Crystal Structures, Magnetic Properties, and Redox Behaviors of Carboxylato-Bridged Mn(II) Complexes with Ditopic Ligands Featuring N3-Coordination Sites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.2.1. [Mn2F(OAc)2(tphn)]BF4 (1)
2.2.2. [{Mn2(OAc)3(tpon)}2](BPh4)2 (2)
2.2.3. [{Mn2(OAc)2(tpxn)(CH3OH)(H2O)2}2][{Mn2(OAc)3(tpxn)(CH3OH)(H2O)}2](PF6)6 (3)
2.3. Measurements
2.4. Single Crystal X-ray Diffraction
2.5. Study of Catalase Activity
3. Results and Discussion
3.1. Synthetic Aspects
3.2. Crystal Structures
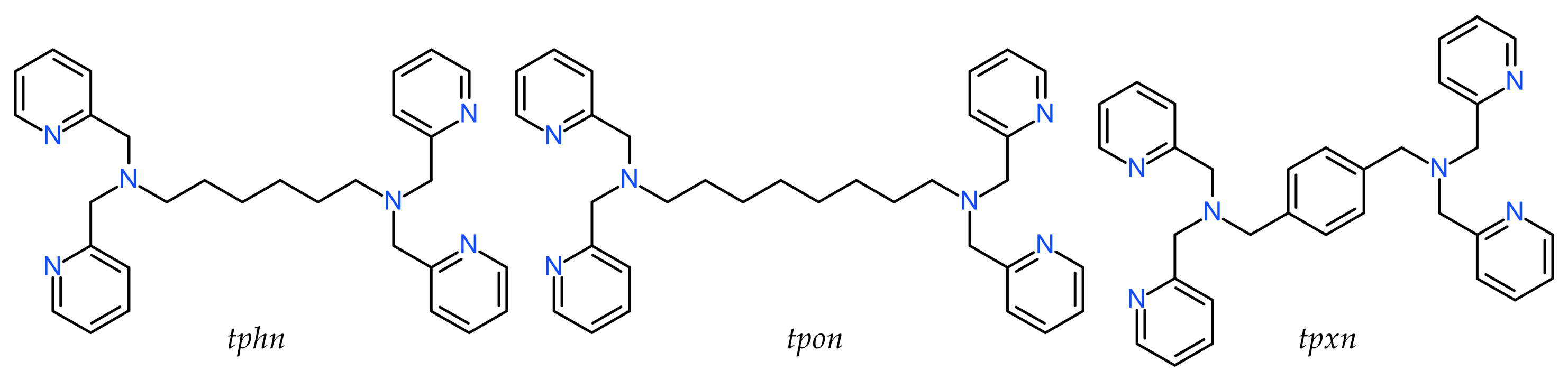
3.2.1. Complex 1
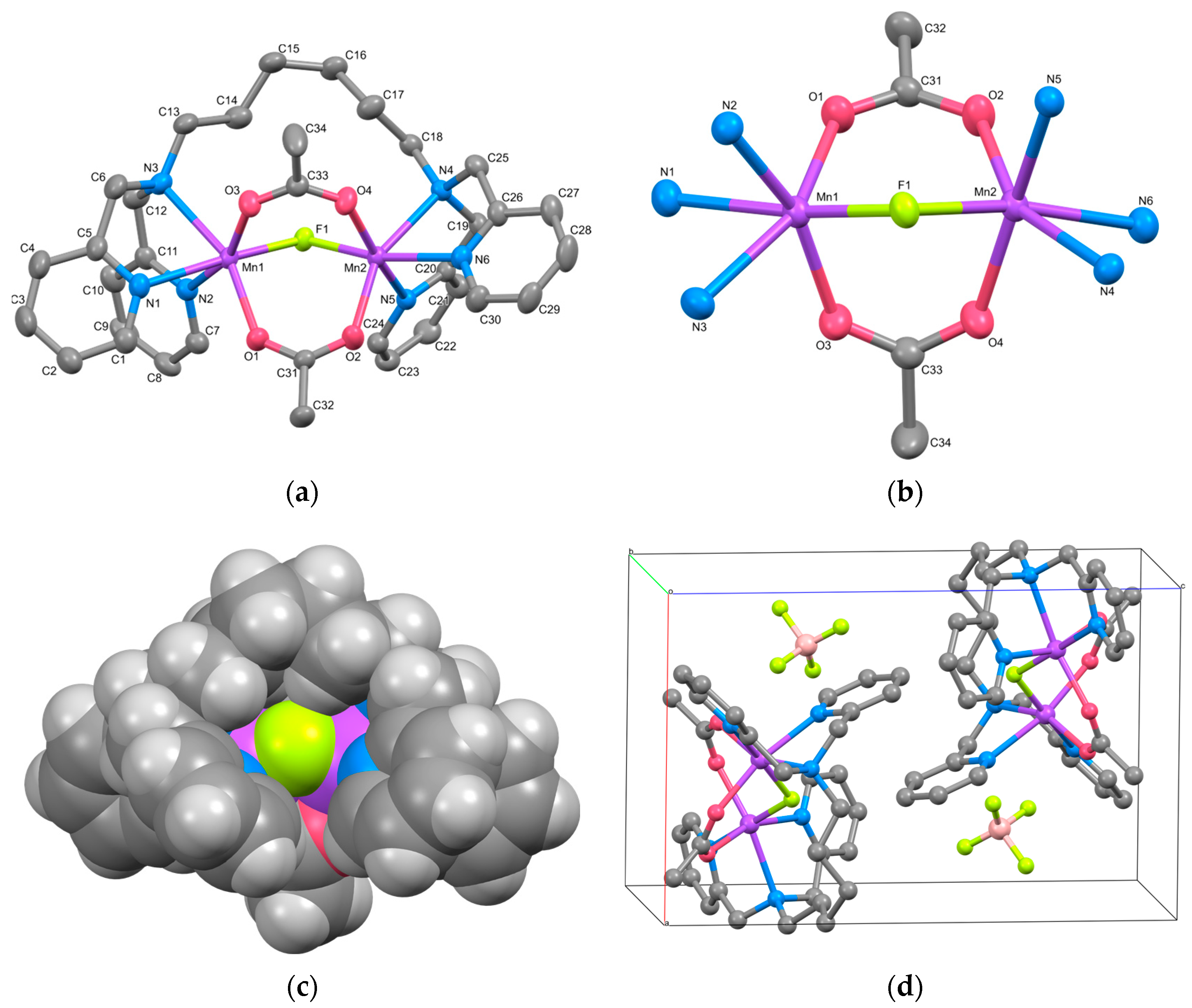
3.2.2. Complex 2
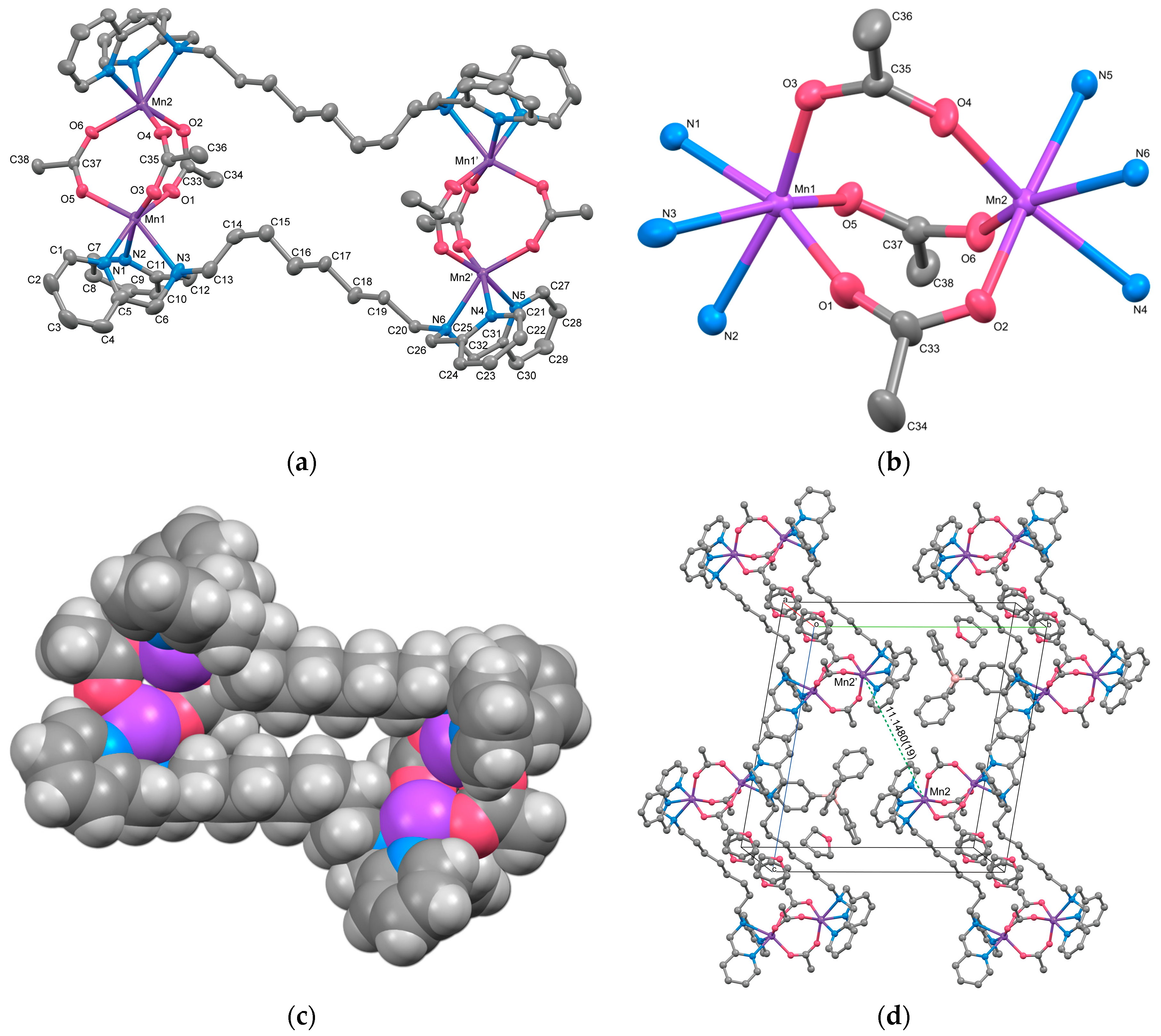
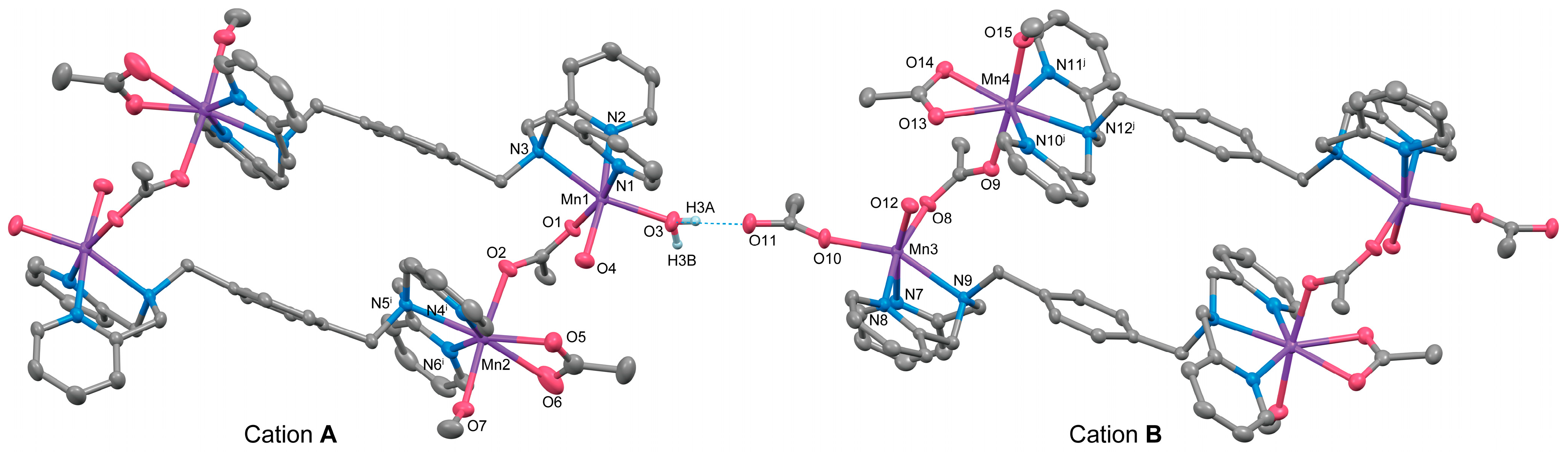
3.2.3. Complex 3
3.3. IR Spectroscopy
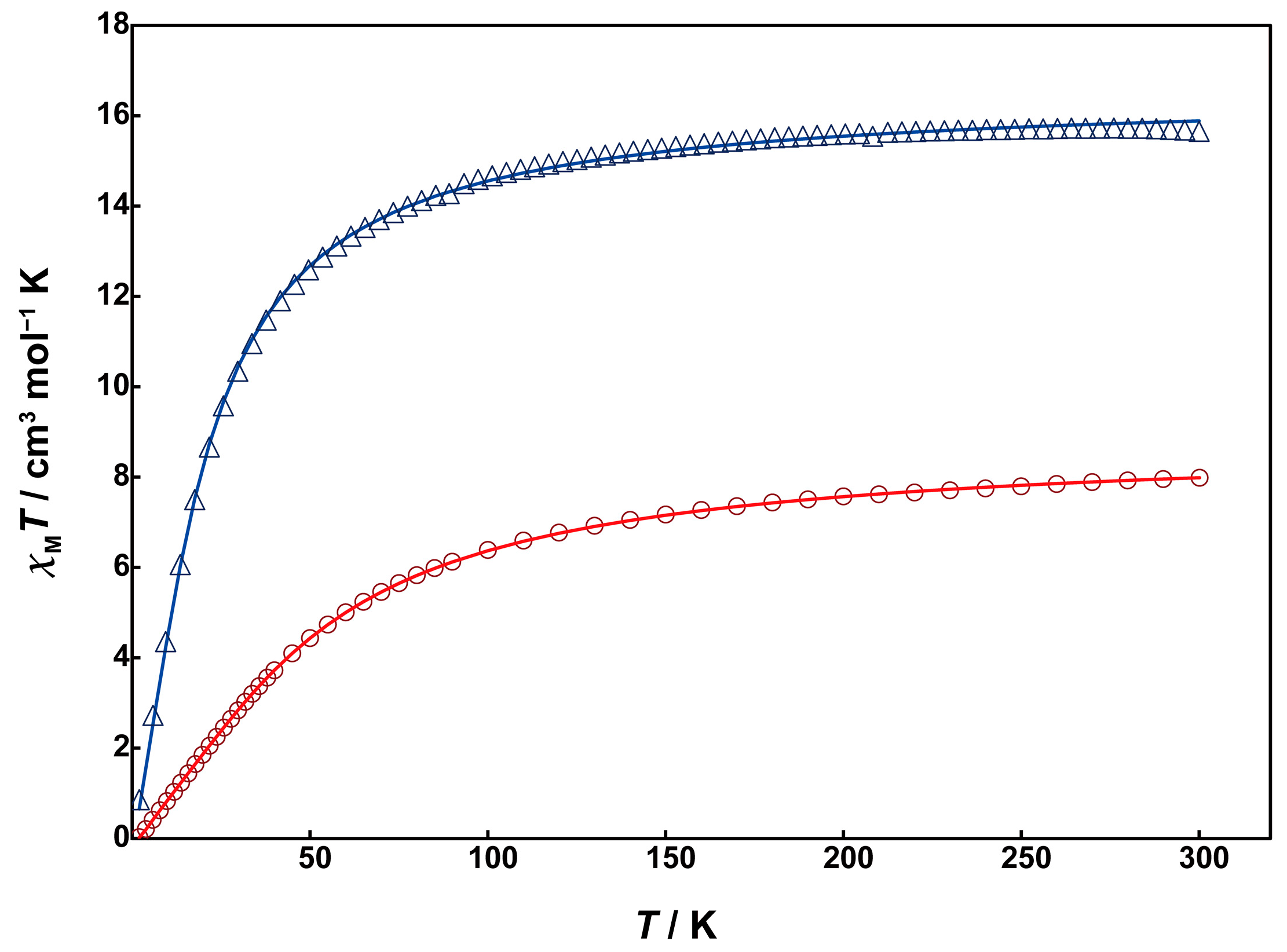
3.4. Magnetic Measurements
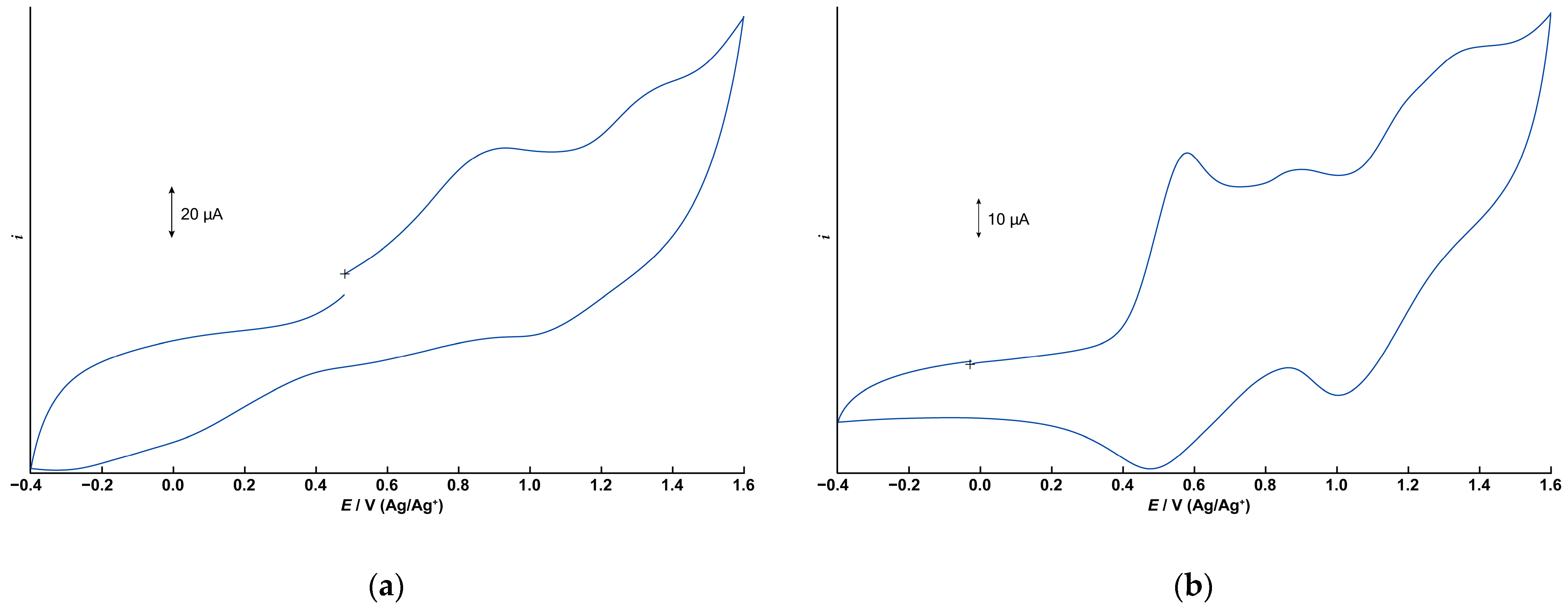
3.5. Electrochemistry
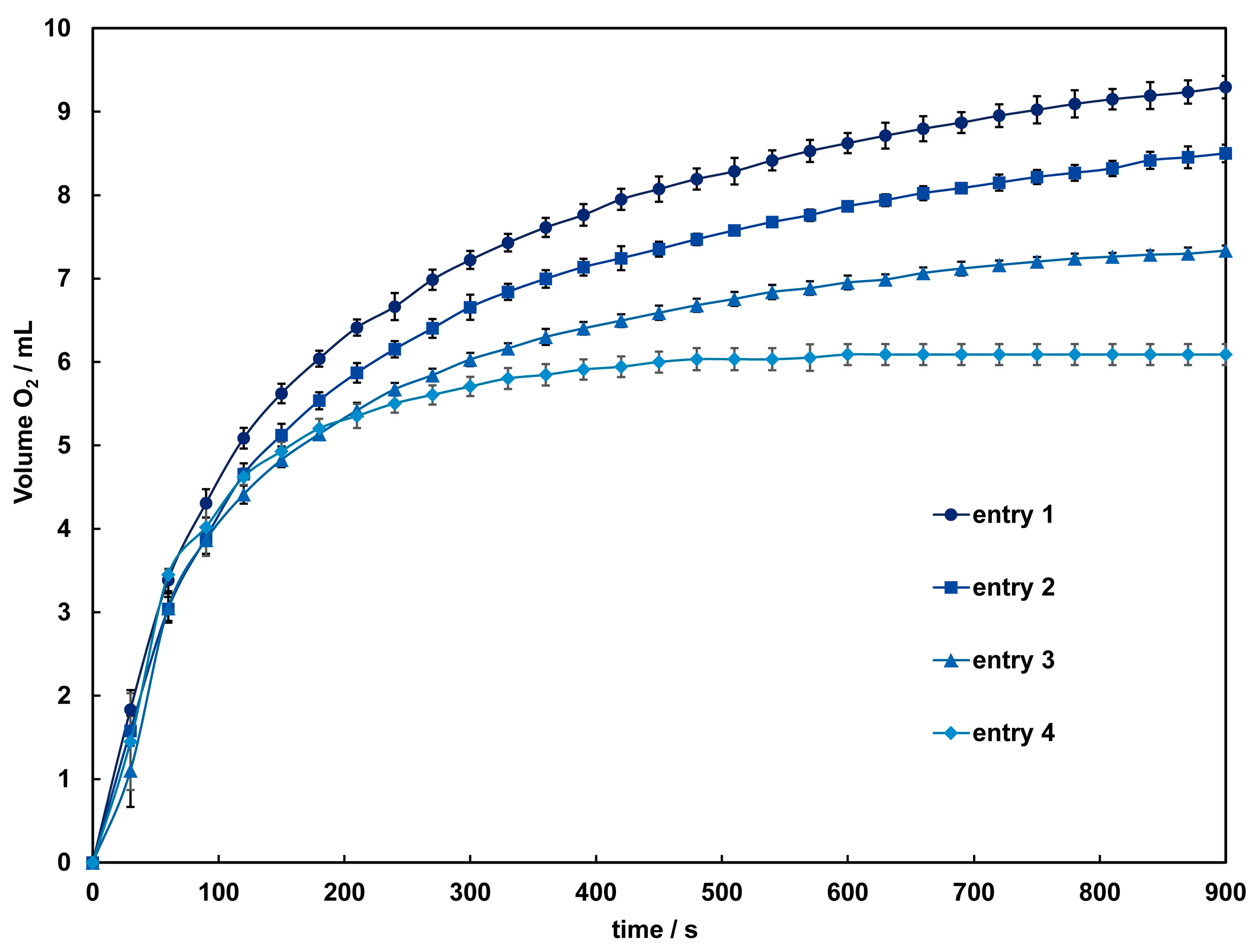
3.6. Catalase Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Price, D.J.; Batten, S.R.; Moubaraki, B.; Murray, K.S. Synthesis, structure and magnetism of a new manganese carboxylate cluster: [Mn16O16(OMe)6(OAc)16(MeOH)3(H2O)3]·6H2O. Chem. Commun. 2002, 762–763. [Google Scholar] [CrossRef]
- Mullins, C.S.; Pecoraro, V.L. Reflections on small molecule manganese models that seek to mimic photosynthetic water oxidation chemistry. Coord. Chem. Rev. 2008, 252, 416–443. [Google Scholar] [CrossRef]
- Asghar, A.; Iqbal, N.; Noor, T.; Kariuki, B.M.; Kidwell, L.; Easun, T.L. Efficient electrochemical synthesis of a manganese-based metal–organic framework for H2 and CO2 uptake. Green Chem. 2021, 23, 1220–1227. [Google Scholar] [CrossRef]
- Ribas, J.; Albela, B.; Stoeckli-Evans, H.; Christou, G. Synthesis and magnetic properties of six new trinuclear oxo-centered manganese complexes of general formula [Mn3O(X-benzoato)6L3] (X = 2-F, 2-Cl, 2-Br, 3-F, 3-Cl, 3-Br; L = pyridine or water) and crystal structures of the 2-F, 3-Cl, and 3-Br complexes. Inorg. Chem. 1997, 36, 2352–2360. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Ghosh, A. Synthesis, structure and alkene epoxidation activity of an alternating phenoxido and formato bridged manganese(III)–salen complex. Inorg. Chim. Acta 2013, 395, 67–71. [Google Scholar] [CrossRef]
- Durot, S.; Policar, C.; Pelosi, G.; Bisceglie, F.; Mallah, T.; Mahy, J.-P. Structural and magnetic properties of carboxylato-bridged manganese(II) complexes involving tetradentate ligands: Discrete complex and 1D polymers. Dependence of J on the nature of the carboxylato bridge. Inorg. Chem. 2003, 42, 8072–8080. [Google Scholar] [CrossRef] [PubMed]
- Triller, M.U.; Hsieh, W.-Y.; Pecoraro, V.L.; Rompel, A.; Krebs, B. Preparation of highly efficient manganese catalase mimics. Inorg. Chem. 2002, 41, 5544–5554. [Google Scholar] [CrossRef]
- Gómez, V.; Corbella, M. Crystal structure and magnetic properties of the dinuclear MnII compound [Mn2(bpy)4(2-ClC6H4COO)2](ClO4)2∙2EtOH. J. Chem. Crystallogr. 2011, 41, 843–846. [Google Scholar] [CrossRef]
- Singh, U.P.; Singh, R.; Hikichi, S.; Akita, M.; Moro-oka, Y. Characterization of a dinuclear Mn(II) tri(μ-carboxylato) complex with the hindered hydrotris(3,5-diisopropyl-1-pyrazolyl)borate (=TpiPr2) ligand: Intramolecular hydrogen bonding interaction between the protonated TpiPr2 and Mn-coordinating carboxylate ligands. Inorg. Chim. Acta 2000, 310, 273–278. [Google Scholar] [CrossRef]
- Romero, I.; Dubois, L.; Collomb, M.-N.; Deronzier, A.; Latour, J.-M.; Pécaut, J. A Dinuclear manganese(II) complex with the {Mn2(μ-O2CCH3)3}+ core: Synthesis, structure, characterization, electroinduced transformation, and catalase-like activity. Inorg. Chem. 2002, 41, 1795–1806. [Google Scholar] [CrossRef]
- Fernández, G.; Corbella, M.; Alfonso, M.; Stoeckli-Evans, H.; Castro, I. A comparative XAS and X-ray diffraction study of new binuclear Mn(III) complexes with catalase activity. Indirect effect of the counteranion on magnetic properties. Inorg. Chem. 2004, 43, 6684–6698. [Google Scholar] [CrossRef] [PubMed]
- Sessoli, R.; Gatteschi, D.; Caneschi, A.; Novak, M.A. Magnetic bistability in a metal-ion cluster. Nature 1993, 365, 141–143. [Google Scholar] [CrossRef]
- Kanyo, Z.F.; Scolnick, L.R.; Ash, D.E.; Christianson, D.W. Structure of a unique binuclear manganese cluster in arginase. Nature 1996, 383, 554–557. [Google Scholar] [CrossRef]
- Barynin, V.V.; Whittaker, M.M.; Antonyuk, S.V.; Lamzin, V.S.; Harrison, P.M.; Artymiuk, P.J.; Whittaker, J.W. Crystal structure of manganese catalase from Lactobacillus plantarum. Structure 2001, 9, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Sakiyama, H.; Ōkawa, H.; Isobe, R. A functional model of manganese catalase. Mass spectrometric and visible spectral evidence for {MnIV(=O)}2 and MnIIMnIV(=O) intermediates. J. Chem. Soc. Chem. Commun. 1993, 882–884. [Google Scholar] [CrossRef]
- de Boer, J.W.; Browne, W.R.; Feringa, B.L.; Hage, R. Carboxylate-bridged dinuclear manganese systems—From catalases to oxidation catalysis. Comptes Rendus Chim. 2007, 10, 341–354. [Google Scholar] [CrossRef]
- Wieghardt, K.; Bossek, U.; Nuber, B.; Weiss, J.; Bonvoisin, J.; Corbella, M.; Vitols, S.E.; Girerd, J.J. Synthesis, crystal structures, reactivity, and magnetochemistry of a series of binuclear complexes of manganese(II), -(III), and -(IV) of biological relevance. The crystal structure of [L’MnIV(μ-O)3MnIVL’](PF6)2·H2O containing an unprecedented short Mn···Mn distance of 2.296 Å. J. Am. Chem. Soc. 1988, 110, 7398–7411. [Google Scholar] [CrossRef]
- Singh, R.; Haukka, M.; McKenzie, C.J.; Nordlander, E. High turnover catalase activity of a mixed-valence MnIIMnIII complex with terminal carboxylate donors. Eur. J. Inorg. Chem. 2015, 2015, 3485–3492. [Google Scholar] [CrossRef]
- Toftlund, H.; Markiewicz, A.; Murray, K.S. Synthesis and magnetic properties of a μ-oxo-di(μ-acetato)manganese(III) complex of a strapped tripodal pyridylamine ligand N,N,N′,N′-tetrakis(2-pyridylmethyl)-1,3-propanediamine. A model for the Mn2 site of Mn-catalase enzymes. Acta Chem. Scand. 1990, 44, 443–446. [Google Scholar] [CrossRef]
- Ertürk, H.; Hofmann, A.; Puchta, R.; van Eldik, R. Influence of the bridging ligand on the substitution behavior of dinuclear Pt(II) complexes. An experimental and theoretical approach. Dalton Trans. 2007, 2295–2301. [Google Scholar] [CrossRef]
- Lombardo, V.; Bonomi, R.; Sissi, C.; Mancin, F. Phosphate diesters and DNA hydrolysis by dinuclear Zn(II) complexes featuring a disulfide bridge and H-bond donors. Tetrahedron 2010, 66, 2189–2195. [Google Scholar] [CrossRef]
- Kawahara, S.; Uchimaru, T. Dinucleotide hydrolysis promoted by dinuclear Zn complexes—The effect of the distance between Zn ions in the complexes on the hydrolysis rate. Eur. J. Inorg. Chem. 2001, 2001, 2437–2442. [Google Scholar] [CrossRef]
- Selwood, P.W. Magnetochemistry; Interscience Publishers: New York, NY, USA, 1956; pp. 78–91. [Google Scholar]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- Rigaku Corporation. CrystalClear: Data Collection and Processing Software; Rigaku Corporation: Tokyo, Japan, 1998. [Google Scholar]
- Rigaku Oxford Diffraction. CrysAlis Pro; Rigaku Oxford Diffraction: Tokyo, Japan, 2022. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Wei, P.-R.; Li, Q.; Leung, W.-P.; Makt, T.C.W. A dinuclear manganese(ll) complex doubly bridged by skew-skew μ-carboxylato groups: Synthesis and X-ray structure of [{Mn(bpy)2(p-Me2N+C5H4NCH2CO2−)}2](C1O4)4. Polyhedron 1997, 16, 897–902. [Google Scholar] [CrossRef]
- Matsushima, H.; Ishiwa, E.; Koikawa, M.; Nakashima, M.; Tokii, T. Synthesis, structure, and magnetic properties of triply carboxylato-bridged dimanganese(II) complexes. Chem. Lett. 1995, 24, 129–130. [Google Scholar] [CrossRef]
- Jeong, E.; Ito, T.; Takahashi, K.; Koganezawa, T.; Hayashi, H.; Aratani, N.; Suzuki, M.; Yamada, H. Exploration of alkyl group effects on the molecular packing of 5,15-disubstituted tetrabenzoporphyrins toward efficient charge-carrier transport. ACS Appl. Mater. Interfaces 2022, 14, 32319–32329. [Google Scholar] [CrossRef] [PubMed]
- Brinksma, J.; Hage, R.; Kerschner, J.; Feringa, B.L. The dinuclear manganese complex Mn2O(OAc)2(TPTN) as a catalyst for epoxidations with hydrogen peroxide. Chem. Commun. 2000, 537–538. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Gómez, V.; Corbella, M. Catalase activity of dinuclear MnIII compounds with chlorobenzoato bridges. Eur. J. Inorg. Chem. 2012, 2012, 3147–3155. [Google Scholar] [CrossRef]
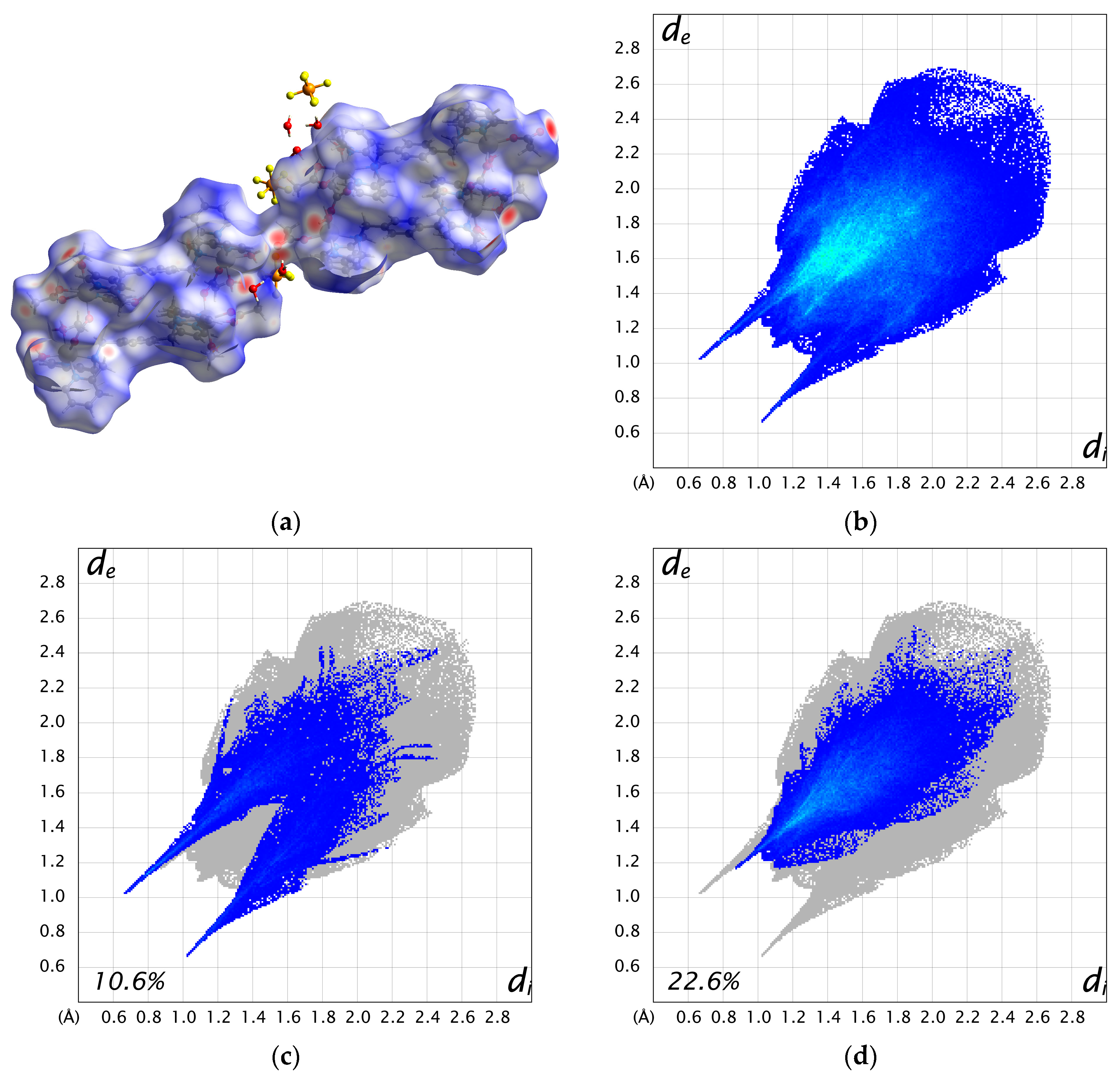
| 1 | 2 | 3 | |
|---|---|---|---|
| Empirical formula | C34H42BF5Mn2N6O4 | C136H162B2Mn4N12O15 | C152H210F36Mn8N24O42P6 |
| Formula weight | 814.42 | 2446.15 | 4354.77 |
| Temperature/K | 301 | 113 | 113 |
| Crystal system | triclinic | triclinic | monoclinic |
| Space group | P21/c | ||
| a/Å | 10.3107(2) | 9.257(2) | 24.719(5) |
| b/Å | 11.4913(2) | 18.174(4) | 20.878(5) |
| c/Å | 15.7802(3) | 19.196(4) | 18.209(4) |
| α/° | 84.2060(10) | 99.904(3) | 90 |
| β/° | 88.346(2) | 101.628(4) | 97.229(4) |
| γ/° | 81.786(2) | 96.449(4) | 90 |
| V/Å3 | 1840.86(6) | 3079.7(12) | 9323(4) |
| Z | 2 | 1 | 2 |
| Dcalc/g cm−3 | 1.469 | 1.319 | 1.551 |
| μ(MoKα)/mm−1 | 0.757 | 0.470 | 0.691 |
| F(0 0 0) | 840.0 | 1292.0 | 4480.0 |
| Crystal dimensions/mm3 | 0.61 × 0.56 × 0.55 | 0.20 × 0.20 × 0.20 | 0.61 × 0.20 × 0.20 |
| Radiation | MoKα (λ = 0.71073) | MoKα (λ = 0.71075) | MoKα (λ = 0.71075) |
| 2θ range for data collection/° | 3.992 to 61.604 | 6.112 to 54.948 | 6.038 to 55.036 |
| Reflections collected | 16,511 | 25,676 | 74,634 |
| Independent reflections | 9644 (Rint = 0.0116) | 13,566 (Rint = 0.0276) | 21,041 (Rint = 0.0626) |
| Data/Restraints/Params | 9644/170/518 | 13,566/75/788 | 21,041/8/1279 |
| Goodness of fit indicator | 1.053 | 1.044 | 1.074 |
| R indices [I > 2.00σ(I)] | R1 = 0.0327 | R1 = 0.0582 | R1 = 0.0581 |
| wR2 = 0.0892 | wR2 = 0.1493 | wR2 = 0.1459 | |
| R indices (all data) | R1 = 0.0355 | R1 = 0.0740 | R1 = 0.0805 |
| wR2 = 0.0917 | wR2 = 0.1673 | wR2 = 0.1644 | |
| Largest diff. peak, hole/e Å−3 | 0.31, −0.24 | 1.52, −0.58 | 1.29, −0.98 |
| CCDC deposition number | 2361225 | 2361226 | 2361227 |
| Bond | Distance/Å | Angle | Angle/° |
|---|---|---|---|
| Mn1–F1 | 2.0637(7) | F1–Mn1–O1 | 100.11(4) |
| Mn1–O1 | 2.1199(10) | F1–Mn1–O3 | 89.92(4) |
| Mn1–O3 | 2.1536(11) | F1–Mn1–N1 | 168.45(4) |
| Mn1–N1 | 2.2883(12) | F1–Mn1–N3 | 98.49(3) |
| Mn1–N2 | 2.2753(11) | O1–Mn1–N3 | 154.74(4) |
| Mn1–N3 | 2.3910(11) | O3–Mn1–N2 | 167.61(4) |
| Mn2–F1 | 2.0429(8) | F1–Mn2–O2 | 94.70(4) |
| Mn2–O2 | 2.1334(10) | F1–Mn2–O4 | 93.04(4) |
| Mn2–O4 | 2.1312(10) | F1–Mn2–N4 | 105.64(4) |
| Mn2–N4 | 2.4314(12) | F1–Mn2–N5 | 91.58(4) |
| Mn2–N5 | 2.2593(11) | O2–Mn2–N4 | 157.97(4) |
| Mn2–N6 | 2.3271(13) | O4–Mn2–N5 | 160.67(4) |
| Mn1⋯Mn2 | 3.4309(3) | Mn1–F1–Mn2 | 113.33(3) |
| Bridging Acetate | Bond Length/Å Mn–O | Bond Angle/° Mn–O–C | Torsion Angle/° Mn–O–C–C | Torsion Angle/° Mn–O∙∙∙O–Mn | Bridging Mode |
|---|---|---|---|---|---|
| A (O1, O2) | 2.1199(10) | 130.52(9) | 175.15(11) | 6.67(6) | syn-syn |
| 2.1334(10) | 136.17(10) | −165.71(12) | coplanar | ||
| B (O3, O4) | 2.1536(11) | 134.20(10) | 174.47(15) | 3.07(6) | syn-syn |
| 2.1312(10) | 131.43(9) | −170.66(14) | coplanar |
| Bond | Distance/Å | Angle | Angle/° |
|---|---|---|---|
| Mn1–O1 | 2.095(2) | O1–Mn1–O3 | 99.68(9) |
| Mn1–O3 | 2.116(2) | O1–Mn1–O5 | 100.64(9) |
| Mn1–O5 | 2.1339(18) | O1–Mn1–N1 | 162.88(6) |
| Mn1–N1 | 2.253(2) | O3–Mn1–O5 | 104.96(8) |
| Mn1–N2 | 2.308(2) | O3–Mn1–N2 | 164.64(8) |
| Mn1–N3 | 2411(2) | O5–Mn1–N3 | 154.00(9) |
| Mn2–O2 | 2.137(2) | O2–Mn2–O4 | 94.02(9) |
| Mn2–O4 | 2.122(2) | O2–Mn2–O6 | 100.10(8) |
| Mn2–O6 | 2.1176(19) | O2–Mn2–N5i | 168.08(8) |
| Mn2–N4i | 2.319(2) | O4–Mn2–O6 | 109.64(8) |
| Mn2–N5i | 2.268(2) | O4–Mn2–N4i | 163.57(8) |
| Mn2–N6i | 2.381(2) | O6–Mn2–N6i | 151.47(7) |
| Mn1···Mn2 | 4.0293(9) | ||
| Mn1···Mn1i | 13.368(3) | ||
| Mn1···Mn2i | 13.348(2) | ||
| Mn2···Mn2i | 14.496(3) |
| Bridging Acetate | Bond Length/Å Mn–O | Bond Angle/° Mn–O–C | Torsion Angle/° M–O–C–C | Torsion Angle/° M–O∙∙∙O–M | Bridging Mode |
|---|---|---|---|---|---|
| A (O1, O2) | 2.095(2) | 162.1(2) | 170.4(6) | 3.01(17) | syn-syn |
| 2.137(2) | 123.91(19) | −169.8(3) | coplanar | ||
| B (O3, O4) | 2.116(2) | 125.32(17) | 147.2(2) | 17.51(12) | syn-skew |
| 2.122(2) | 156.3(2) | −165.2(4) | nonplanar | ||
| C (O5, O6) | 2.1339(19) | 132.27(17) | 137.7(2) | 29.71(11) | syn-skew |
| 2.1176(19) | 142.41(18) | −176.3(2) | nonplanar |
| Bond | Distance/Å | Angle | Angle/° |
|---|---|---|---|
| Cation A | |||
| Mn1–O1 | 2.098(2) | O1–Mn1–N2 | 167.38(9) |
| Mn1–O3 | 2.182(2) | O3–Mn1–N3 | 162.33(9) |
| Mn1–O4 | 2.195(2) | O4–Mn1–N1 | 172.72(9) |
| Mn1–N1 | 2.270(2) | N1–Mn1–N2 | 96.10(9) |
| Mn1–N2 | 2.289(2) | N1–Mn1–N3 | 73.02(9) |
| Mn1–N3 | 2.348(2) | N2–Mn1–N3 | 74.59(9) |
| Mn2–O2 | 2.160(2) | O2–Mn2–O7 | 174.37(10) |
| Mn2–O5 | 2.238(3) | O5–Mn2–O6 | 54.74(10) |
| Mn2–O6 | 2.505(4) | O5–Mn2–N4i | 81.63(10) |
| Mn2–O7 | 2.233(2) | O6–Mn2–N5i | 83.60(10) |
| Mn2–N4i | 2.309(3) | N4i–Mn2–N6i | 71.33(9) |
| Mn2–N5i | 2.331(3) | N5i–Mn2–N6i | 70.68(9) |
| Mn2–N6i | 2.400(3) | ||
| Mn1···Mn2 | 5.3487(13) | ||
| Mn1···Mn1i | 15.039(2) | ||
| Mn1···Mn2i | 11.981(2) | ||
| Mn2···Mn2i | 10.868(2) | ||
| Cation B | |||
| Mn3–O8 | 2.114(2) | O8–Mn3–N8 | 164.21(9) |
| Mn3–O10 | 2.121(2) | O10–Mn3–N9 | 154.96(8) |
| Mn3–O12 | 2.197(2) | O12–Mn3–N7 | 170.74(9) |
| Mn3–N7 | 2.283(2) | N7–Mn3–N8 | 98.75(9) |
| Mn3–N8 | 2.328(2) | N7–Mn3–N9 | 72.58(9) |
| Mn3–N9 | 2.360(2) | N8–Mn3–N9 | 72.92(8) |
| Mn4–O9 | 2.163(2) | O9–Mn4–O15 | 175.04(9) |
| Mn4–O13 | 2.307(2) | O13–Mn4–O14 | 56.40(8) |
| Mn4–O14 | 2.333(2) | O13–Mn4–N10j | 80.03(9) |
| Mn4–O15 | 2.209(2) | O14–Mn4–N11j | 83.96(8) |
| Mn4–N10j | 2.320(2) | N10j–Mn4–N12j | 71.01(9) |
| Mn4–N11j | 2.369(3) | N11j–Mn4–N12j | 70.23(8) |
| Mn4–N12j | 2.387(3) | ||
| Mn3···Mn4 | 5.3389(13) | ||
| Mn3···Mn3j | 14.976(2) | ||
| Mn3···Mn4j | 11.927(2) | ||
| Mn4···Mn4j | 10.826(2) | ||
| Mn1···Mn3 | 8.6610(17) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugiyama, J.; Umemoto, Y.; Sato, S.; Yoneda, K.; Koikawa, M. Crystal Structures, Magnetic Properties, and Redox Behaviors of Carboxylato-Bridged Mn(II) Complexes with Ditopic Ligands Featuring N3-Coordination Sites. Chemistry 2024, 6, 601-617. https://doi.org/10.3390/chemistry6040036
Sugiyama J, Umemoto Y, Sato S, Yoneda K, Koikawa M. Crystal Structures, Magnetic Properties, and Redox Behaviors of Carboxylato-Bridged Mn(II) Complexes with Ditopic Ligands Featuring N3-Coordination Sites. Chemistry. 2024; 6(4):601-617. https://doi.org/10.3390/chemistry6040036
Chicago/Turabian StyleSugiyama, Junya, Yusuke Umemoto, Sota Sato, Ko Yoneda, and Masayuki Koikawa. 2024. "Crystal Structures, Magnetic Properties, and Redox Behaviors of Carboxylato-Bridged Mn(II) Complexes with Ditopic Ligands Featuring N3-Coordination Sites" Chemistry 6, no. 4: 601-617. https://doi.org/10.3390/chemistry6040036
APA StyleSugiyama, J., Umemoto, Y., Sato, S., Yoneda, K., & Koikawa, M. (2024). Crystal Structures, Magnetic Properties, and Redox Behaviors of Carboxylato-Bridged Mn(II) Complexes with Ditopic Ligands Featuring N3-Coordination Sites. Chemistry, 6(4), 601-617. https://doi.org/10.3390/chemistry6040036






