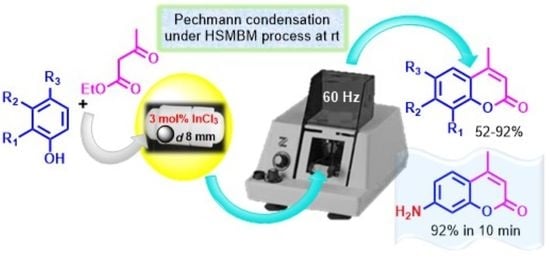
A Simple and Effective Protocol for the Pechmann Reaction to Obtain 4-Methylcoumarin Derivatives Using a High-Speed Mixer Ball Mill Process
Abstract
1. Introduction
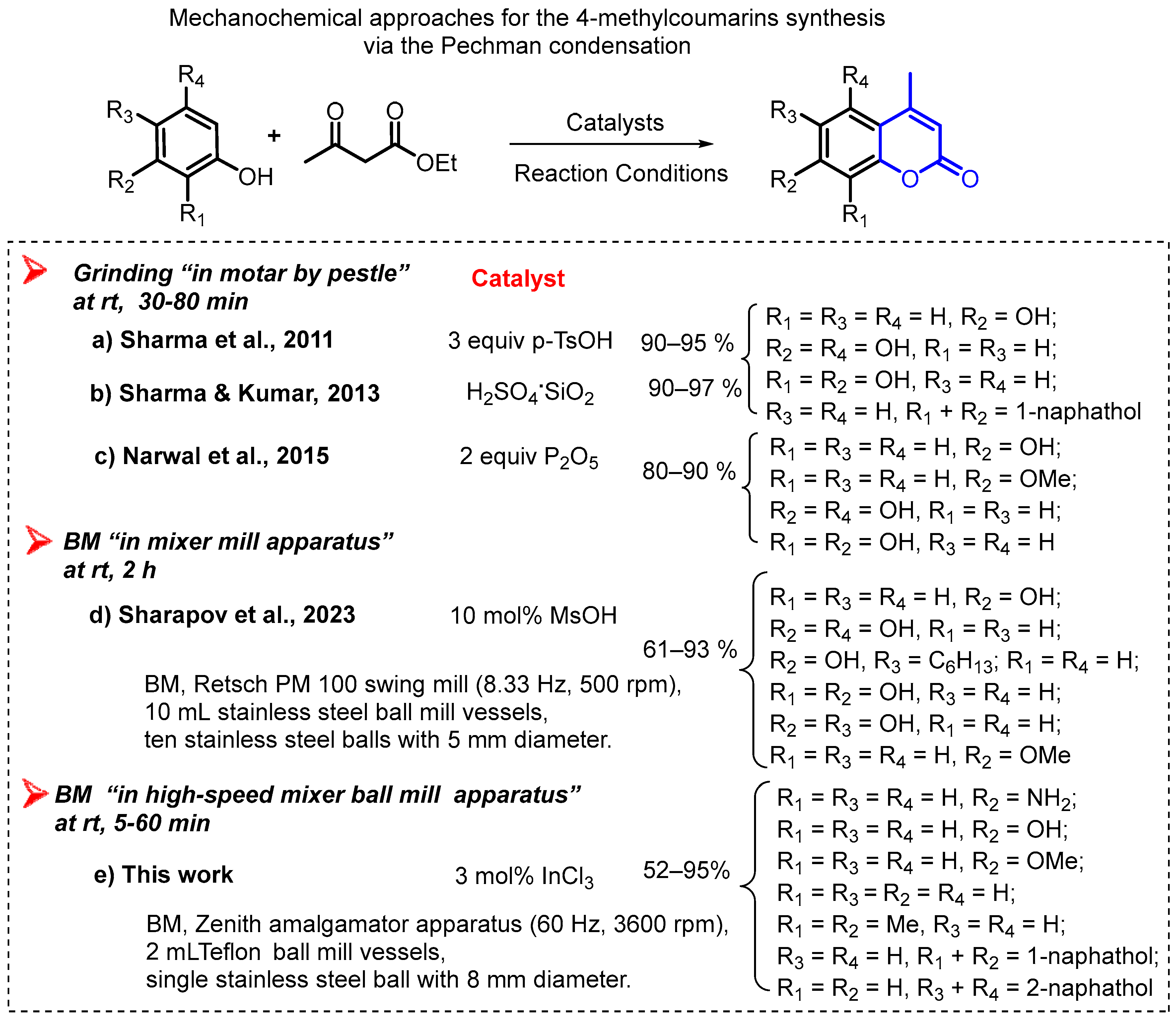
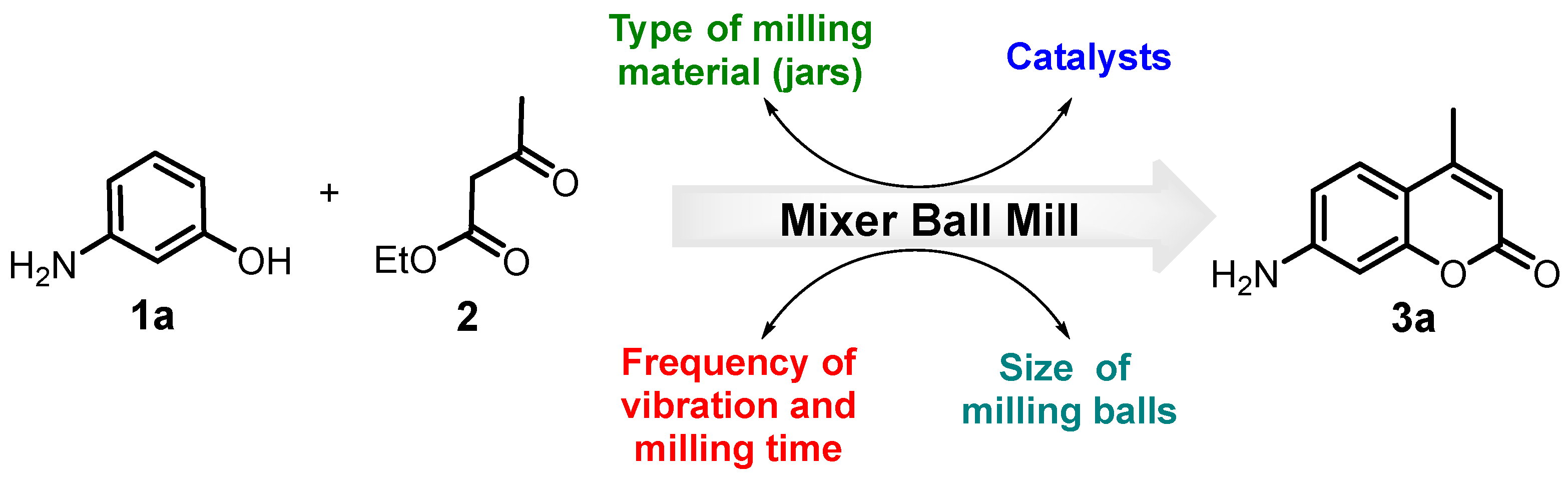
2. Materials and Methods
2.1. Materials and Instruments
2.2. General Procedure for the Synthesis of 4-Methylcoumarin Derivatives 3a–g
2.2.1. The Characterization of 7-Amino-4-methyl-2H-chromen-2-one (7-Amino-4-methylcoumarin, 3a)
2.2.2. The Characterization of 7-Hydroxy-4-methyl-2H-chromen-2-one
2.2.3. The Characterization of 7-Methoxy-4-methyl-2H-chromen-2-one
2.2.4. The Characterization of 4-Methyl-2H-chromen-2-one
2.2.5. The Characterization of 4,7,8-Trimethyl-2H-chromen-2-one
2.2.6. The Characterization of 4-Methyl-2H-benzo[h]chromen-2-one
2.2.7. The Characterization of 1-Methyl-3H-benzo[f]chromen-3-one
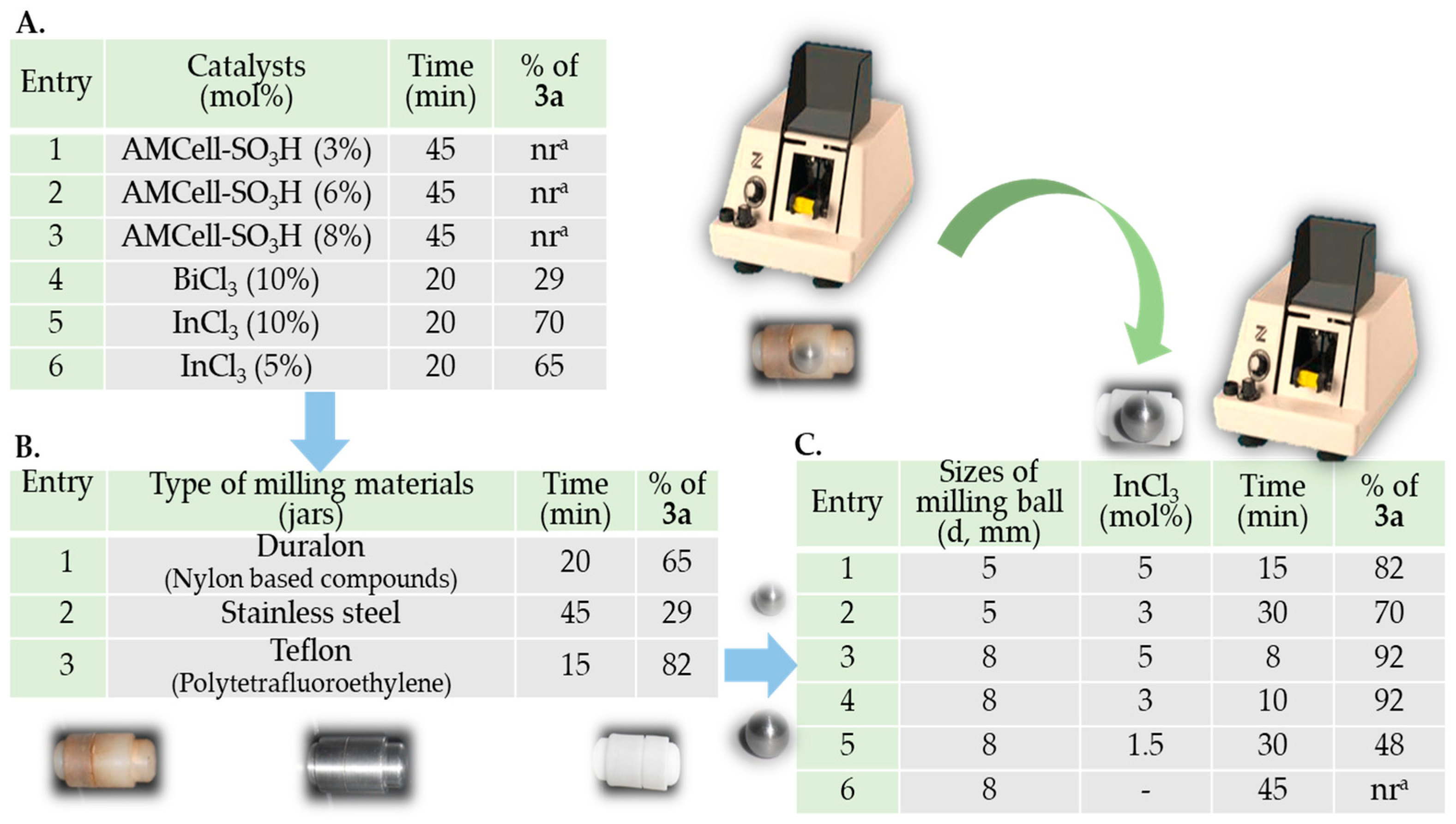
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An important class of phytochemicals. In Phytochemicals-Isolation, Characterisation and Role in Human Health; Rao, A.V., Rao, L.G., Eds.; InTech: Rijeka, Croatia, 2015; pp. 533–538. [Google Scholar]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed]
- Barot, K.P.; Jain, S.V.; Kremer, L.; Singh, S.; Ghate, M.D. Recent advances and therapeutic journey of coumarins: Current status and perspectives. Med. Chem. Res. 2015, 24, 2771–2798. [Google Scholar] [CrossRef]
- Ortiz Villamizar, M.C.; Puerto Galvis, C.E.; Vargas Méndez, L.Y.; Kouznetsov, V.V. Coumarin-Based Molecules as Suitable Models for Developing New Neuroprotective Agents Through Structural Modification. In Discovery and Development of Neuroprotective Agents from Natural Products, Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 149–235. [Google Scholar]
- Hu, X.L.; Gao, C.; Xu, Z.; Liu, M.L.; Feng, L.S.; Zhang, G.D. Recent development of coumarin derivatives as potential antiplasmodial and antimalarial agents. Curr. Top. Med. Chem. 2018, 18, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Liu, Z.; Verwilst, P.; Koo, S.; Jangjili, P.; Kim, J.S.; Lin, W. Coumarin-based small-molecule fluorescent chemosensors. Chem. Rev. 2019, 119, 10403–10519. [Google Scholar] [CrossRef] [PubMed]
- Breidenbach, J.; Bartz, U.; Gütschow, M. Coumarin as a structural component of substrates and probes for serine and cysteine proteases. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140445. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.S.; Gupta, J.; Sharma, S.; Sahu, D. An insight into the therapeutic applications of coumarin compounds and their mechanisms of action. Eur. J. Pharm. Sci. 2020, 152, 105424. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, G.A.; Spillere, A.R.; das Neves, G.M.; Kagami, L.P.; von Poser, G.L.; Canto, R.F.S.; Eifler-Lima, V. Natural and synthetic coumarins as antileishmanial agents: A review. Eur. J. Med. Chem. 2020, 203, 112514. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Albohy, A.; Abdulrazik, B.S.; Bayoumi, S.A.; Malak, L.G.; Khallaf, I.S.; Bringmann, G.; Farag, S.F. Natural coumarins as potential anti-SARS-CoV-2 agents supported by docking analysis. RSC Adv. 2021, 11, 16970–16979. [Google Scholar] [CrossRef]
- Song, F.; Huo, X.; Guo, Z. Anti-breast cancer potential of natural and synthetic coumarin derivatives. Curr. Top. Med. Chem. 2021, 21, 1692–1709. [Google Scholar] [CrossRef]
- Salem, M.A.; Helal, M.H.; Gouda, M.A.; Ammar, Y.A.; ElGaby, M.S.A.; Abbas, S.Y. An Overview on Synthetic Strategies to Coumarins. Synth. Commun. 2018, 48, 1534–1550. [Google Scholar] [CrossRef]
- Lončarić, M.; Gašo-Sokač, D.; Jokić, S.; Molnar, M. Recent advances in the synthesis of coumarin derivatives from different starting materials. Biomolecules 2020, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, Y.F. Classical Approaches and their Creative Advances in the Synthesis of Coumarins: A Brief Review. J. Med. Chem. Sci. 2021, 4, 612–625. [Google Scholar]
- Adimule, V.M.; Nandi, S.S.; Kerur, S.S.; Khadapure, S.A.; Chinnam, S. Recent advances in the one-pot synthesis of coumarin derivatives from different starting materials using nanoparticles: A review. Top. Catal. 2022, 65, 1669–1674. [Google Scholar] [CrossRef]
- Vekariya, R.H.; Patel, H.D. Recent Advances in the Synthesis of Coumarin Derivatives via Knoevenagel Condensation: A Review. Synth. Commun. 2014, 44, 2756–2788. [Google Scholar] [CrossRef]
- Johnson, J.R. The Perkin Reaction and Related Reactions. In Organic Reactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 210–265. [Google Scholar]
- Shriner, R.L. The Reformatsky Reaction. In Organic Reactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 1–37. [Google Scholar]
- Cairns, N.; Harwood, L.M.; Astles, D.P. Tandem Thermal Claisen−Cope Rearrangements of Coumarate Derivatives. Total Syntheses of the Naturally Occurring Coumarins: Suberosin, Demethylsuberosin, Ostruthin, Balsamiferone and Gravelliferone. J. Chem. Soc. Perkin Trans. 1 1994, 3101–3107. [Google Scholar] [CrossRef]
- Kouznetsov, V.V.; Puerto-Galvis, C.E.; Ortiz Villamizar, M.C.; Vargas-Méndez, L.Y. Insights into the metal-catalyzed alkyne hydroarylation reactions and related processes for the synthesis of coumarins. Curr. Org. Chem. 2017, 21, 949–963. [Google Scholar] [CrossRef]
- Bhatia, R.; Pathania, S.; Singh, V.; Rawal, R.K. Metal-catalyzed synthetic strategies toward coumarin derivatives. Chem. Heterocycl. Compd. 2018, 54, 280–291. [Google Scholar] [CrossRef]
- Farid, S.M.; Seifinoferest, B.; Gholamhosseyni, M.; Larijani, B.; Mahdavi, M. Modern metal-catalyzed and organocatalytic methods for synthesis of coumarin derivatives: A review. Org. Biomol. Chem. 2022, 20, 4846–4883. [Google Scholar] [CrossRef]
- Bouhaoui, A.; Eddahmi, M.; Dib, M.; Khouili, M.; Aires, A.; Catto, M.; Bouissane, L. Synthesis and Biological Properties of Coumarin Derivatives. A Review. ChemistrySelect 2021, 6, 5848–5870. [Google Scholar] [CrossRef]
- Zambare, A.S.; Kalam Khan, A.F.; Zambare, S.P.; Shinde, S.D.; Sangshetti, J.N. Recent advances in the synthesis of coumarin derivatives via Pechmann condensation. Curr. Org. Chem. 2016, 20, 798–828. [Google Scholar] [CrossRef]
- Gulati, S.; Singh, R.; Sangwan, S. A review on convenient synthesis of substituted coumarins using reuseable solid acid catalysts. RSC Adv. 2021, 11, 29130–29155. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.P.; Shivasankar, K.; Sivappa, R.; Kale, R. Zinc mediated transesterification of β-ketoesters and coumarin synthesis. Tetrahedron Lett. 2002, 43, 8583–8586. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Mishra, R.K.; Kumar, A. A convenient synthesis of some coumarin derivatives using SnCl2·2H2O as catalyst. Catal. Lett. 2008, 121, 118–120. [Google Scholar] [CrossRef]
- Smitha, G.; Sanjeeva Reddy, C. ZrCl4-catalyzed Pechmann reaction: Synthesis of coumarins under solvent-free conditions. Synth. Comm. 2004, 34, 3997–4003. [Google Scholar] [CrossRef]
- Kumar, S.; Saini, A.; Sandhu, J.S. LiBr-Mediated, solvent free von Pechmann reaction: Facile and efficient method for the synthesis of 2H-chromen-2-ones. Arkivoc 2007, 15, 18–23. [Google Scholar] [CrossRef]
- Borah, K.J.; Borah, R. Poly (4-vinylpyridine)-supported sulfuric acid: An efficient solid acid catalyst for the synthesis of coumarin derivatives under solvent-free conditions. Monatsh. Chem. 2011, 142, 1253–1257. [Google Scholar] [CrossRef]
- Montazeri, N.; Khaksar, S.; Nazari, A.; Alavi, S.S.; Vahdat, S.M.; Tajbakhsh, M. Pentafluorophenylammonium triflate (PFPAT): An efficient, metal-free and reusable catalyst for the von Pechmann reaction. J. Fluor. Chem. 2011, 132, 450–452. [Google Scholar] [CrossRef]
- Heravi, M.M.; Khaghaninejad, S.; Mostofi, M. Pechmann reaction in the synthesis of coumarin derivatives. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 112, pp. 1–50. [Google Scholar]
- Samiei, Z.; Soleimani-Amiri, S.; Azizi, Z. Fe3O4@C@OSO3H as an efficient, recyclable magnetic nanocatalyst in Pechmann condensation: Green synthesis, characterization, and theoretical study. Mol. Divers. 2021, 25, 67–86. [Google Scholar] [CrossRef]
- Rather, I.A.; Ali, R. An efficient and versatile deep eutectic solvent-mediated green method for the synthesis of functionalized coumarins. ACS Omega 2022, 7, 10649–10659. [Google Scholar] [CrossRef]
- Molnar, M.; Lončarić, M.; Kovač, M. Green chemistry approaches to the synthesis of coumarin derivatives. Curr. Org. Chem. 2020, 24, 4–43. [Google Scholar] [CrossRef]
- Gonçalves, G.A.; Eifler-Lima, V.L. Coumarin synthesis via Pechmann condensation: Toward a greener era (microreview). Chem. Heterocycl. Compd. 2021, 57, 734–736. [Google Scholar] [CrossRef]
- Borah, B.; Dwivedi, K.D.; Kumar, B.; Chowhan, L.R. Recent advances in the microwave-and ultrasound-assisted green synthesis of coumarin-heterocycles. Arab. J. Chem. 2021, 15, 103654. [Google Scholar] [CrossRef]
- Zarei, F.; Soleimani-Amiri, S.; Azizi, Z. Heterogeneously catalyzed Pechmann condensation employing the HFe(SO4)2·4 H2O-Chitosan nano-composite: Ultrasound-accelerated green synthesis of coumarins. Polycycl. Aromat. Compd. 2022, 42, 6072–6089. [Google Scholar] [CrossRef]
- Feizpour Bonab, M.; Soleimani-Amiri, S.; Mirza, B. Fe3O4@C@PrS-SO3H: A Novel Efficient Magnetically Recoverable Heterogeneous Catalyst in the Ultrasound-Assisted Synthesis of Coumarin Derivatives. Polycycl. Aromat. Compd. 2022, 42, 1628–1643. [Google Scholar] [CrossRef]
- Sharma, D.; Kumar, S.; Makrandi, J.K. Modified Pechmann condensation using grinding technique under solvent-free condition at room temperature. Green Chem. Lett. Rev. 2011, 4, 127–129. [Google Scholar] [CrossRef]
- Sharma, D.; Kumar, S. A facile synthesis of 2H-chromen-2-ones via Pechmann condensation under solvent free conditions using grinding technique. Green Process. Synth. 2013, 2, 151–155. [Google Scholar] [CrossRef]
- Narwal, J.K.; Malik, R.K.; Kumari, N. An efficient solvent free synthesis of coumarins via solid phase Pechmann reaction. Chem. Sci. Trans. 2015, 4, 1092–1094. [Google Scholar]
- Sharapov, A.D.; Fatykhov, R.F.; Khalymbadzha, I.A.; Sharutin, V.V.; Santra, S.; Zyryanov, G.V.; Chupakhin, O.N.; Ranu, B.C. Mechanochemical synthesis of coumarins via Pechmann condensation under solvent-free conditions: An easy access to coumarins and annulated pyrano [2,3-f] and [3,2-f] indoles. Green Chem. 2022, 24, 2429–2437. [Google Scholar] [CrossRef]
- Stolle, A.; Szuppa, T.; Leonhardt, S.E.; Ondruschka, B. Ball milling in organic synthesis: Solutions and challenges. Chem. Soc. Rev. 2011, 40, 2317–2329. [Google Scholar] [CrossRef]
- Wang, G.W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013, 42, 7668–7700. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.; Villacampa, M.; Menéndez, J.C. Multicomponent mechanochemical synthesis. Chem. Sci. 2018, 9, 2042–2064. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.L.; Cao, C.; Browne, D.L. Mechanochemistry as an emerging tool for molecular synthesis: What can it offer? Chem. Sci. 2018, 9, 3080–3094. [Google Scholar] [CrossRef] [PubMed]
- Ardila-Fierro, K.J.; Hernández, J.G. Sustainability assessment of mechanochemistry by using the twelve principles of green chemistry. ChemSusChem 2021, 14, 2145–2162. [Google Scholar] [CrossRef]
- Cuccu, F.; De Luca, L.; Delogu, F.; Colacino, E.; Solin, N.; Mocci, R.; Porcheddu, A. Mechanochemistry: New Tools to Navigate the Uncharted Territory of “Impossible” Reactions. ChemSusChem 2022, 15, e202200362. [Google Scholar] [CrossRef]
- Banerjee, M.; Panjikar, P.C.; Das, D.; Iyer, S.; Bhosle, A.A.; Chatterjee, A. Grindstone chemistry: A “green” approach for the synthesis and derivatization of heterocycles. Tetrahedron 2022, 112, 132753. [Google Scholar] [CrossRef]
- Kouznetsov, V.V.; Merchán-Arenas, D.R.; Martínez-Bonilla, C.A.; Macías, M.A.; Roussel, P.; Gauthier, G.H. Grinding and Milling: Two efficient methodologies in the solvent-free phosphomolybdic acid-catalyzed and mechanochemical synthesis of cis-4-Amido-N-yl-2-methyl-tetrahydroquinolines. J. Braz. Chem. Soc. 2016, 27, 2246–2255. [Google Scholar] [CrossRef]
- Ortiz-Villamizar, M.C.; Puerto-Galvis, C.E.; Kouznetsov, V.V. The A3 redox-neutral C1-alkynylation of tetrahydroisoquinolines: A comparative study between visible light photocatalysis and transition-metal catalysis. Synthesis 2021, 53, 547–556. [Google Scholar]
- Peñaranda Gómez, A.; Puerto Galvis, C.E.; Macías, M.A.; Ochoa-Puentes, C.; Kouznetsov, V.V. I2/DMSO-Promoted the synthesis of chromeno[4,3-b]quinolines through an imine formation/aza-Diels-Alder/aromatization tandem reaction under metal-catalyst and photosensitizer-free conditions. Synthesis 2022, 54, 1857–1869. [Google Scholar]
- Villamizar-Mogotocoro, A.F.; Bonilla-Castañeda, S.M.; Kouznetsov, V.V. Green conditions for the efficient two-step synthesis of new 6-arylphenanthridines from 2-bromoacetoanilides based on microwave-assisted Suzuki-Miyaura cross-coupling and modified Pictet-Spengler dehydrogenative cyclization in a zinc chloride/[Bmim]BF4 mixture. Green Chem. 2022, 24, 7996–8004. [Google Scholar]
- Sun, X.; Guo, Y.; Wen, R.; Li, H. A highly sensitive and selective ratiometric sensing platform based on 7-amino-4-methylcoumarin for naked-eye visual fluorescence sensing of Cu2+. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 267, 120627. [Google Scholar] [CrossRef] [PubMed]
- Takase, H.; Murase, T.; Hachisuka, D.; Sakamoto, Y.; Sugiura, M.; Nakano, S.; Fujii, K.; Masaki, A.; Inagaki, H. 7-Amino-4-methylcoumarin as a fluorescent substitute for Schiff’s reagent: A new method that can be combined with hemalum and eosin staining on the same tissue section. Biotech. Histochem. 2023, 98, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Von Pechmann, H.; Schwarz, O. Studien über Cumarine.–III. Ueber das ρ-Amido-β-methylcumarin. Ber. 1899, 32, 3696–3699. [Google Scholar] [CrossRef]
- Zimmerman, M.; Yurewicz, E.; Patel, G. A new fluorogenic substrate for chymotrypsin. Anal. Biochem. 1976, 70, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Bissell, E.R.; Mitchell, A.R.; Smith, R.E. Synthesis and chemistry of 7-amino-4-(trifluoromethyl) coumarin and its amino acid and peptide derivatives. J. Org. Chem. 1980, 45, 2283–2287. [Google Scholar] [CrossRef]
- Bao, X.; Wang, G.; Tian, C.; Dong, X.; Xu, G.; Li, F.; Chen, D. Er(OTf)3-catalyzed synthesis of fluorescent 7-aminocoumarins. Tetrahedron 2022, 123, 132994. [Google Scholar] [CrossRef]
- Pozdnev, V.F. Improved method for synthesis of 7-amino-4-methylcoumarin. Chem. Heterocycl. Compd. 1990, 26, 264–265. [Google Scholar] [CrossRef]
- Reddy, T.S.; Reddy, A.R. Synthesis and fluorescence study of 6,7-diaminocoumarin and its imidazolo derivatives. Dyes Pigm. 2013, 96, 525–534. [Google Scholar] [CrossRef]
- Merchan Arenas, D.R.; Kouznetsov, V.V. Diastereoselective synthesis of dihydroisoindolo [2,1-a] quinolin-11-ones by solvent-free AMCell-SO3H-catalyzed imino Diels–Alder/intramolecular amide cyclization cascade reactions. J. Org. Chem. 2014, 79, 5327–5333. [Google Scholar] [CrossRef]
- Senda, N.; Miwa, Y.; Tanaka, J.; Momotake, A.; Arai, T. Tsukuba-green: A fluorescent dye that emits green fluorescence useful for live-cell imaging. Chem. Lett. 2010, 39, 308–310. [Google Scholar] [CrossRef]
- Bose, D.S.; Rudradas, A.P.; Babu, M.H. The indium (III) chloride-catalyzed von Pechmann reaction: A simple and effective procedure for the synthesis of 4-substituted coumarins. Tetrahedron Lett. 2002, 43, 9195–9197. [Google Scholar] [CrossRef]
- Fischer, F.; Fendel, N.; Greiser, S.; Rademann, K.; Emmerling, F. Impact Is Important-Systematic Investigation of the Influence of Milling Balls in Mechanochemical Reactions. Org. Process Res. Dev. 2017, 21, 655–659. [Google Scholar] [CrossRef]
- Robertson, A.; Sandrock, W.F.; Henry, C.B. Hydroxy-carbonyl compounds, part V: The preparation of coumarins and 1:4-pyrones from phenol, p-cresol, quinol, and α-naphthol. J. Chem. Soc. 1931, 2426–2432. [Google Scholar] [CrossRef]
- Goswami, P. Dually activated organo-and nano-cocatalyzed synthesis of coumarin derivatives. Synth. Commun. 2009, 39, 2271–2278. [Google Scholar] [CrossRef]
- Yamada, K.; Takada, S.; Nakamura, S.; Hirata, Y. The structures of anisatin and neoanisatin: Toxic sesquiterpenes from Illicium anisatum L. Tetrahedron 1968, 24, 199–229. [Google Scholar] [CrossRef]
- Soleimani, E.; Khodaei, M.M.; Batooie, N.; Samadi, S. Tetrakis (acetonitrile) copper (I) hexafluorophosphate catalyzed coumarin synthesis via Pechmann condensation under solvent-free condition. J. Heterocycl. Chem. 2012, 49, 409–412. [Google Scholar] [CrossRef]
- Khalafi-Nezhad, A.; Mowlazadeh Haghighi, S.; Panahi, F. Nano-TiO2 on dodecyl-sulfated silica: As an efficient heterogeneous Lewis acid–surfactant-combined catalyst (HLASC) for reaction in aqueous media. ACS Sustain. Chem. Eng. 2013, 1, 1015–1023. [Google Scholar] [CrossRef]


| Coumarin a | Milling Time (min) | Isolated Yields, % | Mp (°C) Found b | Mp (°C) Reported |
|---|---|---|---|---|
| 3a | 10 | 92 | 220–224 | 220–224 [68] |
| 3b | 5 | 95 | 184–186 | 185–187 [69] |
| 3c | 20 | 80 | 170–172 | 172 [69] |
| 3d | 60 | 52 | 77–79 | 78–80 [69] |
| 3e | 45 | 56 | 140–144 | 143–144 [70] |
| 3f | 12 | 88 | 154–156 | 152–154 [71] |
| 3g | 15 | 68 | 176–178 | 177−179 [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becerra-Anaya, S.J.; Merchán Arenas, D.R.; Kouznetsov, V.V. A Simple and Effective Protocol for the Pechmann Reaction to Obtain 4-Methylcoumarin Derivatives Using a High-Speed Mixer Ball Mill Process. Chemistry 2023, 5, 1077-1088. https://doi.org/10.3390/chemistry5020073
Becerra-Anaya SJ, Merchán Arenas DR, Kouznetsov VV. A Simple and Effective Protocol for the Pechmann Reaction to Obtain 4-Methylcoumarin Derivatives Using a High-Speed Mixer Ball Mill Process. Chemistry. 2023; 5(2):1077-1088. https://doi.org/10.3390/chemistry5020073
Chicago/Turabian StyleBecerra-Anaya, Silvia J., Diego R. Merchán Arenas, and Vladimir V. Kouznetsov. 2023. "A Simple and Effective Protocol for the Pechmann Reaction to Obtain 4-Methylcoumarin Derivatives Using a High-Speed Mixer Ball Mill Process" Chemistry 5, no. 2: 1077-1088. https://doi.org/10.3390/chemistry5020073
APA StyleBecerra-Anaya, S. J., Merchán Arenas, D. R., & Kouznetsov, V. V. (2023). A Simple and Effective Protocol for the Pechmann Reaction to Obtain 4-Methylcoumarin Derivatives Using a High-Speed Mixer Ball Mill Process. Chemistry, 5(2), 1077-1088. https://doi.org/10.3390/chemistry5020073