Catalytic Ozonation of Diclofenac Using CuO/Al2O3- and MnO2/Al2O3-Supported Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Catalyst
2.2. Characterization of Catalyst
2.3. Ozone Catalytic Degradation of DCF
3. Results
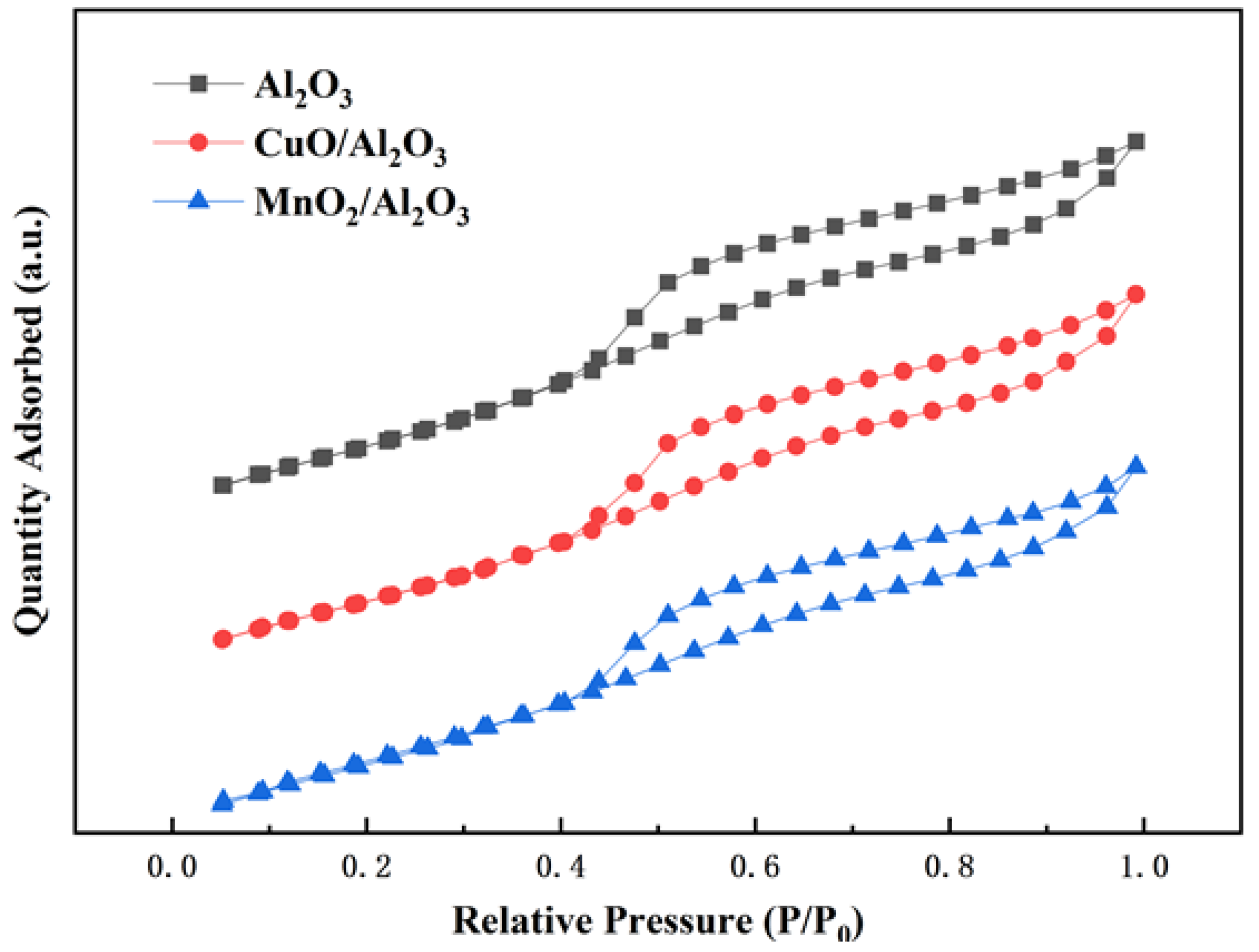
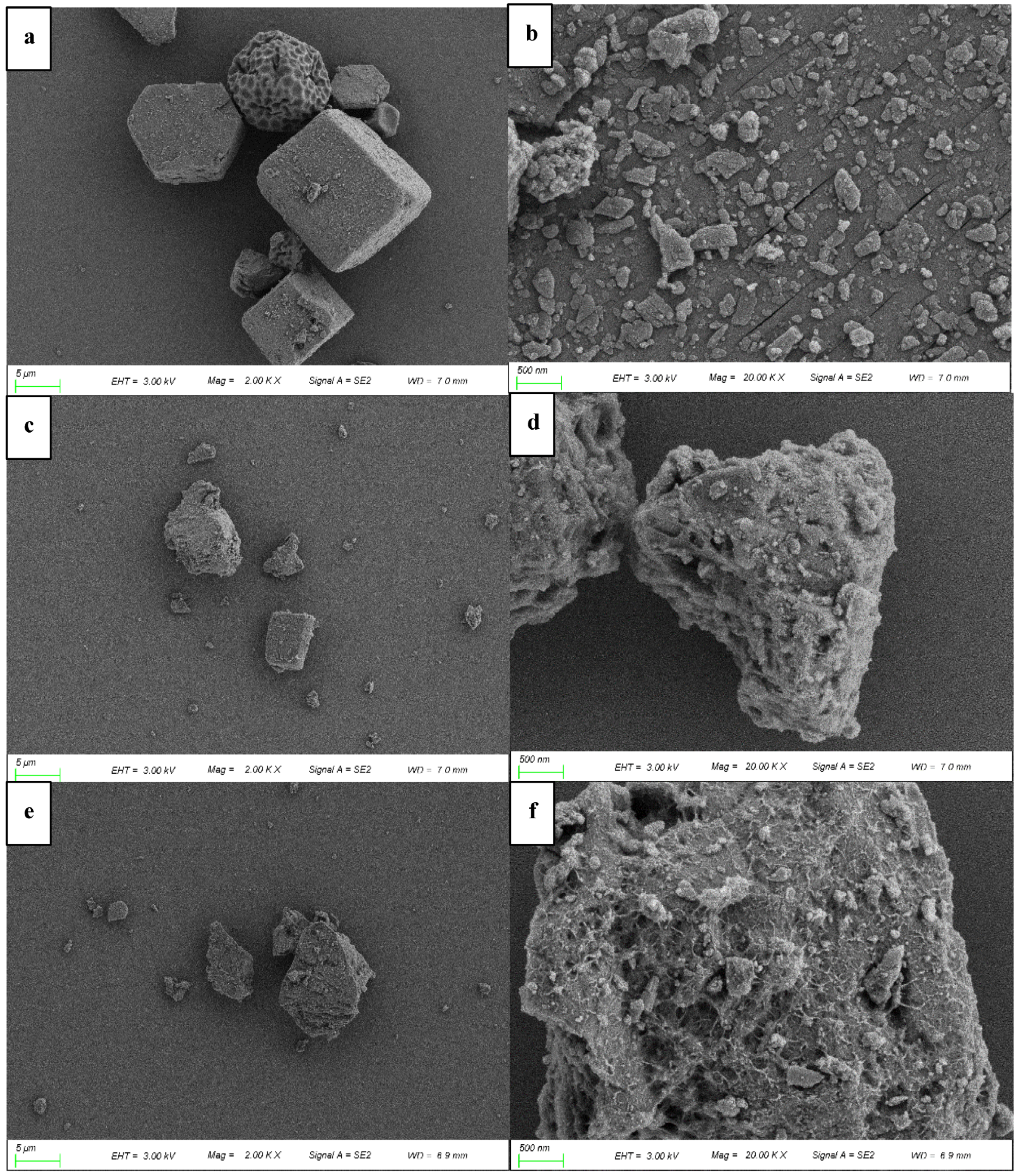
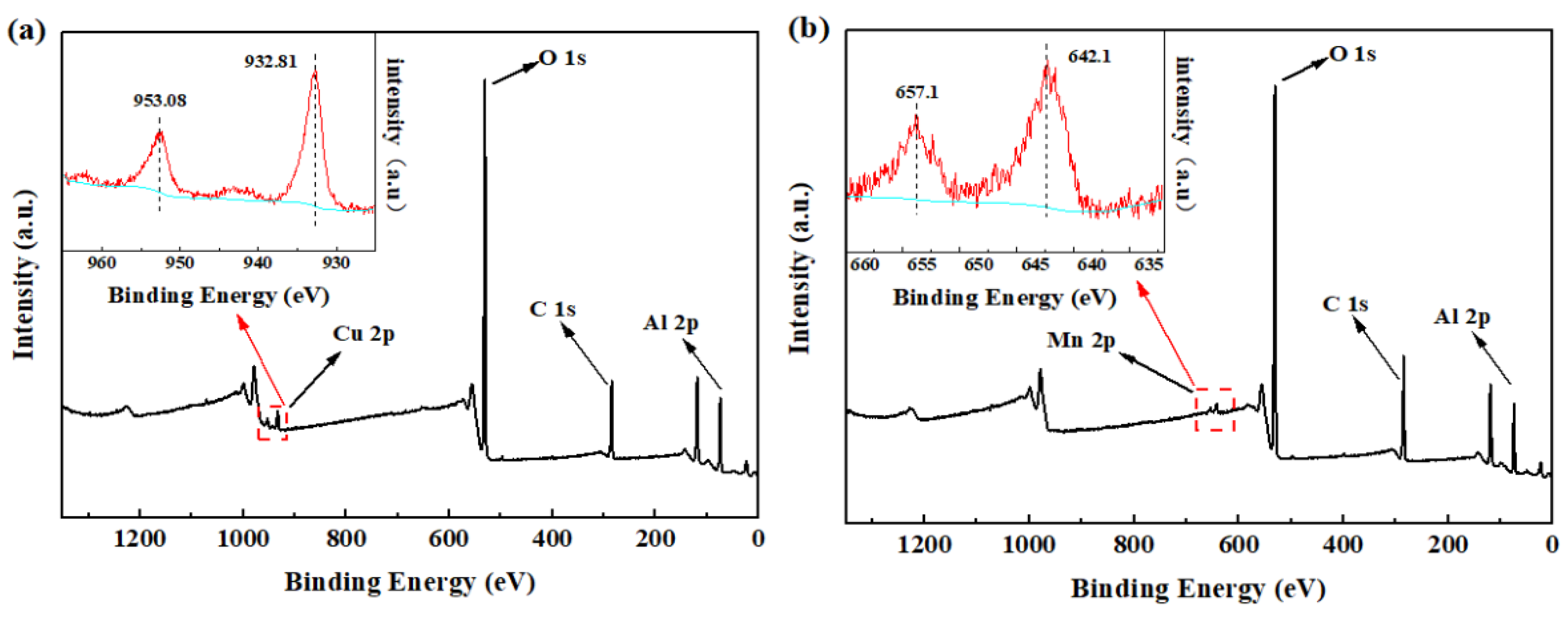
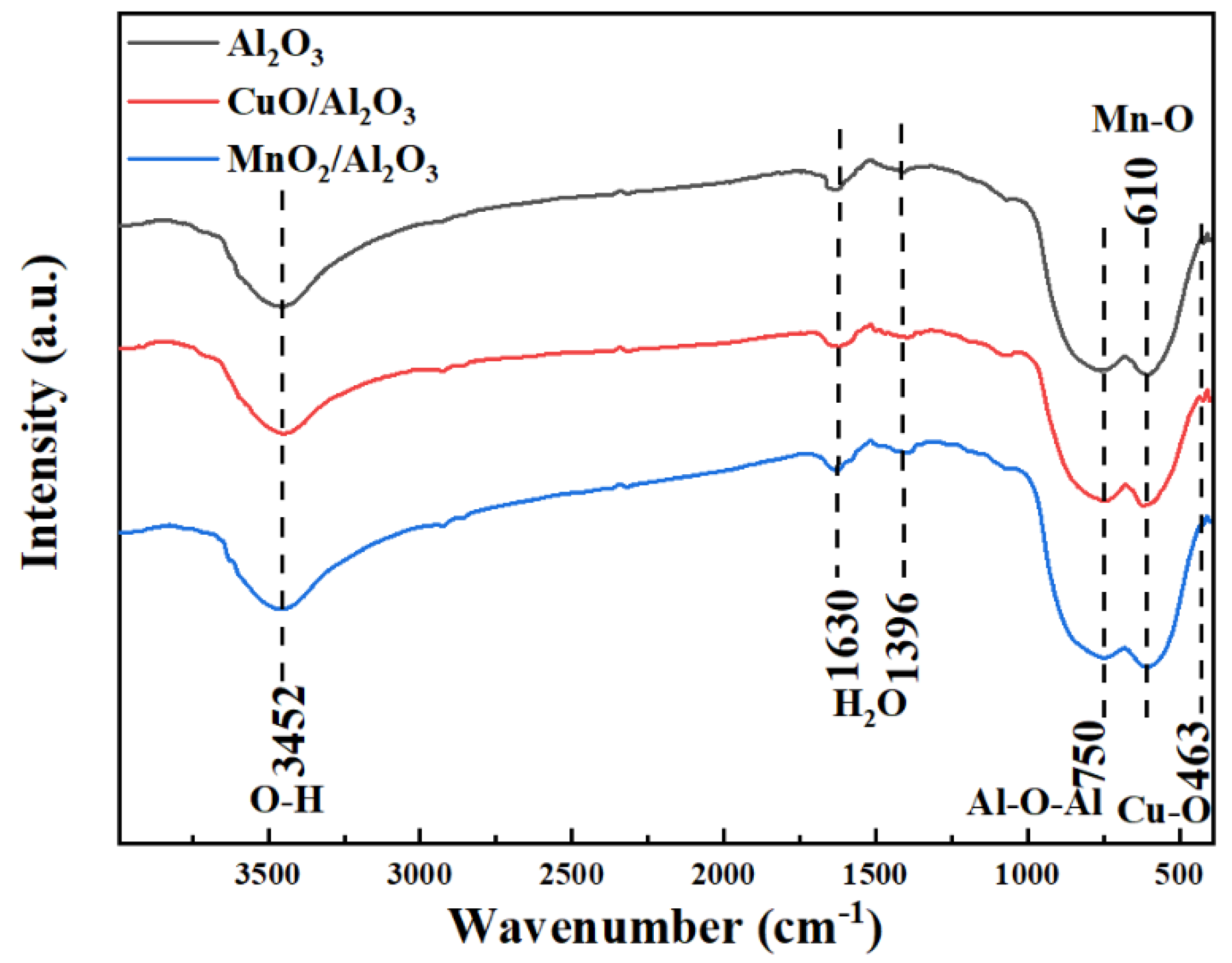
3.1. Characterization of Bimetallic Catalysts CuO/Al2O3 and MnO2/Al2O3
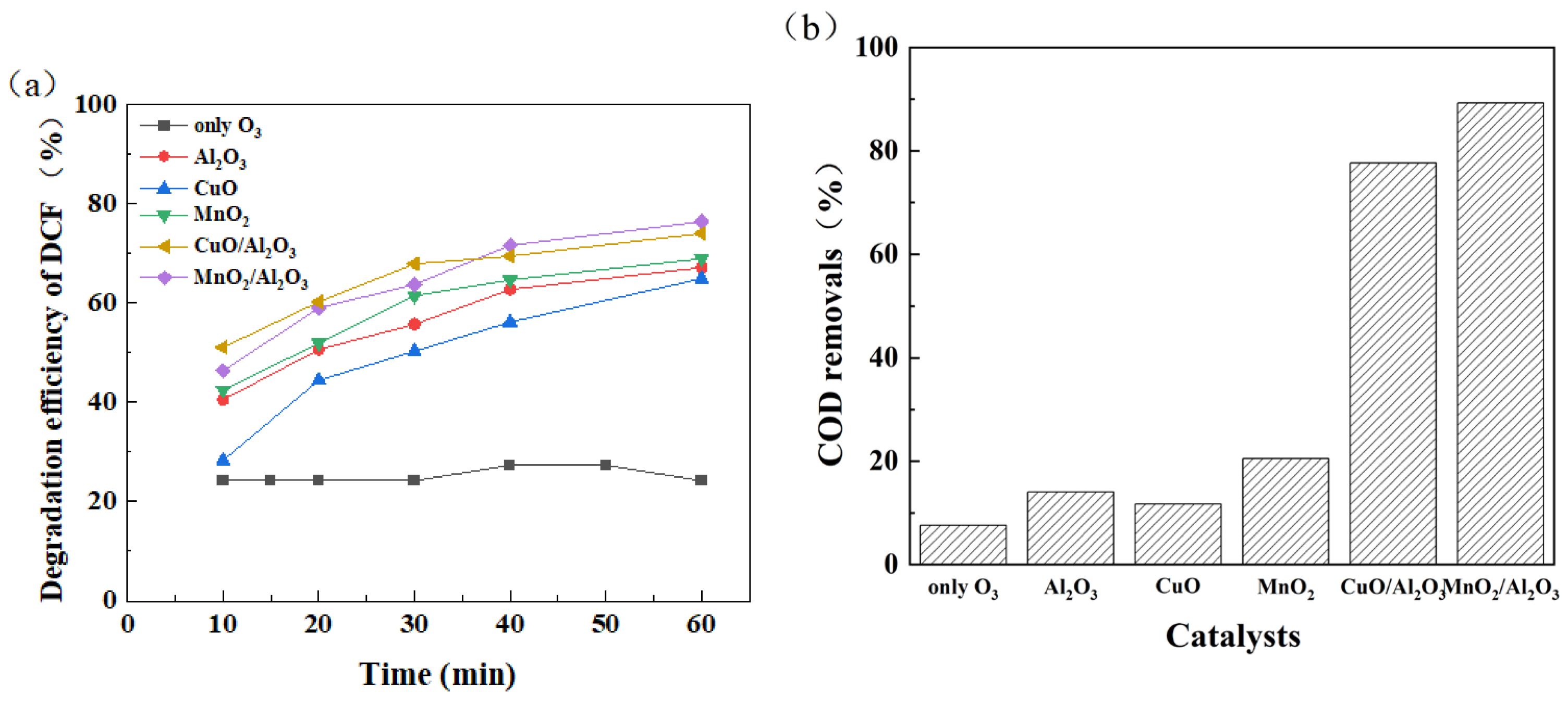
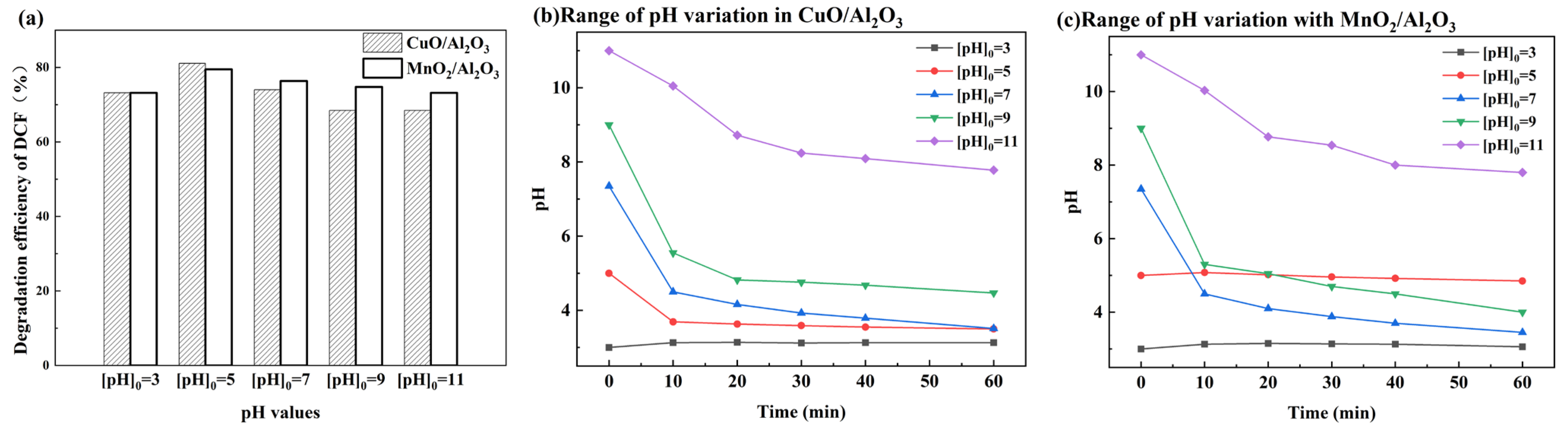
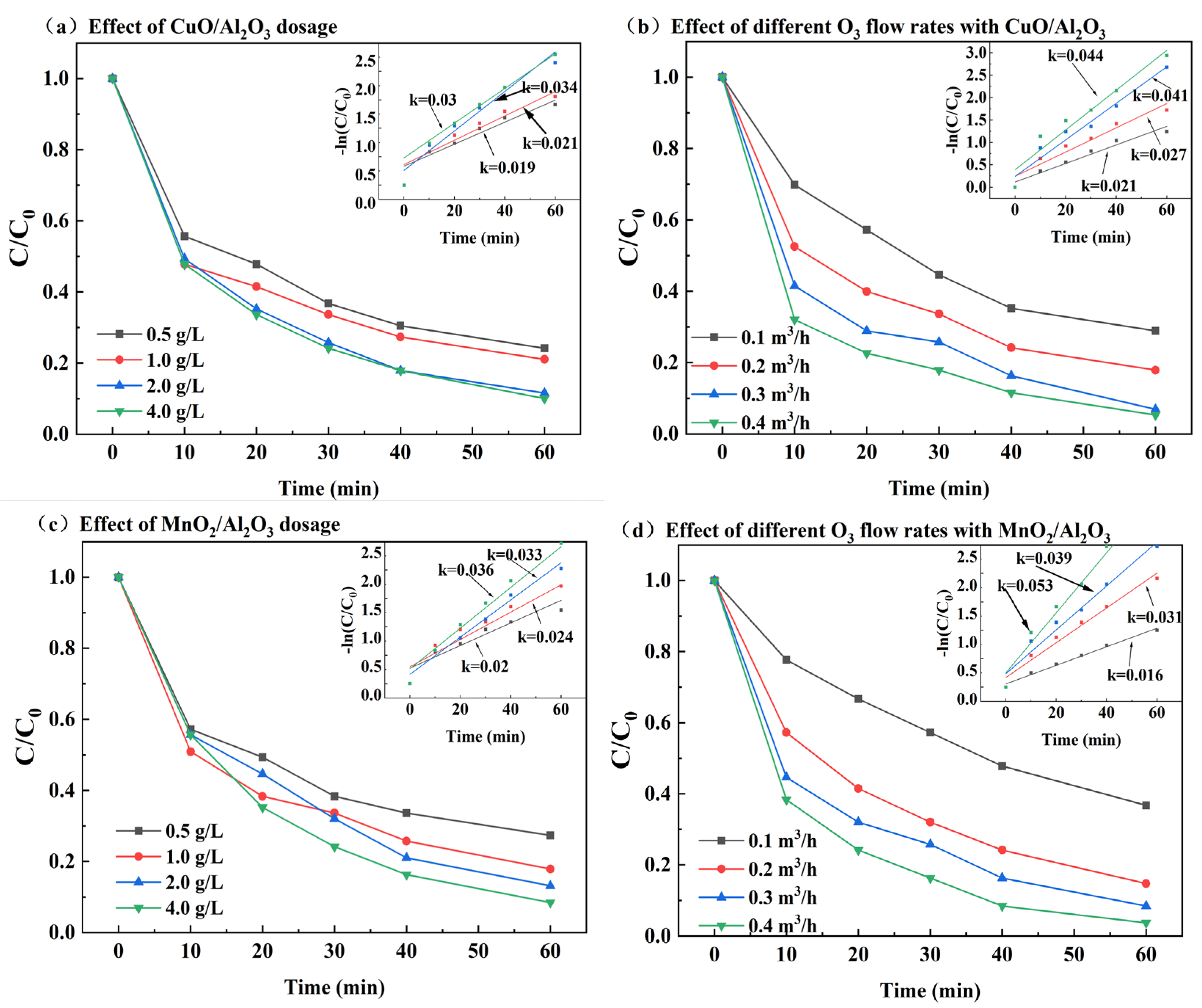
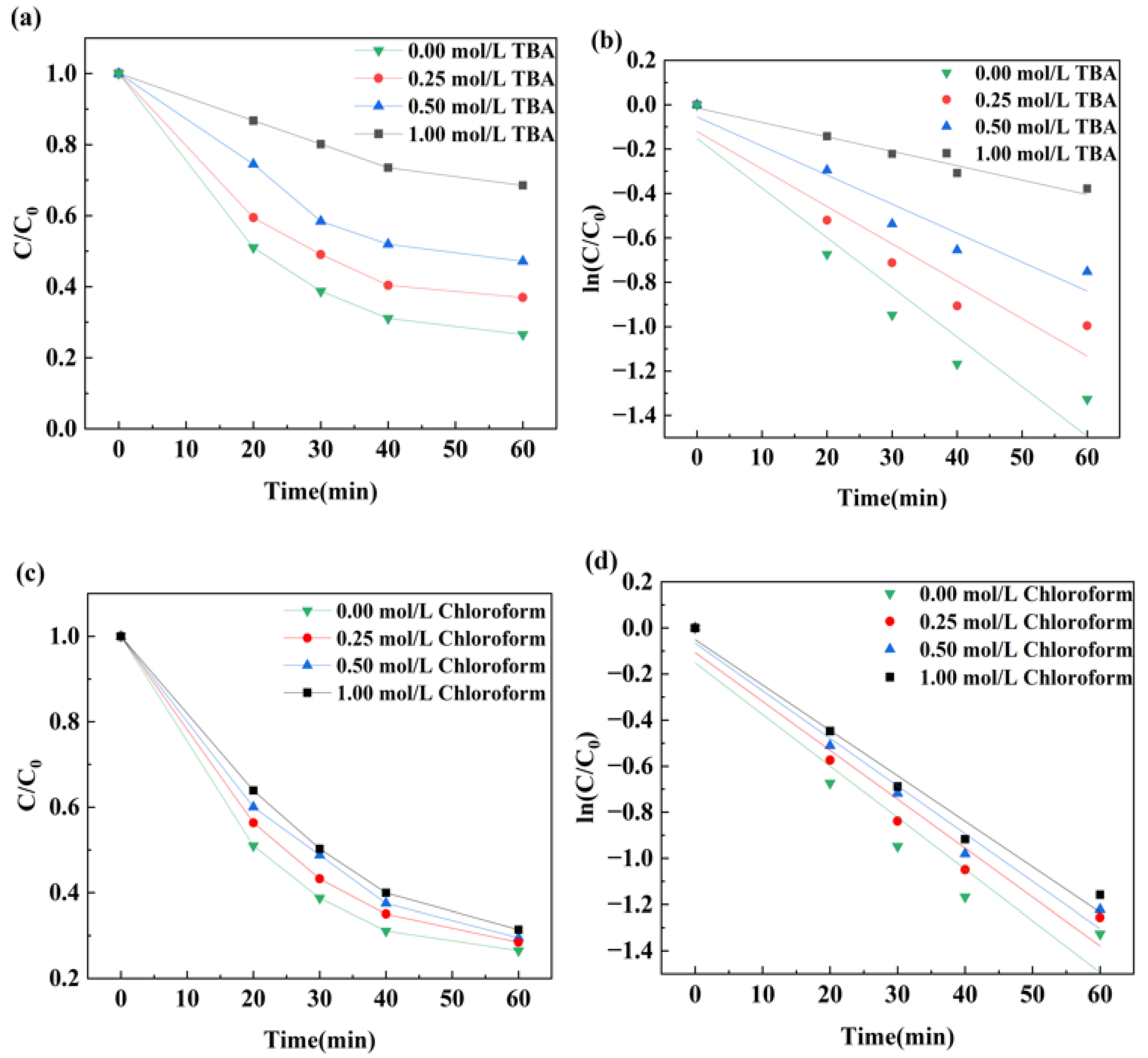
3.2. Bimetallic Catalyst Catalyzed Ozone Degradation of DCF
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xue, Y.E.; Sun, W.J.; Shao, P.H.; Yuan, Y.X.; Cui, F.Y.; Shi, W.X. Degradation of contaminants of PPCPs by photocatalysis for water purification: Kinetics, mechanisms, and cytotoxicity analysis. Chem. Eng. J. 2023, 454, 140505. [Google Scholar] [CrossRef]
- Li, S.; Lin, F.X.; Zheng, H.S.; Zheng, Y.J.; Zhang, B.G.; Ma, J.; Nan, J. Efficient PPCPs degradation by self-assembly Ag/Ti3C2@BiPO4 activated peroxydisulfate with microwave irradiation: Enhanced adsorptive binding and radical generation. Chem. Eng. J. 2023, 452, 139298. [Google Scholar] [CrossRef]
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Biruntha, M.; Govarthanan, M.; Karmegam, N. Removal of emerging micropollutants originating from pharmaceuticals and personal care products (PPCPs) in water and wastewater by advanced oxidation processes: A review. Environ. Technol. Innov. 2021, 23, 101757. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Y.X.; Chen, Y.X.; Yao, B.; Chen, X.; Yu, Y.E.; Yang, J.; Zhou, Y.Y. Pharmaceuticals and personal care products (PPCPs) in the aquatic environment: Biotoxicity, determination and electrochemical treatment. J. Clean. Prod. 2023, 388, 135923. [Google Scholar] [CrossRef]
- Yang, R.M.; Zeng, G.L.; Xu, Z.Q.; Zhou, Z.Y.; Huang, J.Y.; Fu, R.B.; Lyu, S.G. Comparison of naphthalene removal performance using H2O2, sodium percarbonate and calcium peroxide oxidants activated by ferrous ions and degradation mechanism. Chemosphere 2021, 283, 131209. [Google Scholar] [CrossRef]
- Peghin, M.; Vena, A.; Graziano, E.; Giacobbe, D.R.; Tascini, C.; Bassetti, M. Improving management and antimicrobial stewardship for bacterial and fungal infections in hospitalized patients with COVID-19. Ther. Adv. Infect. Dis. 2022, 9, 20499361221095732. [Google Scholar] [CrossRef]
- Avetta, P.; Fabbri, D.; Minella, M.; Brigante, M.; Maurino, V.; Minero, C.; Pazzi, M.; Vione, D. Assessing the phototransformation of diclofenac, clofibric acid and naproxen in surface waters: Model predictions and comparison with field data. Water Res. 2016, 105, 383–394. [Google Scholar] [CrossRef]
- Lonappan, L.; Brar, S.K.; Das, R.K.; Verma, M.; Surampalli, R.Y. Diclofenac and its transformation products: Environmental occurrence and toxicity—A review. Environ. Int. 2016, 96, 127–138. [Google Scholar] [CrossRef]
- Tian, Y.Y.; Liu, M.X.; Sang, Y.X.; Kang, C.Y.; Wang, X.H. Degradation of prometryn in Ruditapes philippinarum using ozonation: Influencing factors, degradation mechanism, pathway and toxicity assessment. Chemosphere 2020, 248, 126018. [Google Scholar] [CrossRef]
- Xiong, Z.K.; Lai, B.; Yang, P. Quantitative analysis the role of reactive oxygen species (ROS) in a Fe/Cu/O3 process for p-nitrophenol treatment. In Proceedings of the 8th International Conference on Environment Science and Engineering (ICESE), Barcelona, Spain, 11–13 March 2018. [Google Scholar]
- Qi, W.C.; Wang, J.; Quan, X.; Chen, S.; Yu, H.T. Catalytic Ozonation by Manganese, Iron and Cerium Oxides on γ-Al2O3 Pellets for the Degradation of Organic Pollutants in Continuous Fixed-Bed Reactor. Ozone Sci. Eng. 2020, 42, 136–145. [Google Scholar] [CrossRef]
- Liu, L.; Wu, N.; Ouyang, M.; Fu, M.L. Enhancement Effect Induced by the Second Metal to Promote Ozone Catalytic Oxidation of VOCs. Environ. Sci. Technol. 2024, 58, 6725–6735. [Google Scholar] [CrossRef] [PubMed]
- Ambroz, F.; Macdonald, T.J.; Martis, V.; Parkin, I.P. Evaluation of the BET Theory for the Characterization of Meso and Microporous MOFs. Small Methods 2018, 2, 1800173. [Google Scholar] [CrossRef]
- Dong, L.H.; Liu, W.J.; Jiang, R.F.; Wang, Z.S. Physicochemical and porosity characteristics of thermally regenerated activated carbon polluted with biological activated carbon process. Bioresour. Technol. 2014, 171, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Subramani, R.H.H.; Ramakrishnan, T.; Sivabalan, A.; Dhanalakshmi, V.; Nair, M.R.G.; Anbarasan, R. Ultrasound assisted one pot synthesis of nano-sized CuO and its nanocomposite with poly(vinyl alcohol). J. Mater. Sci. 2010, 45, 1688–1694. [Google Scholar] [CrossRef]
- Sivakumar, S.; Prabu, L.N. Synthesis and Characterization of α-MnO2 nanoparticles for Supercapacitor application. In Proceedings of the 12th National Web Conference on Recent Advancements in Biomedical Engineering (NCRABE), Electrical Network, Chennai, India, 3 December 2020; pp. 52–55. [Google Scholar]
- Ma, D.; Xue, Q.; Liu, Y.; Liang, F.; Li, W.; Liu, T.; Zhuang, C.; Zhao, Z.; Li, S. Manipulating interfacial charge redistribution in Mn0.5Cd0.5S/N-rich C3N5 S-scheme heterojunction for high-performance photocatalytic removal of emerging contaminants. J. Mater. Sci. Technol. 2025, 243, 265–274. [Google Scholar] [CrossRef]
- Chen, W.R.; Li, X.K.; Tang, Y.M.; Zhou, J.L.; Wu, D.; Wu, Y.; Li, L.S. Mechanism insight of pollutant degradation and bromate inhibition by Fe-Cu-MCM-41 catalyzed ozonation. J. Hazard. Mater. 2018, 346, 226–233. [Google Scholar] [CrossRef]
- Tong, X.Y.; Li, Z.D.; Chen, W.R.; Wang, J.; Li, X.K.; Mu, J.X.; Tang, Y.M.; Li, L.S. Efficient catalytic ozonation of diclofenac by three-dimensional iron (Fe)-doped SBA-16 mesoporous structures. J. Colloid Interface Sci. 2020, 578, 461–470. [Google Scholar] [CrossRef]
- Zhu, G.X.; Zhu, J.G.; Jiang, W.J.; Zhang, Z.J.; Wang, J.; Zhu, Y.F.; Zhang, Q.F. Surface oxygen vacancy induced α-MnO2 nanofiber for highly efficient ozone elimination. Appl. Catal. B Environ. 2017, 209, 729–737. [Google Scholar] [CrossRef]
- Meng, Y.T.; Song, W.Q.; Huang, H.; Ren, Z.; Chen, S.Y.; Suib, S.L. Structure-Property Relationship of Bifunctional MnO2 Nanostructures: Highly Efficient, Ultra-Stable Electrochemical Water Oxidation and Oxygen Reduction Reaction Catalysts Identified in Alkaline Media. J. Am. Chem. Soc. 2014, 136, 11452–11464. [Google Scholar] [CrossRef]
- Yin, M.M.; Miao, H.; Hu, R.G.; Sun, Z.X.; Li, H. Manganese dioxides for oxygen electrocatalysis in energy conversion and storage systems over full pH range. J. Power Sources 2021, 494, 229779. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical-Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen-Atoms and Hydroxyl Radicals (.OH/.O-) in Aqueous-Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate Constants for Reactions of Inorganic Radicals in Aqueous-Solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
| CATALYSTS. | BET Surface Area | Average Pore Width | Pore Volume |
|---|---|---|---|
| m2/g | nm | cm3/g | |
| Al2O3 | 156 | 5.47 | 0.22 |
| CuO/Al2O3 | 158 | 5.37 | 0.21 |
| MnO2/Al2O3 | 157 | 5.33 | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Wang, X.; Xu, Y.; Xu, Q.; Shen, Z.; Qiao, J. Catalytic Ozonation of Diclofenac Using CuO/Al2O3- and MnO2/Al2O3-Supported Catalysts. Chemistry 2025, 7, 107. https://doi.org/10.3390/chemistry7040107
Zhou W, Wang X, Xu Y, Xu Q, Shen Z, Qiao J. Catalytic Ozonation of Diclofenac Using CuO/Al2O3- and MnO2/Al2O3-Supported Catalysts. Chemistry. 2025; 7(4):107. https://doi.org/10.3390/chemistry7040107
Chicago/Turabian StyleZhou, Wenli, Xiaoxia Wang, Yanghong Xu, Qingsong Xu, Zheng Shen, and Junlian Qiao. 2025. "Catalytic Ozonation of Diclofenac Using CuO/Al2O3- and MnO2/Al2O3-Supported Catalysts" Chemistry 7, no. 4: 107. https://doi.org/10.3390/chemistry7040107
APA StyleZhou, W., Wang, X., Xu, Y., Xu, Q., Shen, Z., & Qiao, J. (2025). Catalytic Ozonation of Diclofenac Using CuO/Al2O3- and MnO2/Al2O3-Supported Catalysts. Chemistry, 7(4), 107. https://doi.org/10.3390/chemistry7040107





