Green Synthesis of Activated Carbons from Coconut Coir Dust via Steam Activation for Supercapacitor Electrode Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
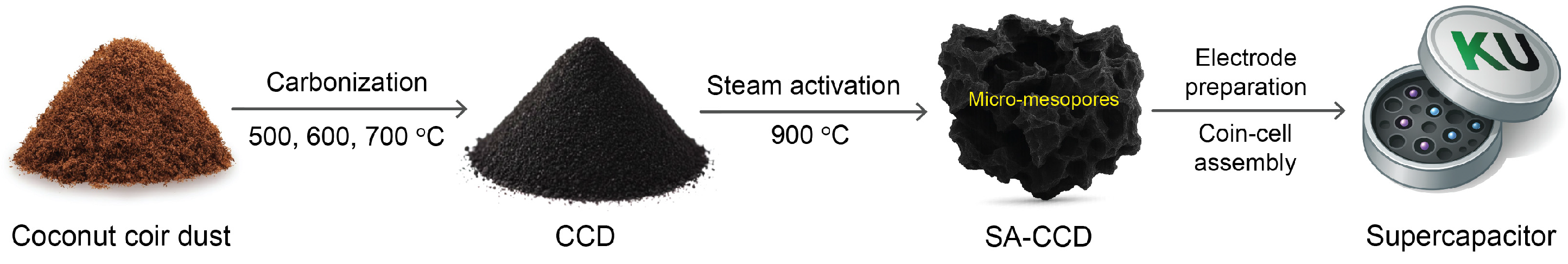
2.2. Preparation of Steam-Activated Carbons from Coconut Coir Dust
2.3. Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
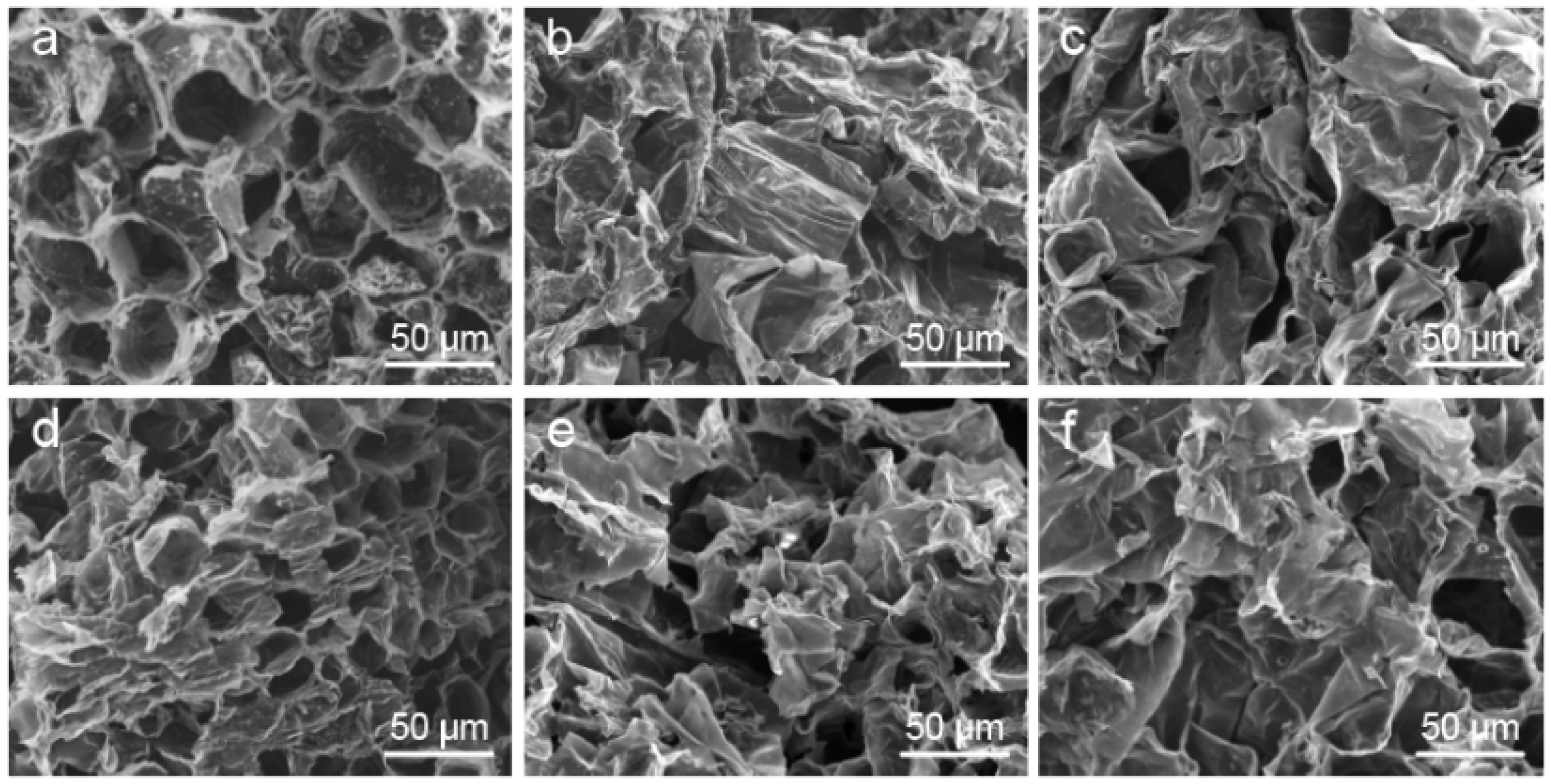
3.1. Morphology
3.2. Surface Area and Porosity
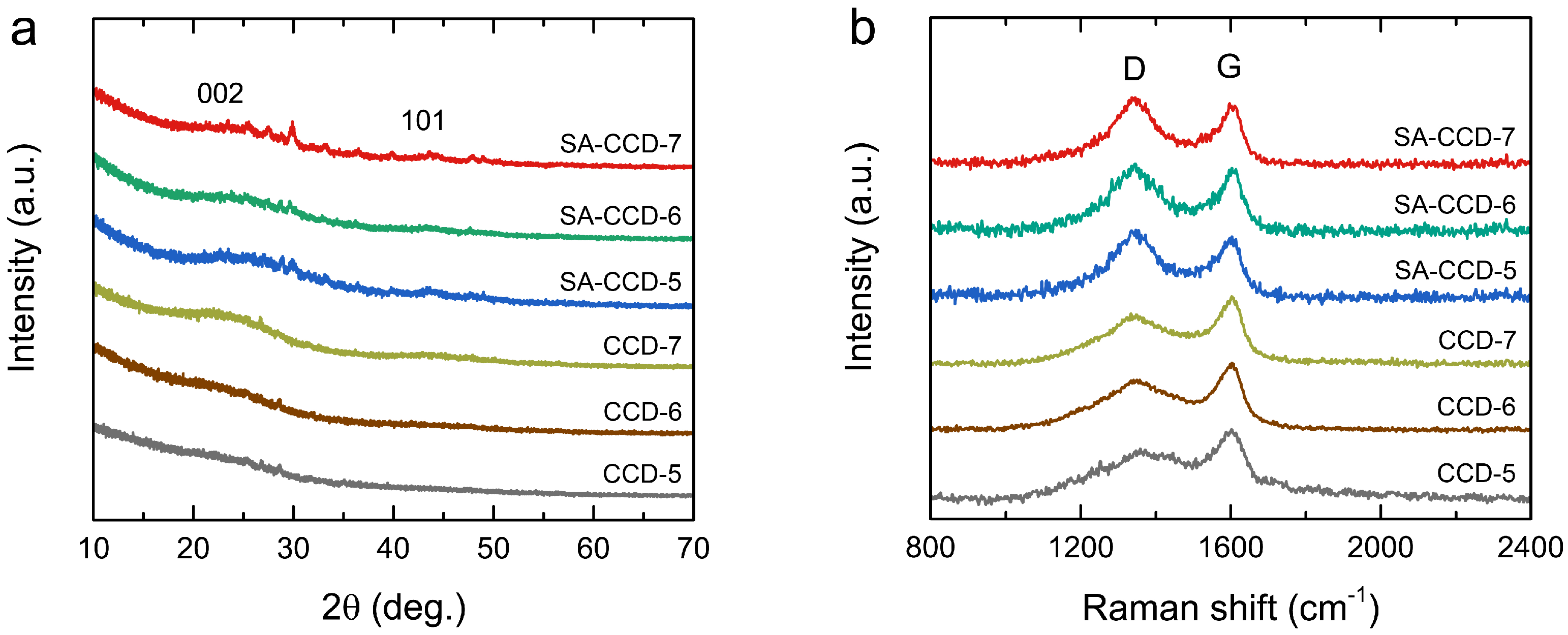
3.3. Structural Properties
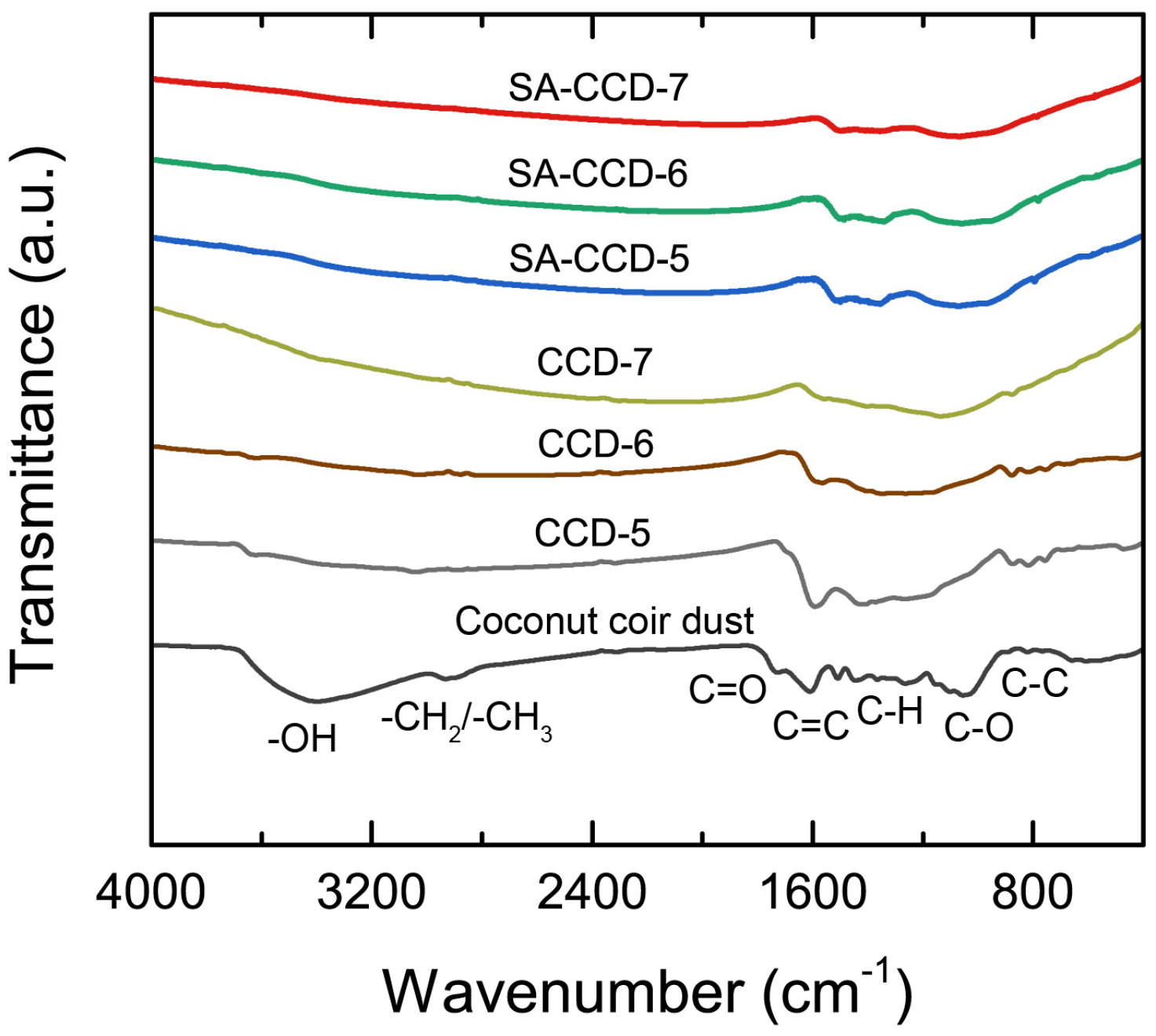
3.4. Chemical Functionality
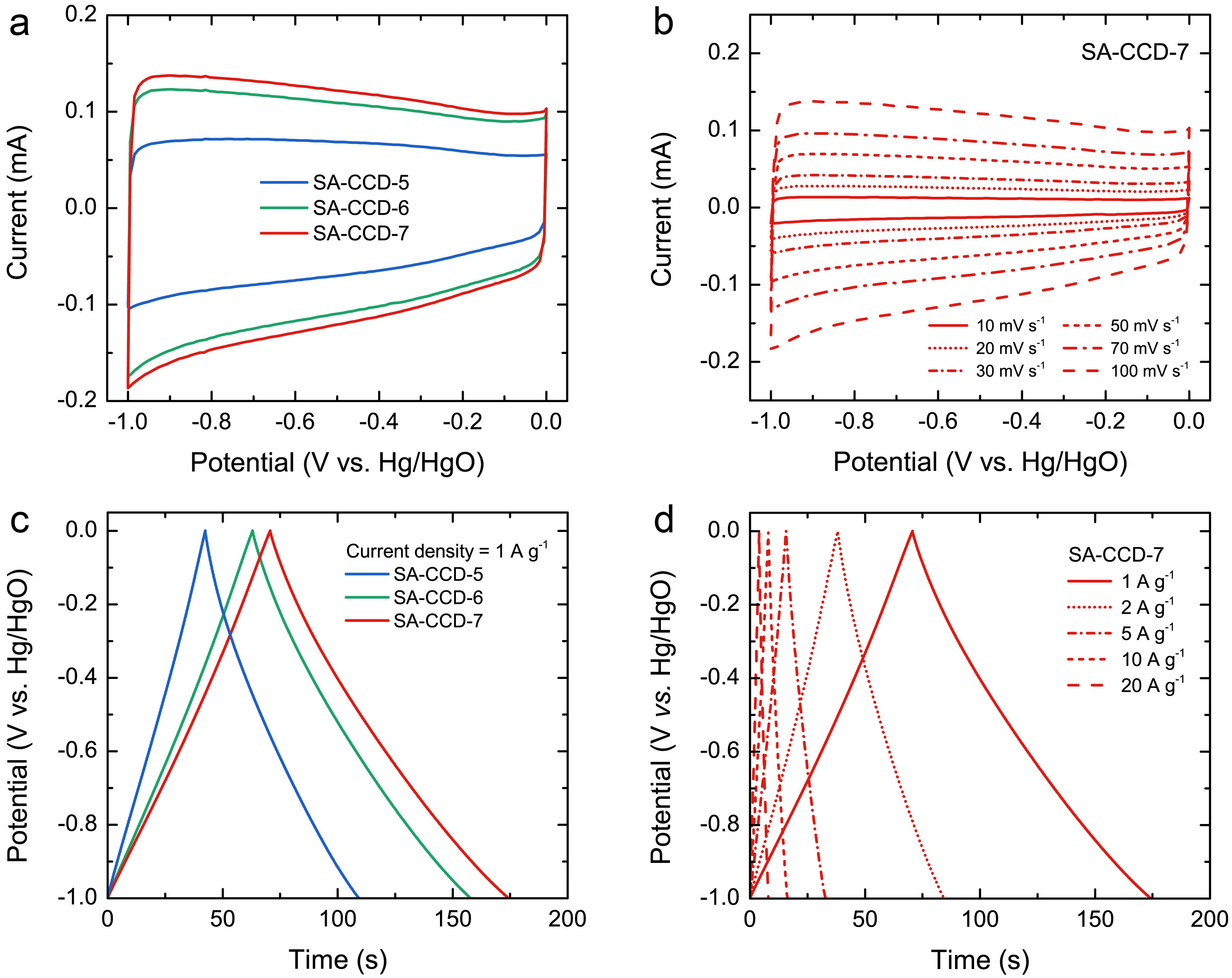
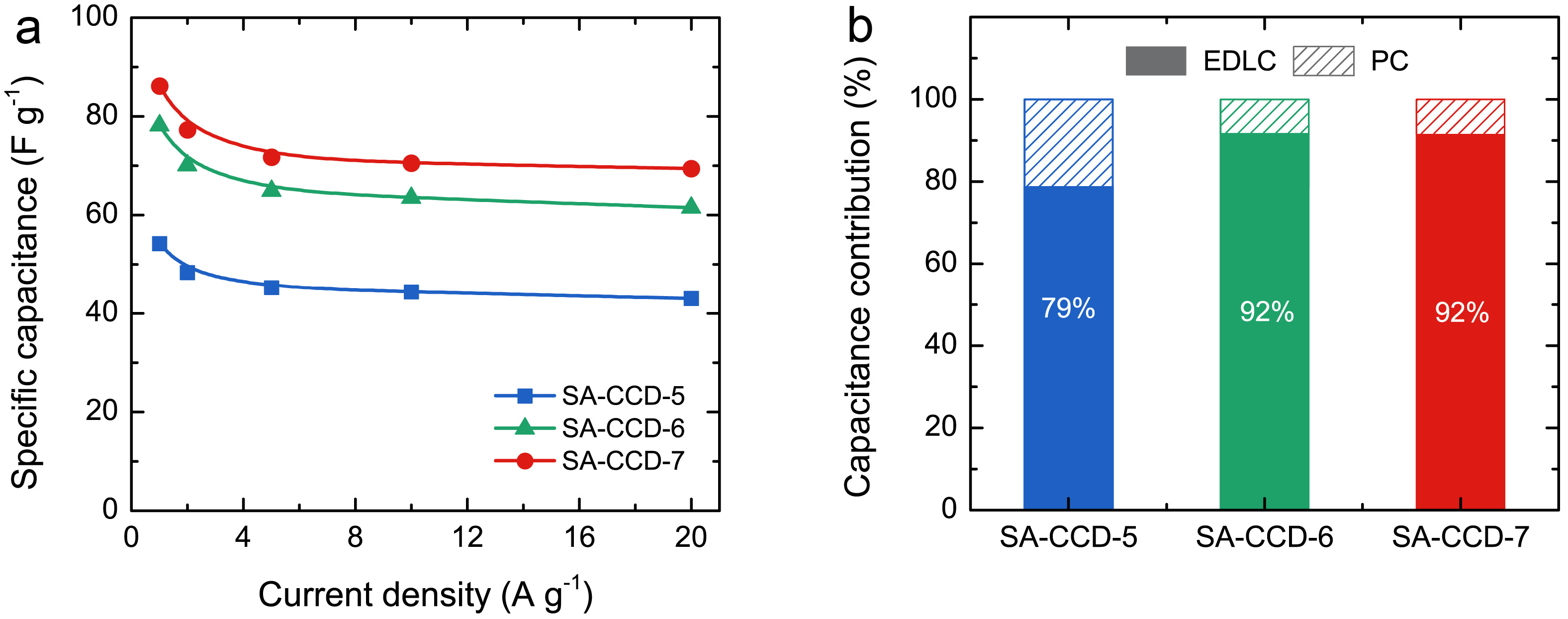
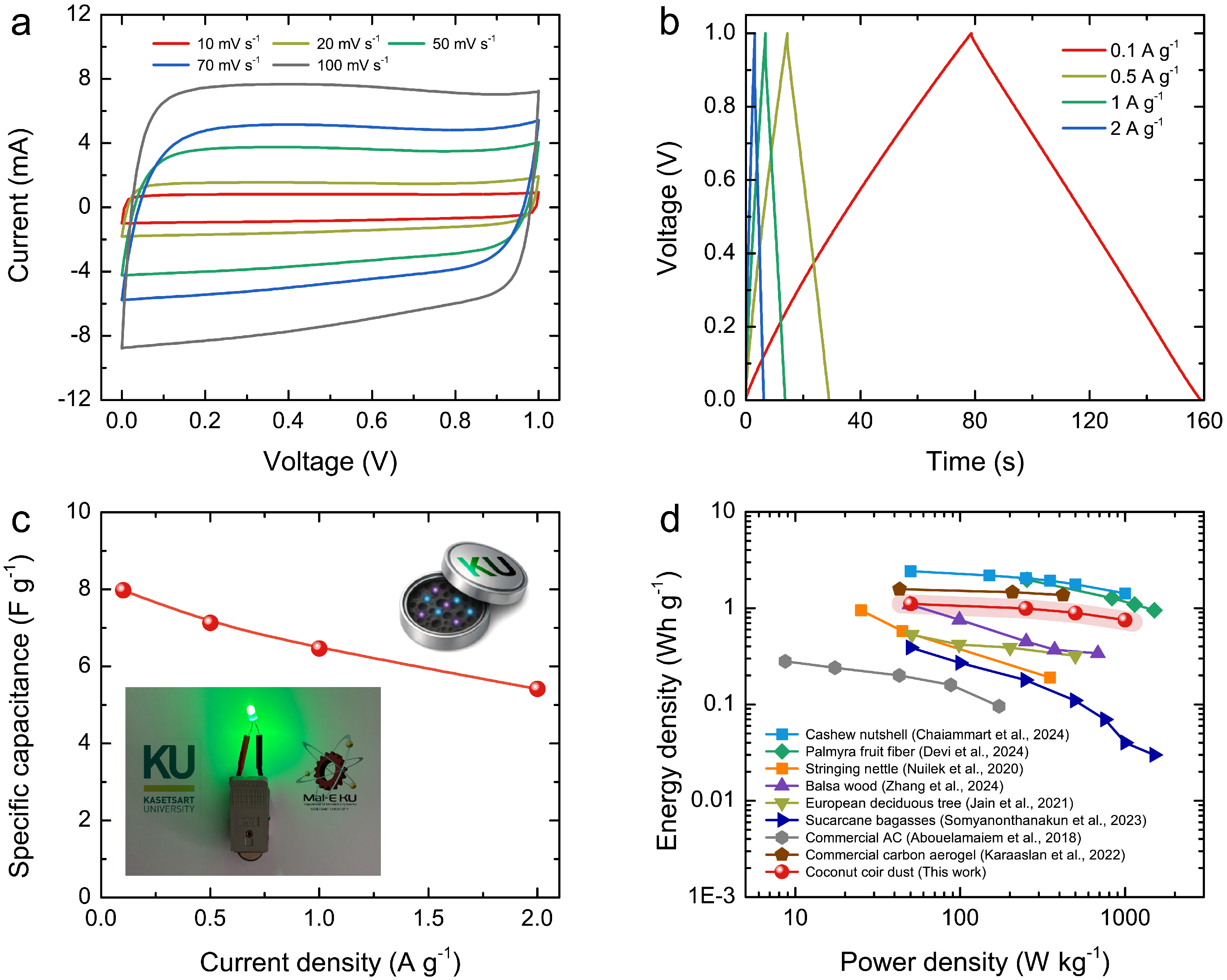
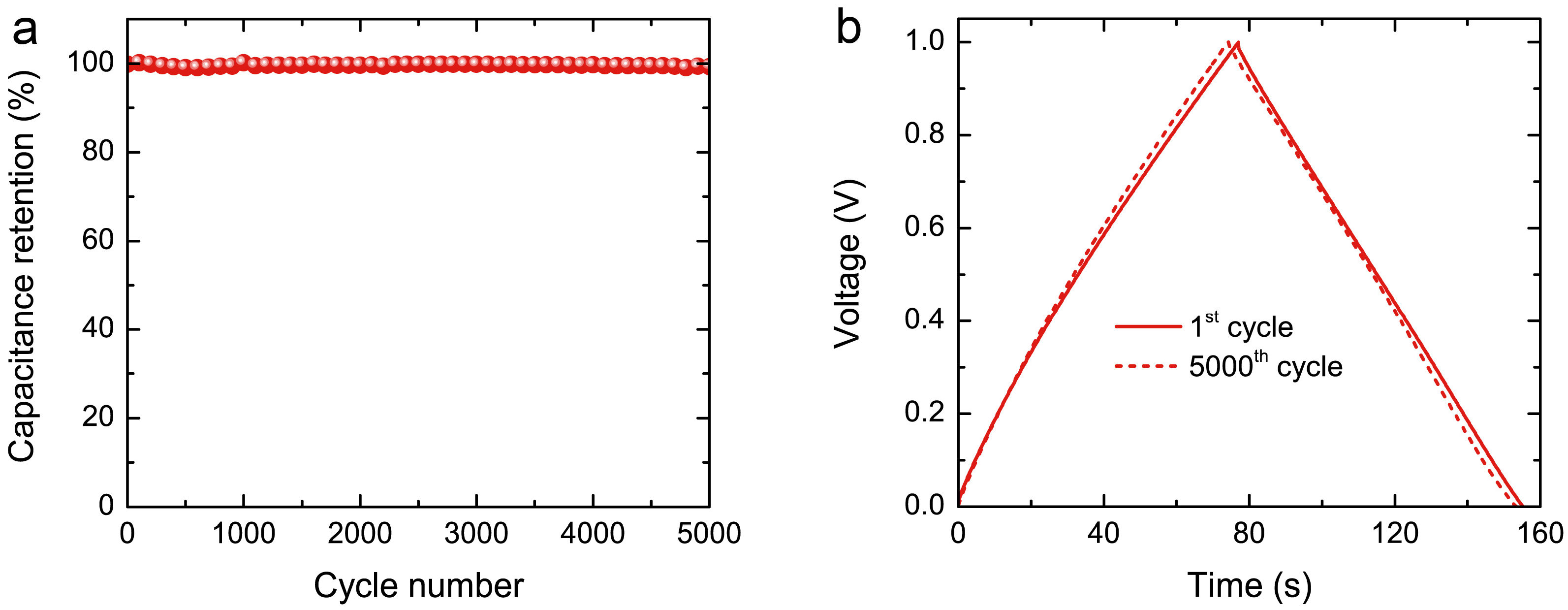
3.5. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elalfy, D.A.; Gouda, E.; Kotb, E.F.; Bureš, V.; Sedhom, B.E. Comprehensive review of energy storage systems technologies, objectives, challenges, and future trends. Energy Strategy Rev. 2024, 54, 101482. [Google Scholar] [CrossRef]
- Dutta, A.; Mitra, S.; Basak, M.; Banerjee, T. A comprehensive review on batteries and supercapacitors: Development and challenges since their inception. Energy Storage 2023, 5, e339. [Google Scholar] [CrossRef]
- Ngoy, K.R.; Lukong, V.T.; Yoro, K.O.; Makambo, J.B.; Chukwuati, N.C.; Ibegbulam, C.; Eterigho-Ikelegbe, O.; Ukoba, K.; Jen, T.-C. Lithium-ion batteries and the future of sustainable energy: A comprehensive review. Rev. Sustain. Energy Rev. 2025, 223, 115971. [Google Scholar] [CrossRef]
- Lu, Z.; Xiao, J.; Xie, H.; Song, W.; Fujishige, M.; Takeuchi, K.; Endo, M.; Li, Z.; Niu, J.; Wang, F. From waste to wealth: Cd-adsorbed rapeseed meal towards CdS-decorated nanocarbons for high-performance sodium metal batteries. Energy Storage Mater. 2025, 80, 104393. [Google Scholar] [CrossRef]
- Şahin, M.E.; Blaabjerg, F.; Sangwongwanich, A. A comprehensive review on supercapacitor applications and developments. Energies 2022, 15, 674. [Google Scholar] [CrossRef]
- Banerjee, S.; Mordina, B.; Sinha, P.; Kar, K.K. A current era of supercapacitor devices through the development of electrical double layer, pseudo and their hybrid supercapacitor electrodes. J. Energy Storage 2025, 108, 115075. [Google Scholar] [CrossRef]
- Zhao, J.; Burke, A.F. Review on supercapacitors: Technologies and performance evaluation. J. Energy Chem. 2021, 59, 276–291. [Google Scholar] [CrossRef]
- Shah, S.S.; Niaz, F.; Ehsan, M.A.; Das, H.T.; Younas, M.; Khan, A.S.; Rahman, H.U.; Nayem, S.M.A.; Oyama, M.; Aziz, M.A. Advanced strategies in electrode engineering and nanomaterial modifications for supercapacitor performance enhancement: A comprehensive review. J. Energy Storage 2024, 79, 110152. [Google Scholar] [CrossRef]
- Meena, D.; Kumar, R.; Gupta, S.; Khan, O.; Gupta, D.; Singh, M. Energy storage in the 21st century: A comprehensive review on factors enhancing the next-generation supercapacitor mechanisms. J. Energy Storage 2023, 72, 109323. [Google Scholar] [CrossRef]
- Reenu; Ahlawat, S.; Phor, L.; Kumar, A.; Chahal, S. Electrode materials for supercapacitors: A comprehensive review of advancements and performance. J. Energy Storage 2024, 84, 110698. [Google Scholar] [CrossRef]
- Forouzandeh, P.; Kumaravel, V.; Pillai, S.C. Electrode Materials for Supercapacitors: A Review of Recent Advances. Catalysts 2020, 10, 969. [Google Scholar] [CrossRef]
- Manimekala, T.; Sivasubramanian, R.; Dar, M.A.; Dharmalingam, G. Crafting the architecture of biomass-derived activated carbon via electrochemical insights for supercapacitors: A review. RSC Adv. 2025, 15, 2490–2522. [Google Scholar] [CrossRef] [PubMed]
- Sagadevan, S.; Balakrishnan, T.; Rahman, M.Z.; Soga, T.; Randriamahazaka, H.; Kakavandi, B.; Johan, M.R. Agricultural biomass-based activated carbons for efficient and sustainable supercapacitors. J. Energy Storage 2024, 97, 112878. [Google Scholar] [CrossRef]
- Duraisamy, N.; SK, K.; Dhandapani, E.; Kandiah, K. A review on biomass-derived activated carbon for next-generation supercapacitors: Cutting-edge advances and future prospects. Energy Fuels 2025, 39, 2306–2347. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, Y.; Liang, X.; Tang, L.; Sun, L.; Wang, R.; Wei, S.; Huang, H.; Yang, P.; Hu, H. Hierarchical porous activated carbon derived from coconut shell for ultrahigh-performance supercapacitors. Molecules 2023, 28, 7187. [Google Scholar] [CrossRef]
- Chaiammart, N.; Vignesh, V.; Thu, M.M.; Eiad-ua, A.; Maiyalagan, T.; Panomsuwan, G. Chemically activated carbons derived from cashew nut shells as potential electrode materials for electrochemical supercapacitors. Carbon Resour. Convers. 2025, 8, 100267. [Google Scholar] [CrossRef]
- Torrarit, P.; Poompradub, S.; Mohammadifar, M.; Pattananuwat, P.; Jayaraman, T.; Jeong, Y.; Chanlek, N.; Choi, M.Y.; Kasemchainan, J. Highly porous activated carbon from betel palm shells as the prospective electrode for high-performance supercapacitors. Mater. Sci. Energy Technol. 2025, 8, 143–153. [Google Scholar] [CrossRef]
- Dong, L.; Pan, C.; Ji, Y.; Ren, S.; Lei, T. Corncob-derived activated carbon as electrode material for high-performance supercapacitor. Materials 2024, 17, 4341. [Google Scholar] [CrossRef]
- Chang, J.H.; Arunpandian, R.; Nagarani, S.; Kumar, M.; Balachandran, S.; Sivasubramanian, P.; Lenin, R.A. Activated carbon derived from rice husk for highly enhanced symmetric supercapacitor application. Mater. Lett. 2025, 384, 138117. [Google Scholar] [CrossRef]
- Wang, L.; Ma, X.; Ma, Z.; Li, P.; Li, W. Mild chemical-activated hydrothermal porous carbon derived from durian peel biomass for an electrochemical supercapacitor. New J. Chem. 2025, 49, 61–71. [Google Scholar] [CrossRef]
- Singh, A.; Ojha, A.K. Orange peel derived activated carbon for supercapacitor electrode material. J. Mater. Sci. Mater. Electron. 2023, 34, 1003. [Google Scholar] [CrossRef]
- Tripathy, A.; Mohanty, S.; Nayak, S.K.; Ramadoss, A. Renewable banana-peel-derived activated carbon as an inexpensive and efficient electrode material showing fascinating supercapacitive performance. J. Environ. Chem. Eng. 2021, 9, 106398. [Google Scholar] [CrossRef]
- Ncube, A.; Mtetwa, S.; Bukhari, M.; Fiorentino, G.; Passaro, R. Circular economy and green chemistry: The need for radical innovative approaches in the design for new products. Energies 2023, 16, 1752. [Google Scholar] [CrossRef]
- Madhusha, C.; Jayasundara, T.; Munaweera, I.; Perera, C.; Wijesinghe, G.; Weerasekera, M.; Sandaruwan, C.; Meiyazhagan, A.; Robles Hernandez, F.C.; Ajayan, P.M.; et al. Synthesis and structural characterization of copper nanoparticles doped activated carbon derived from coconut coir for drinking water purification. Mater. Today Chem. 2023, 27, 101312. [Google Scholar] [CrossRef]
- Macedo, J.S.; Costa Júnior, N.B.; Almeida, L.E.; Viera, E.F.S.; Cestari, A.R.; Gimenez, I.F.; Carreño, N.L.V.; Barreto, L.S. Kinetic and calorimetric study of the adsorption of dyes on mesoporous activated carbon prepared from coconut coir dust. J. Colloid Interface Sci. 2006, 298, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, R.; Mondal, N.K. Adsorption of fluoride from aqueous solution by a new low-cost adsorbent: Thermally and chemically activated coconut fibre dust. Clean Technol. Environ. Policy 2015, 17, 2157–2172. [Google Scholar] [CrossRef]
- Tetteh, I.K.; Issahaku, I.; Tetteh, A.Y. Recent advances in synthesis, characterization, and environmental applications of activated carbons and other carbon derivatives. Carbon Trends 2024, 14, 100328. [Google Scholar] [CrossRef]
- Zhang, L.; Zuo, S. The significance of lignocellulosic raw materials on the pore structure of activated carbons prepared by steam activation. Molecules 2024, 29, 3197. [Google Scholar] [CrossRef]
- Roy, M.; Barai, H.R. Chapter 13: Steam-activated Carbon for Supercapacitors. In Biomass-Based Supercapacitors: Design, Fabrication and Sustainability, 1st ed.; Aziz, M.A., Shah, S.S., Eds.; Wiley: Hoboken, NJ, USA, 2023. [Google Scholar]
- Yi, H.; Nakabayashi, K.; Yoon, S.H.; Miyawaki, J. Pressurized physical activation: A simple production method for activated carbon with a highly developed pore structure. Carbon 2021, 183, 735–742. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.-Y.; Egiebora, N.O. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2019, 35, 735–776. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Román-Martínez, M.d.C.; Lillo-Ródenas, M.Á. Chemical Activation of Lignocellulosic Precursors and Residues: What Else to Consider? Molecules 2022, 27, 1630. [Google Scholar] [CrossRef]
- Shiryaev, A.A.; Pré, P.; Pardanaud, C.; Murzin, V.; Averin, A.; Rouzaud, J.N. Microporosity and nanostructure of activated carbons: Characterization by X-ray diffraction and scattering, Raman spectroscopy and transmission electron microscopy. Adsorption 2023, 29, 275–289. [Google Scholar] [CrossRef]
- Ramirez, N.; Sardella, F.; Deiana, C.; Schlosser, A.; Müller, D.; Kißling, P.A.; Klepzig, L.F.; Bigall, N.C. Capacitive behavior of activated carbons obtained from coffee husk. RSC Adv. 2020, 10, 38097–38106. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Guo, Y.; Gurgan, I.; Siddique, N.; Li, Y.S.; Jang, S.; Noh, G.A.; Kim, S.H. Raman spectroscopy analysis of disordered and amorphous carbon materials: A review of empirical correlations. Carbon 2025, 238, 120214. [Google Scholar] [CrossRef]
- Liu, X.; Choi, J.; Xu, Z.; Grey, C.; Fleischmann, S.; Forse, A.C. Raman spectroscopy measurements support disorder-driven capacitance in nanoporous carbons. J. Am. Chem. Soc. 2024, 146, 30748–30752. [Google Scholar] [CrossRef]
- Xu, F.; Wang, D. Chapter 2: Analysis of Lignocellulosic Biomass Using Infrared Methodology. In Pretreatment of Biomass: Processes and Technologies, 1st ed.; Pandey, A., Negi, S., Binod, P., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 7–25. [Google Scholar]
- Sangian, H.F.; Widjaja, A. Effect of pretreatment method on structural changes of coconut coir dust. BioResources 2017, 12, 8030–8046. [Google Scholar] [CrossRef]
- Elnour, A.Y.; Alghyamah, A.A.; Shaikh, H.M.; Poulose, A.M.; Al-Zahrani, S.M.; Anis, A.; Al-Wabel, M.I. Effect of pyrolysis temperature on biochar microstructural evolution, physicochemical characteristics, and its influence on biochar/polypropylene composites. Appl. Sci. 2019, 9, 1149. [Google Scholar] [CrossRef]
- Mopoung, S.; Dejang, M. Activated carbon preparation from eucalyptus wood chips using continuous carbonization–steam activation process in a batch intermittent rotary kiln. Sci. Rep. 2021, 11, 1394. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J. Definitions of pseudocapacitive materials: A brief review. Energy Environ. Mater. 2019, 2, 30–37. [Google Scholar] [CrossRef]
- Sharma, S.; Chand, P. Supercapacitor and electrochemical techniques: A brief review. Results Chem. 2023, 5, 100885. [Google Scholar] [CrossRef]
- Supiyeva, Z.; Pan, X.; Abbas, Q. The critical role of nanostructured carbon pores in supercapacitors. Curr. Opin. Electrochem. 2023, 39, 101249. [Google Scholar] [CrossRef]
- Sayed, M.S.; Aman, D.; Fayed, M.G.; Omrane, M.M.; Zaki, T.; Mohamed, S.G. Unravelling the role of pore structure of biomass-derived porous carbon in charge storage mechanisms for supercapacitors. RSC Adv. 2024, 14, 24631–24642. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, F.; Songa, Y.; Li, Y. Revitalizing carbon supercapacitor electrodes with hierarchical porous structures. J. Mater. Chem. A 2017, 5, 17705–17733. [Google Scholar] [CrossRef]
- Yu, M.; Han, Y.; Li, J.; Wang, L. CO2-activated porous carbon derived from cattail biomass for removal of malachite green dye and application as supercapacitors. Chem. Eng. J. 2017, 317, 493–502. [Google Scholar] [CrossRef]
- Alhebshi, N.A.; Salah, N.; Hussain, H.; Salah, Y.N.; Yin, J. Structural and electrochemical properties of physically and chemically activated carbon nanoparticles for supercapacitors. Nanomaterials 2022, 12, 122. [Google Scholar] [CrossRef]
- Adan-Mas, A.; Alcaraz, L.; Arévalo-Cid, P.; López-Gómez, F.A.; Montemor, F. Coffee-derived activated carbon from second biowaste for supercapacitor applications. Waste Manag. 2021, 120, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Misnon, I.I.; Zain, N.K.M.; Aziz, R.A.; Vidyadharan, B.; Jose, R. Electrochemical properties of carbon from oil palm kernel shell for high performance supercapacitors. Electrochim. Acta 2015, 174, 78–86. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, D.; Zhang, G.; Li, B.; Wang, A.; Sun, K. Oaks-derived activated carbon by trace alkali-induced catalytic steam activation for electrochemical capacitor applications. J. Energy Storage 2022, 53, 105090. [Google Scholar] [CrossRef]
- Noh, J.-H.; Lee, K.Y.; Kim, J.-H.; Lee, H.-M.; Radhakrishnan, S.; Kim, B.S. Green synthesis of Kenaf-based activated carbons with excellent rate capability and cycle-life via hydrothermal-co-activation process for high-performance capacitive energy storage. Colloids Surf. A Physicochem. Eng. Asp. 2024, 682, 132874. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, X.; Chen, W.; Chen, M.; Liu, C. Enhancement of the electrochemical properties of commercial coconut shell-based activated carbon by H2O dielectric barrier discharge plasma. R. Soc. Open Sci. 2019, 6, 180872. [Google Scholar] [CrossRef]
- Forgahni, T.M.; Donne, S.W. Method comparison for deconvoluting capacitive and pseudo-capacitive contributions to electrochemical capacitor electrode behavior. J. Electrochem. Soc. 2018, 165, A664–A673. [Google Scholar] [CrossRef]
- Li, Z.; Wu, H.; Zhang, D.; Wang, Q.; Sun, H.; Sun, Q.; Wang, B. Revealing the mechanism of oxygen-containing functional groups on the capacitive behavior of activated carbon. Appl. Surf. Sci. 2024, 657, 159744. [Google Scholar] [CrossRef]
- Jerigová, M.; Odziomek, M.; López-Salas, N. “We are here!” oxygen functional groups in carbons for electrochemical applications. ACS Omega 2022, 7, 11544–11554. [Google Scholar]
- Kim, J.-H.; Kim, S.-H.; Kim, B.-J.; Lee, H.-M. Effects of Oxygen-Containing Functional Groups on the Electrochemical Performance of Activated Carbon for EDLCs. Nanomaterials 2023, 13, 262. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.S.; Vivekanandhan, S. Effect of carbonization temperatures on the synthesis of biocarbon from Borassus flabellifer fruit fiber for capacitive energy storage. Appl. Res. 2024, 3, e202400005. [Google Scholar] [CrossRef]
- Nuilek, K.; Wongwiriyapan, W.; Sattayarut, V.; Simon, A.; Koncz-Horváth, D.; Ferenczi, T.; Kristály, F.; Baumli, P. Comparison of acid exfoliators in carbon nanosheets synthesis from stinging nettle (Urtica dioica) for electrochemical applications. Sci. Rep. 2020, 10, 17270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Pang, Y.; Wang, B.; Guo, H.; Liu, L. Wood-derived carbon electrodes prepared via simple surface treatment for high performance supercapacitors. Diam. Relat. Mater. 2024, 41, 110684. [Google Scholar] [CrossRef]
- Jain, A.; Ghosh, M.; Krajewski, M.; Kurungot, S.; Michalska, M. Biomass-derived activated carbon material from native European deciduous trees as an inexpensive and sustainable energy material for supercapacitor application. J. Energy Storage 2021, 34, 102178. [Google Scholar] [CrossRef]
- Somyanonthanakun, W.; Greszta, A.; Roberts, A.J.; Thongmee, S. Sugarcane bagasse-derived activated carbon as a potential material for lead ions removal from aqueous solution and supercapacitor energy storage application. Sustainability 2023, 15, 5566. [Google Scholar] [CrossRef]
- Abouelamaiem, D.I.; Rasha, L.; He, G.; Neville, T.P.; Millichamp, J.; Mason, T.J.; Jorge, A.B.; Parkin, I.P.; Titirici, M.-M.; Wang, R.; et al. Integration of supercapacitors into printed circuit boards. J. Energy Storage 2018, 19, 28–34. [Google Scholar] [CrossRef]
- Karaaslan, M.A.; Lin, L.T.; Ko, F.; Renneckar, S. Carbon aerogels from softwood kraft lignin for high performance supercapacitor electrodes. Front. Mater. 2022, 9, 894061. [Google Scholar] [CrossRef]
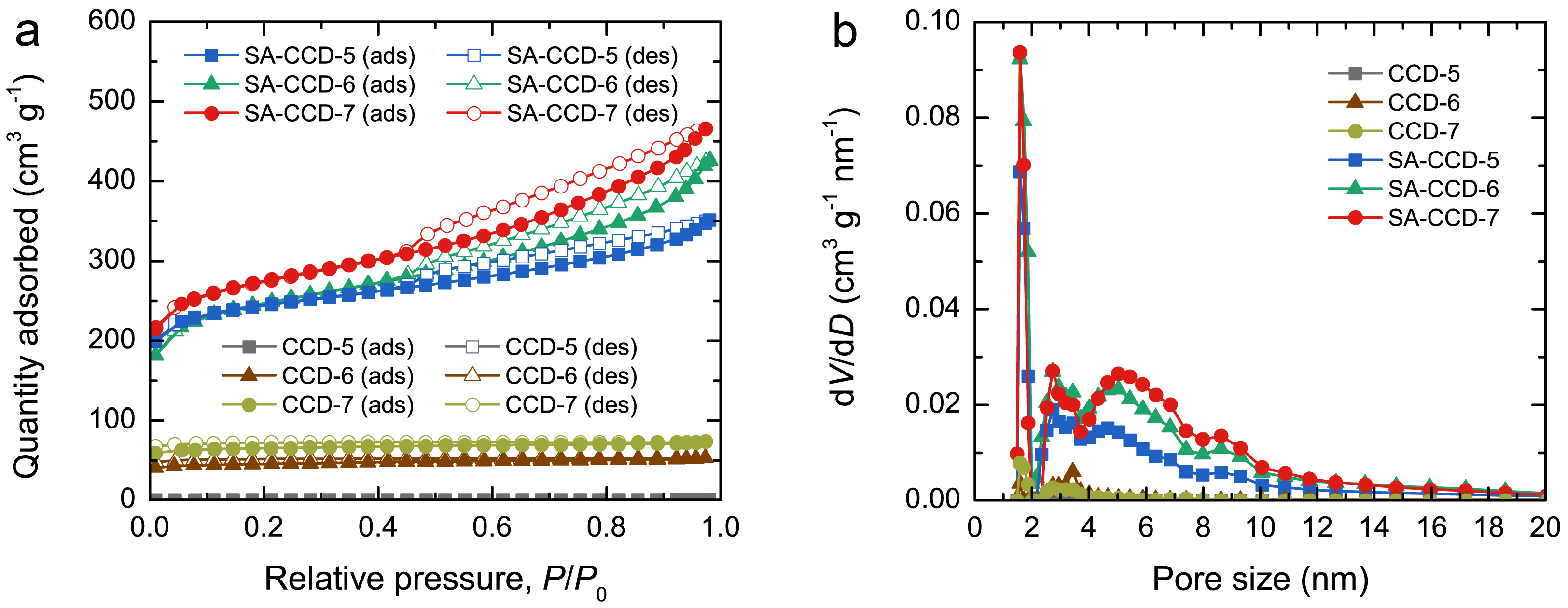
| Sample | SBET (m2 g−1) | Smicro (m2 g−1) | Smeso (m2 g−1) | Vtotal (cm3 g−1) | Vmicro (cm3 g−1) | Vmeso (cm3 g−1) |
|---|---|---|---|---|---|---|
| CCD-5 | 7 | 0 | 7 | 0.012 | 0 | 0.012 |
| CCD-6 | 140 | 111 | 29 | 0.082 | 0.058 | 0.024 |
| CCD-7 | 203 | 172 | 31 | 0.113 | 0.087 | 0.026 |
| SA-CCD-5 | 779 | 526 | 253 | 0.536 | 0.268 | 0.268 |
| SA-CCD-6 | 812 | 507 | 305 | 0.644 | 0.232 | 0.412 |
| SA-CCD-7 | 889 | 531 | 358 | 0.715 | 0.269 | 0.446 |
| Sample | Source | Activating Agent | Activation Condition | SBET (m2 g−1) | Cs (F g−1) | Electrolyte | Ref. |
|---|---|---|---|---|---|---|---|
| CAC | Cattail | CO2 | 850 °C, 2 h | 441 | 110 | 6 M KOH | [46] |
| CO2 activated | Date palm frond | CO2 | 900 °C, 90 min | 604 | 57 | 1 M H2SO4 | [47] |
| PA-3 | Spent coffee grounds | Steam | 800 °C, 60 min | 981 | 72 | 1 M Na2SO4 | [48] |
| AC-P | Oil palm kernel shell | Steam | 500 °C, 4 h | 727 | 94 | 1 M KOH | [49] |
| AC-S | Oak | Steam | 800 °C, 40 min | 581 | 105 | 6 M KOH | [50] |
| p-KF-9A | Kenaf | Steam | 900 °C, 30 min | 1375 | 85 | 6 M KOH | [51] |
| CSAC | Coconut shell | - | - | 780 | 94 | 6 M KOH | [52] |
| SA-CCD-7 | Coconut coir | Steam | 900 °C, 2 h | 889 | 86 | 6 M KOH | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kongtip, J.; Kanjulkeat, N.; Ninneit, T.; Phanapadipong, N.; Chaiammart, N.; Eiad-ua, A.; Munprom, R.; Panomsuwan, G. Green Synthesis of Activated Carbons from Coconut Coir Dust via Steam Activation for Supercapacitor Electrode Applications. Chemistry 2025, 7, 184. https://doi.org/10.3390/chemistry7060184
Kongtip J, Kanjulkeat N, Ninneit T, Phanapadipong N, Chaiammart N, Eiad-ua A, Munprom R, Panomsuwan G. Green Synthesis of Activated Carbons from Coconut Coir Dust via Steam Activation for Supercapacitor Electrode Applications. Chemistry. 2025; 7(6):184. https://doi.org/10.3390/chemistry7060184
Chicago/Turabian StyleKongtip, Jirayu, Natapol Kanjulkeat, Thanapol Ninneit, Norapat Phanapadipong, Nattapat Chaiammart, Apiluck Eiad-ua, Ratiporn Munprom, and Gasidit Panomsuwan. 2025. "Green Synthesis of Activated Carbons from Coconut Coir Dust via Steam Activation for Supercapacitor Electrode Applications" Chemistry 7, no. 6: 184. https://doi.org/10.3390/chemistry7060184
APA StyleKongtip, J., Kanjulkeat, N., Ninneit, T., Phanapadipong, N., Chaiammart, N., Eiad-ua, A., Munprom, R., & Panomsuwan, G. (2025). Green Synthesis of Activated Carbons from Coconut Coir Dust via Steam Activation for Supercapacitor Electrode Applications. Chemistry, 7(6), 184. https://doi.org/10.3390/chemistry7060184









