Packed Bed Microreactors for Sustainable Chemistry and Process Development
Abstract
1. Introduction
2. Application of Packed Bed Microreactors
2.1. Synthesis of Value-Added Chemicals
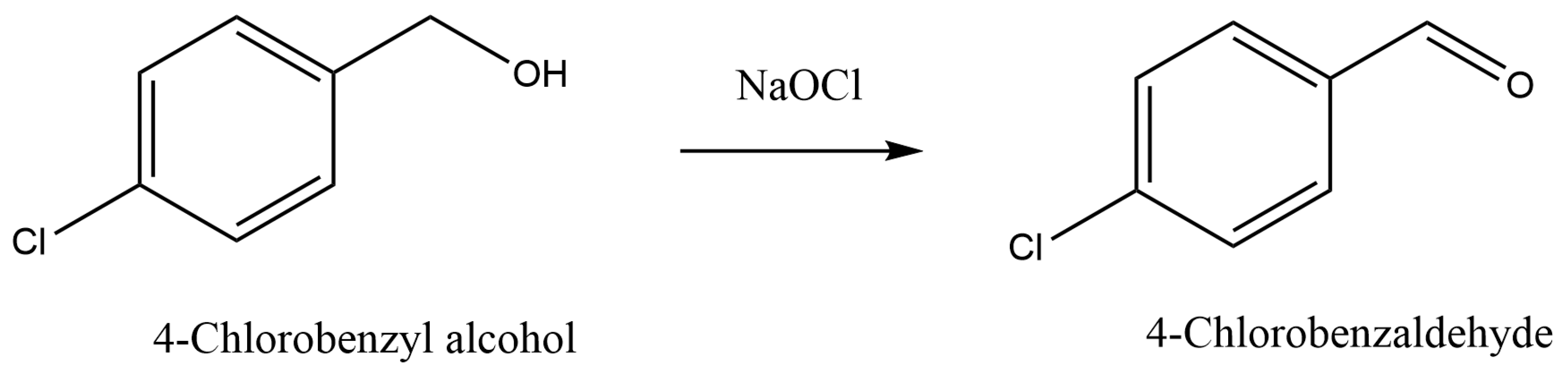
2.1.1. Selective Alcohol Oxidation to Aldehydes and Ketones
2.1.2. Safe Synthesis of Hydrogen Peroxide and Phosgene
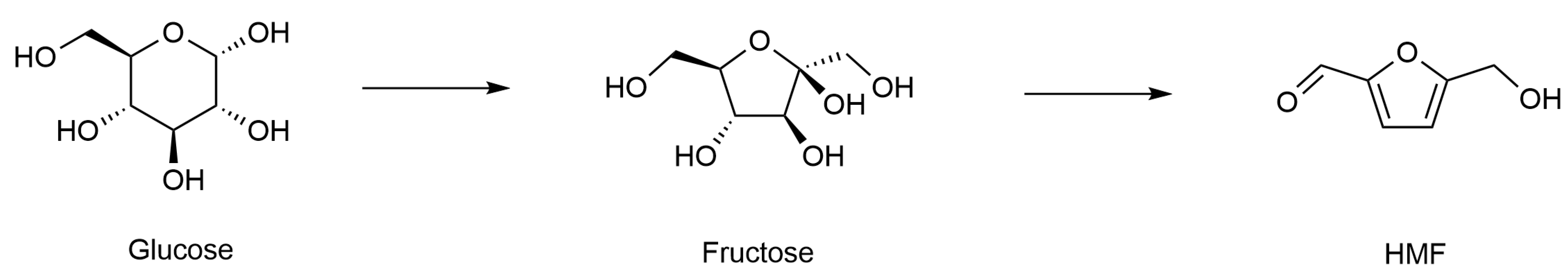
2.1.3. Bio-Based Platform Chemical Production
2.1.4. Enzymatic Chemical Synthesis
2.1.5. Organic Synthesis via Oxidation of Aromatics and Alkenes
2.2. Liquid Fuel Production
2.2.1. Biodiesel Production
2.2.2. Fischer–Tropsch Synthesis
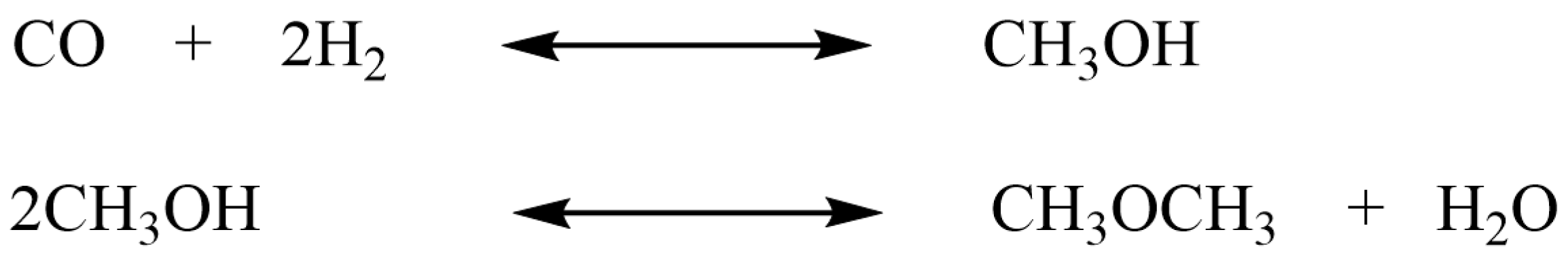
2.2.3. Methanol Synthesis
2.2.4. Dimethyl Ether Synthesis
2.3. Catalyst Screening and Kinetic Study
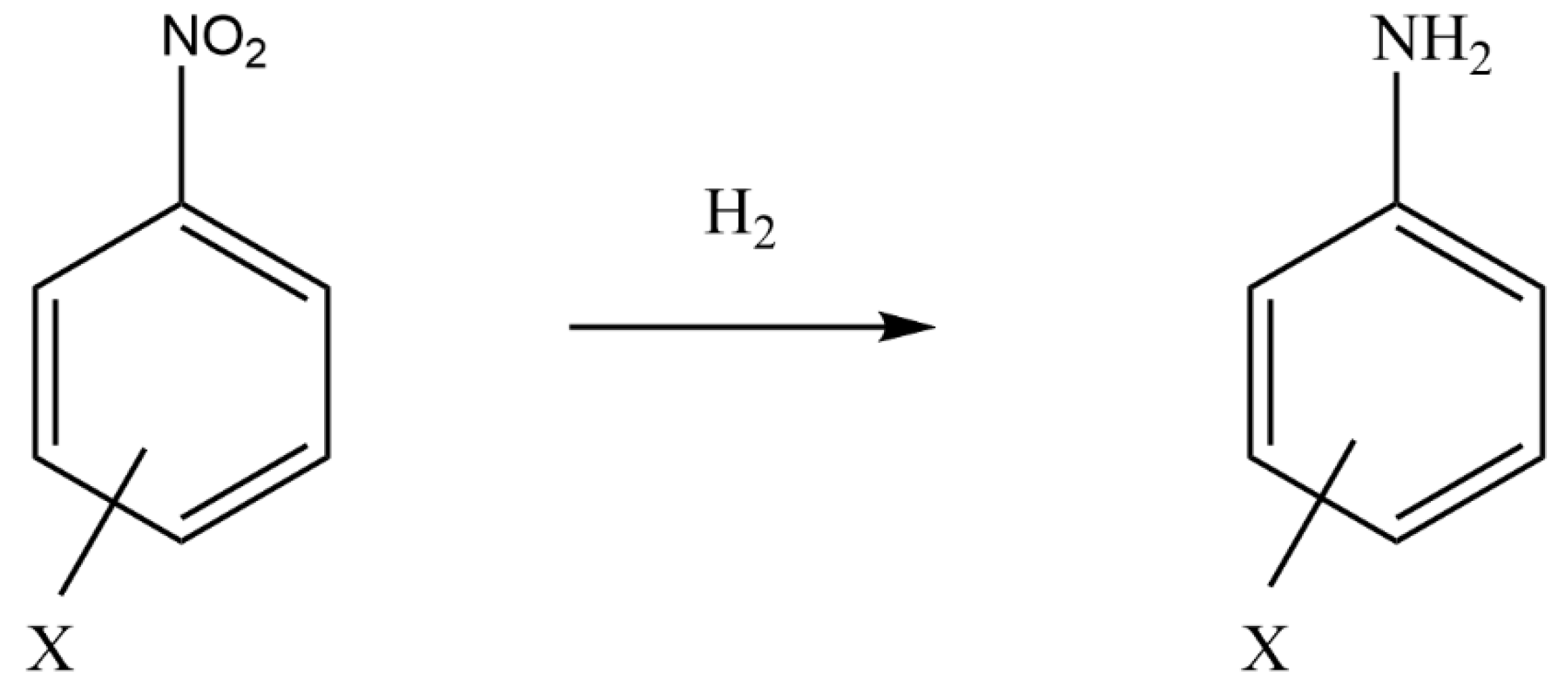
2.3.1. Hydrogenation Reactions
2.3.2. Oxidation Reactions
2.3.3. Hydrolysis, Dehydration, and Chlorination Reactions

| Entry | Reactor a | Catalyst | Reaction | Operational Conditions b | Results | Reference |
|---|---|---|---|---|---|---|
| 1 | Fluoroelastomeric capillary, D = 1.6 mm, L = 60 cm | TEMPO/AO | Oxidation of primary and secondary alcohols | Aqueous phase: NaOCl and KBr, Organic phase: alcohol in CH2Cl2 or EtOAc. Qaq = 56 µL/min, Qorg = 44 μL/min, ~4.8 min residence time, T: 0 °C | >99% conversion of 4-chlorobenzyl alcohol and 93% yield of 4-chlorobenzaldehyde could be obtained, long-term catalyst stability within 9 h | [23] |
| 2 | Capillary, D = 1.65 mm, L = 15–30 cm | Amberlyst-15, 200 and 700 μm | Fructose dehydration to HMF | Organic phase: fructose in 1,4-dioxane and DMSO, Qaq = 0.1–0.35 mL/min, residence time: 3 min, T: 80–110 °C, atmospheric pressure | HMF yield of 92%, an STY of 9.2 × 10−5 mol/(mL·min) being 75 times higher than that in batch, good catalyst stability within 96 h | [46] |
| 3 | Stainless steel capillary, D = 4.35–5 mm, L = 10 cm | HND-580 260, 510, 770 μm | Production of fructose, HMF, and LA from inulin | Aqueous phase: inulin in (DMSO–)water solution, Qaq = 0.1–0.9 mL/min T: 70–190 °C, P: 2 MPa | Maximum yields of 89% for fructose, 81% for HMF, and 84% for LA obtained within a residence time of 7.26 min | [47] |
| 4 | PFA tubing, D = 4 mm, L = 2–8 cm | P-TiO2, 300–425 μm | Synthesis of HMF from glucose | Aqueous phase: glucose in NaCl solution, Organic phase: mTHF, Qorg/Qaq = 4:1, T: 150 °C, P: 10 bar | Glucose conversion of 96% and 72% HMF yield from 0.1 M glucose, STY at 145.64 mol/(m3·min) | [48] |
| 5 | Aluminum alloy plate-type microreactor, H × W × L = 0.5 mm × 1 mm × 60 mm | CaO | Biodiesel synthesis | Alcohol phase: methanol, Oil phase: refined palm oil (with or without iso-propanol as co-solvent), T = 65 °C, ambient pressure at outlet | >98% yield of methyl esters, with a purity of methyl esters of 99% at 6.5 min residence time | [19] |
| 6 | Stainless steel capillary, D = 4.8 mm, L = 5.1 cm | Lipozyme TL IM | Biodiesel synthesis | Alcohol: ethanol, Oil phase: fatty acid oil, water content: 0–6%, T: 0–40 °C, molar ratio of fatty acid oil to ethanol: 1:3–1:6 | The highest biodiesel yield of 92% at a mean residence time of 4 h | [72] |
| 7 | Stainless steel capillary, D = 3 mm, L = 10 cm | Pd/Al2O3, 480 μm | Hydrogenation of α-methylstyrene and nitrobenzene | Organic phase: α-methylstyrene or nitrobenzene in methanol, a gas–liquid contactor for gas dissolution before the packed bed T: 26–46 °C, P: 1.0–1.3 Mpa | Accurate reaction kinetics was obtained in line with the literature findings | [92] |
| Entry | Reactor a | Catalyst | Reaction | Operational Conditions b | Results | Reference |
|---|---|---|---|---|---|---|
| 1 | Teflon capillary, D = 1.65 mm | TEMPO/silica, 160–240 μm | Oxidation of different alcohols | Liquid: alcohol and HNO3 in DCE, Gas: O2 QL = 0.1–0.4 mL/min, QG = 1.3–5 mL/min, T: 55–80 °C, P: 5 bar | 98% benzyl alcohol conversion and 99% benzaldehyde yield within a residence time of 0.5 min, STY of 4.1 × 105 mol benzaldehyde/(molcat·L·h) | [33] |
| 2 | Silicon-glass chip, H × W × L = 0.3 mm × 0.6 mm × 3.2 mm | Au–Pd/TiO2, 64 ± 14 μm | Benzyl alcohol oxidation | Liquid: benzyl alcohol, Gas: O2, QL = 0–5 μL/min, QG = 0–0.6 NmL/min T: 120 °C, P: 2 bar | >90% selectivity to benzaldehyde in the partially wetted gas-continuous flow; 93% conversion and 80% selectivity at fully wetted gas continuous flow | [31] |
| 3 | Capillary, D = 4.35 mm | No solid catalyst, glass beads as packing, 300–350 μm | Benzyl alcohol oxidation | Liquid: benzyl alcohol and Cu/TEMPO in acetonitrile solution, Gas: 9% O2 in N2, molar ratio of O2 to alcohol = 0.65:1 T: 20–45 °C, P: 5–35 bar | Almost 100% yield of benzaldehyde within 30 s, STY = 7318.4 mol/(m3 h) at optimum condition, which is ca. 2 to 20 times higher than that in conventional flow reactors under similar conditions | [35] |
| 4 | Tempax glass chips, H × W × L = 0.9 mm × 0.6 mm × 40/120 mm | Pd–Au/TiO2, Pd/TiO2, Pd/Al2O3, | Direct synthesis of H2O2 | Liquid: aqueous solution (of diluted sulfuric acid, phosphoric acid, sodium bromide), Gas: O2 and H2, QG = 5 NmL/min, QL = 0.1 mL/min, T: 20 °C, P: 0.95 MPa, | 11.3 wt.% H2O2 with a yield of at 42%, H2O2 productivityat 17 × 10−4 mol/h | [39] |
| 5 | Glass chip, H × W × L = 0.9 mm × 0.6 mm × 4 0 mm | Pd–Au/TiO2, Pd/TiO2, 60 μm | Direct synthesis of H2O2 | Liquid: aqueous solution (of diluted sulfuric acid, phosphoric acid, sodium bromide), Gas: O2 and H2, QG = 5 sccm, QL = 0.01–0.16 mL/min, T: 23–31 °C, P: 0.95 MPa | 10.4 wt.% H2O2 and selectivity of 48% in 16-channel microreactor, ca. 1 kg/day H2O2 produced from parallel operation of 4 microreactors | [38] |
| 6 | PMMA chip, H × W = 0.8 mm × 0.8 mm | Pd/Al2O3, 180–250 μm | Direct synthesis of H2O2 | Liquid: aqueous solution (of methanol, sulfuric acid, sodium bromide), Gas: O2 and H2, QG = 3–10 mL/min, QL = 0.01–0.05 mL/min, H2/O2 molar ratio: 0.2–1 T: 10–30 °C, P: atmospheric pressure (0.1 Mpa) | 0.388 wt.% H2O2 produced under optimum conditions | [41] |
| 7 | PMMA chip, H × W = 0.8 mm × 0.8 mm | Pd-Sn/Al2O3, 180–250 μm | Direct synthesis of H2O2 | Liquid: aqueous solution (of methanol, sulfuric acid, sodium bromide), Gas: O2 and H2, QG = 6 NmL/min, QL = 0.02 mL/min, H2/O2 molar ratio: 0.5, atmospheric T and P | 0.4929 wt.% H2O2 produced under optimum conditions | [42] |
| 8 | Capillary, D = 4 mm L = 10 cm | SO42−/Al2O3 and HND-580, 300–580 μm | Dehydration of xylose | Liquid: aqueous xylose solution, Gas: N2/CO2, QL = 0.1–1 mL/min, QG = 0–50 mL/min, T: 140–170 °C, P: 1.5–3.5 MPa, | STY of 5 × 10−3 g/(mL·min), increase in the reaction rate at 1–2 and STY at 1–3 orders of magnitude higher than the batch reactor employing other acid catalysts | [50] |
| 9 | Teflon capillary, D = 1.65 mm | TEMPO/silica, 160–240 μm | Oxidation of HMF | Liquid: HMF and HNO3 in DCE, Gas: O2, QL = 0.1–0.4 mL/min, QG = 1.3–5 mL/min, T: 55 °C, P: 5 bar | 97% conversion of HMF and 98% selectivity towards DFF within a residence time of 2 min | [33] |
| 10 | Stainless steel capillary, D = 2 mm, L = 2–6 cm | Au/CeO2 | Oxidation of HMF | Liquid: HMF and NaOH (molar ratio at 1:4) in water, Gas: O2, QG/QL = 30–130, T: 90–130 °C, P: 0.1–2 MPa | 100% HMF conversion and 90% FDCA selectivity were achieved within 41 s, an STY of 1–2 orders of magnitude higher than that of traditional reactors under similar conditions | [51] |
| 11 | Capillary, D = 3.87–5.35 mm L = 5.35–25 cm | Cu-/SiO2, Ni/SiO2, Pd/C, Pt/C, 425–600 μm | Hydrogenation of furfural | Liquid: furfural in isopropanol, Gas: H2 or N2, QL = 0.1–1 mL/min, QG = 10–50 mL/min, T: 60–120 °C, P: 0.1–3.1 MPa, | Furfural converted completely to FA under 80 °C and 0.6 MPa over Cu/SiO2 catalyst, an STY of 1–2 orders of magnitude higher than that of traditional reactors under similar conditions | [52] |
| 12 | PFA capillary, D = 1.6 mm, L = 40–80 cm | Ru/C 300 and 450 μm | Hydrogenation of levulinic acid | Liquid: levulinic acid in 1, 4-dioxane, Gas: H2 or N2, QL = 0.05–0.17 mL/min, QG = 0.16–0.33 mL/min, T: 70–130 °C, P: 9–13 bar | 100% levulinic acid conversion and 84% γ-valerolactone yield were obtained | [25] |
| 13 | Stainless steel capillary, D = 4 mm, L = 3 mm | Au/TiO2, 90–180 μm | Oxidation of glycerol | Liquid: glycerol and NaOH in water, Gas: O2, QL = 0.2–4 mL/min, QG = 5–30 mL/min T: 30–70 °C, P: 5–15 bar | Selectivity towards secondary oxidation products like oxalic acid and tartronic acid was higher (>30%) than that in a batch slurry reactor (<5%) under similar conditions | [53] |
| 14 | Stainless steel capillary, D = 4.35 mm, L = 20 cm | NHPI/AC, 200–250 μm | Oxidation of ethylbenzene | Liquid: EB and TBN (molar ratio of TBN/EB = 0.6–1.5) in MeCN, Gas: O2, QG/QL = 25–125, T: 50–110 °C, P: 2–7 bar | 93% EB conversion and 90.1% selectivity to AP within a reaction time of 2.4 min | [57] |
| 15 | Stainless steel capillary, D = 4.6 mm, L = 150 mm | No catalyst, soda lime glass beads as packing, 210–250 μm | Oxidation of alkenes with nitrous oxide | Liquid: dodecenes mixture, Gas: vaporized N2O, QL = 12.2 μL/min, QG = 6.1 μL/min, T: 300–375 °C, P: 6205–10,342 kPa | Conversions of dodecene mixture up to 77% and yields of primary product (ketones) up to 45%. | [61] |
| 16 | Stainless steel microreactors, D = 4 mm, L = 30 mm | Au/Al2O3, 125–500 μm | Hydrogenation of functionalized nitroaromatics | Liquid: substituted nitrobenzene in toluene, Gas: H2, QL = 0.5 mL/min, QG = 8–60 mL/min T: 60–110 °C and P: 10–20 bar | A high selectivity to the target product (>92%) and almost 100% conversion of substrate were obtained | [93] |
| 17 | 6 parallel stainless steel microreactors, D = 3.5 mm | Pt-HHDMA/C, 200–400 μm | Hydrogenation of functionalized nitroaromatics | Liquid: functionalized nitroaromatics in THF, Gas: H2 QL = 0.3–3 mL/min, QG = 3–60 mL/min T: 30–90 °C, P: 1–60 bar, | Pt-HHDMA/C exhibited superior activity, chemo selectivity, and leaching resistance compared with Pt−Pb/CaCO3 | [97] |
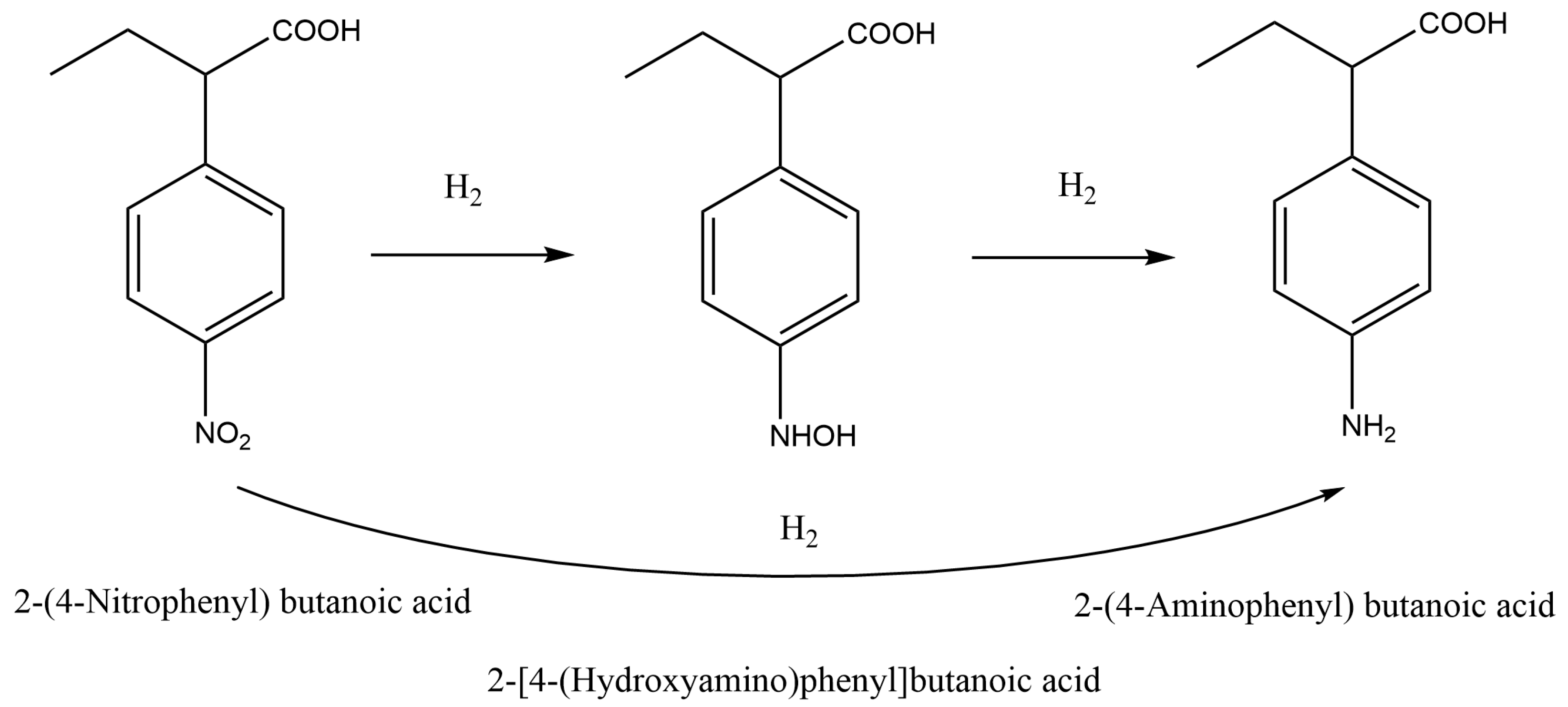
| 18 | Stainless steel capillary, D = 4.6 mm, L = 50 mm | Pd/C powder, 6–20 μm | Hydrogenation of 2-(4-nitrophenyl) butanoic acid | Liquid: NBA, Gas: H2, QL = 0.8–1.2 mL/min, QG = 11.3 NmL/min, T: 0–30 °C, P: 1–2.5 Mpa | Reaction kinetics were determined | [3] |
| 19 | Capillary, D = 2.2 mm, L = 200 mm | Pt-Pd/Al2O3 catalyst, 70–200 μm | Hydrogenation of biphenyl | Liquid: biphenyl in tetradecane, Gas: H2, QL = 0.5–5 g/h, QG = 5–50 mL/min T: 140 °C, P: 5 MPa | Intrisic kinetic values were obtained in microreactors, similar to those from the autoclave experiments | [88] |
| 20 | PTFE capillary, D = 0.8 mm, L = 30 cm | Au–Pd/TiO2, 53–63 μm | Oxidation of cinnamyl alcohol | Liquid: cinnamyl alcohol in toluene, Gas: O2 (in N2), T: 80–120 °C, P: 4 bar | Catalyst performance was tested and deactivation chracteristics were revealed | [91] |
| 21 | Silicon/glass chip, H × W × L = 0.3 mm × 0.6 mm × 190 mm | Au-Pd/TiO2, 53–63 μm | Aerobic oxidation of benzyl alcohol | Liquid: benzyl alcohol, Gas: O2 QL = 0.003 mL/min, QG = 0.6 mL/min, T: 120 °C | MIm-prepared catalyst demonstrated long-term stability for over 75 h and achieved around 80% conversion of benzyl alcohol with 60% selectivity to benzaldehyde | [20] |
| Entry | Reactor a | Catalyst | Reaction | Operational Conditions | Results | Reference |
|---|---|---|---|---|---|---|
| 1 | Silicon chip, H × W × L = 300 μm × 625 μm × 20 mm | Activated carbon, 53–73 μm | Phosgene synthesis | Gas: mixed gas with molar ratio at CO/Cl2 = 2:1, T: 220 °C, P: 132 KPa at inlet | Kinetic operation and data were obtained, phosgene productivity at 9.3 kg/year | [43] |
| 2 | Stainless steel slit-type microreactor, W = 0.508 mm | Co-Re/Al2O3, 45, 150 μm | FT synthesis | Gas: syngas with molar ratio at H2/CO = 1–2.5, T: 224 °C, P = 10–35 atm, | CO conversion of 90.3%, CH4 selectivity of 3.4%, productivity of 2.14 g C2+/(gcat·h) were obrained under P = 35 atm, H2/CO = 2, and GHSV = 22,886 h−1 | [75] |
| 3 | Stainless steel capillary, D = 1.4 mm, L = 320 mm | Co-Pt/Al2O3 | FT synthesis | Gas: syngas with molar ratio at H2/CO = 2, T: 493 K, P: 20 bar | Chain growth probability was 0.92 with C5+ productivity of 1.1–1.4 g/(gcat·h), slow catalyst deactivation occured at 1% per day | [24] |
| 4 | Stainless steel plate-type microreactor, H × W × L = 0.8 mm × 8 mm × 60 mm | Cu-ZnO/Al2O3, 50–120 μm | Methanol synthesis from syngas | Gas: syngas with a molar ratio: H2/CO/CO2/N2 = 65:25:5:5, T: 235–265 °C, P: 80 bar | Methanol selectivity exceeding 98.7% | [82] |
| 5 | Not specified | Pd/In2O3/SBA-15 catalyst | Methanol synthesis from CO2 | Gas: mixed gas with a molar ratio: H2/CO2/N2 = 64:16:20, T: 260–360 °C, P: 5 MPa, GHSV = 15,000 cm3/(gcat·h) | Methanol selectivity of 83.9%, CO2 conversion of 12.6%, and STY of 1.1 × 10−2 mol/(gcat·h) | [85] |
| 6 | Stainless steel slit-type micoreactor, L = 6 mm | CuO–ZnO– Al2O3 and γ-Al2O3, 50–80 μm | DME synthesis | Gas: syngas with a molar ratio at H2/CO/CO2/N2/ CH4 = 56:28:5:5:6, T: 220–320 °C, P: 50–70 bar | An DME yield up to ca. 50% at ca. 90% CO conversion was achieved | [87] |
| 7 | Silicon-glass chip, H × W = 0.42 mm × 2 mm | Pd/Al2O3, 53–90 μm | Methanol complete oxidation | Gas: mixed gas (O2, CH4, N2, He) with molar ratio at O2/CH4 = 2–4, T: 250–350 °C, P: 1.3 bar at outlet | Rapid and reliable kinetic model screening within 48 h | [106] |
3. Challenges and Outlook
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anastas, P.T.; Kirchhoff, M.M. Origins, Current Status, and Future Challenges of Green Chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Zimmerman, J.B. Peer Reviewed: Design Through the 12 Principles of Green Engineering. Environ. Sci. Technol. 2003, 37, 94A–101A. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Ren, M.; Li, P.; Zhou, J.; Li, N.; Li, X.; Fan, H. Continuous hydrogenation of 2-(4-nitrophenyl) butanoic acid: Kinetics study in a micropacked-bed reactor. Chem. Eng. Sci. 2023, 271, 118565. [Google Scholar] [CrossRef]
- Moulijn, J.A.; Makkee, M.; Berger, R.J. Catalyst testing in multiphase micro-packed-bed reactors; criterion for radial mass transport. Catal. Today 2015, 259, 354–359. [Google Scholar] [CrossRef]
- Jensen, K.F. Flow chemistry—Microreaction technology comes of age. AIChE J. 2017, 63, 858–869. [Google Scholar] [CrossRef]
- Hommes, A.; Heeres, H.J.; Yue, J. Catalytic Transformation of Biomass Derivatives to Value-Added Chemicals and Fuels in Continuous Flow Microreactors. Chemcatchem 2019, 11, 4671–4708. [Google Scholar] [CrossRef]
- Yue, J. Green process intensification using microreactor technology for the synthesis of biobased chemicals and fuels. Chem. Eng. Process. 2022, 177, 109002. [Google Scholar] [CrossRef]
- Yue, J.; Chen, G.; Yuan, Q.; Luo, L.; Gonthier, Y. Hydrodynamics and mass transfer characteristics in gas–liquid flow through a rectangular microchannel. Chem. Eng. Sci. 2007, 62, 2096–2108. [Google Scholar] [CrossRef]
- Sun, J.; Ju, J.; Ji, L.; Zhang, L.; Xu, N. Synthesis of Biodiesel in Capillary Microreactors. Ind. Eng. Chem. Res. 2008, 47, 1398–1403. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Sia, S.K.; Whitesides, G.M. Microfluidic devices fabricated in Poly(dimethylsiloxane) for biological studies. Electrophoresis 2003, 24, 3563–3576. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.M.K.; Gitlin, I.; Stroock, A.D.; Whitesides, G.M. Components for integrated poly(dimethylsiloxane) microfluidic systems. Electrophoresis 2002, 23, 3461–3473. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Liebner, C.; Hieronymus, H.; Klemm, E. Maximum safe diameters of microcapillaries for a stoichiometric ethene/oxygen mixture. Chem. Eng. Sci. 2009, 64, 2951–2956. [Google Scholar] [CrossRef]
- Hafeez, S.; Manos, G.; Al-Salem, S.M.; Aristodemou, E.; Constantinou, A. Liquid fuel synthesis in microreactors. React. Chem. Eng. 2018, 3, 414–432. [Google Scholar] [CrossRef]
- Porta, R.; Benaglia, M.; Puglisi, A. Flow Chemistry: Recent Developments in the Synthesis of Pharmaceutical Products. Org. Process Res. Dev. 2016, 20, 2–25. [Google Scholar] [CrossRef]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.A.; Whitesides, G.M. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- McDonald, J.C.; Whitesides, G.M. Poly(dimethylsiloxane) as a Material for Fabricating Microfluidic Devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef]
- Chueluecha, N.; Kaewchada, A.; Jaree, A. Enhancement of biodiesel synthesis using co-solvent in a packed-microchannel. J. Ind. Eng. Chem. 2017, 51, 162–171. [Google Scholar] [CrossRef]
- Morad, M.; Sankar, M.; Cao, E.; Nowicka, E.; Davies, T.E.; Miedziak, P.J.; Morgan, D.J.; Knight, D.W.; Bethell, D.; Gavriilidis, A.; et al. Solvent-free aerobic oxidation of alcohols using supported gold palladium nanoalloys prepared by a modified impregnation method. Catal. Sci. Technol. 2014, 4, 3120–3128. [Google Scholar] [CrossRef]
- Inoue, T.; Schmidt, M.; Jensen, K. Microfabricated Multiphase Reactors for the Direct Synthesis of Hydrogen Peroxide from Hydrogen and Oxygen. Ind. Eng. Chem. Res. 2007, 46, 1153–1160. [Google Scholar] [CrossRef]
- Tanimu, A.; Jaenicke, S.; Alhooshani, K. Heterogeneous catalysis in continuous flow microreactors: A review of methods and applications. Chem. Eng. J. 2017, 327, 792–821. [Google Scholar] [CrossRef]
- Bogdan, A.; McQuade, D. A biphasic oxidation of alcohols to aldehydes and ketones using a simplified packed-bed microreactor. Beilstein J. Org. Chem. 2009, 5. [Google Scholar] [CrossRef] [PubMed]
- Chambrey, S.; Fongarland, P.; Karaca, H.; Piché, S.; Griboval-Constant, A.; Schweich, D.; Luck, F.; Savin, S.; Khodakov, A.Y. Fischer–Tropsch synthesis in milli-fixed bed reactor: Comparison with centimetric fixed bed and slurry stirred tank reactors. Catal. Today 2011, 171, 201–206. [Google Scholar] [CrossRef]
- Hommes, A.; Horst, A.; Koeslag, M.; Heeres, H.J.; Yue, J. Experimental and modeling studies on the Ru/C catalyzed levulinic acid hydrogenation to γ–valerolactone in packed bed microreactors. Chem. Eng. J. 2020, 399. [Google Scholar] [CrossRef]
- Inoue, T.; Ohtaki, K.; Adachi, J.; Lu, M.; Murakami, S. Direct synthesis of hydrogen peroxide using glass fabricated microreactor—Multichannel operation and catalyst comparison. Catal. Today 2015, 248, 169–176. [Google Scholar] [CrossRef]
- Liu, X.; Ünal, B.; Jensen, K.F. Heterogeneous catalysis with continuous flow microreactors. Catal. Sci. Technol. 2012, 2, 2134–2138. [Google Scholar] [CrossRef]
- Yue, J. Multiphase flow processing in microreactors combined with heterogeneous catalysis for efficient and sustainable chemical synthesis. Catal. Today 2018, 308, 3–19. [Google Scholar] [CrossRef]
- Munirathinam, R.; Huskens, J.; Verboom, W. Supported Catalysis in Continuous-Flow Microreactors. Adv. Synth. Catal. 2015, 357, 1093–1123. [Google Scholar] [CrossRef]
- Zong, J.; Yue, J. Continuous Solid Particle Flow in Microreactors for Efficient Chemical Conversion. Ind. Eng. Chem. Res. 2022, 61, 6269–6291. [Google Scholar] [CrossRef]
- Al-Rifai, N.; Galvanin, F.; Morad, M.; Cao, E.; Cattaneo, S.; Sankar, M.; Dua, V.; Hutchings, G.; Gavriilidis, A. Hydrodynamic effects on three phase micro-packed bed reactor performance—Gold–palladium catalysed benzyl alcohol oxidation. Chem. Eng. Sci. 2016, 149, 129–142. [Google Scholar] [CrossRef]
- Hone, C.A.; Kappe, C.O. The Use of Molecular Oxygen for Liquid Phase Aerobic Oxidations in Continuous Flow. Top. Curr. Chem. 2018, 377, 2. [Google Scholar] [CrossRef] [PubMed]
- Aellig, C.; Scholz, D.; Conrad, S.; Hermans, I. Intensification of TEMPO-mediated aerobic alcohol oxidations under three-phase flow conditions. Green Chem. 2013, 15, 1975–1980. [Google Scholar] [CrossRef]
- Waldron, C.; Cao, E.; Cattaneo, S.; Brett, G.L.; Miedziak, P.J.; Wu, G.; Sankar, M.; Hutchings, G.J.; Gavriilidis, A. Three step synthesis of benzylacetone and 4-(4-methoxyphenyl)butan-2-one in flow using micropacked bed reactors. Chem. Eng. J. 2019, 377, 119976. [Google Scholar] [CrossRef]
- Zhang, C.; Duan, X.; Yin, J.; Lou, F.; Zhang, J. Copper/TEMPO-catalyzed continuous aerobic alcohol oxidation in a micro-packed bed reactor. React. Chem. Eng. 2022, 7, 1289–1296. [Google Scholar] [CrossRef]
- Voloshin, Y.; Lawal, A. Overall kinetics of hydrogen peroxide formation by direct combination of H2 and O2 in a microreactor. Chem. Eng. Sci. 2010, 65, 1028–1036. [Google Scholar] [CrossRef]
- Dittmeyer, R.; Grunwaldt, J.D.; Pashkova, A. A review of catalyst performance and novel reaction engineering concepts in direct synthesis of hydrogen peroxide. Catal. Today 2015, 248, 149–159. [Google Scholar] [CrossRef]
- Inoue, T.; Adachi, J.; Ohtaki, K.; Lu, M.; Murakami, S.; Sun, X.; Wang, D.F. Direct hydrogen peroxide synthesis using glass microfabricated reactor—Paralleled packed bed operation. Chem. Eng. J. 2015, 278, 517–526. [Google Scholar] [CrossRef]
- Inoue, T.; Ohtaki, K.; Murakami, S.; Matsumoto, S. Direct synthesis of hydrogen peroxide based on microreactor technology. Fuel Process. Technol. 2013, 108, 8–11. [Google Scholar] [CrossRef]
- Hirama, H.; Yoshioka, H.; Matsumoto, Y.; Amada, T.; Hori, Y.; Ohtaki, K.; Lu, M.; Inoue, T. Design, Fabrication, and Performance of an Optimized Flow Reactor with Parallel Micropacked Beds. Ind. Eng. Chem. Res. 2017, 56, 14200–14206. [Google Scholar] [CrossRef]
- Yang, Z.; Wei, Z.; Zhou, S.; Bao, B.; Zhao, S.; Gong, F. Direct thermal catalytic synthesis of hydrogen peroxide by using microchip reactor. Chem. Eng. J. 2023, 456, 140915. [Google Scholar] [CrossRef]
- Yang, Z.; Hao, Z.; Zhou, S.; Xie, P.; Wei, Z.; Zhao, S.; Gong, F. Pd–Sn Alloy Catalysts for Direct Synthesis of Hydrogen Peroxide from H2 and O2 in a Microchannel Reactor. ACS Appl. Mater. Interfaces 2023, 15, 23058–23067. [Google Scholar] [CrossRef] [PubMed]
- Ajmera, S.K.; Losey, M.W.; Jensen, K.F.; Schmidt, M.A. Microfabricated packed-bed reactor for phosgene synthesis. AIChE J. 2001, 47, 1639–1647. [Google Scholar] [CrossRef]
- Hughes, R.; Rossi, G.E.; Lennon, D. Operational parameters relevant to the examination of phosgene synthesis catalysis. React. Chem. Eng. 2023, 8, 3150–3161. [Google Scholar] [CrossRef]
- Jiang, Z.; Zeng, Y.; Hu, D.; Guo, R.; Yan, K.; Luque, R. Chemical transformations of 5-hydroxymethylfurfural into highly added value products: Present and future. Green Chem. 2023, 25, 871–892. [Google Scholar] [CrossRef]
- Aellig, C.; Hermans, I. Continuous D-Fructose Dehydration to 5-Hydroxymethylfurfural Under Mild Conditions. Chemsuschem 2012, 5, 1737–1742. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, W.; Zhang, H.; Sang, L.; Zhao, Z. Selective Conversion of Inulin into 5-Hydroxymethylfurfural and Levulinic Acid in Micropacked Bed Reactors. Ind. Eng. Chem. Res. 2024, 63, 13134–13144. [Google Scholar] [CrossRef]
- Guo, W.; Kortenbach, T.; Qi, W.; Hensen, E.; Jan Heeres, H.; Yue, J. Selective tandem catalysis for the synthesis of 5-hydroxymethylfurfural from glucose over in-situ phosphated titania catalysts: Insights into structure, bi-functionality and performance in flow microreactors. Appl. Catal. B Environ. 2022, 301, 120800. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Lu, H.-X.; Yang, W.-Y.; Shi, Y.-X.; Wang, H.-B.; Mao, H.; Sang, L.; Zhao, Z.-P. Fast and continuous conversion of xylose to furfural in micropacked bed reactors. Chem. Eng. Sci. 2023, 266, 118256. [Google Scholar] [CrossRef]
- Yang, W.; Tang, X.; Li, W.; Luo, X.; Zhang, C.; Shen, C. Fast and continuous synthesis of 2,5-furandicarboxylic acid in a micropacked-bed reactor. Chem. Eng. J. 2022, 442, 136110. [Google Scholar] [CrossRef]
- Duan, L.; Huang, M.; Peng, Z.; Sang, L.; Zhang, J. Efficient and continuous furfural hydrogenation to furfuryl alcohol in a micropacked bed reactor. React. Chem. Eng. 2023, 8, 1719–1728. [Google Scholar] [CrossRef]
- Zope, B.N.; Davis, R.J. Influence of Reactor Configuration on the Selective Oxidation of Glycerol over Au/TiO2. Top. Catal. 2009, 52, 269–277. [Google Scholar] [CrossRef]
- Denčić, I.; de Vaan, S.; Noël, T.; Meuldijk, J.; de Croon, M.; Hessel, V. Lipase-Based Biocatalytic Flow Process in a Packed-Bed Microreactor. Ind. Eng. Chem. Res. 2013, 52, 10951–10960. [Google Scholar] [CrossRef]
- Brás, E.J.S.; Domingues, C.; Chu, V.; Fernandes, P.; Conde, J.P. Microfluidic bioreactors for enzymatic synthesis in packed-bed reactors—Multi-step reactions and upscaling. J. Biotechnol. 2020, 323, 24–32. [Google Scholar] [CrossRef]
- Strniša, F.; Bajić, M.; Panjan, P.; Plazl, I.; Sesay, A.M.; Žnidaršič-Plazl, P. Characterization of an enzymatic packed-bed microreactor: Experiments and modeling. Chem. Eng. J. 2018, 350, 541–550. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, J.; Xie, B.; Liu, W.; Zhang, J. Green and continuous aerobic oxidation of ethylbenzene over homogeneous and heterogeneous NHPI in a micro-packed bed reactor. Chem. Eng. J. 2023, 468, 143674. [Google Scholar] [CrossRef]
- Lou, F.; Cao, Q.; Zhang, C.; Ai, N.; Wang, Q.; Zhang, J. Continuous synthesis of benzaldehyde by ozonolysis of styrene in a micro-packed bed reactor. J. Flow Chem. 2022, 12, 307–315. [Google Scholar] [CrossRef]
- Cao, Q.; Sang, L.; Tu, J.; Xiao, Y.; Liu, N.; Wu, L.; Zhang, J. Rapid degradation of refractory organic pollutants by continuous ozonation in a micro-packed bed reactor. Chemosphere 2021, 270, 128621. [Google Scholar] [CrossRef]
- Cao, Q.; Lou, F.; Liu, N.; Zhang, J.; Wu, L. Continuous Catalytic Ozonation of Antibiotics Using Mn and Cu Oxides on γ-Al2O3 Pellets in a Micropacked Bed Reactor. ACS ES&T Water 2021, 1, 1911–1920. [Google Scholar] [CrossRef]
- Newman, S.G.; Lee, K.; Cai, J.; Yang, L.; Green, W.H.; Jensen, K.F. Continuous Thermal Oxidation of Alkenes with Nitrous Oxide in a Packed Bed Reactor. Ind. Eng. Chem. Res. 2015, 54, 4166–4173. [Google Scholar] [CrossRef]
- Lerou, J.J.; Tonkovich, A.L.; Silva, L.; Perry, S.; McDaniel, J. Microchannel reactor architecture enables greener processes. Chem. Eng. Sci. 2010, 65, 380–385. [Google Scholar] [CrossRef]
- Yadav, D.; Lu, X.; Vishwakarma, C.B.; Jing, D. Advancements in microreactor technology for hydrogen production via steam reforming: A comprehensive review of experimental studies. J. Power Sources 2023, 585, 233621. [Google Scholar] [CrossRef]
- Chueluecha, N.; Kaewchada, A.; Jaree, A. Biodiesel synthesis using heterogeneous catalyst in a packed-microchannel. Energy Convers. Manag. 2016, 141, 145–154. [Google Scholar] [CrossRef]
- Myrstad, R.; Eri, S.; Pfeifer, P.; Rytter, E.; Holmen, A. Fischer–Tropsch synthesis in a microstructured reactor. Catal. Today 2009, 147, S301–S304. [Google Scholar] [CrossRef]
- Todić, B.; Ordomsky, V.V.; Nikačević, N.M.; Khodakov, A.Y.; Bukur, D.B. Opportunities for intensification of Fischer–Tropsch synthesis through reduced formation of methane over cobalt catalysts in microreactors. Catal. Sci. Technol. 2015, 5, 1400–1411. [Google Scholar] [CrossRef]
- Bakhtiary-Davijany, H.; Hayer, F.; Kim Phan, X.; Myrstad, R.; Pfeifer, P.; Venvik, H.J.; Holmen, A. Performance of a multi-slit packed bed microstructured reactor in the synthesis of methanol: Comparison with a laboratory fixed-bed reactor. Chem. Eng. Sci. 2011, 66, 6350–6357. [Google Scholar] [CrossRef]
- Bakhtiary-Davijany, H.; Hayer, F.; Kim Phan, X.; Myrstad, R.; Venvik, H.J.; Pfeifer, P.; Holmen, A. Modelling and simulation of a single slit micro packed bed reactor for methanol synthesis. Catal. Today 2020, 343, 226–233. [Google Scholar] [CrossRef]
- Hayer, F.; Bakhtiary-Davijany, H.; Myrstad, R.; Holmen, A.; Pfeifer, P.; Venvik, H.J. Modeling and simulation of an integrated micro packed bed reactor-heat exchanger configuration for direct dimethyl ether synthesis. Top. Catal. 2011, 54, 817–827. [Google Scholar] [CrossRef]
- Hayer, F.; Bakhtiary-Davijany, H.; Myrstad, R.; Holmen, A.; Pfeifer, P.; Venvik, H. Synthesis of dimethyl ether from syngas in a microchannel reactor—Simulation and experimental study. Chem. Eng. J. 2011, 167, 610–615. [Google Scholar] [CrossRef]
- Stamenković, O.S.; Todorović, Z.B.; Lazić, M.L.; Veljković, V.B.; Skala, D.U. Kinetics of sunflower oil methanolysis at low temperatures. Bioresour. Technol. 2008, 99, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.; Kim, Y.; Lee, J.-S.; Kwak, J.; Lee, J.; Kim, I.-H. Synthesis of Fatty Acid Ethyl Ester from Acid Oil in a Continuous Reactor via an Enzymatic Transesterification. J. Am. Oil Chem. Soc. 2016, 93, 311–318. [Google Scholar] [CrossRef]
- Pandey, S.; Narayanan, I.; Selvaraj, R.; Varadavenkatesan, T.; Vinayagam, R. Biodiesel production from microalgae: A comprehensive review on influential factors, transesterification processes, and challenges. Fuel 2024, 367, 131547. [Google Scholar] [CrossRef]
- Zhou, L.; Lawal, A. Evaluation of Presulfided NiMo/γ-Al2O3 for Hydrodeoxygenation of Microalgae Oil To Produce Green Diesel. Energy Fuels 2015, 29, 262–272. [Google Scholar] [CrossRef]
- Cao, C.; Hu, J.; Li, S.; Wilcox, W.; Wang, Y. Intensified Fischer–Tropsch synthesis process with microchannel catalytic reactors. Catal. Today 2009, 140, 149–156. [Google Scholar] [CrossRef]
- Potgieter, J.H.; Moodley, D.; Botha, T.; Visagie, J.; Manong, T.; Frank, M.; Claeys, M.; van Steen, E.; Böltken, T.; Pfeifer, P. Development of promoted cobalt/alumina Fischer-Tropsch catalysts for increased activity and selectivity: Micro-reactor to piloting scale. Catal. Today 2024, 432, 114554. [Google Scholar] [CrossRef]
- Loewert, M.; Francesconi, V.Z.; Brübach, L.T.; Pfeifer, P. Coupling of Fischer-Tropsch reaction kinetics, enhanced vapor–liquid flash calculation and residence time distribution modeling for time-dependent product determination in load-flexible plants. Chem. Eng. J. 2020, 402, 126032. [Google Scholar] [CrossRef]
- Knobloch, C.; Güttel, R.; Turek, T. Holdup and Pressure Drop in Micro Packed-Bed Reactors for Fischer-Tropsch Synthesis. Chem-Ing-Tech 2013, 85, 455–460. [Google Scholar] [CrossRef]
- Na, J.; Kshetrimayum, K.S.; Jung, I.; Park, S.; Lee, Y.; Kwon, O.; Mo, Y.; Chung, J.; Yi, J.; Lee, U.; et al. Optimal design and operation of Fischer-Tropsch microchannel reactor for pilot-scale compact Gas-to-Liquid process. Chem. Eng. Process. Process Intensif. 2018, 128, 63–76. [Google Scholar] [CrossRef]
- Knochen, J.; Güttel, R.; Knobloch, C.; Turek, T. Fischer–Tropsch synthesis in milli-structured fixed-bed reactors: Experimental study and scale-up considerations. Chem. Eng. Process. Process Intensif. 2010, 49, 958–964. [Google Scholar] [CrossRef]
- Simon Araya, S.; Liso, V.; Cui, X.; Li, N.; Zhu, J.; Sahlin, S.L.; Jensen, S.H.; Nielsen, M.P.; Kær, S.K. A Review of The Methanol Economy: The Fuel Cell Route. Energies 2020, 13, 596. [Google Scholar] [CrossRef]
- Bakhtiary-Davijany, H.; Hayer, F.; Phan, X.K.; Myrstad, R.; Venvik, H.J.; Pfeifer, P.; Holmen, A. Characteristics of an Integrated Micro Packed Bed Reactor-Heat Exchanger for methanol synthesis from syngas. Chem. Eng. J. 2011, 167, 496–503. [Google Scholar] [CrossRef]
- Bakhtiary-Davijany, H.; Dadgar, F.; Hayer, F.; Phan, X.K.; Myrstad, R.; Venvik, H.J.; Pfeifer, P.; Holmen, A. Analysis of External and Internal Mass Transfer at Low Reynolds Numbers in a Multiple-Slit Packed Bed Microstructured Reactor for Synthesis of Methanol from Syngas. Ind. Eng. Chem. Res. 2012, 51, 13574–13579. [Google Scholar] [CrossRef]
- Hafeez, S.; Harkou, E.; Al-Salem, S.M.; Goula, M.A.; Dimitratos, N.; Charisiou, N.D.; Villa, A.; Bansode, A.; Leeke, G.; Manos, G.; et al. Hydrogenation of carbon dioxide (CO2) to fuels in microreactors: A review of set-ups and value-added chemicals production. React. Chem. Eng. 2022, 7, 795–812. [Google Scholar] [CrossRef]
- Jiang, H.; Lin, J.; Wu, X.; Wang, W.; Chen, Y.; Zhang, M. Efficient hydrogenation of CO2 to methanol over Pd/In2O3/SBA-15 catalysts. J. CO2 Util. 2020, 36, 33–39. [Google Scholar] [CrossRef]
- Tidona, B.; Koppold, C.; Bansode, A.; Urakawa, A.; Rudolf von Rohr, P. CO2 hydrogenation to methanol at pressures up to 950bar. J. Supercrit. Fluids 2013, 78, 70–77. [Google Scholar] [CrossRef]
- Hayer, F.; Bakhtiary-Davijany, H.; Myrstad, R.; Holmen, A.; Pfeifer, P.; Venvik, H.J. Characteristics of integrated micro packed bed reactor-heat exchanger configurations in the direct synthesis of dimethyl ether. Chem. Eng. Process. Process Intensif. 2013, 70, 77–85. [Google Scholar] [CrossRef]
- Herk, D.; Castaño, P.; Makkee, M.; Moulijn, J.A.; Kreutzer, M. Catalyst testing in a multiple-parallel, gas-liquid, powder-packed bed microreactor. Appl. Catal. A-Gen. 2009, 365, 199–206. [Google Scholar] [CrossRef]
- Alsolami, B.H.; Berger, R.J.; Makkee, M.; Moulijn, J.A. Catalyst Performance Testing in Multiphase Systems: Implications of Using Small Catalyst Particles in Hydrodesulfurization. Ind. Eng. Chem. Res. 2013, 52, 9069–9085. [Google Scholar] [CrossRef]
- Wiles, C.; Watts, P. Continuous process technology: A tool for sustainable production. Green Chem. 2014, 16, 55–62. [Google Scholar] [CrossRef]
- Wu, G.; Brett, G.L.; Cao, E.; Constantinou, A.; Ellis, P.; Kuhn, S.; Hutchings, G.J.; Bethell, D.; Gavriilidis, A. Oxidation of cinnamyl alcohol using bimetallic Au–Pd/TiO2 catalysts: A deactivation study in a continuous flow packed bed microreactor. Catal. Sci. Technol. 2016, 6, 4749–4758. [Google Scholar] [CrossRef]
- Duan, X.; Tu, J.; Teixeira, A.R.; Sang, L.; Jensen, K.F.; Zhang, J. An automated flow platform for accurate determination of gas–liquid–solid reaction kinetics. React. Chem. Eng. 2020, 5, 1751–1758. [Google Scholar] [CrossRef]
- Nuzhdin, A.; Moroz, B.; Reshetnikov, S.; Pavel, P.; Aleksandrov, P.; Bukhtiyarov, V.; Bukhtiyarova, G. Selective Liquid-Phase Hydrogenation of a Nitro Group in Substituted Nitrobenzenes over Au/Al2O3 Catalyst in a Packed-Bed Flow Reactor. ChemPlusChem 2015, 80, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Teixeira, A.R.; Shi, Y.; Born, S.C.; Lin, H.; Li Song, Y.; Martin, B.; Schenkel, B.; Peer Lachegurabi, M.; Jensen, K.F. Catalytic hydrogenation of N-4-nitrophenyl nicotinamide in a micro-packed bed reactor. Green Chem. 2018, 20, 886–893. [Google Scholar] [CrossRef]
- Al-Rifai, N.; Miedziak, P.J.; Morad, M.; Sankar, M.; Waldron, C.; Cattaneo, S.; Cao, E.; Pattisson, S.; Morgan, D.; Bethell, D.; et al. Deactivation Behavior of Supported Gold Palladium Nanoalloy Catalysts during the Selective Oxidation of Benzyl Alcohol in a Micropacked Bed Reactor. Ind. Eng. Chem. Res. 2017, 56, 12984–12993. [Google Scholar] [CrossRef]
- Yue, J.; Schouten, J.C.; Nijhuis, T.A. Integration of Microreactors with Spectroscopic Detection for Online Reaction Monitoring and Catalyst Characterization. Ind. Eng. Chem. Res. 2012, 51, 14583–14609. [Google Scholar] [CrossRef]
- Vilé, G.; Almora-Barrios, N.; López, N.; Pérez-Ramírez, J. Structure and Reactivity of Supported Hybrid Platinum Nanoparticles for the Flow Hydrogenation of Functionalized Nitroaromatics. ACS Catal. 2015, 5, 3767–3778. [Google Scholar] [CrossRef]
- Duan, X.; Yin, J.; Huang, M.; Feng, A.; Fu, W.; Chen, H.; Huang, Z.; Ding, Y.; Zhang, J. Hydrogenation kinetics of m-dinitrobenzene in a continuous micro-packed bed reactor. Chem. Eng. Sci. 2022, 248, 117113. [Google Scholar] [CrossRef]
- Duan, X.; Yin, J.; Huang, M.; Wang, P.; Zhang, J. Hydrogenation kinetics of halogenated nitroaromatics over Pt/C in a continuous Micro-packed bed reactor. Chem. Eng. Sci. 2022, 251, 117483. [Google Scholar] [CrossRef]
- Tadepalli, S.; Halder, R.; Lawal, A. Catalytic hydrogenation of o-nitroanisole in a microreactor: Reactor performance and kinetic studies. Chem. Eng. Sci. 2007, 62, 2663–2678. [Google Scholar] [CrossRef]
- Ashok, A.; Chen, W.; Zaza, A.; Madrahimov, S.; Al-Rawashdeh, M.m. Packed-Bed Microreactor Under Taylor Flow for MOFs Catalyst Testing Demonstrated on Phenylacetylene Hydrogenation. ChemistrySelect 2024, 9, e202400978. [Google Scholar] [CrossRef]
- Joshi, N.; Lawal, A. Hydrodeoxygenation of acetic acid in a microreactor. Chem. Eng. Sci. 2012, 84, 761–771. [Google Scholar] [CrossRef]
- Joshi, N.; Lawal, A. Hydrodeoxygenation of 4-Propylguaiacol (2-Methoxy-4-propylphenol) in a Microreactor: Performance and Kinetic Studies. Ind. Eng. Chem. Res. 2013, 52, 4049–4058. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, P.; Zhang, J.; Huang, M.; Liu, W.; Xu, Y.; Duan, X.; Li, Y.; Zhang, J. Continuous hydrogenation of N-ethylcarbazole in a micro-packed bed reactor for hydrogen storage. Chem. Eng. J. 2024, 484, 149404. [Google Scholar] [CrossRef]
- Sankar, M.; He, Q.; Morad, M.; Pritchard, J.; Freakley, S.J.; Edwards, J.K.; Taylor, S.H.; Morgan, D.J.; Carley, A.F.; Knight, D.W.; et al. Synthesis of Stable Ligand-free Gold–Palladium Nanoparticles Using a Simple Excess Anion Method. ACS Nano 2012, 6, 6600–6613. [Google Scholar] [CrossRef]
- Bawa, S.G.; Pankajakshan, A.; Waldron, C.; Cao, E.; Galvanin, F.; Gavriilidis, A. Rapid Screening of Kinetic Models for Methane Total Oxidation using an Automated Gas Phase Catalytic Microreactor Platform. Chem. Methods 2023, 3, e202200049. [Google Scholar] [CrossRef]
- Faridkhou, A.; Tourvieille, J.-N.; Larachi, F. Reactions, hydrodynamics and mass transfer in micro-packed beds—Overview and new mass transfer data. Chem. Eng. Process. Process Intensif. 2016, 110, 80–96. [Google Scholar] [CrossRef]
- Yang, L.; Shi, Y.; Abolhasani, M.; Jensen, K.F. Characterization and modeling of multiphase flow in structured microreactors: A post microreactor case study. Lab Chip 2015, 15, 3232–3241. [Google Scholar] [CrossRef]
- Haase, S.; Weiss, M.; Langsch, R.; Bauer, T.; Lange, R. Hydrodynamics and mass transfer in three-phase composite minichannel fixed-bed reactors. Chem. Eng. Sci. 2013, 94, 224–236. [Google Scholar] [CrossRef]
- Zhang, L.; Hommes, A.; Schuring, R.; Yue, J. An experimental study of pressure drop characteristics under single-phase flow through packed bed microreactors. AIChE J. 2025, 71, e18640. [Google Scholar] [CrossRef]
- Shang, M.; Noel, T.; Wang, Q.; Hessel, V. Packed-Bed Microreactor for Continuous-Flow Adipic Acid Synthesis from Cyclohexene and Hydrogen Peroxide. Chem. Eng. Technol. 2013, 36, 1001–1009. [Google Scholar] [CrossRef]
- Dong, Z.; Wen, Z.; Zhao, F.; Kuhn, S.; Noël, T. Scale-up of micro- and milli-reactors: An overview of strategies, design principles and applications. Chem. Eng. Sci. X 2021, 10, 100097. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, K.; Teixeira, A.R.; Jensen, K.F.; Luo, G. Design and Scaling Up of Microchemical Systems: A Review. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 285–305. [Google Scholar] [CrossRef] [PubMed]
- Kockmann, N.; Gottsponer, M.; Roberge, D.M. Scale-up concept of single-channel microreactors from process development to industrial production. Chem. Eng. J. 2011, 167, 718–726. [Google Scholar] [CrossRef]
- Roberge, D.M.; Ducry, L.; Bieler, N.; Cretton, P.; Zimmermann, B. Microreactor Technology: A Revolution for the Fine Chemical and Pharmaceutical Industries? Chem. Eng. Technol. 2005, 28, 318–323. [Google Scholar] [CrossRef]
- Krtschil, U.; Hessel, V.; Kralisch, D.; Kreisel, G.; Kuepper, M.; Schenk, R. Cost analysis of a commercial manufacturing process of a fine chemical using micro process engineering. Chimia 2006, 60, 611–617. [Google Scholar] [CrossRef]
- Natarajan, Y.; Nabera, A.; Salike, S.; Valan, D.T.; Muthukumar, K.; Appusamy, A. An overview on the process intensification of microchannel reactors for biodiesel production. Chem. Eng. Process. Process Intensif. 2018, 136. [Google Scholar] [CrossRef]
- Ekinci, F.; Guzel, M.S.; Acici, K.; Asuroglu, T. The Future of Microreactors: Technological Advantages, Economic Challenges, and Innovative Licensing Solutions with Blockchain. Appl. Sci. 2024, 14, 6673. [Google Scholar] [CrossRef]
- Hessel, V.; Kralisch, D.; Krtschil, U. Sustainability through green processing—Novel process windows intensify micro and milli process technologies. Energy Environ. Sci. 2008, 1, 467–478. [Google Scholar] [CrossRef]
- Chakrabortty, S.; Kumar, R.; Nayak, J.; Jeon, B.-H.; Dargar, S.K.; Tripathy, S.K.; Pal, P.; Ha, G.-S.; Kim, K.H.; Jasiński, M. Green synthesis of MeOH derivatives through in situ catalytic transformations of captured CO2 in a membrane integrated photo-microreactor system: A state-of-art review for carbon capture and utilization. Renew. Sustain. Energy Rev. 2023, 182, 113417. [Google Scholar] [CrossRef]
- Hardwick, T.; Ahmed, N. Advances in electro- and sono-microreactors for chemical synthesis. RSC Adv. 2018, 8, 22233–22249. [Google Scholar] [CrossRef] [PubMed]
- Goyal, H.; Chen, T.-Y.; Chen, W.; Vlachos, D.G. A review of microwave-assisted process intensified multiphase reactors. Chem. Eng. J. 2022, 430, 133183. [Google Scholar] [CrossRef]
- Chen, Z.; Pei, Z.; Zhao, X.; Zhang, J.; Wei, J.; Hao, N. Acoustic microreactors for chemical engineering. Chem. Eng. J. 2022, 433, 133258. [Google Scholar] [CrossRef]
- Chen, X.; Kim, H.-H.; Nozaki, T. Plasma catalytic technology for CH4 and CO2 conversion: A review highlighting fluidized-bed plasma reactor. Plasma Process. Polym. 2024, 21, 2200207. [Google Scholar] [CrossRef]
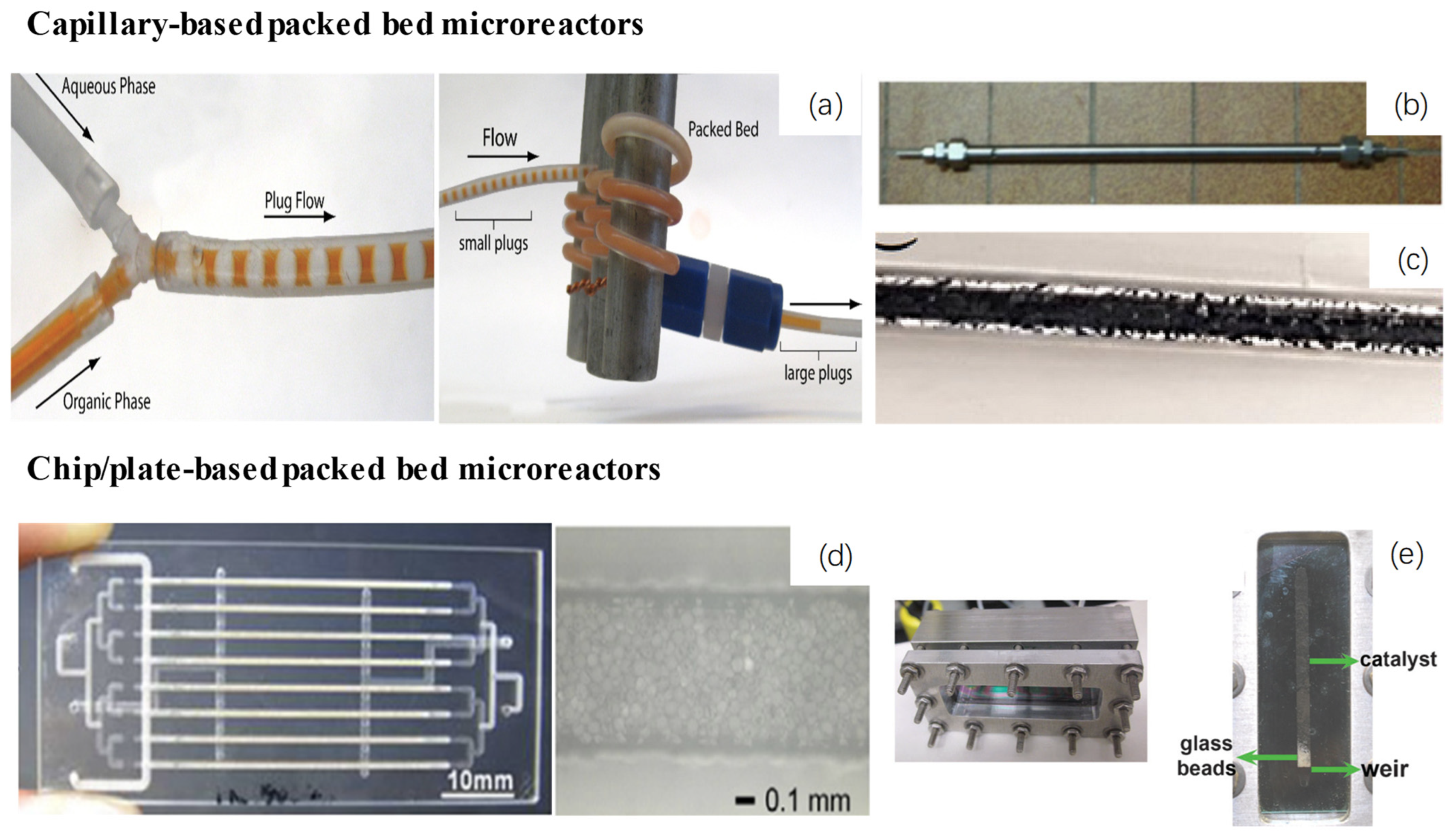

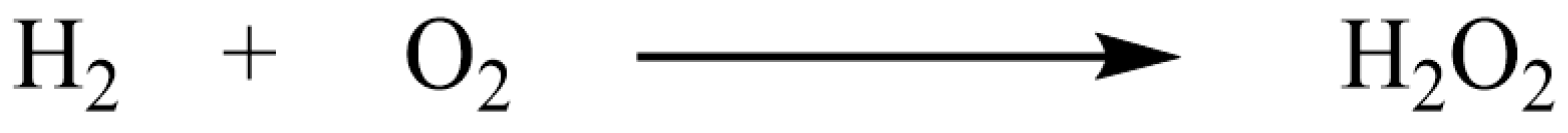
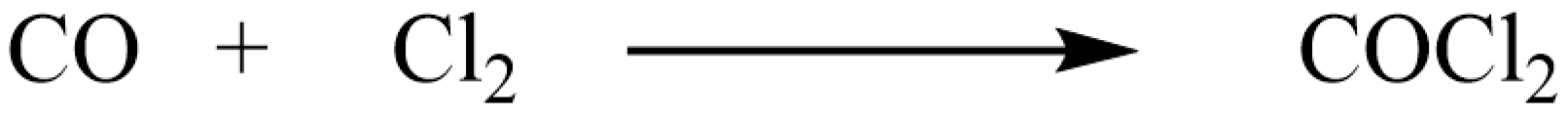




| Reactor Type | Reactor Sketch a | Configuration | Advantages | Disadvantages |
|---|---|---|---|---|
| Packed bed microreactor |   | Solid catalysts are packed in capillary- or chip-based microreactors in the form of powder/particles |
|
|
| Wall-coated microreactor | 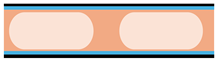  | A thin layer of catalyst is coated onto the microreactor inner wall surface |
|
|
| Slurry-based microreactors | 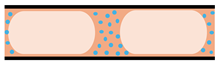  | Solid catalysts are suspended in the liquid phase (to form a slurry) and continuously flow through the microreactor |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Yue, J. Packed Bed Microreactors for Sustainable Chemistry and Process Development. Chemistry 2025, 7, 29. https://doi.org/10.3390/chemistry7020029
Zhang L, Yue J. Packed Bed Microreactors for Sustainable Chemistry and Process Development. Chemistry. 2025; 7(2):29. https://doi.org/10.3390/chemistry7020029
Chicago/Turabian StyleZhang, Lu, and Jun Yue. 2025. "Packed Bed Microreactors for Sustainable Chemistry and Process Development" Chemistry 7, no. 2: 29. https://doi.org/10.3390/chemistry7020029
APA StyleZhang, L., & Yue, J. (2025). Packed Bed Microreactors for Sustainable Chemistry and Process Development. Chemistry, 7(2), 29. https://doi.org/10.3390/chemistry7020029









