1. Introduction
Graphene quantum dots have attracted considerable attention due to their unique optical and electronic properties [
1,
2]. The attachment of various functional groups to GQDs represents an effective strategy for enhancing optical absorption intensity [
3]. Specifically, the addition of an epoxy group to carbon atoms induces a change in their hybridization state from sp
2 to sp
3. This alteration disrupts the delocalization of π-electrons within the benzene rings by modifying the spatial distribution of the electronic wavefunction [
4], which can lead to a change in the energy gap and a redshift of the absorption band into the visible region of the electromagnetic spectrum. Consequently, epoxy group functionalization emerges as a particularly promising tool for tuning the optical properties of GQDs compared to other oxygen-containing functional groups [
5].
Moreover, benzene rings located near the functionalization site undergo significant structural deformation, leading to a loss of planarity. Consequently, the change in carbon hybridization from sp
2 to sp
3, combined with pronounced structural curvature, disrupts the formation of resonance energy within the benzene rings. This results in a decrease in structural stability [
6] due to the loss of aromaticity.
An effective method for precisely evaluating the influence of the epoxy group at different sites on GQDs is to perform quantum-chemical calculations. However, the main drawback of this approach lies in its high computational complexity and cost.
As an alternative approach, Clar’s rule [
7,
8,
9] may be employed to assess structural stability without requiring additional computational efforts. Moreover, this rule potentially enables the identification of trends in energy gap modulation and the prediction of the most reactive sites for functionalization.
However, the applicability and predictive potential of Clar’s rule for functionalized GQDs—particularly under conditions of structural non-planarity and sp3 hybridization—remain insufficiently explored. The primary objective of this work is to evaluate the validity of Clar’s rule for predicting both the stability and energy gap modulation of GQDs upon epoxy group functionalization.
2. Models and Methods
As a GQD model structure, the coronene molecule was selected—a polycyclic aromatic hydrocarbon (PAH) with chemical formula C
24H
12 and point symmetry group D
6h—due to its high stability [
10] and suitability for analysis using Clar’s rule.
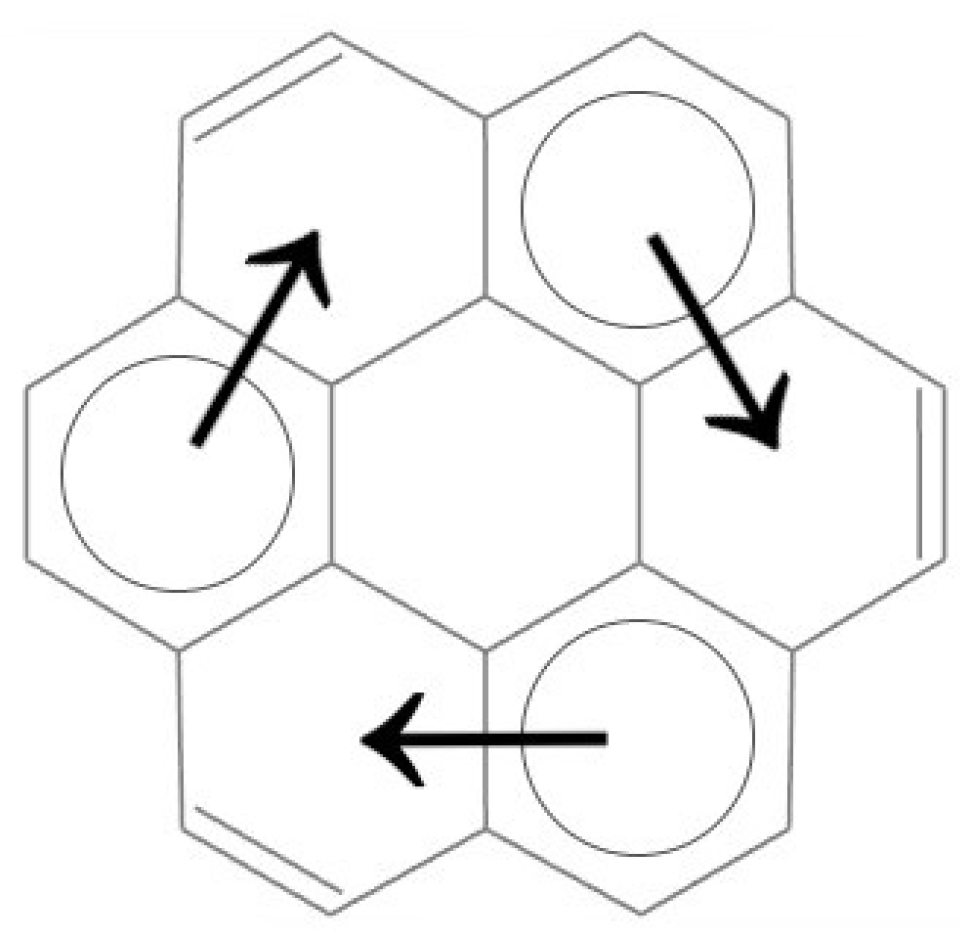
The coronene molecule possesses three migrating aromatic sextets, indicating a high degree of π-electron delocalization and making it a suitable model for analyzing the effects of functionalization. The coronene molecule, with its aromatic sextets and their migration pathways highlighted, is illustrated in
Figure 1. The positions of the aromatic sextets are marked by circles, while the directions of their migration are indicated by arrows.
To investigate the applicability of Clar’s rule for evaluating the influence of the epoxy group, quantum-chemical calculations were performed within the framework of density functional theory (DFT) [
11], employing numerical orbitals for the basis functions (Double Numerical plus Polarization basis set), which has demonstrated high accuracy in nanoscale structure calculations [
12].
The geometries of both pristine and functionalized coronene molecules were optimized using the hybrid B3LYP functional [
13], which is widely employed in quantum-chemical calculations of GQDs. To account for van der Waals dispersion interactions, Grimme’s dispersion correction (G06) was applied [
14]. Vibrational frequency calculations were performed for all optimized functionalized structures using B3LYP functional to confirm the absence of imaginary frequencies.
For assessing the overall stability of a molecules and comparing isomers we calculated the oxygen binding energy for the epoxy-functionalized coronene derivatives:
where
Etotal—total energy of the system,
Ecor—total energy of the pristine coronene molecule,
EO—the energy of a single, isolated oxygen atom in triplet ground state.
To identify the sites on coronene most susceptible to epoxy group attachment, the Fukui function (
), which quantifies susceptibility to free radical attack, was calculated based on the frontier molecular orbital theory [
15] using the GGA-PBE functional [
16]:
where
ρN±1(
r)—electron density of the anion/cation.
Optical properties were computed using time-dependent density functional theory (TD-DFT) with the B3LYP/def2-SVP level of theory [
17] and the Grimme D3 dispersion correction [
18].
3. Results and Discussion
To qualitatively assess the applicability of Clar’s rule, it is first necessary to identify the structural sites most susceptible to functionalization.
Figure 2 presents the computed
isosurface for coronene, highlighting the most probable sites for functionalization. Regions marked in blue correspond to the highest reactivity; the larger the Fukui function value at a given site, the higher the probability of functionalization at that position on the GQD.
As evident from
Figure 2, the most reactive region of coronene is located at the edge, involving carbon atoms belonging exclusively to a single benzene ring. Attaching the oxygen atom of the epoxy group to such edge carbons minimizes energetic penalties associated with the disruption of aromatic sextets, as the sp
3 hybridization change affects only one ring. Furthermore, positioning the epoxy group away from the molecular center effectively localizes and isolates structural strain, thereby minimizing overall curvature and preserving the maximum possible number of aromatic sextets.
Due to the high symmetry of the coronene molecule (D
6h point group), four unique positions for epoxy group attachment can be identified: two at edge sites and two at interior sites. The optimized geometries of coronene functionalized at these positions are illustrated in
Figure 3. Aromatic sextets are indicated by circles, and their migration pathways are marked by arrows. The realistic ball-stick model structures are provided in the
Supplementary Information (Figure S1).
For all optimized functionalized structures, vibrational frequency analysis was performed, confirming the absence of imaginary frequencies and thus verifying that each geometry corresponds to a local minimum on the potential energy surface. The simulated IR spectra are provided in the
Supporting Information (Figure S2).
The oxygen binding energy, energy gap, and point symmetry group for epoxy-functionalized coronene derivatives are summarized in
Table 1.
Figure 4 presents the spatial distributions of the frontier molecular orbitals—HOMO (shown at the bottom) and LUMO (shown at the top)—for pristine coronene and its functionalized variants, with the corresponding energy gap values displayed in the center. The frontier molecular orbital distribution of graphene quantum dots is shown in the
Supplementary Information (Figure S3).
Optical absorption spectra for pristine coronene and its epoxy-functionalized forms at different positions are provided in the
Supporting Information (Figure S4). Additionally, fluorescence wavelengths for pristine and all functionalized GQDs were calculated (
see Table S1 in the Supporting Information). This was accomplished by optimizing the geometry of the first singlet excited state for each of these structures.
The epoxy group at position α attaches to carbon atoms belonging exclusively to a single benzene ring, thereby minimizing local structural strain. As a result, the overall planarity of the structure is preserved, and the number of aromatic sextets remains maximal. Consequently, position α exhibits the highest stability and is, therefore, the most probable site for functionalization.
Moreover, for position α, a significant reduction in the energy gap by 0.56 eV compared to pristine coronene is observed, which potentially results in a redshift of the optical absorption toward longer wavelengths in the electromagnetic spectrum.
Attachment of the epoxy group at position β leads to localization of π-electrons in adjacent benzene rings, resulting in the disruption of one aromatic sextet. Nevertheless, due to the preservation of two migrating sextets, position β remains relatively stable, exhibiting oxygen binding energy second only to that of position α.
It is also important to note that position β exhibits the largest reduction in energy gap—by 1.22 eV compared to pristine coronene—indicating the strongest influence on optical properties among all considered functionalization sites.
Thus, edge sites of GQDs exhibit the highest reactivity, which is fully consistent with the results of the Fukui function () calculations. It can therefore be concluded that functionalization at edge positions represents a promising strategy for tuning the optical properties of GQDs, owing to the significant reduction in energy gap observed at these sites.
Attachment of the epoxy group at position γ induces significant structural deformation of the quantum dot, leading to a pronounced loss of planarity. Although the valence angles of carbon atoms in adjacent benzene rings remain within the range of 118–121°—formally permitting the identification of two migrating aromatic sextets—the disruption of ring planarity substantially reduces the overlap of 2p-orbitals. This diminished orbital overlap results in a decrease in the resonance energy of the aromatic sextets. Consequently, the coronene molecule functionalized at position γ exhibits the lowest stability among all considered configurations.
Nevertheless, despite its low stability, pronounced structural deformation, and disruption of one aromatic sextet, position γ exhibits only a minor reduction in energy gap—merely 0.19 eV compared to pristine coronene.
Attachment of the epoxy group at position δ was initially expected to disrupt the local aromaticity of four adjacent benzene rings, leading to the destruction of two aromatic sextets. However, in this specific configuration, it proves energetically more favorable for the C–C bond between the carbon atoms bonded to the oxygen atom to undergo cleavage [
19]. This bond rupture results in an ether-like configuration with increased valence angles, thereby enhancing the oxygen binding energy. Thus, C–C bond scission upon epoxy functionalization acts as an effective self-stabilizing mechanism, preserving the structural integrity of the GQD and preventing the loss of a larger number of aromatic sextets.
Thus, the carbon atoms regain sp2 hybridization, allowing resonance stabilization to be preserved within the benzene rings. Structural planarity is also partially maintained due to the presence of CS symmetry at this functionalization site, resulting in symmetric distortion of the two adjacent benzene rings. The valence angles of the carbon atoms in these rings range from 115° to 121°, which is sufficient to ensure effective p-orbital overlap and sustain resonance energy.
Similar to position γ, the aromatic sextets in the distorted benzene rings at position δ exhibit reduced resonance energy due to the overall loss of structural planarity. For this reason, despite possessing three sextets, position δ demonstrates a lower oxygen binding energy than position β, which retains only two sextets.
It is also noteworthy that the energy gap value for position δ remains unchanged compared to pristine coronene. This can be explained by the fact that the oxygen atom after the C–C bond cleavage is practically uninvolved in the formation of the frontier molecular orbitals, as can be seen from
Figure S2e. Consequently, it can be concluded that functionalization at interior sites of GQDs exerts minimal influence on their optical properties. Additionally, the presence of a larger number of benzene rings surrounding the functionalization site is hypothesized to stabilize the structure by mitigating the disruptive effect of the epoxy group on overall planarity. As demonstrated in reference [
5], attachment of an epoxy group to the central region of a larger graphene quantum dot with chemical formula C
48H
18 and point symmetry group D
3h does not induce C–C bond cleavage. This suggests that the probability of C–C bond rupture decreases proportionally with increasing GQD size.
Based on the obtained results, a clear correlation between energy gap modulation and Clar’s rule can be established. When comparing edge functionalization sites, position α—which retains a greater number of aromatic sextets compared to position β—exhibits a wider energy gap. A similar trend is observed for interior functionalization sites: position δ demonstrates a larger energy gap than position γ, owing to C–C bond cleavage and the consequent preservation of a higher number of aromatic sextets. To generalize the findings obtained using the coronene model, we also performed calculations employing ovalene as an alternative molecular model of a graphene quantum dot (GQD). The results for various non-equivalent epoxidation sites are provided in the
Supporting Information (Figure S5 and Table S2). Importantly, the outcomes derived from both models proved to be consistent.
The ability to predict energy gap modulation enables precise tuning of optical properties, which is particularly crucial for the application of GQDs as efficient photovoltaic materials. As shown in
Figure S3 (Supporting Information), the optical absorption spectra demonstrate a distinct redshift in absorption bands for functionalization at positions α and β, corresponding to transitions toward longer wavelengths in the electromagnetic spectrum.