A Novel Galantamine–Curcumin Hybrid Inhibits Butyrylcholinesterase: A Molecular Dynamics Study
Abstract
:1. Introduction
2. Models and Methods
2.1. Modeled Ligands and Complexes
2.2. Molecular Docking Protocol
2.3. Molecular Dynamics (MD) Protocol
3. Results
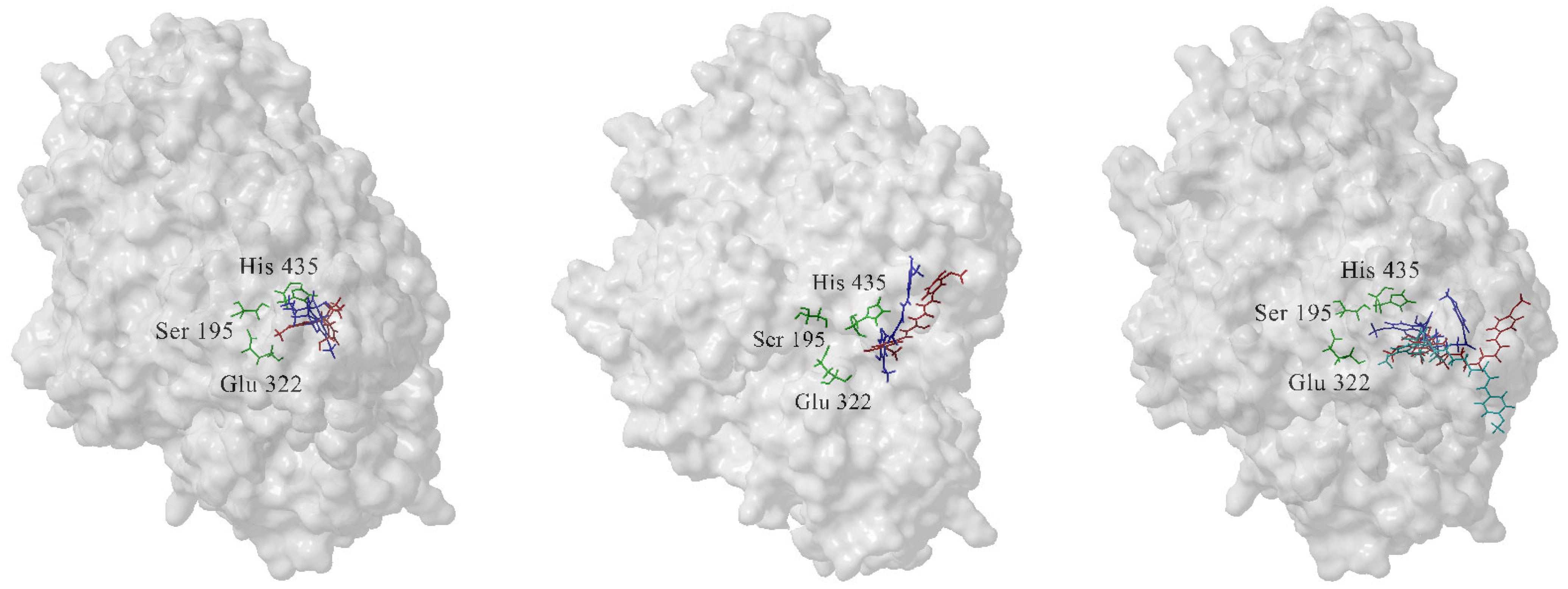
3.1. The Most Populated Conformations of the Complexes Were Identified by Cluster Analysis
3.2. GAL, CCN, and 4b Form Stable Complexes with the Enzyme BChE
3.3. Compound 4b Forms More Hydrogen Bonds with BChE than GAL and CCN
3.4. Compound 4b Forms More Interactions with BChE than GAL and CCN
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Y.; Wang, S.; Zhang, Y. Catalytic Reaction Mechanism of Acetylcholinesterase Determined by Born–Oppenheimer Ab Initio QM/MM Molecular Dynamics Simulations. J. Phys. Chem. B 2010, 114, 8817–8825. [Google Scholar] [CrossRef]
- McHardy, S.; Wang, H.; McCowen, S.; Valdez, M. Recent Advances in Acetylcholinesterase Inhibitors and Reactivators: An Update on the Patent Literature (2012–2015). Expert Opin. Ther. Pat. 2017, 27, 455–476. [Google Scholar] [CrossRef] [PubMed]
- Mack, A.; Robitzki, A. The Key Role of Butyrylcholinesterase during Neurogenesis and Neural Disorders: An Antisense-5′Butyrylcholinesterase-DNA Study. Prog. Neurobiol. 2000, 60, 607–628. [Google Scholar] [CrossRef] [PubMed]
- Hackett, P.; Sakai, T. Pseudocholinesterase Deficiency: A Case Report and Literature Review. Open J. Anesthesiol. 2012, 2, 188–194. [Google Scholar] [CrossRef]
- Thomsen, J.; Gätke, M. Patient Information Sheet for Plasma Cholinesterase Deficiency. Anaesthesia 2013, 69, 1287–1288. [Google Scholar] [CrossRef] [PubMed]
- Moreta, M.; Burgos-Alonso, N.; Torrecilla, M.; Marco-Contelles, J.; Bruzos-Cidón, C. Efficacy of Acetylcholinesterase Inhibitors on Cognitive Function in Alzheimer’s Disease: Review of Reviews. Biomedicines 2021, 9, 1689. [Google Scholar] [CrossRef]
- Rejc, L.; Gómez-Vallejo, V.; Joya, A.; Moreno, O.; Egimendia, A.; Castellnou, P.; Ríos-Anglada, X.; Cossío, U.; Baz, Z.; Passannante, R.; et al. Longitudinal Evaluation of a Novel BChE PET Tracer as an Early In Vivo Biomarker in the Brain of a Mouse Model for Alzheimer Disease. Theranostics 2021, 11, 6542–6559. [Google Scholar] [CrossRef]
- Wilcock, G.; Lilienfeld, S.; Gaens, E. Efficacy and Safety of Galantamine in Patients with Mild to Moderate Alzheimer’s Disease: Multicentre Randomised Controlled Trial. BMJ 2000, 321, 1445. [Google Scholar] [CrossRef]
- Potasiewicz, A.; Krawczyk, M.; Gzielo, K.; Popik, P.; Nikiforuk, A. Positive Allosteric Modulators of Alpha 7 Nicotinic Acetylcholine Receptors Enhance Procognitive Effects of Conventional Anti-Alzheimer Drugs in Scopolamine-Treated Rats. Behav. Brain Res. 2020, 385, 112547. [Google Scholar] [CrossRef]
- Kowal, N.; Ahring, P.; Liao, V.; Indurti, D.; Harvey, B.; O’Connor, S.; Chebib, M.; Olafsdottir, E.; Balle, T. Galantamine Is Not a Positive Allosteric Modulator of Human α4β2 or α7 Nicotinic Acetylcholine Receptors. Br. J. Pharmacol. 2018, 175, 2911–2925. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Rayess, Y.; Rizk, A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 1021. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Drużga, A.; Katarzyna, J.; Skonieczna-Żydecka, K. Antioxidant Potential of Curcumin—A Meta-Analysis of Randomized Clinical Trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef]
- Buhrmann, C.; Brockmueller, A.; Mueller, A.; Shayan, P.; Shakibaei, M. Curcumin Attenuates Environment-Derived Osteoarthritis by Sox9/NF-κB Signaling Axis. Int. J. Mol. Sci. 2021, 22, 7645. [Google Scholar] [CrossRef] [PubMed]
- Stavrakov, G.; Philipova, I.; Lukarski, A.; Atanasova, M.; Zheleva, D.; Zhivkova, Z.D.; Ivanov, S.; Atanasova, T.; Konstantinov, S.; Doytchinova, I. Galantamine-Curcumin Hybrids as Dual-Site Binding Acetylcholinesterase Inhibitors. Molecules 2020, 25, 3341. [Google Scholar] [CrossRef] [PubMed]
- Simeonova, R.; Zheleva, D.; Valkova, I.; Stavrakov, G.; Philipova, I.; Atanasova, M.; Doytchinova, I. A Novel Galantamine-Curcumin Hybrid as a Potential Multi-Target Agent Against Neurodegenerative Disorders. Molecules 2021, 26, 1865. [Google Scholar] [CrossRef] [PubMed]
- Mladenova, K.; Stavrakov, G.; Philipova, I.; Atanasova, M.; Petrova, S.; Doumanov, J.; Doytchinova, I. A Galantamine-Curcumin Hybrid Decreases the Cytotoxicity of Amyloid-Beta Peptide on SH-SY5Y Cells. Int. J. Mol. Sci. 2021, 22, 7592. [Google Scholar] [CrossRef] [PubMed]
- Simeonova, R.; Atanasova, M.; Stavrakov, G.; Philipova, I.; Doytchinova, I. Ex Vivo Antioxidant and Cholinesterase-Inhibiting Effects of a Novel Galantamine–Curcumin Hybrid on Scopolamine-Induced Neurotoxicity in Mice. Int. J. Mol. Sci. 2022, 23, 14843. [Google Scholar] [CrossRef] [PubMed]
- Kostadinova, I.; Atanasova, M.; Stavrakov, G.; Philipova, I.; Doytchinova, I. A Galantamine-Curcumin Hybrid Lacks the Depressant Side Effect of Acetylcholinesterase Inhibitors. Biotechnol. Biotechnol. Equip. 2024, 38, 2305903. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 Update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Zheng, B.; McClements, D.J. Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef] [PubMed]
- Meden, A.; Knez, D.; Jukič, M.; Brazzolotto, X.; Gršič, M.; Pišlar, A.; Zahirović, A.; Kos, J.; Nachon, F.; Svete, J.; et al. Tryptophan-Derived Butyrylcholinesterase Inhibitors as Promising Leads Against Alzheimer’s Disease. Chem. Commun. 2019, 55, 3765–3768. [Google Scholar] [CrossRef]
- Case, D.A.; Aktulga, H.M.; Belfon, K.; Cerutti, D.S.; Cisneros, G.A.; Cruzeiro, V.W.D.; Forouzesh, N.; Giese, T.J.; Götz, A.W.; Gohlke, H.; et al. AmberTools. J. Chem. Inf. Model. 2023, 63, 6183–6191. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General AMBER Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Kagami, L.; Wilter, A.; Diaz, A.; Vranken, W. The ACPYPE Web Server for Small-Molecule MD Topology Generation. Bioinformatics 2023, 39, btad350. [Google Scholar] [CrossRef] [PubMed]
- Roe, D.R.; Cheatham, T.E. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and Applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Tanner, S.W.; Thompson, N.; Cheatham, T.E. Clustering Molecular Dynamics Trajectories: 1. Characterizing the Performance of Different Clustering Algorithms. J. Chem. Theory Comput. 2007, 3, 2312–2334. [Google Scholar] [CrossRef]
- Xing, S.; Li, Q.; Xiong, B.; Chen, Y.; Feng, F.; Liu, W.; Sun, H. Structure and Therapeutic Uses of Butyrylcholinesterase: Application in Detoxification, Alzheimer’s Disease, and Fat Metabolism. Med. Res. Rev. 2020, 41, 858–901. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.M.; Potkin, S.G.; Enz, A. Targeting Acetylcholinesterase and Butyrylcholinesterase in Dementia. Int. J. Neuropsychopharmacol. 2006, 9, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E. Cholinesterases: New Roles in Brain Function and in Alzheimer’s Disease. Neurochem. Res. 2003, 28, 515–522. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent Advances in Alzheimer’s Disease: Mechanisms, Clinical Trials, and New Drug Development Strategies. Sig. Transduct. Target Ther. 2024, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Darvesh, S. Butyrylcholinesterase as a Diagnostic and Therapeutic Target for Alzheimer’s Disease. Curr. Alzheimer Res. 2016, 13, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhen, T.; Harakandi, C.H.; Wang, L.; Guo, H.; Chen, Y.; Sun, H. New Insights into Butyrylcholinesterase: Pharmaceutical Applications, Selective Inhibitors and Multitarget-Directed Ligands. Eur. J. Med. Chem. 2024, 275, 116569. [Google Scholar] [CrossRef]
- Saxena, A.; Redman, A.; Jiang, X.; Lockridge, O.; Doctor, B. Differences in active-site gorge dimensions of cholinesterases revealed by binding of inhibitors to human butyrylcholinesterase. Chem. Biol. Interact. 1999, 119–120, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Rosenberry, T.; Brazzolotto, X.; Macdonald, I.; Wandhammer, M.; Trovaslet-Leroy, M.; Darvesh, S.; Nachon, F. Comparison of the binding of reversible inhibitors to human butyrylcholinesterase and acetylcholinesterase: A crystallographic, kinetic, and calorimetric study. Molecules 2017, 22, 2098. [Google Scholar] [CrossRef] [PubMed]
- Simeon-Rudolf, V.; Kovarik, Z.; Radić, Z.; Reiner, E. Reversible inhibition of acetylcholinesterase and butyrylcholinesterase by 4,4′-bipyridine and by a coumarin derivative. Chem. Biol. Interact. 1999, 119–120, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Kharlamova, A.; Lushchekina, S.; Petrov, K.; Kots, E.; Nachon, F.; Villard-Wandhammer, M.; Zueva, I.; Krejci, E.; Reznik, V.; Zobov, V.; et al. Slow-binding inhibition of acetylcholinesterase by an alkylammonium derivative of 6-methyluracil: Mechanism and possible advantages for myasthenia gravis treatment. Biochem. J. 2016, 473, 1225–1236. [Google Scholar] [CrossRef]
- Alvarez, A.; Opazo, C.; Alarcon, R.; Garrido, J.; Inestrosa, N.C. Acetylcholinesterase promotes the aggregation of amyloid-beta-peptide fragments by forming a complex with the growing fibrils. J. Mol. Biol. 1997, 272, 348–361. [Google Scholar] [CrossRef]
- Diamant, S.; Podoly, E.; Friedler, A.; Ligumsky, H.; Livnah, O.; Soreq, H. Butyrylcholinesterase Attenuates Amyloid Fibril Formation In Vitro. Proc. Natl. Acad. Sci. USA 2006, 103, 8628–8633. [Google Scholar] [CrossRef]
- Camps, P.; Formosa, X.; Galdeano, C.; Gómez, T.; Muñoz-Torrero, D.; Ramírez, L.; Viayna, E.; Gómez, E.; Isambert, N.; Lavilla, R.; et al. Tacrine-Based Dual Binding Site Acetylcholinesterase Inhibitors as Potential Disease-Modifying Anti-Alzheimer Drug Candidates. Chem. Biol. Interact. 2010, 187, 411–415. [Google Scholar] [CrossRef]
- Gupta, S.; Mohan, C.G. Dual Binding Site and Selective Acetylcholinesterase Inhibitors Derived from Integrated Pharmacophore Models and Sequential Virtual Screening. Biomed Res. Int. 2014, 2014, 291214. [Google Scholar] [CrossRef]
- Chierrito, T.P.C.; Pedersoli-Mantoani, S.; Roca, C.; Requena, C.; Sebastian-Perez, V.; Castillo, W.O.; Moreira, N.C.S.; Pérez, C.; Sakamoto-Hojo, E.T.; Takahashi, C.S.; et al. From Dual Binding Site Acetylcholinesterase Inhibitors to Allosteric Modulators: A New Avenue for Disease-Modifying Drugs in Alzheimer’s Disease. Eur. J. Med. Chem. 2017, 139, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; Catto, M.; De Palma, A.; Farina, R.; Cellamare, S.; Altomare, C.D. Discovery of Potent Dual Binding Site Acetylcholinesterase Inhibitors via Homo- and Heterodimerization of Coumarin-Based Moieties. ChemMedChem 2017, 12, 1349. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, W.; Wang, Y.; Zhang, R.; Hou, L.; Xu, J.; Qiu, Z.; Xie, Q.; Chen, H.; Zhang, Y. Bis(9)-(−)-Meptazinol, a Novel Dual-Binding AChE Inhibitor, Rescues Cognitive Deficits and Pathological Changes in APP/PS1 Transgenic Mice. Transl. Neurodegener. 2018, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Zueva, I.; Dias, J.; Lushchekina, S.; Semenov, V.; Mukhamedyarov, M.; Pashirova, T.; Babaev, V.; Nachon, F.; Petrova, N.; Nurullin, L.; et al. New Evidence for Dual Binding Site Inhibitors of Acetylcholinesterase as Improved Drugs for Treatment of Alzheimer’s Disease. Neuropharmacology 2019, 155, 131–141. [Google Scholar] [CrossRef]
- Suwanhom, P.; Nualnoi, T.; Khongkow, P.; Tipmanee, V.; Lomlim, L. Novel Lawsone–Quinoxaline Hybrids as New Dual Binding Site Acetylcholinesterase Inhibitors. ACS Omega 2023, 8, 32498–32511. [Google Scholar] [CrossRef] [PubMed]
- Bajda, M.; Wieckowska, A.; Hebda, M.; Szałaj, N.; Sotriffer, C.; Malawska, B. Structure-Based Search for New Inhibitors of Cholinesterases. Int. J. Mol. Sci. 2013, 14, 5608–5632. [Google Scholar] [CrossRef]
- Hamuľaková, S.; Janovec, L.; Soukup, O.; Jun, D.; Janocková, J.; Hrabinová, M.; Sepsová, V.; Kuča, K. Tacrine-coumarin and Tacrine-7-chloroquinoline Hybrids with Thiourea Linkers: Cholinesterase Inhibition Properties, Kinetic Study, Molecular Docking, and Permeability Assay for Blood–Brain Barrier. Curr. Alzheimer Res. 2018, 15, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhu, K.; Zhang, J.; Song, J.; Muehlmann, L.; Jiang, C.; Liu, C.; Zhang, H. Molecular-Docking-Guided Design and Synthesis of New IAA-Tacrine Hybrids as Multifunctional AChE/BChE Inhibitors. Bioorg. Chem. 2019, 83, 277–288. [Google Scholar] [CrossRef]
- Elkina, N.; Grishchenko, M.; Shchegolkov, E.; Makhaeva, G.; Kovaleva, N.; Rudakova, E.; Boltneva, N.; Lushchekina, S.; Astakhova, T.; Radchenko, E.; et al. New Multifunctional Agents for Potential Alzheimer’s Disease Treatment Based on Tacrine Conjugates with 2-Arylhydrazinylidene-1,3-Diketones. Biomolecules 2022, 12, 1551. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Peng, D.; Yang, S.; Zhu, X.; Yang, W.; Yang, G. Syntheses of Coumarin-Tacrine Hybrids as Dual-Site Acetylcholinesterase Inhibitors and Their Activity Against Butyrylcholinesterase, Aβ Aggregation, and β-Secretase. Bioorg. Med. Chem. 2014, 22, 4784–4791. [Google Scholar] [CrossRef] [PubMed]
- Makhaeva, G.; Kovaleva, N.; Rudakova, E.; Boltneva, N.; Grishchenko, M.; Lushchekina, S.; Astakhova, T.; Serebryakova, O.; Timokhina, E.; Zhilina, E.; et al. Conjugates of Tacrine and Salicylic Acid Derivatives as New Promising Multitarget Agents for Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 2285. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamanova, E.; Atanasova, M.; Doytchinova, I. A Novel Galantamine–Curcumin Hybrid Inhibits Butyrylcholinesterase: A Molecular Dynamics Study. Chemistry 2024, 6, 1645-1657. https://doi.org/10.3390/chemistry6060100
Salamanova E, Atanasova M, Doytchinova I. A Novel Galantamine–Curcumin Hybrid Inhibits Butyrylcholinesterase: A Molecular Dynamics Study. Chemistry. 2024; 6(6):1645-1657. https://doi.org/10.3390/chemistry6060100
Chicago/Turabian StyleSalamanova, Evdokiya, Mariyana Atanasova, and Irini Doytchinova. 2024. "A Novel Galantamine–Curcumin Hybrid Inhibits Butyrylcholinesterase: A Molecular Dynamics Study" Chemistry 6, no. 6: 1645-1657. https://doi.org/10.3390/chemistry6060100
APA StyleSalamanova, E., Atanasova, M., & Doytchinova, I. (2024). A Novel Galantamine–Curcumin Hybrid Inhibits Butyrylcholinesterase: A Molecular Dynamics Study. Chemistry, 6(6), 1645-1657. https://doi.org/10.3390/chemistry6060100






