The Magnetic Properties of Fluorenyl and tert-Butyl-nitroxyl Acene-Based Derivatives: A Quantum Chemical Insight
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Compounds 1–3
3.2. Compounds 4–6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anthony, J.E. Functionalized acenes and heteroacenes for organic electronics. Chem. Rev. 2006, 106, 5028–5048. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.G. Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds; John Wiley & Sons Ltd.: Chichester, UK, 2010. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, J. Open-Shell Polycyclic Aromatic Hydrocarbons. J. Mater. Chem. 2012, 22, 4151–4160. [Google Scholar] [CrossRef]
- Abe, M. Diradicals. Chem. Rev. 2013, 113, 7011–7088. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S. Organic- and Molecule-Based Magnets. Mater. Today 2014, 17, 224–235. [Google Scholar] [CrossRef]
- Kubo, T. Recent progress in quinoidal singlet biradical molecules. Chem. Lett. 2014, 44, 111–122. [Google Scholar] [CrossRef]
- Liu, J.; Xia, J.; Song, P.; Ding, Y.; Cui, Y.; Liu, X.; Dai, Y.; Ma, F. Organic Nonlinear Optical Materials: The Mechanism of Intermolecular Covalent Bonding Interactions of Kekulé Hydrocarbons with Significant Singlet Biradical Character. ChemPhysChem 2014, 15, 2626–2633. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, Y.; Kumar Keshri, S.; Mukhopadhyay, P. Recent Advances in Organic Radicals and Their Magnetism. Magnetochemistry 2016, 2, 42. [Google Scholar] [CrossRef]
- Hu, X.; Wang, W.; Wang, D.; Zheng, Y. The Electronic Applications of Stable Diradicaloids: Present and Future. J. Mater. Chem. C 2018, 6, 11232–11242. [Google Scholar] [CrossRef]
- Stuyver, T.; Chen, B.; Zeng, T.; Geerlings, P.; De Proft, F.; Hoffmann, R. Do Diradicals Behave like Radicals? Chem. Rev. 2019, 119, 11291–11351. [Google Scholar] [CrossRef]
- Jousselin-Oba, T.; Mamada, M.; Marrot, J.; Maignan, A.; Adachi, C.; Yassar, A.; Frigoli, M. Excellent Semiconductors Based on Tetracenotetracene and Pentacenopentacene: From Stable Closed-Shell to Singlet Open-Shell. J. Am. Chem. Soc. 2019, 141, 9373–9381. [Google Scholar] [CrossRef]
- Dressler, J.J.; Haley, M.M. Learning How to Fine-tune Diradical Properties by Structure Refinement. J. Phys. Org. Chem. 2020, 33, e4114. [Google Scholar] [CrossRef]
- Kamada, K.; Ohta, K.; Kubo, T.; Shimizu, A.; Morita, Y.; Nakasuji, K.; Kishi, R.; Ohta, S.; Furukawa, S.; Takahashi, H.; et al. Strong Two-Photon Absorption of Singlet Diradical Hydrocarbons. Angew. Chem. Int. Ed. 2007, 46, 3544–3546. [Google Scholar] [CrossRef] [PubMed]
- Lukman, S.; Richter, J.M.; Yang, L.; Hu, P.; Wu, J.; Greenham, N.C.; Musser, A.J. Efficient Singlet Fission and Triplet-Pair Emission in a Family of Zethrene Diradicaloids. J. Am. Chem. Soc. 2017, 139, 18376–18385. [Google Scholar] [CrossRef]
- Casanova, D. Theoretical Modeling of Singlet Fission. Chem. Rev. 2018, 118, 7164–7207. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tom, R.; Gao, S.; Marom, N. Assessing Zethrene Derivatives as Singlet Fission Candidates Based on Multiple Descriptors. J. Phys. Chem. C 2020, 124, 26134–26143. [Google Scholar] [CrossRef]
- Tonami, T.; Nagami, T.; Okada, K.; Yoshida, W.; Miyamoto, H.; Nakano, M. Quantum design for singlet-fission-induced nonlinear optical systems: Effects of π-conjugation length and molecular packing of butterfly-shaped acenes. J. Chem. Phys. 2020, 153, 084304. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M. Open-Shell-Character-Based Molecular Design Principles: Applications to Nonlinear Optics and Singlet Fission. Chem. Rec. 2017, 17, 27–62. [Google Scholar] [CrossRef]
- Teki, Y.; Toichi, T.; Nakajima, S. π Topology and Spin Alignment in Unique Photoexcited Triplet and Quintet States Arising from Four Unpaired Electrons of an Organic Spin System. Chem. Eur. J. 2006, 12, 2329–2336. [Google Scholar] [CrossRef]
- Kawanaka, Y.; Shimizu, A.; Shinada, T.; Tanaka, R.; Teki, Y. Using Stable Radicals To Protect Pentacene Derivatives from Photodegradation. Angew. Chem. Int. Ed. 2013, 52, 6643–6647. [Google Scholar] [CrossRef]
- Shimizu, A.; Ito, A.; Teki, Y. Photostability enhancement of the pentacene derivative having two nitronyl nitroxide radical substituents. Chem. Commun. 2016, 52, 2889–2892. [Google Scholar] [CrossRef]
- Minami, N.; Yoshida, K.; Maeguchi, K.; Kato, K.; Shimizu, A.; Kashima, G.; Fujiwara, M.; Uragami, C.; Hashimoto, H.; Teki, Y. p-Topology and ultrafast excited-state dynamics of remarkably photochemically stabilized pentacene derivatives with radical substituents. Phys. Chem. Chem. Phys. 2022, 24, 13514–13518. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Teki, Y. Theoretical investigation of multi-spin excited states of anthracene radical-linked p-conjugated spin systems by computational chemistry. Phys. Chem. Chem. Phys. 2024, 26, 8106–8114. [Google Scholar] [CrossRef]
- Shinozuka, T.; Shimizu, D.; Matsuda, K. Theoretical investigation of the effect of radical substituents on the open-shell character of polycyclic aromatic hydrocarbons. New J. Chem. 2024, 48, 8683–8689. [Google Scholar] [CrossRef]
- Sanvito, S. Molecular spintronics. Chem. Soc. Rev. 2011, 40, 3336–3355. [Google Scholar] [CrossRef]
- Pilevarshahri, R.; Rungger, I.; Archer, T.; Sanvito, S.; Shahtahmassebi, N. Spin transport in higher n-acene molecules. Phys. Rev. B 2011, 84, 174437. [Google Scholar] [CrossRef]
- Ratera, I.; Veciana, J. Playing with organic radicals as building blocks for functional molecular material. Chem. Soc. Rev. 2012, 41, 303–349. [Google Scholar] [CrossRef]
- Cano, J.; Lloret, F.; Julve, M. Theoretical design of magnetic wires from acene and nanocorone derivatives. Dalton Trans. 2016, 45, 16700–16708. [Google Scholar] [CrossRef]
- Zhang, H.; Miao, F.; Liu, X.; Wang, D.; Zheng, Y. Recent Advances of Stable Phenoxyl Diradicals. Chem. Res. Chin. Univ. 2023, 39, 170–175. [Google Scholar] [CrossRef]
- Kurata, H.; Tanaka, T.; Oda, M. Dibenzoannulated 3,5,3″,5″-Tetra(t-butyl)-p-terphenoquinone. A Reversible, Photochemical-Thermal Switching System Involving Restricted Conformational Change. Chem. Lett. 1999, 28, 749–750. [Google Scholar] [CrossRef]
- Wentrup, C.; Regimbald-Krnel, M.J.; Müller, D.; Comba, P.A. Thermally Populated, Perpendicularly Twisted Alkene Triplet Diradical. Angew. Chem. Int. Ed. 2016, 55, 14600–14605. [Google Scholar] [CrossRef]
- Yin, X.; Low, J.Z.; Fallon, K.J.; Paley, D.W.; Campos, L.M. The Butterfly Effect in Bisfluorenylidene-Based Dihydroacenes: Aggregation Induced Emission and Spin Switching. Chem. Sci. 2019, 10, 10733–10739. [Google Scholar] [CrossRef]
- Hamamoto, Y.; Hirao, Y.; Kubo, T. Biradicaloid Behavior of a Twisted Double Bond. J. Phys. Chem. Lett. 2021, 12, 4729–4734. [Google Scholar] [CrossRef] [PubMed]
- Ravat, P.; Baumgarten, M. “Tschitschibabin type Biradicals”: Benzenoid or Quinoid? Phys. Chem. Chem. Phys. 2015, 17, 983–991. [Google Scholar] [CrossRef]
- Ten, Y.A.; Troshkova, N.M.; Tretyakov, E.V. From spin-labelled fused polyaromatic compounds to magnetically active graphene nanostructures. Russ. Chem. Rev. 2020, 89, 693–712. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Ovcharenko, V.I.; Terent’ev, A.O.; Krylov, I.B.; Magdesieva, T.V.; Mazhukin, D.G.; Gritsan, N.P. Conjugated nitroxides. Russ. Chem. Rev. 2022, 91, RCR5025. [Google Scholar] [CrossRef]
- Ishigaki, Y.; Harimoto, T.; Shimajiri, T.; Suzuki, T. Carbon-based Biradicals: Structural and Magnetic Switching. Chem. Rev. 2023, 123, 13952–13965. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 (Revision C.01); Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Starikova, A.A.; Chegerev, M.G.; Starikov, A.G.; Metelitsa, A.V.; Minkin, V.I.; Aldoshin, S.M. Size matters: Computational insight into magnetic properties of extended acenes. Chem. Phys. Lett. 2023, 833, 140965. [Google Scholar] [CrossRef]
- Starikov, A.G.; Chegerev, M.G.; Starikova, A.A.; Minkin, V.I. Organic Polyradicals Based on Acenes. Computational Modeling. Dokl. Chem. 2022, 503, 51–55. [Google Scholar] [CrossRef]
- Starikova, A.A.; Starikov, A.G.; Minyaev, R.M.; Boldyrev, A.I.; Minkin, V.I. Magnetic Properties of Acenes and Their o-Quinone Derivatives: Computer Simulation. Dokl. Chem. 2018, 478, 21–25. [Google Scholar] [CrossRef]
- Minkin, V.I.; Starikov, A.G.; Starikova, A.A.; Gapurenko, O.A.; Minyaev, R.M.; Boldyrev, A.I. Electronic structure and magnetic properties of the triangular nanographenes with radical substituents: A DFT study. Phys. Chem. Chem. Phys. 2020, 22, 1288–1298. [Google Scholar] [CrossRef]
- Minkin, V.I.; Starikov, A.G.; Starikova, A.A. Acene-Linked Zethrenes and Bisphenalenyls: A DFT Search for Organic Tetraradicals. J. Phys. Chem. A 2021, 125, 6562–6570. [Google Scholar] [CrossRef]
- Noodleman, L. Valence Bond Description of Antiferromagnetic Coupling in Transition Metal Dimers. J. Chem. Phys. 1981, 74, 5737–5743. [Google Scholar] [CrossRef]
- Minyaev, R.M. Gradient lines on multidimensional potential energy surfaces and chemical reaction mechanisms. Russ. Chem. Rev. 1994, 63, 883–903. [Google Scholar] [CrossRef]
- Harvey, J.N.; Aschi, M.; Schwarz, H.; Koch, W. The singlet and triplet states of phenyl cation. A hybrid approach for locating minimum energy crossing points between non-interacting potential energy surfaces. Theor. Chem. Acc. 1998, 99, 95–99. [Google Scholar] [CrossRef]
- Shoji, M.; Koizumi, K.; Kitagawa, Y.; Kawakami, T.; Yamanaka, S.; Okumura, M.; Yamaguchi, K. A general algorithm for calculation of Heisenberg exchange integrals J in multispin systems. Chem. Phys. Lett. 2006, 432, 343–347. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2017, 8, e1327. [Google Scholar] [CrossRef]
- Chemcraft, Version 1.8. 2014. Available online: http://www.chemcraftprog.com (accessed on 5 July 2021).
- Nishiuchi, T.; Ito, R.; Stratmann, E.; Kubo, T. Switchable Conformational Isomerization of an Overcrowded Tristricyclic Aromatic Ene. J. Org. Chem. 2020, 85, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bendikov, M.; Duong, H.M.; Starkey, K.; Houk, K.N.; Carter, E.A.; Wudl, F. Oligoacenes: Theoretical Prediction of Open-Shell Singlet Diradical Ground States. J. Am. Chem. Soc. 2004, 126, 7416–7417. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Dai, S. Electronic ground state of higher acenes. J. Phys. Chem. A 2008, 112, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Zade, S.S.; Bendikov, M. Heptacene and Beyond: The Longest Characterized Acenes. Angew. Chem. Int. Ed. 2010, 49, 4012–4015. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Hodgson, J.L.; Jiang, D.; Zhang, S.B.; Nagase, S.; Miller, G.P.; Chen, Z. Open-shell singlet character of stable derivatives of nonacene, hexacene and teranthene. Org. Lett. 2011, 13, 3316–3319. [Google Scholar] [CrossRef]
- Rivero, P.; Jiménez-Hoyos, C.A.; Scuseria, G.E. Entanglement and Polyradical Character of Polycyclic Aromatic Hydrocarbons Predicted by Projected Hartree-Fock Theory. J. Phys. Chem. B 2013, 117, 12750–12758. [Google Scholar] [CrossRef]
- Trinquier, G.; David, G.; Malrieu, J.-P. Qualitative Views on the Polyradical Character of Long Acenes. J. Phys. Chem. A 2018, 122, 6926–6933. [Google Scholar] [CrossRef]
- Hachmann, J.; Dorando, J.J.; Aviĺs, M.; Chan, G.K.-L. The Radical Character of the Acenes: A Density Matrix Renormalization Group Study. J. Chem. Phys. 2007, 127, 134309. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Panda, A.; Misra, A.; Klein, D.J. Clar Theory Extended for Polyacenes and Beyond. J. Phys. Chem. A 2014, 118, 4325–4338. [Google Scholar] [CrossRef]
- Tönshoff, C.; Bettinger, H.F. Pushing the Limits of Acene Chemistry: The Recent Surge of Large Acenes. Chem. Eur. J. 2021, 27, 3193–3212. [Google Scholar] [CrossRef]
- Ali, M.d.E.; Datta, S.N. Polyacene Spacers in Intramolecular Magnetic Coupling. J. Phys. Chem. A 2006, 110, 13232–13237. [Google Scholar] [CrossRef]
- Ravat, P.; Teki, Y.; Ito, Y.; Gorelik, E.; Baumgarten, M. Breaking the semi-quinoid structure: Spin-switching from strongly coupled singlet to polarized triplet state. Chem. Eur. J. 2014, 20, 12041–12045. [Google Scholar] [CrossRef]
- Kruszewski, J.; Krygowski, T.M. Definition of aromaticity basing on the harmonic oscillator model. Tetrahedron Lett. 1972, 13, 3839–3842. [Google Scholar] [CrossRef]
- Krygowski, T.M. Crystallographic studies of inter- and intramolecular interactions reflected in aromatic character of.pi.-electron systems. J. Chem. Inf. Comput. Sci. 1993, 33, 70–78. [Google Scholar] [CrossRef]
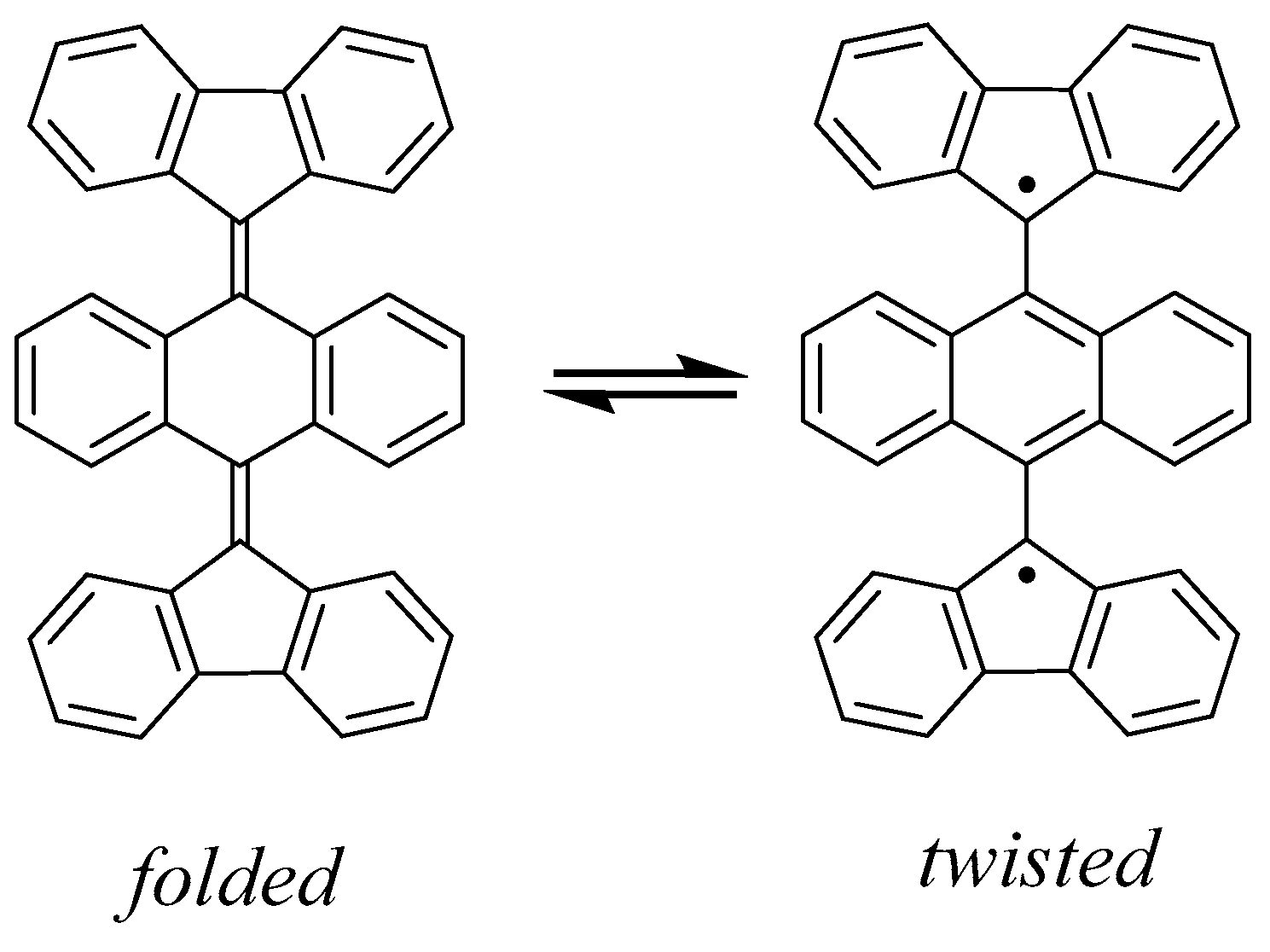

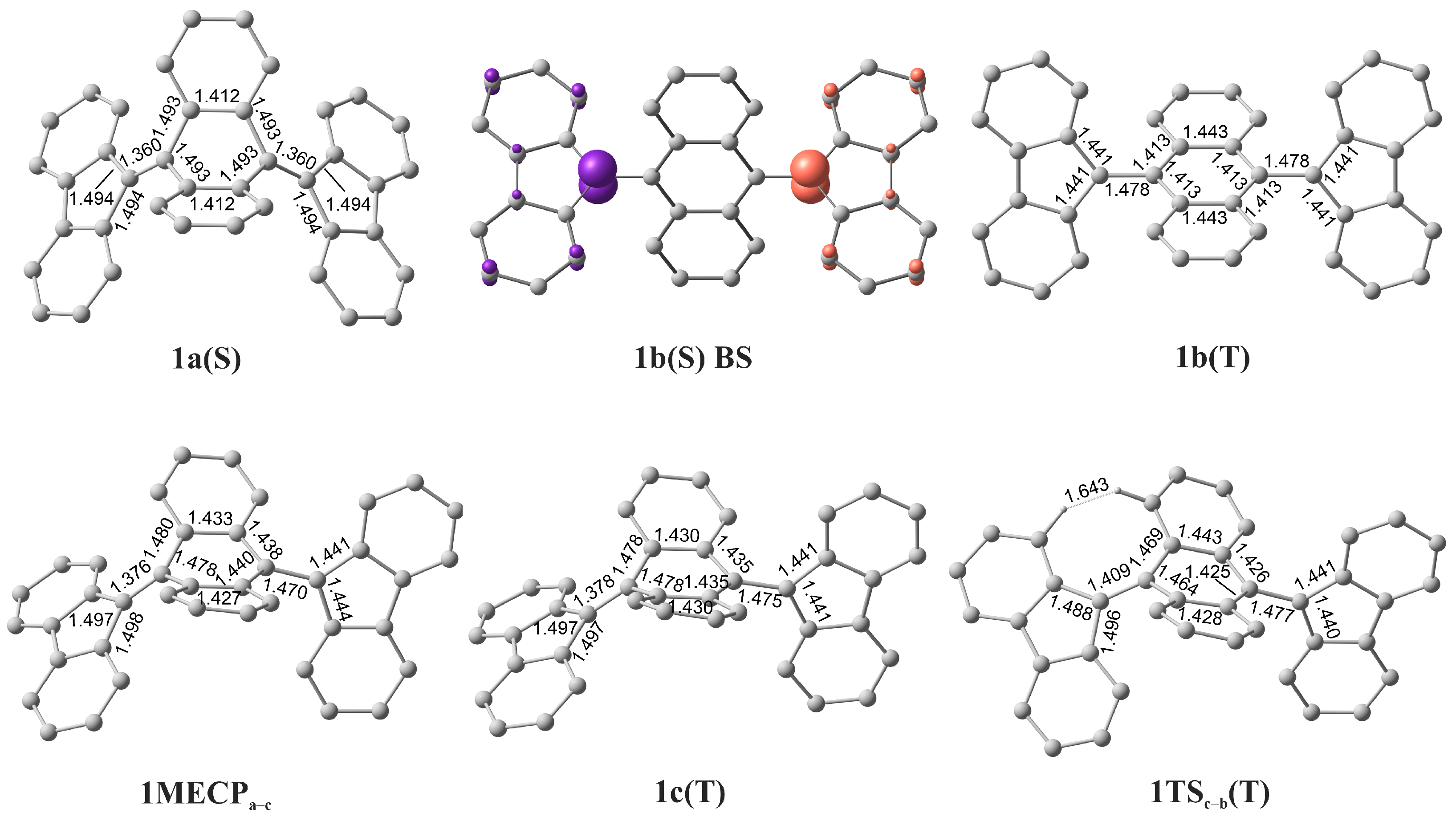


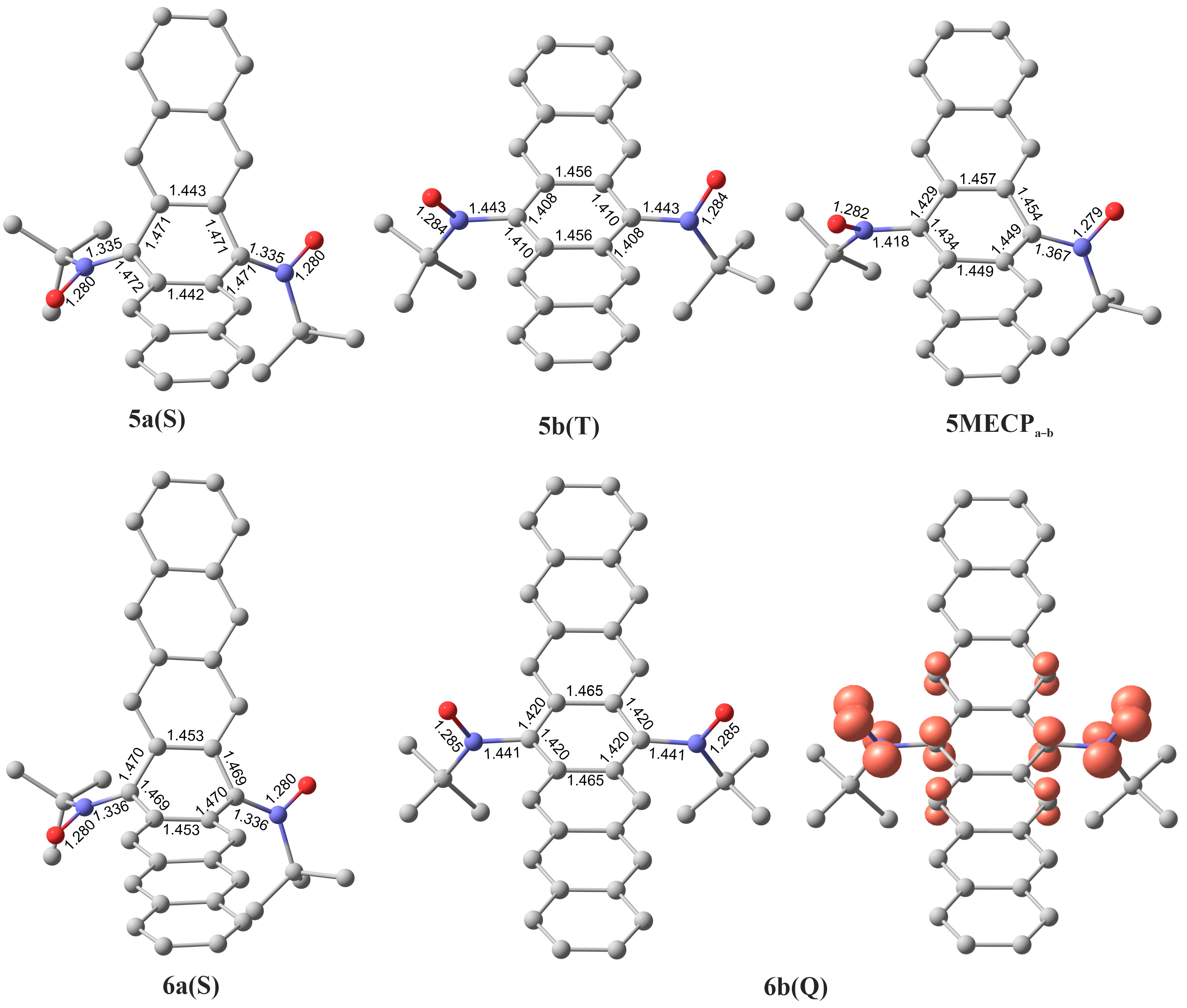
| Structure | S | ΔE, kcal mol−1 | ||
|---|---|---|---|---|
| UB3LYP | wB97XD | LC-wPBE | ||
| 1a(S) 1 folded | 0 | 0.0 | 0.0 | 0.0 |
| 1b(T) twisted | 1 | 6.1 | 10.7 | 8.3 |
| 1b(S) BS | 0 | 5.9 | 10.4 | 8.2 |
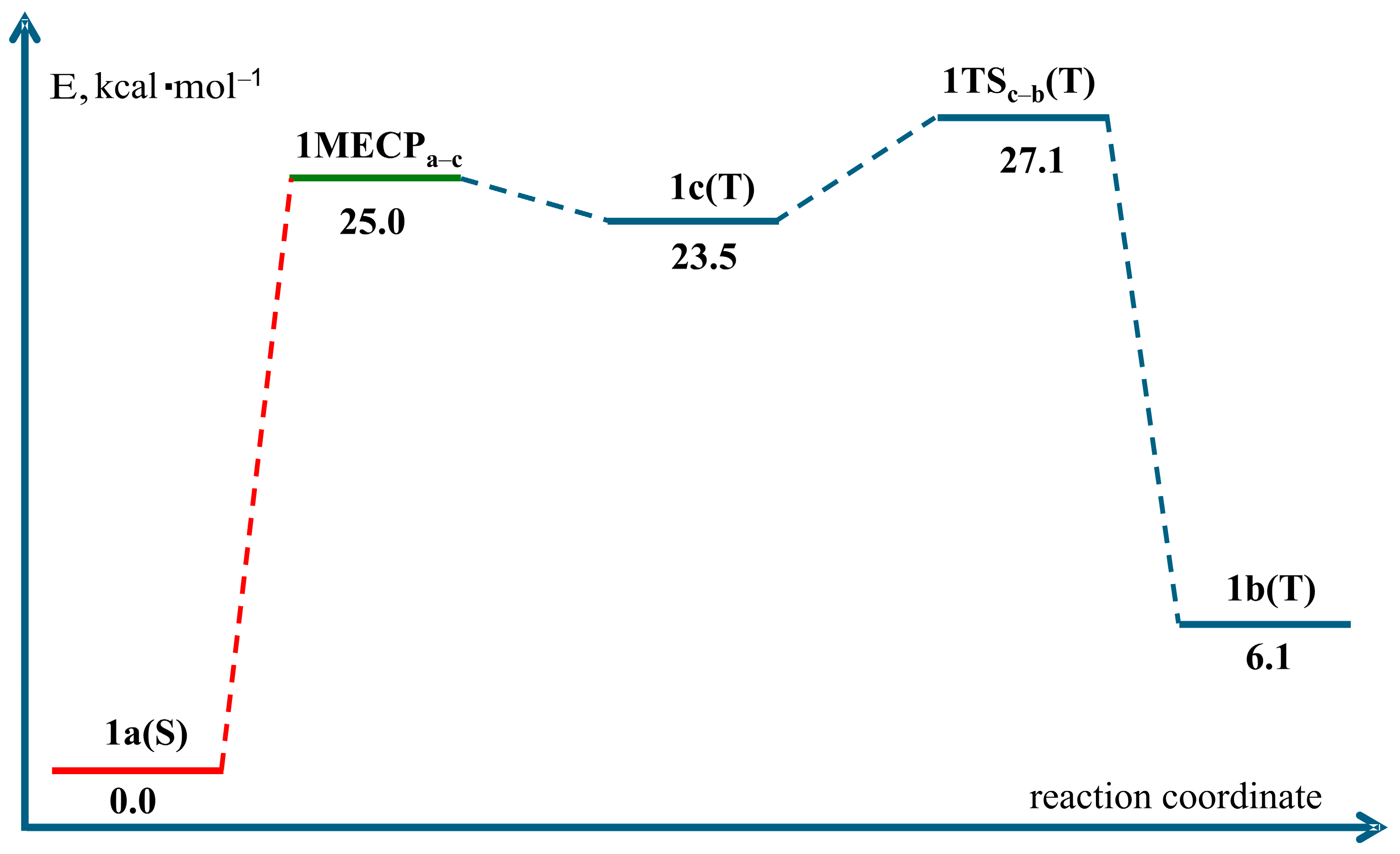
| 1MECPa–c | – | 25.0 | 28.7 | 28.1 |
| 1c(T) | 1 | 23.5 | 26.4 | 23.3 |
| 1TSc–b(T) | 1 | 27.1 | 31.8 | 29.6 |
| Structure | S | ΔE, kcal mol−1 |
|---|---|---|
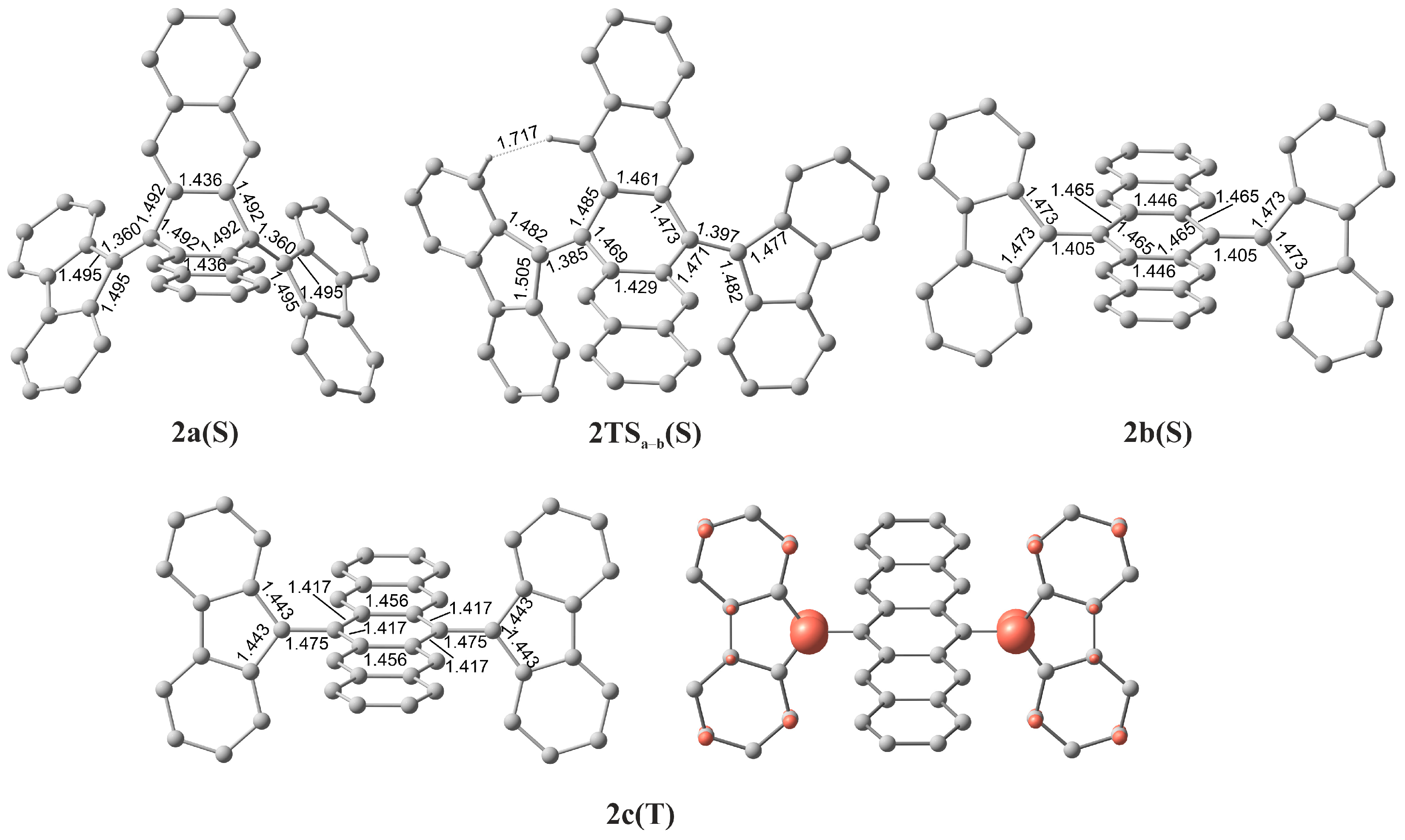
| 2a(S)1 folded | 0 | 0.0 |
| 2TSa–b(S) | 0 | 18.0 |
| 2b(S) twisted | 0 | 13.5 |
| 2c(T) twisted | 1 | 17.5 |
| 3a(S) folded | 0 | 0.0 |
| 3TSa–b(S) | 0 | 17.8 |
| 3b(S) twisted | 0 | 14.4 |
| 3c(Q) | 2 | 29.6 |
| Structure | S | ΔE, kcal mol−1 |
|---|---|---|
| 4a(S) 1 folded | 0 | 0.0 |
| 4b(T)twisted | 1 | −1.5 |
| 4b(S) BS | 0 | −1.5 |
| 4MECPb–a | - | 10.4 |
| 5a(S)folded | 0 | 0.0 |
| 5b(T)twisted | 1 | 11.0 |
| 5b(S) BS | 0 | 10.7 |
| 5MECPa–b | - | 16.8 |
| 6a(S) folded | 0 | 0.0 |
| 6b(Q) | 2 | 22.6 |
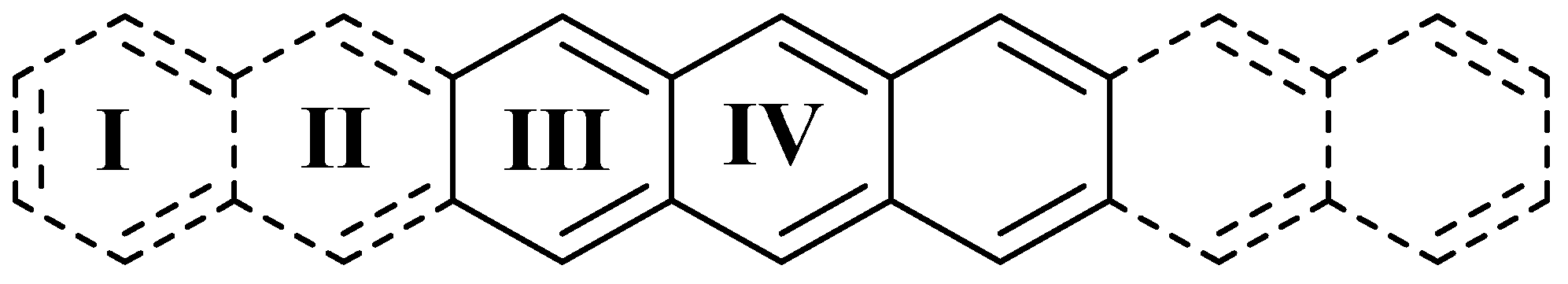
| Compound | I 1 | II | III | IV |
|---|---|---|---|---|
| Anthracene | - | - | 0.629 | 0.720 |
| Pentacene | - | 0.473 | 0.575 | 0.597 |
| Heptacene | 0.731 | 0.682 | 0.484 | 0.411 |
| 1 | - | - | 0.623 | 0.628 |
| 2 | - | 0.527 | 0.586 | 0.458 |
| 3 | 0.728 | 0.702 | 0.504 | 0.268 |
| 4 | - | - | 0.635 | 0.680 |
| 5 | - | 0.516 | 0.587 | 0.530 |
| 6 | 0.727 | 0.704 | 0.506 | 0.316 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starikova, A.A.; Chegerev, M.G.; Starikov, A.G.; Minkin, V.I. The Magnetic Properties of Fluorenyl and tert-Butyl-nitroxyl Acene-Based Derivatives: A Quantum Chemical Insight. Chemistry 2024, 6, 816-829. https://doi.org/10.3390/chemistry6050049
Starikova AA, Chegerev MG, Starikov AG, Minkin VI. The Magnetic Properties of Fluorenyl and tert-Butyl-nitroxyl Acene-Based Derivatives: A Quantum Chemical Insight. Chemistry. 2024; 6(5):816-829. https://doi.org/10.3390/chemistry6050049
Chicago/Turabian StyleStarikova, Alyona A., Maxim G. Chegerev, Andrey G. Starikov, and Vladimir I. Minkin. 2024. "The Magnetic Properties of Fluorenyl and tert-Butyl-nitroxyl Acene-Based Derivatives: A Quantum Chemical Insight" Chemistry 6, no. 5: 816-829. https://doi.org/10.3390/chemistry6050049
APA StyleStarikova, A. A., Chegerev, M. G., Starikov, A. G., & Minkin, V. I. (2024). The Magnetic Properties of Fluorenyl and tert-Butyl-nitroxyl Acene-Based Derivatives: A Quantum Chemical Insight. Chemistry, 6(5), 816-829. https://doi.org/10.3390/chemistry6050049








