Ethosomes: A Promising Drug Delivery Platform for Transdermal Application
Abstract
1. Introduction
2. The Type of the Ethosomes
2.1. Classical Ethosomes
2.2. Binary Ethosomes
2.3. Transethosomes (TEs)
2.4. Composite Phospholipid Ethosomes (CE)
2.5. Actively Targeted Ethosomes
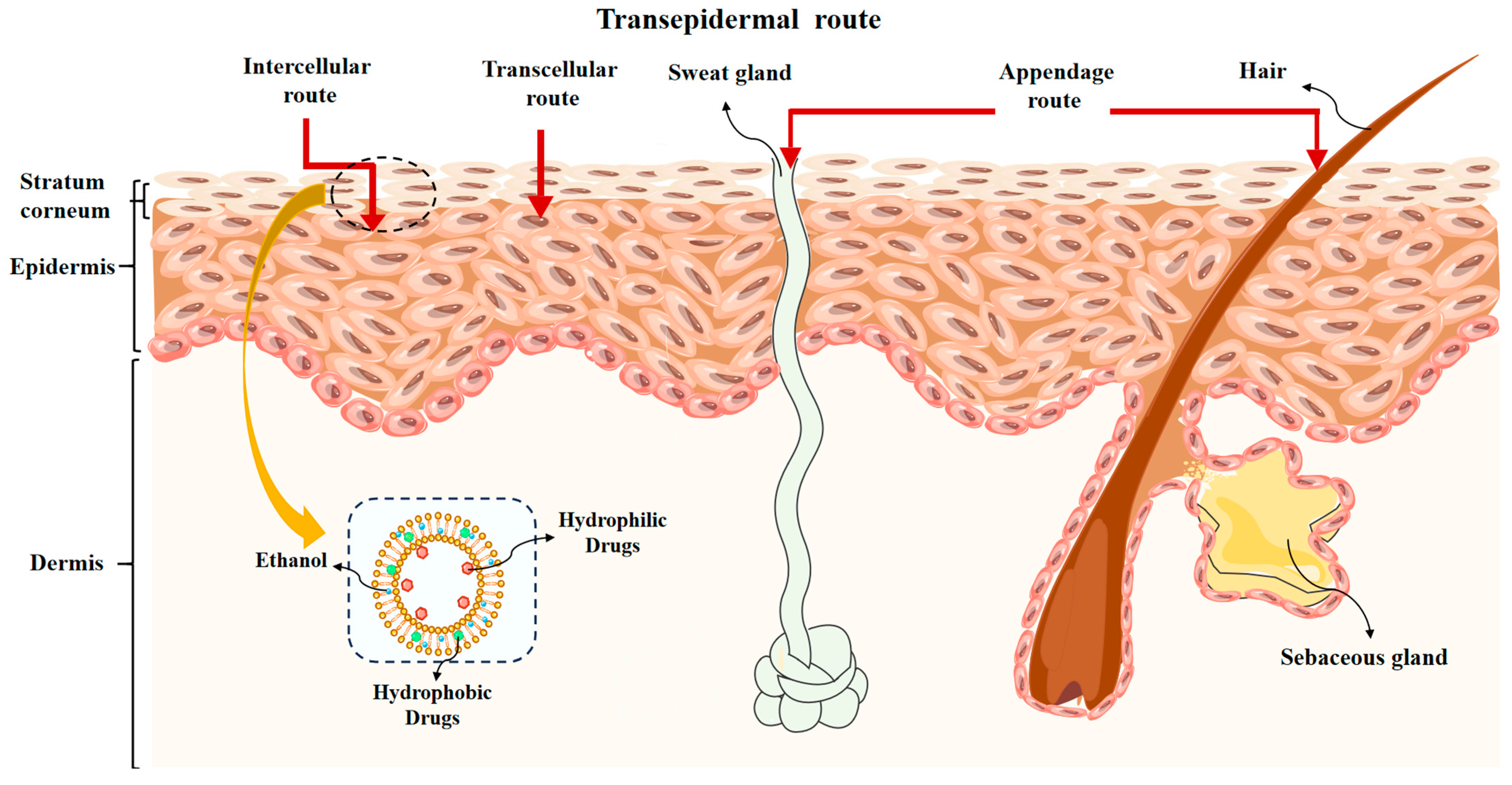
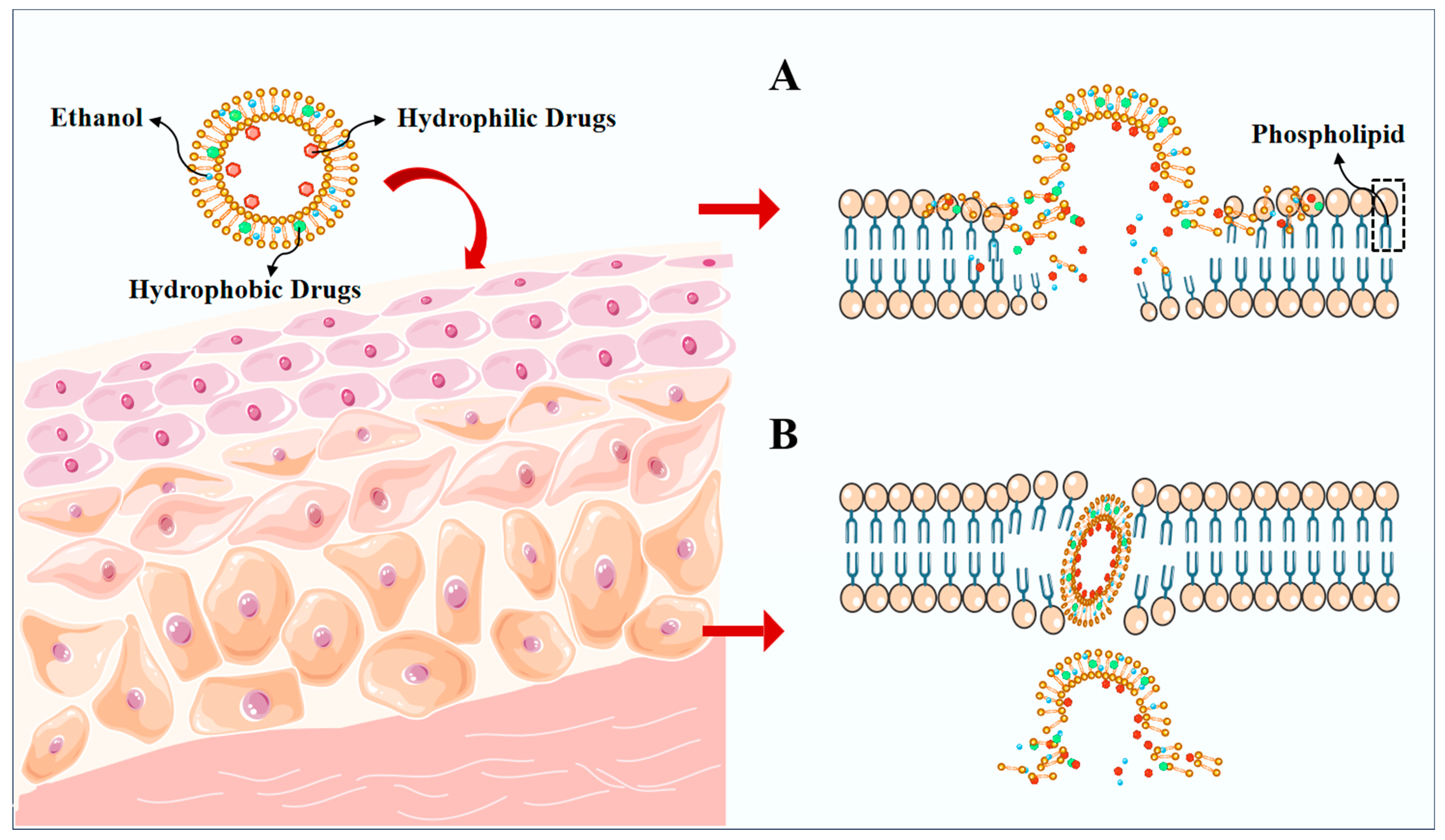
3. Mechanisms for Penetration of Ethosomes
4. Methods of Ethosomal Penetration Mechanisms
4.1. ATR-FTIR and Raman Spectroscopy
4.2. DSC
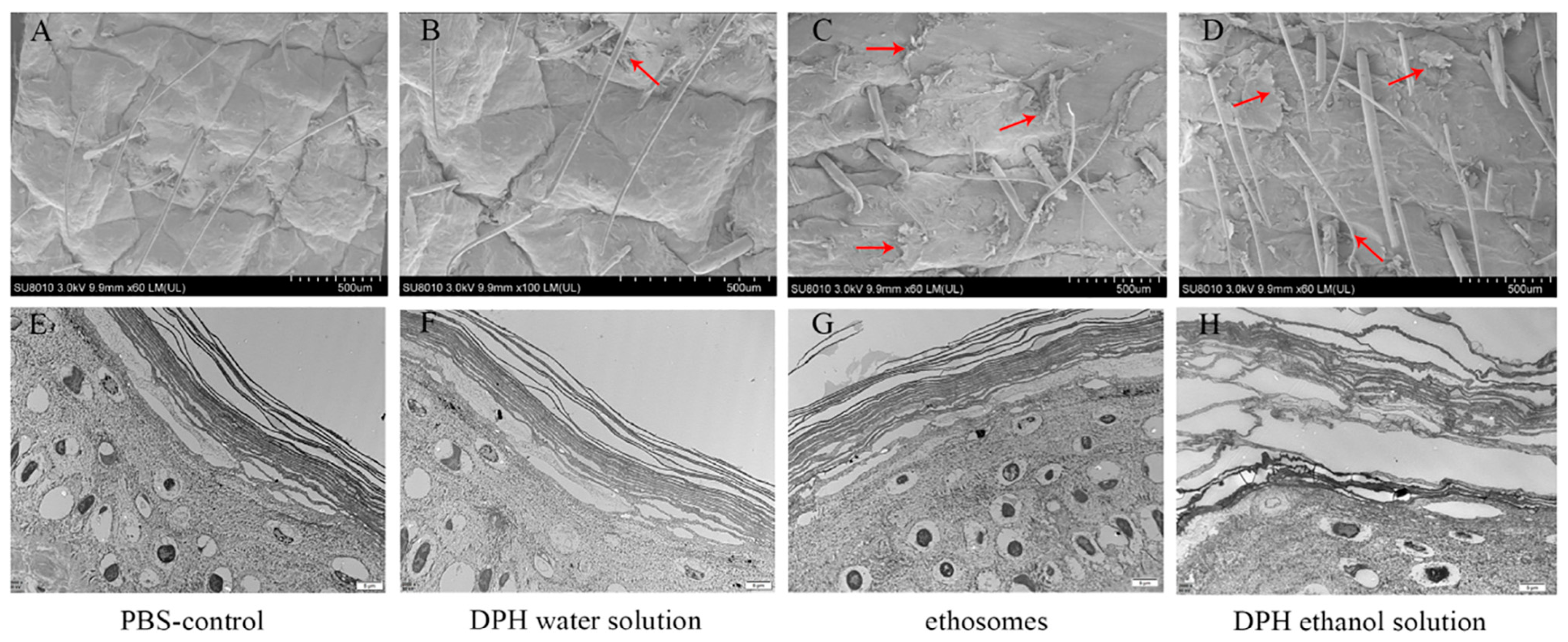
4.3. SEM and TEM
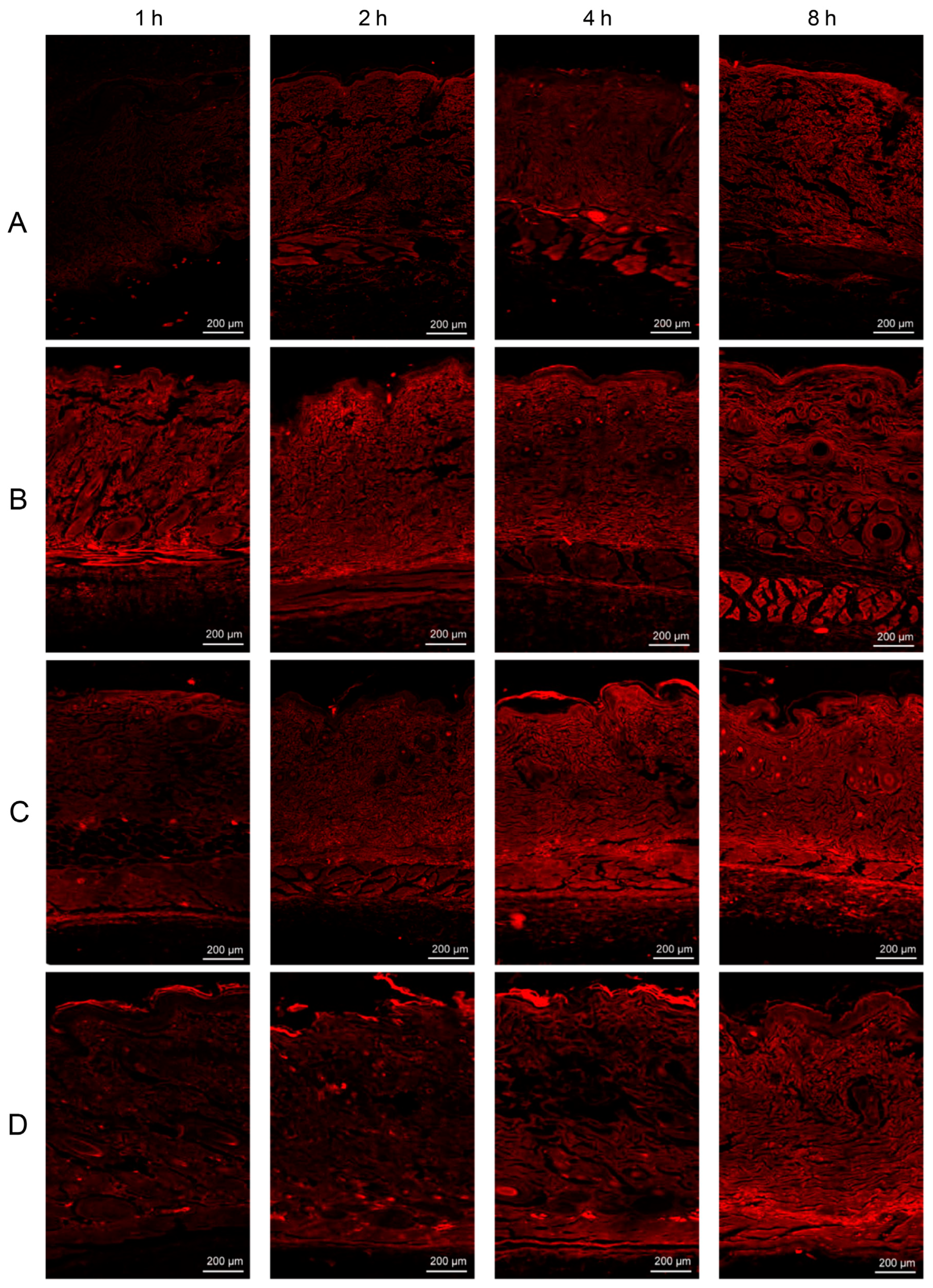
4.4. CLSM
5. The Method of Preparation of Ethosomes
5.1. Thin Film Dispersion Method
5.2. Ethanol Injection Method
5.3. Injection–Ultrasound Combination Method
5.4. pH Gradient Method
5.5. Microfluidic Techniques
6. Factors Affecting Properties of Ethosomes
6.1. Effect of Ethanol
6.2. Effect of Phospholipids
6.3. Effect of Propylene Glycol
6.4. Effect of Cholesterol
6.5. Effect of Edge Activators
7. Characterization of the Ethosomes
8. Different Applications of Ethosomes
8.1. Delivery of Antifungal and Antibacterial Drug
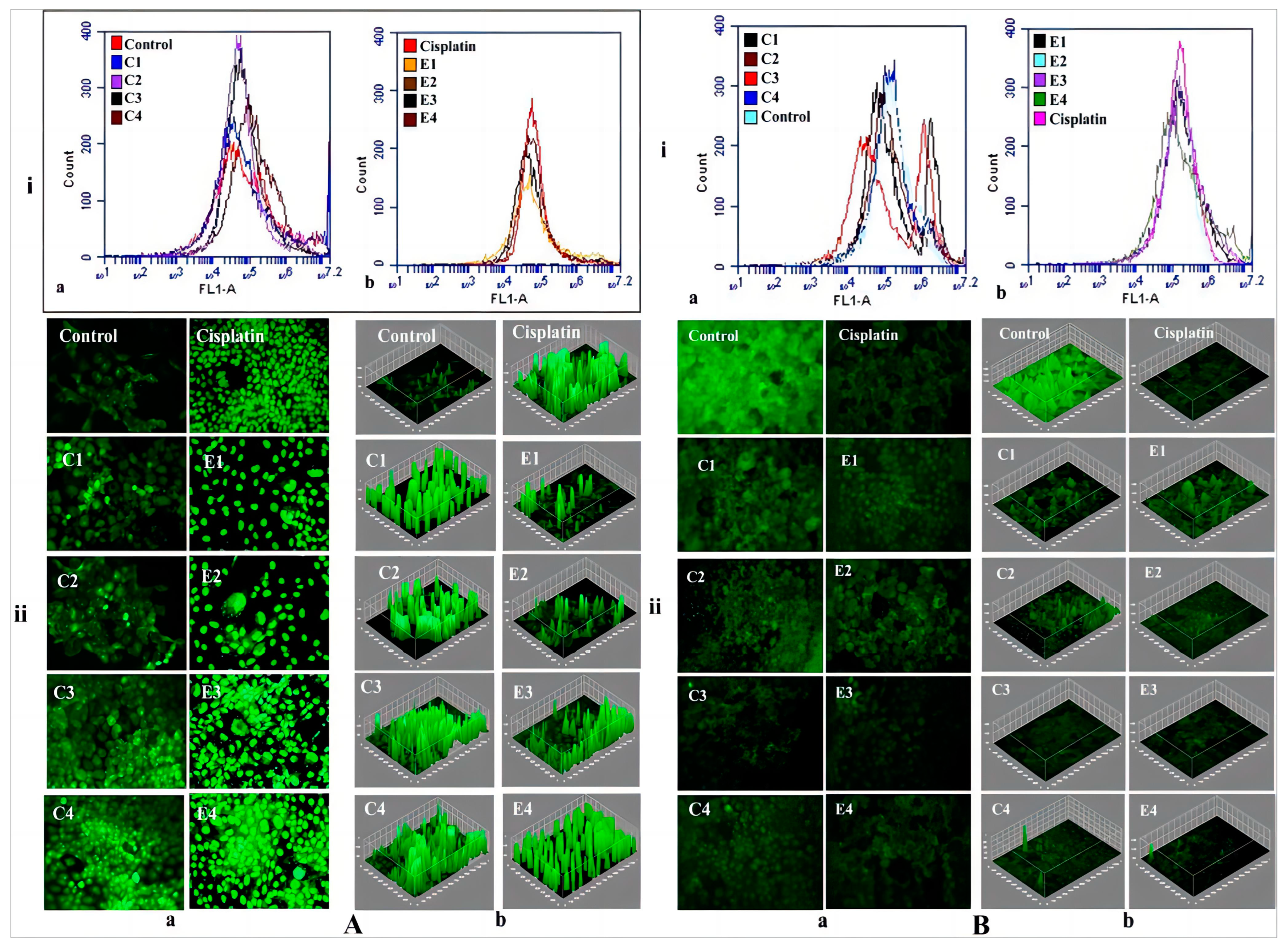
8.2. Delivery of Anticancer Drug
8.3. Delivery of Anti-Psoriasis Drug
8.4. Delivery of Anti-Hypertrophic Scar (HS) Drug
8.5. Delivering Drugs for Chronic Diseases
8.6. Delivery of Other Drug from Ethosomal Systems
9. Discussion and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jafari, A.; Daneshamouz, S.; Ghasemiyeh, P.; Mohammadi-Samani, S. Ethosomes as dermal/transdermal drug delivery systems: Applications, preparation and characterization. J. Liposome Res. 2023, 33, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Sguizzato, M.; Ferrara, F.; Hallan, S.S.; Baldisserotto, A.; Drechsler, M.; Malatesta, M.; Costanzo, M.; Cortesi, R.; Puglia, C.; Valacchi, G.; et al. Ethosomes and Transethosomes for Mangiferin Transdermal Delivery. Antioxidants 2021, 10, 768. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.S.; Billa, N.; Leong, C.O.; Morris, A.P. An evaluation of tocotrienol ethosomes for transdermal delivery using Strat-M(®) membrane and excised human skin. Pharm. Dev. Technol. 2021, 26, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.; Singh, H.; Bimbrawh, S.; Singh, S.K.; Gulati, M.; Vaidya, Y.; Kaur, P. Ethosomes and Transfersomes: Principles, Perspectives and Practices. Curr. Drug Deliv. 2017, 14, 613–633. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Makky, A.M.; Teaima, M.H.; Abdellatif, M.M.; Hamzawy, M.A.; Khalil, M.A. Transdermal delivery of vancomycin hydrochloride using combination of nano-ethosomes and iontophoresis: In vitro and in vivo study. Drug Deliv. 2016, 23, 1558–1564. [Google Scholar] [PubMed]
- Huang, M.; Liu, J.; Fan, Y.; Sun, J.; Cheng, J.X.; Zhang, X.F.; Zhai, B.T.; Guo, D.Y. Development of curcumin-loaded galactosylated chitosan-coated nanoparticles for targeted delivery of hepatocellular carcinoma. Int. J. Biol. Macromol. 2023, 253, 127219. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhai, B.; Fan, Y.; Sun, J.; Cheng, J.; Zou, J.; Zhang, X.; Shi, Y.; Guo, D. Development of Paeonol Liposomes: Design, Optimization, in vitro and in vivo Evaluation. Int. J. Nanomed. 2022, 17, 5027–5046. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Lv, H.; Han, G.; Ma, K. Ethosomes Loaded with Cryptotanshinone for Acne Treatment through Topical Gel Formulation. PLoS ONE 2016, 11, e0159967. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Y.; Zhai, B.; Cheng, J.; Sun, J.; Zhang, X.; Guo, D. Phloretin Transfersomes for Transdermal Delivery: Design, Optimization, and In Vivo Evaluation. Molecules 2023, 28, 6790. [Google Scholar] [CrossRef]
- Carita, A.C.; Eloy, J.O.; Chorilli, M.; Lee, R.J.; Leonardi, G.R. Recent Advances and Perspectives in Liposomes for Cutaneous Drug Delivery. Curr. Med. Chem. 2018, 25, 606–635. [Google Scholar] [CrossRef]
- Lu, J.; Guo, T.; Fan, Y.; Li, Z.; He, Z.; Yin, S.; Feng, N. Recent Developments in the Principles, Modification and Application Prospects of Functionalized Ethosomes for Topical Delivery. Curr. Drug Deliv. 2021, 18, 570–582. [Google Scholar] [CrossRef]
- Dumitriu Buzia, O.; Păduraru, A.M.; Stefan, C.S.; Dinu, M.; Cocoș, D.I.; Nwabudike, L.C.; Tatu, A.L. Strategies for Improving Transdermal Administration: New Approaches to Controlled Drug Release. Pharmaceutics 2023, 15, 1183. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Y.H.; Zhang, G.Q.; Wu, X.A. Synergistic penetration of ethosomes and lipophilic prodrug on the transdermal delivery of acyclovir. Arch. Pharm. Res. 2010, 33, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Caberlotto, E.; Ruiz, L.; Miller, Z.; Poletti, M.; Tadlock, L. Effects of a skin-massaging device on the ex-vivo expression of human dermis proteins and in-vivo facial wrinkles. PLoS ONE 2017, 12, e0172624. [Google Scholar] [CrossRef]
- Sharon, A. Gynecare Morcellex Sigma(®): Manufacturer: ETHICON Women’s Health & Urology, A Division of ETHICON, INC., a Johnson & Johnson company, Somerville, NJ 08876-0151, USA, © ETHICON, INC. 2005. J. Obstet. Gynaecol. India 2014, 64, 226–227. [Google Scholar]
- Anderson, P. Assessment and development of executive function (EF) during childhood. Child Neuropsychol. 2002, 8, 71–82. [Google Scholar] [CrossRef]
- Zhang, J.P.; Wei, Y.H.; Zhou, Y.; Li, Y.Q.; Wu, X.A. Ethosomes, binary ethosomes and transfersomes of terbinafine hydrochloride: A comparative study. Arch. Pharm. Res. 2012, 35, 109–117. [Google Scholar] [CrossRef]
- Yucel, C.; Seker Karatoprak, G.; Degim, I.T. Anti-aging formulation of rosmarinic acid-loaded ethosomes and liposomes. J. Microencapsul. 2019, 36, 180–191. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Shen, L.N.; Wu, Z.H.; Zhao, J.H.; Feng, N.P. Comparison of ethosomes and liposomes for skin delivery of psoralen for psoriasis therapy. Int. J. Pharm. 2014, 471, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wei, Y.; Liu, H.; Zhang, G.; Wu, X. Preparation and in vitro evaluation of ethosomal total alkaloids of Sophora alopecuroides loaded by a transmembrane pH-gradient method. AAPS PharmSciTech 2010, 11, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Jiang, W.; Tan, M.; Yang, X.; He, C.; Huang, W.; Xing, J. Optimization of the process variables of tilianin-loaded composite phospholipid liposomes based on response surface-central composite design and pharmacokinetic study. Eur. J. Pharm. Sci. 2016, 85, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.M.; Abdallah, O.Y.; Naggar, V.F.; Khalafallah, N.M. Deformable liposomes and ethosomes: Mechanism of enhanced skin delivery. Int. J. Pharm. 2006, 322, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Verma, A.; Pathak, K. Feasibility of binary composition in development of nanoethosomal glycolic vesicles of triamcinolone acetonide using Box-behnken design: In vitro and ex vivo characterization. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Song, C.K.; Balakrishnan, P.; Shim, C.K.; Chung, S.J.; Chong, S.; Kim, D.D. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: Characterization and in vitro/in vivo evaluation. Colloids Surf. B Biointerfaces 2012, 92, 299–304. [Google Scholar] [CrossRef]
- Ascenso, A.; Raposo, S.; Batista, C.; Cardoso, P.; Mendes, T.; Praca, F.G.; Bentley, M.V.; Simoes, S. Development, characterization, and skin delivery studies of related ultradeformable vesicles: Transfersomes, ethosomes, and transethosomes. Int. J. Nanomed. 2015, 10, 5837–5851. [Google Scholar] [CrossRef] [PubMed]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Iqbal, B.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Development of transethosomes formulation for dermal fisetin delivery: Box-Behnken design, optimization, in vitro skin penetration, vesicles-skin interaction and dermatokinetic studies. Artif. Cells Nanomed. Biotechnol. 2018, 46, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Albash, R.; Abdelbary, A.A.; Refai, H.; El-Nabarawi, M.A. Use of transethosomes for enhancing the transdermal delivery of olmesartan medoxomil: In vitro, ex vivo, and in vivo evaluation. Int. J. Nanomed. 2019, 14, 1953–1968. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, L.; Wu, D.; Shi, D.; Wang, T.; Zhu, X. Mechanism of transdermal permeation promotion of lipophilic drugs by ethosomes. Int. J. Nanomed. 2017, 12, 3357–3364. [Google Scholar] [CrossRef]
- Li, Y.; Xu, F.; Li, X.; Chen, S.Y.; Huang, L.Y.; Bian, Y.Y.; Wang, J.; Shu, Y.T.; Yan, G.J.; Dong, J.; et al. Development of curcumin-loaded composite phospholipid ethosomes for enhanced skin permeability and vesicle stability. Int. J. Pharm. 2021, 592, 119936. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, X.; Wu, J.; Zhang, D.; Zhang, L.; Song, X.; Hong, H.; He, C.; Mo, X.; Wu, S.; et al. Polyethylenimine and sodium cholate-modified ethosomes complex as multidrug carrier for the treatment of melanoma through transdermal delivery. Nanomedicine 2019, 14, 2395–2408. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, Q.; Li, Y.; He, Z.; Li, Z.; Guo, T.; Wu, Z.; Feng, N. CD44 Assists the Topical Anti-Psoriatic Efficacy of Curcumin-Loaded Hyaluronan-Modified Ethosomes: A New Strategy for Clustering Drug in Inflammatory Skin. Theranostics 2019, 9, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Mishra, P.K.; Dubey, V.; Nahar, M.; Dabadghao, S.; Jain, N.K. Systemic and mucosal immune response induced by transcutaneous immunization using Hepatitis B surface antigen-loaded modified liposomes. Eur. J. Pharm. Sci. 2008, 33, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Rattanapak, T.; Young, K.; Rades, T.; Hook, S. Comparative study of liposomes, transfersomes, ethosomes and cubosomes for transcutaneous immunisation: Characterisation and in vitro skin penetration. J. Pharm. Pharmacol. 2012, 64, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, J.; Zhang, Y.; Cao, J.; Wang, X.; Chu, X. Study on the transdermal penetration mechanism of ibuprofen nanoemulsions. Drug Dev. Ind. Pharm. 2019, 45, 465–473. [Google Scholar]
- Champeau, M.; Vignoud, S.; Mortier, L.; Mordon, S. Photodynamic therapy for skin cancer: How to enhance drug penetration? J. Photochem. Photobiol. B Biol. 2019, 197, 111544. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, Y.; Le, G. [Antibacterial activity and mechanisms of a new peptide derived from cell-penetrating peptide]. Wei Sheng Wu Xue Bao = Acta Microbiol. Sin. 2013, 53, 950–956. [Google Scholar] [PubMed]
- Jia, Z.; Wu, A.; Tam, M.; Spain, J.; McKinney, J.M.; Wang, W. Caval Penetration by Inferior Vena Cava Filters: A Systematic Literature Review of Clinical Significance and Management. Circulation 2015, 132, 944–952. [Google Scholar] [CrossRef]
- Niu, X.Q.; Zhang, D.P.; Bian, Q.; Feng, X.F.; Li, H.; Rao, Y.F.; Shen, Y.M.; Geng, F.N.; Yuan, A.R.; Ying, X.Y.; et al. Mechanism investigation of ethosomes transdermal permeation. Int. J. Pharm. X 2019, 1, 100027. [Google Scholar] [CrossRef]
- Shen, S.; Liu, S.Z.; Zhang, Y.S.; Du, M.B.; Liang, A.H.; Song, L.H.; Ye, Z.G. Compound antimalarial ethosomal cataplasm: Preparation, evaluation, and mechanism of penetration enhancement. Int. J. Nanomed. 2015, 10, 4239–4253. [Google Scholar] [CrossRef]
- Mbah, C.C.; Builders, P.F.; Attama, A.A. Nanovesicular carriers as alternative drug delivery systems: Ethosomes in focus. Expert Opin. Drug Deliv. 2014, 11, 45–59. [Google Scholar] [CrossRef]
- Sala, M.; Diab, R.; Elaissari, A.; Fessi, H. Lipid nanocarriers as skin drug delivery systems: Properties, mechanisms of skin interactions and medical applications. Int. J. Pharm. 2018, 535, 1–17. [Google Scholar] [CrossRef]
- Binder, L.; Kulovits, E.M.; Petz, R.; Ruthofer, J.; Baurecht, D.; Klang, V.; Valenta, C. Penetration monitoring of drugs and additives by ATR-FTIR spectroscopy/tape stripping and confocal Raman spectroscopy—A comparative study. Eur. J. Pharm. Biopharm. 2018, 130, 214–223. [Google Scholar] [CrossRef]
- Amin, S.; Kohli, K.; Khar, R.K.; Mir, S.R.; Pillai, K.K. Mechanism of in vitro percutaneous absorption enhancement of carvedilol by penetration enhancers. Pharm. Dev. Technol. 2008, 13, 533–539. [Google Scholar] [CrossRef]
- Mollica, F.; Biondi, M.; Muzzi, S.; Ungaro, F.; Quaglia, F.; La Rotonda, M.I.; Netti, P.A. Mathematical modelling of the evolution of protein distribution within single PLGA microspheres: Prediction of local concentration profiles and release kinetics. J. Mater. Sci. Mater. Med. 2008, 19, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Cevc, G.; Vierl, U. Nanotechnology and the transdermal route: A state of the art review and critical appraisal. J. Control. Release 2010, 141, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Sekine, A.; Ogura, T.; Tsuchiya, K.; Ohishi, K.; Masubuchi, Y.; Akamatsu, M.; Sakai, K.; Abe, M.; Sakai, H. Effect of polyols on membrane structures of liposomes: A study using small-angle X-ray scattering data and generalized indirect Fourier transformation. Chem. Phys. Lipids 2022, 249, 105253. [Google Scholar] [CrossRef]
- Ji, H.; Zhao, W.; Yu, Z. Interaction mechanism of three egg protein derived ACE inhibitory tri-peptides and DPPC membrane using FS, FTIR, and DSC studies. Food Chem. X 2022, 15, 100366. [Google Scholar] [CrossRef]
- Xiaowei, J.; Lijuan, Y.; Yanling, L.; Qiuxiao, L.; Bohong, G. Design and Development of Functionalized Single-walled Carbon Nanotube-ethosomes for Transdermal Delivery of Ketoprofen. Die Pharm. 2023, 78, 31–36. [Google Scholar]
- Xie, W.J.; Zhang, Y.P.; Xu, J.; Sun, X.B.; Yang, F.F. The Effect and Mechanism of Transdermal Penetration Enhancement of Fu’s Cupping Therapy: New Physical Penetration Technology for Transdermal Administration with Traditional Chinese Medicine (TCM) Characteristics. Molecules 2017, 22, 525. [Google Scholar] [CrossRef]
- Elamin, A.; Enomoto, H.; Watanabe, M.; Sakuda, S. The Mechanism of Ochratoxin Contamination of Artificially Inoculated Licorice Roots. Toxins 2023, 15, 219. [Google Scholar] [CrossRef] [PubMed]
- Kaliyavaradhan, S.K.; Ling, T.C.; Guo, M.Z.; Mo, K.H. Waste resources recycling in controlled low-strength material (CLSM): A critical review on plastic properties. J. Environ. Manag. 2019, 241, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Gleichgerrcht, E.; Fridriksson, J.; Rorden, C.; Bonilha, L. Connectome-based lesion-symptom mapping (CLSM): A novel approach to map neurological function. NeuroImage Clin. 2017, 16, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Alim, S.H.; Kassem, A.A.; Basha, M.; Salama, A. Comparative study of liposomes, ethosomes and transfersomes as carriers for enhancing the transdermal delivery of diflunisal: In vitro and in vivo evaluation. Int. J. Pharm. 2019, 563, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.P.; Huang, Y.B.; Wu, P.C.; Tsai, Y.H. Topical delivery of 5-aminolevulinic acid-encapsulated ethosomes in a hyperproliferative skin animal model using the CLSM technique to evaluate the penetration behavior. Eur. J. Pharm. Biopharm. 2009, 73, 391–398. [Google Scholar] [CrossRef]
- Wang, S.; Li, W.; Sun, K.; Zhang, R.; Wang, S.; Geng, L. Study of release kinetics and degradation thermodynamics of ferric citrate liposomes. Chem. Phys. Lipids 2019, 225, 104811. [Google Scholar] [CrossRef]
- Ibrahim, T.M.; Abdallah, M.H.; El-Megrab, N.A.; El-Nahas, H.M. Transdermal ethosomal gel nanocarriers; a promising strategy for enhancement of anti-hypertensive effect of carvedilol. J. Liposome Res. 2019, 29, 215–228. [Google Scholar] [CrossRef]
- Yu, X.; Du, L.; Li, Y.; Fu, G.; Jin, Y. Improved anti-melanoma effect of a transdermal mitoxantrone ethosome gel. Biomed. Pharmacother. 2015, 73, 6–11. [Google Scholar] [CrossRef]
- Maestrelli, F.; Capasso, G.; Gonzalez-Rodriguez, M.L.; Rabasco, A.M.; Ghelardini, C.; Mura, P. Effect of preparation technique on the properties and in vivo efficacy of benzocaine-loaded ethosomes. J. Liposome Res. 2009, 19, 253–260. [Google Scholar] [CrossRef]
- Chen, Y.; Wiu, Q.; Zhang, Z.; Zhou, L.; Liu, X.; Du, M.; Jia, X. [Preparation and transdermal diffusion of ursolic acid ethosomes]. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Medica 2011, 36, 988–991. [Google Scholar]
- Ma, H.; Guo, D.; Fan, Y.; Wang, J.; Cheng, J.; Zhang, X. Paeonol-Loaded Ethosomes as Transdermal Delivery Carriers: Design, Preparation and Evaluation. Molecules 2018, 23, 1756. [Google Scholar] [CrossRef] [PubMed]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ou, R.; Guan, S.; Ye, X.; Hu, B.; Zhang, Y.; Lu, S.; Zhou, Y.; Yuan, Z.; Zhang, J.; et al. A novel drug delivery gel of terbinafine hydrochloride with high penetration for external use. Drug Deliv. 2015, 22, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Balata, G.F.; Faisal, M.M.; Elghamry, H.A.; Sabry, S.A. Preparation and Characterization of Ivabradine HCl Transfersomes for Enhanced Transdermal Delivery. J. Drug Deliv. Sci. Technol. 2020, 60, 101921. [Google Scholar] [CrossRef]
- Fathalla, D.; Youssef, E.M.K.; Soliman, G.M. Liposomal and Ethosomal Gels for the Topical Delivery of Anthralin: Preparation, Comparative Evaluation and Clinical Assessment in Psoriatic Patients. Pharmaceutics 2020, 12, 446. [Google Scholar] [CrossRef]
- Pilch, E.; Musial, W. Liposomes with an Ethanol Fraction as an Application for Drug Delivery. Int. J. Mol. Sci. 2018, 19, 3806. [Google Scholar] [CrossRef]
- Fan, C.; Li, X.; Zhou, Y.; Zhao, Y.; Ma, S.; Li, W.; Liu, Y.; Li, G. Enhanced topical delivery of tetrandrine by ethosomes for treatment of arthritis. BioMed Res. Int. 2013, 2013, 161943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y.; Xu, H.; Wo, Y.; Zhang, Z.; Liu, Y.; Su, W.; Cui, D.; Zhang, Y. 5-Aminolevulinic acid loaded ethosomal vesicles with high entrapment efficiency for in vitro topical transdermal delivery and photodynamic therapy of hypertrophic scars. Nanoscale 2016, 8, 19270–19279. [Google Scholar] [CrossRef]
- Levy, E.S.; Yu, J.; Estevez, A.; Mao, J.; Liu, L.; Torres, E.; Leung, D.; Yen, C.W. A Systematic Approach for Liposome and Lipodisk Preclinical Formulation Development by Microfluidic Technology. AAPS J. 2021, 23, 111. [Google Scholar] [CrossRef]
- Al-Amin, M.D.; Bellato, F.; Mastrotto, F.; Garofalo, M.; Malfanti, A.; Salmaso, S.; Caliceti, P. Dexamethasone Loaded Liposomes by Thin-Film Hydration and Microfluidic Procedures: Formulation Challenges. Int. J. Mol. Sci. 2020, 21, 1611. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J. Self-assembly of colloids based on microfluidics. Nanoscale 2019, 11, 16708–16722. [Google Scholar] [CrossRef]
- Ainbinder, D.; Paolino, D.; Fresta, M.; Touitou, E. Drug delivery applications with ethosomes. J. Biomed. Nanotechnol. 2010, 6, 558–568. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.; Assi, R.A.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef]
- Dayan, N.; Touitou, E. Carriers for skin delivery of trihexyphenidyl HCl: Ethosomes vs. liposomes. Biomaterials 2000, 21, 1879–1885. [Google Scholar] [CrossRef]
- Majeed, I.; Raza, S.A.; Akhtar, N.; Siddiqui, F.A.; Iqbal, B. Formulation and in-vitro characterization of Capsaicin loaded ethosomes. Pak. J. Pharm. Sci. 2019, 32, 2849–2857. [Google Scholar]
- Mishra, A.D.; Patel, C.N.; Shah, D.R. Formulation and optimization of ethosomes for transdermal delivery of ropinirole hydrochloride. Curr. Drug Deliv. 2013, 10, 500–516. [Google Scholar] [CrossRef] [PubMed]
- El-Shenawy, A.A.; Mahmoud, R.A.; Mahmoud, E.A.; Mohamed, M.S. Intranasal In Situ Gel of Apixaban-Loaded Nanoethosomes: Preparation, Optimization, and In Vivo Evaluation. AAPS PharmSciTech 2021, 22, 147. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, B.; Gupta, R.; Singh, S.K.; Gulati, M.; Singh, S. Prodrugs, phospholipids and vesicular delivery—An effective triumvirate of pharmacosomes. Adv. Colloid Interface Sci. 2018, 253, 35–65. [Google Scholar] [CrossRef] [PubMed]
- Kawar, D.; Abdelkader, H. Hyaluronic acid gel-core liposomes (hyaluosomes) enhance skin permeation of ketoprofen. Pharm. Dev. Technol. 2019, 24, 947–953. [Google Scholar] [CrossRef]
- López-Pinto, J.M.; González-Rodríguez, M.L.; Rabasco, A.M. Effect of cholesterol and ethanol on dermal delivery from DPPC liposomes. Int. J. Pharm. 2005, 298, 1–12. [Google Scholar] [CrossRef]
- Li, G.; Fan, Y.; Fan, C.; Li, X.; Wang, X.; Li, M.; Liu, Y. Tacrolimus-loaded ethosomes: Physicochemical characterization and in vivo evaluation. Eur. J. Pharm. Biopharm. 2012, 82, 49–57. [Google Scholar] [CrossRef]
- El Zaafarany, G.M.; Awad, G.A.; Holayel, S.M.; Mortada, N.D. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int. J. Pharm. 2010, 397, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Natsheh, H.; Touitou, E. Phospholipid Vesicles for Dermal/Transdermal and Nasal Administration of Active Molecules: The Effect of Surfactants and Alcohols on the Fluidity of Their Lipid Bilayers and Penetration Enhancement Properties. Molecules 2020, 25, 2959. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, S.; Arami, S. Enhanced transdermal delivery of diclofenac sodium via conventional liposomes, ethosomes, and transfersomes. BioMed Res. Int. 2013, 2013, 616810. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Wu, Q.; Wang, W.; Zhang, M.; Han, X.; Li, Q.; Chen, P.; Chen, X.; Huang, X.; Li, G.; et al. Preparation, characterization, pharmacokinetics and anticancer effects of PEGylated beta-elemene liposomes. Cancer Biol. Med. 2020, 17, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Golhani, D.; Shukla, R. Ethosomes: Versatile vesicular carriers for efficient transdermal delivery of therapeutic agents. Drug Deliv. 2015, 22, 988–1002. [Google Scholar] [CrossRef]
- Shields, B.E.; Rosenbach, M.; Brown-Joel, Z.; Berger, A.P.; Ford, B.A.; Wanat, K.A. Angioinvasive fungal infections impacting the skin: Background, epidemiology, and clinical presentation. J. Am. Acad. Dermatol. 2019, 80, 869–880 e865. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Jose, J.; Kumar, L.; Charyulu, R.N. Novel ethosomal gel of clove oil for the treatment of cutaneous candidiasis. J. Cosmet. Dermatol. 2019, 18, 862–869. [Google Scholar] [CrossRef]
- Maheshwari, R.G.; Tekade, R.K.; Sharma, P.A.; Darwhekar, G.; Tyagi, A.; Patel, R.P.; Jain, D.K. Ethosomes and ultradeformable liposomes for transdermal delivery of clotrimazole: A comparative assessment. Saudi Pharm. J. 2012, 20, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Dutta, S.; Singh, P.; Chaudhuri, S.; Pal, S.K. Essential Dynamics of an Effective Phototherapeutic Drug in a Nanoscopic Delivery Vehicle: Psoralen in Ethosomes for Biofilm Treatment. ACS Omega 2017, 2, 1850–1857. [Google Scholar] [CrossRef]
- Wang, Y.; Song, J.; Zhang, F.; Zeng, K.; Zhu, X. Antifungal Photodynamic Activity of Hexyl-Aminolevulinate Ethosomes Against Candida albicans Biofilm. Front. Microbiol. 2020, 11, 2052. [Google Scholar] [CrossRef]
- Marto, J.; Vitor, C.; Guerreiro, A.; Severino, C.; Eleuterio, C.; Ascenso, A.; Simoes, S. Ethosomes for enhanced skin delivery of griseofulvin. Colloids Surf. B Biointerfaces 2016, 146, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Wang, J.; Ma, M.; Tan, F.; Li, N. Skin targeted lipid vesicles as novel nano-carrier of ketoconazole: Characterization, in vitro and in vivo evaluation. J. Mater. Sci. Mater. Med. 2015, 26, 175. [Google Scholar] [CrossRef]
- Dave, V.; Bhardwaj, N.; Gupta, N.; Tak, K. Herbal ethosomal gel containing luliconazole for productive relevance in the field of biomedicine. 3 Biotech 2020, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Pathak, K. Cavamax W7 composite ethosomal gel of clotrimazole for improved topical delivery: Development and comparison with ethosomal gel. AAPS PharmSciTech 2012, 13, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Yao, R.; Qin, D.; Chen, Q.; Du, Q. Enhancement in Antibacterial Activities of Eugenol-Entrapped Ethosome Nanoparticles via Strengthening Its Permeability and Sustained Release. J. Agric. Food Chem. 2019, 67, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.; Partoazar, A.; Takzaree, N.; Fasihi-Ramandi, M.; Bahador, A.; Darvishi, M.H. Silver sulfadiazine nanoethogel for burn healing: Characterization and investigation of its in vivo effects. Nanomedicine 2018, 13, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Gamal, F.A.; Sayed, O.M.; Abo El-Ela, F.I.; Kharshoum, R.M.; Salem, H.F. Treatment of Basal Cell Carcinoma Via Binary Ethosomes of Vismodegib: In Vitro and In Vivo Studies. AAPS PharmSciTech 2020, 21, 51. [Google Scholar]
- Raj, R.; Raj, P.M.; Ram, A. Lipid based noninvasive vesicular formulation of cytarabine: Nanodeformable liposomes. Eur. J. Pharm. Sci. 2016, 88, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Lin, L.; Choi, Y.; Michniak-Kohn, B. Development and in-vitro evaluation of co-loaded berberine chloride and evodiamine ethosomes for treatment of melanoma. Int. J. Pharm. 2020, 581, 119278. [Google Scholar] [CrossRef]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M. Preparation and optimization of fisetin loaded glycerol based soft nanovesicles by Box-Behnken design. Int. J. Pharm. 2020, 578, 119125. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Froiio, F.; Spaccapelo, R.; Mancuso, A.; Nisticò, S.P.; Udongo, B.P.; Fresta, M.; Paolino, D. Sulforaphane-Loaded Ultradeformable Vesicles as A Potential Natural Nanomedicine for the Treatment of Skin Cancer Diseases. Pharmaceutics 2019, 12, 6. [Google Scholar] [CrossRef]
- El Kayal, M.; Nasr, M.; Mortada, N.; Elkheshen, S. Optimization of the colloidal properties of different vesicular systems aiming to encapsulate (-)-epigallocatechin-3-gallate. Farmacia 2020, 68, 97–110. [Google Scholar] [CrossRef]
- Bragagni, M.; Mennini, N.; Maestrelli, F.; Cirri, M.; Mura, P. Comparative study of liposomes, transfersomes and ethosomes as carriers for improving topical delivery of celecoxib. Drug Deliv. 2012, 19, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Paolino, D.; Celia, C.; Trapasso, E.; Cilurzo, F.; Fresta, M. Paclitaxel-loaded ethosomes®: Potential treatment of squamous cell carcinoma, a malignant transformation of actinic keratoses. Eur. J. Pharm. Biopharm. 2012, 81, 102–112. [Google Scholar] [CrossRef]
- Song, X.; Jiang, Y.; Zhang, W.; Elfawal, G.; Wang, K.; Jiang, D.; Hong, H.; Wu, J.; He, C.; Mo, X.; et al. Transcutaneous tumor vaccination combined with anti-programmed death-1 monoclonal antibody treatment produces a synergistic antitumor effect. Acta Biomater. 2022, 140, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Thathapudi, N.C.; Ashe, S.; Nayak, B. Bioengineered ethosomes encapsulating AgNPs and Tasar silk sericin proteins for non melanoma skin carcinoma (NMSC) as an alternative therapeutics. Int. J. Pharm. 2021, 596, 120265. [Google Scholar] [CrossRef]
- Raj, R.; Raj, P.M.; Ram, A. Nanosized ethanol based malleable liposomes of cytarabine to accentuate transdermal delivery: Formulation optimization, in vitro skin permeation and in vivo bioavailability. Artif. Cells Nanomed. Biotechnol. 2018, 46, 951–963. [Google Scholar] [CrossRef]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Fisetin loaded binary ethosomes for management of skin cancer by dermal application on UV exposed mice. Int. J. Pharm. 2019, 560, 78–91. [Google Scholar] [CrossRef]
- El-Kayal, M.; Nasr, M.; Elkheshen, S.; Mortada, N. Colloidal (-)-epigallocatechin-3-gallate vesicular systems for prevention and treatment of skin cancer: A comprehensive experimental study with preclinical investigation. Eur. J. Pharm. Sci. 2019, 137, 104972. [Google Scholar] [CrossRef]
- Negi, P.; Sharma, I.; Hemrajani, C.; Rathore, C.; Bisht, A.; Raza, K.; Katare, O.P. Thymoquinone-loaded lipid vesicles: A promising nanomedicine for psoriasis. BMC Complement. Altern. Med. 2019, 19, 334. [Google Scholar] [CrossRef]
- Guo, T.; Lu, J.; Fan, Y.; Zhang, Y.; Yin, S.; Sha, X.; Feng, N. TPGS assists the percutaneous administration of curcumin and glycyrrhetinic acid coloaded functionalized ethosomes for the synergistic treatment of psoriasis. Int. J. Pharm. 2021, 604, 120762. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Meng, X.; Zhang, S.; Wang, X.; Chen, Y.; Min, P.; Zhang, Z.; Zhang, Y. IR-808 loaded nanoethosomes for aggregation-enhanced synergistic transdermal photodynamic/photothermal treatment of hypertrophic scars. Biomater. Sci. 2021, 10, 158–166. [Google Scholar] [CrossRef]
- Gu, J.; Mao, X.; Li, C.; Ao, H.; Yang, X. A Novel Therapy for Laryngotracheal Stenosis: Treatment With Ethosomes Containing 5-Fluorouracil. Ann. Otol. Rhinol. Laryngol. 2015, 124, 561–566. [Google Scholar] [CrossRef]
- Zhang, Z.; Wo, Y.; Zhang, Y.; Wang, D.; He, R.; Chen, H.; Cui, D. In vitro study of ethosome penetration in human skin and hypertrophic scar tissue. Nanomedicine 2012, 8, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Wo, Y.; Zhang, Z.; Zhang, Y.; Zhang, Z.; Wang, K.; Mao, X.; Su, W.; Li, K.; Cui, D.; Chen, J. Enhanced in vivo delivery of 5-fluorouracil by ethosomal gels in rabbit ear hypertrophic scar model. Int. J. Mol. Sci. 2014, 15, 22786–22800. [Google Scholar] [CrossRef]
- Huang, J.; Chen, J.; Wo, Y.; Wang, X.; Zhang, Y.; Chen, X.; Zhang, Z.; Biskup, E. CO2 Fractional Laser Combined with 5-Fluorouracil Ethosomal Gel Treatment of Hypertrophic Scar Macro-, Microscopic, and Molecular Mechanism of Action in a Rabbit Animal Model. Rejuvenation Res. 2021, 24, 131–138. [Google Scholar] [CrossRef]
- Xie, J.; Ji, Y.; Xue, W.; Ma, D.; Hu, Y. Hyaluronic acid-containing ethosomes as a potential carrier for transdermal drug delivery. Colloids Surf. B Biointerfaces 2018, 172, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Jankowska-Polanska, B.; Karniej, P.; Polanski, J.; Sen, M.; Swiatoniowska-Lonc, N.; Grochans, E. Diabetes Mellitus Versus Hypertension-Does Disease Affect Pharmacological Adherence? Front. Pharmacol. 2020, 11, 1157. [Google Scholar] [CrossRef]
- Ammar, H.O.; Tadros, M.I.; Salama, N.M.; Ghoneim, A.M. Ethosome-Derived Invasomes as a Potential Transdermal Delivery System for Vardenafil Hydrochloride: Development, Optimization and Application of Physiologically Based Pharmacokinetic Modeling in Adults and Geriatrics. Int. J. Nanomed. 2020, 15, 5671–5685. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Zeng, Z.; Zhou, W.; Liu, J.; He, Z. Pharmacokinetics of ligustrazine ethosome patch in rats and anti-myocardial ischemia and anti-ischemic reperfusion injury effect. Int. J. Nanomed. 2011, 6, 1391–1398. [Google Scholar] [CrossRef]
- Bodade, S.S.; Shaikh, K.S.; Kamble, M.S.; Chaudhari, P.D. A study on ethosomes as mode for transdermal delivery of an antidiabetic drug. Drug Deliv. 2013, 20, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Y.; Luo, G. Ligustrazine phosphate ethosomes for treatment of Alzheimer’s disease, in vitro and in animal model studies. AAPS PharmSciTech 2012, 13, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.; Liu, J.; He, Z.; Ding, C.; Huang, G.; Zhou, W.; Zhou, L. Preparation of a ligustrazine ethosome patch and its evaluation in vitro and in vivo. Int. J. Nanomed. 2011, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.S.; Avachat, A.M. Design and development of ethosomal transdermal drug delivery system of valsartan with preclinical assessment in Wistar albino rats. J. Liposome Res. 2013, 23, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Sarim Imam, S.; Zafar, A.; Ali, A.; Aqil, M.; Gull, A. In vitro and preclinical assessment of factorial design based nanoethosomes transgel formulation of an opioid analgesic. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Zhang, K.; Li, Z.; Guo, T.; Wu, T.; Hou, X.; Feng, N. Co-hybridized composite nanovesicles for enhanced transdermal eugenol and cinnamaldehyde delivery and their potential efficacy in ulcerative colitis. Nanomedicine 2020, 28, 102212. [Google Scholar] [CrossRef] [PubMed]
- Wilson, V.; Siram, K.; Rajendran, S.; Sankar, V. Development and evaluation of finasteride loaded ethosomes for targeting to the pilosebaceous unit. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1892–1901. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Chen, Z.; Yang, L.; Zhang, X.; Guo, J.; Li, M.; Li, J. High-efficient nano-carrier gel systems for testosterone propionate skin delivery. Pharm. Dev. Technol. 2015, 20, 724–729. [Google Scholar] [CrossRef]
- Shelke, S.; Shahi, S.; Jalalpure, S.; Dhamecha, D. Poloxamer 407-based intranasal thermoreversible gel of zolmitriptan-loaded nanoethosomes: Formulation, optimization, evaluation and permeation studies. J. Liposome Res. 2016, 26, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Babaie, S.; Ghanbarzadeh, S.; Davaran, S.; Kouhsoltani, M.; Hamishehkar, H. Nanoethosomes for Dermal Delivery of Lidocaine. Adv. Pharm. Bull. 2015, 5, 549–556. [Google Scholar] [CrossRef]
- Fu, X.; Shi, Y.; Wang, H.; Zhao, X.; Sun, Q.; Huang, Y.; Qi, T.; Lin, G. Ethosomal Gel for Improving Transdermal Delivery of Thymosin β-4. Int. J. Nanomed. 2019, 14, 9275–9284. [Google Scholar] [CrossRef] [PubMed]
- El-Menshawe, S.F.; Ali, A.A.; Halawa, A.A.; Srag El-Din, A.S. A novel transdermal nanoethosomal gel of betahistine dihydrochloride for weight gain control: In-vitro and in-vivo characterization. Drug Des. Dev. Ther. 2017, 11, 3377–3388. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, Y.K.; Hyun, S.Y.; Kim, H.T.; Kim, C.K.; Oh, J.M. Transdermal delivery of low molecular weight heparin loaded in flexible liposomes with bioavailability enhancement: Comparison with ethosomes. J. Microencapsul. 2011, 28, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Shumilov, M.; Bercovich, R.; Duchi, S.; Ainbinder, D.; Touitou, E. Ibuprofen transdermal ethosomal gel: Characterization and efficiency in animal models. J. Biomed. Nanotechnol. 2010, 6, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Campani, V.; Biondi, M.; Mayol, L.; Cilurzo, F.; Franze, S.; Pitaro, M.; De Rosa, G. Nanocarriers to Enhance the Accumulation of Vitamin K1 into the Skin. Pharm. Res. 2016, 33, 893–908. [Google Scholar] [CrossRef] [PubMed]
- Shumilov, M.; Touitou, E. Buspirone transdermal administration for menopausal syndromes, in vitro and in animal model studies. Int. J. Pharm. 2010, 387, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Limsuwan, T.; Boonme, P.; Khongkow, P.; Amnuaikit, T. Ethosomes of Phenylethyl Resorcinol as Vesicular Delivery System for Skin Lightening Applications. Biomed. Res. Int. 2017, 2017, 8310979. [Google Scholar] [CrossRef]
- Ansari, S.A.; Qadir, A.; Warsi, M.H.; Mujeeb, M.; Aqil, M.; Mir, S.R.; Sharma, S. Ethosomes-based gel formulation of karanjin for treatment of acne vulgaris: In vitro investigations and preclinical assessment. 3 Biotech 2021, 11, 456. [Google Scholar] [CrossRef]
- Goindi, S.; Dhatt, B.; Kaur, A. Ethosomes-based topical delivery system of antihistaminic drug for treatment of skin allergies. J. Microencapsul. 2014, 31, 716–724. [Google Scholar] [CrossRef]
- Shukla, R.; Tiwari, G.; Tiwari, R.; Rai, A.K. Formulation and evaluation of the topical ethosomal gel of melatonin to prevent UV radiation. J. Cosmet. Dermatol. 2020, 19, 2093–2104. [Google Scholar] [CrossRef]


| Drug | Preparation Methods | Application | Form/Type | Remarks | References |
|---|---|---|---|---|---|
| Terbinafine hydrochloride | pH gradient method | Antifungal | Ethosomes, binary ethosomes | The penetration depth and fluorescence intensity ↑ (binary ethosomes compared with other formulations) | [17] |
| Vancomycin hydrochloride | Ethanol injection method, cold method | Antibiotic | Ethosomes | The minimum inhibitory concentration of infected pathogen ↑ | [5] |
| Clove oil | Mechanical-dispersion method | Antifungal | Ethosomal gel | Antifungal activity against the fungus C. albicans ↑ (compared with pure clove oil) | [87] |
| Clotrimazole | Mechanical-dispersion method | Antifungal | Ethosomes | Zone of inhibition (34.6 ± 0.57 mm) ↑ (compared with ultradeformable liposome) | [88] |
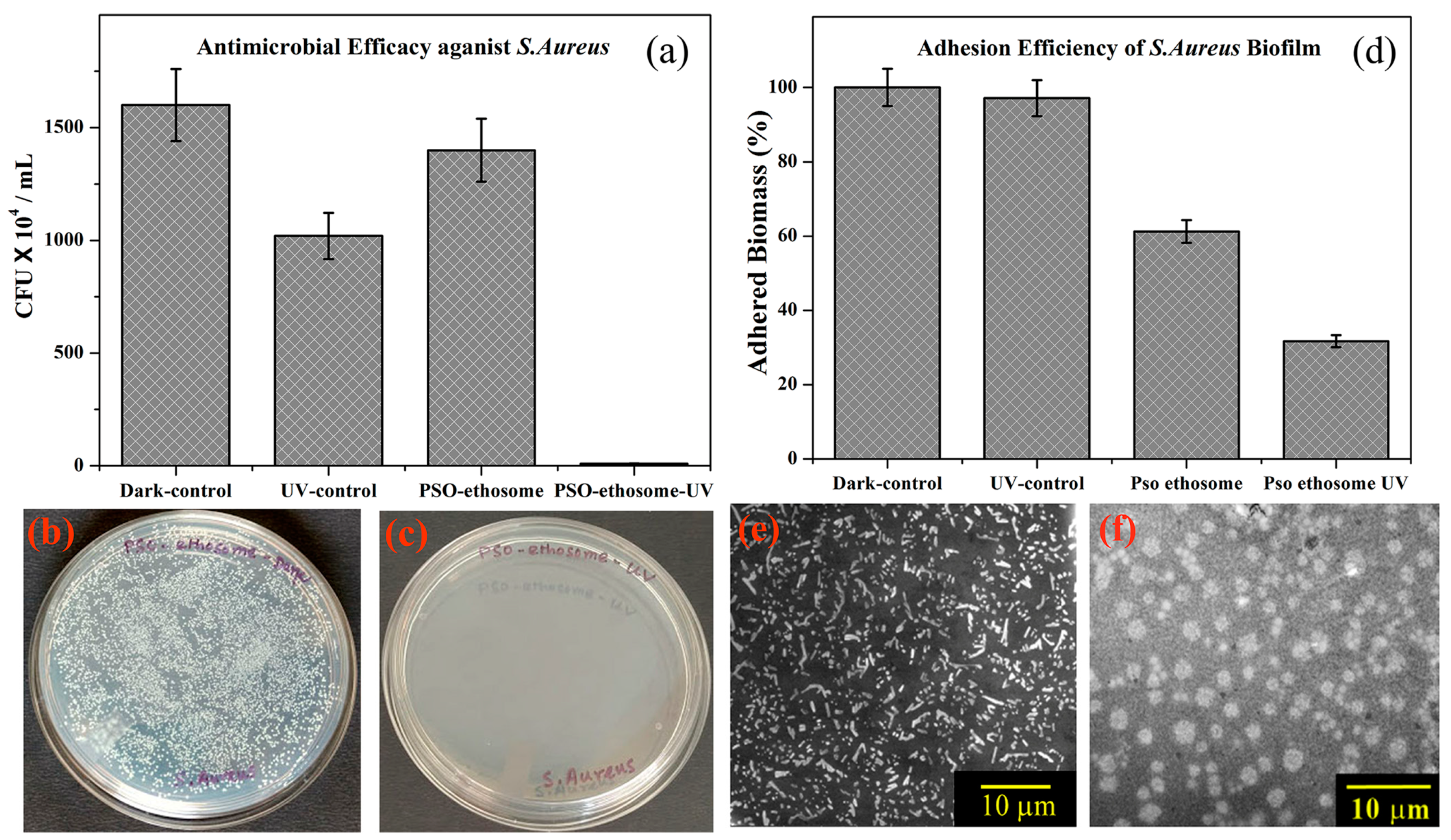
| Psoralen | NA | Antimicrobial | Ethosomes | Improved antimicrobial activity ↑, biomass for both bacteria 30% ↓ | [89] |
| Hexyl-Aminolevulinate | NA | Recurrent Candida albicans infections | Ethosomes | The sessile minimum inhibitory concentration 50 ↓ and survival rate of C. albicans biofilm-infected mice ↑ (compared with Hexyl-aminolevulinate) | [90] |
| Griseofulvin | Mechanical-dispersion method | Antifungal | Ethosomes | GRF-loaded ethosomes have an adequate profile and drug skin retention ↑ | [91] |
| Ketoconazole | Thin-film evaporation and hydration method | Antifungal | Ethosomes | Improved antifungal activity ↑ | [92] |
| Luliconazole | Ultrasound injection method | Antifungal | Ethosomal gel | Inhibits the activity of Candida parapsilosis ↑ (compared with Aspergillus niger) | [93] |
| Clotrimazole encapsulated Cavamax W7 | Ethanol injection method | Antifungal | Ethosomal gel | Stable and efficacious ↑ (composite ethosomes than ethosomal formulation) | [94] |
| Eugenol | Injection homogenization method | Antibacterial | Ethosomes | Antibacterial activity (>93%) ↑ (compared with free eugenol) | [95] |
| Silver sulfadiazine | Cold method | Antibacterial | Ethosomal gel | Wound contraction rate of 96.83% (compared with free drug) | [96] |
| Drug | Preparation Methods | Application | Form/Type | Remarks | References |
|---|---|---|---|---|---|
| Mitoxantrone (MTO) | Thin film dispersion method | Anti-melanoma | Ethosomal gel | The calreticulin membrane translocation of B16 cells ↑ (compared with MTO solutions) | [57] |
| Vismodegib (VMD) | Hot method | Basal cell carcinoma | Binary ethosomal gel | Anti-tumour activity (papilloma number and diameter) ↑ (compared with oral VMD and VMD-loaded ethosome gel containing 0% IPA) VMD permeated in the epidermis ↑ (compared with VMD-loaded ethosomal gel containing 0% IPA) | [97] |
| Cytarabine | Thin layer evaporation | Acute myeloid leukemia (AML) | Ethosomes | Percutaneous penetration of cytarabine ↑ | [107] |
| Berberine chloride and evodiamine | The single step injection technique | Melanoma | Ethosomes | The inhibitory effect on B16 melanoma cells ↑ | [99] |
| Fisetin | The thin-film hydration method | Skin cancer | Binary ethosomal gel | TNF-α ↓, IL-1α ↓, tumour incidences ↓ (compared to the mice exposed to UV only) | [108] |
| Sulforaphane | The ethanol injection method | Skin Cancer | Ethosomes | Anticancer activity on SK-MEL 28 after 24 h ↑ (compared with free drug) | [101] |
| (-)-epigallocatechin-3-gallate | Thin film hydration technique | Skin cancer | Ethosomes and transethosomes | Tumor sizes, glutathione, superoxide dismutase, catalase and lipid peroxidation ↓ an inhibitory effect on epidermoid carcinoma cell line A431 | [109] |
| Celecoxib | Thin layer evaporation technique | Anticancer | Transfersomes and ethosomes | Drug penetration ↑ (compared with suspension and liposomes) | [103] |
| Paclitaxel | The ethanol injection method | Squamous cell carcinoma | Ethosomes | The anti-proliferative and anti-apoptotic activity ↑ (compared with the free drug) | [104] |
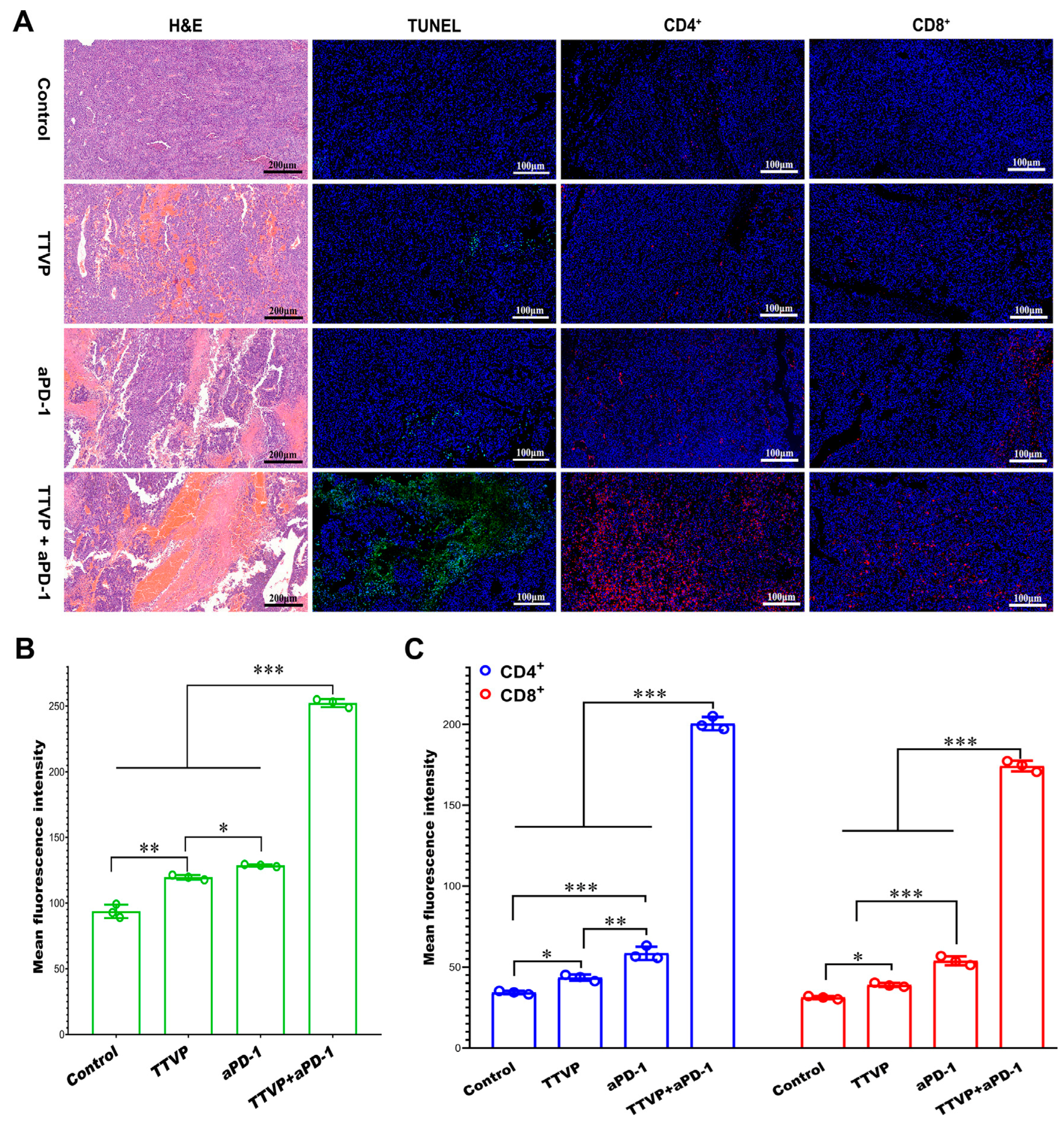
| Transcutaneous tumor vaccine/anti-programmed death-1 monoclonal antibody | The thin layer evaporation Method | Transcutaneous immunization (inhibit melanoma) | Electrospun silk fibroin and polyvinyl alcohol composite nanofibrous patch loaded with mannosylated polyethyleneimine-modified ethosome (Eth-PEIman) (TTVP) | Target dendritic cells (DCs) and induce DC maturation; inhibit the growth of melanoma; the combined of the TTVP and anti-programmed death-1 monoclonal antibody (aPD-1) produced a synergistic antitumor effect (the infiltration of more CD4+ and CD8+ T cells in the tumor tissues ↑, IL-12 ↑) | [105] |
| AgNPs and tasar silk sericin proteins | The ethanol injection method | Non-melanoma skin carcinoma | Bioengineered ethosomes | AgNPs stimulate the production of ROS, alter the ΔΨm and trigger a cascade of events for apoptotic and necrotic cell death, break the DNA double helix | [106] |
| Drug | Preparation Methods | Application | Form/Type | Remarks | References |
|---|---|---|---|---|---|
| Psoralen | The ethanol injection method | Psoriasis | Ethosomes | Biocompatibility ↑ (compared with free drug) | [19] |
| Anthralin | The thin-film hydration method | Psoriatic | Ethosomal gel | Psoriasis Area and Severity Index (PASI), liposomes: 68.66%, ethosomes: 81.84%; no adverse effects | [64] |
| Thymoquinone | Cold method | Psoriasis | Ethosomal gel | The % Orthokeratosis and % drug activity ↑ (compared with other studied test formulations) | [110] |
| Curcumin | The ethanol injection method | Psoriasis | Multifunctional ethosomes | Synergistic anti-inflammatory effects, glucocorticoid-like effects and anti-oxidative effects | [111] |
| Drug | Preparation Methods | Application | Form/Type | Remarks | References |
|---|---|---|---|---|---|
| Near-infrared heptamethine cyanine dye (IR-808) | Thin layer evaporation technique | Hypertrophic scar | Ethosomal gel | The special structure of IR-808 aggregate distribution in the ES lipid membrane enhances ROS gene- ration and hyperthermia. | [112] |
| 5-fluorouracil | The ethanol injection method | Hypertrophic scar | Ethosomes | E-Scar > H-Scar > E-Skin > H- Skin | [114] |
| 5-fluorouracil | The ethanol injection method | Hypertrophic scar | Ethosomal gel | SEI ↓ (compared with 5-FU Phosphate Buffered Saline gel). | [115] |
| 5-fluorouracil | The film dispersion | Hypertrophic scar | Ethosomal gel | collagen I/III ↓ TGF-β1 expression ↓ | [116] |
| 5-fluorouracil | The ethanol injection method | Hypertrophic scar (HS) | Ethosome | ALA and AuNPs penetration ↑ (compared with hydroethanolic solution). | [117] |
| Drug | Preparation Methods | Application | Form/Type | Remarks | References |
|---|---|---|---|---|---|
| Vardenafil hydrochloride | Ethanol injection technique | Pulmonary arterial hypertension | Ethosomes | Cmax values ↓, delayed Tmax estimates | [119] |
| Carvedilol | The ethanol injection Method | Antihypertensive | Ethosomal gel | Mean arterial pressure of rats ↓ (146.11 mmHg) after 6 h ↓ (98.88 mmHg). | [56] |
| Ligustrazine | The ethanol injection Method | Anti-myocardial ischemia | Ethosomal patch | The scope of myocardial infarction induced by long-term ischemia ↓. | [120] |
| Repaglinide | The cold method | Antidiabetic | Ethosomes | Antidiabetic effect of time ↑ (compared with the equivalent oral dose). | [121] |
| Ligustrazine phosphate | The ethanol injection method | Alzheimer | Ethosome | Recover the activities of the antioxidant enzymes and MDA (compared with the aqueous one) | [122] |
| Ligustrazine | The ethanol injection-sonication method | Angina pectoris | Ethosomal patch | Drug absorption and bioavailability ↑ (Compared with conventional ligustrazine) | [123] |
| Valsartan | The ethanol injection method | Antihypertensive | Ethosomes | Antihypertensive activity ↑ (comparison to orally administered VLT suspension) | [124] |
| Drug | Preparation Methods | Application | Form/Type | Remarks | References |
|---|---|---|---|---|---|
| Cryptotanshinone | Ethanol injection technique | Acne | Ethosomal gel | Transdermal flux 2.5 times and skin deposition 2.1 times (compared with conventional gel) | [8] |
| Finasteride (FIN) | Cold method | Androgenic alopecia | Ethosomes | Permeation across rat skin and frontal scalp skin of human cadaver ↑ (compared with the unencapsulated FIN) | [127] |
| Triamcinolone acetonide | Infusion method | Atopic dermatitis | Binary nanoethosomes | Topical delivery ↑, EE ↑, and stability ↑ (compared with reference ethosomal vesicles) | [23] |
| Artemisinin | Injection method. | Antimalarial | Ethosomal cataplasm | The accumulated permeation quantity 1.57 times (compared with conventional cataplasm) | [39] |
| Tramadol | By thin layer evaporation technique | Analgesic | Ethosomal gel | Increase bioavailability (7.51 times) | [125] |
| Testosterone propionate (TP) | Ethanol injection method | Testosterone deficient therapy | Ethosomal gel | Transdermal flux: 7.64 ± 1.4 mg/cm2/h ↑ Lag time of across rat skin ↓ (compared with other formulations) | [128] |
| Zolmitriptan | The ethanol injection Method | Migraine | Nanoethosomes | Size: (171.67 nm) EE: (66%) | [129] |
| Rosmarinic acid | The thin-film hydration Method | Anti-aging | Ethosomes | Size: (138 ± 1.11 nm) EE: (55 ± 1.8)% | [53] |
| Lidocaine | The modified ethanol injection method | Anesthetics | Nanoethosomes | Size: 105.4 ± 7.9 nm, Zeta: −33.6 ± 2.4 mv, EE: 40.14 ± 2.5% | [130] |
| Thymosin β-4 | Ethanol infusion | Wound repair | Ethosomal gel | The percutaneous absorption protein drugs ↑ recovery time ↓ (compared with the free drug) | [131] |
| Eugenol and cinnamaldehyde | The ethanol injection method | Ulcerative colitis | Ethosomes | increasing the number of ICCs and restoring the function of ICCs ↑ (compared with ethosomes) | [126] |
| Betahistine dihydrochloride | The ethanol injection method | Weight gain control | Ethosomal gel | H3 antagonist action and H1 agonist action, brain histamine ↑ (compared with control, placebo, and BDH gel) | [132] |
| Heparin | The ethanol injection method | Anticoagulant | Flexosomes | Skin permeation and bioavailability ↑ (compared with ethosomes) | [133] |
| Ibuprofen | The ethanol injection method | Fever and pain | Ethosomal gel | Relative bioavailability ↑ present in plasma of time ↑ (compared to the oral administration) | [134] |
| Vitamin K1 | The ethanol injection method | Suppress pigmentation and resolve bruising | Ethosomes | Accumulation into/permeation through the skin ↑. | [135] |
| Buspirone | The ethanol injection method | Menopausal syndromes | Ethosomes | A Frel value of 0.89 was estimated for transdermal vs. Oral buspirone. | [136] |
| Phenylethyl resorcinol | Thin-film hydration method | Skin lightening | Ethosomes | Tyrosinase activity ↓ and melanin content ↓ | [137] |
| karanjin | The film hydration method | Acne vulgaris | Ethosome-based gel | Compared to the hydro-ethanolic solution: penetration ↑, against Propionibacterium acnes and Staphylococcus epidermidis inhibition 30.0 ± 1.52 mm and 36.22 ± 0.57 mm | [138] |
| Cetirizine | The ethanol injection method | Atopic dermatitis | Ethosomes | Permeation flux and skin retention ↑ (compared to conventional formulations) | [139] |
| Melatonin | Na | Prevention of UV radiation | Ethosomal gel | Zeta: −12.4 mV to −27.4 mV EE: 49.61–78.047% | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, B.; Wang, J.; Li, H.; Xiao, K.; Fang, X.; Shi, Y.; Jia, Y. Ethosomes: A Promising Drug Delivery Platform for Transdermal Application. Chemistry 2024, 6, 993-1019. https://doi.org/10.3390/chemistry6050058
Zhan B, Wang J, Li H, Xiao K, Fang X, Shi Y, Jia Y. Ethosomes: A Promising Drug Delivery Platform for Transdermal Application. Chemistry. 2024; 6(5):993-1019. https://doi.org/10.3390/chemistry6050058
Chicago/Turabian StyleZhan, Bo, Jiawen Wang, Hongyu Li, Kexin Xiao, Xiaohua Fang, Yajun Shi, and Yanyan Jia. 2024. "Ethosomes: A Promising Drug Delivery Platform for Transdermal Application" Chemistry 6, no. 5: 993-1019. https://doi.org/10.3390/chemistry6050058
APA StyleZhan, B., Wang, J., Li, H., Xiao, K., Fang, X., Shi, Y., & Jia, Y. (2024). Ethosomes: A Promising Drug Delivery Platform for Transdermal Application. Chemistry, 6(5), 993-1019. https://doi.org/10.3390/chemistry6050058






