Alaska pollack (Theragra chalcogramma) skin. Process Biochem. 2001, 36, 1155–1162. [Google Scholar] [CrossRef]
- Najafian, L.; Babji, A.S. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides 2012, 33, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Ejiri, M.; Mizuno, S. Biogenic peptides and their potential use. Curr. Pharm. Des. 2003, 9, 1345–1355. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Savijoki, K.; Ingmer, H.; Varmanen, P. Proteolytic systems of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2006, 71, 394–406. [Google Scholar] [CrossRef]
- Christensen, J.E.; Dudley, E.G.; Pederson, J.A.; Steele, J.L. Peptidases and amino acid catabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 1999, 76, 217–246. [Google Scholar] [CrossRef]
- Piccolomini, A.; Iskandar, M.; Lands, L.; Kubow, S. High hydrostatic pressure pre-treatment of whey proteins enhances whey protein hydrolysate inhibition of oxidative stress and IL-8 secretion in intestinal epithelial cells. Food Nutr. Res. 2012, 56, 17549. [Google Scholar] [CrossRef]
- Lands, L.C.; Iskandar, M.; Beaudoin, N.; Meehan, B.; Dauletbaev, N.; Berthiuame, Y. Dietary supplementation with pressurized whey in patients with cystic fibrosis. J. Med. Food 2010, 13, 77–82. [Google Scholar] [CrossRef]
- Kong, B.; Peng, X.; Xiong, Y.L.; Zhao, X. Protection of lung fibroblast MRC-5 cells against hydrogen peroxide-induced oxidative damage by 0.1–2.8 kDa antioxidative peptides isolated from whey protein hydrolysate. Food Chem. 2012, 135, 540–547. [Google Scholar] [CrossRef]
- Takayanagi, T.; Sasaki, H.; Kawashima, A.; Mizuochi, Y.; Hirate, H.; Sugiura, T.; Sobue, K. A New Enteral Diet, MHN-02, Which Contains Abundant Antioxidants and Whey Peptide, Protects Against Carbon Tetrachloride–Induced Hepatitis. J. Parenter. Enter. Nutr. 2011, 35, 516–522. [Google Scholar] [CrossRef]
- Ross, E.K.; Gray, J.J.; Winter, A.N.; Linseman, D.A. Immunocal® and preservation of glutathione as a novel neuroprotective strategy for degenerative disorders of the nervous system. Recent Pat. Cns Drug Discov. 2012, 7, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Paik, H.D.; Yoon, Y.C.; Park, E. Whey protein inhibits iron overload-induced oxidative stress in rats. J. Nutr. Sci. Vitaminol. 2013, 59, 198–205. [Google Scholar] [CrossRef]
- Lyczak, J.B.; Cannon, C.L.; Pier, G.B. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 2002, 15, 194–222. [Google Scholar] [CrossRef]
- Kishta, O.A.; Iskandar, M.; Dauletbaev, N.; Kubow, S.; Lands, L.C. Pressurized whey protein can limit bacterial burden and protein oxidation in Pseudomonas aeruginosa lung infection. Nutrition 2013, 29, 918–924. [Google Scholar] [CrossRef]
- Athira, S.; Mann, B.; Sharma, R.; Kumar, R. Ameliorative potential of whey protein hydrolysate against paracetamol-induced oxidative stress. J. Dairy Sci. 2013, 96, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Zhao, Z. Effect of whey protein hydrolysates with different molecular weight on fatigue induced by swimming exercise in mice. J. Sci. Food Agric. 2014, 94, 126–130. [Google Scholar] [CrossRef]
- Haque, E.; Chand, R.; Kapila, S. Biofunctional properties of bioactive peptides of milk origin. Food Rev. Int. 2008, 25, 28–43. [Google Scholar] [CrossRef]
- Mullally, M.M.; Meisel, H.; FitzGerald, R.J. Angiotensin-I-converting enzyme inhibitory activities of gastric and pancreatic proteinase digests of whey proteins. Int. Dairy J. 1997, 7, 299–303. [Google Scholar] [CrossRef]
- Pihlanto-Leppälä, A. Bioactive peptides derived from bovine whey proteins: Opioid and ace-inhibitory peptides. Trends Food Sci. Technol. 2000, 11, 347–356. [Google Scholar] [CrossRef]
- Eliseeva, I.E. Angiotensin-converting enzyme and its physiological role. Voprosy Meditsinskoi Khimii 2001, 47, 43–54. [Google Scholar]
- Estévez, N.; Fuciños, P.; Sobrosa, A.C.; Pastrana, L.; Pérez, N.; Luisa Rúa, M. Modeling the angiotensin-converting enzyme inhibitory activity of peptide mixtures obtained from cheese whey hydrolysates using concentration–response curves. Biotechnol. Prog. 2012, 28, 1197–1206. [Google Scholar] [CrossRef]
- Chiba, H.; Yoshikawa, M. Biologically Functional Peptides from Food Proteins: New Opioid Peptides from Milk Proteins. In Protein Tailoring for Food and Medical Uses; Feeney, R.E., Whitaker, J.R., Eds.; Marcel Dekker: New York, NY, USA, 1986; pp. 123–153. [Google Scholar]
- Teschemacher, H.; Brantl, V. Milk Protein Derived Atypical Opioid Peptides and Related Compounds with Opioid Antagonist Activity. In β-Casomorphins and Related Peptides: Recent Developments; Brantl, V., Teschemacher, H., Eds.; VCH: Weinheim, Germany, 1994; pp. 3–17. [Google Scholar]
- Paakkari, I.; Järvinen, A.; Antila, P.; Mattila, M.J.; Pihlanto-Leppälä, A. Opioid effect of the milk whey protein-derived peptides alpha-and betalactorphin. In Beta-Casomorphins and Related Peptides: Recent Development; Brantl, V., Teschemacher, H., Eds.; VCH: Weinheim, Germany, 1994; pp. 33–37. [Google Scholar]
- Casqueiro, J.; Casqueiro, J.; Alves, C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J. Endocrinol. Metab. 2012, 16 (Suppl. 1), S27. [Google Scholar]
- Jain, S.K. L-cysteine supplementation as an adjuvant therapy for type-2 diabetes. Can. J. Physiol. Pharm. 2012, 90, 1061–1064. [Google Scholar] [CrossRef]
- Badr, G.; Badr, B.M.; Mahmoud, M.H.; Mohany, M.; Rabah, D.M.; Garraud, O. Treatment of diabetic mice with undenatured whey protein accelerates the wound healing process by enhancing the expression of MIP-1α, MIP-2, KC, CX3CL1 and TGF-β in wounded tissue. BMC Immunol. 2012, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.; Gunnerud, U.; Muhammed, S.J.; Östman, E.; Holst, J.J.; Björck, I.; Rorsman, P. The insulinogenic effect of whey protein is partially mediated by a direct effect of amino acids and GIP on β-cells. Nutr. Metab. 2012, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, L.S.; Holmer-Jensen, J.; Hartvigsen, M.L.; Jensen, V.K.; Astrup, A.; De Vrese, M.; Hermansen, K. Effects of different fractions of whey protein on postprandial lipid and hormone responses in type 2 diabetes. Eur. J. Clin. Nutr. 2012, 66, 799–805. [Google Scholar] [CrossRef]
- Toedebusch, R.G.; Childs, T.E.; Hamilton, S.R.; Crowley, J.R.; Booth, F.W.; Roberts, M.D. Postprandial leucine and insulin responses and toxicological effects of a novel whey protein hydrolysate-based supplement in rats. J. Int. Soc. Sports Nutr. 2012, 9, 24. [Google Scholar] [CrossRef]
- Akhavan, T.; Luhovyy, B.L.; Panahi, S.; Kubant, R.; Brown, P.H.; Anderson, G.H. Mechanism of action of pre-meal consumption of whey protein on glycemic control in young adults. J. Nutr. Biochem. 2014, 25, 36–43. [Google Scholar] [CrossRef]
- Tong, X.; Li, W.; Xu, J.Y.; Han, S.; Qin, L.Q. Effects of whey protein and leucine supplementation on insulin resistance in non-obese insulin-resistant model rats. Nutrition 2014, 30, 1076–1080. [Google Scholar] [CrossRef]
- Attaallah, W.; Yılmaz, A.M.; Erdoğan, N.; Yalçın, A.S.; Aktan, A.Ö. Whey protein versus whey protein hydrolyzate for the protection of azoxymethane and dextran sodium sulfate induced colonic tumors in rats. Pathol. Oncol. Res. 2012, 18, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Castro, G.A.; Maria, D.A.; Bouhallab, S.; Sgarbieri, V.C. In vitro impact of a whey protein isolate (WPI) and collagen hydrolysates (CHs) on B16F10 melanoma cells proliferation. J. Dermatol. Sci. 2009, 56, 51–57. [Google Scholar] [CrossRef]
- Takata, T.; Tanaka, F.; Yamada, T.; Yanagihara, K.; Otake, Y.; Kawano, Y.; Wada, H. Clinical significance of caspase-3 expression in pathologic-stage I, nonsmall-cell lung cancer. Int. J. Cancer 2001, 96, 54–60. [Google Scholar] [CrossRef]
- Dillon, E.L.; Basra, G.; Horstman, A.M.; Casperson, S.L.; Randolph, K.M.; Durham, W.J.; Willis, M. Cancer cachexia and anabolic interventions: A case report. J. Cachexia Sarcopenia Muscle 2012, 3, 253–263. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Ling, Y.F.; Sun, Z.; Zhang, L.; Yu, H.X.; Kamau, S.M.; Lu, R.R. Protective effect of whey protein hydrolysates against hydrogen peroxide-induced oxidative stress on PC12 cells. Biotechnol. Lett. 2012, 34, 2001–2006. [Google Scholar] [CrossRef]
- Pérez-Cano, F.J.; Marin-Gallen, S.; Castell, M.; Rodríguez-Palmero, M.; Rivero, M.; Franch, A.; Castellote, C. Bovine whey protein concentrate supplementation modulates maturation of immune system in suckling rats. Br. J. Nutr. 2007, 98, S80–S84. [Google Scholar] [CrossRef][Green Version]
- Alexander, D.D.; Schmitt, D.F.; Tran, N.L. Partially hydrolyzed 100% whey protein infant formula and atopic dermatitis risk reduction: A systematic review of the literature. Nutr. Rev. 2010, 68, 232–245. [Google Scholar] [CrossRef]
- Prussick, R.; Prussick, L.; Gutman, J. Psoriasis improvement in patients using glutathione-enhancing, nondenatured whey protein isolate: A pilot study. J. Clin. Aesthetic Dermatol. 2013, 6, 23. [Google Scholar]
- Morton, J.P.; Kayani, A.C.; McArdle, A.; Drust, B. The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med. 2009, 39, 643–662. [Google Scholar] [CrossRef]
- Freidenreich, D.J.; Volek, J.S. Immune responses to resistance exercise. Exerc. Immunol. Rev. 2012, 18, 8–41. [Google Scholar]
- Morato, P.N.; Lollo, P.C.B.; Moura, C.S.; Batista, T.M.; Carneiro, E.M.; Amaya-Farfan, J. A dipeptide and an amino acid present in whey protein hydrolysate increase translocation of GLUT-4 to the plasma membrane in Wistar rats. Food Chem. 2013, 139, 853–859. [Google Scholar] [CrossRef]
- Martin, V.; Ratel, S.; Siracusa, J.; Le Ruyet, P.; Savary-Auzeloux, I.; Combaret, L.; Dardevet, D. Whey proteins are more efficient than casein in the recovery of muscle functional properties following a casting induced muscle atrophy. PLoS ONE 2013, 8, e75408. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Breen, L.; Di Donato, D.M.; Hector, A.J.; Mitchell, C.J.; Moore, D.R.; Phillips, S.M. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: A double-blind, randomized trial. Am. J. Clin. Nutr. 2014, 99, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Lollo, P.C.B.; Amaya-Farfan, J.; Faria, I.C.; Salgado, J.V.V.; Chacon-Mikahil, M.P.T.; Cruz, A.G.; Arruda, M. Hydrolysed whey protein reduces muscle damage markers in Brazilian elite soccer players compared with whey protein and maltodextrin. A twelve-week in-championship intervention. Int. Dairy J. 2014, 34, 19–24. [Google Scholar] [CrossRef]
- Volek, J.S.; Volk, B.M.; Gómez, A.L.; Kunces, L.J.; Kupchak, B.R.; Freidenreich, D.J.; Quann, E.E. Whey protein supplementation during resistance training augments lean body mass. J. Am. Coll. Nutr. 2013, 32, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Roblet, C.; Amiot, J.; Lavigne, C.; Marette, A.; Lessard, M.; Jean, J.; Bazinet, L. Screening of in vitro bioactivities of a soy protein hydrolysate separated by hollow fiber and spiral-wound ultrafiltration membranes. Food Res. Int. 2012, 46, 237–249. [Google Scholar] [CrossRef]
- Del Mar Contreras, M.; Lpez-Expsito, I.; Hernndez-Ledesma, B.; Ramos, M.; Recio, I. Application of mass spectrometry to the characterization and quantification of food-derived bioactive peptides. J. AOAC Int. 2008, 91, 981–994. [Google Scholar] [CrossRef]
- Pownall, T.L.; Udenigwe, C.C.; Aluko, R.E. Amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) enzymatic protein hydrolysate fractions. J. Agric. Food Chem. 2010, 58, 4712–4718. [Google Scholar] [CrossRef]
- Haileselassie, S.S.; Lee, B.H.; Gibbs, B.F. Purification and identification of potentially bioactive peptides from enzyme-modified cheese. J. Dairy Sci. 1999, 82, 1612–1617. [Google Scholar] [CrossRef]
- Chiang, W.D.; Tsou, M.J.; Tsai, Z.Y.; Tsai, T.C. Angiotensin I-converting enzyme inhibitor derived from soy protein hydrolysate and produced by using membrane reactor. Food Chem. 2006, 98, 725–732. [Google Scholar] [CrossRef]
- Hayes, M.; Ross, R.P.; Fitzgerald, G.F.; Hill, C.; Stanton, C. Casein-derived antimicrobial peptides generated by Lactobacillus acidophilus DPC6026. Appl. Environ. Microbiol. 2006, 72, 2260–2264. [Google Scholar] [CrossRef]
- Liu, R.; Zheng, W.; Li, J.; Wang, L.; Wu, H.; Wang, X.; Shi, L. Rapid identification of bioactive peptides with antioxidant activity from the enzymatic hydrolysate of Mactra veneriformis by UHPLC–Q-TOF mass spectrometry. Food Chem. 2015, 167, 484–489. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Dziuba, J.; Iwaniak, A.; Dziuba, M.; Darewicz, M. BIOPEP database and other programs for processing bioactive peptide sequences. J. AOAC Int. 2008, 91, 965–980. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, J.E.; Whitehead, D.M. Proteins in Whey: Chemical, Physical, and Functional Properties. In Advances in Food and Nutrition Research; Academic Press, Inc.: Cambridge, MA, USA, 1989; Volume 33, pp. 343–438. [Google Scholar]
- Kella, N.K.D.; Kinsella, J.E. Enhanced thermodynamic stability of β-lactoglobulin at low pH. A possible mechanism. Biochem. J. 1988, 255, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Haque, Z.; Kinsella, J.E. Interaction between κ-casein and β-lactoglobulin: Effect of calcium. Agric. Biol. Chem. 1987, 51, 1997–1998. [Google Scholar] [CrossRef]
- Modler, H.W.; Emmons, D.B. Properties of whey protein concentrate prepared by heating under acidic conditions. J. Dairy Sci. 1977, 60, 177–184. [Google Scholar] [CrossRef]
- Rüegg, M.; Moor, U.; Blanc, B. A calorimetric study of the thermal denaturation of whey proteins in simulated milk ultrafiltrate. J. Dairy Res. 1977, 44, 509–520. [Google Scholar] [CrossRef]
- Kronman, M.J.; Sinha, S.K.; Brew, K. Characteristics of the binding of Ca2+ and other divalent metal ions to bovine alpha-lactalbumin. J. Biol. Chem. 1981, 256, 8582–8587. [Google Scholar]
- Kinsella, J.E.; Morr, C.V. Milk proteins: Physicochemical and functional properties. Crit. Rev. Food Sci. Nutr. 1984, 21, 197–262. [Google Scholar] [CrossRef]
- Kinsella, J.E.; Fox, P.F.; Rockland, L.B. Water sorption by proteins: Milk and whey proteins. Crit. Rev. Food Sci. Nutr. 1986, 24, 91–139. [Google Scholar] [CrossRef]
- Neff, E.; Morris, H.A.L. Agglomeration of milk powder and its influence on reconstitution properties. J. Dairy Sci. 1968, 51, 330–338. [Google Scholar] [CrossRef]
- Townend, R.; Gyuricsek, D.M. Heat denaturation of whey and model protein systems. J. Dairy Sci. 1974, 57, 1152–1158. [Google Scholar] [CrossRef]
- Kohnhorst, A.L.; Mangino, M.E. Prediction of the strength of whey protein gels based on composition. J. Food Sci. 1985, 50, 1403–1405. [Google Scholar] [CrossRef]
- Mulvihill, D.M.; Kinsella, J.E. Gelation of β-lactoglobulin: Effects of sodium chloride and calcium chloride on the rheological and structural properties of gels. J. Food Sci. 1988, 53, 231–236. [Google Scholar] [CrossRef]
- Bernal, V.; Jelen, P. Thermal stability of whey proteins—A calorimetric study. J. Dairy Sci. 1985, 68, 2847–2852. [Google Scholar] [CrossRef]
- Clark, A.H.; Saunderson, D.H.P.; Suggett, A. Infrared and laser-Raman spectroscopic studies of thermally-induced globular protein gels. Int. J. Pept. Protein Res. 1981, 17, 353–364. [Google Scholar] [CrossRef]
- Harwalkar, V.R.; Kalab, M. Thermal denaturation and aggregation of blactoglobulin at pH 2.5. Effect of ionic strength and protein concentration. Milchwissenschaft 1985, 40, 31–34. [Google Scholar]
- Farooq, Z.; Boye, J.I. Novel Food and Industrial Applications of Pulse Flours and Fractions. In Pulse Foods: Processing, Quality and Nutraceutical Applications; Tiwari, B.K., Gowen, A., McKenna, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 283–323. [Google Scholar]
- Shimizu, M.; Kamiya, T.; Yamauchi, K. The adsorption of whey proteins on the surface of emulsified fat. Agric. Biol. Chem. 1981, 45, 2491–2496. [Google Scholar]
- Yamauchi, K.; Shimizu, M.; Kamiya, T. Emulsifying properties of whey protein. J. Food Sci. 1980, 45, 1237–1242. [Google Scholar] [CrossRef]
- Tornberg, E. Functional characterization of protein stabilized emulsions: Creaming stability. J. Food Sci. 1978, 43, 1559–1562. [Google Scholar] [CrossRef]
- Slack, A.W.; Amundson, C.H.; Hill, C.G., Jr. Foaming and emulsifying characteristics of fractionated whey protein. J. Food Process. Preserv. 1986, 10, 81–88. [Google Scholar] [CrossRef]
- Kato, A.; Osako, Y.; Matsudomi, N.; Kobayashi, K. Changes in the emulsifying and foaming properties of proteins during heat denaturation. Agric. Biol. Chem. 1983, 47, 33–37. [Google Scholar]
- Hernández-Ledesma, B.; García-Nebot, M.J.; Fernández-Tomé, S.; Amigo, L.; Recio, I. Dairy protein hydrolysates: Peptides for health benefits. Int. Dairy J. 2014, 38, 82–100. [Google Scholar] [CrossRef]
- LaClair, C.E.; Etzel, M.R. Ingredients and pH are key to clear beverages that contain whey protein. J. Food Sci. 2010, 75, C21–C27. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.K.I.O. Industrial applications of Maillard-type protein-polysaccharide conjugates. Food Sci. Technol. Res. 2002, 8, 193–199. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Zhou, K. Chelating and radical scavenging activities of soy protein hydrolysates prepared from microbial proteases and their effect on meat lipid peroxidation. Bioresour. Technol. 2010, 101, 2084–2089. [Google Scholar] [CrossRef]
- Oliver, C.M.; Melton, L.D.; Stanley, R.A. Functional properties of caseinate glycoconjugates prepared by controlled heating in the ‘dry’state. J. Sci. Food Agric. 2006, 86, 732–740. [Google Scholar] [CrossRef]
- Krzeminski, A.; Prell, K.A.; Busch-Stockfisch, M. Whey protein–pectin complexes as new texturising elements in fat-reduced yoghurt systems. Int. Dairy J. 2014, 36, 118–127. [Google Scholar] [CrossRef]
- Kuhn, K.R.; Cunha, R.L. Flaxseed oil–whey protein isolate emulsions: Effect of high pressure homogenization. J. Food Eng. 2012, 111, 449–457. [Google Scholar] [CrossRef]
- Akalın, A.S.; Unal, G.; Dinkci, N.; Hayaloglu, A.A. Microstructural, textural, and sensory characteristics of probiotic yogurts fortified with sodium calcium caseinate or whey protein concentrate. J. Dairy Sci. 2012, 95, 3617–3628. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Muhammad Anjum, F.; Murtaza, M.A.; Mueen-ud-Din, G. Development, characterization, and optimization of protein level in date bars using response surface methodology. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leksrisompong, P.; Gerard, P.; Lopetcharat, K.; Drake, M. Bitter taste inhibiting agents for whey protein hydrolysate and whey protein hydrolysate beverages. J. Food Sci. 2012, 77, S282–S287. [Google Scholar] [CrossRef] [PubMed]
- Gad, A.S.; Khadrawy, Y.A.; El-Nekeety, A.A.; Mohamed, S.R.; Hassan, N.S.; Abdel-Wahhab, M.A. Antioxidant activity and hepatoprotective effects of whey protein and Spirulina in rats. Nutrition 2011, 27, 582–589. [Google Scholar] [CrossRef]
- Kattan, J.D.; Cocco, R.R.; Järvinen, K.M. Milk and soy allergy. Pediatr. Clin. N. Am. 2011, 58, 407–426. [Google Scholar] [CrossRef]
- Botteman, M.; Detzel, P. Cost-effectiveness of partially hydrolyzed whey protein formula in the primary prevention of atopic dermatitis in high-risk urban infants in Southeast Asia. Ann. Nutr. Metab. 2015, 66 (Suppl. 1), 26–32. [Google Scholar] [CrossRef]
- Ameratunga, R.; Woon, S.T. Anaphylaxis to hyperallergenic functional foods. Allergy Asthma Clin. Immunol. 2010, 6, 33. [Google Scholar] [CrossRef]
- Hochwallner, H.; Schulmeister, U.; Swoboda, I.; Spitzauer, S.; Valenta, R. Cow’s milk allergy: From allergens to new forms of diagnosis, therapy and prevention. Methods 2014, 66, 22–33. [Google Scholar] [CrossRef]
- Bu, G.; Luo, Y.; Zheng, Z.; Zheng, H. Effect of heat treatment on the antigenicity of bovine α-lactalbumin and β-lactoglobulin in whey protein isolate. Food Agric. Immunol. 2009, 20, 195–206. [Google Scholar] [CrossRef]
- Duan, C.C.; Yang, L.J.; Li, A.L.; Zhao, R.; Huo, G.C. Effects of Enzymatic Hydrolysis on the Allergenicity of Whey Protein Concentrates. Iran J. Allergy Asthma Immunol. 2014, 13, 231–239. [Google Scholar]
- Nedovic, V.A.; Obradovic, B.; Leskosek-Cukalovic, I.; Vunjak-Novakovic, G. Immobilized yeast bioreactor systems for brewing—Recent achievements. In Engineering and Manufacturing for Biotechnology; Springer: Berlin/Heidelberg, Germany, 2001; pp. 277–292. [Google Scholar]
- De Vos, P.; Faas, M.M.; Spasojevic, M.; Sikkema, J. Encapsulation for preservation of functionality and targeted delivery of bioactive food components. Int. Dairy J. 2010, 20, 292–302. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Jin Park, H. Recent developments in microencapsulation of food ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Wandrey, C.; Bartkowiak, A.; Harding, S.E. Materials for Encapsulation. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Springer: New York, NY, USA, 2010; pp. 31–100. [Google Scholar]
- Martin, A.H.; De Jong, G.A.H. Enhancing the in vitro Fe2+ bio-accessibility using ascorbate and cold-set whey protein gel particles. Dairy Sci. Technol. 2012, 92, 133–149. [Google Scholar] [CrossRef]
- Pérez-Masiá, R.; López-Nicolás, R.; Periago, M.J.; Ros, G.; Lagaron, J.M.; López-Rubio, A. Encapsulation of folic acid in food hydrocolloids through nanospray drying and electrospraying for nutraceutical applications. Food Chem. 2015, 168, 124–133. [Google Scholar] [CrossRef]
- Gülseren, İ.; Fang, Y.; Corredig, M. Complexation of high methoxyl pectin with ethanol desolvated whey protein nanoparticles: Physico-chemical properties and encapsulation behaviour. Food Funct. 2012, 3, 859–866. [Google Scholar] [CrossRef]
- Parris, N.; Cooke, P.H.; Hicks, K.B. Encapsulation of essential oils in zein nanospherical particles. J. Agric. Food Chem. 2005, 53, 4788–4792. [Google Scholar] [CrossRef]
- Mehyar, G.F.; Al-Isamil, K.M.; Al-Ghizzawi, H.A.M.; Holley, R.A. Stability of cardamom (Elettaria Cardamomum) essential oil in microcapsules made of whey protein isolate, guar gum, and carrageenan. J. Food Sci. 2014, 79, C1939–C1949. [Google Scholar] [CrossRef] [PubMed]
- Janjarasskul, T.; Tananuwong, K.; Krochta, J.M. Whey protein film with oxygen scavenging function by incorporation of ascorbic acid. J. Food Sci. 2011, 76, E561–E568. [Google Scholar] [CrossRef]
- Cinelli, P.; Schmid, M.; Bugnicourt, E.; Wildner, J.; Bazzichi, A.; Anguillesi, I.; Lazzeri, A. Whey protein layer applied on biodegradable packaging film to improve barrier properties while maintaining biodegradability. Polym. Degrad. Stab. 2014, 108, 151–157. [Google Scholar] [CrossRef]
- Tippetts, M.; Martini, S.; Brothersen, C.; McMahon, D.J. Fortification of cheese with vitamin D3 using dairy protein emulsions as delivery systems. J. Dairy Sci. 2012, 95, 4768–4774. [Google Scholar] [CrossRef]
- Li, M.; Ma, Y.; Cui, J. Whey-protein-stabilized nanoemulsions as a potential delivery system for water-insoluble curcumin. LWT-Food Sci. Technol. 2014, 59, 49–58. [Google Scholar] [CrossRef]
| Whey Protein Constituent | Concentration (g/L) b,e | Molecular Weight in kDa c,d | Number of Amino Acid Residues c |
|---|---|---|---|
| α-Lactalbumin | 1.2 | 14,175 | 123 |
| β-Lactoglobulin | 1.3 | 18,277 | 162 |
| Bovine serum albumin | 0.4 | 66,267 | 582 |
| Immunoglobulins (A, M, and C) | 0.7 | 25,000 (light chain) and 50,000–70,000 (heavy chain) | - |
| Bovine lactoferrin | 0.1 | 80,000 | 700 |
| Glycomacropeptide | 1.2 | 6700 | 64 |
| bovine Lactoperoxidase | 0.03 | 70,000 | 612 |
| Whey Protein Constituent | Biological Activities | References |
|---|---|---|
| α-Lactalbumin | Anticancer activity | [18] |
| Lactose metabolism and synthesis | [39] | |
| Treatment of chronic stress-induced disease | [40] | |
| β-Lactoglobulin | Transporter of retinol, fatty acids, palmitate, vitamin D and cholesterol | [41,42,43] |
| Increase in pregastric esterase activity | [44] | |
| Mammary gland phosphorus synthesis and metabolism | [45] | |
| Passive immunity transfer | [46] | |
| Bovine serum albumin | Bind fatty acids | [47] |
| Anti-mutagenic activity | [48] | |
| Anti-cancer activity | [49] | |
| Immune system modulation through passive immunity | [50,51] | |
| Immunoglobulins (A, M, and C) | Antimicrobial activity | [52] |
| Antifungal activity | [53] | |
| Opioid activity | [54] |
| Processing conditions | Heating |
| Acidification | |
| Counter ions | |
| Ionic strength | |
| Reducing conditions | |
| Drying | |
| Storage conditions | |
| Modifications related to physical, chemical, enzymatic, and genetic | |
| Extrinsic parameters | Temperature |
| pH | |
| Oxidation-reduction potential | |
| Salts or ions | |
| Water | |
| Carbohydrates | |
| Lipids | |
| Gums | |
| Surfactants | |
| Tannins | |
| Intrinsic parameters | Protein composition |
| Monomeric oligomeric | |
| Protein blends | |
| Rigidity/flexibility | |
| Hydrophobicity or hydrophilicity | |
| Surface charge | |
| Bound flavor ligands |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minj, S.; Anand, S. Whey Proteins and Its Derivatives: Bioactivity, Functionality, and Current Applications. Dairy 2020, 1, 233-258. https://doi.org/10.3390/dairy1030016
Minj S, Anand S. Whey Proteins and Its Derivatives: Bioactivity, Functionality, and Current Applications. Dairy. 2020; 1(3):233-258. https://doi.org/10.3390/dairy1030016
Chicago/Turabian StyleMinj, Shayanti, and Sanjeev Anand. 2020. "Whey Proteins and Its Derivatives: Bioactivity, Functionality, and Current Applications" Dairy 1, no. 3: 233-258. https://doi.org/10.3390/dairy1030016
APA StyleMinj, S., & Anand, S. (2020). Whey Proteins and Its Derivatives: Bioactivity, Functionality, and Current Applications. Dairy, 1(3), 233-258. https://doi.org/10.3390/dairy1030016





