A Structural Colored Epoxy Resin Sensor for the Discrimination of Methanol and Ethanol
Abstract
1. Introduction
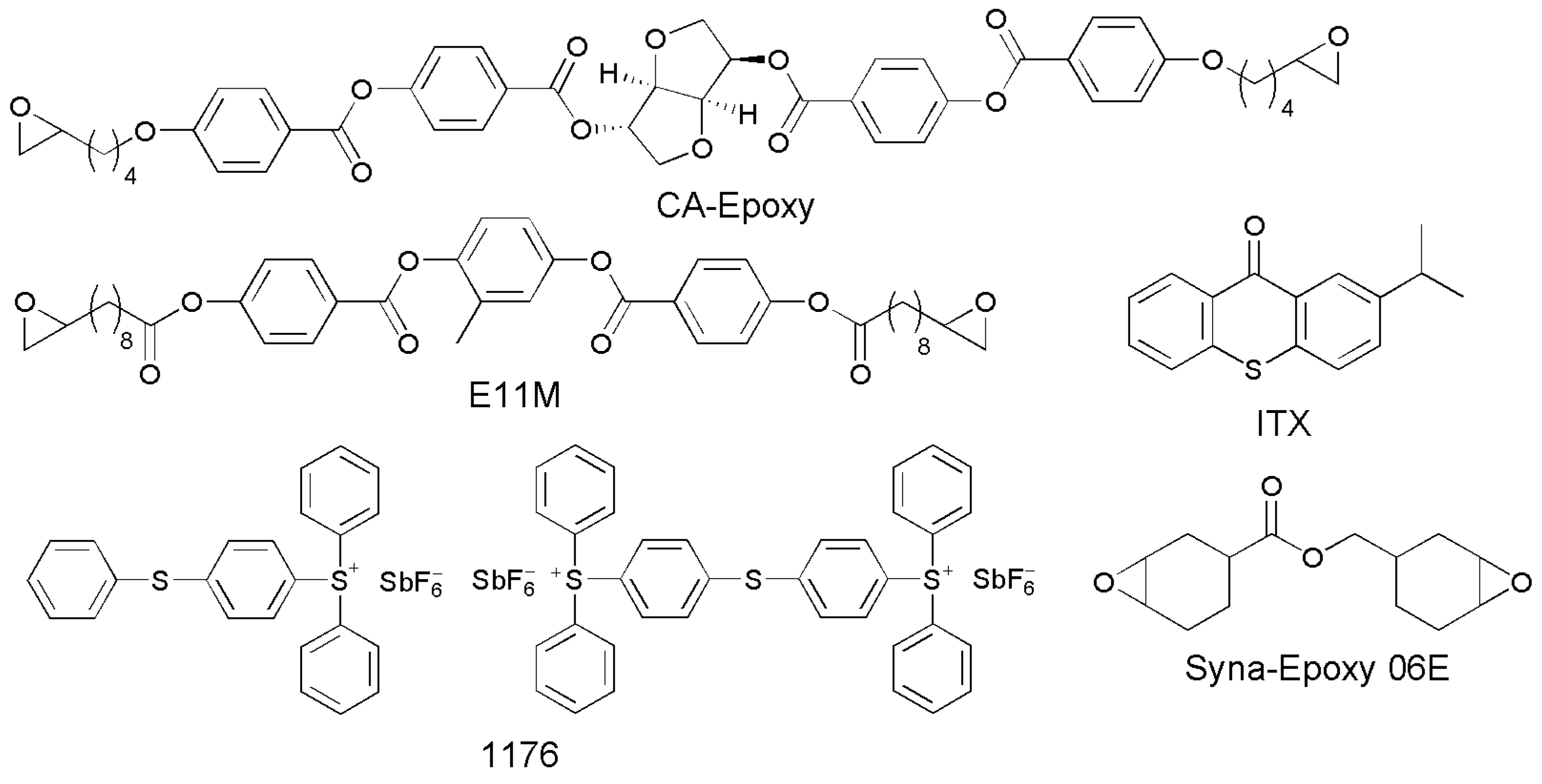
2. Materials and Methods
2.1. Chemical Reagents and Instruments
2.2. Preparation of the Structurally Colored CLCN Films
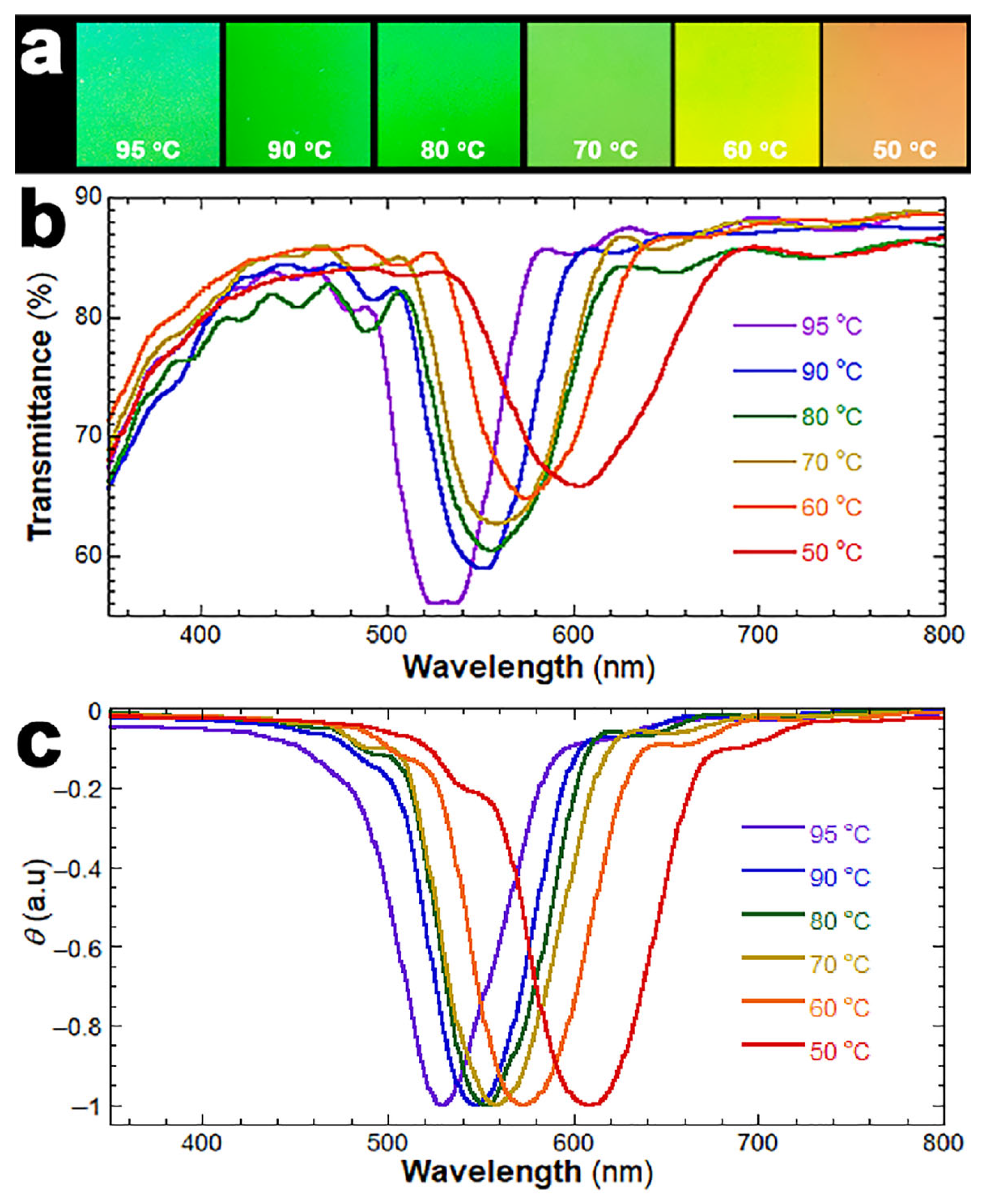
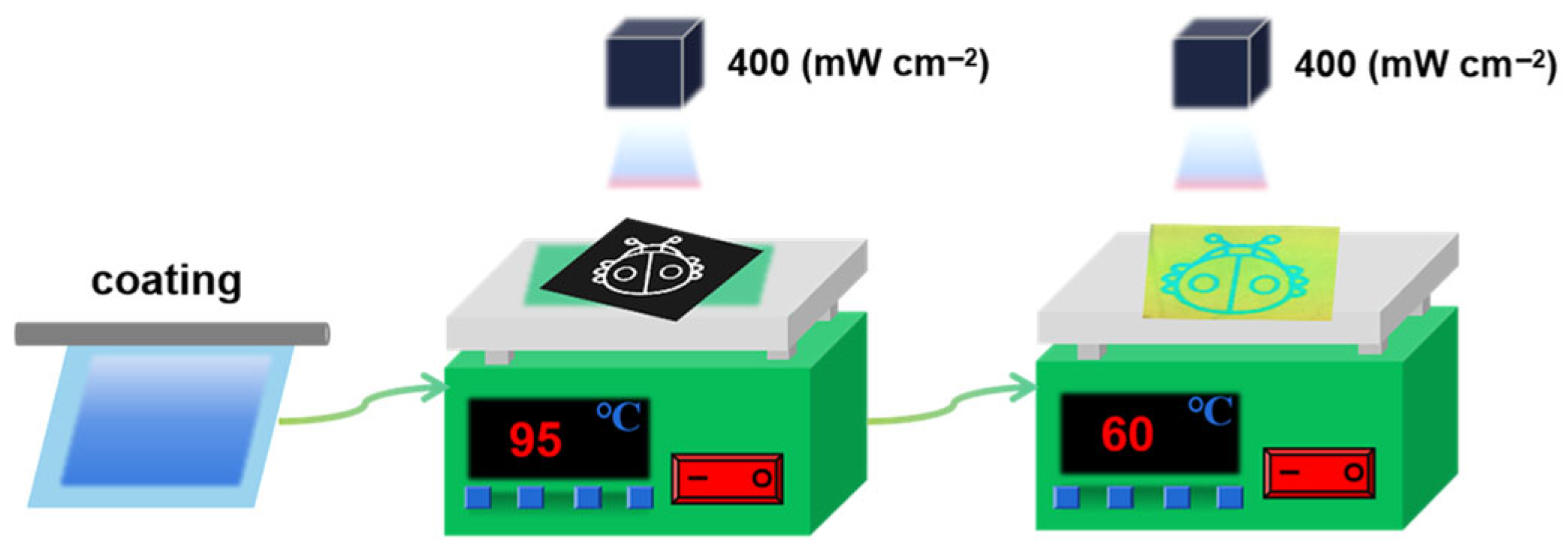
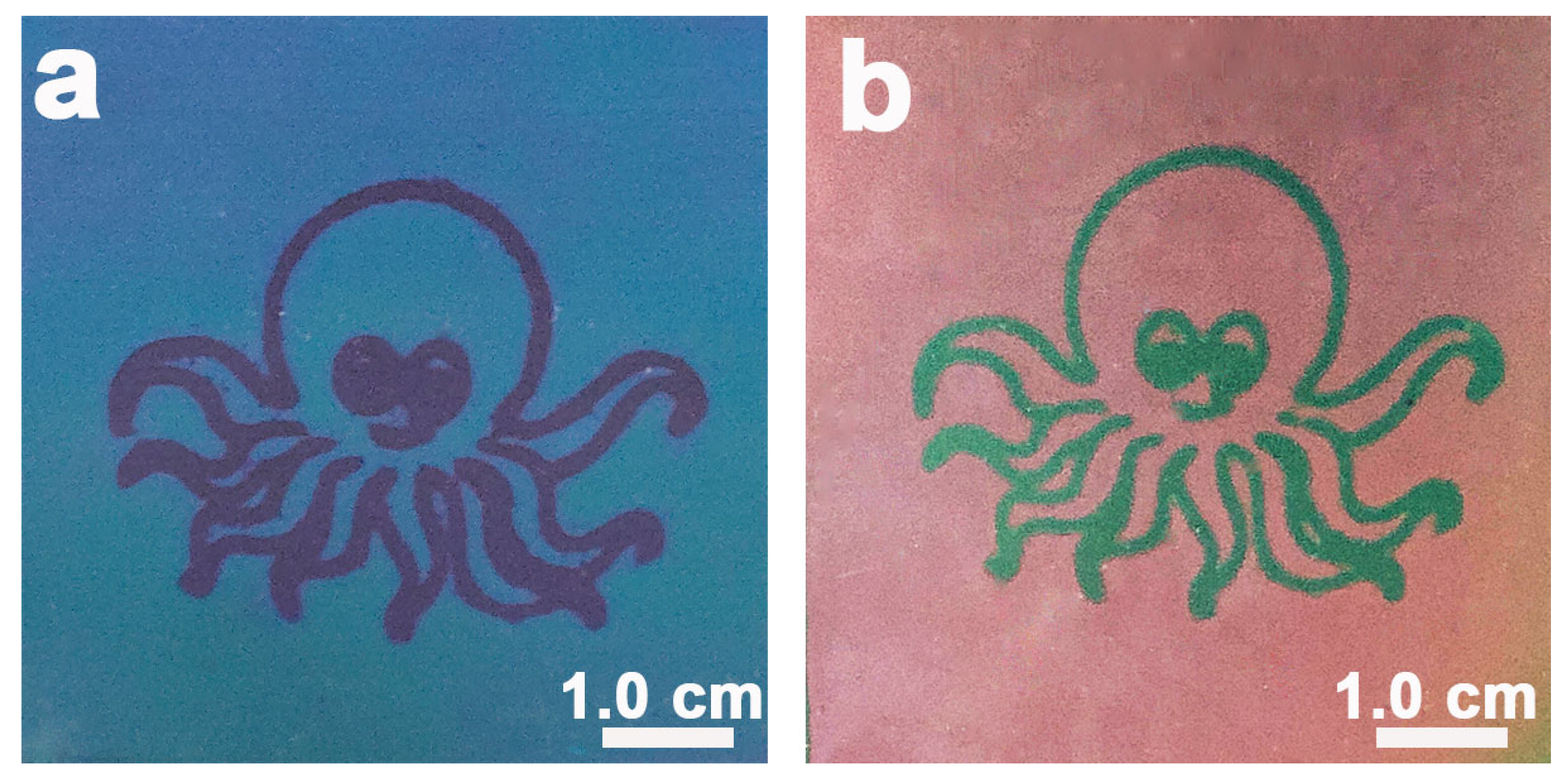
2.3. Preparation of the CLCN Patterns at Different Temperatures
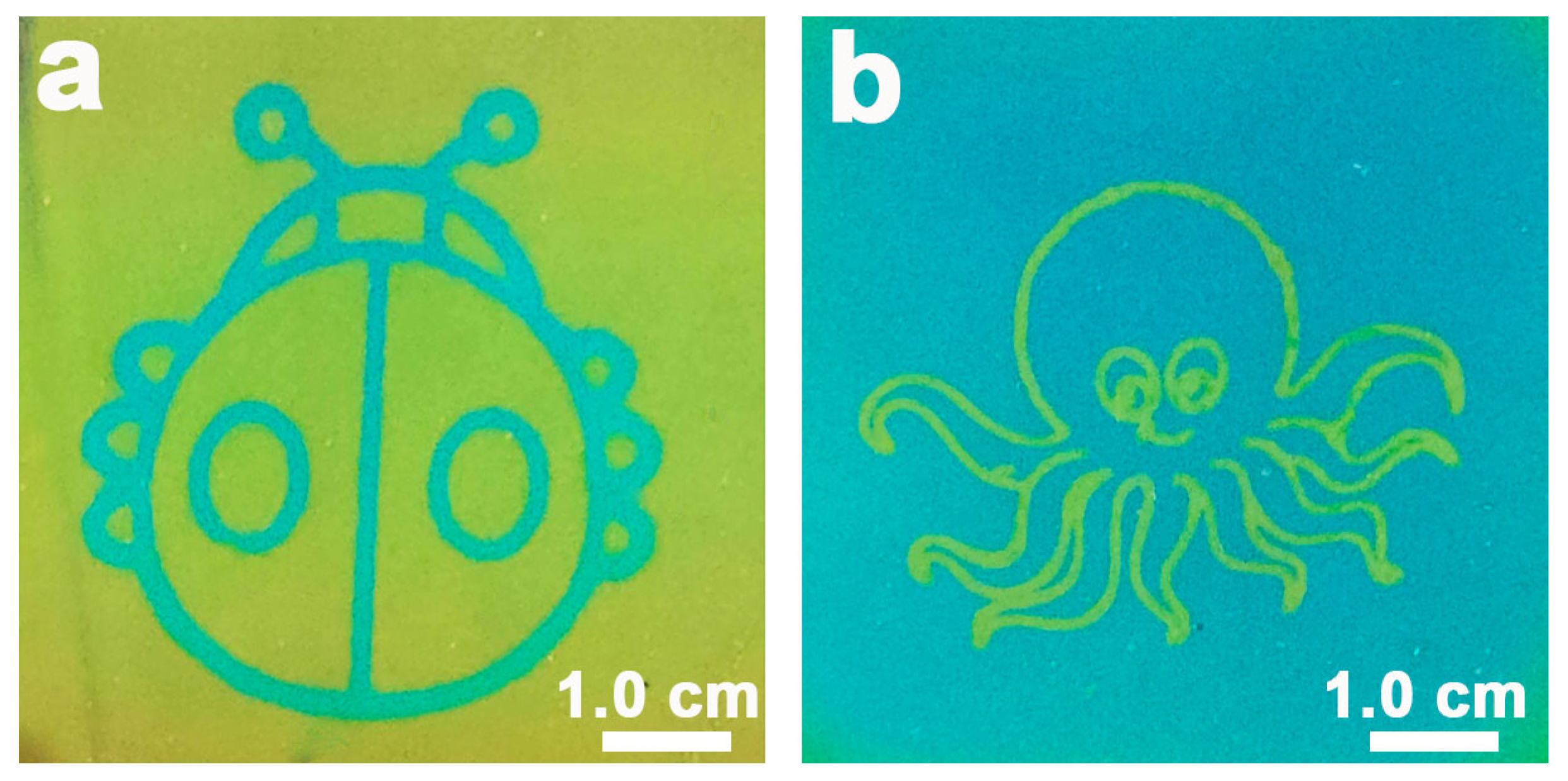
2.4. Preparation of the Lizard Pattern Using the Inkjet Printer
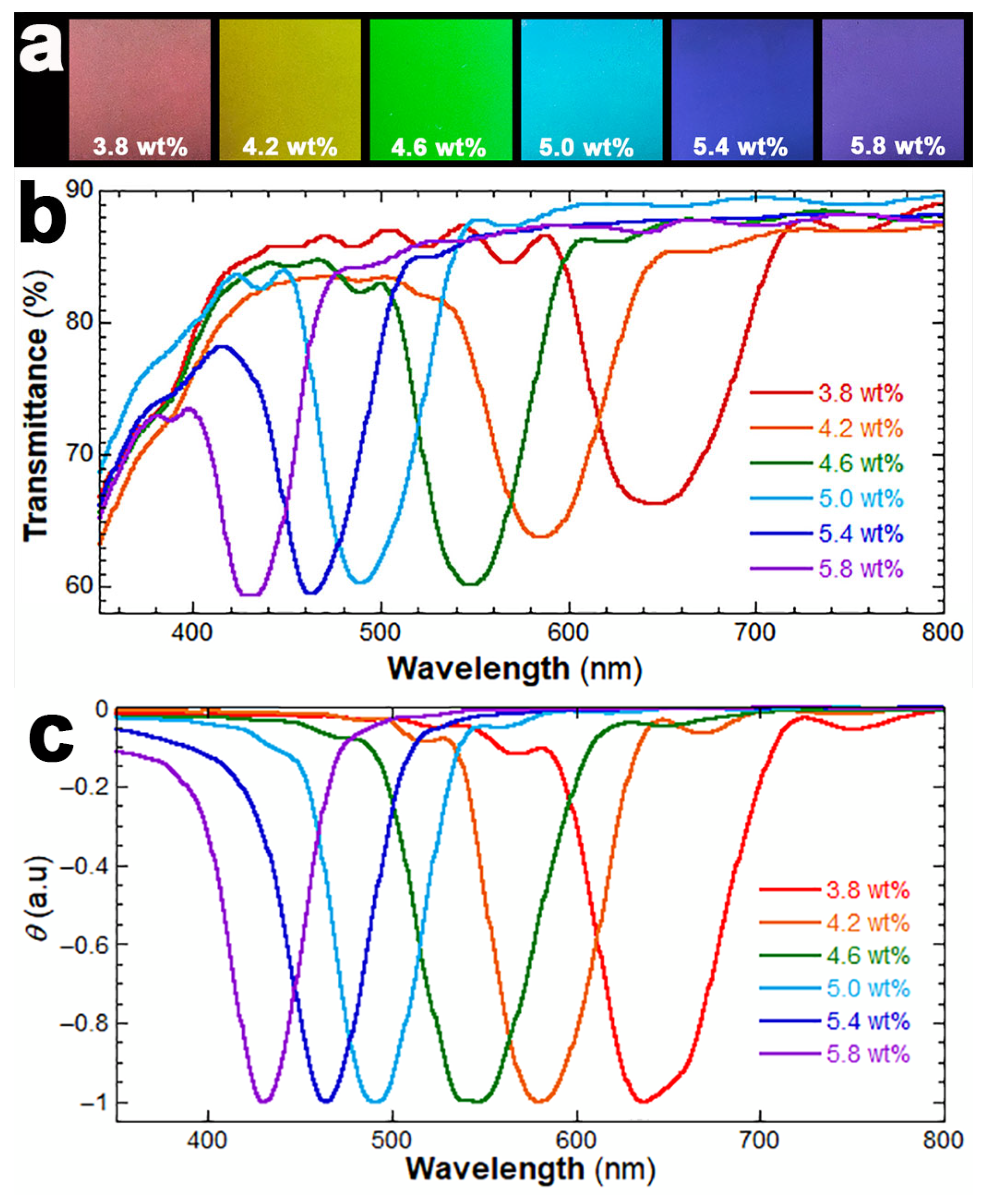
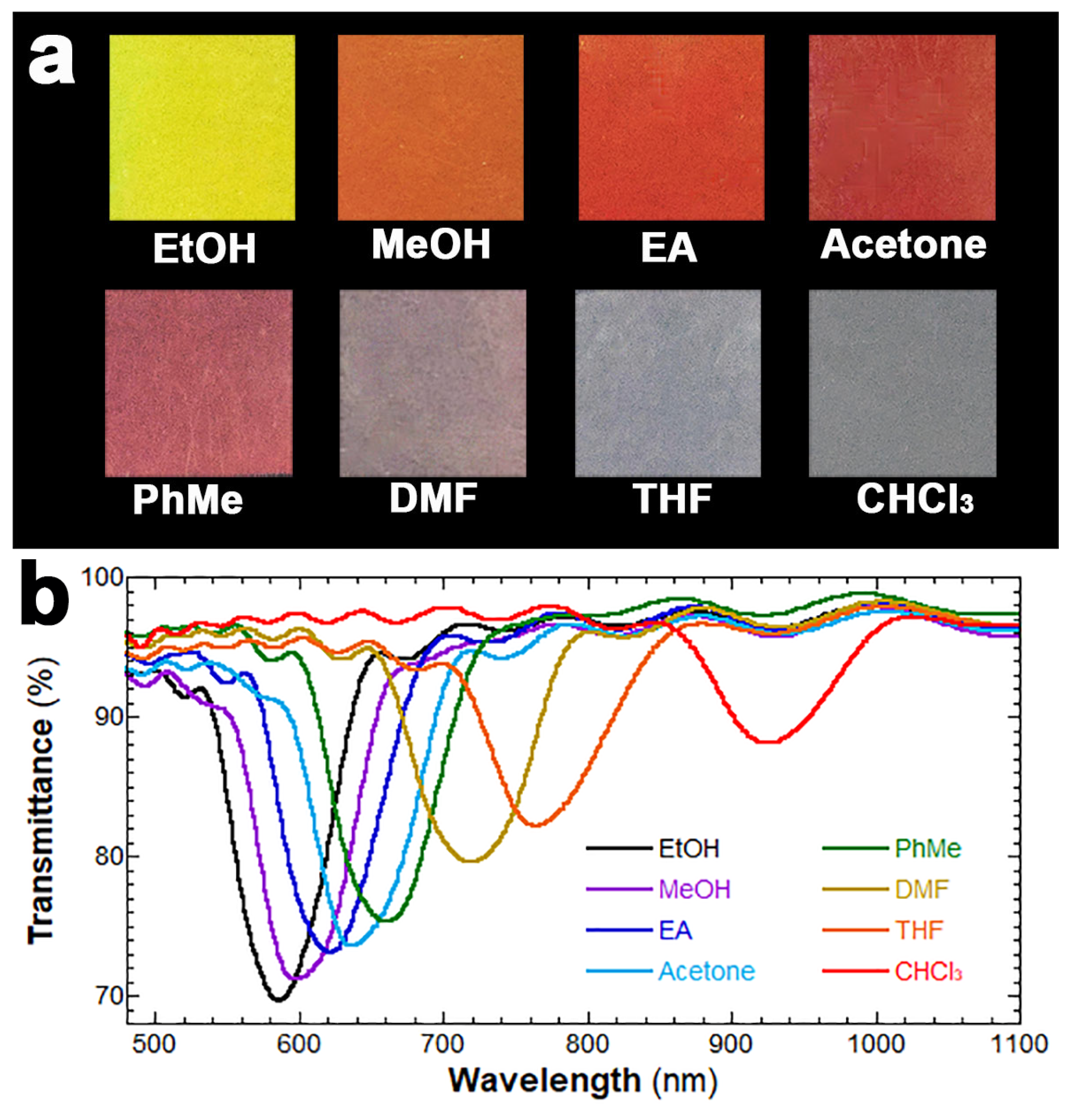
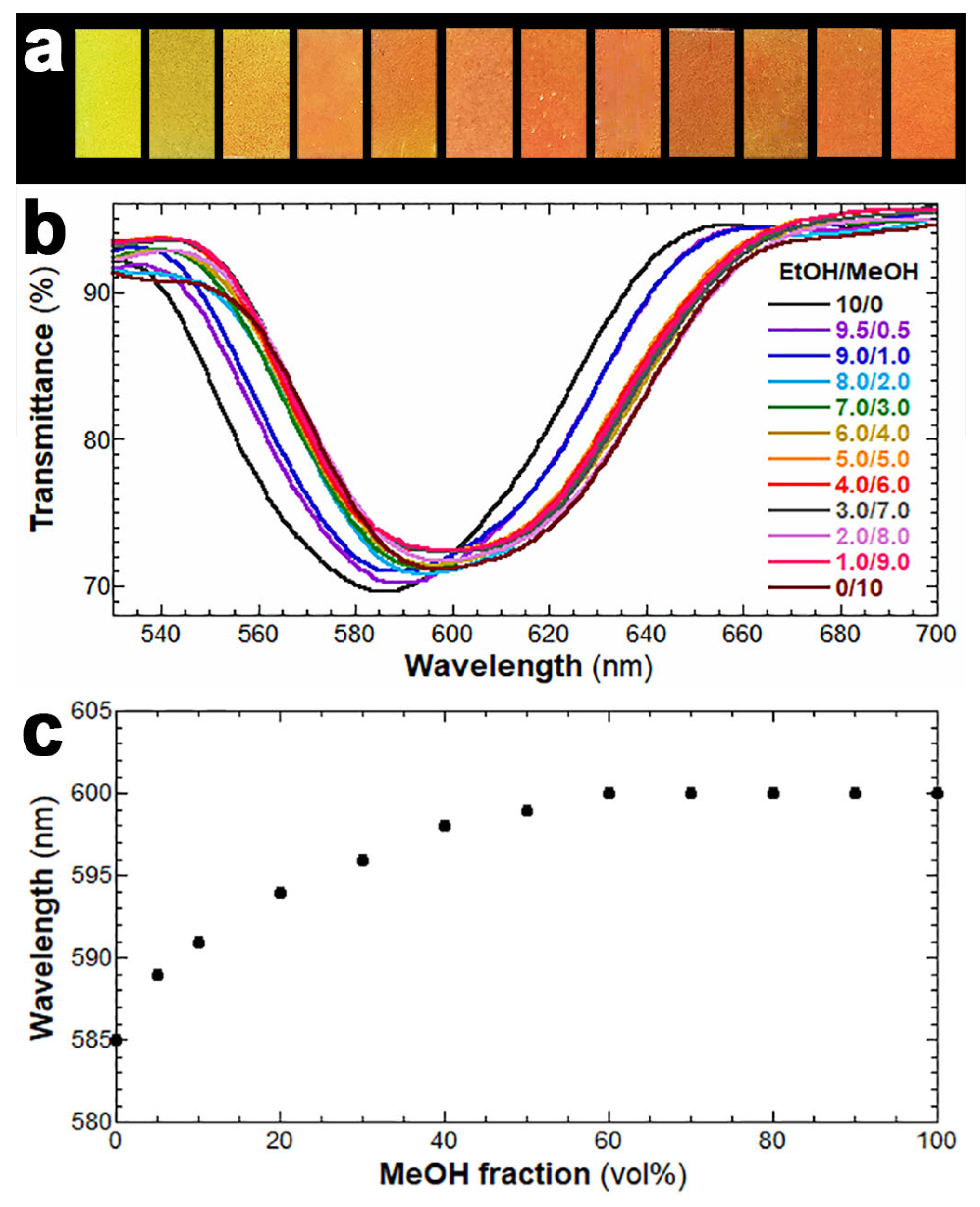
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Holtz, J.H.; Asher, S.A. Polymerized Colloidal Crystal Hydrogel Films as Intelligent Chemical Sensing Materials. Nature 1997, 389, 829–832. [Google Scholar] [CrossRef]
- Noh, K.-G.; Park, S.-Y. Biosensor Array of Interpenetrating Polymer Network with Photonic Film Templated from Reactive Cholesteric Liquid Crystal and Enzyme-Immobilized Hydrogel Polymer. Adv. Funct. Mater. 2018, 28, 1707562. [Google Scholar] [CrossRef]
- Fujii, Y.; Honma, S.; Morisawa, M.; Muto, S. Development of New Optical Fiber Toluene Sensor. Adv. Mater. Devices Sens. Imaging III 2007, 6829, 68291Z. [Google Scholar]
- Bikbaev, R.G.; Timofeev, I.V.; Shabanov, V.F. Strain Sensor via Wood Anomalies in 2D Dielectric Array. Nanomaterials 2021, 11, 1022. [Google Scholar] [CrossRef] [PubMed]
- Batir, O.; Bat, E.; Bukusoglu, E. Strain-Enhanced Sensitivity of Polymeric Sensors Templated from Cholesteric Liquid Crystals. Soft Matter 2020, 16, 6794–6802. [Google Scholar] [CrossRef] [PubMed]
- Boyon, C.; Soldan, V.; Mitov, M. Bioinspired, Cholesteric Liquid-Crystal Reflectors with Time-Controlled Coexisting Chiral and Achiral Structures. ACS Appl. Mater. Interfaces 2021, 13, 30118–30126. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, K.-Q. Photonic Crystal Structures with Tunable Structure Color as Colorimetric Sensors. Sensors 2013, 13, 4192–4213. [Google Scholar] [CrossRef]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O.S. Photonic Crystals for Chemical Sensing and Biosensing. Angew. Chem. Int. Ed. 2014, 53, 3318–3335. [Google Scholar] [CrossRef]
- Ge, J.; Yin, Y. Responsive Photonic Crystals. Angew. Chem. Int. Ed. 2011, 50, 1492–1522. [Google Scholar] [CrossRef]
- Zhang, D.; Cao, H.; Duan, M.; Wang, H.; Chen, Y.; Zong, C.; Gan, P.; Zhao, L.; Yang, Z.; Wang, D.; et al. Effect of Monomer Composition on the Performance of Polymer-Stabilized Liquid Crystals with Two-Step Photopolymerization. J. Polym. Sci. Pol. Phys. 2019, 57, 1126–1132. [Google Scholar] [CrossRef]
- Mitov, M. Cholesteric Liquid Crystals with a Broad Light Reflection Band. Adv. Mater. 2012, 24, 6260–6276. [Google Scholar] [CrossRef]
- Dierking, I. Recent Developments in Polymer Stabilised Liquid Crystals. Polym. Chem. 2010, 1, 1153–1159. [Google Scholar] [CrossRef]
- Ye, Y.; Guo, L.; Zhong, T. A Review of Developments in Polymer Stabilized Liquid Crystals. Polymers 2023, 15, 2962. [Google Scholar] [CrossRef]
- Tang, J.; Fang, J.; Liang, Y.; Zhang, B.; Chen, Z.; Liu, X.; Li, Z.; Cai, X.; Xian, J.; Lin, H.; et al. All-Fiber-Optic Voc Gas Sensor Based on Side-Polished Fiber Wavelength Selectively Coupled with Cholesteric Liquid Crystal Film. Sens. Actuat. B-Chem. 2018, 273, 1816–1826. [Google Scholar] [CrossRef]
- Chang, C.-K.; Bastiaansen, C.W.M.; Broer, D.J.; Kuo, H.-L. Discrimination of Alcohol Molecules Using Hydrogen-Bridged Cholesteric Polymer Networks. Macromolecules 2012, 45, 4550–4555. [Google Scholar] [CrossRef]
- Zhang, P.; Kragt, A.J.J.; Schenning, A.P.H.J.; de Haan, L.T.; Zhou, G. An Easily Coatable Temperature Responsive Cholesteric Liquid Crystal Oligomer for Making Structural Colour Patterns. J. Mater. Chem. C 2018, 6, 7184–7187. [Google Scholar] [CrossRef]
- Chang, C.-K.; Bastiaansen, C.M.W.; Broer, D.J.; Kuo, H.-L. Alcohol-Responsive, Hydrogen-Bonded, Cholesteric Liquid-Crystal Networks. Adv. Funct. Mater. 2012, 22, 2855–2859. [Google Scholar] [CrossRef]
- Lugger, S.J.D.; Houben, S.J.A.; Foelen, Y.; Debije, M.G.; Schenning, A.P.H.J.; Mulder, D.J. Hydrogen-Bonded Supramolecular Liquid Crystal Polymers: Smart Materials with Stimuli-Responsive, Self-Healing, and Recyclable Properties. Chem. Rev. 2022, 122, 4946–4975. [Google Scholar] [CrossRef]
- Herzer, N.; Guneysu, H.; Davies, D.J.D.; Yildirim, D.; Vaccaro, A.R.; Broer, D.J.; Bastiaansen, C.W.M.; Schenning, A.P.H.J. Printable Optical Sensors Based on H-Bonded Supramolecular Cholesteric Liquid Crystal Networks. J. Am. Chem. Soc. 2012, 134, 7608–7611. [Google Scholar] [CrossRef]
- Liu, B.; Yang, T.; Mu, X.; Mai, Z.; Li, H.; Wang, Y.; Zhou, G. Smart Supramolecular Self-Assembled Nanosystem: Stimulus-Responsive Hydrogen-Bonded Liquid Crystals. Nanomaterials 2021, 11, 448. [Google Scholar] [CrossRef]
- Raynes, E.P.; Jia, X. The Measurement of Weak Chirality Using Nematic Liquid Crystals. Liq. Cryst. 2017, 44, 1960–1967. [Google Scholar] [CrossRef]
- Popov, P.; Honaker, L.W.; Mirheydari, M.; Mann, E.K.; Jákli, A. Chiral Nematic Liquid Crystal Microlenses. Sci. Rep. 2017, 7, 1603. [Google Scholar] [CrossRef]
- Shibaev, P.V.; Schaumburg, K.; Plaksin, V. Responsive Chiral Hydrogen-Bonded Polymer Composites. Chem. Mater. 2002, 14, 959–961. [Google Scholar] [CrossRef]
- Shen, C.; Wang, Z.; Huang, R.; Bao, J.; Li, Z.; Zhang, L.; Lan, R.; Yang, H. Humidity-Responsive Photonic Crystals with pH and SO2 Gas Detection Ability Based on Cholesteric Liquid Crystalline Networks. ACS Appl. Mater. Interfaces 2022, 14, 16764–16771. [Google Scholar] [CrossRef]
- Chen, F.; Guo, J.; Jin, O.; Wei, J. A Temperature and pH Double Sensitive Cholesteric Polymer Film from a Photopolymerizable Chiral Hydrogen-Bonded Assembly. Chin. J. Polym. Sci. 2013, 31, 630–640. [Google Scholar] [CrossRef]
- Stumpel, J.E.; Broer, D.J.; Schenning, A.P.H.J. Water-Responsive Dual-Coloured Photonic Polymer Coatings Based on Cholesteric Liquid Crystals. RSC Adv. 2015, 5, 94650–94653. [Google Scholar] [CrossRef]
- Chen, F.; Guo, J.; Qu, Z.; Wei, J. Novel Photo-Polymerizable Chiral Hydrogen-Bonded Self-Assembled Complexes: Preparation, Characterization and the Utilization as a Thermal Switching Reflective Color Film. J. Mater. Chem. 2011, 21, 8574–8582. [Google Scholar] [CrossRef]
- Myung, D.-B.; Hussain, S.; Park, S.-Y. Photonic Calcium and Humidity Array Sensor Prepared with Reactive Cholesteric Liquid Crystal Mesogens. Sens. Actuat. B-Chem. 2019, 298, 126894. [Google Scholar] [CrossRef]
- Moirangthem, M.; Arts, R.; Merkx, M.; Schenning, A.P.H.J. An Optical Sensor Based on a Photonic Polymer Film to Detect Calcium in Serum. Adv. Funct. Mater. 2016, 26, 1154–1160. [Google Scholar] [CrossRef]
- Stroganov, V.; Ryabchun, A.; Bobrovsky, A.; Shibaev, V. A Novel Type of Crown Ether-Containing Metal Ions Optical Sensors Based on Polymer-Stabilized Cholesteric Liquid Crystalline Films. Macromol. Rapid Commun. 2012, 33, 1875–1881. [Google Scholar] [CrossRef]
- Han, Y.; Pacheco, K.; Bastiaansen, C.W.M.; Broer, D.J.; Sijbesma, R.P. Optical Monitoring of Gases with Cholesteric Liquid Crystals. J. Am. Chem. Soc. 2010, 132, 2961–2967. [Google Scholar] [CrossRef]
- Niu, X.; Zhong, Y.; Chen, R.; Wang, F.; Luo, D. Highly Sensitive and Selective Liquid Crystal Optical Sensor for Detection of Ammonia. Opt. Express 2017, 25, 13549–13556. [Google Scholar] [CrossRef]
- Mujahid, A.; Stathopulos, H.; Lieberzeit, P.A.; Dickert, F.L. Solvent Vapour Detection with Cholesteric Liquid Crystals-Optical and Mass-Sensitive Evaluation of the Sensor Mechanism. Sensors 2010, 10, 4887–4897. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.-Y.; Liu, M.-F.; Lin, R.-D.; Hwang, S.-J. Alcohol Selective Optical Sensor Based on Porous Cholesteric Liquid Crystal Polymer Networks. Molecules 2022, 27, 773. [Google Scholar] [CrossRef] [PubMed]
- Tiscione, N.B.; Alford, I.; Yeatman, D.T.; Shan, X. Ethanol Analysis by Headspace Gas Chromatography with Simultaneous Flame-Ionization and Mass Spectrometry Detection. J. Anal. Toxicol. 2011, 35, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ye, Q.; Cai, H.; Qu, R. Determination of Methanol Ratio in Methanol-Doped Biogasoline by a Fiber Raman Sensing System. Sens. Actuat. B-Chem. 2010, 146, 75–78. [Google Scholar] [CrossRef]
- Su, Y.; Lan, Z.; Wang, J.; Zeng, L.; Zhou, D.; Peng, Z.; Sun, W.; Liu, Y. Optical Fiber Sensor for Determination of Methanol Ratio in Methanol-Doped Ethanol Based on Two Cholesteric Liquid Crystal Droplets Embedded in Chitosan. J. Light. Technol. 2021, 39, 5170–5176. [Google Scholar] [CrossRef]
- Iqbal, F.; Biswas, S.; Bulbul, A.A.-M.; Rahaman, H.; Hossain, M.B.; Rahaman, M.E.; Awal, M.A. Alcohol Sensing and Classification Using PCF-based Sensor. Sens. Bio-Sens. Res. 2020, 30, 100384. [Google Scholar] [CrossRef]
- Liu, D.; Kumar, R.; Wei, F.; Han, W.; Mallik, A.K.; Yuan, J.; Wan, S.; He, X.; Kang, Z.; Li, F.; et al. High Sensitivity Optical Fiber Sensors for Simultaneous Measurement of Methanol and Ethanol. Sens. Actuat. B-Chem. 2018, 271, 1–8. [Google Scholar] [CrossRef]
- Ishihara, S.; Iyi, N.; Labuta, J.; Deguchi, K.; Ohki, S.; Tansho, M.; Shimizu, T.; Yamauchi, Y.; Sahoo, P.; Naito, M.; et al. Naked-Eye Discrimination of Methanol from Ethanol Using Composite Film of Oxoporphyrinogen and Layered Double Hydroxide. ACS Appl. Mater. Interfaces 2013, 5, 5927–5930. [Google Scholar] [CrossRef]
- Yan, W.; Chen, Y.; Zeng, X.; Wu, G.; Jiang, W.; Wei, D.; Ling, M.; Ng, K.W.; Qin, Y. Ultrasensitive Ethanol Sensor Based on Segregated ZnO-In2O3 Porous Nanosheets. Appl. Surf. Sci. 2021, 535, 147697. [Google Scholar] [CrossRef]
- Lub, J.; Nijssen, W.P.M.; Wegh, R.T.; De Francisco, I.; Ezquerro, M.P.; Malo, B. Photoisomerizable Chiral Compounds Derived from Isosorbide and Cinnamic Acid. Liq. Cryst. 2005, 32, 1031–1044. [Google Scholar] [CrossRef]
- O’Brien, A.K.; Cramer, N.B.; Bowman, C.N. Oxygen Inhibition in Thiol-Acrylate Photopolymerizations. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 2007–2014. [Google Scholar] [CrossRef]
- Lub, J.; Nijssen, W.P.M.; Wegh, R.T.; Vogels, J.P.A.; Ferrer, A. Synthesis and Properties of Photoisomerizable Derivatives of Isosorbide and Their Use in Cholesteric Filters. Adv. Funct. Mater. 2005, 15, 1961–1972. [Google Scholar] [CrossRef]
- Hoekstra, D.C.; van der Lubbe, B.P.A.C.; Bus, T.; Yang, L.; Grossiord, N.; Debije, M.G.; Schenning, A.P.H.J. Wavelength-Selective Photopolymerization of Hybrid Acrylate-Oxetane Liquid Crystals. Angew. Chem. Int. Ed. 2021, 60, 10935–10941. [Google Scholar] [CrossRef]
- Shen, W.; Wang, L.; Cao, Y.; Zhang, L.; Yang, Z.; Yuan, X.; Yang, H.; Jiang, T.; Chen, H. Cationic Photopolymerization of Liquid Crystalline Epoxide in Mesogenic Solvents and Its Application in Polymer-Stabilized Liquid Crystals. Polymer 2019, 172, 231–238. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Y.; Li, Y.; Liu, W.; Li, H.; Yang, Y. Colorfully Patterned Epoxy Resin Films with a Cholesteric Structure Prepared through a Photopolymerization Approach. ACS Appl. Polym. Mater. 2023, 5, 193–200. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, J.; Li, Y.; Liu, W.; Yang, Y. Preparation and Applications of the Epoxy Resin-Based Polymer- Stabilised Cholesteric Liquid Crystal Films with Microcracks at the Surface. Liq. Cryst. 2023, 50, 2047–2058. [Google Scholar] [CrossRef]
- Hua, Y.; Crivello, J.V. Synergistic Interaction of Epoxides and N-Vinylcarbazole During Photoinitiated Cationic Polymerization. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 3697–3709. [Google Scholar] [CrossRef]
- Morita, Y. Cationic Polymerization of Hydrogenated Bisphenol-a Glycidyl Ether with Cycloaliphatic Epoxy Resin and Its Thermal Discoloration. J. Appl. Polym. Sci. 2005, 97, 1395–1400. [Google Scholar] [CrossRef]
- Walker, F.H.; Dickenson, J.B.; Hegedus, C.R.; Pepe, F.R.; Keller, R. New Polymeric Polyol for Thermoset Coatings: Superacid-Catalyzed Copolymerization of Water and Epoxy Resins. J. Coat. Technol. 2002, 74, 33–47. [Google Scholar] [CrossRef]
- Ge, X.; Ye, Q.; Song, L.; Misra, A.; Spencer, P. The Influence of Water on Visible-Light Initiated Free-Radical/Cationic Ring-Opening Hybrid Polymerization of Methacrylate/Epoxy: Polymerization Kinetics, Crosslinking Structure and Dynamic Mechanical Properties. RSC Adv. 2015, 5, 77791–77802. [Google Scholar] [CrossRef] [PubMed]
- Golaz, B.; Michaud, V.; Leterrier, Y.; Månson, J.-A.E. UV Intensity, Temperature and Dark-Curing Effects in Cationic Photo-Polymerization of a Cycloaliphatic Epoxy Resin. Polymer 2012, 53, 2038–2048. [Google Scholar] [CrossRef]
- Burke, J. Solubility Parameters: Theory and Application; The Book and Paper Group Annual, The American Institute for Conservation: Washington, DC, USA, 1984; Volume 3, Available online: https://cool.culturalheritage.org/byauth/burke/solpar/ (accessed on 30 May 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Yi, Y.; Wu, L.; Liu, W.; Li, Y.; Yang, Y. A Structural Colored Epoxy Resin Sensor for the Discrimination of Methanol and Ethanol. Chemistry 2025, 7, 122. https://doi.org/10.3390/chemistry7040122
Guo Y, Yi Y, Wu L, Liu W, Li Y, Yang Y. A Structural Colored Epoxy Resin Sensor for the Discrimination of Methanol and Ethanol. Chemistry. 2025; 7(4):122. https://doi.org/10.3390/chemistry7040122
Chicago/Turabian StyleGuo, Yongxing, Yingying Yi, Limin Wu, Wei Liu, Yi Li, and Yonggang Yang. 2025. "A Structural Colored Epoxy Resin Sensor for the Discrimination of Methanol and Ethanol" Chemistry 7, no. 4: 122. https://doi.org/10.3390/chemistry7040122
APA StyleGuo, Y., Yi, Y., Wu, L., Liu, W., Li, Y., & Yang, Y. (2025). A Structural Colored Epoxy Resin Sensor for the Discrimination of Methanol and Ethanol. Chemistry, 7(4), 122. https://doi.org/10.3390/chemistry7040122







