Efficient Two-Step Synthesis of Novel Pyrimido[4,5-d] Pyrimidines with Potent Neuroprotective, Antioxidant, and Aβ Anti-Aggregation Properties
Abstract
1. Introduction
2. Results
2.1. Synthesis
2.2. Biological Evaluation
2.2.1. In Cellulo Evaluation of the Neuroprotective Activity of Compounds 4a–k
2.2.2. Antioxidant Analysis
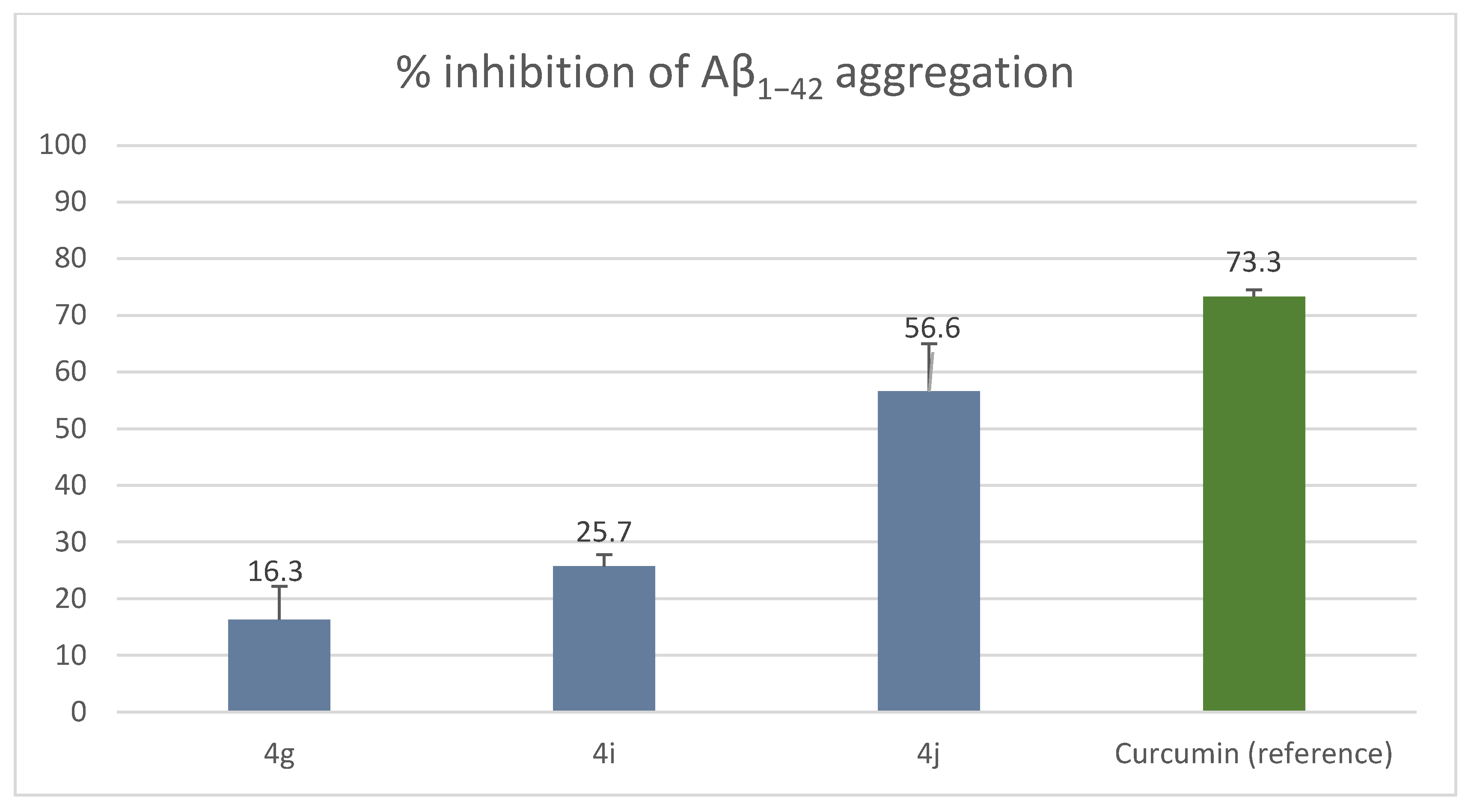
2.2.3. Inhibition of Self-Induced Aβ1–42 Aggregation by Compounds 4g, 4i and 4j
2.2.4. ADME Studies
3. Materials and Methods
3.1. Synthesis of Compounds 2a–k
3.2. Synthesis of Compounds 4a–k
3.3. Biological Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Benchekroun, M.; Pachón-Angona, I.; Luzet, V.; Martin, H.; Oset-Gasque, M.-J.; Marco-Contelles, J.; Ismaili, L. Synthesis, Antioxidant and Aβ Anti-Aggregation Properties of New Ferulic, Caffeic and Lipoic Acid Derivatives Obtained by the Ugi Four-Component Reaction. Bioorganic Chem. 2019, 85, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Ismaili, L.; Bernard, P.J.; Refouvelet, B. Latest Progress in the Development of Multitarget Ligands for Alzheimer’s Disease Based on the Hantzsch Reaction. Future Med. Chem. 2022, 14, 943–946. [Google Scholar] [CrossRef]
- Bernard, P.J.; Bellili, D.; Ismaili, L. Calcium Channel Blockers’ Contribution to Overcoming Current Drug Discovery Challenges in Alzheimer’s Disease. Expert Opin. Drug Discov. 2024, 19, 21–32. [Google Scholar] [CrossRef]
- Mkaouar, K.; Iriepa, I.; Diez-Iriepa, D.; Marco-Contelles, J.; Ismaili, L.; Chabchoub, F. Synthesis of (±)-cis-1-Aryl-3-Oxo-2,3-Dihydro-1H-benzo[f]chromene-2-carbonitriles and (±)-trans-4-Aryl-2-oxo-3,4-Dihydro-2H-benzo[h]chromene-3-carbonitriles. ChemistrySelect 2019, 4, 12902–12905. [Google Scholar] [CrossRef]
- Choura, E.; El Ghali, F.; Bernard, P.J.; Marco-Contelles, J.; Aifa, S.; Ismaili, L.; Chabchoub, F. Synthesis and Biological Evaluation of New Benzochromenopyrimidines for the Therapy of Colon and Lung Cancer. ChemistrySelect 2024, 9, e202303632. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s Disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Christen, Y. Oxidative Stress and Alzheimer Disease. Am. J. Clin. Nutr. 2000, 71, 621s–629s. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, M.; Coppede, F.; Migliore, L.; Siciliano, G.; Murri, L. Mitochondrial Dysfunction, Oxidative Stress and Neurodegeneration. J. Alzheimers Dis. 2006, 10, 59–73. [Google Scholar] [CrossRef] [PubMed]
- von Bernhardi, R.; Eugenín, J. Alzheimer’s Disease: Redox Dysregulation as a Common Denominator for Diverse Pathogenic Mechanisms. Antioxid. Redox Signal. 2011, 16, 974–1031. [Google Scholar] [CrossRef]
- Giasson, B.I.; Ischiropoulos, H.; Lee, V.M.-Y.; Trojanowski, J.Q. The Relationship between Oxidative/Nitrative Stress and Pathological Inclusions in Alzheimer’s and Parkinson’s Diseases. Free. Radic. Biol. Med. 2002, 32, 1264–1275. [Google Scholar] [CrossRef]
- McBean, G.J.; López, M.G.; Wallner, F.K. Redox-Based Therapeutics in Neurodegenerative Disease. Br. J. Pharmacol. 2017, 174, 1750–1770. [Google Scholar] [CrossRef] [PubMed]
- Guzior, N.; Bajda, M.; Skrok, M.; Kurpiewska, K.; Lewiński, K.; Brus, B.; Pišlar, A.; Kos, J.; Gobec, S.; Malawska, B. Development of Multifunctional, Heterodimeric Isoindoline-1,3-Dione Derivatives as Cholinesterase and β-Amyloid Aggregation Inhibitors with Neuroprotective Properties. Eur. J. Med. Chem. 2015, 92, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Albertini, C.; Salerno, A.; de Sena Murteira Pinheiro, P.; Bolognesi, M.L. From Combinations to Multitarget-Directed Ligands: A Continuum in Alzheimer’s Disease Polypharmacology. Med. Res. Rev. 2021, 41, 2606–2633. [Google Scholar] [CrossRef] [PubMed]
- Pathak, C.; Kabra, U.D. A Comprehensive Review of Multi-Target Directed Ligands in the Treatment of Alzheimer’s Disease. Bioorganic Chem. 2024, 144, 107152. [Google Scholar] [CrossRef] [PubMed]
- Dgachi, Y.; Ismaili, L.; Knez, D.; Benchekroun, M.; Martin, H.; Szałaj, N.; Wehle, S.; Bautista-Aguilera, O.M.; Luzet, V.; Bonnet, A.; et al. Synthesis and Biological Assessment of Racemic Benzochromenopyrimidinimines as Antioxidant, Cholinesterase, and Aβ1–42 Aggregation Inhibitors for Alzheimer’s Disease Therapy. ChemMedChem 2016, 11, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Dgachi, Y.; Bautista-Aguilera, O.M.; Benchekroun, M.; Martin, H.; Bonet, A.; Knez, D.; Godyń, J.; Malawska, B.; Gobec, S.; Chioua, M.; et al. Synthesis and Biological Evaluation of Benzochromenopyrimidinones as Cholinesterase Inhibitors and Potent Antioxidant, Non-Hepatotoxic Agents for Alzheimer’s Disease. Molecules 2016, 21, 634. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Maddeboina, K.; Bachu, R.D.; Boddu, S.H.S.; Trippier, P.C.; Tiwari, A.K. Pivotal Role of Nitrogen Heterocycles in Alzheimer’s Disease Drug Discovery. Drug Discov. Today 2022, 27, 103322. [Google Scholar] [CrossRef]
- Martorana, A.; Giacalone, V.; Bonsignore, R.; Pace, A.; Gentile, C.; Pibiri, I.; Buscemi, S.; Lauria, A.; Piccionello, A.P. Heterocyclic Scaffolds for the Treatment of Alzheimer’s Disease. Curr. Pharm. Des. 2016, 22, 3971–3995. [Google Scholar] [CrossRef]
- Rastegari, A.; Nadri, H.; Mahdavi, M.; Moradi, A.; Mirfazli, S.S.; Edraki, N.; Moghadam, F.H.; Larijani, B.; Akbarzadeh, T.; Saeedi, M. Design, Synthesis and Anti-Alzheimer’s Activity of Novel 1,2,3-Triazole-Chromenone Carboxamide Derivatives. Bioorganic Chem. 2019, 83, 391–401. [Google Scholar] [CrossRef]
- Xu, M.; Peng, Y.; Zhu, L.; Wang, S.; Ji, J.; Rakesh, K.P. Triazole Derivatives as Inhibitors of Alzheimer’s Disease: Current Developments and Structure-Activity Relationships. Eur. J. Med. Chem. 2019, 180, 656–672. [Google Scholar] [CrossRef]
- Zribi, L.; Pachòn-Angona, I.; Bautista-Aguilera, Ò.M.; Diez-Iriepa, D.; Marco-Contelles, J.; Ismaili, L.; Iriepa, I.; Chabchoub, F. Triazolopyridopyrimidine: A New Scaffold for Dual-Target Small Molecules for Alzheimer’s Disease Therapy. Molecules 2020, 25, 3190. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Silakari, O. Multifunctional Compounds: Smart Molecules for Multifactorial Diseases. Eur. J. Med. Chem. 2014, 76, 31–42. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Rushell, E.; Kumar, M. 9—Use of Sustainable Organic Transformations in the Construction of Heterocyclic Scaffolds. In Green Approaches in Medicinal Chemistry for Sustainable Drug Design; Banik, B.K., Ed.; Advances in Green and Sustainable Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 245–352. ISBN 978-0-12-817592-7. [Google Scholar]
- González-Muñoz, G.C.; Arce, M.P.; López, B.; Pérez, C.; Romero, A.; Barrio, L.d.; Martín-de-Saavedra, M.D.; Egea, J.; León, R.; Villarroya, M.; et al. N-Acylaminophenothiazines: Neuroprotective Agents Displaying Multifunctional Activities for a Potential Treatment of Alzheimer’s Disease. Eur. J. Med. Chem. 2011, 46, 2224–2235. [Google Scholar] [CrossRef] [PubMed]
- Denizot, F.; Lang, R. Rapid Colorimetric Assay for Cell Growth and Survival. Modifications to the Tetrazolium Dye Procedure Giving Improved Sensitivity and Reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC–Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Benchekroun, M.; Bartolini, M.; Egea, J.; Romero, A.; Soriano, E.; Pudlo, M.; Luzet, V.; Andrisano, V.; Jimeno, M.-L.; López, M.G.; et al. Novel Tacrine-Grafted Ugi Adducts as Multipotent Anti-Alzheimer Drugs: A Synthetic Renewal in Tacrine-Ferulic Acid Hybrids. ChemMedChem 2015, 10, 523–539. [Google Scholar] [CrossRef]
- Kam, T.-I.; Gwon, Y.; Jung, Y.-K. Amyloid Beta Receptors Responsible for Neurotoxicity and Cellular Defects in Alzheimer’s Disease. Cell. Mol. Life Sci. 2014, 71, 4803–4813. [Google Scholar] [CrossRef]
- Levine III, H. Thioflavine T Interaction with Synthetic Alzheimer’s Disease β-Amyloid Peptides: Detection of Amyloid Aggregation in Solution. Protein Sci. 1993, 2, 404–410. [Google Scholar] [CrossRef]
- Aronov, A.M. Predictive in Silico Modeling for hERG Channel Blockers. Drug Discov. Today 2005, 10, 149–155. [Google Scholar] [CrossRef]
- Aronov, A.M. Ligand Structural Aspects of hERG Channel Blockade. Curr. Top. Med. Chem. 2008, 8, 1113–1127. [Google Scholar] [CrossRef] [PubMed]

| Compound | R1 | R2 | R3 | R4 | R5 | R6 | Yield (%) |
|---|---|---|---|---|---|---|---|
| 4a | CH3 | H | H | H | H | H | 57 |
| 4b | C2H5 | H | H | H | H | H | 50 |
| 4c | CH3 | C2H5 | H | H | H | H | 35 |
| 4d | CH3 | H | CH3 | H | H | H | 16 |
| 4e | CH3 | CH3 | CH3 | H | H | H | 20 |
| 4f | CH3 | CH3 | H | H | H | H | 50 |
| 4g | CH3 | CH3 | H | H | CH3 | H | 28 |
| 4h | CH3 | C2H5 | H | H | CH3 | H | 30 |
| 4i | CH3 | CH3 | H | H | Cl | H | 22 |
| 4j | CH3 | C2H5 | H | OCH3 | OCH3 | H | 20 |
| 4k | CH3 | H | H | OCH3 | H | OCH3 | 47 |
| Compound | Neuroprotection (%) against H2O2 a at 0.1, 1 and 5µM | ORAC c (TE) | ||
|---|---|---|---|---|
| 0.1 µM | 1 µM | 5 µM | ||
| 4a | - b | - b | - b | 0.07 ± 0.00 |
| 4b | 6.17 ± 0.01 | 4.95 ± 0.01 | 14.66 ± 0.04 | - b |
| 4c | 6.96 ± 0.02 | 3.09 ± 0.2 | 8.64 ± 0.05 | 0.24 ± 0.02 |
| 4d | 9.40 ± 0.01 | - b | - b | 0.14 ± 0.01 |
| 4e | - b | 34.00 ± 0.09 * | 8.45 ± 0.2 | 0.17 ± 0.01 |
| 4f | 4.75 ± 0.01 | 6.98 ± 0.02 | 24.30 ± 0.02 * | 0.19 ± 0.02 |
| 4g | 47.03 ± 0.12 ** | 68.06 ± 0.22 ** | 77.55 ± 0.21 ** | 0.24 ± 0.02 |
| 4h | 66.13 ± 0.17 ** | 38.77 ± 0.07 ** | 26.12 ± 0.16 ** | - b |
| 4i | 68.35 ± 0.23 ** | 117.89 ± 0.04 *** | 94.54 ± 0.33 *** | 0.34 ± 0.01 |
| 4j | 66.94 ± 0.23 ** | 45.89 ± 0187 ** | 11.14 ± 0.15 | 1.64 ± 0.02 |
| 4k | 0.13 ± 0.01 | - b | - b | - b |
| Trolox | - d | - d | - d | 0.99 ± 0.01 |
| Melatonin | - d | - d | - d | 2.45 ± 0.09 |
| Compound | Molweight (g/mol) | CLogP | H-Acceptors | H-Donors | Topological Polar Surface Area (Å2) | hERG Inhibition |
|---|---|---|---|---|---|---|
| 4a | 312.375 | 3.8793 | 4 | 0 | 51.56 | low_risk |
| 4b | 326.402 | 4.2949 | 4 | 0 | 51.56 | low_risk |
| 4c | 340.429 | 4.6928 | 4 | 0 | 51.56 | medium_risk |
| 4d | 326.402 | 4.2232 | 4 | 0 | 51.56 | medium_risk |
| 4e | 340.429 | 4.6211 | 4 | 0 | 51.56 | medium_risk |
| 4f | 326.402 | 4.2772 | 4 | 0 | 51.56 | low_risk |
| 4g | 340.429 | 4.6211 | 4 | 0 | 51.56 | medium_risk |
| 4h | 354.456 | 5.0367 | 4 | 0 | 51.56 | medium_risk |
| 4i | 360.847 | 4.8832 | 4 | 0 | 51.56 | medium_risk |
| 4j | 400.481 | 4.5528 | 6 | 0 | 70.02 | medium_risk |
| 4k | 372.427 | 3.7393 | 6 | 0 | 70.02 | medium_risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Ameur, G.; Maalej, E.; Martin, H.; Jacquinot, A.-S.; Barbanneau, N.; Bernard, P.J.; Marco-Contelles, J.; Chabchoub, F.; Ismaili, L. Efficient Two-Step Synthesis of Novel Pyrimido[4,5-d] Pyrimidines with Potent Neuroprotective, Antioxidant, and Aβ Anti-Aggregation Properties. Chemistry 2024, 6, 695-705. https://doi.org/10.3390/chemistry6040041
Ben Ameur G, Maalej E, Martin H, Jacquinot A-S, Barbanneau N, Bernard PJ, Marco-Contelles J, Chabchoub F, Ismaili L. Efficient Two-Step Synthesis of Novel Pyrimido[4,5-d] Pyrimidines with Potent Neuroprotective, Antioxidant, and Aβ Anti-Aggregation Properties. Chemistry. 2024; 6(4):695-705. https://doi.org/10.3390/chemistry6040041
Chicago/Turabian StyleBen Ameur, Ghada, Emna Maalej, Helene Martin, Anne-Sophie Jacquinot, Nadine Barbanneau, Paul J. Bernard, José Marco-Contelles, Fakher Chabchoub, and Lhassane Ismaili. 2024. "Efficient Two-Step Synthesis of Novel Pyrimido[4,5-d] Pyrimidines with Potent Neuroprotective, Antioxidant, and Aβ Anti-Aggregation Properties" Chemistry 6, no. 4: 695-705. https://doi.org/10.3390/chemistry6040041
APA StyleBen Ameur, G., Maalej, E., Martin, H., Jacquinot, A.-S., Barbanneau, N., Bernard, P. J., Marco-Contelles, J., Chabchoub, F., & Ismaili, L. (2024). Efficient Two-Step Synthesis of Novel Pyrimido[4,5-d] Pyrimidines with Potent Neuroprotective, Antioxidant, and Aβ Anti-Aggregation Properties. Chemistry, 6(4), 695-705. https://doi.org/10.3390/chemistry6040041







