Beyond L-Proline: Investigation into the Catalytic Properties of Other Natural Amino Acids in an Organocatalytic Warfarin Synthesis
Abstract
1. Introduction
2. Materials and Methods
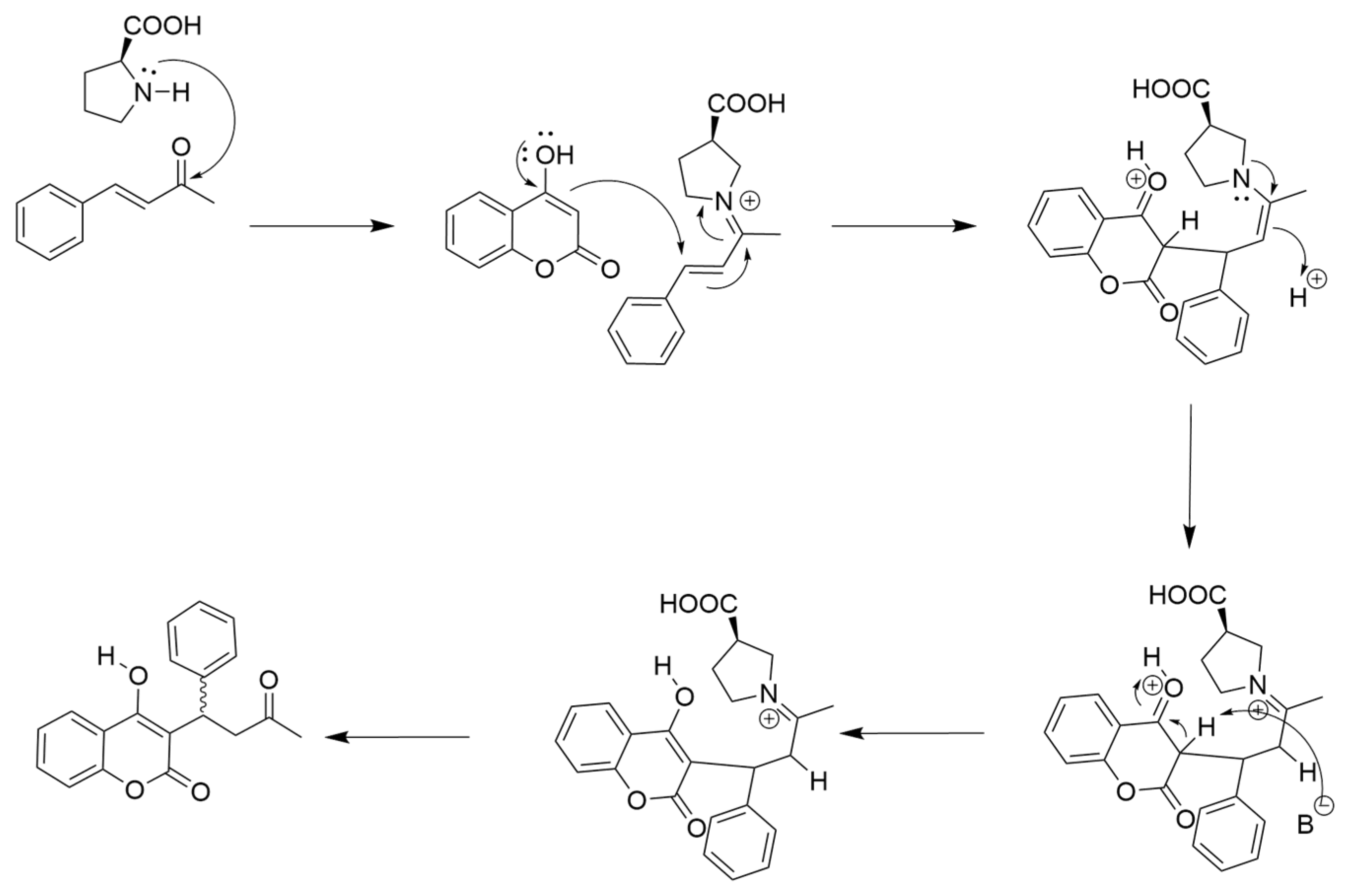
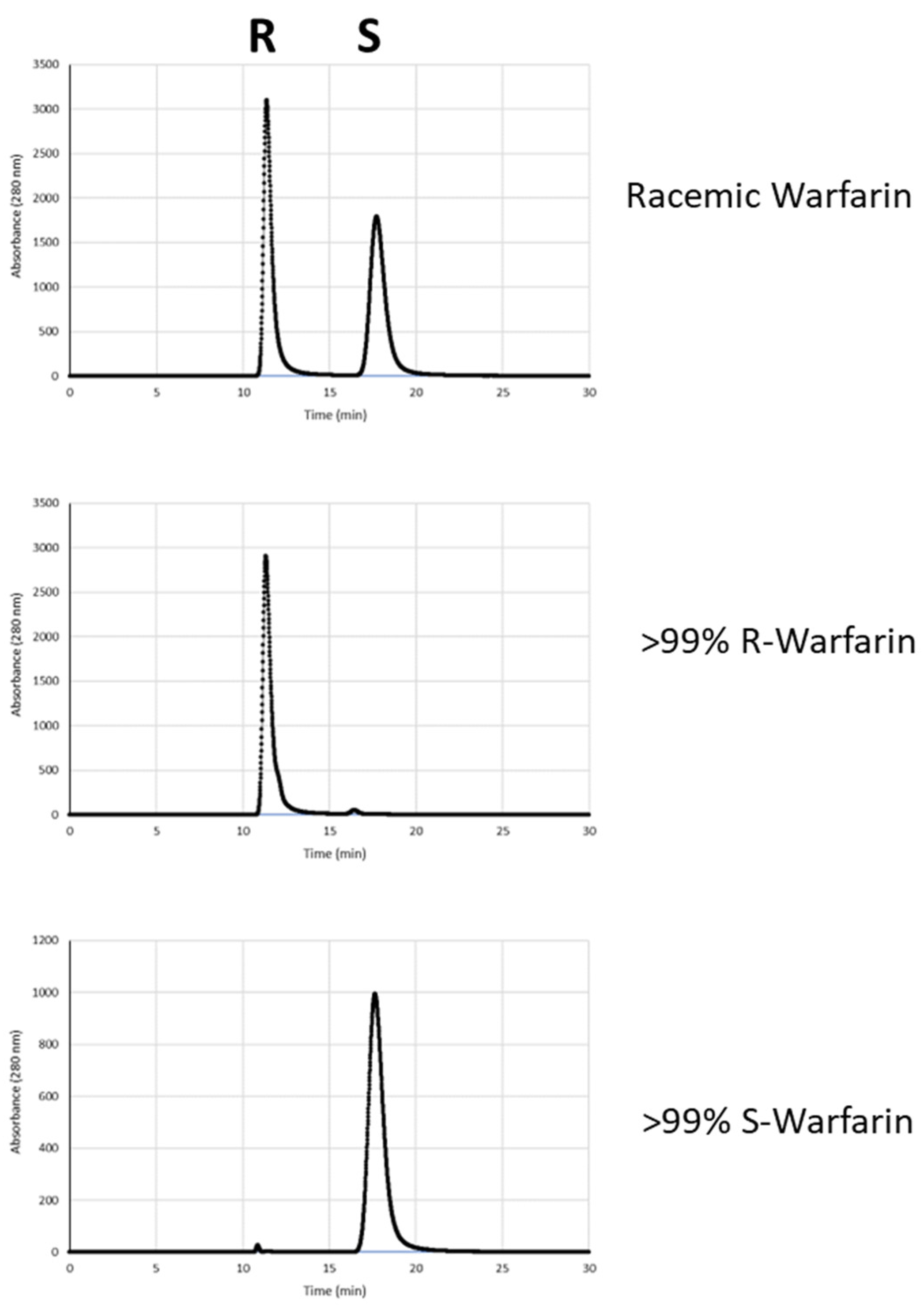
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, S.; Singh, R.; Preuss, C.V.; Patel, N. Warfarin; 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470313/ (accessed on 9 December 2024).
- Crader, M.F.; Johns, T.; Arnold, J.K. Warfarin Drug Interactions; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kaminsky, L.S.; Zhang, Z.-Y. Human P450 metabolism of warfarin. Pharmacol. Ther. 1997, 73, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wigle, T.J.; Jansen, L.E.; Teft, W.A.; Kim, R.B. Pharmacogenomics Guided-Personalization of Warfarin and Tamoxifen. J. Pers. Med. 2017, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Rulcova, A.; Prokopova, I.; Krausova, L.; Bitman, M.; Vrzal, R.; Dvorak, Z.; Blahos, J.; Pavek, P. Stereoselective interactions of warfarin enantiomers with the pregnane X nuclear receptor in gene regulation of major drug-metabolizing cytochrome P450 enzymes. J. Thromb. Haemost. 2010, 8, 2708–2717. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Frutos, R.P.; Tampone, T.; Senanayake, C.H.; Krishnamurthy, D. 9.3 Industrial Applications of Asymmetric Synthesis: Asymmetric Synthesis as an Enabler of Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2012; pp. 46–72. [Google Scholar] [CrossRef]
- Mondal, A.; Bhowmick, K.C. Asymmetric Organocatalyzed Warfarin Synthesis in Aqueous and Nonaqueous Media: A Discussion in the Era of COVID-19 Pandemic. Curr. Organocatalysis 2021, 8, 109–125. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, L.; Chen, X.; Liu, X.; Lin, L.; Feng, X. Organocatalytic Enantioselective Michael Addition of 4-Hydroxycoumarin to α,β-Unsaturated Ketones: A Simple Synthesis of Warfarin. Eur. J. Org. Chem. 2009, 2009, 5192–5197. [Google Scholar] [CrossRef]
- Halland, N.; Hansen, T.; Jørgensen, K.A. Organocatalytic Asymmetric Michael Reaction of Cyclic 1,3-Dicarbonyl Compounds and α,β-Unsaturated Ketones—A Highly Atom-Economic Catalytic One-Step Formation of Optically Active Warfarin Anticoagulant. Angew. Chem. Int. Ed. 2003, 42, 4955–4957. [Google Scholar] [CrossRef]
- Kim, H.; Yen, C.; Preston, P.; Chin, J. Substrate-Directed Stereoselectivity in Vicinal Diamine-Catalyzed Synthesis of Warfarin. Org. Lett. 2006, 8, 5239–5242. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-W.; Yue, L.; Chen, W.; Du, W.; Zhu, J.; Deng, J.-G.; Chen, Y.-C. Highly Enantioselective Michael Addition of Cyclic 1,3-Dicarbonyl Compounds to α,β-Unsaturated Ketones. Org. Lett. 2007, 9, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Panday, S.K. Advances in the chemistry of proline and its derivatives: An excellent amino acid with versatile applications in asymmetric synthesis. Tetrahedron Asymmetry 2011, 22, 1817–1847. [Google Scholar] [CrossRef]
- Thorat, R.B.; Mali, N.S.; Wavhal, S.S.; Bhagat, S.D.; Borade, M.R.; Chapolikar, A.; Gandhi, A.; Shinde, P. L-Proline: A Versatile Organo-Catalyst in Organic Chemistry. Comb. Chem. High Throughput Screen. 2023, 26, 1108–1140. [Google Scholar] [CrossRef] [PubMed]
- List, B.; Lerner, R.A.; Barbas, C.F., III. Proline-Catalyzed Direct Asymmetric Aldol Reactions. J. Am. Chem. Soc. 2000, 122, 2395–2396. [Google Scholar]
- Wong, T.C.; Sultana, C.M.; Vosburg, D.A. A Green, Enantioselective Synthesis of Warfarin for the Undergraduate Organic Laboratory. J. Chem. Educ. 2010, 87, 194–195. [Google Scholar] [CrossRef]
- Dong, S.; Feng, X.; Liu, X. Chiral guanidines and their derivatives in asymmetric synthesis. Chem. Soc. Rev. 2018, 47, 8525–8540. [Google Scholar] [CrossRef] [PubMed]
- Odagi, M.; Nagasawa, K. Exploring Guanidinium Organocatalysts for Hypoiodite-Mediated Reactions. Chem. Rec. 2023, 23, e202300030. [Google Scholar] [CrossRef] [PubMed]
- Vachan, B.S.; Karuppasamy, M.; Vinoth, P.; Vivek Kumar, S.; Perumal, S.; Sridharan, V.; Menéndez, J.C. Proline and its Derivatives as Organocatalysts for Multi-Component Reactions in Aqueous Media: Synergic Pathways to the Green Synthesis of Heterocycles. Adv. Synth. Catal. 2020, 362, 87–110. [Google Scholar] [CrossRef]


| Catalyst | Yield (%) |
|---|---|
| Uncatalyzed | 4 a |
| Tyrosine (L-Tyr) | 14 a |
| Glutamic Acid (L-Glu) | 14 a |
| Leucine (L-Leu) | 18 a |
| Isoleucine (L-Ile) | 20 a |
| Glycine (Gly) | 34 a, 38 b |
| Phenylalanine (L-Phe) | 35 a, 33 b |
| Lysine (L-Lys) | 44 a, 56 b |
| Methionine (L-Met) | 47 a, 47 b |
| Histidine (L-His) | 52 a, 48 b |
| Tryptophan (L-Trp) | 77 a, 53 b |
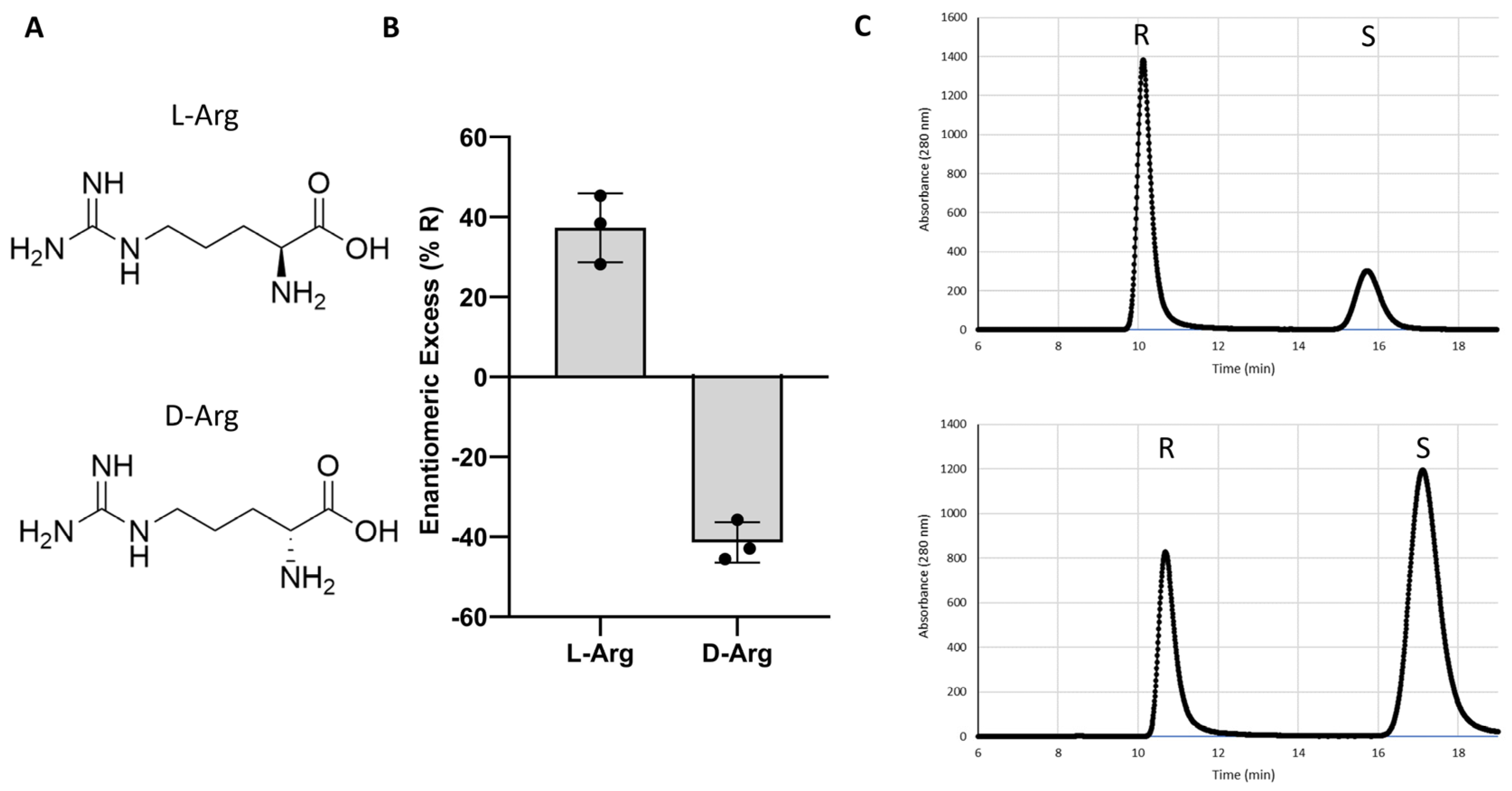
| Arginine (L-Arg) | 78 a, 52 b |
| Proline (L-Pro) | 86 a, 47 b |
| Catalyst | ee (%R) |
|---|---|
| Glycine (Gly) | 0 |
| Proline (L-Pro) | 0 |
| Tryptophan (L-Trp) | +23 |
| Lysine (L-Lys) | +30 |
| Phenylalanine (L-Phe) | +35 |
| Histidine (L-His) | +37 |
| Methionine (L-Met) | +44 |
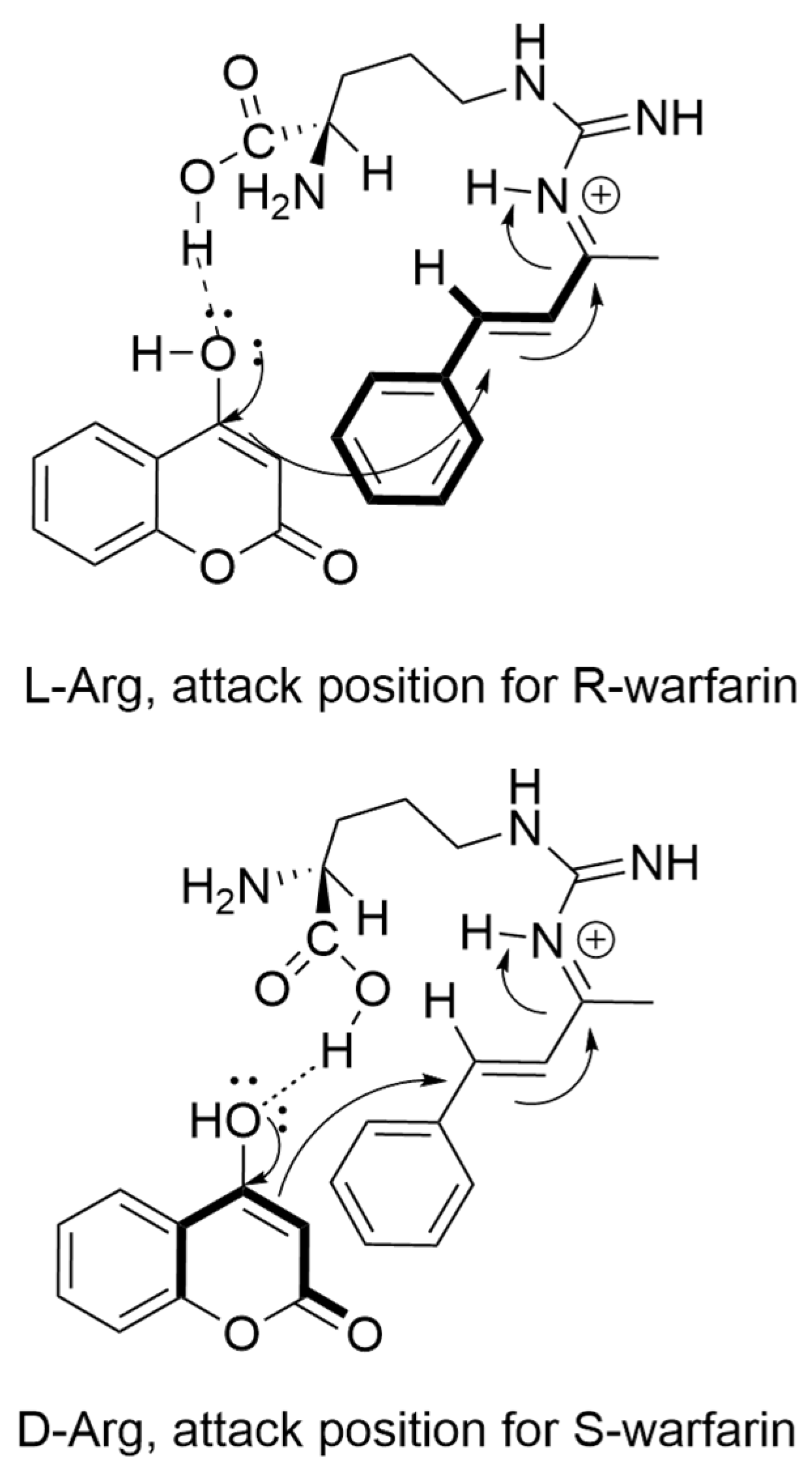
| Arginine (L-Arg) | +48 |
| R,R-DPEN | +48 |
| S,S-DPEN | −44 |
| Catalyst | Yield (%) | ee (%R) |
|---|---|---|
| Uncatalyzed | 4 | n.d. |
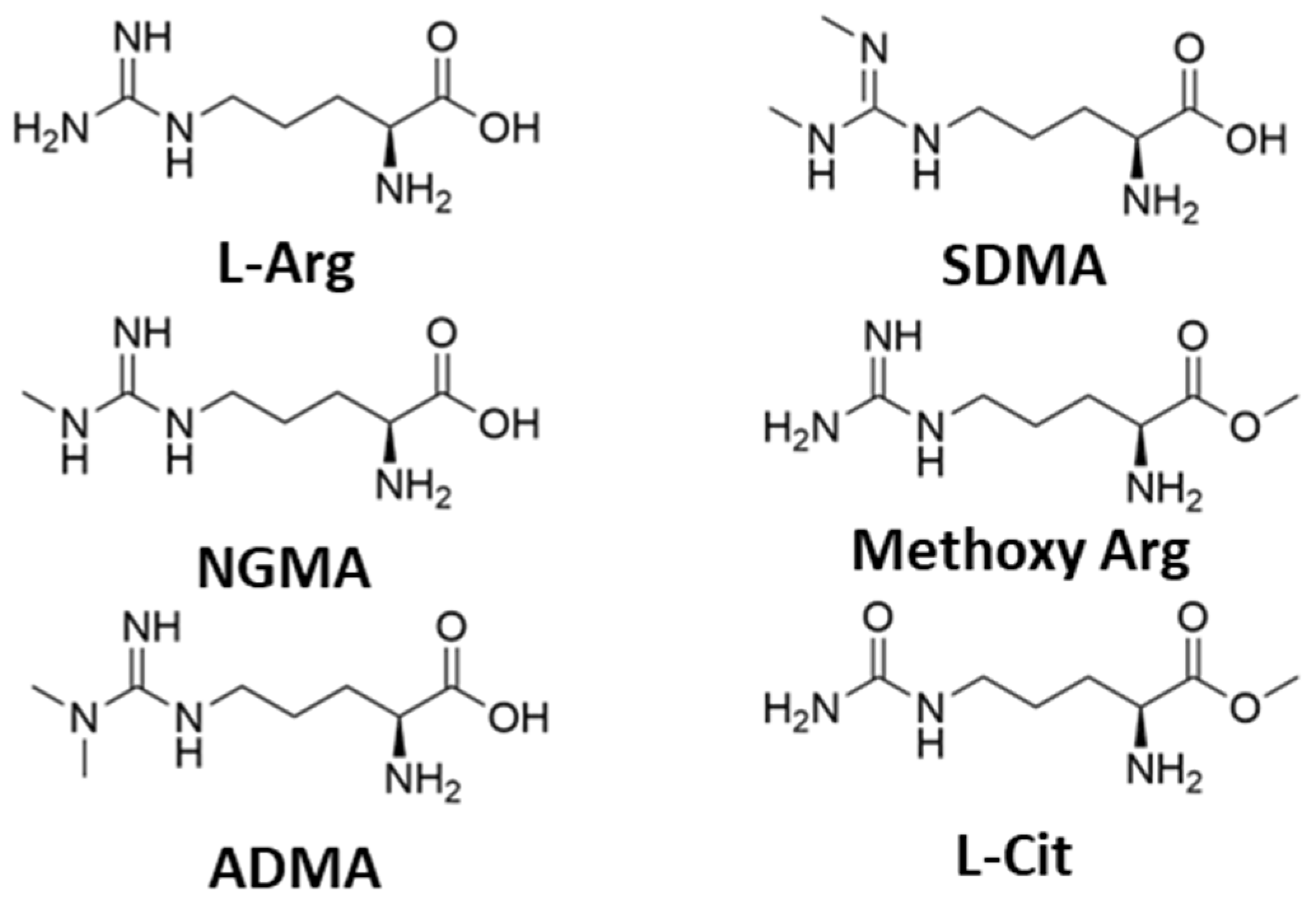
| L-Arginine (L-Arg) | 78 a, 52 b | +48 |
| NGMA | 68 a, 47 b | +52 |
| ADMA | 36 | +41 |
| SDMA | 4 | n.d. |
| Methoxy Arginine | 7 | −2 |
| L-citrulline (L-Cit) | 13 | +20 |
| Catalyst | Yield (%) | ee (%R) |
|---|---|---|
| Boc-Arg-OH | 9 | 22 |
| N-alpha-p-tosyl-L-Arg OMe | 2 | n.d. |
| H-Arg(Tos)-OH | 56 a, 27 b | 38 |
| H-Arg(Mtr)-OH | 41 a, 21 b | 32 |
| N-w-Nitro-L-Arg | 16 | 28 |
| Boc-N-omega-(nitro)-L-homoArg | 2 | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wurz, A.I.; Franco-Gonzalez, A.; Benson, N.R.; Jankowski, H.L.; Carr, S.N.; Chamakura, K.; Chirinos, L.; Coll, S.P.; Ivory, K.F.; Lamb, T.J.; et al. Beyond L-Proline: Investigation into the Catalytic Properties of Other Natural Amino Acids in an Organocatalytic Warfarin Synthesis. Chemistry 2025, 7, 59. https://doi.org/10.3390/chemistry7020059
Wurz AI, Franco-Gonzalez A, Benson NR, Jankowski HL, Carr SN, Chamakura K, Chirinos L, Coll SP, Ivory KF, Lamb TJ, et al. Beyond L-Proline: Investigation into the Catalytic Properties of Other Natural Amino Acids in an Organocatalytic Warfarin Synthesis. Chemistry. 2025; 7(2):59. https://doi.org/10.3390/chemistry7020059
Chicago/Turabian StyleWurz, Anna I., Arhemy Franco-Gonzalez, Naomi R. Benson, Hope L. Jankowski, Sierra N. Carr, Ketan Chamakura, Lizbeth Chirinos, Sydney P. Coll, Kayla F. Ivory, Trinity J. Lamb, and et al. 2025. "Beyond L-Proline: Investigation into the Catalytic Properties of Other Natural Amino Acids in an Organocatalytic Warfarin Synthesis" Chemistry 7, no. 2: 59. https://doi.org/10.3390/chemistry7020059
APA StyleWurz, A. I., Franco-Gonzalez, A., Benson, N. R., Jankowski, H. L., Carr, S. N., Chamakura, K., Chirinos, L., Coll, S. P., Ivory, K. F., Lamb, T. J., LeBauer, S., McPherson, G. L., Nguyen, T., Nolasco Guevara, J., Parsad, L. N., Pham, P., Piner, E. G., Richardson, K., Bendjellal, A., ... Hughes, R. M. (2025). Beyond L-Proline: Investigation into the Catalytic Properties of Other Natural Amino Acids in an Organocatalytic Warfarin Synthesis. Chemistry, 7(2), 59. https://doi.org/10.3390/chemistry7020059









