Phytochemistry, Anti-Tyrosinase, and Anti-Diabetes Studies of Extracts and Chemical Constituents of Dicerothamnus rhinocerotis Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Equipment and Chemical Reagents
2.3. Extraction and Fractionation of the Plant Material
2.4. Anti-Tyrosinase Inhibition Assay
2.5. Alpha-Glucosidase Inhibition Assay
2.6. Alpha-Amylase Inhibition Assay
3. Results and Discussion
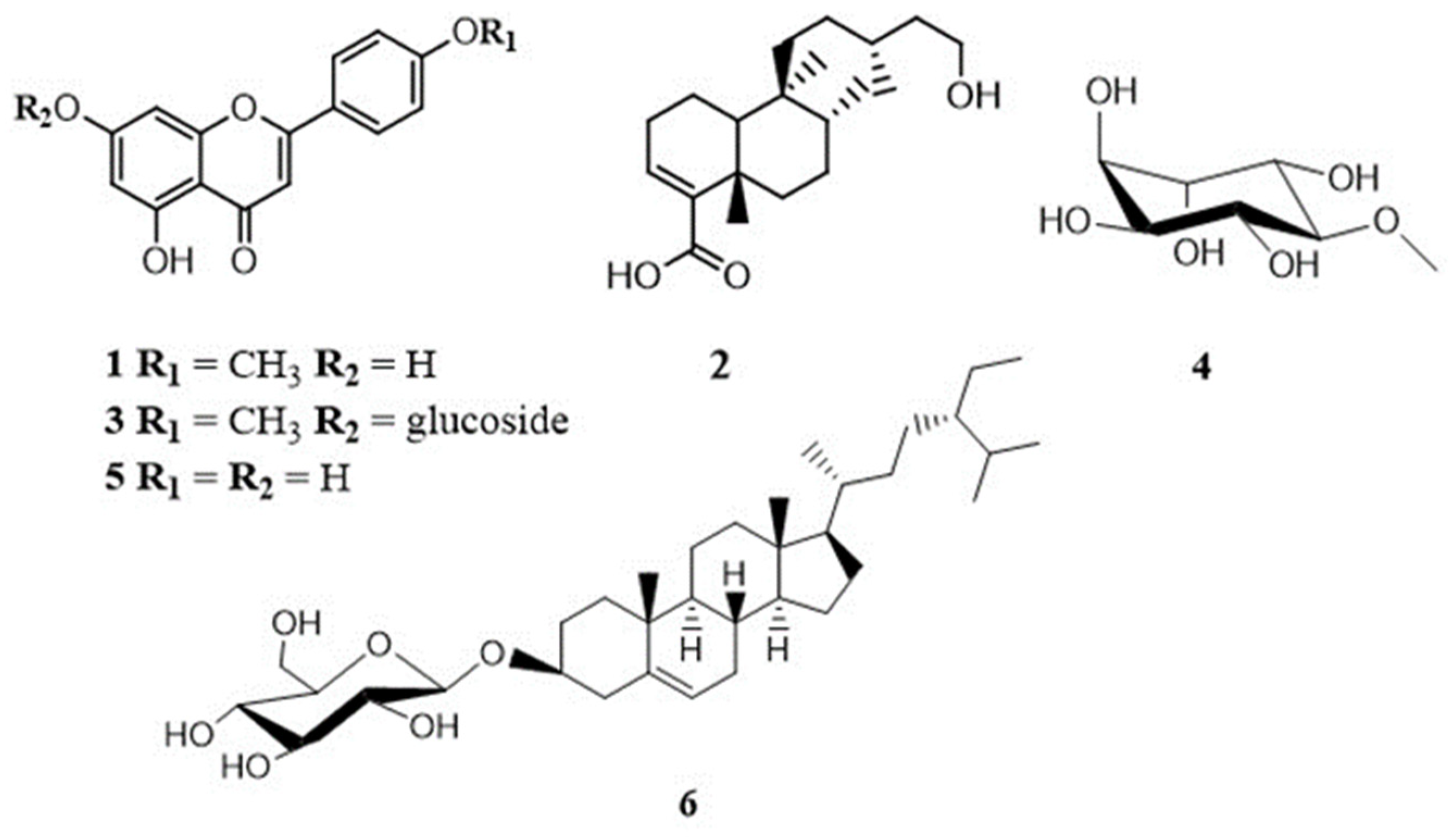
3.1. Structural Elucidation of the Isolated Compounds
3.2. Tyrosinase Inhibitory Activities of Fractions and Isolated Compounds
3.3. Alpha-Glucosidase Assay
3.4. Alpha-Amylase Inhibition Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, P. Angiosperm Phylogeny Website. 2001. Available online: http://www.mobot.org/MOBOT/research/APweb/ (accessed on 12 April 2024).
- Jeffrey, C. Compositae: Introduction with key to tribes. Fam. Genera Vasc. Plants 2007, 8, 61–87. [Google Scholar]
- WFO 2024: Asteraceae Giseke. Published on the Internet. Available online: http://www.worldfloraonline.org/taxon/wfo-7000000146 (accessed on 25 June 2024).
- Chiari, M.E.; Joray, M.B.; Ruiz, G.; Palacios, S.M.; Carpinella, M.C. Tyrosinase inhibitory activity of native plants from central Argentina: Isolation of an active principle from Lithrea molleoides. Food Chem. 2010, 120, 10–14. [Google Scholar] [CrossRef]
- Aghraz, A.; Gonçalves, S.; Rodríguez-Solana, R.; Ait Dra, L.; Di Stefano, V.; Dugo, G.; Cicero, N.; Larhsini, M.; Markouk, M.; Romano, A. Antioxidant activity and enzymes’ inhibitory properties of several extracts from two Moroccan Asteraceae species. S. Afr. J. Bot. 2018, 118, 58–64. [Google Scholar] [CrossRef]
- Pool, E.; Klaasen, A.; Shoko, Y. The environmental toxicity of Dicerothamnus rhinocerotis and Galenia africana. Afr. J. Biotechnol. 2009, 8, 4465–4468. [Google Scholar]
- Proksch, P.; Proksch, M.; Rundel, P.; Rodriguez, E. Ecological significance of the chemistry of the leaf resin of Elytropappus rhinocerotis. Biochem. Syst. Ecol. 1982, 10, 49–53. [Google Scholar] [CrossRef]
- Levyns, M. A revision of Elytropappus Cass. S. Afr. J. Bot. 1935, 1, 89–103. [Google Scholar]
- Mitchell-Watt, J.; Breyer-Brandwijk, M. The Medical and Poisonous Plants of Southern and Eastern Africa, 2nd ed.; Livingston: London, UK, 1962; p. 226. [Google Scholar]
- Davids, D.; Gibson, D.; Johnson, Q. Ethnobotanical survey of medicinal plants used to manage high blood pressure and type 2 diabetes mellitus in Bitterfontein, Western Cape Province, South Africa. J. Ethnopharmacol. 2016, 194, 755–766. [Google Scholar] [CrossRef]
- Bremer, K. Asteraceae, Cladistic and Classification; Timber Press, Inc.: Portland, OR, USA, 1994; pp. 435–458. [Google Scholar]
- Wadkar, K.; Magdum, C.; Patil, S.; Naikwade, N. Antidiabetic potential and Indian medicinal plants. J. Herb. Med. Toxicol. 2008, 2, 45–50. [Google Scholar]
- Baena-Díez, J.; Peñafiel, J.; Subirana, I.; Ramos, R.; Elosua, R.; Marín-Ibañez, A.; Guembe, M.; Rigo, F.; Tormo-Díaz, J.; Moreno-Iribas, C.; et al. Risk of cause—Specific death in individuals with diabetes: A competing risks analysis. Diabetes Care 2016, 39, 1987–1995. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation (IDF). Diabetes Atlas, 7th ed.; International Diabetes Federation: Brussels, Belgium, 2015. [Google Scholar]
- Kengne, A.P.; Echouffo-Tcheugui, J.B.; Sobngwi, E. New insights on diabetes mellitus and obesity in Africa-part 1: Prevalence, pathogenesis and comorbidities. Heart 2013, 99, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Peer, N.; Kengne, A.; Motala, A.; Mbanya, J. Diabetes in the Africa Region: An update. Diabetes Res. Clin. Pract. 2014, 103, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Bertram, M.; Jaswal, A.; Van Wyk, V. The Non-Fatal Disease Burden Caused by Type 2 Diabetes in South Africa. Glob. Health Action 2013, 6, 19244. [Google Scholar] [CrossRef] [PubMed]
- Shouip, H. Diabetes mellitus: Signs and symptoms. Open J. Nurs. 2014, 10, 1–9. [Google Scholar]
- America Diabetes Association. Pharmacologic Approaches to Glycemic Treatment. Diabetes Care 2017, 40, S64–S74. [Google Scholar] [CrossRef] [PubMed]
- Keskin, A.; Bilge, U. Mental disorders frequency alternative and complementary medicine usage among patients with hypertension and type 2 diabetes mellitus. Niger. J. Clin. Pract. 2014, 17, 717–722. [Google Scholar] [PubMed]
- Bhalerao, M.; Bolshete, P.; Swar, B.; Bangera, T.; Kolhe, V.; Tambe, M.; Wade, M.; Bhowate, S.; Sonje, U.; Gogtay, N.; et al. Use of and satisfaction with complementary and alternative medicine in four chronic diseases: A cross-sectional study from India. Natl. Med. J. India 2013, 26, 75–78. [Google Scholar] [PubMed]
- Zolghadri, S.; Bahrami, A.; Khan, M.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.; Sathisha, U.; Dharmesh, S.; Rao, A.; Singh, S. Interaction of sesamol (3, 4-methylenedioxyphenol) with tyrosinase and its effect on melanin synthesis. Biochimie 2011, 93, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Chang, T. An Updated Review of Tyrosinase Inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Chai, W.; Wei, M.; Wang, R.; Deng, R.; Zou, Z.; Peng, Y. Avocado Proanthocyanidins as a Source of Tyrosinase Inhibitors: Structure Characterization, Inhibitory Activity, and Mechanism. J. Agric. Food. Chem. 2015, 63, 7381–7387. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Natural products in cosmetics. Nat. Prod. Bioprospect. 2022, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Germishuizen, G.; Meyer, N. Plants of Southern Africa: An annotated Checklist; National Botanical Institute: Pretoria, South Africa, 2003; Volume 14, p. 186. [Google Scholar]
- Dekker, T.; Fourie, T.; Matthee, E.; Snyckers, F.; Van der Schyf, C.; Boeyens, J.; Denner, L. Studies of South African medicinal plants: Pt. 7. Rhinocerotinoic acid: A labdane diterpene with anti-inflammatory properties from Elytropappus rhinocerotis. S. Afr. J. Chem. 1988, 41, 33–35. [Google Scholar]
- Mshengu, B.; Gakuba, E.; Van Heerden, F. Chemical constituents from Elytropappus rhinocerotis (Lf) Less. Biochem. Syst. Ecol. 2017, 75, 18–20. [Google Scholar] [CrossRef]
- Hulley, I.; Van Vuuren, S.; Sadgrove, N.; Van Wyk, B. Antimicrobial activity of Elytropappus rhinocerotis (Asteraceae) against micro-organisms associated with foot odour and skin ailments. J. Ethnopharmacol. 2019, 228, 92–98. [Google Scholar] [CrossRef]
- Yalo, M.; Makhaba, M.; Hussein, A.A.; Sharma, R.; Koki, M.; Nako, N.; Mabusela, W.T. Characterization of Four New Compounds from Protea cynaroides Leaves and Their Tyrosinase Inhibitory Potential. Plants 2022, 11, 1751. [Google Scholar] [CrossRef]
- Yamaki, K.; Mori, Y. Evaluation of a-glucosidase inhibitory activity in colored foods: A trial using slope factors of regression curves. J. Jpn. Soc. Food. Sci. 2006, 53, 229–231. [Google Scholar] [CrossRef]
- Miyazawa, M.; Hisama, M. Antimutagenic activity of flavonoids from Chrysanthemum morifolium. Biosci. Biotechnol. Biochem. 2003, 67, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Kalpoutzakis, E.; Aligiannis, N.; Skaltsounis, A.L.; Mitakou, S. cis-Clerodane type diterpenes from Cistus monspeliensis. J. Nat. Prod. 2003, 66, 316–319. [Google Scholar] [CrossRef]
- Sofrenić, I.; Anđelković, B.; Gođevac, D.; Ivanović, S.; Simić, K.; Ljujić, J.; Tešević, V.; Milosavljević, S. Metabolomics as a Potential Chemotaxonomical Tool: Application on the Selected Euphorbia Species Growing Wild in Serbia. Plants 2023, 12, 262. [Google Scholar] [CrossRef]
- Raya-Gonzalez, D.; Pamatz-Bolanos, T.; Rio-Torres, R.; Martinez-Munoz, R.; Ron-Echeverria, O.; Martinez-Pacheco, M. D-(+)-pinitol, a component of the heartwood of Enterolobium cyclocarpum (Jacq.) Griseb. Z. Naturforschung C 2008, 63, 922–924. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, G.; Sundaraganesan, N.; Manoharan, S. The spectroscopic properties of anticancer drug Apigenin investigated by using DFT calculations, FT-IR, FT-Raman and NMR analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 95, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Flamini, G.; Antognoli, E.; Morelli, I. Two flavonoids and other compounds from the aerial parts of Centaurea bracteata from Italy. Phytochemistry 2001, 57, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, J. Comparative evaluation of phenolic phytochemicals from perilla seeds of diverse species and screening for their tyrosinase inhibitory and antioxidant properties. S. Afr. J. Bot 2019, 123, 341–350. [Google Scholar] [CrossRef]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Flavonols and flavones as BACE-1 inhibitors: Structure–activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim. Biophys. Acta 2008, 1780, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.; Han, J.; Kim, J.; Hwang, J. Inhibitory effects of active compounds isolated from safflower (Carthamus tinctorius L.) seeds for melanogenesis. Biol. Pharm. Bull. 2004, 27, 1976–1978. [Google Scholar] [CrossRef] [PubMed]
- Cespedes, C.; Balbontin, C.; Avila, J.; Dominguez, M.; Alarcon, J.; Paz, C.; Burgos, V.; Ortiz, L.; Penaloza-Castro, I.; Seigler, D.; et al. Inhibition on cholinesterase and tyrosinase by alkaloids and phenolics from Aristotelia chilensis leaves. Food. Chem. Toxicol. 2017, 109, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.; Xiaoqing, C. Structures required of flavonoids for inhibiting digestive enzymes. Anti-Cancer Agents Med. Chem. 2012, 12, 929–939. [Google Scholar]
- Narayanan, C.; Joshi, D.; Mujumdar, A.; Dhekne, V. Pinitol—A new anti-diabetic compound from the leaves of Bougainvillea spectabilis. Curr. Sci. 1987, 56, 139–141. [Google Scholar]
- Abo-Elghiet, F.; Ahmed, A.; Aly, H.; Younis, E.; Rabeh, M.; Alshehri, S.; Alshahrani, K.S.; Mohamed, S. D-Pinitol Content and Antioxidant and Antidiabetic Activities of Five Bougainvillea spectabilis Willd. Cultivars. Pharmaceuticals 2023, 16, 1008. [Google Scholar] [CrossRef]
- Le Nguyen, T.T.; Pham, T.T.; Hansen, P.E.; Nguyen, P.K. In vitro α-glucosidase inhibitory activity of compounds isolated from mangrove Lumnitzera littorea leaves. Sci. Tech. Dev. J. 2019, 106–113. [Google Scholar] [CrossRef]
- Ivorra, M.; D’ocon, M.; Paya, M.; Villar, A. Antihyperglycemic and insulin-releasing effects of beta-sitosterol 3-beta-D-glucoside and its aglycone, beta-sitosterol. Int. Pharmacodyn. Ther. 1988, 296, 224–231. [Google Scholar]
| Extracts/Compounds | % Inhibition | IC50 | |
|---|---|---|---|
| (µg/mL) | µM | ||
| DRC (Crude) | 43.41 | 42.2 | |
| DRH (Hexane) | 36.26 | 200.1 | |
| DRD (DCM) | 40.36 | 35.1 | |
| DRE (EtOAc) | 67.87 | 11.6 | |
| DRB (BuOH) | 44.04 | 13.7 | |
| DRM (crude MeOH) | 50.11 | 57 ± 2.48 | |
| 1 | nd | - | 1011 |
| 2 | 12.72 | - | 1552 |
| 3 | 30.42 | - | 583.3 |
| 4 | 30.24 | - | 995.6 |
| 5 | 67.51 | - | 14.58 |
| 6 | 37.24 | - | 273 |
| Kojic acid | 100 | - | 17.26 |
| Extracts/Compounds | % Inhibition | Alpha-Glucosidase IC50 (µg/mL) ± SD | % Inhibition | Alpha-Amylase IC50 (µg/mL) ± SD |
|---|---|---|---|---|
| DRC (Crude) | nd | - | nd | - |
| DRH (Hexane) | 6.13 | - | nd | - |
| DRD (DCM) | 41.49 | 201.8 ± 2.12 | nd | - |
| DRE (EtOAc) | 44.45 | 199.8 ± 2.57 | 3.44 | - |
| DRB (BuOH) | 9.33 | - | nd | - |
| DRM (crude MeOH) | 53.50 | 198.4 ± 2.48 | 5.59 | - |
| 1 | 13.88 | - | nd | - |
| 2 | 3.74 | - | nd | - |
| 3 | nd | - | nd | - |
| 4 | 7.05 | - | 0.67 | - |
| 5 | 94.17 | 83.0 ± 2.16 | 7.04 | - |
| 6 | 3.86 | - | 28.56 | - |
| Acarbose | 63.94 | 130.2 ± 1.84 | 88.86 | 20.25 ± 1.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watti, O.I.; Yalo, M.; Sharma, R.; Makhaba, M.; Hussein, A.A.; Mabusela, W.T. Phytochemistry, Anti-Tyrosinase, and Anti-Diabetes Studies of Extracts and Chemical Constituents of Dicerothamnus rhinocerotis Leaves. Chemistry 2024, 6, 546-554. https://doi.org/10.3390/chemistry6040032
Watti OI, Yalo M, Sharma R, Makhaba M, Hussein AA, Mabusela WT. Phytochemistry, Anti-Tyrosinase, and Anti-Diabetes Studies of Extracts and Chemical Constituents of Dicerothamnus rhinocerotis Leaves. Chemistry. 2024; 6(4):546-554. https://doi.org/10.3390/chemistry6040032
Chicago/Turabian StyleWatti, Olusola Ifedolapo, Masande Yalo, Rajan Sharma, Masixole Makhaba, Ahmed A. Hussein, and Wilfred T. Mabusela. 2024. "Phytochemistry, Anti-Tyrosinase, and Anti-Diabetes Studies of Extracts and Chemical Constituents of Dicerothamnus rhinocerotis Leaves" Chemistry 6, no. 4: 546-554. https://doi.org/10.3390/chemistry6040032
APA StyleWatti, O. I., Yalo, M., Sharma, R., Makhaba, M., Hussein, A. A., & Mabusela, W. T. (2024). Phytochemistry, Anti-Tyrosinase, and Anti-Diabetes Studies of Extracts and Chemical Constituents of Dicerothamnus rhinocerotis Leaves. Chemistry, 6(4), 546-554. https://doi.org/10.3390/chemistry6040032













