Abstract
Aaptamine (8,9-dimethoxy-1H-benzo[de][1,6]naphthyridine), an alkaloid obtained from marine sponges of the genus Aaptos (Demospongiae, Suberitida, Suberitidae), has attracted significant attention as a promising scaffold for the development of antioxidant, antibacterial, and anticancer agents. This review offers an extensive overview of updated research on aaptamine, focusing on its multifaceted pharmacological properties. The antioxidant potential of aaptamine reflects its potential ability for use in the DPPH free radical scavenging assay, for suppressing ROS, and subsequently deactivating the MAPK and AP-1 signaling pathway. Moreover, it demonstrates notable antibacterial activity against pathogenic bacteria, including mycobacterial active and dormant states, making it a potential candidate for combating bacterial infections. Additionally, aaptamine shows promising anticancer activity by inhibiting cancer cell proliferation, apoptosis induction, and suppressing tumor growth through various signaling pathways, including the regulation of PTEN/PI3K/Akt and CDK2/4, and the regulation of cyclin D1/E in cell cycle arrest. The unique chemical structure of aaptamine offers opportunities for structural modifications aimed at enhancing its antioxidant, antibacterial, and anticancer activities. The exploration of aaptamine as a scaffold in the development of novel therapeutic agents offers great promise for addressing various challenges associated with oxidative stress, bacterial infections, and cancer. This article underscores the potential of aaptamine as a valuable marine-derived scaffold in the fields of antioxidant, antibacterial, and anticancer therapy.
1. Introduction
Natural products (NPs) play a vital role in medicine, with many pharmaceuticals derived from compounds found in nature. These natural products, which include substances like plants, fungi, bacteria, and marine organisms, have been integral components of traditional medicinal practices [1]. They serve as an abundant source of potential drugs and therapeutic agents due to their diverse structural framework and pharmacological activities [2]. These natural components have emerged as novel drug candidates, particularly in the case of life-threatening diseases which lack effective treatments [3,4]. Despite the development of synthetic drugs, which tend to be more potent, approximately 60% of drugs available today originate from natural sources. These natural drugs offer lower risks of side effects and better absorption rates, making them the preferred choice for lead structure identification and optimization [5,6]. The advantages of natural products in medicine include their often well-established safety profiles, lower incidence of adverse effects compared to synthetic drugs, and the potential for synergistic interactions among multiple compounds within a natural source. Additionally, NPs provide inspiration for drug discovery efforts, with scientists continually exploring their chemical diversity and pharmacological properties to develop novel therapies [3,7,8]. Oceans cover 70% of the earth and host a vast array of diverse marine life [9,10]. Marine organisms including algae, bacteria, fungi, invertebrates, and vertebrates bear an abundant source of compounds with promising pharmaceutical potential [11,12]. These organisms acquire unique chemical defenses and adaptations to survive in challenging marine environments, making them promising candidates for new drug discovery and development. Marine-derived compounds have demonstrated diverse biological activities, including antimicrobial, anti-inflammatory, anticancer, analgesic, and neuroprotective activities [13,14,15,16,17]. The investigation of marine-derived NPs began in the 1950s with the discovery of nucleoside derivatives in marine sponges, Tethya crypta, marking the emergence of promising research areas of interest [18]. By the 1960s, research on marine products predominantly centered on their chemical analysis, with fewer biological studies [19,20]. The isolation of prostaglandin (PG) derivatives from the Caribbean Sea whip (coral), Plexaura homomalla, marked a significant milestone in utilizing marine bioactive metabolites [21,22]. The unique structural frameworks and diverse pharmacological activities of marine compounds have captured researchers’ interest in describing novel pharmaceutical potentials [7,23]. These investigations have demonstrated that the marine environment harbors a wealth of new metabolites with intriguing chemical structures, many of which hold promise to develop multiple targeted drug candidates [24,25]. Among the plethora of marine invertebrates, sponges stand out for their substantial production of metabolites, boasting unprecedented structures and notable activity. Consequently, sponges have been the focal point of natural product studies for numerous years [26]. Apart from aaptamine, several marine alkaloids, including Pityriacitrin, Cyclizidine J, Neopetrosins A-D, Fascaplysin, and Lepadin A, have shown potential anticancer activities. Notably, anticancer agents from marine sources, such as didemnin B, aplidine, dolastatin-10, bryostatin-1, and ecteinascidin-743 (trabectedin), have reached clinical trial stages. Aaptamine (1), a natural product isolated from marine sponges, has garnered significant attention from researchers due to its intriguing chemical structure and diverse pharmacological properties (Figure 1). This article provides an overview on the current understanding of aaptamine, including its isolation, structural elucidation, synthesis, and biological activities. Aaptamine exhibits a broad spectrum of biological activities against cancer, inflammation, and microorganisms. Moreover, its unique chemical architecture makes it an attractive target for synthetic efforts aimed at developing analogues with improved therapeutic properties. The exploration of aaptamine and its derivatives holds promise to discover new drug candidates for various medical applications including cancer. This review article has been planned to elaborate on aaptamine as a versatile therapeutic agent, emphasizing the antioxidant, antibacterial, and anticancer properties of aaptamine derivatives. Future research should focus on structural modifications and unexplored molecular targets to develop aaptamine as a promising candidate in drug discovery and development.
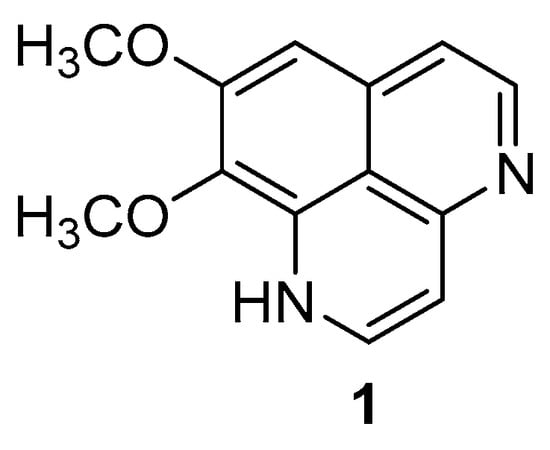
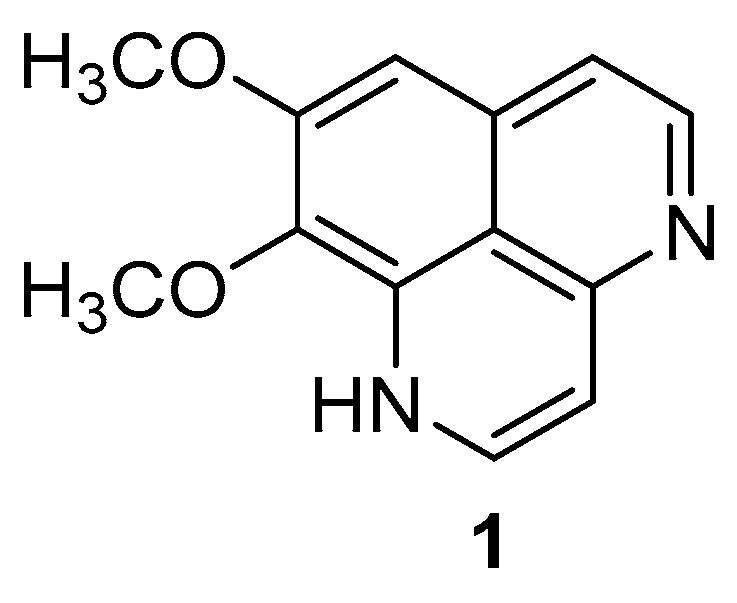
Figure 1.
Chemical structure of aaptamine.
2. Isolation and Total Synthesis of Aaptamine
2.1. Isolation of Aaptamine
In 1982, Nakamura et al. efficiently isolated aaptamine from the marine sponge A. aaptos. They prepared a methanolic extract of the sea sponge and fractionated it with various solvents. The purification of the ethanol soluble fraction was carried out using column chromatography (CHCl3:MeOH = 8:2). The resulting product underwent three-time recrystallization with a methanol: acetone solvent system, yielding bright yellow crystals of aaptamine with a melting point of 110–113 °C [27].
2.2. Total Synthesis of Aaptamine
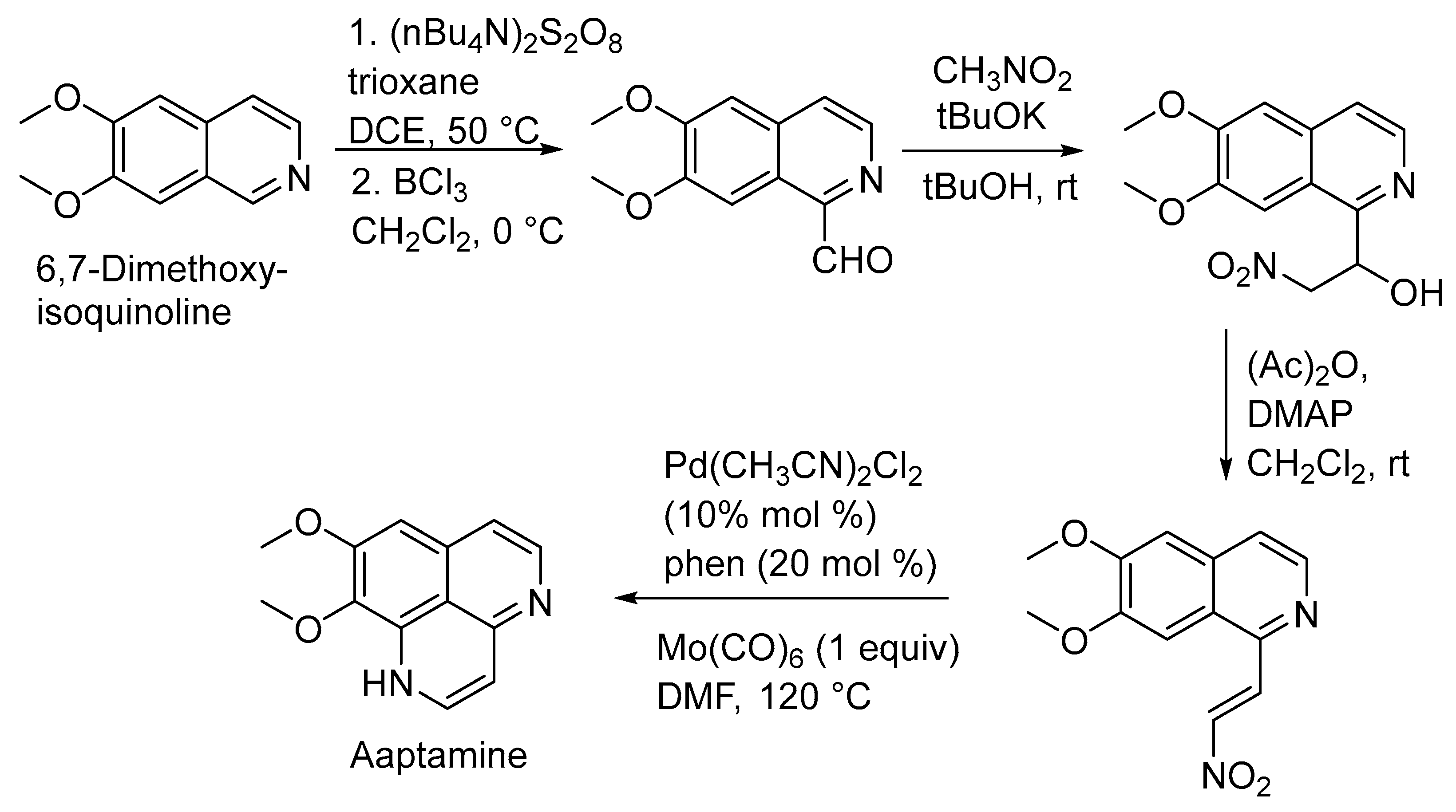
In 2019, Yaun Gao et al. achieved aaptamine’s total synthesis efficiently. They treated 6,7-dimethoxyisoquinoline with trioxane and (nBu4N)2S2O8, leading to a coupling reaction. The subsequent hydrolysis with BF3 yielded the corresponding aldehyde. Further steps involved a Henry reaction and elimination reaction to produce the nitro-alkene, followed by a palladium-catalyzed reductive cyclization, ultimately resulting in aaptamine synthesis (Scheme 1) [28].
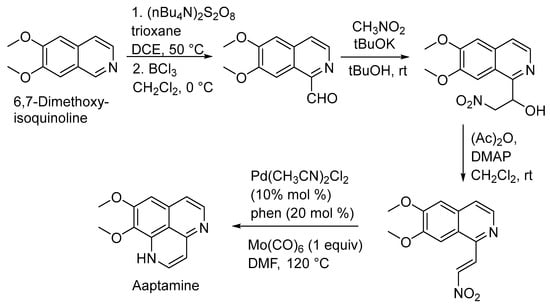
Scheme 1.
Total synthesis of aaptamine.
3. Biological Activities
3.1. Antioxidant Activities
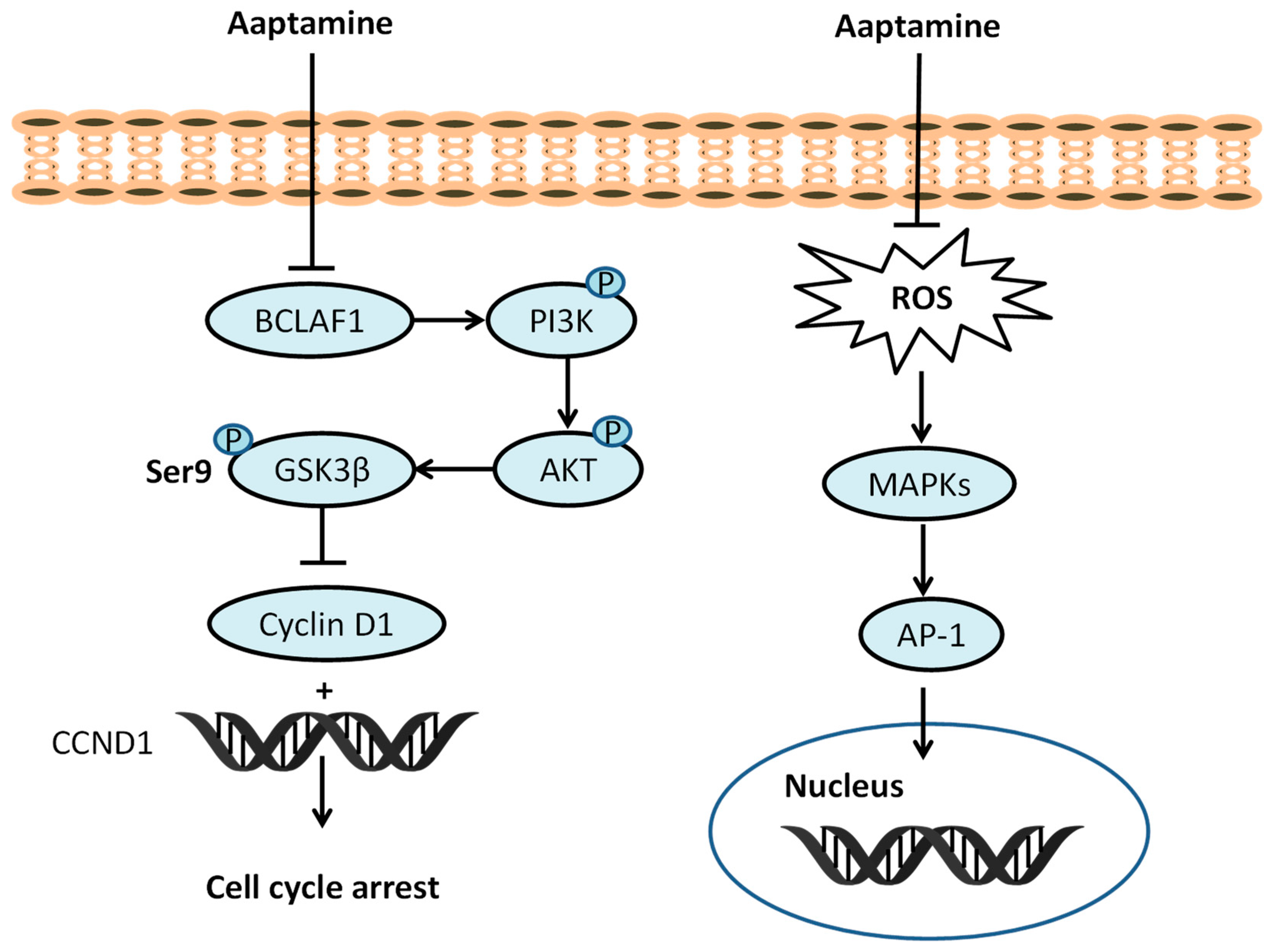
Exposure to ionizing radiation at the cellular level leads to increase levels of reactive oxygen species (ROS), including hydroxyl radicals (HO•), hydrogen peroxide (H2O2), and superoxide anions (O2•−); this exposure causes oxidative stress and results in the development of oxidative stress-induced diseases [29,30,31]. ROS have deleterious effects on cells, primarily targeting macromolecules such as DNA, proteins, and lipids. At lower concentrations, ROS function as intercellular signaling messengers. However, at higher levels, ROS can induce oxidative stress, resulting in damage to cellular structures and functions. This oxidative stress is associated with various diseases, including cancer, inflammation, Alzheimer’s disease, Parkinson’s disease, and cardiovascular diseases [29,31,32,33]. Superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), glutathione-S-transferase (GST), and reduced glutathione (GSH) represent endogenous mechanisms crucial for preserving cellular redox balance. Exogenous antioxidants, such as vitamins A, C, and E neutralize the effect of ROS generated from various sources [34,35]. In 2009, Utkina et al. evaluated compounds 1 (IC50 18 μM) and 2 (IC50 16 μM) for their ability to trap DPPH radicals, reduce the Folin–Ciocalteau reagent (FCR), and inhibit peroxide radical-induced linoleic acid (LA) oxidation. They reacted strongly with DPPH, comparable to trolox (IC50 16 μM), and showed a high FCR reducing ability (GAE 0.50 and 0.52 mmol/L) compared to trolox (GAE 0.70 mmol/L). The inhibition of LA oxidation by compounds 1 and 2 (0.91 μm/min and 0.78 μm/min, respectively) was comparable to ionol (BHT) at 0.84 μm/min [36]. The correlation between number and hydroxyl (OH) functionality and antioxidant potential was established by Takamatus et al. in 2003 [37]. Aaptamine and isoaaptamine displayed antioxidant potential (DPPH), while compounds remained inactive towards Dichloro-dihydro-fluorescein diacetate (DCFH-DA), with IC50 > 55 μM compared to ascorbic acid IC50 9.7 μM. The antioxidant potential of aaptamine depends on the quenching ability and subsequent penetration to cellular membranes by using the 2′7′-dichlorodihydrofluorescein diacetate cellular-based assay [37]. Aaptamine (10 µM) can increase levels of antioxidant enzymes such as CAT (71.87%), SOD (26.2%), and GPx (95.43%) as compared to a UVB control. Aaptamine (1, 5 or 10 µM) can suppress ROS, the subsequent deactivation of mitogen-activated protein kinases (MAPKs) and the activator protein-1 (AP-1) signaling pathway [38]. Aaptamine (10 µM) decreases pro-inflammatory cytokines including cyclooxygenase-2 (COX-2) (518.75%), tumor necrosis factor-α (TNF-α) (665.57%), interleukin-1β (IL-1β) (579.66%), and nuclear factor-kappa B (NF-κB) subunits (453.33%), and p65 (352.89%) in UVB-irradiated human keratinocytes (Figure 2).
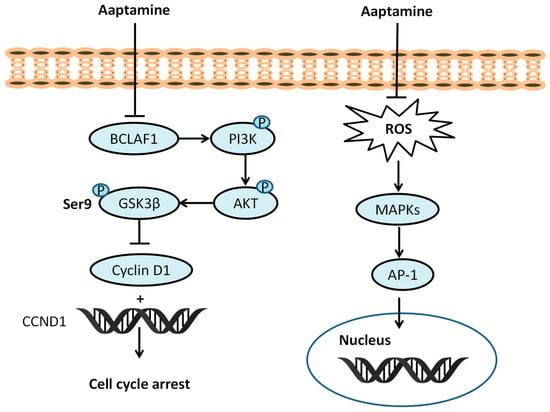
Figure 2.
Effect of aaptamine on PI3K/AKT/GSK3β, ROS/MAPKs/AP-1 pathway.
3.2. Antimicrobial Activity
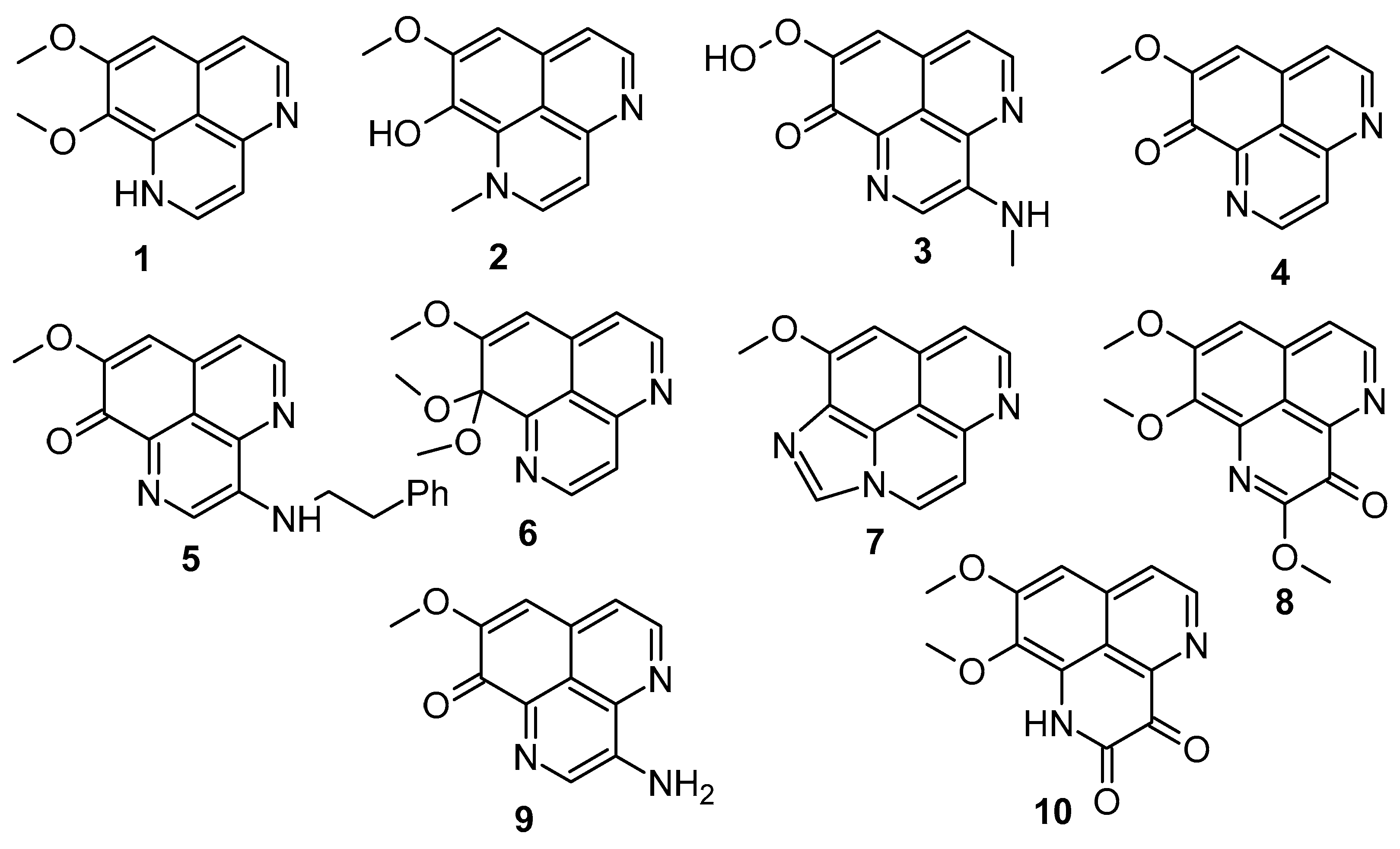
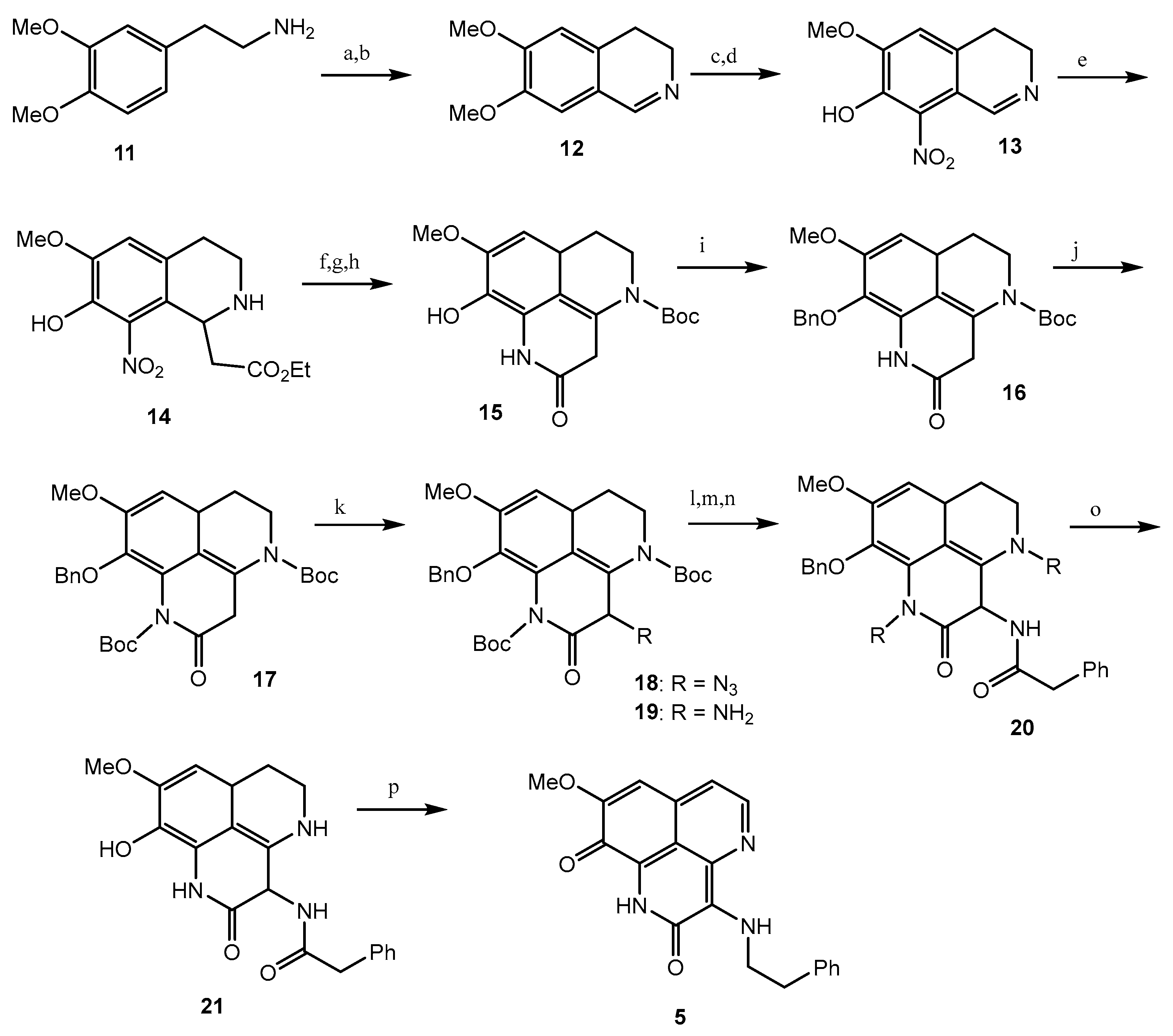
Tuberculosis (TB) is a prevalent cause of mortality and is linked with HIV-positive candidates, with millions of new TB cases and deaths every year [39,40,41]. It is difficult to eradicate non-replicating persistent Mycobacterium tuberculosis (Mtb), and the development of dormancy requires a 6-month treatment regimen [42,43]. Therefore, there is still a significant demand for the development of new anti-mycobacterial compounds which are effective against both active and dormant tubercular states. Among all the screened test compounds (Figure 3), 3-(phenethylamino)demethyl(oxy)aaptamine (5) exhibited potential anti-mycobacterium bovis BCG activity with an MIC value of 0.75 μg/mL, while the reference compound, isoniazid, showed an MIC value of 0.05 μg/mL under aerobic conditions (Table 1) (Scheme 2) [44]. Compound 1 showed potential towards M. tuberculosis H37Rv strains, with the MIC value ranging from 0.5 to 2.0 μg/mL. The insertion of rifluoromethyl diazirine as a probe moiety in the 3-(phenethylamino)demethyl(oxy)aaptamine (5) product retains equivalent anti-mycobacterium activity compared to the parent compound 5 [45].
Figure 3.
Aaptamines screened against Mycobacterium bovis BCG.

Table 1.
MICs of aaptamines against M. smegmatis and M. bovis BCG under aerobic and hypoxic conditions. Adapted with permission from Ref. [44]. 2020, Elsevier.
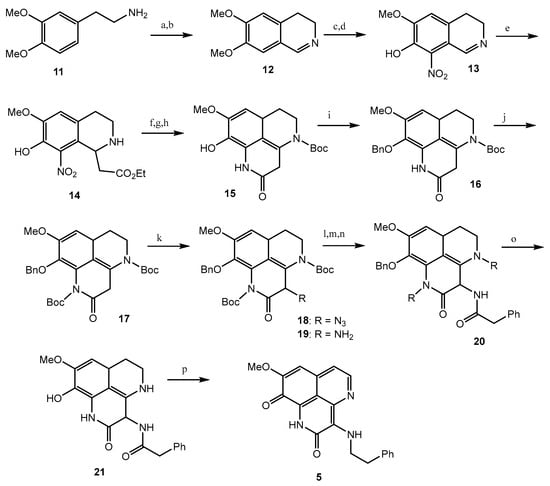
Scheme 2.
Total synthesis of 3-(Phenethylamino)demethyl(oxy)aaptamine. Reagents and conditions: (a) HCO2H, 175 °C; (b) POCl3, toluene, 95 °C; (c) 48% HBr, 95 °C, 46% (3 steps); (d) 40% HNO3, NaNO2, EtOH, −15 °C to 0 °C, 59%; (e) monoethyl malonate, reflux, 89%; (f) H2, Pd-C, AcOH, 71%; (g) Boc2O, CHCl3, reflux; (h) NaOMe, MeOH-CH2Cl2, 88% (2 steps); (i) BnBr, K2CO3; (j) Boc2O, Et3N, DMAP, CHCl3, reflux, (89%); (k) KHMDS, trisylazide, −78 °C, then AcOH, 0 °C, 70%; (l) Zn, NH4HCO2, CH2Cl2-MeOH; (m) PhCH2COCl, pyridine; (n) TFA, CH2Cl2, 46% (3 steps); (o) H2, Pd-C, THF-MeOH, 80%; (p) (i) BH3·THF, THF, 45 °C, (ii) 5% HCl, THF; (iii) O2, 20% TFA, 85 °C, 45%.
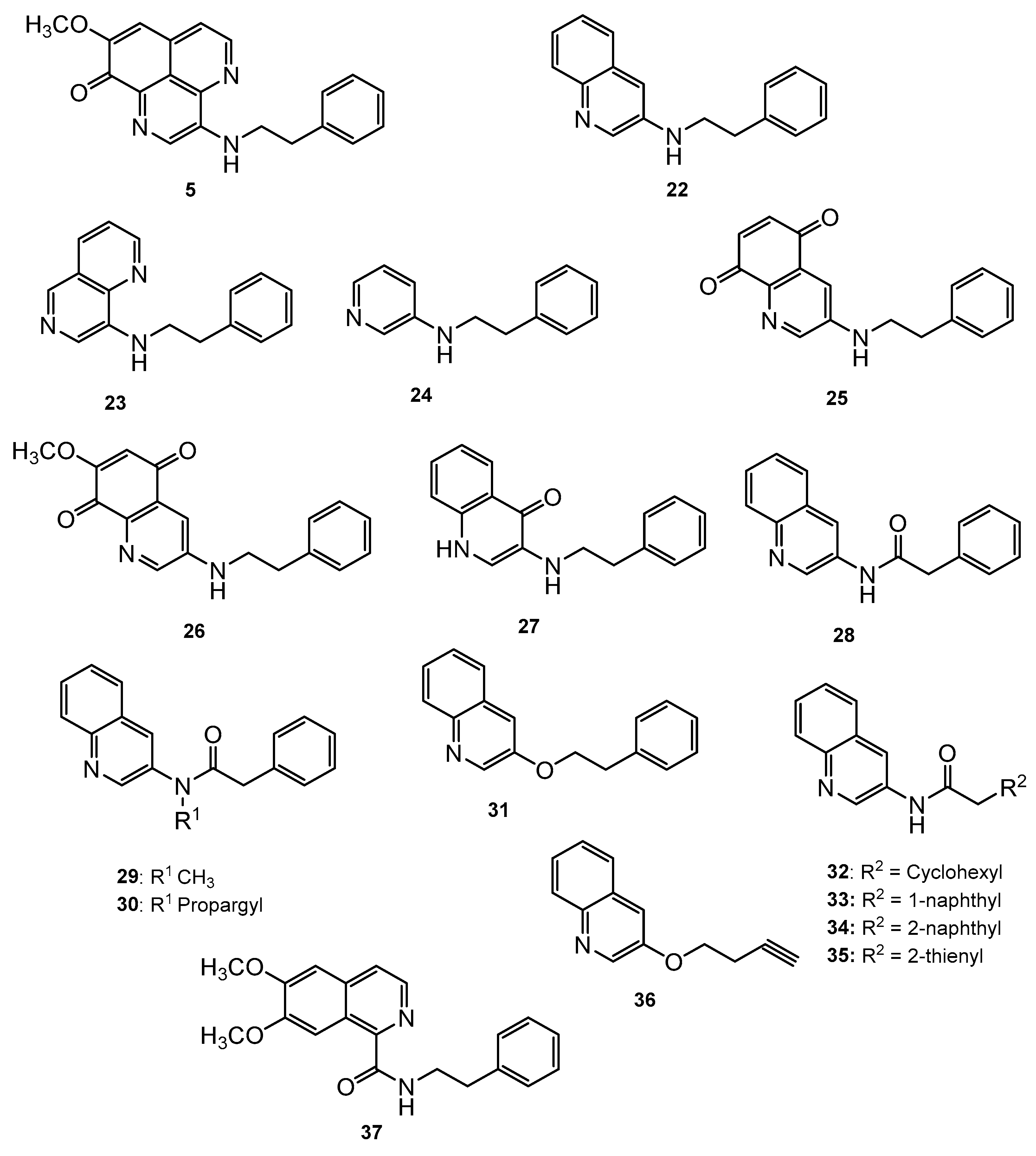
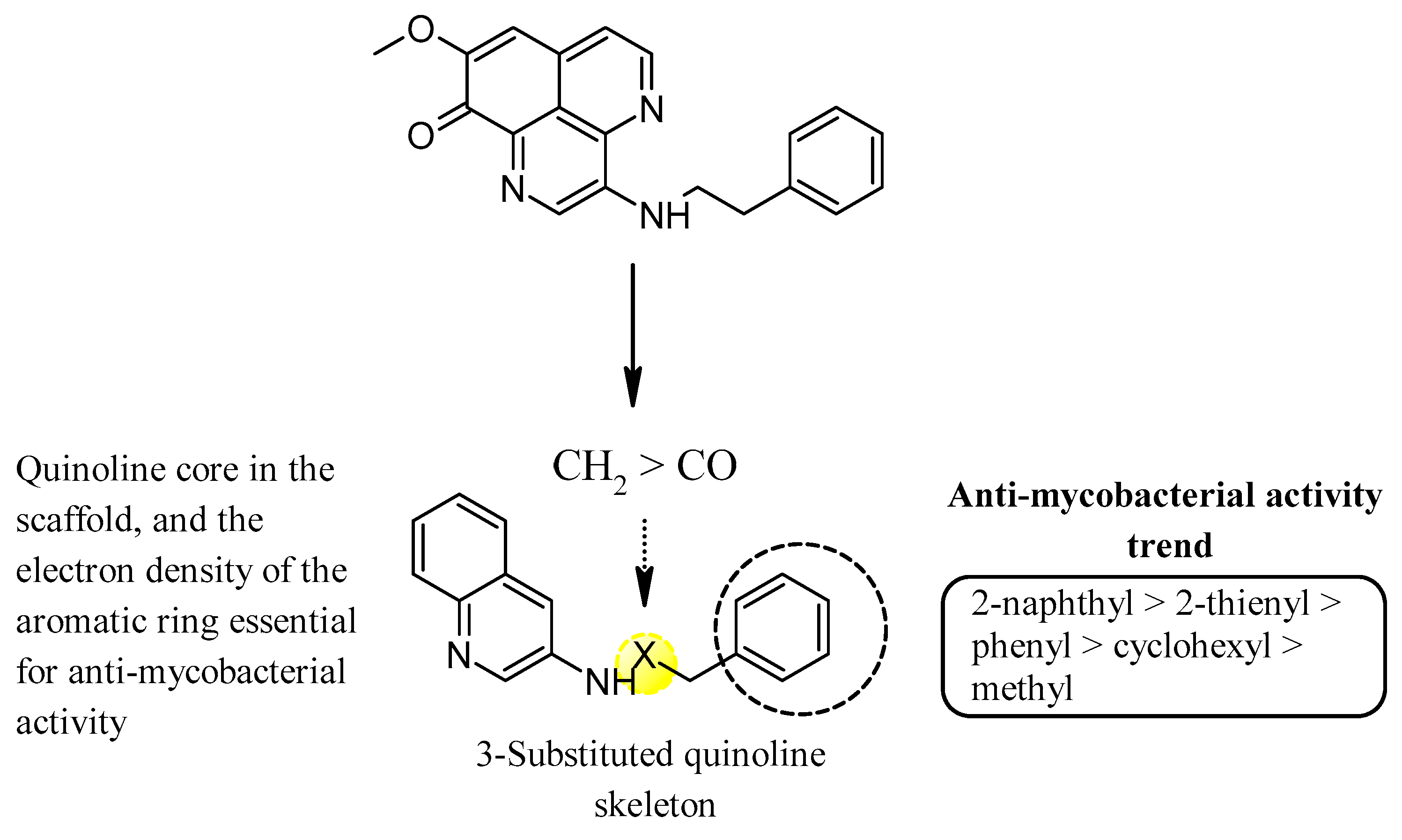
Mukomura et al. have synthesized several quinolone derivatives of aaptamine and observed that 3-(phenethylamino)demethyl(oxy)aaptamine compound 5 showed potential to develop potential anti-mycobacterial agents (Figure 4) (Table 2) [46]. The structure activity relationship (SAR) study indicates that the quinoline ring and the π-electron density over the aromatic ring is essential for anti-mycobacterial potential. Anti-mycobacterial activity trends were observed in the sequence of 2-naphthyl > 2-thienyl > phenyl > cyclohexyl > methyl. In brief, the N-(2-arylethyl) quinolin-3-amine moiety showed a promising framework for anti-mycobacterial candidates (Figure 5).
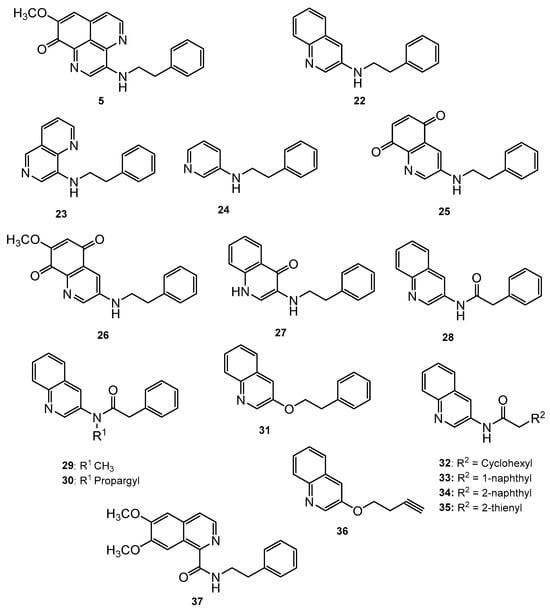
Figure 4.
N-(2-Arylethyl)quinolin-3-amines inspired aaptamines with anti-mycobacterial activity.

Table 2.
Anti-mycobacterial activity and cytotoxicity of PDOA analogues [46].
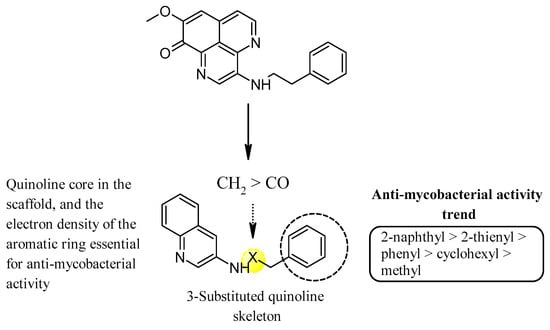
Figure 5.
SAR of N-(2-arylethyl)quinolin-3-amines inspired marine alkaloids.
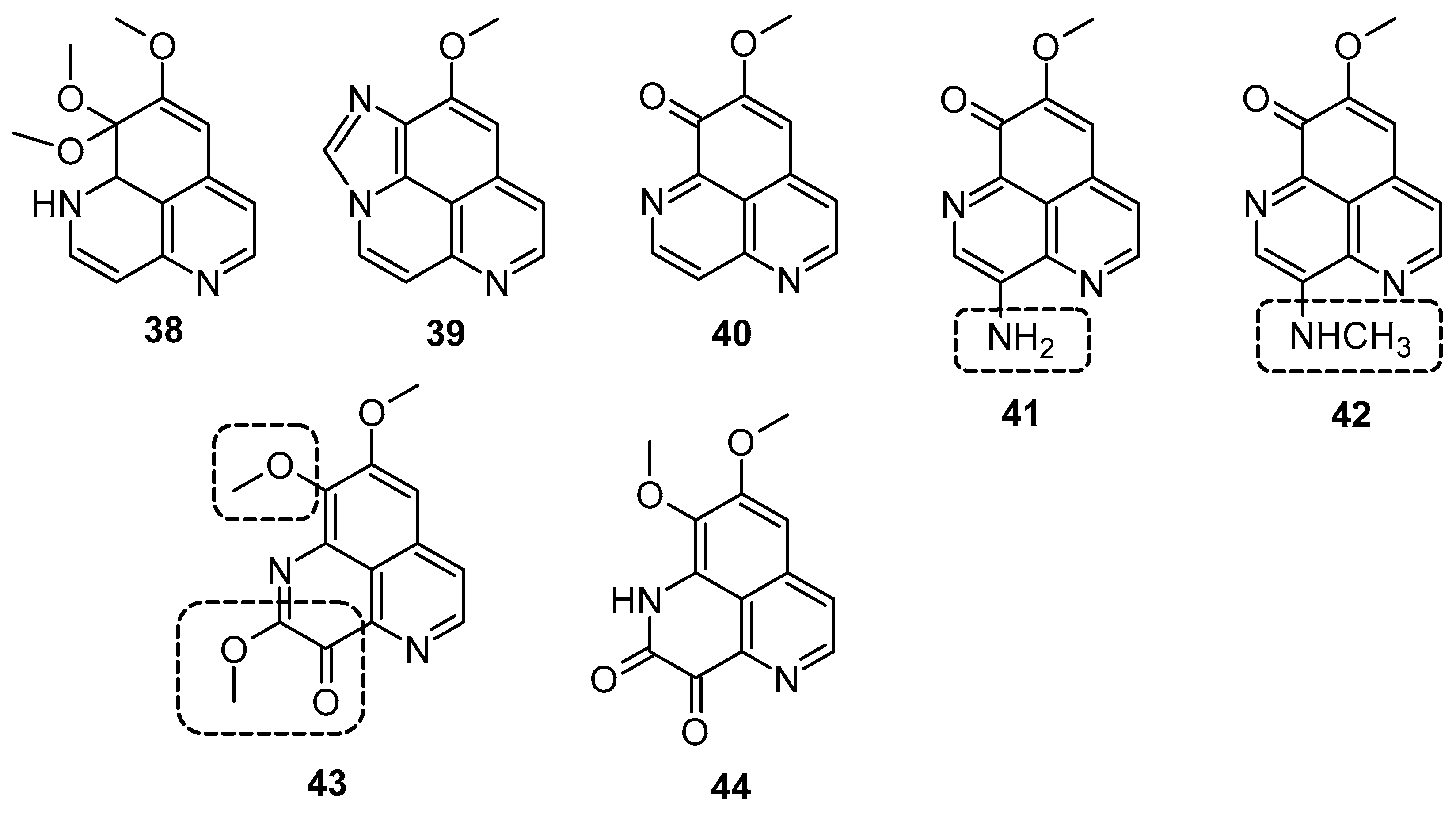
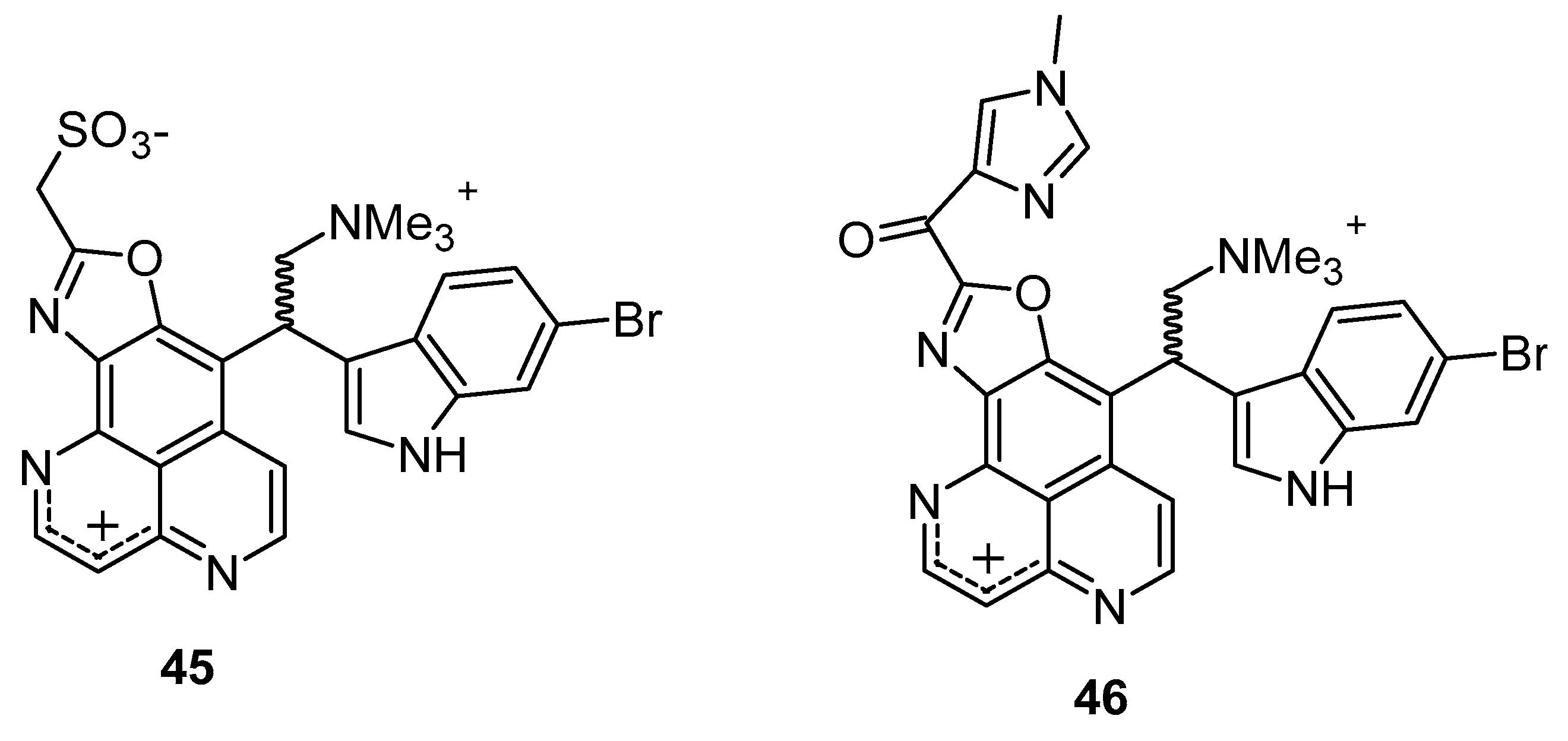
Hypoxia is recognized as a significant factor that triggers a state of non-replicating persistence in tubercle bacilli. The depletion of oxygen initiates a dormancy response in TB, including resistance to isoniazid, in Mycobacterial bacilli [46,47]. In active aerobic growth conditions, compounds 41, 42, and 43 exhibited strong antimicrobial effects, displaying MIC values of 6.25 µg/mL (aerobic environment), while the reference isoniazid showed an MIC value of 2.5 µg/mL against M. smegmatis, and it moves to 25 µg/mL in a 0.2% O2 containing N2 atmosphere (Figure 6) (Table 3). The results indicated that under active growing conditions, the presence of carbonyl functionality at position (C-3 or 9) imparts incremental potential against M. smegmatis. Compounds 40, 41, 42, and 44 displayed antimicrobial efficacies towards M. smegmatis (dormancy-induced), with MIC values of 6.25, 6.25, 1.5, and 1.5 µg/mL, respectively. In aerobic and O2 deficient conditions, compound 41 (MIC value: 6.25 µg/mL) and 42 (MIC value: 1.25 µg/mL) demonstrated the highest potency along with selectivity [48]. In 2011, Takahashi et al. synthesized bromoindole derivatives of aaptamine. Amongst these bromoindole derivatives, nakijinamines C (45) and E (46) containing a 1H-oxazolo[4′,5′:4,5]benzo[1,2,3-de][1,6]naphthyridine ring system (Figure 7) demonstrated potential towards A. niger (MIC value of 16 μg/mL) [49]. Isoaaptamine inhibited Sortase A (SrtA) and enhanced virulence in S. aureus (IC50 value: 3.7 ± 0.2 µg/mL) [50].
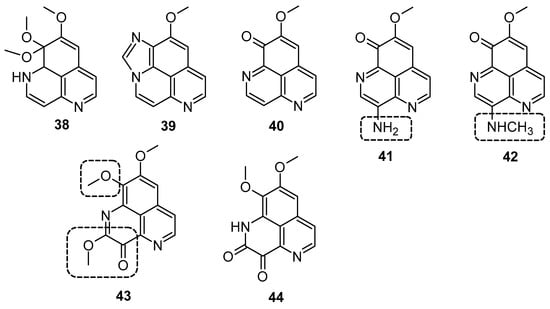
Figure 6.
Aaptamine and derivatives screened for M. smegmatis.

Table 3.
MIC of aaptamines against M. smegmatis under aerobic and hypoxic conditions [48].
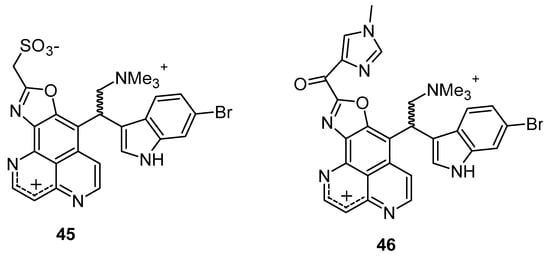
Figure 7.
Bromoindole derivatives of aaptamine.
3.3. Anticancer Activity
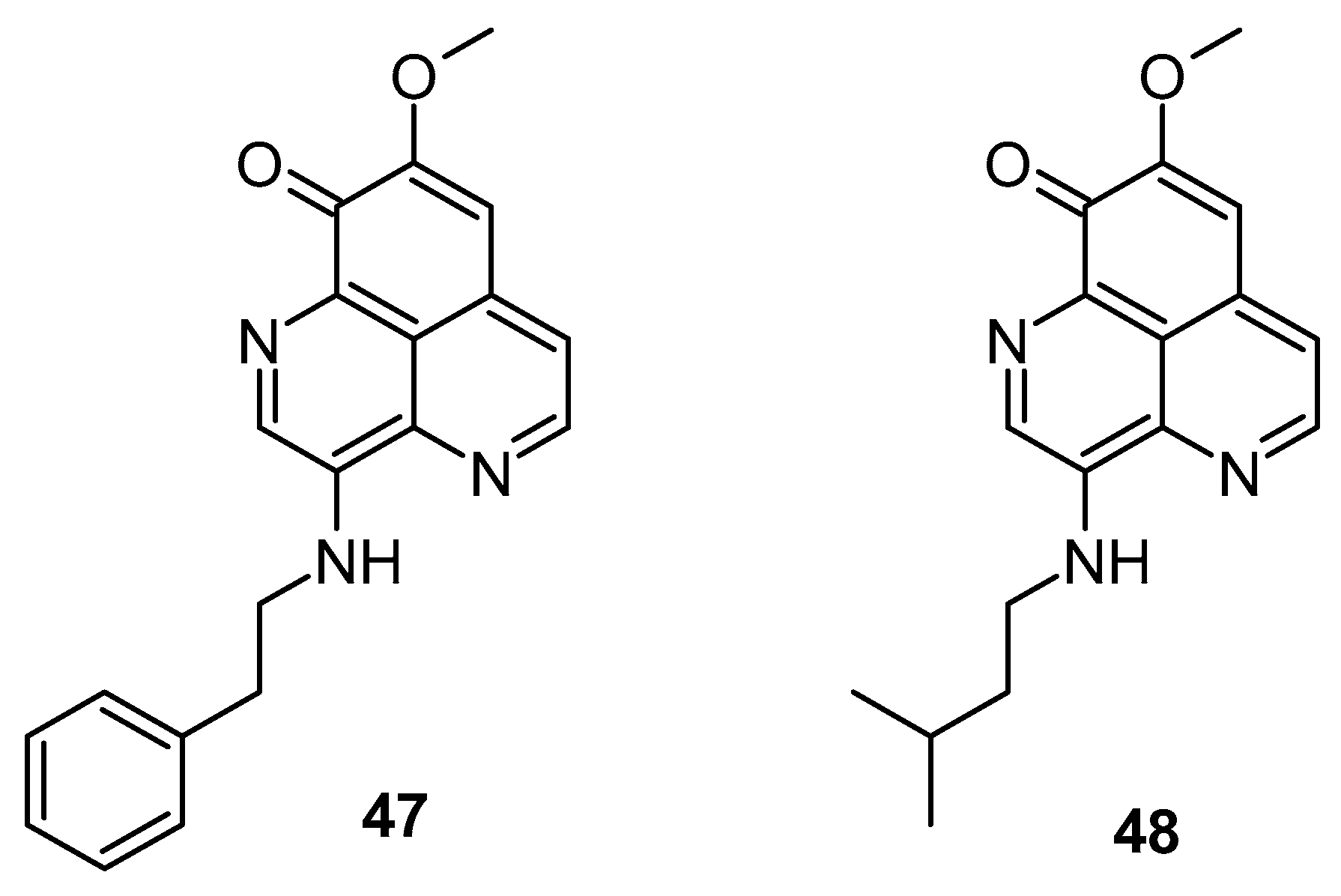
Cyclins and cyclin-dependent kinases (CDKs) play pivotal roles in governing the progression of the cell cycle [51,52]. Cell progression via the G1 phase is crucially regulated by cyclin D1, which functions as a subunit component of Cdk4 and Cdk6 [53,54]. Cyclin E binds to cdk2, forming a kinase complex necessary for the G1 to S phase transition. Cyclin A, when paired with Cdk2 or Cdk1, regulates the S phase, whereas pairing the cyclin B complex to Cdk1 regulates the M phase [55]. Aberrations in cell cycle checkpoints along with the overexpression of growth-promoting factors including cyclin D1 and cyclin E results in tumor genesis [56,57]. Aoki et al. found that aaptamine inhibits growth in human osteosarcoma MG63 cells by activating the p21 promoter, leading to cell cycle arrest at the G2/M phase, independent of p53 [58]. Jin et al. evaluated the anti-proliferative efficacy of aaptamine on K562 cells, a type of chronic myeloid leukemia (CML) cells [59]. Aoki et al. conducted the first isolation and structural elucidation of aaptamine from A. suberitoides from Java, Indonesia [58]. The isolated compound exhibited significant anti-proliferative activity against MG63 cells by modulating the p21 promoter, leading to cell cycle arrest at the G2/M phase independently of p53. Additionally, aaptamine induced G2/M phase cell cycle arrest through the stimulation of p21 gene expression at a concentration of 10 mM. In lung and prostate cancer cells, aaptamine demonstrated GI50 values of 7 and 10 μM, respectively. Li et al. (2015) demonstrated aaptamine’s potential against hepatocellular carcinoma HepG2 and LM3 cells in vitro and in subcutaneous xenograft models in vivo [60]. Their findings indicated that cell cycle arrest was associated with the reduced expression of CDK2 and SOX9, along with enhanced p21 levels due to inhibitory activity on the CDK2 kinase. The phosphatidylinositol 3 kinase (PI3K)/protein kinase B (AKT) signaling pathway regulates numerous cellular functions such as cell cycle regulation, growth, and proliferation by activating downstream effector molecules [61,62]. The activation of the PI3K/Akt pathway contributes to malignant transformation and resistance to apoptosis induced by conventional anticancer therapies [63,64]. The inhibition of the PI3K/AKT pathway can disrupt tumor cell proliferation and growth, making them more susceptible to programmed cell death. mTOR, a key component of the PTEN/PI3K/AKT pathway, is frequently disrupted in cancer cells [65,66]. The simultaneous suppression of mTOR and the PTEN/PI3K/AKT pathway is proposed as an effective strategy against cancer [67,68]. Lung cancer has gained notoriety for being known worldwide as an aggressive form of cancer and causing cancer-related deaths [69,70]. Lung cancer continues to hold its position as the most aggressive form of malignant tumor, with low survival rates [71,72,73]. The PI3K/AKT/GSK3β signaling pathway and its downstream regulation is one of most promising targets of cancer management. GSK3β, a key player in cellular growth regulation and tumorigenesis, is phosphorylated at the Ser9 residue by various protein kinases including AKT, MAPK/ERK, and PKA pathways, promoting cellular survival [74,75,76,77,78]. The activation of the PI3K/AKT pathways regulates growth, survival, and cellular metabolism, with AKT phosphorylation at Ser473 and Thr308 leading to GSK3β phosphorylation at Ser9 [79,80]. Key regulators of cell cycle progression, including CDK2/4 and cyclin D1/E, are modulated by GSK3β, which is crucial for G1 phase progression and stability [81]. Gong et al. observed the potential of aaptamine against non-small cell lung carcinoma (NSCLC) proliferation and progression in A549 and H1299 cells [82]. Aaptamine inhibited NSCLC A549 cell (IC50: 13.91 µg/mL) and H1299 cell (IC50: 10.47 µg/mL) proliferation, arresting G1 phase cell cycle progression by targeting CDK2/4 and cyclin D1/E. Furthermore, aaptamine arrested G1 phase cell cycle progression by targeting key regulators of cell cycle progression, namely CDK2/4 and cyclin D1/E. A Western blot analysis revealed reduced levels of MMP-7 and -9 proteins alongside the upregulation of cleaved-PARP and cleaved-caspase 3 expression. Additionally, aaptamine suppressed the PI3K/AKT/GSK3β signaling pathway by degrading phosphorylated AKT and GSK3β specifically. Trang et al. reported that demethyl(oxy)aaptamine exhibited anticancer potential over lung carcinoma (SK-LU-1, IC50: 9.2 ± 1.0 µM), breast carcinoma (MCF-7, IC50: 7.8 ± 0.6 µM), hepatocellular carcinoma (HepG2, IC50: 8.4 ± 0.8 µM), and melanoma (SK-Mel-2, IC50: 7.7 ± 0.8 µM) [83]. In 2009, Shaari K et al. observed that both 3-(phenethylamino)demethyl(oxy)aaptamine (47) and 3-(isopentylamino)demethyl(oxy)aaptamine (48) inhibited T-lymphoblastic leukemia (CEM-SS), with IC50 values of 5.32 and 6.73 µg/mL, respectively, compared to aaptamine (IC50 15.03 µg/mL) (Figure 8). These findings suggest that substitution at the C-3 position enhances cytotoxic effects [83].
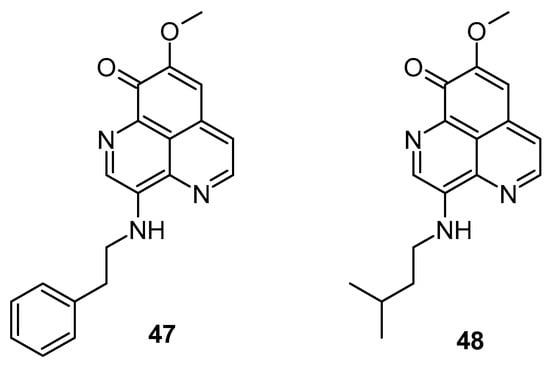
Figure 8.
Demethyl(oxy)aaptamine screened as anticancer agents.
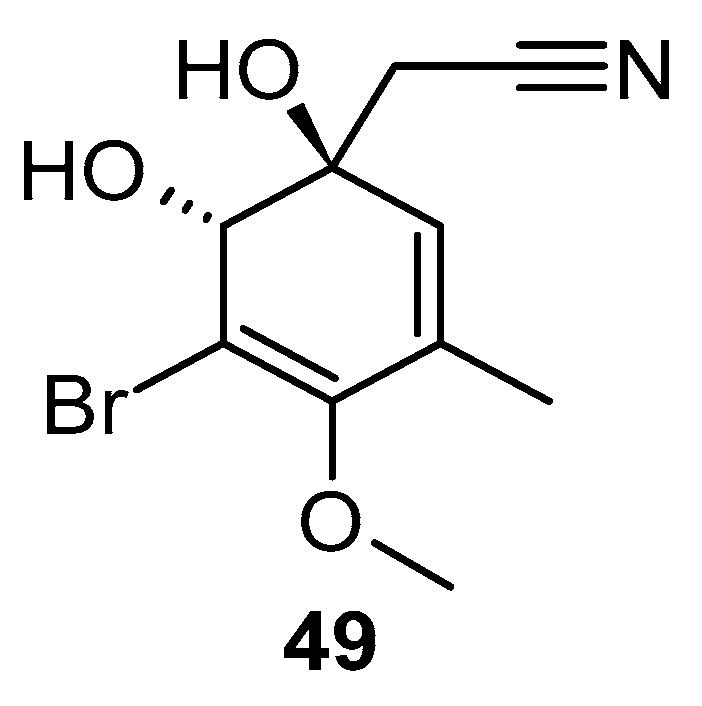
Stuhldreier et al. examined the impact of two sponge-derived alkaloids, aaptamine and aeroplysinin-1 (49) over acute myeloid leukemia (AML) cells (Figure 9). They found that the viability of AML cells decreased significantly, with an IC50 value ranging from 10 to 20 µM. Moreover, they observed increased levels of p21 and p16 expression, decreased levels of p-chk-2 phosphorylation expression, and a noticeable cellular accumulation of the S phase of the cell cycle [84].
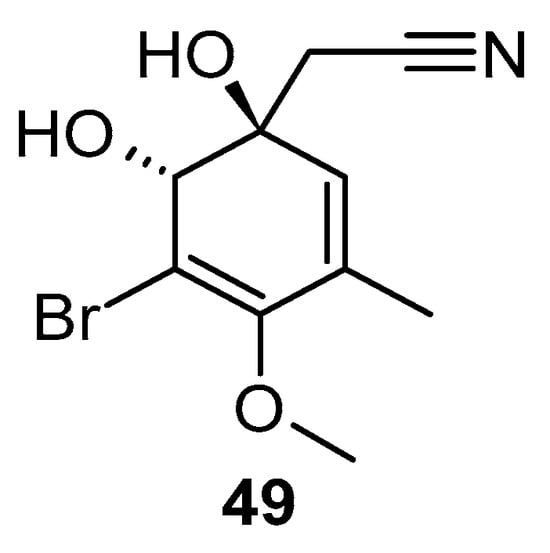
Figure 9.
Structure of aeroplysinin-1.
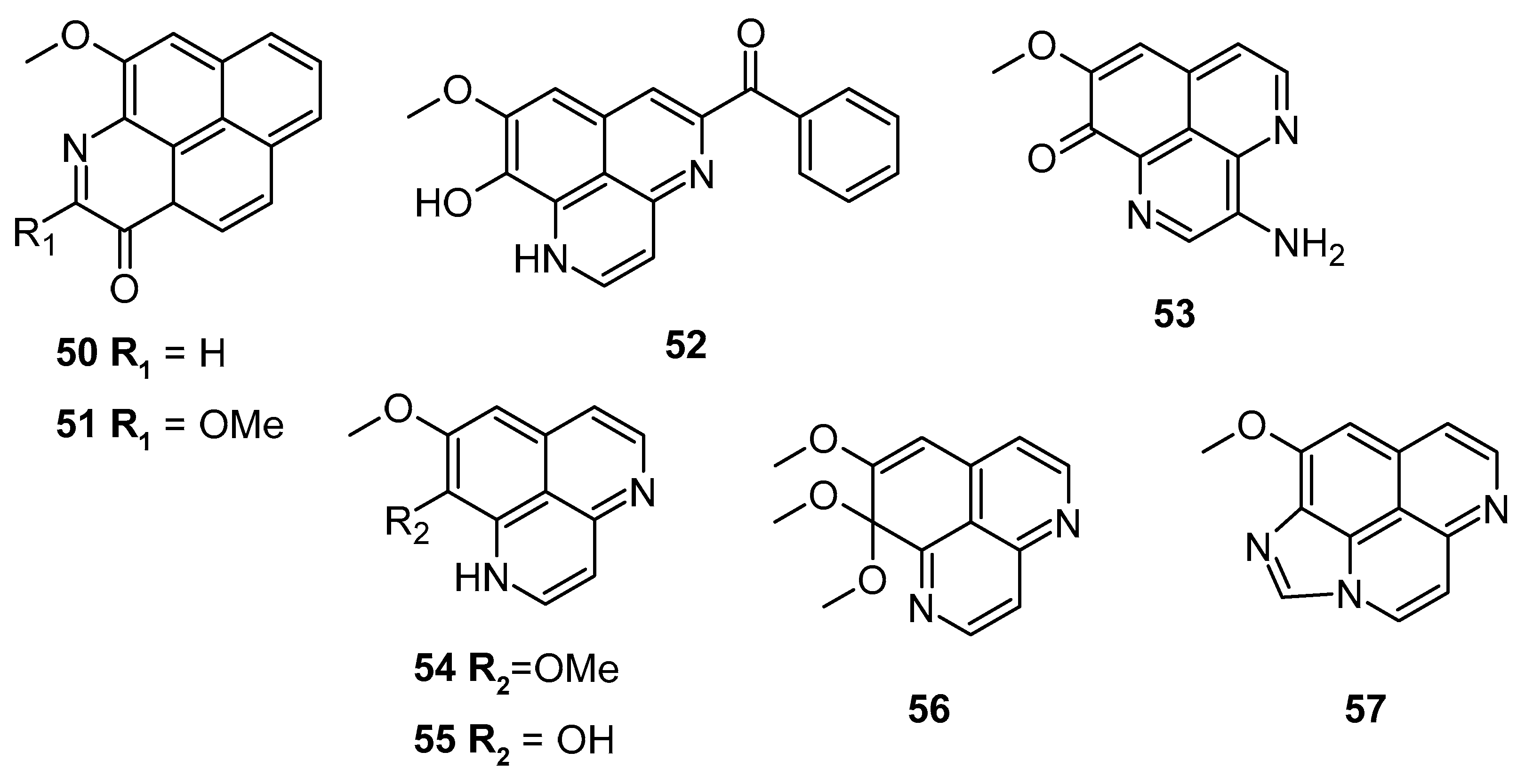
Pham et al. screened several aaptamine derivatives (Figure 10) over the L5178Y cell line (murine lymphoma). Compound 55 exhibited a notable cytotoxic potential with an IC50: 0.9 μM, compared to the reference kahalalide F (IC50: 4.3 μM). Compound 52 showed comparable efficacy with an IC50 of 5.5 μM (Table 4) [85].
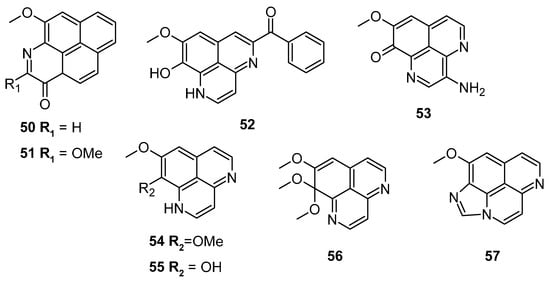
Figure 10.
Aaptamine derivatives screened against the murine lymphoma L5178Y cell line.

Table 4.
Cytotoxic activities of compounds 50–57. Adapted with permission from Ref. [85]. 2013, American Chemical Society.
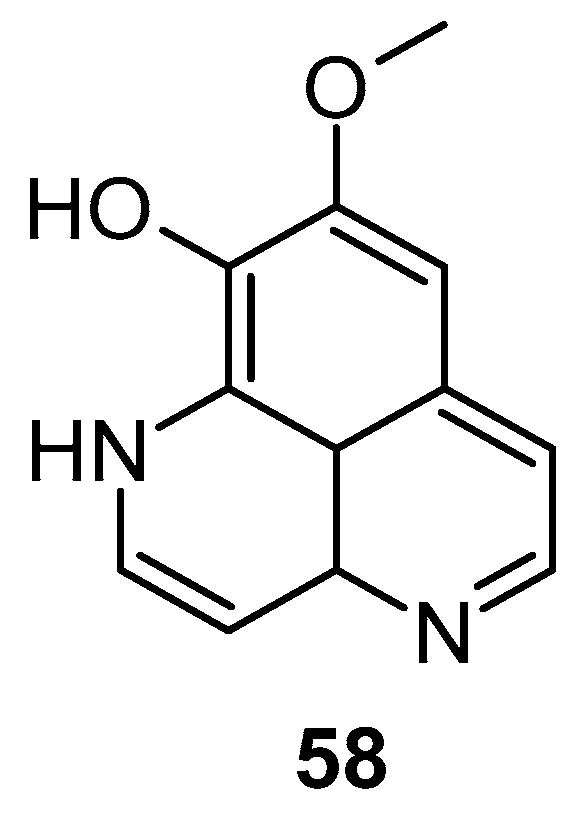
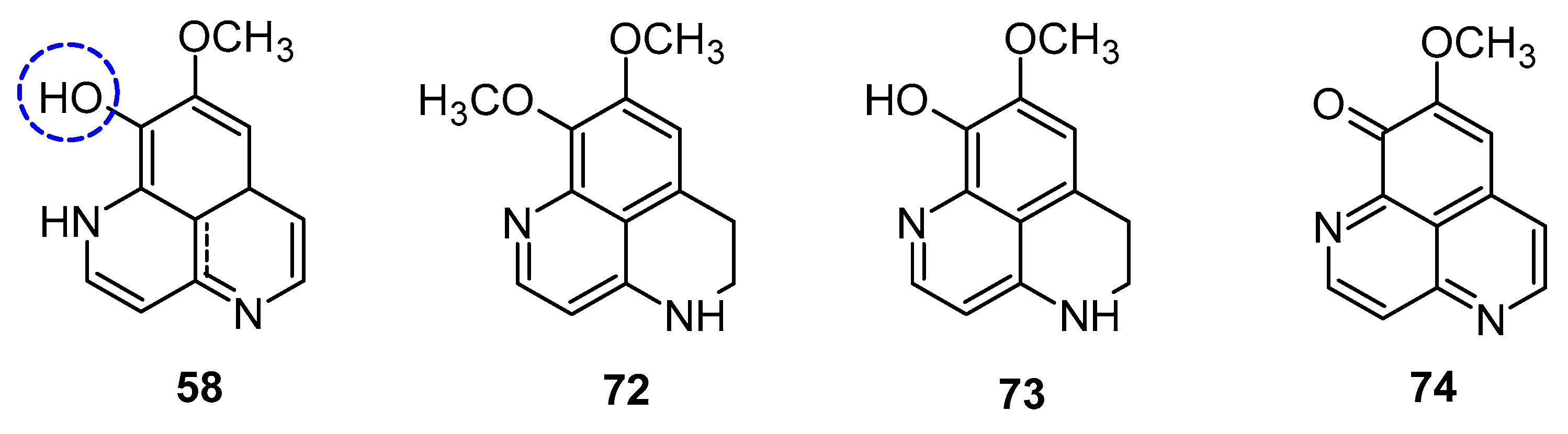
Aoki et al. noted that aaptamine could upregulate p21 promoter expression and induce G2/M phase cell cycle arrest in human osteosarcoma MG63 cells (MG63luc+), independent of p53. Aaptamine (1), isoaaptamine (2), and demethylaaptamine (58) were reported as potential inhibitors of proteasomes (Figure 11). These compounds inhibited chymotrypsin (IC50: 1.6 to 4.6 µg/mL) and caspase-like activities without affecting trypsin-like activity, and exhibited cytotoxic effects against HeLa cells, though their proteasome inhibition potency did not correlate with cytotoxicity [58].
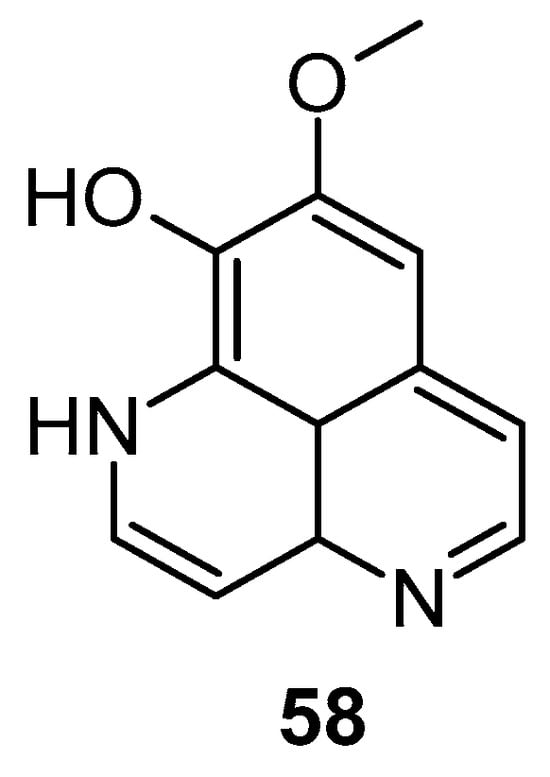
Figure 11.
Structures of demethylaaptamine.
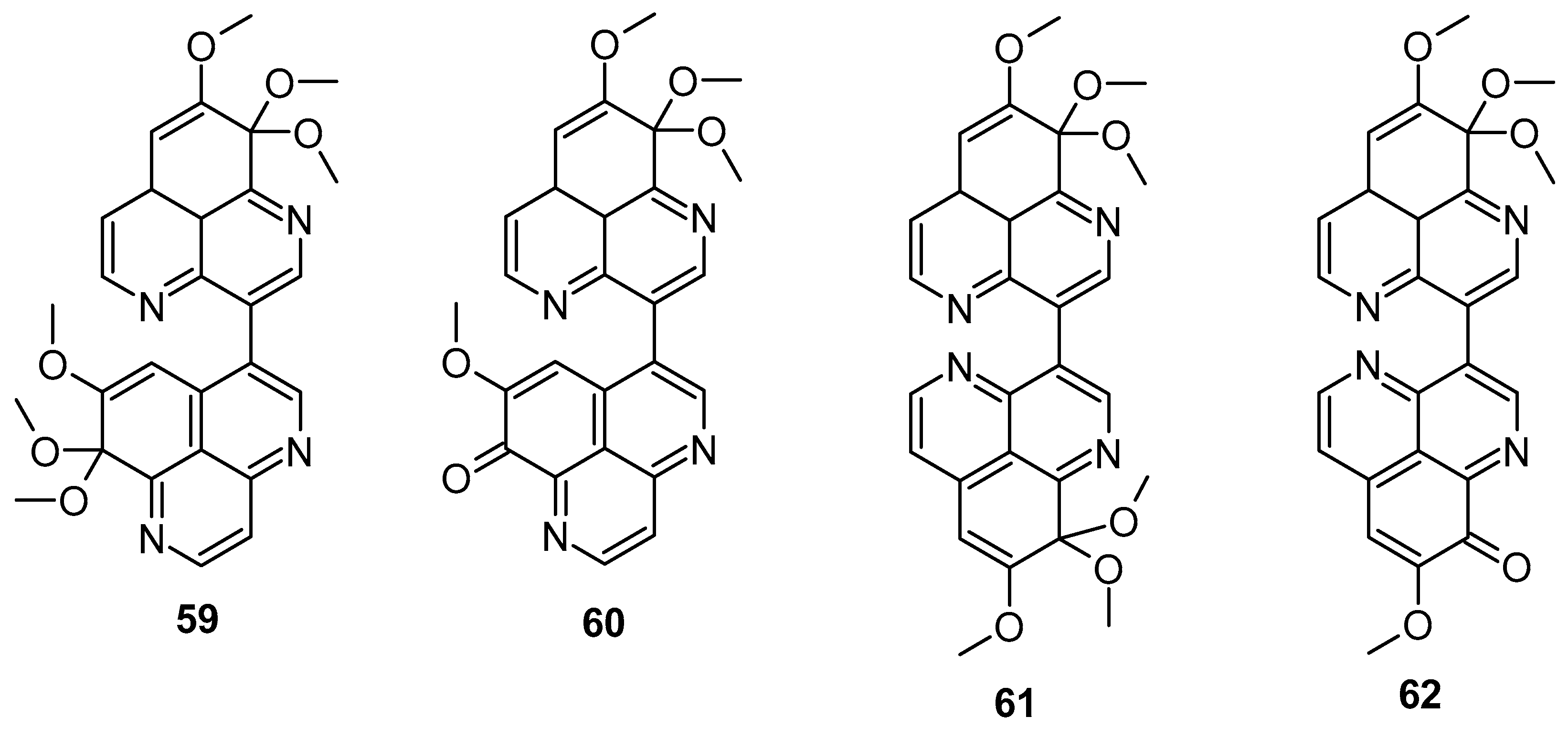
In 2018, Wu et al. demonstrated that isoaaptamine displayed significant cytotoxic effects against T-47D cells (breast cancer), inducing apoptosis through DNA ladder formation, caspase-7 activation, XIAP inhibition, and PARP cleavage, achieving up to 90% cell death by impairing the Nrf2/p62 pathway. Isoaaptamine also affected cleaved caspases-3 and -7, PARP, p62, LC3-II, XIAP, p-Akt, and mTOR [86]. Dyshlovoy et al. found that aaptamine inhibited the proliferation of NT2 human embryonal carcinoma cells (IC50 of 50 μM) and induced the G2/M phase cell cycle arrest [87]. Bis-aaptamine alkaloids, named suberitines A-D (Figure 12), were discovered, featuring two aaptamine skeleton units. These compounds contain 8,9,9-trimethoxy-9H-benzo[de][1,6]-naphthyridine and demethyl(oxy)-aaptamine, 1,6-naphthyridine rings linked via the C-3-C-3′ or C-3-C-6′ σ-bond. Compounds 59 (IC50: 1.8 μM) and 62 (IC50: 3.5 μM) exhibited significant cytotoxic activity against P388 cell lines [88].
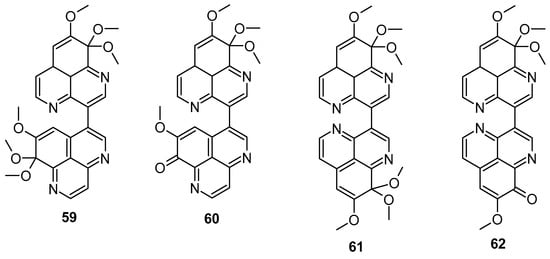
Figure 12.
Structure of suberitines A–D.
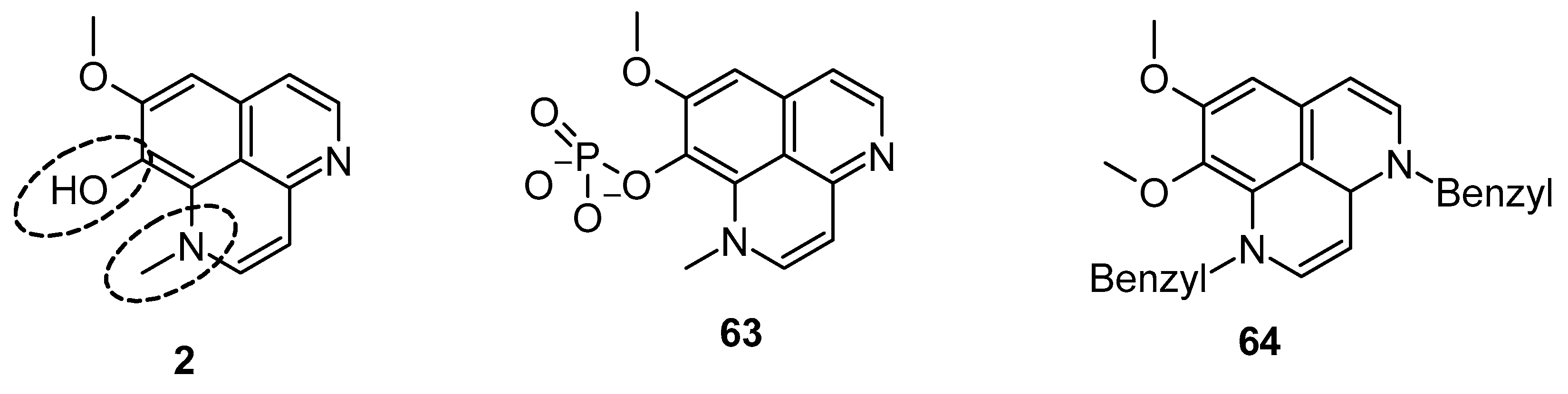
Pettit et al. reported the synthetic transformation of aaptamine to isoaaptamine (2), 9-demethylaaptamine (63), and 4-methylaaptamine (64) and their anticancer and antibacterial activity (Figure 13). The SAR study indicated that presence of the C-9 methoxy group and N-1 methylation are essential for their anticancer and antibacterial activity. Again, Pettit et al. reported that isoaaptamine (2) showed better anticancer activity than aaptamine and demethyloxy aaptamine. The instability of the potential isoaaptamine can be improved with a conversion to the phosphate prodrug hystatin 1 (63), but the anticancer and antimicrobial activity profile of hystatin 1 (63) was inferior to the parent compound. The insertion of a bulky group at the N-1 and N-4 position improved activity. 4-methylaaptamine (64) inhibited growth in the S phase of cell cycle [89].
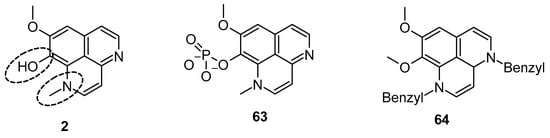
Figure 13.
Structure of isoaaptamine, 9-demethylaaptamine, and 4-methylaaptamine.
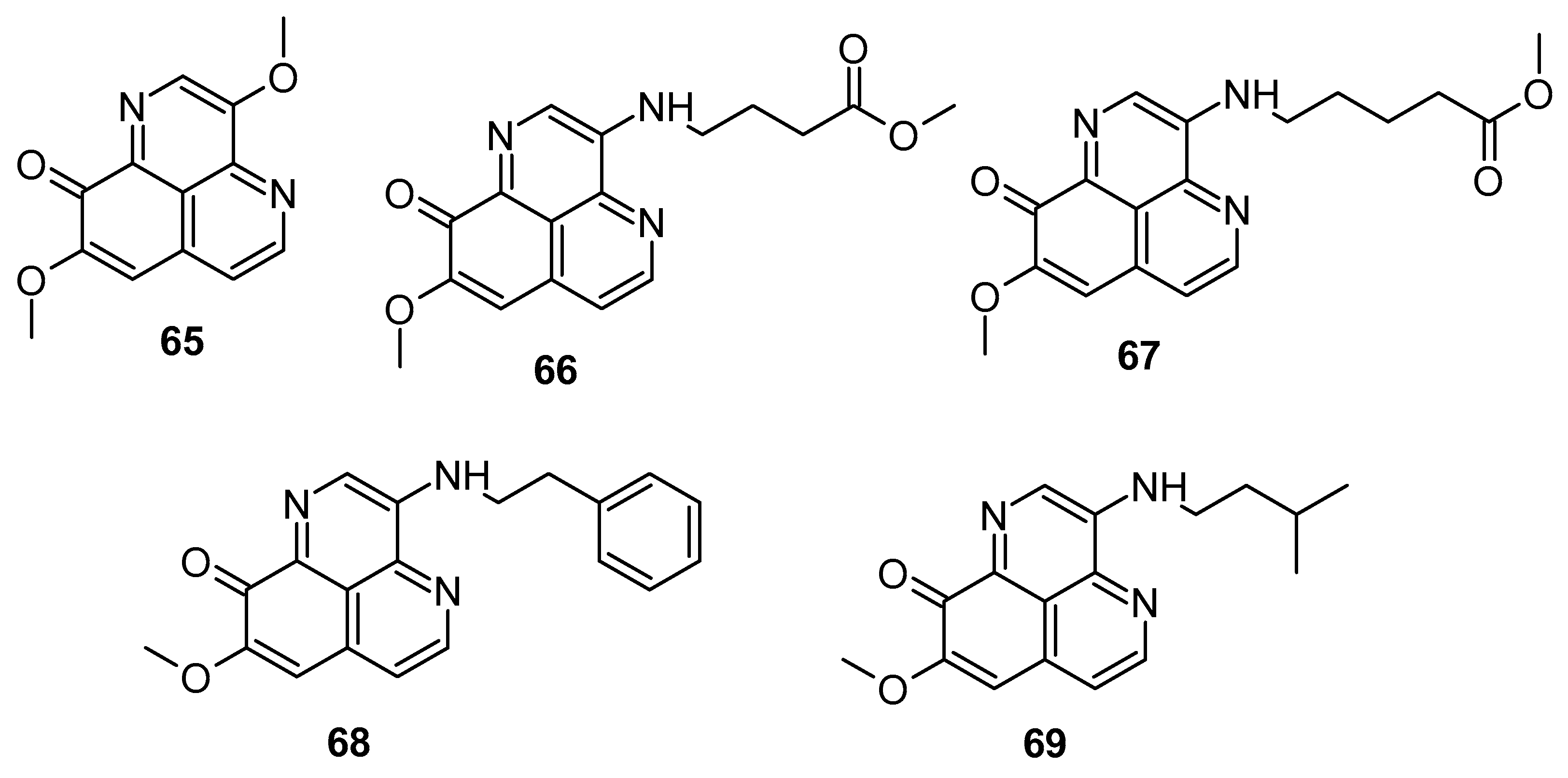
He et al. (2022) screened aaptamine and its analogues (Figure 14) for cytotoxicity against H1299, SCG7901, H520, SW680, and CNE-2 cancer cells. Compounds 67–69 exhibited cytotoxicity over H1299 and H520 cells (IC50: 12.9 to 20.6 μg/mL). Compounds 67–69 (Figure 14) demonstrated potent inhibitory activity against CDK2, with IC50 values of 14.3, 3.0, and 6.0 μg/mL, respectively. Moreover, compounds 67–69 exhibited significant G1 arrests in H1299 cells. Compound 67 also efficiently bound to the CDK2 protein, protecting it from degradation [90].
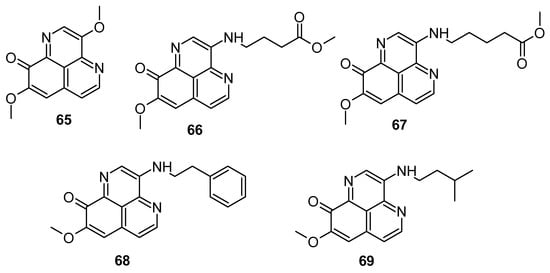
Figure 14.
Aaptamine derivatives screened for CDK2 inhibitory activity.
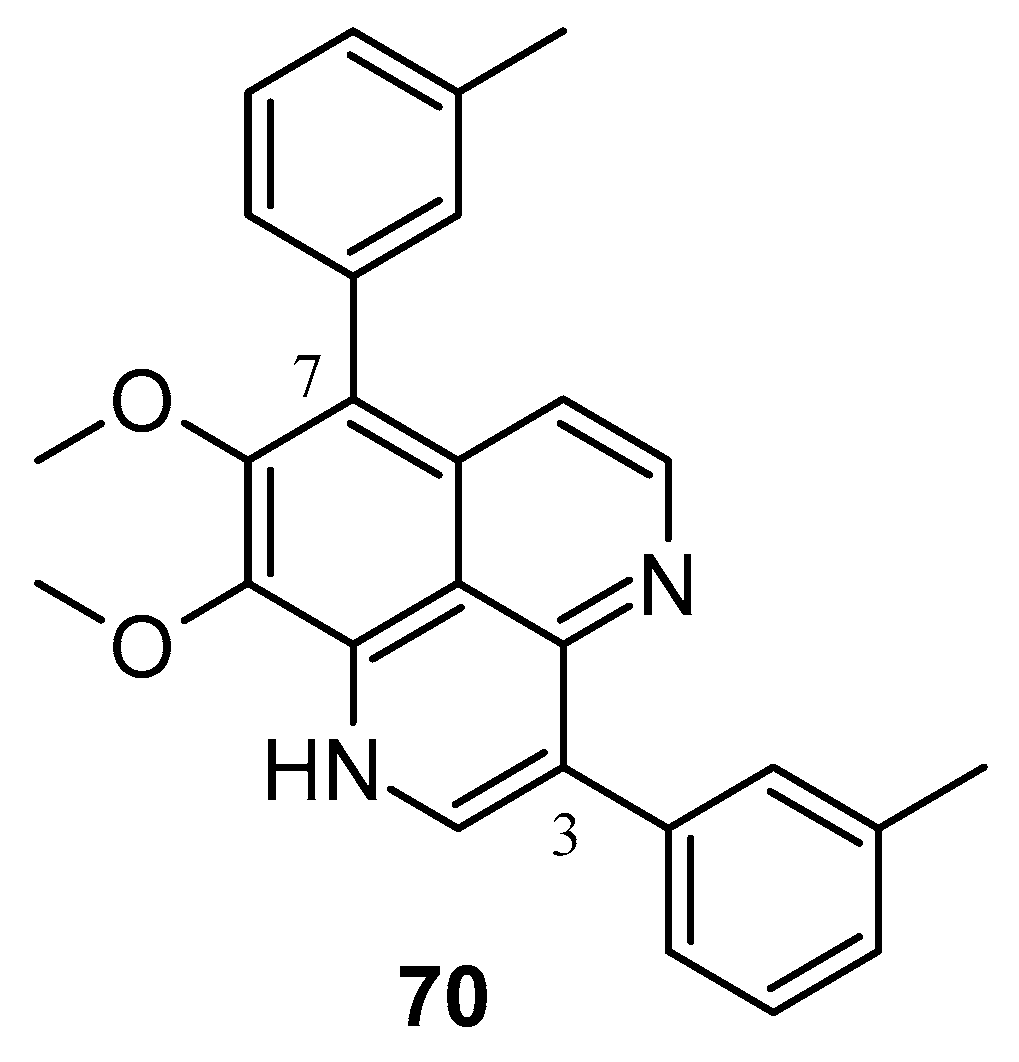
In 2020, Yang et al. synthesized numerous derivatives of aaptamine by introducing aromatic rings at the 3- and 7-positions; compound 70 (Figure 15) exhibited notable anti-proliferative activity against the extranodal natural killer/T-cell lymphoma cell line SNK-6 (IC50: 0.6 μM). Furthermore, compound 70 demonstrated cytotoxic effects on various lymphoma cell lines, such as Ramos, Raji, WSU-DLCL2, and SU-DHL-4 cells [91]. Shen et al. synthesized various 9-O-acylisoaaptamine and 4-N-acyl-dihydroaaptamine derivatives, which were then assessed for their anti-tumor activity against murine P-388 cells and various human tumor cell lines such as KB16, A549, and HT-29. All compounds exhibited a greater sensitivity towards P-388 cells [92]. Shubina et al. (2010) found that 2,3-dihydro-2,3-dioxoaaptamine, 6-(N-morpholinyl)-4,5-dihydro-5-oxo-demethyl(oxy) aaptamine, and 3-(methylamino) dimethyl (oxy) aaptamine have the potential to trigger apoptosis in THP-1 cells (human leukemia) [93].
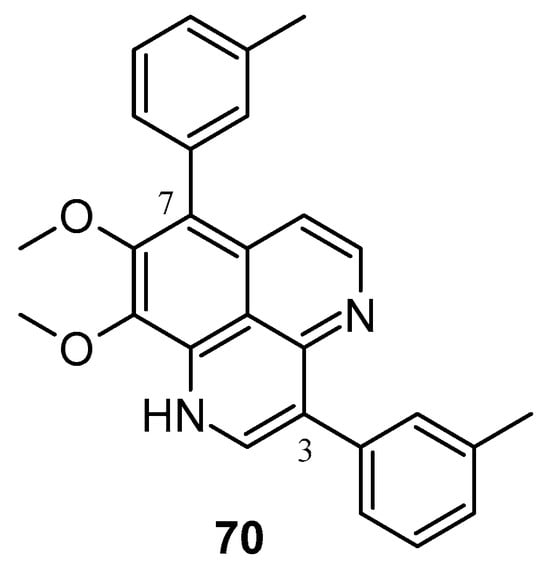
Figure 15.
Aaptamine derivatives as cytotoxic agents against extranodal natural killer/T-cell lymphoma.
3.4. Miscellaneous Activity
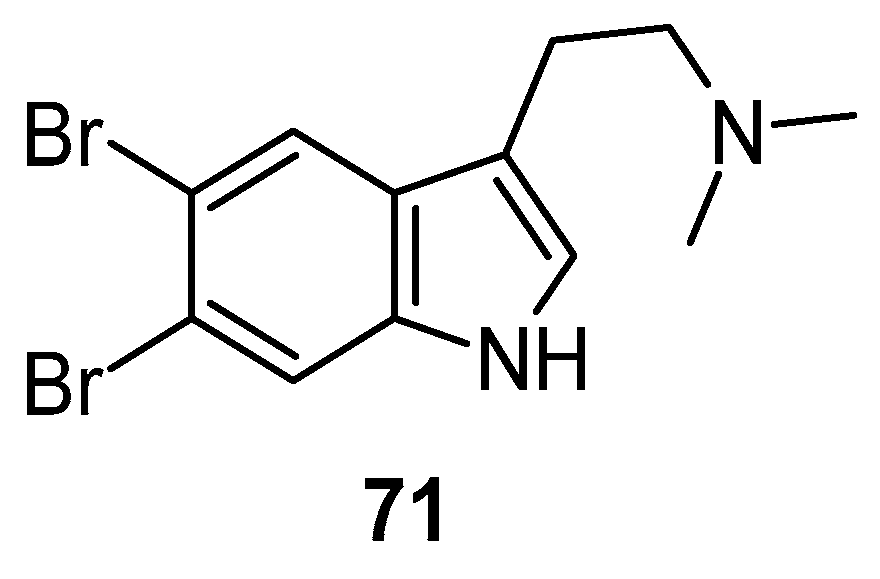
Depression ranks in the top four of the major contributors among the globally recognized burdens of disease and one of the top most prevalent psychiatric disorders, impacting approximately 120 million individuals globally. Natural compounds found in marine sources, including peptides, alkaloids, polyphenols, diterpenes, glycosides, vitamins, and minerals, have been under investigation for their therapeutic potential for treating depression [94,95]. In a forced swim test, 5,6-dibromo-N,N-dimethyltryptamine 71 (Figure 16) showed significant antidepressant activity [96].
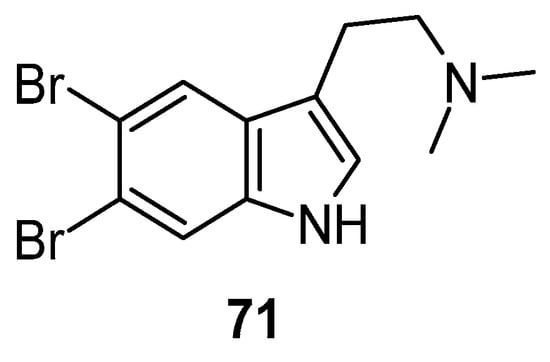
Figure 16.
Structure of 5,6-dibromo-N,N-dimethyltryptamine.
In 1984, Ohizu et al. isolated hetero-aromatic compounds (Figure 17) from the sea sponge A. aaptos and assessed them for their potential as inhibitors of the α-adrenoceptor in vascular smooth muscles [97]. Their investigation revealed that biological activity requires the presence of a C-11 methyl group. Additionally, the reduction of aaptamine to dihydroaaptamine significantly diminishes the compound’s effectiveness. Cholinesterase inhibitors play an important role in neurological conditions such as dementia, Alzheimer’s disease, myasthenia gravis, and glaucoma. Marine organisms spanning from algae to ascidians showed potential anti-cholinesterase activity [98,99]. Miao et al. observed that aaptamine has the potential to display dual-targeted AChE and BuChE inhibitors for AD treatment via binding with PAS and CAS sites [100]. Sung et al. documented that the neuropathic pain management potential of aaptamine was a result of its delta-opioid agonist properties. The intrathecal administration of aaptamine effectively mitigated chronic constriction injury (CCI)-induced nociceptive sensitization, allodynia, and hyperalgesia. Furthermore, aaptamine notably decreased the expression of vascular endothelial growth factor (VEGF), the cluster of differentiation 31 (CD31), and LDHA induced by CCI in the spinal cord [101]. Aaptamine exhibits potent effects on specific G-protein coupled receptors (GPCRs), including antagonistic activity on the α-adrenoreceptor (ADRA2C, IC50: 11.9 µM), β-adrenoreceptor (ADRB2, IC50: 0.20 µM), and dopamine receptor D4 (DRD4, IC50: 6.9 µM). Furthermore, it demonstrates agonistic effects on certain chemokine receptors either independently (CCR1, EC50: 11.8 µM; CXCR7, EC50: 6.2 µM) or as an enhancer of agonist activity (CCR3, EC50: 16.2 µM; CXCR3, EC50: 31.8 µM) [102,103,104].
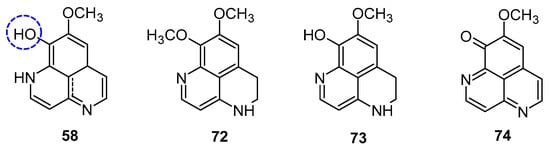
Figure 17.
Aaptamine derivatives as adrenoceptor blocking agents.
4. Conclusions
In summary, aaptamine and its derivatives showed a wide range of pharmacological activities including anticancer, antioxidant, antimicrobial, and anti-neurological disorder activities, etc. The key findings from the study of aaptamine include its potential to inhibit cancer cell proliferation, induce apoptosis, and suppress tumor growth through various signaling pathways, including PTEN/PI3K/Akt and CDK2/4 and cyclin D1/E regulation in cell cycle arrest. Its antioxidant potential is evident in its ability to scavenge free radicals in DPPH assays, suppress ROS, and deactivate the MAPK and AP-1 signaling pathways. Aaptamine also shows notable antibacterial activity against pathogenic bacteria, including active and dormant mycobacterial states, making it a promising candidate for combating bacterial infections. Presently, the biological activities and structural optimization of aaptamine have not been extensively explored. There is still a great need for researchers to explore structural modifications and to establish aaptamine as a multi-targeted entity. Future research should explore additional molecular targets, conduct extensive preclinical studies to evaluate the efficacy and safety of aaptamine in animal models, assess its synergistic effects in multi-drug regimens, and test its effectiveness against a broad spectrum of resistant pathogens. Apart from challenges associated with drug development and approval, aaptamine could have a significant impact on public health if its anticancer, antioxidant, and antimicrobial properties are effectively harnessed and translated for clinical applications. Based on the literature reviews, and with the possibility of diverse structural modifications in aaptamine and its analogues, aaptamine can be used for preparing anti-tumor drugs (especially in cases of lung cancer) as well as anti-fungal drugs.
Author Contributions
Conceptualization, N.K.T.; writing—original draft preparation, N.K.T.; writing—review and editing, G.D. and A.S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to sincerely thank Chandigarh University, Mohali, Punjab and Guru Gobind Singh College of Pharmacy, Yamuna Nagar, Haryana for offering the tools and academic atmosphere that were necessary to finish this review study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Maleš, I.; Pedisić, S.; Zorić, Z.; Elez-Garofulić, I.; Repajić, M.; You, L.; Vladimir-Knežević, S.; Butorac, D.; Dragović-Uzelac, V. The medicinal and aromatic plants as ingredients in functional beverage production. J. Funct. Foods 2022, 96, 105210. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Beutler, J.A. Natural Products as a foundation for drug discovery. Curr. Protoc. Pharmacol. 2019, 86, e67. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Eichler, B.; Klausner, E.A.; Duffy-Matzner, J.; Zheng, W. Lead/Drug Discovery from Natural Resources. Molecules 2022, 27, 8280. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of early drug discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dong, G.; Sheng, C. Structural simplification: An efficient strategy in lead optimization. Acta Pharm. Sin. B 2019, 9, 880–901. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2023, 40, 275–325. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Kannappan, A.; Shi, C.; Lin, X. Marine Bacterial Secondary Metabolites: A Treasure House for Structurally Unique and Effective Antimicrobial Compounds. Mar. Drugs 2021, 19, 530. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, W.H.; Fenner, A.M. Drug discovery from marine microbes. Microb. Ecol. 2013, 65, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.Y.; Li, H.J.; Li, Q.Y.; Wu, Y.C. Application of marine natural products in drug research. Bioorg. Med. Chem. 2021, 35, 116058. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Kumla, D.; Kijjoa, A. Chemical Diversity and Biological Activities of Meroterpenoids from Marine Derived-Fungi: A Comprehensive Update. Mar. Drugs 2020, 18, 317. [Google Scholar] [CrossRef] [PubMed]
- Saini, N.; Sirohi, R.; Anuradha, A.; Saini, N.; Wadhwa, P.; Kaur, P.; Sharma, V.; Singh, G.; Singh, I.; Sahu, S.K. Marine-derived Natural Products as Anticancer Agents. Med. Chem. 2023, 19, 538–555. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Wei, B.; Wang, S.; Ke, S.; Chen, J.; Zhang, H.; Wang, H. The Antioxidant Activity of Polysaccharides Derived from Marine Organisms: An Overview. Mar. Drugs 2019, 17, 674. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Luo, D.; Luesch, H. Advances in exploring the therapeutic potential of marine natural products. Pharmacol. Res. 2019, 147, 104373. [Google Scholar] [CrossRef] [PubMed]
- Nadar, V.M.; Manivannan, S.; Chinnaiyan, R.; Govarthanan, M.; Ponnuchamy, K. Review on marine sponge alkaloid, aaptamine: A potential antibacterial and anticancer drug. Chem. Biol. Drug. Des. 2022, 99, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Mehbub, M.F.; Lei, J.; Franco, C.; Zhang, W. Marine sponge derived natural products between 2001 and 2010: Trends and opportunities for discovery of bioactives. Mar. Drugs 2014, 12, 4539–4577. [Google Scholar] [CrossRef] [PubMed]
- Lindequist, U. Marine-Derived Pharmaceuticals—Challenges and Opportunities. Biomol. Ther. 2016, 24, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, D.X.; Castellanos, F.A.; Coy-Barrera, E.; Tello, E. Prostaglandins Isolated from the Octocoral Plexaura homomalla: In silico and in Vitro studies against different enzymes of cancer. Mar. Drugs 2020, 18, 141. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Manier, M.L.; Hachey, D.L.; Brash, A.R. Detection of the 15-acetate of prostaglandin E2 methyl ester as a prominent component of the prostaglandins in the gorgonian coral Plexaura homomalla. Lipids 2002, 37, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef] [PubMed]
- Larghi, E.L.; Bohn, M.L.; Kaufman, T.S. Aaptamine and related products. Their isolation, chemical syntheses, and biological activity. Tetrahedron 2009, 65, 4257–4282. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Joseph, A.; Nair, B.G. Promising bioactive compounds from the marine environment and their potential effects on various diseases. J. Genet. Eng. Biotechnol. 2022, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Proksch, P.; Ebel, R.; Edrada, R.A.; Schupp, P.; Lin, W.H.; Sudarsono; Wray, V.; Steube, K. Detection of pharmacologically active natural products using ecology. Selected examples from Indopacific marine invertebrates and sponge-derived fungi. Pure Appl. Chem. 2003, 75, 343–352. [Google Scholar] [CrossRef]
- Nakamura, H.; Kobayashi, J.I.; Ohizumi, Y.; Hirata, Y. Isolation and structure of aaptamine a novel heteroaromatic substance possessing α-blocking activity from the sea sponge Aaptos aaptos. Tetrahedron Lett. 1982, 23, 5555–5558. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, F.; Sun, F.; Liu, L.; Liu, B.; Wang, S.P.; Cheng, C.W.; Liao, H.; Lin, H.W. Total synthesis of aaptamine, demethyloxyaaptamine, and their 3-alkylamino derivatives. Org. Lett. 2019, 21, 1430–1433. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M. Reactive oxygen species in tumor metastasis. Cancer Lett. 2008, 266, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Gao, Y.; Feng, Z.; Zhang, B.; Na, Z.; Li, D. Reactive oxygen species and ovarian diseases: Antioxidant strategies. Redox Biol. 2023, 62, 102659. [Google Scholar] [CrossRef] [PubMed]
- Averill-Bates, D. Reactive oxygen species and cell signaling. Review. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119573. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V.; Valacchi, G. Free radicals and antioxidants: How to reestablish redox homeostasis in chronic diseases? Curr. Med. Chem. 2013, 20, 3397–3415. [Google Scholar] [CrossRef] [PubMed]
- Utkina, N.K. Antioxidant activity of aromatic alkaloids from the marine sponges Aaptos aaptos and Hyrtios sp. Chem. Nat. Compd. 2009, 45, 849–853. [Google Scholar] [CrossRef]
- Takamatsu, S.; Hodges, T.W.; Rajbhandari, I.; Gerwick, W.H.; Hamann, M.T.; Nagle, D.G. Marine natural products as novel antioxidant prototypes. J. Nat. Prod. 2003, 66, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Woo, S.W.; Kim, M.S.; Park, J.E.; Hwang, J.K. Anti-photoaging effect of aaptamine in UVB-irradiated human dermal fibroblasts and epidermal keratinocytes. J. Asian Nat. Prod. Res. 2014, 16, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Kole, A. Tuberculosis. JAAPA 2023, 36, 43–44. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Han, J.; Shen, J.; Peng, X.; Zhou, L.; Yin, X. Diagnosis and treatment of tuberculosis in adults with HIV. Medicine 2022, 101, e30405. [Google Scholar] [CrossRef] [PubMed]
- Bouzeyen, R.; Javid, B. Therapeutic Vaccines for Tuberculosis: An Overview. Front. Immunol. 2022, 13, 878471. [Google Scholar] [CrossRef] [PubMed]
- Günther, G.; Ruswa, N.; Keller, P.M. Drug-resistant tuberculosis: Advances in diagnosis and management. Curr. Opin. Pulm. Med. 2022, 28, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Tiberi, S.; Utjesanovic, N.; Galvin, J.; Centis, R.; D’Ambrosio, L.; van den Boom, M.; Zumla, A.; Migliori, G.B. Drug resistant TB-latest developments in epidemiology, diagnostics and management. Int. J. Infect. Dis. 2022, 124, S20–S25. [Google Scholar] [CrossRef] [PubMed]
- Sumii, Y.; Kotoku, N.; Han, C.; Kamiya, K.; Setiawan, A.; Vilchèze, C.; Jacobs Jr, W.R.; Arai, M. 3-(Phenethylamino) dimethyl (oxy) aaptamine as an anti-dormant mycobacterial substance: Isolation, evaluation and total synthesis. Tetrahedron Lett. 2020, 61, 151924. [Google Scholar] [CrossRef] [PubMed]
- Sumii, Y.; Kamiya, K.; Nakamura, T.; Tanaka, K.; Kaji, T.; Mukomura, J.; Kotoku, N.; Arai, M. Study of the structure-activity relationship of an anti-dormant mycobacterial substance 3-(phenethylamino) dimethyl (oxy) aaptamine to create a probe molecule for detecting its target protein. Mar. Drugs 2022, 20, 98. [Google Scholar] [CrossRef] [PubMed]
- Mukomura, J.; Nonaka, H.; Sato, H.; Kishimoto, M.; Arai, M.; Kotoku, N. Anti-Mycobacterial N-(2-Arylethyl) quinolin-3-amines Inspired by Marine Sponge-Derived Alkaloid. Molecules 2022, 27, 8701. [Google Scholar] [CrossRef] [PubMed]
- Wayne, L.G.; Sohaskey, C.D. Nonreplicating persistence of Mycobacterium tuberculosis. Annu. Rev. Microbiol. 2001, 55, 139–163. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Han, C.; Yamano, Y.; Setiawan, A.; Kobayashi, M. Aaptamines, marine spongean alkaloids, as anti-dormant mycobacterial substances. J. Nat. Med. 2014, 68, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Kubota, T.; Shibazaki, A.; Gonoi, T.; Fromont, J.; Kobayashi, J. Nakijinamines C-E, new heteroaromatic alkaloids from the sponge Suberites species. Org. Lett. 2011, 13, 3016–3019. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.H.; Chung, S.C.; Shin, J.; Lee, S.H.; Kim, T.I.; Lee, H.S.; Oh, K.B. Aaptamines as sortase A inhibitors from the tropical sponge Aaptos aaptos. Bioorg. Med. Chem. Lett. 2007, 17, 5366–5369. [Google Scholar] [CrossRef] [PubMed]
- Pluta, A.J.; Studniarek, C.; Murphy, S.; Norbury, C.J. Cyclin-dependent kinases: Masters of the eukaryotic universe. Wiley Interdiscip. Rev. RNA 2023, 15, e1816. [Google Scholar] [CrossRef] [PubMed]
- Zabihi, M.; Lotfi, R.; Yousefi, A.M.; Bashash, D. Cyclins and cyclin-dependent kinases: From biology to tumorigenesis and therapeutic opportunities. J. Cancer Res. Clin. Oncol. 2023, 149, 1585–1606. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, J.A.; Crncec, A.; Afifi, M.M.; Tang, K.; Amin, R.; Cappell, S.D. Loss of CDK4/6 activity in S/G2 phase leads to cell cycle reversal. Nature 2023, 619, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kang, M.K.; Seong, S.Y.; Jo, J.H.; Kim, M.J.; Shin, E.K.; Lee, C.G.; Han, S.J. Meiotic Cell Cycle Progression in Mouse Oocytes: Role of Cyclins. Int. J. Mol. Sci. 2023, 24, 13659. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Geng, Y.; Zhou, Y.; Sicinski, P. Cyclin E in normal physiology and disease states. Trends Cell Biol. 2021, 31, 732–746. [Google Scholar] [CrossRef] [PubMed]
- Bendris, N.; Lemmers, B.; Blanchard, J.M. Cell cycle, cytoskeleton dynamics and beyond: The many functions of cyclins and CDK inhibitors. Cell Cycle 2015, 14, 1786–1798. [Google Scholar] [CrossRef]
- Loukil, A.; Cheung, C.T.; Bendris, N.; Lemmers, B.; Peter, M.; Blanchard, J.M. Cyclin A2: At the crossroads of cell cycle and cell invasion. World J. Biol. Chem. 2015, 6, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Kong, D.; Suna, H.; Sowa, Y.; Sakai, T.; Setiawan, A.; Kobayashi, M. Aaptamine, a spongean alkaloid, activates p21 promoter in a p53-independent manner. Biochem. Biophys. Res. Commun. 2006, 342, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Zhao, W.; Zhang, Y.; Kobayashi, M.; Duan, H.; Kong, D. Antiproliferative effect of aaptamine on human chronic myeloid leukemia K562 cells. Int. J. Mol. Sci. 2011, 12, 7352–7359. [Google Scholar] [CrossRef]
- Li, Q.L.; Zhang, P.P.; Wang, P.Q.; Yu, H.B.; Sun, F.; Hu, W.Z.; Wu, W.H.; Zhang, X.; Chen, F.; Chu, Z.Y.; et al. The cytotoxic and mechanistic effects of aaptamine on hepatocellular carcinoma. Anti-Cancer Agents Med. Chem. 2015, 15, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Slingerland, J.M. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle 2003, 2, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, B.A.; Restuccia, D.F. The PI3K-PKB/Akt pathway. Cold Spring Harb. Perspect. Biol. 2015, 7, a011189. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, B.T.; Smith, D.L.; Ram, P.T.; Lu, Y.; Mills, G.B. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005, 4, 988–1004. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Patni, P.; Bishayee, A.; Sah, A.N.; Bishayee, A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Semin. Cancer Biol. 2022, 80, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Braglia, L.; Zavatti, M.; Vinceti, M.; Martelli, A.M.; Marmiroli, S. Deregulated PTEN/PI3K/AKT/mTOR signaling in prostate cancer: Still a potential druggable target? Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118731. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, F.; Evangelisti, C.; McCubrey, J.A.; Martelli, A.M. Current treatment strategies for inhibiting mTOR in cancer. Trends Pharmacol. Sci. 2015, 36, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Hlozkova, K.; Hermanova, I.; Safrhansova, L.; Alquezar-Artieda, N.; Kuzilkova, D.; Vavrova, A.; Sperkova, K.; Zaliova, M.; Stary, J.; Trka, J.; et al. PTEN/PI3K/Akt pathway alters sensitivity of T-cell acute lymphoblastic leukemia to L-asparaginase. Sci. Rep. 2022, 12, 4043. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Etemad, S.; Rezaei, S.; Ziaolhagh, S.; Rajabi, R.; Rahmanian, P.; Abdi, S.; Koohpar, Z.K.; Rafiei, R.; Raei, B.; et al. Progress in targeting PTEN/PI3K/Akt axis in glioblastoma therapy: Revisiting molecular interactions. Biomed. Pharmacother. 2023, 158, 114204. [Google Scholar] [CrossRef] [PubMed]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.J.; Stone, E.; Baldwin, D.R.; Vliegenthart, R.; Lee, P.; Fintelmann, F.J. Lung cancer screening. Lancet 2023, 401, 390–408. [Google Scholar] [CrossRef] [PubMed]
- Nooreldeen, R.; Bach, H. Current and Future Development in Lung Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J.; Wu, Y.L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current challenges in cancer treatment. Clin. Ther. 2016, 38, 1551–1566. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Ren, K.H.; He, H.W.; Shao, R.G. Involvement of PI3K/AKT/GSK3beta pathway in tetrandrine-induced G1 arrest and apoptosis. Cancer Biol. Ther. 2008, 7, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Doble, B.W.; Woodgett, J.R. GSK-3: Tricks of the trade for a multi-tasking kinase. J. Cell Sci. 2003, 116, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Forde, J.E.; Dale, T.C. Glycogen synthase kinase 3: A key regulator of cellular fate. Cell. Mol. Life Sci. 2007, 64, 1930–1944. [Google Scholar] [CrossRef] [PubMed]
- Domoto, T.; Pyko, I.V.; Furuta, T.; Miyashita, K.; Uehara, M.; Shimasaki, T.; Nakada, M.; Minamoto, T. Glycogen synthase kinase-3β is a pivotal mediator of cancer invasion and resistance to therapy. Cancer Sci. 2016, 107, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Walz, A.; Ugolkov, A.; Chandra, S.; Kozikowski, A.; Carneiro, B.A.; O’Halloran, T.V.; Giles, F.J.; Billadeau, D.D.; Mazar, A.P. Molecular pathways: Revisiting glycogen synthase kinase-3β as a target for the treatment of cancer. Clin. Cancer Res. 2017, 23, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.M.; Hemmings, B.A. Inhibition of protein kinase B/Akt: Implications for cancer therapy. Pharmacol. Ther. 2002, 93, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Majewska, E.; Szeliga, M. AKT/GSK3β signaling in glioblastoma. Neurochem. Res. 2017, 42, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Takahashi-Yanaga, F.; Sasaguri, T. GSK-3beta regulates cyclin D1 expression: A new target for chemotherapy. Cell. Signal. 2008, 20, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Miao, S.; Yang, L.; Wu, Y.; Guo, J.; Chen, W.; Dai, J.; Du, J.; Xi, S. Aaptamine attenuates the proliferation and progression of non-small cell lung carcinoma. Pharm. Biol. 2020, 58, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Trang, D.T.; Tai, B.H.; Hang, D.T.T.; Yen, P.H.; Nhiem, N.X.; Kiem, P.V. Four new aaptamine alkaloids from marine sponge Aaptos aaptos. Nat. Prod. Res. 2022, 36, 5022–5031. [Google Scholar] [CrossRef] [PubMed]
- Stuhldreier, F.; Kassel, S.; Schumacher, L.; Wesselborg, S.; Proksch, P.; Fritz, G. Pleiotropic effects of spongean alkaloids on mechanisms of cell death, cell cycle progression and DNA damage response (DDR) of acute myeloid leukemia (AML) cells. Cancer Lett. 2015, 361, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.D.; Hartmann, R.; Müller, W.E.; de Voogd, N.; Lai, D.; Proksch, P. Aaptamine derivatives from the Indonesian sponge Aaptos suberitoides. J. Nat. Prod. 2013, 76, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.F.; Lee, M.G.; El-Shazly, M.; Lai, K.H.; Ke, S.C.; Su, C.W.; Shih, S.P.; Sung, P.J.; Hong, M.C.; Wen, Z.H.; et al. Isoaaptamine induces T-47D cells apoptosis and autophagy via oxidative stress. Mar. Drugs 2018, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Venz, S.; Shubina, L.K.; Fedorov, S.N.; Walther, R.; Jacobsen, C.; Stonik, V.A.; Bokemeyer, C.; Balabanov, S.; Honecker, F. Activity of aaptamine and two derivatives, demethyloxyaaptamine and isoaaptamine, in cisplatin-resistant germ cell cancer. J. Proteom. 2014, 96, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tang, X.; Li, P.; Li, G. Suberitine A-D, four new cytotoxic dimeric aaptamine alkaloids from the marine sponge Aaptos suberitoides. Org. Lett. 2012, 14, 1994–1997. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Hoffmann, H.; Herald, D.L.; McNulty, J.; Murphy, A.; Higgs, K.C.; Hamel, E.; Lewin, N.E.; Pearce, L.V.; Blumberg, P.M.; et al. Antineoplastic agents 491. Synthetic conversion of aaptamine to isoaaptamine, 9-demethylaaptamine, and 4-methylaaptamine. J. Org. Chem. 2004, 69, 2251–2256. [Google Scholar] [CrossRef] [PubMed]
- He, Q.Q.; Man, Y.Q.; Sun, K.L.; Yang, L.J.; Wu, Y.; Du, J.; Chen, W.W.; Dai, J.J.; Ni, N.; Miao, S.; et al. Aaptamine derivatives with CDK2 inhibitory activities from the South China Sea sponge Aaptos suberitoides. Nat. Prod. Res. 2022, 36, 6215–6223. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Gao, Y.; Chang, Y.T.; Zou, Y.; Houk, K.N.; Lu, J.R.; He, J.; Tang, W.Z.; Liao, H.Z.; Han, H.; et al. Aromatic ring substituted aaptamine analogues as potential cytotoxic agents against extranodal natural killer/T-cell lymphoma. J. Nat. Prod. 2020, 83, 3758–3763. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.C.; Lin, T.T.; Sheu, J.H.; Duh, C.Y. Structures and cytotoxicity relationship of isoaaptamine and aaptamine derivatives. J. Nat. Prod. 1999, 62, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Shubina, L.K.; Makarieva, T.N.; Dyshlovoy, S.A.; Fedorov, S.N.; Dmitrenok, P.S.; Stonik, V.A. Three new aaptamines from the marine sponge Aaptos sp. and their proapoptotic properties. Nat. Prod. Commun. 2010, 5, 1881–1884. [Google Scholar] [CrossRef] [PubMed]
- Shejul, P.P.; Raheja, R.K.; Doshi, G.M. An update on potential antidepressants derived from marine natural products. Cent. Nerv. Syst. Agents Med. Chem. 2023, 23, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; El-Alfy, A.T.; Ezel, K.; Radwan, M.O.; Shilabin, A.G.; Kochanowska-Karamyan, A.J.; Abd-Alla, H.I.; Otsuka, M.; Hamann, M.T. Marine inspired 2-(5-halo-1H-indol-3-yl)-N,N-dimethylethanamines as modulators of serotonin receptors: An example illustrating the power of bromine as part of the uniquely marine chemical space. Mar. Drugs 2017, 15, 248. [Google Scholar] [CrossRef] [PubMed]
- Diers, J.A.; Ivey, K.D.; El-Alfy, A.; Shaikh, J.; Wang, J.; Kochanowska, A.J.; Stoker, J.F.; Hamann, M.T.; Matsumoto, R.R. Identification of antidepressant drug leads through the evaluation of marine natural products with neuropsychiatric pharmacophores. Pharmacol. Biochem. Behav. 2008, 89, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Ohizumi, Y.; Kajiwara, A.; Nakamura, H.; Kobayashi, J. Alpha-adrenoceptor blocking action of aaptamine, a novel marine natural product, in vascular smooth muscle. J. Pharm. Pharmacol. 1984, 36, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Moodie, L.W.K.; Sepčić, K.; Turk, T.; Frangež, R.; Svenson, J. Natural cholinesterase inhibitors from marine organisms. Nat. Prod. Rep. 2019, 36, 1053–1092. [Google Scholar] [CrossRef] [PubMed]
- Lins Alves, L.K.; Cechinel Filho, V.; de Souza, R.L.R.; Furtado-Alle, L. BChE inhibitors from marine organisms—A review. Chem. Biol. Interact. 2022, 367, 110136. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; He, Q.; Li, C.; Wu, Y.; Liu, M.; Chen, Y.; Qi, S.; Gong, K. Aaptamine—A dual acetyl—And butyrylcholinesterase inhibitor as potential anti-Alzheimer’s disease agent. Pharm. Biol. 2022, 60, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.S.; Cheng, H.J.; Chen, N.F.; Tang, S.H.; Kuo, H.M.; Sung, P.J.; Chen, W.F.; Wen, Z.H. Antinociceptive effects of aaptamine, a sponge component, on peripheral neuropathy in rats. Mar. Drugs 2023, 21, 113. [Google Scholar] [CrossRef] [PubMed]
- Luyao, H.; Luesch, H.; Uy, M. GPCR pharmacological profiling of aaptamine from the Philippine sponge Stylissa sp. extends its therapeutic potential for noncommunicable diseases. Molecules 2021, 26, 5618. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122. [Google Scholar] [CrossRef] [PubMed]
- Chabowska, G.; Barg, E.; Wójcicka, A. Biological Activity of Naturally Derived Naphthyridines. Molecules 2021, 26, 4324. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).