Abstract
Pyrazolopyridines and pyrazolopyrimidines are 5:6 aza-fused N-heteroaromatic compounds (NHACs) comprising a pyrazole ring fused to a pyridine or pyrimidine ring. They exhibit dipolar behavior due to their π-excessive and π-deficient characteristics conferred by their five- and six-membered rings. These features favor their stability, reactivity, and structural diversity, offering numerous modular and functional derivatives (e.g., pyrazolo[1,2-a]pyridines, pyrazolo[1,5-a]pyrimidines, etc.). They have been utilized to obtain relevant chemicals in pharmaceuticals, photophysics, industry, and materials science; thus, their synthesis is highly desirable for discovering novel or improved applications. Therefore, this review focuses on recent advances in the synthesis and applications of these compounds, considering reports from the last decade (2015–2024), with particular emphasis on photophysics, as they contain dipolar 5:6 aza-fused rings as essential scaffolds for this purpose.
1. Introduction
N-heteroaromatic compounds (NHACs) address numerous societal and environmental needs due to their stability and recurrence, as heteroatoms confer biological relevance and structural and synthetic versatility; thus, the chemistry and properties of this compound class have been studied for several decades. They usually bear five- or six-membered rings (5-MRs or 6-MRs) of π-excessive or π-deficient nature, respectively, which is ruled by the two types of nitrogen atoms they possess: one pyrrole-like (–NR–, donor) and the other pyridine-like (=N–, acceptor), allowing for relevant applications [1,2,3,4,5,6,7,8]. Pyrazoles (five-membered rings) or pyridines and pyrimidines (six-membered rings) are among the most distinctive NHACs, exhibiting diverse combinations, as there are various functionalized derivatives and some are based on 5:5, 5:6, or 6:6 aza-fused rings [9,10,11,12,13,14,15,16,17] (Figure 1).
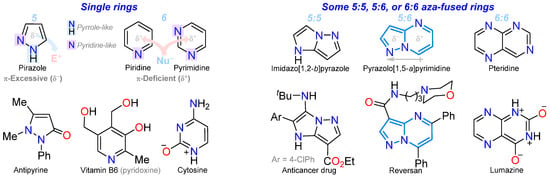
Figure 1.
Structure of pyrazole, pyridine, pyrimidine, their fused derivatives, and some biologically relevant derivatives.
Remarkably, 5:6 aza-fused motifs such as pyrazolopyridines and pyrazolopyrimidines offer several derivatives with diverse substitution and noticeable electronic properties, e.g., pyrazolo[1,5-a]pyridines, pyrazolo[3,4-b]pyridines, pyrazolo[4,3-c]pyridines, pyrazolo[1,5-a]pyrimidines, pyrazolo[1,5-c]pyrimidines, and pyrazolo[3,4-d]pyrimidines. These compounds contain an N-heteroaromatic core with a planar and rigid structure, exhibiting pertinent dipolar behavior due to the fusion and electronic nature of the separate rings, namely the five- (π-excessive) and six-membered (π-deficient) rings. These characteristics favor both their synthetic and supramolecular properties, which are relevant for the applications of their derivatives, considering the dual reactivity (i.e., with electrophiles and nucleophiles) and dipolar nature of these 5:6 aza-fused rings. The last property was analyzed by density functional theory (DFT) calculations (i.e., the pyrazolo[1,5-a]pyrimidine ring by us [18]) and is crucial in photophysics; for example, several derivatives are sensitive to the environment, generating supramolecular structures that can be stabilized or destabilized both in the excited state and the ground state [16,17,18,19,20] (Figure 2).
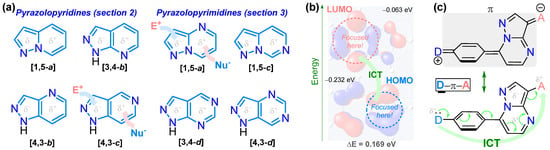
Figure 2.
(a) 5:6 Aza-fused rings of interest in this work. (b) HOMO, LUMO, and (c) ICT processes in pyrazolo[1,5-a]pyrimidines.
The dipolarity of pyrazolopyridine and pyrazolopyrimidine rings confers exceptional supramolecular properties to their derivatives in biological and photophysical fields, explicitly when they bear donor–π–acceptor (D–π–A or push-pull) molecular architecture. However, unlike their applicability in the biological field, their photophysics have been poorly explored, considering the noticeable results achieved in various derivatives and microenvironments (e.g., solvatofluorochromism, chemodetection, bioimaging, etc.) via typical intramolecular charge transfer (ICT) phenomena, for example, using pyrazolo[1,5-a]pyrimidines (Figure 2c). Indeed, this is the primary motivation for conducting this review, as it is one line of research pursued by our group, and, to date, there have been few examples involving the development of fluorophores based on 5:6 aza-fused rings, though there are examples worth analyzing (Figure 3) [6,21,22,23,24]. These scaffolds possess structural features that favor their performance in organic, inorganic (N-donor ligands), medicinal, and physical chemistry, mainly in drug discovery, biological routes, and materials science [18,21,22,23,24,25,26,27]. Pyrazolo[1,5-a]pyridine, pyrazolo[3,4-b]pyridine, and pyrazolo[1,5-a]pyrimidine are the most commonly used 5:6 aza-fused rings in photophysics, and most of the studies presented herein are focused on them.
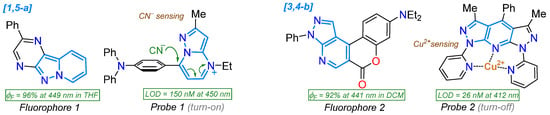
Figure 3.
Photophysically relevant molecules based on pyrazolopyridines and pyrazolopyrimidines.
Developing small-molecule organic fluorophores based on NHACs is a common research objective, as these compounds exhibit exceptional photophysical properties that facilitate diverse applications in materials science and detection chemistry. In addition, they possess crucial synthetically modulable structural and electronic properties, governed by their π-conjugation and the electronic nature of the heteroatom as a donor (D) or acceptor (A) center [28,29,30,31,32] (Figure 3). Thus, synthesizing pyrazolopyridines and pyrazolopyrimidines is highly desirable for discovering novel applications. Access to functionalized derivatives is achieved through ring construction from strategically selected substrates or successive functionalizations. The cyclization protocols for accessing the ring focus on forming the pyrazole on the pyridine or pyrimidine ring or generating the 6-MRs on the pyrazole moiety, typically through cyclocondensation reactions, and to a lesser extent, through 1,3-dipolar or aza-Diels–Alder cycloaddition reactions (Scheme 1) [18,21,22,23,24,25,26,27].
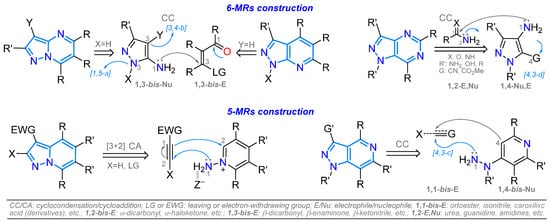
Scheme 1.
General strategies for synthesizing pyrazolopyridines and pyrazolopyrimidines.
Pyrazole (1,2-diazole), the central ring in this contribution, has two adjacent nitrogen atoms and high biological and synthetic relevance, despite naturally occurring pyrazole-based compounds being relatively scarce. Some derivatives, including fused systems, have exhibited antimicrobial, anticancer, anti-inflammatory, analgesic, and agrochemical properties [33], while others have been used as fluorescent probes [34] and energetic materials for the development of explosives [35]. Pyrazole derivatives exhibit high synthetic flexibility for delivering crucial chemicals with diverse applications [33,34,35,36,37,38]. In particular, 5:6 aza-fused motifs with π-deficient rings such as pyridine (e.g., fusion [1,5-a], [3,4-b], etc.) or pyrimidine (e.g., fusion [1,5-a], [3,4-d], etc.) are common and have noticeable features due to their previously investigated dipolar electronic nature. However, pyrazolo[1,5-a]pyridines, pyrazolo[1,5-a]pyrimidines, and pyrazolo[3,4-b]pyridine are the most common motifs in different fields; [1,5-a] fusion has a pyrrole-like nitrogen atom as a bridge, conferring optimal aromatic characteristics to the pyrazole moiety (Figure 2 and Figure 4a) [18,27,39,40,41,42,43].
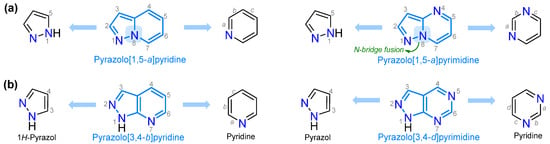
Figure 4.
Examples of forming pyrazoles fused to pyridine or pyrimidine with (a) N-bridge fusion, and (b) [3,4-b]- and [3,4-d]-type fusion.
Considering the numerous reports on the synthesis of pyrazolopyridines and pyrazolopyrimidines, it is challenging to cover the most relevant examples herein. However, strategic and representative works were selected chronologically, encompassing examples from the ten-year period observed. For instance, in addition to the Introduction (1.), the manuscript is organized into two sections, covering Pyrazolopyridines (2.) and Pyrazolopyrimidines (3.). Each section includes two to three papers selected annually according to the ring, resulting in 52 studies being analyzed (Figure 2). These sections include studies on synthesis and photophysics, highlighting key aspects in this regard—i.e., cyclization methods, functionalizations, optical applications, and solvatofluorochromism. Due to the prevalence of pyrazolo[1,5-a]pyridines, pyrazolo[3,4-b]pyridines, and pyrazolo[1,5-a]pyrimidines in photophysics, examples of compounds that are not photophysically relevant should be included. However, many of the selected examples are biologically active motifs, and this crucial approach is also highlighted; likewise, we propose to display most of the combinations found regarding the corresponding fused pyrazole. Thus, the studies for each year were selected indiscriminately to complete the timeline of studies covering most rings.
2. Pyrazolopyridines
Below are some general properties of pyrazolopyridines, whose chemistry and biological effects have been well-documented for many derivatives [43,44,45,46,47,48,49,50,51]. Their general synthetic approaches are established in Scheme 1. For instance, pyrazolo[1,5-a]pyridines have garnered significant attention due to their anti-inflammatory, antitumor, antimicrobial, antifungal, and antiviral properties; some drugs, such as the p8 kinase inhibitor and the adenosine receptor antagonist, exemplify this structure [40,52]. Likewise, pyrazolo[3,4-b]pyridines have been extensively studied for their diverse biological activities; an example is found in the anxiolytic drug Tracazolate and the analgesic N-acylhydrazone [41,53]. In addition, pyrazolo[4,3-c]pyridines have been reported for their anti-HIV-1 activity [54] or inhibitory activity against CK1d induced by 3-aminoderivatives [55], and pyrazolo[4,3-b]pyridines have been reported as having PD-1/PD-L1 inhibitory activity [45] (Figure 5).
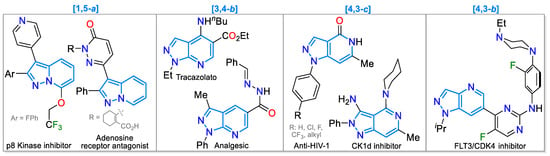
Figure 5.
Examples of drugs based on pyrazolopyridine derivatives.
On the other hand, the electronic properties and luminescent nature stand out in some pyrazolopyridines [38,56,57,58], enabling their use as organic fluorophores in detection chemistry or for probing lipid droplets [59]. They have been used for cellular bioimaging [60,61], cyanide ion detection [62], recognition of sulfite ions in dry white wine [56], organic optoelectronics [38], the fluorimetric detection of BF3 with applications in bioimaging [63], and chemosensors for the detection of Cu+2 [24], among others (Figure 3 and Figure 6). As a result, the combination of pyrazole and pyridine rings is valuable in discovering and researching new bioactive compounds and molecular systems with potential in photophysics. Notably, the biological field is well-documented, with several specialized reviews [18,27,43,64,65,66,67,68]; however, the photophysical aspect is emerging, and there are no contributions that gather relevant information on any pyrazolopyridine derivative, considering dipolar 5:6 aza-fused rings. Thus, we hope this review may help several researchers propose novel investigations on organic fluorophores based on pyrazoles.

Figure 6.
Pyrazolopyridine derivatives with photophysical properties.
We begin the discussion by considering a 2015 study by Wang et al. [58], who reported the synthesis of 5-cyanopyrazolo[1,5-a]pyridines 3a–u in yields of up to 78%, which have high biological and photophysical potential, as the authors highlight. Indeed, the 2,4-disubstituted dyes 4f–u are highly fluorescent in both solution and solid state (Scheme 2a). The synthesis of compounds 3a–e proceeds via the cyclocondensation reaction of 3(5)-formylpyrazole 2a–e with 4-bromobutenenitrile (3), which form the six-membered ring; however, 1a–e also lead to ketones 4f–u to similarly give rise to the 4-(hetero)aryl-substituted dyes 4f–u in poor yields due to steric effects through intermediates 5 and 5′. Notably, unlike the R group at position 4, the aryl substituent at position 2 in products has minimal impact on absorption and emission spectra. These dyes exhibited redshifted emission as the solvent polarity increased—fluorescence quantum yields (ɸF) of 10% to 95%—and showed intense emission in the powdered state, making them suitable for use as optoelectronic materials. Specifically, dyes with Ar = 4-FC6H4 and 4-ClC6H4 showed relevant results when R = Ph (blue fluorescence at 462 and 464 nm) or 2-thienyl (blue-green at 486 and 495 nm) due to the steric congestion in their trisubstituted rings [58] (Scheme 2a; Table 1, Entry 1).
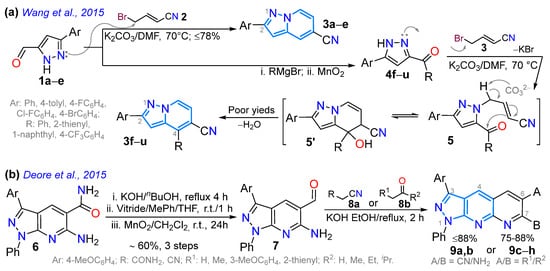
Scheme 2.
Synthesis of optically relevant (a) 5-cyanopyrazolo[1,5-a]pyridines and (b) pyrido-pyrazolo[3,4-b]pyridines [58,69].
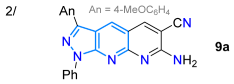
That same year, Deore’s group reported the 5-formylpyrazolo[3,4-b]pyridines 7 as precursors for synthesizing the pyrido-fused derivatives 9a–h in high yields via the Friedlander condensation reactions with active methylene reagents 8a (nitrile) or 8b (ketone) and KOH under reflux in EtOH. Heteroaldehyde 7 was obtained by hydrolysis under reflux in an n-butanol solution of the 1,2-aminocarboxamide 6, with later reduction using Vitride, and, finally, mild oxidation of the respective intermediate primary alcohol using MnO2 [69]. Through photophysical studies, they observed that the absorption and emission intensity of these dyes increased with electron-donating groups (EDGs) as substituents at positions 6 and 7 of the tricyclic core, improving absorption and fluorescence in the ranges of 467–476 nm and 545–559 nm, respectively, with ΦF values in the range of 30–34%; however, the results decreased with electro-withdrawing groups (EWGs), exhibiting absorption in the range of 441–444 nm and fluorescence maxima at 513–516 nm, with ɸF values of up to 29%. Notably, these dyes emitted high levels of blue, yellow, and green fluorescence, indicating potential applications in the manufacturing of optoelectronic devices (Scheme 2b; Table 1, Entry 2).
Grošelj et al. [52] reported the 1,5,7-trisubstituted pyrazolo[4,3-c]pyridine derivatives 14 as having no photophysical relevance, but it was worth showing how this ring type can be accessed, precisely through a pyrazole diester 10a,b, which was treated with t-butoxy-bis(dimethylamino)methane (TBDMAM) and then primary alkylamines to give rise to enaminoesters 11a,b in good yields. Subsequently, 11a,b underwent intramolecular cyclization to become dihydropyrazolo[4,3-c]pyridine esters 12a,b under standard conditions (tBuOK or DBU), whose later hydrolysis or reduction resulted in the corresponding carboxylic acids 13a,b. Ultimately, acids 13a,b were activated with bis(pentafluorophenyl)carbonate (BPC) and Et3N (1 equiv), and the desired amine was added to form the final amides 14a–j in good yields (Scheme 3a).
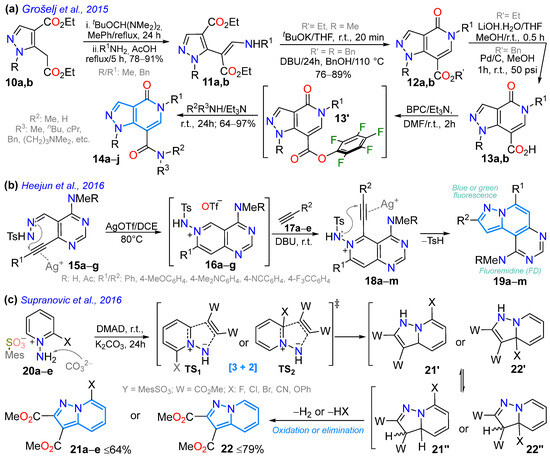
Scheme 3.
Synthesis of (a) pyrazolo[4,3-c]pyridines 14a–j and pyrazolo[1,5-a]pyridines (b) 19a–m and (c) 21a–e or 22 [52,59,70].
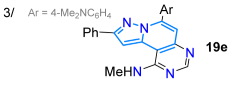
A year later, Heejum et al. [59] investigated various pyrazolo[1,5-a]pyridines bearing the fluoremidine (FD) scaffold in moderate yields through Ag-promoted domino cyclization, implying pyrazole ring formation from the pyrimidin-3-yl-p-tosylhydrazone 15a–g. First, this led to the 6-endocyclization of 15a–g into bicyclic pyridinium 16a–g, which was attacked by the terminal alkynes 17a–e to give rise to the intermediates 18a–m, which, finally, were subjected to 5-endocyclization to become 19a–m through aromatization with p-tosyl group elimination. Photophysical studies of these dyes have shown that the N,N-dimethylamino group at position 6 favors higher ΦF values, from 12% to 73%, and double the molar absorptivity coefficient (ε); the same occurred with this group at position 9; thus, the authors suggest that the significant redshift in the emission wavelength could be due to the ICT between these substituents and the fused ring (Scheme 3b; Table 1, Entry 3). They also found that an N-acetyl group at position 1 promotes redshifted emissions and that dyes exhibit excellent solvatochromism with active emission in living cells. In the same year, 2016, Supranovich et al. [70] reported synthesizing the 7-substituted pyrazolo[1,5-a]pyridines 21a–e (via the ST1) and the unsubstituted derivative 22 (via ST1), employing the [3 + 2] cycloaddition of the 2-substituted pyridinium-N-imines 20a–e with dimethyl acetylenedicarboxylate (DMAD). The authors observed that reactions with 1,3-dipoles containing EDGs offer higher yields, and polar solvents favor elimination products, resulting in a 30% increase (Scheme 3c).
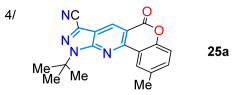
In 2017, Miliutina et al. [71] synthesized the chromeno-fused pyrazolo[3,4-b]pyridines 25a–k in high yields using a cyclocondensation reaction of the chromen-4-one-3-carboxylic acids 23a–f with the N-substituted 5-amino-3-methylpyrazoles 24a–c in acetic acid. The reaction started with conjugate addition of the enamine group of 24 (acting as a 1,3-bis-nucleophile) to the double C=C bond, which were then transferred into the pyranone ring of 23 (1,3-bis-electrophilic substrate), and it proceeded with the opening of this ring and later lactonization between the phenol and acid groups of the open intermediate (via 25′). Ultimately, the condensation between amino and carbonyl groups gave rise to the fluorescent tetracyclic derivatives 25a–k, which exhibited ɸF values ranging from 6.9% to 14.9%. The authors found that the diverse substitution of the pyrazole ring changed the photophysical results (Scheme 4a; Table 1, Entry 4). In the same year, Charris-Molina et al. [72] regioselectively synthesized fully substituted pyrazolo[3,4-b]pyridines 27a–j using the reaction between the N-substituted 5-aminopyrazoles 24a–j and the 3-(3-oxo-2-benzofuran-1(3H)-ylidene)pentane-2,4-dione (26). This reaction resulted in good yields under microwave (MW) conditions, resulting in furane ring opening (by 25′) to deliver rings functionalized with acetyl and carboxyl groups (Scheme 4b).
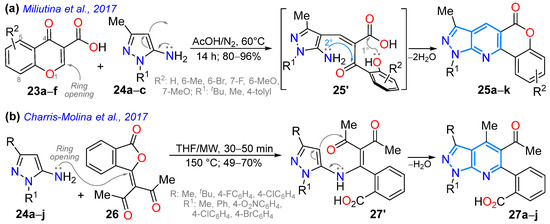
Scheme 4.
Synthesis of (a) fluorescent chromeno-fused 25a–k and (b) highly functionalized 27a–j pyrazolo[4,3-c]pyridines [71,72].
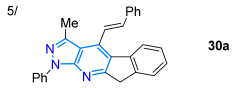
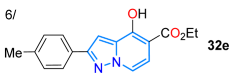
In 2018, Manickam et al. [73] obtained highly conjugated indeno-fused dyes 30a–e in high yields, through 4-styrylpyrazolo[3,4-b]pyridines, using a SnCl2-mediated three-component reaction of 24a, 2-indenone (28), and cinnamaldehydes 29a–e. These dyes exhibited charge transfer (CT) absorption bands at around 360 nm, which were redshifted for derivatives bearing EDGs (MeO and NMe2) versus those with EWGs (F and NO2). The emission maxima of 30a–e in chloroform are distributed in the range of 430–620 nm, with a redshift of the order of substituents NO2 < MeO < F < H < NMe2, indicating a redistribution of electron density in the excited state. Due to its high electron-donating characteristic, the dimethylamino group confers the highest ε and ɸF values to the dye, meaning that it exhibits a positive solvatofluorochromism (redshift) by increasing solvent polarity due to ICT phenomena (Scheme 5a; Table 1, Entry 5). This dye also shows positive acidochromism in chloroform (by absorption and emission) upon TFA addition due to protonation of the pyridine ring nitrogen atom. In the same year, Yan et al. [60] reported dyes 32a–f derived from 4-hydroxypyrazolo[1,5-a]pyridines obtained via the K2CO3-mediated cyclization reaction of the pyrazole esters 1a–f with the bromoalkenyl ester 31; these dyes exhibited UV-vis absorption in the range of 300–380 nm and fluorescence maxima at around 415, with ΦF values ranging from 17 to 60% (Scheme 5b; Table 1, Entry 6).
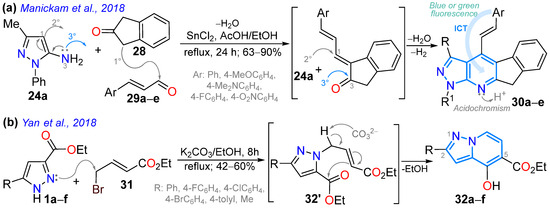
Scheme 5.
Synthesis of fluorescent (a) 4-styrylindenopyrazolo[3,4-b]pyridines 30 and (b) 4-hydroxypyrazolo[1,5-a]pyridines 32 [60,73].
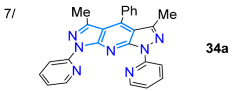
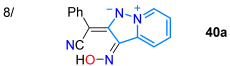
In 2019, Garcia et al. [24] reported obtaining the 4-aryl-1,7-di(2-pyridyl)bispyrazolo[3,4-b:4′,3′-e]pyridines 34a–e in high yields via the MW-assisted reaction of 5-amino-3-methyl-N-(2-pyridyl)pyrazole (24b, 2 equiv) with arylaldehydes 33a–e (1 equiv); three of these dyes (Ar: Ph, 4-MeOC6H4, 4-Py) were photophysically studied to establish their potential as tridentate ligands for metal ion detection. These dyes exhibited large Stokes shifts (9623–13,700 cm−1), strong blue light emission with ΦF values of up to 88% due to twisted ICT (TICT) phenomena, and a low tendency toward solvatofluorochromism; notably, the 4-phenyl derivative was used to detect Cu2+ with an LOD of 26 nM through a “turn-off” fluorescence process (Scheme 6a; Table 1, Entry 7). On the other hand, Agheli et al. [57] prepared the pyrazolo[1,5-a]pyridines 40a–c via pyrazole ring formation using the [3 + 2] cycloaddition of 1-aminopyridinium iodine (35) with ethyl propiolate (36) in a standard medium containing 37; this ester was hydrolyzed, decarboxylated, and nitrated to produce 3-nitropyrazolo[1,5-a]pyridine (38), which then reacted with arylacetonitriles 39a–c, also under standard conditions, forming the desired products. These dyes exhibited modest redshift in polar solvents, with absorption bands at ~315 nm (sharp peak) and ~525 nm (broad band), attributed to π-π* and CT transitions (OH → CN), respectively (Scheme 6b; Entry 8).
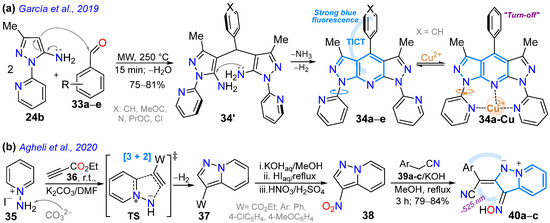
Scheme 6.
Synthesis of fluorescent (a) bispyrazolo[3,4-b:4′,3′-e]pyridines 34a–e and (b) conjugated pyrazolo[1,5-a]pyridines 40a–c [24,57].

Table 1.
Summary of some synthetic and photophysical data for representative pyrazolopyridine-based dyes.
Table 1.
Summary of some synthetic and photophysical data for representative pyrazolopyridine-based dyes.
| Entry/Fluorophore | Yield (%) | labs (nm) | lem (nm) | ΦF (%)/Solvent | Ref. |
|---|---|---|---|---|---|
 | 73 | 270 | 440 | 49/DCM | Wang et al., 2015 [58] Scheme 2a |
 | 86 | 462 | 541 | 34/DMSO | Deore et al., 2015 [69] Scheme 2b |
 | 62 | 367 | 452 | 73/DCM | Heejum et al., 2016 [59] Scheme 3b |
 | 70 | 277 | 372 | 15/MeCy | Miliutina et al., 2017 [71] Scheme 4a |
 | 87 | 365 | 440 | 25/CDCl3 | Manickam et al., 2018 [73] Scheme 5a |
 | 48 | 267 | 418 | 61/DCM | Yan et al., 2018 [60] Scheme 5b |
 | 75 | 250 | 412 | 88/DMSO | García et al., 2019 [24] Scheme 6a |
 | 79 | 352 | 530 | -- | Agheli et al., 2020 [57] Scheme 6b |
 | 70 | 330 | 465 | 34/CDCl3 | Alizadeh et al., 2020 [74] Scheme 7a |
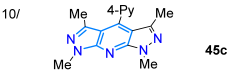 | 52 | 321 | 446 | 82/CDCl3 | Ma et al., 2020 [75] Scheme 7b |
 | 77 | 357 | 499 | -- | Ma et al., 2021 [56] Scheme 8a |
 | 50 | 261 | 463 | 75/THF | Razmiené et al., 2021 [55] Scheme 8b |
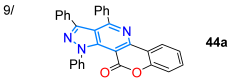
Alizadeh’s work in 2020 [74] described the one-pot synthesis of fluorescent chromeno-fused pyrazolo[3,4-d]pyridines 44a–h in high yields through [3 + 2] cycloaddition and, later, a pyridine ring-closing reaction with ammonia (by 43) starting from the enone coumarin derivative 41 and hydrazonoyl chloride 42 (Scheme 7a). Except for the nitro-substituted derivative, these dyes exhibited high fluorescence intensity, possibly due to increased internal conversion, which decreases the transition of the fluorescence pathway. The 4-methoxyphenyl-substituted derivative was the one that gave the best fluorescence intensity (ΦF = 70% in MeCN) due to its electron-donating nature (Table 1, Entry 9); in addition, the 1,3,11-triphenyl derivative was used for bioimaging in MG-63 cells, exhibiting low cytotoxicity, pH tolerance, and excellent penetration into the cell membrane to mark the intracellular space. Ma et al. [75] contributed by synthesizing the bispirazolo[3,4-b:4′,3′-e]pyridines 45a–e in good yields using a FeCl3-mediated reaction of 5-amino-1,3-dimethylpyrazole (24c) with aldehydes 33a–e (Scheme 7b). They investigated the photophysics of these dyes using five organic solvents and aqueous solutions, observing absorptions and emissions in the ranges of 310–375 nm and 400–580 nm, respectively, in all solvents. The 4-dimethylamino derivative exhibited excellent photostability and notable solvatofluorochromism (positive) and fluorescence (ΦF = 40–84% in organic solvents and 69–89% in aqueous solutions), with emissions ranging from 429 to 500 nm, due to the excellent CT offered by this highly electron-donating group (Table 1, Entry 10).
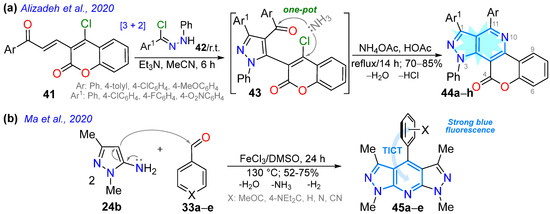
Scheme 7.
Synthesis of (a) chromeno-fused pyrazolo[3,4-d]pyridines 44a–h and (b) bispyrazolo[3,4-b:4′,3′-e]pyridines 45a–e [74,75].
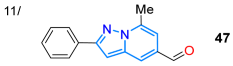
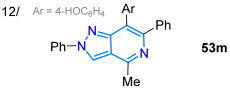
In 2021, Ma and co-workers synthesized the fluorescent probe 47 for the detection of sulfite ions in dry white wine, using the reduction reaction of the pyrazolo[1,5-a]pyridine ester 46 to alcohol (with NaBH4/EtOH) and, later, mild oxidation (MnO2/THF) to the 5-formyl-2-phenylpyrazolo[1,5-a]pyridine (47) [56]. Notably, upon the addition of SO3−2 ions to a solution of 47 in EtOH/PBS (3:7 v/v, pH = 7.4), the emission at 499 nm gradually decreased, and a Stokes shift of 119 nm was observed due to the formation of 48 (Scheme 8a). This probe showed high sensitivity and selectivity and a fast response (30 s), with good linearity between the fluorescence intensity and SO3−2 concentration in the range of 30 to 90 μM (Table 1, Entry 11). That same year, the Razmiené group reported 2,4,6,7-tetrasubstituted pyrazolo[4,3-c]pyridines 53a–u and studied their antiproliferative activity against cancer cell lines (i.e., K562, MV4-11, and MCF-7), as well as their photophysics. These dyes were synthesized in good yields starting from 4-formyl-1-phenyl-3-phenylethynylpyrazole (49) to produce azides 50a–e, which cyclized into the pyridine derivatives 51a–e; finally, these (hetero)aryl iodides were subjected to Suzuki–Miyaura coupling with arylboronic acids 52a–k to produce the desired 7-aryl derivatives 53a–u in moderate to excellent yields (Scheme 8b). These dyes exhibited emission bands in the range of 437–487 nm in THF, with both high Stokes shifts (199–205 nm) and ΦF values (60–85%). The results indicate that compounds with the most polar 7-aryl (i.e., 4-HOC6H4) substituents possess the most prominent fluorescent properties; in addition, the pH dependence of emission was evaluated, finding that derivatives with 7-(4-hydroxyphenyl) or 7-(4-methoxyphenyl) showed emission maxima and ΦF values that increase under standard pH [55] (Table 1, Entry 12).
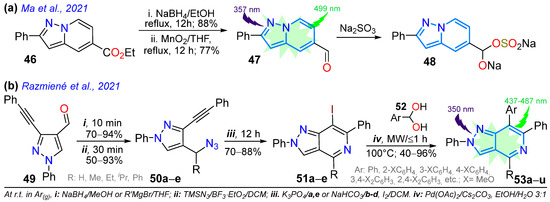
Scheme 8.
Synthesis of (a) 6-formyl-2-phenylpyrazolo[1,5-a]pyridine (47) and (b) pyrazolo[4,3-c]pyridines 53a–u [55,56].
A year later, Abumelha’s group reported the hybrid dye donor–π-acceptor (D–π-A) 57 bearing a pyrazolo[3,4-b]pyridine and an acceptor moiety from 3-cyano-2-(dicyanomethylene)-4,5,5-trimethylfuran (TCF). This dye was synthesized by diazotizing the (hetero)arylamine 54 to the respective diazonium salt 55, which finally reacted with TCF (56) to produce the desired dye 57 (Scheme 9a). The solvatofluorochromism of this dye evidenced the two characteristic absorption bands attributed to π-π* transitions of the conjugated system (~315 nm) and the ICT from the donor heteroaryl core to the nitrile-rich TCF acceptor (451–493 nm). This dye showed a greater dependence on solvent polarity in the fluorescence spectra, exhibiting a redshift in the more polar solvents (from 575 nm in cyclohexane to 632 nm in acetone, Table 2, Entry 1) [61]. Vidali’s group reported, also in 2022 [76], pyrazolo[3,4-b]pyridines hybridized with the characteristic fluorophores triphenylamine (TPA, 60a), anthracene (60b), and pyrene (60c); these dyes were synthesized in high yields by the ZrCl4-catalyzed reaction of the appropriate enone 58a–c (prepared from the formylated fluorophore 58′a–c through the Wittig reaction) with 5-amino-1-phenylpyrazole (59) (Scheme 9b). The TPA derivative 60a displayed significant Stokes shifts and a higher absorption wavelength maximum than 60b,c due to the strong electron-donating nature of the TPA moiety (Table 2, Entry 2). Dyes 60a and 60b showed potential as amyloid plaque probes for diagnosing Alzheimer’s disease (AD) due to their high and selective binding to amyloid plaques in brain slices from AD patients using fluorescence confocal microscopy.
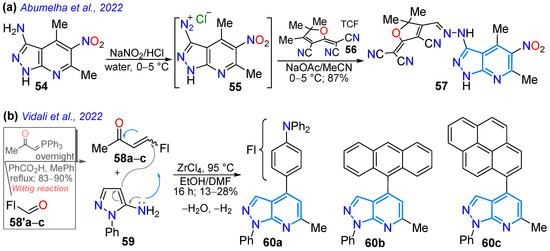
Scheme 9.
Synthesis of highly conjugated pyrazolo[3,4-b]pyridines bearing (a) TCF 57 and (b) other typical fluorophores 60a–c [61,76].
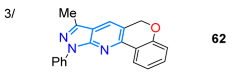
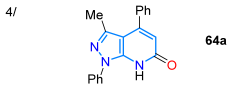
In 2023, Krishnan and co-workers reported the synthesis of a probe for the detection of BF3 (in CH3CN/H2O, 8:2 v/v) chromene-fused pyrazolo[3,4-b]pyridine 62 via the Cu(I)-catalyzed Povarov reaction of O-propargylsalicylaldehyde (61) with heteroamine 24a. This dye exhibited a redshifted fluorescence upon the addition of BF3 (from 440 to 473 nm), with a limit of detection (LOD) of 1.6 nM, enabling the detection of BF3 with good repeatability when switching between BF3 and Et3N (Scheme 10a; Table 2, Entry 3). Probe 62 was also tested for BF3 bioimaging in E. coli and HeLa cells, as well as for BF3 measurement in real water samples [63]. Vladislav’s group reported the 4-arylpyrazolo[3,4-b]pyridinones 64a–i obtained through the cyclization reaction of 24a–c with the 4-arylidene-2-phenyloxazol-5(4H)-ones (azalactones) 63a–i under solvent-free conditions, followed by the tBuOK-promoted elimination of a benzamide molecule through heating from 90 to 150 °C. Dyes 63a–i exhibit luminescence at 409–440 nm with ΦF values of 9–23% and large Stokes shifts (107–152 nm) when irradiated with UV light (Scheme 10b; Table 2, Entry 4) [77].
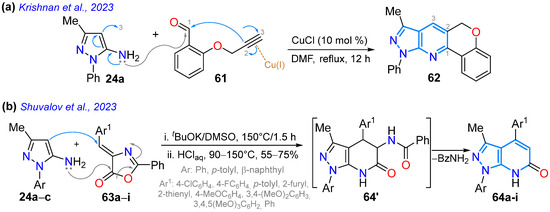
Scheme 10.
The synthesis of (a) the chromene-fused pyrazolo[3,4-b]pyridine 62 and (b) 4-arylpyrazolo[3,4-b]pyridinones 64a–I [63,77].

Table 2.
Summary of some synthetic and photophysical data for other representative pyrazolopyridine-based dyes.
Table 2.
Summary of some synthetic and photophysical data for other representative pyrazolopyridine-based dyes.
| Entry/Fluorophore | Yield (%) | labs (nm) | lem (nm) | ΦF (%)/Solvent | Ref. |
|---|---|---|---|---|---|
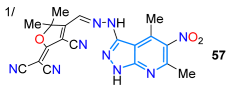 | 87 | 493 | 633 | 89/EtOH | Abumelha et al., 2022 [61] Scheme 9a |
 | 28 | 364 | 465 | -- | Shuvalov et al., 2022 [76] Scheme 9b |
 | 85 | 336 | 440 | 35/MeCN:H2O | Krishnan et al., 2023 [63] Scheme 10a |
 | 73 | 260, 302 | 419 | 22/EtOH | Vladislav et al., 2023 [77] Scheme 10b |
 | 79 | 395 | 454 | 13/hexane | Kolbus et al., 2024 [78] Scheme 11a |
 | 55 | 395 | 458 | 8.5/hexane | Kolbus et al., 2024 [79] Scheme 11b |
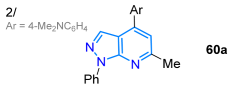
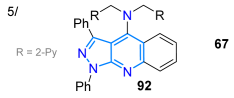
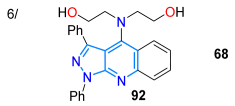
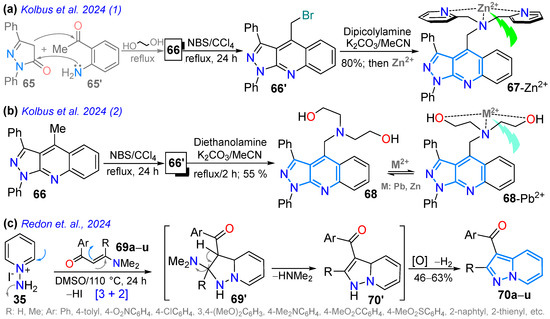
In 2024, the Kolbus group reported synthesizing the tridentate ligand 67, with diphenylpyrazolo[3,4-b]quinoline-based dipicolylamine substituted at position 4, starting from diphenylpyrazolone 65, which underwent a Friedländer reaction with o-aminoacetophenone 65′ to produce the tricyclic dye 66; then, 66 was brominated with NBS in carbon tetrachloride, and the resulting bromoderivative 66′ then reacted with dipicolylamine in a standard medium, delivering probe 67 in a high yield (Scheme 11a). The solvatofluorochromism of 67 revealed absorption bands at around 390 nm with a slight blueshift upon increasing the solvent polarity, while a redshifted fluorescence (460–480 nm) was observed as solvent polarity increased due to an excited state with charge transfer. The highest ΦF value was observed in n-hexane (13%) and the lowest in acetonitrile (0.8%), indicating that the emission is suppressed in polar solvents. Notably, the maximum emission was redshifted (~30 nm) and the fluorescence intensity was increased by 13-fold only in the presence of Zn2+ versus other metal ions with an LOD of 193 nM (Table 2, Entry 5); likewise, this probe localizes in cells near the membrane and cytoplasm and can be used to detect Zn2+ in eukaryotic cells [78].
The same Kolbus group, in a similar way, prepared an analogue ligand substituted with diethanolamine (probe 68), used to detect Zn2+ and Pb2+ with an LOD of ~1000 nM through ‘turned-on’ fluorescence [79]. This probe also exhibited positive solvatofluorochromism with the highest ɸF value in n-hexane (8.5%) and the lowest in acetonitrile (0.1%). It was used to identify Pb2+ in river water solutions (Scheme 11b; Table 2, Entry 6). In parallel, Rendon’s group prepared 3-aroylpyrazolo[1,5-a]pyridines 70a–u in a moderate yield through the [3 + 2] cycloaddition of N-aminoyridinium iodide (35) with β-enaminones 69 in DMSO used as a solvent and oxidant, without the need to add a base or any other kind of oxidizing reagent (Scheme 11c). The authors propose that the reaction proceeds through the cycloadduct 69′ with later dimethylamine elimination to produce the intermediates 70′; finally, aerobic or DMSO oxidation leads to the formation of 3-aroylpyrazolo[1,5-a]pyridines 70a–u [80].
Scheme 11.
Synthesis of pyrazolo[3,4-b]quinolines (a) 67-Zn2+ and (b) 68-M2+ and (c) pyrazolo[1,5-a]pyridines 70a–u [78,79,80].
3. Pyrazolopyrimidines
The general synthetic routes of pyrazolopyrimidines are also drawn in Scheme 1; this family of compounds perhaps contains the more recognized 5:6 aza-fused pyrazole ring, that is, the pyrazolo[1,5-a]pyrimidine scaffold [18,39,81,82,83,84]. Still, the chemistry and biological effects of different pyrazolopyrimidine derivatives have been well-documented [85,86,87]. For example, pyrazolo[4,3-d]pyrimidines have been used as anticancer agents, phosphodiesterase inhibitors, cytokinin antagonists, anti-infectious agents, and central nervous system (CNS) agents, such as phosphodiesterase-5 inhibitors and adenosine receptor antagonists [88,89,90]; they have also presented antimicrobial and anti-inflammatory (against LPS-induced NO) activity [91,92]. The [3,4-d] derivatives possess anticancer, anti-inflammatory, antituberculosis, antimicrobial, and antiviral activity [93,94,95,96,97,98,99], while pyrazolo[1,5-a]pyrimidines also stand out for their anticancer activity [100,101,102,103,104,105,106], among others [39,106,107,108]. Figure 7 shows some drugs with a pyrazolopyrimidine ring, such as Sildenafil (Viagra) [88] or the tyrosine kinase inhibitor Ibrutinib [109] bearing the [4,3-d] system, the hypnotic and anxiolytic agents Indiplon and Ocinaplon based on pyrazolo[1,5-a]pyrimidine [18,39], and an anti-inflammatory agent with [3,4-d] fusion [110].
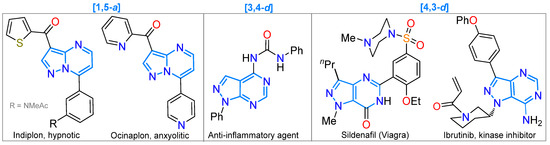
Figure 7.
Examples of drugs containing a pyrazolopyrimidine ring.
In addition to their varied biological activity, pyrazolopyrimidines have been studied as fluorophoric molecules, particularly those with [1,5-a] fusion, as these derivatives possess an emergent and functional fluorophoric scaffold characterized by high synthetic viability and exceptional photophysics driven by ICT mechanisms; indeed, this N-heterocyclic core is a standard scaffold used in our group for this purpose [18,22,68]. In this context, some developed fluorophores based on pyrazolo[1,5-a]pyrimidines (PPs) have been identified [19,111,112,113,114,115], exhibiting significant solvatofluorochromism in organic solvents and applications as fluorescent probes for cyanide and water detection [19,112,113,114] and as fluorescent biomarkers [87,108] (Figure 3 and Figure 8). Thus, in addition to PPs, this section will cover the syntheses of several dyes that have not been photophysically studied, providing relevant examples of how the other rings ([1,5-c], [3,4-d], or [4,3-d]) are accessed.
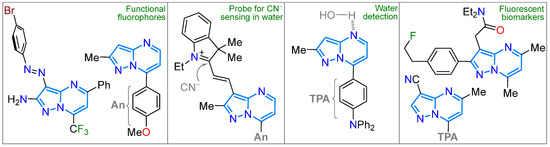
Figure 8.
Compounds with photophysical properties containing a pyrazolo[1,5-a]pyrimidine ring.
We begin the discussion of this section by considering work by Mohammed et al. [116], who synthesized pyrazolo[4,3-d]pyrimidin-7(6H)-ones 73a–c using a I2-catalyzed oxidative coupling in the cyclocondensation of 4-amino-1-methyl-3-propyl-1H-pyrazole-5-carboxamide (71) with acetophenones 72a–c (Scheme 12a). As mentioned, articles on photophysically relevant pyrazolopyrimidines focus on [1,5-a] fusion; however, dyes 73a–c could be relevant for this purpose due to their rigid structure and high π-conjugation. That same year (2015), Nassar’s group described a protocol to obtain pyrazolo[3,4-d]pyrimidines 78a–c in a good yield through the cyclocondensation reaction of ethyl 5-amino-1-tosyl-1H-pyrazole-4-carboxylate (76) with formamide (77a), urea (77b), or thiourea (77c), giving rise to the desired products 78a–c (Scheme 12b). The pyrazole precursor 76 was obtained through the reaction of the substituted acrylonitrile 74 with the hydrazine derivative 75. Compounds 78a–c were evaluated as having antitumor properties, and it was found that compound 15a showed moderate anticancer activity (IC50 = 29.58 µm/mL) in the cancer cell line (EAC), so they also evaluated its antitumor activity in two human cell lines (HCT-29 and MCF-7) with a cytotoxicity assay with crystal violet, showing good activity (24.34 µm/mL) [95].
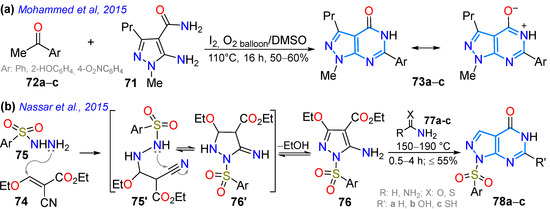
Scheme 12.
Synthesis of (a) pyrazolo[4,3-d]pyrimidinones 73a–c and (b) pyrazolo[3,4-d]pyridines 78a–c [95,116].
In 2016, Squarcialupi et al. [89] synthesized 7-acylaminopyrazolo[4,3-d]pyrimidines 81a–i bearing groups with different steric bulk, flexibility, and lipophilicity (Me, Ar, hetaryl, and Bn) at positions 2 and 5, which turned out to be potent antagonists of the human adenosine A3 receptor (AR) (h). All 7-amino and 7-acylamino-2-phenylpyrazolo[4,3-d]pyrimidines with 4-methoxyphenyl or 2-thienyl groups at position 5 showed high affinity and selectivity for hA3. The 7-(4-methoxybenzoylamino)-2-phenyl-5-(2-thienyl) derivative (Ki = 0.027 nM) was one of the most potent and selective hA3 antagonists of the series studied. For this purpose, 4-amino-1-aryl-3-cyanopyrazoles 79a,b reacted with ethyl iminoesters and ammonium acetate to produce the 5:6-fused heteroamines 80a–i, which were then acylated, producing 81a–k in moderate to good yields (Scheme 13a).
Abdelgawad et al. [94] obtained 4-alkoxypyrazolo[3,4-d]pyrimidines 87a–g by reacting the 4-hydroxypyrazolo[3,4-d]pyrimidine 84 with ethyl chloroacetate (85), producing the respective ester that then underwent hydrazinolysis to form 86, which finally condensed with arylaldehydes 33a–g, producing the desired products in good yields (Scheme 13b). Compounds 87a–g were evaluated for their cytotoxic activity against breast carcinoma (MCF-7), non-small-cell lung cancer (A549), and human colorectal adenocarcinoma (HT-29) cell lines through MTT and colony formation assays; 87g was the most potent anticancer agent, with IC50 values of 5.36–9.09 μM; likewise, 87g and 87c,d were tested against fibroblast growth factor receptor (FGFR), insulin receptor (IR), and vascular endothelial growth factor receptor (VEGFR), observing potent inhibitory activity. That same year, Hafez et al. [91] obtained the pyrazolo[4,3-d]pyrimidinone 92, starting with the formation of hydrazide 88′ from the aminoester 88, which reacted with arylaldehydes 33a–d, producing 89a–d, which were acylated and cyclized to produce 90, which was chlorinated, leading to the key intermediate 90′. From 90′, coupling reactions with the stannane reagent 91 produced 92a, while with sodium azide or ethyl chloroacetate (85), the tetrazole-fused product 92b or the alkoxy derivative 92c was obtained (Scheme 13c). Compounds 92a–c exhibited good to excellent antimicrobial activity, and 92b showed greater anticancer activity than the reference doxorubicin.
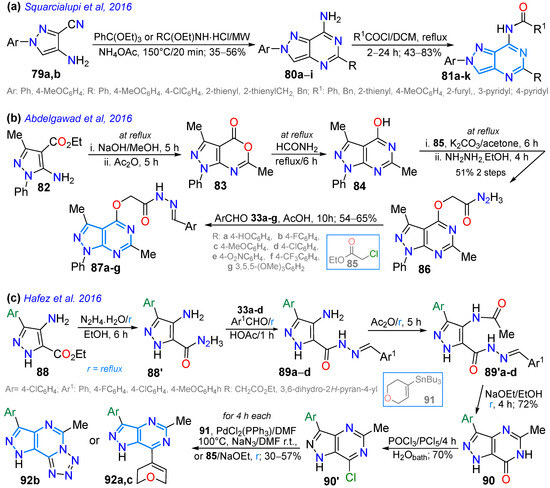
Scheme 13.
Synthesis of pyrazolopyrimidines (a) [4,3-d] 81a–k, (b) [3,4-d] 87a–g, and (c) [4,3-d] 92a–c [89,91,94].
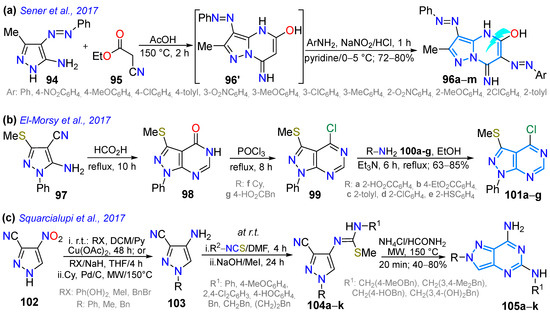
In 2017, Sener et al. [117] studied the solvatochromism of 3,6-bisaryldiazenylpyrazolo[1,5-a]pyrimidines 96a–m obtained by reacting the 5-amino-4-phenylazopyrazole 94 with ethyl cyanoacetate (95) to yield a 3-phenylazo intermediate, which was then diazotized to produce the desired products 96a–m. The absorption spectra of 96a–m correlated with solvent polarity. Upon adding HCl (0.1 M) to the methanol solution, a blueshift with two absorption maxima was observed, whereas the addition of KOH (0.1 M) resulted in a redshift. For example, the 2-methoxyphenyl derivative exhibited λabs at 406 and 430 nm; upon the addition of HCl, these values shifted to 337 and 448 nm, and upon the addition of KOH, they shifted to 444 and 414 nm (Scheme 14a; Table 3, Entry 1). The authors also explored the antimicrobial properties of these dyes, finding that they all exhibited good activity against both Gram-positive and Gram-negative bacteria, as well as against fungi.

Table 3.
Summary of some synthetic and photophysical data for representative pyrazolopyrimidines.
Table 3.
Summary of some synthetic and photophysical data for representative pyrazolopyrimidines.
| Entry/Fluorophore | Yield (%) | labs (nm) | lem (nm) | ΦF (%)/Solvent | Ref. |
|---|---|---|---|---|---|
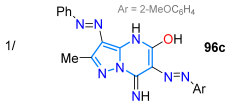 | 76 | 406/430 | -- | -- | Sener et al., 2017 [117] Scheme 14a |
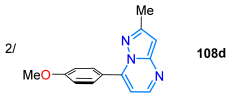 | 90 | 353 | 479 | 44/DCM | Castillo et al., 2018 [118] Scheme 15a |
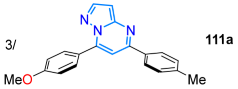 | 83 | 332 | 386/470/504 | 84/benzonitrile | Singsardar et al., 2018 [119] Scheme 15b |
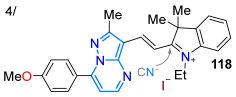 | 74 | 464 | 525 | 42/H2O | Tigreros et al., 2019 [113] Scheme 16a |
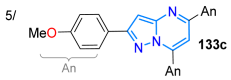 | 80 | 354/301/295 | 474 | 54/THF:H2O | Tigreros et al., 2019 [112] Scheme 17a |
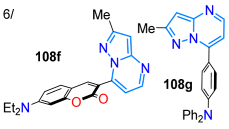 | 8790 | 440385 | 494488 | 53/THF97/DCM | Tigreros et al., 2020 [120] Scheme 17b |
 | 58 | 414 | 548 | 88/DMSO | Ma et al., 2021 [121] Scheme 18b |
 | 94 | 284/478325/478 | 500447 | 0.0/EtOH:H2O>20/EtOH:H2O | Tigreros et al., 2021 [22] Scheme 18c |
Scheme 14.
Synthesis of pyrazolopyrimidines (a) [1,5-a] 96a–m, (b) [3,4-d] 101a–g, and (c) [4,3-d] 105a–k [96,117,122].
Scheme 15.
Synthesis of pyrazolopyrimidines with fusion (a) [1,5-a] 109a–k, (b) [1,5-a] 111, and (c) [3,4-d] 116a–z [118,119,123].
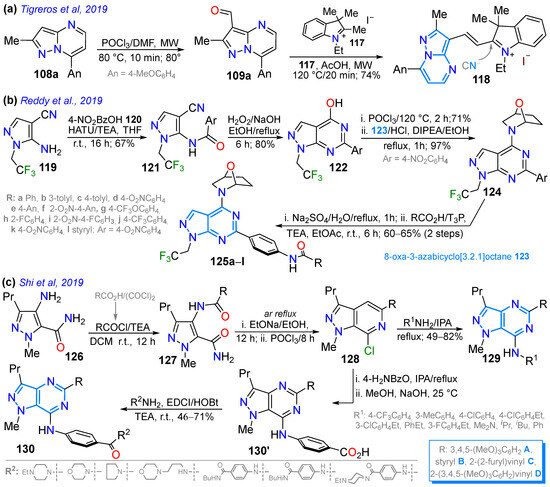
Scheme 16.
Synthesis of pyrazolopyrimidines with fusion (a) [1,5-a] 118, (b) [3,4-d] 125, and (c) [4,3-d] 128 and 130 [97,113,124].
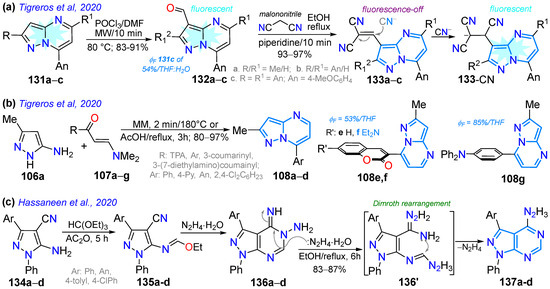
Scheme 17.
Synthesis of pyrazolopyrimidines with fusion (a) [1,5-a] 133a–c, (b) [1,5-a] 108a–g, and (c) [3,4-d] 137a–d [112,120,125].
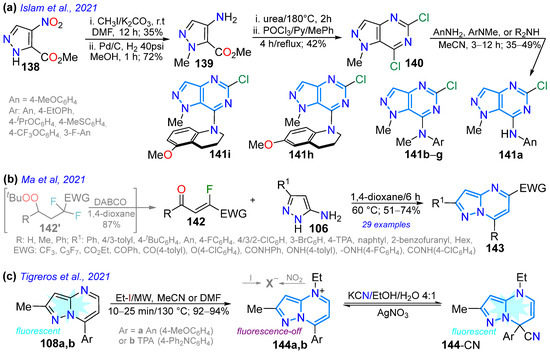
Scheme 18.
Synthesis of pyrazolopyrimidines with fusion (a) [4,3-d] 141a–1, (b) [1,5-a] 143, and (c) [1,5-a] 144a,b [22,90,121].
El-Morsy et al. [96] obtained 3-methylthiopyrazolo[3,4-d]pyrimidines 101a–g, starting with a reaction of the 5-aminopyrazole 97 with formic acid to produce the intermediate 98; this intermediate was chlorinated with POCl3, forming the derivative 99, which, when reacted with aromatic 100a–e or aliphatic 100f,g amines, produced 101a–g (Scheme 14b). These compounds were evaluated for antitumor activity against the human breast adenocarcinoma cell line MCF7, finding that 10 of them showed mild to moderate activity compared to doxorubicin (reference drug). Likewise, Squarcialupi et al. [122] obtained 5,7-diaminopyrazolo[4,3-d]pyrimidines 105a–k to identify novel antagonists targeting the (h) A1 or A2A receptors, starting from 4-nitro-1H-pyrazole-3-carbonitrile (102), which was N-substituted (alkylated or arylated) and then reduced, yielding amines 103a–c. These amines were treated with isothiocyanates and then with CH3I, resulting in the S-methylisothiourea derivatives 104a–k. Upon cyclization, these derivatives were converted into 105a–k by treatment with formic acid (Scheme 14c). Structure–affinity studies and molecular modeling studies of 105a–k were performed, finding that the 5-(4-hydroxyphenethylamino) derivative showed good affinity (Ki = 150 nM) and the best selectivity for the hA2A receptor, while the 5-benzylamino-substituted derivative showed the best combined affinity for the hA2A (Ki = 123 nM) and A1 (Ki = 25 nM) receptors.
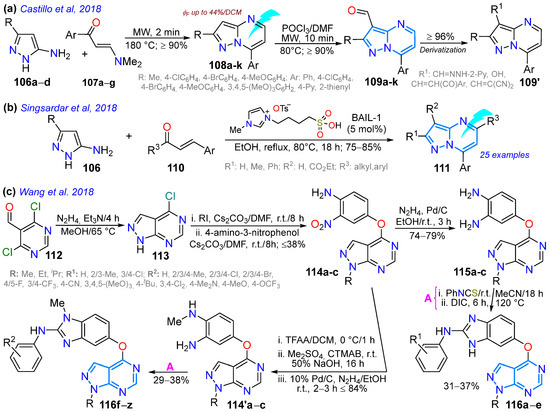
A year later, Castillo et al. [118] reported 3-formylpyrazolo[1,5-a]pyrimidines 109a–k as functional fluorophores, obtained in high yields through the microwave-assisted reaction of 3-amino-NH-pyrazoles 106 with β-enaminones 107 to the deliver free dyes 108a–k, followed by a Vilsmeyer–Haack formylation reaction. These aldehydes were used in post-functionalization reactions, and four 7-aryl derivatives of 108, bearing acceptor (Ar = 4-Py), neutral (Ar = Ph), and donor (Ar = 4-MeOC6H4 and 3,4,5-(MeO)3C6H2) groups, were photophysically explored; these dyes exhibited a large Stokes shift (7187–9249 cm−1) in different solvents, with the 4-methoxyphenyl derivative 108a being the most promising dye (ΦF = 34% in toluene, 44%/DCM, 17%/MeCN, 23%/DMSO, 19%/EtOH) due to an ICT process from the methoxy group to the pyrimidine ring (Scheme 15a; Table 3, Entry 2).
Similarly, Singsardar et al. [119] obtained dyes 111a–y in high yields using the ionic liquid BAIL as a Bronsted acid catalyst in the reaction of 5-amino-NH-pyrazoles 106 with enones 110. The photophysics of these dyes proved that they were all highly fluorescent, with the unsubstituted dyes in the pyrazole moiety and those substituted with EDGs in the pyrimidine moiety derivatives showing the highest ΦF values; indeed, the maximum ΦF value (84% in benzonitrile) was obtained for 7-(p-anysyl)-5-(p-tolyl)pyrazolo[1,5-a]pyrimidine (Scheme 15b; Table 3, Entry 3). As a third example, Wang et al. [123] synthesized biologically relevant pyrazolo[3,4-d]pyrimidines 116a–z, starting with pyrazole ring formation on the 5-formylpyrimidine 112 to produce the fused derivative 113, which was N-alkylated and C-aryloxylated, producing o-nitroanilines 114; the o-nitroanilines 114 were reduced to obtain NH-benzimidazol-5-yl derivatives 116a–e via the o-diamine 115 on one side or methylated and reduced to access N-methylbenzimidazol-5-yl derivatives 116f–z through 115′ on the other (Scheme 15c). These compounds demonstrated activity in enzymatic and cell proliferation assays; in particular, those substituted with haloaryl groups showed high inhibitory activity against the BRAFV600E and VEGFR-2 kinases, comparable to that of the positive control, sorafenib.
In 2019, Tigreros et al. [113] reported the application of the dye 108a to obtain the hybrid probe pyrazolo[1,5-a]pyrimidine−hemicyanine 118 in a 74% yield using the microwave-assisted reaction of the 3-formyl derivative 109a with the N-ethylindolinium salt 117. This probe was helpful for the colorimetric and fluorimetric chemodetection of cyanide (CN−) in water, with LODs of 600 and 86 nM, respectively, through the nucleophilic addition of cyanide to the C=N+ bond of the hemicyanine group (Scheme 16a; Table 3, Entry 4). That same year, Reddy et al. [97] obtained the 6-(4-phenylamido)pyrazolo[3,4-d]pyrimidine derivatives 125a–l, starting from the 5-aminopyrazole 119, and evaluated their in vitro α-amylase inhibitory activity. The amidation of 119 with 4-nitrobenzoic acid (120) yields 121, which is cyclized using H2O2 to produce 122. Once halogenated, 122 reacts with 8-oxa-3-azabicyclo[3.2.1]octane hydrochloride (123), resulting in 124. Then, the nitro group of 123 was reduced to the respective arylamine, allowing for amidation with benzoic acids to finally form products 125a–l (Scheme 16b). The authors found that all compounds were active, with IC50 values ranging from 1.60 ± 0.48 to 2.04 ± 1.20 μM, versus the standard acarbose (1.73 ± 0.05 μM).
In parallel, Shi et al. [124] obtained four series (A, B, C, and D) of a total of ninety pyrazolo[4,3-d]pyrimidines 129 and 130 in moderate to good yields as anti-inflammatory agents for the treatment of arthritis, starting from the 4-amino-5-carbamoylpyrazole 126 (Scheme 16c). First, the amidation of 126 yields the diamide 127, which cyclizes and subsequently undergoes chlorination with POCl3, providing the intermediate 128 for accessing target products through nucleophilic aromatic substitution (NAS) reactions with amines. The authors found that the D series had the most active scaffold, delivering the R1 = iPrNH group with the most potent anti-inflammatory agent (IC50 = 3.17 μM) with low toxicity and the potent inhibition of NO release (IR = 90.4% at 10 μM). This compound also exhibited the potent inhibition of iNOS (inducible nitric oxide synthase), with an IC50 value of 1.12 μM.
A year later, Tigreros et al. [112] obtained probes 133a–c for sensing CN− based on 3-dicyanovinylpyrazolo[1,5-a]pyrimidines substituted with 4-anisyl groups through the formylation of 131a–c and then the piperidine-catalyzed condensation reaction of 132a–c with malononitrile. Probes 133a–c demonstrated high selectivity and sensitivity toward CN−, with LOD values of up to 610 nM and 170 nM, as determined by absorption and emission spectroscopy. Upon the addition of CN−, the solution changed color from yellow to colorless (at ~390 nm) and exhibited an 82-fold increase in fluorescence at ~465 nm, emitting intense blue light. Moreover, the photophysics of aldehyde intermediates 132a–c showed that the tri-(4-methoxyphenyl) derivative 132c showed very high ε values at ~300 nm due to major π-conjugation; likewise, these heteroaldehydes exhibited emission bands at ~470 nm with high ΦF values, from 40% for 132a to 54% for 132c (Scheme 17a; Table 3, Entry 5).
The notable photophysics of 7-arylpyrazolo[1,5-a]pyrimidines prompted these authors to conduct a theoretical and experimental study of dyes 108a–g, bearing substituents with EDGs and EWGs at position 7, to investigate the influence of these groups on this property. These compounds were obtained by reacting 3-amino-5-methyl-NH-pyrazoles (106a) with β-enaminones 107a–g, which exhibited absorption bands between 340 and 440 nm (assigned to an ICT process) and emission bands between 474 and 541 nm. Derivatives with EDGs revealed high ΦF values (up to 85% in THF), and, through DFT calculation, the electronic properties of these dyes were established (Scheme 17b; Table 3, Entry 6) [120]. Finally, Hassaneen’s group synthesized pyrazolo[3,4-d]pyrimidines 137a–d by treating the 5-amino-3-cyanopyrazoles 134a–d with triethyl orthoformate and then with hydrazine monohydrate, yielding the imino–amino derivatives 136a–d, which were converted to 4-hydrazino derivatives 137a–d, involving a Dimroth rearrangement (Scheme 17c) [125].
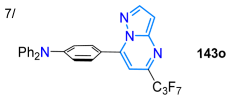
In 2021, Islam et al. [90] reported N-methylpyrazolo[4,3-d]pyrimidines 141a–i as inhibitors of tubulin polymerization and colchicine binding, which were obtained in moderate yields; they used the cyclization of the pyrazole aminoester 138 with urea, followed by treatment with POCl3 to form the 5,7-dichloropyrazolopyrimidine 139. Upon reacting 139 with primary 140a and secondary 140b–i anilines in acetonitrile through NAS reactions, the desired products were obtained. Notably, all compounds, except 141a, inhibited the binding of [3H]colchicine to tubulin, and it was found that they strongly inhibit tubulin polymerization, with IC50 values of ~0.4 μM (Scheme 18a). Ma’s group synthesized, in good yields, 5,7-disubstituted pyrazolo[1,5-a]pyrimidines 143 with EWGs in the pyrimidine moiety using the cyclocondensation reaction of β-fluoroenones 142 (obtained from β,β-difluoroperoxides 142′ using DABCO) with 3-aminopyrazoles 106 under standard conditions. These dyes showed large Stokes shifts (up to 211 nm) and tunable fluorescence, with emission for the dye bearing R1 = H, R = TPA, and EWG = C3F7 shifted from 459 to 548 nm in different solvents due to the typical ICT process from the TPA moiety to the EWG, which was confirmed by DFT calculations [121] (Scheme 18b; Table 3, Entry 7).
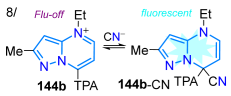
That same year, Tigreros et al. [22] reported the synthesis, in high yields, of probes 144a,b for cyanide detection based on 7-aryl-N4-ethylpyrazolo[1,5-a]pyrimidinium salts obtained through the microwave-assisted N4-alkylation of 108a,b with ethyl iodide. The authors determined that the TPA-substituted probe 144b is ideal, with improved absorption and emission properties compared to 144a due to the characteristic ICT process in this probe type; indeed, both probes detected CN−, with 144b exhibiting better LOD values (0.30 and 0.34 μM for 144a → 0.15 and 0.25 μM for 144b), despite it being a more time-consuming process than with 144b due to the lower reactivity from the strong electron-donating characteristic of the TPA moiety in 144b (Scheme 18c; Table 3, Entry 8). It is important to note that the practical applicability of 144b was demonstrated through the determination of small amounts of CN− (0.5 μmol/L) in tap water and the successful use of test strips; likewise, through emission in solid-state measurements of this probe supported on silica, cyanide ions were detected in the solid state and in bitter almonds at concentrations as low as 50 ppm.
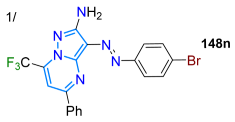
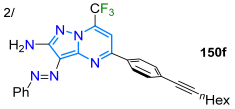
In 2022, Stefanello et al. [126] prepared 3-aryldiazenyl-7-trifluoromethylpyrazolo[1,5-a]pyrimidines 148a–q in up to a 90% yield through the reaction of the 3,5-diamino-NH-pyrazoles 146a–g (obtained from aryldiazenylmalononitriles 145) with β-methoxyenones 147. These dyes exhibited the characteristic absorption bands of π-π* and n-π* transitions with emission bands in the blue range (in diverse polarity solvents) and low ΦF values (Scheme 19a; Table 4, Entry 1). Subsequently, they used 4-bromophenyl-substituted dyes as substrates in preparing the alkynyl derivatives 150a–m through Sonogashira coupling reactions with the terminal alkynes 149a–e; the authors found that arylacetylenes bearing EWGs offer low yields (up to 20% with 4-cyanophenyl), while the highest yields were obtained with alkylacetylenes (up to 87%), followed by arylacetylenes with EDGs (up to 79%). Dyes 150a–m exhibited strong absorption bands in the range of 250–500 nm, with their emission properties (λem ~490 nm) being unaffected by EDGs or EWGs in the diazenyl moiety, but this wavelength was shifted by ~30 nm when the substitution occurred on the opposite side of the diazenyl unit; a major effect was observed in doubly substituted dyes, where the electronic effects were enhanced and a larger shift was observed (Scheme 19b; Table 4, Entry 2) [115].

Scheme 19.
Synthesis of pyrazolopyrimidines with fusion (a) [1,5-a] 148a–q, (b) [1,5-a] 150a–m, and (c) [3,4-d] 155a–n [115,126,127].
That same year, Sun et al. [127] reported synthesizing 4,6-diaminopyrazolo[3,4-d]pyrimidines 156a–n in moderate yields, starting from barbituric acid (151), which was trichlorinated and formylated to produce 152, which then reacted with hydrazine derivatives to give rise to 4,6-dichloropyrazolopyrimidines 153a–h; these intermediates were monosubstituted with the 3-amino-NH-pyrazole 106a to form 154a–h, on which NAS reactions with benzylamines 155a,b occurred to produce 156a–n. Most compounds showed potent suppressive activity against PLK4, with IC50 values <10 nM; among them, the derivative with R = tetrahydropyran-4-yl had a PLK4 IC50 of 0.2 nM, which showed potent enzyme inhibition and good selectivity across a panel of 35 kinases and displayed more potent antiproliferative activity than centrinone against MCF-7, BT474, and MDA-MB-231 cells, with IC50 values of 0.36, 1.35, and 2.88 μM, respectively (Scheme 19c).
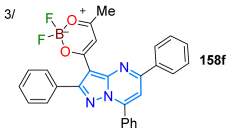
In 2023, Tigreros et al. [128] obtained pyrazolo[1,5-a]pyrimidine-dioxaborinine hybrid dyes 158a–f by directly constructing the dioxaborinine ring on the N-heterocyclic core of substrate 157a–f, using excess acylating reagent (Ac2O/BF3·OEt2); the reaction consisted of a double acetylation reaction of 157, forming the enolized β-diketone 158′ via the methyl ketone 157′ [129], which resulted in 158a–f through complexation with BF3OEt2. These dyes and their precursors were photophysically studied in THF, observing the typical absorption bands of π-π* (260–330 nm) and n-π* (340–391 nm) transitions, with emissions in the range of 400–491 nm; improved ΦF values for 158a–f (up to 69%) compared to their precursors 157a–f (up to 30%) were found as the EWG (i.e., dioxaborinine ring) in the hybrid dyes favors ICT phenomena, which was confirmed with DFT calculations (Scheme 20a; Table 4, Entry 3). Similarly, Stefanello et al. [111] reused 3-aryldiazenyl derivatives 148 as functional dyes in preparing triazole-fused pyrazolo[1,5-a]pyrimidines 159a–h in up to an 85% yield through two different methods: the thermal decomposition of the azide intermediate 159′ (which comes from the diazonium salt 148′) and the Cu(II)-promoted oxidative cyclization of 148a–h through single-electron transfer (SET) to a metal cation via radical species. Dyes 159a–h in THF exhibited the typical absorption bands of π-π* (260 nm) and n-π* (380 nm) transitions, with negligible substituent effects on shifting the emission peaks, which were centered in the green region and had low to moderate ΦF values (Scheme 20b; Table 4, Entry 4).
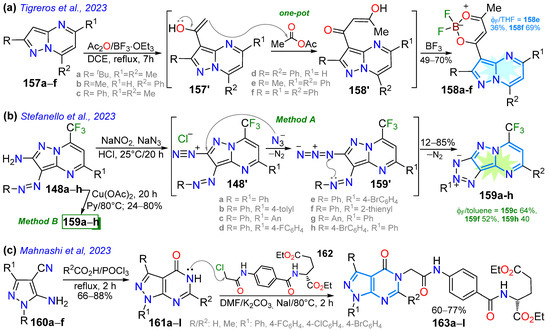
Scheme 20.
Synthesis of pyrazolopyrimidines with fusion (a) [1,5-a] 158a–f, (b) [1,5-a] 159a–h, and (c) [3,4-d] 163a–l [111,128,130].
On the other hand, Mahnashi et al. [130] reported glutamate analogues 163a–l based on pyrazolo[3,4-d]pyrimidine as antifolate anticancer agents, which were prepared through the POCl3-catalyzed cyclization of 5-amino-4-cyanopyrazole derivatives 160a–f with aliphatic acids, forming pyrazolo[3,4-d]pyrimidine-4-ones 161a–l. The desired products were obtained through the N5-alkylation of 161a–l with diethyl(4-(2-chloroacetamido)benzoyl)-D-glutamate (162). The compound with R1 = Me, R2 = 4-BrC6H4, and R3 = H displayed the most potent antiproliferative activity against non-small-cell lung cancer (NSCLC), issues with the central nervous system (CNS), ovarian cancer, prostate cancer, colon cancer, melanoma, breast cancer, and renal cancer, with good to weak cytostatic activity and non-lethal effects; this compound showed greater selectivity for cancer cells than for normal cells (Scheme 20c).
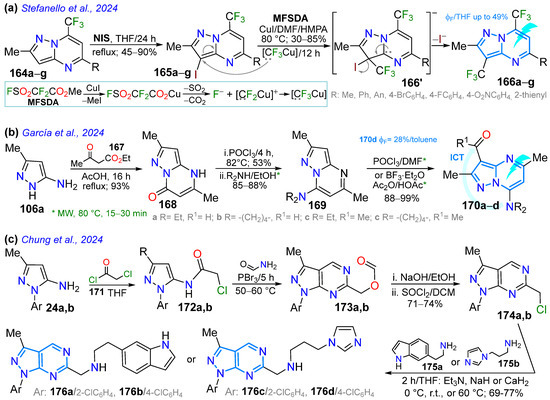
In the last year presented in this contribution (i.e., 2024), Stefanello et al. [131] synthesized 5-substituted 3,7-bis(trifluoromethyl)-2-methylpyrazolo[1,5-a]pyrimidines 166a–g through a sequential process involving the iodination of substrates 164a–g with N-iodosuccinimide (NIS) via an electrophilic aromatic substitution (EAS) reaction, resulting in the 3-iodo derivatives 165a–g in high yields, followed by trifluoromethylation with methylfluorosulfonyl-difluoroacetate ([MFSDA]); this last reaction involves the in situ production of a nucleophilic species [:CF3−], which replaces the iodine via an addition–elimination mechanism at C3. The photophysics of three (with R: Ph, 4-MeOC6H4, 4-O2NC6H4) of these dyes (in DCM, THF, and DMSO) showed the typical absorption bands of π-π* (268–267 nm) and n-π* (325–347 nm) transitions, emissions in the range of 434–497 nm, and ɸF values of up to 49% (by R = 4-MeOC6H4) (Scheme 21a; Table 4, Entry 5).
On the other hand, García et al. [109] reported four dyes based on 3-acyl-7-dialkylaminopyrazolo[1,5-a]pyrimidines 170a–d with a D-π-A nature similar to that of the fluorescence standard Prodan (6-dimethylamino-2-propionylnaphthalene), starting with the reaction of the heteroamine 106a with ethyl acetoacetate (167). This reaction produced the pyrazolo[1,5-a]pyrimidinone 168 in good yield, which was chlorinated and then substituted with secondary amines through an NAS reaction, leading to the 7-amino derivatives 169a,b. Finally, MW-assisted acylation reactions of 170a,b produced aldehydes 170a,b and ketones 170c,d. The solvatofluorochromism of these dyes in ten solvents of different polarities revealed that the ketone with pyrrolidine 171d exhibited superior results, with negative (blueshift) behavior for all compounds due to the larger dipole moment in the ground state of these ICT dyes. These dyes, based on very small molecules, showed the characteristic absorption bands of n-π* transitions at 321–356 nm with ε values (in M⁻1 cm⁻1) following the trend (in EtOH) 170a (10,200) < Prodan (14,400) < 170c (18,500) < 170b (24,500) < 170d (27,200); their emissions in all the evaluated solvents were in the range of 374–406 nm, with ɸF values of up to 28% for 170d vs. 418–488 nm and 56% for Prodan, which is perhaps the most used dye in biological systems, thanks to its small size. Prodan is susceptible to solvatofluorochromism, which enables its use in studying various biological systems with excellent results, despite being part of the polycyclic arene family [132].
The final study discussed in this review is by Chung’s group, who reported a synthetic strategy for obtaining the biologically relevant 1,3,6-trisubstituted pyrazolo[3,4-d]pyrimidines 177a–d, starting with the N-substituted 5-aminopyrazoles 24a,b (Scheme 21c). The acylation reaction of these heteroamines with 2-chloroacetyl chloride (172) produced the amide derivatives 173a,b, which cyclocondensed with formamide in the presence of PBr3, producing the formyl ester intermediates 174a,b containing the fused ring. Later, this formyl ester was hydrolyzed with NaOH/EtOH and then treated with thionyl chloride (SOCl2) to give rise to the 6-chloromethylpyrazolo[3,4-b]pyrimidines 175a,b. Finally, the alkyl chlorides 175a,b were used as substrates in a nucleophilic substitution reaction with 6-(2-aminoethyl)indole (176a) and 1-(3-aminopropyl)imidazole (176b), producing the desired products 177a–d in good yields. These compounds exhibited increased inhibitory activity against different leukemia cell lines; in particular, the 2-chlorophenyl-substituted compounds 177a and 177c were found to present the highest inhibitory activity, IC50, for the different leukemia cell lines, especially for Jurkat, K-562, and HL-60 [133].
Scheme 21.
Synthesis of pyrazolopyrimidines with fusion (a) [1,5-a] 166a–g, (b) [1,5-a] 170a–d, and (c) [3,4-d] 176a–d [109,131,133].

Table 4.
Summary of some synthetic and photophysical data for other representative pyrazolopyrimidines.
Table 4.
Summary of some synthetic and photophysical data for other representative pyrazolopyrimidines.
| Entry/Fluorophore | Yield (%) | labs (nm) | lem (nm) | ΦF (%)/Solvent | Ref. |
|---|---|---|---|---|---|
 | 85 | 276/393/456 | 520 | 18/DMSO | Stefanello et al. 2022 [126] Scheme 19a |
 | 25 | 301/337 | 483 | 8/THF | Stefanello et al. 2022 [115] Scheme 19b |
 | 70 | 269/288/347 | 477 | 69/THF | Tigreros et al. 2023 [128] Scheme 20a |
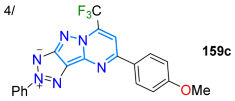 | 45 | 327/385 | 535 | 64/toluene | Stefanello et al. 2023 [111] Scheme 20b |
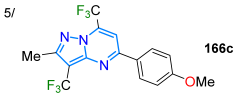 | 85 | 281/345 | 434 | 49/THF | Stefanello et al. 2024 [131] Scheme 21a |
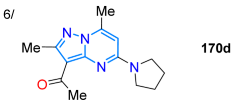 | 99 | 334 | 388 | 28/toluene | García et al. 2024 [109] Scheme 21b |
4. Conclusions and Outlooks
In summary, most strategies for synthesizing pyrazolopyridines and pyrazolopyrimidines involve typical cyclocondensation reactions using conveniently functionalized aminopyrazoles to form the pyridine or pyrimidine ring on the five-membered ring, that is, a 5-to-6 strategy. Some derivatives were synthesized using multicomponent reactions (MCRs) or via a one-pot strategy, in which, for example, the 1,3-biselectrophilic intermediate was generated in situ. Examples of 6-to-5 cyclizations starting from substrates with a pyridine or pyrimidine ring were also found; e.g., aminopyridinium salts were used to access the pyrazole ring through [3 + 2] cycloadditions. Likewise, some pyrazolopyridines or pyrazolopyrimidines were obtained from 4-alkynylpyridines, hydrazinopyridines, and formylated or chlorinated pyridines or pyrimidines. In general, access to functionalized rings is achieved through ring construction, using standard reactions to transform substituents on the ring or leveraging the dual reactivity of the fused ring to electrophiles and nucleophiles via EAS or NAS reactions. However, functionalization protocols must be expanded, as the existing ones are somewhat limited, especially when the fused ring has a lower number of CH groups on the periphery and the basic or chelating nature of pyridine-like (=N–) nitrogen atoms (=N:) on the periphery of the ring increases.
On the other hand, we found that the pyrazolo[1,5-a]pyridine and pyrazolo[1,5-a]pyrimiddine rings are the most representative of the N-heterocyclic core in photophysics among the pyrazolopyridines and pyrazolopyrimidines reported in the last decade, followed by pyrazolo[3,4-b]pyridines. Only a few examples of other rings were identified in this context (i.e., pyrazolo[4,3-c]pyridines and pyrazolo[4,3-e]pyridines). The photophysics of the active dyes indicate that those with π-extended conjugation or bearing EDGs (the 4-MeOC6H4 group is the most common as it improves ICT processes) exhibit enhanced behavior, implying positive solvatofluorochromism, large Stokes shifts, high quantum yields, and low-energy transitions due to photophysics driven by the dipolar nature of the respective 5:6 aza-fused ring. Likewise, the applications of these dyes include ion chemodetection, cell markers, or bioimaging, as well as antitumor scaffolds. Due to the noticeable synthetic viability and electronic properties of these pyrazole derivatives, various reactions have been developed that enable the modification of their modular structural; still, the scope of fluorophores based on pyrazolo[1,5-a]pyridine, pyrazolo[1,5-a]pyrimiddine, and pyrazolo[3,4-b]pyridine rings remains limited, and even more so in derivatives with the other fusion types found. Therefore, researchers interested in this area are expected to work on filling the existing gaps in obtaining pyrazole 5:6 aza-fused rings over the next decade.
Finally, we found that many research groups not only focused on synthesis methods to obtain the molecular systems studied herein but also on the application of these compounds in biological and/or photophysical fields. In general, investigators explored photophysics through absorption and emission spectroscopy and experiments on thermo- and photostabilities, fluorescence time, solvatofluorochromism, and acidochromism; likewise, selectivity, sensitivity, cytotoxicity, detection limits, and, in one study, cellular penetration, were used to evaluate the dyes’ possible application as fluorescent probes in chemodetection or the biomarking of diverse species (i.e., ions, molecules, cells, etc.) with biological or environmental relevance. In addition, DFT calculations performed on several derivatives have enabled us to understand their electronic behavior better, thereby enhancing their optical applications. Therefore, we hope this contribution helps to understand the synthetic protocols and photophysical behavior of the 5:6 aza-fused N-heteroaromatic motif, enabling researchers to carry out novel investigations from the efficient production of these compounds. However, the structural and photophysical properties of these dye families require further investigation via DFT calculations, as do their potential applications in bioimaging.
Author Contributions
Two individuals listed as authors contributed significantly to the development of this manuscript, and no other person was involved with its composition. The authors’ contributions are as follows: literature review, analysis of articles, and composition of the original draft for the chapter on pyrazole derivatives, S.C.; conceptualization, analysis of articles, writing, manuscript review and editing, supervision, and resources, J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the science faculty at the Universidad de los Andes, project INV-2023-162-2810.
Acknowledgments
We thank the Chemistry Department and Vicerrectoría de Investigaciones at the Universidad de los Andes for financial support. J.P. wishes to acknowledge the current and former members of the Bioorganic Compounds Research Group, whose names appear in the reference section; this mention is for their valuable contributions to the various investigations on the synthesis and functionalization of diazolopyridines and diazolopyrimidines, including some relevant photophysical results on this important class of N-heterocyclic compounds. S.C. wishes to acknowledge the Universidad de Nariño for financial support to carry out her research stay.
Conflicts of Interest
The authors declare no conflicts of interest, as this literature review was conducted without any commercial or financial relationships.
References
- Dhayalan, V.; Dodke, V.S.; Pradeep Kumar, M.; Korkmaz, H.S.; Hoffmann-Röder, A.; Amaladass, P.; Dandela, R.; Dhanusuraman, R.; Knochel, P. Recent Synthetic Strategies for the Functionalization of Fused Bicyclic Heteroaromatics Using Organo-Li, -Mg and -Zn Reagents. Chem. Soc. Rev. 2024, 53, 11045–11099. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, G.; Sohail, M.; Bilal, M.; Rasool, N.; Qamar, M.U.; Ciurea, C.; Marceanu, L.G.; Misarca, C. N-Heterocycles as Promising Antiviral Agents: A Comprehensive Overview. Molecules 2024, 29, 2232. [Google Scholar] [CrossRef]
- Flanigan, D.M.; Romanov-Michailidis, F.; White, N.A.; Rovis, T. Organocatalytic Reactions Enabled by N-Heterocyclic Carbenes. Chem. Rev. 2015, 115, 9307–9387. [Google Scholar] [CrossRef] [PubMed]
- Maji, M.; Panja, D.; Borthakur, I.; Kundu, S. Recent Advances in Sustainable Synthesis of N-Heterocycles Following Acceptorless Dehydrogenative Coupling Protocol Using Alcohols. Org. Chem. Front. 2021, 8, 2673–2709. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, M.; Tan, W.; Zheng, L.; Tao, K.; Fan, X. Developments towards Synthesis of N-Heterocycles from Amidines: Via C-N/C-C Bond Formation. Org. Chem. Front. 2019, 6, 2120–2141. [Google Scholar] [CrossRef]
- Rios, M.C.; Bravo, N.F.; Sanchez, C.C.; Portilla, J. Chemosensors Based on N-Heterocyclic Dyes: Advances in Sensing Highly Toxic Ions Such as CN- and Hg2+. RSC Adv. 2021, 11, 34206–34234. [Google Scholar] [CrossRef]
- Jali, B.R.; Barick, A.K.; Mohapatra, P.; Sahoo, S.K. A Comprehensive Review on Quinones Based Fluoride Selective Colorimetric and Fluorescence Chemosensors. J. Fluor. Chem. 2021, 244, 109744. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M. Shamsuzzaman Review: Biologically Active Pyrazole Derivatives. New J. Chem. 2016, 41, 16–41. [Google Scholar] [CrossRef]
- Katritzky, A.; Vakulenko, A.; Sivapackiam, J.; Draghici, B.; Damavarapu, R. Synthesis of Dinitro-Substituted Furans, Thiophenes, and Azoles. Synthesis 2008, 2008, 699–706. [Google Scholar] [CrossRef]
- Sarmiento, J.T.; Portilla, J. Current Advances in Diazoles-Based Chemosensors for CN- and FDetection. Curr. Org. Synth. 2023, 20, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, P.; Penaloza, A.; Gris, M. Pyridoxine in Clinical Toxicology: A Review. Eur. J. Emerg. Med. 2005, 12, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Jennings, O. Antipyrin and the prevailing epidemic. Lancet 1890, 135, 105–106. [Google Scholar] [CrossRef]
- Arias-Gómez, A.; Macías, M.A.; Portilla, J. Synthesis of Structural Analogues of Reversan by Ester Aminolysis: An Access to Pyrazolo[1,5-a]Pyrimidines from Chalcones. RSC Adv. 2023, 13, 16377–16386. [Google Scholar] [CrossRef] [PubMed]
- Schwärzer, K.; Rout, S.K.; Bessinger, D.; Lima, F.; Brocklehurst, C.E.; Karaghiosoff, K.; Bein, T.; Knochel, P. Selective Functionalization of the 1H-Imidazo[1,2-b]Pyrazole Scaffold. A New Potential Non-Classical Isostere of Indole and a Precursor of Push–Pull Dyes. Chem. Sci. 2021, 12, 12993–13000. [Google Scholar] [CrossRef]
- Azuma, Y.; Edwardson, T.G.W.; Hilvert, D. Tailoring Lumazine Synthase Assemblies for Bionanotechnology. Chem. Soc. Rev. 2018, 47, 3543–3557. [Google Scholar] [CrossRef]
- Ortiz, M.-C.; Portilla, J. Access to Five-Membered N-Heteroaromatic Compounds: Current Appoach Based on Microwave-Assisted Synthesis. In Targets Heterocyclic Systems; Attanasi, O.A.S.D., Ed.; Società Chimica Italiana: Italy, 2021; Volume 25, pp. 436–462. [Google Scholar]
- Joule, J.A.; Mills, K. 1,2-Azoles: Pyrazoles, Isothiazoles, Isoxazoles: Reactions and Synthesis. In Heterocyclic Chemistry, 5th ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2010; pp. 485–501. ISBN 9781405193658. [Google Scholar]
- Portilla, J. Recent Advances in the Chemistry of Pyrazolo[1,5-a]Pyrimidines. In Advances Heterocyclic Chemistry; Scriven, E.F.V., Ramsden, C.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; Volume 142, pp. 71–138. [Google Scholar]
- Tigreros, A.; Macías, M.; Portilla, J. Structural, Photophysical, and Water Sensing Properties of Pyrazolo[1,5-a]Pyrimidine-Triphenylamine Hybrid Systems. ChemPhotoChem 2022, 6, e202200133. [Google Scholar] [CrossRef]
- Quiroga, J.; Trilleras, J.; Insuasty, B.; Abonía, R.; Nogueras, M.; Cobo, J. Regioselective Formylation of Pyrazolo[3,4-b]Pyridine and Pyrazolo[1,5-a]Pyrimidine Systems Using Vilsmeier-Haack Conditions. Tetrahedron Lett. 2008, 49, 2689–2691. [Google Scholar] [CrossRef]
- Bravo, N.-F.; Cifuentes, C.; Ríos, M.-C.; Pérez, L.-J.; Portilla, J. Synthesis and Photophysical Properties of Conjugated Fluorophores with N-Heteroaromatic Rings. Asian J. Org. Chem. 2025, 14, e202400742. [Google Scholar] [CrossRef]
- Tigreros, A.; Zapata-Rivera, J.; Portilla, J. Pyrazolo[1,5-a]Pyrimidinium Salts for Cyanide Sensing: A Performance and Sustainability Study of the Probes. ACS Sustain. Chem. Eng. 2021, 9, 12058–12069. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.; Ma, J.; Xu, H.; Wu, J.; Tang, X.; Fan, Z.; Wang, P. Synthesis and Properties of Fluorescence Dyes: Tetracyclic Pyrazolo[3,4-b]Pyridine-Based Coumarin Chromophores with Intramolecular Charge Transfer Character. J. Org. Chem. 2012, 77, 3475–3482. [Google Scholar] [CrossRef]
- García, M.; Romero, I.; Portilla, J. Synthesis of Fluorescent 1,7-Dipyridyl-Bis-Pyrazolo[3,4-b:4′,3′-e]Pyridines: Design of Reversible Chemosensors for Nanomolar Detection of Cu 2+. ACS Omega 2019, 4, 6757–6768. [Google Scholar] [CrossRef] [PubMed]
- Abet, V.; Nuñez, A.; Mendicuti, F.; Burgos, C.; Alvarez-Builla, J. A New Class of Pyrazolopyridine Nucleus with Fluorescent Properties, Obtained through Either a Radical or a Pd Arylation Pathway from N-Azinylpyridinium N-Aminides. J. Org. Chem. 2008, 73, 8800–8807. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Jain Kumar, S. A Comprehensive Review of N-Heterocycles as Cytotoxic Agents. Curr. Med. Chem. 2016, 23, 4338–4394. [Google Scholar] [CrossRef] [PubMed]
- Asati, V.; Anant, A.; Patel, P.; Kaur, K.; Gupta, G.D. Pyrazolopyrimidines as Anticancer Agents: A Review on Structural and Target-Based Approaches. Eur. J. Med. Chem. 2021, 225, 113781. [Google Scholar] [CrossRef]
- Cao, D.; Liu, Z.; Verwilst, P.; Koo, S.; Jangjili, P.; Kim, J.S.; Lin, W. Coumarin-Based Small-Molecule Fluorescent Chemosensors. Chem. Rev. 2019, 119, 10403–10519. [Google Scholar] [CrossRef]
- Terai, T.; Nagano, T. Small-Molecule Fluorophores and Fluorescent Probes for Bioimaging. Pflug. Arch. Eur. J. Physiol. 2013, 465, 347–359. [Google Scholar] [CrossRef]
- Kim, H.M.; Cho, B.R. Small-Molecule Two-Photon Probes for Bioimaging Applications. Chem. Rev. 2015, 115, 5014–5055. [Google Scholar] [CrossRef]
- Wu, L.; Liu, J.; Li, P.; Tang, B.; James, T.D. Two-Photon Small-Molecule Fluorescence-Based Agents for Sensing, Imaging, and Therapy within Biological Systems. Chem. Soc. Rev. 2021, 50, 702–734. [Google Scholar] [CrossRef]
- Jiao, X.; Li, Y.; Niu, J.; Xie, X.; Wang, X.; Tang, B. Small-Molecule Fluorescent Probes for Imaging and Detection of Reactive Oxygen, Nitrogen, and Sulfur Species in Biological Systems. Anal. Chem. 2018, 90, 533–555. [Google Scholar] [CrossRef]
- Ríos, M.-C.; Portilla, J. Recent Advances in Synthesis and Properties of Pyrazoles. Chemistry 2022, 4, 940–968. [Google Scholar] [CrossRef]
- Tigreros, A.; Portilla, J. Recent Progress in Chemosensors Based on Pyrazole Derivatives. RSC Adv. 2020, 10, 19693–19712. [Google Scholar] [CrossRef] [PubMed]
- Portilla, J. Current Advances in Synthesis of Pyrazole Derivatives: An Approach Toward Energetic Materials. J. Heterocycl. Chem. 2024, 61, 2026–2039. [Google Scholar] [CrossRef]
- Hemalatha K., K. R.H.; Pal, R.; Matada, G.S.P.; B, K.; I, A.; Aishwarya, N.V.S.S. Pyrazole, Pyrazoline, and Fused Pyrazole Derivatives: New Horizons in EGFR-Targeted Anticancer Agents. Chem. Biodivers. 2024, 21, e2024008. [Google Scholar] [CrossRef]
- Shaabani, A.; Nazeri, M.T.; Afshari, R. 5-Amino-Pyrazoles: Potent Reagents in Organic and Medicinal Synthesis. Mol. Divers. 2019, 23, 751–807. [Google Scholar] [CrossRef]
- Nair, A.R.; Sunil Kumar, Y.C.; Sivan, A. Synthesis and In-Depth Investigation of the Photophysical and Electrochemical Properties of Novel Pyrazole Cored D-A-D Molecules. Opt. Mater. 2022, 134, 113117. [Google Scholar] [CrossRef]
- Arias-Gómez, A.; Godoy, A.; Portilla, J. Functional Pyrazolo[1,5-a]Pyrimidines: Current Approaches in Synthetic Transformations and Uses as an Antitumor Scaffold. Molecules 2021, 26, 2708. [Google Scholar] [CrossRef]
- Devi Priya, D.; Nandhakumar, M.; Mohana Roopan, S. Pyrazolo[1,5-a]Pyridine: Recent Synthetic View on Crucial Heterocycles. Synth. Commun. 2020, 50, 3535–3562. [Google Scholar] [CrossRef]
- Donaire-Arias, A.; Montagut, A.M.; de la Bellacasa, R.P.; Estrada-Tejedor, R.; Teixidó, J.; Borrell, J.I. 1H-Pyrazolo[3,4-b]Pyridines: Biomedical Applications Synthesis and Biomedical Applications. Molecules 2022, 27, 2237. [Google Scholar] [CrossRef]
- Danel, A.; Gondek, E.; Kucharek, M.; Szlachcic, P.; Gut, A. 1H-Pyrazolo[3,4-b]Quinolines: Synthesis and Properties over 100 Years of Research. Molecules 2022, 27, 2775. [Google Scholar] [CrossRef]
- Atukuri, D. Pyrazolopyridine: An Efficient Pharmacophore in Recent Drug Design and Development. Chem. Biol. Drug Des. 2022, 100, 376–388. [Google Scholar] [CrossRef]
- Basir, N.H.; Ramle, A.Q.; Ng, M.P.; Tan, C.H.; Tiekink, E.R.T.; Sim, K.S.; Basirun, W.J.; Khairuddean, M. Discovery of Indoleninyl-Pyrazolo[3,4-b]Pyridines as Potent Chemotherapeutic Agents against Colorectal Cancer Cells. Bioorg. Chem. 2024, 146, 107256. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, T.; Hu, M.; Yang, Y.; Tang, M.; Deng, D.; Liu, K.; Fu, S.; Tan, Y.; Wang, H.; et al. Synthesis and Biological Evaluation of 6-(Pyrimidin-4-Yl)-1H-Pyrazolo[4,3-b]Pyridine Derivatives as Novel Dual FLT3/CDK4 Inhibitors. Bioorg. Chem. 2022, 121, 105669. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.A.; Halu, B.; Saundane, A.R.; Meti, R.S. Synthesis, Biological Validation, and Docking Studies of Novel Purine Derivatives Containing Pyridopyrimidine, Pyrazolopyridine, and Pyranonapthyridine Rings. Polycycl. Aromat. Compd. 2022, 42, 3694–3716. [Google Scholar] [CrossRef]
- Milišiūnaitė, V.; Arbačiauskienė, E.; Řezníčková, E.; Jorda, R.; Malínková, V.; Žukauskaitė, A.; Holzer, W.; Šačkus, A.; Kryštof, V. Synthesis and Anti-Mitotic Activity of 2,4- or 2,6-Disubstituted- and 2,4,6-Trisubstituted-2H-Pyrazolo[4,3-c]Pyridines. Eur. J. Med. Chem. 2018, 150, 908–919. [Google Scholar] [CrossRef]
- Sabat, M.; Wang, H.; Scorah, N.; Lawson, J.D.; Atienza, J.; Kamran, R.; Hixon, M.S.; Dougan, D.R. Design, Synthesis and Optimization of 7-Substituted-Pyrazolo[4,3-b]Pyridine ALK5 (Activin Receptor-like Kinase 5) Inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 1955–1961. [Google Scholar] [CrossRef]
- Metwally, N.H.; Deeb, E.A. Synthesis, Anticancer Assessment on Human Breast, Liver and Colon Carcinoma Cell Lines and Molecular Modeling Study Using Novel Pyrazolo[4,3-c]Pyridine Derivatives. Bioorg. Chem. 2018, 77, 203–214. [Google Scholar] [CrossRef]
- Braganza, J.F.; Bernier, L.; Botrous, I.; Collins, M.R.; Li, B.; McAlpine, I.; Ninkovic, S.; Ren, S.; Sach, N.; Tran-Dubé, M.; et al. Improved Cyclization Conditions to Prepare 6-Substituted Pyrazolo[1,5-a]Pyridines and Pyrazolo[1,5-a]Pyrazines Using Catalytic Ag(I) and Au(III) Salts. Tetrahedron Lett. 2015, 56, 5757–5760. [Google Scholar] [CrossRef]
- Mohan, D.C.; Ravi, C.; Rao, S.N.; Adimurthy, S. Copper-Mediated Synthesis of Pyrazolo[1,5-a]Pyridines through Oxidative Linkage of C–C/N–N Bonds. Org. Biomol. Chem. 2015, 13, 3556–3560. [Google Scholar] [CrossRef]
- Grošelj, U.; Pušavec, E.; Golobič, A.; Dahmann, G.; Stanovnik, B.; Svete, J. Synthesis of 1,5-Disubstituted-4-Oxo-4,5-Dihydro-1H-Pyrazolo[4,3-c]Pyridine-7-Carboxamides. Tetrahedron 2015, 71, 109–123. [Google Scholar] [CrossRef]
- Patel, J.B.; Malick, J.B. Pharmacological Properties of Tracazolate: A New Non-Benzodiazepine Anxiolytic Agent. Eur. J. Pharmacol. 1982, 78, 323–333. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, S.; Abadi, L.F.; Gaikwad, S.; Desai, D.; Bhutani, K.K.; Kulkarni, S.; Singh, I.P. Synthesis and in–Vitro Anti–HIV–1 Evaluation of Novel Pyrazolo[4,3-c]Pyridin–4–One Derivatives. Eur. J. Med. Chem. 2019, 183, 111714. [Google Scholar] [CrossRef] [PubMed]
- Razmienė, B.; Řezníčková, E.; Dambrauskienė, V.; Ostruszka, R.; Kubala, M.; Žukauskaitė, A.; Kryštof, V.; Šačkus, A.; Arbačiauskienė, E. Synthesis and Antiproliferative Activity of 2,4,6,7-Tetrasubstituted-2H-Pyrazolo[4,3-c]Pyridines. Molecules 2021, 26, 6747. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhang, L.; Hou, L.; Chen, F.; Liu, A.; Ji, R.; Wang, Q.; Yuan, C.; Ge, Y. A Fluorescent Probe with a Large Stokes Shift for Sensing Sulfite in Dry White Wine Based on Pyrazolo[1,5-a]Pyridine Fluorophore. Tetrahedron Lett. 2021, 76, 153210. [Google Scholar] [CrossRef]
- Agheli, Z.; Pordel, M.; Beyramabadi, S.A. Synthesis, Characterization, Optical Properties, Computational Characterizations, QTAIM Analysis and Cyclic Voltammetry of New Organic Dyes for Dye-Sensitized Solar Cells. J. Mol. Struct. 2020, 1202, 127228. [Google Scholar] [CrossRef]
- Wang, B.; Su, F.; Jia, J.; Wu, F.; Zhang, S.-Y.; Ge, Y.-Q.; Wang, J.; Wang, J.-W. Synthesis of 5-Cyanopyrazolo[1,5-a]Pyridine Derivatives via Tandem Reaction and Their Optical Properties. Tetrahedron Lett. 2015, 56, 425–429. [Google Scholar] [CrossRef]
- Kim, H.; Jo, A.; Ha, J.; Lee, Y.; Hwang, Y.S.; Park, S.B. A Pyrazolo[1,5-a]Pyridine-Fused Pyrimidine Based Novel Fluorophore and Its Bioapplication to Probing Lipid Droplets. Chem. Comm. 2016, 52, 7822–7825. [Google Scholar] [CrossRef]
- Yan, P.; Duan, G.; Ji, R.; Ge, Y. A Simple and Efficient Synthesis of New Fluorophore 4-Hydroxy Pyrazolo[1,5-a]Pyridines through a Tandem Reaction. Tetrahedron Lett. 2018, 59, 2426–2429. [Google Scholar] [CrossRef]
- Abumelha, H.M.; Bayazeed, A.; Alaysuy, O.; Alsoliemy, A.; Alharbi, A.; Habeebullah, T.M.; El-Metwaly, N.M. Synthesis, Photophysical Properties and DFT Studies of 2-(3-Cyano-4-((2-(4,6-Dimethyl-5-Nitro-1H-Pyrazolo[3,4-b]Pyridin-3-Yl)Hydrazono)Methyl)-5,5-Dimethylfuran-2(5H)-Ylidene)Malononitrile Dye. J. Saudi Chem. Soc. 2022, 26, 101502. [Google Scholar] [CrossRef]
- Orrego-Hernández, J.; Lizarazo, C.; Cobo, J.; Portilla, J. Pyrazolo-Fused 4-Azafluorenones as Key Reagents for the Synthesis of Fluorescent Dicyanovinylidene-Substituted Derivatives. RSC Adv. 2019, 9, 27318–27323. [Google Scholar] [CrossRef]
- Krishnan, U.; Manickam, S.; Iyer, S.K. BF3 Detection by Pyrazolo-Pyridine Based Fluorescent Probe and Applications in Bioimaging and Paper Strip Analysis. J. Mol. Liq. 2023, 385, 122413. [Google Scholar] [CrossRef]
- Nam, N.L.; Grandberg, I.I.; Sorokin, V.I. Pyrazolopyrimidines Based on 5-Aminopyrazoles Unsubstituted at the Position 1. Chem. Heterocycl. Compd. 2002, 38, 1371–1374. [Google Scholar] [CrossRef]
- Hassanzadeh-Afruzi, F.; Amiri-Khamakani, Z.; Saeidirad, M.; Salehi, M.M.; Taheri-Ledari, R.; Maleki, A. Facile Synthesis of Pyrazolopyridine Pharmaceuticals under Mild Conditions Using an Algin-Functionalized Silica-Based Magnetic Nanocatalyst (Alg@SBA-15/Fe3O4). RSC Adv. 2023, 13, 10367–10378. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, H.A.; Saleh, T.S.; El-Zahabi, H.S.A. Facile Synthesis and In-Vitro Antitumor Activity of Some Pyrazolo[3,4-b]Pyridines and Pyrazolo[1,5-a]Pyrimidines Linked to a Thiazolo[3,2-a]Benzimidazole Moiety. Arch. Pharm. 2010, 343, 24–30. [Google Scholar] [CrossRef]
- Ghaedi, A.; Bardajee, G.R.; Mirshokrayi, A.; Mahdavi, M.; Shafiee, A.; Akbarzadeh, T. Facile, Novel and Efficient Synthesis of New Pyrazolo[3,4-b]Pyridine Products from Condensation of Pyrazole-5-Amine Derivatives and Activated Carbonyl Groups. RSC Adv. 2015, 5, 89652–89658. [Google Scholar] [CrossRef]
- Tigreros, A.; Portilla, J. Ecological and Economic Efforts in the Development of Molecular Sensors for the Optical Detection of Cyanide Ions. Eur. J. Org. Chem. 2022, 2022, e202200249. [Google Scholar] [CrossRef]
- Deore, R.; Dingore, K.; Jachak, M. An Efficient Synthesis and Photophysical Properties of 1H-Pyrazolo[3,4-b]Pyridine and Pyrazolo[3,4-b][1,8]Naphthyridines. J. Fluoresc. 2015, 25, 1549–1557. [Google Scholar] [CrossRef]
- Supranovich, V.I.; Vorob’ev, A.Y.; Borodkin, G.I.; Gatilov, Y.V.; Shubin, V.G. Study on Selectivity in the Reaction of 2-Substituted Pyridinium-N-Imines with Dimethyl Acetylenedicarboxylate. Tetrahedron Lett. 2016, 57, 1093–1096. [Google Scholar] [CrossRef]
- Miliutina, M.; Janke, J.; Chirkina, E.; Hassan, S.; Ejaz, S.A.; Khan, S.U.; Iqbal, J.; Friedrich, A.; Lochbrunner, S.; Ivanov, A.; et al. Domino Reactions of Chromone-3-carboxylic Acids with Aminoheterocycles: Synthesis of Heteroannulated Pyrido[2,3-c]Coumarins and Their Optical and Biological Activity. Eur. J. Org. Chem. 2017, 2017, 7148–7159. [Google Scholar] [CrossRef]
- Charris-Molina, A.; Castillo, J.C.; Macías, M.; Portilla, J. One-Step Synthesis of Fully Functionalized Pyrazolo[3,4-b]Pyridines via Isobenzofuranone Ring Opening. J. Org. Chem. 2017, 82, 12674–12681. [Google Scholar] [CrossRef]
- Manickam, S.; Balijapalli, U.; Sawminathan, S.; Samuelrajamani, P.; Kamaraj, S.; Shanmugam, V.; Ramalingam, S.; Iyer, S.K. One-Pot Synthesis and Photophysical Studies of Styryl-Based Benzo[f]Pyrazolo[3,4-b]Quinoline and Indeno[2,1-b]Pyrazolo[4,3-e]Pyridines. Eur. J. Org. Chem. 2018, 2018, 6204–6216. [Google Scholar] [CrossRef]
- Alizadeh, A.; Farajpour, B.; Mohammadi, S.S.; Sedghi, M.; Naderi-Manesh, H.; Janiak, C.; Knedel, T. Design and Synthesis of Coumarin-Based Pyrazolopyridines as Biocompatible Fluorescence Dyes for Live-Cell Imaging. ChemistrySelect 2020, 5, 9362–9369. [Google Scholar] [CrossRef]
- Ma, W.; Wang, Y.; Gao, J.; Sun, R.; Ge, J.-F. Synthesis and Optical Properties of Bispyrazolopyridine Derivatives. Dyes Pigm. 2020, 181, 108569. [Google Scholar] [CrossRef]
- Vidali, V.P.; Nigianni, G.; Athanassopoulou, G.D.; Canko, A.; Mavroidi, B.; Matiadis, D.; Pelecanou, M.; Sagnou, M. Synthesis of Novel Pyrazolo[3,4-b]Pyridines with Affinity for β-Amyloid Plaques. Molbank 2022, 2022, M1343. [Google Scholar] [CrossRef]
- Shuvalov, V.Y.; Vlasova, E.Y.; Zheleznova, T.Y.; Fisyuk, A.S. New One-Pot Synthesis of 4-Arylpyrazolo[3,4-b]Pyridin-6-Ones Based on 5-Aminopyrazoles and Azlactones. Beilstein J. Org. Chem. 2023, 19, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Kolbus, A.; Uchacz, T.; Danel, A.; Gałczyńska, K.; Moskwa, P.; Kolek, P. Fluorescent Sensor Based on 1H-Pyrazolo[3,4-b]Quinoline Derivative for Detecting Zn2+ Cations. Molecules 2024, 29, 823. [Google Scholar] [CrossRef]
- Kolbus, A.; Danel, A.; Moskwa, P.; Szary, K.; Uchacz, T. Pyrazoloquinoline-Based Fluorescent Sensor for the Detection of Pb2+, Zn2+ and the Realization of an OR-Type Optical Logic Gate. Dyes Pigm. 2024, 223, 111956. [Google Scholar] [CrossRef]
- Redon, S.; Vanelle, P. Simple and Green Synthesis of 3-acyl-pyrazolo[1,5-a]Pyridines: [3+2] through Cycloaddition of N-aminopyridines on Enaminones. J. Heterocycl. Chem. 2024, 61, 1200–1205. [Google Scholar] [CrossRef]
- Al-Azmi, A. Pyrazolo[1,5-a]Pyrimidines: A Close Look into Their Synthesis and Applications. Curr. Org. Chem. 2019, 23, 721–743. [Google Scholar] [CrossRef]
- Salem, M.A.; Helal, M.H.; Gouda, M.A.; Abd EL-Gawad, H.H.; Shehab, M.A.M.; El-Khalafawy, A. Recent Synthetic Methodologies for Pyrazolo[1,5-a]Pyrimidine. Synth. Commun. 2019, 49, 1750–1776. [Google Scholar] [CrossRef]
- Ghelani, S.M.; Naliapara, Y.T. Design, Multicomponent Synthesis and Characterization of Diversely Substituted Pyrazolo[1,5-a]Pyrimidine Derivatives. J. Heterocycl. Chem. 2016, 53, 1843–1851. [Google Scholar] [CrossRef]
- Cherukupalli, S.; Karpoormath, R.; Chandrasekaran, B.; Hampannavar, G.A.; Thapliyal, N.; Palakollu, V.N. An Insight on Synthetic and Medicinal Aspects of Pyrazolo[1,5-a]Pyrimidine Scaffold. Eur. J. Med. Chem. 2017, 126, 298–352. [Google Scholar] [CrossRef] [PubMed]
- George, C.F.P. Pyrazolopyrimidines. Lancet 2001, 358, 1623–1626. [Google Scholar] [CrossRef] [PubMed]
- Elnagdi, M.H.; Elmoghayar, M.R.H.; Elgemeie, G.E.H. Chemistry of Pyrazolopyrimidines. In Advances Heterocyclic Chemistry; Katritzky, A.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1987; Volume 41, pp. 319–376. [Google Scholar]
- Yang, X.-Z.; Sun, R.; Guo, X.; Wei, X.-R.; Gao, J.; Xu, Y.-J.; Ge, J.-F. The Application of Bioactive Pyrazolopyrimidine Unit for the Construction of Fluorescent Biomarkers. Dyes Pigm. 2020, 173, 107878. [Google Scholar] [CrossRef]
- Cherukupalli, S.; Hampannavar, G.A.; Chinnam, S.; Chandrasekaran, B.; Sayyad, N.; Kayamba, F.; Reddy Aleti, R.; Karpoormath, R. An Appraisal on Synthetic and Pharmaceutical Perspectives of Pyrazolo[4,3-d]Pyrimidine Scaffold. Bioorg. Med. Chem. 2018, 26, 309–339. [Google Scholar] [CrossRef]
- Squarcialupi, L.; Catarzi, D.; Varano, F.; Betti, M.; Falsini, M.; Vincenzi, F.; Ravani, A.; Ciancetta, A.; Varani, K.; Moro, S.; et al. Structural Refinement of Pyrazolo[4,3-d]Pyrimidine Derivatives to Obtain Highly Potent and Selective Antagonists for the Human A3 Adenosine Receptor. Eur. J. Med. Chem. 2016, 108, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Quadery, T.M.; Bai, R.; Luckett-Chastain, L.R.; Hamel, E.; Ihnat, M.A.; Gangjee, A. Novel Pyrazolo[4,3-d]Pyrimidine Microtubule Targeting Agents (MTAs): Synthesis, Structure–Activity Relationship, in Vitro and in Vivo Evaluation as Antitumor Agents. Bioorg. Med. Chem. Lett. 2021, 41, 127923. [Google Scholar] [CrossRef]
- Hafez, H.N.; El-Gazzar, A.-R.B.A.; Al-Hussain, S.A. Novel Pyrazole Derivatives with Oxa/Thiadiazolyl, Pyrazolyl Moieties and Pyrazolo[4,3-d]Pyrimidine Derivatives as Potential Antimicrobial and Anticancer Agents. Bioorg. Med. Chem. Lett. 2016, 26, 2428–2433. [Google Scholar] [CrossRef]
- Wang, B.S.; Huang, X.; Chen, L.Z.; Liu, M.M.; Shi, J.B. Design and Synthesis of Novel Pyrazolo[4,3- d ]Pyrimidines as Potential Therapeutic Agents for Acute Lung Injury. J. Enzyme Inhib. Med. Chem. 2019, 34, 1121–1130. [Google Scholar] [CrossRef]
- Abdellatif, K.R.A.; Bakr, R.B. New Advances in Synthesis and Clinical Aspects of Pyrazolo[3,4-d]Pyrimidine Scaffolds. Bioorg. Chem. 2018, 78, 341–357. [Google Scholar] [CrossRef]
- Abdelgawad, M.A.; Bakr, R.B.; Alkhoja, O.A.; Mohamed, W.R. Design, Synthesis and Antitumor Activity of Novel Pyrazolo[3,4-d]Pyrimidine Derivatives as EGFR-TK Inhibitors. Bioorg. Chem. 2016, 66, 88–96. [Google Scholar] [CrossRef]
- Nassar, I.F.; Atta-Allah, S.R.; Elgazwy, A.-S.S.H. A Convenient Synthesis and Molecular Modeling Study of Novel Pyrazolo[3,4-d]Pyrimidine and Pyrazole Derivatives as Anti-Tumor Agents. J. Enzyme Inhib. Med. Chem. 2015, 30, 396–405. [Google Scholar] [CrossRef] [PubMed]
- El-Morsy, A.M.; El-Sayed, M.S.; Abulkhair, H.S. Synthesis, Characterization and In Vitro Antitumor Evaluation of New Pyrazolo[3,4-d]Pyrimidine Derivatives. Open J. Med. Chem. 2017, 7, 1–17. [Google Scholar] [CrossRef]
- Reddy, B.N.; Ruddarraju, R.R.; Kiran, G.; Pathak, M.; Reddy, A.R.N. Novel Pyrazolo[3,4-d]Pyrimidine-Containing Amide Derivatives: Synthesis, Molecular Docking, In Vitro and In Vivo Antidiabetic Activity. ChemistrySelect 2019, 4, 10072–10078. [Google Scholar] [CrossRef]
- Atatreh, N.; Youssef, A.M.; Ghattas, M.A.; Al Sorkhy, M.; Alrawashdeh, S.; Al-Harbi, K.B.; El-Ashmawy, I.M.; Almundarij, T.I.; Abdelghani, A.A.; Abd-El-Aziz, A.S. Anti-Inflammatory Drug Approach: Synthesis and Biological Evaluation of Novel Pyrazolo[3,4-d]Pyrimidine Compounds. Bioorg. Chem. 2019, 86, 393–400. [Google Scholar] [CrossRef]
- Gilles, P.; Kashyap, R.S.; Freitas, M.J.; Ceusters, S.; Van Asch, K.; Janssens, A.; De Jonghe, S.; Persoons, L.; Cobbaut, M.; Daelemans, D.; et al. Design, Synthesis and Biological Evaluation of Pyrazolo[3,4-d]Pyrimidine-Based Protein Kinase D Inhibitors. Eur. J. Med. Chem. 2020, 205, 112638. [Google Scholar] [CrossRef]
- Ballesteros-Casallas, A.; Paulino, M.; Vidossich, P.; Melo, C.; Jiménez, E.; Castillo, J.-C.; Portilla, J.; Miscione, G. Pietro Synthesis of 2,7-Diarylpyrazolo [1,5-a] Pyrimidine Derivatives with Antitumor Activity. Theoretical Identification of Targets. Eur. J. Med. Chem. Rep. 2022, 4, 100028. [Google Scholar] [CrossRef]
- Dwyer, M.P.; Keertikar, K.; Paruch, K.; Alvarez, C.; Labroli, M.; Poker, C.; Fischmann, T.O.; Mayer-Ezell, R.; Bond, R.; Wang, Y.; et al. Discovery of Pyrazolo[1,5-a]Pyrimidine-Based Pim Inhibitors: A Template-Based Approach. Bioorg. Med. Chem. Lett. 2013, 23, 6178–6182. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zhou, Y.; Zhang, Q.; Han, T.; Tang, C.; Fan, W. Pyrazolo[1,5-a]Pyrimidine Based Trk Inhibitors: Design, Synthesis, Biological Activity Evaluation. Bioorg. Med. Chem. Lett. 2021, 31, 127712. [Google Scholar] [CrossRef]
- Ismail, N.S.M.; Ali, G.M.E.; Ibrahim, D.A.; Elmetwali, A.M. Medicinal Attributes of Pyrazolo[1,5-a]Pyrimidine Based Scaffold Derivatives Targeting Kinases as Anticancer Agents. Futur. J. Pharm. Sci. 2016, 2, 60–70. [Google Scholar] [CrossRef]
- Phillipson, L.J.; Segal, D.H.; Nero, T.L.; Parker, M.W.; Wan, S.S.; De Silva, M.; Guthridge, M.A.; Wei, A.H.; Burns, C.J. Discovery and SAR of Novel Pyrazolo[1,5-a]Pyrimidines as Inhibitors of CDK9. Bioorg. Med. Chem. 2015, 23, 6280–6296. [Google Scholar] [CrossRef]
- Naik, N.S.; Shastri, L.A.; Chougala, B.M.; Samundeeswari, S.; Holiyachi, M.; Joshi, S.D.; Sunagar, V. Synthesis of Novel Aryl and Coumarin Substituted Pyrazolo[1,5-a]Pyrimidine Derivatives as Potent Anti-Inflammatory and Anticancer Agents. Chem. Comm. 2020, 30, 100550. [Google Scholar] [CrossRef]
- Deshmukh, S.; Dingore, K.; Gaikwad, V.; Jachak, M. An Efficient Synthesis of Pyrazolo[1,5-a]Pyrimidines and Evaluation of Their Antimicrobial Activity. J. Chem. Sci. 2016, 128, 1459–1468. [Google Scholar] [CrossRef]
- Gregg, B.T.; Tymoshenko, D.O.; Razzano, D.A.; Johnson, M.R. Pyrazolo[1,5-a]Pyrimidines. Identification of the Privileged Structure and Combinatorial Synthesis of 3-(Hetero)Arylpyrazolo[1,5-a]Pyrimidine-6- Carboxamides. J. Comb. Chem. 2007, 9, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Damont, A.; Médran-Navarrete, V.; Cacheux, F.; Kuhnast, B.; Pottier, G.; Bernards, N.; Marguet, F.; Puech, F.; Boisgard, R.; Dollé, F. Novel Pyrazolo[1,5-a]Pyrimidines as Translocator Protein 18 KDa (TSPO) Ligands: Synthesis, in Vitro Biological Evaluation, [18F]-Labeling, and in Vivo Neuroinflammation PET Images. J. Med. Chem. 2015, 58, 7449–7464. [Google Scholar] [CrossRef]
- García-Olave, M.; Tigreros, A.; Iglesias, B.A.; Portilla, J. Synthesis and Photophysical Properties of Pyrazolo[1,5-a]Pyrimidines Containing D−π−A Architecture Similar to Prodan. J. Mol. Struct. 2024, 1317, 139142. [Google Scholar] [CrossRef]
- Abdelazeem, A.H.; Abdelatef, S.A.; El-Saadi, M.T.; Omar, H.A.; Khan, S.I.; McCurdy, C.R.; El-Moghazy, S.M. Novel Pyrazolopyrimidine Derivatives Targeting COXs and INOS Enzymes; Design, Synthesis and Biological Evaluation as Potential Anti-Inflammatory Agents. Eur. J. Pharm. Sci. 2014, 62, 197–211. [Google Scholar] [CrossRef]
- Stefanello, F.S.; Kappenberg, Y.G.; Luz, F.M.; Araújo, J.N.; Zanatta, N.; Martins, M.A.P.; Iglesias, B.A.; Bonacorso, H.G. Thermal and Oxidative Syntheses, Structure, and Optical Properties of 2,6,8-Trisubstituted 1,2,3-Triazolo[4′,5′:3,4]Pyrazolo[1,5-a]Pyrimidin-2-Ium-1(3)-Ides. J. Mol. Struct. 2023, 1293, 136220. [Google Scholar] [CrossRef]
- Tigreros, A.; Castillo, J.C.; Portilla, J. Cyanide Chemosensors Based on 3-Dicyanovinylpyrazolo[1,5-a]Pyrimidines: Effects of Peripheral 4-Anisyl Group Substitution on the Photophysical Properties. Talanta 2020, 215, 120905. [Google Scholar] [CrossRef]
- Tigreros, A.; Rosero, H.A.; Castillo, J.C.; Portilla, J. Integrated Pyrazolo[1,5-a]Pyrimidine–Hemicyanine System as a Colorimetric and Fluorometric Chemosensor for Cyanide Recognition in Water. Talanta 2019, 196, 395–401. [Google Scholar] [CrossRef]
- Tigreros, A.; Macías, M.; Portilla, J. Photophysical and Crystallographic Study of Three Integrated Pyrazolo[1,5-a]Pyrimidine–Triphenylamine Systems. Dyes Pigm. 2021, 184, 108730. [Google Scholar] [CrossRef]
- Stefanello, F.S.; Kappenberg, Y.G.; Araújo, J.N.; Dozza, B.; Nogara, P.A.; Rocha, J.B.T.; Zanatta, N.; Martins, M.A.P.; Bonacorso, H.G.; Iglesias, B.A. Bromo-Substituted Diazenyl-pyrazolo[1,5-a]Pyrimidin-2-amines: Sonogashira Cross-Coupling Reaction, Photophysical Properties, Bio-interaction and HSA Light-Up Sensor. ChemBioChem 2022, 23, e202200248. [Google Scholar] [CrossRef]
- Mohammed, S.; Vishwakarma, R.A.; Bharate, S.B. Iodine Catalyzed Oxidative Synthesis of Quinazolin-4(3H-Ones and Pyrazolo[4,3-d]Pyrimidin-7(6H)-Ones via Amination of Sp3 C–H Bond. J. Org. Chem. 2015, 80, 6915–6921. [Google Scholar] [CrossRef] [PubMed]
- Şener, N.; Erişkin, S.; Yavuz, S.; Şener, İ. Synthesis, Characterization, Solvatochromic Properties, and Antimicrobial-radical Scavenging Activities of New Diazo Dyes Derived from Pyrazolo[1,5-a]Pyrimidine. J. Heterocycl. Chem. 2017, 54, 3538–3548. [Google Scholar] [CrossRef]
- Castillo, J.-C.; Tigreros, A.; Portilla, J. 3-Formylpyrazolo[1,5-a]Pyrimidines as Key Intermediates for the Preparation of Functional Fluorophores. J. Org. Chem. 2018, 83, 10887–10897. [Google Scholar] [CrossRef] [PubMed]
- Singsardar, M.; Sarkar, R.; Majhi, K.; Sinha, S.; Hajra, A. Brønsted Acidic Ionic Liquid-Catalyzed Regioselective Synthesis of Pyrazolopyrimidines and Their Photophysical Properties. ChemistrySelect 2018, 3, 1404–1410. [Google Scholar] [CrossRef]
- Tigreros, A.; Aranzazu, S.-L.; Bravo, N.-F.; Zapata-Rivera, J.; Portilla, J. Pyrazolo[1,5-a]Pyrimidines Based Fluorophores: A Comprehensive Theoretical-Experimental Study. RSC Adv. 2020, 10, 39542–39552. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Y.; Lv, L.; Li, Z. Regioselective Synthesis of Emission Color-Tunable Pyrazolo[1,5-a]Pyrimidines with β,β-Difluoro Peroxides as 1,3-Bis-Electrophiles. Adv. Synth. Catal. 2021, 363, 3233–3239. [Google Scholar] [CrossRef]
- Squarcialupi, L.; Betti, M.; Catarzi, D.; Varano, F.; Falsini, M.; Ravani, A.; Pasquini, S.; Vincenzi, F.; Salmaso, V.; Sturlese, M.; et al. The Role of 5-Arylalkylamino- and 5-Piperazino- Moieties on the 7-Aminopyrazolo[4,3-d]Pyrimidine Core in Affecting Adenosine A1 and A2 Receptor Affinity and Selectivity Profiles. J. Enzyme Inhib. Med. Chem. 2017, 32, 248–263. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, S.; Li, Z.; Fu, Y.; Wang, G.; Zhang, J.; Wu, X. Design, Synthesis, Biological Evaluation and Molecular Modeling of Novel 1H-Pyrazolo[3,4-d]Pyrimidine Derivatives as BRAFV600E and VEGFR-2 Dual Inhibitors. Eur. J. Med. Chem. 2018, 155, 210–228. [Google Scholar] [CrossRef]
- Shi, J.B.; Chen, L.Z.; Wang, B.S.; Huang, X.; Jiao, M.M.; Liu, M.M.; Tang, W.J.; Liu, X.H. Novel Pyrazolo[4,3-d]Pyrimidine as Potent and Orally Active Inducible Nitric Oxide Synthase (INOS) Dimerization Inhibitor with Efficacy in Rheumatoid Arthritis Mouse Model. J. Med. Chem. 2019, 62, 4013–4031. [Google Scholar] [CrossRef]
- Hassaneen, H.M.; Saleh, F.M.; Abdallah, T.A.; Mohamed, Y.S.; Awad, E.M. Synthesis, Reactions, and Antimicrobial Activity of Some Novel Pyrazolo[3,4-d]Pyrimidine, Pyrazolo[4,3-e][1,2,4]Triazolo[1,5-c]Pyrimidine, and Pyrazolo[4,3-e][1,2,4]Triazolo[3,4-c]Pyrimidine Derivatives. J. Heterocycl. Chem. 2020, 57, 892–912. [Google Scholar] [CrossRef]
- Stefanello, F.S.; Kappenberg, Y.G.; Araújo, J.N.; Franceschini, S.Z.; Martins, M.A.P.; Zanatta, N.; Iglesias, B.A.; Bonacorso, H.G. Trifluoromethyl-Substituted Aryldiazenyl-Pyrazolo[1,5-a]Pyrimidin-2-Amines: Regioselective Synthesis, Structure, and Optical Properties. J. Fluor. Chem. 2022, 255–256, 109967. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, Y.; Wang, L.; Wu, T.; Yin, W.; Wang, J.; Xue, Y.; Qin, Q.; Sun, Y.; Yang, H.; et al. Design, Synthesis, and Biological Evaluation of Novel Pyrazolo [3,4-d]Pyrimidine Derivatives as Potent PLK4 Inhibitors for the Treatment of TRIM37-Amplified Breast Cancer. Eur. J. Med. Chem. 2022, 238, 114424. [Google Scholar] [CrossRef]
- Tigreros, A.; Aranzazu, S.-L.; Ríos, M.-C.; Portilla, J. Pyrazolo[1,5-a]Pyrimidine–Dioxaborinine Hybrid Dyes: Synthesis and Substituent Effect in the Photophysical Properties. Eur. J. Org. Chem. 2023, 26, e202300089. [Google Scholar] [CrossRef]
- Aranzazu, S.L.; Tigreros, A.; Arias-Gómez, A.; Zapata-Rivera, J.; Portilla, J. BF3-Mediated Acetylation of Pyrazolo[1,5-a]Pyrimidines and Other π-Excedent (N-Hetero)Arenes. J. Org. Chem. 2022, 87, 9839–9850. [Google Scholar] [CrossRef]
- Mahnashi, M.; Alshahrani, M.M.; Al Ali, A.; Asiri, A.; Abou-Salim, M.A. Novel Glu-Based Pyrazolo[3,4-d]Pyrimidine Analogues: Design, Synthesis and Biological Evaluation as DHFR and TS Dual Inhibitors. Enzym. Inhib. Med. Chem. 2023, 38, 2203879. [Google Scholar] [CrossRef]
- Stefanello, F.S.; Kappenberg, Y.G.; Araújo, J.N.; Gritzenco, F.; Luz, F.M.; Martins, M.A.P.; Zanatta, N.; Iglesias, B.A.; Bonacorso, H.G. 3,7-Bis(Trifluoromethyl)-2-Methylpyrazolo[1,5-a]Pyrimidines: Sequential Synthesis via Iodination (NIS)/Trifluoromethylation (MFSDA) Reactions and Photoluminescent Behavior. J. Mol. Struct. 2024, 1311, 138350. [Google Scholar] [CrossRef]
- Niko, Y.; Kawauchi, S.; Konishi, G. Solvatochromic Pyrene Analogues of Prodan Exhibiting Extremely High Fluorescence Quantum Yields in Apolar and Polar Solvents. Chem. Eur. J. 2013, 19, 9760–9765. [Google Scholar] [CrossRef]
- Chung, C.-Y.; Li, S.-M.; Zeng, W.-Z.; Uramaru, N.; Huang, G.-J.; Juang, S.-H.; Wong, F.F. Synthesis, Design, and Antiproliferative Evaluation of 6-(N-Substituted-Methyl)Pyrazolo[3,4-d]Pyrimidines as the Potent Anti-Leukemia Agents. Bioorg. Chem. 2024, 148, 107424. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).