Alkoxy Substituted Brominated closo-Dodecaborates with Functionalized Aliphatic Spacers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Materials
2.3. Experimental Procedures
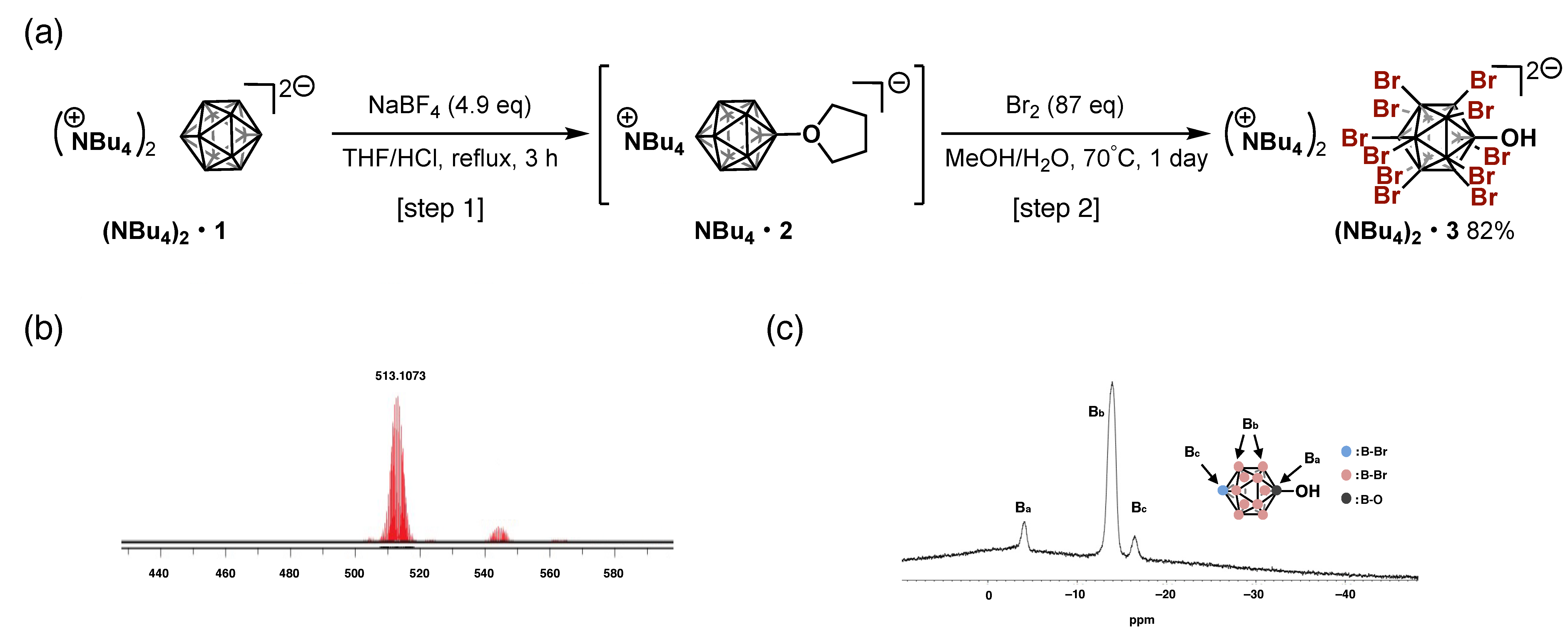
2.3.1. Procedure for One-Pot Synthesis of [NBu4+]2[B12Br11OH2−]
Compound: [NBu4+]2[B12Br11OH2−] ((NBu4)2•3)
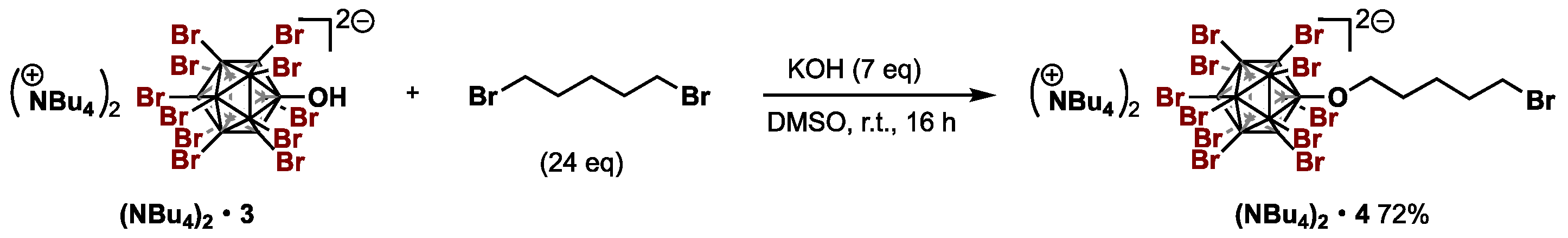
2.3.2. Procedure for the Alkoxylation of [B12Br11OH2−]
Compound: [NBu4+]2[B12Br11O(CH2)4Br2−] ((NBu4)2•4)
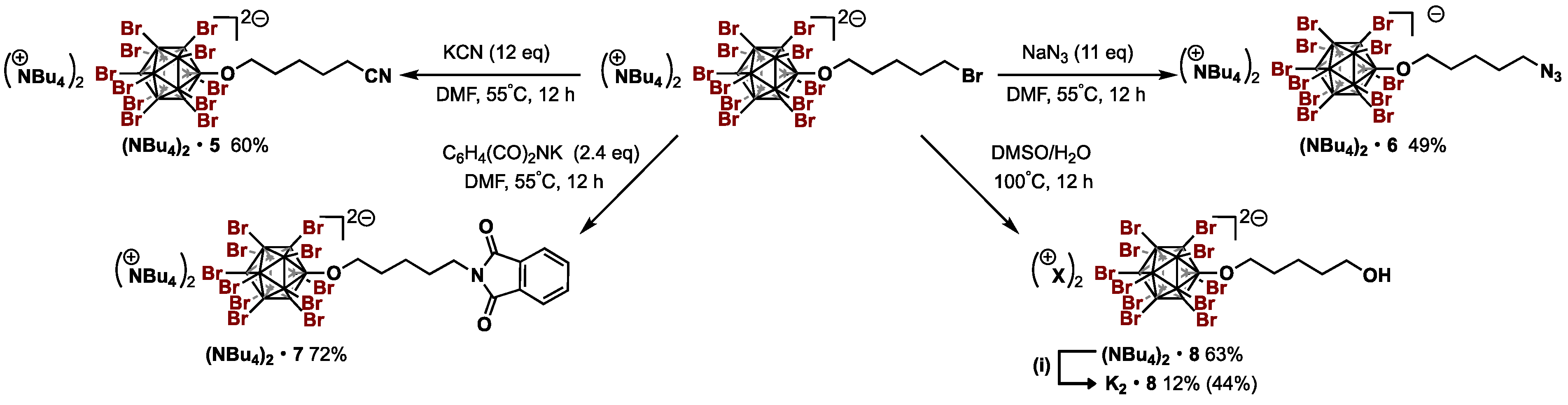
2.3.3. General Procedure for the Synthesis of [NBu4+]2[B12Br11O(CH2)5X2−] (X = CN, N3, NC6H4(CO)2)
Compound: [NBu4+]2[B12Br11O(CH2)4CN2−] ((NBu4)2•5)
Compound: [NBu4+]2[B12Br11O(CH2)4N32−] ((NBu4)2•6)
Compound: [NBu4+]2[B12Br11O(CH2)4NC6H4(CO)22−] ((NBu4)2•7)
2.3.4. Procedure for the Hydroxylation of Terminal Bromine of (NBu4)2•4
Compound: [NBu4+]2[B12Br11O(CH2)4OH2−] ((NBu4)2•8)
2.3.5. Procedure for the Cation Exchange from NBu4+ to K+
Compound: [K+]2[B12Br11O(CH2)4OH2−] ((K)2•8)
2.4. Materials and Methods for Cell Viability Assay
2.4.1. Cell Culture and Imaging
2.4.2. Cell Viability Assay
3. Results
3.1. Preparation of [B12Br11OH]2−
3.2. Preparation of [B12Br11OH]2−
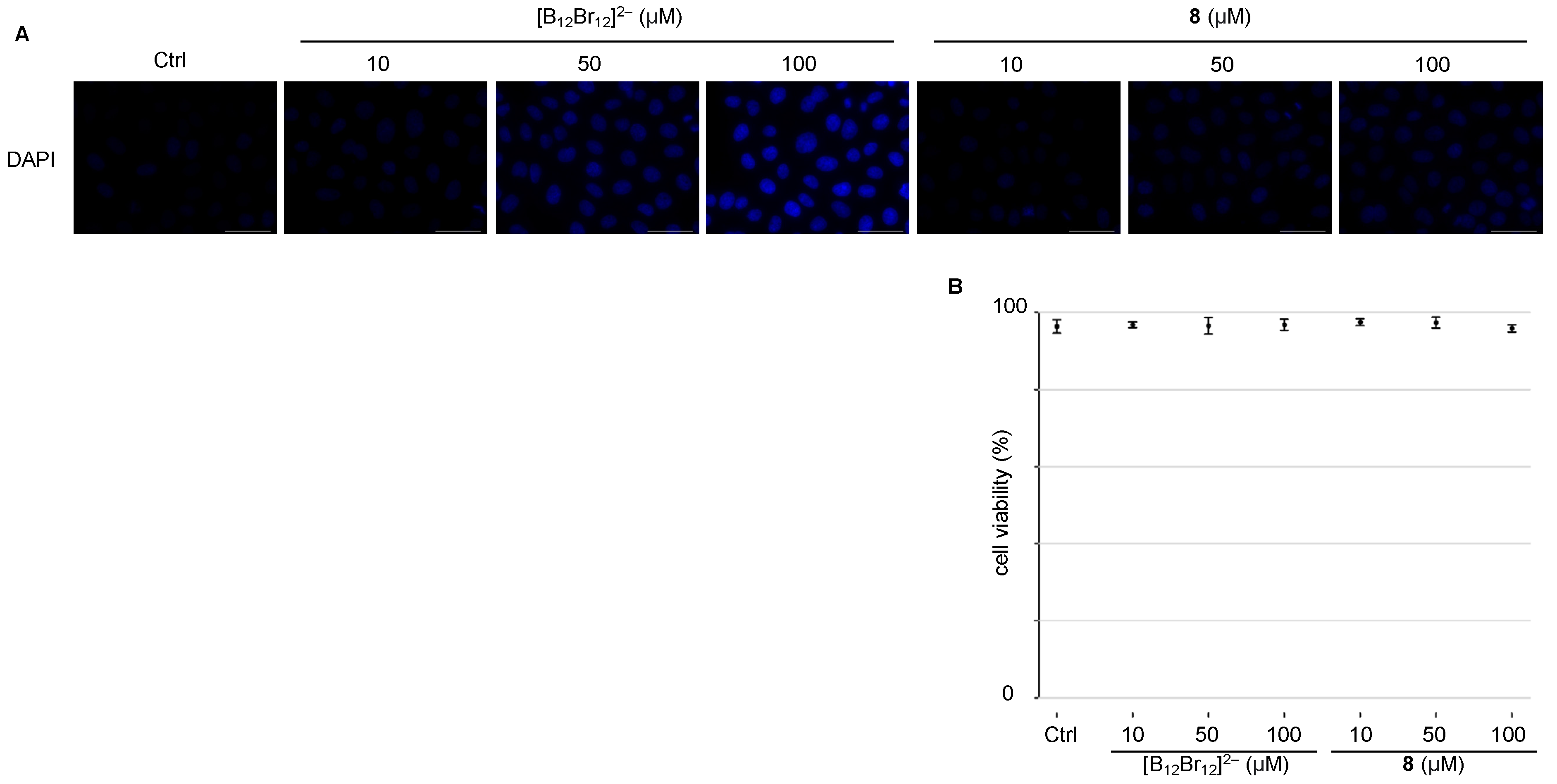
3.3. Cell Viability Assay and Membrane Translocation in Living Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sivaev, I.B.; Bregadze, V.I.; Sjöberg, S. Chemistry of closo-Dodecaborate Anion [B12H12]2-: A Review. Collect. Czech. Chem. Commun. 2002, 67, 679–727. [Google Scholar] [CrossRef]
- Axtell, J.C.; Saleh, L.M.A.; Qian, E.A.; Wixtrom, A.I.; Spokoyny, A.M. Synthesis and Applications of Perfunctionalized Boron Clusters. Inorg. Chem. 2018, 57, 2330–2350. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, Y.; Duttwyler, S.; Lin, F.; Zhang, Y. Chemistry of Three-Dimensional Icosahedral Boron Clusters Anions: Closo-Dodecaborate (2-) [B12H12]2- and Carba-closo-Dodecaborate(-) [CB11H12]-. Coord. Chem. Rev. 2024, 516, 215974. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Z.; Chen, H.; Wang, Z.; Zhou, X.; Zhang, H. Progress in Three-Dimensional Aromatic-like closo-Dodecaborate. Coord. Chem. Rev. 2021, 444, 214042. [Google Scholar] [CrossRef]
- Assaf, K.I.; Ural, M.S.; Pan, F.; Georgiev, T.; Simova, S.; Rissanen, K.; Gabel, D.; Nau, W.M. Water Structure Recovery in Chaotropic Anion Recognition: High-Affinity Binding of Dodecaborate Clusters to γ-Cyclodextrin. Angew. Chem. Int. Ed. 2015, 54, 6852–6856. [Google Scholar] [CrossRef] [PubMed]
- Barba-Bon, A.; Salluce, G.; Lostalé-Seijo, I.; Assaf Khaleel, I.; Hennig, A.; Montenegro, J.; Nau, W.M. Boron Clusters as Broadband Membrane Carriers. Nature 2022, 603, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Guo, D. Superchaotropic Boron Clusters as Membrane Carriers for the Transport of Hydrophilic Cargos. Angew. Chem. Int. Ed. 2022, 61, e202204979. [Google Scholar] [CrossRef]
- Chen, Y.; Barba-Bon, A.; Grüner, B.; Winterhalter, M.; Aksoyoglu, M.A.; Pangeni, S.; Ashjari, M.; Brix, K.; Salluce, G.; Folgar-Cameán, Y.; et al. Metallacarborane Cluster Anions of the Cobalt Bisdicarbollide-Type as Chaotropic Carriers for Transmembrane and Intracellular Delivery of Cationic Peptides. J. Am. Chem. Soc. 2023, 145, 13089–13098. [Google Scholar] [CrossRef] [PubMed]
- Hirai, Y.; Makita, Y.; Asaoka, J.; Aoyagi, Y.; Nomoto, A.; Okamura, H.; Fujiwara, S. Boron Clusters Alter the Membrane Permeability of Dicationic Fluorescent DNA-Staining Dyes. ACS Omega 2023, 8, 35321–35327. [Google Scholar] [CrossRef]
- Salluce, G.; Folgar-Cameán, Y.; Barba-Bon, A.; Nikšić-Franjić, I.; El Anwar, S.; Grüner, B.; Lostalé-Seijo, I.; Nau, W.M.; Montenegro, J. Size and Polarizability of Boron Cluster Carriers Modulate Chaotropic Membrane Transport. Angew. Chem. Int. Ed. 2024, 63, e202404286. [Google Scholar] [CrossRef]
- Barba-Bon, A.; El Haitami, A.; Pasquier, C.; Nikšić-Franjić, I.; Diat, O.; Bauduin, P.; Cantin, S.; Nau, W.M. Boron Cluster Anions Dissolve En Masse in Lipids Causing Membrane Expansion and Thinning. Angew. Chem. 2024, 2024, e202412834. [Google Scholar]
- Ma, X.; Zhang, Z.; Barba-Bon, A.; Han, D.; Qi, Z.; Ge, B.; He, H.; Huang, F.; Nau, W.M.; Wang, X. A Small-Molecule Carrier for the Intracellular Delivery of a Membrane-Impermeable Protein with Retained Bioactivity. Proc. Natl. Acad. Sci. USA 2024, 121, e2407515121. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Yamamoto, S.; Kawamura, Y.K.; Kitazawa, T.; Kimura, M.; Kitazawa, Y. Enhancing Membrane Permeability of Fluorescein-Type Chromophore Through Covalent Attachment of Chlorinated Dodecaborate. Molecules 2024, 29, 5416. [Google Scholar] [CrossRef] [PubMed]
- Jenne, C.; Kirsch, C. Alkoxy Substituted Halogenated closo-Dodecaborates as Anions for Ionic Liquids. Dalton Trans. 2015, 44, 13119–13124. [Google Scholar] [CrossRef]
- Marei, T.; Al-Joumhawy, M.K.; Alnajjar, M.A.; Nau, W.M.; Assaf, K.I.; Gabel, D. Binding Affinity of Aniline-Substituted Dodecaborates to Cyclodextrins. Chem. Commun. 2022, 58, 2363–2366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gabel, D.; Assaf, K.I.; Nau, W.M. A Fluorescein-Substituted Perbrominated Dodecaborate Cluster as an Anchor Dye for Large Macrocyclic Hosts and Its Application in Indicator Displacement Assays. Org. Lett. 2022, 24, 9184–9188. [Google Scholar] [CrossRef]
- Yamamoto, S.; Nakamura, H.; Kawamura, Y.K.; Kitazawa, T.; Kimura, M.; Kitazawa, Y. Alkoxy Substituted Brominated closo-Dodecaborates with Functionalized Aliphatic Spacers. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Rasband, W.S.; ImageJ, U.S. National Institutes of Health: Bethesda, MD, USA, 1997–2018. Available online: https://imagej.net/ij/ (accessed on 15 January 2024).
- Peymann, T.; Knobler, C.B.; Hawthorne, M.F. A Study of the Sequential Acid-Catalyzed Hydroxylation of Dodecahydro-closo-Dodecaborate(2−). Inorg. Chem. 2000, 39, 1163–1170. [Google Scholar] [CrossRef]
- Al-Joumhawy, M.; Cendoya, P.; Shmalko, A.; Marei, T.; Gabel, D. Improved Synthesis of Halo- and Oxonium Derivatives of Dodecahydrido-closo-Dodecaborate(2-). J. Organomet. Chem. 2021, 949, 121967. [Google Scholar] [CrossRef]
- Nelson, Y.A.; Irshad, A.; Kim, S.; Waddington, M.A.; Salamat, C.Z.; Gembicky, M.; Rheingold, A.L.; Carta, V.; Tolbert, S.H.; Narayan, S.R.; et al. Vertex Differentiation Strategy for Tuning the Physical Properties of closo-Dodecaborate Weakly Coordinating Anions. Inorg. Chem. 2023, 62, 15084–15093. [Google Scholar] [CrossRef] [PubMed]
- Bakardjiev, M.; El Anwar, S.; Bavol, D.; Růžičková, Z.; Grűner, B. Focus on Chemistry of the 10-Dioxane-Nido-7,8-Dicarba-Undecahydrido Undecaborate Zwitterion; Exceptionally Easy Abstraction of Hydrogen Bridge and Double-Action Pathways Observed in Ring Cleavage Reactions with OH− as Nucleophile. Molecules 2020, 25, 814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Duttwyler, S. Synthesis and Structural Characterization of Ammonio/Hydroxo Undecachloro-closo-Dodecaborates [B12Cl11NH3]−/[B12Cl11OH]2− and Their Derivatives. Eur. J. Inorg. Chem. 2015, 2015, 5158–5162. [Google Scholar] [CrossRef]
- Arseneault, M.; Wafer, C.; Morin, J.-F. Recent Advances in Click Chemistry Applied to Dendrimer Synthesis. Molecules 2015, 20, 9263–9294. [Google Scholar] [CrossRef]
- Hawker, C.J.; Frechet, J.M.J. Preparation of Polymers with Controlled Molecular Architecture. A New Convergent Approach to Dendritic Macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Chougala, B.M.; Samundeeswari, S.; Holiyachi, M.; Shastri, L.A. Mild, Efficient and Catalyst-Free Hydroxylation of Alkyl Halides in Water: Significant Enhancement of Water Nucleophilicity in Dipolar Solvents. ChemistrySelect 2017, 2, 1290–1296. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, S.; Nakamura, H.; Kawamura, Y.K.; Kitazawa, T.; Kimura, M.; Kitazawa, Y. Alkoxy Substituted Brominated closo-Dodecaborates with Functionalized Aliphatic Spacers. Chemistry 2024, 6, 1635-1644. https://doi.org/10.3390/chemistry6060099
Yamamoto S, Nakamura H, Kawamura YK, Kitazawa T, Kimura M, Kitazawa Y. Alkoxy Substituted Brominated closo-Dodecaborates with Functionalized Aliphatic Spacers. Chemistry. 2024; 6(6):1635-1644. https://doi.org/10.3390/chemistry6060099
Chicago/Turabian StyleYamamoto, Satoshi, Hibiki Nakamura, Yumiko K. Kawamura, Taro Kitazawa, Mutsumi Kimura, and Yu Kitazawa. 2024. "Alkoxy Substituted Brominated closo-Dodecaborates with Functionalized Aliphatic Spacers" Chemistry 6, no. 6: 1635-1644. https://doi.org/10.3390/chemistry6060099
APA StyleYamamoto, S., Nakamura, H., Kawamura, Y. K., Kitazawa, T., Kimura, M., & Kitazawa, Y. (2024). Alkoxy Substituted Brominated closo-Dodecaborates with Functionalized Aliphatic Spacers. Chemistry, 6(6), 1635-1644. https://doi.org/10.3390/chemistry6060099







