Abstract
Proline is considered the model organocatalytic amino acid. However, other naturally occurring amino acids remain a potent and perhaps overlooked source of organocatalytic potential. In this work, we investigated the capacity of various natural amino acids to promote enantioselectivity in a synthesis of warfarin. We have identified L- and d-arginine as enantioselective catalysts for this reaction and have developed a recrystallization method to isolate the enantiomers of warfarin with high enantiopurity. In addition, we used methylated derivatives of arginine to provide insight into the reaction mechanism.
1. Introduction
Warfarin is a commonly prescribed anti-coagulant compound that is administered in racemic form. However, the biological activities of (R)- and (S)-warfarin are quite different (S enantiomer is ~5 fold more active than the R enantiomer), which can further complicate warfarin administration regimen for sensitive patients [1,2]. Warfarin enantiomers are metabolized by different human cytochromes (CYP); (R)-warfarin by CYP1A2, CYP3A4, and carbonyl reductases, and (S)-warfarin by CYP2C9 [3]. This results in an array of different metabolic byproducts with variable efficacies and possible interactions with other drugs [3]. To circumvent these side effects, personalized medicine approaches to warfarin administration have been proposed [4]. Such strategies include the administration of enantiopure warfarins [5]. While the clinical relevance of these approaches is still a matter of debate [4], the enantioselective synthesis of warfarin from simple starting materials remains a useful proving ground for organocatalytic molecules.
Organocatalytic approaches to the synthesis of warfarin have been known for quite some time [6,7,8,9]. Some of these approaches have both high yields and enantioselectivities (Diphenylethylenediamine (DPEN) catalysts: (99% yield, 92% ee (R)-warfarin) [10], Cinchona alkaloids: (88% yield, 96% ee (S)-warfarin [11])), while others have good yields but are not enantioselective (L-proline: 85% yield, 0% ee [9]). Organocatalytic applications of L-proline are widespread throughout the chemical literature, with particular utility in the catalysis of carbon–carbon bond-forming reactions such as Aldol additions, Michael additions, Knoevenagel condensation, and Robinson Annulation [12]. The elevated pKa of the secondary amine and unique cyclic structure of L-proline are typically important contributors to these enamine-based reaction mechanisms [13]. By contrast, while L-proline and L-proline-like molecules are often an early avenue of exploration for the catalysis of new carbon–carbon bond-forming reactions [12], the organocatalytic properties of other natural amino acids are frequently found to be inferior to those of L-proline. Seminal work in the organocatalysis field demonstrates that this is often a correct assumption [14]. However, for many organocatalytic reactions, the superiority of L-proline versus other natural amino acids has not been explicitly demonstrated. We propose that these overlooked amino acids should be re-examined, as they are an untapped reservoir of organocatalytic potential.
In this work, we investigated the ability of amino acids other than L-proline to catalyze an organocatalytic synthesis of warfarin. While we confirm that L-proline is an effective catalyst for this reaction, we find that it is not uniquely capable of catalyzing this transformation, as all amino acids investigated in this study produced measurable yields of warfarin. Importantly, we discovered that L-arginine is also an effective catalyst of this transformation, with an enantioselectivity surpassing that of other natural amino acids. We also found that L- and d-isomers of arginine have opposing enantioselectivities in the production of (R)- and (S)-warfarin. Although these enantioselectivities do not approach those previously reported for DPEN catalysts [9,10], we demonstrate that a modified recrystallization procedure results in highly enriched samples of (R)- and (S)-warfarin from L- and d-Arg-catalyzed reactions, respectively.
2. Materials and Methods
General Procedure for Warfarin Synthesis
In 2 mL screw top reaction vials, we combined the following: 0.081 g (81 mg) of 4-hydroxycoumarin (Sigma Aldrich, St. Louis, MO, USA), 0.088 g (88 mg) of benzylideneacetone (Sigma Aldrich, St. Louis, MO, USA), 0.020 g (20 mg) of L-proline (Fisher Scientific, Waltham, MA, USA; or other amino acid catalyst, see Supplementary Materials for full list), 900 μL DMSO (ThermoFisher Scientific, Waltham, MA, USA), and 100 μL distilled, deionized H2O. After the addition of all reactants, we secured screw top lids on the vials and placed reactions in an end-over-end rotator for seven days at room temperature.
Warfarin Reactions: Amino acids (0.17 mmol) were added to a reaction of 81 mg (0.5 mmol) of 4-hydroxycoumarin and 88 mg (0.6 mmol) of benzylideneacetone (BZA) with 900 µL DMSO AND 100 µL distilled water. All reactions were incubated in an end-over-end rotator at room temperature for 7 days.
Thin-Layer Chromatography (TLC): TLC analysis was performed on each reaction using silica gel plates with 2:1 Hexanes:Ethyl Acetate as the mobile phase. Plates were visualized with UV illumination followed by anisaldehyde staining with heating until purple warfarin spots were visible.
High-Performance Liquid Chromatography (HPLC) for reaction yields: Diluted samples (1:200 dilution of crude reaction in ethanol) were analyzed using a Hitachi HPLC (Hitachi High-Tech, Schaumburg, IL, USA) equipped with a Zorbax SB-C18 (Agilent, Santa Clara, CA, USA), 3.5 μm, 4.6 × 150 mm column; 40% 0.1% formic acid and 60% methanol mobile phase; 1 mL/min flow rate; 280 nm wavelength UV detector (Hitachi High-Tech, Schaumburg, IL, USA); 20 min run time; and PC/Chrom software (H&A Scientific, Greenville, NC, USA). Yields were determined using a standard curve.
Flash Chromatography: Warfarin was isolated using silica gel flash column chromatography with Hexanes:Ethyl acetate mobile phase (4:1, 2:1, 0:1).
Chiral Chromatography for % enantiomeric excess: The purified sample was analyzed using a Shimadzu HPLC (Shimadzu Corp, Kyoto, Japan) equipped with a Chiralcel OD-H (Daicel Corp, Tokyo, Japan) chiral column: 50% Heptane, 50% IPA, 0.1% acetic acid mobile phase. Samples were monitored by UV/VIS detection at 220 and 280 nm.
3. Results and Discussion
We first investigated the ability of various L-amino acids to catalyze the synthesis of warfarin from hydroxycoumarin and benzylideneacetone (Scheme 1) in a DMSO/water (90% DMSO/10% water) mixture. L-Pro has previously been characterized as an effective catalyst for this reaction [9], and was used as a positive control.
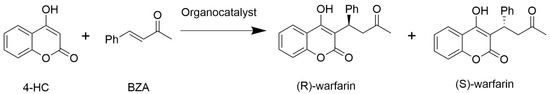
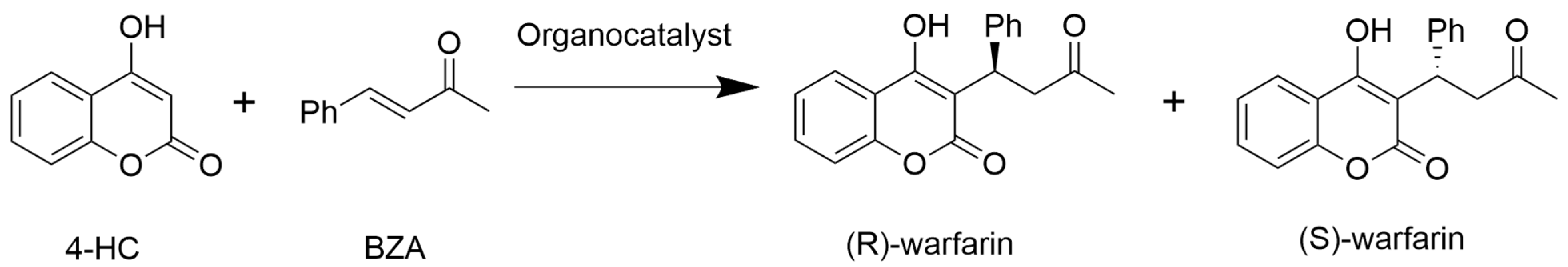
Scheme 1.
Organocatalytic warfarin synthesis from 4-hydroxycoumarin (4-HC) and benzylideneacetone (BZA). Organocatalysts (0.17 mmol) were added to a reaction of 81 mg (0.5 mmol) of 4-hydroxycoumarin and 88 mg (0.6 mmol) of benzylideneacetone (BZA) with 900 µL DMSO and 100 µL distilled water. All reactions were incubated in an end-over-end rotator at room temperature for 7 days.
Among the twelve amino acids tested (Table 1), three (L-Trp, L-Arg, L-Pro) produced good yields of warfarin, ranging from 75 to 85%, with HPLC analysis. By contrast, Gly, L-Ala, L-Lys, L-Met, and L-His produced moderate yields of warfarin (35–52%), while low yields were achieved with L-Tyr, L-Glu, L-Leu, and L-Ile (10–20%). Generally, these yields fell within expectations for an iminium/enamine-catalyzed route in which a primary or secondary amine-bearing sidechain with an elevated pKa promotes catalysis (L-Lys, L-His, L-Trp, L-Arg, L-Pro) through the attack of the benzylideneacetone carbonyl (Scheme 2). However, it is somewhat surprising that Gly, which lacks a sidechain, is a modestly competent catalyst for this reaction. Furthermore, L-Met, which contains a sulfur-bearing sidechain, also produced moderate yields of warfarin, on par with that of L-Lys and L-His.

Table 1.
Yields of L-amino acid-catalyzed warfarin reactions.
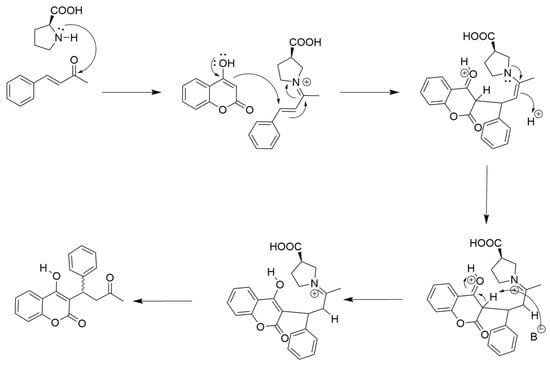
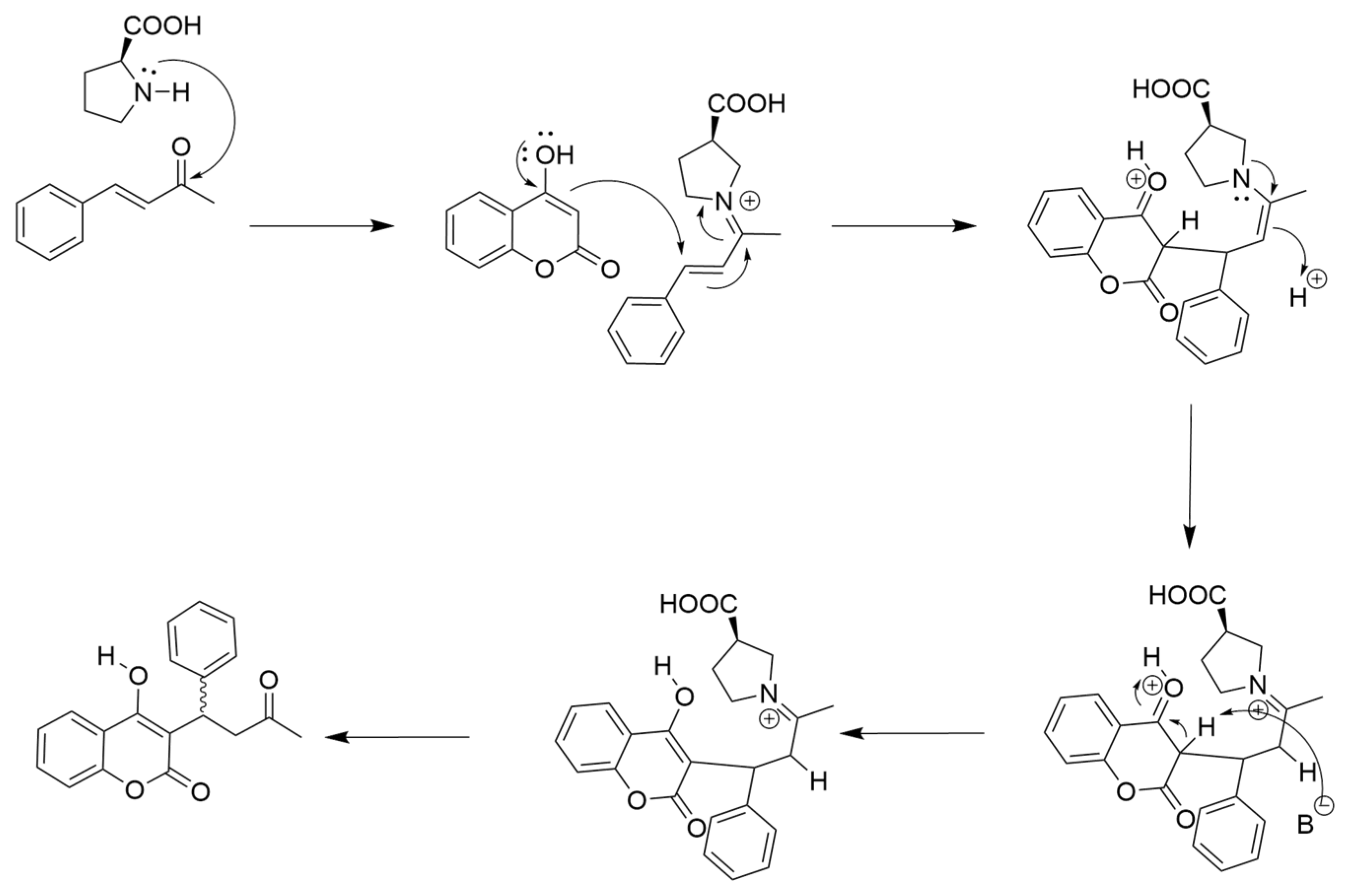
Scheme 2.
L-proline catalyzed synthesis of warfarin from 4-hydroxycoumarin (4-HC) and benzylideneacetone (BZA). An iminium ion formed by L-proline and BZA promotes conjugate addition of 4-HC to BZA.
We subsequently characterized the enantioselectivities of a subset of these amino acids (Table 2). These amino acid catalysts were selected based on their high warfarin yields or, in the case of Gly, the absence of a sidechain. We also compared these enantioselectivities to R,R- and S,S-DPEN, catalysts previously shown to promote the enantioselective synthesis of warfarin [10,15]. L-amino acids favored the production of (R)-warfarin, with the exceptions of L-Pro and achiral Gly, which demonstrated no selectivity. Surprisingly, L-Arg had the highest enantioselectivity among the amino acids investigated, on par with R,R-DPEN in the DMSO/H2O solvent system. As anticipated, R,R and S,S-DPEN produced opposing enantioselectivities. We note that while higher enantioselectivities are observed with DPEN catalysts under THF/AcOH solvent conditions, the solubility limitations of our natural amino acid catalysts prevented a direct comparison under such conditions.

Table 2.
Enantiomeric excess values for selected catalysts a.
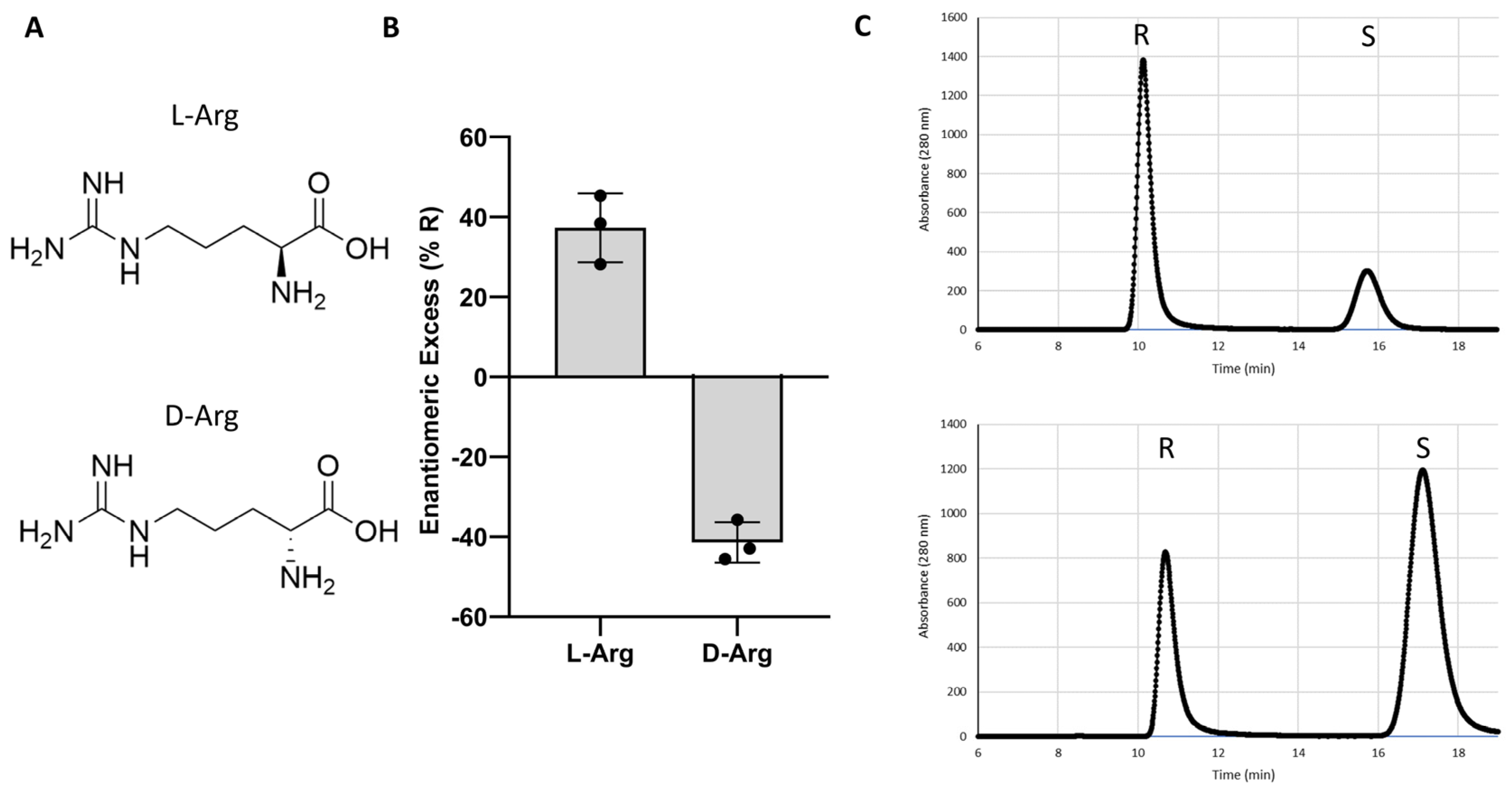
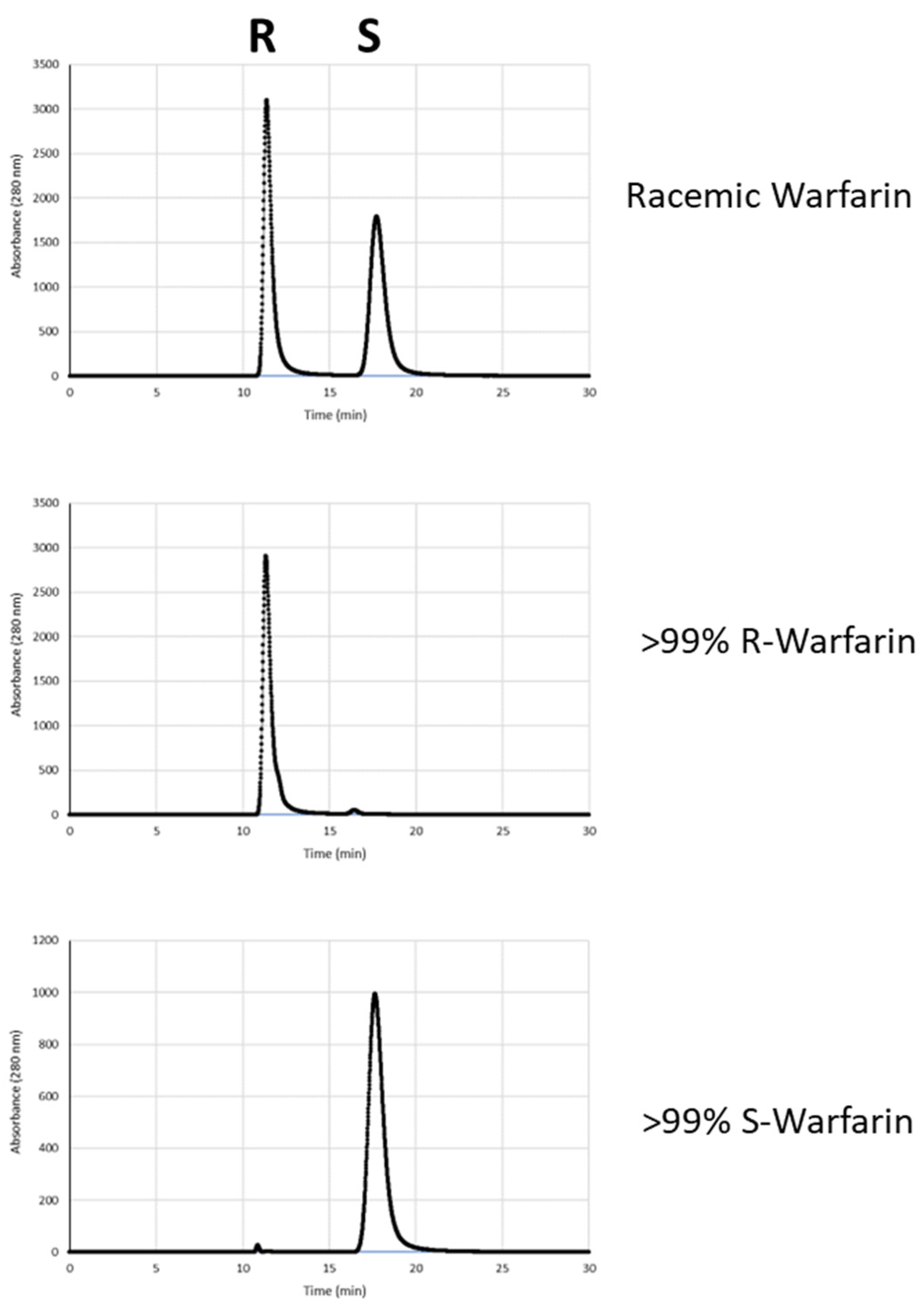
Having identified L-Arg as a potent and moderately enantioselective catalyst for warfarin synthesis, we next investigated whether a d-Arg catalyst might impose the opposing stereoselectivity (Figure 1). For this experiment, independent trials of either d- or L-Arg-catalyzed warfarin reactions were conducted. The reactions from the L-Arg trials favored the R-enantiomer (37 (+/−9)% ee), while the d-Arg trials favored synthesis of the S-enantiomer of warfarin (−41 (+/−5)%).
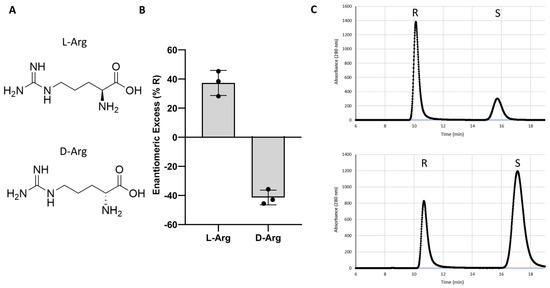
Figure 1.
Enantioselectivity of L-arginine versus d-arginine. (A) Structures of L- and d-arginine. (B) Results of trials of L- and d-arginine catalysts. (C) Representative chiral HPLC traces from L-arg (top) and d-arginine (bottom) warfarin reactions.
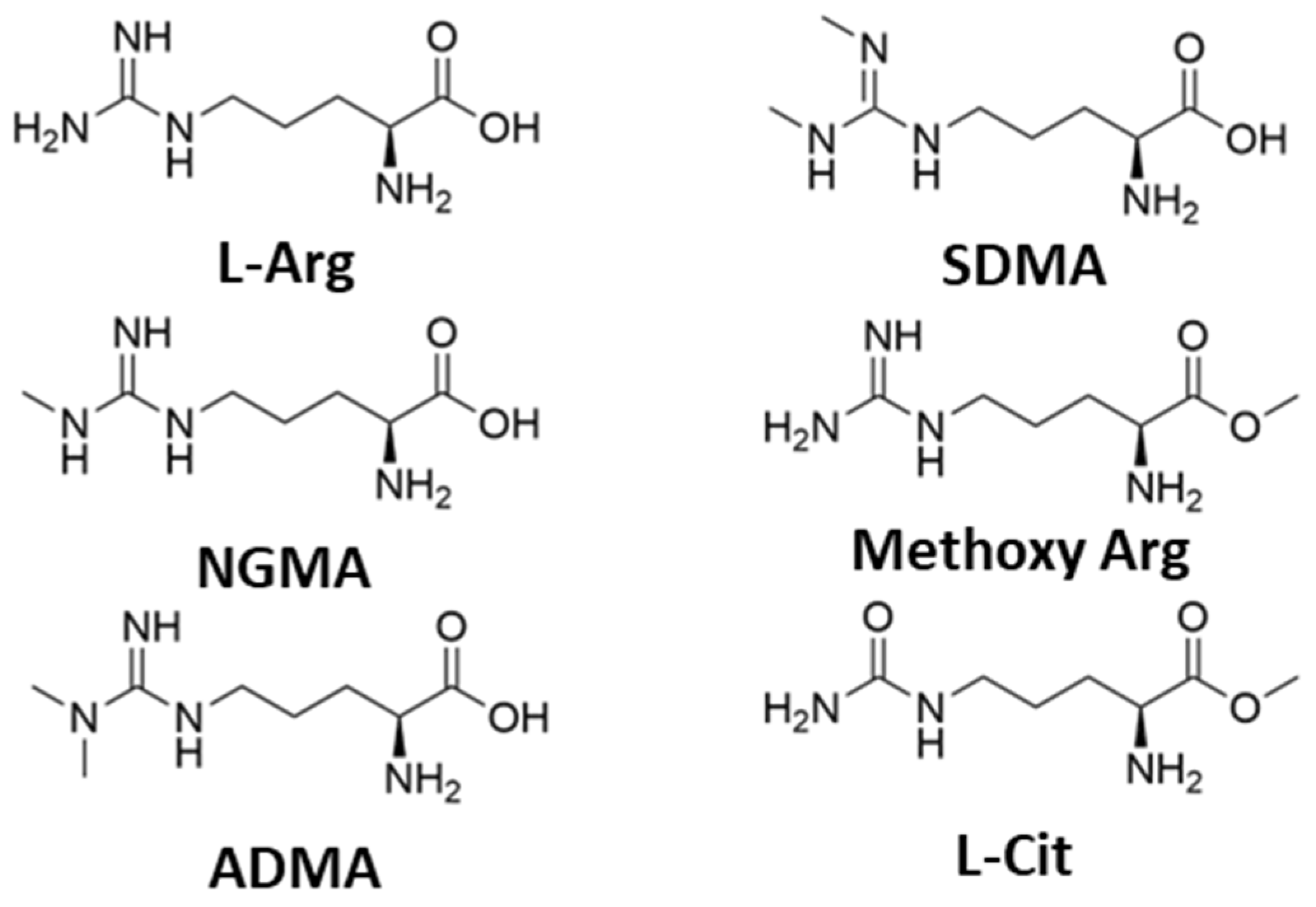
To provide additional insight into the mechanism of the arginine catalyst, we subsequently investigated the ability of methylated derivatives of L-Arg to catalyze the warfarin transformation (Table 3 and Figure 2). In comparison to L-Arg, a single-methylated arginine (NG-Monomethyl-L-arginine; NGMA) produced a good yield of warfarin and demonstrated a slightly elevated production of the R-enantiomer. By contrast, a double-methylated variant (NG, NG-asymmetric Dimethylarginine; ADMA) produced a diminished yield of warfarin yet retained a similar induction of stereoselectivity, and the symmetric methylated variant (NG-NG′-Dimethyl-L-arginine; SDMA) was catalytically incompetent. This result demonstrates an important property of L-arginine as an organocatalyst in this synthesis; while it tolerates NG-sidechain functionalization, dual methylation either reduces or eliminates catalytic activity. While these derivatives did not promote enantioselective catalysis over unmodified L-Arg, we do note that other highly derivatized chiral guanidinium-containing catalysts have been used extensively in asymmetric organocatalytic synthesis [16,17].

Table 3.
Yields and ee values for arginine derivatives.
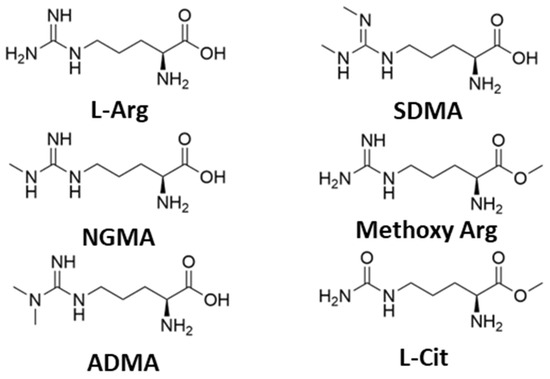
Figure 2.
Structures of arginine and methylated derivatives (L-Arg = L-arginine; NGMA = NG-Monomethyl-L-arginine; ADMA = NG, NG-Dimethylarginine; SDMA = NG, NG′-Dimethylarginine; Methoxy Arg = L-Arg, methoxy ester; L-Cit = L-citrulline).
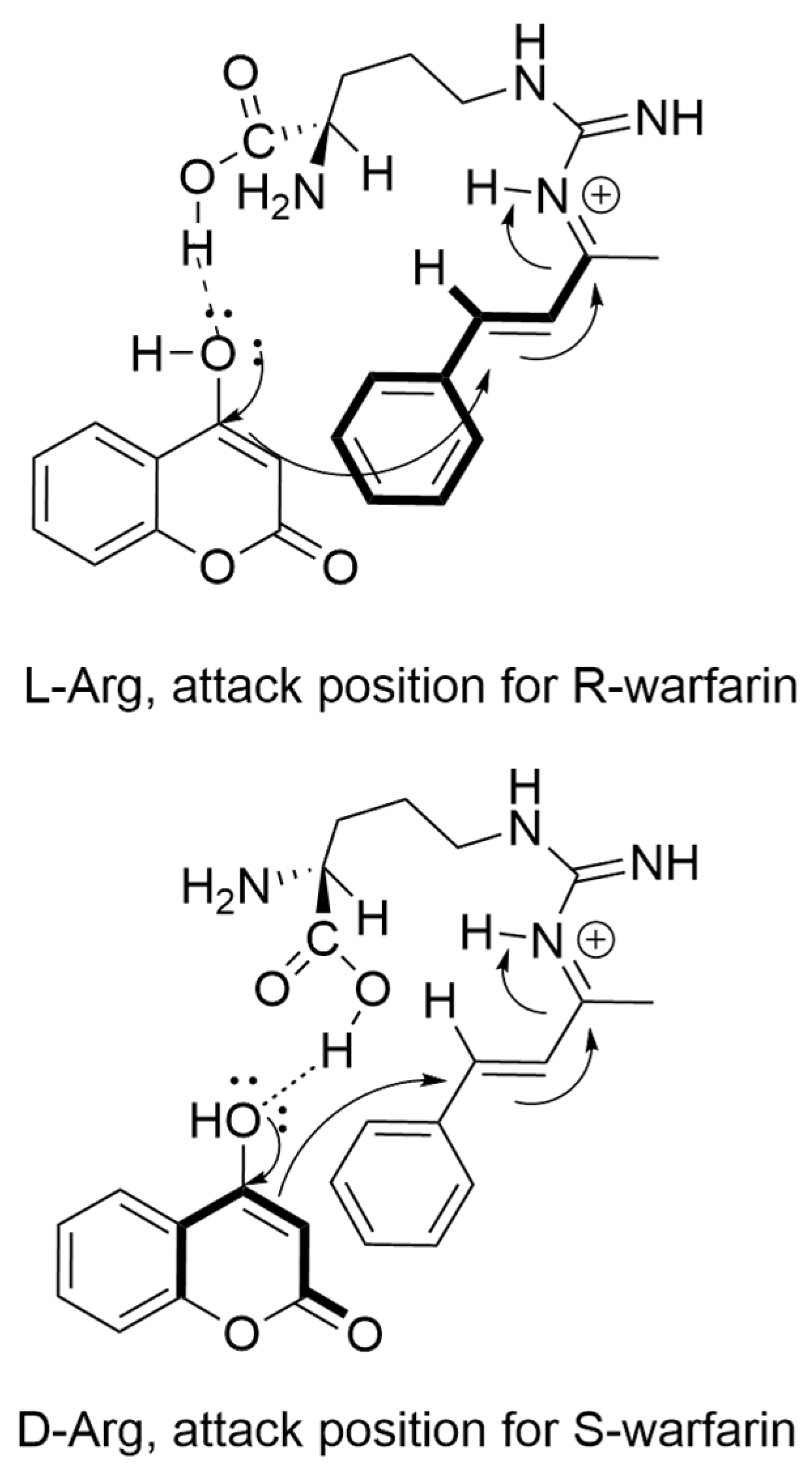
In addition to these methylated derivatives, other L-arginine derivatives and analogs were also investigated in warfarin synthesis. Interestingly, a methoxy-ester arginine (Methoxy Arginine, Table 3) derivative had near-background activity. This result demonstrates that the carboxy portion of the amino acid plays a critical role in catalysis, and strongly implicates Bronsted acid-type catalysis as a component of the reaction mechanism [18]. Bronsted acid catalysis would explain the general catalytic effect observed with most of the amino acids investigated in this study (Table 1). Finally, L-citrulline, which lacks the guanidinium group present in arginine, had significantly diminished catalytic activity, demonstrating the criticality of the guanidine sidechain for organocatalysis. Taken together, we propose that the interaction of L-Arg and D-Arg with BZA occurs through iminium ion formation with an NG guanidinium nitrogen, with selectivity for R- and S-warfarin promoted by hydrogen bonding of the Arg carboxylic acid with 4-HC (Scheme 3).
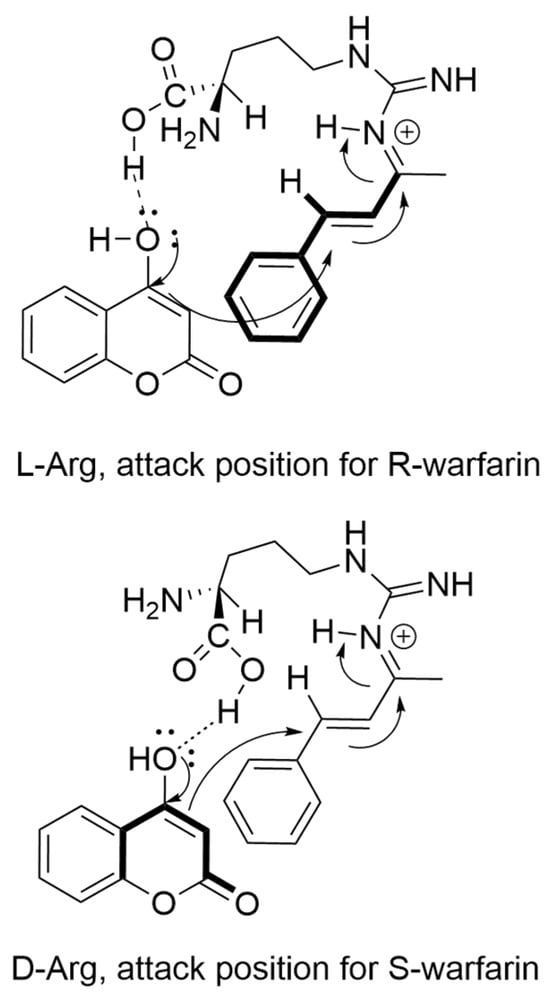
Scheme 3.
Model of enantioselective mechanistic step catalyzed by L-Arg (top) and D-Arg (bottom). Bold lines indicate forwardmost structures in mechanism. Binding of L-Arg carboxylic acid promotes the addition of 4-HC from underneath the BZA–iminium complex. Binding of D-Arg carboxylic acid promotes the addition of 4-HC from above the BZA–iminium complex.
We subsequently screened commercially available derivatives of L-arginine typically used in solid phase peptide synthesis for their ability to catalyze warfarin synthesis (Table 4). While some of these (the closely related derivatives H-Arg(Tos)-OH and H-Arg(Mtr)-OH) were catalytically competent, none of them produced an enhancement in enantioselectivity over L-arginine.

Table 4.
Yields and ee values for additional arginine derivatives from SPPS.
Finally, to further enhance the enantiopurity of the mixtures of (R)- and (S)-warfarin obtained from d- and L-Arg-catalyzed reactions, we sought to enrich these enantiomers by recrystallization. However, we found that simple acetone–water recrystallization was an unreliable method for enantiomeric enrichment. Instead, following flash column isolation of warfarin (hexanes/ethyl acetate) and the removal of solvent via rotary evaporation, the resulting yellowish oil was placed under hexanes and allowed to sit for at least 48 h under reduced pressure. During this interval, the hexane layer evaporated and warfarin began to crystallize. Under these conditions, warfarin crystallizes in a roughly 1:1 ratio of R:S warfarin, leaving behind a portion of the product as an enantiomerically enriched yellow liquid. This enriched yellow liquid was then separated from the crystals by pipette (crystals can also be removed by centrifugation and pipetting away the yellow liquid) and recrystallized using 80% acetone/20% water, yielding highly enriched warfarin (Figure 3 and Figure 4). For example, from scaled up (7X) L- or d-Arg-catalyzed reactions, we obtained 132 mg of (R)-Warfarin (12% overall yield) and 173 mg of (S)-Warfarin (16% overall yield), both in >99% ee. These overall yields are in line with those obtained from the resolution of warfarin enantiomers with quinidine–diastereomer approaches, with fewer recrystallization steps [6].
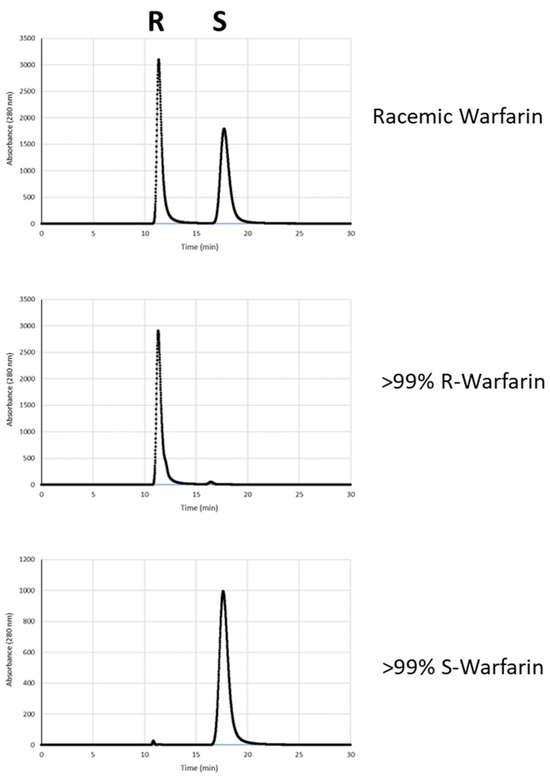
Figure 3.
Comparison of chiral HPLC traces of recrystallized products from L- and d-Arg-catalyzed reactions (middle and bottom) and commercially available racemic warfarin (top).

Figure 4.
Example recrystallization protocol for the isolation of enantiopure warfarin from enantiomerically enriched organocatalytic preparations.
4. Conclusions
This work demonstrates the range of yields and enantioselectivities accessible with commercially available amino acids in a mixed solvent-based synthesis of warfarin. Surprisingly, L- and d-arginine have opposing enantioselectivities in this synthesis. While these enantioselectivities may be considered modest by current standards, recrystallization approaches can be used to obtain both (R)- and (S)-warfarin enantiomers in excess of 99% purity. Furthermore, an analysis of sidechain methylated arginine derivatives suggests that synthetic arginine-based organocatalysts with enhanced enantioselectivities are within reach.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/chemistry7020059/s1, High-resolution mass spectrometry, 1H NMR, and FTIR of R- and S-warfarins, commercial sources of catalysts.
Author Contributions
Methodology, A.I.W. and R.M.H.; investigation, A.I.W., A.F.-G., N.R.B., S.N.C., K.C., L.C., S.P.C., K.F.I., H.L.J., T.J.L., S.L., G.L.M., T.N., J.N.G., L.N.P., P.P., E.G.P., K.R., A.B., C.M., and R.M.H.; resources, R.M.H.; writing—original draft preparation, A.I.W., A.F.-G., N.R.B., S.N.C., K.C., L.C., S.P.C., K.F.I., H.L.J., T.J.L., S.L., G.L.M., T.N., J.N.G., L.N.P., P.P., E.G.P., K.R., A.B., C.M., and R.M.H.; resources, R.M.H.; writing—review and editing, A.I.W. and R.M.H.; supervision, R.M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the US National Science Foundation (Award 2013358).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors wish to thank Joi Walker and the ECU StemCore for their support of this research and CUREs (course-based undergraduate research experiences) at ECU. We also thank the University of North Carolina’s Department of Chemistry Mass Spectrometry Core Laboratory, especially Lazaro Toledo, for their assistance with mass spectrometry analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Patel, S.; Singh, R.; Preuss, C.V.; Patel, N. Warfarin; 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470313/ (accessed on 9 December 2024).
- Crader, M.F.; Johns, T.; Arnold, J.K. Warfarin Drug Interactions; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kaminsky, L.S.; Zhang, Z.-Y. Human P450 metabolism of warfarin. Pharmacol. Ther. 1997, 73, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wigle, T.J.; Jansen, L.E.; Teft, W.A.; Kim, R.B. Pharmacogenomics Guided-Personalization of Warfarin and Tamoxifen. J. Pers. Med. 2017, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Rulcova, A.; Prokopova, I.; Krausova, L.; Bitman, M.; Vrzal, R.; Dvorak, Z.; Blahos, J.; Pavek, P. Stereoselective interactions of warfarin enantiomers with the pregnane X nuclear receptor in gene regulation of major drug-metabolizing cytochrome P450 enzymes. J. Thromb. Haemost. 2010, 8, 2708–2717. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Frutos, R.P.; Tampone, T.; Senanayake, C.H.; Krishnamurthy, D. 9.3 Industrial Applications of Asymmetric Synthesis: Asymmetric Synthesis as an Enabler of Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2012; pp. 46–72. [Google Scholar] [CrossRef]
- Mondal, A.; Bhowmick, K.C. Asymmetric Organocatalyzed Warfarin Synthesis in Aqueous and Nonaqueous Media: A Discussion in the Era of COVID-19 Pandemic. Curr. Organocatalysis 2021, 8, 109–125. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, L.; Chen, X.; Liu, X.; Lin, L.; Feng, X. Organocatalytic Enantioselective Michael Addition of 4-Hydroxycoumarin to α,β-Unsaturated Ketones: A Simple Synthesis of Warfarin. Eur. J. Org. Chem. 2009, 2009, 5192–5197. [Google Scholar] [CrossRef]
- Halland, N.; Hansen, T.; Jørgensen, K.A. Organocatalytic Asymmetric Michael Reaction of Cyclic 1,3-Dicarbonyl Compounds and α,β-Unsaturated Ketones—A Highly Atom-Economic Catalytic One-Step Formation of Optically Active Warfarin Anticoagulant. Angew. Chem. Int. Ed. 2003, 42, 4955–4957. [Google Scholar] [CrossRef]
- Kim, H.; Yen, C.; Preston, P.; Chin, J. Substrate-Directed Stereoselectivity in Vicinal Diamine-Catalyzed Synthesis of Warfarin. Org. Lett. 2006, 8, 5239–5242. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-W.; Yue, L.; Chen, W.; Du, W.; Zhu, J.; Deng, J.-G.; Chen, Y.-C. Highly Enantioselective Michael Addition of Cyclic 1,3-Dicarbonyl Compounds to α,β-Unsaturated Ketones. Org. Lett. 2007, 9, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Panday, S.K. Advances in the chemistry of proline and its derivatives: An excellent amino acid with versatile applications in asymmetric synthesis. Tetrahedron Asymmetry 2011, 22, 1817–1847. [Google Scholar] [CrossRef]
- Thorat, R.B.; Mali, N.S.; Wavhal, S.S.; Bhagat, S.D.; Borade, M.R.; Chapolikar, A.; Gandhi, A.; Shinde, P. L-Proline: A Versatile Organo-Catalyst in Organic Chemistry. Comb. Chem. High Throughput Screen. 2023, 26, 1108–1140. [Google Scholar] [CrossRef] [PubMed]
- List, B.; Lerner, R.A.; Barbas, C.F., III. Proline-Catalyzed Direct Asymmetric Aldol Reactions. J. Am. Chem. Soc. 2000, 122, 2395–2396. [Google Scholar]
- Wong, T.C.; Sultana, C.M.; Vosburg, D.A. A Green, Enantioselective Synthesis of Warfarin for the Undergraduate Organic Laboratory. J. Chem. Educ. 2010, 87, 194–195. [Google Scholar] [CrossRef]
- Dong, S.; Feng, X.; Liu, X. Chiral guanidines and their derivatives in asymmetric synthesis. Chem. Soc. Rev. 2018, 47, 8525–8540. [Google Scholar] [CrossRef] [PubMed]
- Odagi, M.; Nagasawa, K. Exploring Guanidinium Organocatalysts for Hypoiodite-Mediated Reactions. Chem. Rec. 2023, 23, e202300030. [Google Scholar] [CrossRef] [PubMed]
- Vachan, B.S.; Karuppasamy, M.; Vinoth, P.; Vivek Kumar, S.; Perumal, S.; Sridharan, V.; Menéndez, J.C. Proline and its Derivatives as Organocatalysts for Multi-Component Reactions in Aqueous Media: Synergic Pathways to the Green Synthesis of Heterocycles. Adv. Synth. Catal. 2020, 362, 87–110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).