Synthesis and Characterization of a New Hydrogen-Bond-Stabilized 1,10-Phenanthroline–Phenol Schiff Base: Integrated Spectroscopic, Electrochemical, Theoretical Studies, and Antimicrobial Evaluation
Abstract
1. Introduction
2. Results and Discussion
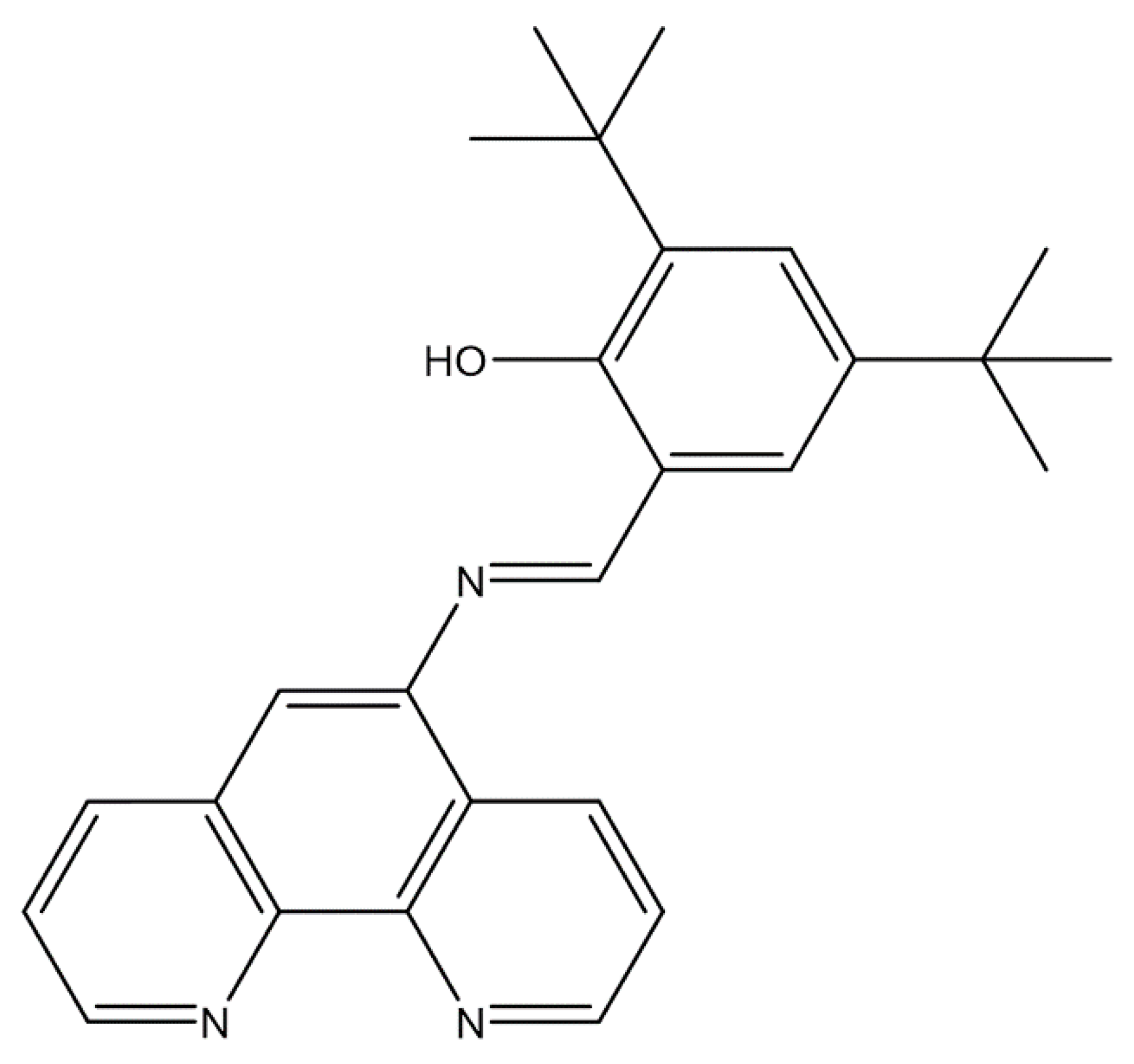
2.1. Synthesis and Structural Features of Fen-IHB
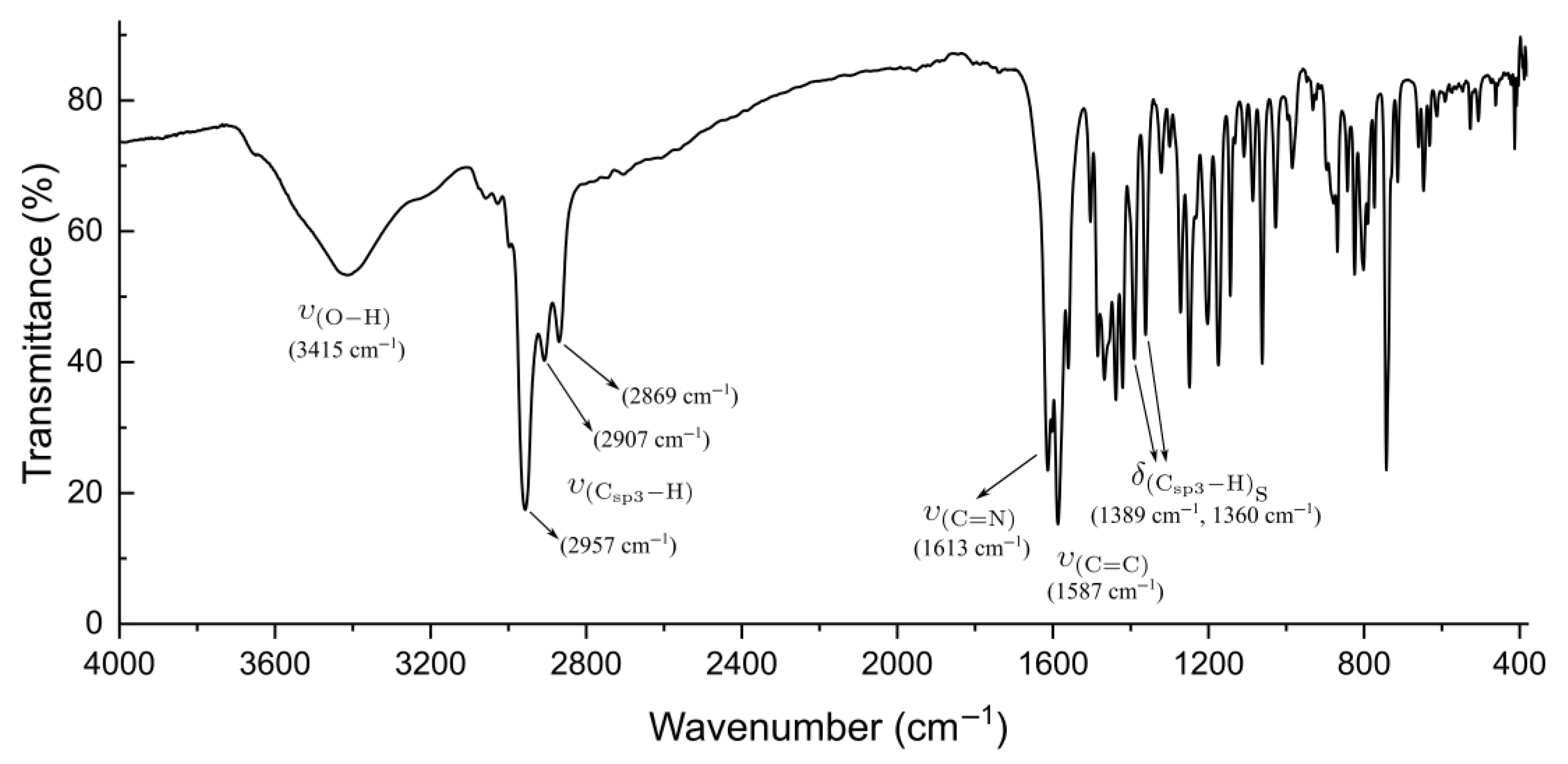
2.2. High-Resolution Mass Spectrometry, FTIR, UV–Vis Spectroscopy, and NMR Analysis
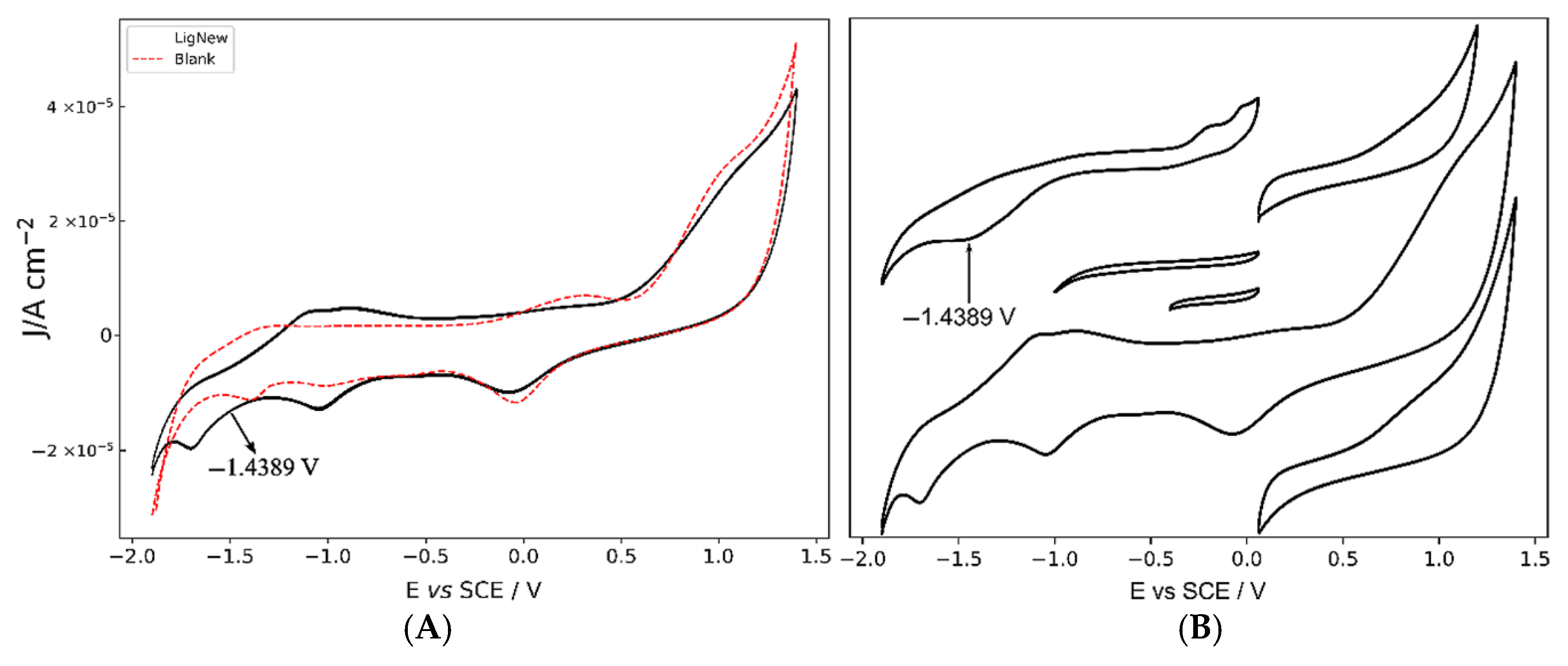
2.3. Cyclic Voltammetry
2.4. Quantum Chemistry: Geometry Optimizations, TD-DFT, Reactivity Descriptors, and Noncovalent Interaction (NCI) Index
2.4.1. Comparative Analysis of DFT-Based Reactivity Descriptors
2.4.2. Noncovalent Interaction (NCI) Index
2.5. Biocidal Activity of Fen-IHB
3. Experimental
3.1. Materials and Methods
3.2. Synthesis of (E)-2-(((1,10-Phenanthrolin-5-yl)imino)methyl)-4,6-di-tert-butylphenol (Fen-IHB)
3.3. Computational Methods
3.4. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mushtaq, I.; Ahmad, M.; Saleem, M.; Ahmed, A. Pharmaceutical significance of Schiff bases: An overview. F J. Pharm. Sci. 2024, 10, 16. [Google Scholar] [CrossRef]
- Raczuk, E.; Dmochowska, B.; Samaszko-Fiertek, J.; Madaj, J. Different Schiff Bases-Structure, Importance and Classification. Molecules 2022, 27, 787. [Google Scholar] [CrossRef]
- De, S.; Jain, A.; Barman, P. Recent Advances in the Catalytic Applications of Chiral Schiff-Base Ligands and Metal Complexes in Asymmetric Organic Transformations. ChemistrySelect 2022, 7, e202104334. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Ko, Y.G. Schiff base complexes and their versatile applications as catalysts in oxidation of organic compounds: Part I. Appl. Organomet. Chem. 2016, 31, e3574. [Google Scholar] [CrossRef]
- Senthil Kumar, R.; Archana, S.; Archana, T.; Divya, R.; Praveen, S.; Shridharshini, K. Synthetic approaches of medicinally important Schiff bases: An updated Review. World J. Adv. Res. Rev. 2022, 16, 838–852. [Google Scholar] [CrossRef]
- Soliman, A.I.A.; Sayed, M.; Elshanawany, M.M.; Younis, O.; Ahmed, M.; Kamal El-Dean, A.M.; Abdel-Wahab, A.A.; Wachtveitl, J.; Braun, M.; Fatehi, P.; et al. Base-Free Synthesis and Photophysical Properties of New Schiff Bases Containing Indole Moiety. ACS Omega 2022, 7, 10178–10186. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Saturnino, C.; Bonomo, M.G.; Franchini, C.; Sinicropi, M.S. Schiff Bases: Interesting Scaffolds with Promising Antitumoral Properties. Appl. Sci. 2021, 11, 1877. [Google Scholar] [CrossRef]
- Krupka, K.M.; Pochec, M.; Panek, J.J.; Jezierska, A. Comprehensive Empirical Model of Substitution-Influence on Hydrogen Bonding in Aromatic Schiff Bases. Int. J. Mol. Sci. 2022, 23, 12439. [Google Scholar] [CrossRef]
- Irfan, A.; Al-Sehemi, A.G.; Kalam, A. Tuning the Electronic and Charge Transport Properties of Schiff Base Compounds by Electron Donor and/or Acceptor Groups. Materials 2022, 15, 8590. [Google Scholar] [CrossRef]
- Sarkar, C.G. Theoretical Explorations of Structural, Electronic, and Spectroscopic Properties of a Series of Ruthenium Schiff-Base Complexes. J. Sci. Res. 2023, 15, 489–508. [Google Scholar] [CrossRef]
- Jezierska-Mazzarello, A.; Panek, J.J.; Vuilleumier, R.; Koll, A.; Ciccotti, G. Direct observation of the substitution effects on the hydrogen bridge dynamics in selected Schiff bases--a comparative molecular dynamics study. J. Chem. Phys. 2011, 134, 034308. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.R.; Rajeshwar, S.; Satyanarayana, B. Synthesis, characterization of new copper (ii) Schiff base and 1,10 phenanthroline complexes and study of their bioproperties. J. Photochem. Photobiol. B 2016, 160, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Accorsi, G.; Listorti, A.; Yoosaf, K.; Armaroli, N. ChemInform Abstract: 1,10-Phenanthrolines: Versatile Building Blocks for Luminescent Molecules, Materials and Metal Complexes. ChemInform 2009, 38, 1690–1700. [Google Scholar] [CrossRef]
- Ashrafuzzaman, M.; Camellia, F.K.; Hossain, M.D.; Shovon, M.G.M.; Rahman, H.; Hossain, M.T.; Rahman, M.H.; Kudrat-E-Zahan, M.; Haque, M.M. Mixed Ligand Complexes of Ni2+, Cu2+ and Zn2+ Ions Containing N-(4-methoxybenzylidene)isonicotinohydrazone Schiff Base and 1,10-Phenanthroline: Synthesis, Characterization, Antimicrobial and Antioxidant Properties. Asian J. Chem. Sci. 2023, 13, 46–57. [Google Scholar] [CrossRef]
- Sogukomerogullari, H.G.; Başaran, E.; Sarıoğlu, A.O.; Köse, A.; Akkoç, S. Synthesis, Characterization, Photoluminescence Properties and Antiproliferative Activity of New Pd(II), Ni(II) and Cu(II) Mixed Complexes Bearing Schiff Base Ligand and 1,10-phenanthroline. ChemistrySelect 2023, 8, e202301014. [Google Scholar] [CrossRef]
- Chang, C.; Shieh, T.L.; Floss, H.G. Model studies of pyridoxal Schiff’s bases. Coplanarity and intramolecular hydrogen bonding. J. Med. Chem. 1977, 20, 176–178. [Google Scholar] [CrossRef]
- Carreno, A.; Morales-Guevara, R.; Cepeda-Plaza, M.; Paez-Hernandez, D.; Preite, M.; Polanco, R.; Barrera, B.; Fuentes, I.; Marchant, P.; Fuentes, J.A. Synthesis, Physicochemical Characterization, and Antimicrobial Evaluation of Halogen-Substituted Non-Metal Pyridine Schiff Bases. Molecules 2024, 29, 4726. [Google Scholar] [CrossRef]
- Sidir, I.; Gulseven Sidir, Y.; Gobi, S.; Berber, H.; Fausto, R. Structural Relevance of Intramolecular H-Bonding in Ortho-Hydroxyaryl Schiff Bases: The Case of 3-(5-bromo-2-hydroxybenzylideneamino) Phenol. Molecules 2021, 26, 2814. [Google Scholar] [CrossRef]
- Szady-Chelmieniecka, A.; Kolodziej, B.; Morawiak, M.; Kamienski, B.; Schilf, W. Spectroscopic studies of the intramolecular hydrogen bonding in o-hydroxy Schiff bases, derived from diaminomaleonitrile, and their deprotonation reaction products. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 189, 330–341. [Google Scholar] [CrossRef]
- Yıldız, M.; Kılıç, Z.; Hökelek, T. Intramolecular hydrogen bonding and tautomerism in Schiff bases. Part I. Structure of 1,8-di[N-2-oxyphenyl-salicylidene]-3,6-dioxaoctane. J. Mol. Struct. 1998, 441, 1–10. [Google Scholar] [CrossRef]
- Flores-Leonar, M.; Esturau-Escofet, N.; Méndez-Stivalet, J.M.; Marín-Becerra, A.; Amador-Bedolla, C. Factors determining tautomeric equilibria in Schiff bases. J. Mol. Struct. 2011, 1006, 600–605. [Google Scholar] [CrossRef]
- Schilf, W.; Kamienski, B.; Szady-Chelmieniecka, A.; Kolodziej, B.; Grech, E.; Zarzeczanska, D.; Wcislo, A.; Ossowski, T. Structure investigation of intramolecular hydrogen bond in some substituted salicylaldehydes and 4-aminoantipyrine derivatives in solution and in the solid state. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 109, 47–54. [Google Scholar] [CrossRef]
- Sıdır, İ.; Sıdır, Y.G.; Berber, H.; Fausto, R. Effects of Enol-imine/Keto-amine tautomerism and conformational changes on the electronic spectra of a novel 1,2,4-triazole ortho-hydroxyaryl Schiff base in different solvents. J. Mol. Struct. 2023, 1292, 136191. [Google Scholar] [CrossRef]
- Kujawski, J.; Drabinska, B.; Dettlaff, K.; Skotnicki, M.; Olszewska, A.; Ratajczak, T.; Napierala, M.; Chmielewski, M.K.; Kasprzak, M.; Kujawski, R.; et al. Structural and Spectroscopic Properties of Magnolol and Honokiol-Experimental and Theoretical Studies. Int. J. Mol. Sci. 2025, 26, 6085. [Google Scholar] [CrossRef] [PubMed]
- Ditler, E.; Luber, S. Vibrational spectroscopy by means of first-principles molecular dynamics simulations. WIREs Comput. Mol. Sci. 2022, 12, e1605. [Google Scholar] [CrossRef]
- Shimakoshi, H.; Hisaeda, Y. Synthesis of Cyclic and Acyclic Schiff-base Compounds and Development of Unique Dicobalt Complexes with Supramolecular Functions. J. Synth. Org. Chem. Jpn. 2012, 70, 60–70. [Google Scholar] [CrossRef]
- Dong, Y.W.; Fan, R.Q.; Chen, W.; Zhang, H.J.; Song, Y.; Du, X.; Wang, P.; Wei, L.G.; Yang, Y.L. Different conjugated system Zn(ii) Schiff base complexes: Supramolecular structure, luminescent properties, and applications in the PMMA-doped hybrid materials. Dalton Trans. 2017, 46, 1266–1276. [Google Scholar] [CrossRef]
- Tesfaye, D.; Linert, W.; Gebrezgiabher, M.; Bayeh, Y.; Elemo, F.; Sani, T.; Kalarikkal, N.; Thomas, M. Iron(II) Mediated Supramolecular Architectures with Schiff Bases and Their Spin-Crossover Properties. Molecules 2023, 28, 1012. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, Y.; Zhang, W. Development of organic porous materials through Schiff-base chemistry. CrystEngComm 2013, 15, 1484–1499. [Google Scholar] [CrossRef]
- Hansen, P.E.; Spanget-Larsen, J. NMR and IR Investigations of Strong Intramolecular Hydrogen Bonds. Molecules 2017, 22, 552. [Google Scholar] [CrossRef]
- Szady-Chełmieniecka, A.; Rozwadowski, Z.; Dziembowska, T.; Grech, E.; Wojciechowski, G.; Brzezinski, B. Intramolecular hydrogen bonds in 3-diethylaminomethyl-5-R-salicylic aldehydes. J. Mol. Struct. 2000, 520, 39–43. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, N.; Silwal, M.; Adhikari, A.; Yadav, P.N. Synthesis, characterization, anticancer activity, molecular docking and DFT calculation of 3-acetylcoumarin thiosemicarbazones and Schiff’s bases. Results Chem. 2024, 11, 101794. [Google Scholar] [CrossRef]
- Yousif, E.; Hasan, A.; El-Hiti, G.A. Spectroscopic, Physical and Topography of Photochemical Process of PVC Films in the Presence of Schiff Base Metal Complexes. Polymers 2016, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Filarowski, A.; Koll, A.; Karpfen, A.; Wolschann, P. Intramolecular hydrogen bond in molecular and proton-transfer forms of Schiff bases. Chem. Phys. 2004, 297, 323–332. [Google Scholar] [CrossRef]
- Carreno, A.; Paez-Hernandez, D.; Cantero-Lopez, P.; Zuniga, C.; Nevermann, J.; Ramirez-Osorio, A.; Gacitua, M.; Oyarzun, P.; Saez-Cortez, F.; Polanco, R.; et al. Structural Characterization, DFT Calculation, NCI, Scan-Rate Analysis and Antifungal Activity against Botrytis cinerea of (E)-2-{[(2-Aminopyridin-2-yl)imino]-methyl}-4,6-di-tert-butylphenol (Pyridine Schiff Base). Molecules 2020, 25, 2741. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Q.; Yang, S.H.; Li, Y.; Ye, W.X.; Liao, Z.Y.; Lu, J.Q.; Wang, Z.Y. Schiff Base Compounds Derived from 5-Methyl Salicylaldehyde as Turn-On Fluorescent Probes for Al(3+) Detection: Experimental and DFT Calculations. Molecules 2025, 30, 1128. [Google Scholar] [CrossRef]
- Reddy, K.R.; Raghu, A.V.; Jeong, H.M. Siddaramaiah, Synthesis and Characterization of Pyridine-Based Polyurethanes. Des. Monomers Polym. 2012, 12, 109–118. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Salassa, G.; Kleij, A.W. Recent advances with pi-conjugated salen systems. Chem. Soc. Rev. 2012, 41, 622–631. [Google Scholar] [CrossRef]
- Al-Hakimi, A.N.; Alresheedi, T.M.; Albarrak, R.A.; Albadri, A.E.A.E.; Abd El-Hady, M.M.; Saeed, S.E.-S. Synthesis and Characterization of a Fluorinated Schiff Base from Benzimidazole and Its Metal Complexes for Antimicrobial and UV-Protective Cotton Fabrics. Coatings 2025, 15, 380. [Google Scholar] [CrossRef]
- Caviglia, M.; Li, Z.; Santini, C.; Del Gobbo, J.; Cimarelli, C.; Du, M.; Dolmella, A.; Pellei, M. New Cu(II), Cu(I) and Ag(I) Complexes of Phenoxy-Ketimine Schiff Base Ligands: Synthesis, Structures and Antibacterial Activity. Molecules 2025, 30, 1893. [Google Scholar] [CrossRef]
- Houjou, H.; Shingai, H.; Yagi, K.; Yoshikawa, I.; Araki, K. Mutual interference between intramolecular proton transfer sites through the adjoining pi-conjugated system in Schiff bases of double-headed, fused salicylaldehydes. J. Org. Chem. 2013, 78, 9021–9031. [Google Scholar] [CrossRef]
- Sigalov, M.V.; Shainyan, B.A.; Chipanina, N.N.; Oznobikhina, L.P. Molecular Structure, Intramolecular Hydrogen Bonding, Solvent-Induced Isomerization, and Tautomerism in Azolylmethylidene Derivatives of 2-Indanone. Eur. J. Org. Chem. 2017, 2017, 1353–1364. [Google Scholar] [CrossRef]
- Mabesoone, M.F.J.; Palmans, A.R.A.; Meijer, E.W. Solute-Solvent Interactions in Modern Physical Organic Chemistry: Supramolecular Polymers as a Muse. J. Am. Chem. Soc. 2020, 142, 19781–19798. [Google Scholar] [CrossRef] [PubMed]
- Ashok, U.P.; Kollur, S.P.; Anil, N.; Arun, B.P.; Jadhav, S.N.; Sarsamkar, S.; Helavi, V.B.; Srinivasan, A.; Kaulage, S.; Veerapur, R.; et al. Preparation, Spectroscopic Characterization, Theoretical Investigations, and In Vitro Anticancer Activity of Cd(II), Ni(II), Zn(II), and Cu(II) Complexes of 4(3H)-Quinazolinone-Derived Schiff Base. Molecules 2020, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- Bal, M.; Köse, A. Schiff bases containing 1,2,3-triazole group and phenanthroline: Synthesis, characterization, and investigation of DNA binding properties. J. Photochem. Photobiol. A 2024, 448, 115320. [Google Scholar] [CrossRef]
- Issa, Y.M.; Hassib, H.B.; Abdelaal, H.E. 1H NMR, 13C NMR and mass spectral studies of some Schiff bases derived from 3-amino-1,2,4-triazole. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 74, 902–910. [Google Scholar] [CrossRef]
- Gurusamy, S.; Sankarganesh, M.; Revathi, N.; Asha, R.N.; Mathavan, A. Synthesis and structural investigation of o-Vanillin scaffold Schiff base metal complexes: Biomolecular interaction and molecular docking studies. J. Mol. Liq. 2023, 369, 120941. [Google Scholar] [CrossRef]
- Fabbrizzi, L. Beauty in Chemistry: Making Artistic Molecules with Schiff Bases. J. Org. Chem. 2020, 85, 12212–12226. [Google Scholar] [CrossRef]
- Arumugam, A.; Shanmugam, R.; Munusamy, S.; Muhammad, S.; Algarni, H.; Sekar, M. Study of the Crystal Architecture, Optoelectronic Characteristics, and Nonlinear Optical Properties of 4-Amino Antipyrine Schiff Bases. ACS Omega 2023, 8, 15168–15180. [Google Scholar] [CrossRef]
- Akitsu, T.; Nakane, D.; Miroslaw, B. Viewpoints Concerning Crystal Structure from Recent Reports on Schiff Base Compounds and Their Metal Complexes. Symmetry 2024, 16, 1525. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Mohamed, M.B.I.; Bedair, M.A.; El-Zomrawy, A.A.; Bakr, M.F. A new Schiff base-fabricated pencil lead electrode for the efficient detection of copper, lead, and cadmium ions in aqueous media. RSC Adv. 2023, 13, 15651–15666. [Google Scholar] [CrossRef]
- Aytac, S.; Gundogdu, O.; Bingol, Z.; Gulcin, I. Synthesis of Schiff Bases Containing Phenol Rings and Investigation of Their Antioxidant Capacity, Anticholinesterase, Butyrylcholinesterase, and Carbonic Anhydrase Inhibition Properties. Pharmaceutics 2023, 15, 779. [Google Scholar] [CrossRef]
- Chen, K.Y.; Tsai, H.Y. Synthesis, X-ray structure, spectroscopic properties and DFT studies of a novel Schiff base. Int. J. Mol. Sci. 2014, 15, 18706–18724. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, H.C.; Hernandes, I.S.; De Almeida, W.B. Modeling Solvent Effects in Quantum Chemical Calculation of Relative Energies and NMR Chemical Shifts for Azithromycin. J. Phys. Chem. A 2025, 129, 2200–2216. [Google Scholar] [CrossRef] [PubMed]
- Malloum, A.; Conradie, J. Accurate binding energies of ammonia clusters and benchmarking of hybrid DFT functionals. Comput. Theor. Chem. 2021, 1200, 113236. [Google Scholar] [CrossRef]
- Hughes, C.E.; Reddy, G.N.M.; Masiero, S.; Brown, S.P.; Williams, P.A.; Harris, K.D.M. Determination of a complex crystal structure in the absence of single crystals: Analysis of powder X-ray diffraction data, guided by solid-state NMR and periodic DFT calculations, reveals a new 2’-deoxyguanosine structural motif. Chem. Sci. 2017, 8, 3971–3979. [Google Scholar] [CrossRef]
- Barbas, R.; Portell, A.; Prohens, R.; Frontera, A. DFT–Assisted Structure Determination from Powder X-ray Diffraction Data of a New Zonisamide/ϵ-Caprolactam Cocrystal. Crystals 2022, 12, 1020. [Google Scholar] [CrossRef]
- Mancinelli, M.; Franzini, R.; Renzetti, A.; Marotta, E.; Villani, C.; Mazzanti, A. Determination of the absolute configuration of conformationally flexible molecules by simulation of chiro-optical spectra: A case study. RSC Adv. 2019, 9, 18165–18175. [Google Scholar] [CrossRef]
- Rad, A.S.; Ardjmand, M.; Esfahani, M.R.; Khodashenas, B. DFT calculations towards the geometry optimization, electronic structure, infrared spectroscopy and UV-vis analyses of Favipiravir adsorption on the first-row transition metals doped fullerenes; a new strategy for COVID-19 therapy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 247, 119082. [Google Scholar] [CrossRef]
- Carreno, A.; Ladeira, S.; Castel, A.; Vega, A.; Chavez, I. (E)-2-{[(2-Amino-pyridin-3-yl)imino]-meth-yl}-4,6-di-tert-butyl-phenol. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68 Pt 8, o2507–o2508. [Google Scholar] [CrossRef]
- Gacitúa, M.; Carreño, A.; Morales-Guevara, R.; Páez-Hernández, D.; Martínez-Araya, J.I.; Araya, E.; Preite, M.; Otero, C.; Rivera-Zaldívar, M.M.; Silva, A.; et al. Physicochemical and Theoretical Characterization of a New Small Non-Metal Schiff Base with a Differential Antimicrobial Effect against Gram-Positive Bacteria. Int. J. Mol. Sci. 2022, 23, 2553. [Google Scholar] [CrossRef]
- Raza, M.A.; Dege, N.; DoĞAn, O.E.; AĞAr, T.; Sumrra, S.H. Synthesis of two new Schiff bases; crystal structure, Hirshfeld surface analysis, density functional theory and molecular docking. J. Mol. Struct. 2021, 1226, 129330. [Google Scholar] [CrossRef]
- Thakor, P.M.; Patel, J.D.; Patel, R.J.; Chaki, S.H.; Khimani, A.J.; Vaidya, Y.H.; Chauhan, A.P.; Dholakia, A.B.; Patel, V.C.; Patel, A.J.; et al. Exploring New Schiff Bases: Synthesis, Characterization, and Multifaceted Analysis for Biomedical Applications. ACS Omega 2024, 9, 35431–35448. [Google Scholar] [CrossRef] [PubMed]
- Panova, E.V.; Safin, D.A. Novel cyclohexyl-containing N-salicylidene aniline Schiff bases: Synthesis, characterization, DFT studies, photophysical properties and potency against SARS-CoV-2 proteins. J. Mol. Struct. 2025, 1323, 140443. [Google Scholar] [CrossRef]
- de Toledo, T.A.; Pizani, P.S.; da Silva, L.E.; Teixeira, A.M.R.; Freire, P.T.C. Spectroscopy studies on Schiff base N,N′-bis(salicylidene)-1,2-phenylenediamine by NMR, infrared, Raman and DFT calculations. J. Mol. Struct. 2015, 1097, 106–111. [Google Scholar] [CrossRef]
- Sánchez-Pacheco, A.D.; Hernández-Vergara, M.; Jaime-Adán, E.; Hernández-Ortega, S.; Valdés-Martínez, J. Schiff bases as possible hydrogen bond donors and acceptors. J. Mol. Struct. 2021, 1234, 130136. [Google Scholar] [CrossRef]
- Shaner, S.E.; Stone, K.L. Determination of Stretching Frequencies by Isotopic Substitution Using Infrared Spectroscopy: An Upper-Level Undergraduate Experiment for an In-Person or Online Laboratory. J. Chem. Educ. 2023, 100, 2347–2352. [Google Scholar] [CrossRef]
- Henschel, H.; Andersson, A.T.; Jespers, W.; Mehdi Ghahremanpour, M.; van der Spoel, D. Theoretical Infrared Spectra: Quantitative Similarity Measures and Force Fields. J. Chem. Theory Comput. 2020, 16, 3307–3315. [Google Scholar] [CrossRef]
- Giechaskiel, B.; Clairotte, M. Fourier Transform Infrared (FTIR) Spectroscopy for Measurements of Vehicle Exhaust Emissions: A Review. Appl. Sci. 2021, 11, 7416. [Google Scholar] [CrossRef]
- Tirri, B.; Mazzone, G.; Ottochian, A.; Gomar, J.; Raucci, U.; Adamo, C.; Ciofini, I. A combined Monte Carlo/DFT approach to simulate UV-vis spectra of molecules and aggregates: Merocyanine dyes as a case study. J. Comput. Chem. 2021, 42, 1054–1063. [Google Scholar] [CrossRef]
- Safi, Z.S.; Wazzan, N.; Aqel, H. Calculation of vertical and adiabatic ionization potentials for some benzaldehydes using hybrid DFT, multilevel G3B3 and MP2 methods. Chem. Phys. Lett. 2022, 791, 139349. [Google Scholar] [CrossRef]
- Skyner, R.E.; McDonagh, J.L.; Groom, C.R.; van Mourik, T.; Mitchell, J.B. A review of methods for the calculation of solution free energies and the modelling of systems in solution. Phys. Chem. Chem. Phys. 2015, 17, 6174–6191. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, J.; Cammi, R.; Mennucci, B.; Cappelli, C.; Corni, S. Molecular properties in solution described with a continuum solvation model. Phys. Chem. Chem. Phys. 2002, 4, 5697–5712. [Google Scholar] [CrossRef]
- Yusof, E.N.M.; Latif, M.A.M.; Tahir, M.I.M.; Sakoff, J.A.; Simone, M.I.; Page, A.J.; Veerakumarasivam, A.; Tiekink, E.R.T.; Ravoof, T. o-Vanillin Derived Schiff Bases and Their Organotin(IV) Compounds: Synthesis, Structural Characterisation, In-Silico Studies and Cytotoxicity. Int. J. Mol. Sci. 2019, 20, 854. [Google Scholar] [CrossRef]
- Pis-Diez, R.; Echeverría, G.A.; Piro, O.E.; Jios, J.L.; Parajón-Costa, B.S. A structural, spectroscopic and theoretical study of an o-vanillin Schiff base derivative involved in enol-imine and keto-amine tautomerism. New J. Chem. 2016, 40, 2730–2740. [Google Scholar] [CrossRef]
- Gray, M.; Bowling, P.E.; Herbert, J.M. Comment on “Benchmarking Basis Sets for Density Functional Theory Thermochemistry Calculations: Why Unpolarized Basis Sets and the Polarized 6-311G Family Should Be Avoided”. J. Phys. Chem. A 2024, 128, 7739–7745. [Google Scholar] [CrossRef]
- Loco, D.; Chataigner, I.; Piquemal, J.P.; Spezia, R. Efficient and Accurate Description of Diels-Alder Reactions Using Density Functional Theory. Chemphyschem 2022, 23, e202200349. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. The fundamental nature and role of the electrostatic potential in atoms and molecules. Theor. Chem. Acc. 2002, 108, 134–142. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. The electrostatic potential: An overview. WIREs Comput. Mol. Sci. 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Lakshminarayanan, S.; Jeyasingh, V.; Murugesan, K.; Selvapalam, N.; Dass, G. Molecular electrostatic potential (MEP) surface analysis of chemo sensors: An extra supporting hand for strength, selectivity & non-traditional interactions. J. Photochem. Photobiol. 2021, 6, 100022. [Google Scholar] [CrossRef]
- Martinez-Araya, J.I. An intermediate level of approximation for computing the dual descriptor. J. Mol. Model. 2013, 19, 2811–2820. [Google Scholar] [CrossRef]
- Martinez-Araya, J.I. The Dual Descriptor Reveals the Janus-Faced Behaviour of Diiodine. Front. Chem. 2022, 10, 869110. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Araya, J.I. Explaining reaction mechanisms using the dual descriptor: A complementary tool to the molecular electrostatic potential. J. Mol. Model. 2013, 19, 2715–2722. [Google Scholar] [CrossRef] [PubMed]
- Balawender, R.; Komorowski, L. Atomic Fukui function indices and local softness ab initio. J. Chem. Phys. 1998, 109, 5203–5211. [Google Scholar] [CrossRef]
- Morell, C.; Grand, A.; Toro-Labbe, A. New dual descriptor for chemical reactivity. J. Phys. Chem. A 2005, 109, 205–212. [Google Scholar] [CrossRef]
- Morell, C.; Grand, A.; Toro-Labbé, A. Theoretical support for using the Δf(r) descriptor. Chem. Phys. Lett. 2006, 425, 342–346. [Google Scholar] [CrossRef]
- Cardenas, C.; Tiznado, W.; Ayers, P.W.; Fuentealba, P. The Fukui potential and the capacity of charge and the global hardness of atoms. J. Phys. Chem. A 2011, 115, 2325–2331. [Google Scholar] [CrossRef]
- Martínez-Araya, J.I. The dual descriptor potential. J. Math. Chem. 2024, 62, 1094–1112. [Google Scholar] [CrossRef]
- Wu, P.; Chaudret, R.; Hu, X.; Yang, W. Noncovalent Interaction Analysis in Fluctuating Environments. J. Chem. Theory Comput. 2013, 9, 2226–2234. [Google Scholar] [CrossRef]
- Jablonski, M. Determining Repulsion in Cyclophane Cages. Molecules 2022, 27, 3969. [Google Scholar] [CrossRef]
- Tu, D.; Li, J.; Sun, F.; Yan, H.; Poater, J.; Sola, M. Cage(-)...Cage(-) Interaction: Boron Cluster-Based Noncovalent Bond and Its Applications in Solid-State Materials. JACS Au 2021, 1, 2047–2057. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.B.; de Fátima, Â. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density functional approach to the frontier-electron theory of chemical reactivity. J. Am. Chem. Soc. 2002, 106, 4049–4050. [Google Scholar] [CrossRef]
- Ceramella, J.; Iacopetta, D.; Catalano, A.; Cirillo, F.; Lappano, R.; Sinicropi, M.S. A Review on the Antimicrobial Activity of Schiff Bases: Data Collection and Recent Studies. Antibiotics 2022, 11, 191. [Google Scholar] [CrossRef]
- Lei, S.; Hu, Y.; Yuan, C.; Sun, R.; Wang, J.; Zhang, Y.; Zhang, Y.; Lu, D.; Fu, L.; Jiang, F. Discovery of Sortase A covalent inhibitors with benzofuranene cyanide structures as potential antibacterial agents against Staphylococcus aureus. Eur. J. Med. Chem. 2022, 229, 114032. [Google Scholar] [CrossRef]
- Spirig, T.; Weiner, E.M.; Clubb, R.T. Sortase enzymes in Gram-positive bacteria. Mol. Microbiol. 2011, 82, 1044–1059. [Google Scholar] [CrossRef]
- Zhou, H.X.; Pang, X. Electrostatic Interactions in Protein Structure, Folding, Binding, and Condensation. Chem. Rev. 2018, 118, 1691–1741. [Google Scholar] [CrossRef]
- Saar-Dover, R.; Bitler, A.; Nezer, R.; Shmuel-Galia, L.; Firon, A.; Shimoni, E.; Trieu-Cuot, P.; Shai, Y. D-alanylation of lipoteichoic acids confers resistance to cationic peptides in group B streptococcus by increasing the cell wall density. PLoS Pathog. 2012, 8, e1002891. [Google Scholar] [CrossRef]
- Alias, Y.; Bhangwar, S.; Ali, A.; Sherino, B.; Bahron, H.; Sarwar, A. A review of current trends of antibacterial Schiff base complexes: Lower and higher transition metal complexes. Malays. J. Microbiol. 2023, 19, 333–347. [Google Scholar] [CrossRef]
- Rojas-Poblete, M.; Carreño, A.; Gacitúa, M.; Páez-Hernández, D.; Rabanal-León, W.A.; Arratia-Pérez, R. Electrochemical behaviors and relativistic DFT calculations to understand the terminal ligand influence on the [Re6(μ3-Q)8X6]4− clusters. New J. Chem. 2018, 42, 5471–5478. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.L.X.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Chai, J.D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.D.; Head-Gordon, M. Systematic optimization of long-range corrected hybrid density functionals. J. Chem. Phys. 2008, 128, 084106. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Realization of Conceptual Density Functional Theory and Information-Theoretic Approach in Multiwfn Program. In Conceptual Density Functional Theory: Towards a New Chemical Reactivity Theory; Liu, S., Ed.; Wiley: Hoboken, NJ, USA, 2022; Volume 2. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Cuenca-Estrella, M.; Moore, C.B.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Denning, D.W.; Donnelly, J.P.; Dromer, F.; Dupont, B.; Rex, J.H.; et al. Multicenter evaluation of the reproducibility of the proposed antifungal susceptibility testing method for fermentative yeasts of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST). Clin. Microbiol. Infect. 2003, 9, 467–474. [Google Scholar] [CrossRef]
- Marchant, P.; Carreno, A.; Vivanco, E.; Silva, A.; Nevermann, J.; Otero, C.; Araya, E.; Gil, F.; Calderon, I.L.; Fuentes, J.A. “One for All”: Functional Transfer of OMV-Mediated Polymyxin B Resistance From Salmonella enterica sv. Typhi DtolR and DdegS to Susceptible Bacteria. Front. Microbiol. 2021, 12, 672467. [Google Scholar] [CrossRef]
- Marchant, P.; Vivanco, E.; Silva, A.; Nevermann, J.; Fuentes, I.; Barrera, B.; Otero, C.; Calderon, I.L.; Gil, F.; Fuentes, J.A. b-lactam-induced OMV release promotes polymyxin tolerance in Salmonella enterica sv. Typhi. Front. Microbiol. 2024, 15, 1389663. [Google Scholar] [CrossRef]
- Parra, F.; Carreño, A.; Ancede-Gallardo, E.; Majluf, D.; Soto, J.A.; Sepúlveda, R.V.; Aguayo, D.; Otero, M.C.; Calderón, I.L.; Gil, F.; et al. Benzimidazole-Derived B2 as a Fluorescent Probe for Bacterial Outer Membrane Vesicle (OMV) Labeling: Integrating DFT, Molecular Dynamics, Flow Cytometry, and Confocal Microscopy. Int. J. Mol. Sci. 2025, 26, 4682. [Google Scholar] [CrossRef]
- Fuentes, I.; Parra, F.; Rojas, D.; Silva, A.; Nevermann, J.; Otero, M.C.; Gil, F.; Calderon, I.L.; Fuentes, J.A. Hypervesiculation Meets Sec-Targeting: Enhancing Heterologous Protein Loading in Salmonella Typhi Outer Membrane Vesicles for Delivery and Immune Response. Int. J. Mol. Sci. 2025, 26, 4223. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, K.; Nie, K.; Deng, M.; Luo, W.; Wu, X.; Huang, Y.; Wang, X. Assessment of the safety and probiotic properties of Roseburia intestinalis: A potential “Next Generation Probiotic”. Front. Microbiol. 2022, 13, 973046. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, A.D.; Schaefer, T.; Schneider, W.G. Solvent Effects in Nuclear Magnetic Resonance Spectra. J. Chem. Phys. 1960, 32, 1227–1233. [Google Scholar] [CrossRef]
- Masnabadi, N.; Thalji, M.R.; Alhasan, H.S.; Mahmoodi, Z.; Soldatov, A.V.; Ali, G.A.M. Structural, Electronic, Reactivity, and Conformational Features of 2,5,5-Trimethyl-1,3,2-diheterophosphinane-2-sulfide, and Its Derivatives: DFT, MEP, and NBO Calculations. Molecules 2022, 27, 4011. [Google Scholar] [CrossRef] [PubMed]
- Gadre, S.R.; Suresh, C.H.; Mohan, N. Electrostatic Potential Topology for Probing Molecular Structure, Bonding and Reactivity. Molecules 2021, 26, 3289. [Google Scholar] [CrossRef]
- Liu, S.; Pedersen, L.G. Estimation of molecular acidity via electrostatic potential at the nucleus and valence natural atomic orbitals. J. Phys. Chem. A 2009, 113, 3648–3655. [Google Scholar] [CrossRef]
- Zamora, P.P.; Bieger, K.; Cuchillo, A.; Tello, A.; Muena, J.P. Theoretical determination of a reaction intermediate: Fukui function analysis, dual reactivity descriptor and activation energy. J. Mol. Struct. 2021, 1227, 129369. [Google Scholar] [CrossRef]
- Kenouche, S.; Sandoval-Yañez, C.; Martínez-Araya, J.I. The antioxidant capacity of myricetin. A molecular electrostatic potential analysis based on DFT calculations. Chem. Phys. Lett. 2022, 801, 139708. [Google Scholar] [CrossRef]
- Martínez-Araya, J.I. Why is the dual descriptor a more accurate local reactivity descriptor than Fukui functions? J. Math. Chem. 2014, 53, 451–465. [Google Scholar] [CrossRef]
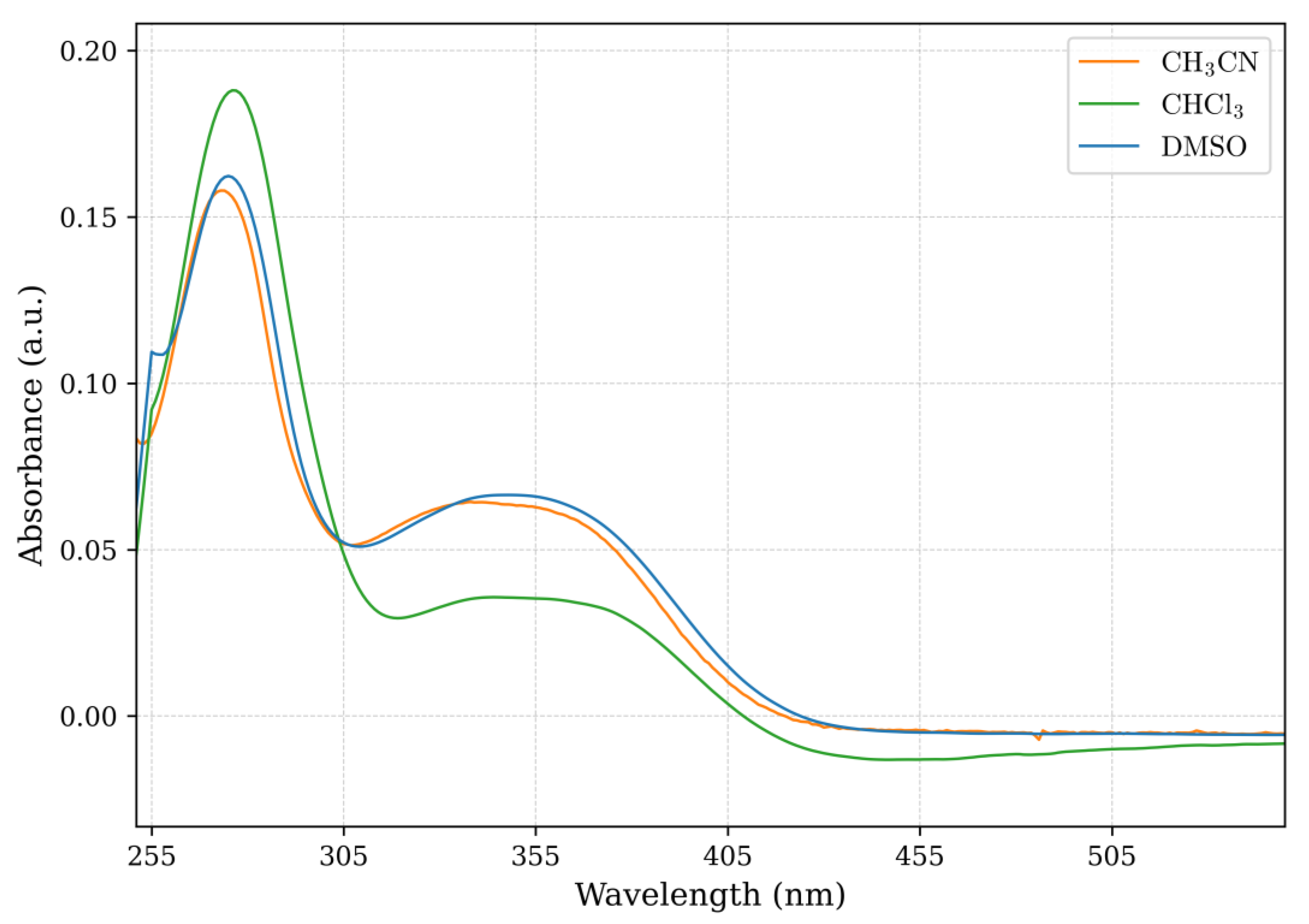
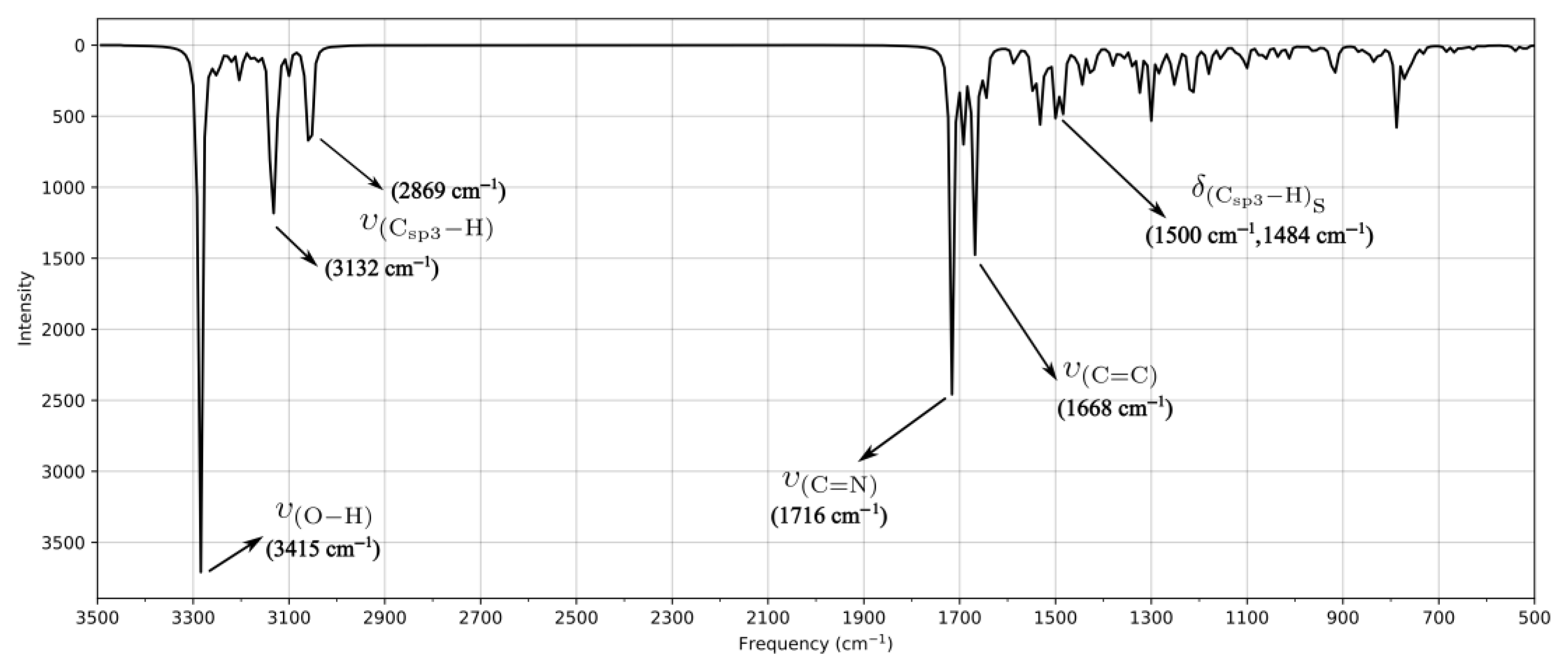
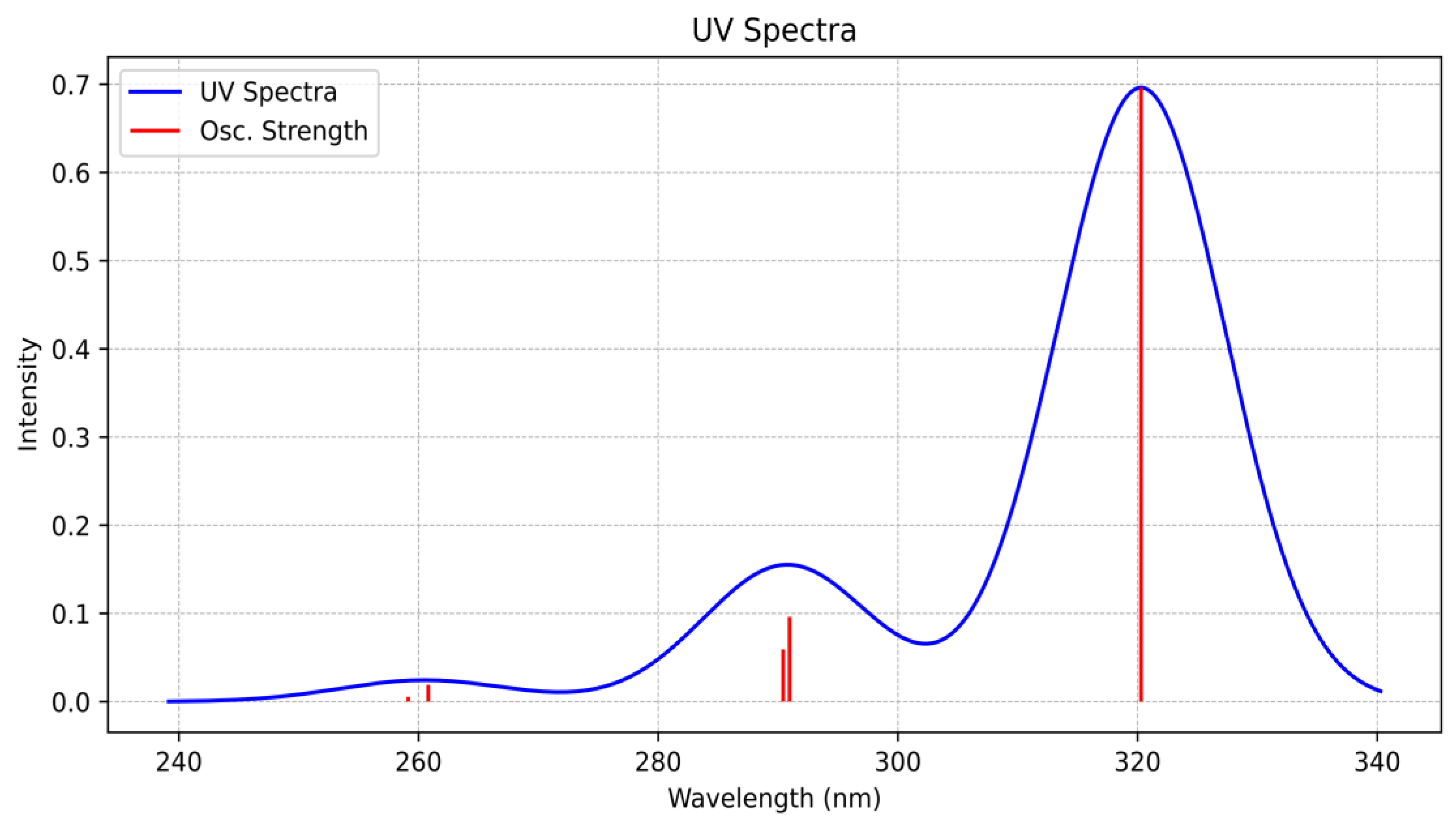
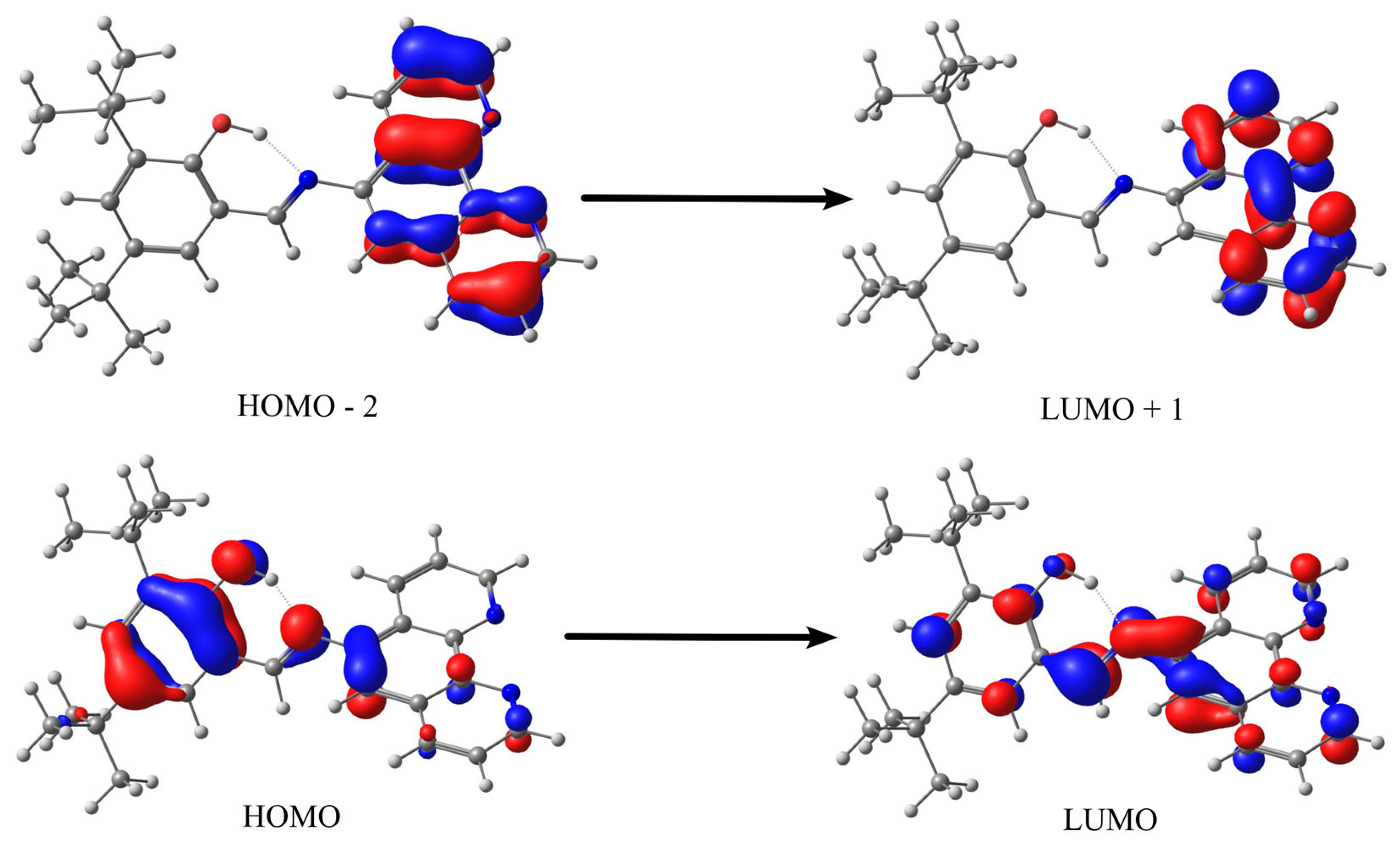
| Schiff Base | Solvent | λ abs (nm) | ε (L/mol × cm) | Assignment |
|---|---|---|---|---|
| Fen-IHB | Chloroform | 276 | 33,774.41 | π → π* |
| 345 | 14,009.92 | n → π*, π → π* | ||
| Acetonitrile | 273 | 33,469.59 | π → π* | |
| 340 | 14,155.42 | n → π*, π → π* | ||
| DMSO | 275 | 333,618.74 | π → π* | |
| 345 | 14,035.21 | n → π*, π → π* |
| Gram Classification | Oxygen Requirement | Species | MIC (µM) |
|---|---|---|---|
| Negative | Facultative anaerobe | Salmonella enterica subsp. Enterica sv Typhimurium ATCC 14028s | No effect ** |
| Negative | Facultative anaerobe | Salmonella enterica subsp. Enterica sv Typhi STH2370 | No effect |
| Negative | Facultative anaerobe | Escherichia coli | No effect |
| Positive | Strict aerobe | Bacillus subtilis | 18.9 ± 3.6 * |
| Positive | Aerotolerant anaerobe | Streptococcus pyogenes | 3.2 ± 0.0 * |
| Positive | Aerotolerant anaerobe | Enterococcus faecalis | 22.1 ± 3.1 * |
| Positive | Aerotolerant anaerobe | Staphylococcus aureus | 12.6 ± 0.0 * |
| Positive | Aerotolerant anaerobe | Staphylococcus haemolyticus | 18.9 ± 3.6 * |
| Positive | Strict anaerobe | Clostridioides difficile R20291 | No effect |
| Positive | Strict anaerobe | Roseburiainulinivorans | No effect |
| Positive | Strict anaerobe | Blautia coccoides | No effect |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreño, A.; Ancede-Gallardo, E.; Suárez, A.G.; Cepeda-Plaza, M.; Duque-Noreña, M.; Arce, R.; Gacitúa, M.; Lavín, R.; Inostroza, O.; Gil, F.; et al. Synthesis and Characterization of a New Hydrogen-Bond-Stabilized 1,10-Phenanthroline–Phenol Schiff Base: Integrated Spectroscopic, Electrochemical, Theoretical Studies, and Antimicrobial Evaluation. Chemistry 2025, 7, 135. https://doi.org/10.3390/chemistry7040135
Carreño A, Ancede-Gallardo E, Suárez AG, Cepeda-Plaza M, Duque-Noreña M, Arce R, Gacitúa M, Lavín R, Inostroza O, Gil F, et al. Synthesis and Characterization of a New Hydrogen-Bond-Stabilized 1,10-Phenanthroline–Phenol Schiff Base: Integrated Spectroscopic, Electrochemical, Theoretical Studies, and Antimicrobial Evaluation. Chemistry. 2025; 7(4):135. https://doi.org/10.3390/chemistry7040135
Chicago/Turabian StyleCarreño, Alexander, Evys Ancede-Gallardo, Ana G. Suárez, Marjorie Cepeda-Plaza, Mario Duque-Noreña, Roxana Arce, Manuel Gacitúa, Roberto Lavín, Osvaldo Inostroza, Fernando Gil, and et al. 2025. "Synthesis and Characterization of a New Hydrogen-Bond-Stabilized 1,10-Phenanthroline–Phenol Schiff Base: Integrated Spectroscopic, Electrochemical, Theoretical Studies, and Antimicrobial Evaluation" Chemistry 7, no. 4: 135. https://doi.org/10.3390/chemistry7040135
APA StyleCarreño, A., Ancede-Gallardo, E., Suárez, A. G., Cepeda-Plaza, M., Duque-Noreña, M., Arce, R., Gacitúa, M., Lavín, R., Inostroza, O., Gil, F., Fuentes, I., & Fuentes, J. A. (2025). Synthesis and Characterization of a New Hydrogen-Bond-Stabilized 1,10-Phenanthroline–Phenol Schiff Base: Integrated Spectroscopic, Electrochemical, Theoretical Studies, and Antimicrobial Evaluation. Chemistry, 7(4), 135. https://doi.org/10.3390/chemistry7040135








