Synthesis and Characterization of Polymethylhydrosiloxane-Modified Phenol–Formaldehyde Resin
Abstract
1. Introduction
2. Chemicals and Methods
2.1. Chemicals
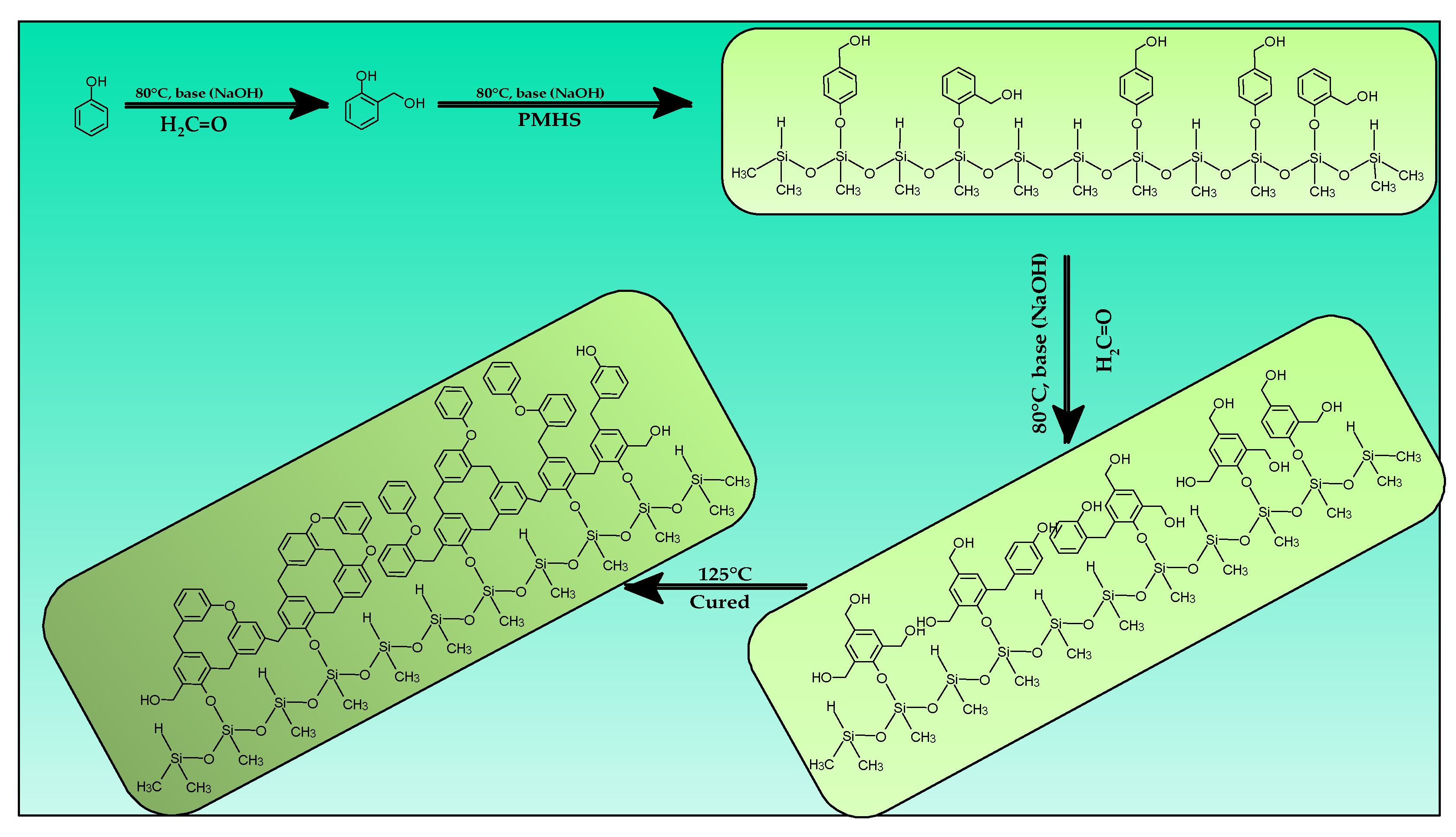
2.2. Synthesis of Materials
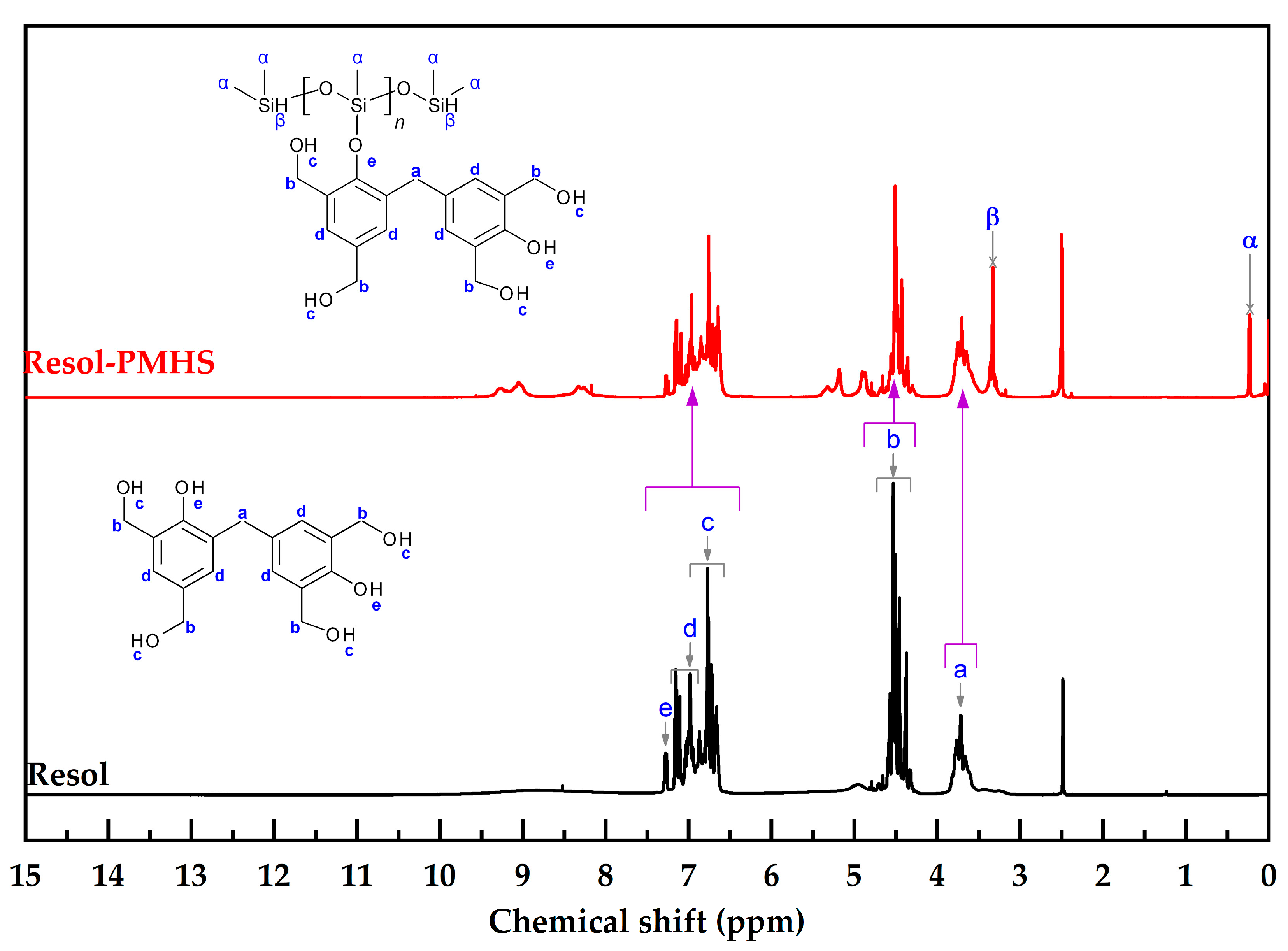
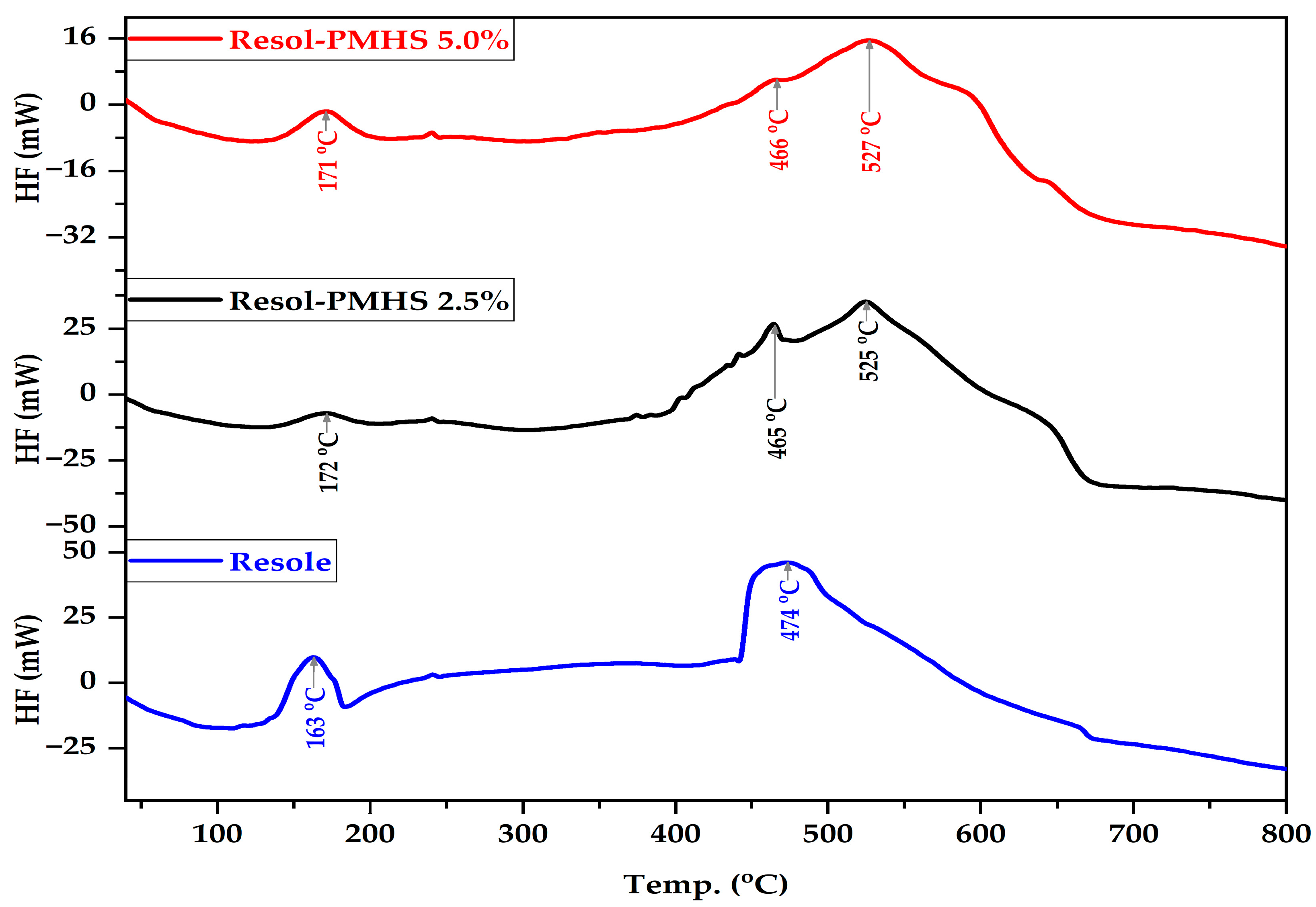
2.3. Characterization of Materials
3. Results and Discussion
3.1. Synthesis of PMHS-Modified PF Resin
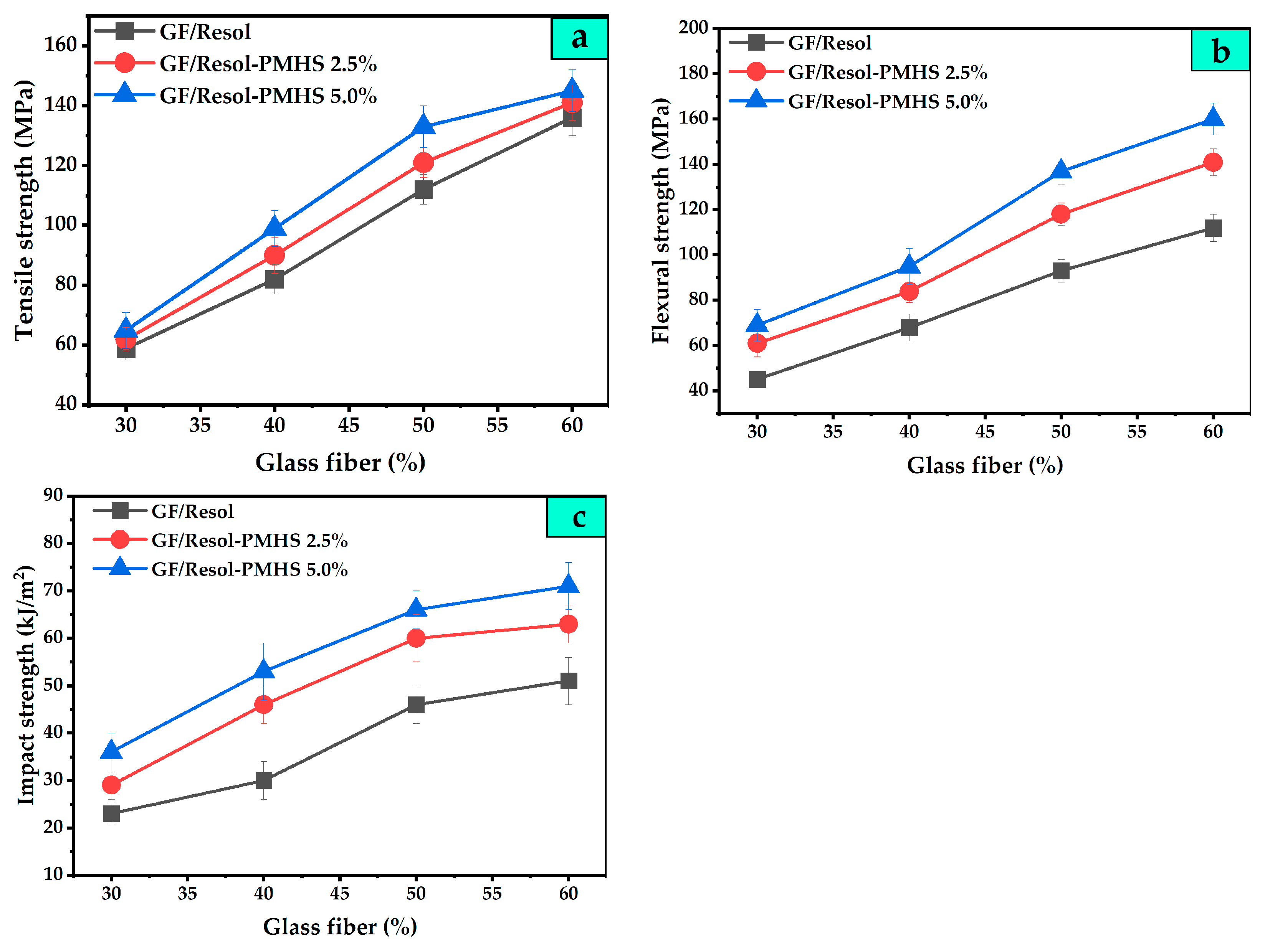
3.2. Mechanical Properties of Composites from GF/PMHS-Modified PF Composites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PF | Phenol-formaldehyde |
| PMHS | Polymethylhydrosiloxane |
| IPA | Isopropanol |
| GF | Glass Fiber |
| CNSL | Cashew Nut Shell Liquid |
| FTIR | Fourier-transform infrared |
| 1H NMR | Proton Nuclear Magnetic Resonance |
| DSC | Differential Scanning Calorimetry |
| TGA | Thermogravimetric Analysis |
References
- Pizzi, A.; Ibeh, C.C. Chapter 2: Phenol–Formaldehydes. In Handbook of Thermoset Plastics, 3rd ed.; Dodiuk, H., Goodman, S.H., Eds.; William Andrew Publishing: Boston, MA, USA, 2014; pp. 13–44. [Google Scholar]
- Xu, Y.; Guo, L.; Zhang, H.; Zhai, H.; Ren, H. Research status, industrial application demand and prospects of phenolic resin. RSC Adv. 2019, 9, 28924–28935. [Google Scholar] [CrossRef]
- Lang, J.; Cornick, M. Resole Production. In Phenolic Resins: A Century of Progress; Pilato, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 139–146. [Google Scholar]
- Monni, J.; Alvila, L.; Rainio, J.; Pakkanen, T.T. Novel two-stage phenol–formaldehyde resol resin synthesis. J. Appl. Polym. Sci. 2007, 103, 371–379. [Google Scholar] [CrossRef]
- Domínguez, J.C.; Oliet, M.; Alonso, M.V.; Rojo, E.; Rodríguez, F. Structural, thermal and rheological behavior of a bio-based phenolic resin in relation to a commercial resol resin. Ind. Crops Prod. 2013, 42, 308–314. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Jiang, D.; Shalash, M.; El-Bahy, S.M.; Wu, Z.; Ren, J.; Guo, Z.; El-Bahy, Z.M. A comprehensive review on modified phenolic resin composites for enhanced performance across various applications. Polym. Compos. 2025, 1–39. [Google Scholar] [CrossRef]
- Ahmed, S.; Mohamed, M.; Mansour, N.; El-Komy, D.; Sadek, E. Modified resol type phenolic resin nanocomposites as surface metal coatings. SPE Polym. 2021, 2, 28–37. [Google Scholar] [CrossRef]
- Cardona, F.; Aravinthan, T.; Moscou, C. Modified PF Resins for Composite Structures with Improved Mechanical Properties. Polym. Polym. Compos. 2010, 18, 297–306. [Google Scholar] [CrossRef]
- Huong, N.L.; Nieu, N.H.; Tan, T.T.M.; Griesser, U.J. Cardanol-phenol-formaldehyde resins. Thermal analysis and characterization. Die Angew. Makromol. Chem. 1996, 243, 77–85. [Google Scholar] [CrossRef]
- Sarika, P.R.; Nancarrow, P.; Khansaheb, A.; Ibrahim, T. Bio-Based Alternatives to Phenol and Formaldehyde for the Production of Resins. Polymers 2020, 12, 2237. [Google Scholar] [CrossRef]
- Sarkar, S.; Adhikari, B. Lignin-modified phenolic resin: Synthesis optimization, adhesive strength, and thermal stability. J. Adhes. Sci. Technol. 2000, 14, 1179–1193. [Google Scholar] [CrossRef]
- Galdino, D.S.; Kondo, M.Y.; De Araujo, V.A.; Ferrufino, G.L.A.A.; Faustino, E.; Santos, H.F.d.; Christoforo, A.L.; Luna, C.M.R.; Campos, C.I.d. Thermal and Gluing Properties of Phenol-Based Resin with Lignin for Potential Application in Structural Composites. Polymers 2023, 15, 357. [Google Scholar] [CrossRef]
- Patel, M.; Yadav, M.; Raj, M. Development in the Modification of Phenolic Resin by Renewable Resources: (A-Review). Orient. J. Chem. 2023, 39, 867. [Google Scholar] [CrossRef]
- Unnikrishnan, K.P.; Thachil, E.T. The Modification of Commercial Epoxy Resin Using Cardanol—Formaldehyde Copolymers. Int. J. Polym. Mater. Polym. Biomater. 2006, 55, 323–338. [Google Scholar] [CrossRef]
- Kaynak, C.; Cagatay, O. Rubber toughening of phenolic resin by using nitrile rubber and amino silane. Polym. Test. 2006, 25, 296–305. [Google Scholar] [CrossRef]
- Tang, K.; Tang, X.; Liu, X.; Zhang, A.; Ge, T.; Li, Y. Phenolic foams toughened with triethylene glycol by in situ polymerization and prepolymerization processes. ACS Appl. Polym. Mater. 2022, 4, 8303–8314. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Xu, P.; Chang, J. Preparation and characterization of phenolic foam modified with bio-oil. Materials 2018, 11, 2228. [Google Scholar] [CrossRef]
- Xie, J.; Sun, H.; Yang, Y.; Liang, J.; Li, Y.; Hou, D.; Lin, X.; Zhang, J.; Shi, Z.; Liu, C. Preparation of High-Toughness Lignin Phenolic Resin Biomaterials Based via Polybutylene Succinate Molecular Intercalation. Int. J. Mol. Sci. 2023, 24, 6418. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, P.; Chang, M.; Chang, J. Aging Properties of Phenol-Formaldehyde Resin Modified by Bio-Oil Using UV Weathering. Polymers 2018, 10, 1183. [Google Scholar] [CrossRef]
- Yu, Y.; Qiu, X.; Li, C.; Bao, D.; Chang, J. Performance and characterization of phenol-formaldehyde resin with crude bio-oil by model compound method. PLoS ONE 2023, 18, e0271478. [Google Scholar] [CrossRef]
- Feng, L.B.; Li, H.; Hao, X.Z.; Chai, C.S.; Xue, X.J.; Hao, X.X. Structure and thermal resistance of phosphoric acid-modified phenol-formaldehyde resin. Cailiao Gongcheng/J. Mater. Eng. 2013, 3, 75–78. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, W.-H. Properties of phenol formaldehyde resin modified with silane coupling agent (KH550). Int. J. Adhes. Adhes. 2018, 84, 166–172. [Google Scholar] [CrossRef]
- Lian, S.; Lin, H.; Zhang, W.; Lei, H.; Cao, M.; Mao, J.; Li, T.; Chen, S.; Yang, L. Effects of the Addition of Amino-Terminated Highly Branched Polyurea on Curing Properties of Phenol-Formaldehyde Resin. Materials 2023, 16, 3620. [Google Scholar] [CrossRef]
- Senapati, K.K. Polymethylhydrosiloxane (PMHS). Synlett 2005, 2005, 1960–1961. [Google Scholar] [CrossRef]
- Mujahid Alam, M.; Seema, V.; Dubasi, N.; Kurra, M.; Varala, R. Applications of Polymethylhydrosiloxane (PMHS) in Organic Synthesis- Covering up to March 2022. Mini-Rev. Org. Chem. 2023, 20, 708–734. [Google Scholar] [CrossRef]
- Nagappan, S.; Joo, P.J.; Soo, P.S.; Sang-Hyun, H.; Sik, J.Y.; Won-Ki, L.; Ha, C.-S. Polymethylhydrosiloxane-based organic–inorganic hybrids for amphiphobic coatings. Compos. Interfaces 2013, 20, 33–43. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, W. Sol–gel composite coatings from methyltriethoxysilane and polymethylhydrosiloxane. J. Sol-Gel Sci. Technol. 2010, 55, 261–268. [Google Scholar] [CrossRef]
- He, M.; Zhang, L.; Ruan, K.; Zhang, J.; Zhang, H.; Lv, P.; Guo, Y.; Shi, X.; Guo, H.; Kong, J.; et al. Functionalized Aluminum Nitride for Improving Hydrolysis Resistances of Highly Thermally Conductive Polysiloxane Composites. Nano-Micro Lett. 2025, 17, 134. [Google Scholar] [CrossRef]
- Yang, G.; Chen, Z.; Lv, C.; Deng, L.; Luo, X.; Li, Y.; He, S.; Liu, Q. Preparation and Performance of H-PDMS/PMHS/OTS Hybrid Nanosilica Hydrophobic and Self-Cleaning Coatings on Phosphogypsum Surface. Polymers 2023, 15, 3574. [Google Scholar] [CrossRef]
- Hu, H.; Wang, W.; Jiang, L.; Liu, L.; Zhang, Y.; Yang, Y.; Wang, J. Curing mechanism of resole phenolic resin based on variable temperature FTIR spectra and thermogravimetry-mass spectrometry. Polym. Polym. Compos. 2022, 30, 09673911221102114. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Wang, K.; He, D.; Diao, W.; Yang, H.; Yuan, G.Y.; Wu, Y.; Shi, J.; Wu, K. Perfluoropolyether methyl propionate polysiloxane coating with high hydrophobicity and low dielectric property. J. Appl. Polym. Sci. 2023, 140, e53784. [Google Scholar] [CrossRef]
- Syabani, M.W.; Indra, P.; Rochmadi, R. Modified phenol formaldehyde resin with sodium silicate as a low-cure wood adhesive. J. Wood Chem. Technol. 2024, 44, 164–171. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, Z.; Yang, Z.; Yao, J.; Mu, Q.; Peng, D.; Zhao, H. Study on the synthesis and thermal stability of silicone resins reinforced by Si–O–Ph cross-linking. RSC Adv. 2021, 11, 30971–30979. [Google Scholar] [CrossRef]
- Zahra, N.F.d.; Auliya, D.G.; Arini, V.F.; Fitrilawati, F.; Safriani, L.; Risdiana, R. NMR Characterization of Polymethylhydrosiloxane Synthesized using Dichloromethane and Diethyl Ether as Solvent for Vitreous Substitute. Indones. J. Appl. Phys. 2023, 13, 226–232. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Zhang, X.; Wu, H.; Wang, Y.; Zhou, N.; Zhao, Z.; Wang, C.; Zhang, X.; Wang, X.; et al. Synthesis and Characterization of an Environmentally Friendly Phenol-Formaldehyde Resin Modified with Waste Plant Protein. Polymers 2023, 15, 2975. [Google Scholar] [CrossRef]
- Li, S.; Han, Y.; Chen, F.; Luo, Z.; Li, H.; Zhao, T. The effect of structure on thermal stability and anti-oxidation mechanism of silicone modified phenolic resin. Polym. Degrad. Stab. 2016, 124, 68–76. [Google Scholar] [CrossRef]
- Debnath, S.; Wunder, S.L.; McCool, J.I.; Baran, G.R. Silane treatment effects on glass/resin interfacial shear strengths. Dent. Mater. 2003, 19, 441–448. [Google Scholar] [CrossRef]
- Morii, T.; Ivens, J.; Verpoest, I. Interfacial Effects on the Mechanical Properties of Glass/Phenolic Composites. Adv. Compos. Lett. 1999, 8, 295–301. [Google Scholar] [CrossRef]
- Kimura, T.; Nakamoto, Y.; Konishi, G.-I. Preparation of Organic/Inorganic Polymer Hybrid from a New Class of Novolac. Polym. J. 2006, 38, 606–609. [Google Scholar] [CrossRef]
- Chruściel, J.J.; Fejdyś, M.; Fortuniak, W. Synthesis, Characterization and Microstructure of New Liquid Poly(methylhydrosiloxanes) Containing Branching Units SiO4/2. Polymers 2018, 10, 484. [Google Scholar] [CrossRef]
- Mohapatra, C. Revealing of Failure Modes of FRP Composites by Microscopic Technique; National Institute of Technology: Rourkela, India, 2009. [Google Scholar]
- Lee, C.H.; Khalina, A.; Lee, S.H. Importance of Interfacial Adhesion Condition on Characterization of Plant-Fiber-Reinforced Polymer Composites: A Review. Polymers 2021, 13, 438. [Google Scholar] [CrossRef]
- Schauperl, Z.; Ivanković, L.; Bauer, L.; Šolić, S.; Ivanković, M. Effects of Different Surface Treatments of Woven Glass Fibers on Mechanical Properties of an Acrylic Denture Base Material. Int. J. Mol. Sci. 2023, 24, 909. [Google Scholar] [CrossRef]
- Yang, L.; Thomason, J.L. Effect of silane coupling agent on mechanical performance of glass fibre. J. Mater. Sci. 2013, 48, 1947–1954. [Google Scholar] [CrossRef]
- Minty, R.F. The role of residual stress at the fibre-matrix interface in composite materials. In Proceedings of the ScotCHEM Polymer and Soft Materials Conference II, Edinburgh, UK, 4 May 2023. [Google Scholar]
- Holmes, G.A.; Hunston, D.L.; McDonough, W.G.; Peterson, R.C. A Test Method for Assessing Interfacial Shear Strength in Composites. In Proceedings of the International Sampe Symposium and Exhibition, Long Beach, CA, USA, 21–25 May 2000; pp. 1114–1124. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hai, L.N.; Thao, N.V.; Long, P.T.; Anh, N.X.; Tiep, L.T.; Nam, H.Q.; Viet, N.M.; Dinh, T.T.; Binh, L.D.; Hien, T.K.T.; et al. Synthesis and Characterization of Polymethylhydrosiloxane-Modified Phenol–Formaldehyde Resin. Chemistry 2025, 7, 112. https://doi.org/10.3390/chemistry7040112
Hai LN, Thao NV, Long PT, Anh NX, Tiep LT, Nam HQ, Viet NM, Dinh TT, Binh LD, Hien TKT, et al. Synthesis and Characterization of Polymethylhydrosiloxane-Modified Phenol–Formaldehyde Resin. Chemistry. 2025; 7(4):112. https://doi.org/10.3390/chemistry7040112
Chicago/Turabian StyleHai, Luong Nhu, Nguyen Van Thao, Pham The Long, Nguyen Xuan Anh, Le Tran Tiep, Hoang Quoc Nam, Nguyen Minh Viet, Tran The Dinh, Le Duy Binh, Ta Kim Thanh Hien, and et al. 2025. "Synthesis and Characterization of Polymethylhydrosiloxane-Modified Phenol–Formaldehyde Resin" Chemistry 7, no. 4: 112. https://doi.org/10.3390/chemistry7040112
APA StyleHai, L. N., Thao, N. V., Long, P. T., Anh, N. X., Tiep, L. T., Nam, H. Q., Viet, N. M., Dinh, T. T., Binh, L. D., Hien, T. K. T., & Dung, C. T. (2025). Synthesis and Characterization of Polymethylhydrosiloxane-Modified Phenol–Formaldehyde Resin. Chemistry, 7(4), 112. https://doi.org/10.3390/chemistry7040112





