Selenium Methylation: Insights into Chemical Reactions and Enzymatic Pathways
Abstract
1. Introduction
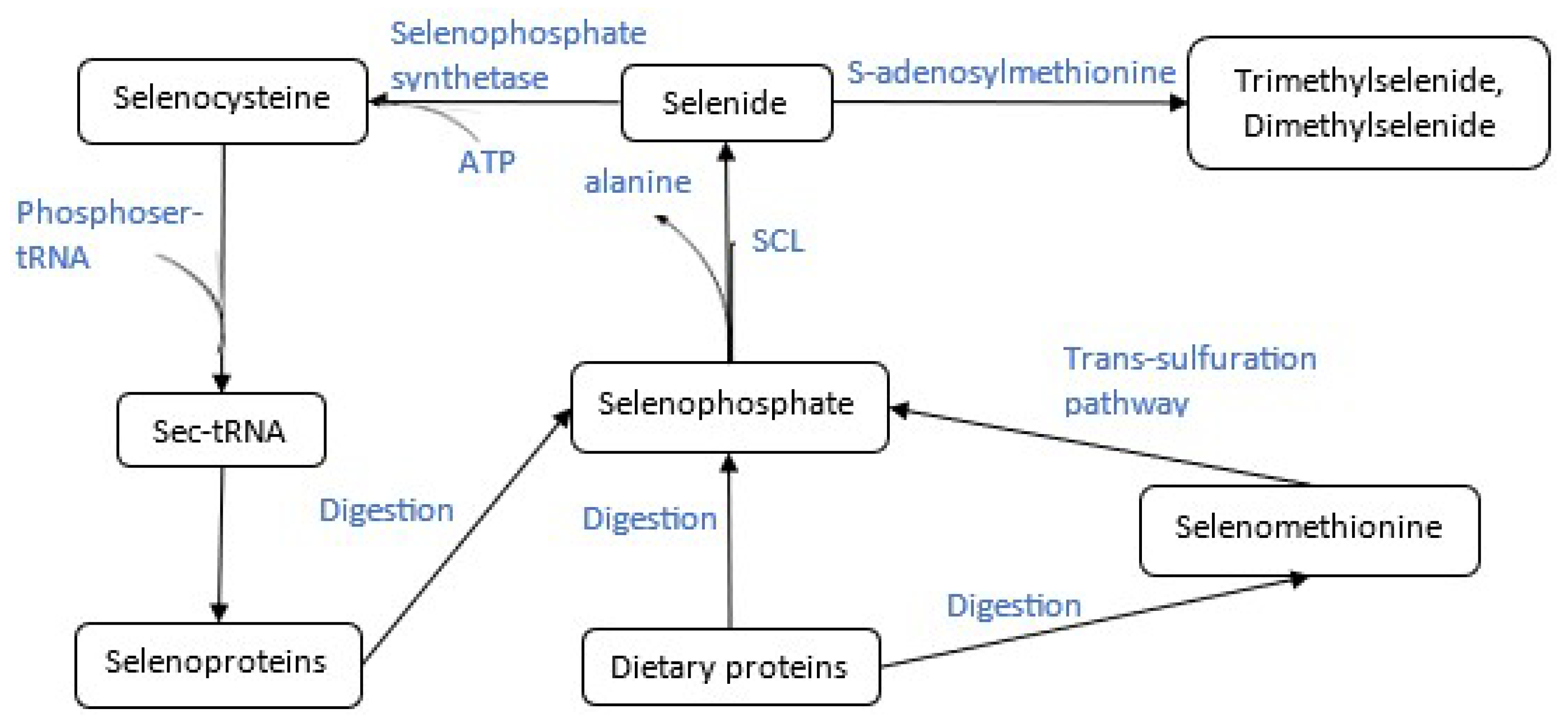
2. Selenium Metabolism and Methylation
2.1. Reaction Mechanisms and Pathways
2.1.1. Primary Chemical Reactions in Selenium Methylation
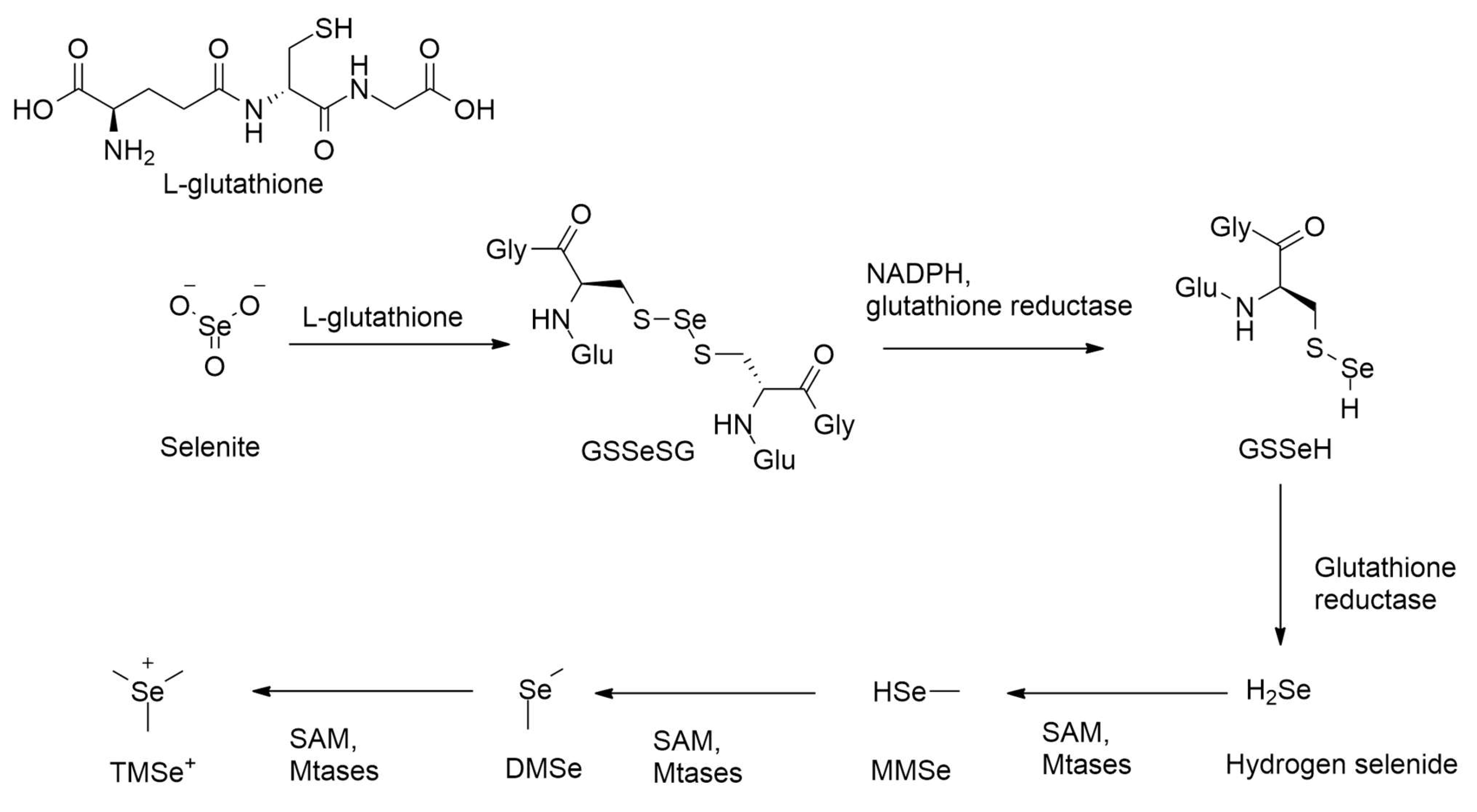
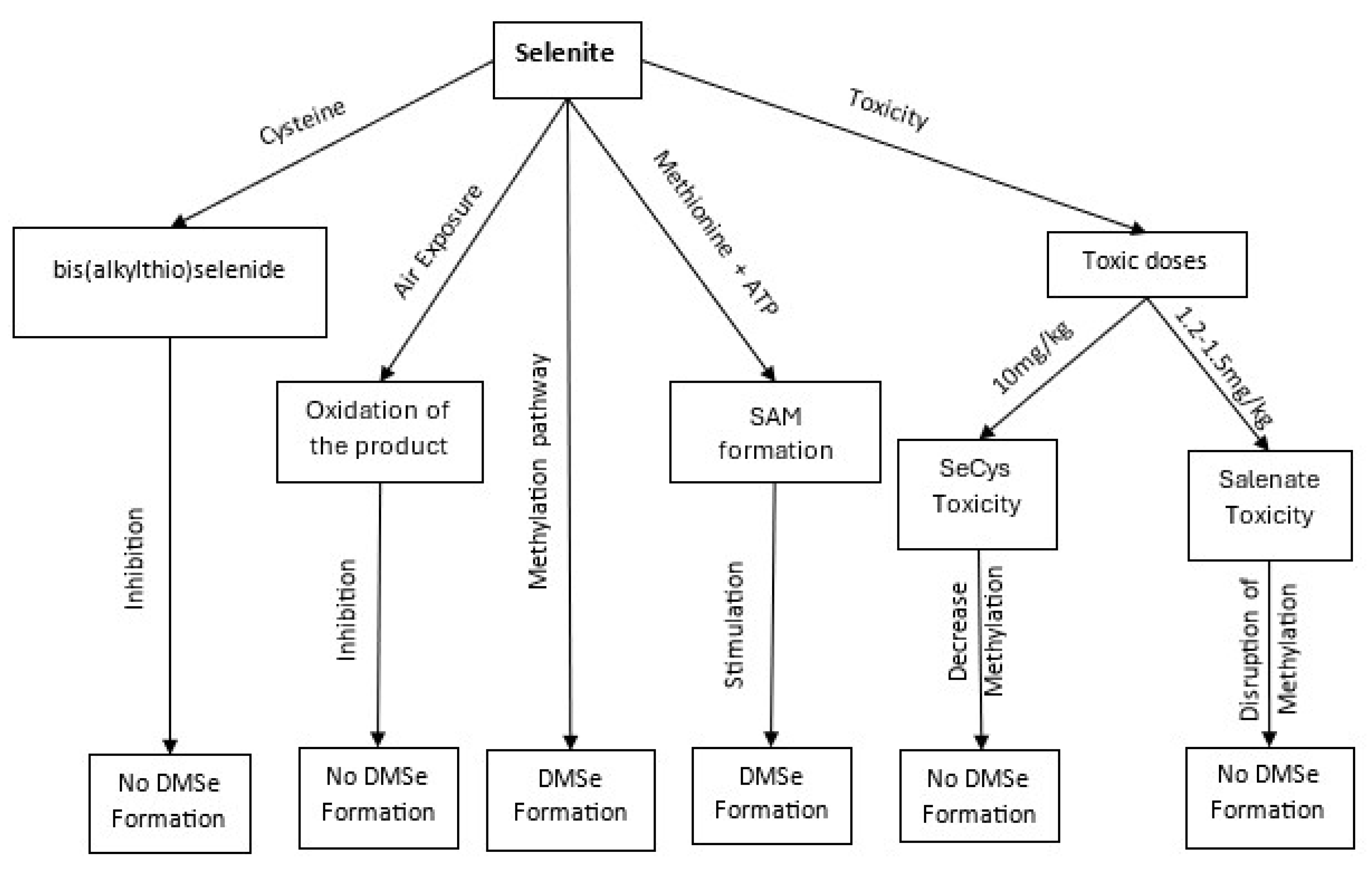
2.1.2. Detoxification of Inorganic Forms of Selenium
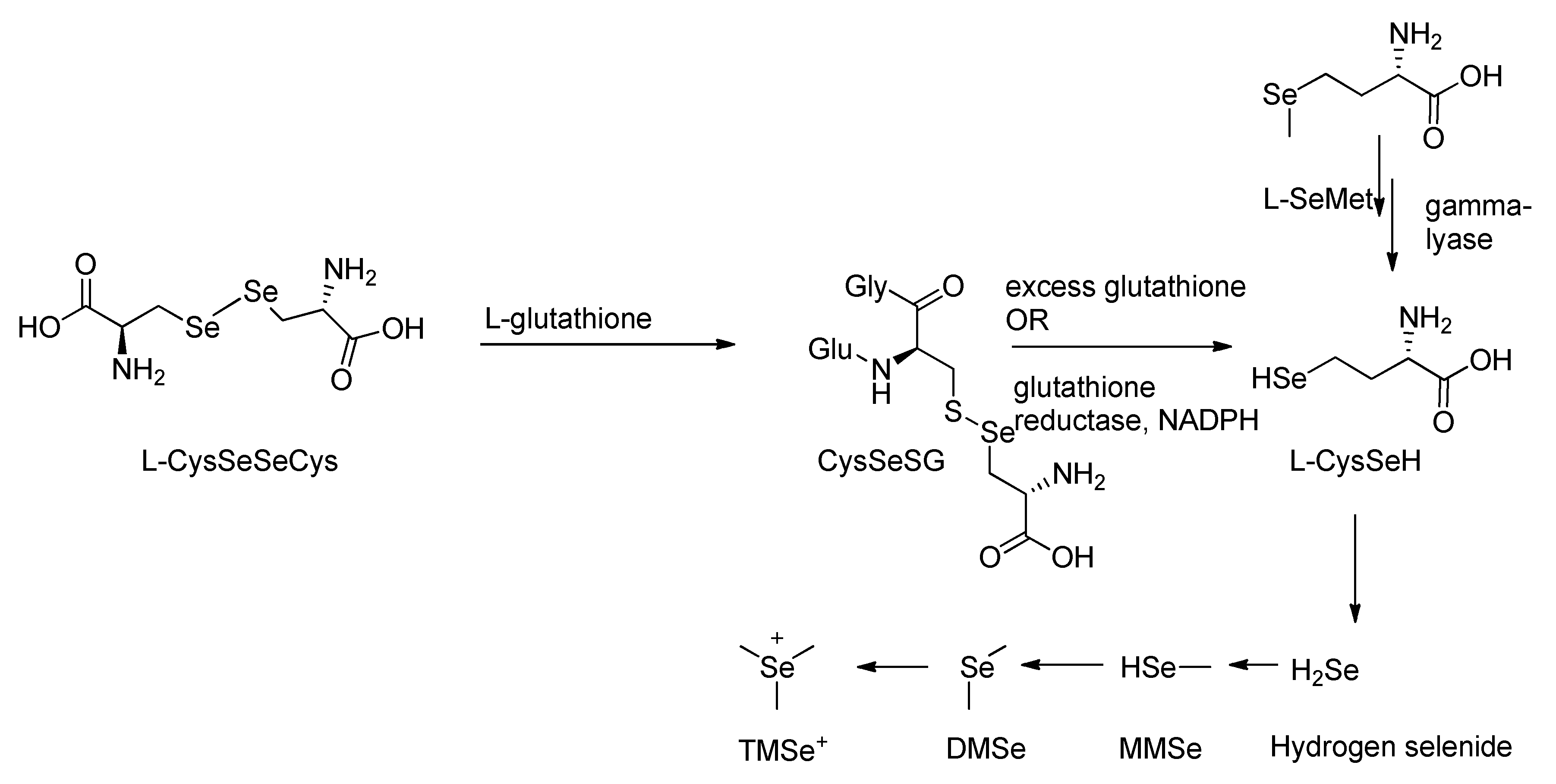
2.1.3. Intermediate and Excretory Metabolites in Selenium Methylation
2.2. Enzymes in Selenium Methylation
2.2.1. Role of Enzyme Glutathione Reductase
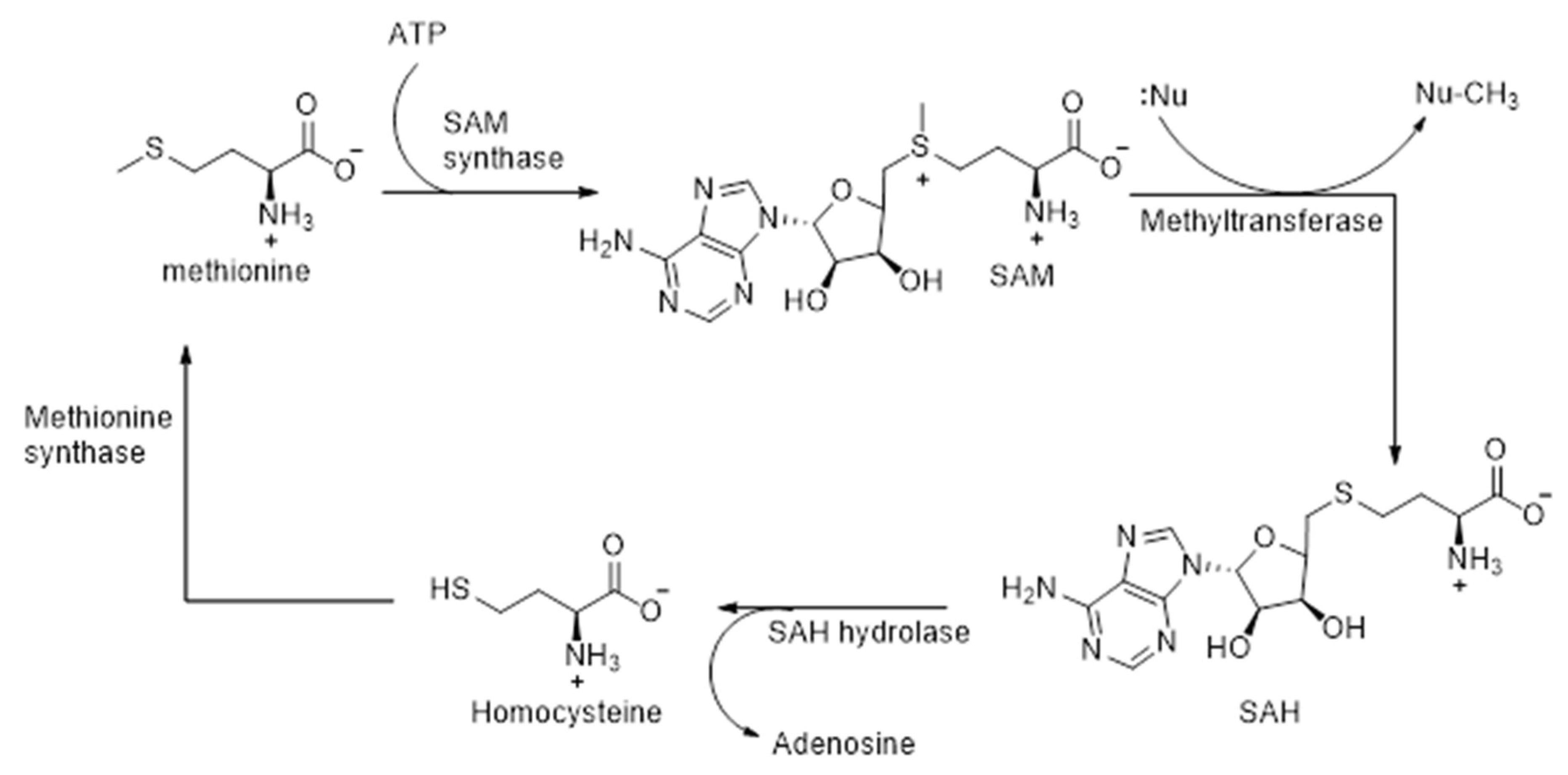
2.2.2. Role of SAM-Dependent Methyltransferases
2.2.3. Role of Selenocysteine Lyase Enzyme
2.3. Factors Influencing Rate and Selectivity
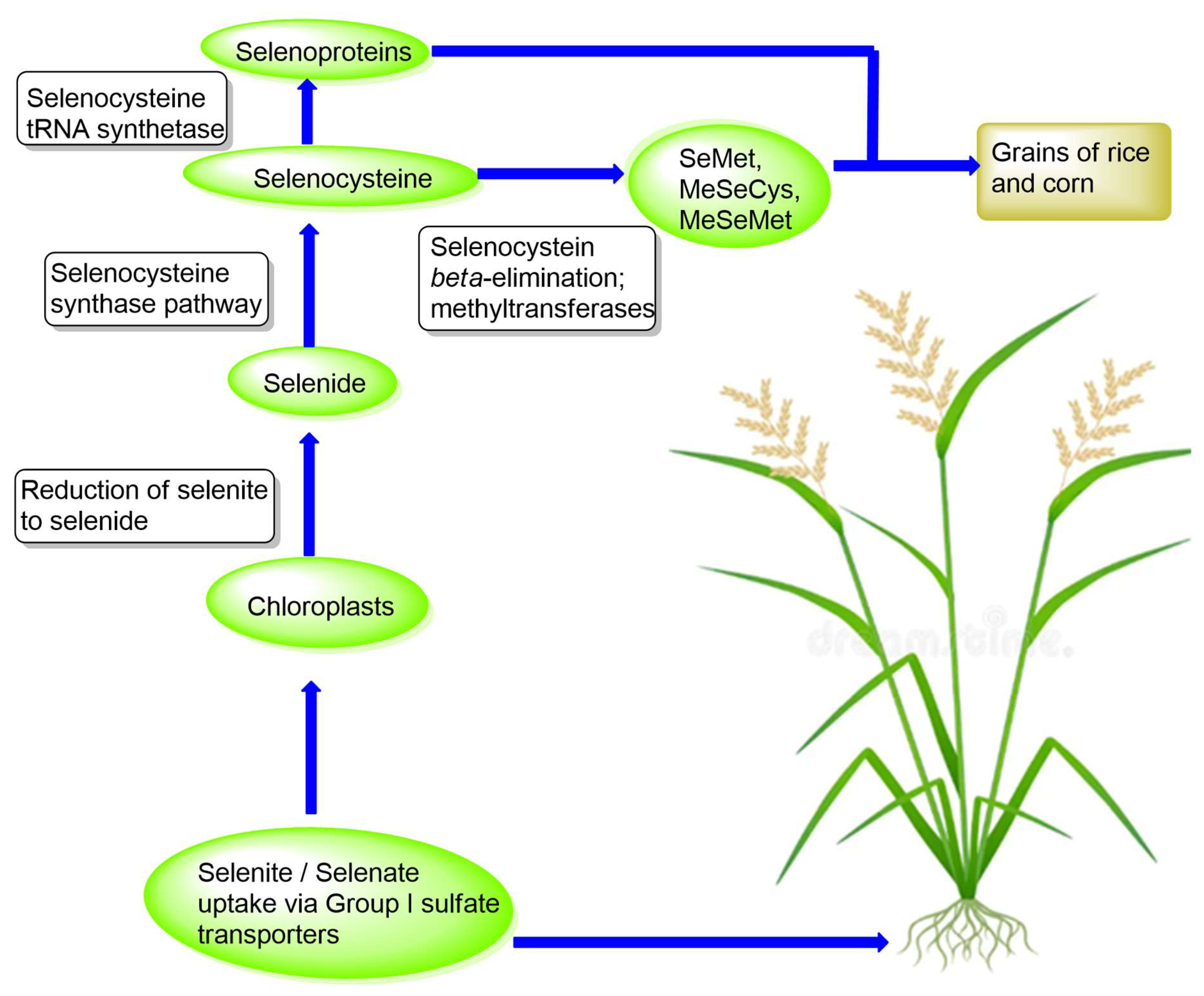
2.4. Pathways of Selenium Compounds Methylation in Rice and Corn
2.5. Chemical Structures’ Impact on Stability and Reactivity
2.6. Roles of Biomolecules in Selenium Methylation
2.7. Environmental and Species-Specific Factors
2.7.1. Participation of Different Selenium Species in Methylation
2.7.2. Environmental Conditions for Selenium Methylation in Rice and Corn
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwarz, K.; Foltz, C.M. Selenium as an Integral Part of Factor 3 Against Dietary Necrotic Liver Degeneration. J. Am. Chem. Soc. 1957, 79, 3292–3293. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Shahidin; Wang, Y.; Wu, Y.; Chen, T.; Wu, X.; Yuan, W.; Zhu, Q.; Wang, X.; Zi, C. Selenium and Selenoproteins: Mechanisms, Health Functions, and Emerging Applications. Molecules 2025, 30, 437. [Google Scholar] [CrossRef] [PubMed]
- Batyrova, G.; Taskozhina, G.; Umarova, G.; Umarov, Y.; Morenko, M.; Iriskulov, B.; Kudabayeva, K.; Bazargaliyev, Y. Unveiling the Role of Selenium in Child Development: Impacts on Growth, Neurodevelopment and Immunity. J. Clin. Med. 2025, 14, 1274. [Google Scholar] [CrossRef] [PubMed]
- Cilla, A.; Barberá, R.; Lagarda, M.J.; Farré, R. Nutriential Hazards: Micronutrients: Vitamins and Minerals. Encycl. Food Saf. 2014, 3, 86–94. [Google Scholar] [CrossRef]
- Zhang, F.; Li, X.; Wei, Y. Selenium and Selenoproteins in Health. Biomolecules 2023, 13, 799. [Google Scholar] [CrossRef]
- Kosnett, M.; Chan, L.; Faustman, E.; Foldy, S.; Miller, G.; Takaro, T.; Ahmed, S.; Allen, P.; Boehme, J.; Gabriel, M.; et al. A Review of Human Health Impacts of Selenium in Aquatic Systems. A Report Submitted to the International Joint Commission by the Health Professionals Advisory Board. 2020. Available online: https://ijc.org/sites/default/files/2020-09/HPAB_SeleniumHealthReview_2020.pdf (accessed on 15 July 2025).
- Antonyak, H.; Malavolta, M.; Mocchegiani, E. Healthy Ageing and Longevity. In Trace Elements and Minerals in Health and Longevity; Malavolta, M., Mocchegiani, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; Volume 8. [Google Scholar]
- Agarwal, P.; Mehta, R.; Panse, G.; Patil, S. Selenium toxicity: A rare diagnosis. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 690–693. [Google Scholar] [CrossRef]
- Mehdi, Y.; Hornick, J.-L.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef]
- Misra, S.; Boylan, M.; Selvam, A.K.; Spallholz, J.E.; Björnstedt, M.; Fernandes, A.P. Redox-active selenium compounds—From toxicity and cell death to cancer treatment. Nutrients 2015, 7, 3536–3556. [Google Scholar] [CrossRef]
- Grosicka-Maciąg, E.; Kurhaluk, N.; Zielińska, A.; Skrajnowska, D. Biomedical effects of selenium in a human organism. J. Elem. 2017, 22, 1269–1284. [Google Scholar] [CrossRef]
- Qi, Z.; Duan, A.; Ng, K. Selenoproteins in Health. Molecules 2023, 29, 136. [Google Scholar] [CrossRef]
- Hodges, R.E.; Minich, D.M. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J. Nutr. Metab. 2015, 2015, 760689. [Google Scholar] [CrossRef]
- Hassoun, B.S.; Stohs, S.J.; Bagchi, D. Selenium detoxification by methylation. Res. Commun. Mol. Pathol. Pharmacol. 1995, 90, 133–142. [Google Scholar] [PubMed]
- Fukumoto, Y.; Rin Kyono, R.; Shibukawa, Y.; Tanaka, Y.; Suzuki, N.; Ogra, Y. Differential molecular mechanisms of substrate recognition by selenium methyltransferases, INMT and TPMT, in selenium detoxification and excretion. J. Biol. Chem. 2024, 300, 105599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; He, F.; Li, L.; Guo, L.; Zhang, B.; Yu, S.; Zhao, W. Bioavailability Based on the Gut Microbiota: A New Perspective. Microbiol. Mol. Biol. Rev. 2020, 84, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.L.U.; Sena-Evangelista, K.C.M.; de Azevedo, E.P.; Pinheiro, F.I.; Ney Cobucci, R.; Pedrosa, L.F.C. Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship With Diseases. Front. Nutr. 2021, 8, 685317. [Google Scholar] [CrossRef]
- Ferenc, K.; Śliżewska, K.; Czech, A.; Grela, E.R. Modulation of the Gut Microbiota by Nutrition and Its Relationship to Epigenetics. Int. J. Mol. Sci. 2024, 25, 1228. [Google Scholar] [CrossRef]
- Paul, B.; Barnes, S.; Demark-Wahnefried, W.; Morrow, C.D.; Salvador, C.; Skibola, C.F.; Tollefsbol, T.O. Influences of Diet and the Gut Microbiome on Epigenetic Modulation in Cancer and Other Diseases. Clin. Epigenetics 2015, 7, 112. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Biological Activity of Selenium and Its Impact on Human Health. Int. J. Mol. Sci. 2023, 24, 2663. [Google Scholar] [CrossRef]
- Xu, M.; Li, S.; Chen, X.; Wang, J.; Qian, W.; Zhang, Y. Pivotal Biological Processes and Proteins for Selenite Reduction and Methylation in Ganoderma lucidum. J. Hazard. Mater. 2023, 444, 130409. [Google Scholar] [CrossRef]
- Chen, M.; Nie, Y.; Xu, Y.; Li, X.; Xu, Y.; Zang, J.; Zhang, Y.; Zhang, H.; Wu, Z.; Yang, J. Identification and Functional Characterization of a Novel Selenocysteine Methyltransferase from Brassica juncea. J. Exp. Bot. 2019, 70, 6401–6416. [Google Scholar] [CrossRef]
- Ullah, H.; Ullah, W.; Hussain, A.; Khan, M.; Bilal, M.; Mehmood, S.; Mahmood, Q. A Comprehensive Review on Environmental Transformation of Selenium: Recent Advances and Research Perspectives. Environ. Geochem. Health 2019, 41, 1003–1035. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Masuda, H.; Kamiyama, S.; Matsumoto, A.; Nakanishi, H.; Endo, Y.; Ohkubo, T.; Terao, T. Production of a Urinary Selenium Metabolite, Trimethylselenonium, by Thiopurine S-Methyltransferase and Indolethylamine N-Methyltransferase. Chem. Res. Toxicol. 2021, 33, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liu, C.; He, W.; Zhang, X.; Wang, H.; Jin, C.; Liu, J. Unveiling the Vital Role of Soil Microorganisms in Selenium Cycling: A Review. Front. Microbiol. 2024, 15, 1448539. [Google Scholar] [CrossRef] [PubMed]
- Mlangeni, A.T.; Jagot, F.; Namaumbo, S.; Kapito, N.J.; Tsukuluza, D.C.; Botha, L.; Ndovi, P.; Kumambala, P. Analytical Strategies for Quantifying Methylated Selenium Species in Staple Crops: Methods and Emerging Techniques. Chin. J. Anal. Chem. 2025, 53, 100511. [Google Scholar] [CrossRef]
- Reich, H.J.; Hondal, R.J. Why Nature Chose Selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef]
- Madabeni, A.; Bortoli, M.; Nogara, P.A.; Ribaudo, G.; Dalla Tiezza, M.; Flohé, L.; Rocha, J.B.T.; Orian, L. 50 years of organoselenium chemistry, biochemistry and reactivity: Mechanistic understanding, successful and controversial stories. Chem. A Eur. J. 2024, 30, e202403003. [Google Scholar] [CrossRef]
- Orian, L.; Flohé, L. Selenium-catalyzed reduction of hydroperoxides in chemistry and biology. Antioxidants 2021, 10, 1560. [Google Scholar] [CrossRef]
- Naga Jyothi, M.S.V.; Ramaiah, B.J.; Maliyekkal, S.M. Occurrence, contamination, speciation and analysis of selenium in the environment. In Measurement, Analysis and Remediation of Environmental Pollutants; Springer Nature: Berlin/Heidelberg, Germany, 2020; pp. 245–269. [Google Scholar] [CrossRef]
- Sigel, H.; Sigel, A. The bio-relevant metals of the periodic table of the elements. Z. Für Naturforschung B 2019, 74, 461–471. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones 2020, 19, 9–14. [Google Scholar] [CrossRef]
- Sun, H.J.; Rathinasabapathi, B.; Wu, B.; Luo, J.; Pu, L.-P.; Ma, L.Q. Arsenic and selenium toxicity and their interactive effects in humans. Environ. Int. 2014, 69, 148–158. [Google Scholar] [CrossRef]
- Mangiapane, E.; Pessione, A.; Pessione, E. Selenium and Selenoproteins: An Overview on Different Biological Systems. Curr. Protein Pept. Sci. 2014, 15, 598–607. [Google Scholar] [CrossRef]
- Dolgova, N.V.; Hackett, M.J.; MacDonald, T.C.; Nehzati, S.; James, A.K.; Krone, P.H.; George, G.N.; Pickering, I.J. Distribution of selenium in zebrafish larvae after exposure to organic and inorganic selenium forms. Metallomics 2016, 8, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Velichko, O.A. Selenium in poultry nutrition: From sodium selenite to organic selenium sources. J. Poult. Sci. 2018, 55, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Vriens, B.P. The Role of Methylation in the Biogeochemical Selenium Cycle. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2015. Available online: https://www.research-collection.ethz.ch/bitstream/handle/20.500.11850/155269/2/ETH22919.pdf (accessed on 25 February 2025).
- Eswayah, A.S.; Smith, T.J.; Gardiner, P.H.E. Microbial Transformations of Selenium Species of Relevance to Bioremediation. Appl. Environ. Microbiol. 2016, 82, 4848–4859. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, U.D.; Rana, M.; Chakrapani, H. Phenacylselenoesters allow facile selenium transfer and hydrogen selenide generation. Chem. Sci. 2024, 15, 19315–19321. [Google Scholar] [CrossRef]
- Shalini, S.; Bansal, M.P. Co-operative effect of glutathione depletion and selenium induced oxidative stress on AP1 and NFκB expression in testicular cells in vitro: Insights to regulation of spermatogenesis. Biol. Res. 2007, 40, 307–317. [Google Scholar] [CrossRef]
- Paydary, P.; Schellenger, A.E.P.; Teli, M.; Jaisi, D.P.; Onnis-Hayden, A.; Larese-Casanova, P. Chemical oxidation of selenite to selenate: Evaluation of reactive oxygen species and O transfer pathways. Chem. Geol. 2021, 575, 120229. [Google Scholar] [CrossRef]
- Ting, K.K.Y.; Black, A.J.; Zheng, S.; Nguyen, T.; Lui, J.; Le, V.; Day, B.J.; Stocker, R.; Sobey, C.G.; Drummond, G.R.; et al. Measuring the rate of NADPH consumption by glutathione reductase in the cytosol and mitochondria. PLoS ONE 2024, 19, e0309886. [Google Scholar] [CrossRef]
- Koju, N.; Song, J.; You, J.; Lim, S.; Park, S.; Jo, J.; Kim, D.K.; Lee, J. Reduced nicotinamide adenine dinucleotide phosphate in redox balance and diseases: A friend or foe? Acta Pharmacol. Sin. 2022, 43, 1889–1904. [Google Scholar] [CrossRef]
- Gansemer, E.R.; Rutkowski, D.T. Pathways Linking Nicotinamide Adenine Dinucleotide Phosphate Production to Endoplasmic Reticulum Protein Oxidation and Stress. Front. Mol. Biosci. 2022, 9, 858142. [Google Scholar] [CrossRef]
- Spagnoletta, A.; Pastore, D.; Tozzi, M.G.; Pierri, C.L. Modulatory Effect of Nicotinamide Adenine Dinucleotide Phosphate (NADPH) on the 2-Oxoglutarate Mitochondrial Carrier. Molecules 2024, 29, 5154. [Google Scholar] [CrossRef] [PubMed]
- Kuganesan, M.; Haque, M.E.; Kageyama, Y.; Kar, S.; Saif, S.R.; Shimizu, T.; Tomita, K.; Fukuto, J.M.; Nagasawa, H.; Akaike, T. Selenium and hydrogen selenide: Essential micronutrient and the fourth gasotransmitter? Intensive Care Med. Exp. 2019, 7, 71. [Google Scholar] [CrossRef]
- Peyroche, G.; Saveanu, C.; Dauplais, M.; Lazard, M.; Beuneu, F.; Decourty, L.; Malabat, C.; Jacquier, A.; Blanquet, S.; Plateau, P. Sodium selenide toxicity is mediated by O2-dependent DNA breaks. PLoS ONE 2012, 7, e36343. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I.; Zaporowska, H. Selenium interactions and toxicity: A review. Cell Biol. Toxicol. 2012, 28, 31–46. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Z.; Du, S.; Tan, Y.; Wang, L.; Zhang, Z.; Li, Y.; Li, L. Selenium volatilization in plants, microalgae, and microorganisms. Heliyon 2024, 10, e26023. [Google Scholar] [CrossRef]
- Nguyen, T.-Q.; Nicolet, Y. Structure and Catalytic Mechanism of Radical SAM Methylases. Life 2022, 12, 1732. [Google Scholar] [CrossRef]
- Zhang, C.; Weng, J.; Liu, X.; Liu, H.; Deng, Z.; Qu, X.; Zhang, Q. Biotechnological applications of S-adenosyl-methionine-dependent methyltransferases for natural products biosynthesis and diversification. Bioresour. Bioprocess. 2021, 8, 72. [Google Scholar] [CrossRef]
- Lin, H. S-Adenosylmethionine-dependent alkylation reactions: When are radical reactions used? Bioorganic Chem. 2021, 39, 161–170. [Google Scholar] [CrossRef]
- Taylor, D.; Reilly, C.; Burk, R. Recent developments in selenium research. Br. J. Biomed. Sci. 2009, 66, 107–116. [Google Scholar] [CrossRef]
- Rayman, M.P.; Infante, H.G.; Sargent, M. Food-chain selenium and human health: Spotlight on speciation. Br. J. Nutr. 2008, 100, 238–253. [Google Scholar] [CrossRef]
- Kang, D.; Lee, J.; Jung, Y.; Zhang, X.; Lee, Y.J.; Choi, Y.; Lee, S.; Kim, Y.; Lee, E.B.; Bae, S.C.; et al. The role of selenium metabolism and selenoproteins in cartilage homeostasis and arthropathies. Exp. Mol. Med. 2020, 52, 1198–1208. [Google Scholar] [CrossRef]
- Minich, W.B. Selenium Metabolism and Biosynthesis of Selenoproteins in the Human Body. Biochemistry 2022, 87, S168–S177. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, C.; Yao, Q.; Li, J. Role of selenoproteins in redox regulation of signaling and the antioxidant system: A review. Antioxidants 2020, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Wang, L.; Li, J.; Zhang, Y. Effects and Impact of Selenium on Human Health, A Review. Molecules 2024, 30, 50. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Wang, C. Factors affecting selenium-enrichment efficiency, metabolic mechanisms and physiological functions of selenium-enriched lactic acid bacteria. J. Future Foods 2022, 2, 285–293. [Google Scholar] [CrossRef]
- Schneider, H.A. Selenium in Nutrition. Science 1936, 83, 32–34. [Google Scholar] [CrossRef]
- Li, Z.; Liang, D.; Peng, Q.; Cui, Z.; Huang, Q. Interaction between selenium and soil organic matter and its impact on soil selenium bioavailability: A review. Geoderma 2017, 295, 69–79. [Google Scholar] [CrossRef]
- Shini, S.; Sultan, A.; Bryden, W.L.; Downing, J. Selenium Biochemistry and Bioavailability: Implications for Animal Agriculture. Agriculture 2015, 5, 1277–1288. [Google Scholar] [CrossRef]
- Mutonhodza, B.; Joy, E.J.M.; Broadley, M.R.; King, J.C.; Watts, M.J. Linkages between soil, crop, livestock, and human selenium status in Sub-Saharan Africa: A scoping review. Int. J. Food Sci. Technol. 2022, 57, 6336–6349. [Google Scholar] [CrossRef]
- Chasteen, T.G.; Bentley, R. Biomethylation of Selenium and Tellurium: Microorganisms and Plants. Chem. Rev. 2003, 103, 1–26. [Google Scholar] [CrossRef]
- Hasegawa, T.; Suzuki, K.T. Inorganic compounds Mechanisms of Selenium Methylation and Toxicity in Mice Treated with Selenocystine. Arch. Toxicol. 1996, 71, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Nakamuro, K.; Sayato, Y.; Yamanaka, K.; Yamane, Y. Metabolism of Selenoamino Acids and Their Contributionof Selenium Methylation to Their Toxicity. J. Health Sci. 2000, 46, 418–421. [Google Scholar] [CrossRef]
- Fernandes, A.P.; Wallenberg, M.; Gandin, V.; Misra, S.; Tisato, F.; Marzano, C.; Rigobello, M.P.; Kumar, S.; Björnstedt, M. Methylselenol Formed by Spontaneous Methylation of Selenide Is a Superior Selenium Substrate to the Thioredoxin and Glutaredoxin Systems. PLoS ONE 2012, 7, e50727. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.K.; Wong, P.T.S.; Silverberg, B.A.; Luxon, P.L.; Bengert, G.A. Methylation of selenium in the aquatic environment. Science 1976, 192, 1130–1131. [Google Scholar] [CrossRef]
- Ohta, Y.; Suzuki, K.T. Methylation and demethylation of intermediates selenide and methylselenol in the metabolism of selenium. Toxicol. Appl. Pharmacol. 2008, 226, 169–177. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef]
- Vaškov, J.; Trudičová, M.; Dobrota, D. Glutathione-Related Enzymes and Proteins: A Review. Molecules 2023, 28, 1447. [Google Scholar] [CrossRef]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Properties and functions of glutathione reductase in plants. Physiol. Plant. 1989, 77, 449–456. [Google Scholar] [CrossRef]
- Nuttall, K.L. Elemental selenium and glutathione reductase. Med. Hypotheses 1985, 16, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.M. Toxicity of glutathione-binding metals: A review of targets and mechanisms. Toxics 2015, 3, 20–62. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, K.; Nakaki, T. Glutathione in Cellular Redox Homeostasis: Association with the Excitatory Amino Acid Carrier 1 (EAAC1). Molecules 2015, 20, 8742–8758. [Google Scholar] [CrossRef]
- Lushchak, V.I. Glutathione Homeostasis and Functions: Potential Targets for Medical Interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef] [PubMed]
- Csiszár, J.; Horváth, E.; Bela, K.; Gallé, Á.; Erdei, L.; Györgyey, J.; Brunner, S.; Tari, I. Glutathione-Related Enzyme System: Glutathione Reductase (GR), Glutathione Transferases (GSTs) and Glutathione Peroxidases (GPXs). In Redox State as a Central Regulator of Plant-Cell Stress Responses; Springer: Berlin/Heidelberg, Germany, 2016; pp. 137–158. [Google Scholar] [CrossRef]
- Zhi, Y.; Qin, Y.; Wang, L.; Chen, Y.; Hu, X.; Yu, Z.; Li, Y. Glutathione reductase modulates endogenous oxidative stress and affects growth and virulence in Avibacterium paragallinarum. Vet. Res. 2025, 56, 1. [Google Scholar] [CrossRef]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmocology 2014, 5, 196. [Google Scholar] [CrossRef]
- Armutcu, F.; Akyol, S.; Ustunsoy, S.; Turan, F.F. The interaction of glutathione and thymoquinone and their antioxidant properties. Electron. J. Gen. Med. 2018, 15, 4–11. [Google Scholar] [CrossRef]
- Matuz-Mares, D.; Hernández-López, V.M.; Becerra, A.; Vázquez-Meza, H. Glutathione participation in the prevention of cardiovascular diseases. Antioxidants 2021, 10, 1220. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione Peroxidase-1 in Health and Disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Klinkhammer, C.; Matthes, R.; Stufler, S.; Barton, A.; von Woedtke, T.; Weltmann, K.-D.; Partecke, L.I.; Masur, K.; Harms, M. Elucidation of Plasma-induced Chemical Modifications on Glutathione and Glutathione Disulphide. Sci. Rep. 2017, 7, 13828. [Google Scholar] [CrossRef]
- Abdelraheem, E.M.M.; Busch, F.; Hanefeld, U.; Hollmann, F. Methyltransferases: Functions and applications. ChemBioChem 2022, 23, e202200212. [Google Scholar] [CrossRef] [PubMed]
- Liscombe, D.K.; Louie, G.V.; Noel, J.P. Architectures, mechanisms and molecular evolution of natural product methyltransferases. Nat. Prod. Rep. 2012, 29, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Grillo, M.A.; Colombatto, S. S-adenosylmethionine and its products. Amino Acids 2008, 34, 187–193. [Google Scholar] [CrossRef]
- Esaki, N.; Soda, K.; Tanaka, H.; Kojima, Y.; Yamauchi, K.; Tani, Y.; Suda, H. Selenocysteine lyase, a novel enzyme that specifically acts on selenocysteine. Mammalian distribution and purification and properties of pig liver enzyme. J. Biol. Chem. 1982, 257, 4386–4391. [Google Scholar] [CrossRef]
- Esaki, N.; Soda, K.; Suda, H. Mechanism of Reactions Catalyzed by Selenocysteine β-Lyase. Arch. Biochem. Biophys. 1985, 238, 418–423. [Google Scholar] [CrossRef]
- Mihara, H.; Esaki, N. Selenocysteine lyase: Mechanism, structure, and biological role. In Selenium: Its Molecular Biology and Role in Human Health; Springer: New York, NY, USA, 2012; pp. 95–105. [Google Scholar]
- Mihara, H.; Esaki, N. Mechanism, structure, and biological role of selenocysteine lyase. In Selenium: Its Molecular Biology and Role in Human Health, 4th ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 113–123. [Google Scholar]
- Suzuki, K.T.; Ogra, Y.; Imura, N. Selenocysteine β-lyase and methylselenol demethylase in the metabolism of Se-methylated selenocompounds into selenide. Biochim. Et Biophys. Acta Gen. Subj. 2007, 1770, 1053–1061. [Google Scholar] [CrossRef]
- Seale, L.A. Selenocysteine β-lyase: Biochemistry, regulation and physiological role of the selenocysteine decomposition enzyme. Antioxidants 2019, 8, 357. [Google Scholar] [CrossRef]
- Omi, R.; Sakakibara, Y.; Yamaguchi, Y.; Hatakeyama, T.; Fujisawa, K.; Esaki, N.; Suzuki, T. Reaction mechanism and molecular basis for selenium/sulfur discrimination of selenocysteine lyase. J. Biol. Chem. 2010, 285, 12133–12139. [Google Scholar] [CrossRef]
- Seale, L.A.; Torres, S.; Berry, M.J.; Burk, R.F.; Van Den Veyver, I.B.; Ogra, Y.; Hoffmann, V.; Schweizer, U.; Sunde, R.A. Relationship between selenoprotein P and selenocysteine lyase: Insights into selenium metabolism. Free Radic. Biol. Med. 2018, 127, 182–189. [Google Scholar] [CrossRef]
- Seale, L.A.; Torres, S.; Schweizer, U.; Berry, M.J.; Burk, R.F.; Sunde, R.A. Disruption of the Selenocysteine Lyase-Mediated Selenium Recycling Pathway Leads to Metabolic Syndrome in Mice. Mol. Cell. Biol. 2012, 32, 4141–4154. [Google Scholar] [CrossRef]
- De Souza, M.P.; Pilon-Smits, E.A.; Terry, N. Rate-Limiting Steps in Selenium Assimilation and Volatilization by Indian Mustard 1. Plant Physiol. 1988, 117, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E.A.; Quinn, C.F.; Tappero, R.; Cotter-Howells, J.; Hwang, S.; Lytle, C.M.; Pilon, M. Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 2009, 12, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Skrypnik, L.; Skrypnik, K.; Medvedeva, I.; Ederli, L.; Inzaghi, C.; Pardossi, A. The Integral Boosting Effect of Selenium on the Secondary Metabolism of Higher Plants. Plants 2022, 11, 3432. [Google Scholar] [CrossRef] [PubMed]
- Ganther, H.E. Enzymic Synthesis of Dimethyl Selenide from Sodium Selenite in Mouse Liver Extracts. Biochemistry 1966, 5, 1089–1098. [Google Scholar] [CrossRef]
- Cummins, L.M.; Kimura, E.T. Safety evaluation of selenium sulfide antidandruff shampoos. Toxicol. Appl. Pharmacol. 1971, 20, 89–96. [Google Scholar] [CrossRef]
- Pletnikova, I.P. Biological effect and tolerance level of Se in drinking water. Gig. Sanit. 1970, 2, 14–19. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Selenium; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2003. Available online: https://www.ncbi.nlm.nih.gov/books/NBK600364/ (accessed on 12 February 2025).
- Sayato, Y.; Fukuda, M.; Fujii, M.; Kurokawa, Y.; Saito, H. Acute and subacute oral toxicity of selenocystine in mice. Eisei Kagaku 1993, 39, 289–296. [Google Scholar] [CrossRef]
- Power, R.; Lyons, T.P.; Jacques, K.A. (Eds.) English, Book chapter Conference paper, UK, Stamford, Nutritional biotechnology in the feed and food industries. In Proceedings of the Alltech’s 21st Annual Symposium, Lexington, KY, USA, 22–25 May 2005; pp. 135–145. [Google Scholar]
- McConnell, K.P.; Portman, O.W. Toxicity of dimethyl selenide in the rat and mouse. Proc. Soc. Exp. Biol. Med. 1952, 79, 230–231. [Google Scholar] [CrossRef]
- Obermeyer, B.D.; Sultatos, L.G.; Ganther, H.E.l. Toxicity of trimethylselenonium chloride in the rat with and without arsenite. Toxicol. Appl. Pharmacol. 1971, 20, 135–146. [Google Scholar] [CrossRef]
- Wilber, C.G. Toxicology of selenium: A review. Clin. Toxicol. 1980, 17, 171–230. [Google Scholar] [CrossRef]
- Hoffman, J.L. Selenite Toxicity, Depletion of Liver S-Adenosylmethionine, and Inactivation of Methionine Adenosyltransferase. Arch. Biochem. Biophys. 1977, 179, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Ravn-Haren, G. Acute human toxicity and mortality after selenium ingestion: A review. J. Trace Elem. Med. Biol. 2020, 58, 126435. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Ravn-Haren, G. Toxicity of repeated oral intake of organic selenium, inorganic selenium, and selenium nanoparticles: A review. J. Trace Elem. Med. Biol. 2023, 79, 127235. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jia, X. Safety evaluation of Se-methylselenocysteine as nutritional selenium supplement: Acute toxicity, genotoxicity and subchronic toxicity. Regul. Toxicol. Pharmacol. 2014, 70, 720–727. [Google Scholar] [CrossRef]
- Jacevic, V.; Djordjevic, A.; Petrovic, D.; Vucinic, S. Acute toxicity of sodium selenite in rodents: Pathomorphological study. Mil. Med. Sci. Lett. 2011, 80, 90–96. [Google Scholar] [CrossRef]
- Xie, M.; Wang, Y.; Zhang, F.; Mao, J.; Zhang, Y.; Li, Y. Selenium in cereals: Insight into species of the element from total amount. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2914–2940. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Zhu, Y.G.; Pilon-Smits, E.A.H.; Zhao, F.J.; Williams, P.N.; Meharg, A.A. Selenium in higher plants: Understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci. 2009, 14, 436–442. [Google Scholar] [CrossRef]
- Zhang, L.; Chu, C. Selenium Uptake, Transport, Metabolism, Reutilization, and Biofortification in Rice. Rice 2022, 15, 30. [Google Scholar] [CrossRef]
- Trippe, R.C.; Pilon-Smits, E.A.H. Selenium transport and metabolism in plants: Phytoremediation and biofortification implications. J. Hazard. Mater. 2021, 404, 124178. [Google Scholar] [CrossRef]
- El Mehdawi, A.F.; Jiang, Y.; Guignardi, Z.S.; Esmat, A.; Pilon, M.; Pilon-Smits, E.A.H. Influence of sulfate supply on selenium uptake dynamics and expression of sulfate/selenate transporters in selenium hyperaccumulator and nonhyperaccumulator Brassicaceae. New Phytol. 2018, 217, 194–205. [Google Scholar] [CrossRef]
- Cabannes, E.; Buchner, P.; Broadley, M.R.; Hawkesford, M.J. A comparison of sulfate and selenium accumulation in relation to the expression of sulfate transporter genes in Astragalus species. Plant Physiol. 2011, 157, 2227–2239. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. Selenoproteins. J. Biol. Chem. 2009, 284, 723–727. [Google Scholar] [CrossRef]
- Johansson, L.; Gafvelin, G.; Arnér, E.S.J. Selenocysteine in proteins—Properties and biotechnological use. Biochim. Biophys. Acta Gen. Subj. 2005, 1726, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.S.; Bindi, A.B.; Buzzelli, J.; Stolz, J.F.; Oremland, R.S. Reduction of elemental selenium to selenide: Experiments with anoxic sediments and bacteria that respire se-oxyanions. Geomicrobiol. J. 2003, 20, 587–602. [Google Scholar] [CrossRef]
- Guo, J.; Lin, Z.; Wang, L.; Zhang, X.; Ma, Y.; Wang, D.; Zhang, L.; Ma, J. Contributions of selenium-oxidizing bacteria to selenium biofortification and cadmium bioremediation in a native seleniferous Cd-polluted sandy loam soil. Ecotoxicol. Environ. Saf. 2024, 272, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Laliberte, M.; De Ladurantaye-Noel, M. Removal of Selenium by Reduction to Selenite and Surface Complexation. In Proceedings of the Mine Water Solutions, Vancouver, BC, Canada, 14–16 June 2022; pp. 295–301. [Google Scholar]
- Perrone, D.; Lavorgna, M.; Preedy, V.R. Selenium: Chemistry, Analysis, Function and Effects; Preedy, V.R., Ed.; The Royal Society of Chemistry: London, UK, 2015; Available online: www.rsc.org (accessed on 12 June 2025).
- Pyrzynska, K. Speciation Analysis of Some Organic Selenium Compounds: A Review. Analyst 1996, 121, 77R–83R. [Google Scholar] [CrossRef]
- Pyrzynska, K. Speciation of Selenium Compounds. Anal. Sci. 1998, 14, 479–483. [Google Scholar] [CrossRef][Green Version]
- Weekley, C.M.; Harris, H.H. Which form is that? the importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem. Soc. Rev. 2013, 42, 8870–8894. [Google Scholar] [CrossRef]
- Tanini, D.; Capperucci, A. Synthesis and Applications of Organic Selenols. Adv. Synth. Catal. 2021, 363, 5360–5385. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błazejak, S. Selenium: Significance, and outlook for supplementation. Nutrition 2013, 29, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Forstrom, J.W.; Zakowski, J.J.; Tappel, A.L. Identification of the Catalytic Site of Rat Liver Glutathione Peroxidase as Selenocysteine. Biochemistry 1978, 17, 2639–2644. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.G.; Kunwar, A. Redox reactions of organoselenium compounds: Implication in their biological activity. Free Radic. Res. 2021, 55, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Liu, Q.; Zhang, S.; Guo, H.; Song, X.; Chen, W. Synthesis and Potential Anticancer Activity of Some Novel Selenocyanates and Diselenides. Chem. Biodivers. 2020, 17, e1900603. [Google Scholar] [CrossRef]
- Lu, S.C. S-adenosylmethionine. Int. J. Biochem. Cell Biol. 2000, 32, 391–395. [Google Scholar] [CrossRef]
- Fontecave, M.; Atta, M.; Mulliez, E. S-adenosylmethionine: Nothing goes to waste. Trends Biochem. Sci. 2004, 9, 243–249. [Google Scholar] [CrossRef]
- Ueland, P.M. Pharmacological and Biochemical Aspects of S-Adenosylhomocysteine and S-Adenosylhomocysteine Hydrolase. Pharmacol. Rev. 1982, 34, 223–253. [Google Scholar] [CrossRef]
- Brown, K.; Arthur, J. Selenium, selenoproteins and human health: A review. Public Health Nutr. 2001, 4, 593–599. [Google Scholar] [CrossRef]
- Lacourciere, G.M.; Stadtman, T.C. Utilization of Selenocysteine as a Source of Selenium for Selenophosphate Biosynthesis. BioFactors 2001, 14, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Dodig, S.; Cepelak, I. The facts and controverses about selenium. Acta Pharm. 2004, 54, 261–276. [Google Scholar] [PubMed]
- Driscoll, D.M.; Copeland, P.R. Mechanism and regulation of selenoprotein synthesis. Annu. Rev. Nutr. 2003, 23, 17–40. [Google Scholar] [CrossRef] [PubMed]
- Winkel, L.H.E.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Bañuelos, G.S. Selenium cycling across soil-plant-atmosphere interfaces: A critical review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef]
- Muleya, M.; Watts, M.J.; Nyirenda, G.K.C.; Chilimba, A.D.C.; Phiri, F.P.; Broadley, M.R.; Siyeni, D.; Chilima, B.; Chilima, B.Z.; Joy, E.J.M. Selenium speciation and bioaccessibility in Se-fertilised crops of dietary importance in Malawi. J. Food Compos. Anal. 2021, 98, 103841. [Google Scholar] [CrossRef]
- Cooke, T.D.; Bruland, K.W. Aquatic Chemistry of Selenium: Evidence of Biomethylationt. Environ. Sci. Technol. 1987, 21, 1214–1219. [Google Scholar] [CrossRef]
- Khanam, A.; Platel, K. Bioaccessibility of selenium, selenomethionine and selenocysteine from foods and influence of heat processing on the same. Food Chem. 2016, 194, 1293–1299. [Google Scholar] [CrossRef]
- Williams, P.N.; Lombi, E.; Sun, G.X.; Scheckel, K.; Zhu, Y.G.; Feng, X.; Zhu, J.; Carey, A.M.; Adomako, E.; Lawgali, Y.Y.; et al. Selenium characterization in the global rice supply chain. Environ. Sci. Technol. 2009, 43, 6024–6030. [Google Scholar] [CrossRef]
- Tani, L.S.K.; Dhanarajan, A.; Nair, R.; Gaman, M.A.; Efficace, E.; Sarac, I.; Aon, M.A. Selenium deficiency-from soil to thyroid cancer. Appl. Sci. 2020, 10, 5386. [Google Scholar]
- Abdalla, M.A.; Alotaibi, S.H.; Khattab, A.R.; El Sayed, A.M.; Elnakady, Y.A.; Shalaby, A.S.G.; Badr, G.; Abdel-Hady, H.; Alharthy, B.; Abdelmohsen, U.R. Regulation of Selenium/Sulfur Interactions to Enhance Chemopreventive Effects: Lessons to Learn from Brassicaceae. Molecules 2020, 25, 5846. [Google Scholar] [CrossRef]
- Sun, G.X.; Williams, P.N.; Carey, A.M.; Zhu, Y.G.; Deacon, C.; Raab, A.; Feldmann, J.; Islam, R.M.; Meharg, A.A. Distribution and translocation of selenium from soil to grain and its speciation in Paddy Rice (Oryza sativa L.). Environ. Sci. Technol. 2010, 44, 6706–6711. [Google Scholar] [CrossRef]
- Lyons, M.P.; Papazyan, T.T.; Surai, P.F. Selenium in food chain and animal nutrition: Lessons from nature-review. Asian Australas. J. Anim. Sci. 2007, 20, 1135–1155. [Google Scholar] [CrossRef]
- Bodnar, M.; Konieczka, P.; Namiesnik, J. The properties, functions, and use of selenium compounds in living organisms. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2012, 30, 225–252. [Google Scholar] [CrossRef] [PubMed]
- Mayland, H.F. Selenium in Plant and Animal Nutrition. In Selenium in the Environment, 1st ed.; CRC Press: Boca Raton, FL, USA, 1994; pp. 29–45. [Google Scholar]
- Pyrzyńska, K. Determination of selenium species in environmental samples. Mikrochim. Acta 2002, 140, 55–62. [Google Scholar] [CrossRef]
- Bocchini, M.; D’Amato, R.; Ciancaleoni, S.; Fontanella, M.C.; Palmerini, C.A.; Beone, G.M.; Onofri, A.; Negri, V.; Pilon-Smits, E.A.H.; Iannelli, M.A.; et al. Soil selenium (Se) biofortification changes the physiological, biochemical and epigenetic responses to water stress in Zea mays L. by inducing a higher drought tolerance. Front. Plant Sci. 2018, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Proietti, P.; Nasini, L.; Del Buono, D.; D’Amato, R.; Tedeschini, E.; Businelli, D. Selenium protects olive (Olea europaea L.) from drought stress. Sci. Hortic. 2013, 164, 165–171. [Google Scholar] [CrossRef]
- Liu, J.X.; Howell, S.H. Endoplasmic reticulum protein quality control and its relationship to environmental stress responses in plants. Plant Cell 2010, 22, 2930–2942. [Google Scholar] [CrossRef]
- Bohnert, H.J.; Nelson, D.E.; Jensen, R.G. Adaptations to Environmental Stresses. Plant Cell 1995, 7, 1099–1111. [Google Scholar] [CrossRef]
- Mareri, L.; Romi, M.; Cai, G. Environmental Stress and Plants. Int. J. Mol. Sci. 2022, 23, 5416. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Prášil, I.T.; Renaut, J. Plant proteoforms under environmental stress: Functional proteins arising from a single gene. Front. Plant Sci. 2021, 12, 793113. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A.; Khan, S.Z.; Ehsan, A. Selenium supplementation affects physiological and biochemical processes to improve fodder yield and quality of maize (Zea mays L.) under water deficit conditions. Front. Plant Sci. 2016, 7, 1438. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.; Ahmad, F.; Wang, X.; Ahmad, M.; Wu, D.; Hassan, M.J.; Chen, X. Influence of selenium on growth, physiology, and antioxidant responses in maize varies in a dose-dependent manner. J. Food Qual. 2021, 2021, 6642018. [Google Scholar] [CrossRef]
- Andrade, F.R.; Carvalho, R.F.; Campos, M.L.; Azevedo, R.A. Selenium protects rice plants from water deficit stress. Ecotoxicol. Environ. Saf. 2018, 164, 562–570. [Google Scholar] [CrossRef]
- Patnaik, G.P.; Dash, S.; Sahoo, A.; Mohapatra, S.; Tripathy, S.K.; Bastia, D.N. Selenium Application Improves Drought Tolerance during Reproductive Phase of Rice. Sustainability 2023, 15, 2730. [Google Scholar] [CrossRef]
- Ghouri, F.; Nawaz, M.A.; Farooq, N.; Javed, M.T.; Hussain, H.A.; Waraich, E.A.; Rasheed, R.; Irshad, M.K. Effects of Silicon and Selenium in Alleviation of Drought Stress in Rice. Silicon 2022, 14, 5453–5461. [Google Scholar] [CrossRef]
- Liu, M.; Li, Y.; Yin, R.; Zhang, H.; Zhang, L.; Wang, M.; Sommar, J.; Feng, X. Rice Life Cycle-Based Global Mercury Biotransport and Human Methylmercury Exposure. Nat. Commun. 2019, 10, 5164. [Google Scholar] [CrossRef]
- Palmieri, J.R.; Edelstein, M.R.; Angeloni, S.V. Implications and Significance of Mercury in Rice. J. Food Nutr. Metab. 2020, 3, 1–5. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H.; Wang, Q.; Wang, Y.; Wang, M.; Zhang, M.; Liang, Y.; Zhu, Y. Mercury contents in rice and potential health risks across China. Environ. Int. 2019, 126, 406–412. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, X.; Wang, X.; Larssen, T.; Shang, L.; Li, P. Selenium in soil inhibits mercury uptake and translocation in rice (Oryza sativa L.). Environ. Sci. Technol. 2012, 46, 10040–10046. [Google Scholar] [CrossRef]
| Selenium Compound | Organism/Species | Exposure Route | LD50 (mg Se/kg Body Weight) | Reference |
|---|---|---|---|---|
| Sodium selenite (Na2SeO3) | Rat (Sprague Dawley) | Oral | 7.0 | [102] |
| Mouse | Oral | 3.2 | [103] | |
| Rabbit | Oral | 1.0 | [103] | |
| Sodium selenate (Na2SeO4) | Rat | Oral | 7.0 | [103] |
| Mouse | Oral | 4.0 | [103] | |
| Selenium dioxide (SeO2) | Rat | Oral | 48.0 | [104] |
| Elemental selenium (Se) | Rat | Oral | 6700 | [102] |
| Selenocystine | Mouse | Oral | 35.9 | [105] |
| Selenomethionine | Rat | Oral | 37.3 | [106] |
| Dimethyl selenide ((CH3)2Se) | Mouse | Intraperitoneal | 1600 | [107] |
| Trimethylselenonium chloride | Rat | Intraperitoneal | 49.4 | [108] |
| Hydrogen selenide (H2Se) | Guinea pig | Inhalation | 0.02 mg/L (LC50) | [109] |
| Selenium sulfide (SeS2) | Rat | Oral | 138.0 | [102] |
| Selenourea | Rat | Oral | 50.0 | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagot, F.; Botha, L.K.; Namaumbo, S.; Kapito, N.J.; Ndovie, P.; Tsukuluza, D.C.; Mlangeni, A.T. Selenium Methylation: Insights into Chemical Reactions and Enzymatic Pathways. Chemistry 2025, 7, 169. https://doi.org/10.3390/chemistry7050169
Jagot F, Botha LK, Namaumbo S, Kapito NJ, Ndovie P, Tsukuluza DC, Mlangeni AT. Selenium Methylation: Insights into Chemical Reactions and Enzymatic Pathways. Chemistry. 2025; 7(5):169. https://doi.org/10.3390/chemistry7050169
Chicago/Turabian StyleJagot, Fatema, Loti Kasegza Botha, Sydney Namaumbo, Noel Jabesi Kapito, Patrick Ndovie, Deboral Charles Tsukuluza, and Angstone Thembachako Mlangeni. 2025. "Selenium Methylation: Insights into Chemical Reactions and Enzymatic Pathways" Chemistry 7, no. 5: 169. https://doi.org/10.3390/chemistry7050169
APA StyleJagot, F., Botha, L. K., Namaumbo, S., Kapito, N. J., Ndovie, P., Tsukuluza, D. C., & Mlangeni, A. T. (2025). Selenium Methylation: Insights into Chemical Reactions and Enzymatic Pathways. Chemistry, 7(5), 169. https://doi.org/10.3390/chemistry7050169







