Synthesis, Reductive Reactivity and Anticancer Activity of Cobalt(III)– and Manganese(III)–Salen Complexes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Mn-1
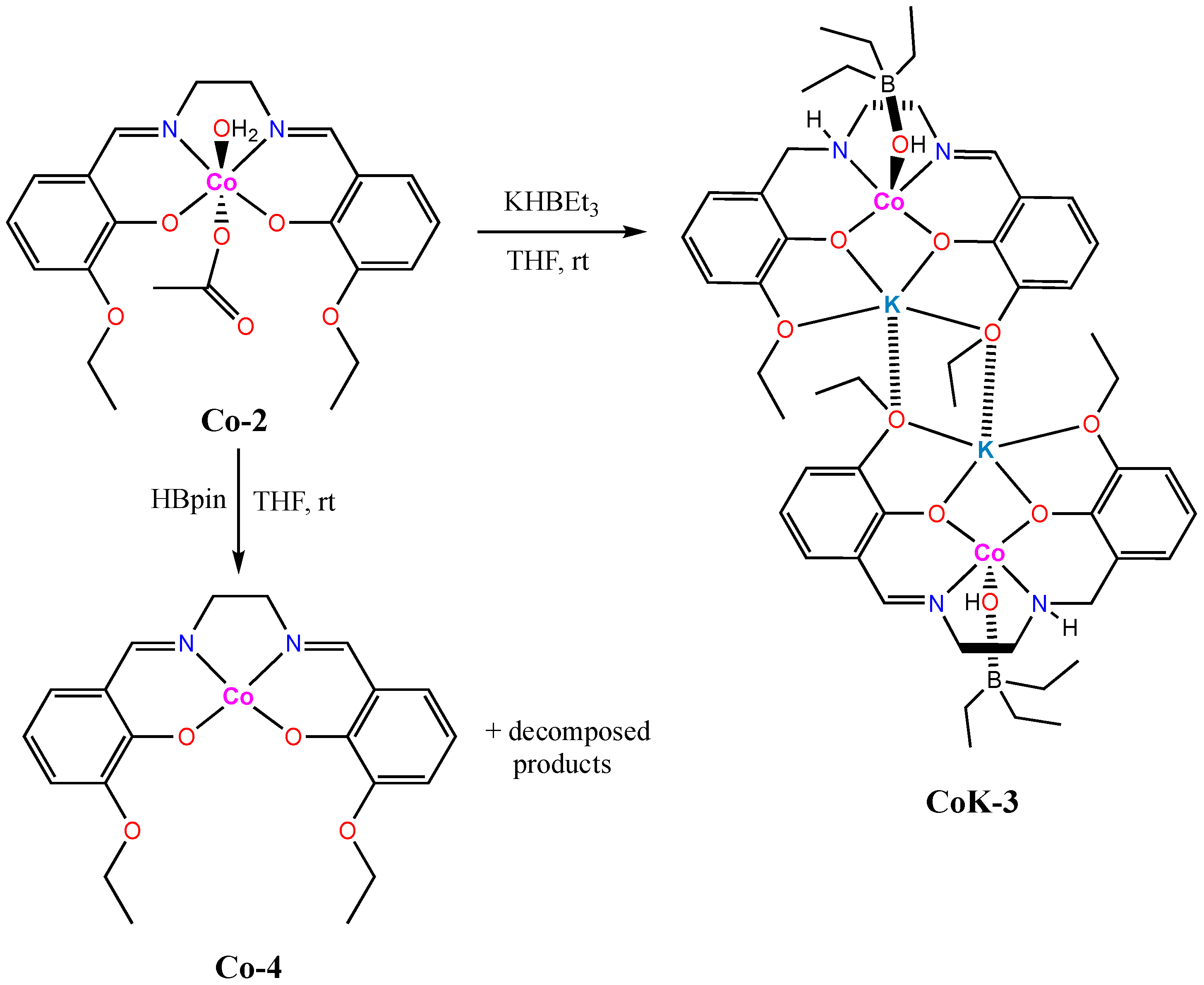
2.3. Synthesis of Co-2
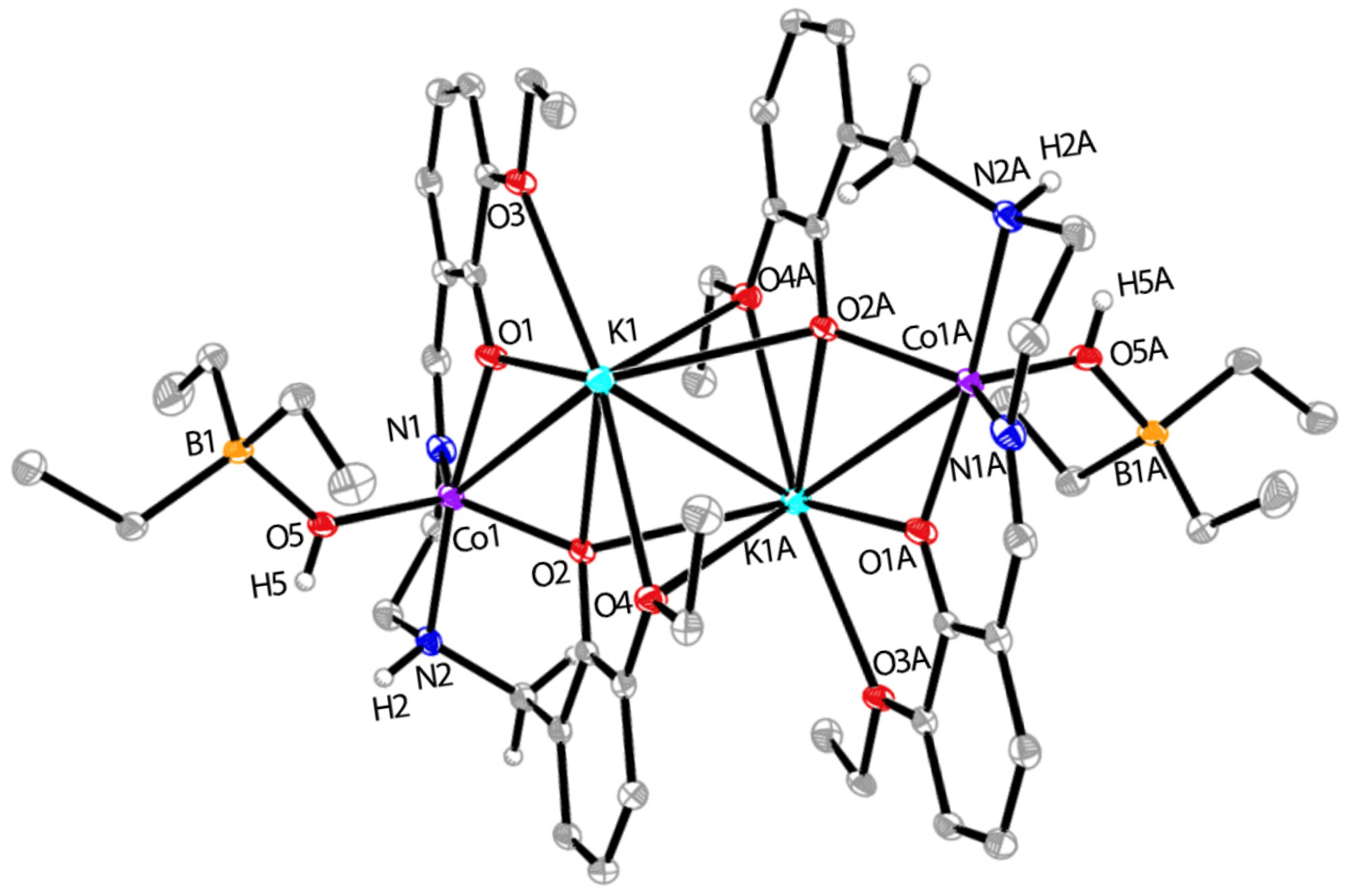
2.4. Synthesis of CoK-3
2.5. Synthesis of Co-4
2.6. UV-Vis Absorption Measurement
2.7. Cytotoxicity Measurement
2.8. X-Ray Crystallography
3. Results and Discussion
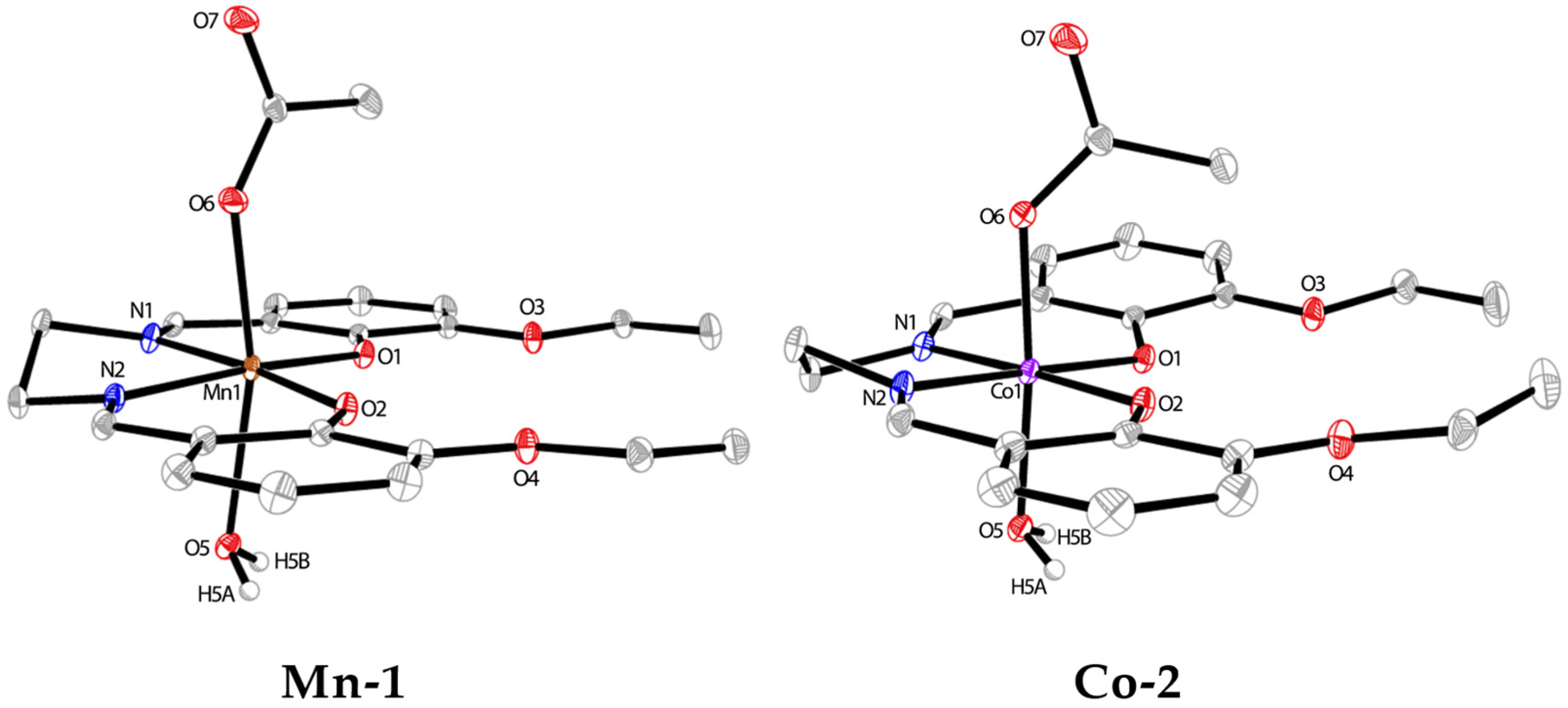
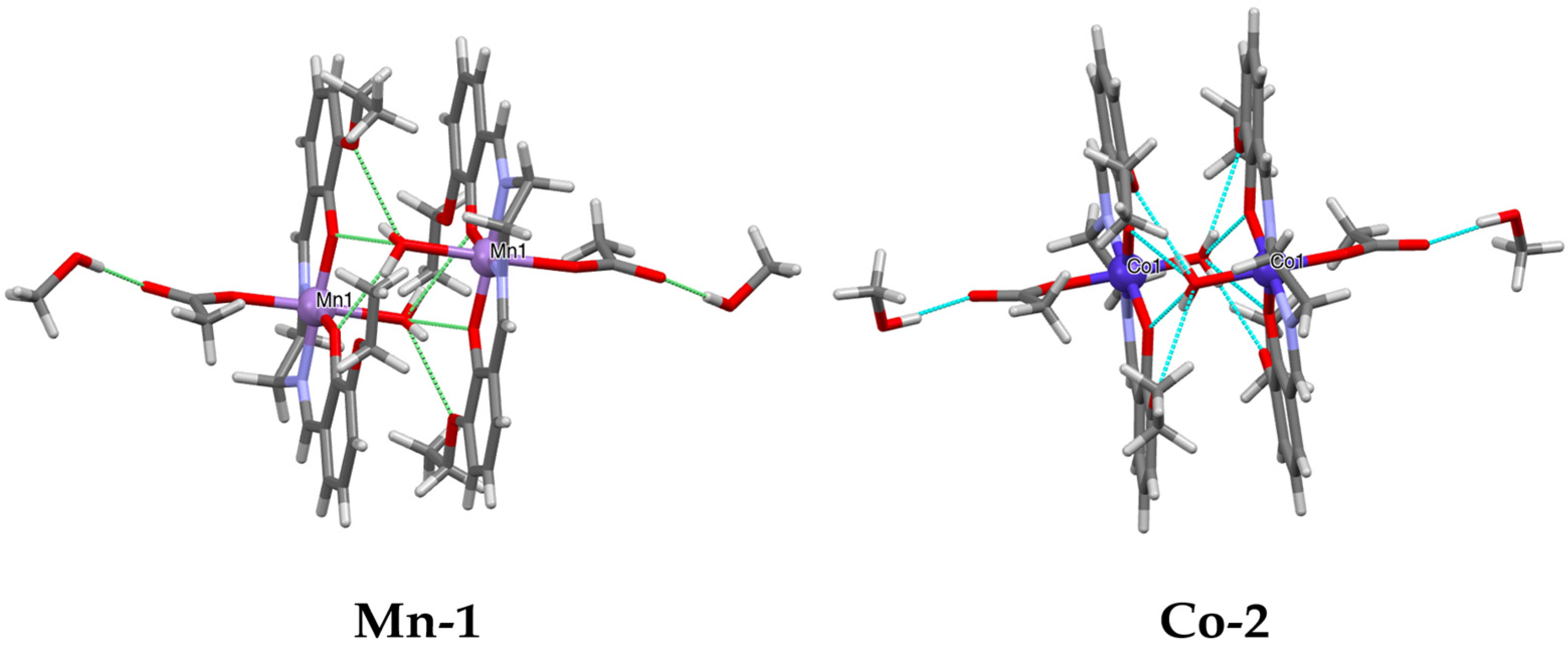
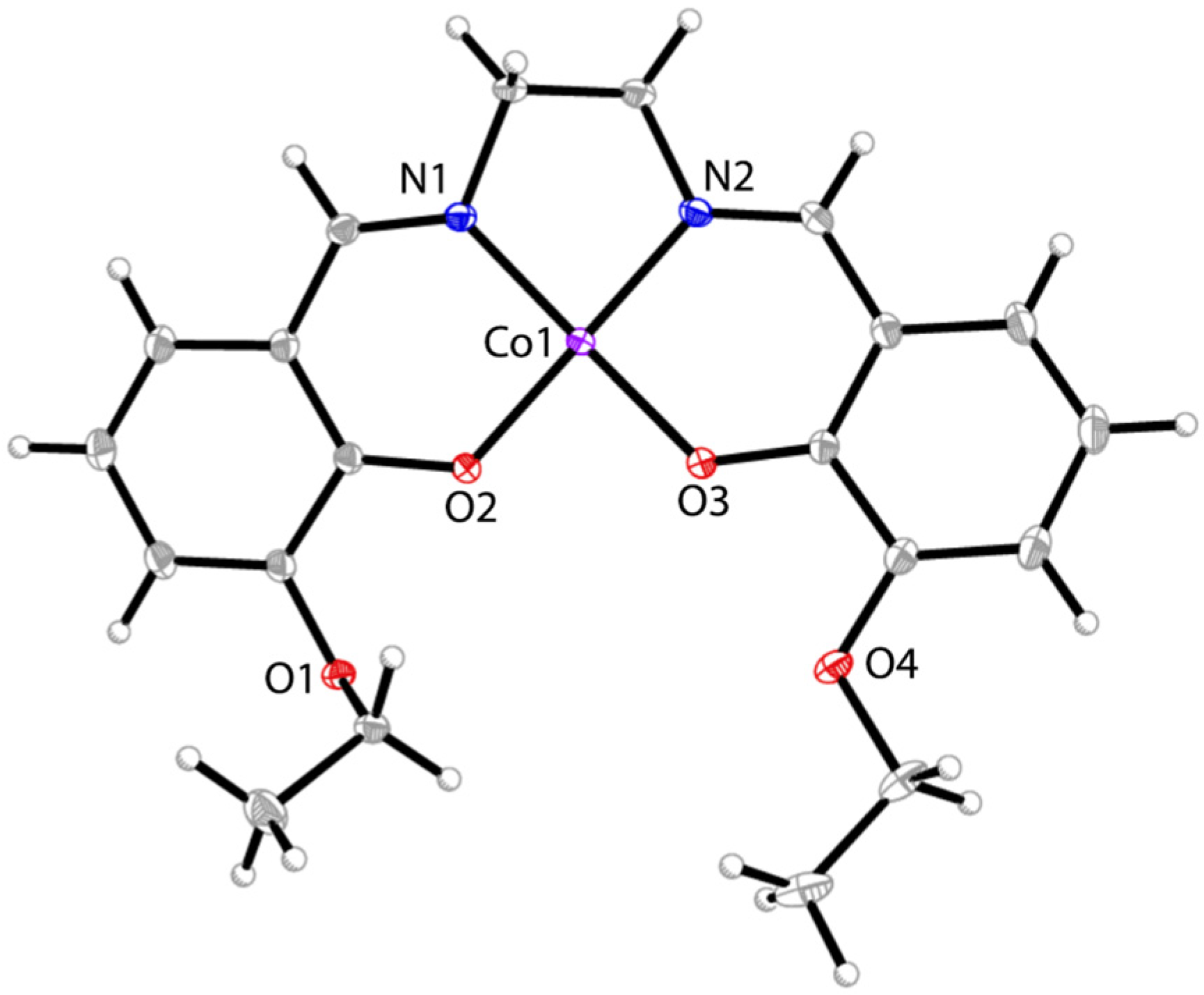
3.1. Synthesis and Crystal Structures of Mn-1 and Co-2
3.2. Reductive Reactivity of Co-2
3.3. Stability of Drug Molecules
3.4. In Vitro Anticancer Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dilruba, S.; Kalayda, G.V. Platinum-Based Drugs: Past, Present, and Future. Cancer Chemother. Pharmacol. 2016, 77, 1103–1124. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.M. Cisplatin in Cancer Treatment. Biochem. Pharmacol. 2022, 206, 115323. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Cisplatin: The First Metal-Based Anticancer Drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef]
- Gou, Y.; Huang, G.; Li, J.; Yang, F.; Liang, H. Versatile Delivery Systems for Non-Platinum Metal-Based Anticancer Therapeutic Agents. Coord. Chem. Rev. 2021, 441, 213975. [Google Scholar] [CrossRef]
- Raymond, E.; Chaney, S.G.; Taamma, A.; Cvitkovic, E. Oxaliplatin: A Review of Preclinical and Clinical Studies. Ann. Oncol. 1998, 9, 1053–1071. [Google Scholar] [CrossRef]
- O’Dwyer, P.J.; Stevenson, J.P.; Johnson, S.W. Clinical Status of Cisplatin, Carboplatin, and Other Platinum-Based Antitumor Drugs. In Cisplatin–Chemistry and Biochemistry of a Leading Anticancer Drug; Lippert, B., Ed.; Helvetica Chimica Acta: Zürich, Switzerland, 1999; pp. 29–69. [Google Scholar]
- Siddik, Z.H. Cisplatin: Mode of Cytotoxic Action and Molecular Basis of Resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef]
- Florea, A.M.; Büsselberg, D. Cisplatin as an Anti-Tumor Drug: Cellular Mechanisms of Activity, Drug Resistance, and Induced Side Effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Lucaciu, R.L.; Hangan, A.C.; Sevastre, B.; Oprean, L.S. Metallo-Drugs in Cancer Therapy: Past, Present, and Future. Molecules 2022, 27, 6485. [Google Scholar] [CrossRef] [PubMed]
- Komeda, S.; Casini, A. Next-Generation Anticancer Metallodrugs. Curr. Top. Med. Chem. 2012, 12, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Ott, I.; Gust, R. Non-Platinum Metal Complexes as Anti-Cancer Drugs. Arch. Pharm. 2007, 340, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Kar, K.; Ghosh, D.; Kabi, B.; Chandra, A. A Concise Review on Cobalt Schiff Base Complexes as Anticancer Agents. Polyhedron 2022, 222, 115890. [Google Scholar] [CrossRef]
- Munteanu, C.R.; Suntharalingam, K. Advances in Cobalt Complexes as Anticancer Agents. Dalton Trans. 2015, 44, 13796–13808. [Google Scholar] [CrossRef]
- Tisato, F.; Marzano, C.; Porchia, M.; Pellei, M.; Santini, C. Copper in Diseases and Treatments, and Copper-Based Anticancer Strategies. Med. Res. Rev. 2010, 30, 708–749. [Google Scholar] [CrossRef]
- Wani, W.A.; Baig, U.; Shreaz, S.; Shiekh, R.A.; Iqbal, P.F.; Jameel, E.; Ahmad, A.; Mohd-Setapar, S.H.; Mushtaque, M.; Hun, L.T. Recent Advances in Iron Complexes as Potential Anticancer Agents. New J. Chem. 2016, 40, 1063–1090. [Google Scholar] [CrossRef]
- Bouché, M.; Hognon, C.; Grandemange, S.; Monari, A.; Gros, P.C. Recent Advances in Iron-Complexes as Drug Candidates for Cancer Therapy: Reactivity, Mechanism of Action, and Metabolites. Dalton Trans. 2020, 49, 11451–11466. [Google Scholar] [CrossRef]
- Zhang, P.; Sadler, P.J. Redox-Active Metal Complexes for Anticancer Therapy. Eur. J. Inorg. Chem. 2017, 12, 1541–1548. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Zhang, Q.; Tang, Q.; Neary, M.C.; Zheng, S. Diverse Cobalt(II) and Iron(II/III) Coordination Complexes/Polymers Based on 4′-Pyridyl:2,2′;6′,2″-Terpyridine: Synthesis, Structures, Catalytic and Anticancer Activities. Chemistry 2024, 6, 1099–1110. [Google Scholar] [CrossRef]
- Zhang, G.; Zeng, H.; Zadori, N.; Marino, C.; Zheng, S.; Neary, M.C. Homoleptic Octahedral CoII Complexes as Precatalysts for Regioselective Hydroboration of Alkenes with High Turnover Frequencies. RSC Adv. 2023, 13, 28089–28096. [Google Scholar] [CrossRef] [PubMed]
- Erxleben, A. Transition Metal Salen Complexes in Bioinorganic and Medicinal Chemistry. Inorg. Chim. Acta 2018, 472, 40–57. [Google Scholar] [CrossRef]
- Alfonso-Herrera, L.A.; Hernández-Romero, D.; Cruz-Navarro, J.A.; Ramos-Ligonio, Á.; López-Monteon, A.; Rivera-Villanueva, J.M.; Morales-Morales, D.; Colorado-Peralta, R. Transition Metal Complexes with Tetradentate Schiff Bases (N2O2) Obtained from Salicylaldehyde: A Review of Their Possible Anticancer Properties. Coord. Chem. Rev. 2024, 505, 215698. [Google Scholar] [CrossRef]
- Alfonso-Herrera, L.A.; Rosete-Luna, S.; Hernández-Romero, D.; Rivera-Villanueva, J.M.; Olivares-Romero, J.L.; Cruz-Navarro, J.A.; Soto-Contreras, A.; Arenaza-Corona, A.; Morales-Morales, D.; Colorado-Peralta, R. Transition Metal Complexes with Tridentate Schiff Bases (ONO and ONN) Derived from Salicylaldehyde: An Analysis of Their Potential Anticancer Activity. ChemMedChem 2022, 17, e202200367. [Google Scholar] [CrossRef]
- Doctrow, S.R.; Liesa, M.; Melov, S.; Shirihai, O.S.; Tofilon, P. Salen Mn Complexes Are Superoxide Dismutase/Catalase Mimetics That Protect the Mitochondria. Curr. Inorg. Chem. 2012, 2, 325–334. [Google Scholar] [CrossRef]
- Segat, B.B.; Menezes, L.B.; Cervo, R.; Cargnelutti, R.; Tolentino, H.; Latini, A.; Horn, A., Jr.; Fernandes, C. Scavenging of Reactive Species Probed by EPR and Ex-Vivo Nanomolar Reduction of Lipid Peroxidation of Manganese Complexes. J. Inorg. Biochem. 2023, 239, 112060. [Google Scholar] [CrossRef]
- Nistri, S.; Boccalini, G.; Bencini, A.; Becatti, M.; Valtancoli, B.; Conti, L.; Lucarini, L.; Bani, D. A New Low Molecular Weight, Mn(II)-Containing Scavenger of Superoxide Anion Protects Cardiac Muscle Cells from Hypoxia/Reoxygenation Injury. Free Radic. Res. 2015, 49, 67–77. [Google Scholar] [CrossRef]
- Nayak, M.; Koner, R.; Lin, H.-H.; Flörke, U.; Wei, H.-H.; Mohanta, S. Syntheses, Structures, and Magnetic Properties of Mononuclear Cu(II) and Tetranuclear CuII3MII (M = Cu, Co, or Mn) Compounds Derived from N,N′-Ethylenebis(3-ethoxysalicylaldimine): Cocrystallization Due to Potential Encapsulation of Water. Inorg. Chem. 2006, 45, 10764–10773. [Google Scholar] [CrossRef]
- Santos, L.M.; Shimabuko, D.Y.; Sipert, C.R. Dimethyl Sulfoxide Affects the Viability and Mineralization Activity of Apical Papilla Cells in vitro. Braz. Dent. J. 2024, 35, e24-6054. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXTL, An Integrated System for Solving, Refining, and Displaying Crystal Structures from Diffraction Data; University of Göttingen: Göttingen, Germany, 1981. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Chang, T.; Jing, H.; Jin, L.; Qiu, W. Quaternary Onium Tribromide Catalyzed Cyclic Carbonate Synthesis from Carbon Dioxide and Epoxides. J. Mol. Catal. A Chem. 2007, 264, 241–247. [Google Scholar] [CrossRef]
- Casellato, U.; Tamburini, S.; Tomasin, P.; Vigato, P.A. Cyclic and Acyclic Compartmental Schiff Bases, Their Reduced Analogues, and Related Mononuclear and Heterodinuclear Complexes. Inorg. Chim. Acta 2004, 357, 4191–4207. [Google Scholar] [CrossRef]
- Costes, J.P.; Tuchagues, J.P.; Vendier, L.; Garcia-Tojal, J. A Strictly Dinuclear MnIII–GdIII Complex: Synthesis and Magnetic Properties. Eur. J. Inorg. Chem. 2013, 19, 3307–3311. [Google Scholar] [CrossRef]
- Tripathi, N.; Sharma, V.K. Synthesis, Spectroscopic and Structural Characterization of Trivalent Chromium, Manganese, Iron, and Cobalt Complexes with Schiff Bases Derived from o-Vanillin. Pol. J. Chem. 2008, 82, 523–535. [Google Scholar]
- Gutierrez, A.; Perpiñán, M.F.; Sanchez, A.E.; Torralba, M.C.; Gonzalez, V. Water Inclusion Mediated Structural Diversity and the Role of H-Bonds in Molecular Assemblies of Manganese(III) Bicompartmental Schiff-Base Complexes. Inorg. Chim. Acta 2016, 453, 169–178. [Google Scholar] [CrossRef]
- Chakraborty, P.; Mohanta, S. Syntheses, Structures, and Catecholase Activity of Two Cobalt(III) Complexes Derived from N,N′-Ethylenebis(3-ethoxysalicylaldiimine): A Special Host–Guest System from a Special Ligand. Inorg. Chim. Acta 2015, 435, 38–45. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, C.Y.; Shi, D.H.; You, Z.L. Unprecedented Preparation of Bis-Schiff Bases and Their Manganese(III) Complexes with Urease Inhibitory Activity. Inorg. Chem. Commun. 2011, 14, 1636–1639. [Google Scholar] [CrossRef]
- Sutradhar, M.; Carrella, L.M.; Rentschler, E. Mononuclear Mn(III) and Dinuclear Mn(III,III) Schiff Base Complexes: Influence of π–π Stacking on Magnetic Properties. Polyhedron 2012, 38, 297–303. [Google Scholar] [CrossRef]
- Chakraborty, P.; Majumder, S.; Jana, A.; Mohanta, S. Syntheses, Structures, Catecholase Activity, Spectroscopy, and Electrochemistry of a Series of Manganese(III) Complexes: Role of Auxiliary Anionic Ligand on Catecholase Activity. Inorg. Chim. Acta 2014, 410, 65–75. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Correia, I. Salan vs. Salen Metal Complexes in Catalysis and Medicinal Applications: Virtues and Pitfalls. Coord. Chem. Rev. 2019, 388, 227–247. [Google Scholar] [CrossRef]
- Zhang, C.; Sutherland, M.; Herasymchuk, K.; Clarke, R.M.; Thompson, J.R.; Chiang, L.; Walsby, C.J.; Storr, T. Octahedral Co(III) Salen Complexes: The Role of Peripheral Ligand Electronics on Axial Ligand Release upon Reduction. Can. J. Chem. 2018, 96, 110–118. [Google Scholar] [CrossRef]
- Miranda, J.A.; Wade, C.J.; Little, R.D. Indirect Electroreductive Cyclization and Electrohydrocyclization Using Catalytic Reduced Nickel(II) Salen. J. Org. Chem. 2005, 70, 8017–8026. [Google Scholar] [CrossRef] [PubMed]
- Gehring, H.; Metzinger, R.; Herwig, C.; Intemann, J.; Harder, S.; Limberg, C. Hydride Reactivity of Ni(II)–X–Ni(II) Entities: Mixed-Valent Hydrido Complexes and Reversible Metal Reduction. Chem. Eur. J. 2013, 19, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Gehring, H.; Metzinger, R.; Braun, B.; Herwig, C.; Harder, S.; Ray, K.; Limberg, C. An Iron(II) Hydride Complex of a Ligand with Two Adjacent β-Diketiminate Binding Sites and Its Reactivity. Dalton Trans. 2016, 45, 2989–2996. [Google Scholar] [CrossRef]
- Ding, K.; Brennessel, W.W.; Holland, P.L. Three-Coordinate and Four-Coordinate Cobalt Hydride Complexes That React with Dinitrogen. J. Am. Chem. Soc. 2009, 131, 10804–10805. [Google Scholar] [CrossRef]
- Wang, G.X.; Yan, X.; Yin, J.; Yin, Z.B.; Wei, J.; Xi, Z. Cobalt Cyclopentadienyl-Phosphine Dinitrogen Complexes. Chem. Eur. J. 2022, 28, e202202803. [Google Scholar] [CrossRef]
- Earnshaw, A.; Hewlett, P.C.; King, E.A.; Larkworthy, L.F. Transition Metal–Schiff’s Base Complexes. Part III. The Magnetic Properties of Some Oxygen-Carrying Cobalt(II) Complexes. J. Chem. Soc. A Inorg. Phys. Theor. 1968, 241–246. [Google Scholar] [CrossRef]
- Park, S.; Mathur, V.K.; Planalp, R.P. Syntheses, Solubilities, and Oxygen Absorption Properties of New Cobalt(II) Schiff-Base Complexes. Polyhedron 1998, 17, 325–330. [Google Scholar] [CrossRef]
- Jiang, G.B.; Zhang, S.H.; Zeng, M.H. Aqua{6,6′-Dimethoxy-2,2′-[Ethane-1,2-Diylbis(Nitrilomethylidyne)]Diphenolato-κ4O,N,N′,O′}Cobalt(II). Struct. Rep. 2007, 63, m2383. [Google Scholar] [CrossRef]
- Reath, A.H.; Ziller, J.W.; Tsay, C.; Ryan, A.J.; Yang, J.Y. Redox Potential and Electronic Structure Effects of Proximal Nonredox Active Cations in Cobalt Schiff Base Complexes. Inorg. Chem. 2017, 56, 3713–3718. [Google Scholar] [CrossRef]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting p53 Pathways: Mechanisms, Structures and Advances in Therapy. Signal Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Law, B.Y.K.; Qu, Y.Q.; Mok, S.W.F.; Liu, H.; Zeng, W.; Han, Y.; Gordillo-Martinez, F.; Chan, W.K.; Wong, K.M.C.; Wong, V.K.W. New Perspectives of Cobalt Tris(bipyridine) System: Anti-Cancer Effect and Its Collateral Sensitivity towards Multidrug-Resistant (MDR) Cancers. Oncotarget 2017, 8, 55003. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Liu, D.; Hu, H.; Qiu, Z.; Liang, Y.; Chen, Z. Anticancer Activity of Four Trinuclear Cobalt Complexes Bearing Bis(salicylidene)-1,3-propanediamine Derivatives. J. Inorg. Biochem. 2022, 233, 111860. [Google Scholar] [CrossRef]


| Compound | MCF-7 | MDA-MB 468 | MCF-10A |
|---|---|---|---|
| Mn-1 | 115.86 | 19.77 | 15.10 |
| Co-2 | 0.425 | 17.44 | 214.54 |
| CoK-3 | 31.46 | 14.84 | 116.22 |
| cisplatin | 11.46 | 0.309 | 17.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanina, A.; Mei, H.; Palma, C.; Neary, M.C.; Cheng, S.-Y.; Zhang, G. Synthesis, Reductive Reactivity and Anticancer Activity of Cobalt(III)– and Manganese(III)–Salen Complexes. Chemistry 2025, 7, 85. https://doi.org/10.3390/chemistry7030085
Kanina A, Mei H, Palma C, Neary MC, Cheng S-Y, Zhang G. Synthesis, Reductive Reactivity and Anticancer Activity of Cobalt(III)– and Manganese(III)–Salen Complexes. Chemistry. 2025; 7(3):85. https://doi.org/10.3390/chemistry7030085
Chicago/Turabian StyleKanina, Amy, Haiyu Mei, Cheska Palma, Michelle C. Neary, Shu-Yuan Cheng, and Guoqi Zhang. 2025. "Synthesis, Reductive Reactivity and Anticancer Activity of Cobalt(III)– and Manganese(III)–Salen Complexes" Chemistry 7, no. 3: 85. https://doi.org/10.3390/chemistry7030085
APA StyleKanina, A., Mei, H., Palma, C., Neary, M. C., Cheng, S.-Y., & Zhang, G. (2025). Synthesis, Reductive Reactivity and Anticancer Activity of Cobalt(III)– and Manganese(III)–Salen Complexes. Chemistry, 7(3), 85. https://doi.org/10.3390/chemistry7030085







