Graphene Oxide-Assisted Aptamer-Based Fluorescent Detection of Tetracycline Antibiotics
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fluorescence Spectroscopy
2.3. Adsorption Kinetics
2.4. TEM
3. Results and Discussion
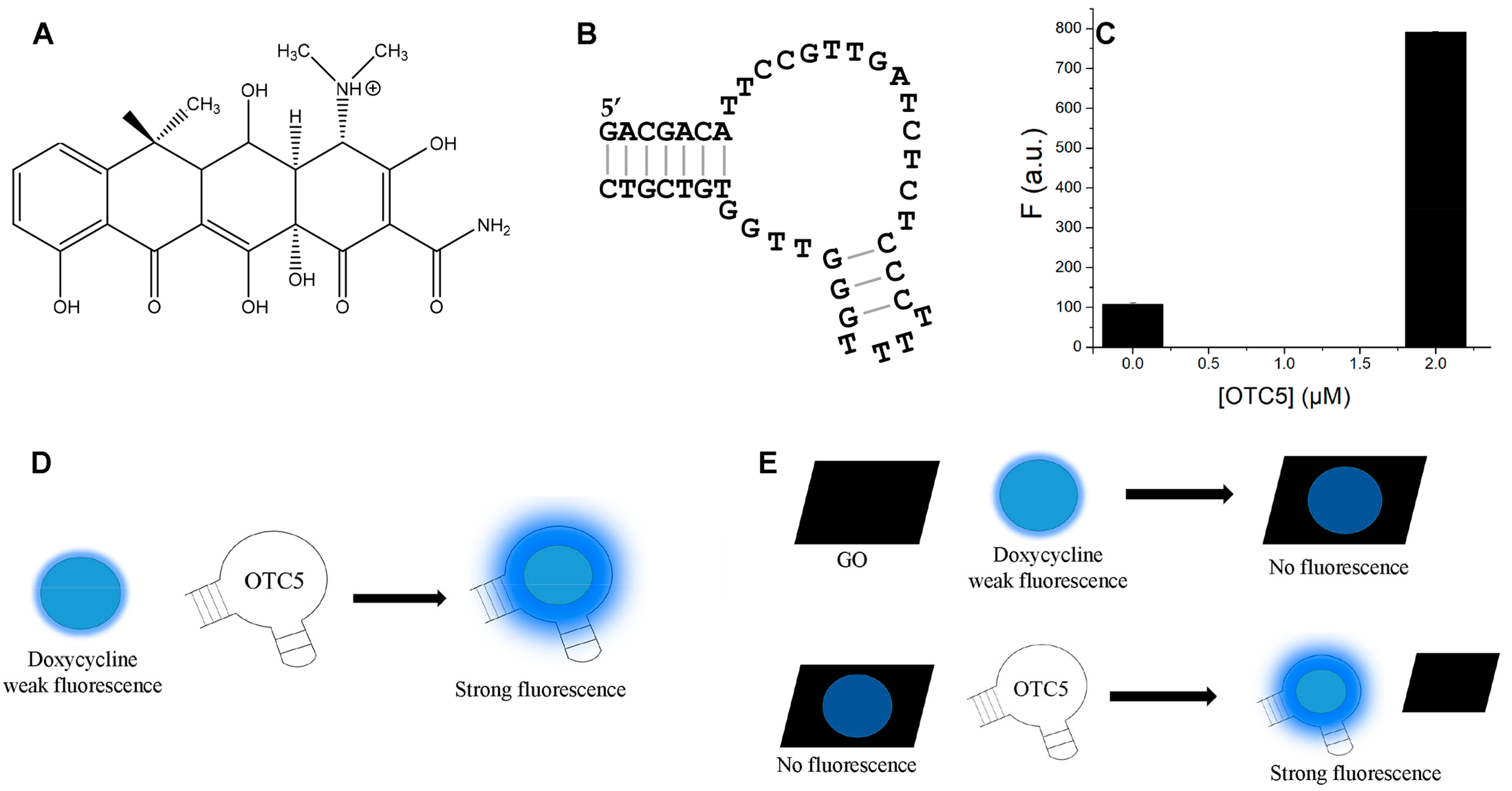
3.1. Biosensor Design
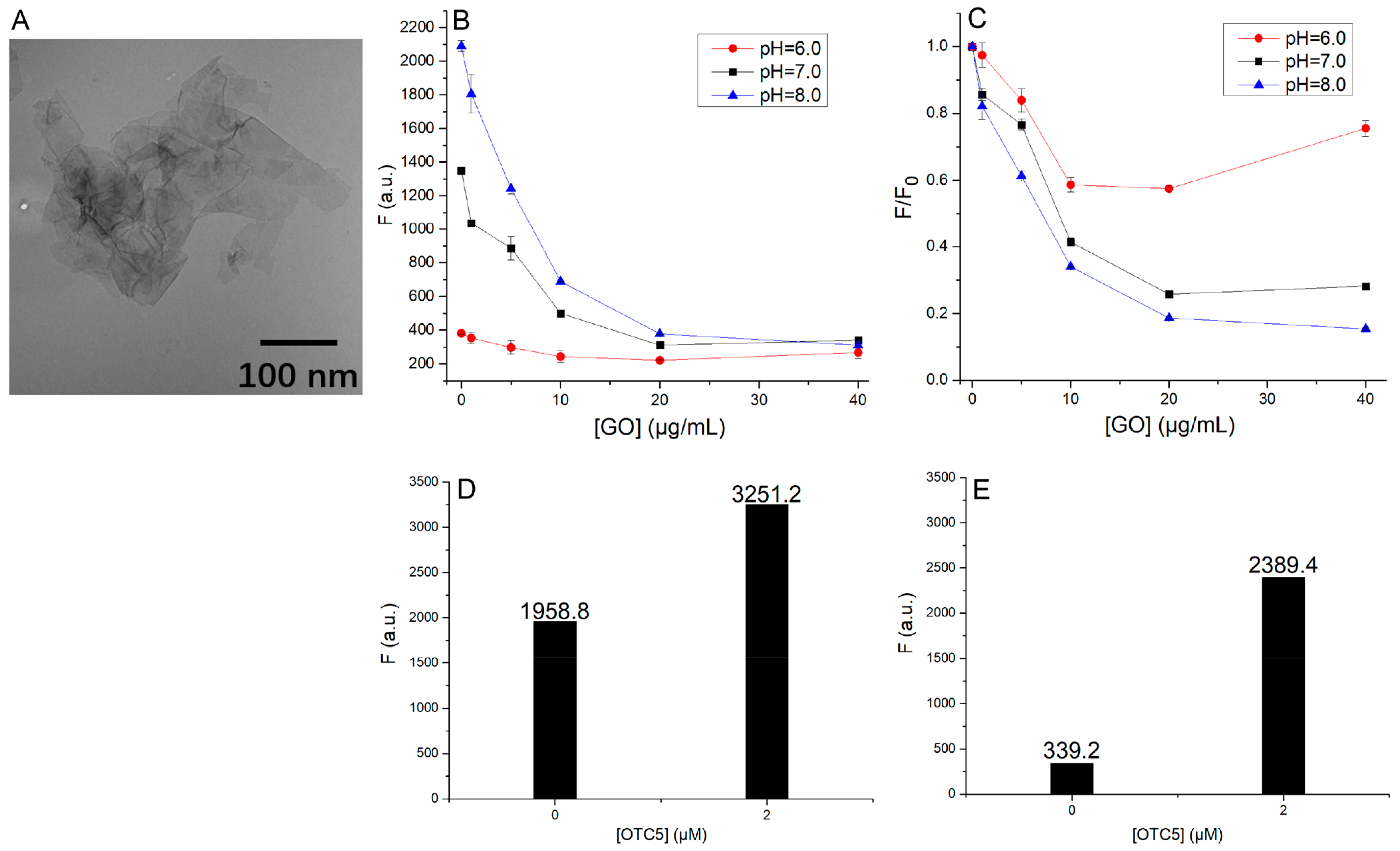
3.2. Adsorption of Doxycycline to GO
3.3. Suppression of Background Fluorescence by GO
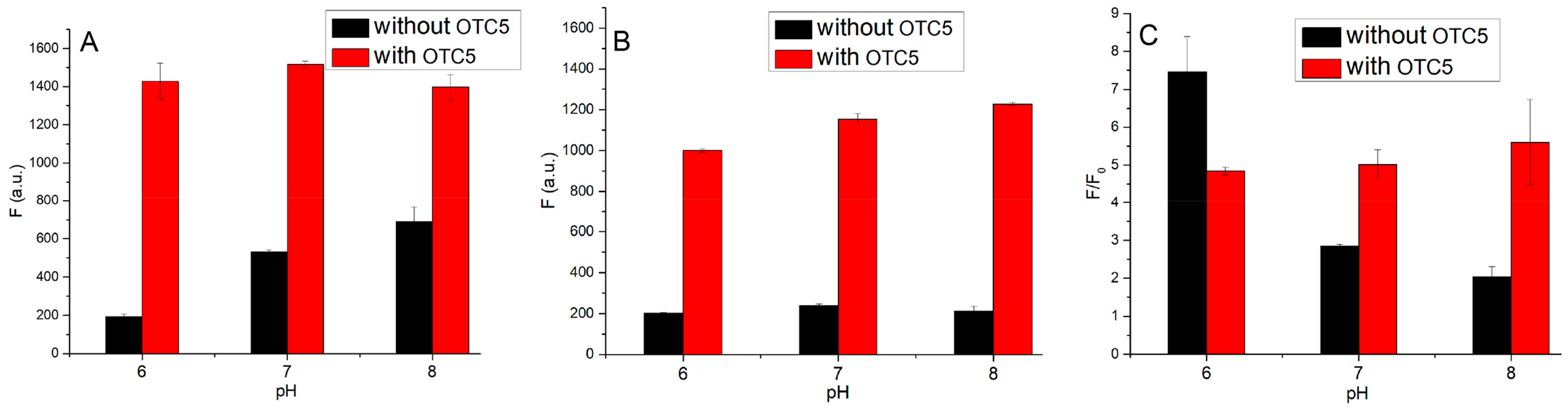
3.4. Optimization of Sensing Conditions
3.5. Detection of Doxycycline
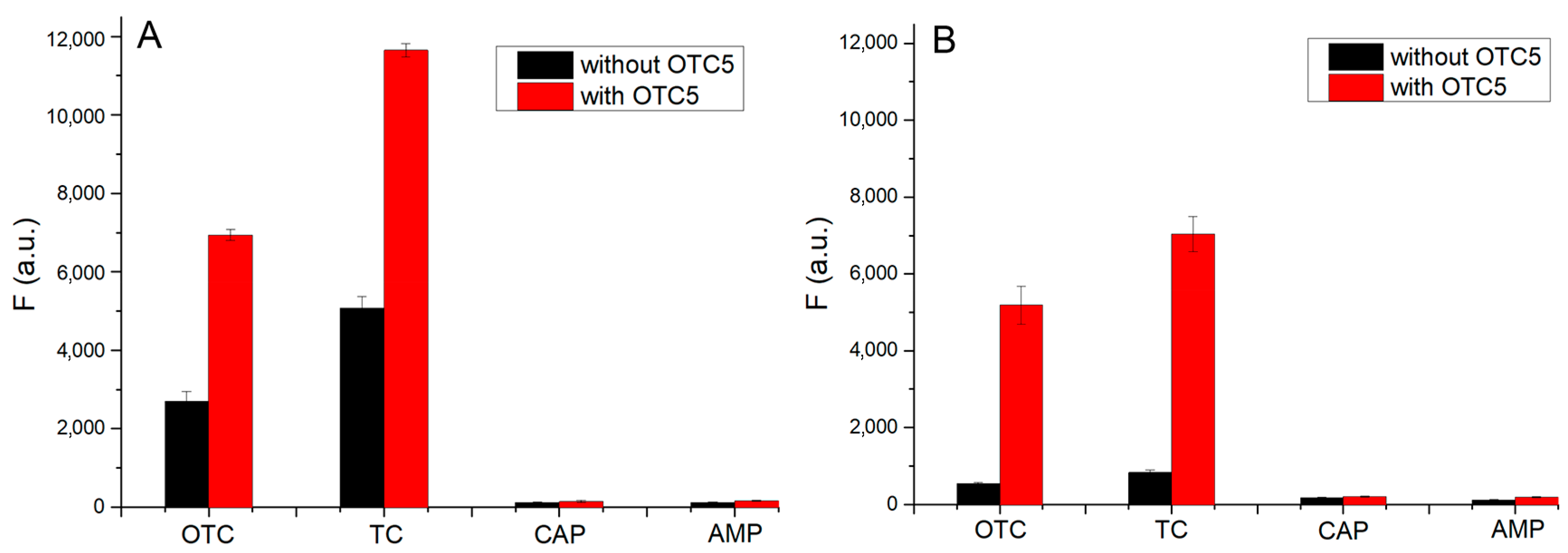
3.6. Selectivity Test
3.7. Further Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed]
- Schnappinger, D.; Hillen, W. Tetracyclines: Antibiotic action, uptake, and resistance mechanisms. Arch. Microbiol. 1996, 165, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Niazi, J.H.; Lee, S.J.; Kim, Y.S.; Gu, M.B. ssDNA aptamers that selectively bind oxytetracycline. Biorg. Med. Chem. 2008, 16, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Niazi, J.H.; Lee, S.J.; Gu, M.B. Single-stranded DNA aptamers specific for antibiotics tetracyclines. Biorg. Med. Chem. 2008, 16, 7245–7253. [Google Scholar] [CrossRef] [PubMed]
- Tickner, Z.J.; Zhong, G.; Sheptack, K.R.; Farzan, M. Selection of High-Affinity RNA Aptamers That Distinguish between Doxycycline and Tetracycline. Biochemistry 2020, 59, 3473–3486. [Google Scholar] [CrossRef]
- Berens, C.; Thain, A.; Schroeder, R. A tetracycline-binding RNA aptamer. Biorg. Med. Chem. 2001, 9, 2549–2556. [Google Scholar] [CrossRef]
- Yu, H.; Alkhamis, O.; Canoura, J.; Liu, Y.; Xiao, Y. Advances and Challenges in Small-Molecule DNA Aptamer Isolation, Characterization, and Sensor Development. Angew. Chem. Int. Ed. 2021, 60, 16800–16823. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Y.; Xu, X.; Liu, Y.; Lin, B.; Zhang, M.; Zhang, J.; Wan, S.; Yang, C.; Tan, W. Aptamer-Based Detection of Circulating Targets for Precision Medicine. Chem. Rev. 2021, 121, 12035–12105. [Google Scholar] [CrossRef]
- He, L.; Huang, R.; Xiao, P.; Liu, Y.; Jin, L.; Liu, H.; Li, S.; Deng, Y.; Chen, Z.; Li, Z.; et al. Current signal amplification strategies in aptamer-based electrochemical biosensor: A review. Chin. Chem. Lett. 2021, 32, 1593–1602. [Google Scholar] [CrossRef]
- Li, Y.; Gao, H.; Qi, Z.; Huang, Z.; Ma, L.; Liu, J. Freezing-Assisted Conjugation of Unmodified Diblock DNA to Hydrogel Nanoparticles and Monoliths for DNA and Hg2+ Sensing. Angew. Chem. Int. Ed. 2021, 60, 12985–12991. [Google Scholar] [CrossRef]
- McConnell, E.M.; Nguyen, J.; Li, Y. Aptamer-Based Biosensors for Environmental Monitoring. Front. Chem. 2020, 8, 434. [Google Scholar] [CrossRef]
- Zhao, Y.; Yavari, K.; Wang, Y.; Pi, K.; Van Cappellen, P.; Liu, J. Deployment of functional DNA-based biosensors for environmental water analysis. TrAC Trends Anal. Chem. 2022, 153, 116639. [Google Scholar] [CrossRef]
- Müller, M.; Weigand, J.E.; Weichenrieder, O.; Suess, B. Thermodynamic characterization of an engineered tetracycline-binding riboswitch. Nucleic Acids Res. 2006, 34, 2607–2617. [Google Scholar] [CrossRef]
- Zhao, Y.; Ong, S.; Chen, Y.; Jimmy Huang, P.-J.; Liu, J. Label-free and Dye-free Fluorescent Sensing of Tetracyclines Using a Capture-Selected DNA Aptamer. Anal. Chem. 2022, 94, 10175–10182. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, B.; Sun, P.; Liu, J.; Liu, J. Metal and pH-Dependent Aptamer Binding of Tetracyclines Enabling Highly Sensitive Fluorescence Sensing. Biosensors 2022, 12, 717. [Google Scholar] [CrossRef]
- Lu, C.H.; Yang, H.H.; Zhu, C.L.; Chen, X.; Chen, G.N. A Graphene Platform for Sensing Biomolecules. Angew. Chem. Int. Ed. 2009, 48, 4785–4787. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef]
- Liu, B.; Salgado, S.; Maheshwari, V.; Liu, J. DNA adsorbed on graphene and graphene oxide: Fundamental interactions, desorption and applications. Curr. Opin. Colloid Interface Sci. 2016, 26, 41–49. [Google Scholar] [CrossRef]
- Liu, X.Q.; Aizen, R.; Freeman, R.; Yehezkeli, O.; Willner, I. Multiplexed Aptasensors and Amplified DNA Sensors Using Functionalized Graphene Oxide: Application for Logic Gate Operations. ACS Nano 2012, 6, 3553–3563. [Google Scholar] [CrossRef]
- He, S.J.; Song, B.; Li, D.; Zhu, C.F.; Qi, W.P.; Wen, Y.Q.; Wang, L.H.; Song, S.P.; Fang, H.P.; Fan, C.H. A Graphene Nanoprobe for Rapid, Sensitive, and Multicolor Fluorescent DNA Analysis. Adv. Funct. Mater. 2010, 20, 453–459. [Google Scholar] [CrossRef]
- Lopez, A.; Liu, J. Covalent and Noncovalent Functionalization of Graphene Oxide with DNA for Smart Sensing. Adv. Intell. Syst. 2020, 2, 2000123. [Google Scholar] [CrossRef]
- Hassani, M.; Lázaro, R.; Pérez, C.; Condón, S.; Pagán, R. Thermostability of Oxytetracycline, Tetracycline, and Doxycycline at Ultrahigh Temperatures. J. Agric. Food. Chem. 2008, 56, 2676–2680. [Google Scholar] [CrossRef] [PubMed]
- Heaton, P.C.; Fenwick, S.R.; Brewer, D.E. Association between tetracycline or doxycycline and hepatotoxicity: A population based case–control study1. J. Clin. Pharm. Ther. 2007, 32, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Kogawa, A.C.; Salgado, H.R.N. Quantification of Doxycycline Hyclate in Tablets by HPLC–UV Method. J. Chromatogr. Sci. 2012, 51, 919–925. [Google Scholar] [CrossRef]
- Kushalkar, M.P.; Liu, B.; Liu, J. Promoting DNA Adsorption by Acids and Polyvalent Cations: Beyond Charge Screening. Langmuir 2020, 36, 11183–11195. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Z.; Han, J.; Xie, G.; Liu, J. Polyvalent Metal Ion Promoted Adsorption of DNA Oligonucleotides by Montmorillonite. Langmuir 2021, 37, 1037–1044. [Google Scholar] [CrossRef]
- Huang, P.-J.J.; Liu, J. Signaling Kinetics of DNA and Aptamer Biosensors Revealing Graphene Oxide Surface Heterogeneity. J. Anal. Test. 2022, 6, 20–27. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Nanobiotechnology: Integrated nanoparticle-biomolecule hybrid systems: Synthesis, properties, and applications. Angew. Chem. Int. Ed. 2004, 43, 6042–6108. [Google Scholar] [CrossRef]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in Biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef]
- Li, L.L.; Xing, H.; Zhang, J.J.; Lu, Y. Functional DNA Molecules Enable Selective and Stimuli-Responsive Nanoparticles for Biomedical Applications. Acc. Chem. Res. 2019, 52, 2415–2426. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, J. Label-Free Colorimetric Biosensors Based on Aptamers and Gold Nanoparticles: A Critical Review. Anal. Sens. 2021, 1, 30–43. [Google Scholar] [CrossRef]
- Lopez, A.; Liu, J. Nanomaterial and Aptamer-Based Sensing: Target Binding versus Target Adsorption Illustrated by the Detection of Adenosine and ATP on Metal Oxides and Graphene Oxide. Anal. Chem. 2021, 93, 3018–3025. [Google Scholar] [CrossRef]
- Li, F.; Huang, Y.; Yang, Q.; Zhong, Z.T.; Li, D.; Wang, L.H.; Song, S.P.; Fan, C.H. A graphene-enhanced molecular beacon for homogeneous DNA detection. Nanoscale 2010, 2, 1021–1026. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Liu, J. Graphene Oxide-Assisted Aptamer-Based Fluorescent Detection of Tetracycline Antibiotics. Chemistry 2023, 5, 789-799. https://doi.org/10.3390/chemistry5020056
Zhou Y, Liu J. Graphene Oxide-Assisted Aptamer-Based Fluorescent Detection of Tetracycline Antibiotics. Chemistry. 2023; 5(2):789-799. https://doi.org/10.3390/chemistry5020056
Chicago/Turabian StyleZhou, Yang, and Juewen Liu. 2023. "Graphene Oxide-Assisted Aptamer-Based Fluorescent Detection of Tetracycline Antibiotics" Chemistry 5, no. 2: 789-799. https://doi.org/10.3390/chemistry5020056
APA StyleZhou, Y., & Liu, J. (2023). Graphene Oxide-Assisted Aptamer-Based Fluorescent Detection of Tetracycline Antibiotics. Chemistry, 5(2), 789-799. https://doi.org/10.3390/chemistry5020056





