Advancements and Challenges of Cobalt–Zeolite Composite Catalysts in Heterogeneous Catalysis
Abstract
1. Introduction
2. Synthesis of Cobalt–Zeolite Composite Catalysts
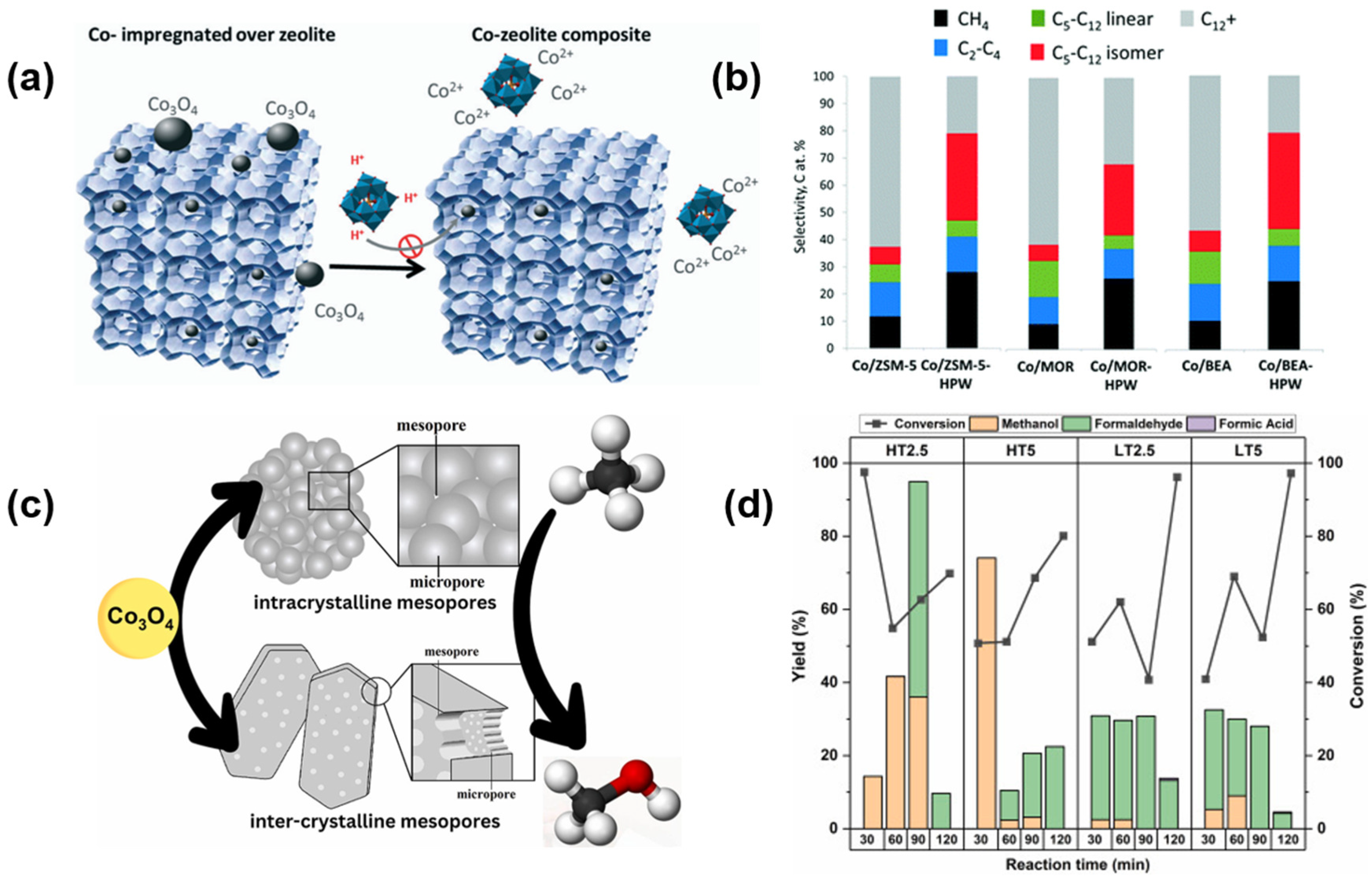
2.1. Synthesis Method
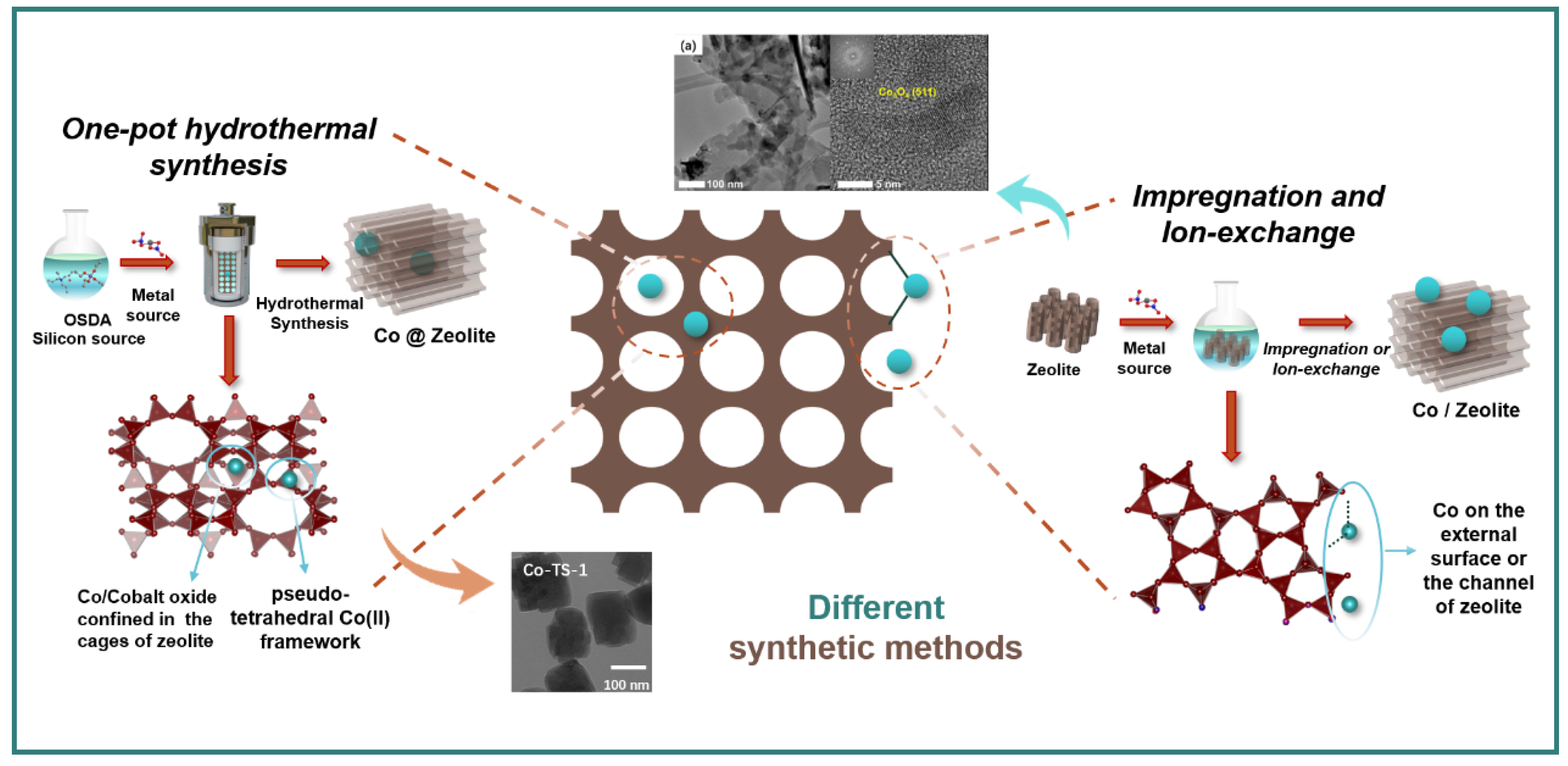
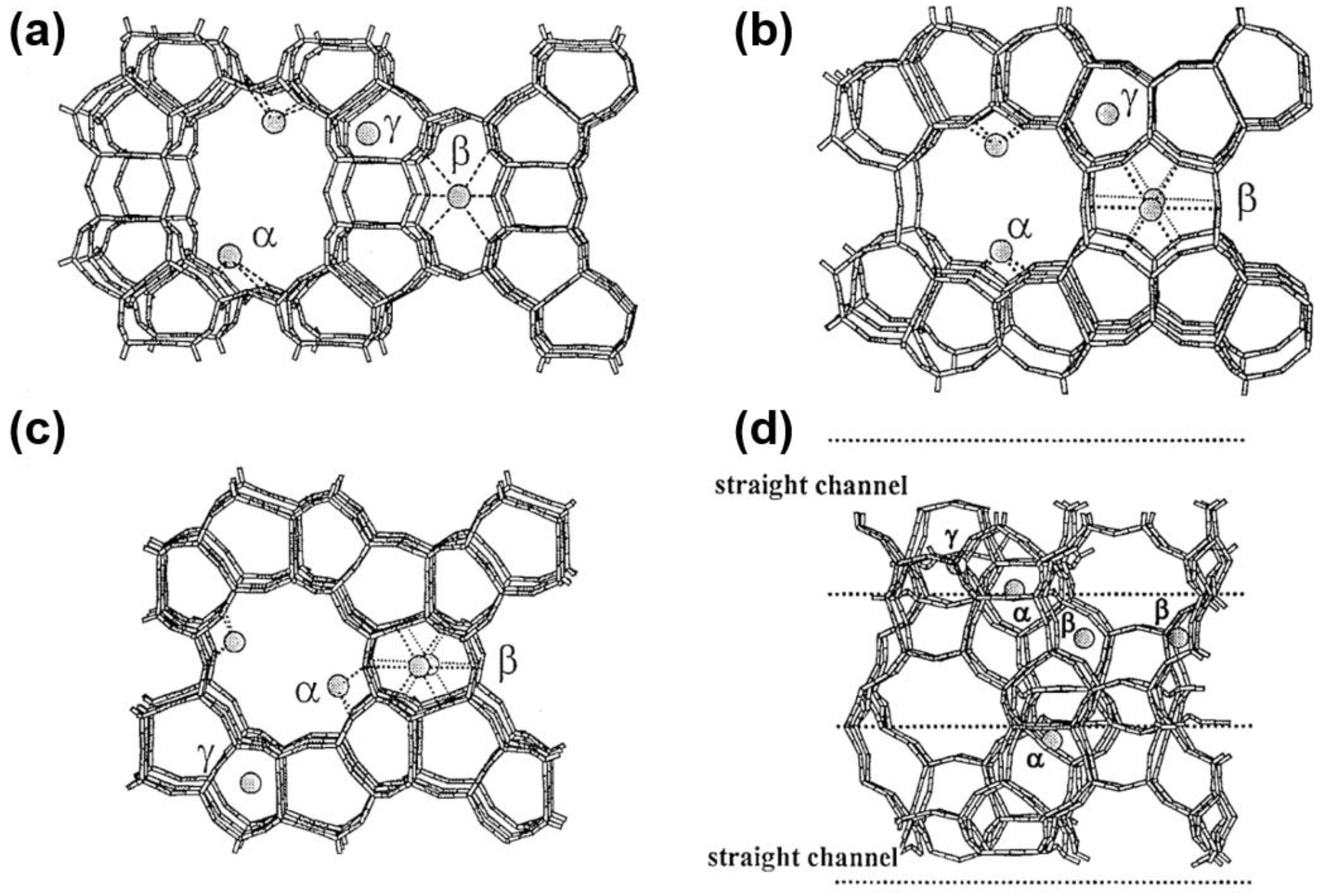
2.2. Multiple Valence States of Co
2.3. Bimetallic Synergy
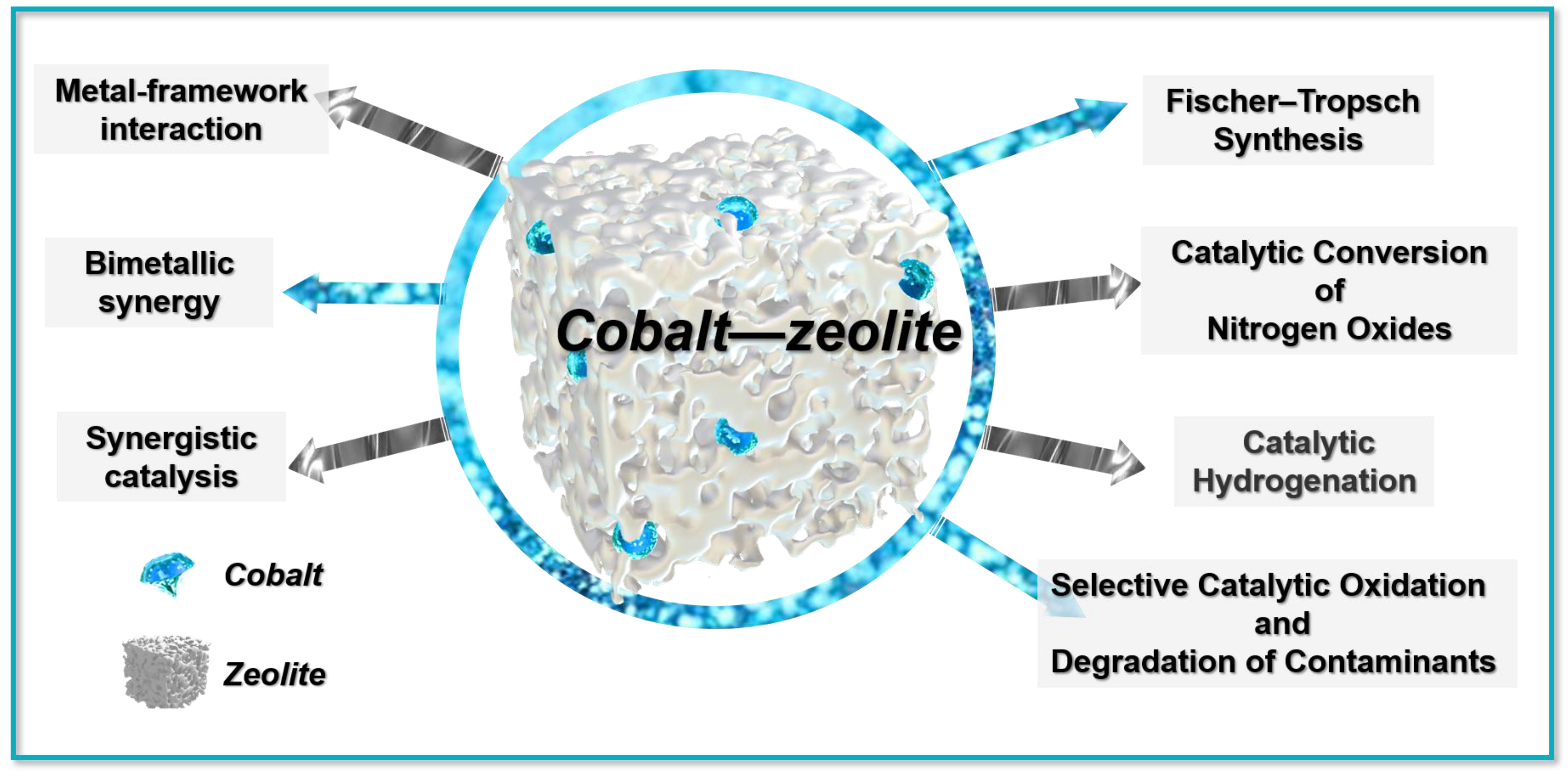
3. Application of Cobalt–Zeolite
3.1. Fischer–Tropsch Synthesis
| Catalyst | T (K) | Experimental Parameters | CO Conv. (%) | Product Selectivity (%) | Ref. |
|---|---|---|---|---|---|
| PtCoAl2O3/SiO2 | 423 | H2/CO=2; GHSV 1000 h−1 | 87.2 | C5+: 70.6 | [44] |
| Co/HZSM-5 | 513 | H2/CO=2; GHSV 0.5 h−1 | 87 | C5+: 40 | [52] |
| Co3O4/Beta | 503 | H2/CO=2; GHSV 1705–1852 h−1 | 12.6 | C5+: 88.2 | [54] |
| Co/NaBEA | 523 | H2/CO=2; GHSV 40–65 L·h−1·gCo−1 | 68 | C5+: 71.9 | [55] |
| Co/H-ZSM-5 | 503 | H2/CO=2; GHSV 8 SL·g−1·h−1 | 14.3 | C5–12: 67.8 | [56] |
| Co-MCM-22 | 523 | H2/CO=2; W/F = 5.0 g·h·mol−1 | 60 | CH4: 33.3 | [57] |
| Co@ZSM-5/SiC | 673 | H2/CO=2; W/F = 10 g·h·mol−1 | 100 | C5–12: 60 | [58] |
| Co/BEA | 523 | H2/CO=2; GHSV 1.7–5 L·g−1·h−1 | 22 | Ciso(5–12): 35 | [59] |
| NiCoAlBeta | 533 | H2/CO=2; P = 30 atm | 100 | C5+: 100 | [60] |
| Co/SBA-15 | 463 | H2/CO=2; P = 1 atm | 4 | C5+: 71.8 | [64] |
| Co/MOR | 523 | H2/CO=2; GHSV 34 L·h−1·gCo−1 | 40.1 | C12+: 60.9 | [65] |
3.2. Catalytic Conversion of Nitrogen Oxides
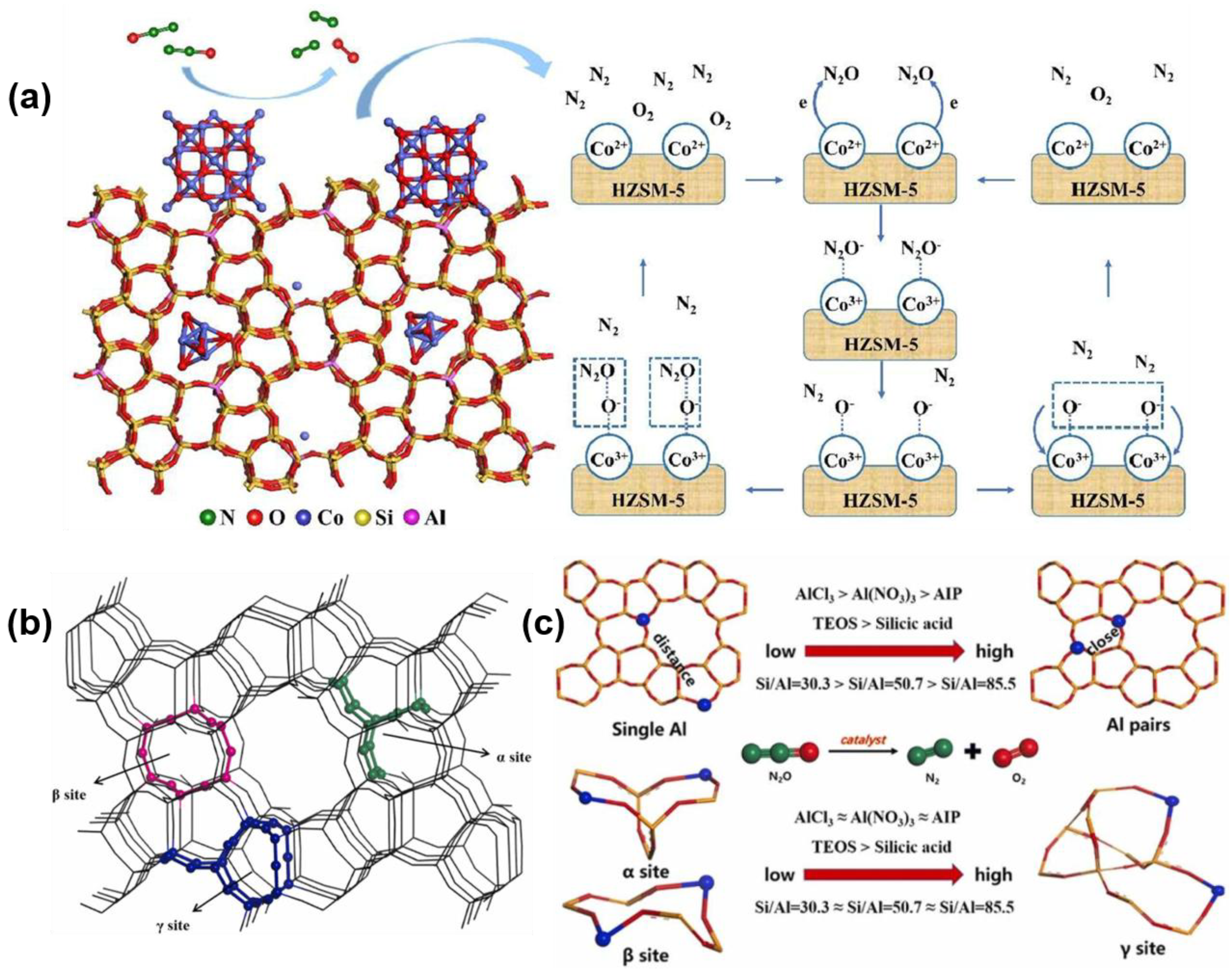
3.2.1. Nitrous Oxide (N2O) Decomposition
3.2.2. Selective Catalytic Reduction (SCR) of NOx
| Catalyst | T (K) | Reaction Mixture a | Experimental Parameters | NO Conv. (%) | Ref. |
|---|---|---|---|---|---|
| 0.15 wt% Pd/4.8 wt% Co-HMOR | 773 | 1000 ppm NO, 2700 ppm CH4, | GHSV 30,000 h−1 | 60% | [83] |
| 0.31 wt% Pd/5.35 wt% Co-FER | 773 | 6% O2, 8% H2O | GHSV 14,000 h−1 | ~80% | [84] |
| 0.4 wt% Pd/2.3 wt% Co-HZSM-5 | 723 | 1200 ppm NO, 2400 ppm CH4, | GHSV 20,000 h−1 | ~80–90% | [85] |
| 0.4 wt% Pd/3.3 wt% Co/HZSM-5 | 773 | 2.6% O2, 10% H2O | cat 0.1 g; flow rate | (first 30 h) | [86] |
| 9.4 wt% Co/SBA-15 | 873 | 500 ppm NO, 2500 ppm CH4, | 100 cm3 min−1 | ~60% * | [91] |
| 2.16 wt% CoSiBEA | 673 | 5% O2, 5% H2O | GHSV 15,000 h−1 | ~65% | [92] |
| 0.21 wt% Ba/1.28 wt% Co/ZSM-5 | 773 | 100 ppm NO, 2000 ppm CH4, | cat 0.2 g; flow rate | ~80% | [95] |
| 6.9 wt% CoO-IM5 | 723 | 700 ppm NO, 380 ppm C3H8, | cat 1.0 g; flow rate | ~79% | [97] |
| 1.13 wt% Co/BEA | 723 | 2% O2 | 650 cm3 min−1 | ~80% | [98] |
| Co-BEA | 723 | 1000 ppm NO, 1000 ppm C3H8, | GHSV 15,000 h−1 | ~90% | [99] |
| 2.87 wt% CoNH4-MFI | 698 | 850 ppm NO, 550 ppm C3H8, | 650 cm3 min−1 | ~90% * | [100] |
| CoNa-ZSM-5 (Co/Al 0.22) | 723 | 2.5% O2 | GHSV 30,000 h−1 | ~90% * | [102] |
| 3.3 wt% Co-CHA | 750 | 4000 ppm NO, 4000 ppm CH4, | GHSV 7500 h−1 | ~95% * | [103] |
| CoSiBEA | 650 | 2% O2 | GHSV 7500 h−1 | ~80% | [110] |
| Co-HZSM-5 | 573 | 900 ppm NO, 1200 ppm CH4, | GHSV 14,000 h−1 | ~90% | [111] |
3.3. Catalytic Hydrogenation
3.4. Selective Catalytic Oxidation and the Degradation of Contaminants
3.5. Other Applications
4. Conclusions and Outlook
- (a)
- Precise identification of active species and mechanistic elucidation:
- (b)
- The creation of innovative synthesis strategies and catalysts:
- (c)
- Machine learning-guided catalyst optimization
- (d)
- The advancement of eco-friendly catalysts:
Author Contributions
Funding
Conflicts of Interest
References
- Meng, Y.; Liu, Y.Q.; Wang, C.; Si, Y.; Wang, Y.J.; Xia, W.Q.; Liu, T.; Cao, X.; Guo, Z.Y.; Chen, J.J.; et al. Nanoconfinement steers nonradical pathway transition in single atom fenton-like catalysis for improving oxidant utilization. Nat. Commun. 2024, 15, 5314. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.H.; Tian, S.N.; Liang, H.L.; Wang, H.; Gao, S.; Dai, W. Oxidative cleavage and ammoxidation of organosulfur compounds via synergistic Co-Nx sites and Co nanoparticles catalysis. Nat. Commun. 2023, 14, 2981. [Google Scholar] [CrossRef] [PubMed]
- Parastaev, A.; Muravev, V.; Osta, E.H.; Kimpel, T.F.; Simons, J.F.M.; van Hoof, A.J.F.; Uslamin, E.; Zhang, L.; Struijs, J.J.C.; Burueva, D.B.; et al. Breaking structure sensitivity in CO hydrogenation by tuning metal-oxide interfaces in supported cobalt nanoparticles. Nat. Catal. 2022, 5, 1051–1060. [Google Scholar] [CrossRef]
- Scharnagl, F.K.; Hertrich, M.F.; Ferretti, F.; Kreyenschulte, C.; Lund, H.; Jackstell, R.; Beller, M. Hydrogenation of terminal and internal olefins using a biowaste-derived heterogeneous cobalt catalyst. Sci. Adv. 2018, 4, eaau1248. [Google Scholar] [CrossRef]
- Zhou, P.; Jiang, L.; Wang, F.; Deng, K.J.; Lv, K.L.; Zhang, Z.H. High performance of a cobalt-nitrogen complex for the reduction and reductive coupling of nitro compounds into amines and their derivatives. Sci. Adv. 2017, 3, e1601945. [Google Scholar] [CrossRef]
- Faculak, M.S.; Veatch, A.M.; Alexanian, E.J. Cobalt-catalyzed synthesis of amides from alkenes and amines promoted by light. Science 2024, 383, 77–81. [Google Scholar] [CrossRef]
- Alamoudi, O.M.; Khan, W.U.; Hantoko, D.; Bakare, I.A.; Ali, S.A.; Hossain, M.M. Catalytic activity of Co/γ-Al2O3 catalysts for decomposition of ammonia to produce hydrogen. Fuel 2024, 372, 132230. [Google Scholar] [CrossRef]
- Jeletic, M.S.; Helm, M.L.; Hulley, E.B.; Mock, M.T.; Appel, A.M.; Linehan, J.C. A Cobalt Hydride Catalyst for the Hydrogenation of CO: Pathways for Catalysis and Deactivation. ACS Catal. 2014, 4, 3755–3762. [Google Scholar] [CrossRef]
- Xia, W.; Salmeia, K.A.; Vagin, S.I.; Rieger, B. Concerning the Deactivation of Cobalt(III)-Based Porphyrin and Salen Catalysts in Epoxide/CO Copolymerization. Chem.-Eur. J. 2015, 21, 4384–4390. [Google Scholar] [CrossRef]
- Eschemann, T.O.; de Jong, K.P. Deactivation Behavior of Co/TiO2 Catalysts during Fischer-Tropsch Synthesis. ACS Catal. 2015, 5, 3181–3188. [Google Scholar] [CrossRef]
- Gorshkov, A.S.; Sineva, L.V.; Gryaznov, K.O.; Asalieva, E.Y.; Mordkovich, V.Z. Deactivation and Regeneration of a Zeolite-Containing Cobalt Catalyst in a Fisher-Tropsch Synthesis Reactor. Catal. Ind. 2023, 15, 152–164. [Google Scholar] [CrossRef]
- Moodley, D.J.; van de Loosdrecht, J.; Saib, A.M.; Overett, M.J.; Datye, A.K.; Niemantsverdriet, J.W. Carbon deposition as a deactivation mechanism of cobalt-based Fischer-Tropsch synthesis catalysts under realistic conditions. Appl. Catal. A-Gen. 2009, 354, 102–110. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J.H. Emerging applications of zeolites in catalysis, separation and host-guest assembly. Nat. Rev. Mater. 2021, 6, 1156–1174. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, S.Q.; Yu, J.H. Metal Sites in Zeolites: Synthesis, Characterization, and Catalysis. Chem. Rev. 2023, 123, 6039–6106. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Xiao, F.S. Metal@Zeolite Hybrid Materials for Catalysis. ACS Cent. Sci. 2020, 6, 1685–1697. [Google Scholar] [CrossRef]
- Gomez-Hortiguela, L.; Perez-Pariente, J. Synthesis and Properties of Zeolite Materials Guided by Periodic Considerations. In Periodic Table II: Catalytic, Materials, Biological and Medical Applications; Mingos, D.M.P., Ed.; Springer Nature: Berlin, Germany, 2019; pp. 53–88. [Google Scholar]
- Wu, S.M.; Yang, X.Y.; Janiak, C. Confinement Effects in Zeolite-Confined Noble Metals. Angew. Chem.-Int. Ed. 2019, 58, 12340–12354. [Google Scholar] [CrossRef]
- Otto, T.; Zones, S.I.; Hong, Y.C.; Iglesia, E. Synthesis of highly dispersed cobalt oxide clusters encapsulated within LTA zeolites. J. Catal. 2017, 356, 173–185. [Google Scholar] [CrossRef]
- Han, S.; Tang, X.; Ma, Y.; Wu, Q.; Shi, J.; Li, J.; Meng, X.; Zheng, A.; Xiao, F.-S. Design of Cobalt-Amine Complex as an Efficient Structure-Directing Agent for One-Pot Synthesis of Co-SSZ-13 Zeolite. J. Phys. Chem. C 2021, 125, 16343–16349. [Google Scholar] [CrossRef]
- Jin, S.M.; Lee, K.-Y.; Lee, D.-W. Ozone-induced lean methane oxidation over cobalt ion-exchanged BEA catalyst under dry reaction conditions. J. Ind. Eng. Chem. 2022, 112, 296–306. [Google Scholar] [CrossRef]
- Guo, H.; He, H.; Miao, C.; Hua, W.; Yue, Y.; Gao, Z. Ethane conversion in the presence of CO2 over Co-based ZSM-5 zeolite: Co species controlling the reaction pathway. Mol. Catal. 2022, 519, 112155. [Google Scholar] [CrossRef]
- Crawford, J.M.; Smoljan, C.S.; Lucero, J.; Carreon, M.A. Deoxygenation of Stearic Acid over Cobalt-Based NaX Zeolite Catalysts. Catalysts 2019, 9, 42. [Google Scholar] [CrossRef]
- Jentys, A.; Lugstein, A.; Vinek, H. Co-containing zeolites prepared by solid-state ion exchange. J. Chem. Soc. Faraday Trans. 1997, 93, 4091–4094. [Google Scholar] [CrossRef]
- van der Meer, J.; Bardez, I.; Bart, F.; Albouy, P.A.; Wallez, G.; Davidson, A. Dispersion of CoO nanoparticles within SBA-15 using alkane solvents. Microporous Mesoporous Mater. 2009, 118, 183–188. [Google Scholar] [CrossRef]
- Liang, W.; Xu, G.; Fu, Y. Selective photocatalytic oxidation of furfural to C4 compounds with metal-TS-1 zeolite. Appl. Catal. B Environ. 2024, 340, 123220. [Google Scholar] [CrossRef]
- Khemthong, P.; Klysubun, W.; Prayoonpokarach, S.; Wittayakun, J. Reducibility of cobalt species impregnated on NaY and HY zeolites. Mater. Chem. Phys. 2010, 121, 131–137. [Google Scholar] [CrossRef]
- Bania, K.K.; Deka, R.C. Influence of Zeolite Framework on the Structure, Properties, and Reactivity of Cobalt Phenanthroline Complex: A Combined Experimental and Computational Study. J. Phys. Chem. C 2011, 115, 9601–9607. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, Y.; Shamzhy, M.; Molitorisova, S.; Opanasenko, M.; Giroir-Fendler, A. Total Oxidation of Toluene and Propane over Supported Co3O4 Catalysts: Effect of Structure/Acidity of MWW Zeolite and Cobalt Loading. ACS Appl. Mater. Interfaces 2021, 13, 15143–15158. [Google Scholar] [CrossRef]
- Lónyi, F.; Solt, H.E.; Valyon, J.; Boix, A.; Gutierrez, L.B. The activation of NO and CH for NO-SCR reaction over In- and Co-containing H-ZSM-5 catalysts. J. Mol. Catal. A-Chem. 2011, 345, 75–80. [Google Scholar] [CrossRef]
- Xu, Y.B.; Yu, W.D.; Zhang, H.; Xin, J.; He, X.H.; Liu, B.; Jiang, F.; Liu, X.H. Suppressing C–C Bond Dissociation for Efficient Ethane Dehydrogenation over the Isolated Co(II) Sites in SAPO-34. ACS Catal. 2021, 11, 13001–13019. [Google Scholar] [CrossRef]
- Sadek, R.; Chalupka-Spiewak, K.; Krafft, J.-M.; Millot, Y.; Valentin, L.; Casale, S.; Gurgul, J.; Dzwigaj, S. The Synthesis of Different Series of Cobalt BEA Zeolite Catalysts by Post-Synthesis Methods and Their Characterization. Catalysts 2022, 12, 1644. [Google Scholar] [CrossRef]
- Lee, J.H.; Bonte, W.; Corthals, S.; Krumeich, F.; Ruitenbeek, M.; van Bokhoven, J.A. Zeolite Nanoreactor for Investigating Sintering Effects of Cobalt-Catalyzed Fischer-Tropsch Synthesis. Ind. Eng. Chem. Res. 2019, 58, 5140–5145. [Google Scholar] [CrossRef]
- Tang, Q.H.; Zhang, Q.H.; Wang, P.; Wang, Y.; Wan, H.L. Characterizations of cobalt oxide nanoparticles within faujasite zeolites and the formation of metallic cobalt. Chem. Mater. 2004, 16, 1967–1976. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Wang, D.G.; Feng, P.; Shi, S.; Wang, C.X.; Zheng, A.D.; Lu, G.; Tian, Z.J. Synthesis of zeolite Beta containing ultra-small CoO particles for ethylbenzene oxidation. Chin. J. Catal. 2017, 38, 1207–1215. [Google Scholar] [CrossRef]
- Alhashmi, E.; Ebri, G.; Hellgardt, K. Highly Active and Stable Single-Atom Cobalt in Zeolite for Acetylene Semihydrogenation. ACS Catal. 2015, 15, 4121–4140. [Google Scholar] [CrossRef]
- Zhou, H.; Huan, L.; Wang, L.; Chu, S.; Liu, L.; Qi, J.; Ren, Z.; Cai, A.; Hui, Y.; Qin, Y.; et al. Cobaltosilicate zeolite beyond platinum catalysts for propane dehydrogenation. Nat. Catal. 2025, 8, 357–367. [Google Scholar] [CrossRef]
- Lira, E.; López, C.M.; Oropeza, F.; Bartolini, M.; Alvarez, J.; Goldwasser, M.; Linares, F.; Lamonier, J.; Zurita, M. HMS mesoporous silica as cobalt support for the Fischer–Tropsch Synthesis: Pretreatment, cobalt loading and particle size effects. J. Mol. Catal. A Chem. 2008, 281, 146–153. [Google Scholar] [CrossRef]
- Liang, J.; Fu, L.; Gao, K.; Duan, X. Accelerating radical generation from peroxymonosulfate by confined variable Co species toward ciprofloxacin mineralization: ROS quantification and mechanisms elucidation. Appl. Catal. B Environ. 2022, 315, 121542. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Fan, X.; Zhang, F.; Zhang, G.; Peng, W. Photo-accelerated Co3+/Co2+ transformation on cobalt and phosphorus co-doped g-C3N4 for Fenton-like reaction. J. Mater. Chem. A 2021, 9, 22399–22409. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, D.; Lu, Y.; Li, Y.; Yang, Q.; Di, J.; Liang, H.; Qiao, Y.; Tian, Y.; Gai, X. Embedded catalysts prepared by grinding crystallization and their catalytic performance in dry reforming of methane: Domain-limited nanostructures for high activity and stability. Int. J. Hydrogen Energy 2024, 73, 895–904. [Google Scholar] [CrossRef]
- Zhang, J.; Bo, S.; Liao, W.; Yang, K.; Su, T.; Lue, H.; Zhu, Z. Zeolitic framework Sn boosts the 2,5-dimethylfuran selectivity for the hydrodeoxygenation of 5-hydroxymethylfurfural over Co/Sn-Beta catalyst. Chem. Eng. J. 2024, 484, 149511. [Google Scholar] [CrossRef]
- Wei, Z.; Bai, X.; Maximov, A.L.; Wu, W. Ultrasound-assisted preparation of PdCo bimetallic nanoparticles loaded on beta zeolite for efficient catalytic hydrogen production from dodecahydro-N-ethylcarbazole. Ultrason. Sonochem. 2024, 103, 106793. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, A.; Shi, C. Enhanced Low-Temperature Activity of Ag-Promoted Co-ZSM-5 for the CH4-SCR of NO. Catal. Lett. 2011, 141, 207–212. [Google Scholar] [CrossRef]
- Yakovenko, R.E.; Zubkov, I.N.; Papeta, O.P.; Kataria, Y.V.; Bakun, V.G.; Svetogorov, R.D.; Savost’yanov, A.P. The Influence of Platinum on the Catalytic Properties of Bifunctional Cobalt Catalysts for the Synthesis of Hydrocarbons from CO and H2. Catalysts 2024, 14, 351. [Google Scholar] [CrossRef]
- Telaar, P.; Diehl, P.; Muhler, M. Bridging Homogeneously and Heterogeneously Catalyzed Higher-Alcohol Synthesis via Cobalt-Based Catalysts. Chem. Ing. Tech. 2024, 9, 1244–1255. [Google Scholar] [CrossRef]
- Sineva, L.; Mordkovich, V.; Asalieva, E.; Smirnova, V. Zeolite-Containing Co Catalysts for Fischer-Tropsch Synthesis with Tailor-Made Molecular-Weight Distribution of Hydrocarbons. Reactions 2023, 4, 22. [Google Scholar] [CrossRef]
- Mazurova, K.; Miyassarova, A.; Eliseev, O.; Stytsenko, V.; Glotov, A.; Stavitskaya, A. Fischer-Tropsch Synthesis Catalysts for Selective Production of Diesel Fraction. Catalysts 2023, 13, 1215. [Google Scholar] [CrossRef]
- Martínez, A.; Rollán, J.; Arribas, M.A.; Cerqueira, H.S.; Costa, A.F.; Aguiar, E.F.S. A detailed study of the activity and deactivation of zeolites in hybrid Co/SiO2-zeolite Fischer-Tropsch catalysts. J. Catal. 2007, 249, 162–173. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Kang, J.C.; Wang, Y. Development of Novel Catalysts for Fischer-Tropsch Synthesis: Tuning the Product Selectivity. Chemcatchem 2010, 2, 1030–1058. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Y.; Wang, C. Research progress on the direct catalytic conversion of syngas to light olefins. Chem. Ind. Eng. Prog. 2022, 41, 4754–4766. [Google Scholar] [CrossRef]
- Xulu Martinez-Vargas, D.; Sandoval-Rangel, L.; Campuzano-Calderon, O.; Romero-Flores, M.; Lozano, F.J.; Nigam, K.D.P.; Mendoza, A.; Montesinos-Castellanos, A. Recent Advances in Bifunctional Catalysts for the Fischer-Tropsch Process: One-Stage Production of Liquid Hydrocarbons from Syngas. Ind. Eng. Chem. Res. 2019, 58, 15872–15901. [Google Scholar] [CrossRef]
- Dalil, M.; Sohrabi, M.; Royaee, S.J. Application of nano-sized cobalt on ZSM-5 zeolite as an active catalyst in Fischer-Tropsch synthesis. J. Ind. Eng. Chem. 2012, 18, 690–696. [Google Scholar] [CrossRef]
- Liu, J.Y.; Wang, D.; Chen, J.F.; Zhang, Y. Cobalt nanoparticles imbedded into zeolite crystals: A tailor-made catalyst for one-step synthesis of gasoline from syngas. Int. J. Hydrogen Energy 2016, 41, 21965–21978. [Google Scholar] [CrossRef]
- Nakanishi, M.; Uddin, M.A.; Kato, Y.; Nishina, Y.; Hapipi, A.M. Effects of preparation method on the properties of cobalt supported β-zeolite catalysts for Fischer-Tropsch synthesis. Catal. Today 2017, 291, 124–132. [Google Scholar] [CrossRef]
- Flores, C.; Batalha, N.; Marcilio, N.R.; Ordomsky, V.V.; Khodakov, A.Y. Influence of Impregnation and Ion Exchange Sequence on Metal Localization, Acidity and Catalytic Performance of Cobalt BEA Zeolite Catalysts in Fischer-Tropsch Synthesis. Chemcatchem 2019, 11, 568–574. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Zhao, Y.; Lyu, S.; Wei, L.; Li, X.; Zhang, Y.; Li, J. Nano-ZSM-5-supported cobalt for the production of liquid fuel in Fischer-Tropsch synthesis: Effect of preparation method and reaction temperature. Zurita 2020, 263, 116619. [Google Scholar] [CrossRef]
- Ren, H.-P.; Xie, Z.-X.; Tian, S.-P.; Ding, S.-Y.; Ma, Q.; Zhao, Y.-Z.; Zhang, Z.; Fu, J.-J.; Hao, Q.-Q. Physical Grinding of Prefabricated Co3O4 and MCM-22 Zeolite for Fischer-Tropsch Synthesis: Impact of Pretreatment Procedure on the Dispersion and Catalytic Performance. Molecules 2024, 29, 1283. [Google Scholar] [CrossRef]
- Qi, H.; Xing, C.; Huang, W.; Li, M.; Jiang, Y.; Sun, X.; Liu, H.; Lu, P.; Chen, J.; Chen, S. Design of a hierarchical Co@ZSM-5/SiC capsule catalyst for direct conversion of syngas to middle olefin. Microporous Mesoporous Mater. 2022, 343, 112134. [Google Scholar] [CrossRef]
- Carvalho, A.; Marinova, M.; Batalha, N.; Marcilio, N.R.; Khodakov, A.Y.; Ordomsky, V.V. Design of nanocomposites with cobalt encapsulated in the zeolite micropores for selective synthesis of isoparaffins in Fischer-Tropsch reaction. Catal. Sci. Technol. 2017, 7, 5019–5027. [Google Scholar] [CrossRef]
- Khatrin, I.; Abdullah, I.; McCue, A.J.; Krisnandi, Y.K. Mesoporous configuration effects on the physicochemical features of hierarchical ZSM-5 supported cobalt oxide as catalysts in methane partial oxidation. Microporous Mesoporous Mater. 2024, 365, 112896. [Google Scholar] [CrossRef]
- Sadek, R.; Chalupka, K.A.; Mierczynski, P.; Maniukiewicz, W.; Rynkowski, J.; Gurgul, J.; Lason-Rydel, M.; Casale, S.; Brouri, D.; Dzwigaj, S. The Catalytic Performance of Ni-Co/Beta Zeolite Catalysts in Fischer-Tropsch Synthesis. Catalysts 2020, 10, 112. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Griboval-Constant, A.; Bechara, R.; Zholobenko, V.L. Pore size effects in Fischer Tropsch synthesis over cobalt-supported mesoporous silicas. J. Catal. 2002, 206, 230–241. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Bechara, R.; Griboval-Constant, A. Fischer-Tropsch synthesis over silica supported cobalt catalysts: Mesoporous structure versus cobalt surface density. Appl. Catal. A-Gen. 2003, 254, 273–288. [Google Scholar] [CrossRef]
- Subramanian, V.; Zholobenko, V.L.; Cheng, K.; Lancelot, C.; Heyte, S.; Thuriot, J.; Paul, S.; Ordomsky, V.V.; Khodakov, A.Y. The Role of Steric Effects and Acidity in the Direct Synthesis of Paraffins from Syngas on Cobalt Zeolite Catalysts. Chemcatchem 2016, 8, 380–389. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S.; Vazzana, F.; Marella, M.; Tomaselli, M.; Mantegazza, M. Novel catalysts and catalytic technologies for NO removal from industrial emissions containing O2, H2O and SO2. Adv. Environ. Res. 2000, 4, 325–338. [Google Scholar] [CrossRef]
- Roy, S.; Hegde, M.S.; Madras, G. Catalysis for NO abatement. Appl. Energy 2009, 86, 2283–2297. [Google Scholar] [CrossRef]
- Iwamoto, M.; Hamada, H. Removal of Nitrogen Monoxide from Exhaust Gases through Novel Catalytic Processes. Catal. Today 1991, 10, 57–71. [Google Scholar] [CrossRef]
- Li, Y.J.; Armor, J.N. Catalytic Decomposition of Nitrous-Oxide on Metal Exchanged Zeolites. Appl. Catal. B-Environ. 1992, 1, 21–29. [Google Scholar] [CrossRef]
- Chang, Y.F.; Mccarty, J.G.; Wachsman, E.D.; Wong, V.L. Catalytic Decomposition of Nitrous-Oxide over Ru-Exchanged Zeolites. Appl. Catal. B-Environ. 1994, 4, 283–299. [Google Scholar] [CrossRef]
- De Correa, C.M.; Villa, A.L.; Zapata, M. Decomposition of nitrous oxide in excess oxygen over Co- and Cu-exchanged MFI zeolites. Catal. Lett. 1996, 38, 27–32. [Google Scholar] [CrossRef]
- Armor, J.N.; Farris, T.S. The Unusual Hydrothermal Stability of Co-Zsm-5. Appl. Catal. B-Environ. 1994, 4, 11–17. [Google Scholar] [CrossRef]
- da Cruz, R.S.; Mascarenhas, A.J.S.; Andrade, H.M.C. Co-ZSM-5 catalysts for NO decomposition. Appl. Catal. B-Environ. 1998, 18, 223–231. [Google Scholar] [CrossRef]
- Ghahri, A.; Golbabaei, F.; Vafajoo, L.; Mireskandari, S.M.; Yaseri, M.; Shahtaheri, S.J. Removal of Greenhouse Gas (NO) by Catalytic Decomposition on Natural Clinoptilolite Zeolites Impregnated with Cobalt. Int. J. Environ. Res. 2017, 11, 327–337. [Google Scholar] [CrossRef]
- Smeets, P.J.; Meng, Q.G.; Corthals, S.; Leeman, H.; Schoonheydt, R.A. Co-ZSM-5 catalysts in the decomposition of NO and the SCR of NO with CH: Influence of preparation method and cobalt loading. Appl. Catal. B-Environ. 2008, 84, 505–513. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Shen, Q.; He, C.; Wang, Y.F.; Cheng, J.; Hao, Z.P. CoMOR zeolite catalyst prepared by buffered ion exchange for effective decomposition of nitrous oxide. J. Hazard. Mater. 2011, 192, 1756–1765. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Shen, Q.; He, C.; Ma, C.Y.; Cheng, J.; Liu, Z.M.; Hao, Z.P. Decomposition of nitrous oxide over Cobalt-zeolite catalysts: Role of zeolite structure and active site. Catal. Sci. Technol. 2012, 2, 1249–1258. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Schwieger, W.; Unger, A. Nitrous oxide decomposition over transition metal exchanged ZSM-5 zeolites prepared by the solid-state ion-exchange method. Appl. Catal. B-Environ. 2008, 84, 277–288. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Schwieger, W. Self oscillatory behaviour in NO decomposition over Co-ZSM-5 catalysts. Appl. Catal. B-Environ. 2009, 85, 120–130. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Shang, R.; Zhao, J.; Xu, Q.; Wang, H.; Liu, J. Multiple cobalt species on HZSM-5 cooperative catalyzing N2O decomposition. Mol. Catal. 2024, 552, 113706. [Google Scholar] [CrossRef]
- Kang, B.; Li, M.; Di, Z.; Guo, X.; Wei, Y.; Jia, J.; Zhang, R. Role of Al pairs on effective N2O decomposition over the ZSM-5 zeolite catalyst. Catal. Today 2022, 402, 17–26. [Google Scholar] [CrossRef]
- Tabata, T.; Kokitsu, M.; Ohtsuka, H.; Okada, O.; Sabatino, L.M.F.; Bellussi, G. Study on catalysts of selective reduction of NOx using hydrocarbons for natural gas engines. Catal. Today 1996, 27, 91–98. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.Y.; Sachtler, W.M.H. Mechanism of the selective reduction of NO over Co/MFI: Comparison with Fe/MFI. J. Catal. 2001, 197, 281–291. [Google Scholar] [CrossRef]
- Bustamante, F.; Córdoba, F.; Yates, M.; de Correa, C.M. The promotion of cobalt mordenite by palladium for the lean CH-SCR of NO in moist streams. Appl. Catal. A-Gen. 2002, 234, 127–136. [Google Scholar] [CrossRef]
- Lee, T.J.; Nam, I.S.; Ham, S.W.; Baek, Y.S.; Shin, K.H. Effect of Pd on the water tolerance of Co-ferrierite catalyst for NO reduction-by CH. Appl. Catal. B-Environ. 2003, 41, 115–127. [Google Scholar] [CrossRef]
- Pieterse, J.A.Z.; van den Brink, R.W.; Booneveld, S.; de Bruijn, F.A. Influence of zeolite structure on the activity and durability of Co-Pd-zeolite catalysts in the reduction of NO with methane. Appl. Catal. B-Environ. 2003, 46, 239–250. [Google Scholar] [CrossRef]
- Ogura, M.; Kage, S.; Shimojo, T.; Oba, J.; Hayashi, M.; Matsukata, M.; Kikuchi, E. Co cation effects on activity and stability of isolated Pd(II) cations in zeolite matrices for selective catalytic reduction of nitric oxide with methane. J. Catal. 2002, 211, 75–84. [Google Scholar] [CrossRef]
- Lee, J.; Chen, J.; Giewont, K.; Mon, T.; Liu, C.-H.; Walker, E.A.; Kyriakidou, E.A. Effect of cobalt incorporation on the stability of ionic Pd in the presence of carbon monoxide over Pd/BEA passive NOx adsorbers. Chem. Eng. J. 2022, 440, 135834. [Google Scholar] [CrossRef]
- Pieterse, J.A.Z.; van den Brink, R.W.; Booneveld, S.; de Bruijn, F.A. Durability of ZSM5-supported Co-Pd catalysts in the reduction of NO with methane. Appl. Catal. B-Environ. 2002, 39, 167–179. [Google Scholar] [CrossRef]
- Ogura, M.; Sugiura, Y.; Hayashi, M.; Kikuchi, E. Reduction of nitric oxide with methane on Pd/Co/H-ZSM-5 catalysts: Cooperative effects of Pd and Co. Catal. Lett. 1996, 42, 185–189. [Google Scholar] [CrossRef]
- Guo, A.Q.; Liu, H.B.; Li, Y.T.; Luo, Y.H.; Ye, D.Q.; Jiang, J.X.; Chen, P.R. Recent progress in novel zeolite catalysts for selective catalytic reduction of nitrogen oxides. Catal. Today 2023, 422, 114212. [Google Scholar] [CrossRef]
- Bin, F.; Song, C.L.; Lv, G.; Song, J.O.; Cao, X.F.; Pang, H.T.; Wang, K.P. Structural Characterization and Selective Catalytic Reduction of Nitrogen Oxides with Ammonia: A Comparison Between Co/ZSM-5 and Co/SBA-15. J. Phys. Chem. C 2012, 116, 26262–26274. [Google Scholar] [CrossRef]
- Baran, R.; Krafft, J.M.; Onfroy, T.; Grzybek, T.; Dzwigaj, S. Influence of the nature and environment of cobalt on the catalytic activity of Co-BEA zeolites in selective catalytic reduction of NO with ammonia. Microporous Mesoporous Mater. 2016, 225, 515–523. [Google Scholar] [CrossRef]
- Boron, P.; Chmielarz, L.; Gil, B.; Marszalek, B.; Dzwigaj, S. Experimental evidence of NO SCR mechanism in the presence of the BEA zeolite with framework and extra-framework cobalt species. Appl. Catal. B-Environ. 2016, 198, 457–470. [Google Scholar] [CrossRef]
- Mrad, R.; Aissat, A.; Cousin, R.; Courcot, D.; Siffert, S. Catalysts for NO selective catalytic reduction by hydrocarbons (HC-SCR). Appl. Catal. A-Gen. 2015, 504, 542–548. [Google Scholar] [CrossRef]
- Stakheev, A.Y.; Lee, C.W.; Park, S.J.; Chong, P.J. Selective catalytic reduction of NO with propane over CoZSM-5 containing alkaline earth cations. Appl. Catal. B-Environ. 1996, 9, 65–76. [Google Scholar] [CrossRef]
- Ohtsuka, H.; Tabata, T.; Okada, O.; Sabatino, L.M.F.; Bellussi, G. A study on the roles of cobalt species in NO reduction by propane on Co-Beta. Catal. Today 1998, 42, 45–50. [Google Scholar] [CrossRef]
- Palomares, A.E.; Prato, J.G.; Corma, A. Co-exchanged IM5, a stable zeolite for the selective catalytic reduction of NO in the presence of water and SO. Ind. Eng. Chem. Res. 2003, 42, 1538–1542. [Google Scholar] [CrossRef]
- Chen, H.H.; Shen, S.C.; Chen, X.Y.; Kawi, S. Selective catalytic reduction of NO over Co/beta-zeolite: Effects of synthesis condition of beta-zeolites, Co precursor, Co loading method and reductant. Appl. Catal. B-Environ. 2004, 50, 37–47. [Google Scholar] [CrossRef]
- Palomares, A.E.; Franch, C.; Corma, A. Determining the characteristics of a Cobalt-zeolite to be active for the selective catalytic reduction of NO with hydrocarbons. Catal. Today 2011, 176, 239–241. [Google Scholar] [CrossRef]
- Resini, C.; Montanari, T.; Nappi, L.; Bagnasco, G.; Turco, M.; Busca, G.; Bregani, F.; Notaro, M.; Rocchini, G. Selective catalytic reduction of NO by methane over Co-H-MFI and Co-H-FER zeolite catalysts: Characterisation and catalytic activity. J. Catal. 2003, 214, 179–190. [Google Scholar] [CrossRef]
- Kaucky, D.; Vondrová, A.; Dedecek, J.; Wichterlová, B. Activity of Co ion sites in ZSM-5, ferrierite, and mordenite in selective catalytic reduction of NO with methane. J. Catal. 2000, 194, 318–329. [Google Scholar] [CrossRef]
- Dedecek, J.; Kaucky, D.; Wichterlová, B. Does density of cationic sites affect catalytic activity of Co zeolites in selective catalytic reduction of NO with methane? Top. Catal. 2002, 18, 283–290. [Google Scholar] [CrossRef]
- Lim, J.B.; Shin, J.; Ahn, N.H.; Heo, I.; Hong, S.B. Selective catalytic reduction of NO with CH over cobalt-exchanged cage-based, small-pore zeolites with different framework structures. Appl. Catal. B-Environ. 2020, 267, 118710. [Google Scholar] [CrossRef]
- Lónyi, F.; Solt, H.E.; Pászti, Z.; Valyon, J. Mechanism of NO-SCR by methane over Co,H-ZSM-5 and Co,H-mordenite catalysts. Appl. Catal. B-Environ. 2014, 150, 218–229. [Google Scholar] [CrossRef]
- Sazama, P.; Pilar, R.; Mokrzycki, L.; Vondrova, A.; Kaucky, D.; Plsek, J.; Sklenak, S.; Stastny, P.; Klein, P. Remarkably enhanced density and specific activity of active sites in Al-rich Cu-, Fe- and Co-beta zeolites for selective catalytic reduction of NO. Appl. Catal. B-Environ. 2016, 189, 65–74. [Google Scholar] [CrossRef]
- Bellmann, A.; Atia, H.; Bentrup, U.; Brückner, A. Mechanism of the selective reduction of NO by methane over Co-ZSM-5. Appl. Catal. B-Environ. 2018, 230, 184–193. [Google Scholar] [CrossRef]
- Adelman, B.J.; Beutel, T.; Lei, G.D.; Sachtler, W.M.H. Mechanistic cause of hydrocarbon specificity over Cu/ZSM-5 and Co/ZSM-5 catalysts in the selective catalytic reduction of Nox. J. Catal. 1996, 158, 327–335. [Google Scholar] [CrossRef]
- Cant, N.W.; Liu, I.O.Y. The mechanism of the selective reduction of nitrogen oxides by hydrocarbons on zeolite catalysts. Catal. Today 2000, 63, 133–146. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.F.; Qin, M.Y.; Li, Q.B. Selective catalytic reduction of NO with CH over zeolite catalysts: Research progress, challenges and perspectives. J. Environ. Chem. Eng. 2022, 10, 107270. [Google Scholar] [CrossRef]
- Janas, J.; Machej, T.; Gurgul, J.; Socha, R.P.; Che, M.; Dzwigaj, S. Effect of Co content on the catalytic activity of CoSiBEA zeolite in the selective catalytic reduction of NO with ethanol: Nature of the cobalt species. Appl. Catal. B-Environ. 2007, 75, 239–248. [Google Scholar] [CrossRef]
- Niu, J.H.; Yang, X.F.; Zhu, A.M.; Shi, L.L.; Sun, Q.; Xu, Y.; Shi, C. Plasma-assisted selective catalytic reduction of NO by CH over Co-HZSM-5 catalyst. Catal. Commun. 2006, 7, 297–301. [Google Scholar] [CrossRef]
- Praserthdam, P.; Chaisuk, C.; Panit, A.; Kraiwattanawong, K. Some aspects about the nature of surface species on Pt-based and MFI-based catalysts for the selective catalytic reduction of NO by propene under lean-burn condition. Appl. Catal. B-Environ. 2002, 38, 227–241. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.Y.; Sachtler, W.M.H. Catalytic reduction of NO by hydrocarbons over Co/ZSM-5 catalysts prepared with different methods. Appl. Catal. B-Environ. 2000, 26, 227–239. [Google Scholar] [CrossRef]
- Suib, S.L.; Zhang, Z.C. Structure-Sensitive Reactions of Cyclopropane with Cobalt and Iron Zeolite Catalysts. ACS Symp. Ser. 1988, 368, 569–578. [Google Scholar] [CrossRef]
- Pedrosa, A.M.G.; Souza, M.J.B.; Melo, D.M.A.; Araujo, A.S. Cobalt and nickel supported on HY zeolite: Synthesis, characterization and catalytic properties. Mater. Res. Bull. 2006, 41, 1105–1111. [Google Scholar] [CrossRef]
- Upare, D.P.; Park, S.; Kim, M.S.; Kim, J.; Lee, D.; Lee, J.; Chang, H.; Choi, W.; Choi, S.; Jeon, Y.P.; et al. Cobalt promoted Mo/beta zeolite for selective hydrocracking of tetralin and pyrolysis fuel oil into monocyclic aromatic hydrocarbons. J. Ind. Eng. Chem. 2016, 35, 99–107. [Google Scholar] [CrossRef]
- Ahmed, M.H.M.; Muraza, O.; Jamil, A.K.; Shafei, E.N.; Yamani, Z.H.; Choi, K.H. Steam Catalytic Cracking of -Dodecane over Ni and Ni/Co Bimetallic Catalyst Supported on Hierarchical BEA Zeolite. Energy Fuels 2017, 31, 5482–5490. [Google Scholar] [CrossRef]
- Zula, M.; Grilc, M.; Likozar, B. Hydrocracking, hydrogenation and hydro-deoxygenation of fatty acids, esters and glycerides: Mechanisms, kinetics and transport phenomena. Chem. Eng. J. 2022, 444, 136564. [Google Scholar] [CrossRef]
- Ayodele, O.B. Influence of oxalate ligand functionalization on Co/ZSM-5 activity in Fischer Tropsch synthesis and hydrodeoxygenation of oleic acid into hydrocarbon fuels. Sci. Rep. 2017, 7, 10008. [Google Scholar] [CrossRef]
- Wu, G.J.; Zhang, N.; Dai, W.L.; Guan, N.J.; Li, L.D. Construction of Bifunctional Co/H-ZSM-5 Catalysts for the Hydrodeoxygenation of Stearic Acid to Diesel-Range Alkanes. Chemsuschem 2018, 11, 2179–2188. [Google Scholar] [CrossRef]
- Weilbeer, C.; Selent, D.; Dyballa, K.M.; Franke, R.; Spannenberg, A.; Börner, A. Evaluation of Organoselenium Based Compounds as Co-Catalysts in Rhodium-Catalyzed Hydroformylation. Chemistryselect 2016, 1, 5421–5429. [Google Scholar] [CrossRef]
- Qi, L.; Das, S.; Zhang, Y.; Nozik, D.; Gates, B.C.; Bell, A.T. Ethene Hydroformylation Catalyzed by Rhodium Dispersed with Zinc or Cobalt in Silanol Nests of Dealuminated Zeolite Beta. J. Am. Chem. Soc. 2023, 145, 2911–2929. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Guo, L.S.; Cui, Y.; Zhang, P.P.; Yoneyama, Y.; Yang, G.H.; Tsubaki, N. NaBH4 Reduced Cobalt Catalyst Supported on Zeolite A for 1-Hexene Hydroformylation. Chemistryselect 2019, 4, 10447–10451. [Google Scholar] [CrossRef]
- Lee, S.; Byun, S.W.; Kweon, S.; Shin, H.; Min, H.K.; Park, M.B.; Kang, S.B. Bi-functional two-dimensional cobalt silicate catalyst for selective catalytic oxidation of ammonia. Appl. Catal. B-Environ. 2024, 340, 123264. [Google Scholar] [CrossRef]
- Han, Z.; Chu, Q.; Wang, H.; Jia, X.; Jiang, P.; Li, T. Synthesis of Novel Catalytic Styrene Aerobic Oxidation Catalysts via Embedding Co and Ce. Catal. Lett. 2024, 154, 3787–3797. [Google Scholar] [CrossRef]
- Joseph, T.; Sawant, D.P.; Gopinath, C.S.; Halligudi, S.B. Zeolite encapsulated ruthenium and cobalt schiff base complexes catalyzed allylic oxidation of α-pinene. J. Mol. Catal. A-Chem. 2002, 184, 289–299. [Google Scholar] [CrossRef]
- Dang, J.; Li, W.; Qin, B.; Chai, Y.; Wu, G.; Li, L. Self-adjusted reaction pathway enables efficient oxidation of aromatic C-H bonds over zeolite-encaged single-site cobalt catalyst. Chin. J. Catal. 2024, 57, 133–142. [Google Scholar] [CrossRef]
- Xu, D.; Lv, H.; Liu, B. Encapsulation of Metal Nanoparticle Catalysts Within Mesoporous Zeolites and Their Enhanced Catalytic Performances: A Review. Front. Chem. 2018, 6, 550. [Google Scholar] [CrossRef]
- Liu, J.Y.; Wang, Z.H.; Jian, P.M.; Jian, R.Q. Highly selective oxidation of styrene to benzaldehyde over a tailor-made cobalt oxide encapsulated zeolite catalyst. J. Colloid Interface Sci. 2018, 517, 144–154. [Google Scholar] [CrossRef]
- Tang, Q.H.; Wang, Y.; Liang, J.; Wang, P.; Zhang, Q.H.; Wan, H.L. CO-Exchanged faujasite zeolites as efficient heterogeneous catalysts for epoxidation of styrene with molecular oxygen. Chem. Commun. 2004, 4, 440–441. [Google Scholar] [CrossRef]
- Wang, K.; Wang, F.; Zhai, Y.; Zhang, X.; Jiang, L.; Xu, Z.; Li, M.; Li, M.; Zhang, X.; Sun, M. Alkali metal ion assisted preparation of zeolite encapsulated small cobalt cluster CoOx for direct styrene oxidation to benzaldehyde. Microporous Mesoporous Mater. 2022, 345, 112259. [Google Scholar] [CrossRef]
- Khan, N.A.; Kennedy, E.M.; Dlugogorski, B.Z.; Adesina, A.A.; Stockenhuber, M. Partial oxidation of methane with nitrous oxide forms synthesis gas over cobalt exchanged ZSM-5. Catal. Commun. 2014, 53, 42–46. [Google Scholar] [CrossRef]
- Hou, Y.H.; Nagamatsu, S.; Asakura, K.; Fukuoka, A.; Kobayashi, H. Trace mono-atomically dispersed rhodium on zeolite-supported cobalt catalyst for the efficient methane oxidation. Commun. Chem. 2018, 1, 41. [Google Scholar] [CrossRef]
- Tabor, E.; Lemishka, M.; Sobalik, Z.; Mlekodaj, K.; Andrikopoulos, P.C.; Dedecek, J.; Sklenak, S. Low-temperature selective oxidation of methane over distant binuclear cationic centers in zeolites. Commun. Chem. 2019, 2, 71. [Google Scholar] [CrossRef]
- Beznis, N.V.; van Laak, A.N.C.; Weckhuysen, B.M.; Bitter, J.H. Oxidation of methane to methanol and formaldehyde over Co-ZSM-5 molecular sieves: Tuning the reactivity and selectivity by alkaline and acid treatments of the zeolite ZSM-5 agglomerates. Microporous Mesoporous Mater. 2011, 138, 176–183. [Google Scholar] [CrossRef]
- Beznis, N.V.; Weckhuysen, B.M.; Bitter, J.H. Partial Oxidation of Methane Over Co-ZSM-5: Tuning the Oxygenate Selectivity by Altering the Preparation Route. Catal. Lett. 2010, 136, 52–56. [Google Scholar] [CrossRef]
- Vanoppen, D.L.; Devos, D.E.; Genet, M.J.; Rouxhet, P.G.; Jacobs, P.A. Cobalt-Containing Molecular-Sieves as Catalysts for the Low Conversion Autoxidation of Pure Cyclohexane. Angew. Chem.-Int. Ed. Engl. 1995, 34, 560–563. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.P.; Yan, Y. High efficiency of isopropanol combustion over cobalt oxides modified ZSM-5 zeolite membrane catalysts on paper-like stainless steel fibers. J. Solid State Chem. 2017, 251, 55–60. [Google Scholar] [CrossRef]
- Blanch-Raga, N.; Palomares, A.E.; Martínez-Triguero, J.; Valencia, S. Cu and Co modified beta zeolite catalysts for the trichloroethylene oxidation. Appl. Catal. B-Environ. 2016, 187, 90–97. [Google Scholar] [CrossRef]
- Rokicinska, A.; Drozdek, M.; Dudek, B.; Gil, B.; Michorczyk, P.; Brouri, D.; Dzwigaj, S.; Kustrowski, P. Cobalt-containing BEA zeolite for catalytic combustion of toluene. Appl. Catal. B-Environ. 2017, 212, 59–67. [Google Scholar] [CrossRef]
- Aziz, A.; Sajjad, M.; Kim, M.; Kim, K.S. An efficient Co-ZSM-5 catalyst for the abatement of volatile organics in air: Effect of the synthesis protocol. Int. J. Environ. Sci. Technol. 2018, 15, 707–718. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, W.; Xu, Z.; Xu, Z.; Wang, X.; Wei, Q.; Zhou, Y. In situ synthesis of Co modified SAPO-5 molecular sieves and the application in quinoline hydrodenitrogenation of their NiWS supported catalysts. Chem. Eng. Sci. 2024, 284, 119428. [Google Scholar] [CrossRef]
- Franken, J.; Kirschhock, C.E.A.; Mathys, G.M.; Martens, J.A. Design of a Cobalt-Zeolite Catalyst for Semi-Linear Higher-Olefin Synthesis. Chemcatchem 2012, 4, 1245–1248. [Google Scholar] [CrossRef]
- Matsubara, H.; Tsuji, E.; Moriwaki, Y.; Okumura, K.; Yamamoto, K.; Nakamura, K.; Suganuma, S.; Katada, N. Selective Formation of Active Cobalt Species for Direct Methylation of Benzene with Methane on MFI Zeolite by Co-presence of Secondary Elements. Catal. Lett. 2019, 149, 2627–2635. [Google Scholar] [CrossRef]
- Cao, Y.; Zhuang, T.T.; Yang, J.; Liu, H.D.; Huang, W.; Zhu, J.H. Promoting zeolite NaY as efficient nitrosamines trap by cobalt oxide modification. J. Phys. Chem. C 2007, 111, 538–548. [Google Scholar] [CrossRef]
- Tran, H.L.; Kuo, M.S.; Yang, W.D.; Huang, Y.C. Hydrogen sulfide adsorption by thermally treated cobalt (II)-exchanged NaX zeolite. Adsorpt. Sci. Technol. 2016, 34, 275–286. [Google Scholar] [CrossRef]
- Xue, B.; Zong, X.M.; Wang, C.; Zhang, H.Y.; Luo, J. Corrosion Inhibition of a Sol-Gel Coating Modified with Cobalt-Enriched Zeolite on AA2024-T3 Aluminum Alloy. Int. J. Electrochem. Sc. 2019, 14, 10966–10982. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, W.; Xu, G. Advancements and Challenges of Cobalt–Zeolite Composite Catalysts in Heterogeneous Catalysis. Chemistry 2025, 7, 81. https://doi.org/10.3390/chemistry7030081
Liang W, Xu G. Advancements and Challenges of Cobalt–Zeolite Composite Catalysts in Heterogeneous Catalysis. Chemistry. 2025; 7(3):81. https://doi.org/10.3390/chemistry7030081
Chicago/Turabian StyleLiang, Wanying, and Guangyue Xu. 2025. "Advancements and Challenges of Cobalt–Zeolite Composite Catalysts in Heterogeneous Catalysis" Chemistry 7, no. 3: 81. https://doi.org/10.3390/chemistry7030081
APA StyleLiang, W., & Xu, G. (2025). Advancements and Challenges of Cobalt–Zeolite Composite Catalysts in Heterogeneous Catalysis. Chemistry, 7(3), 81. https://doi.org/10.3390/chemistry7030081





