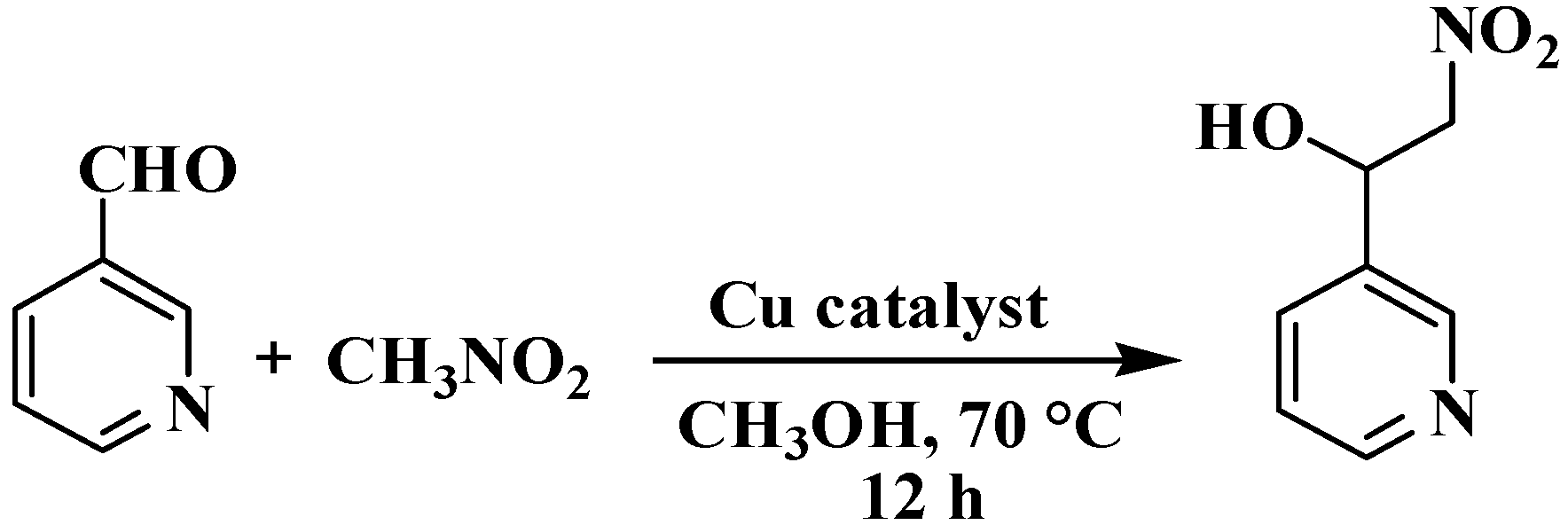Abstract
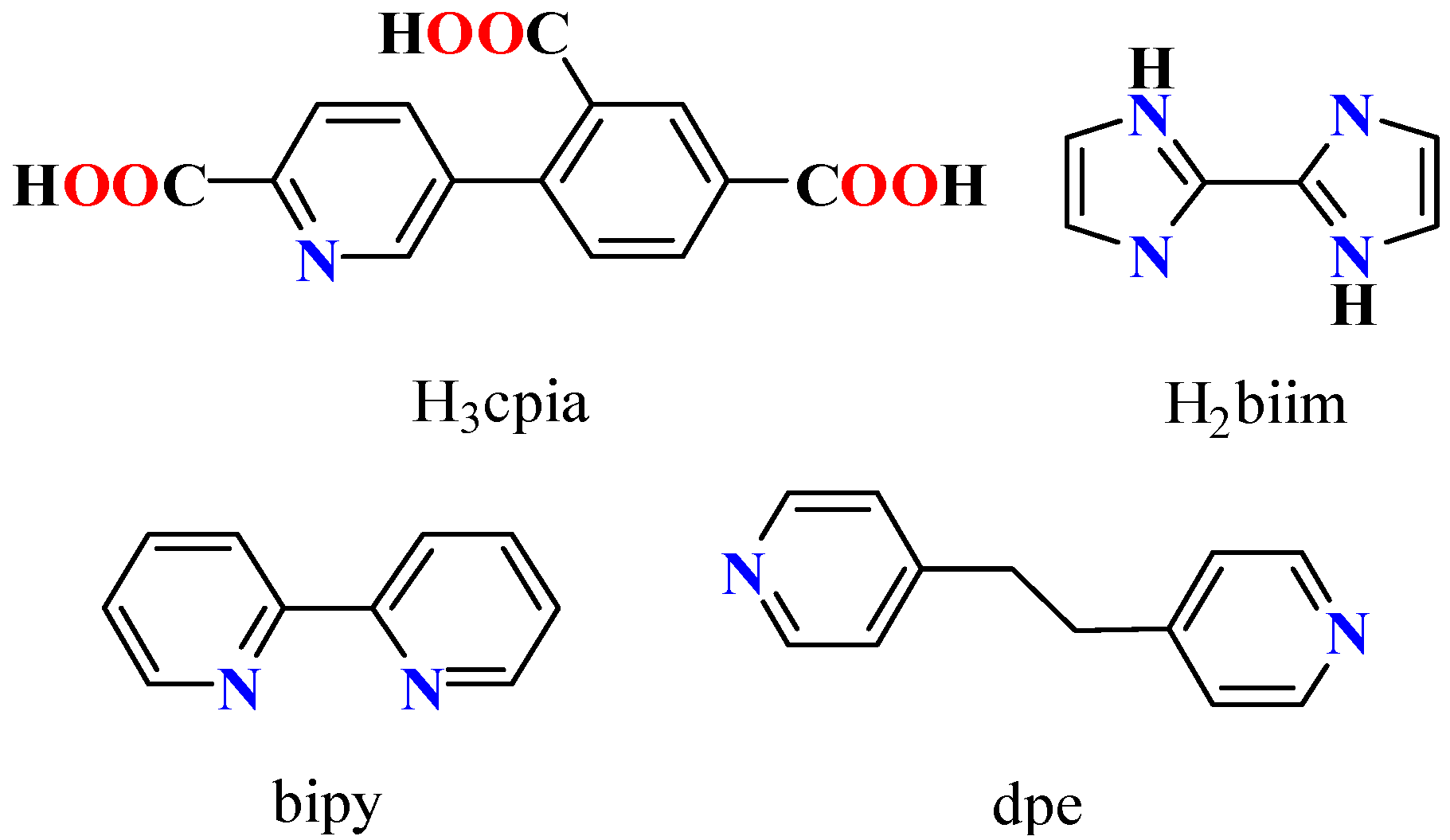
A pyridine–tricarboxylic acid, 4-(6-carboxy-pyridin-3-yl)-isophthalic acid (H3cpia), was used as a versatile building block to synthesize three novel coordination polymers under hydrothermal conditions and formulated as [Mn8(μ3-Hcpia)2 (μ6-cpia)4(Hbiim)2(H2O)6]n·6nH2O (1), [Cu3(μ4-cpia)2(bipy)2(H2O)2]n·4nH2O (2), and [Ni3(μ3-cpia)2(dpe)3(H2O)2]n·4nH2O (3). Three supporting ligands, 2,2′-biimidazole (H2biim), 2,2′-bipyridine (bipy), and 1,2-di(4-pyridyl)ethane (dpe), were used in the synthesis. The structures of the studied products 1–3 varied significantly, ranging from a 1D chain (2) to 2D sheets (1 and 3). Furthermore, these compounds were evaluated as heterogeneous catalysts for the Henry reaction, achieving high product yields under optimized conditions. In addition, we investigated various reaction parameters and substrate scopes, and assessed the feasibility of catalyst recycling. This thorough investigation’s results highlight the versatility of H3cpia as a tricarboxylate building block in the formation of functional coordination polymers.
1. Introduction
The generation of coordination polymers (CPs) through the self-assembly of metal ions with organic bridging ligands using a hydrothermal (solvothermal) method is a key objective in the field of crystal engineering [1,2,3]. Coordination polymers are a unique class of crystal materials [4,5,6]. Their versatile topologies and chemical properties [7,8] make them ideal candidates for diverse applications such as gas adsorption and separation [9,10,11,12], heterogeneous catalysis [13,14,15], sensing [11,16,17], and biomaterials [18].To study the process of mineral formation under supercritical conditions in the laboratory, researchers initially employed hydrothermal and solvothermal synthesis methods. These days, hydrothermal synthesis is extensively utilized in the construction of CPs, especially for the preparation of CPs crystals [19,20,21,22]. The self-assembly in these reactions is highly sensitive to several variables, including the types of metal ions, the concentration of reactants, as well as the pH value of the solution [23,24,25,26]. Polycarboxylate ligands are commonly used as versatile organic linkers in the synthesis of metal clusters and polymers. This preference stems from their partially or fully deprotonated sites, which enable the development of different structural topologies and exhibit a variety of coordination modalities [27]. These ligands, in particular, can function as terminal unidentate, chelating, bridging bidentate, and bridging tridentate ligands [28].
During the last few years, our research has focused on designing new functional coordination polymers using polycarboxylic ligands [13,21,29]. In this study, we chose4-(6-carboxy-pyridin-3-yl)-isophthalicacid (H3cpia, Scheme 1) as a pyridine–tricarboxylate building block.
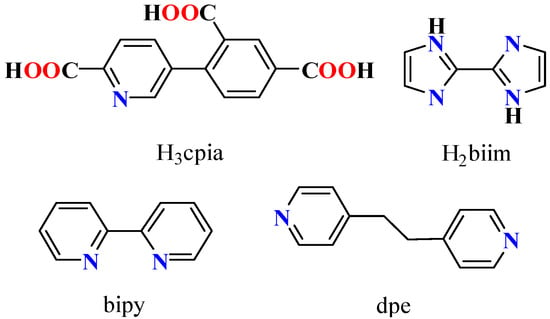
Scheme 1.
Structures of H3cpia linker and auxiliary ligands.
H3cpia possesses several intriguing properties that make it an attractive linker. H3cpia has seven coordination sites, including one pyridine nitrogen and six carboxyl oxygen atoms [30,31,32]. The free pyridine N atom can act as a base site for catalysis, facilitating reactions such as the Henry reaction, cyanosilylation, and Knoevenagel condensation [15,33,34].
Here, we report the synthesis and characterization of three novel CPs derived from metal(II) salts, H3cpia, and three second ligands. These compounds exhibit diverse structures, ranging from a 1D chain (2) to 2D sheets (1 and 3), specifically, [Mn8(μ3-Hcpia)2(μ6-cpia)4(Hbiim)2(H2O)6]n·6nH2O (1), [Cu3(μ4-cpia)2(bipy)2(H2O)2]n·4nH2O (2), and [Ni3(μ3-cpia)2(dpe)3(H2O)2]n·4nH2O (3).
In addition, we evaluated the catalytic activities of these compounds in the Henry reaction between pyridine-3-aldehydeand nitromethane.
2. Experimental Section
2.1. Materials and Measurements
All chemicals were purchased commercially and utilized exactly as supplied. To record FTIR spectra (KBr discs), a Bruker EQUINOX 55 spectrometer was utilized. Elemental analyses (EAs) for carbon, hydrogen, and nitrogen in CPs 1–3 were conducted using an Elementar Vario EL elemental analyzer. Thermogravimetric analyses (TGAs) were conducted using a LINSEIS STA PT1600 thermal analyzer under a nitrogen flow and heating rate of 10 °C/min. The powder X-ray diffraction (PXRD) patterns of the compounds were collected using a Rigaku-Dmax 2400 diffractometer (Cu-Kα radiation, λ = 1.54060 Å, Rigaku Corporation, Tokyo, Japan). For 1H NMR experiments, a JNM ECS 400 M spectrometer was used, with CDCl3 as the solvent.
2.2. Synthesis of [Mn8(μ3-Hcpia)2(μ6-cpia)4(H2biim)2(H2O)6]n·6nH2O (1)
A 20 mL Teflon cup was sealed with the following contents: H3cpia (0.057 g, 0.20 mmol), H2biim (0.040 g, 0.30 mmol), MnCl2∙4H2O (0.060 g, 0.30 mmol), NaOH (0.024 g, 0.60 mmol), and H2O (10 mL).After three days of heating the mixture at 160 °C, it was allowed to cool to ambient temperature. Yellow block-shaped crystals were extracted and rinsed with water. Yield: 41% based on H3cpia. Computed forC96H73Mn8N14O48 (%): C 43.84, H 2.80, N 7.46; found: C 44.13, H 2.78, N 7.42. IR (KBr, cm−1): 3078w, 2898w, 1690w, 1617s, 1594s, 1544s, 1482w, 1440m, 1406s, 1332w, 1256w, 1217w, 1174w, 1106w, 1044w, 1009w, 940w, 886w, 859w, 817w, 786m, 748m, 694m, 667w, 613w, 540w.
2.3. Synthesis of [Cu3(μ3-cpia)2(bipy)2(H2O)2]n·4nH2O (2)
A 20 mL Teflon cup was sealed with the following contents: H3cpia (0.057 g, 0.20 mmol), bipy (0.047 g, 0.30 mmol), CuCl2∙2H2O (0.051 g, 0.30 mmol), NaOH (0.024 g, 0.60 mmol), and H2O (10 mL). After three days of heating the mixture at 160 °C, it was allowed to cool to ambient temperature. Blue block-shaped crystals were extracted and rinsed with water. Yield: 47% based on H3cpia. Computed for C48H40Cu3N6O18 (%): C 48.88, H 3.42, N 7.13; found: C 48.53, H 3.44, N 7.15. IR (KBr, cm−1): 3426w, 3072w, 1649m, 1609s, 1540w, 1420w, 1360s, 1301w, 1246w, 1164w, 1040w, 920w, 865w, 814w, 786w, 764w, 714w, 658w, 562w.
2.4. Synthesis of [Ni3(μ3-cpia)2(μ-dpe)3(H2O)2]n·4nH2O (3)
A 20 mL Teflon cup was sealed with the following contents: H3cpia (0.057 g, 0.20 mmol), dpe (0.055 g, 0.30 mmol), NaOH (0.024 g, 0.60 mmol), NiCl2∙6H2O (0.071 g, 0.30 mmol), and H2O (10 mL).After three days of heating the mixture at 160 °C, it was allowed to cool to ambient temperature. Green block-shaped crystals were extracted and rinsed with water. Yield: 45%based on H3cpia. Computed forC64H60Ni3N8O18 (%): C 54.70, H 4.30, N 7.97; found: C 54.46, H 4.27, N 7.93. IR (KBr, cm−1): 3391w, 2950w, 2850w, 1650w, 1618s, 1532m, 1431w, 1395s, 1286w, 1232w, 1168w, 1095w, 1072w, 1045w, 1023w, 940w, 873w, 814m, 790w, 723w, 668w, 613w, 549w.
2.5. Single Crystal X-ray Diffraction and Topological Analysis
A Bruker APEX-II CCD diffractometer was used to collect the crystals’data of CPs 1–3 using graphite-monochromated MoKα radiation; λ = 0.71073 Å). SHELXS-97 and SHELXL-97(SHELXL-2014/7) software were used to determine their structures [35]. Detailed crystal parameters and structural refinements can be found in Table 1, with selected bond parameters listed in Tables S1 and S2 (Supplementary Materials). Supplementary crystallographic data for CPs 1–3 are available in CCDC 2375707–2375709.

Table 1.
Summary of crystal data for compounds 1–3.
Topological analysis of the obtained CPs was conducted using ToposPro software (version 5.5.2.0). This involved generating a simplified underlying net, wherein bridging ligands were reduced to the centroids [36,37].
2.6. Catalytic Henry Reaction
The following ingredients were mixed in a suspension and stirred at 70 °C for the required reaction time: catalyst (4.0 mol%), aromatic aldehyde (0.50 mmol), nitromethane (2.0 mmol), and solvent (1.0 mL, usually CH3OH). Centrifugation was subsequently used to extract the catalyst. A crude solid product was obtained by evaporating the filtrate using a rotary evaporator. The amount of this solid product was ascertained by 1H NMR spectroscopy (JNM ECS 400M spectrometer, Bruker BioSpin AG, Fällanden, Switzerland) after it was dissolved in CDCl3 (Figure S3). The catalyst was recovered by centrifugation, washed with methanol, allowed to dry at room temperature, and subsequently reused in further reactions, following the same procedure for recycling experiments.
3. Discussion of Results
3.1. Crystal Structure of CP 1
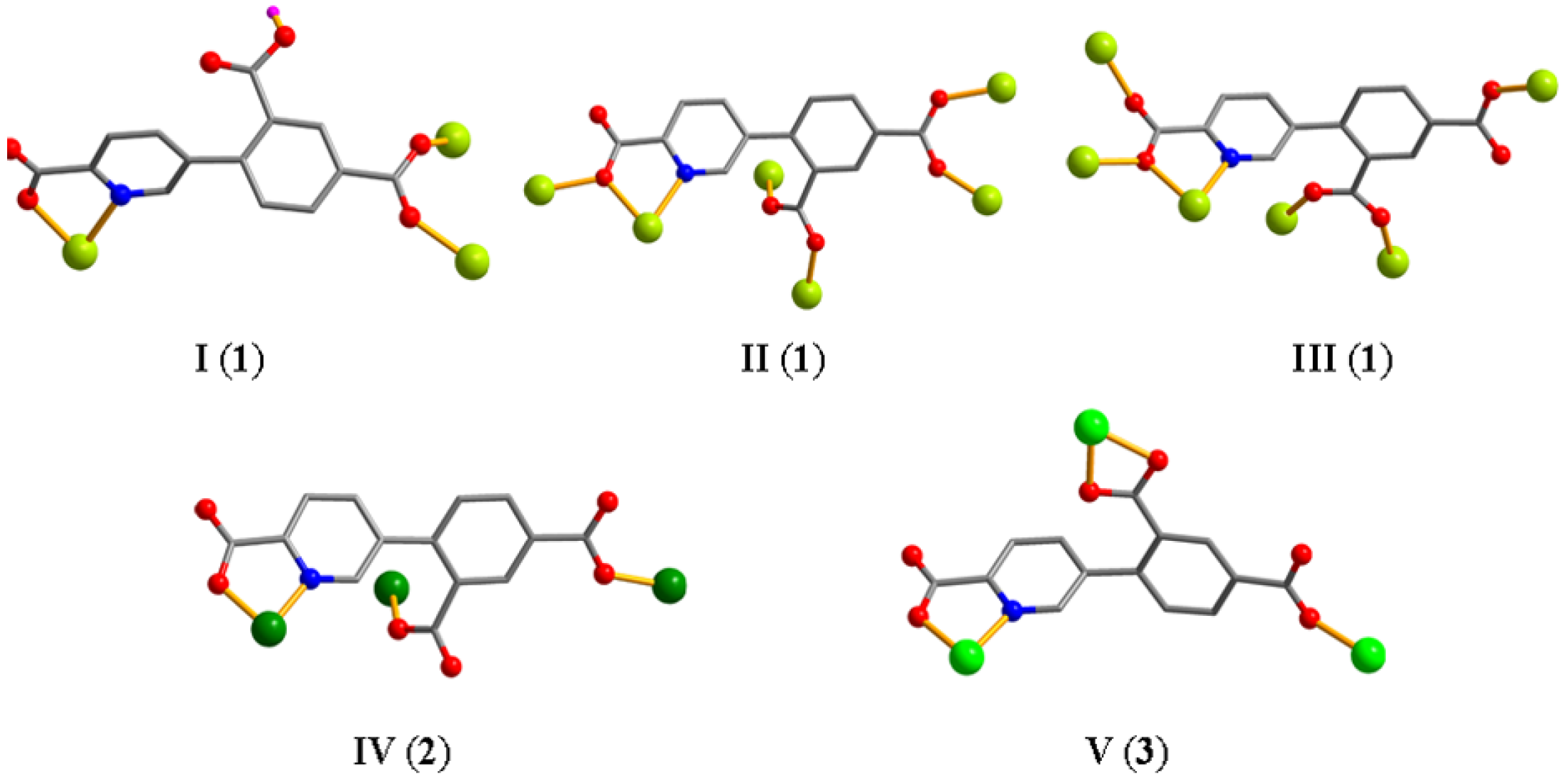
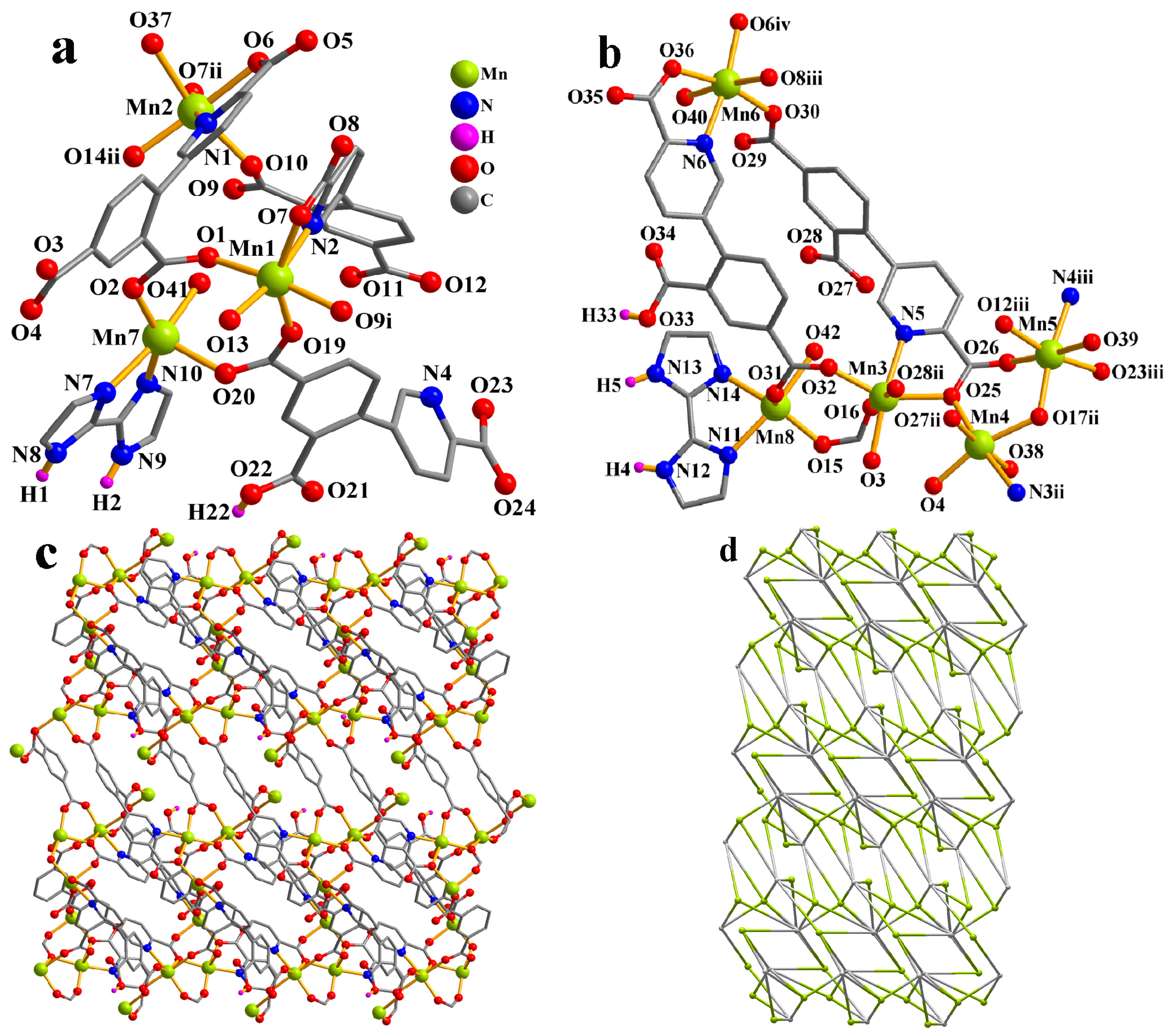
The asymmetric unit of CP 1 contains eight Mn atoms (Mn1–Mn8), two Hcpia2− and four cpia3− blocks, two H2biim second ligands, six H2O ligands, and six free water molecules (Figure 1a). Mn1–Mn6 atoms are six-coordinate and form distorted octahedral {MnNO5} geometries. Mn7 and Mn8 centers are five-coordinate and feature distorted trigonal bipyramid {MnN2O3} environments, which are completed by two carboxyl O atoms from one μ3-Hcpia2− and one μ6-cpia3− block, one O atom from the H2O ligand, and a pair of N donors from the H2biim moiety. Mn–O and Mn–N bond lengths, which measure 2.037(3)–2.322(4) and 2.169(4)–2.337(4) Å, respectively, are within the typical range for Mn(II) compounds [13,32]. In CP 1, the Hcpia2− ligand exhibits the coordination mode I (Scheme 2) with monodentate or bridging bidentate COO− groups. The cpia3− ligands adopt μ6-linkers (modes I and II, Scheme 2), wherein COO− groups are monodentate or bridging bidentate. The H2biim moiety features a terminal coordination fashion. The μ3-Hcpia2− and μ6-cpia3− blocks connect the Mn(II) centers, forming a 2D sheet (Figure 1c). The 2D sheet is composed of the 2-, 4-, 5- and 6-linked Mn7, Mn8/Mn2, Mn4, Mn5, and Mn6/Mn1/Mn3 nodes, and3-, µ3-Hcpia2−,and 6-linked µ6-cpia3− nodes (Figure 1d), which is a new topology, with a point symbol of (42.6)(43.62.8)(46.65.84)(46.66.83)(46)(47.63)(4).
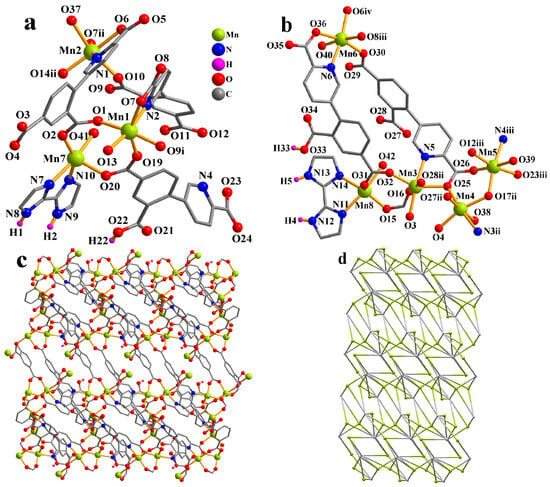
Figure 1.
Structure of CP 1. (a,b) Coordination environment at manganese(II) atoms. (c) Two-dimensional layer viewed along the c axis. (d) Sheet exhibiting a new topology, observed along the c axis.
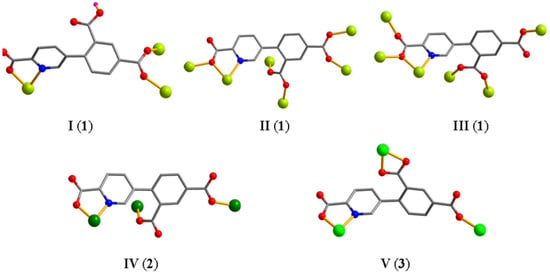
Scheme 2.
Coordination fashions of Hcpia2−/cpia3− linkers.
3.2. Crystal Structure of CP 2
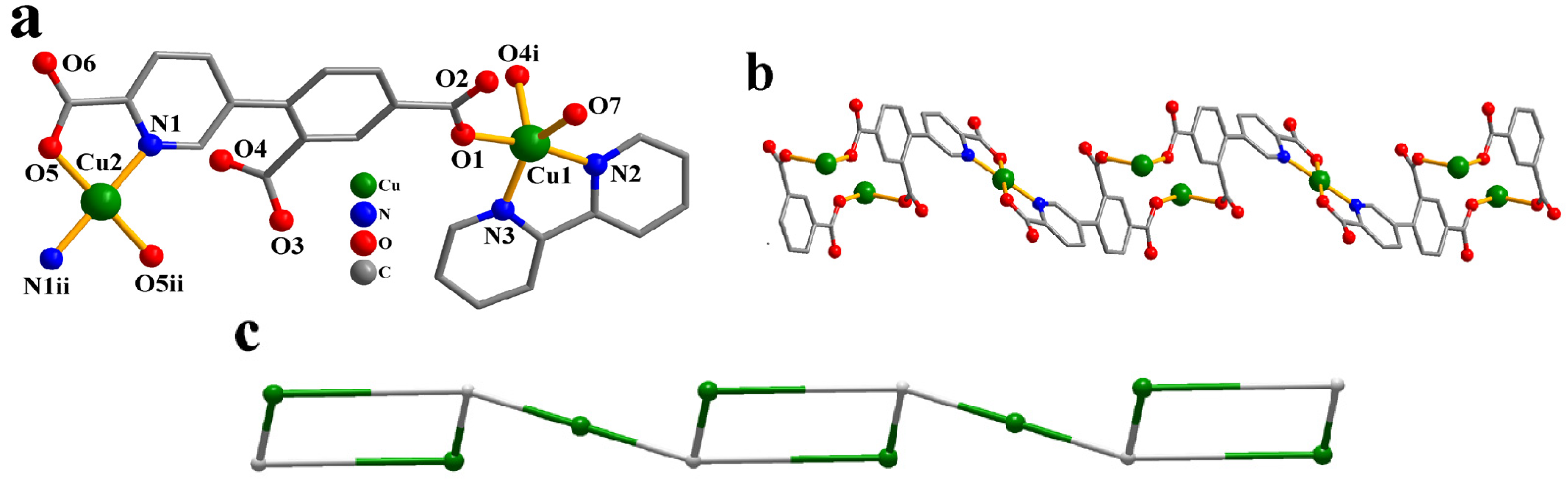
Two Cu(II) atoms (Cu1 with 100% occupancy and Cu2 with 50% occupancy), one μ8-cpia3− block, one bipy moiety, one H2O ligand, and two lattice water molecules form the asymmetric unit of CP 2 (Figure 2a). The five-coordinate Cu1 atom shows a distorted trigonal bipyramid {CuN2O3} environment. It comprises two carboxyl oxygen atoms from two individual µ3-cpia3− linkers, one O atom of the H2O ligand, and two Nbipy donors. The Cu2 atom is tetra-coordinate and adopts a distorted tetrahedral {CuN2O2} environment comprising two carboxyl oxygen and two N atoms of two different µ3-cpia3− linkers. Cu–O [1.935(3)–2.126(3) Å] and Cu–N [1.980(3)–1.998(4)Å] bond lengths fall within expected ranges [15,27]. The cpia3− ligand functions as a μ3-linker, with its carboxylate groups adopting a monodentate mode (mode IV, Scheme 2). The bipy moiety takes a terminal coordination fashion. The μ8-cpia3− linkers connect Cu(II) atoms to form a 1D chain (Figure 2b) with a decorated 2C1 topology (Figure 2c) and a point symbol of (0)(4)4.
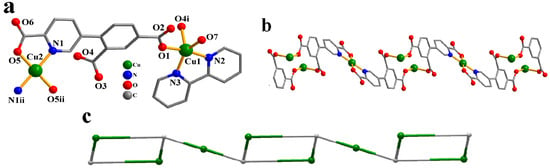
Figure 2.
Structure of CP 2. (a) Coordination environment at copper(II) atoms. (b) One-dimensional chain viewed along the b axis. (c) Chain exhibiting a decorated 2C1 topology, observed along the b axis.
3.3. Crystal Structure of CP 3
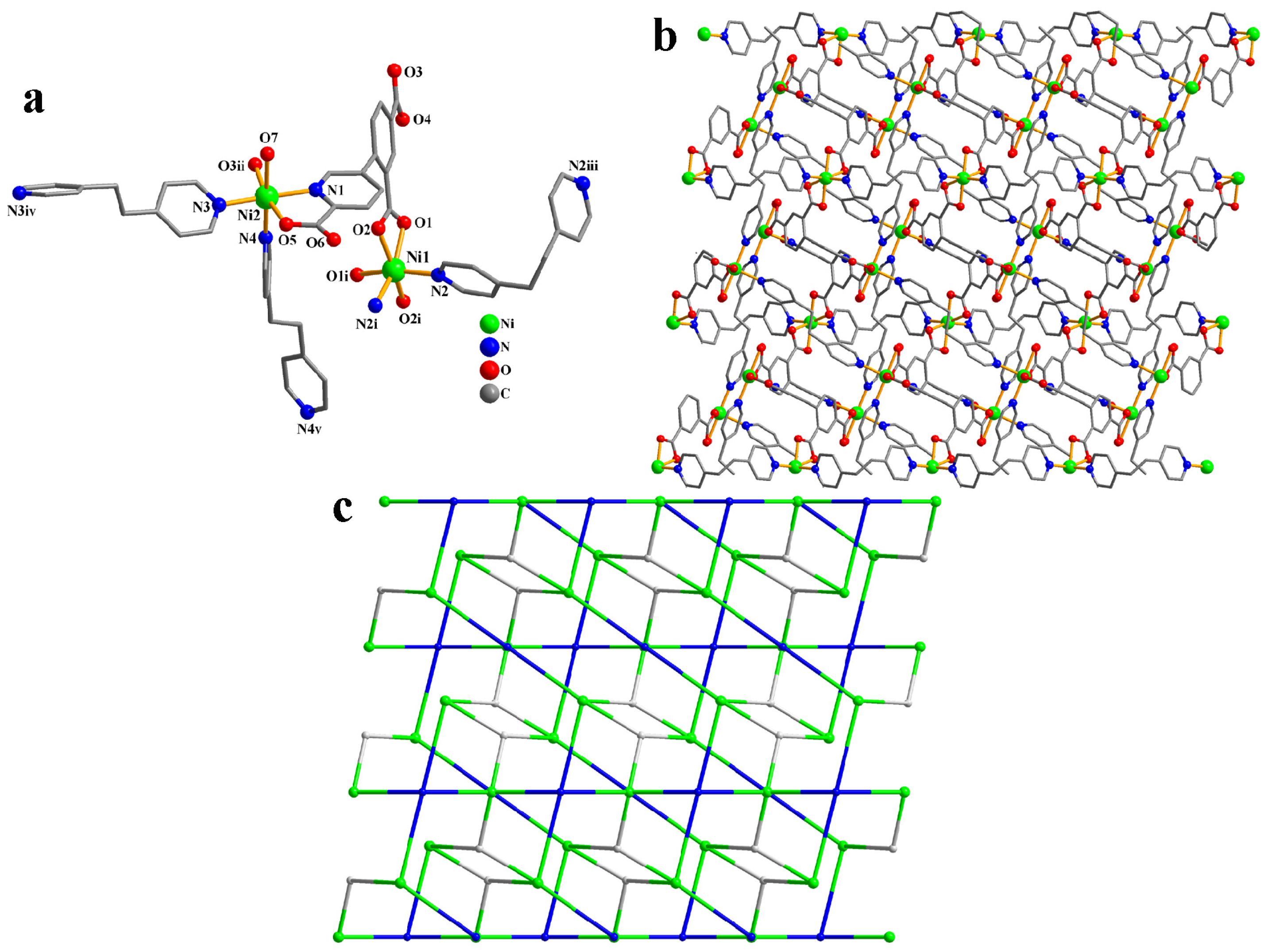
CP 3 is an asymmetric unit with one μ3-cpia3− linker, two nickel(II) atoms (Ni1 with 50% occupancy and Ni2 with 100% occupancy), one and a half μ-dpe second ligands, one H2O ligand, and two lattice H2O (Figure 3a). The six-coordinate Ni1 atom has a distorted octahedral {NiN2O4} geometry, made up of four carboxyl oxygen atoms from two individual µ3-cpia3− linkers and two Ndpe atoms. The Ni2 atom is also six-coordinate and adopts a distorted octahedral {NiN3O3} environment comprising two carboxyl oxygen and nitrogen atoms of two different µ3-cpia3− linkers, one oxygen atom from the H2O ligand, and two N atoms of dpe ligands. Ni–O [2.039(5)–2.188(5) Å] and Ni–N [2.061(6)–2.084(7) Å] distances are comparable to those in Ni(II) derivatives [29,32]. In 3, the cpia3− ligand behaves as a μ3-spacer (mode V, Scheme 2). Meanwhile, the dpe second ligand shows a bridging coordination fashion. The adjacent nickel(II) atoms are linked by μ3-cpia3− and μ-dpe blocks, generating a 2D sheet (Figure 3b). The 2D structure is built from 4-linked Ni1 nodes, 3-linked µ3-cpia3− nodes, and 2-connected µ-dpe linkers (Figure 3c). It is a new topology with a point symbol of (10)(4.8.102.122)2(4.8.10)2(82.103.12)(8)2.
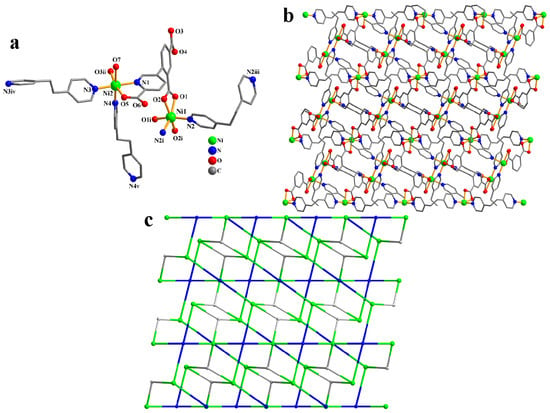
Figure 3.
Structure of CP 3. (a) Coordination environment at nickel(II) atoms. (b) Two-dimensional layer viewed along the b axis. (c)Sheet exhibiting a new topology, observed along the b axis.
3.4. TGA and PXRD Data
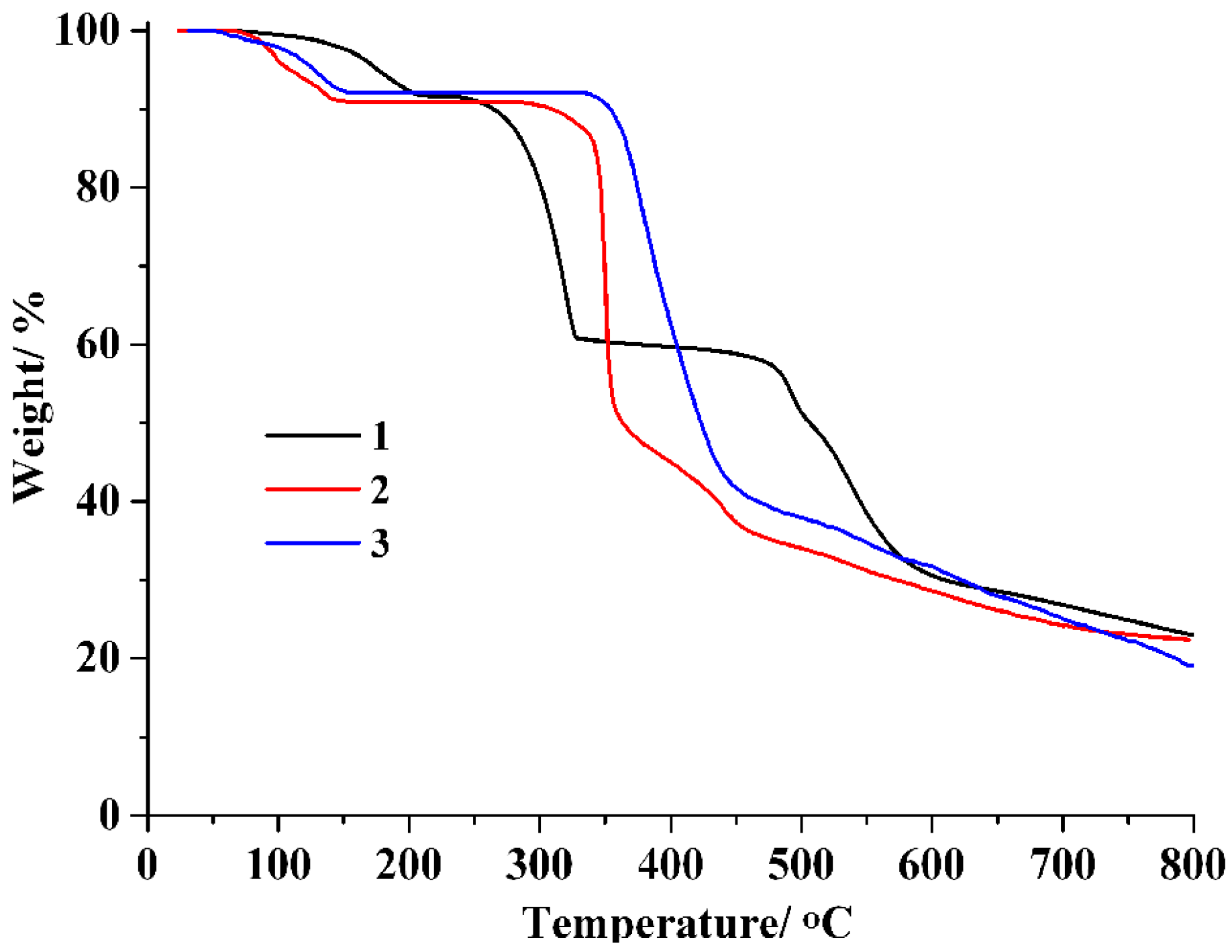
The assessment of thermal stability for CPs 1–3 was carried out using thermogravimetric analysis (TGA) under a N2 atmosphere within a temperature range of 24–800 °C (Figure 4). At temperatures 67 to 215 °C, CP 1 lost six lattice and six coordinated water molecules (exptl 8.3%; calculated 8.2%), whereas the dehydrated sample held steady until 238 °C.For CP 2, a weight loss of 9.0%(calcd9.2%) occurred at temperatures from 65 to 150 °C, associated with the release offour lattice water molecules and two water ligands. In CP 3, four lattice water and two water ligands were lost at 53–153 °C (exptl, 7.9%; calcd, 7.7%), whereas the dehydrated sample was stable up to 334 °C.
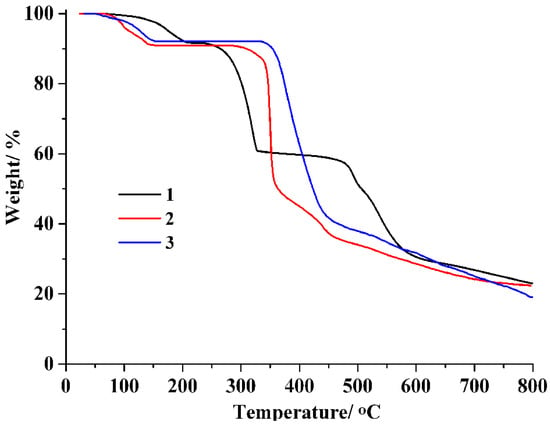
Figure 4.
TGA curves of polymers 1–3.
Power X-ray diffractograms were acquired at 25 °C for CPs 1–3 (Figure S2). The phase purity of all samples was established by analyzing their experimental patterns and comparing them with the simulated ones (based on CIF data).
3.5. Catalytic Henry Reaction
We investigated CPs 1–3 for their potential as heterogeneous catalysts in the Henry reaction, which involves a combination of nitromethane and a variety of aldehydes, considering the possibility of different coordination complexes acting as catalysts [15,38,39]. Using pyridine-3-aldehyde as a model substrate, we reacted it with nitromethane at 70 °C in methanol to synthesize 2-nitro-1-(pyridin-3-yl)ethan-1-ol product (Scheme 3, Table 2). Furthermore, we conducted a thorough investigation of various reaction parameters, which included the duration of the reaction, temperature conditions, the type of solvent used, catalyst loading, potential for catalyst reuse, and the scope of substrates involved. CP 2 exhibited the highest activity, achieving a 98% conversion rate of pyridine-3-aldehyde to 2-nitro-1-(pyridin-3-yl)ethan-1-ol (Table 2). It was then used to study the effects of different reaction parameters. The yield increased from 45% to 98% when the reaction time was extended from 1 to 12 h (Table 2, entries 1–7). The impact of the catalyst amount was also examined, and the results showed that increasing the loading of the catalyst from 3 to 5 mol% increased product yield from 86% to 98% (entries 12 and 13). Entry 10 shows that at 60 °C, CP 2 containing 4 mol% catalyzed the reaction and yielded 77% of product; however, a slightly higher temperature of 70 °C resulted in a 98% yield. It is worth mentioning that various solvents, including methanol, were tested in these reactions. THF, acetonitrile, water, and ethanol all had lower product yields (35–89%). CPs 1 and 3 exhibited reduced activity when compared to CP 2, achieving product yields in the 85–92% range (entries 18 and 19, Table 2). It is worth mentioning that the Henry reaction of pyridine-3-aldehyde was much less efficient without a catalyst (only 3% product yield) or when H3cpia or CuCl2 were used as catalysts (16% yield or 13% yield, respectively) under comparable reaction conditions (entries 20–22, Table 2). The improved performance of CPs 1 and 2 was likely due to the presence of unsaturated coordination sites in the metal centers, while there was no discernible correlation between catalyst structure and activity [15,38,39].
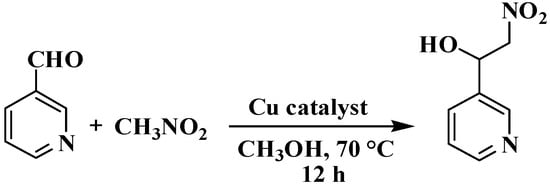
Scheme 3.
Henry reaction between pyridine-3-aldehydeand nitromethane.

Table 2.
Henry reaction of pyridine-3-aldehyde and nitromethane a.
A range of substituted benzaldehyde substrates was evaluated to determine the substrate scope in the Henry reaction and nitromethane. These reactions were carried out under optimized conditions (4.0 mol% catalyst 2, methanol, 70 °C, 12 h). Table S3 shows that the yields of the respective products ranged from 14% to 98%. Benzaldehydes with strong electron-withdrawing groups, such as nitro and chloro substituents, demonstrated the highest efficiency (entries 2–5, Table S3). This increased efficiency was likely due to the higher electrophilicity of these substrates. However, benzaldehydes with electron-donating groups, such as methyl or methoxy, resulted in lower product yields (entries 7 and 8, Table S3). The recyclability of catalyst 2 was evaluated. After every reaction cycle, the catalyst was separated by centrifugation, rinsed in methanol, air-dried at approximately 25 °C, and reused in the subsequent cycle. Data show that CP 2 retained its activity for at least five more reaction cycles (Figure S5).Additionally, PXRD patterns show that the structure of CP 2 remained intact (Figure S6) despite the occurrence of multiple new signals or widened peaks. These changes were likely due to the existence of or a decrease in certain crystallinity after multiple catalytic cycles.
The catalytic efficiency of CP 2 was comparable to, or even surpassed, that of other heterogeneous catalytic systems based on metal–carboxylate coordination compounds, particularly when considering catalyst loading and product yield (Table S4) [40].
4. Conclusions
This study showed the use of H3cpia as a pyridine–tricarboxylate precursor to synthesize three novel coordination polymers using a hydrothermal method. These compounds 1–3 exhibited diverse structural features, ranging from a 1D chain (CP 2) to 2D sheets (CPs 1 and 3). These CPs (based on Hcpia2−/cpia3−blocks) disclosed several types of topologies: sqc2, pcu, SnS, 2C1, sql, and 4,4L34 [32]. In this study, CPs 1 and 3 showed two new topologies. The catalytic potential of CPs 1−3 was evaluated using the Henry reaction, which involved pyridine-3-aldehydeandnitromethane. CP 2 showed significant catalytic efficiency in this reaction.
Supplementary Materials
The following data are available online at https://www.mdpi.com/article/10.3390/chemistry6040048/s1. Figure S1. FT-IR spectra. Figure S2. PXRD patterns. Figures S3–S6. additional catalysis data. Tables S1 and S2. selected bonding and H-bonding parameters for 1–3. Table S3. Substrate scope of catalytic reaction. and Table S4. Comparison of various catalysts for the Henry reaction.
Author Contributions
Conceptualization, H.W. and J.G.; data curation, Z.M.; funding acquisition, Y.Y. and J.G.; investigation, Z.M., X.K. and Y.Y.; methodology, J.G.; visualization, H.W. and J.G.; writing—original draft, Z.M., H.W. and J.G.; writing—review and editing, H.W. and J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Science and Technology Projects of the National Nickel Cobalt New Material Engineering Technology Research Center, Lanzhou, China (grant number GCZX2023JSKF0005).
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lu, J.Y. Crystal engineering of Cu–containing metal—Organic coordination polymers under hydrothermal conditions. Coord. Chem. Rev. 2003, 246, 327–347. [Google Scholar] [CrossRef]
- Chen, X.M.; Tong, M.L. Solvothermal in Situ Metal/Ligand Reactions: A New Bridge between Coordination Chemistry and Organic Synthetic Chemistry. Acc. Chem. Res. 2007, 40, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.R.; Wang, X.Z.; Li, Y.; Du, J.; Liu, S.X.; Luo, Y.; Huo, J.Z.; Liu, Y.Y.; Wu, X.X.; Ding, B. Hydrothermal Preparation of a Series of Luminescent Cadmium(II) and Zinc(II) Coordination Complexes and Enhanced Real-time Photo-luminescent Sensing for Benzaldehyde. Z. Anorg. Allg. Chem. 2018, 644, 357–366. [Google Scholar] [CrossRef]
- Chakraborty, G.; Park, I.H.; Medishetty, R.; Vittal, J.J. Two−Dimensional Metal–Organic Framework Materials: Synthesis, Structures, Properties and Applications. Chem. Rev. 2021, 121, 3751–3891. [Google Scholar] [CrossRef] [PubMed]
- Maurin, G.; Serre, C.; Cooper, A.; Ferey, G. The new age of MOFs and of their porous-related solids. Chem. Soc. Rev. 2017, 46, 3104–3107. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Kirlikovali, K.O.; Li, P.; Farha, O.K. Reticular Chemistry for Highly Porous Metal–Organic Frameworks: The Chemistry and Applications. Acc. Chem. Res. 2022, 55, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Macreadie, L.K.; Babarao, R.; Setter, C.J.; Lee, S.J.; Qazvini, O.T.; Seeber, A.J.; Tsanaktsidis, J.; Telfer, S.G.; Batten, S.R.; Hill, M.R. Enhancing Multicomponent Metal–Organic Frameworks for Low Pressure Liquid Organic Hydrogen Carrier Separations. Angew. Chem. Int. Ed. 2020, 59, 6090–6098. [Google Scholar] [CrossRef]
- Dong, X.Y.; Si, Y.; Yang, S.J.; Zhang, C.; Han, Z.; Luo, P.; Wang, Z.Y.; Zang, S.Q.; Mak, T.C.W. Ligand engineering to achieve enhanced ratiometric oxygen sensing in a silver cluster-based metalorganic framework. Nat. Commun. 2020, 11, 3678. [Google Scholar] [CrossRef]
- Gu, S.F.; Xiong, X.H.; Gong, L.L.; Zhang, H.P.; Xu, Y.; Feng, X.F.; Luo, F. Classified Encapsulation of an Organic Dye and Metal–Organic Complex in Different Molecular Compartments for White-Light Emission and Selective Adsorption of C2H2 over CO2. Inorg. Chem. 2021, 60, 8211–8217. [Google Scholar] [CrossRef]
- Fan, L.; Liu, Z.; Zhang, Y.; Zhao, D.; Yang, J.; Zhang, X. p-Terphenyl-2,2″,5″,5‴–tetracarboxylate acid based bifunctional 1D Zinc(II) metal–organic platform for luminescent sensing and gas adsorption. Inorg. Chem. Commun. 2019, 107, 107463. [Google Scholar] [CrossRef]
- Wu, D.; Liu, J.; Jin, J.; Cheng, J.; Wang, M.; Yang, G.; Wang, Y.Y. New Doubly Interpenetrated MOF with [Zn4O] Clusters and Its Doped Isomorphic MOF: Sensing, Dye, and Gas Adsorption Capacity. Cryst. Growth Des. 2019, 19, 6774–6783. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Fan, N.N.; Han, M.L.; Yang, G.P.; Ma, L.F. Porous Zn(II)–Based Metal–Organic Frameworks Decorated with Carboxylate Groups Exhibiting High Gas Adsorption and Separation of Organic Dyes. Cryst. Growth Des. 2018, 18, 7114–7121. [Google Scholar] [CrossRef]
- Kang, X.Q.; Ren, C.; Mei, Z.Z.; Fan, X.X.; Xue, J.J.; Shao, Y.L.; Gu, J.Z. Hydrothermal Assembly, Structural Multiplicity, and Catalytic Knoevenagel Condensation Reaction of a Series of Coordination Polymers Based on a Pyridine-Tricarboxylic Acid. Molecules 2023, 28, 7474. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Shen, Q.; Li, Z.; Jing, X.; Duan, C. Two Copper–Containing Polyoxometalate–Based Metal−Organic Complexesas Heterogeneous Catalysts for the C−H Bond Oxidation of Benzylic Compounds and Olefin Epoxidation. Inorg. Chem. 2022, 61, 11156–11164. [Google Scholar] [CrossRef] [PubMed]
- Markad, D.; Mandal, S.K. Synthesis and structural characterization of a novel dinuclear Cu(II) complex: An efficient and recyclable bifunctional heterogeneous catalyst for the diastereoselective Henry reaction. Dalton Trans. 2018, 47, 5928–5932. [Google Scholar] [CrossRef] [PubMed]
- Mörtel, M.; Oschwald, J.; Scheurer, A.; Drewello, T.; Khusniyarov, M.M. Molecular Valence Tautomeric Metal Complexes for Chemosensing. Inorg. Chem. 2021, 60, 14230–14237. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Mondal, S.; Ghosh, P. Development and Application of Ruthenium(II) and Iridium(III) Based Complexes for Anion Sensing. Molecules 2023, 28, 1231. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.Q.; Lin, P.Y.; Wang, X.H.; Ho, J.A. Metal–organic frameworks in diagnostics, therapeutics, and other biomedical applications. J. Chin. Chem. Soc. 2023, 70, 1284–1296. [Google Scholar] [CrossRef]
- Qin, Y.; She, P.; Huang, X.; Huang, W.; Zhao, Q. Luminescent manganese(II) complexes: Synthesis, properties and optoelectronic applications. Coord. Chem. Rev. 2020, 416, 213331. [Google Scholar] [CrossRef]
- Hong, L.C.; Lin, L.Y.; Wei, L.; Fei, K.Y. Hydrothermal Synthesis, Crystal Structure and Properties of a New Binuclear Nickel(III) Complex with 3-(Pyridin-2-yl)-1,2,4-triazole. Chin. J. Struct. Chem. 2021, 40, 363–368. [Google Scholar]
- Kang, X.Q.; Wang, J.H.; Gu, J.Z. Syntheses, crystals tructures, and catalytic properties of three zinc(II), nickel(II) and cobalt(II) coordination polymers constructed from 4,4′-(pyridin-3,5-diyl)dibenzoic acid. Chinese. J. Inorg. Chem. 2023, 39, 2385–2392. [Google Scholar]
- Singh, R.S.; Paitandi, R.P.; Gupta, R.K.; Pandey, D.S. Recent developments in metal dipyrrin complexes: Design, synthesis, and applications. Coord. Chem. Rev. 2020, 414, 213269. [Google Scholar] [CrossRef]
- Islam, S.; Tripathi, S.; Hossain, A.; Seth, S.K.; Mukhopadhyay, S. pH-induced structural variations of two new Mg(II)–PDA complexes: Experimental and theoretical studies. J. Mol. Struct. 2022, 1265, 133373. [Google Scholar] [CrossRef]
- Fonseca, D.; Pérez-Torres, A.F.; Cobo, J.; Zapata-Rivera, J.; Hurtado, J.J.; Macías, M.A. Influence of the Lewis basicity hardness of crystallization solvents on the coordinations phere of the complex [Co(3,5–dinitrobenzoate–O,O′)2]: Crystallographic and theoretical analysis. CrystEngComm 2022, 24, 2982–2991. [Google Scholar] [CrossRef]
- Xu, M.; Liang, G.; Wang, S.; Ma, X.; Liang, G.; Ni, Q. Structural variability, topology and luminescent properties of three new cadmium(II) coordination polymers based on 4′,4′,4′–[(trimethylamino)]–tris[(1,1′–biphenyl)–2–carboxylate]. J. Mol. Struct. 2020, 1217, 128411. [Google Scholar] [CrossRef]
- Kong, J.J.; Shao, D.; Zhang, J.C.; Jiang, Y.X.; Ji, C.L.; Huang, X.C. From mononuclear to two-dimensional cobalt(II) complexes based on a mixed benzimidazole-dicarboxylate strategy: Syntheses, structures, and magnetic properties. CrystEngComm 2019, 21, 749–757. [Google Scholar] [CrossRef]
- Huang, R.-W.; Li, B.; Zhang, Y.-Q.; Zhao, Y.; Zang, S.-Q.; Xu, H. Divalent cobalt, zinc, and copper coordination polymers based on a new bifunctional ligand: Syntheses, crystal structures, and properties. Inorg. Chem. Commun. 2014, 39, 106–109. [Google Scholar] [CrossRef]
- Chai, X.; Zhang, H.; Zhang, S.; Cao, Y.; Chen, Y. The tunable coordination architectures of a flexible multicarboxylate N-(4-carboxyphenyl)iminodiacetic acid via different metal ions, pH values and auxiliary ligand. J. Solid State Chem. 2009, 182, 1889–1898. [Google Scholar] [CrossRef]
- Fan, X.X.; Wang, H.Y.; Gu, J.Z.; Lv, D.Y.; Zhang, B.; Xue, J.J.; Kirillova, M.V.; Kirillov, A.M. Coordination Polymers from an Amono-Founctionalized Terphenyl-Tetracarboxylate Linker: Structural Multiplicity and Catalytic Properties. Inorg. Chem. 2023, 62, 17612–17624. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Du, L.Y.; Zhang, W.X.; Li, R.F.; Feng, F.; Feng, X. Syntheses, structures and hirshfeld surface analyses of two 3D supramolecules based on nitrogen-heterocyclictric arboxylate ligand. J. Mol. Struct. 2019, 1194, 138–143. [Google Scholar] [CrossRef]
- You, L.X.; Wang, S.J.; Xiong, G.; Ding, F.; Sun, Y.G. Two Coordination Polymers Generated via insitu Decarboxylation of 3-(2,4-Dicarboxylphenyl)-2,6-dicarboxylpyridine: Structure and Magnetism. Chin. J. Inorg. Chem. 2015, 31, 323–328. [Google Scholar]
- Zhao, N.; Li, Y.; Gu, J.Z.; Kirillova, M.V.; Kirillov, A.M. Hydrothermal generation, structural versatility and properties of metal(Ⅱ)-organic architectures driven by a pyridine-tricarboxylic acid. Dalton Trans. 2019, 48, 8361–8374. [Google Scholar] [CrossRef]
- Gu, J.Z.; Wan, S.M.; Kirillova, M.V.; Kirillov, A.M. H-Bonded and metal(II)–organic architectures assembled from an unexplored aromatic tricarboxylic acid: Structural variety and functional properties. Dalton Trans. 2020, 49, 7197–7209. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Aggarwal, H.; Gupta, R. Three-Dimensional Heterometallic Coordination Networks: Syntheses, Crystal Structures, Topologies, and Heterogeneous Catalysis. Cryst. Growth Des. 2015, 15, 4110–4122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97, Program for X-ray Crystal Structure Determination; University of Gottingen: Göttingen, Germany, 1997. [Google Scholar]
- Blatov, V.A. Multipurpose crystallochemical analysis with the program package TOPOS. IUCrCompComm Newsletter 2006, 7, 4–38. [Google Scholar]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package topospro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Guo, L.R.; Wang, H.Y.; Gu, J.Z.; Yang, Y.; Kirillova, M.V.; Kirillov, A.M. Coordination Polymers from Biphenyl–Dicarboxylate Linkers: Synthesis, Structural Diversity, Interpenetration, and Catalytic Properties. Inorg. Chem. 2022, 61, 12577–12590. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; De, D.; Bharadwaj, P.K. A NbO type Cu(II) metal-organic framework showing efficient catalytic activity in the Friedlander and Henry reactions. Dalton Trans. 2017, 46, 7782–7790. [Google Scholar] [CrossRef] [PubMed]
- Neumann, T.; Ceglarska, M.; Germann, L.S.; Rams, M.; Dinnebier, R.E.; Suckert, S.; Jess, I.; Näther, C. Structures, Thermodynamic Relations, and Magnetism of Stable and Metastable Ni(NCS)2 Coordination Polymers. Inorg. Chem. 2018, 57, 3305–3314. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).