Abstract
This is a short overview of the life and achievements of Justus von Liebig. Clearly, this can only be an incomplete and somewhat personal view of the author, who has been a professor of inorganic chemistry at Justus Liebig University since 2002. Having already been interested in the work of Liebig for many years, and with a strong connection to the Liebig Museum in Giessen, the author hopes to provide some useful information about this great chemist, one of the founders of modern chemistry. The reader should find many interesting, probably new, facts about Liebig’s major impact on chemistry, agriculture, nutrition, and pharmacology.
1. It all Started with a Bang
Justus Liebig was born on 12 May 1803, in Darmstadt (Hesse, Germany). His father owned a business, what today, most likely, you would call a drugstore, selling/preparing paints, and different materials, including chemicals. Early on, Justus had to help his father in the store, which he much preferred to school, where he did not enjoy learning old languages. Several times he had to fetch books for his father about chemistry from the library close by, which, from the age of 13, he read from beginning to end. Already at this early age, Justus wanted to become a chemist. An anecdote demonstrates this, as it was reported that his teacher and his classmates laughed at him when he gave this as an answer being asked in school what he would like to do later in life [1]. Justus enjoyed going to the fair in Darmstadt, where the marketeers (often charlatans/quacksalvers) performed all kinds of chemical tricks to attract people and to sell their wares, e.g., “universal medicine” that was declared to heal nearly every disease. To entertain the audience, they performed small explosions with the silver or mercury salt of fulminic acid (Figure 14). Some readers might be aware of the mercury salt used in the first season of the TV show Breaking Bad (not very realistic). There is an old book (published) for children about this time “Knallsilber—Geschichten aus dem Leben von Justus von Liebigs” (Figure 1a) [2], that describes how Justus watched at the fair how to create these explosions (Figure 10) and how he impressed his father, and especially his classmates, by preparing them himself. Most likely his teachers much less enjoyed this knowledge because all the boys then had easy access to these explosions. Silver fulminate is so unstable that the only application for this compound is in the bang snaps that are still sold today (for children!). Mainly they contain some sand wrapped together in paper with a tiny amount of silver fulminate (Figure 1b), and they explode when thrown on the floor or against a wall.

Figure 1.
(a) The children book “Knallsilber” (Title in English: Silver fulminate—Stories of the life of Justus von Liebig); (b) Commercially sold bang snaps today kept in sawdust.
Due to his problems in school, Liebig did not finish high school. He left school when he was 14 and started an apprenticeship training in a pharmacy in Heppenheim (close to Darmstadt). However, here he also “failed” and left after only a few months. It was reported that he caused an explosion (or a fire) in the pharmacy when performing chemistry experiments (Figure 10) and was thrown out for that reason, but it is more likely that he did not like the work there at all. Back in Darmstadt, he worked again in his father’s shop, produced bang snaps for sale, and even wrote his first publication about their chemistry in a journal. A chemist, Karl Wilhelm Gottlob Kastner, who became a professor in Bonn, was impressed and even wrote an introduction to this publication.
Liebig received the chance to join Kastner in Bonn and started studying chemistry there, despite not having finished high school. He followed Kastner to Erlangen in 1821, and at only 18 years old (Figure 2), he got into some serious trouble due to some student activities. Consequently, he had to escape a police investigation and flee back to Darmstadt. Despite these activities, he obtained a scholarship from the government of the state of Hesse to go to Paris to proceed with his studies. According to Liebig, France was the country where chemistry bloomed then, while Germany was left far behind.

Figure 2.
Drawing of Liebig as a young student (photograph of a drawing at the Liebig Museum).
2. Paris
From October 1822, Liebig stayed in Paris for 17 months (his original stipend for 6 months was extended several times), and he made the acquaintance of some of the most famous chemists of the time, including L. N. Vauquelin, L. J. Thenard, and J. L. Gay-Lussac [1]. Liebig knew some French and studied it further because, in his opinion, it was necessary for every educated scientist. He was extremely impressed by the chemistry in France. He wrote to his very dear friend, the poet August von Platen [3]: “It is a great shame how greatly the reputation of Germans has declined in physics, chemistry, and the other natural sciences. There is scarcely a shadow left, and they fight over this shadow like mad dogs. Contemporary German chemists presume to play at philosophy, thereby losing all their effectiveness.... The French and English proceed in the exact opposite fashion: here science is simply a mechanical stonework, the quasi-mathematical style of treating it allows no play of the mind whatever; but [this method] is at the moment very good, it has led recently to the most magnificent discoveries and is particularly useful for [practical] life”. He admired the lectures by Gay-Lussac and the other professors, which were accompanied by experiments, and he was inspired to apply this later in his own teaching. His research focused again on fulminates, the explosives that had fascinated him as a boy. A short paper on that topic was printed in Annales de chimie and attracted significant attention. When the paper was presented by Gay-Lussac at the Académie des Sciences, Alexander von Humboldt was present and highly impressed by the young Liebig. Therefore, Humboldt recommended Liebig to Grand Duke Ludwig I. for a professorship at Giessen.
3. Giessen
Liebig was appointed in Giessen in May 1824, two weeks after his 21st birthday, as a (Ausserordentlicher) professor and became a full (Ordentlicher) professor in 1825; however, he was not welcomed at Giessen University because he had been put there by the Grand Duke and had not been chosen by the university/the professors [1]. Chemistry at that time was also not an established area of study but was regarded, rather, as a support for pharmacy or medicine. Thus, Liebig started to teach pharmacology, and his institute initially was pharmaceutical chemistry. Furthermore, when Liebig came to Giessen, there was already a full chemistry professor, Ludwig Wilhelm Zimmermann. They soon fell into a competition for students; however, it seemed that students preferred the teaching of Liebig, which must have been very lively and was combined with experimental demonstrations; many of these experiments are still presented in our first-semester chemistry lectures. In Germany, the word for a lecture is “Vorlesung” (a reading) that goes along with a reader for a lecturer. At that time, it was meant literally because professors mainly read from their books in a lecture. Liebig was different and thus attracted many students. The full circumstances are unclear, however, Zimmermann drowned in the river Lahn in 1825 (suicide suspected), and, thus, Liebig became a full professor of chemistry. Looking back, it is interesting to note that Liebig became a professor at 21 without having finished high school, with doubts about a proper doctoral dissertation (he received his doctoral title of Erlangen “in absentia), and having not completed a Habilitation [1]. Pretty amazing!
In Giessen, Liebig received a former guard house of a military barracks where he could set up his laboratory on the first floor and where he lived on the second floor together with his family. Over time, and sometimes pushing hard for money, the building was extended, and he obtained more space for research and teaching. Figure 3 shows the old laboratory with the stove and the front of the building (the door was opened for releasing toxic fumes). There was no heating system and no running water. Water for cooling a distillation was lifted in buckets attached to some tubing. In the newer part of the lab, hoods, quite similar to those used today, were later installed. The modern part of the laboratory and the way of running has been copied many times worldwide, e.g., in Russia or France (see below).

Figure 3.
The stove of the old laboratory; front view of the Liebig Museum (door leading to the old laboratory; distillation setup; one of the hoods in the newer laboratory.
4. Students and Researchers in Giessen
The laboratory of Liebig became famous over time and attracted students and researchers from all over the world (Figure 10). Very early on, international exchange played an important role in the development of chemistry. Unfortunately, at that time, women could not yet participate. It is interesting to see who studied and/or worked with Liebig. Aside from the Germans, there were people from Belgium (1), Denmark (2), Great Britain (88), France (30), the Netherlands (3), Italy (2), Luxembourg (2), Mexico (1), Norway (2), Austria (11), Russia (21), Switzerland (43), Spain (1), Hungary (4), and United States (17) [4]. Some of the important chemists are shown on a genealogical tree in Figure 4, including Fresenius, Erlenmeyer, and Strecker, just to mention a few well-known names in chemistry. From there, it is obvious how chemistry developed further due to the influence of Liebig’s laboratory.
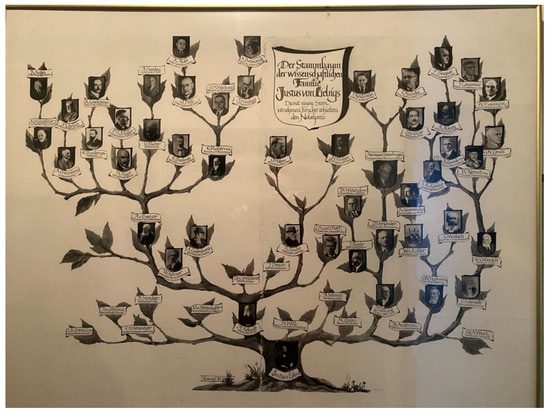
Figure 4.
Photograph of the genealogical tree at the Liebig Museum. Chemists in Figure 4 (from bottom to top and from left to right) Nobel prize winners are assigned with a star and the date of receiving the prize: Trunk: Justus von Liebig; 1. Branch: C. R. Fresenius, H. von Fehling, E. Erlenmeyer, A. Strecker, M. von Pettenkofer, A. Kekulé, H. Will, A. Sobrero, N. Zinin, J. Volhard, Th. Anderson, A. W. Hofmann, Ch. A. Wurtz, C. Schmidt; 2. Branch starting from Kekulé: A. von Baeyer* (1905), W. Körner, A. Ladenburg, Ch. G. Williams, J. Dewar, Th. Zincke, J. H.van’t Hoff* (1901); Branch starting from Volhard: J. Thiele, D. Vorländer, H. Wieland* (1927), H. Staudinger* (1953), T. Reichstein* (1950), L. Ruzicka* (1939); Branch starting from Hofmann: W. von Miller, O. Wallach* (1910), R. Zsigmondy* (1925); Branch starting from Schmidt: W. Ostwald* (1909), S. Arrhenius* (1903), Th. W. Richards* (1914), W. Nernst* (1920), C. N. Lewis, H. von Euler* (1929), R. Abbeg, L. Ruzicka* (1939), F. Bergius* (1931), J. Langmuir* (1932), W. F. Giauque* (1949); Branch starting from von Baeyer: R. Willstätter* (1915), A. von Weinberg, E. Bamberger, H. Rupe, O. Fischer, E. Buchner* (1907), E. Fischer* (1902), R. Kuhn* (1938), P. Ehrlich* (1908), K. Landsteiner* (1930), P. H. Müller* (1948), A. Harden* (1929), O. Diels* (1950), O. H. Warburg* (1931), A. Windaus* (1928), K. Alder* (1950), A. Butenandt* (1939); Branch starting from Zincke: O. Hahn* (1944), H. Fischer* (1930).
A very important chemist from the Liebig laboratory was August Wilhelm von Hofmann, who later became president of the Chemical Society in London (now the Royal Society of Chemistry). After he accepted a position as a professor in Berlin, he was co-founder of the Deutsche Chemische Gesellschaft and became their president. He is well known, especially for his research on aniline (Figure 5).

Figure 5.
Memorial (copy; the original is in the Liebig Museum) of August Wilhelm von Hofmann (Text on the memorial: born in Giessen 8 April 1818; died in Berlin 5 May 1892; “A master and excellent teacher in chemistry; a successful researcher of aniline and aniline dyes”) in Giessen (Frankfurter Strasse); a plaster bust of Nikolay Zinin at the museum of Kazan School of Chemistry.
Nikolay Zinin (Figure 5), a Russian chemist who later became the first president of the Russian Physical and Chemical Society, worked on the so-called benzoin condensation (discovered by Liebig) in Giessen. He later became a professor at the University of Kazan (afterward in St. Petersburg). He discovered that nitrobenzene could be reduced to aniline (general: the Zinin reaction, the reduction of nitro aromates to the corresponding amines). He played an important role in identifying aniline, which was finally confirmed by Hofmann [5]. The laboratory in Kazan was built according to the setup of Liebig’s laboratory in Giessen, and it is now a museum (Museum of Kazan School of Chemistry). Some original samples of aniline prepared by Zinin are shown in the museum [5].
Ascanio Sobrero, a student from Italy, who later became a professor at the University of Turin, was the discoverer of nitroglycerine in 1847. Alfred Nobel (who early on learned chemistry from Zinin in St. Petersburg) became familiar with nitroglycerine from Sobrero in 1850 when he was in Paris studying with Théophile-Jules Pelouze at the age of 17! Sobrero received his Ph.D. 1832 and described his time in Giessen well: “In my last year in Giessen the number of students was 45. There were students of all nations, some with the goal to become a teacher, others with an interest in pharmacology, and many who wanted to do something with pure chemistry. The motivation to learn was very high for all students competing to perform well. However, there was no envy; all were like members of a large family, living under the same roof with the guidance of Liebig as a father” (translation by the author) [4].
Friedrich August Kekulé, as Liebig was born in Darmstadt, started studying architecture in Giessen before switching to chemistry after attending some lectures of Liebig. In 1852, he finished his Ph.D. and later became a professor at the University of Bonn. The lecture notes of Kekulé on experimental chemistry have survived and (while hard to read) give an idea of the content of Liebig’s lectures (Figure 6).

Figure 6.
Photographs of the facsimile of the lecture notes of Kekulé.
Adolphe Wurtz spent one year in Liebig’s laboratory after obtaining his Ph.D. in Strasbourg and was excited about the chemistry developments in Germany. Several French laboratories were later constructed based on the institute in Giessen. Wurtz became the first professor of organic chemistry at the Sorbonne in 1875. Wurtz described the work in Giessen similarly to Sobrero: “What made life in Giessen so pleasant was the inner bond that prevailed among the chemists. United by the same scientific interest, suffused with the same devotion to the teacher, we worked the whole day together and supported each other at every instant with word and deed” [3]. The book “Nationalizing Science—Adolphe Wurtz and the Battle for French Chemistry” by Alan J. Rocke is worth reading concerning chemistry at Liebig’s time [3]. We could learn a lot from history to avoid repeatedly making mistakes.
5. Munich
After 28 years in Giessen, Liebig accepted an offer to move to Munich in 1852. Here, he obtained a new laboratory and had much fewer teaching duties. In Munich he even was in contact with the family of the King of Bavaria, who enjoyed attending spectacular chemistry presentations performed by Liebig. Unfortunately, during one of these lectures an accident occurred. Liebig was performing an experiment called the “Barking Dog” where NO is ignited in the presence of CS2 in a long glass tube. The experiment’s name is a consequence of the sound produced when the flame accelerates through the tube. The experiment was highly appreciated and should therefore be repeated. Then, a mistake occurred and instead of NO, oxygen was introduced into the system that caused an explosion when it was ignited. Despite being injured, Liebig was terrified to see blood on the faces of Queen Therese and Prince Luitpold. Luckily, none of the injuries were serious and there were no consequences. When Liebig realized that aging started to cause health problems he did not complain but instead he wrote to his sister in 1870 [1]: “If one has reached an age of 67 without ever been seriously sick one should be thankful, thank God for it and be content” (translated by the author). Liebig died on April 18, 1873; his grave is at the cemetery in Munich (Figure 7).

Figure 7.
The grave of Liebig in Munich.
6. Achievements
Only a few examples of the many achievements Liebig accomplished can be presented here.
Aside from trying to understand the basics of chemistry, Liebig was always very interested in practical applications. For example, in Liebig’s time, mirrors were made with mercury, a method that seemed to have been developed in Venice (Figure 8) and their production was dangerous due to the toxic mercury fumes. In Germany, especially around Fürth (close to Erlangen) there were several companies producing mirrors based on mercury. Even today, puddles of mercury are still sometimes found from that time when digging at construction sites in the areas of former mirror factories. Liebig developed a method to produce mirrors with silver; however, this business was unsuccessful because people preferred mercury-based mirrors. In contrast to the silver mirrors, these showed people much paler, which was preferred at that time. Only after the application of mercury was prohibited by law did silver mirrors start to be used.

Figure 8.
Photograph of a mirror made with mercury at a museum in Venice; timetable of Horsford.
Furthermore, Liebig invented baking powder that could be used in the place of yeast. However, again, his discovery did not lead to a successful business because people in Germany still preferred to use yeast. Instead, one of his students, the American Eben Norton Horsford, reformulated it later and became rich with it in the United States. After his studies with Liebig, Horsford was appointed as a professor at Harvard (Rumford Chair) where he was an early supporter of higher education for women. It is more than interesting to have a look at his timetable while he was studying with Liebig (Figure 8). Practical work or lectures started at 6 o’clock in the morning!
While Liebig was strongly involved in inorganic chemistry, he and Wöhler are especially important founders of organic chemistry. They introduced the concept of radicals in chemistry, which had nothing to do with molecules that we define today as radicals. The two chemists realized that while some molecule parts could be changed, other parts could be kept constant, for example, the benzoyl group in benzoic acid, benzaldehyde, etc. This finally became the extremely useful concept of functional groups. If cyanide is considered an organic molecule, Liebig also reported the first organocatalysis with the benzoin addition (often wrongly described as benzoin condensation). From the many organic compounds that Liebig investigated, only two more related ones should be mentioned here: chloroform and chloral hydrate. Independently from each other, chloroform was prepared in 1831 for the first time by the American Samuel Guthrie, the French Eugène Subeiran, and Liebig. Several years later, it became extremely useful as an anesthetic. Liebig prepared chloral hydrate around the same time, but its sedative properties were only recognized much later. Initially, it was wrongly assumed that chloral hydrate decomposes in the body to formic acid/formate and chloroform, which would cause the hypnotic effect. It is frightening to note that, according to Wikipedia, in the first 18 months of introducing it commercially in England, 17 million single doses were sold [6]!
Liebig can also be regarded as a pioneer in food chemistry, nutrition, and biochemistry. Many experiments were performed in his laboratory to gain better knowledge of these topics. A significant area of his research was in regard to milk for babies. At the time of Liebig, a baby was in trouble if its mother could not provide milk for feeding. In that case, a nurse had to be found for breastfeeding. Cow milk alone is not suitable for babies. Liebig discovered a way to treat cow milk to make it suitable for infants and, thus, improved the chances of life and life generally for many. Furthermore, while in Munich, a friend’s daughter was very ill with typhus and at the point of dying. Liebig managed to prepare a special soup for her and, thus, kept her alive.
While working on food, Liebig became aware of a problem in South America. Large numbers of cows were slaughtered there without using the meat because the primary interest was in harvesting their skin to produce leather. Without the technologies of today for freezing meat, it was impossible to keep it from going bad while trying to transport it over long distances. Liebig worked out a method to extract the meat as a solid paste that could be kept for a long time without the problem of rotting. He originally thought that he thus extracted the essence of the meat and that it would be a kind of a “superfood”. This was not the case because the extract mainly consists of minerals and can be used to change the taste of your food, like, e.g., Marmite sold in England. However, the extract called “Liebigs Fleischextrakt” became very popular and is still sold today (Figure 9).

Figure 9.
Liebigs Fleischextrakt; a guard book with collector cards inherited from the grandmother of the author’s wife.
What was quite remarkable was how it was sold. Collector cards on many different topics were distributed together with each container of the Fleischextrakt and could be kept in a guard book (Figure 9). Four collector cards are presented in Figure 10. Thousands of these collector cards in many different languages were created.

Figure 10.
Collector cards showing Liebig as a boy at the fair observing a marketer hitting a bang snap; young Liebig causing a fire in the pharmacy in Heppenheim; the laboratory of Liebig (the person on the right with the cylinder is Hofmann); and cows in South America.
Probably the most important research that Liebig completed was related to agriculture. For many different reasons, hunger had become a big problem for the population in Europe. The fields were exhausted and plant growth was only partially understood with many misconceptions. Liebig studied this in detail and realized that fertilizer had to be applied that provided all nutrients for the plants. He realized that if one important component was missing, an excess of all other nutrients will not help. He came up with a very good demonstration for this law of the minimum by showing a barrel that had planks of different heights (Figure 11). The plank that is the lowest would be the minimum.

Figure 11.
The barrel of Liebig shows the law of the minimum. Filling it up with water would lead to a leak at the plank with potassium (“Kali”). So, to improve the plant’s growth, a potassium compounda needs to be added. A reprint of the book Agricultur und Physiologie by Liebig.
One can use this law of the minimum for our own nutrition. We could eat very healthily, however, if one component is too low (or missing), e.g., vitamin C, we still would be unhealthy and become sick over time. With this knowledge that he acquired over time (after many experiments and several failures, see below), Liebig finally could supply different fertilizers that helped to overcome the problem of starvation for many and, thus, enabled the feeding of many more people. Much of this knowledge was published in a book by Liebig “Die Chemie in ihrer Anwendung auf Agricultur und Physiologie” (Figure 11).
Finally, Liebig can be regarded as a pioneer in analytical chemistry. He realized very early on that exact analytical measurements are essential for understanding chemical reactions. In Giessen, he had a carpenter who made the set of scales for him. A photograph of the most accurate balance he used is shown in Figure 12. In contrast to today, such a set of scales took a long time to stabilize and was extremely sensitive to vibration. It was reported that, once, Liebig was smoking a cigar while waiting because that would be about the appropriate time for the set of scales to settle. To avoid any kind of vibration, the measurement result was read by observing the balance through a telescope from the door (Figure 12).

Figure 12.
The best balance of Liebig with an accuracy of 0.3 mg and a maximum of 100 g; the telescope used to read the balance.
At the time of Liebig, there were only very few methods to analyze a compound. Chemists mainly had to rely on elemental analyses. Liebig had learned to perform these analyses well in Gay-Lussac’s laboratory; however, the method was often inaccurate and took a lot of time. Therefore, he improved this method by introducing the Kaliapparat, which allowed a fast and accurate measurement of an organic compound’s carbon and hydrogen content (Figure 13). The compound was burned, the water absorbed by the calcium chloride and the carbon dioxide by a potassium hydroxide solution in the Kaliapparat (the construction of the 5 bulbs ensured that all carbon dioxide was absorbed, and the solution was not pushed out). Afterward, both the tube with the calcium chloride and the Kaliapparat could be weighed, and it was possible to estimate the amount of carbon and hydrogen accurately. If the compound contained oxygen, the amount was just calculated as a difference. Thus, obtaining a molecular formula for glucose with CH2O (“carbon with water, C × H2O”) was possible, leading to the general name for many sugars being carbohydrates. The exact measurement of nitrogen was still a big problem and is discussed in great detail in the book Nationalizing Science, already mentioned above [3].

Figure 13.
The Kaliapparat behind the U-tube with calcium chloride (the setup where the sample is burned is not shown); the author’s membership card with the ACS logo.
This development was extremely important for the advancement of chemistry, now even allowing students to perform elemental analyses fast and accurately [7]. Before using the Kaliapparat this was time-consuming, and an expert was needed to perform the analysis correctly. An American student, Smith, asked Liebig for permission to use the Kaliapparat as a logo for the newly founded American Chemical Society. Many chemists in the US do not know what this symbol means; however, they have been reminded previously in an article published in the membership magazine of the ACS, C&E news [8]. Furthermore, it is not an eagle in the logo but a phoenix rising from the fire/ashes, fitting perfectly well with the idea of elemental analysis (Figure 13).
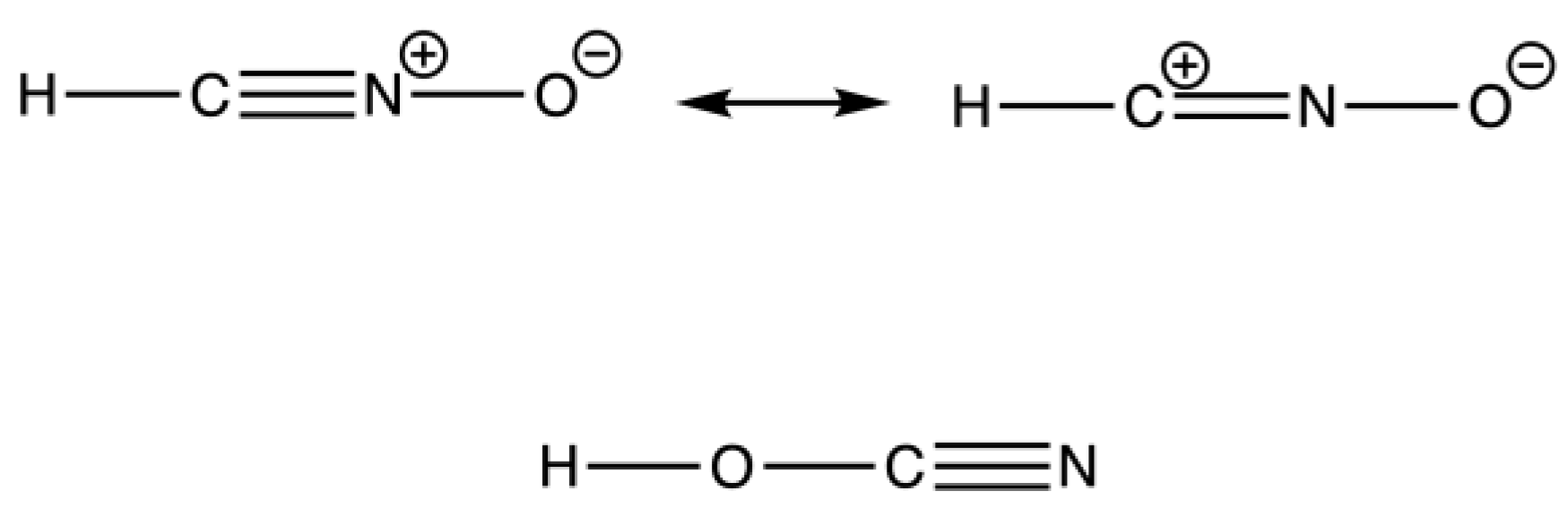
The result of an elemental analysis also led to a verbal fight between Liebig and Wöhler. Still working on the explosive silver fulminate, Liebig learned that Wöhler reported the same molecular formula for a supposedly stable compound. Both accused each other of not being capable of performing an elemental analysis correctly; however, as it turned out, they both were correct. With this dispute, they discovered the phenomenon of isomerism. Readers who have read or watched Harry Potter are fully aware of a so-called anagram ordering letters differently. Thus, in chemistry, it could make a big difference if the compound is written as HCNO or HOCN. The former is Liebig’s fulminic acid and explosive, while the latter is cyanic acid, the compound Wöhler investigated (Figure 14). However, afterwards, Liebig and Wöhler became very good friends.
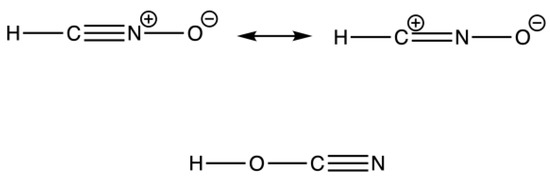
Figure 14.
Isomerism of fulminic acid (top) and cyanic acid (bottom).
7. Failures
Finally, I think it is important to point out that even Liebig, who was an excellent and outstanding chemist, could make mistakes or fail in his work. This is how we all, and Liebig was no different, learn and improve our knowledge. For example, he missed discovering bromine as a new element when he was investigating the mineral waters of different fountains. He was so close but did not recognize it. Only quite late did he finally accept being wrong (as discussed above) that the Fleischextrakt was no superfood. While working on fertilizers he also made many mistakes that he learned to correct over time. Initially, he believed that plants receive nitrogen from the air and did not add nitrogen-containing compounds to the fertilizer. Furthermore, he thought fertilizer should be less soluble to avoid being washed out by rain. Due to these beliefs, there were disastrous results when his first fertilizers were applied. However, this is science: having an idea, testing it with experiments, and learning from the results. Liebig was very good at this. Additionally, Liebig failed to acknowledge the work of Carl Sprengel who recognized the importance of minerals in fertilizer before Liebig.
8. Conclusions
Liebig was an outstanding chemist/scientist. It is amazing what one person can achieve during a lifetime. Not only the many discoveries he made and the achievements for the advancement of chemistry/science, but also the aspects of teaching and the influence he had on other researchers. He also published a great deal of work in scientific journals for other chemists, but he also published in newspapers for the general public as well (Chemische Briefe—Chemistry Letters). It is remarkable that Liebig was already fully aware of the importance of international exchange, which is still relevant today (Figure 15).

Figure 15.
International chemistry students (from Japan, Italy, Russia, and Kosovo) at the Justus Liebig University in the lecture room of Liebig 2021.
To really appreciate the accomplishments of Liebig, the reader is invited to visit the Liebig Museum in Giessen [4]. Furthermore, it is possible to learn more about Liebig (and the development in chemistry) at the museum (Deutsches Museum) in Munich. The museum in Giessen has existed since 1920. Currently, reconstruction is taking place due to a fire in December 2022 (Figure 16). Luckily, it only damaged the lecture room, and rebuilding it to its original state will be possible. On 29 March, a ceremony awarded the Laboratory of Justus Liebig with the “Historical Landmarks Award” of the European Chemical Society (Figure 16). Furthermore, an application process has been started to have it assigned as a UNESCO world cultural heritage site.

Figure 16.
The lecture room at the Liebig Museum after the fire (already cleaned up); Historical Landmarks Award for the Laboratory of Justus Liebig (Prof. Dr. Angela Agostiano, President-elect of the European Chemical Society and Prof. Dr. Gerd Hamscher, Chair of the Justus Liebig Society).
Acknowledgments
The author would like to thank Bernd Commerscheidt (kommisarischer Kurator des Liebig-Museums) for checking the manuscript for mistakes concerning historical facts.
Conflicts of Interest
The author declares no conflict of interest.
References
- Ausstellungskatalog: Justus Liebig (1803–1873) Seine Zeit und Unsere Zeit; Der Präsident der Justus-Liebig-Universität Gießen: Gießen, Germany, 2003.
- Strube, W. Knallsilber—Geschichten aus dem Leben Justus von Liebigs; Kinderbuchverlag Berlin: Berlin, Germany, 1965. [Google Scholar]
- Rocke, A.J. Nationalizing Science: Adolphe Wurtz and the Battle for French Chemistry; MIT Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Website of the Liebig Museum. Available online: https://www.liebig-museum.de or https://www.gdch.de/fileadmin/downloads/GDCh/historische_staetten/liebig.pdf (accessed on 19 April 2023).
- Website of the Museum of Kazan School of Chemistry. Available online: http://www.ksu.museum.ru/chmku/eng/s2.htm (accessed on 19 April 2023).
- Wikipedia on Chloralhydrat. Available online: https://de.wikipedia.org/wiki/Chloralhydrat#cite_note-14 (accessed on 19 April 2023).
- Usselman, M.; Rocke, A.; Reinhart, C.; Foulser, K. Restaging Liebig: A Study in the Replication of Experiments. Ann. Sci. 2012, 62, 1–55. [Google Scholar] [CrossRef]
- Everts, S. A Most Important Artifact. C&E News, 2015, Volume 93. Available online: https://cen.acs.org/articles/93/i35/Important-Artifact.html (accessed on 5 May 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).