Salicylic Acid Derivatives as Antifungal Agents: Synthesis, In Vitro Evaluation, and Molecular Modeling
Abstract
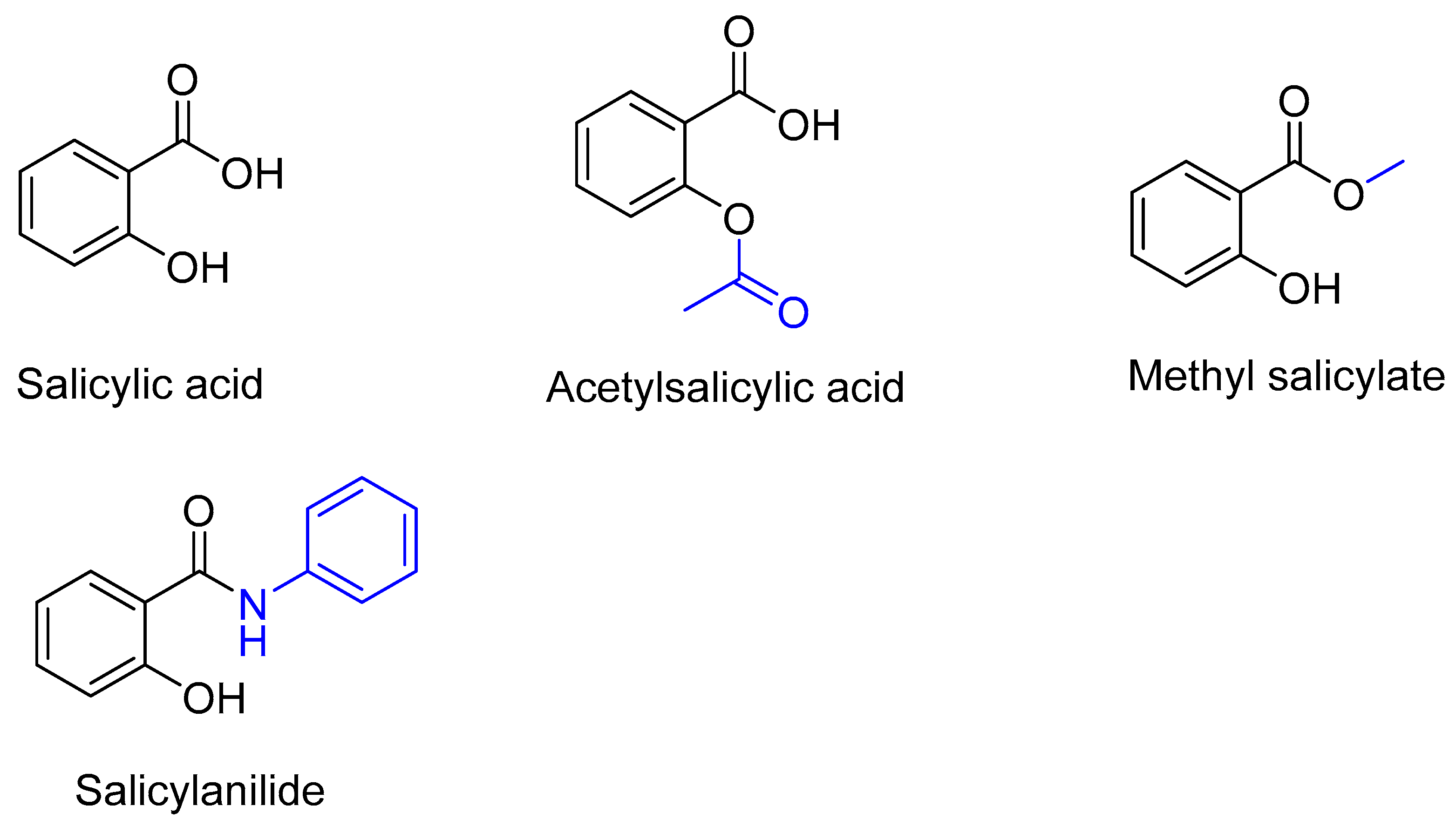
1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. Materials
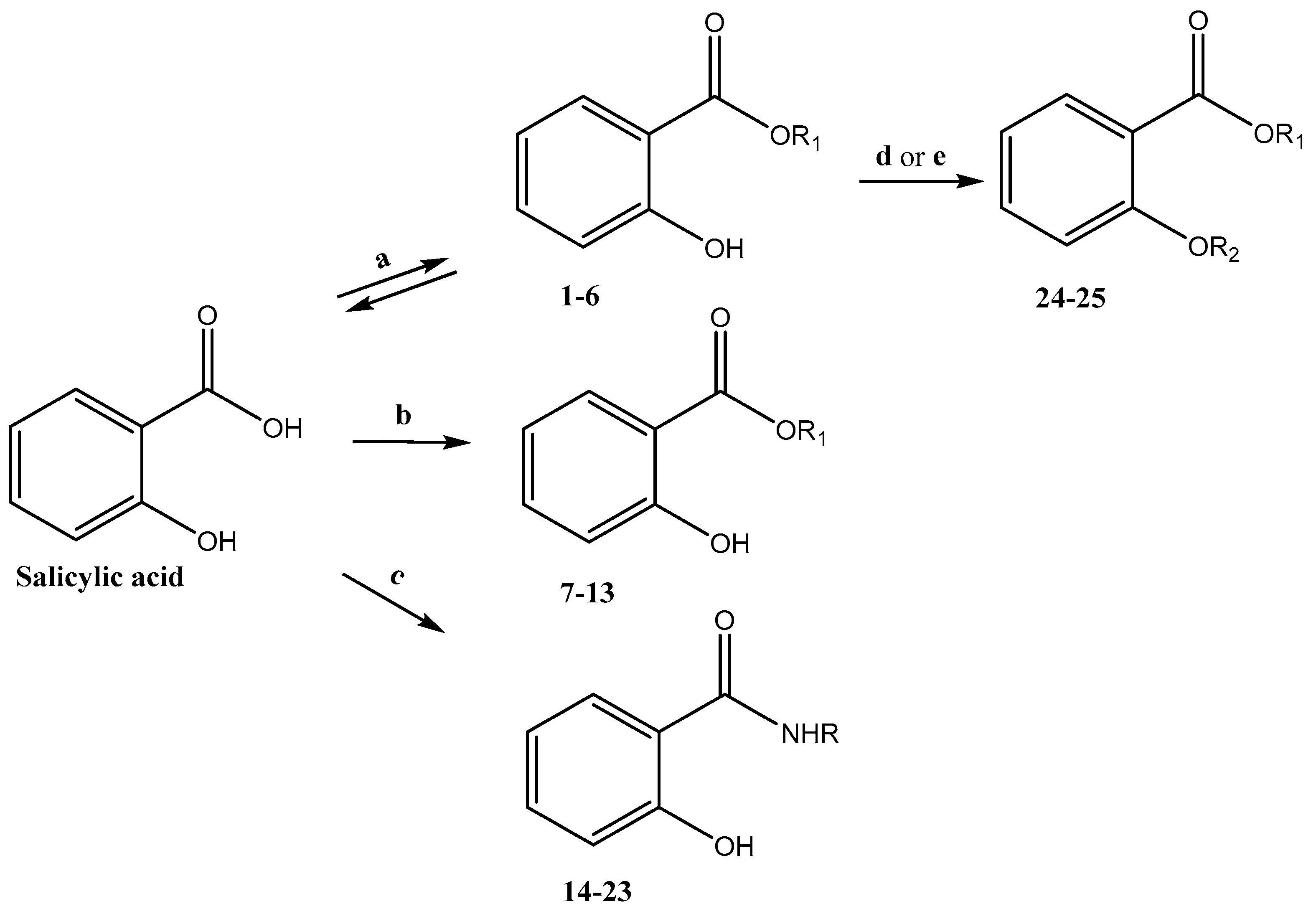
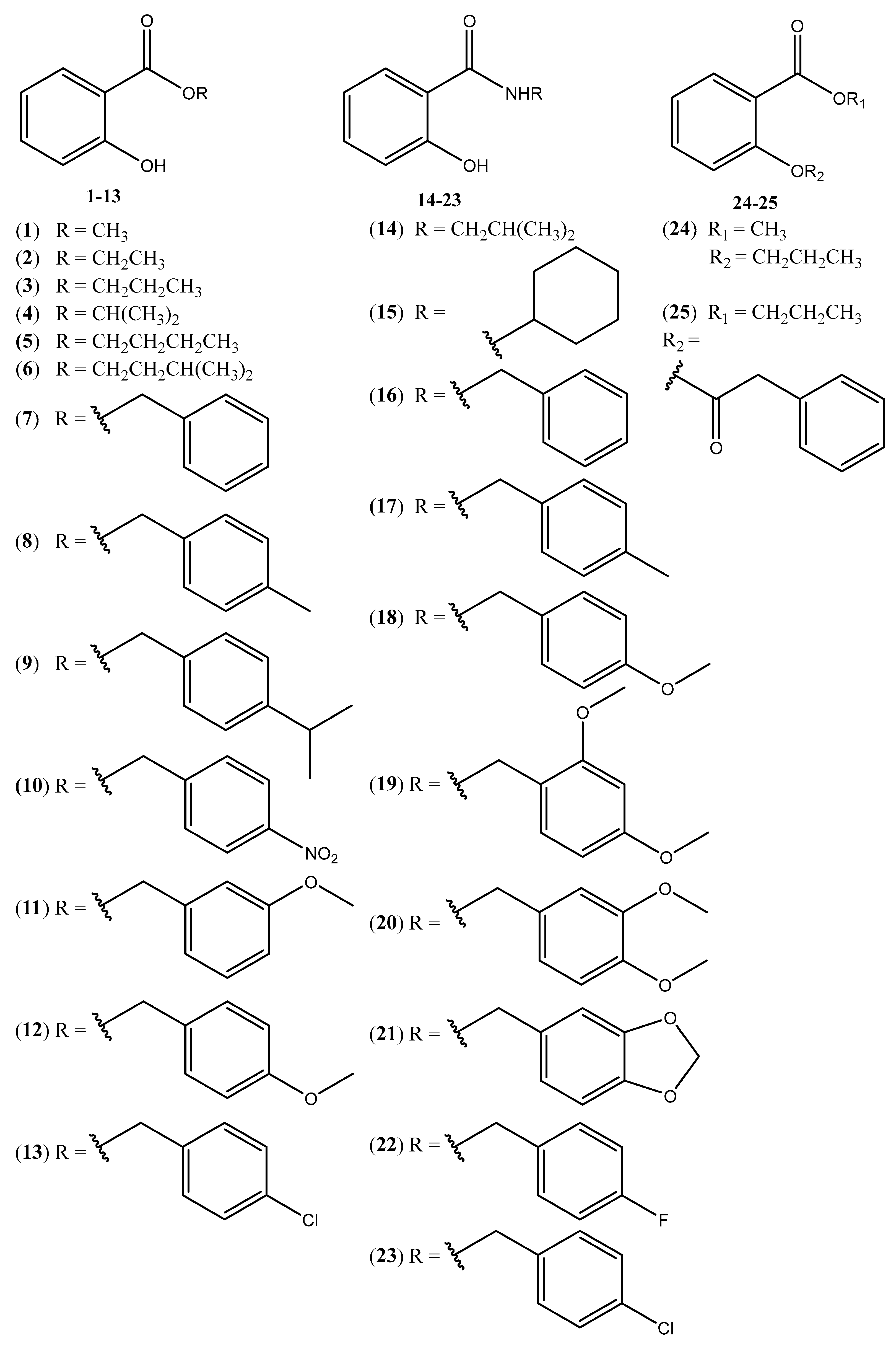
2.1.2. Preparation of Salicylic Acid Derivatives 1–6
2.1.3. Preparation of Salicylic Acid Derivatives 7–13
2.1.4. Preparation of Salicylic Acid Derivatives 14–23
2.1.5. Preparation of Derivative 24
2.1.6. Preparation of Derivative 25
2.2. Antifungal Activity
2.2.1. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Fungicide Concentration (CFM)
2.2.2. Investigation of the Mode of Action on the Fungal Cell Wall and Membrane
2.3. Modeling Methods
2.3.1. Targets Selection
2.3.2. Molecular Docking
2.3.3. Molecular Dynamics (MD) Simulations and Estimation of the Binding Energies
3. Results
3.1. Antifungal Activity
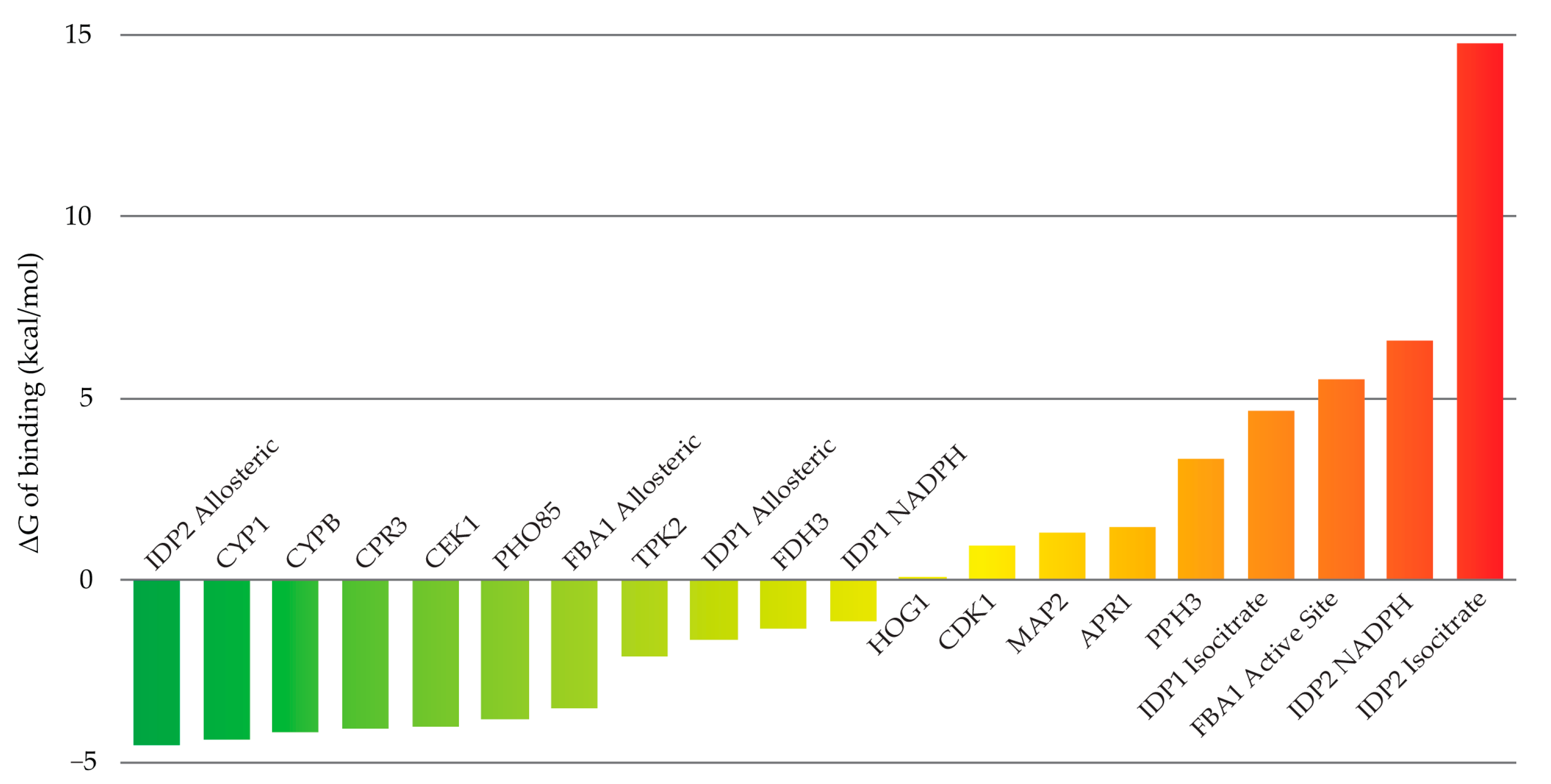
3.2. Molecular Modeling
4. Discussion
4.1. 1H and 13C-APT NMR Spectroscopy Analysis
4.2. Antifungal Activity
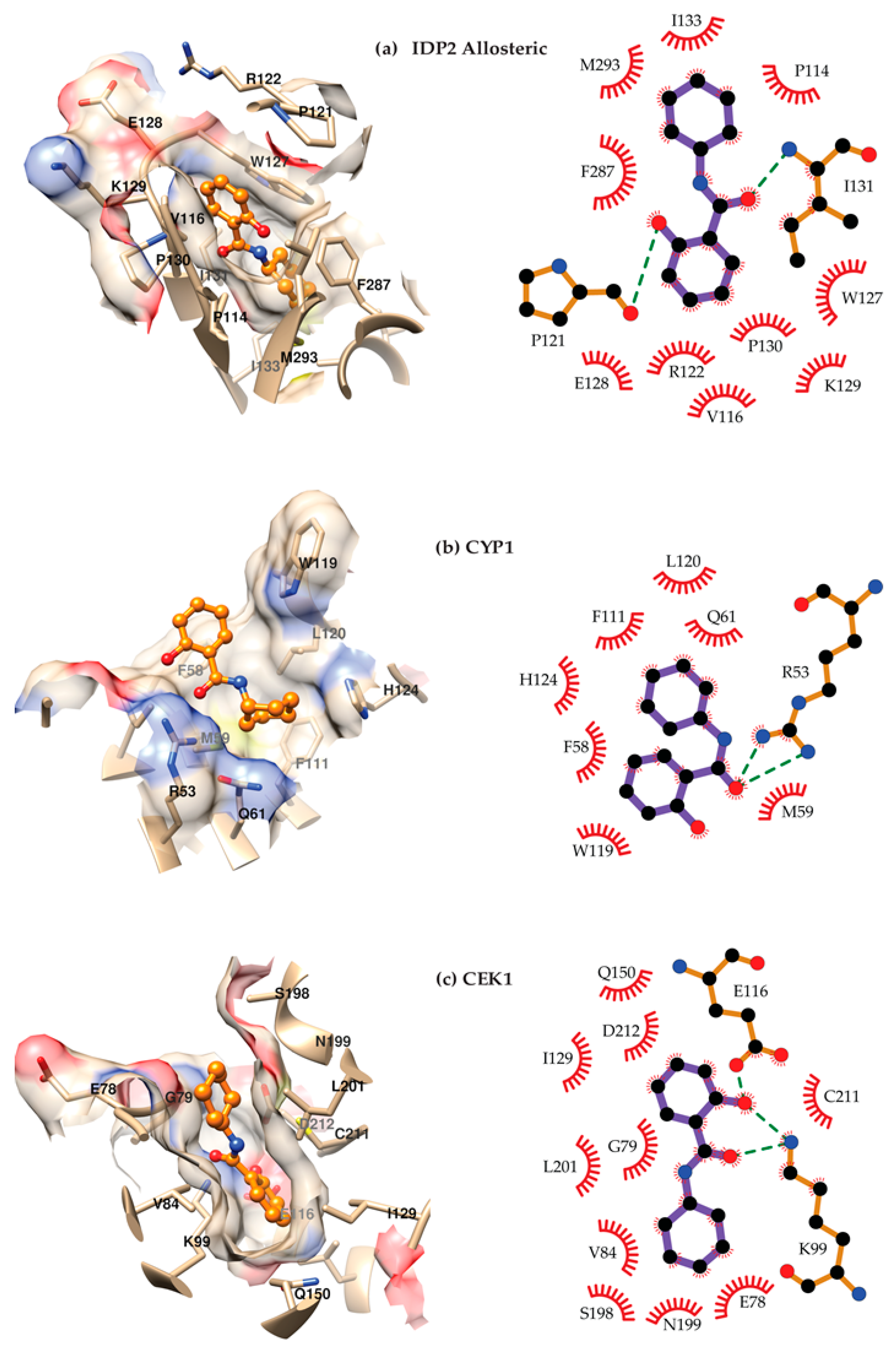
4.3. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bitew, A.; Abebaw, Y. Vulvovaginal Candidiasis: Species Distribution of Candida and Their Antifungal Susceptibility Pattern. BMC Women’s Health 2018, 18, 94. [Google Scholar] [CrossRef]
- Marak, M.B.; Dhanashree, B. Antifungal Susceptibility and Biofilm Production of Candida Spp. Isolated from Clinical Samples. Int. J. Microbiol. 2018, 2018, 7495218. [Google Scholar] [CrossRef]
- Medici, N.P.; Del Poeta, M. New Insights on the Development of Fungal Vaccines: From Immunity to Recent Challenges. Mem. Do Inst. Oswaldo Cruz 2015, 110, 966–973. [Google Scholar] [CrossRef]
- Nami, S.; Mohammadi, R.; Vakili, M.; Khezripour, K.; Mirzaei, H.; Morovati, H. Fungal Vaccines, Mechanism of Actions and Immunology: A Comprehensive Review. Biomed. Pharmacother. 2019, 109, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, C.; Leonardi, D. Candida Infections, Causes, Targets, and Resistance Mechanisms: Traditional and Alternative Antifungal Agents. Biomed. Res. Int. 2013, 2013, 204237. [Google Scholar] [CrossRef] [PubMed]
- Krátký, M.; Vinšová, J. Antifungal Activity of Salicylanilides and Their Esters with 4-(Trifluoromethyl)benzoic Acid. Molecules 2012, 17, 9426–9442. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D. Aspirin and Related Drugs; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9781135732639. [Google Scholar]
- Sahoo, J.; Paidesetty, S.K. Antimicrobial, Analgesic, Antioxidant and in Silico Study of Synthesized Salicylic Acid Congeners and Their Structural Interpretation. Egypt. J. Basic Appl. Sci. 2015, 2, 268–280. [Google Scholar] [CrossRef]
- Djurendić, E.; Vujašković, S.D.; Sakač, M.; Ajduković, J.; Gaković, A.; Kojić, V.; Bogdanović, G.; Klisurić, O.; Gaši, K.P. Synthesis and Biological Evaluation of Some New 2-Oxazoline and Salicylic Acid Derivatives. Arkivoc 2010, 2011, 83–102. [Google Scholar] [CrossRef]
- Sanderson, J.T.; Clabault, H.; Patton, C.; Lassalle-Claux, G.; Jean-François, J.; Paré, A.F.; Hébert, M.J.G.; Surette, M.E.; Touaibia, M. Antiproliferative, Antiandrogenic and Cytotoxic Effects of Novel Caffeic Acid Derivatives in LNCaP Human Androgen-Dependent Prostate Cancer Cells. Bioorg. Med. Chem. 2013, 21, 7182–7193. [Google Scholar] [CrossRef]
- Chapado, L.; Linares-Palomino, P.J.; Salido, S.; Altarejos, J.; Rosado, J.A.; Salido, G.M. Synthesis and Evaluation of the Platelet Antiaggregant Properties of Phenolic Antioxidants Structurally Related to Rosmarinic Acid. Bioorg. Chem. 2009, 38, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.-Y.; Wang, H.-Q.; Hao, L.-H.; He, W.-Y.; Gao, R.-M.; Li, Y.-P.; Li, Y.-H.; Jiang, J.-D.; Li, Z.-R. Synthesis and Antiviral Activity of N-Phenylbenzamide Derivatives, a Novel Class of Enterovirus 71 Inhibitors. Molecules 2013, 18, 3630–3640. [Google Scholar] [CrossRef] [PubMed]
- Coolen, H.K.A.C.; Meeuwis, J.A.M.; van Leeuwen, P.W.M.N.; Nolte, R.J.M. Substrate Selective Catalysis by Rhodium Metallohosts. J. Am. Chem. Soc. 1995, 117, 11906–11913. [Google Scholar] [CrossRef][Green Version]
- Sadeghian, H.; Seyedi, S.M.; Saberi, M.R.; Arghiani, Z.; Riazi, M. Design and Synthesis of Eugenol Derivatives, as Potent 15-Lipoxygenase Inhibitors. Bioorg. Med. Chem. 2008, 16, 890–901. [Google Scholar] [CrossRef]
- Masetto, E.; Lazaroto, A.; Oleinik, G.; Lima, F.; Gallina, A.; Soares, L. Salicilatos Como Inibidores do Processo Oxidativo Mediado por Cobre e Ferro no Biodiesel B100. Quim. Nova 2022, 45. [Google Scholar] [CrossRef]
- Zhao, Q.; Chu, J.; Jiang, T.; Yin, H. Synthesis of Isoamyl Salicylate Using a Novel Mesoporous Titania Superacid as a Catalyst. Korean J. Chem. Eng. 2008, 25, 1008–1013. [Google Scholar] [CrossRef]
- Yamada, K.; Yoshida, S.; Fujita, H.; Kitamura, M.; Kunishima, M. O-Benzylation of Carboxylic Acids Using 2,4,6-Tris(Benzyloxy)-1,3,5-Triazine (TriBOT) under Acidic or Thermal Conditions. Eur. J. Org. Chem. 2015, 2015, 7997–8002. [Google Scholar] [CrossRef]
- Villa, C.; Baldassari, S.; Gambaro, R.; Mariani, E.; Loupy, A. Eco-Friendly Methodologies for the Synthesis of Some Aromatic Esters, Well-Known Cosmetic Ingredients. Int. J. Cosmet. Sci. 2005, 27, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Dev, D.; Palakurthy, N.B.; Thalluri, K.; Chandra, J.; Mandal, B. Ethyl 2-Cyano-2-(2-Nitrobenzenesulfonyloxyimino) Acetate (O-NosylOXY): A Recyclable Coupling Reagent for Racemization-Free Synthesis of Peptide, Amide, Hydroxamate, and Ester. J. Org. Chem. 2014, 79, 5420–5431. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Heravi, M.M.; Roshani, M.; Jahangir, M.; Gharib, A. Preyssler Catalyst, [NaP5W30O110]14−: A Green, Efficient and Reusable Catalyst for Esterification of Salicylic Acid with Aliphatic and Benzylic Alcohols. Appl. Catal. A Gen. 2006, 302, 42–47. [Google Scholar] [CrossRef]
- Carcelli, M.; Rogolino, D.; Bacchi, A.; Rispoli, G.; Fisicaro, E.; Compari, C.; Sechi, M.; Stevaert, A.; Naesens, L. Metal-chelating 2-hydroxyphenyl amide pharmacophore for inhibition of influenza virus endonuclease. Mol Pharm. 2014, 11, 304–316. [Google Scholar] [CrossRef]
- Gao, B.; Chen, K.; Bi, X.; Wang, J. Intramolecular Functionalization of c (Sp3) H Bonds Adjacent to an Amide Nitrogen Atom: Metal-Free Synthesis of 2-Hydroxy-Benzoxazinone Derivatives. Tetrahedron 2017, 73, 7005–7010. [Google Scholar] [CrossRef]
- Madasamy, K.; Balakrishnan, M.H.; Korivi, R.; Mannathan, S. Trifluoroacetic Acid-Mediated Denitrogenative Ortho-Hydroxylation of 1,2,3-Benzotriazin-4(3H)-Ones: A Metal-Free Approach. J. Org. Chem. 2022, 87, 8752–8756. [Google Scholar] [CrossRef]
- Waisser, K.; Peřina, M.; Klimešová, V.; Kaustová, J. On the Relationship between the Structure and Antimycobacterial Activity of Substituted N-Benzylsalicylamides. Coll. Czech. Chem. Commun. 2003, 68, 1275–1294. [Google Scholar] [CrossRef]
- Grintsevich, S.; Sapegin, A.; Reutskaya, E.; Peintner, S.; Erdélyi, M.; Krasavin, M. An Alternative Approach to the Hydrated Imidazoline Ring Expansion (HIRE) of Diarene-Fused [1.4] Oxazepines. Eur. J. Org. Chem. 2020, 2020, 5664–5676. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Ji, Z.; Sun, X.; Tian, Q.; Wei, S.; Ji, Z. Discovery, SAR, and Putative Mode of Action of N-Benzyl-2-Methoxybenzamides as Potential Bleaching Herbicides. Pest Manag. Sci. 2021, 77, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.N.; Ahmad, K.; Yasin, K.A.; Farooq, T.; Khan, B.A.; Roy, S.K. Ligand-Free Cu(Ii)-Catalyzed Aerobic Etherification of Aryl Halides with Silanes: An Experimental and Theoretical Approach. New J. Chem. 2019, 43, 11316–11333. [Google Scholar] [CrossRef]
- Rex, J.H.; CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard, 3rd ed.; CLSI: Wayne, PA, USA, 2008; ISBN 9781562386665. [Google Scholar]
- Siddiqui, Z.N.; Farooq, F.; Musthafa, T.N.M.; Ahmad, A.; Khan, A.U. Synthesis, Characterization and Antimicrobial Evaluation of Novel Halopyrazole Derivatives. J. Saudi Chem. Soc. 2013, 17, 237–243. [Google Scholar] [CrossRef]
- Alves, D.d.N.; Ferreira, A.R.; Duarte, A.B.S.; Melo, A.K.V.; de Sousa, D.P.; Castro, R.D. Breakpoints for the Classification of Anti-Candida Compounds in Antifungal Screening. Biomed. Res. Int. 2021, 2021, e6653311. [Google Scholar] [CrossRef]
- Lima, I.O.; Pereira, F.O.; Oliveira, W.A.; Lima, E.O.; Menezes, E.A.; Cunha, F.A.; Diniz, M.F.F.M. Antifungal Activity and Mode of Action of Carvacrol against Candida albicans Strains. J. Essent. Oil Res. 2013, 25, 138–142. [Google Scholar] [CrossRef]
- Escalante, A.; Gattuso, M.; Pérez, P.; Zacchino, S. Evidence for the Mechanism of Action of the Antifungal Phytolaccoside B Isolated from Phytolacca tetramera Hauman. J. Nat. Prod. 2008, 71, 1720–1725. [Google Scholar] [CrossRef]
- Freires, I.A.; Murata, R.M.; Furletti, V.F.; Sartoratto, A.; Alencar, S.M.; Figueira, G.M.; Rodrigues, J.A.O.; Duarte, M.C.T.; Rosalen, P.L. Coriandrum sativum L. (Coriander) Essential Oil: Antifungal Activity and Mode of Action on Candida Spp., and Molecular Targets Affected in Human Whole-Genome Expression. PLoS ONE 2014, 9, e99086. [Google Scholar] [CrossRef]
- Ferreira, A.R.; Alves, D.N.; Castro, R.D.; Perez-Castillo, Y.; Sousa, D.P. Synthesis of Coumarin and Homoisoflavonoid Derivatives and Analogs: The Search for New Antifungal Agents. Pharmaceuticals 2022, 15, 712. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.P.; Yepes, L.M.; Pérez-Castillo, Y.; Robledo, S.M.; Sousa, D.P. Alkyl and Aryl Derivatives Based on P-Coumaric Acid Modification and Inhibitory Action against Leishmania braziliensis and Plasmodium falciparum. Molecules 2020, 25, 3178. [Google Scholar] [CrossRef] [PubMed]
- Keiser, M.J.; Roth, B.L.; Armbruster, B.N.; Ernsberger, P.; Irwin, J.J.; Shoichet, B.K. Relating Protein Pharmacology by Ligand Chemistry. Nat. Biotechnol. 2007, 25, 197–206. [Google Scholar] [CrossRef]
- Hawkins, P.C.D.; Skillman, A.G.; Warren, G.L.; Ellingson, B.A.; Stahl, M.T. Molecular Modeling Software|OpenEye Scientific. Available online: https://www.eyesopen.com/omega (accessed on 3 June 2025).
- Hawkins, P.C.D.; Skillman, A.G.; Warren, G.L.; Ellingson, B.M.; Stahl, M.T. Conformer Generation with OMEGA: Algorithm and Validation Using High Quality Structures from the Protein Databank and Cambridge Structural Database. J. Chem. Inf. Model. 2010, 50, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Santa Fe, N.M. Molecular Modeling Software|OpenEye Scientific. Available online: https://www.eyesopen.com/quacpac (accessed on 27 May 2025).
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; Beer, T.A.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and Validation of a Genetic Algorithm for Flexible Docking 1 1Edited by F. E. Cohen. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef]
- Case, D.A.; Aktulga, H.M.; Belfon, K.; Ben-Shalom, I.; Berryman, J.T.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E.; Cisneros, G.A.; Cruzeiro, V.W.D.; et al. Amber 2022; University of California: San Francisco, CA, USA, 2022. [Google Scholar]
- Castro, M.; Perez-Castillo, Y.; Silva, V.R.; Santos, L.S.; Botelho, M.; Bezerra, D.P.; Castro, R.D.; Sousa, D.P. Cytotoxic and Antifungal Amides Derived from Ferulic Acid: Molecular Docking and Mechanism of Action. Biomed. Res. Int. 2021, 2021, 3598000. [Google Scholar] [CrossRef]
- Perez-Castillo, Y.; Montes, R.C.; Silva, C.R.; Neto, J.B.A.; Dias, C.S.; Duarte, A.B.S.; Nobre, H.V.; Sousa, D.P. Antifungal Activity of N-(4-Halobenzyl) Amides against Candida Spp. And Molecular Modeling Studies. Int. J. Mol. Sci. 2021, 23, 419. [Google Scholar] [CrossRef]
- AMBER Parameter Database (Bryce Group: Computational Biophysics and Drug Design—University of Manchester). Available online: http://amber.manchester.ac.uk/ (accessed on 7 September 2022).
- Pang, Y.P.; Xu, K.; Yazal, J.E.; Prendergast, F.G. Successful Molecular Dynamics Simulation of the Zinc-Bound Farnesyltransferase Using the Cationic Dummy Atom Approach. Protein Sci. 2000, 9, 1857–1865. [Google Scholar] [CrossRef]
- Li, P.; Merz, K.M. MCPB.py: A Python Based Metal Center Parameter Builder. J. Chem. Inf. Model. 2016, 56, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.R.; Pantano, S. Split the Charge Difference in Two! A Rule of Thumb for Adding Proper Amounts of Ions in MD Simulations. J. Chem. Theory Comput. 2020, 16, 1367–1372. [Google Scholar] [CrossRef]
- Miller, B.R.; McGee, T.D.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.py: An Efficient Program for End-State Free Energy Calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef]
- Czub, J.; Baginski, M. Comparative Molecular Dynamics Study of Lipid Membranes Containing Cholesterol and Ergosterol. Biophys. J. 2006, 90, 2368–2382. [Google Scholar] [CrossRef]
- Vandeputte, P.; Ferrari, S.; Coste, A.T. Antifungal Resistance and New Strategies to Control Fungal Infections. Int. J. Microbiol. 2012, 2012, 713687. [Google Scholar] [CrossRef]
- Poli, G.; Granchi, C.; Rizzolio, F.; Tuccinardi, T. Application of MM-PBSA Methods in Virtual Screening. Molecules 2020, 25, 1971. [Google Scholar] [CrossRef]
- Wang, E.; Sun, H.; Wang, J.; Wang, Z.; Liu, H.; Zhang, J.Z.H.; Hou, T. End-Point Binding Free Energy Calculation with MM/PBSA and MM/GBSA: Strategies and Applications in Drug Design. Chem. Rev. 2019, 119, 9478–9508. [Google Scholar] [CrossRef]
- Lapillo, M.; Tuccinardi, T.; Martinelli, A.; Macchia, M.; Giordano, A.; Poli, G. Extensive Reliability Evaluation of Docking-Based Target-Fishing Strategies. Int. J. Mol. Sci. 2019, 20, 1023. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhao, L.; Hu, J.; Jin, H.; Liu, Z.; Zhang, L. The Scoring Bias in Reverse Docking and the Score Normalization Strategy to Improve Success Rate of Target Fishing. PLoS ONE 2017, 12, e0171433. [Google Scholar] [CrossRef]
- Wen, W.; Cao, H.; Huang, Y.; Tu, J.; Wan, C.; Wan, J.; Han, X.; Chen, H.; Liu, J.; Rao, L.; et al. Structure-Guided Discovery of the Novel Covalent Allosteric Site and Covalent Inhibitors of Fructose-1,6-Bisphosphate Aldolase to Overcome the Azole Resistance of Candidiasis. J. Med. Chem. 2022, 65, 2656–2674. [Google Scholar] [CrossRef] [PubMed]
- Jakob, C.G.; Upadhyay, A.K.; Donner, P.L.; Nicholl, E.; Addo, S.N.; Qiu, W.; Ling, C.; Gopalakrishnan, S.M.; Torrent, M.; Cepa, S.P.; et al. Novel Modes of Inhibition of Wild-Type Isocitrate Dehydrogenase 1 (IDH1): Direct Covalent Modification of His315. J. Med. Chem. 2018, 61, 6647–6657. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.B.S.; Perez-Castillo, Y.; Andrade, P.N.; Castro, R.D.; Sousa, D.P. 3,5-Dinitrobenzoate and 3,5-Dinitrobenzamide Derivatives: Mechanistic, Antifungal, and in Silico Studies. J. Chem. 2022, 2022, e2336175. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand–Protein Interaction Diagrams for Drug Discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- Silva, R.H.N.; Machado, T.Q.; Fonseca, A.C.C.; Tejera, E.; Perez-Castillo, Y.; Robbs, B.K.; Sousa, D.P. Molecular Modeling and in Vitro Evaluation of Piplartine Analogs against Oral Squamous Cell Carcinoma. Molecules 2023, 28, 1675. [Google Scholar] [CrossRef]
- Begum, K.; Shahid, M.S.; Jalil, R.U.J. Topical Nanoemulsion of Rifampicin with Benzoic Acid and Salicylic Acid: Activity against Staphylococcus aureus, Stap. epidermis and Candida albicans. Bangladesh Pharm. J. 2019, 22, 1–6. [Google Scholar] [CrossRef]
- Tavares, W. Manual de Antibióticos E Quimioterápicos Antiinfecciosos, 3rd ed.; Atheneu: São Paulo, Brazil, 2021. [Google Scholar]
- Leite, M.C.A.; Bezerra, A.P.B.; Sousa, J.P.; Lima, E.O. Investigating the Antifungal Activity and Mechanism(S) of Geraniol against Candida albicans Strains. Med. Mycol. 2014, 53, 275–284. [Google Scholar] [CrossRef]

| (a) | ||||||||
| Compounds | C. albicans ATCC 90028 | C. albicans CBS 5602 | ||||||
| MIC (μg/mL) | MIC (μM) | MFC | MFC/ MIC | MIC (μg/mL) | MIC (μM) | MFC | MFC/ MIC | |
| SA | - | - | - | - | - | - | - | - |
| 1 | - | - | - | - | - | - | - | - |
| 2 | - | - | - | - | - | - | - | - |
| 3 | - | - | - | - | - | - | - | - |
| 4 | - | - | - | - | - | - | - | - |
| 5 | - | - | - | - | - | - | - | - |
| 6 | - | - | - | - | - | - | - | - |
| 7 | - | - | - | - | - | - | - | - |
| 8 | - | - | - | - | - | - | - | - |
| 9 | - | - | - | - | - | - | - | - |
| 10 | - | - | - | - | - | - | - | - |
| 11 | - | - | - | - | - | - | - | - |
| 12 | - | - | - | - | - | - | - | - |
| 13 | - | - | - | - | - | - | - | - |
| Nystatin | 1.5 | 1.62 | 1.62 | 1 | 1.5 | 1.62 | 1.62 | 1 |
| Ketoconazole | 0.5 | 0.940 | 0.940 | 1 | 1 | 1.88 | 1.88 | 1 |
| DMSO | - | - | - | - | - | - | - | - |
| (b) | ||||||||
| Compounds | C. tropicalis CBS 94 | C. krusei CBS 573 | ||||||
| MIC (μg/mL) | MIC (μM) | MIC (μg/mL) | MIC (μM) | MIC (μg/mL) | MIC (μM) | MIC (μg/mL) | MIC (μM) | |
| SA | - | - | - | - | - | - | - | - |
| 1 | - | - | - | - | - | - | - | - |
| 2 | - | - | - | - | - | - | - | - |
| 3 | - | - | - | - | - | - | - | - |
| 4 | - | - | - | - | - | - | - | - |
| 5 | - | - | - | - | - | - | - | - |
| 6 | - | - | - | - | - | - | - | - |
| 7 | - | - | - | - | - | - | - | - |
| 8 | - | - | - | - | - | - | - | - |
| 9 | - | - | - | - | - | - | - | - |
| 10 | - | - | - | - | - | - | - | - |
| 11 | - | - | - | - | - | - | - | - |
| 12 | - | - | - | - | - | - | - | - |
| 13 | - | - | - | - | - | - | - | - |
| Nystatin | 1.5 | 1.62 | 1.5 | 1.62 | 1.5 | 1.62 | 1.5 | 1.62 |
| Ketoconazole | 4 | 7.53 | 4 | 7.53 | 4 | 7.53 | 4 | 7.53 |
| DMSO | - | - | - | - | - | - | - | - |
| (c) | ||||||||
| Compounds | C. albicans ATCC 90028 | C. albicans CBS 5602 | ||||||
| MIC (μg/mL) | MIC (μM) | MFC | MFC/ MIC | MIC (μg/mL) | MIC (μM) | MFC | MFC/ MIC | |
| 14 | 250 | 1293.73 | 1293.73 | 1 | 250 | 1293.73 | 1293.73 | 1 |
| 15 | 125 | 570.05 | 570.05 | 1 | 125 | 570.05 | 570.05 | 1 |
| 16 | - | - | - | - | 125 | 550.03 | 550.03 | 1 |
| 17 | 500 | 2072.20 | 2072.20 | 1 | 500 | 2072.20 | 2072.20 | 1 |
| 18 | 125 | 485.83 | 485.83 | 1 | 125 | 485.83 | 485.83 | 1 |
| 19 | - | - | - | - | - | - | - | - |
| 20 | - | - | - | - | - | - | - | - |
| 21 | - | - | - | - | - | - | - | - |
| 22 | 1000 | 4077.47 | 4077.47 | 1 | 500 | 2038.74 | 2038.74 | 1 |
| 23 | 500 | 1910.58 | 1910.58 | 1 | - | - | - | - |
| 24 | - | - | - | - | - | - | - | - |
| 25 | - | - | - | - | - | - | - | - |
| Nystatin | 1.5 | 1.62 | 1.62 | 1 | 1.5 | 1.62 | 1.62 | 1 |
| Ketoconazole | 0.5 | 0.940 | 0.940 | 1 | 1 | 1.88 | 1.88 | 1 |
| DMSO | - | - | - | - | - | - | - | - |
| (d) | ||||||||
| Compounds | C. tropicalis CBS 94 | C. krusei CBS 573 | ||||||
| MIC (μg/mL) | MIC (μM) | MFC | MFC /MIC | MIC (μg/mL) | MIC (μM) | MFC | MFC /MIC | |
| 14 | 500 | 2587.46 | 2587.46 | 1 | 250 | 1293.73 | 1293.73 | 1 |
| 15 | 250 | 1140.10 | 1140.10 | 1 | 125 | 570.05 | 570.05 | 1 |
| 16 | - | - | - | - | 250 | 1100.06 | 1100.06 | 1 |
| 17 | - | - | - | - | 500 | 2072.20 | 2072.20 | 1 |
| 18 | 500 | 1943.33 | 1943.33 | 1 | 125 | 485.83 | 485.83 | 1 |
| 19 | - | - | - | - | 500 | 1740.28 | 1740.28 | 1 |
| 20 | - | - | - | - | - | - | - | - |
| 21 | - | - | - | - | - | - | - | - |
| 22 | 500 | 2038.74 | 2038.74 | 1 | 500 | 2038.74 | 2038.74 | 1 |
| 23 | - | - | - | - | 500 | 1910.58 | 1910.58 | 1 |
| 24 | - | - | - | - | - | - | - | - |
| 25 | - | - | - | - | - | - | - | - |
| Nystatin | 1.5 | 1.62 | 1.62 | 1 | 1.5 | 1.62 | 1.62 | 1 |
| Ketoconazole | 4 | 7.53 | 7.53 | 1 | 0.5 | 0.940 | 0.940 | 1 |
| DMSO | - | - | - | - | - | - | - | - |
| C. albicans ATCC 90028 | |||||
|---|---|---|---|---|---|
| 15 | Nistatin | ||||
| Concentration (μM) | Without Ergosterol | With Ergosterol | Concentration (μM) | Without Ergosterol | With Ergosterol |
| 9120 | − | − | 52 | − | − |
| 4560 | − | − | 26 | − | + |
| 2280 | − | − | 13 | − | + |
| 1140 | − | − | 6.5 | − | + |
| 570 | − | − | 3.2 | − | + |
| 290 | + | + | 1.6 | − | + |
| 140 | + | + | 0.8 | + | + |
| 70 | + | + | 0.4 | + | + |
| C. albicans ATCC 90028 | |||||
|---|---|---|---|---|---|
| 15 | Caspofungin | ||||
| Concentration (μM) | Without Sorbitol | With Sorbitol | Concentration (μM) | Without Sorbitol | With Sorbitol |
| 9120 | − | − | 3.6 | − | − |
| 4560 | − | − | 1.8 | − | − |
| 2280 | − | − | 0.9 | − | + |
| 1140 | − | − | 0.45 | − | + |
| 570 | − | − | 0.228 | − | + |
| 290 | + | + | 0.114 | + | + |
| 140 | + | + | 0.056 | + | + |
| 70 | + | + | 0.028 | + | + |
| UniProt Accession | ID | Description |
|---|---|---|
| A0A1D8PHU1 | TPK2 | cAMP-dependent protein kinase |
| P43063 | CDK1 | Cyclin-dependent kinase 1 |
| A0A1D8PDA6 | PHO85 | Cyclin-dependent serine/threonine-protein kinase |
| Q5A1D3 | CEK1 | Extracellular signal-regulated kinase 1 |
| Q9URB4 | FBA1 | Fructose-bisphosphate aldolase |
| A0A1D8PS79 | IDP2 | Isocitrate dehydrogenase |
| A0A1D8PHH7 | IDP1 | Isocitrate dehydrogenase |
| Q59LF9 | MAP2 | Methionine aminopeptidase 2 |
| Q5ALM6 | CPR3 | Peptidyl-prolyl cis-trans isomerase |
| P22011 | CYP1 | Peptidyl-prolyl cis-trans isomerase |
| A0A8H6F4I1 | CYPB | Peptidyl-prolyl cis-trans isomerase |
| Q59U59 | APR1 | Proteinase A |
| A0A1D8PU61 | FDH3 | S-(hydroxymethyl)glutathione dehydrogenase |
| A0A1D8PSJ8 | PPH3 | Serine/threonine-protein phosphatase |
| Q92207 | HOG1 | Mitogen-activated protein kinase HOG1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, A.J.d.M.S.; Alves, D.d.N.; Duarte, M.C.; Castro, R.D.d.; Perez-Castillo, Y.; de Sousa, D.P. Salicylic Acid Derivatives as Antifungal Agents: Synthesis, In Vitro Evaluation, and Molecular Modeling. Chemistry 2025, 7, 151. https://doi.org/10.3390/chemistry7050151
Oliveira AJdMS, Alves DdN, Duarte MC, Castro RDd, Perez-Castillo Y, de Sousa DP. Salicylic Acid Derivatives as Antifungal Agents: Synthesis, In Vitro Evaluation, and Molecular Modeling. Chemistry. 2025; 7(5):151. https://doi.org/10.3390/chemistry7050151
Chicago/Turabian StyleOliveira, Ana Júlia de Morais Santos, Danielle da N. Alves, Marcelo Cavalcante Duarte, Ricardo Dias de Castro, Yunierkis Perez-Castillo, and Damião Pergentino de Sousa. 2025. "Salicylic Acid Derivatives as Antifungal Agents: Synthesis, In Vitro Evaluation, and Molecular Modeling" Chemistry 7, no. 5: 151. https://doi.org/10.3390/chemistry7050151
APA StyleOliveira, A. J. d. M. S., Alves, D. d. N., Duarte, M. C., Castro, R. D. d., Perez-Castillo, Y., & de Sousa, D. P. (2025). Salicylic Acid Derivatives as Antifungal Agents: Synthesis, In Vitro Evaluation, and Molecular Modeling. Chemistry, 7(5), 151. https://doi.org/10.3390/chemistry7050151








