Synthesis and Photophysics of 5-(1-Pyrenyl)-1,2-Azoles
Abstract
1. Introduction

2. Results and Discussion
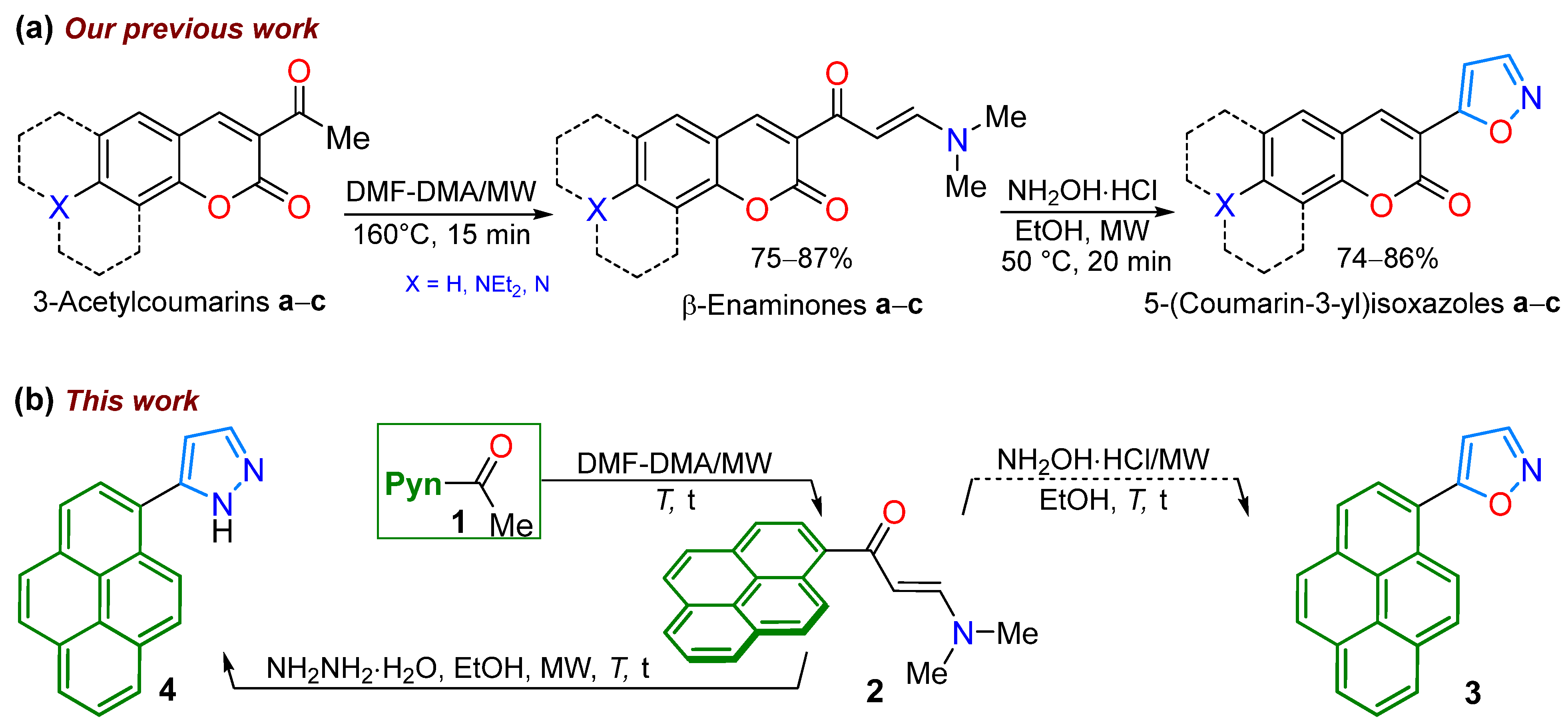
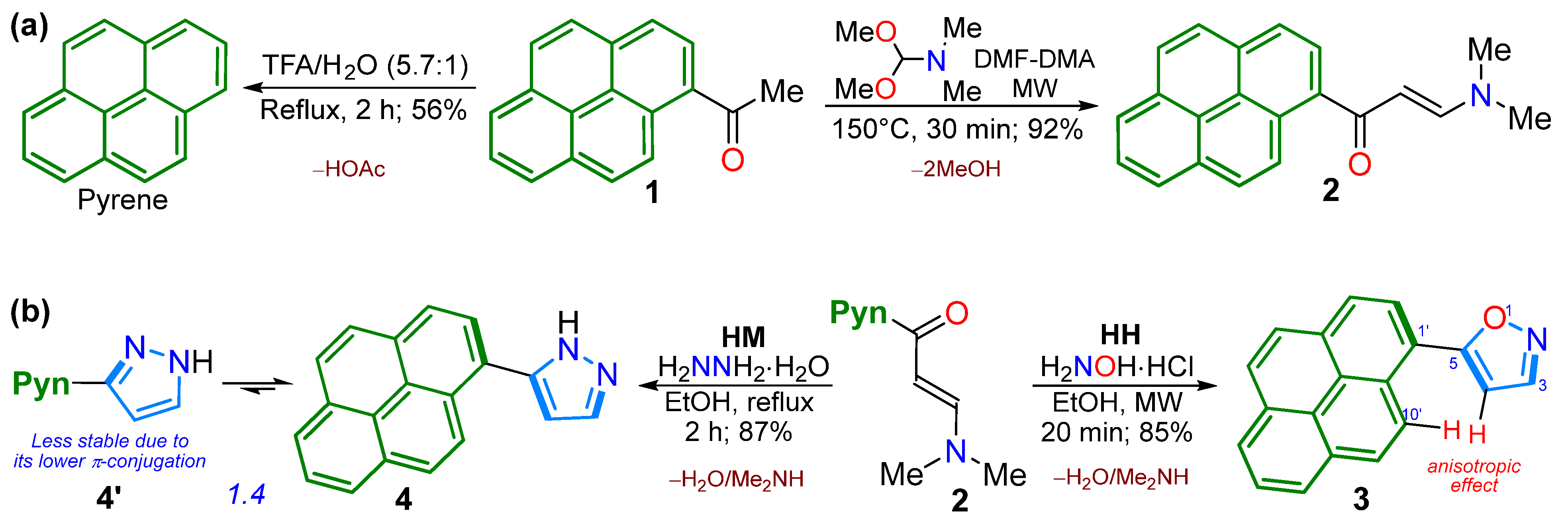
2.1. Synthesis
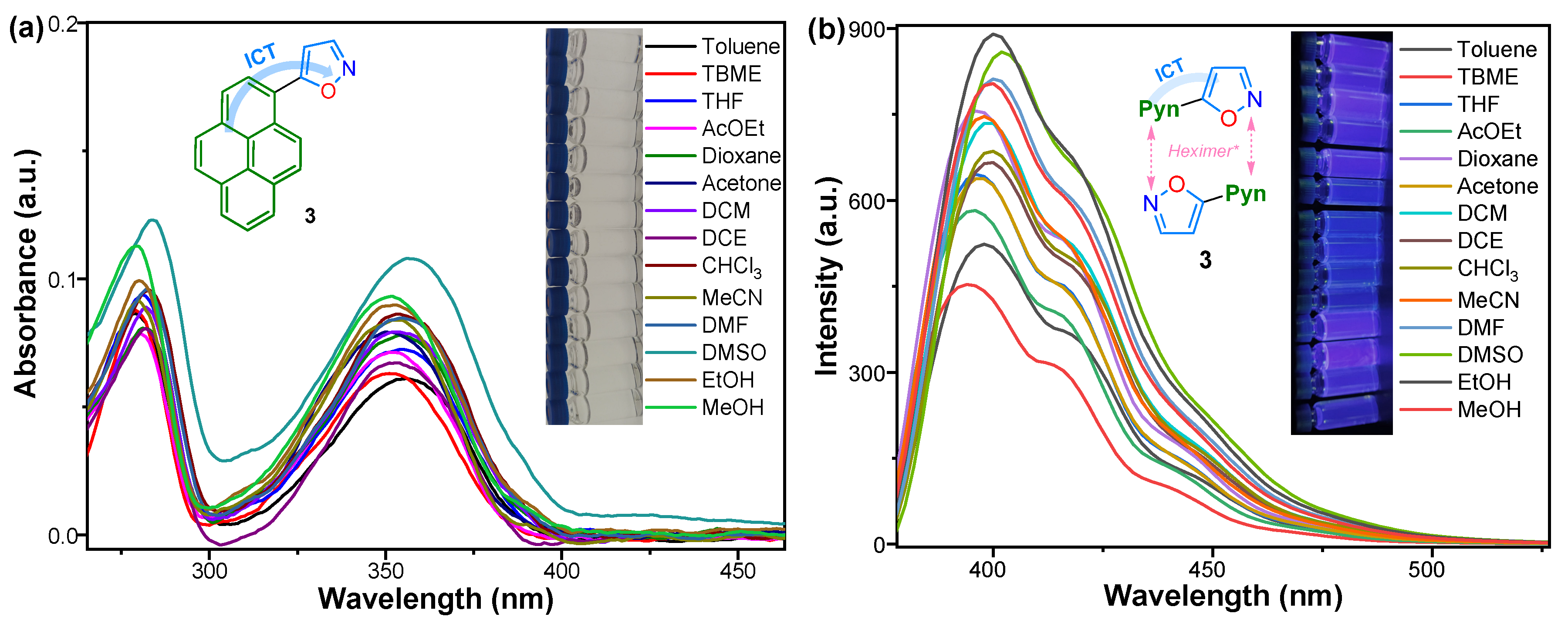
2.2. Photophysical Properties
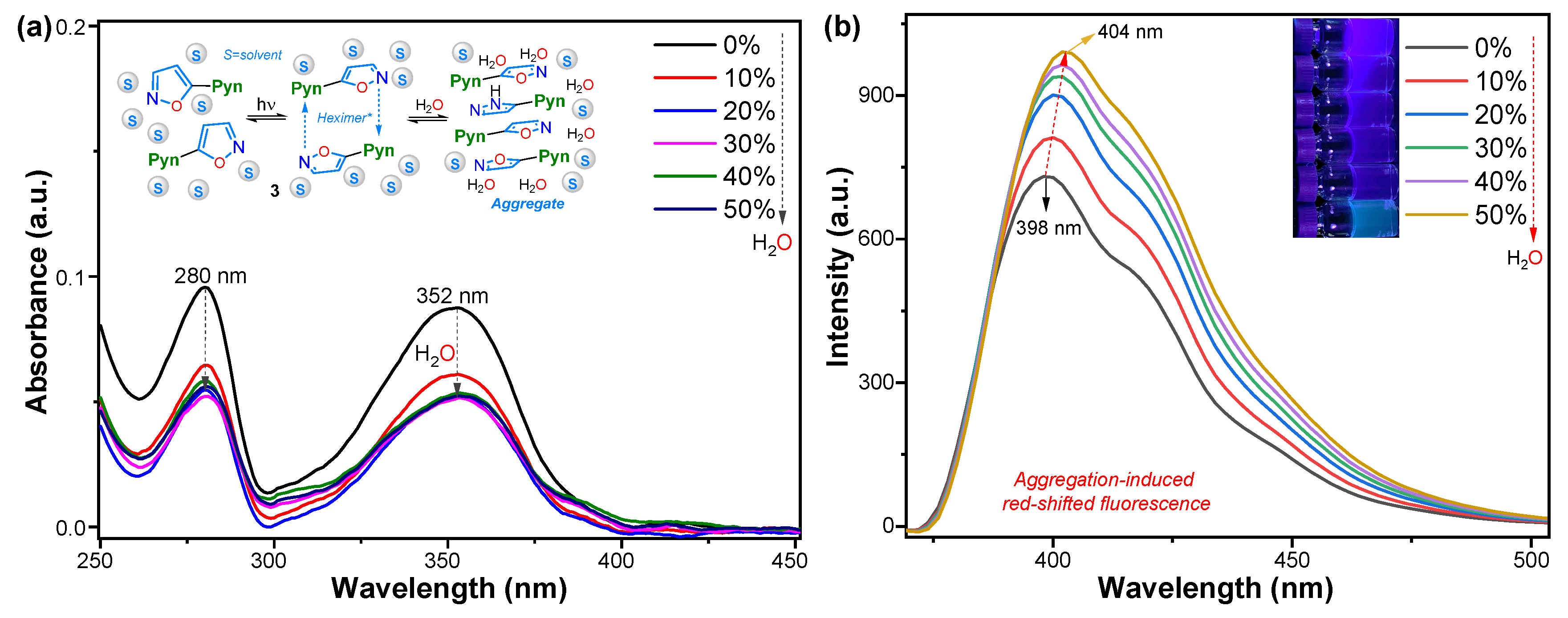
2.3. Properties in the Aggregate State
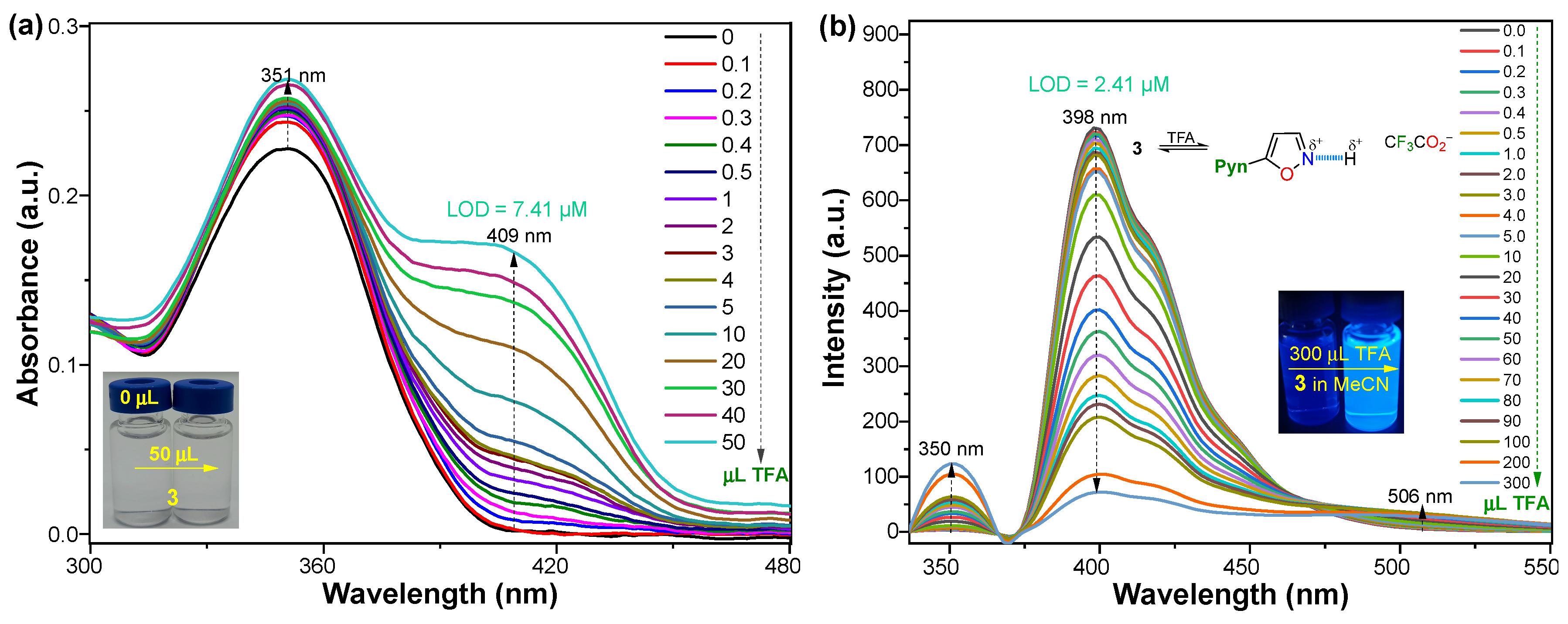
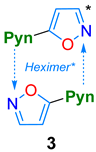
2.4. Acid/Base Properties
3. Conclusions
4. Materials and Methods
4.1. General Information
4.2. Synthesis and Characterization Data
4.2.1. Synthetic Protocols
4.2.2. Characterization Data
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Figueira-Duarte, T.M.; Müllen, K. Pyrene-Based Materials for Organic Electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef]
- Deepika; Juneja, S.; Pandey, S. Fluorescence of Pyrene and Its Derivatives to Reveal Constituent and Composition Dependent Solvation within Hydrophobic Deep Eutectic Solvents. Phys. Chem. Chem. Phys. 2023, 25, 11998–12012. [Google Scholar] [CrossRef]
- Prabakaran, G.; David, C.I.; Nandhakumar, R. A Review on Pyrene Based Chemosensors for the Specific Detection on D-Transition Metal Ions and Their Various Applications. J. Environ. Chem. Eng. 2023, 11, 109701. [Google Scholar] [CrossRef]
- Pensack, R.D.; Ashmore, R.J.; Paoletta, A.L.; Scholes, G.D. The Nature of Excimer Formation in Crystalline Pyrene Nanoparticles. J. Phys. Chem. C 2018, 122, 21004–21017. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Thomas, J.K. Environmental Effects on Vibronic Band Intensities in Pyrene Monomer Fluorescence and Their Application in Studies of Micellar Systems. J. Am. Chem. Soc. 1977, 99, 2039–2044. [Google Scholar] [CrossRef]
- Pokhrel, M.R.; Bossmann, S.H. Synthesis, Characterization, and First Application of High Molecular Weight Polyacrylic Acid Derivatives Possessing Perfluorinated Side Chains and Chemically Linked Pyrene Labels. J. Phys. Chem. B 2000, 104, 2215–2223. [Google Scholar] [CrossRef]
- Kowser, Z.; Rayhan, U.; Akther, T.; Redshaw, C.; Yamato, T. A Brief Review on Novel Pyrene Based Fluorometric and Colorimetric Chemosensors for the Detection of Cu2+. Mater. Chem. Front. 2021, 5, 2173–2200. [Google Scholar] [CrossRef]
- Saha, S.; Paul, S.; Debnath, R.; Dey, N.; Biswas, B. AIE Active Fluorescent Organic Nanoparticles Based Optical Detection of Cu2+ Ions in Pure Water: A Case of Aggregation–Disaggregation Reversibility. Anal. Methods 2024, 16, 1058–1068. [Google Scholar] [CrossRef]
- Ayyavoo, K.; Velusamy, P. Pyrene Based Materials as Fluorescent Probes in Chemical and Biological Fields. New J. Chem. 2021, 45, 10997–11017. [Google Scholar] [CrossRef]
- Köksoy, B. Design, Synthesis, and Chemical Characterization of Pyrene-Substituted BODIPY Dyes. ChemPhotoChem 2025, 9, e202400365. [Google Scholar] [CrossRef]
- Andrews, S.G.J.; Silviya, S.B.J.; Jebaraj, J.W.; Balakrishnan, C. Rhodamine Functionalized Fluorescent Probe Based on Pyrene and Triazole Platform for Hg(II) Ions and Its Application to Bio-Imaging. J. Photochem. Photobiol. A Chem. 2025, 462, 116220. [Google Scholar] [CrossRef]
- Roch, R.; Deschanels, X.; Singaravelu, C.M.; André, N.; Rey, C.; Causse, J. Evidence of the Contribution of Molecular Fluorophores to the Luminescence of Carbon Entities Formed by Solvothermal Treatment of Trinitropyrene. RSC Adv. 2024, 14, 39858–39866. [Google Scholar] [CrossRef]
- Islam, M.M.; Hu, Z.; Wang, Q.; Redshaw, C.; Feng, X. Pyrene-Based Aggregation-Induced Emission Luminogens and Their Applications. Mater. Chem. Front. 2019, 3, 762–781. [Google Scholar] [CrossRef]
- Tigreros, A.; Portilla, J. Ecological and Economic Efforts in the Development of Molecular Sensors for the Optical Detection of Cyanide Ions. Eur. J. Org. Chem. 2022, 2022, e202200249. [Google Scholar] [CrossRef]
- Qiao, M.; Fan, J.; Ding, L.; Fang, Y. Fluorescent Ensemble Sensors and Arrays Based on Surfactant Aggregates Encapsulating Pyrene-Derived Fluorophores for Differentiation Applications. ACS Appl. Mater. Interfaces 2021, 13, 18395–18412. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Zhang, Y.; Sheng, M.; Wang, Y.; Xing, Z.; Yang, L.; Yue, M.; Fu, Y.; Ye, F. A Novel Functional Fluorescent Probe Based on a Pyrene Derivative for the Detection of Multiple Pollutants. J. Mol. Liq. 2023, 382, 121888. [Google Scholar] [CrossRef]
- Wang, M.; Du, J.; Ye, Y.; Lin, G.; Liang, C.; Liu, W. A Pyrene-Based Fluorescent Probe for Cysteine Detection in the Presence of Other Biothiols and Its Application in Living Cells. J. Mol. Struct. 2025, 1337, 142095. [Google Scholar] [CrossRef]
- Pandey, A.; Kumar, D.; Dutta, S.; Manna, S.; Choudhury, S.M.; Sharma, V.; Manna, A.K.; Patra, G.K. A Pyrene Based Novel Di-Schiff Base Colorimetric and Turn-on Fluorescent-Chemosensor for Selective and Sensitive Detection of Hg2+ Ion: Biological, Logic Gate and Real Sample Applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 337, 126104. [Google Scholar] [CrossRef]
- Li, J.; Wei, W.; Qi, X.; Zuo, G.; Fang, J.; Dong, W. Highly Selective Colorimetric/Fluorometric Dual-Channel Sensor for Cyanide Based on ICT off in Aqueous Solution. Sens. Actuators B Chem. 2016, 228, 330–334. [Google Scholar] [CrossRef]
- Fujisaki, H.; Ishizuka, T.; Kotani, H.; Kojima, T. Selective Oxidation of Hydrocarbons by Molecular Iron Catalysts Based on Molecular Recognition through π–π Interaction in Aqueous Medium. ACS Catal. 2024, 14, 2609–2619. [Google Scholar] [CrossRef]
- Bravo, N.-F.; Cifuentes, C.; Ríos, M.-C.; Pérez, L.-J.; Portilla, J. Synthesis and Photophysical Properties of Conjugated Fluorophores with N-Heteroaromatic Rings. Asian J. Org. Chem. 2025, 14, e202400742. [Google Scholar] [CrossRef]
- Idzik, K.R.; Ledwon, P.; Licha, T.; Kuznik, W.; Lapkowski, M.; Frydel, J. Furyl Derivatives of Pyrene: Efficient Synthesis and Relevant Optical Properties. Dye. Pigment. 2014, 103, 55–61. [Google Scholar] [CrossRef]
- Idzik, K.R.; Licha, T.; Lukeš, V.; Rapta, P.; Frydel, J.; Schaffer, M.; Taeuscher, E.; Beckert, R.; Dunsch, L. Synthesis and Optical Properties of Various Thienyl Derivatives of Pyrene. J. Fluoresc. 2014, 24, 153–160. [Google Scholar] [CrossRef]
- Szlapa-Kula, A.; Małecka, M.; Machura, B. Insight into Structure-Property Relationships of Aryl-Substituted 2,2′:6′,2″-Terpyridines. Dye. Pigment. 2020, 180, 108480. [Google Scholar] [CrossRef]
- Zych, D.; Slodek, A. The Impact of a 1,2,3-Triazole Motif on the Photophysical Behavior of Non-K Tetrasubstituted Pyrene with a Substitution Pattern Providing the Long Axial Symmetry. Molecules 2022, 27, 4314. [Google Scholar] [CrossRef]
- Hussein, E.M.; Guesmi, N.E.; Maji, T.K.; Jassas, R.S.; Alsimaree, A.A.; Altass, H.M.; Moussa, Z.; Pal, S.K.; Ahmed, S.A. Synthesis and Photophysical Properties of Benzimidazoles Grafted Pyrazole-Containing Pyrene or Fluorene Moiety: A Combined Spectroscopic and Computational Study. J. Photochem. Photobiol. A Chem. 2021, 419, 113465. [Google Scholar] [CrossRef]
- Palakkeezhillam, V.N.V.; Haribabu, J.; Manakkadan, V.; Rasin, P.; Varughese, R.E.; Gayathri, D.; Bhuvanesh, N.; Echeverria, C.; Sreekanth, A. Synthesis, Spectroscopic Characterizations, Single Crystal X-Ray Analysis, DFT Calculations, in Vitro Biological Evaluation and in Silico Evaluation Studies of Thiosemicarbazones Based 1,3,4-Thiadiazoles. J. Mol. Struct. 2023, 1273, 134309. [Google Scholar] [CrossRef]
- Kumar, V.; Sony, S.; Kaur, N.; Mobin, S.M.; Kaur, P.; Singh, K. Thiazolothiazole Based Donor-π-Acceptor Fluorophore: Protonation/Deprotonation Triggered Molecular Switch, Sensing and Bio-Imaging Applications. Anal. Chim. Acta 2022, 1206, 339776. [Google Scholar] [CrossRef]
- Chidirala, S.; Ulla, H.; Valaboju, A.; Kiran, M.R.; Mohanty, M.E.; Satyanarayan, M.N.; Umesh, G.; Bhanuprakash, K.; Rao, V.J. Pyrene–Oxadiazoles for Organic Light-Emitting Diodes: Triplet to Singlet Energy Transfer and Role of Hole-Injection/Hole-Blocking Materials. J. Org. Chem. 2016, 81, 603–614. [Google Scholar] [CrossRef]
- Feng, X.; Hu, J.; Redshaw, C.; Yamato, T. Functionalization of Pyrene To Prepare Luminescent Materials—Typical Examples of Synthetic Methodology. Chem.–A Eur. J. 2016, 22, 11898–11916. [Google Scholar] [CrossRef]
- Salunke, J.K.; Wong, F.L.; Feron, K.; Manzhos, S.; Lo, M.F.; Shinde, D.; Patil, A.; Lee, C.S.; Roy, V.A.L.; Sonar, P.; et al. Phenothiazine and Carbazole Substituted Pyrene Based Electroluminescent Organic Semiconductors for OLED Devices. J. Mater. Chem. C 2016, 4, 1009–1018. [Google Scholar] [CrossRef]
- Zych, D. 1,3-Di(Hetero)Aryl-7-Substituted Pyrenes—An Undiscovered Area of Important Pyrene Derivatives. Proceedings 2019, 41, 28. [Google Scholar] [CrossRef]
- Zhu, C.; Zeng, H.; Liu, C.; Cai, Y.; Fang, X.; Jiang, H. Regioselective Synthesis of 3-Trifluoromethylpyrazole by Coupling of Aldehydes, Sulfonyl Hydrazides, and 2-Bromo-3,3,3-Trifluoropropene. Org. Lett. 2020, 22, 809–813. [Google Scholar] [CrossRef]
- Wrona-Piotrowicz, A.; Makal, A.; Zakrzewski, J. Highly Fluorescent Dyes Containing Conformationally Restrained Pyrazolylpyrene (Pyrazoolympicene) Chromophore. Molecules 2022, 27, 1272. [Google Scholar] [CrossRef]
- Zych, D.; Zimosz, S.; Kubis, M.; Slodek, A. Pyrene-Pyrazole Systems – Elucidating the Impact of Substitution Patterns in the Group of Mono-, Di-, Tri- and Tetrasubstituted Derivatives on Emission Behaviour through Experimental and Theoretical Approaches. J. Mol. Liq. 2024, 407, 125250. [Google Scholar] [CrossRef]
- Tan, G.; Zhu, L.; Liao, X.; Lan, Y.; You, J. Rhodium/Copper Cocatalyzed Highly Trans-Selective 1,2-Diheteroarylation of Alkynes with Azoles via C–H Addition/Oxidative Cross-Coupling: A Combined Experimental and Theoretical Study. J. Am. Chem. Soc. 2017, 139, 15724–15737. [Google Scholar] [CrossRef]
- Ríos, M.-C.; Bravo, N.-F.; Macías, M.; Iglesias, B.A.; Portilla, J. Synthesis and Photophysical Study of 3-(Isoxazol-5-yl)Coumarins. ChemPhotoChem 2025, 9, e202400389. [Google Scholar] [CrossRef]
- Ríos, M.-C.; Portilla, J. Recent Advances in Synthesis and Properties of Pyrazoles. Chemistry 2022, 4, 940–968. [Google Scholar] [CrossRef]
- Ortiz, M.-C.; Portilla, J. Access to Five-Membered N-Heteroaromatic Compounds: Current Appoach Based on Microwave-Assisted Synthesis. In Targets in Heterocyclic Systems; Attanasi, O.A.S.D., Ed.; Società Chimica Italiana: Rome, Italy, 2021; Volume 25, pp. 436–462. [Google Scholar]
- Martis, G.J.; Gaonkar, S.L. Advances in Isoxazole Chemistry and Their Role in Drug Discovery. RSC Adv. 2025, 15, 8213–8243. [Google Scholar] [CrossRef]
- Shinde, Y.; Khairnar, B.; Bangale, S. Exploring the Diverse Biological Frontiers of Isoxazole: A Comprehensive Review of Its Pharmacological Significance. ChemistrySelect 2024, 9, e202401423. [Google Scholar] [CrossRef]
- Mallik, N.N.; Manasa, C.; Basavanna, V.; Shanthakumar, D.C.; Ningaiah, S.; Lingegowda, N.S. Synthesis of Fused Isoxazoles: A Comprehensive Review. Eng. Proc. 2023, 59, 222. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman. Review: Biologically Active Pyrazole Derivatives. New J. Chem. 2017, 41, 16–41. [Google Scholar] [CrossRef]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-Aizari, F.A.; Ansar, M. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef]
- Mithra, U.; Sarveswari, S. A Review on Pyrazole Moieties as Organic Chemosensors in the Detection of Cations and Anions. Inorganica Chim. Acta 2024, 569, 122118. [Google Scholar] [CrossRef]
- García-Olave, M.; Tigreros, A.; Iglesias, B.A.; Portilla, J. Synthesis and Photophysical Properties of Pyrazolo[1,5-a]Pyrimidines Containing D−π−A Architecture Similar to Prodan. J. Mol. Struct. 2024, 1317, 139142. [Google Scholar] [CrossRef]
- Cao, D.; Liu, Z.; Verwilst, P.; Koo, S.; Jangjili, P.; Kim, J.S.; Lin, W. Coumarin-Based Small-Molecule Fluorescent Chemosensors. Chem. Rev. 2019, 119, 10403–10519. [Google Scholar] [CrossRef]
- Terai, T.; Nagano, T. Small-Molecule Fluorophores and Fluorescent Probes for Bioimaging. Pflügers Arch.-Eur. J. Physiol. 2013, 465, 347–359. [Google Scholar] [CrossRef]
- Kim, H.M.; Cho, B.R. Small-Molecule Two-Photon Probes for Bioimaging Applications. Chem. Rev. 2015, 115, 5014–5055. [Google Scholar] [CrossRef]
- Wu, L.; Liu, J.; Li, P.; Tang, B.; James, T.D. Two-Photon Small-Molecule Fluorescence-Based Agents for Sensing, Imaging, and Therapy within Biological Systems. Chem. Soc. Rev. 2021, 50, 702–734. [Google Scholar] [CrossRef]
- Jiao, X.; Li, Y.; Niu, J.; Xie, X.; Wang, X.; Tang, B. Small-Molecule Fluorescent Probes for Imaging and Detection of Reactive Oxygen, Nitrogen, and Sulfur Species in Biological Systems. Anal. Chem. 2018, 90, 533–555. [Google Scholar] [CrossRef]
- Reger, D.L.; Gardinier, J.R.; Pellechia, P.J.; Smith, M.D.; Brown, K.J. Synthesis and Properties of Rhenium Carbonyl Complexes of α,α‘-Bis[(1-Pyrenyl)Pyrazol-1-Yl]Alkane Ligands. Inorg. Chem. 2003, 42, 7635–7643. [Google Scholar] [CrossRef]
- Keumi, T.; Morita, T.; Inui, Y.; Teshima, N.; Kitajima, H. Selective and Mild Deacylation of Hindered Acylarenes with Aqueous Trifluoroacetic Acid. Synthesis 1985, 1985, 979–980. [Google Scholar] [CrossRef]
- Venkatareddy, V.K.; Kumar, M.; Singh, V.P.; Malakalapalli, R.R. ESIPT-Active Pyrene-imidazole Fluorophores: GSIPT, Dual Solid- and Solution-State Emission plus Counter-Intuitive Crystal Packing. ChemPhotoChem 2023, 7. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Xu, C.; Li, H.; Yang, X. Studies on the Interaction Mechanism of Pyrene Derivatives with Human Tumor-Related DNA. Molecules 2012, 17, 14159–14173. [Google Scholar] [CrossRef]
- Subuddhi, U.; Haldar, S.; Sankararaman, S.; Mishra, A.K. Photophysical Behaviour of 1-(4-N, N-Dimethylaminophenylethynyl)Pyrene (DMAPEPy) in Homogeneous Media. Photochem. Photobiol. Sci. 2006, 5, 459–466. [Google Scholar] [CrossRef]
- Patra, D.; Barakat, C. Synchronous Fluorescence Spectroscopic Study of Solvatochromic Curcumin Dye. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1034–1041. [Google Scholar] [CrossRef]
- Nakagawa, T.; Ishino, S.; Inamori, D.; Masai, H.; Terao, J. Luminescent Thermoresponse via Excimer/Exciplex Transition of Pyrene Derivative in Polymer Networks Containing [3]Rotaxane. ACS Macro Lett. 2023, 12, 751–758. [Google Scholar] [CrossRef]
- Omura, Y.; Tachi, Y.; Okada, K.; Kozaki, M. Synthesis and Properties of Nitrogen-Containing Pyrenes. J. Org. Chem. 2019, 84, 2032–2038. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, J.; Xu, W.; Zhu, D. Novel 2,7-Substituted Pyrene Derivatives: Syntheses, Solid-State Structures, and Properties. Tetrahedron 2011, 67, 3395–3405. [Google Scholar] [CrossRef]
- Jiang, M.-H.; Li, M.-N.; Zhao, Y.; Liu, Y.-H.; Gao, J.; Zhu, C. ESIPT-Induced Intersystem Crossing Leads to Tautomer Fluorescence Quenching for 3-Mercapto-2-(4-(Trifluoromethyl)Phenyl)-4H-Chromen-4-One Molecule. J. Photochem. Photobiol. A Chem. 2025, 459, 116111. [Google Scholar] [CrossRef]
- Larina, L.I. Tautomerism and Structure of Azoles: Nuclear Magnetic Resonance Spectroscopy. Adv. Heterocycl. Chem. 2018, 124, 233–321. [Google Scholar]
- Cui, P.; Gao, H.; Wang, Y.; Tung, C.; Kong, L. The Cation-π Interactions in a Potassium Alkylideneborane Complex. Eur. J. Inorg. Chem. 2022, 2022, e202200400. [Google Scholar] [CrossRef]
- Forbes, C.R.; Sinha, S.K.; Ganguly, H.K.; Bai, S.; Yap, G.P.A.; Patel, S.; Zondlo, N.J. Insights into Thiol–Aromatic Interactions: A Stereoelectronic Basis for S–H/π Interactions. J. Am. Chem. Soc. 2017, 139, 1842–1855. [Google Scholar] [CrossRef]
- Cheng, Y.; Gontard, G.; Khatyr, A.; Knorr, M.; Amouri, H. N-Heterocyclic Carbene Copper (I) Complexes Incorporating Pyrene Chromophore: Synthesis, Crystal Structure, and Luminescent Properties. Molecules 2023, 28, 4025. [Google Scholar] [CrossRef]
- Wen, J.; Tong, J.; Kou, Y.-L.; Wang, J.; Yu, S.-Y. Multi-Responsive Smart Fluorophores from Pyrazole-Functionalized Tetraphenylethene AIEgens in Response to Hg(II) Ion, Temperature, and Mechanical Force. Dye. Pigment. 2024, 223, 111954. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Würth, C.; Grabolle, M.; Pauli, J.; Spieles, M.; Resch-Genger, U. Relative and Absolute Determination of Fluorescence Quantum Yields of Transparent Samples. Nat. Protoc. 2013, 8, 1535–1550. [Google Scholar] [CrossRef]
- Castillo, J.C.; Rosero, H.A.; Portilla, J. Simple Access toward 3-Halo- and 3-Nitro-Pyrazolo[1,5-a] Pyrimidines through a One-Pot Sequence. RSC Adv 2017, 7, 28483–28488. [Google Scholar] [CrossRef]
- Modak, A.; Naveen, T.; Maiti, D. An Efficient Dehydroxymethylation Reaction by a Palladium Catalyst. Chem. Commun. 2013, 49, 252–254. [Google Scholar] [CrossRef]

| Compound | State | λabs (nm) π–π*, n–π*shs | ε (M–1 cm–1) | λem (nm) maxshs | ϕF (%) [b] | Stokes Shift (cm–1) |
|---|---|---|---|---|---|---|
 | Toluene | 279, 356 | 8660, 6110 | 398418,448 | 88 | 2964 |
| TBME | 279, 351 | 8780, 6300 | 394416/442 | 74 | 3109 | |
| THF | 282, 354 | 9330, 7240 | 404416/446 | 92 | 3496 | |
| AcOEt | 280, 351 | 7900, 7130 | 396416/442 | 79 | 3238 | |
| Dioxane | 281, 355 | 8070, 7810 | 396416/446 | 95 | 2916 | |
| Acetone | 282, 350 | 8890, 7920 | 398416/446 | 81 | 3446 | |
| DCM | 282, 352 | 8890, 7930 | 400418/448 | 96 | 3409 | |
| DCE | 282, 354 | 8060, 6720 | 400420/450 | ND | 3249 | |
| CHCl3 | 283, 354 | 9560, 8630 | 400420/450 | 86 | 3249 | |
| MeCN | 280, 352 | 9150, 8390 | 398416/446 | 91 | 3283 | |
| DMF | 282, 351 | 9590, 8470 | 400420/450 | ND | 3490 | |
| DMSO | 284, 356 | 12,300, 10,800 | 402422/452 | 90 | 3214 | |
| EtOH | 280, 353 | 9923, 8970 | 400418/446 | ND | 3329 | |
| MeOH | 279, 352 | 11,270, 9320 | 400418/448 | 95 | 3409 | |
 | TBME | 281, 355359 | 20,630, 17,960 | 398438/466 (514434/548) [c] | 0.1 | 3043 |
| DCM | 285, 359393 | 14,670, 10,980 | 408498/532 | 0.2 | 3345 | |
| MeOH | 281, 356387 | 25,150, 21,580 | 402428/468 | 0.1 | 3314 | |
 | TBME | 281, 347 | 25,570, 21,510 | 394408/436 | 1.3 | 3438 |
| DCM | 281, 346 | 29,140, 23,430 | 394408/436 | 6.9 | 3521 | |
| MeOH | 278, 345 | 39,240, 31,310 | 392408/436 | 1.5 | 3475 | |
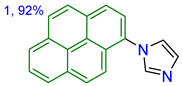
 | TBME | 262, 272, 319305, 334 | 18,000, 34,095, 21,826, 36,660 | 378389 | 16 | 3487 |
| DCM | 263, 274, 321306, 337 | 15,864, 33,546, 24,435, 39,457 | 377392 | 33 | 3146 | |
| MeOH | 261, 272, 318309, 334 | 25,029, 47,577, 29,409, 28,931 | 376390 | 26 | 3349 | |
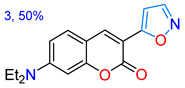
| 3 **[d] | White solid | ND | -- | 490 | 23.4 | -- |
| 4 **[d] | Yellow solid | ND | -- | 504 | 22.6 | -- |
| Fluorophore (Stages, Overall Yield) [a] | State [b] | λabs/λem (nm) | ε (M–1 cm–1) | ϕF (%) | Application, LOD (μM) in Solvent | Ref. |
|---|---|---|---|---|---|---|
 | in DCM -- | 327/379 -- | 30,100 -- | 39 -- | Coordination, NHC-Cu-Cl | Cheng et al., 2023 [65] |
 | in DCM Solid state | 343/423 --/469 | 39,000 -- | 26 33 | -- | Zych et al., 2024 [35] |
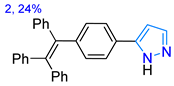 | in DMSO -- | 321/427 --/475 | -- -- | -- 56 | Sensor, 0.09/Hg2+ in DMSO/H2O | Wen et al., 2024 [66] |
 | in TBME Solid state | 438/473 449/535 | 38,130 -- | 21 49 | Acidochromism, 3.38/TFA in MeCN | Ríos et al., 2025 [37] |
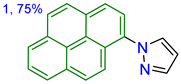
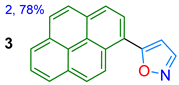 | in TBME Solid state | 351/394 --/490 | 6300 -- | 74 23.4 | Acidochromism, 2.41/TFA in MeCN | This work |
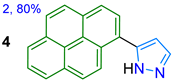 | in TBME Solid state | 347/394 --/504 | 21,510 -- | 1.3 22.6 | Acidochromism, --/CN– in MeCN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ríos, M.-C.; Ladino-Bejarano, A.; Portilla, J. Synthesis and Photophysics of 5-(1-Pyrenyl)-1,2-Azoles. Chemistry 2025, 7, 120. https://doi.org/10.3390/chemistry7040120
Ríos M-C, Ladino-Bejarano A, Portilla J. Synthesis and Photophysics of 5-(1-Pyrenyl)-1,2-Azoles. Chemistry. 2025; 7(4):120. https://doi.org/10.3390/chemistry7040120
Chicago/Turabian StyleRíos, María-Camila, Alexander Ladino-Bejarano, and Jaime Portilla. 2025. "Synthesis and Photophysics of 5-(1-Pyrenyl)-1,2-Azoles" Chemistry 7, no. 4: 120. https://doi.org/10.3390/chemistry7040120
APA StyleRíos, M.-C., Ladino-Bejarano, A., & Portilla, J. (2025). Synthesis and Photophysics of 5-(1-Pyrenyl)-1,2-Azoles. Chemistry, 7(4), 120. https://doi.org/10.3390/chemistry7040120






