Revolutionizing Cancer Vaccine: The Power of Advanced Nanotechnology
Abstract
1. Introduction
2. Cancer Immunotherapy
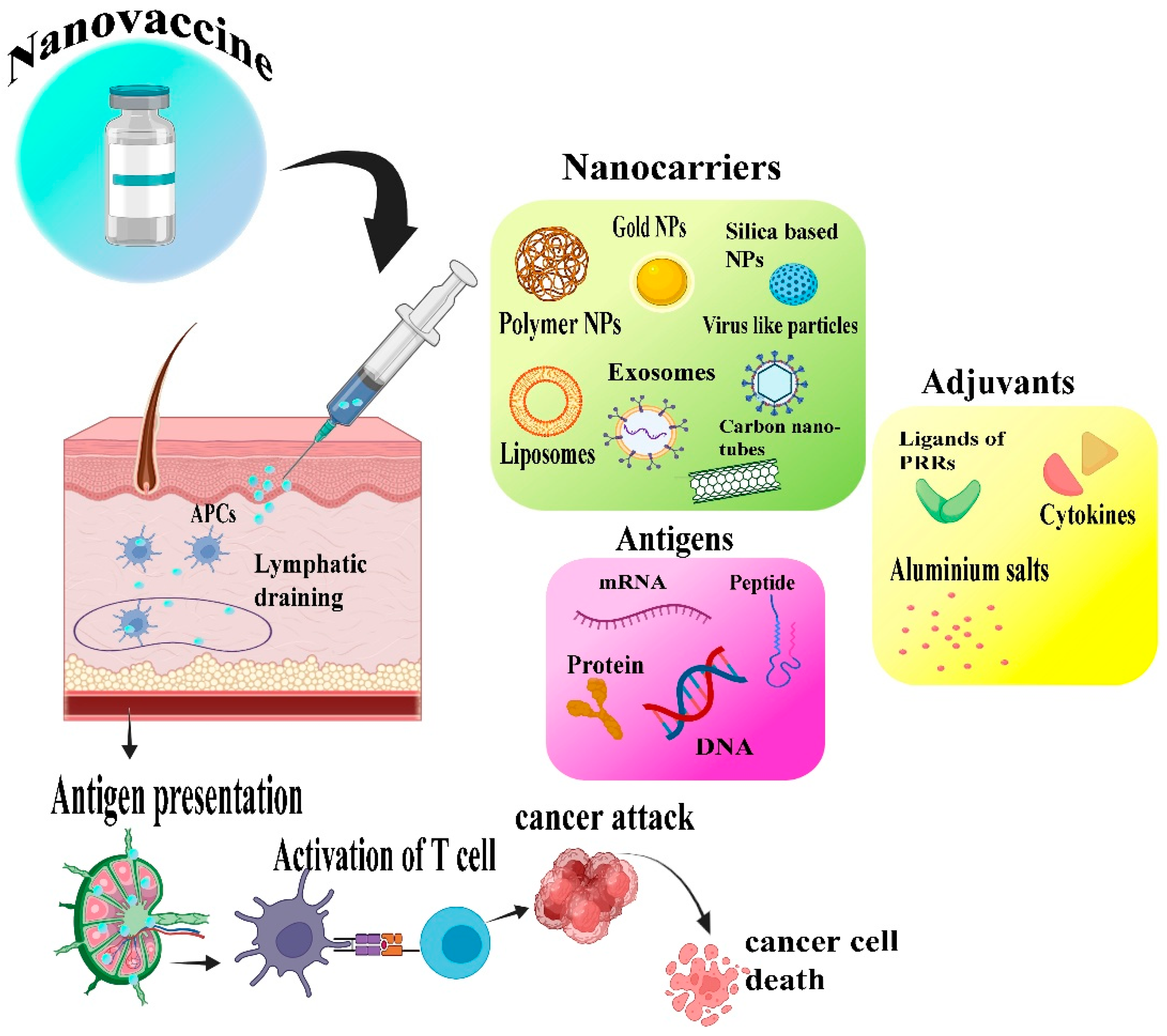
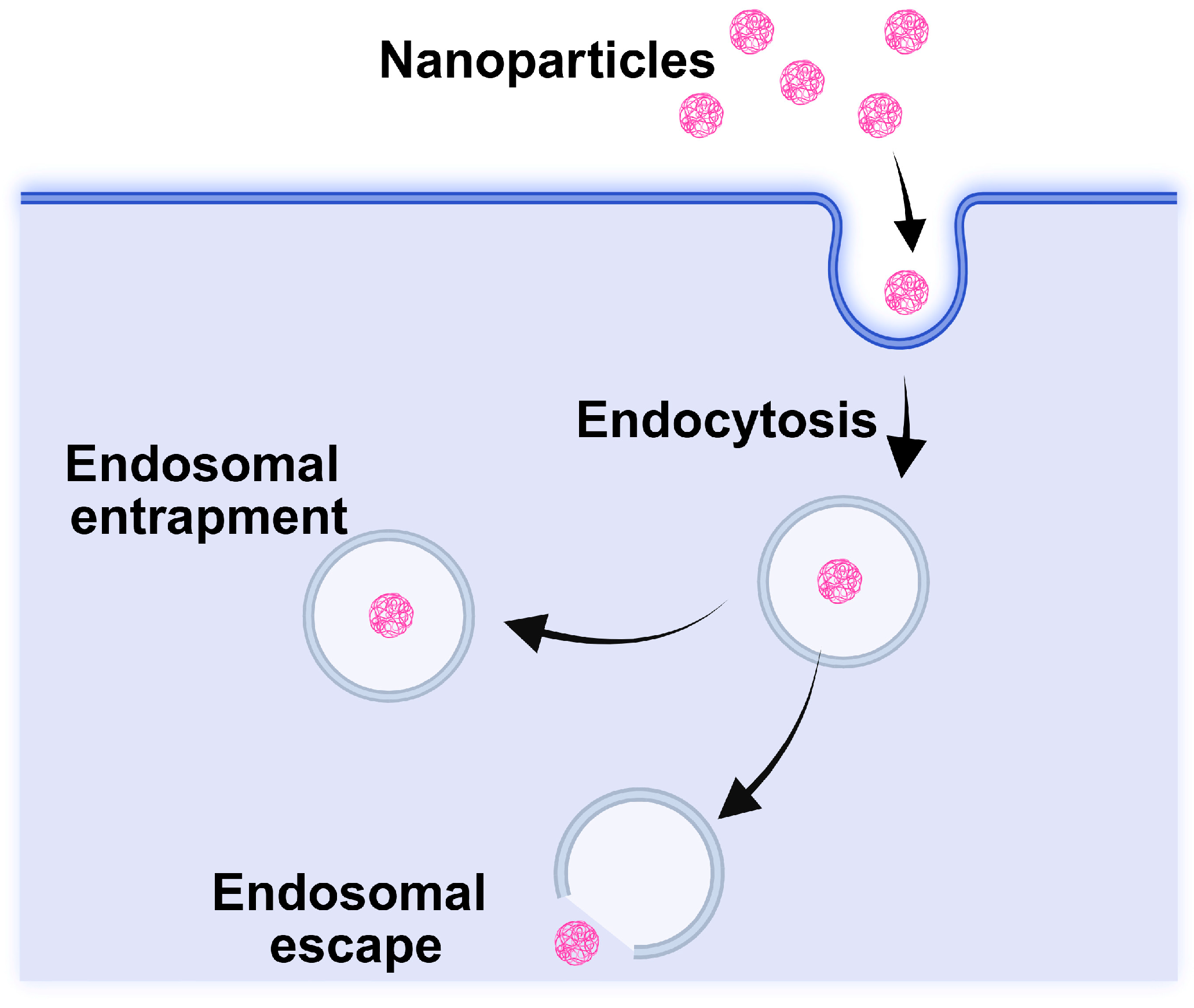
3. Nanotechnology’s Role in the Development of Cancer Vaccine
4. Types of Nanocarriers in Cancer Nanovaccines
4.1. Synthetic Nanocarriers
4.1.1. Lipid- and Polymer-Based Carriers
4.1.2. Inorganic Nanocarriers for Nanovaccines
4.1.3. Dendritic Cell-Based Nanovaccines
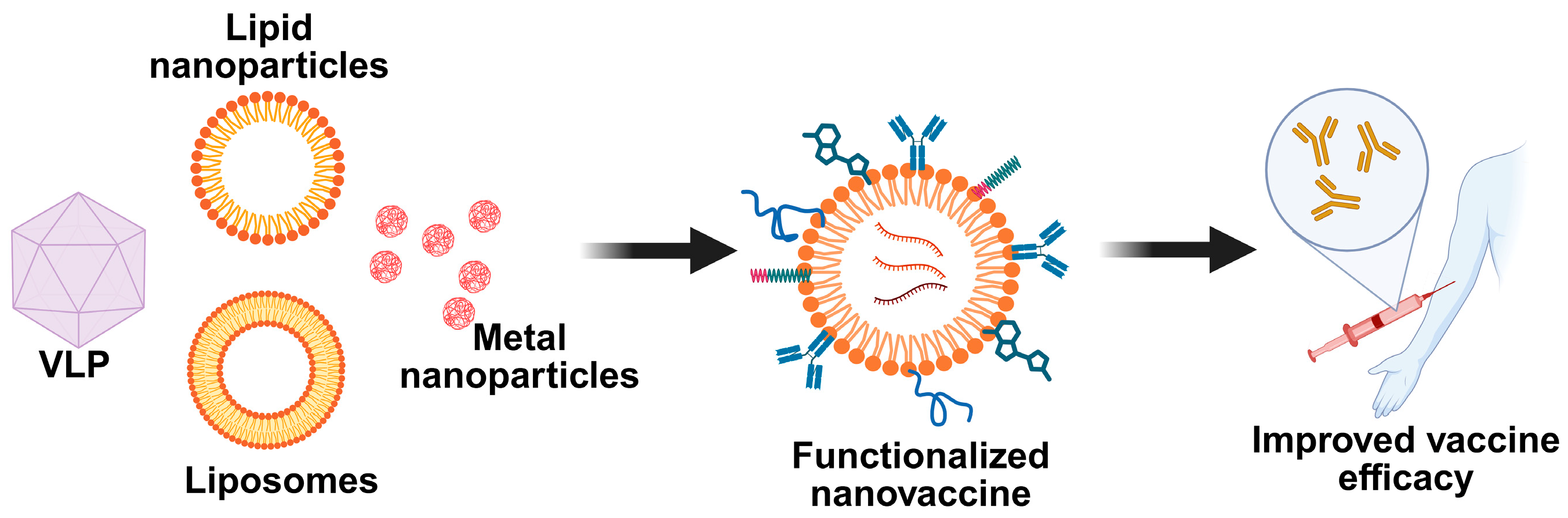
4.2. Semisynthetic Nanocarriers
4.3. Biogenic Nanocarriers (Exosome-Based)
4.4. Functionally Designed Cancer Nanovaccines
4.4.1. Neoantigen-Based Nano Vaccines
4.4.2. STING Agonist Based Nanovaccines
4.4.3. Self Adjuvant Nanovaccines
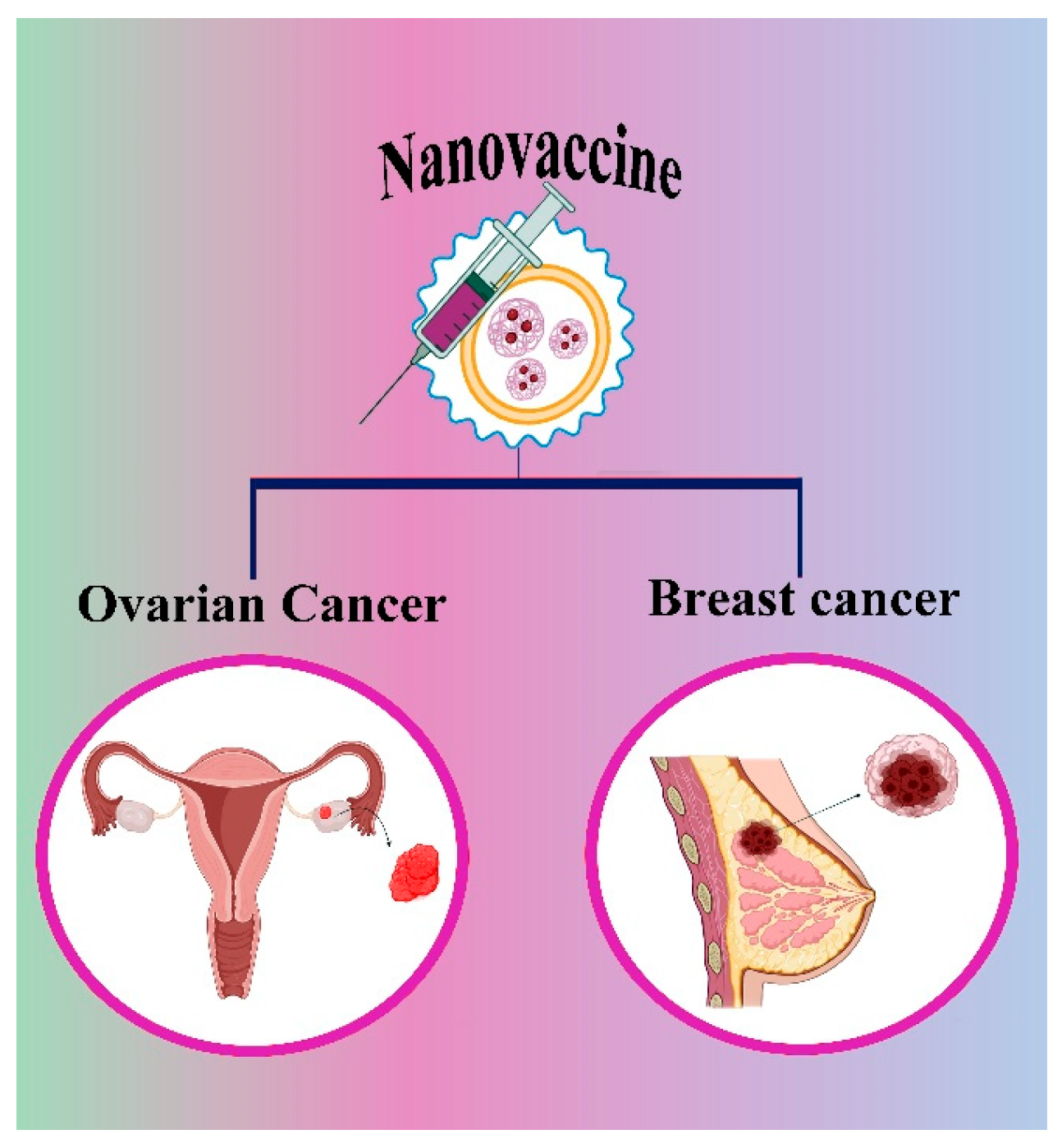
5. Recent Cancer Nanovaccine in the Market
5.1. Nanovaccine for Ovarian Cancer
5.2. Nanovaccines for Breast Cancer
5.3. Applied Strategy for Vaccine Administration
6. Conclusions and Future Direction
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | Antigen-presenting cells |
| FDA | Food and Drug Administration |
| CaP | calcium phosphate nanoparticles |
| DCs | Dendritic cells |
| CTLs | Cytotoxic T lymphocytes |
| MCT | Microcrystalline tyrosine |
| VLPs | Virus like particle |
| LN | Lymph node |
| NK | Natural killer cells |
| MTV | multi-targeted vaccines |
| Mutated-MTV | mutated epitopes |
| GL-MTV | germline epitopes |
| CNTs | Carbon nanotubes |
| PLGA-NPs | Poly (lactic-co-glycolic acid) nanoparticles |
| RNA-LPX | mRNA-lipoplex |
| HLA | Human leukocyte antigens |
| ER | Estrogen receptor |
| DEX | Dendritic cell-derived exosomes |
| TEX | Tumor cell-derived exosomes |
| AEX | Ascitic cell-derived exosomes |
| OC | Ovarian Cancer |
References
- Gopal, R.K.; Ganesh, P.S.; Pathoor, N.N. Functional antibody responses associated with effectiveness of RTS, S malaria vaccine in children: New insights and implications. Lancet Microbe 2025, 6, 101001. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Ahmad, J.; Haque, A.; Alasmary, M.Y.; Abdel-Wahab, B.A.; Akhter, S. Emerging advances in synthetic cancer nano-vaccines: Opportunities and challenges. Expert. Rev. Vaccines 2020, 19, 1053–1071. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, K.; Jagannathan, V.; Rajagopalan, K.; Ganesan, H.; Jeevanandam, M.; Vivekanandam, R.; Christyraj, J.R.S.S.; Mohan, M. Designing of cytotoxic T lymphocyte-based multi-epitope vaccine against SARS-CoV2: A reverse vaccinology approach. J. Biomol. Struct. Dyn. 2022, 40, 13711–13726. [Google Scholar]
- Tan, S.; Li, D.; Zhu, X. Cancer immunotherapy: Pros, cons and beyond. Biomed. Pharmacother. 2020, 124, 109821. [Google Scholar] [CrossRef]
- Deepika, B.; Pallavi, P.; Gowtham, P.; Girigoswami, A.; Girigoswami, K. Anticancer potential of nanoformulated extract of Passiflora incarnata leaves. Biocatal. Agric. Biotechnol. 2024, 57, 103109. [Google Scholar] [CrossRef]
- Janani, G.; Girigoswami, A.; Deepika, B.; Udayakumar, S.; Girigoswami, K. Unveiling the Role of Nano-Formulated Red Algae Extract in Cancer Management. Molecules 2024, 29, 2077. [Google Scholar] [CrossRef] [PubMed]
- Deepika, B.; Janani, G.; Jessy Mercy, D.; Udayakumar, S.; Girigoswami, A.; Girigoswami, K. Inhibitory Effect of Nano-Formulated Extract of Passiflora incarnata on Dalton’s Lymphoma Ascites-Bearing Swiss albino Mice. Pharmaceutics 2025, 17, 270. [Google Scholar] [CrossRef]
- Metkar, S.K.; Udayakumar, S.; Girigoswami, A.; Girigoswami, K. Natural serine proteases and their applications in combating amyloid formation. ADMET DMPK 2024, 12, 797–820. [Google Scholar] [CrossRef]
- Girigoswami, A.; Ghosh, M.; Girigoswami, K. World scenario of earthworms’ prowess as a remedy to a spectrum of diseases–a glimpse on related nanoformulations. Tradit. Med. Res. 2025, 10, 23. [Google Scholar] [CrossRef]
- Bhattacharya, J.B.; Jain, S.L.; Devi, S. The Role of Immunocytochemical Markers to Differentiate Primary from Secondary Neoplastic Hepatic Masses: A Diagnostic Challenge on Cytology. Turk. J. Pathol. 2021, 37, 196. [Google Scholar] [CrossRef]
- Selvaraj, V.; Sudhakar, S.; Sekaran, S.; Rameshkumar, N.; Krishnan, M.; Sekar, S.K.R. Cutting-edge nanotechnology transforming cancer surgery and recovery. Int. J. Surg. 2024, 110, 8207–8209. [Google Scholar] [CrossRef]
- Girigoswami, K.; Prabhu, A.D.; Pallavi, P.; Gowtham, P.; Harini, K.; Thirumalai, A.; Girigoswami, A. Hydrogels of Alginate Derivative-Encased Nanodots Featuring Carbon-Coated Manganese Ferrite Cores with Gold Shells to Offer Antiangiogenesis with Multimodal Imaging-Based Theranostics. Adv. Ther. 2024, 7, 2400054. [Google Scholar]
- Girigoswami, A.; Deepika, B.; Udayakumar, S.; Janani, G.; Mercy, D.J.; Girigoswami, K. Peony-shaped zinc oxide nanoflower synthesized via hydrothermal route exhibits promising anticancer and anti-amyloid activity. BMC Pharmacol. Toxicol. 2024, 25, 101. [Google Scholar] [CrossRef]
- Jayavardhini, B.; Pravin, Y.R.; Kumar, C.; Murugesan, R.; Vedakumari, S.W. Graphene oxide impregnated sericin/collagen scaffolds–Fabrication and characterization. Mater. Lett. 2022, 307, 131060. [Google Scholar] [CrossRef]
- Ameena, M.; Arumugham, M.; Ramalingam, K.; Rajeshkumar, S.; Perumal, E.; Shanmugam, R. Cytocompatibility and wound healing activity of chitosan thiocolchicoside lauric acid nanogel in human gingival fibroblast cells. Cureus 2023, 15, e43727. [Google Scholar]
- Rajkumar, M.; Davis Presley, S.; Thiyagarajulu, N.; Girigoswami, K.; Janani, G.; Kamaraj, C.; Madheswaran, B.; Prajapati, B.; Ali, N.; Khan, M.R. Gelatin/PLA-loaded gold nanocomposites synthesis using Syzygium cumini fruit extract and their antioxidant, antibacterial, anti-inflammatory, antidiabetic and anti-Alzheimer’s activities. Sci. Rep. 2025, 15, 2110. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; Mercy, D.J.; Udayakumar, S.; Girigoswami, A.; Girigoswami, K. Frustrating the Serenity of Bacterial Biofilms by Bristly Reduced Graphene Oxide Sheets. BioNanoScience 2025, 15, 246. [Google Scholar] [CrossRef]
- Devi, N.B.; Satyasri, B.; Ramya, R.; Kanmani, R.; Vinuja, G. Nano-structured Molybdenum Trioxide Nano-hybrid based Conductive Platform for Breast Cancer Detection. J. Environ. Nanotechnol. 2024, 13, 418–426. [Google Scholar] [CrossRef]
- Kroll, A.V.; Jiang, Y.; Zhou, J.; Holay, M.; Fang, R.H.; Zhang, L. Biomimetic nanoparticle vaccines for cancer therapy. Adv. Biosyst. 2019, 3, 1800219. [Google Scholar] [CrossRef]
- Hu, Z.; Ott, P.A.; Wu, C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 2018, 18, 168–182. [Google Scholar] [CrossRef]
- Stolk, D.A.; De Haas, A.; Vree, J.; Duinkerken, S.; Lübbers, J.; Van de Ven, R.; Ambrosini, M.; Kalay, H.; Bruijns, S.; van der Vliet, H.J.; et al. Lipo-based vaccines as an approach to target dendritic cells for induction of T-and iNKT cell responses. Front. Immunol. 2020, 11, 990. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ma, Q.; Cheng, R.; Li, K.; Tang, L.; Luo, S.; Liu, C. Application of nanotechnology in therapeutic cancer vaccines. Adv. NanoBiomed Res. 2023, 3, 2200122. [Google Scholar] [CrossRef]
- Desai, N.; Chavda, V.; Singh, T.R.R.; Thorat, N.D.; Vora, L.K. Cancer nanovaccines: Nanomaterials and clinical perspectives. Small 2024, 20, 2401631. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Yu, M.; Li, W.; Zhu, D.; Mei, L.; Ou, M. Vaccine-like nanomedicine for cancer immunotherapy. J. Control. Release 2023, 355, 760–778. [Google Scholar] [CrossRef]
- Schneider, I.C.; Hartmann, J.; Braun, G.; Stitz, J.; Klamp, T.; Bihi, M.; Sahin, U.; Buchholz, C.J. Displaying tetra-membrane spanning Claudins on enveloped virus-like particles for cancer immunotherapy. Biotechnol. J. 2018, 13, 1700345. [Google Scholar] [CrossRef]
- Geall, A.J.; Verma, A.; Otten, G.R.; Shaw, C.A.; Hekele, A.; Banerjee, K.; Cu, Y.; Beard, C.W.; Brito, L.A.; Krucker, T.; et al. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl. Acad. Sci USA 2012, 109, 14604–14609. [Google Scholar] [CrossRef]
- Gowtham, P.; Pallavi, P.; Harini, K.; Girigoswami, K.; Girigoswami, A. Hydrogelated Virus Nanoparticles in Tissue Engineering. Curr. Nanosci. 2023, 19, 258–269. [Google Scholar]
- Mohsen, M.O.; Bachmann, M.F. Virus-like particle vaccinology, from bench to bedside. Cell. Mol. Immunol. 2022, 19, 993–1011. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Zhang, Z.; Zhu, X.; Tian, M.; Zhang, F.; Zhu, B.; Wang, W.; Ge, Q.; Hong, S.; et al. B cells are the dominant antigen-presenting cells that activate naive CD4+ T cells upon immunization with a virus-derived nanoparticle antigen. Immunity 2018, 49, 695–708.e4. [Google Scholar]
- Yue, Z.; Chen, Z.; Yang, K.; Cheng, Z.; Zhou, X.; Li, S.; Shen, C. Four ounces can move a thousand pounds: The enormous value of nanomaterials in tumor immunotherapy. Adv. Healthc. Mater. 2023, 12, 2300882. [Google Scholar]
- Tornesello, A.L.; Tagliamonte, M.; Buonaguro, F.M.; Tornesello, M.L.; Buonaguro, L. Virus-like particles as preventive and therapeutic cancer vaccines. Vaccines 2022, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Angelicola, S.; Scalambra, L.; Semprini, M.S.; Lollini, P.-L.; Nanni, P.; Palladini, A.; Ruzzi, F.; Cappello, C.; Pittino, O.M. Virus-like particle (VLP) vaccines for cancer immunotherapy. Int. J. Mol. Sci. 2023, 24, 12963. [Google Scholar]
- Gül, D.; Önal Acet, B.; Lu, Q.; Stauber, R.H.; Odabaşı, M.; Acet, Ö. Revolution in cancer treatment: How are intelligently designed nanostructures changing the game? Int. J. Mol. Sci. 2024, 25, 5171. [Google Scholar] [CrossRef]
- Janani, G.; Girigoswami, A.; Deepika, B.; Udayakumar, S.; Mercy, D.J.; Girigoswami, K. Dual Mechanism of Amphiroa anceps: Antiangiogenic and Anticancer Effects in Skin Cancer. Chem. Biodivers. 2025, 10, e202500626. [Google Scholar] [CrossRef]
- Hejabi, F.; Abbaszadeh, M.S.; Taji, S.; O’Neill, A.; Farjadian, F.; Doroudian, M. Nanocarriers: A novel strategy for the delivery of CRISPR/Cas systems. Front. Chem. 2022, 10, 957572. [Google Scholar] [CrossRef]
- Hu, Y.; Xia, D.; Bao, H.; Chen, C.; Xu, Y.; Meng, H.; Li, C. Nano-Oncologic Vaccine for Boosting Cancer Immunotherapy: The Horizons in Cancer Treatment. Nanomaterials 2025, 15, 122. [Google Scholar] [CrossRef]
- Mamuti, M.; Chen, W.; Jiang, X. Nanotechnology-Assisted Immunoengineering for Cancer Vaccines. Adv. NanoBiomed Res. 2023, 3, 2200080. [Google Scholar] [CrossRef]
- Zanganeh, S.; Abbasgholinejad, E.; Doroudian, M.; Esmaelizad, N.; Farjadian, F.; Benhabbour, S.R. The current landscape of glioblastoma biomarkers in body fluids. Cancers 2023, 15, 3804. [Google Scholar] [CrossRef]
- Fan, Y.; Moon, J.J. Nanoparticle drug delivery systems designed to improve cancer vaccines and immunotherapy. Vaccines 2015, 3, 662–685. [Google Scholar] [CrossRef]
- Urbanavicius, D.; Alvarez, T.; Such, G.K.; Johnston, A.P.; Mintern, J.D. The potential of nanoparticle vaccines as a treatment for cancer. Mol. Immunol. 2018, 98, 2–7. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Heath, M.D.; Cabral-Miranda, G.; Lipp, C.; Zeltins, A.; Sande, M.; Stein, J.V.; Riether, C.; Roesti, E.; Zha, L.; et al. Vaccination with nanoparticles combined with micro-adjuvants protects against cancer. J. Immunother. Cancer 2019, 7, 114. [Google Scholar]
- Pandey, A.; Karmous, I. Exploring the Potential of Plant-Based Nanotechnology in Cancer Immunotherapy: Benefits, Limitations, and Future Perspectives. Biol. Trace Elem. Res. 2024, 203, 1746–1763. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H.; Jehng, T.; Li, Y.; Chen, Z.; Lee, K.-D.; Shen, H.-T.; Jones, L.; Huang, X.-F.; Chen, S.Y. A novel anti-PD-L1 vaccine for cancer immunotherapy and immunoprevention. Cancers 2019, 11, 1909. [Google Scholar] [CrossRef]
- Tian, H.; Shi, G.; Wang, Q.; Li, Y.; Yang, Q.; Li, C.; Yang, G.; Wu, M.; Xie, Q.; Zhang, S.; et al. A novel cancer vaccine with the ability to simultaneously produce anti-PD-1 antibody and GM-CSF in cancer cells and enhance Th1-biased antitumor immunity. Signal Transduct. Target. Ther. 2016, 1, 16025. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, Y.; Zhou, X.; Ji, J.; Liu, Z. Nanovaccines with cell-derived components for cancer immunotherapy. Adv. Drug Deliv. Rev. 2022, 182, 114107. [Google Scholar] [CrossRef]
- De Charette, M.; Marabelle, A.; Houot, R. Turning tumour cells into antigen presenting cells: The next step to improve cancer immunotherapy? Eur. J. Cancer 2016, 68, 134–147. [Google Scholar] [CrossRef]
- Wei, Y.; Tian, H.; Yu, D.; Yang, G.; Yang, Q.; Deng, H.; Wang, Q.; Shi, G.; Xiang, R.; Zhang, S.; et al. Nanomedicinal strategies as efficient therapeutic interventions for delivery of cancer vaccines. Semin. Cancer Biol. 2021, 69, 43–51. [Google Scholar]
- Rana, I.; Oh, J.; Baig, J.; Moon, J.H.; Son, S.; Nam, J. Nanocarriers for cancer nano-immunotherapy. Drug Deliv. Transl. Res. 2023, 13, 1936–1954. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, Z.; Wei, Y.; Qian, Z.; Wei, X. Nanovaccines for cancer immunotherapy: Current knowledge and future perspectives. Chin. Chem. Lett. 2023, 34, 108098. [Google Scholar] [CrossRef]
- Fang, X.; Lan, H.; Jin, K.; Gong, D.; Qian, J. Nanovaccines for cancer prevention and immunotherapy: An update review. Cancers 2022, 14, 3842. [Google Scholar] [CrossRef]
- Liu, J.; Miao, L.; Sui, J.; Hao, Y.; Huang, G. Nanoparticle cancer vaccines: Design considerations and recent advances. Asian J. Pharm. Sci. 2020, 15, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Sunoqrot, S.; Abdel Gaber, S.A.; Abujaber, R.; Al-Majawleh, M.; Talhouni, S. Lipid-and polymer-based nanocarrier platforms for cancer vaccine delivery. ACS Appl. Bio Mater. 2024, 7, 4998–5019. [Google Scholar] [CrossRef] [PubMed]
- Irvine, D.J.; Hanson, M.C.; Rakhra, K.; Tokatlian, T. Synthetic nanoparticles for vaccines and immunotherapy. Chem. Rev. 2015, 115, 11109–11146. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; He, Z.; Sun, B.; Jiang, Q.; Luo, C.; Wang, Q.; Wang, Z.; Zhang, S.; Sun, J. Lymph node-targeting nanovaccines for cancer immunotherapy. J. Control. Release 2022, 351, 102–122. [Google Scholar]
- Dewangan, H.K. Rational application of nanoadjuvant for mucosal vaccine delivery system. J. Immunol. Methods 2020, 481, 112791. [Google Scholar] [CrossRef]
- Xie, X.; Song, T.; Feng, Y.; Zhang, H.; Yang, G.; Wu, C.; You, F.; Liu, Y.; Yang, H. Nanotechnology-based multifunctional vaccines for cancer immunotherapy. Chem. Eng. J. 2022, 437, 135505. [Google Scholar] [CrossRef]
- Li, J.; Ren, H.; Zhang, Y. Metal-based nano-vaccines for cancer immunotherapy. Coord. Chem. Rev. 2022, 455, 214345. [Google Scholar] [CrossRef]
- Ahrens, E.T.; Bulte, J.W. Tracking immune cells in vivo using magnetic resonance imaging. Nat. Rev. Immunol. 2013, 13, 755–763. [Google Scholar] [CrossRef]
- Li Li, X.; Zhang, D.; Zhu, Y.; Zheng, X.; Yousef, A.A.; Song, K.; Liu, L.; Cha, D.; Han, Y.; Liu, X.; et al. Direct imaging of tunable crystal surface structures of MOF MIL-101 using high-resolution electron microscopy. J. Am. Chem. Soc. 2019, 141, 12021–12028. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Li, B.; He, J.; Duan, D.; Shao, D.; Nie, M. Fe-MIL-101 exhibits selective cytotoxicity and inhibition of angiogenesis in ovarian cancer cells via downregulation of MMP. Sci. Rep. 2016, 6, 26126. [Google Scholar] [CrossRef]
- Han, T.; Sun, Y.; Jiang, X.; Gong, C.; Kong, F.; Luo, Y.; Ge, C.; Liu, C.; Liu, Y.; Mou, Y.; et al. Air bag-embedded MIL-101 (Fe) metal-organic frameworks for an amplified tumor microenvironment activation loop through strategic delivery of iron ions and lentinan. Theranostics 2024, 14, 5883. [Google Scholar] [CrossRef] [PubMed]
- Najafipour, I.; Emami, N.; Sadeh, P.; Amoli, A.; Mosleh-Shirazi, S.; Amani, A.M.; Kamyab, H.; Chelliapan, S.; Kasaee, S.R.; Vafa, E. Synthesis of Fe3O4@ MIL-101-OH/Chitosan for adsorption and release of doxorubicin. Polym. Test. 2025, 142, 108659. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Chen, H.; Sun, L.-N.; Zhang, B.; Yue, D.-S.; Wang, C.-L.; Zhang, Z.F. Injectable hydrogel with doxorubicin-loaded ZIF-8 nanoparticles for tumor postoperative treatments and wound repair. Sci. Rep. 2024, 14, 9983. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Alizadeh, D.; Zhang, L.; Liu, W.; Farrukh, O.; Manuel, E.; Diamond, D.-J.; Badie, B. Carbon nanotubes enhance CpG uptake and potentiate antiglioma immunity. Clin. Cancer Res. 2011, 17, 771–782. [Google Scholar] [CrossRef]
- Hassan, H.A.; Diebold, S.S.; Smyth, L.A.; Walters, A.A.; Lombardi, G.; Al-Jamal, K.T. Application of carbon nanotubes in cancer vaccines: Achievements, challenges and chances. J. Control. Release 2019, 297, 79–90. [Google Scholar] [CrossRef]
- Achmad, H.; Gabr, G.A.; Ramírez-Coronel, A.A.; Budi, H.S.; Alshahrani, S.H.; Riaz, M.W.; Ibrahim, Y.S.; Al-Taee, M.M.; Sawitri, W.; Jalil, A.T.; et al. Nanovaccines in cancer immunotherapy: Focusing on dendritic cell targeting. Int. Immunopharmacol. 2022, 113, 109434. [Google Scholar] [CrossRef]
- Lu, Y.; Shi, Y.; You, J. Strategy and clinical application of up-regulating cross presentation by DCs in anti-tumor therapy. J. Control. Release 2022, 341, 184–205. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Zhou, L.; Mi, Q.-S.; Jiang, A. DC-Based Vaccines for Cancer Immunotherapy. Vaccines 2020, 8, 706. [Google Scholar] [CrossRef]
- Michael, S.G. Immunoengineering: How Nanotechnology Can Enhance Cancer Immunotherapy. Cell 2015, 161, 201–204. [Google Scholar]
- Han, H.D.; Byeon, Y.; Kang, T.H.; Jung, I.D.; Lee, J.-W.; Shin, B.C.; Lee, Y.J.; Sood, A.K.; Park, Y.-M. Toll-like receptor 3-induced immune response by poly (d, l-lactide-co-glycolide) nanoparticles for dendritic cell-based cancer immunotherapy. Int. J. Nanomed. 2016, 2, 5729–5742. [Google Scholar] [CrossRef]
- Cruz, L.J.; Tacken, P.J.; Fokkink, R.; Joosten, B.; Stuart, M.C.; Albericio, F.; Torensma, R.; Figdor, C.G. Targeted PLGA nano-but not microparticles specifically deliver antigen to human dendritic cells via DC-SIGN in vitro. J. Control. Release 2010, 144, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-D.; Lü, K.-L.; Yu, J.; Du, H.-J.; Fan, C.-Q.; Chen, L. In vitro and in vivo evaluation of DC-targeting PLGA nanoparticles encapsulating heparanase CD4+ and CD8+ T-cell epitopes for cancer immunotherapy. Cancer Immunol. Immunother. 2022, 71, 2969–2983. [Google Scholar]
- Cruz, L.J.; Tacken, P.J.; Eich, C.; Rueda, F.; Torensma, R.; Figdor, C.G. Controlled release of antigen and Toll-like receptor ligands from PLGA nanoparticles enhances immunogenicity. Nanomedicine 2017, 12, 491–510. [Google Scholar] [CrossRef]
- Hamdy, S.; Haddadi, A.; Hung, R.W.; Lavasanifar, A. Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv. Drug Deliv. Rev. 2011, 63, 943–955. [Google Scholar] [CrossRef]
- Paulis, L.E.; Mandal, S.; Kreutz, M.; Figdor, C.G. Dendritic cell-based nanovaccines for cancer immunotherapy. Curr. Opin. Immunol. 2013, 25, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Perica, K.; Medero, A.D.L.; Durai, M.; Chiu, Y.L.; Bieler, J.G.; Sibener, L.; Niemöller, M.; Assenmacher, M.; Richter, A.; Edidin, M.; et al. Nanoscale artificial antigen presenting cells for T cell immunotherapy. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 119–129. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Vogel, M.; Riether, C.; Muller, J.; Salatino, S.; Ternette, N.; Gomes, A.C.; Cabral-Miranda, G.; El-Turabi, A.; Rued, C.; et al. Targeting mutated plus germline epitopes confers pre-clinical efficacy of an instantly formulated cancer nano-vaccine. Front. Immunol. 2019, 10, 1015. [Google Scholar] [CrossRef]
- Al-Hawary, S.I.S.; Almajidi, Y.Q.; Bansal, P.; Ahmad, I.; Kaur, H.; Hjazi, A.; Deorari, M.; Zwamel, A.H.; Hamzah, H.F.; Mohammed, B.A. Dendritic cell-derived exosome (DEX) therapy for digestive system cancers; recent advances and future prospect. Pathol. Res. Pract. 2024, 257, 155288. [Google Scholar] [CrossRef]
- Wan, M.; Ning, B.; Spiegel, S.; Lyon, C.J.; Hu, T.Y. Tumor-derived exosomes (TDEs): How to avoid the sting in the tail. Med. Res. Rev. 2020, 40, 385–412. [Google Scholar] [CrossRef]
- Tran, T.-H.; Mattheolabakis, G.; Aldawsari, H.; Amiji, M. Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clin. Immunol. 2015, 160, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.; Almeida, F. Exosome-based vaccines: History, current state, and clinical trials. Front. Immunol. 2021, 12, 711565. [Google Scholar] [CrossRef] [PubMed]
- Greening, D.W.; Gopal, S.K.; Xu, R.; Simpson, R.J.; Chen, W. Exosomes and their roles in immune regulation and cancer. Semin. Cell Dev. Biol. 2015, 40, 72–81. [Google Scholar] [CrossRef]
- Tan, A.; De La Peña, H.; Seifalian, A.M. The application of exosomes as a nanoscale cancer vaccine. Int. J. Nanomed. 2010, 5, 889–900. [Google Scholar]
- Smith, D.M.; Simon, J.K.; Baker, J.R. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 2013, 13, 592–605. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, R.; Nie, G. Applications of nanomaterials as vaccine adjuvants. Hum. Vaccines Immunother. 2014, 10, 2761–2774. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Speiser, D.E.; Knuth, A.; Bachmann, M.F. Virus-like particles for vaccination against cancer. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1579. [Google Scholar] [CrossRef]
- Zhang, D.; Lin, Z.; Wu, M.; Cai, Z.; Zheng, Y.; He, L.; Li, Z.; Zhou, J.; Sun, L.; Chen, G.; et al. Cytosolic delivery of thiolated neoantigen nano-vaccine combined with immune checkpoint blockade to boost anti-cancer T cell immunity. Adv. Sci. 2021, 8, 2003504. [Google Scholar] [CrossRef]
- Lybaert, L.; Vermaelen, K.; De Geest, B.G.; Nuhn, L. Immunoengineering through cancer vaccines–A personalized and multi-step vaccine approach towards precise cancer immunity. J. Control. Release 2018, 289, 125–145. [Google Scholar] [CrossRef]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhang, F.; Ni, Q.; Niu, G.; Chen, X. Efficient nanovaccine delivery in cancer immunotherapy. ACS Nano 2017, 11, 2387–2392. [Google Scholar] [CrossRef]
- Amouzegar, A.; Chelvanambi, M.; Filderman, J.N.; Storkus, W.J.; Luke, J.J. STING agonists as cancer therapeutics. Cancers 2021, 13, 2695. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, S.; Wang, X.Y.; Zhu, G. Nanovaccines for cancer immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1559. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, J.; Zheng, X.; Liu, Z.; Zhang, X.; Li, Y.; Wilhelm, J.; Cao, J.; Huang, G.; Zhang, J.; et al. Intratumoral administration of STING-activating nanovaccine enhances T cell immunotherapy. J. Immunother. Cancer 2022, 10, e003960. [Google Scholar] [CrossRef]
- Zhou, S.; Cheng, F.; Zhang, Y.; Su, T.; Zhu, G. Engineering and delivery of cGAS-STING immunomodulators for the immunotherapy of cancer and autoimmune diseases. Acc. Chem. Res. 2023, 56, 2933–2943. [Google Scholar] [CrossRef] [PubMed]
- An, M.; Yu, C.; Xi, J.; Reyes, J.; Mao, G.; Wei, W.Z.; Liu, H. Induction of necrotic cell death and activation of STING in the tumor microenvironment via cationic silica nanoparticles leading to enhanced antitumor immunity. Nanoscale 2018, 10, 9311–9319. [Google Scholar] [CrossRef]
- Ghosh, S.; Girigoswami, K.; Girigoswami, A. Membrane-encapsulated camouflaged nanomedicines in drug delivery. Nanomedicine 2019, 14, 2067–2082. [Google Scholar] [CrossRef]
- Tomljenovic, L.; Shaw, C.A. Aluminum vaccine adjuvants: Are they safe? Curr. Med. Chem. 2011, 18, 2630–2637. [Google Scholar] [CrossRef]
- Chen, Z.; Hao, X.; Wang, H.; Zhong, X.; Chen, X.; Zhao, Y.; Zhang, Y.; Du, G.; Sun, X. Smart combination of aluminum hydroxide and MF59 to induce strong cellular immune responses. J. Control. Release 2022, 349, 699–711. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Huang, J.; Lo, P.-C.; Lovell, J.F.; Jin, H.; Yang, K. Self-adjuvanting cancer nanovaccines. J. Nanobiotechnol. 2022, 20, 345. [Google Scholar] [CrossRef]
- Aikins, M.E.; Xu, C.; Moon, J.J. Engineered nanoparticles for cancer vaccination and immunotherapy. Acc. Chem. Res. 2020, 53, 2094–2105. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lv, J.; Zhuang, Q.; Yang, Z.; Cao, Z.; Xu, L.; Pei, P.; Wang, C.; Wu, H.; Dong, Z.; et al. A general strategy towards personalized nanovaccines based on fluoropolymers for post-surgical cancer immunotherapy. Nat. Nanotechnol. 2020, 15, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-L.; Hong, S.; Dong, X.; Zheng, D.-W.; Liang, J.-L.; Bai, X.-F.; Wang, X.-N.; Han, Z.-Y.; Zhang, X.-Z. Bioinspired nano-vaccine construction by antigen pre-degradation for boosting cancer personalized immunotherapy. Biomaterials 2022, 287, 121628. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Xue, T.; Yan, H.; Liu, F.; Liu, R.; Zhang, K.; Chong, Y.; Du, J.; Zhang, H. Radiotherapy combined with nano-biomaterials for cancer radio-immunotherapy. J. Nanobiotechnol. 2023, 21, 395. [Google Scholar] [CrossRef]
- Chao, Y.; Xu, L.; Liang, C.; Feng, L.; Xu, J.; Dong, Z.; Tian, L.; Yi, X.; Yang, K.; Liu, Z. Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses. Nat. Biomed. Eng. 2018, 2, 611–621. [Google Scholar] [CrossRef]
- Mi, Y.; Hagan, I.V.C.T.; Vincent, B.G.; Wang, A.Z. Emerging nano-/microapproaches for cancer immunotherapy. Adv. Sci. 2019, 6, 1801847. [Google Scholar] [CrossRef]
- Loquai, C.; Hassel, J.; Brück, P.; Derhovanessian, E.; Cuk, K.; Lörks, V.; Sikorski, J.; Gold, M.; Maurus, D.; Schwarck-Kokarakis, D.; et al. 549 An RNA-lipoplex (RNA-LPX) vaccine demonstrates strong immunogenicity and promising clinical activity in a phase I trial in cutaneous melanoma patients with no evidence of disease at trial inclusion. BMJ Spec. J. 2021, 585, 107–112. [Google Scholar]
- Lopez, J.; Powles, T.; Braiteh, F.; Siu, L.L.; LoRusso, P.; Friedman, C.F.; Balmanoukian, A.S.; Gordon, M.; Yachnin, J.; Rottey, S.; et al. Autogene cevumeran with or without atezolizumab in advanced solid tumors: A phase 1 trial. Nat. Med. 2025, 31, 152–164. [Google Scholar] [CrossRef]
- Berinstein, N.L.; Karkada, M.; Oza, A.M.; Odunsi, K.; Villella, J.A.; Nemunaitis, J.J.; Morse, M.A.; Pejovic, T.; Bentley, J.; Buyse, M.; et al. Survivin-targeted immunotherapy drives robust polyfunctional T cell generation and differentiation in advanced ovarian cancer patients. Oncoimmunology 2015, 4, e1026529. [Google Scholar] [CrossRef]
- Koerner, J.; Horvath, D.; Herrmann, V.L.; MacKerracher, A.; Gander, B.; Yagita, H.; Rohayem, J.; Groettrup, M. PLGA-particle vaccine carrying TLR3/RIG-I ligand Riboxxim synergizes with immune checkpoint blockade for effective anti-cancer immunotherapy. Nat. Commun. 2021, 12, 2935. [Google Scholar] [CrossRef]
- Xiong, J.; Huang, J.; Xu, H.; Wu, Q.; Zhao, J.; Chen, Y.; Chen, Y.; Fan, G.; Guan, H.; Xiao, R.; et al. CpG-Based Nanovaccines Enhance Ovarian Cancer Immune Response by Gbp2-Mediated Remodeling of Tumor-Associated Macrophages. Adv. Sci. 2025, 12, e2412881. [Google Scholar] [CrossRef] [PubMed]
- Creemers, J.H.; Pawlitzky, I.; Grosios, K.; Gileadi, U.; Middleton, M.R.; Gerritsen, W.R.; Mehra, N.; Rivoltini, L.; Walters, I.; Figdor, C.G.; et al. Assessing the safety, tolerability and efficacy of PLGA-based immunomodulatory nanoparticles in patients with advanced NY-ESO-1-positive cancers: A first-in-human phase I open-label dose-escalation study protocol. BMJ Open 2021, 11, e050725. [Google Scholar] [CrossRef] [PubMed]
- Zacharia, A.; Harberts, E.; Valencia, S.M.; Myers, B.; Sanders, C.; Jain, A.; Larson, N.R.; Middaugh, C.R.; Picking, W.D.; Difilippantonio, S.; et al. Optimization of RG1-VLP vaccine performance in mice with novel TLR4 agonists. Vaccine 2021, 39, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Panda, S.; Bhol, C.S.; Bhutia, S.K.; Mohapatra, S. N-doped carbon quantum dot (NCQD)-Deposited carbon capsules for synergistic fluorescence imaging and photothermal therapy of oral cancer. Langmuir 2019, 35, 15320–15329. [Google Scholar] [CrossRef]
- Zou, Y.; Li, S.; Li, Y.; Zhang, D.; Zheng, M.; Shi, B. Glioblastoma Cell Derived Exosomes as a Potent Vaccine Platform Targeting Primary Brain Cancers and Brain Metastases. ACS Nano 2025, 19, 17309–17322. [Google Scholar] [CrossRef]
- Kim, H.Y.; Min, H.-K.; Song, H.-W.; Yoo, A.; Lee, S.; Kim, K.-P.; Park, J.-O.; Choi, Y.H.; Choi, E. Delivery of human natural killer cell-derived exosomes for liver cancer therapy: An in vivo study in subcutaneous and orthotopic animal models. Drug Deliv. 2022, 29, 2897–2911. [Google Scholar] [CrossRef]
- Zhao, X.; Xuan, F.; Li, Z.; Yin, X.; Zeng, X.; Chen, J.; Fang, C. A KIF20A-based thermosensitive hydrogel vaccine effectively potentiates immune checkpoint blockade therapy for hepatocellular carcinoma. npj Vaccines 2025, 10, 1. [Google Scholar] [CrossRef]
- Bhardwaj, U.; Li, Y.; Ma, A.-H.; Mossanen, M.; Lee, J.S.; Lara, P.N.; Lam, K.S.; Lin, T.-Y.; Zhao, J.; Lerner, L.; et al. A phase I first-in-human clinical trial with PLZ4-coated paclitaxel-loaded micelles (PPM) in therapy-resistant non-muscle-invasive bladder cancer (NMIBC). Am. Soc. Clin. Oncol. 2025, 41, TPS4615. [Google Scholar]
- Ren, H.; Li, J.; Liu, G.; Sun, Y.; Yang, X.; Jiang, Z.; Zhang, J.; Lovell, J.F.; Zhang, Y. Anticancer vaccination with immunogenic micelles that capture and release pristine CD8+ T-cell epitopes and adjuvants. ACS Appl. Mater. Interfaces 2022, 14, 2510–2521. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Kang, S.Y.; Kim, I.H.; Jang, J.S.; Sun, D.-S.; Lee, S.-C.; Lee, H.W.; Jin, M. Phase 2 study of weekly polymeric micelle-formulated paclitaxel plus gemcitabine in patients with recurrent and metastatic adenocarcinoma of the pancreas. J. Clin. Oncol. 2023, 41 (Suppl. S16), 465–472. [Google Scholar]
- Markovic, M.D.; Panic, V.V.; Pjanovic, R.V. Polymeric Nanosystems: A Breakthrough Approach to Treating Inflammation and Inflammation Related Diseases. Biopolymers 2025, 116, e70012. [Google Scholar] [CrossRef]
- Webb, P.M.; Jordan, S.J. Global epidemiology of epithelial ovarian cancer. Nat. Rev. Clin. Oncol. 2024, 21, 389–400. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, W.; Huang, J.; Li, F.; Sheng, J.; Song, H.; Chen, Y. Development of a dendritic cell/tumor cell fusion cell membrane nano-vaccine for the treatment of ovarian cancer. Front. Immunol. 2022, 13, 828263. [Google Scholar] [CrossRef]
- Weigelt, B.; Reis-Filho, J.S. Histological and molecular types of breast cancer: Is there a unifying taxonomy? Nat. Rev. Clin. Oncol. 2009, 6, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Davodabadi, F.; Sarhadi, M.; Arabpour, J.; Sargazi, S.; Rahdar, A.; Díez-Pascual, A.M. Breast cancer vaccines, New insights into immunomodulatory and nano-therapeutic approaches. J. Control. Release 2022, 349, 844–875. [Google Scholar] [CrossRef]
- Herdiana, Y.; Husni, P.; Nurhasanah, S.; Shamsuddin, S.; Wathoni, N. Chitosan-based nano systems for natural antioxidants in breast cancer therapy. Polymers 2023, 15, 2953. [Google Scholar] [CrossRef] [PubMed]
- Phuengkham, H.; Song, C.; Um, S.H.; Lim, Y.T. Implantable synthetic immune niche for spatiotemporal modulation of tumor-derived immunosuppression and systemic antitumor immunity: Postoperative immunotherapy. Adv. Mater. 2018, 30, 1706719. [Google Scholar] [CrossRef]
- Mauldin, I.S.; Wages, N.A.; Stowman, A.M.; Wang, E.; Olson, W.C.; Deacon, D.H.; Smith, K.T.; Galeassi, N.; Teague, J.E.; Smolkin, M.E.; et al. Topical treatment of melanoma metastases with imiquimod, plus administration of a cancer vaccine, promotes immune signatures in the metastases. Cancer Immunol. Immunother. 2016, 65, 1201–1212. [Google Scholar] [CrossRef]
- Maillet, A.; Guilleminault, L.; Lemarié, E.; Lerondel, S.; Azzopardi, N.; Montharu, J.; Congy-Jolivet, N.; Reverdiau, P.; Legrain, B.; Parent, C.; et al. The airways, a novel route for delivering monoclonal antibodies to treat lung tumors. Pharm. Res. 2011, 28, 2147–2156. [Google Scholar] [CrossRef] [PubMed]
- Garbuzenko, O.B.; Kuzmov, A.; Taratula, O.; Pine, S.R.; Minko, T. Strategy to enhance lung cancer treatment by five essential elements: Inhalation delivery, nanotechnology, tumor-receptor targeting, chemo-and gene therapy. Theranostics 2019, 9, 8362. [Google Scholar] [CrossRef] [PubMed]
| Types of Nanovaccine | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Polymeric nanocarrier (PLGA, PEG-PLA) |
|
| [85] |
| Lipid-based nanocarrier (liposomes, Lipid nanoparticles) |
|
| [86] |
| Carbon-based nanocarriers (CNTs) |
|
| [87] |
| Metal-based nanocarrier |
|
| [57] |
| Peptide/protein based nanovaccine |
|
| [88] |
| Types of Nanovaccines | Immunogenicity (T Cell/DC Activation) | Delivery Efficiency | Biocompatibility | Cost Effectiveness | Clinical Trial Number | Clinical Developmental Stages |
|---|---|---|---|---|---|---|
| BNT111 | Strong T cell response | Delivered via RNA-LPX | Biocompatible | Moderate scalability | NCT02410733 | Phase I trial in patients with cutaneous melanoma is ongoing [109] |
| Autogene Cevumeran (RNA lipoplex-based neoantigen vaccine) | Strong cellular uptake and CD4+/CD8+ T cell response | Delivered via lipoplex | Non toxic | Expensive due to the synthesis and sequencing process | NCT03289962 | Phase I trial in patients with solid tumors [110] |
| DPX-survivac (DepoVax-based surviving vaccine) | Excellent antigen-specific T cell response | Enhances APC uptake and activation | Biocompatible at low doses | Long-term stability and cost-effectiveness | NCT01416038 | Phase I in patients with advanced-stage ovarian, fallopian, and peritoneal cancer [111] |
| PLGA-Riboxxim-based vaccine | Enhanced T cell and dendritic cell response | Improved cellular uptake and activation | Biodegradable and biocompatible | Cost effective | - | Preclinical-testes in animal model [112] |
| PLGA-CpG @1D8-M | Strong activation | Improved CpG accumulation via PLGA | Biodegradable and biocompatible | Moderately complex due to encapsulation and membrane extraction | - | Preclinical in ID8 ovarian and 4T1 breast cancer [113] |
| PRECIOUS-01 | Activates invariant natural killer cells | Intravenous administration | Biodegradable | Moderate complexity | NCT04751786 | Phase I clinical trial [114] |
| RG1-VLP | Strong B-cell response | Enhanced lymph node targeting | Safety profiles | Stable formulation and scalable | - | Preclinical [115] |
| Carbon quantum dot capivasertib | Induces dendritic cell activation | Enhanced targeted delivery | Biocompatible | Cost effective | - | Preclinical [116] |
| Exosome | Promotes mature T cell and DC activation | Effectively targets both the lymph node and the brain tumor site | Biocompatible | Cost effective | - | Preclinical in mice model [117] |
| Natural killer cell-derived exosomes | Activates immune response and reduces immunosuppressive signalling | Effectively targets the tumor site | Highly biocompatible | Moderate scalable | - | Preclinical [118] |
| KIF20A (kinesin family member 20A) based thermosenstive hydrogel | Strong T cell and DC activation | Sustained in vivo antigen release | Safety | Moderate complexity | - | Preclinical study in CDX (cell-derived xenograft) and an immune humanized PDX (patient-derived xenograft) models [119] |
| PLZ4—coated paclitaxel loaded micelles | Synergistically works on post BCG | Effectively targets bladder cancer cells | Biocompatible at 25 mg dose | Cost effective | NCT05519241 | Phase I-bladder cancer [120] |
| Micelle | Strong T cell response | Efficient targeting of lymph nodes and active delivery of adjuvants to DC | Biocompatible | Expensive | - | Preclinical [121] |
| GENEXOL-PM | Moderate immune response | Enhanced tumor penetration | Biocompatible | Cost effective | NCT02739633 | Phase II—pancreatic cancer [122] |
| Gold NPs based vaccines | Strong T cell priming and immune response | Targeted delivery to Dectin-1 expressing APC | Highly biocompatible | Scalable | - | Preclinical—mice model [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udayakumar, S.; Pandiarajan, S.; Jessy Mercy, D.; Suresh, J.; Jagadeesh kumar, J.R.; Girigoswami, A.; Girigoswami, K. Revolutionizing Cancer Vaccine: The Power of Advanced Nanotechnology. Chemistry 2025, 7, 97. https://doi.org/10.3390/chemistry7030097
Udayakumar S, Pandiarajan S, Jessy Mercy D, Suresh J, Jagadeesh kumar JR, Girigoswami A, Girigoswami K. Revolutionizing Cancer Vaccine: The Power of Advanced Nanotechnology. Chemistry. 2025; 7(3):97. https://doi.org/10.3390/chemistry7030097
Chicago/Turabian StyleUdayakumar, Saranya, Shangavy Pandiarajan, Devadass Jessy Mercy, Jayaprakash Suresh, Jashwanth Raj Jagadeesh kumar, Agnishwar Girigoswami, and Koyeli Girigoswami. 2025. "Revolutionizing Cancer Vaccine: The Power of Advanced Nanotechnology" Chemistry 7, no. 3: 97. https://doi.org/10.3390/chemistry7030097
APA StyleUdayakumar, S., Pandiarajan, S., Jessy Mercy, D., Suresh, J., Jagadeesh kumar, J. R., Girigoswami, A., & Girigoswami, K. (2025). Revolutionizing Cancer Vaccine: The Power of Advanced Nanotechnology. Chemistry, 7(3), 97. https://doi.org/10.3390/chemistry7030097









