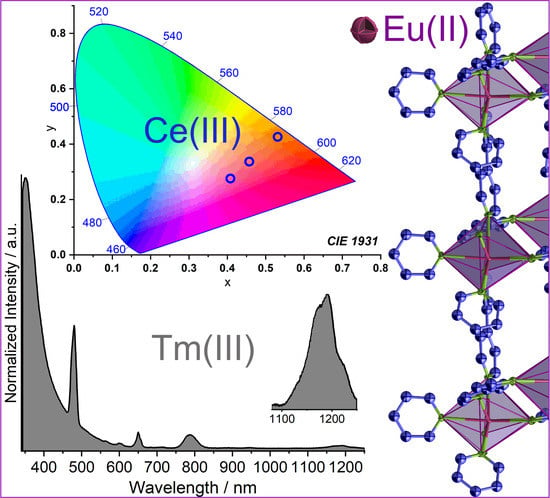
Divalent Europium, NIR and Variable Emission of Trivalent Tm, Ho, Pr, Er, Nd, and Ce in 3D Frameworks and 2D Networks of Ln–Pyridylpyrazolates
Abstract
:1. Introduction
2. Results and Discussion
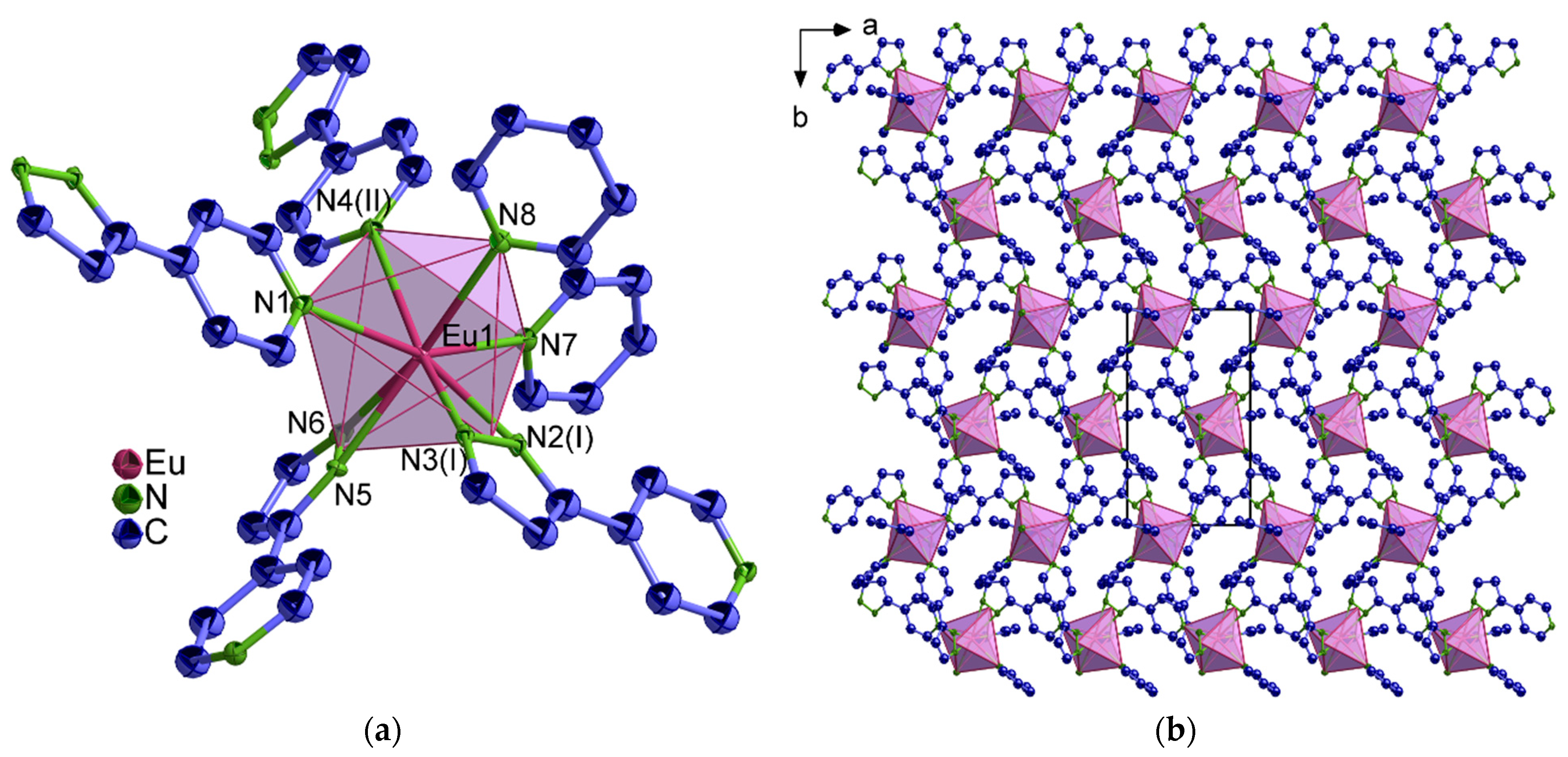
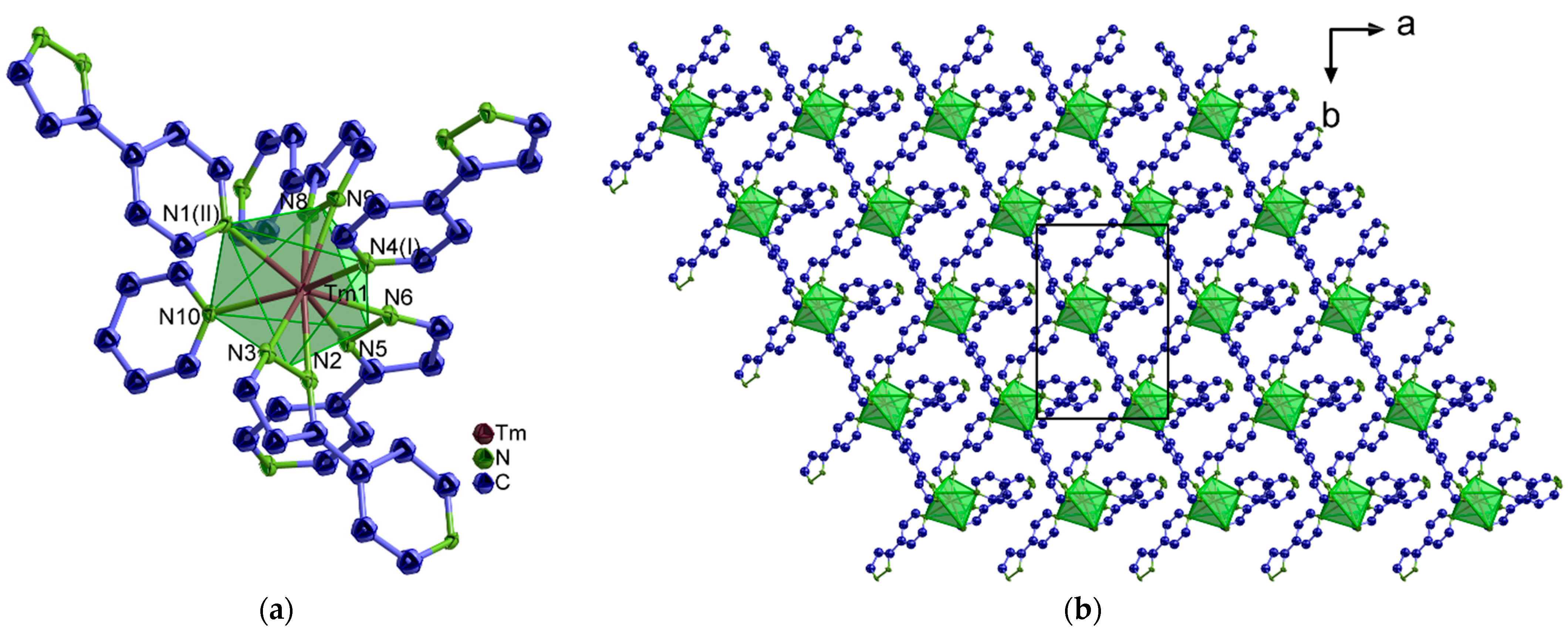
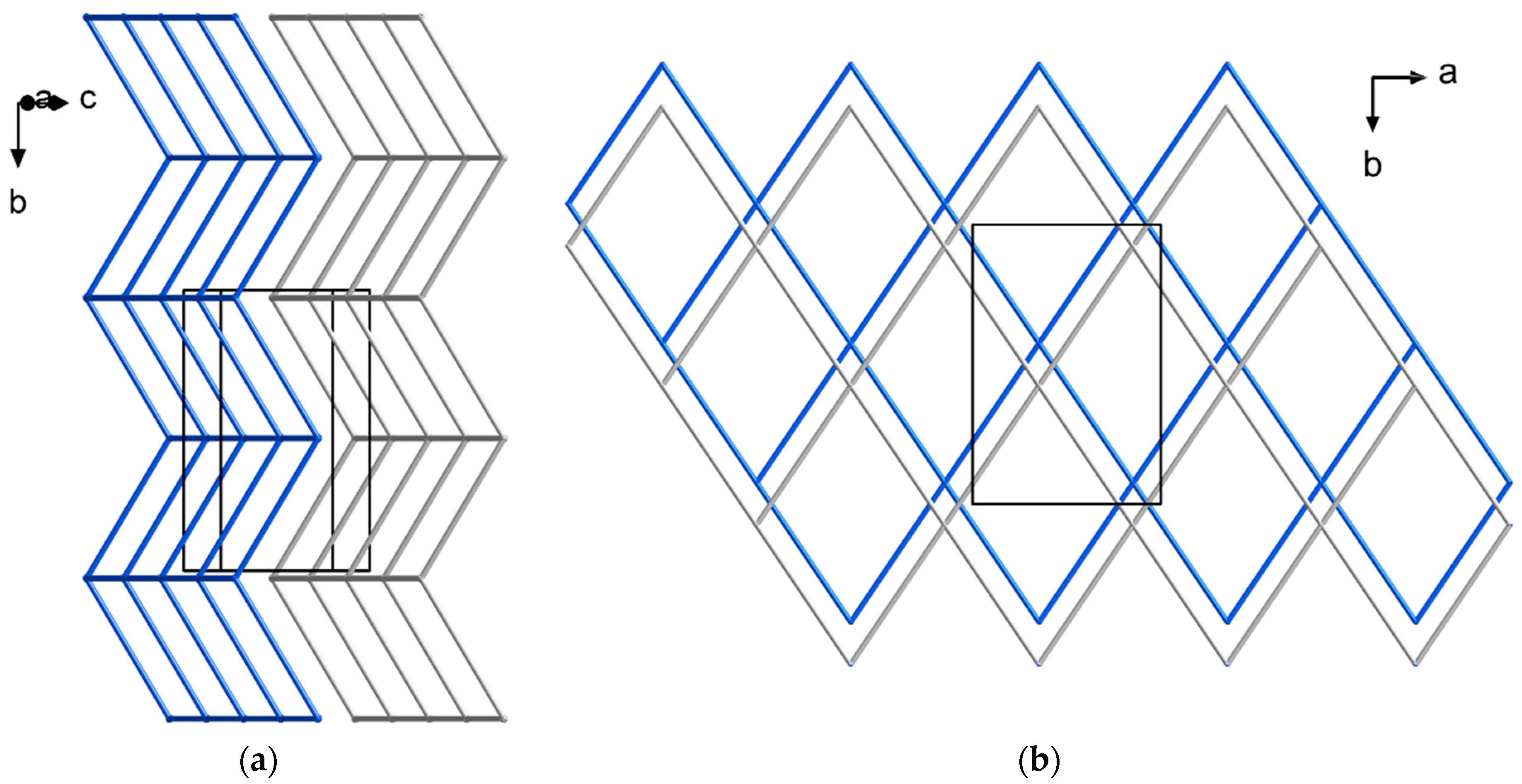
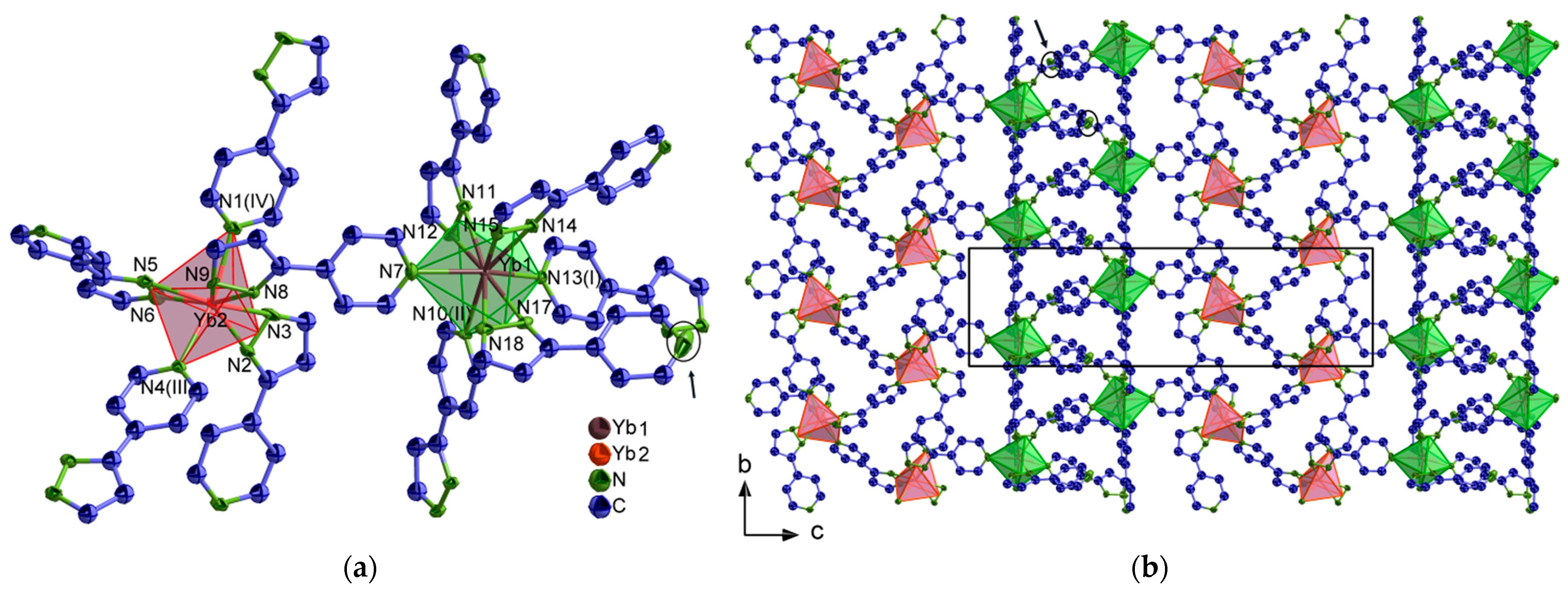
2.1. Structural Analysis
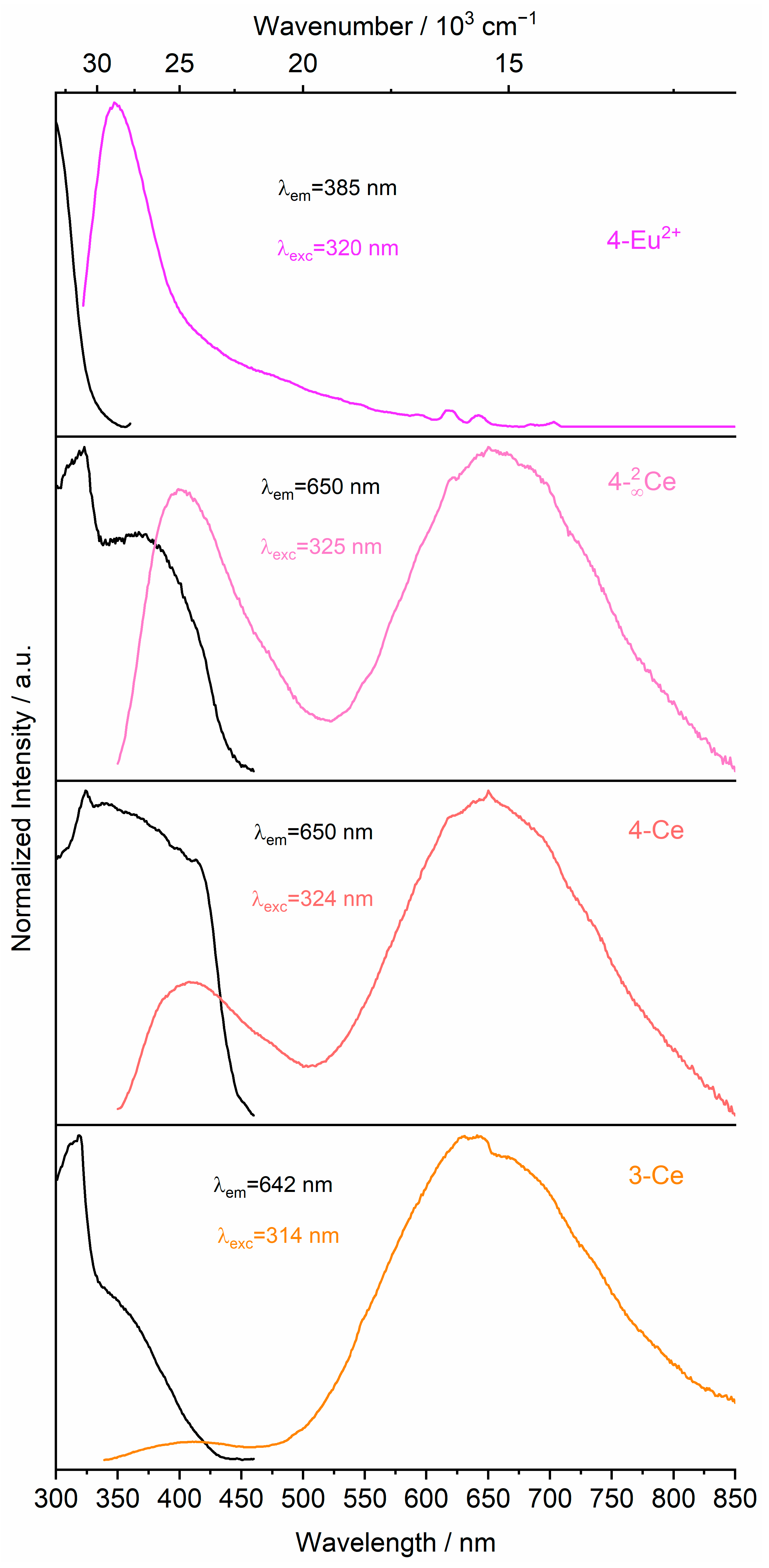
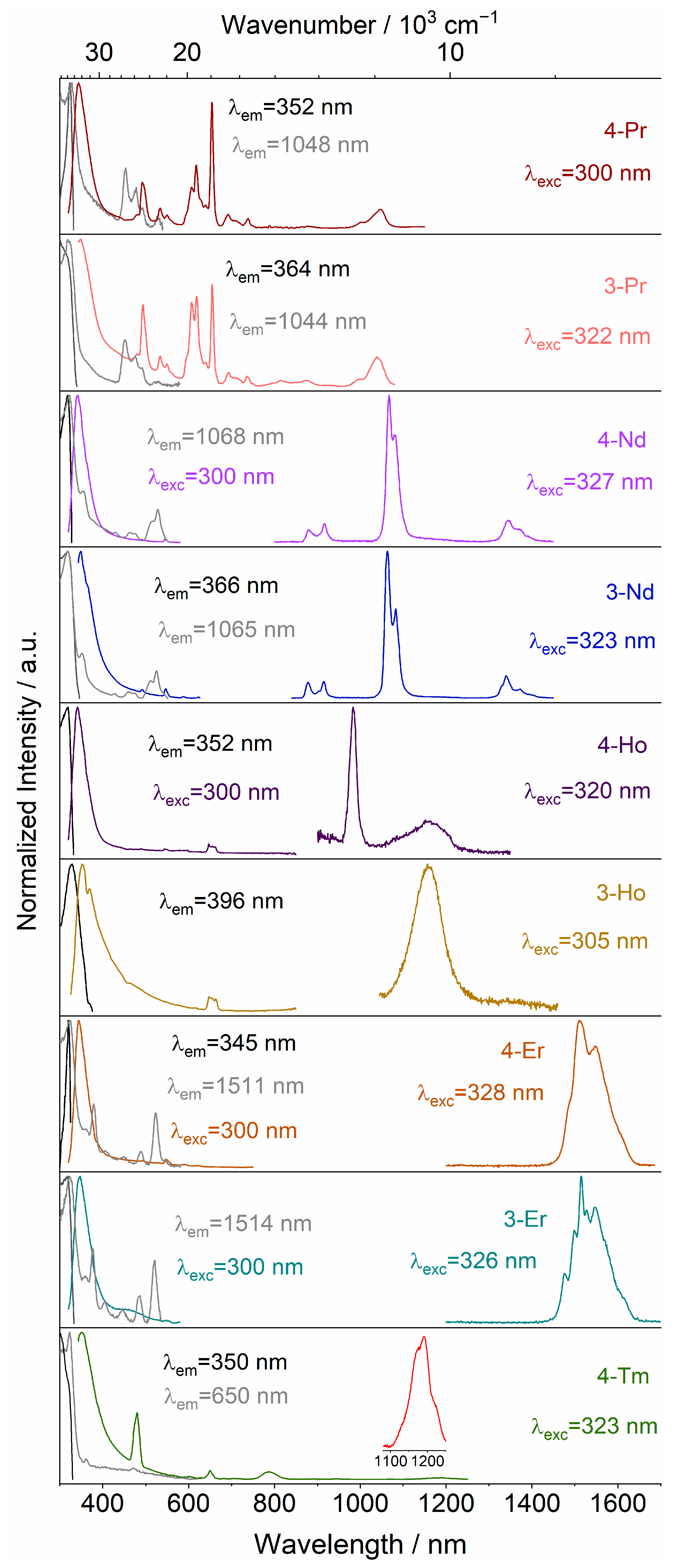
2.2. Photophysical Properties
2.2.1. UV–VIS–NIR Absorption Spectra
2.2.2. Emission and Excitation Spectra
2.2.3. Mechanism of Energy Transfer
2.3. Magnetic Properties
3. Materials and Methods
3.1. General Procedures
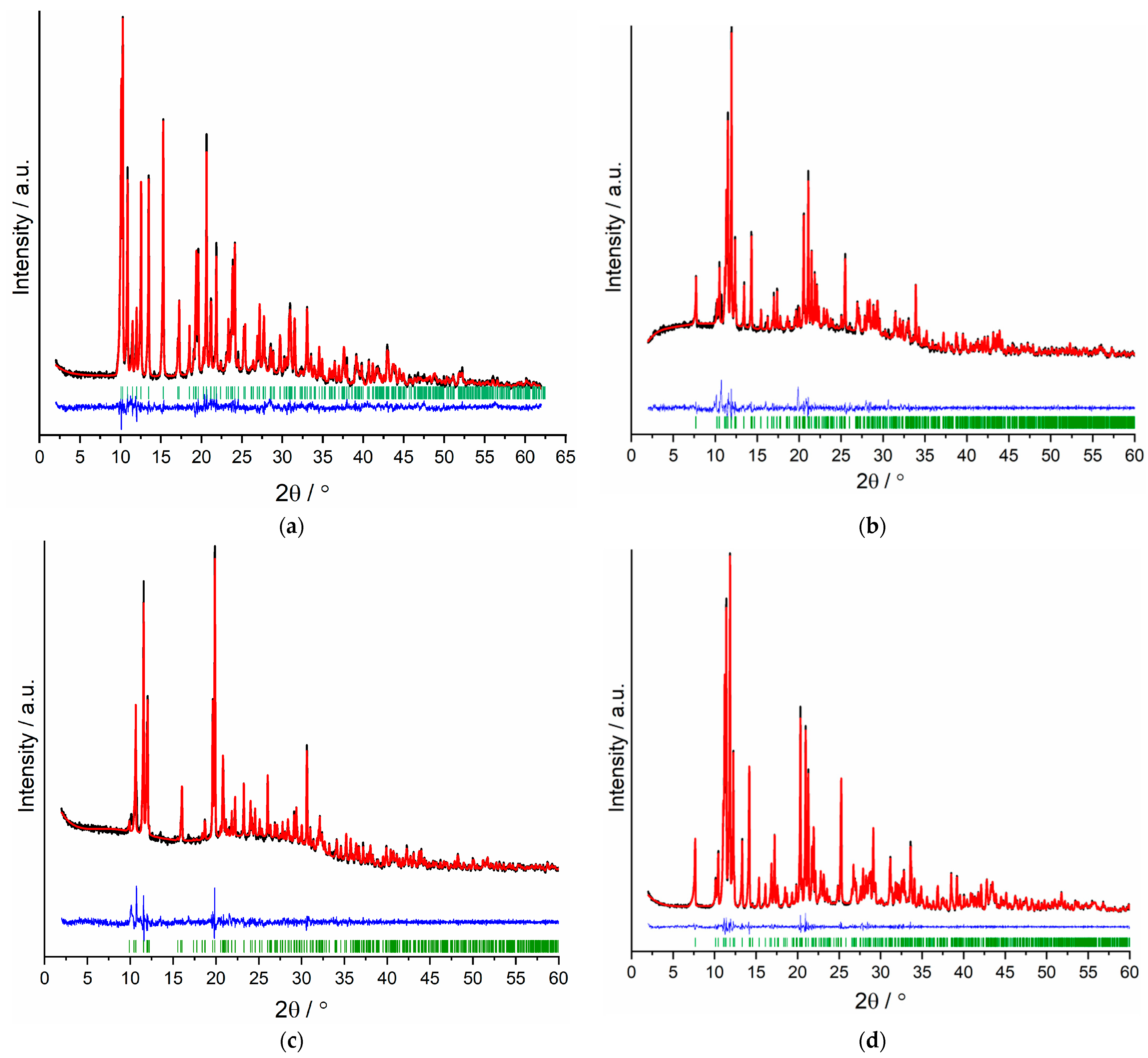
3.2. X-ray Crystallography
3.3. Spectroscopical Investigations
3.3.1. Absorption Spectra
3.3.2. Photoluminescence Spectroscopy
3.4. PPMS Magnetic Measurements
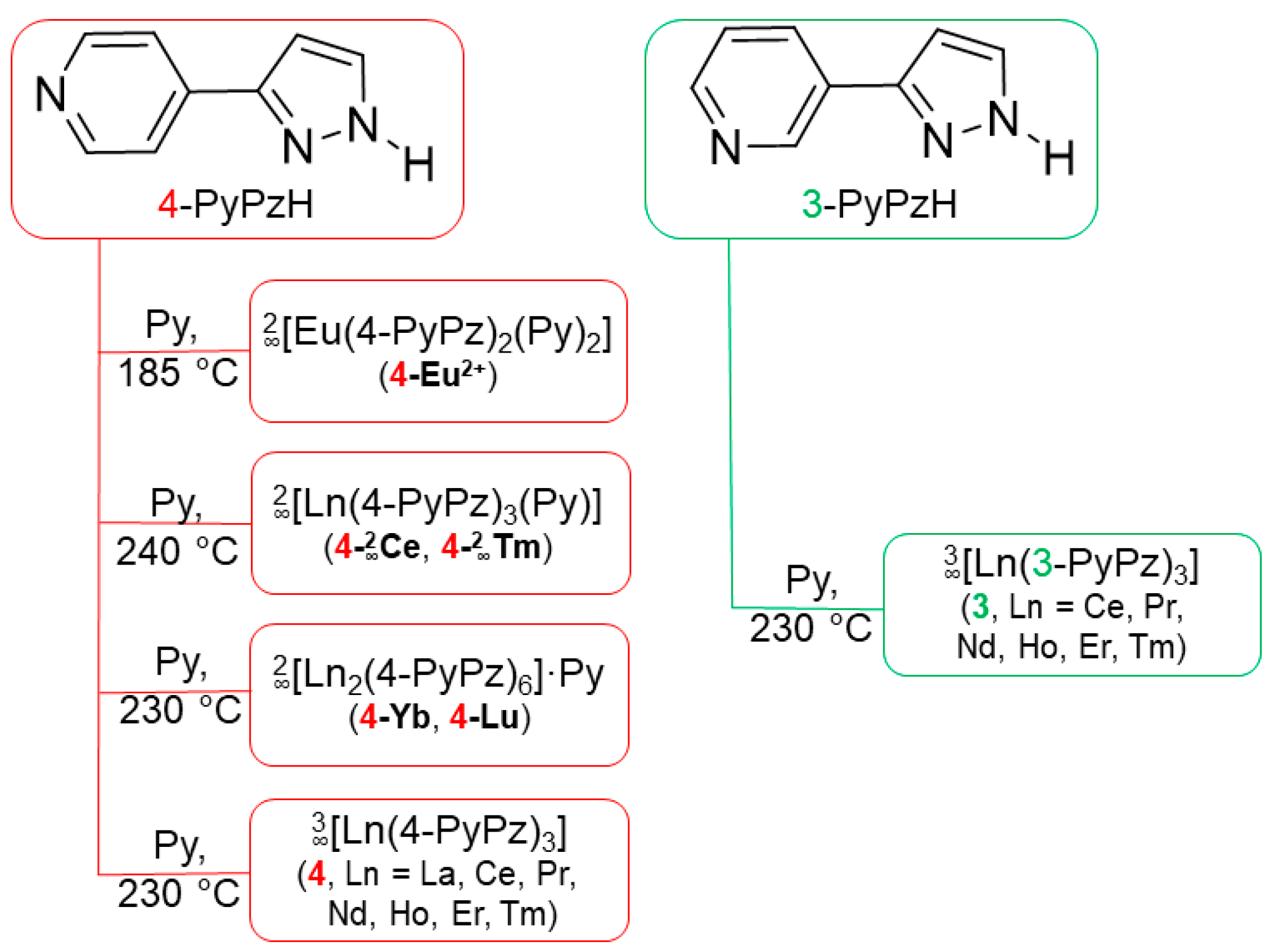
3.5. Synthesis
3.5.1. Synthesis of [Eu(4-PyPz)2(Py)2] (4-Eu2+)
 = 3036 (w), 1065 (s), 1552 (w), 1522 (w), 1456 (w), 1439 (m), 1418 (w), 1404 (w), 1346 (w), 1293 (w), 1213 (m), 1187 (w), 1065 (w), 1044 (m), 999 (s), 963 (w), 951 (w), 925 (w), 871 (w), 830 (s), 761 (s), 690 (s), 659 (w), 614 (m), 532 (m), 451 (m) cm–1.
= 3036 (w), 1065 (s), 1552 (w), 1522 (w), 1456 (w), 1439 (m), 1418 (w), 1404 (w), 1346 (w), 1293 (w), 1213 (m), 1187 (w), 1065 (w), 1044 (m), 999 (s), 963 (w), 951 (w), 925 (w), 871 (w), 830 (s), 761 (s), 690 (s), 659 (w), 614 (m), 532 (m), 451 (m) cm–1.3.5.2. Synthesis of [Ln(4-PyPz)3(Py)] (4-Ce, 4-Tm)
 = 3085 (w), 1698 (w), 1606 (s), 1523 (w), 1459 (w), 1440 (m), 1418 (w), 1347 (w), 1330 (w), 1295 (w), 1212 (s), 1099 (w), 1066 (w), 1047 (m), 1003 (s), 992 (m), 968 (w), 929 (m), 857 (w), 830 (m), 773 (s), 741 (w), 692 (s), 652 (m), 622 (w), 572 (m), 457 (s) cm–1.
= 3085 (w), 1698 (w), 1606 (s), 1523 (w), 1459 (w), 1440 (m), 1418 (w), 1347 (w), 1330 (w), 1295 (w), 1212 (s), 1099 (w), 1066 (w), 1047 (m), 1003 (s), 992 (m), 968 (w), 929 (m), 857 (w), 830 (m), 773 (s), 741 (w), 692 (s), 652 (m), 622 (w), 572 (m), 457 (s) cm–1.3.5.3. Synthesis of [Ln2(4-PyPz)6]ꞏPy (4-Yb, 4-Lu)
3.5.4. Synthesis of [Ln(4-PyPz)3] (4, Ln = La, Ce, Pr, Nd, Ho, Er, Tm)
 = 3096 (w), 1698 (w), 1607 (s), 1550 (w), 1526 (w), 1460 (w), 1447 (m), 1420 (w), 1348 (w), 1329 (w), 1215 (m), 1099 (w), 1066 (w), 1046 (m), 1005 (s), 968 (w), 927 (m), 831 (m), 763 (s), 739 (w), 694 (s), 652 (m), 527 (m), 459 (s) cm–1.
= 3096 (w), 1698 (w), 1607 (s), 1550 (w), 1526 (w), 1460 (w), 1447 (m), 1420 (w), 1348 (w), 1329 (w), 1215 (m), 1099 (w), 1066 (w), 1046 (m), 1005 (s), 968 (w), 927 (m), 831 (m), 763 (s), 739 (w), 694 (s), 652 (m), 527 (m), 459 (s) cm–1. = 3101 (w), 1608 (s), 1526 (w), 1459 (w), 1447 (w), 1420 (w), 1348 (m), 1215 (m), 1066 (w), 1046 (m), 1006 (s), 969 (w), 927 (m), 831 (m), 763 (m), 739 (w), 695 (s), 652 (m), 527 (m), 460 (s) cm–1.
= 3101 (w), 1608 (s), 1526 (w), 1459 (w), 1447 (w), 1420 (w), 1348 (m), 1215 (m), 1066 (w), 1046 (m), 1006 (s), 969 (w), 927 (m), 831 (m), 763 (m), 739 (w), 695 (s), 652 (m), 527 (m), 460 (s) cm–1. = 3095 (w), 1607 (s), 1526 (w), 1460 (w), 1446 (w), 1420 (w), 1348 (w), 1328 (w), 1215 (m), 1099 (w), 1067 (w), 1046 (m), 1006 (s), 969 (w), 927 (m), 831 (m), 762 (s), 739 (w), 694 (s), 652 (m), 527 (m), 460 (s) cm–1.
= 3095 (w), 1607 (s), 1526 (w), 1460 (w), 1446 (w), 1420 (w), 1348 (w), 1328 (w), 1215 (m), 1099 (w), 1067 (w), 1046 (m), 1006 (s), 969 (w), 927 (m), 831 (m), 762 (s), 739 (w), 694 (s), 652 (m), 527 (m), 460 (s) cm–1. = 3093 (w), 1609 (s), 1526 (w), 1459 (w), 1447 (m), 1420 (w), 1348 (w), 1215 (m), 1074 (w), 1047 (m), 1007 (s), 969 (w), 928 (m), 831 (m), 771 (s), 763 (s), 740 (w), 696 (s), 652 (w), 527 (w), 461 (s) cm–1.
= 3093 (w), 1609 (s), 1526 (w), 1459 (w), 1447 (m), 1420 (w), 1348 (w), 1215 (m), 1074 (w), 1047 (m), 1007 (s), 969 (w), 928 (m), 831 (m), 771 (s), 763 (s), 740 (w), 696 (s), 652 (w), 527 (w), 461 (s) cm–1. = 3113 (w), 1697 (w), 1609 (s), 1549 (w), 1526 (w), 1460 (w), 1446 (w), 1419 (w), 1348 (w), 1328 (w), 1297 (w), 1214 (m), 1101 (w), 1075 (w), 1048 (m), 1007 (s), 970 (w), 929 (m), 845 (w), 831 (m), 770 (s), 760 (s), 739 (m), 696 (s), 652 (m), 528 (m), 461 (s) cm–1.
= 3113 (w), 1697 (w), 1609 (s), 1549 (w), 1526 (w), 1460 (w), 1446 (w), 1419 (w), 1348 (w), 1328 (w), 1297 (w), 1214 (m), 1101 (w), 1075 (w), 1048 (m), 1007 (s), 970 (w), 929 (m), 845 (w), 831 (m), 770 (s), 760 (s), 739 (m), 696 (s), 652 (m), 528 (m), 461 (s) cm–1. = 3062 (w), 1696 (w), 1609 (s), 1549 (w), 1526 (w), 1460 (w), 1446 (w), 1419 (w), 1348 (w), 1328 (w), 1214 (m), 1101 (w), 1076 (m), 1048 (m), 1008 (s), 971 (w), 929 (m), 831 (m), 770 (s), 759 (s), 739 (m), 697 (s), 652 (m), 528 (m), 461 (s) cm–1.
= 3062 (w), 1696 (w), 1609 (s), 1549 (w), 1526 (w), 1460 (w), 1446 (w), 1419 (w), 1348 (w), 1328 (w), 1214 (m), 1101 (w), 1076 (m), 1048 (m), 1008 (s), 971 (w), 929 (m), 831 (m), 770 (s), 759 (s), 739 (m), 697 (s), 652 (m), 528 (m), 461 (s) cm–1. = 3117 (w), 1697 (w), 1609 (s), 1550 (w), 1527 (m), 1460 (m), 1447 (m), 1419 (w), 1349 (m), 1329 (w), 1296 (w), 1214 (s), 1102 (w), 1077 (m), 1049 (m), 1008 (s), 971 (w), 930 (m), 845 (w), 830 (s), 770 (s), 759 (s), 740 (m), 696 (s), 652 (m), 528 (m), 461 (s) cm–1.
= 3117 (w), 1697 (w), 1609 (s), 1550 (w), 1527 (m), 1460 (m), 1447 (m), 1419 (w), 1349 (m), 1329 (w), 1296 (w), 1214 (s), 1102 (w), 1077 (m), 1049 (m), 1008 (s), 971 (w), 930 (m), 845 (w), 830 (s), 770 (s), 759 (s), 740 (m), 696 (s), 652 (m), 528 (m), 461 (s) cm–1.3.5.5. Synthesis of [Ln(3-PyPz)3] (3, Ln = Ce, Pr, Nd, Ho, Er)
 = 3085 (w), 1596 (w), 1576 (m), 1509 (w), 1464 (m), 1453 (m), 1407 (m), 1359 (w), 1346 (w), 1250 (w), 1206 (m), 1186 (m), 1122 (w), 1099 (w), 1072 (m), 1039 (s), 963 (m), 928 (m), 859 (w), 818 (m), 779 (s), 716 (w), 702 (s), 656 (w), 635 (s), 510 (w), 461 (s) cm–1.
= 3085 (w), 1596 (w), 1576 (m), 1509 (w), 1464 (m), 1453 (m), 1407 (m), 1359 (w), 1346 (w), 1250 (w), 1206 (m), 1186 (m), 1122 (w), 1099 (w), 1072 (m), 1039 (s), 963 (m), 928 (m), 859 (w), 818 (m), 779 (s), 716 (w), 702 (s), 656 (w), 635 (s), 510 (w), 461 (s) cm–1. = 2897 (w), 1683 (m), 1596 (w), 1576 (w), 1508 (w), 1453 (m), 1407 (m), 1359 (w), 1345 (w), 1260 (w), 1249 (w), 1207 (m), 1186 (m), 1099 (w), 1074 (m), 1041 (s), 963 (m), 928 (m), 817 (w), 781 (s), 702 (s), 657 (w), 636 (s), 515 (w), 462 (s) cm–1.
= 2897 (w), 1683 (m), 1596 (w), 1576 (w), 1508 (w), 1453 (m), 1407 (m), 1359 (w), 1345 (w), 1260 (w), 1249 (w), 1207 (m), 1186 (m), 1099 (w), 1074 (m), 1041 (s), 963 (m), 928 (m), 817 (w), 781 (s), 702 (s), 657 (w), 636 (s), 515 (w), 462 (s) cm–1. = 3085 (w), 1576 (m), 1509 (w), 1465 (m), 1452 (m), 1408 (m), 1360 (m), 1347 (m), 1330 (w), 1249 (w), 1207 (m), 1186 (s), 1122 (w), 1099 (w), 1072 (m), 1041 (s), 963 (m), 928 (s), 818 (m), 779 (s), 717 (w), 702 (s), 656 (m), 636 (s), 509 (w), 463 (s) cm–1.
= 3085 (w), 1576 (m), 1509 (w), 1465 (m), 1452 (m), 1408 (m), 1360 (m), 1347 (m), 1330 (w), 1249 (w), 1207 (m), 1186 (s), 1122 (w), 1099 (w), 1072 (m), 1041 (s), 963 (m), 928 (s), 818 (m), 779 (s), 717 (w), 702 (s), 656 (m), 636 (s), 509 (w), 463 (s) cm–1. = 3086 (w), 1597 (w), 1577 (m), 1508 (w), 1465 (m), 1452 (m), 1408 (m), 1348 (m), 1248 (w), 1210 (m), 1187 (s), 1100 (w), 1075 (m), 1044 (s), 964 (m), 931 (m), 818 (m), 778 (s), 700 (s), 657 (m), 637 (s), 467 (s) cm–1.
= 3086 (w), 1597 (w), 1577 (m), 1508 (w), 1465 (m), 1452 (m), 1408 (m), 1348 (m), 1248 (w), 1210 (m), 1187 (s), 1100 (w), 1075 (m), 1044 (s), 964 (m), 931 (m), 818 (m), 778 (s), 700 (s), 657 (m), 637 (s), 467 (s) cm–1. = 3086 (w), 1577 (w), 1508 (w), 1465 (w), 1453 (w), 1408 (w), 1348 (w), 1248 (w), 1210 (w), 1187 (m), 1100 (w), 1077 (m), 1045 (s), 964 (m), 931 (m), 818 (w), 780 (s), 700 (m), 657 (w), 637 (m), 513 (w), 468 (s) cm–1.
= 3086 (w), 1577 (w), 1508 (w), 1465 (w), 1453 (w), 1408 (w), 1348 (w), 1248 (w), 1210 (w), 1187 (m), 1100 (w), 1077 (m), 1045 (s), 964 (m), 931 (m), 818 (w), 780 (s), 700 (m), 657 (w), 637 (m), 513 (w), 468 (s) cm–1.3.5.6. Single Crystal of [Tm(3-PyPz)3] (3-Tm):
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Millward, J.M.; Ariza de Schellenberger, A.; Berndt, D.; Hanke-Vela, L.; Schellenberger, E.; Waiczies, S.; Taupitz, M.; Kobayashi, Y.; Wagner, S.; Infante-Duarte, C. Application of europium-doped very small iron oxide nanoparticles to visualize neuroinflammation with MRI and fluorescence microscopy. Neuroscience 2017, 403, 136–144. [Google Scholar] [CrossRef]
- Lenora, C.U.; Carniato, F.; Shen, Y.; Latif, Z.; Haacke, E.M.; Martin, P.D.; Botta, M.; Allen, M.J. Structural features of europium(II)-containing cryptates that influence relaxivity. Chem. Eur. J. 2017, 23, 15404–15414. [Google Scholar] [CrossRef]
- Acharjya, A.; Corbin, B.A.; Prasad, E.; Allen, M.J.; Maity, S. Solvation-controlled emission of divalent europium salts. J. Photochem. Photobiol. A 2022, 429, 113892–113899. [Google Scholar] [CrossRef]
- Dorenbos, P. Anomalous luminescence of Eu2+ and Yb2+ in inorganic compounds. J. Phys. Condens. Matter 2003, 15, 2645–2665. [Google Scholar] [CrossRef]
- Qin, X.; Liu, X.; Huang, W.; Bettinelli, M.; Liu, X. Lanthanide-activated phosphors based on 4f-5d optical transitions: Theoretical and experimental aspects. Chem. Rev. 2017, 117, 4488–4527. [Google Scholar] [CrossRef] [PubMed]
- Zurawski, A.; Mai, M.; Baumann, D.; Feldmann, C.; Müller-Buschbaum, K. Homoleptic imidazolate frameworks [Sr1−xEux(Im)2]―hybrid materials with efficient and tuneable luminescence. Chem. Commun. 2011, 47, 496–498. [Google Scholar] [CrossRef]
- Kajdas, C.; Furey, M.J.; Ritter, A.L.; Molina, G.J. Triboemission as a basic part of the boundary friction regime. Lubr. Sci. 2002, 14, 223–254. [Google Scholar] [CrossRef]
- Galimov, D.I.; Yakupova, S.M.; Vasilyuk, K.S.; Bulgakov, R.G. A novel gas assay for ultra-small amounts of molecular oxygen based on the chemiluminescence of divalent europium. J. Photochem. Photobiol. A 2021, 418, 113430–113437. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Bünzli, J.C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef]
- Carnall, W.T.; Fields, P.R.; Rajnak, K. Electronic energy levels in the trivalent lanthanide aquo Ions. I. Pr3+, Nd3+, Pm3+, Sm3+, Dy3+, Ho3+, Er3+, and Tm3+. J. Chem. Phys. 1968, 49, 4424–4442. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, H.-J.; Song, S.-Y.; Li, Z.-F.; Sun, L.-N.; Xing, Y.; Guo, X.-M. Syntheses, crystal structures, visible and near-IR luminescent properties of ternary lanthanide (Dy3+, Tm3+) complexes containing 4,4,4-trifluoro-1-phenyl-1,3-butanedione and 1,10-phenanthroline. J. Lumin. 2008, 128, 1957–1964. [Google Scholar] [CrossRef]
- Serra, O.A.; Nassar, E.J.; Calefi, P.S.; Rosa, I.L.V. Luminescence of a new Tm3+ β-diketonate compound. J. Alloys Compd. 1998, 275–277, 838–840. [Google Scholar] [CrossRef]
- Youssef, H.; Schäfer, T.; Becker, J.; Sedykh, A.E.; Basso, L.; Pietzonka, C.; Taydakov, I.V.; Kraus, F.; Müller-Buschbaum, K. 3D-Frameworks and 2D-networks of lanthanide coordination polymers with 3-pyridylpyrazole: Photophysical and magnetic properties. Dalton Trans. 2022, 51, 14673–14685. [Google Scholar] [CrossRef]
- Ridenour, J.A.; Carter, K.P.; Butcher, R.J.; Cahill, C.L. RE-p-halobenzoic acid–terpyridine complexes, Part II: Structural diversity, supramolecular assembly, and luminescence properties in a series of p-bromobenzoic acid rare-earth hybrid materials. CrystEngComm 2017, 19, 1172–1189. [Google Scholar] [CrossRef]
- Batrice, R.J.; Adcock, A.K.; Cantos, P.M.; Bertke, J.A.; Knope, K.E. Synthesis and characterization of an isomorphous lanthanide-thiophenemonocarboxylate series (Ln = La–Lu, except Pm) amenable to color tuning. Cryst. Growth Des. 2017, 17, 4603–4612. [Google Scholar] [CrossRef]
- Kawamura, Y.; Wada, Y.; Hasegawa, Y.; Iwamuro, M.; Kitamura, T.; Yanagida, S. Observation of neodymium electroluminescence. Appl. Phys. Lett. 1999, 74, 3245–3247. [Google Scholar] [CrossRef]
- Curry, R.; Gillin, W.P. 1.54 μm electroluminescence from erbium (III) tris(8-hydroxyquinoline) (ErQ)-based organic light-emitting diodes. Appl. Phys. Lett. 1999, 75, 1380–1382. [Google Scholar] [CrossRef]
- Mehrdel, B.; Nikbakht, A.; Aziz, A.A.; Jameel, M.S.; Dheyab, M.A.; Khaniabadi, P.M. Upconversion lanthanide nanomaterials: Basics introduction, synthesis approaches, mechanism and application in photodetector and photovoltaic devices. Nanotechnology 2021, 33, 082001. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Ivaturi, A.; Fröhlich, B.; Rüdiger, M.; Richter, A.; Krämer, K.W.; Richards, B.S.; Goldschmidt, J.C. Upconverter silicon solar cell devices for efficient utilization of sub-band-gap photons under concentrated solar radiation. IEEE J. Photovolt. 2013, 4, 183–189. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Eliseeva, S.V. Basics of lanthanide photophysics. In Springer Series on Fluorescence: Lanthanide Luminescence: Photophysical, Analytical and Biological Aspects; Wolfbeis, O.S., Hof, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 7, pp. 1–46. [Google Scholar]
- Davies, G.M.; Aarons, R.J.; Motson, G.R.; Jeffery, J.C.; Adams, H.; Faulkner, S.; Ward, M.D. Structural and near-IR photophysical studies on ternary lanthanide complexes containing poly (pyrazolyl) borate and 1,3-diketonate ligands. Dalton Trans. 2004, 4, 1136–1144. [Google Scholar] [CrossRef]
- Bao, S.; Liang, Y.; Wang, L.; Wang, L.; Xu, L.; Wang, Y.; Liang, X.; Xiang, W. Superhigh-luminance Ce:YAG phosphor in glass and phosphor-in-glass film for laser lighting. ACS Sustain. Chem. Eng. 2022, 10, 8105–8114. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Zhao, Z.; Sun, B.; Zhan, G.; Liu, H.; Bian, Z.; Liu, Z. Highly efficient and air-stable Eu(II)-containing azacryptates ready for organic light-emitting diodes. Nat. Commun. 2020, 11, 5218–5225. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.V.; Schönfeld, F.; Zurawski, A.; Mai, M.; Feldmann, C.; Müller-Buschbaum, K. A blue luminescent MOF as a rapid turn-off/turn-on detector for H2O, O2 and CH2Cl2, MeCN: [Ce(Im)3ImH]·ImH. Dalton Trans. 2015, 44, 4070–4079. [Google Scholar] [CrossRef]
- Matthes, P.R.; Müller-Buschbaum, K. Synthesis and characterization of the cerium(III) UV-emitting 2D-coordination polymer [Ce2Cl6(4,4′-bipyridine)4]·py. Z. Anorg. Allg. Chem. 2014, 640, 2847–2851. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, L.; Zhan, G.; Liu, Z.; Bian, Z.; Huang, C. Efficient rare earth cerium(III) complex with nanosecond d−f emission for blue organic light-emitting diodes. Natl. Sci. Rev. 2021, 8, nwaa193. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.T.; Horrocks Jr., W.D. Complexation, luminescence, and energy transfer of Ce3+ with a series of multidentate amino phosphonic acids in aqueous solution. Inorg. Chem. 1991, 30, 1073–1079. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, W.; Zhang, J.; Zhu, G.; Cong, R. Formation, photoluminescence and ferromagnetic characterization of Ce doped AlN hierarchical nanostructures. J. Alloys Compd. 2019, 775, 498–502. [Google Scholar] [CrossRef]
- Vadan, M.; Popovici, E.J.; Ungur, L.; Vasilescu, M.; Macarovici, D. Synthesis of luminescent strontium-magnesium orthophosphate activated with cerium and manganese. In Proceedings of the SIOEL’99: Sixth Symposium on Optoelectronics, Bucharest, Romania, 22–24 September 1999; pp. 111–116. [Google Scholar]
- Youssef, H.; Sedykh, A.E.; Becker, J.; Taydakov, I.V.; Müller-Buschbaum, K. 3-(2-pyridyl)pyrazole based luminescent 1D-coordination polymers and polymorphic complexes of various lanthanide chlorides including orange-emitting cerium(III). Inorganics 2022, 10, 254. [Google Scholar] [CrossRef]
- Youssef, H.; Becker, J.; Sedykh, A.E.; Schäfer, T.; Taydakov, I.V.; Müller-Buschbaum, K. Red emitting cerium(III) and versatile luminescence chromaticity of 1D-coordination polymers and heterobimetallic Ln/AE pyridylpyrazolate complexes. Z. Anorg. Allg. Chem. 2022, 648, e202200295. [Google Scholar] [CrossRef]
- Youssef, H.; Sedykh, A.E.; Becker, J.; Schäfer, T.; Taydakov, I.V.; Li, H.R.; Müller-Buschbaum, K. Variable luminescence and chromaticity of homoleptic frameworks of the lanthanides together with pyridylpyrazolates. Chem. Eur. J. 2021, 27, 16634–16641. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Guo, Z.; Blair, V.L.; Deacon, G.B.; Junk, P.C. Europium is different: Solvent and ligand effects on oxidation state outcomes and C-F activation in reactions between europium metal and pentafluorophenylsilver. Chem. Eur. J. 2022, 28, e202103865. [Google Scholar] [CrossRef] [PubMed]
- Drew, M.G.B.; Guillaneux, D.; Hudson, M.J.; Iveson, P.B.; Russell, M.L.; Madic, C. Lanthanide(III) complexes of a highly efficient actinide(III) extracting agent—2,6-bis(5,6-dipropyl-1,2,4-triazin-3-yl)pyridine. Inorg. Chem. Commun. 2001, 4, 12–15. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Peskov, M.A.; Ramsden, S.J.; Yaghi, O.M. The reticular chemistry structure resource (RCSR) database of, and symbols for, crystal nets. Acc. Chem. Res. 2008, 41, 1782–1789. [Google Scholar] [CrossRef]
- Wells, A.F. Three-Dimensional Nets and Polyhedra; Wiley-Interscience: New York, NY, USA, 1977. [Google Scholar]
- Guo, Z.; Luu, J.; Blair, V.; Deacon, G.B.; Junk, P.C. Replacing mercury: Syntheses of lanthanoid pyrazolates from free lanthanoid metals, pentafluorophenylsilver, and pyrazoles, aided by a facile synthesis of polyfluoroarylsilver compounds. Eur. J. Inorg. Chem. 2019, 2019, 1018–1029. [Google Scholar] [CrossRef]
- Jiménez, J.R.; Díaz-Ortega, I.F.; Ruiz, E.; Aravena, D.; Pope, S.J.A.; Colacio, E.; Herrera, J.M. Lanthanide tetrazolate complexes combining single-molecule magnet and luminescence properties: The effect of the replacement of tetrazolate N3 by β-diketonate ligands on the anisotropy energy barrier. Chem. Eur. J. 2016, 22, 14548–14559. [Google Scholar] [CrossRef]
- Hughes, I.D.; Däne, M.; Ernst, A.; Hergert, W.; Lüders, M.; Poulter, J.; Staunton, J.B.; Svane, A.; Szotek, Z.; Temmerman, W.M. Lanthanide contraction and magnetism in the heavy rare earth elements. Nature 2007, 446, 650–653. [Google Scholar] [CrossRef]
- Carnall, W.T.; Fields, P.R.; Rajnak, K. Electronic energy levels of the trivalent lanthanide aquo ions. IV. Eu3+. J. Chem. Phys. 1968, 49, 4450–4455. [Google Scholar] [CrossRef]
- Carnall, W.T.; Fields, P.R.; Rajnak, K. Electronic energy levels of the trivalent lanthanide aquo ions. III. Tb3+. J. Chem. Phys. 1968, 49, 4447–4449. [Google Scholar] [CrossRef]
- Seidel, C.; Lorbeer, C.; Cybińska, J.; Mudring, A.-V.; Ruschewitz, U. Lanthanide coordination polymers with tetrafluoroterephthalate as a bridging ligand: Thermal and optical properties. Inorg. Chem. 2012, 51, 4679–4688. [Google Scholar] [CrossRef]
- Huskowska, E.; Turowska-Tyrk, I.; Legendziewicz, J.; Riehl, J.P. The structure and spectroscopy of lanthanide(III) complexes with 2,2′-bipyridine-1,1′-dioxide in solution and in the solid state: Effects of ionic size and solvent on photophysics, ligand structure and coordination. New J. Chem. 2002, 26, 1461–1467. [Google Scholar] [CrossRef]
- Fernández-Fernández, M.d.C.; Bastida, R.; Macías, A.; Pérez-Lourido, P.; Platas-Iglesias, C.; Valencia, L. Lanthanide(III) complexes with a tetrapyridine pendant-armed macrocyclic ligand: 1H NMR structural determination in solution, X-ray diffraction, and density-functional theory calculations. Inorg. Chem. 2006, 45, 4484–4496. [Google Scholar] [CrossRef] [PubMed]
- Bochkarev, M.N.; Khoroshenkov, G.V.; Schumann, H.; Dechert, S. A novel bis(imino)amine ligand as a result of acetonitrile coupling with the diiodides of Dy(II) and Tm(II). J. Am. Chem. Soc. 2003, 125, 2894–2895. [Google Scholar] [CrossRef]
- Sedykh, A.E.; Kurth, D.G.; Müller-Buschbaum, K. Two series of lanthanide coordination polymers and complexes with 4′-phenylterpyridine and their luminescence properties. Eur. J. Inorg. Chem. 2019, 2019, 4564–4571. [Google Scholar] [CrossRef]
- Matthes, P.R.; Eyley, J.; Klein, J.H.; Kuzmanoski, A.; Lambert, C.; Feldmann, C.; Müller-Buschbaum, K. Photoluminescent one-dimensional coordination polymers from suitable pyridine antenna and LnCl3 for visible and near-IR emission. Eur. J. Inorg. Chem. 2015, 2015, 826–836. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Comby, S.; Chauvin, A.-S.; Vandevyver, C.D.B. New Opportunities for Lanthanide Luminescence. J. Rare Earths 2007, 25, 257–274. [Google Scholar] [CrossRef]
- Dexter, D.L. A theory of sensitized luminescence in solids. J. Chem. Phys. 1953, 21, 836–850. [Google Scholar] [CrossRef]
- Dang, S.; Yu, J.; Wang, X.; Sun, L.; Deng, R.; Feng, J.; Fan, W.; Zhang, H. NIR-luminescence from ternary lanthanide [HoIII, PrIII and TmIII] complexes with 1-(2-naphthyl)-4,4,4-trifluoro-1,3-butanedionate. J. Lumin. 2011, 131, 1857–1863. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; Wiley-VCH: Weinheim, Germany, 1993. [Google Scholar]
- Pham, Y.H.; Trush, V.A.; Carneiro Neto, A.N.; Korabik, M.; Sokolnicki, J.; Weselski, M.; Malta, O.L.; Amirkhanov, V.M.; Gawryszewska, P. Lanthanide complexes with N-phosphorylated carboxamide as UV converters with excellent emission quantum yield and single-ion magnet behavior. J. Mater. Chem. C 2020, 8, 9993–10009. [Google Scholar] [CrossRef]
- Benelli, C.; Gatteschi, D. Magnetism of lanthanides in molecular materials with transition-metal ions and organic radicals. Chem. Rev. 2002, 102, 2369–2388. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, M.R.; Mustazza, C.; Borioni, A.; Gatta, F.; Tayebati, K.; Amenta, F.; Tucci, P.; Pieretti, S. Synthesis of 1-methyl-5-(pyrazol-3-and-5-yl- and 1,2,4-triazol-3- and 5-yl)-1,2,3,6-tetrahydropyridine derivatives and their evaluation as muscarinic receptor ligands. Arch. Pharm. Pharm. Med. Chem. 2003, 336, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Bauer, V.J.; Dalalian, H.P.; Fanshawe, W.J.; Safir, S.R.; Tocus, E.C.; Boshart, C.R. 4-[3(5)-Pyrazolyl]pyridinium salts. A new class of hypoglycemic agents. J. Med. Chem. 1968, 11, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Spek, A.L. What makes a crystal structure report valid? Inorg. Chim. Acta 2018, 470, 232–237. [Google Scholar] [CrossRef]
- Spek, A.L. CheckCIF validation ALERTS: What they mean and how to respond. Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 1–11. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package topospro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Pennington, W.T. DIAMOND—Visual crystal structure information system. J. Appl. Crystallogr. 1999, 32, 1028–1029. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
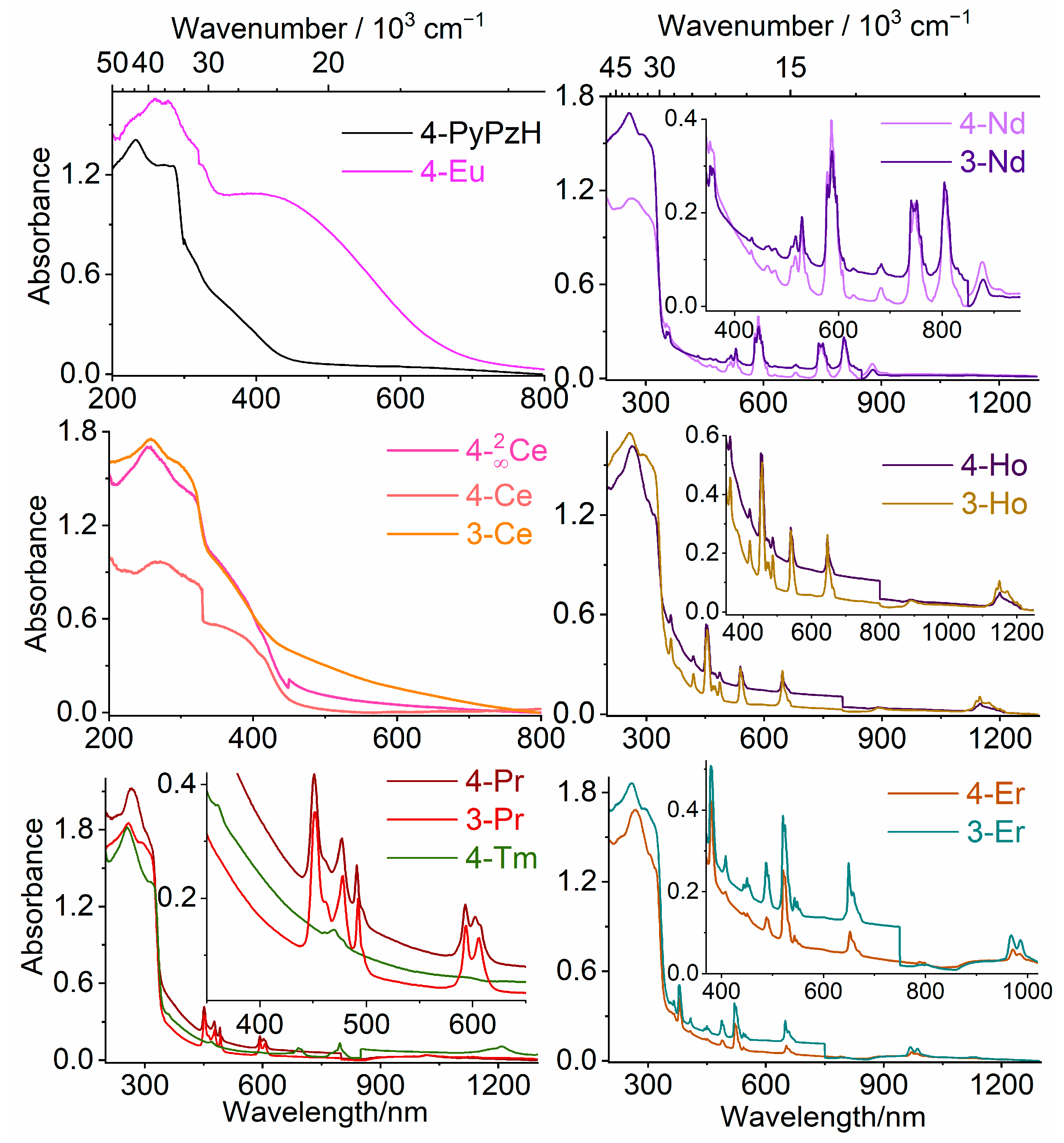
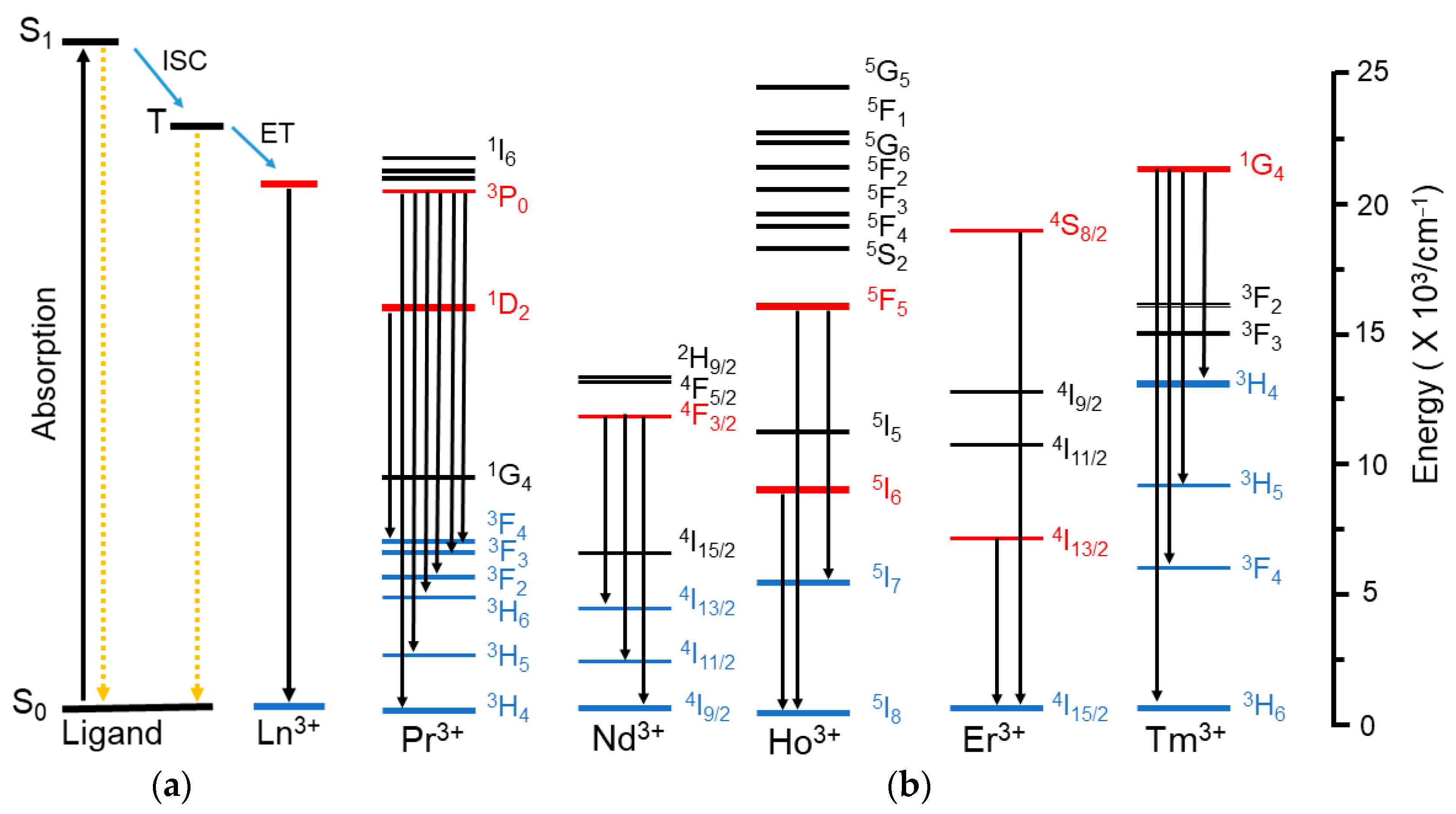

| ID | Intra–4f Absorption Transitions | λmax (nm) | |
|---|---|---|---|
| Ground State | Excited States | ||
| 4-Pr | 3H4→ | 3P2, 3P1, 3P0, 1D2 | 451, 477, 491, 593 nm |
| 3-Pr | 3H4→ | 3P2, 3P1, 3P0, 1D2 | 451, 477, 492, 593 nm |
| 4-Nd | 4I9/2→ | 4D3/2, 2P1/2, 2K15/2, 2K13/2, 4G5/2, 4F9/2, 4F7/2, 4F5/2, 4F3/2 | 353, 432, 478, 529, 586, 681, 746, 803, 878 nm |
| 3-Nd | 4I9/2→ | 2I11/2, 2P1/2, 2K15/2, 2K13/2, 4G5/2, 4F9/2, 4F7/2, 4F5/2, 4F3/2 | 353, 433, 479, 529, 588, 682, 740, 804, 878 nm |
| 4-Ho | 5I8→ | (5G, 3H)5, (5G, 3G)5, 5G6, 5F2, 5F3, 5F4, 5F5, 5I5, 5I6 | 362, 419, 452, 474, 486, 539, 646, 892, 1150 nm |
| 3-Ho | 5I8→ | (5G, 3H)5, (5G, 3G)5, 5G6, 5F2, 5F3, 5F4, 5F5, 5I5, 5I6 | 362, 420, 455, 475, 486, 539, 646, 891, 1149 nm |
| 4-Er | 4I15/2→ | 4G11/2, (2G, 4F)9/2, 4F5/2, 4F7/2, 2H11/2, 4S8/2, 4F9/2, 4I11/2 | 379, 408, 451, 488, 521, 543, 652, 971 nm |
| 3-Er | 4I15/2→ | 4G11/2, (2G, 4F)9/2, 4F5/2, 4F7/2, 2H11/2, 4S8/2, 4F9/2, 4I11/2 | 379, 408, 450, 487, 520, 543, 649, 968 nm |
| 4-Tm | 3H6→ | 1D2, 1G4, 3F3, 3H4, 3H5 | 360, 470, 691, 796, 1209 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, H.; Becker, J.; Pietzonka, C.; Taydakov, I.V.; Kraus, F.; Müller-Buschbaum, K. Divalent Europium, NIR and Variable Emission of Trivalent Tm, Ho, Pr, Er, Nd, and Ce in 3D Frameworks and 2D Networks of Ln–Pyridylpyrazolates. Chemistry 2023, 5, 1006-1027. https://doi.org/10.3390/chemistry5020069
Youssef H, Becker J, Pietzonka C, Taydakov IV, Kraus F, Müller-Buschbaum K. Divalent Europium, NIR and Variable Emission of Trivalent Tm, Ho, Pr, Er, Nd, and Ce in 3D Frameworks and 2D Networks of Ln–Pyridylpyrazolates. Chemistry. 2023; 5(2):1006-1027. https://doi.org/10.3390/chemistry5020069
Chicago/Turabian StyleYoussef, Heba, Jonathan Becker, Clemens Pietzonka, Ilya V. Taydakov, Florian Kraus, and Klaus Müller-Buschbaum. 2023. "Divalent Europium, NIR and Variable Emission of Trivalent Tm, Ho, Pr, Er, Nd, and Ce in 3D Frameworks and 2D Networks of Ln–Pyridylpyrazolates" Chemistry 5, no. 2: 1006-1027. https://doi.org/10.3390/chemistry5020069
APA StyleYoussef, H., Becker, J., Pietzonka, C., Taydakov, I. V., Kraus, F., & Müller-Buschbaum, K. (2023). Divalent Europium, NIR and Variable Emission of Trivalent Tm, Ho, Pr, Er, Nd, and Ce in 3D Frameworks and 2D Networks of Ln–Pyridylpyrazolates. Chemistry, 5(2), 1006-1027. https://doi.org/10.3390/chemistry5020069