Combining Two Types of Boron in One Molecule (To the 60th Anniversary of the First Synthesis of Carborane)
Abstract
1. Introduction
2. Early Work on Carborane Derivatives with exo-Polyhedral Carbon-Boron Bonds
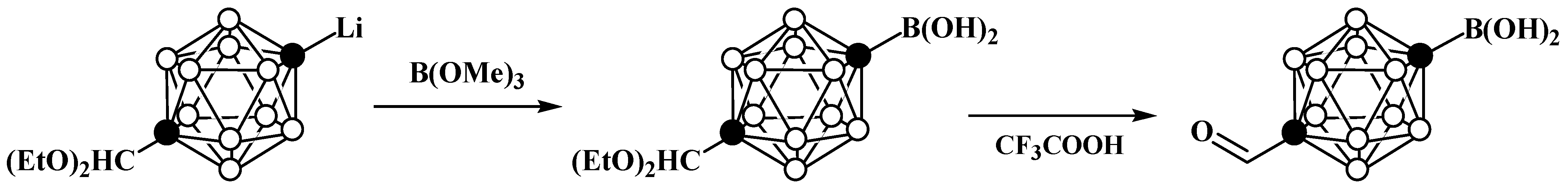
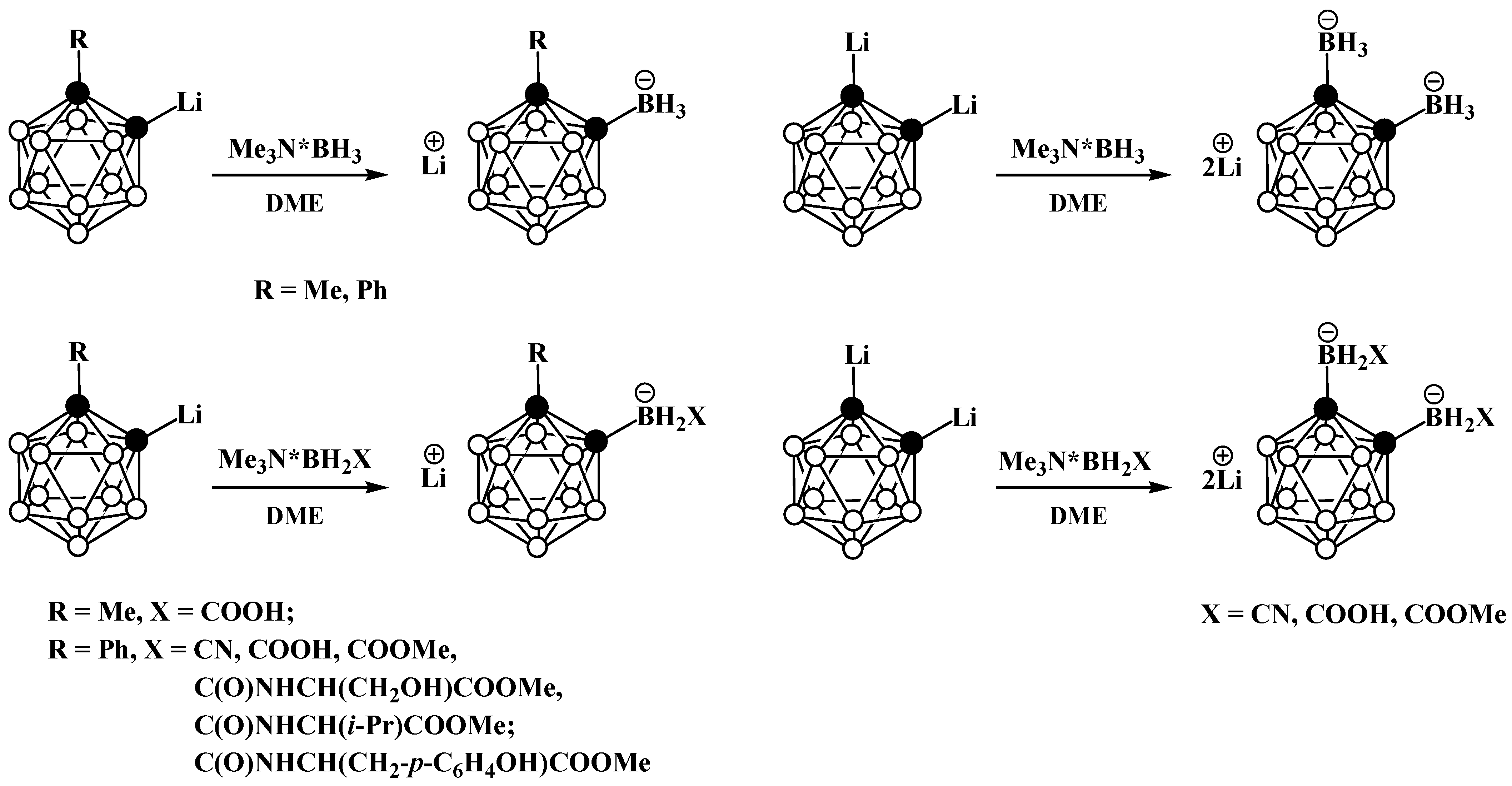
3. Anionic Carboranes with BX3 Substituents and Derivatives Thereof
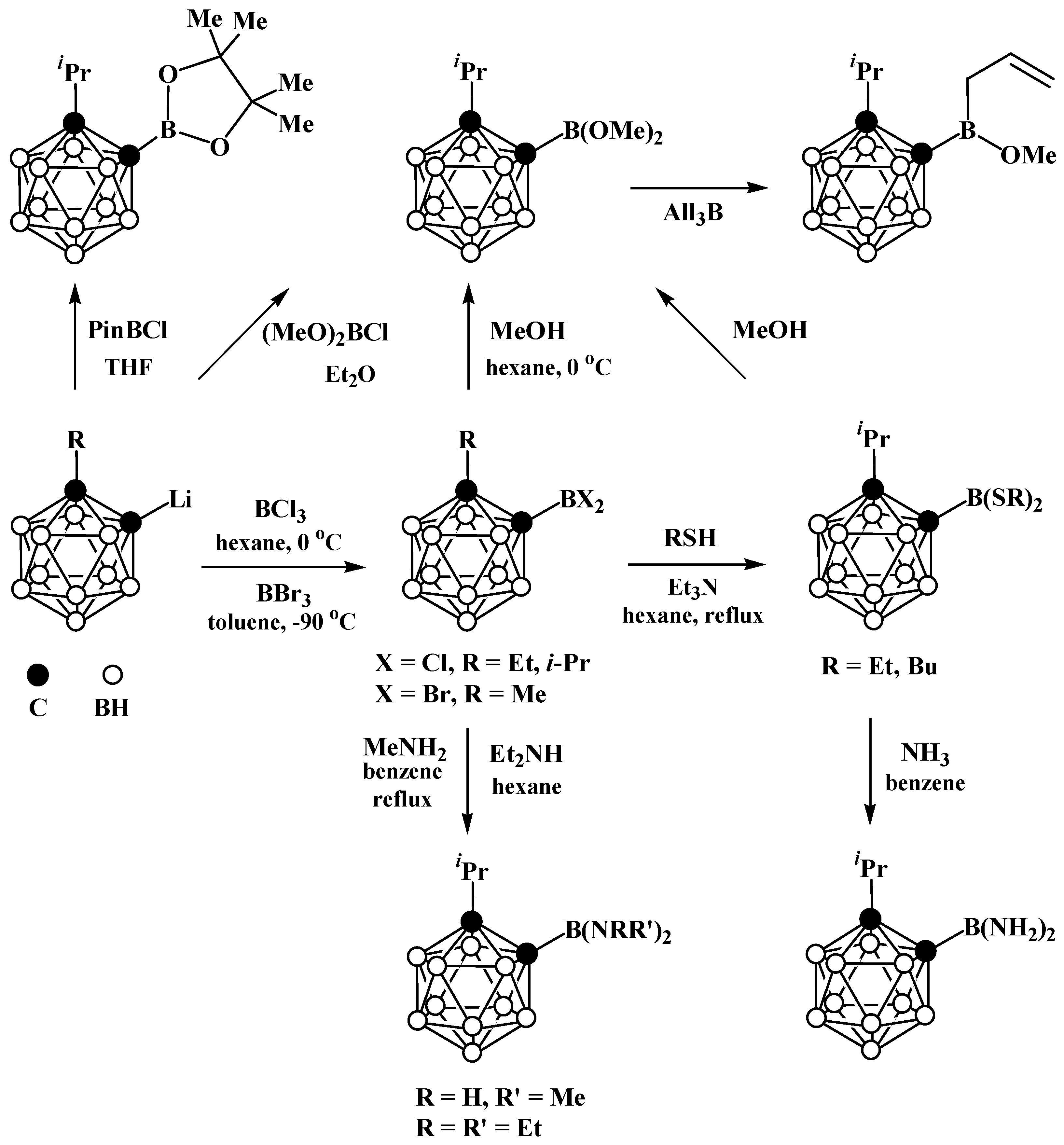
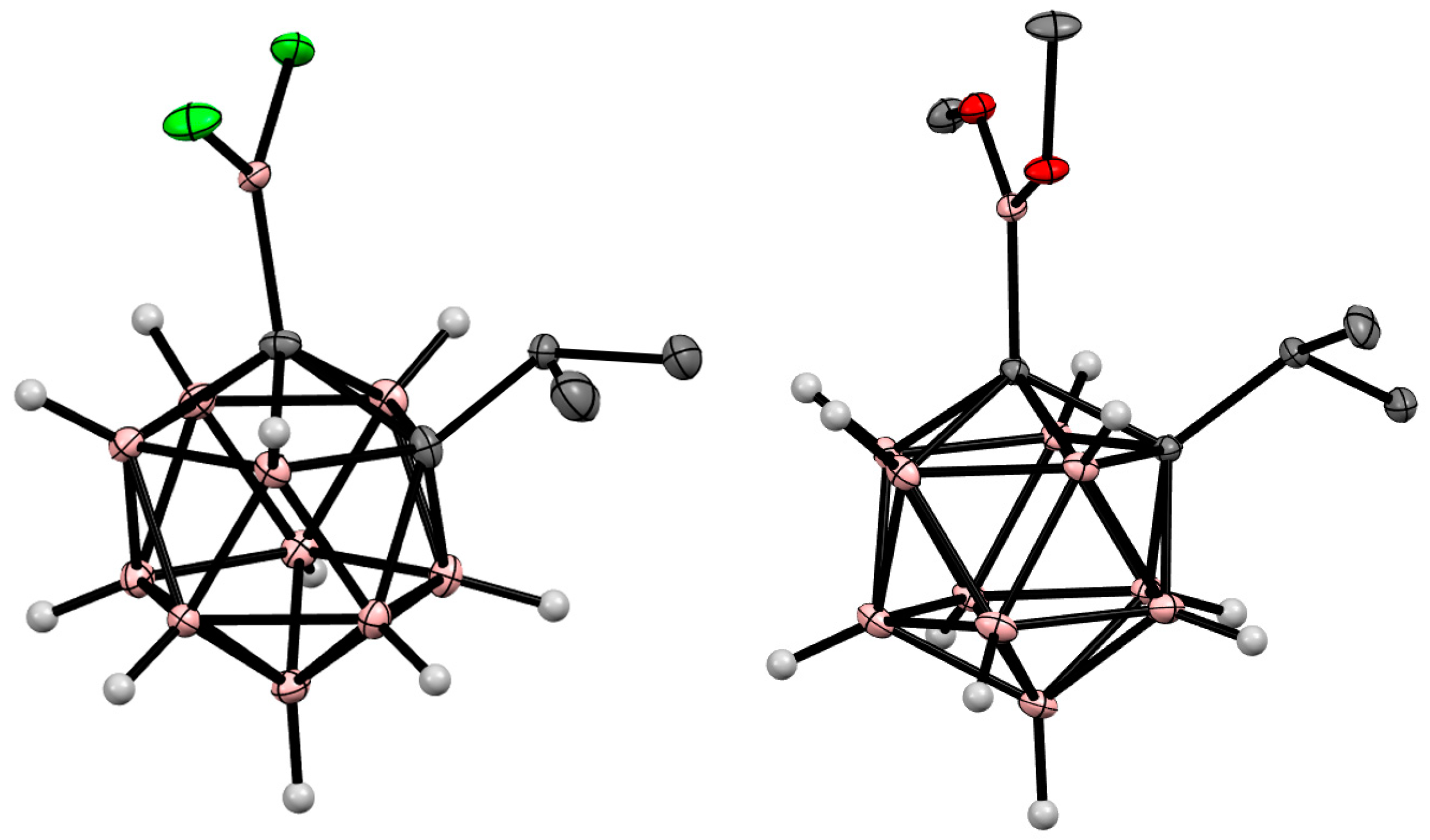
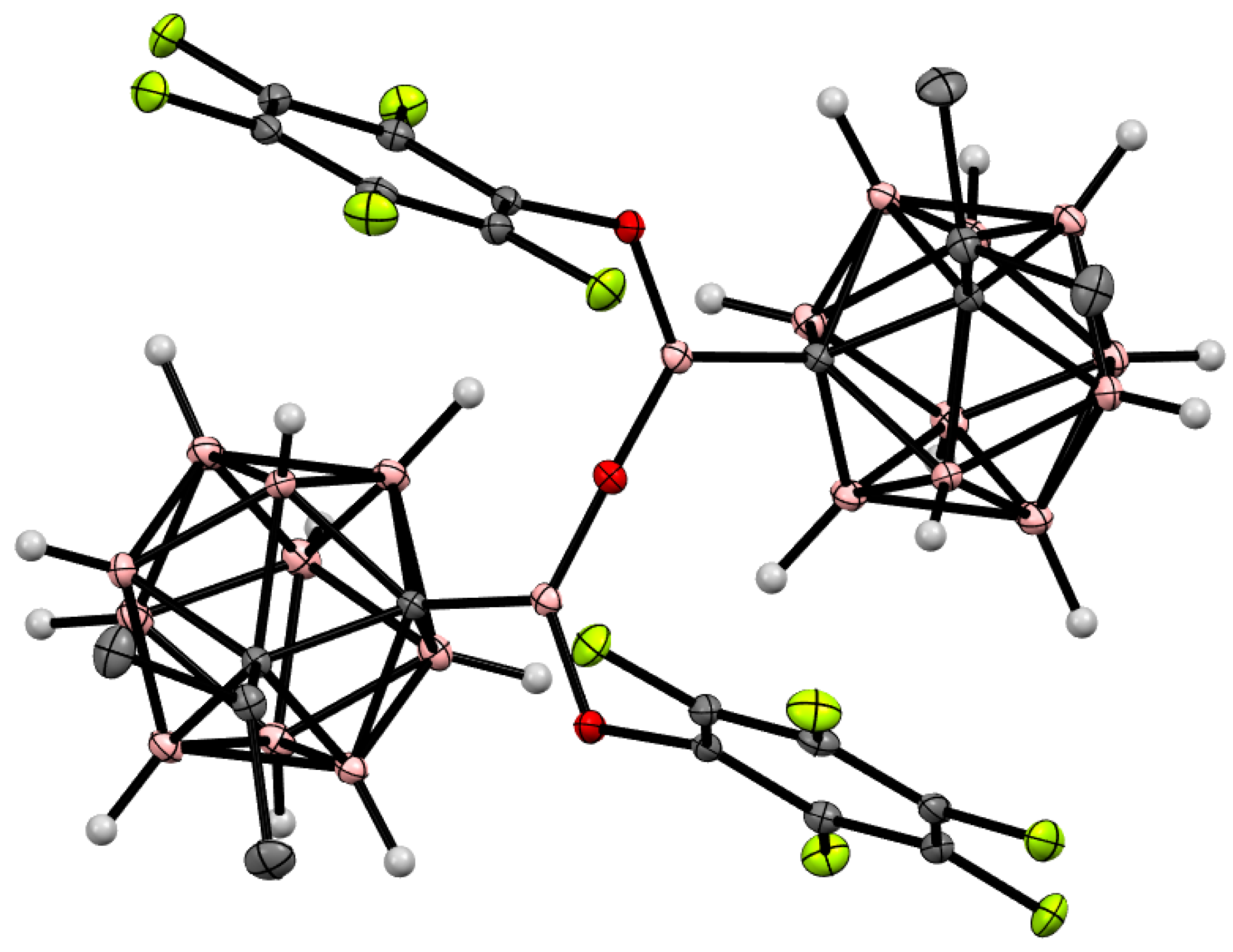
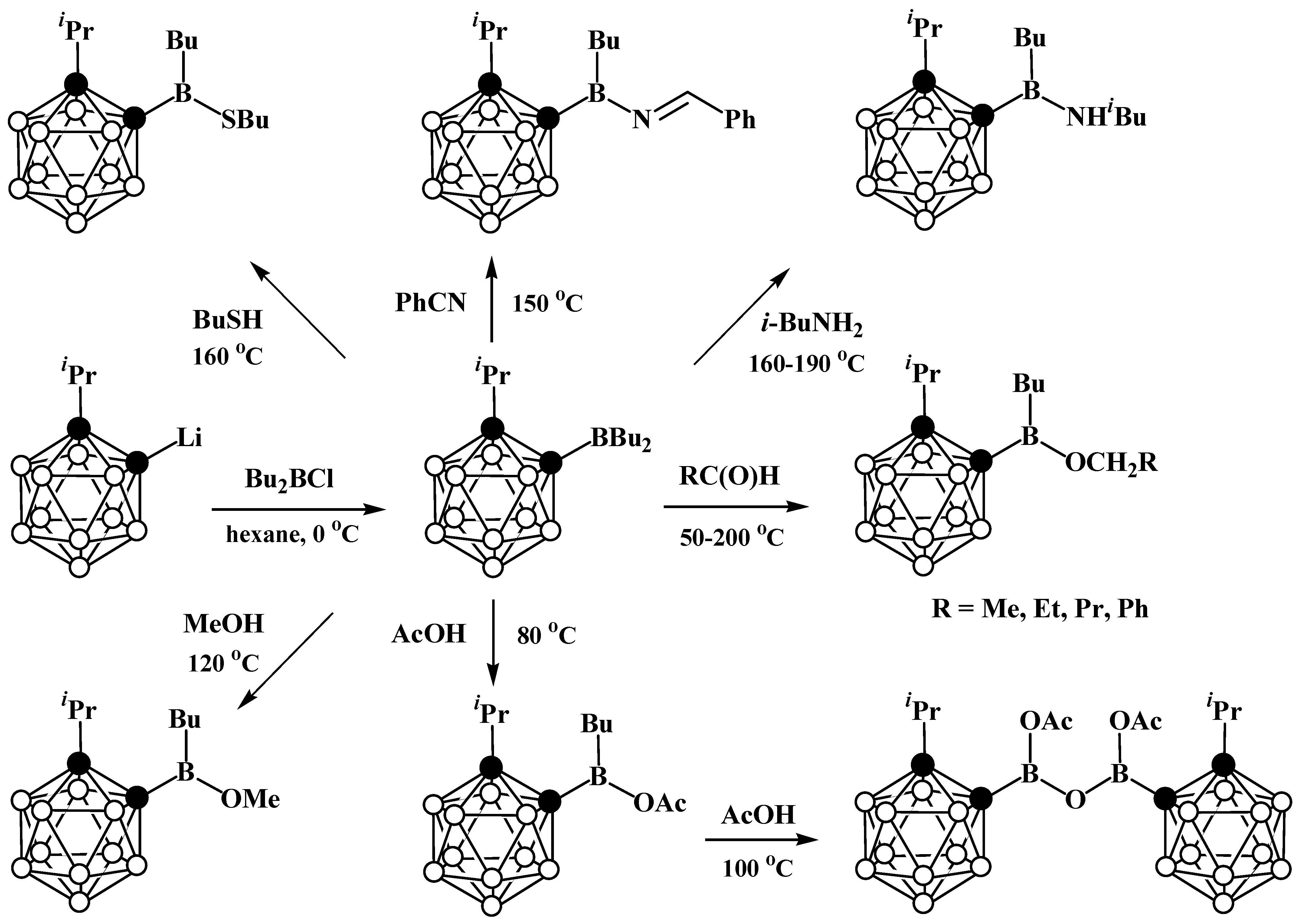
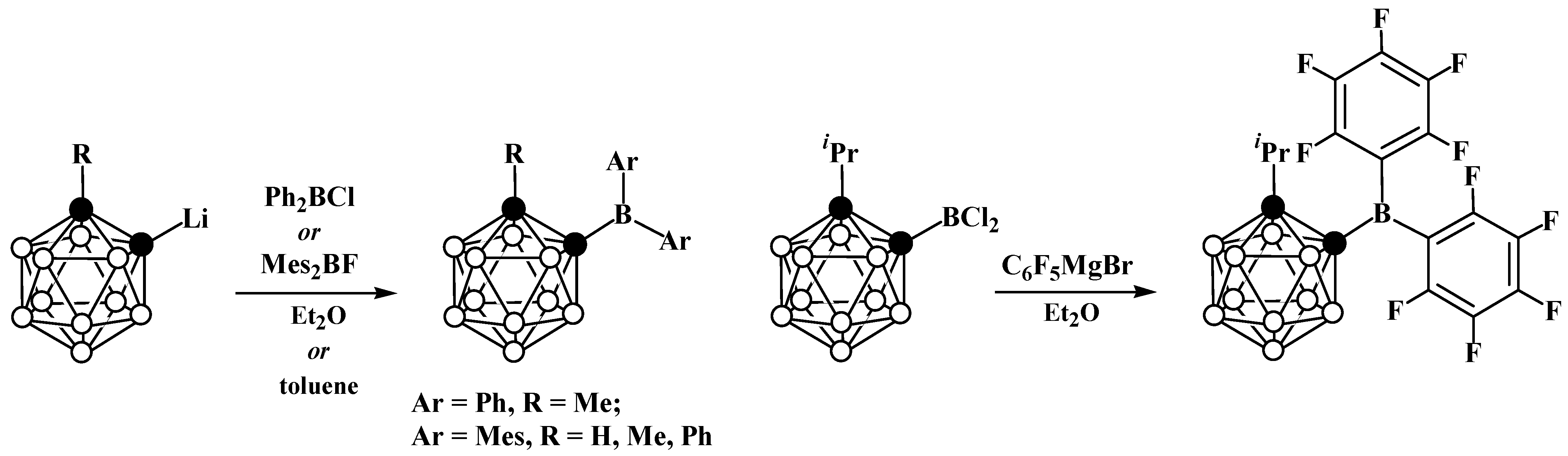
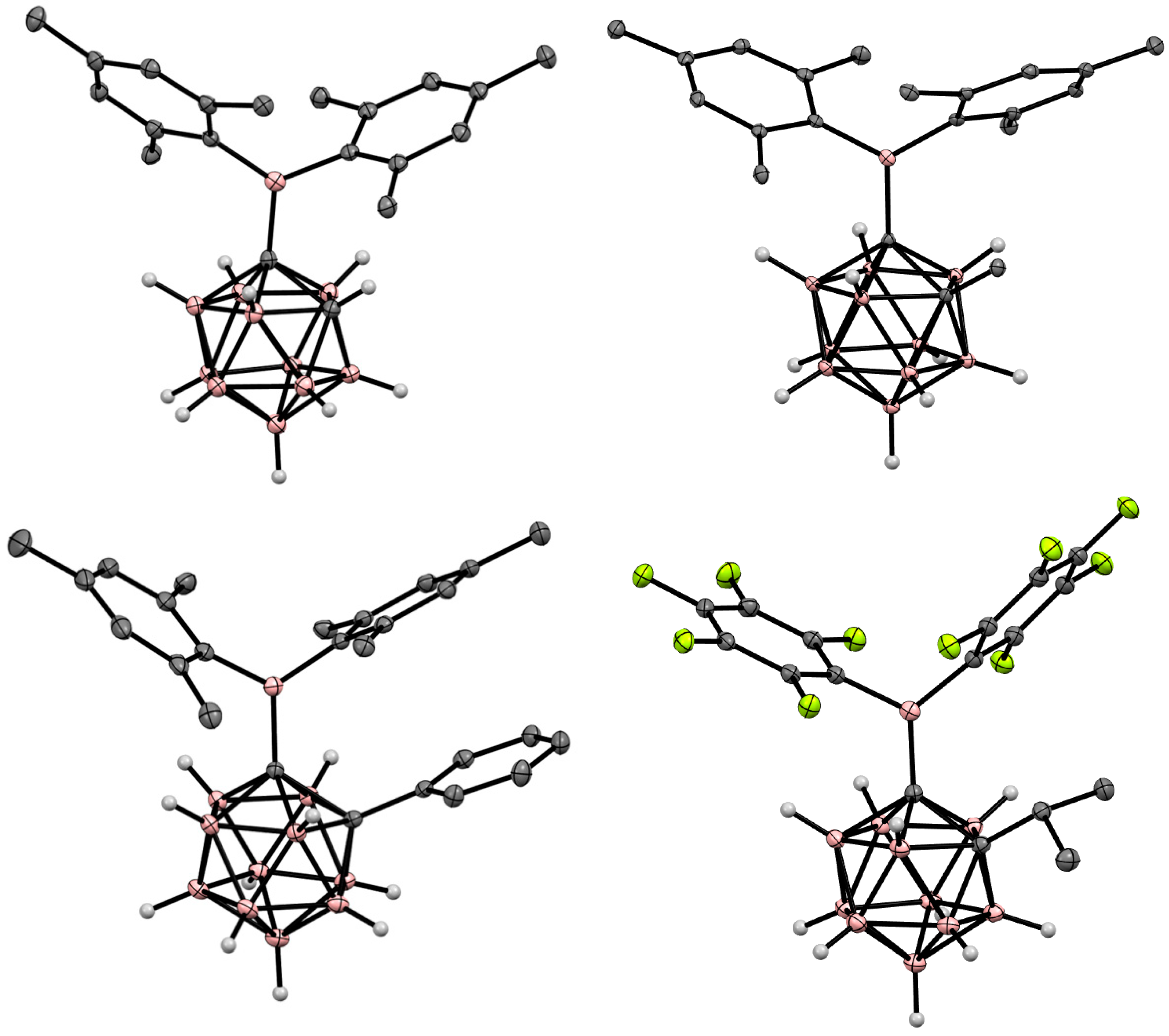
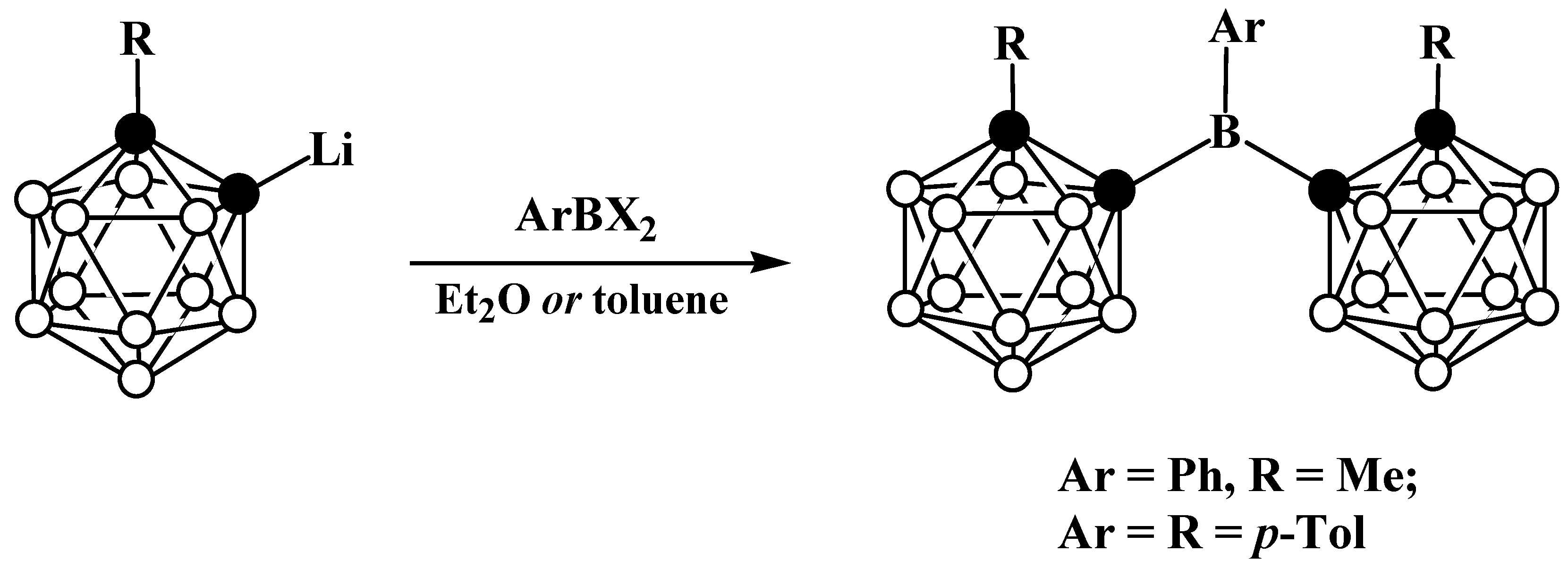
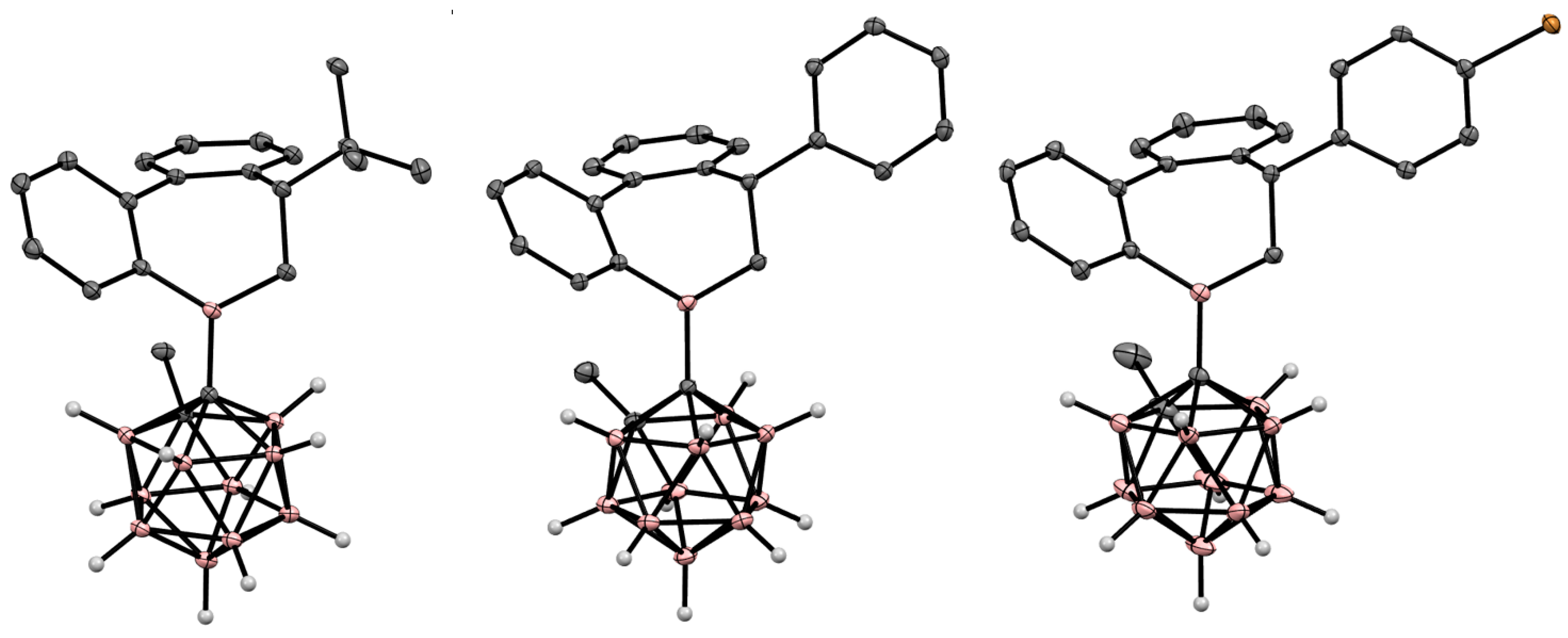
4. Arylboryl Derivatives of Carboranes
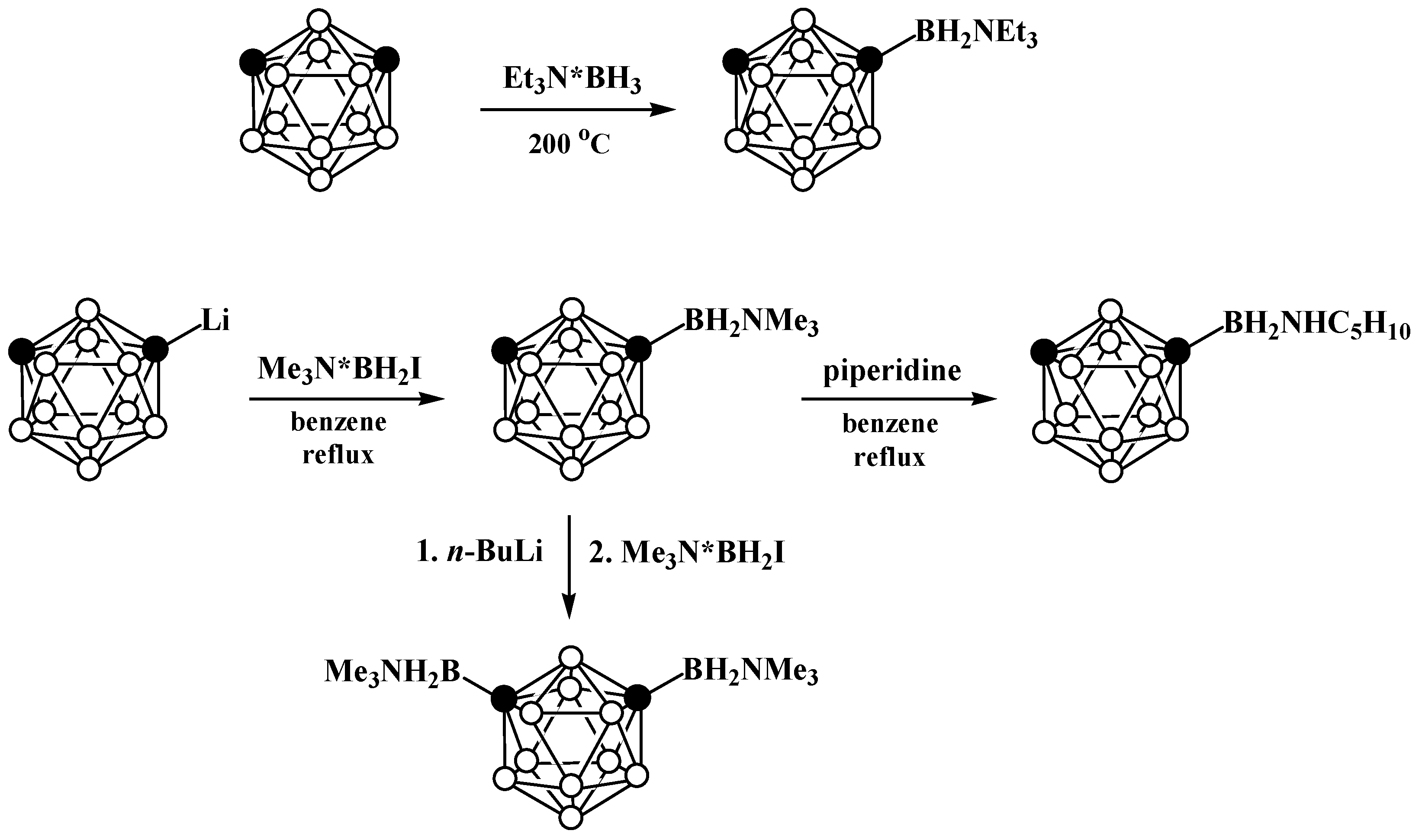
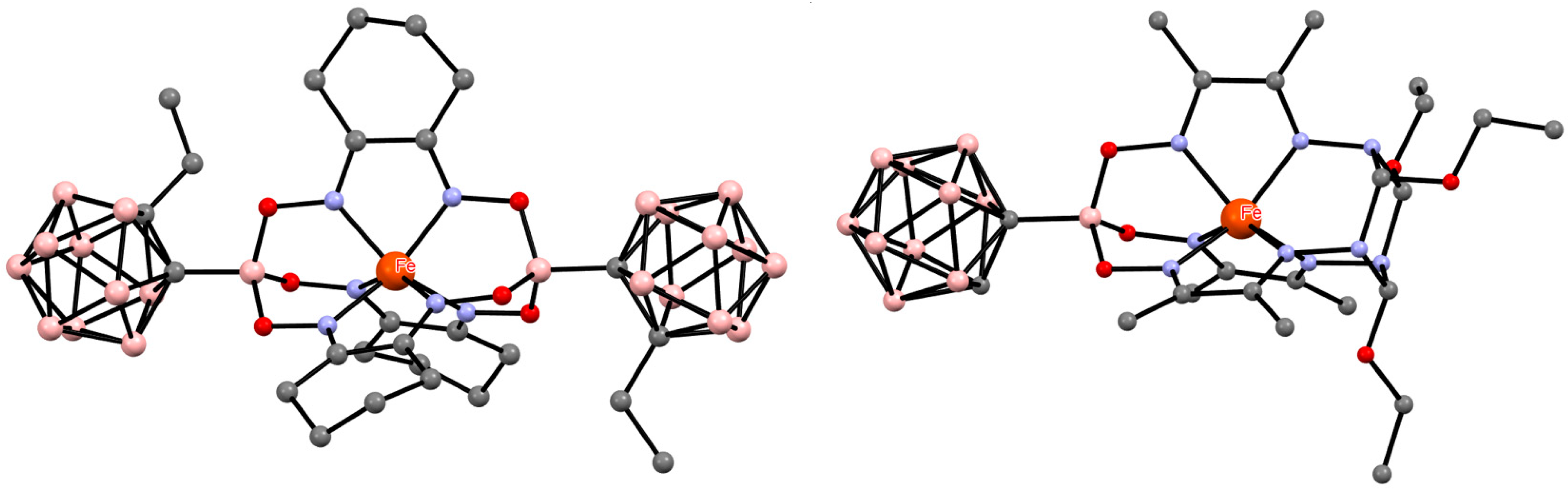
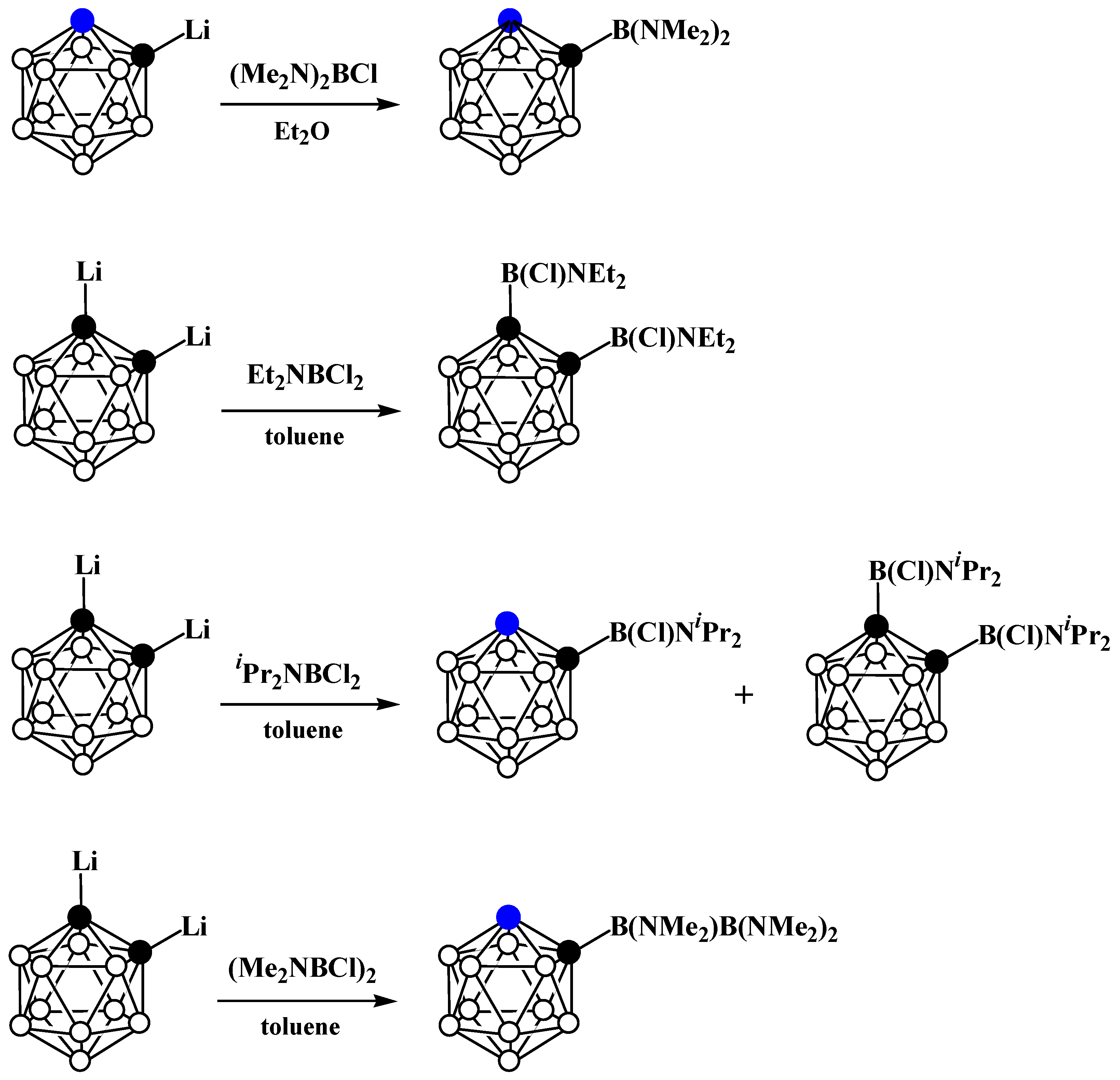
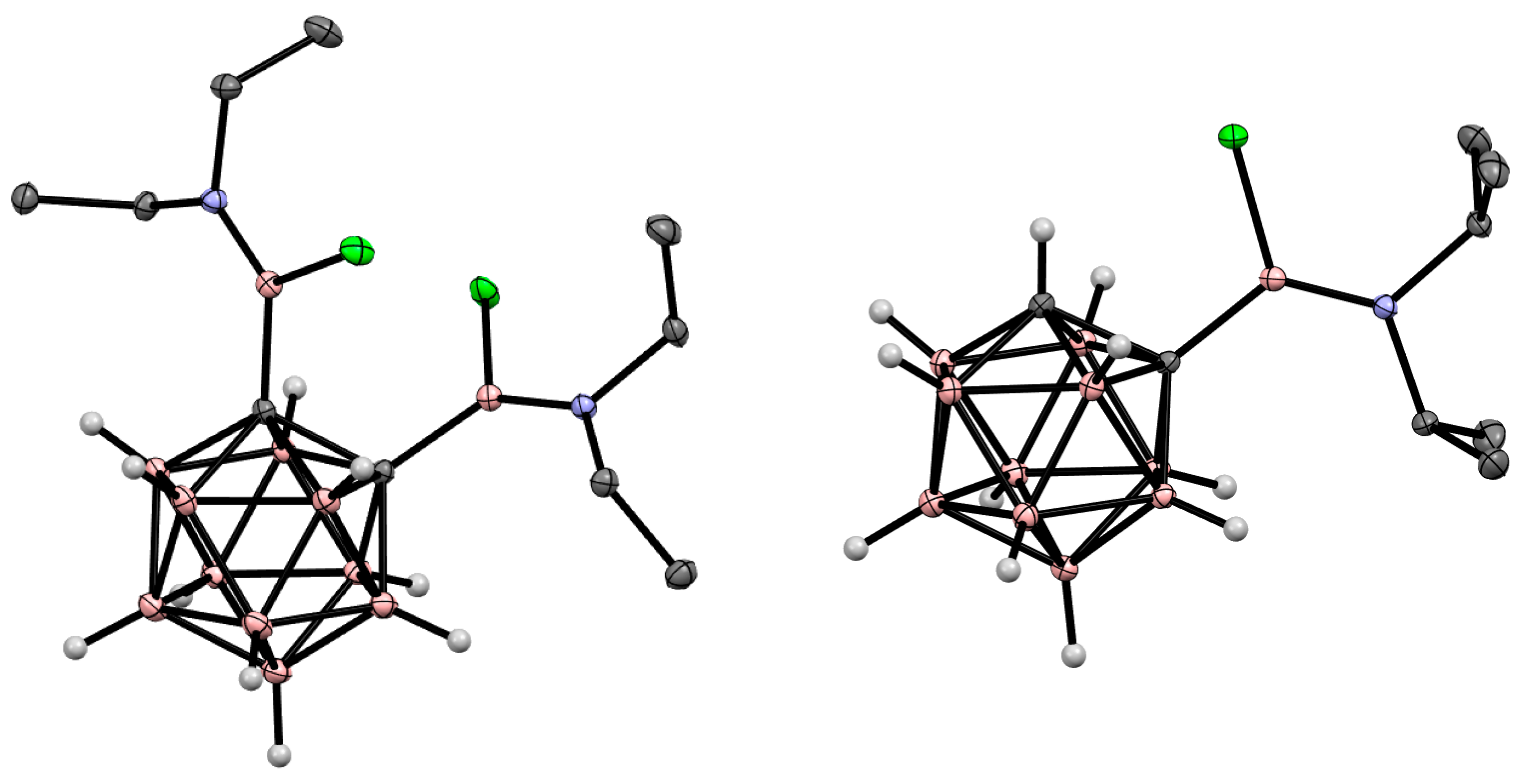
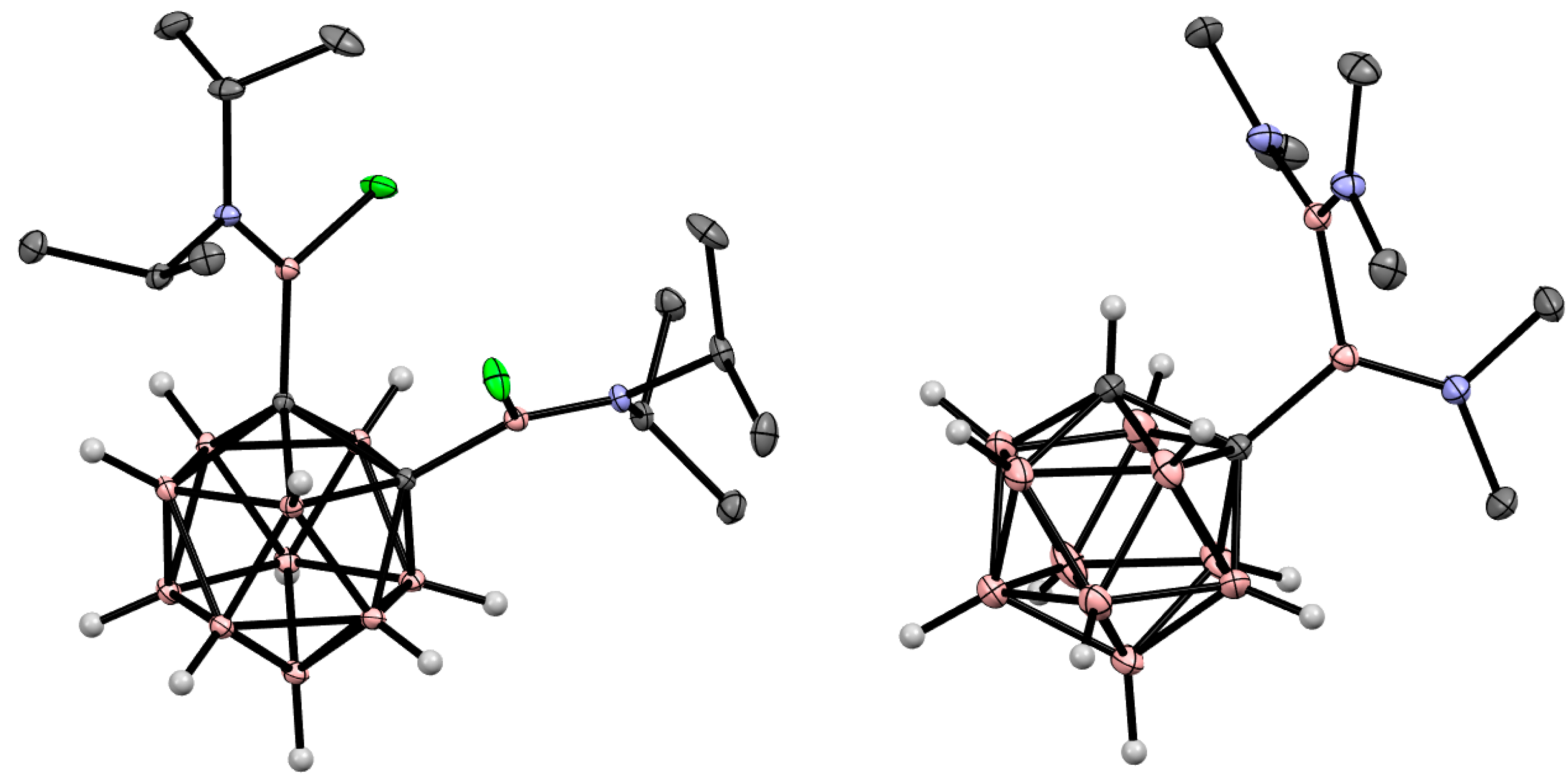
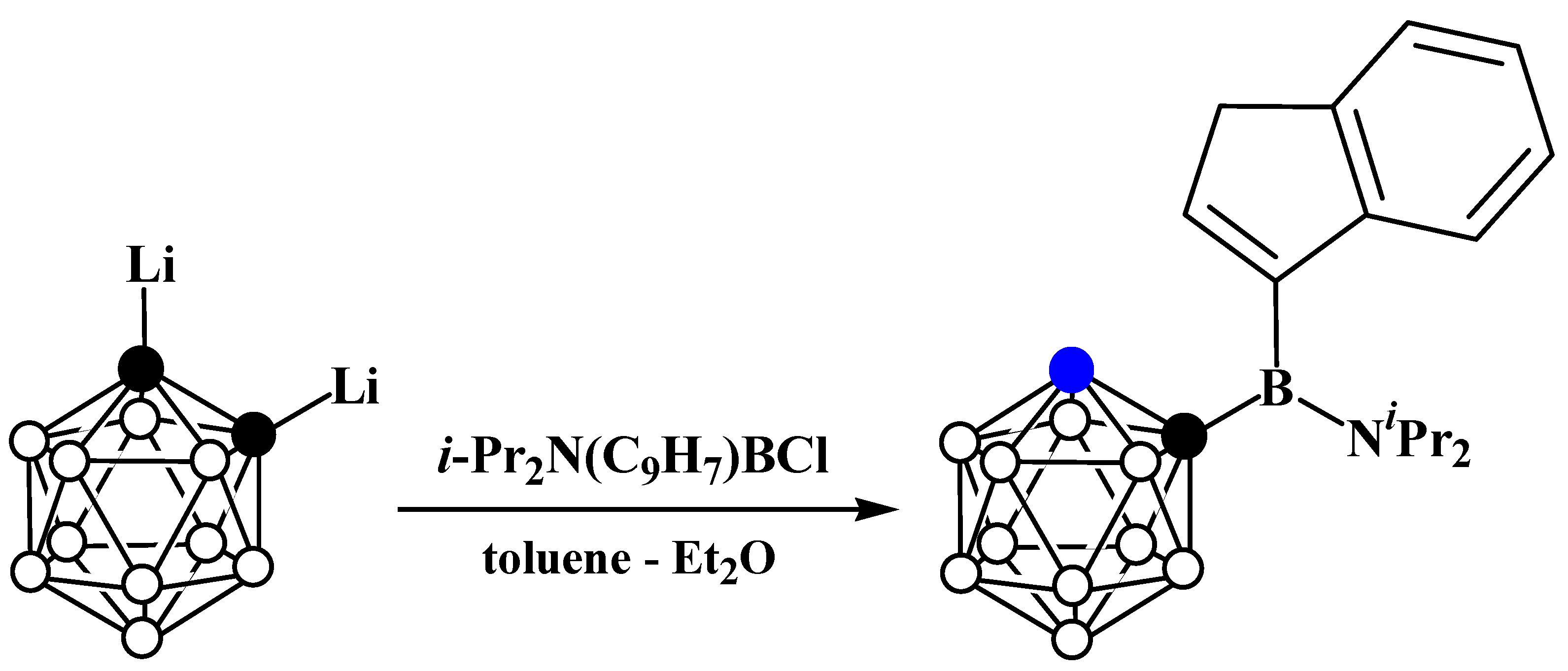
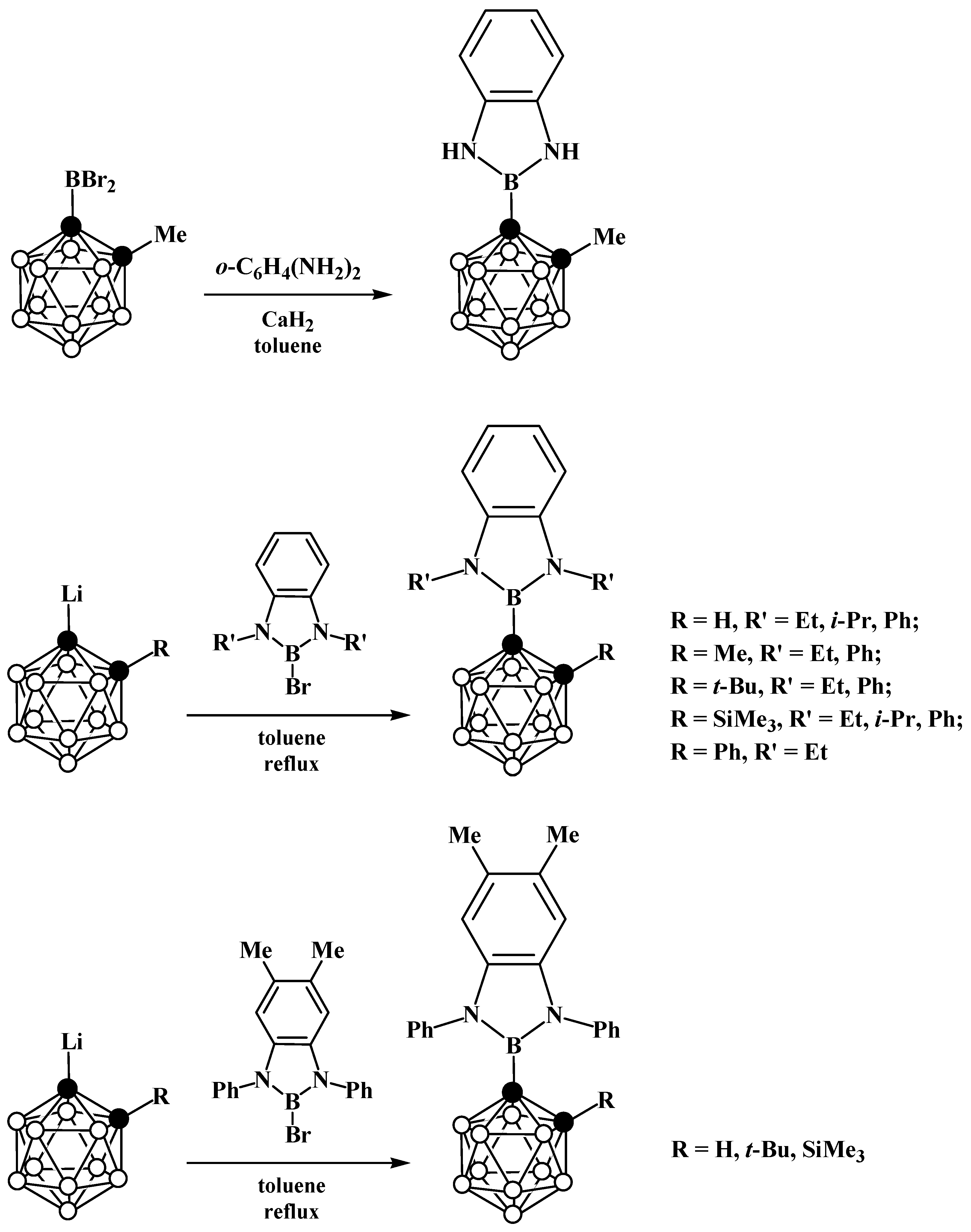
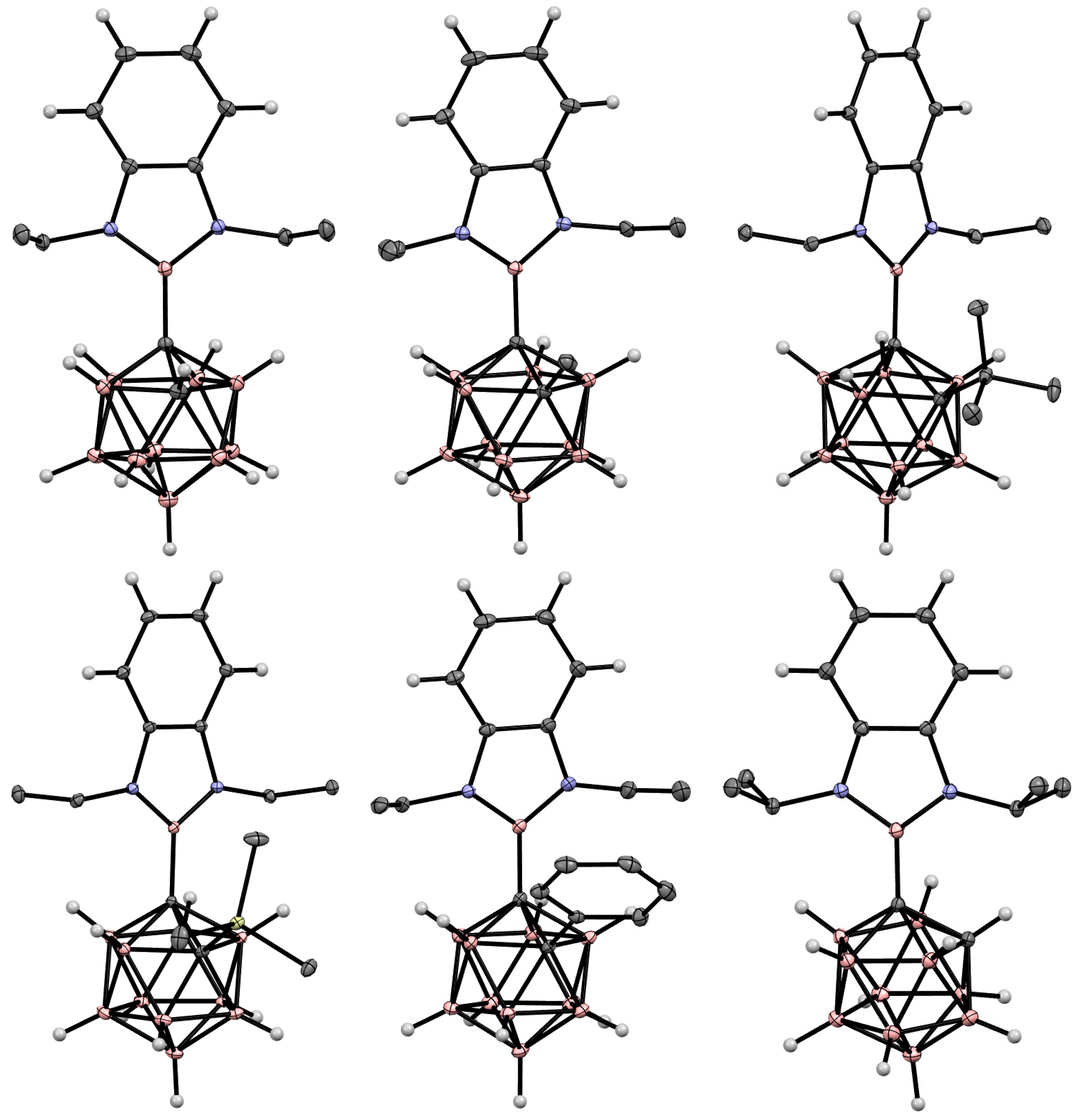
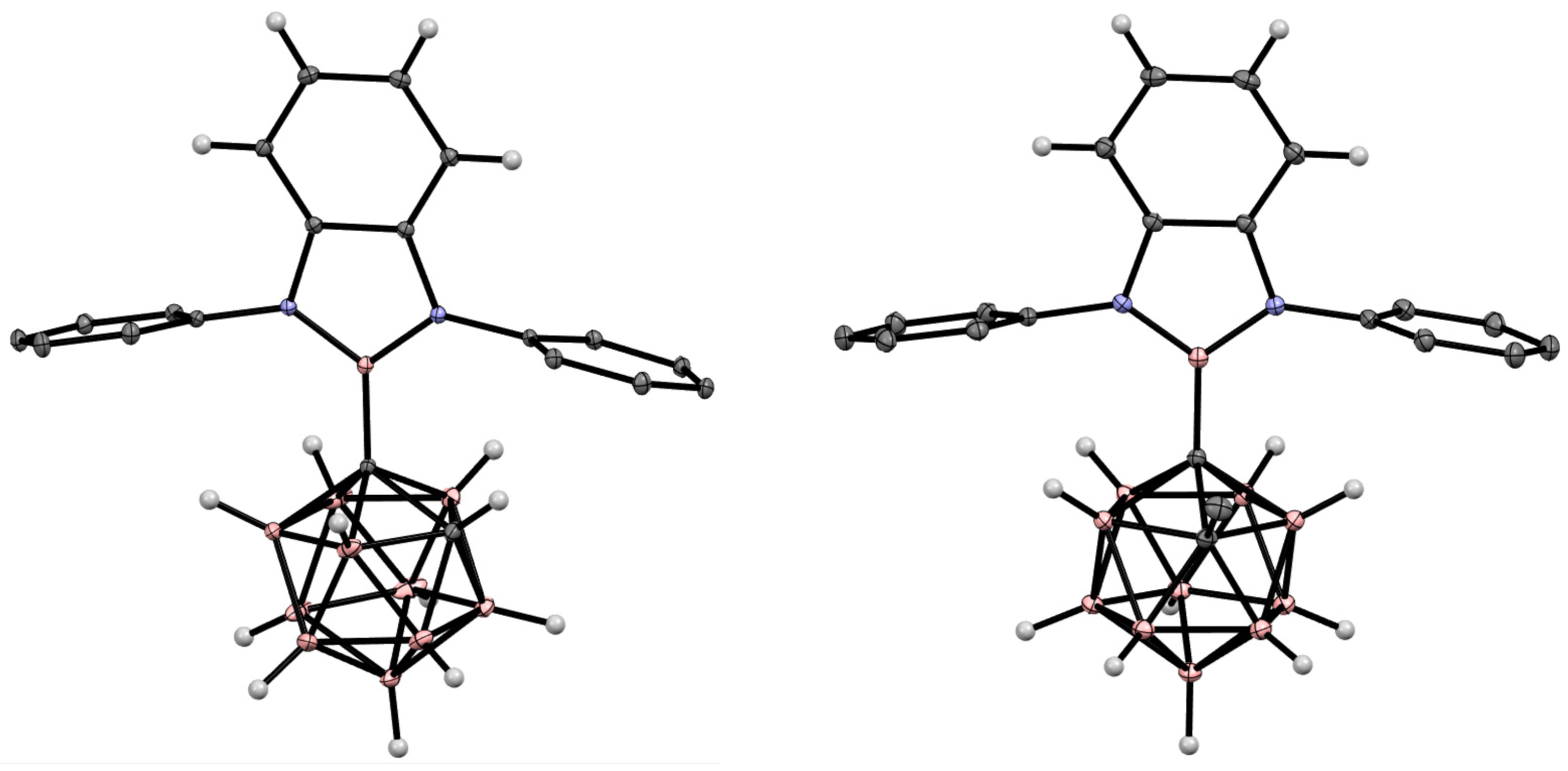
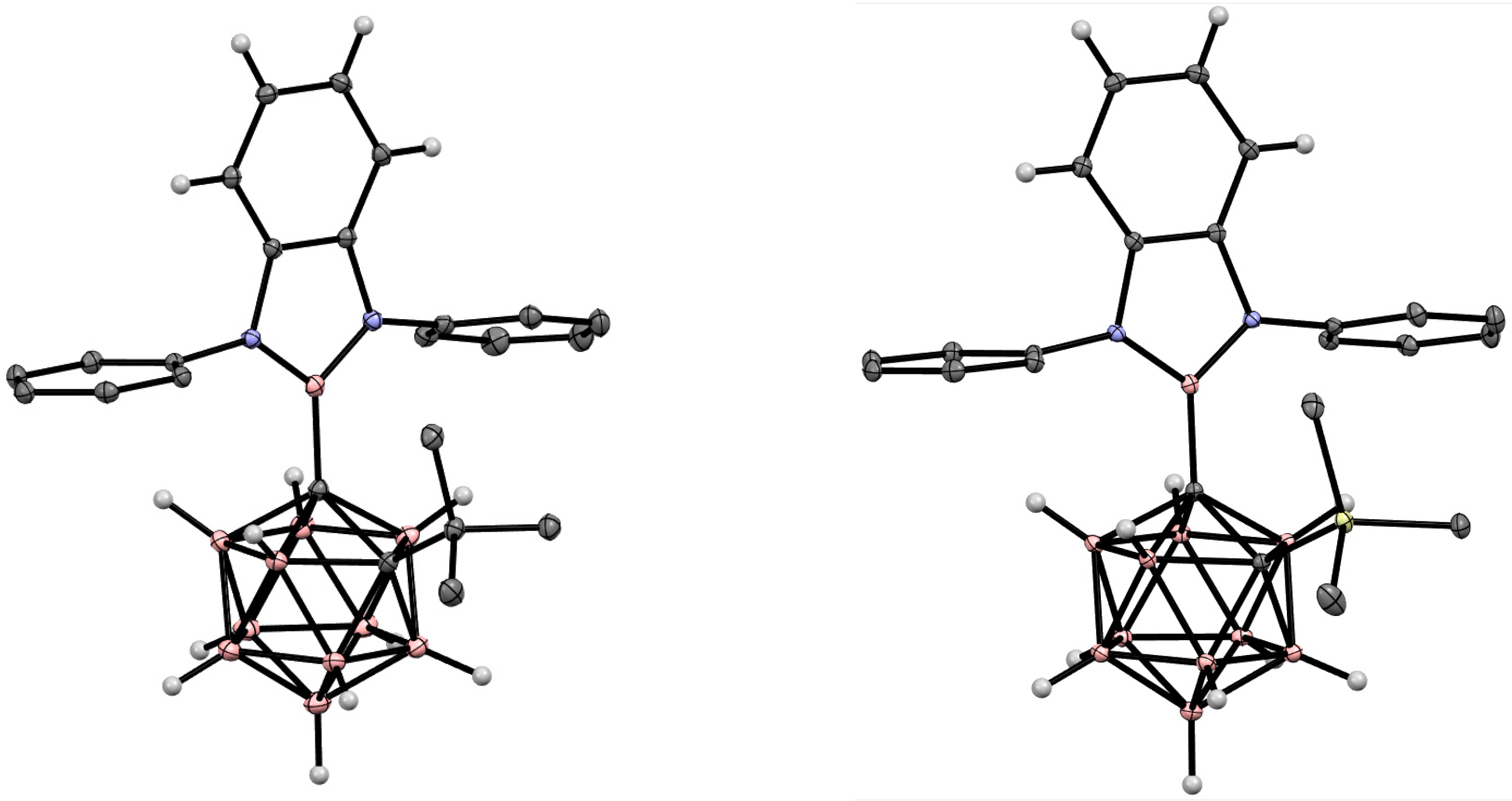
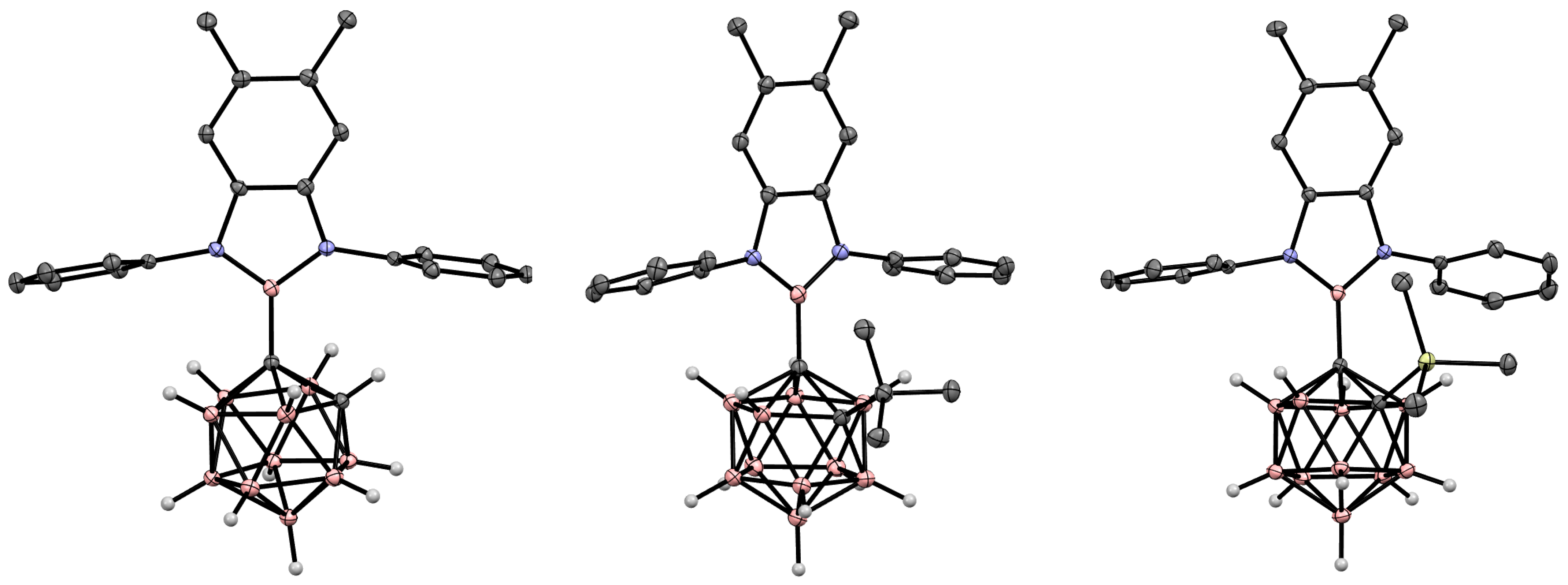
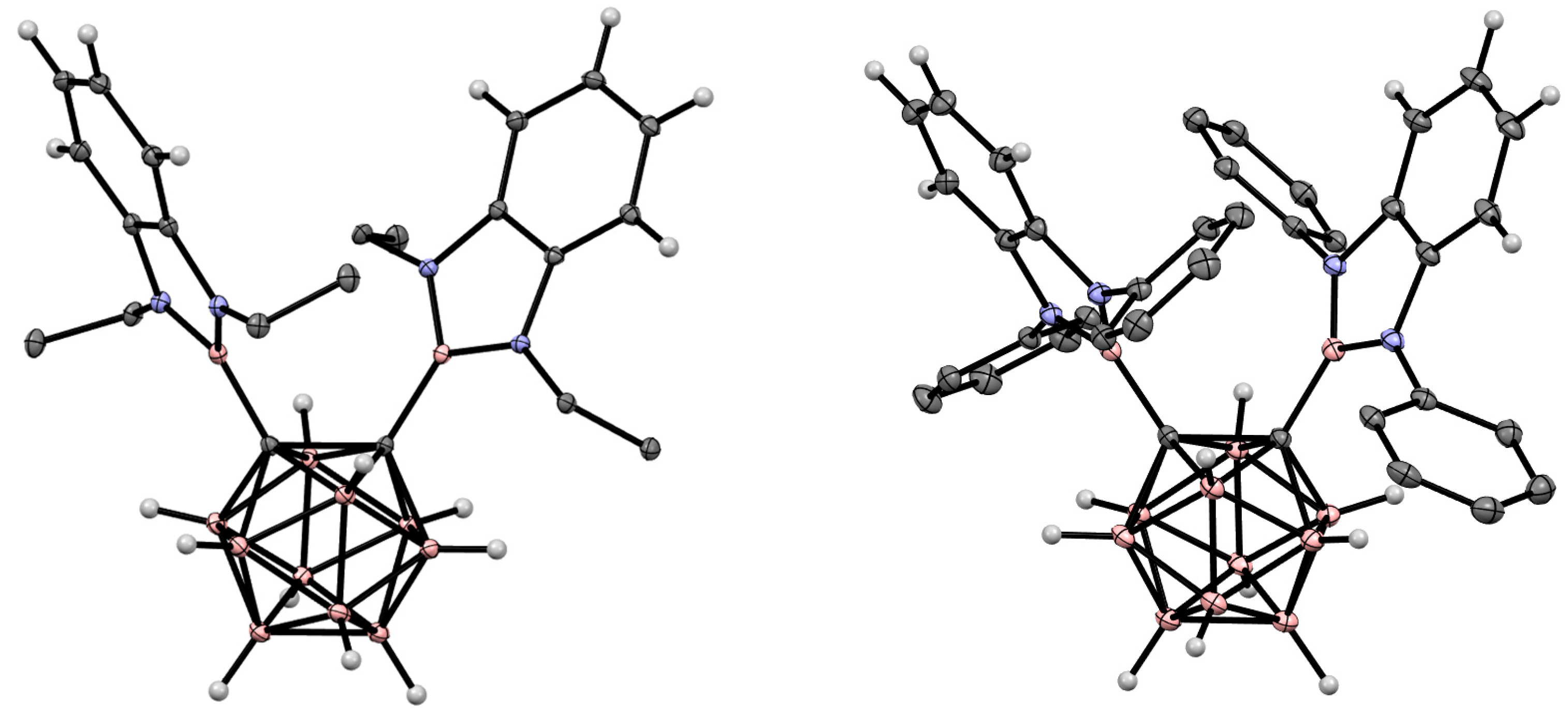
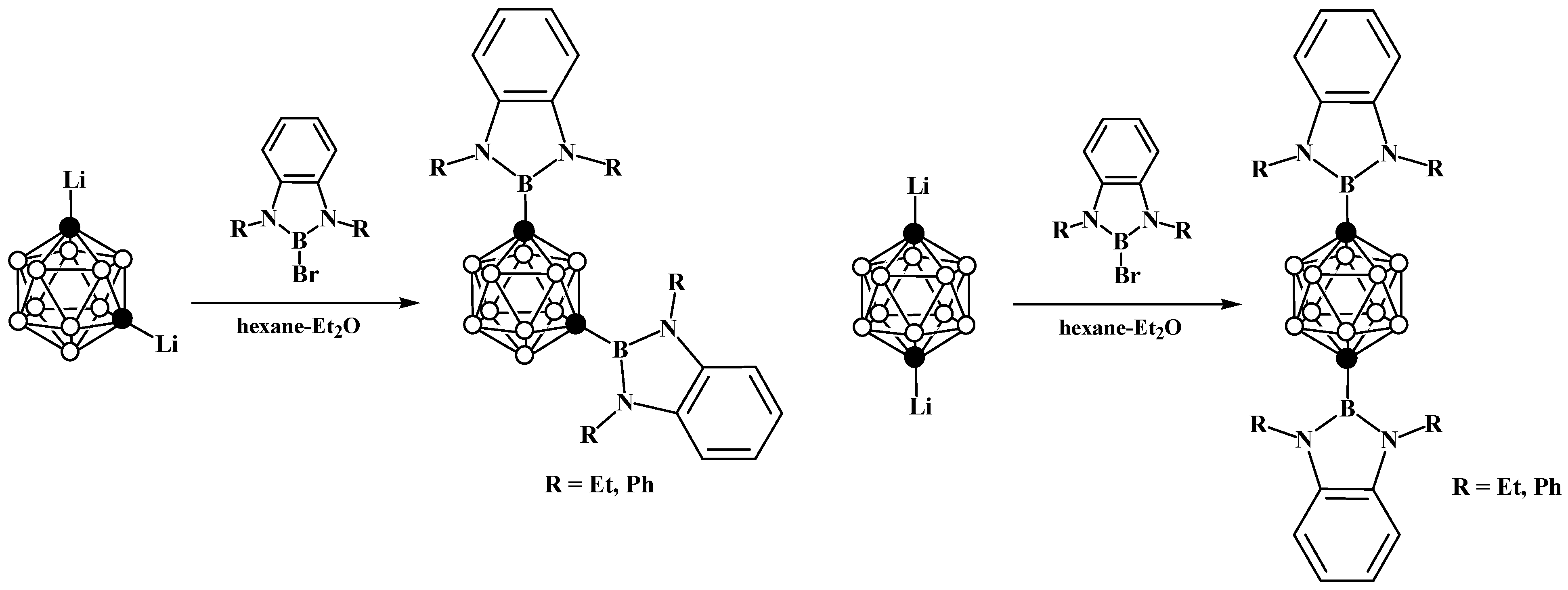
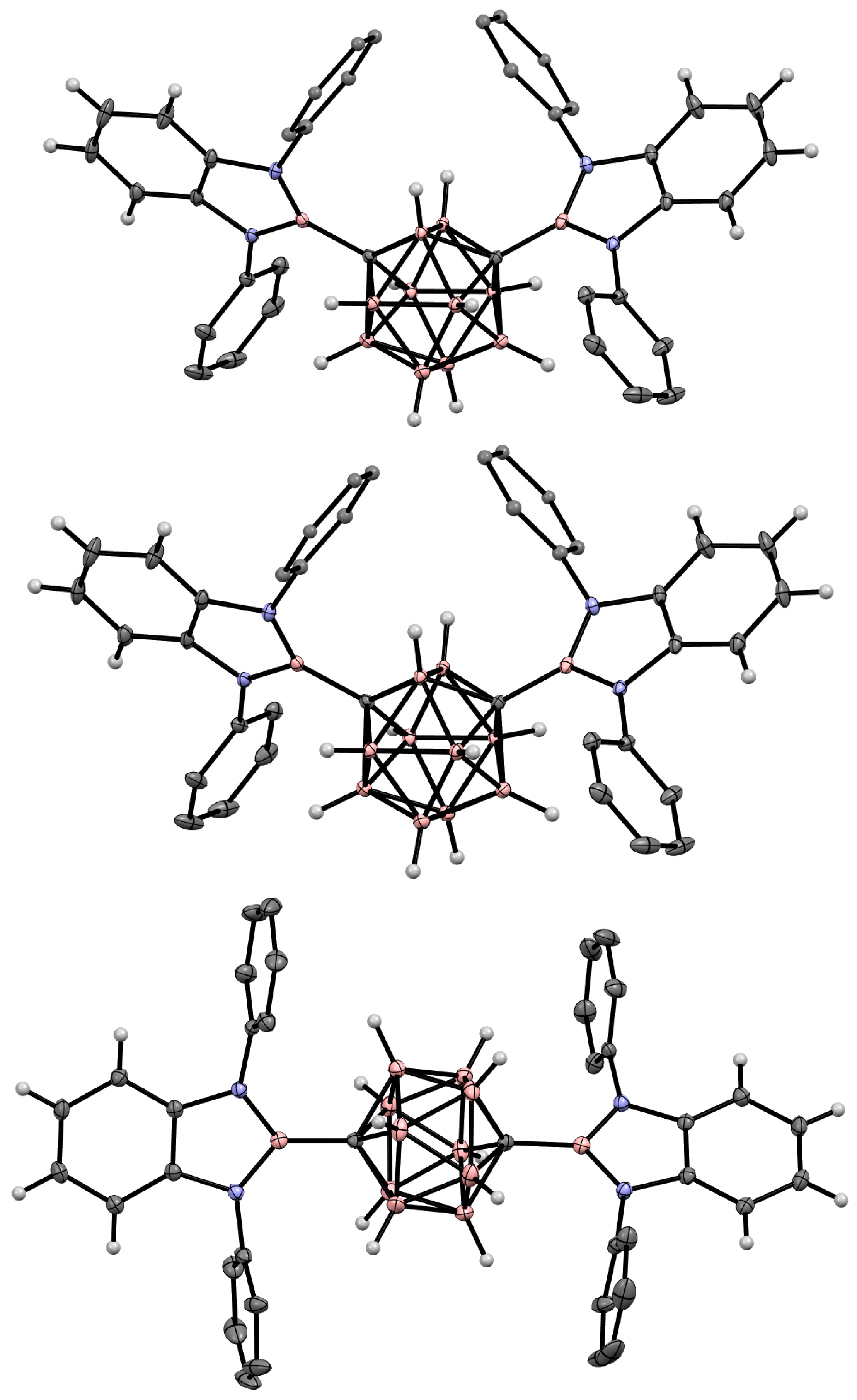
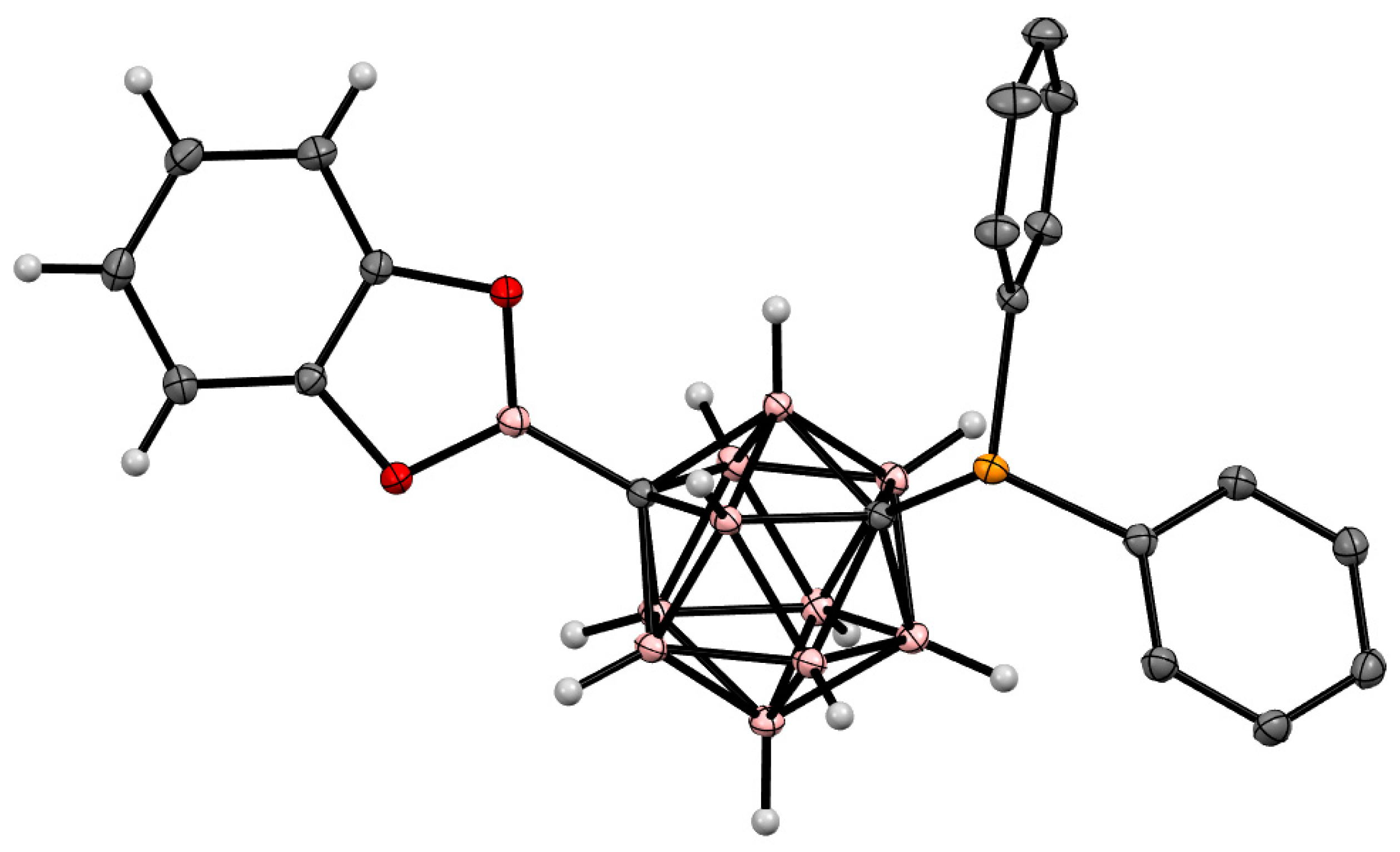
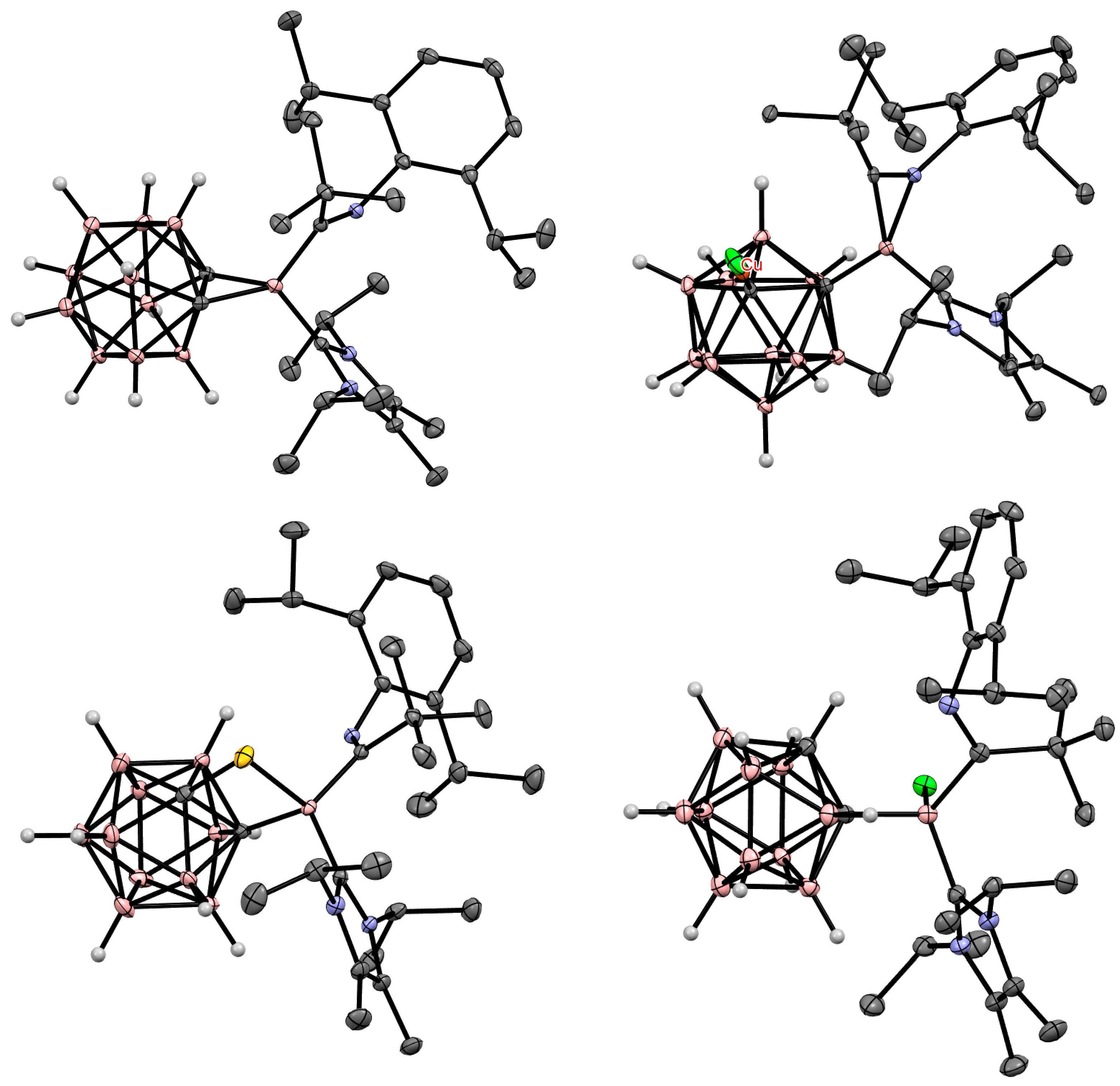
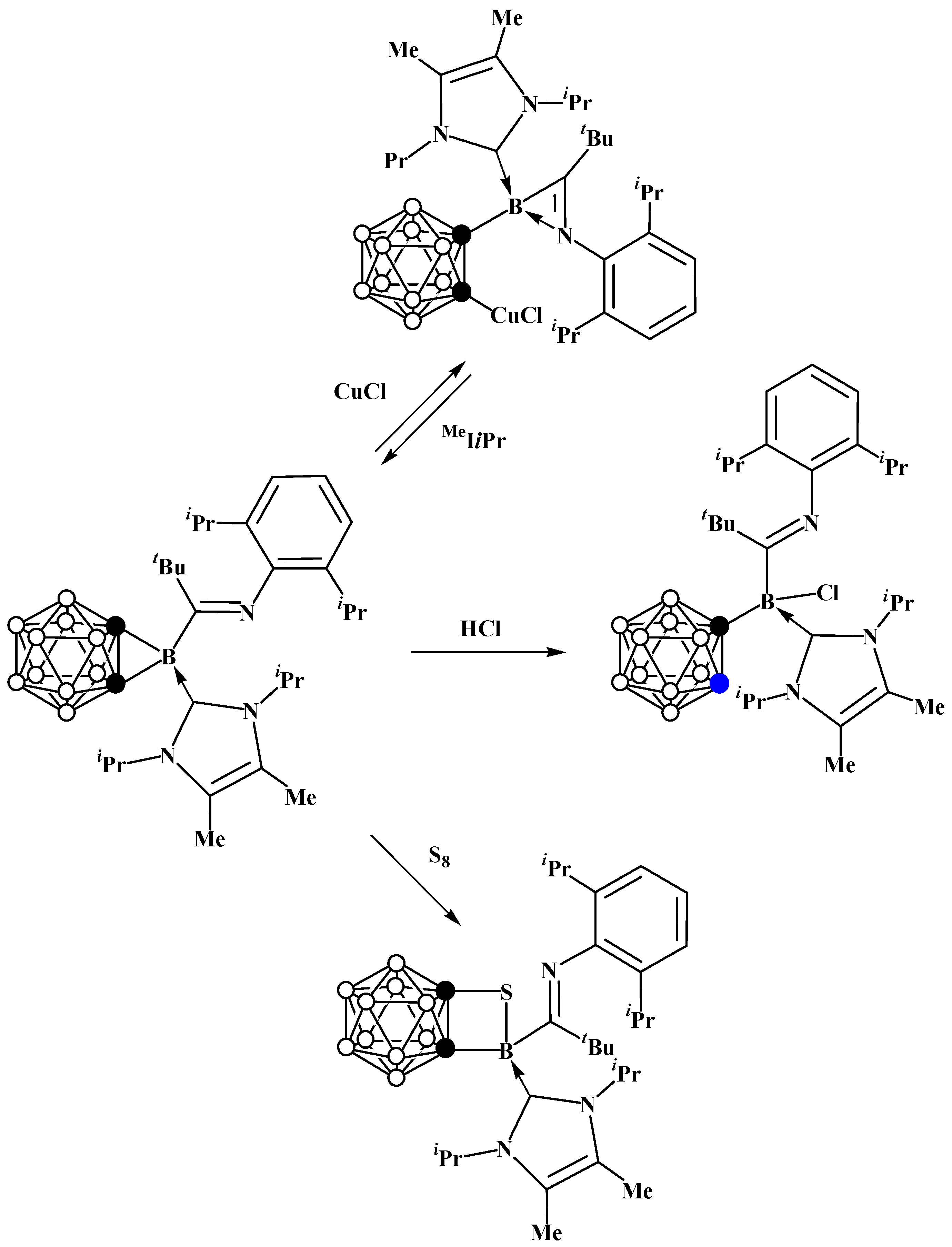
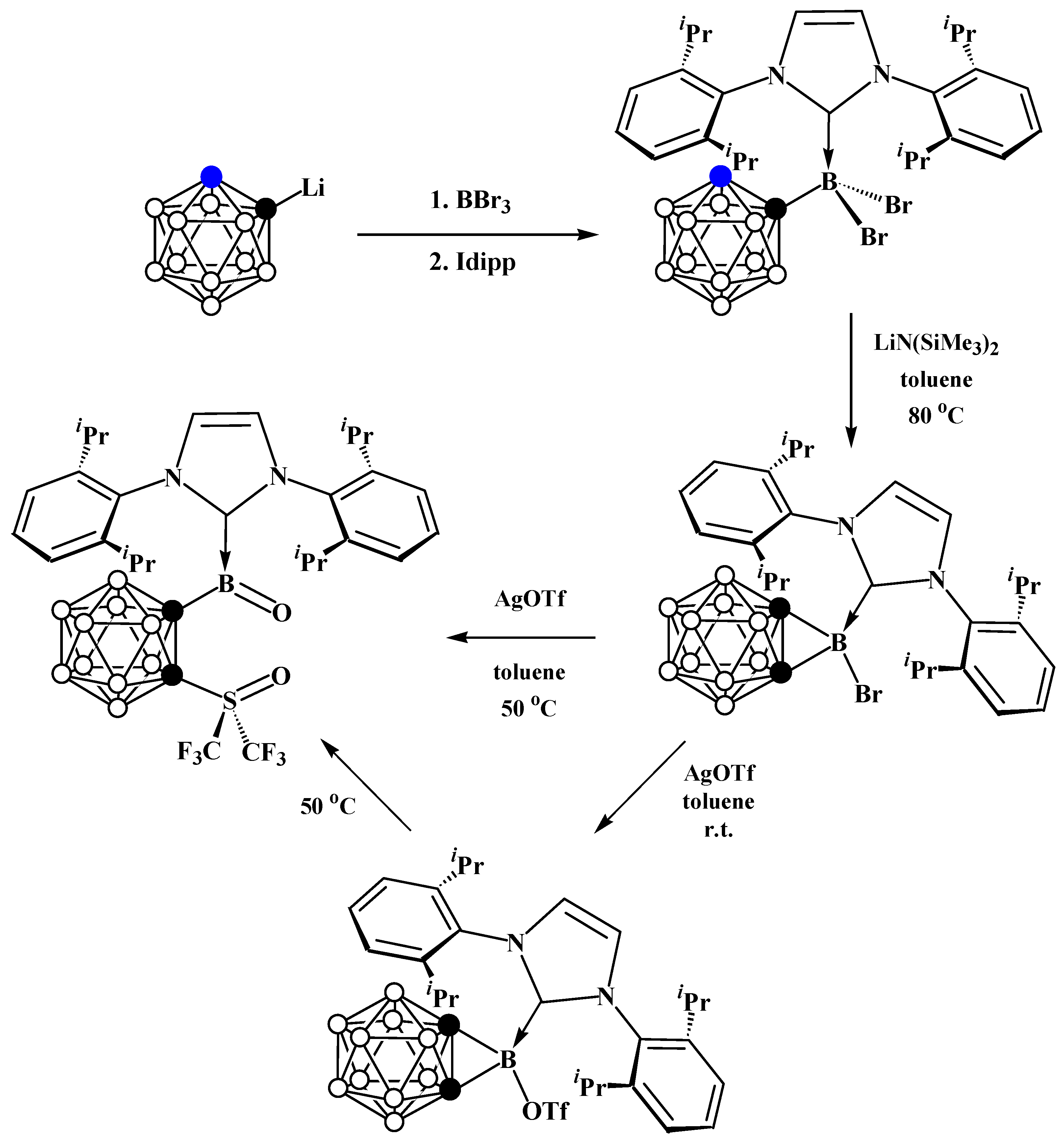
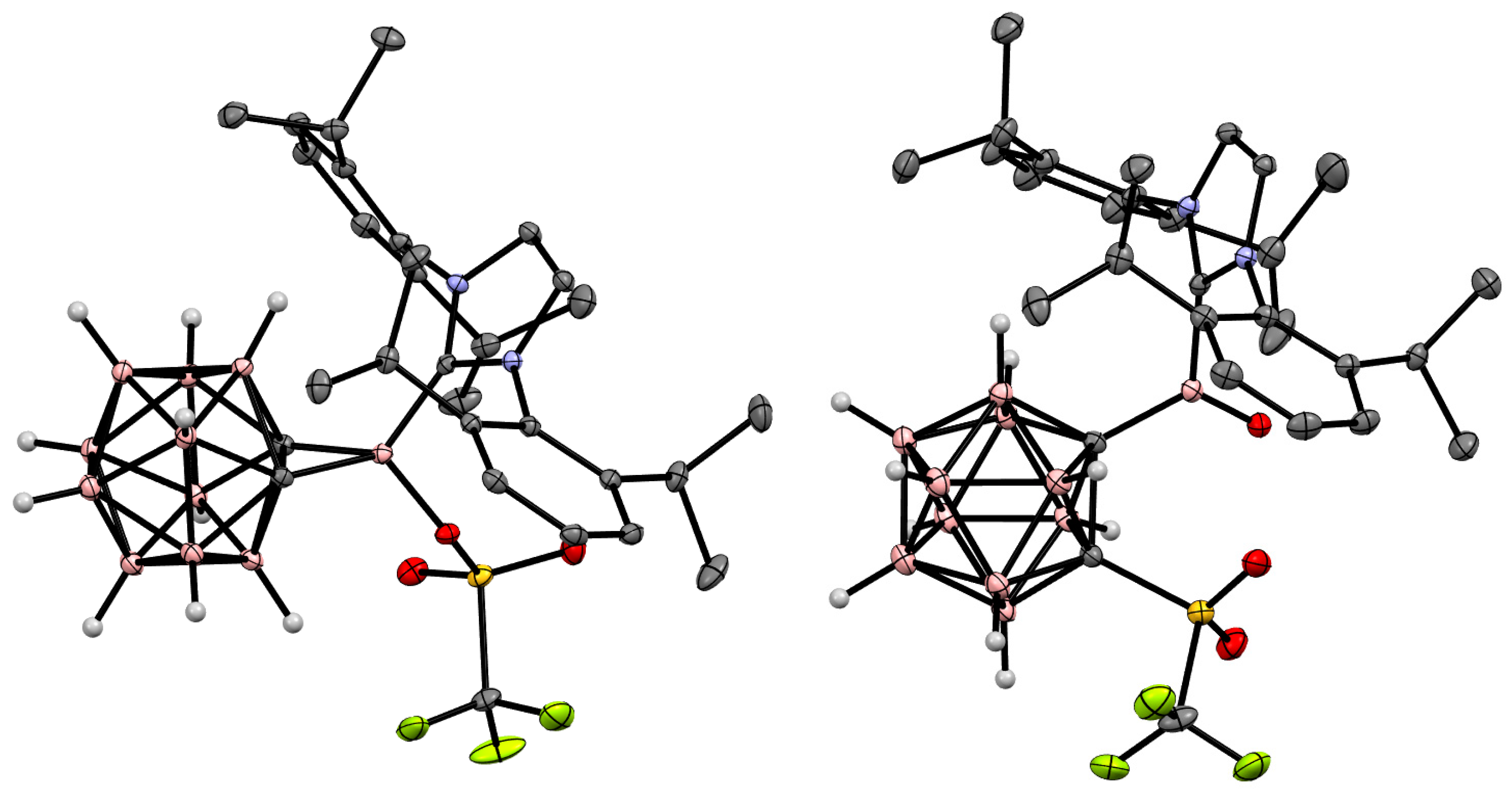
5. Aminoboryl Derivatives of Carboranes and Their Analogs
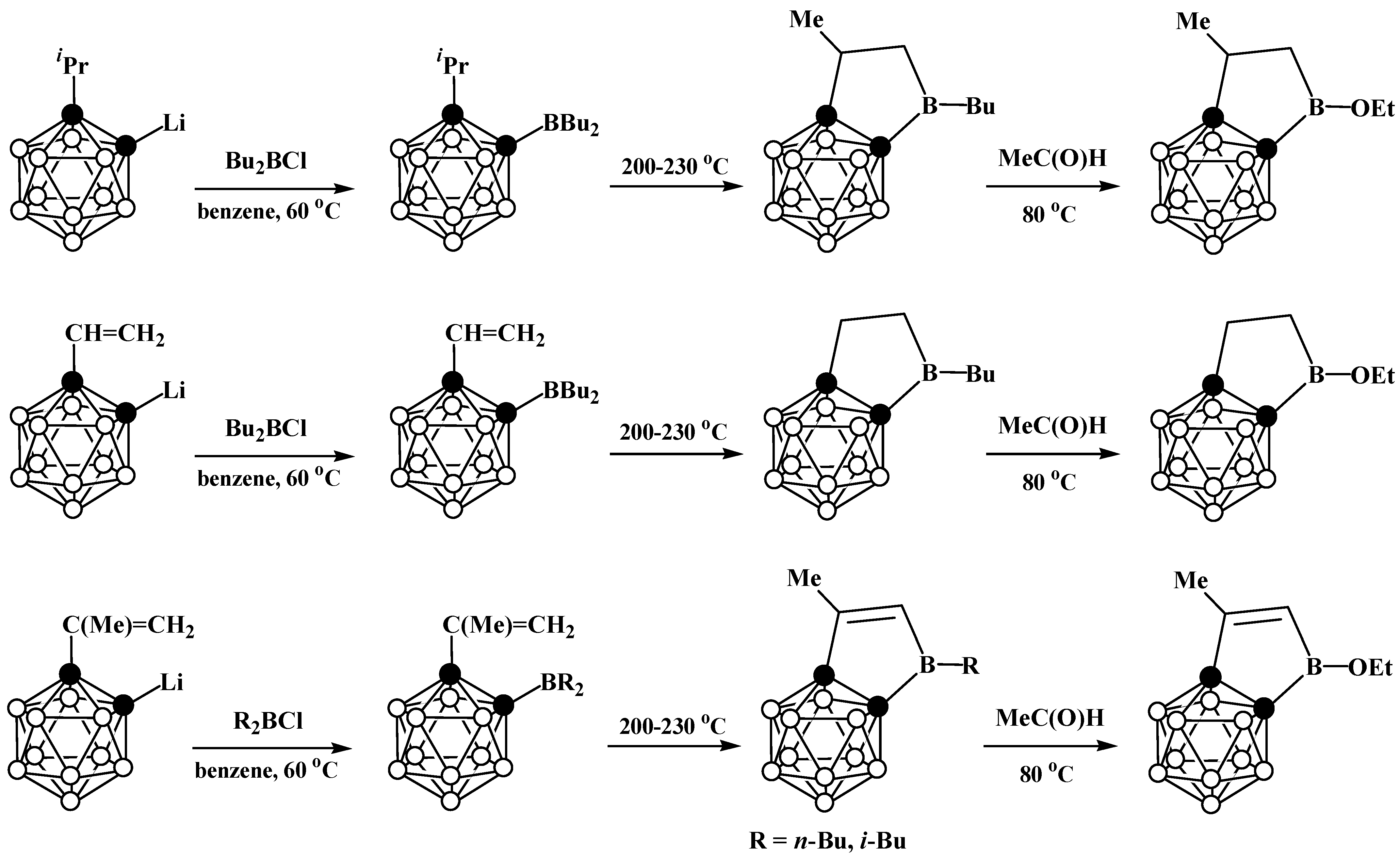
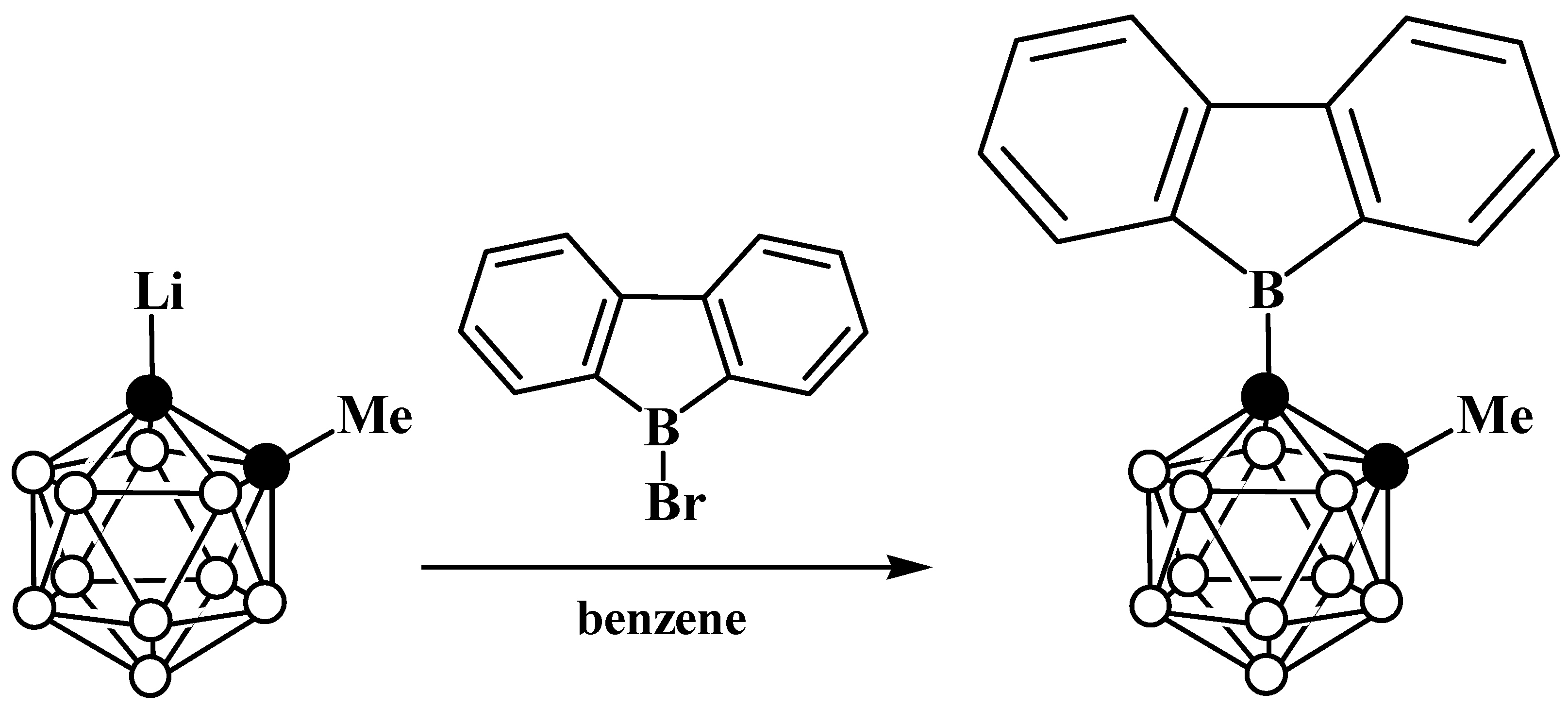
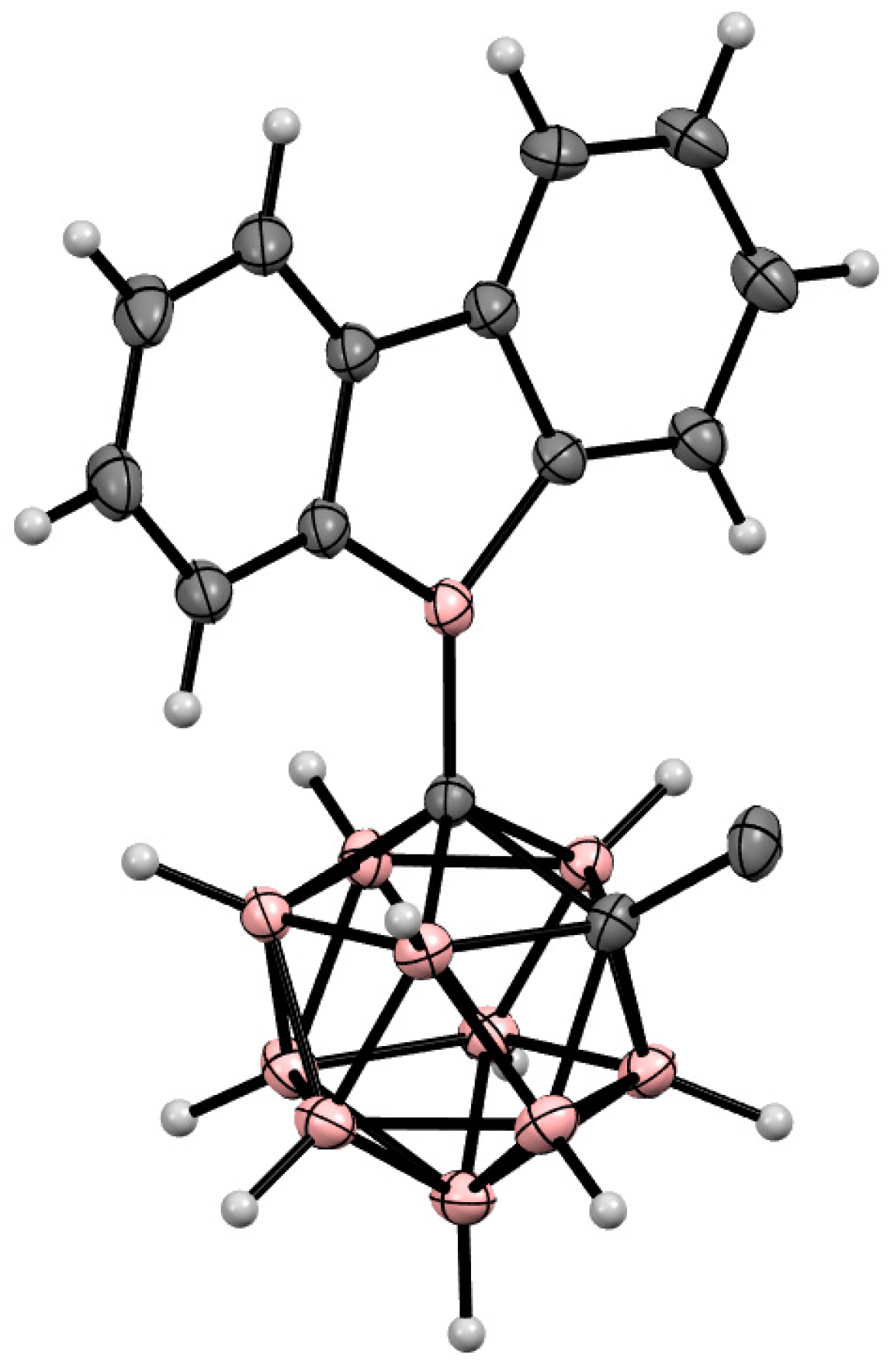
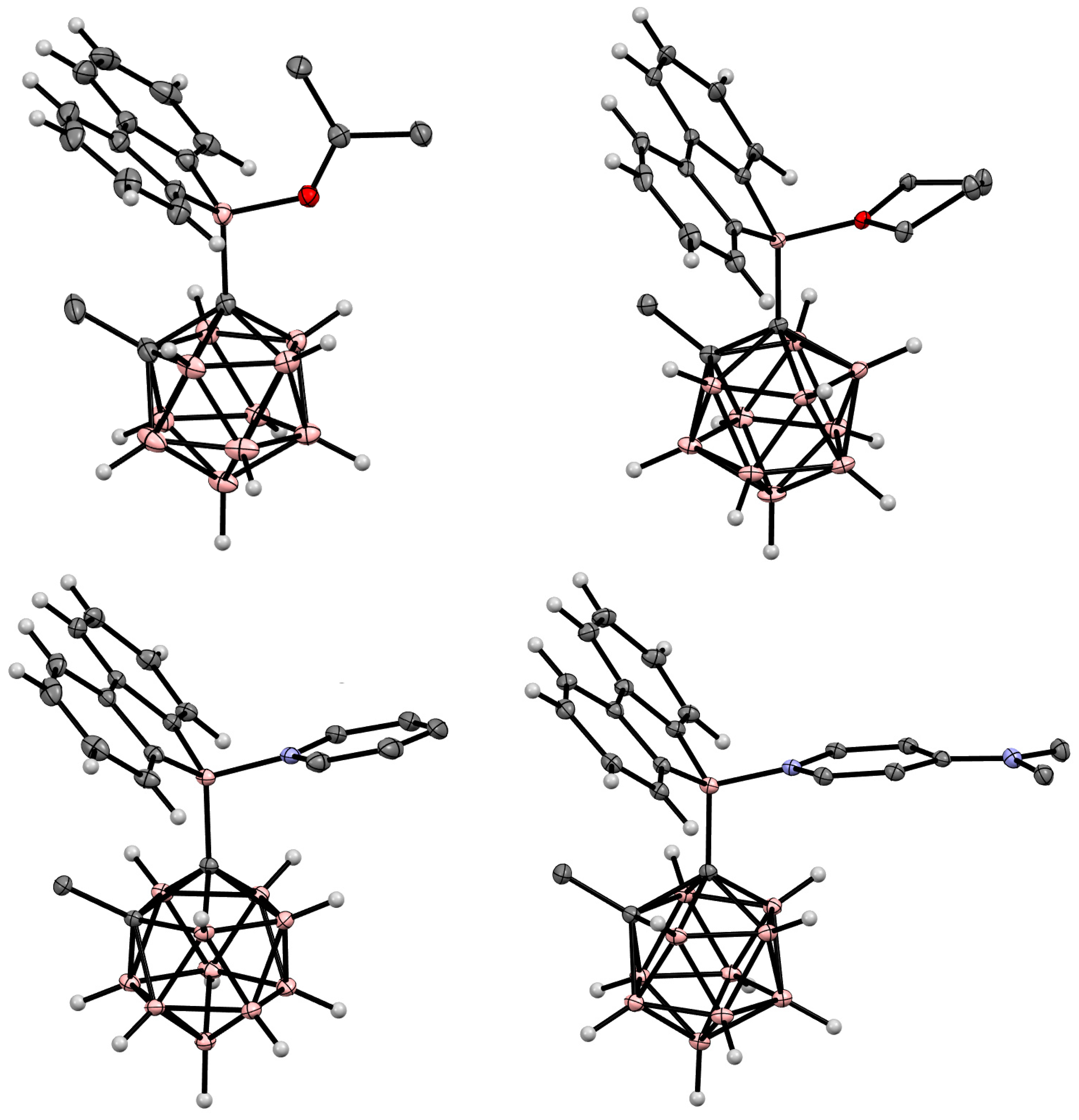
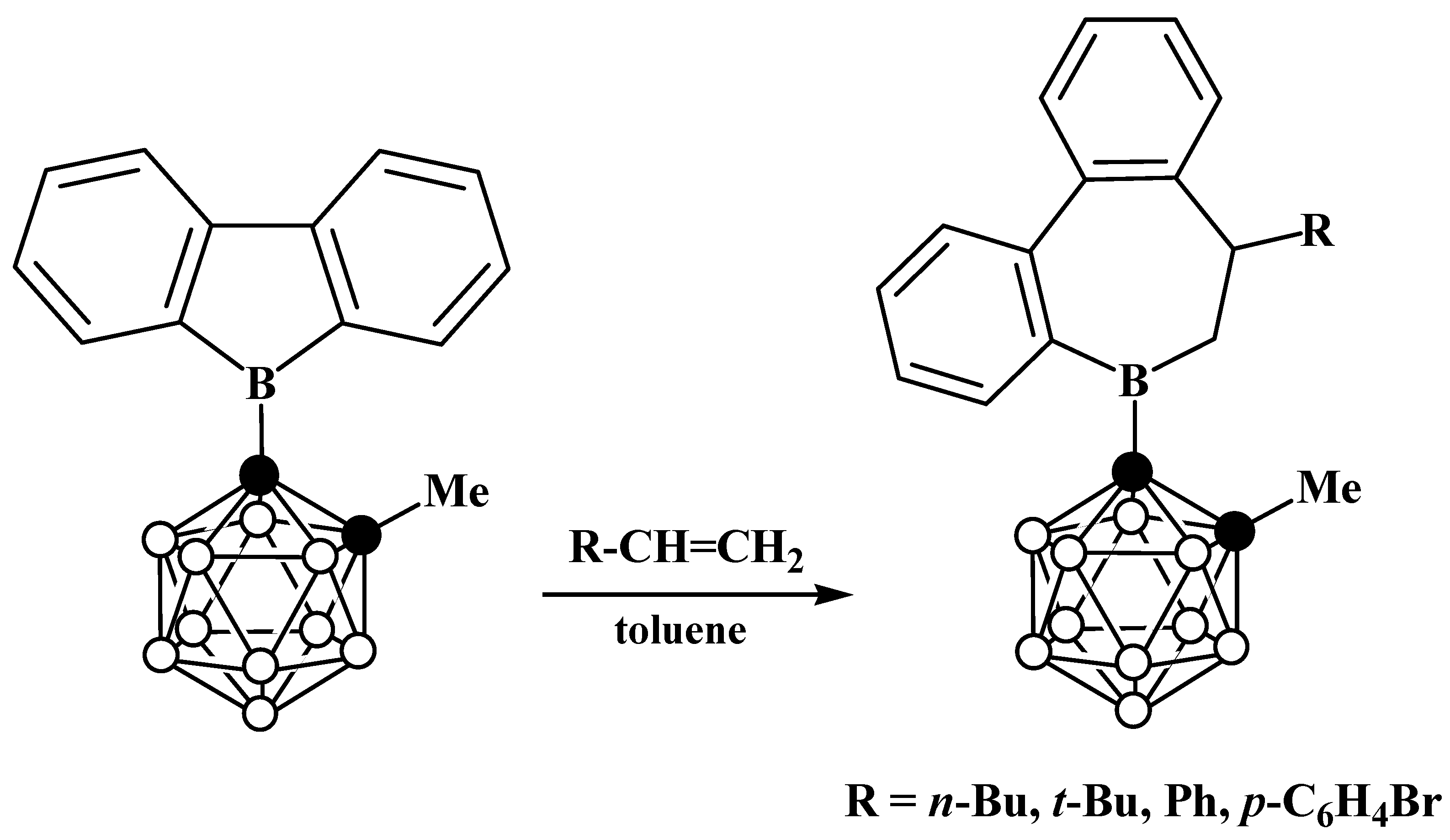
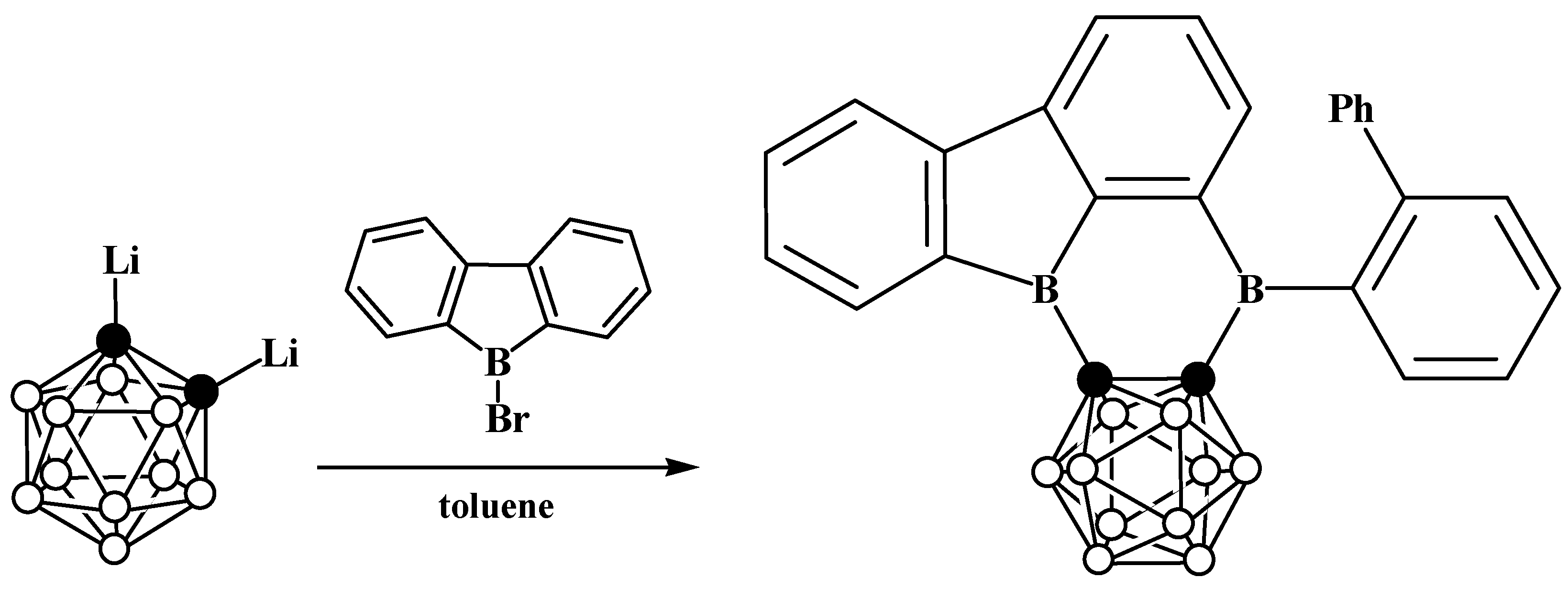
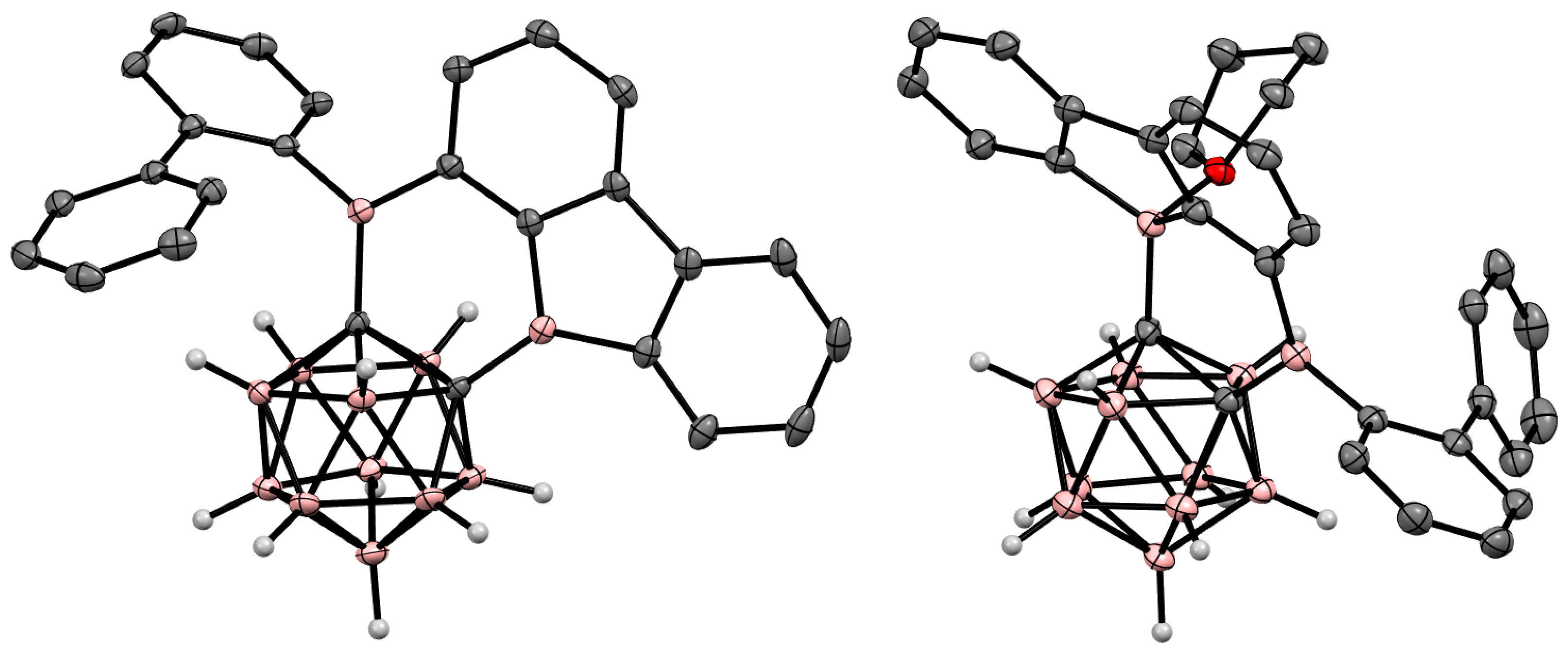
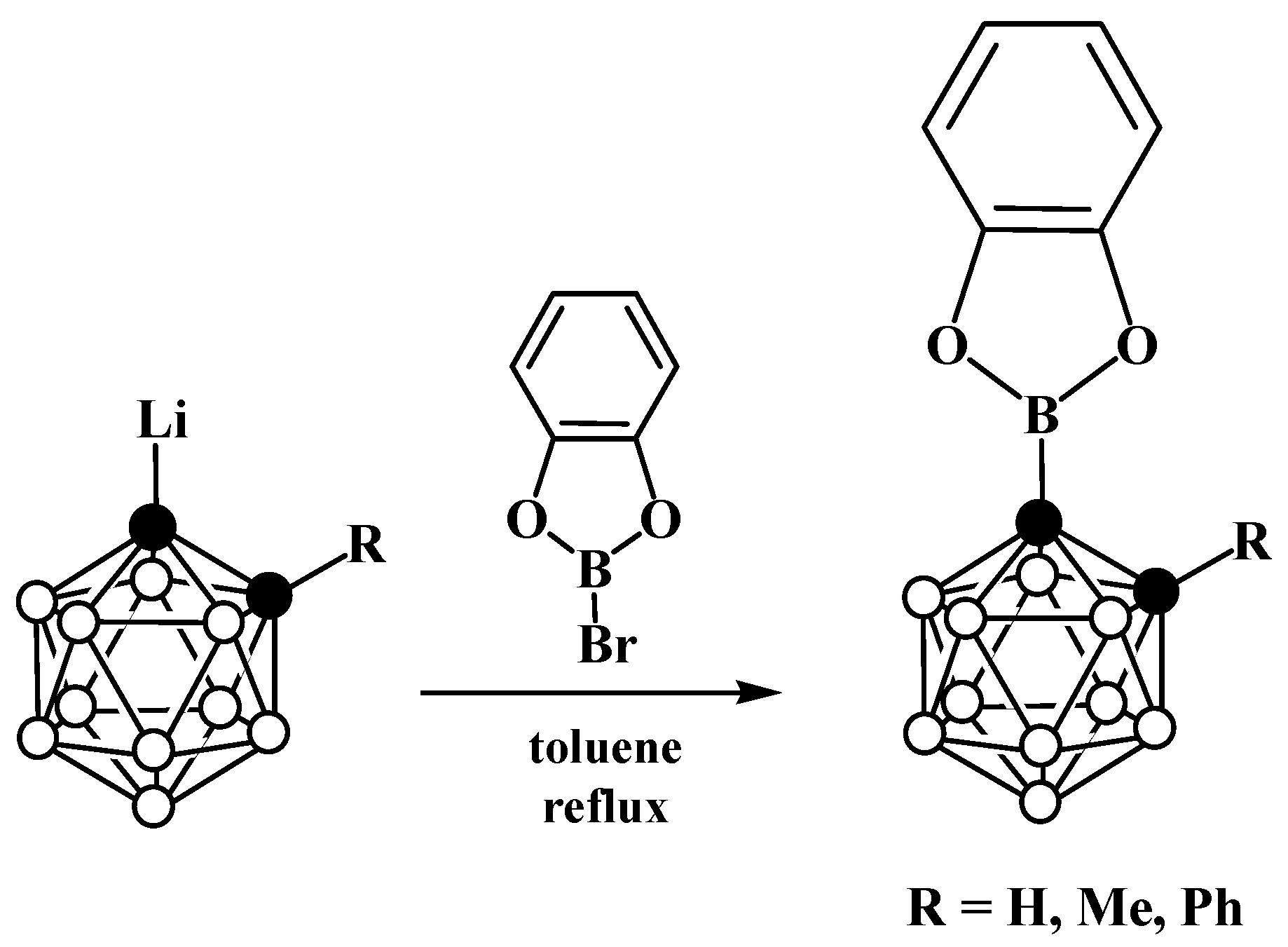
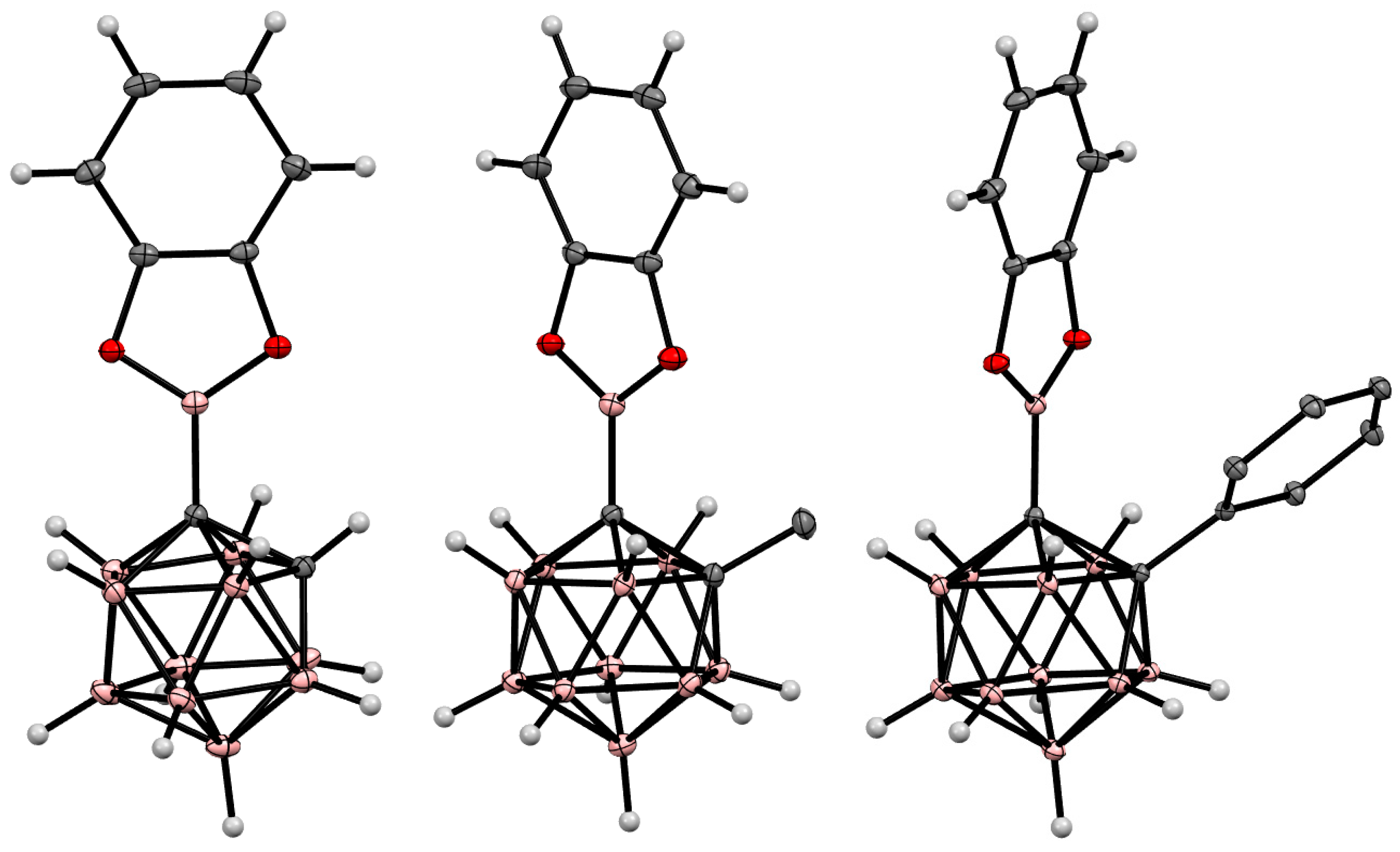
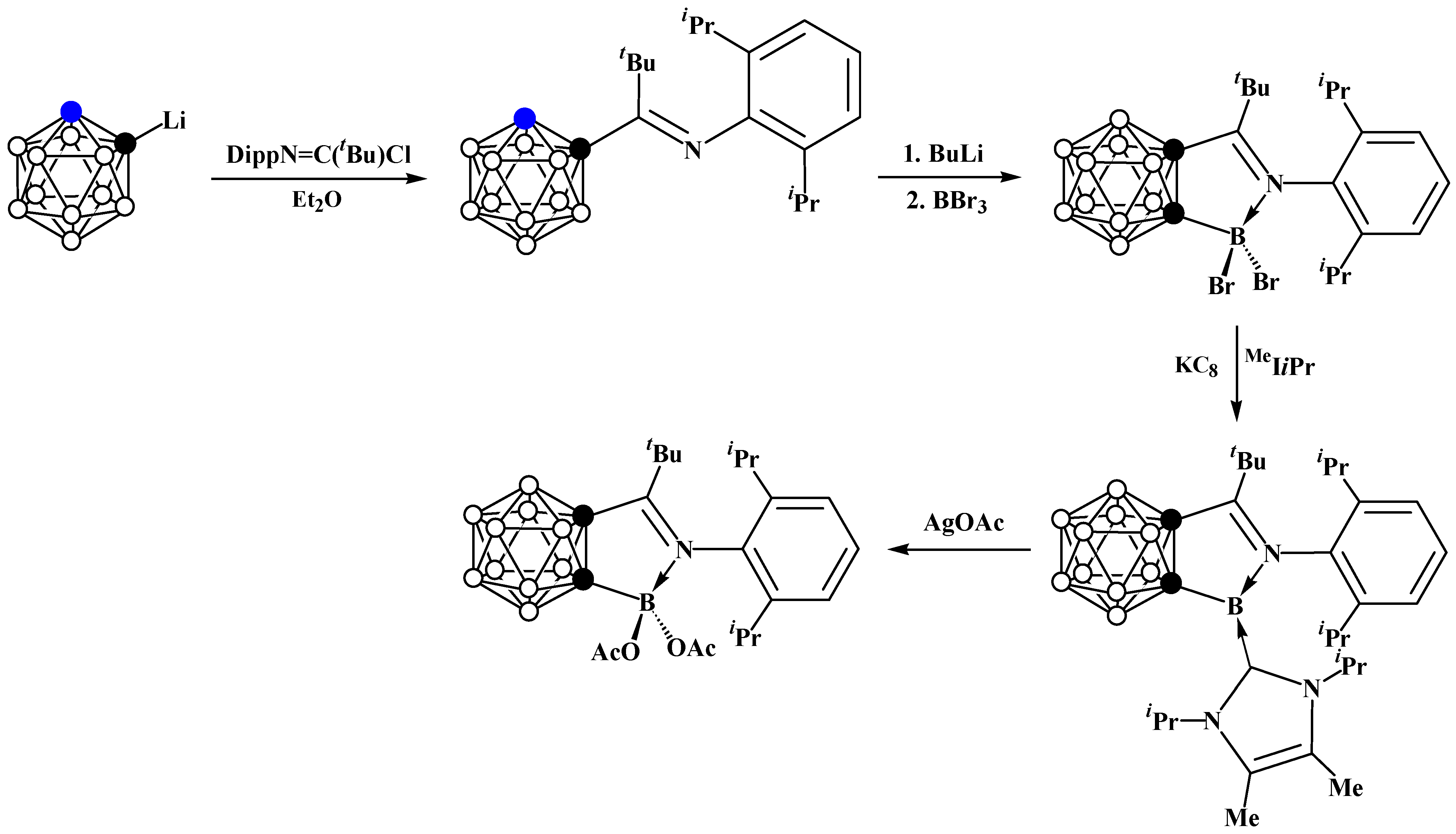
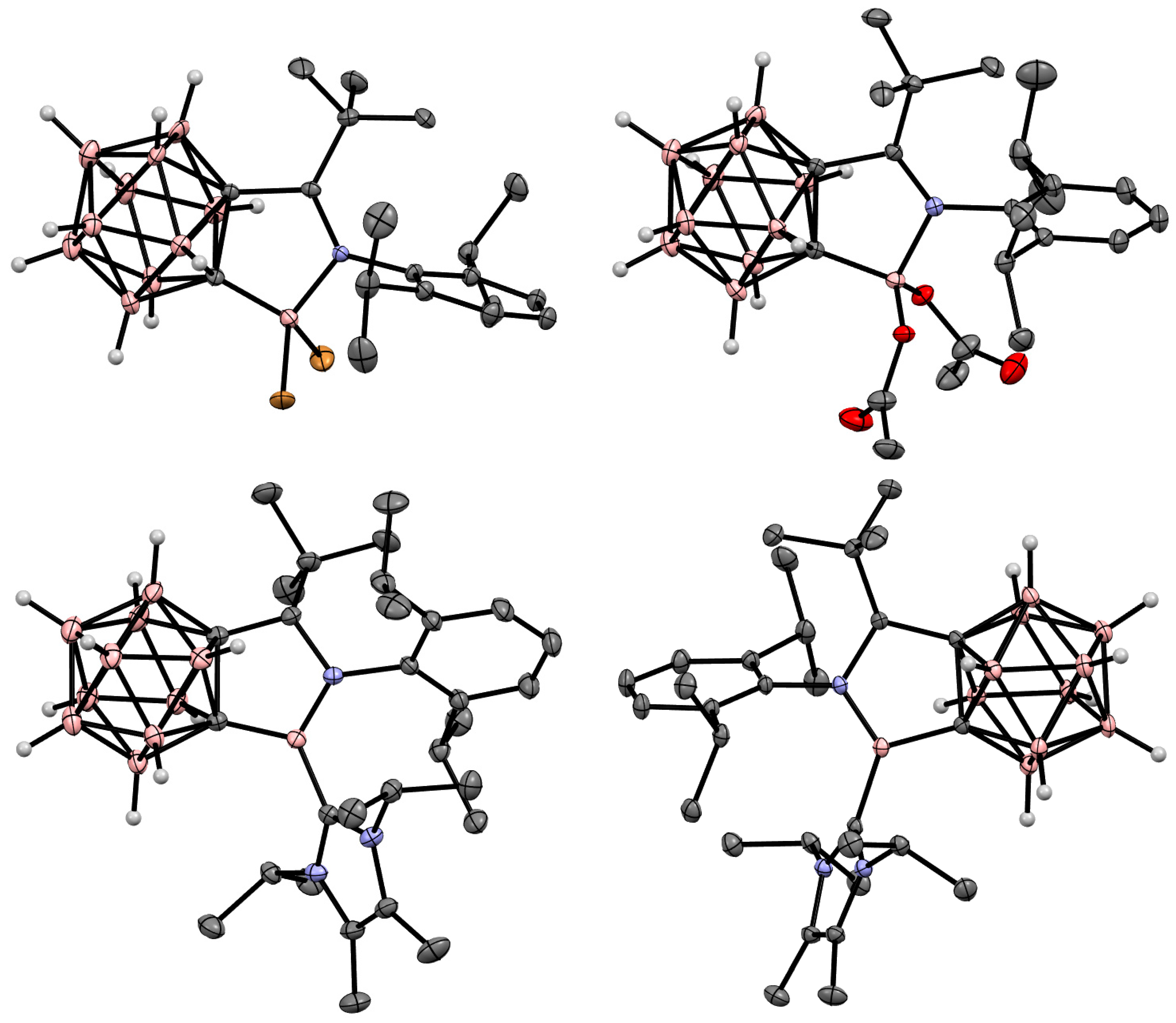
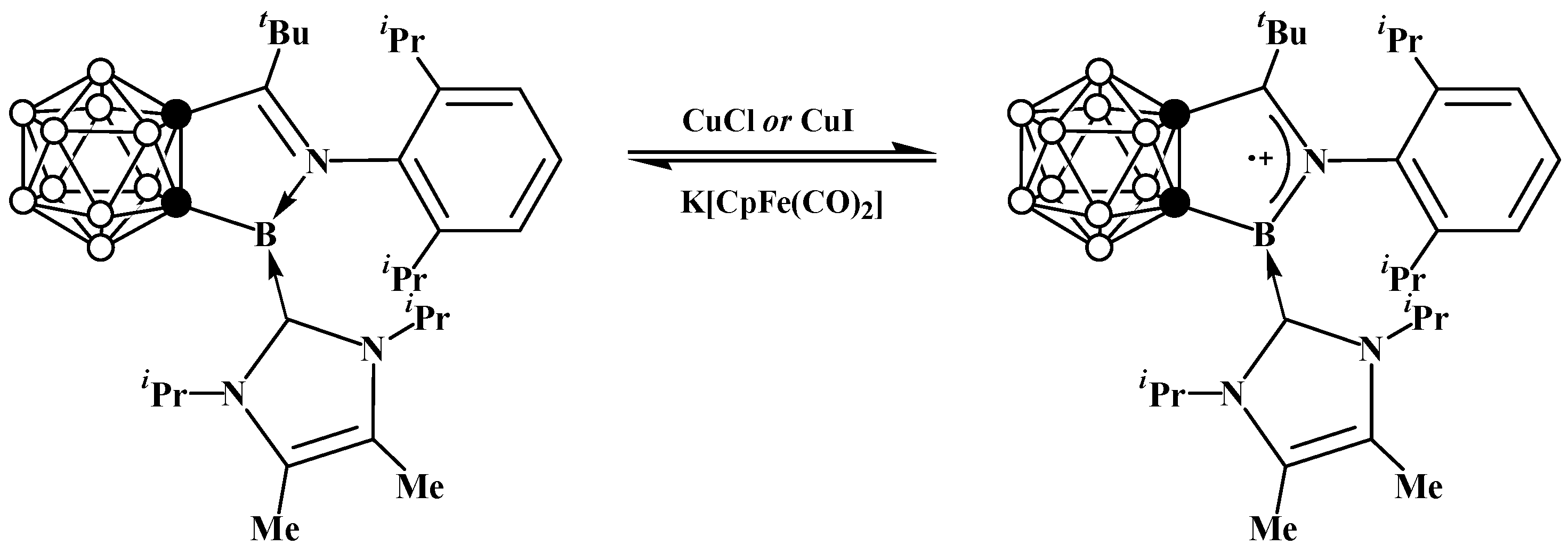
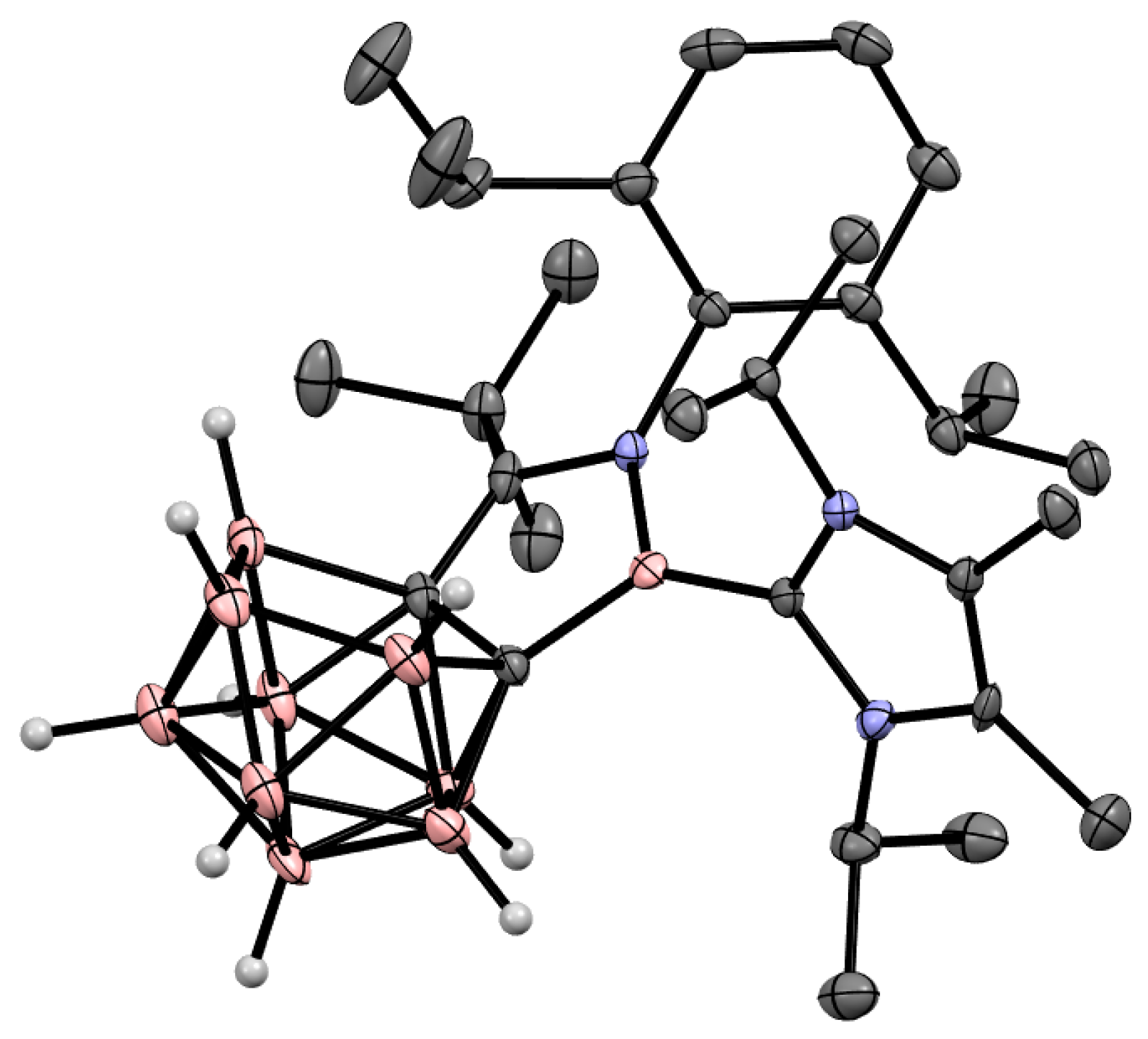
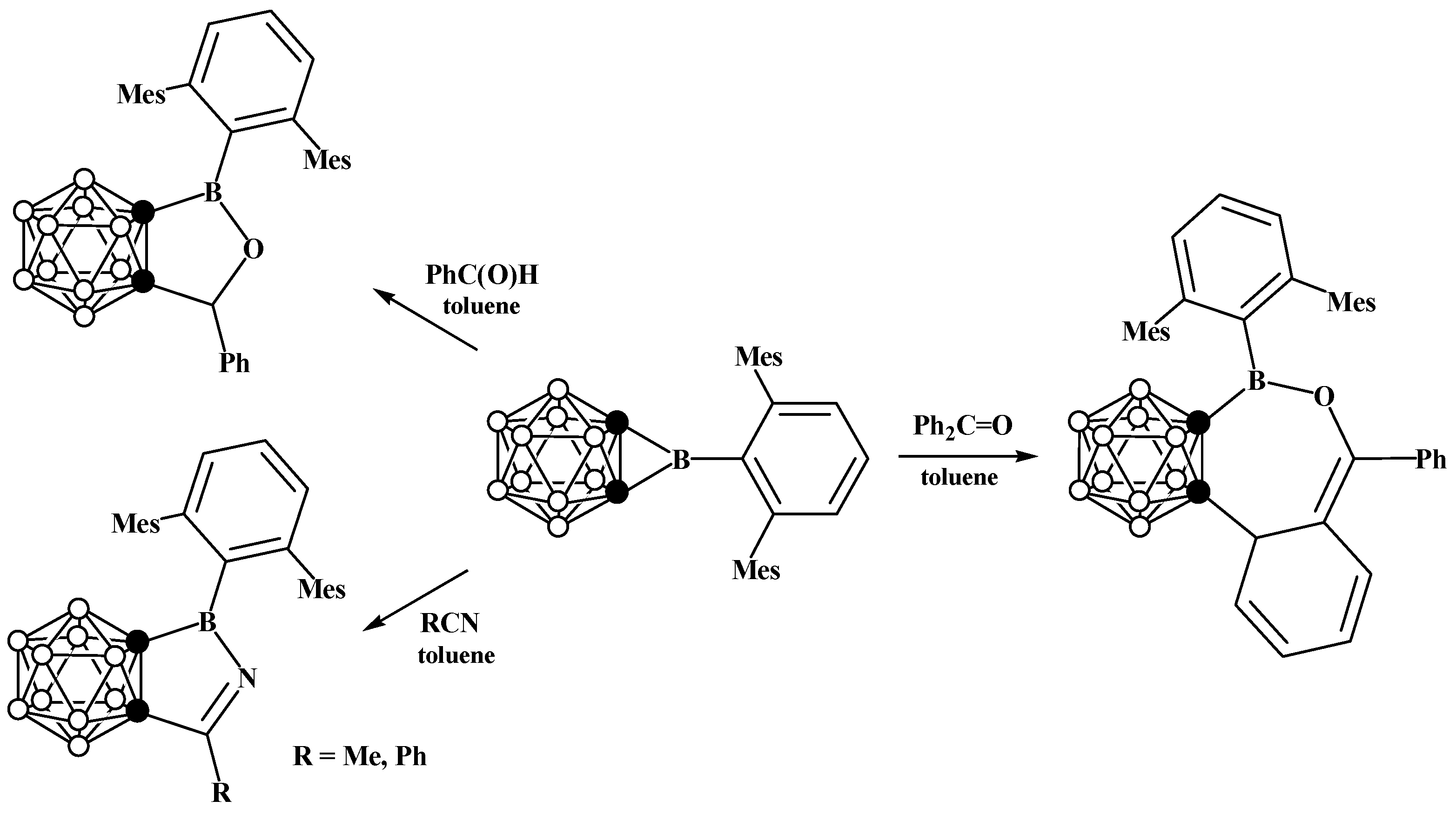
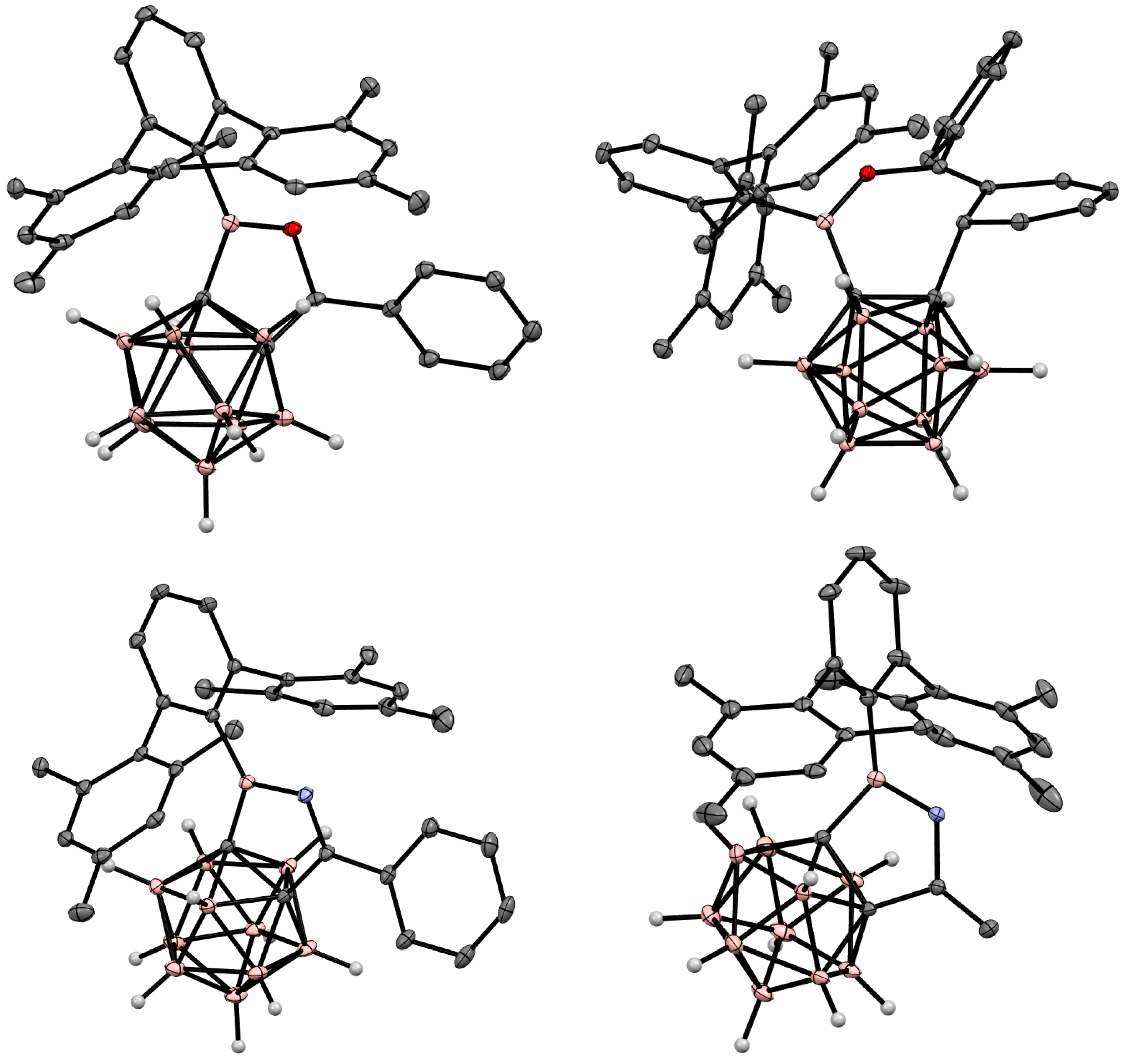
6. Carboranyl-Fused Boron-Containing Heterocycles
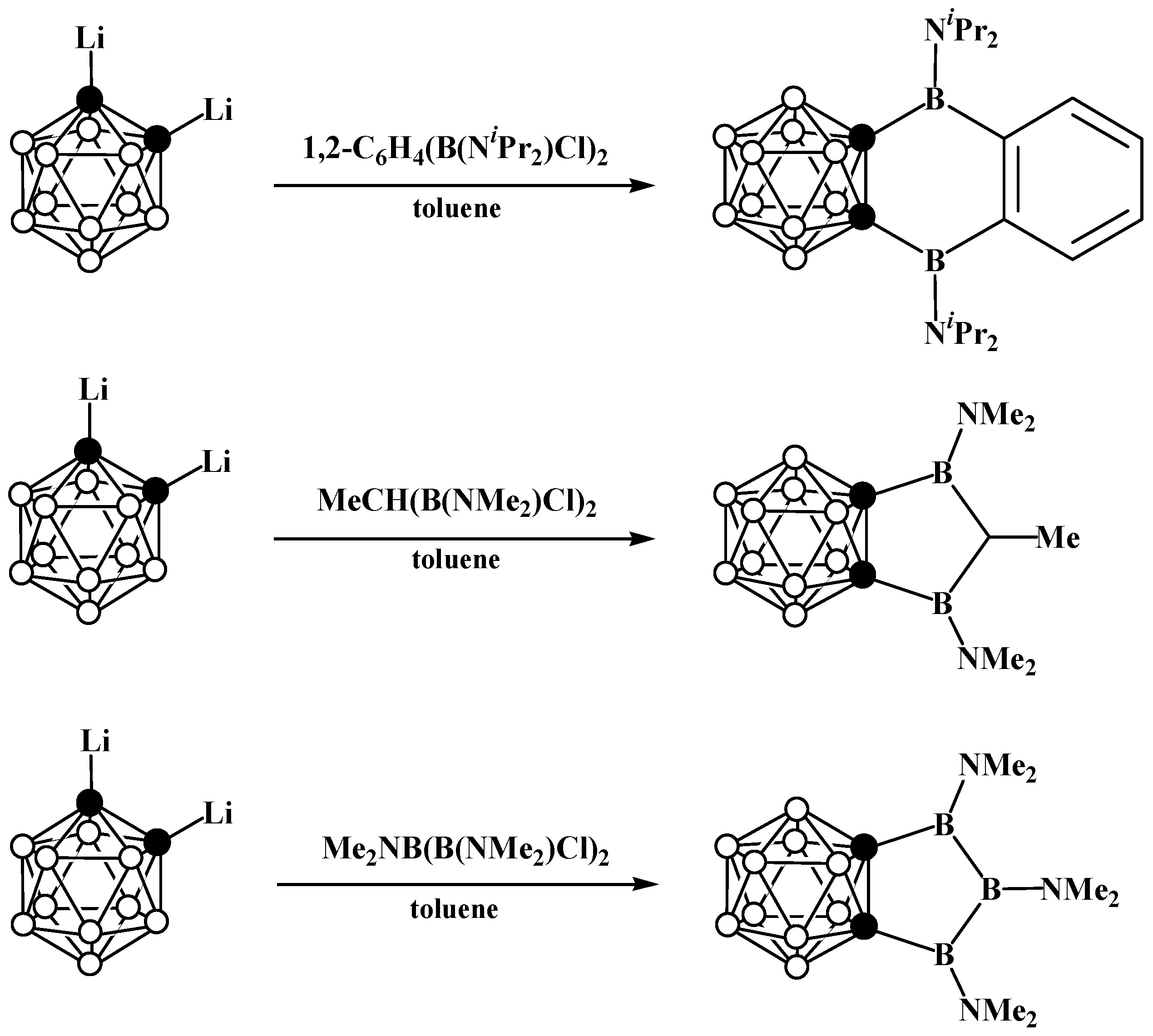
7. Bis(Carboranyl)-Fused Boron-Containing Heterocycles
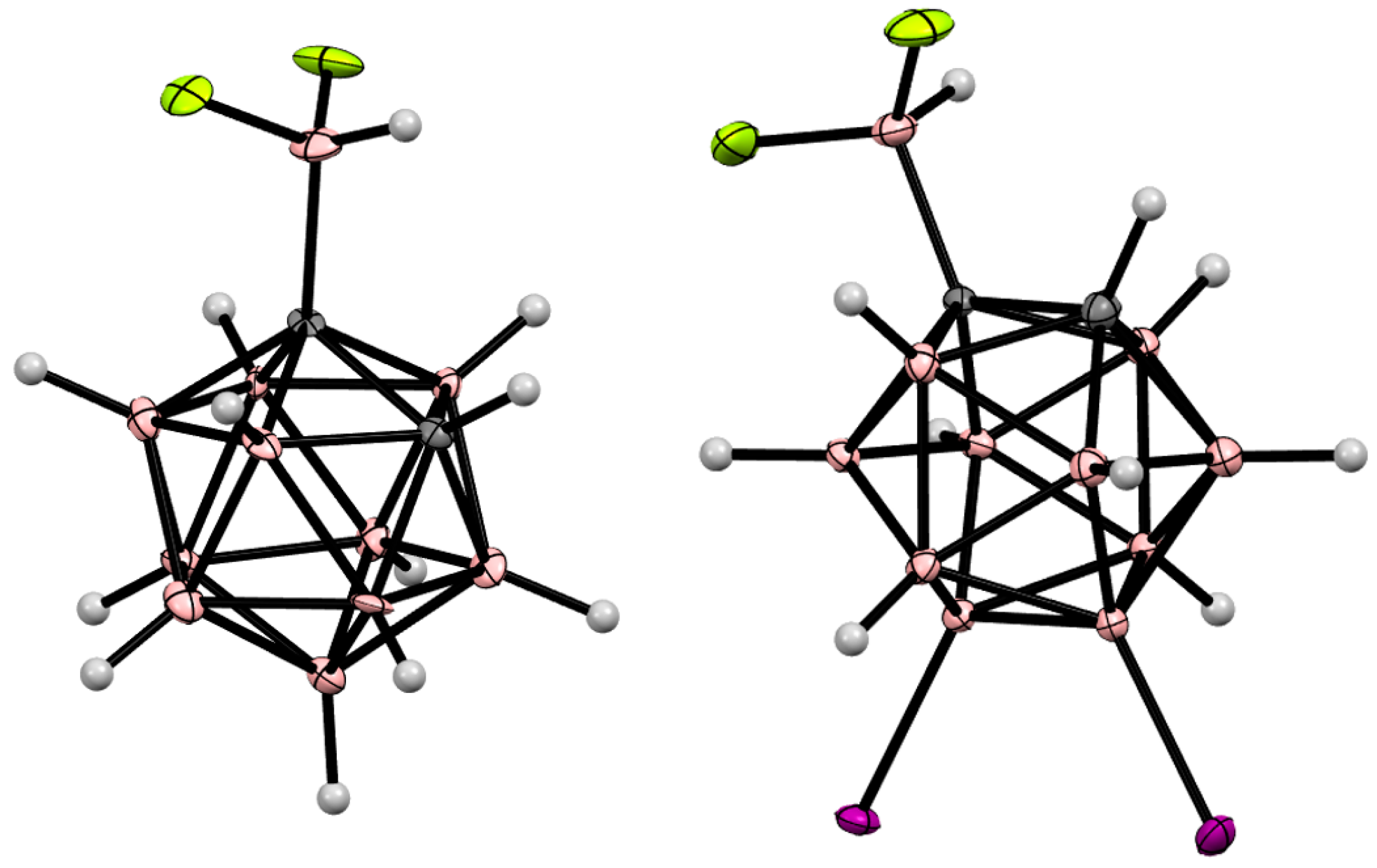
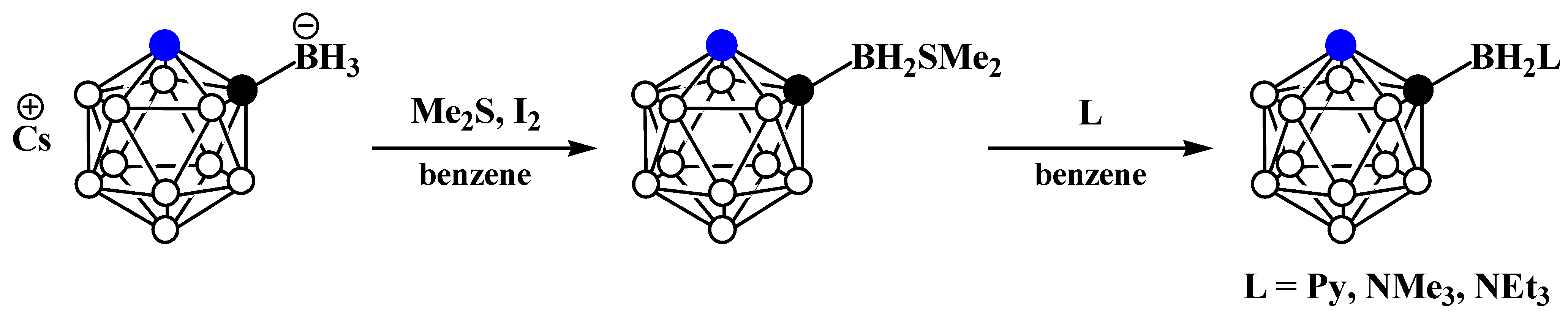
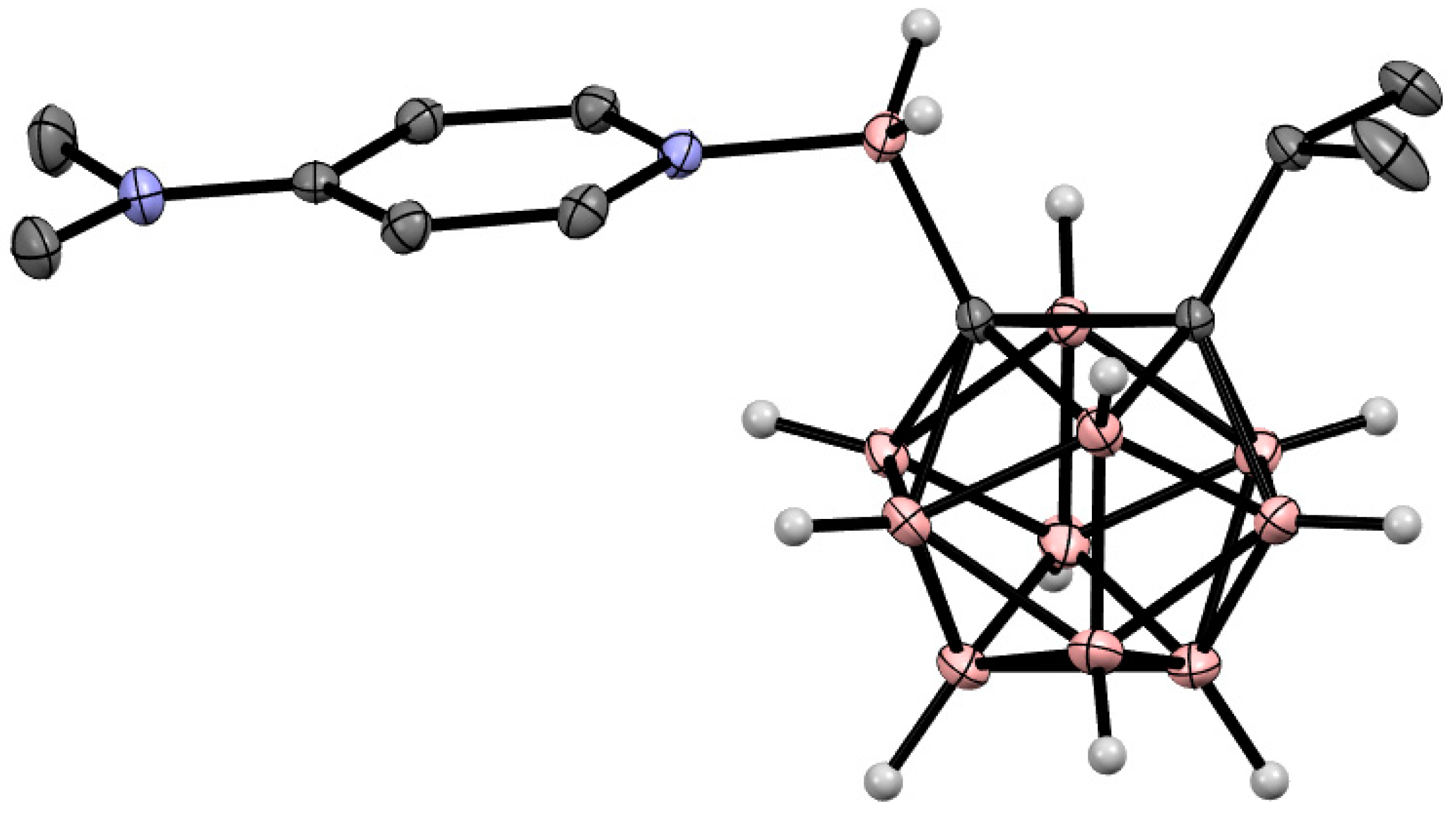
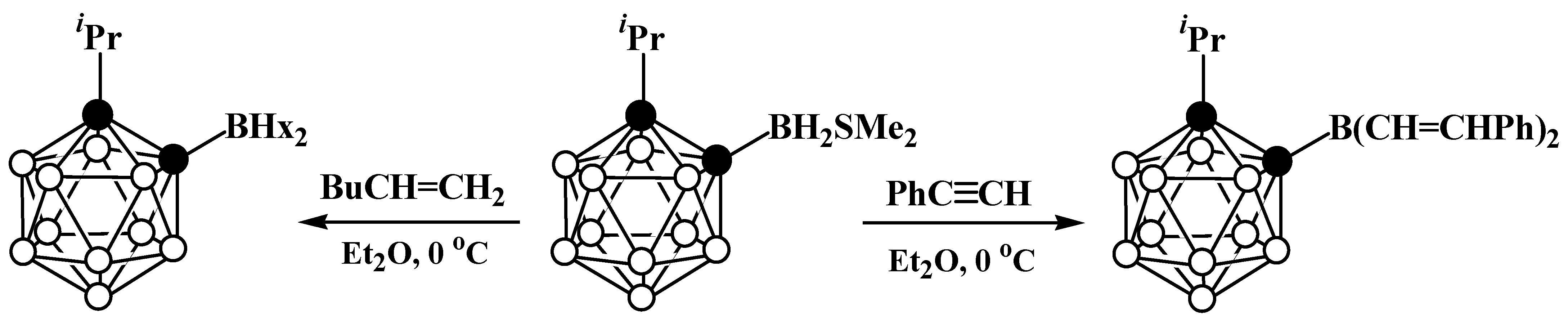
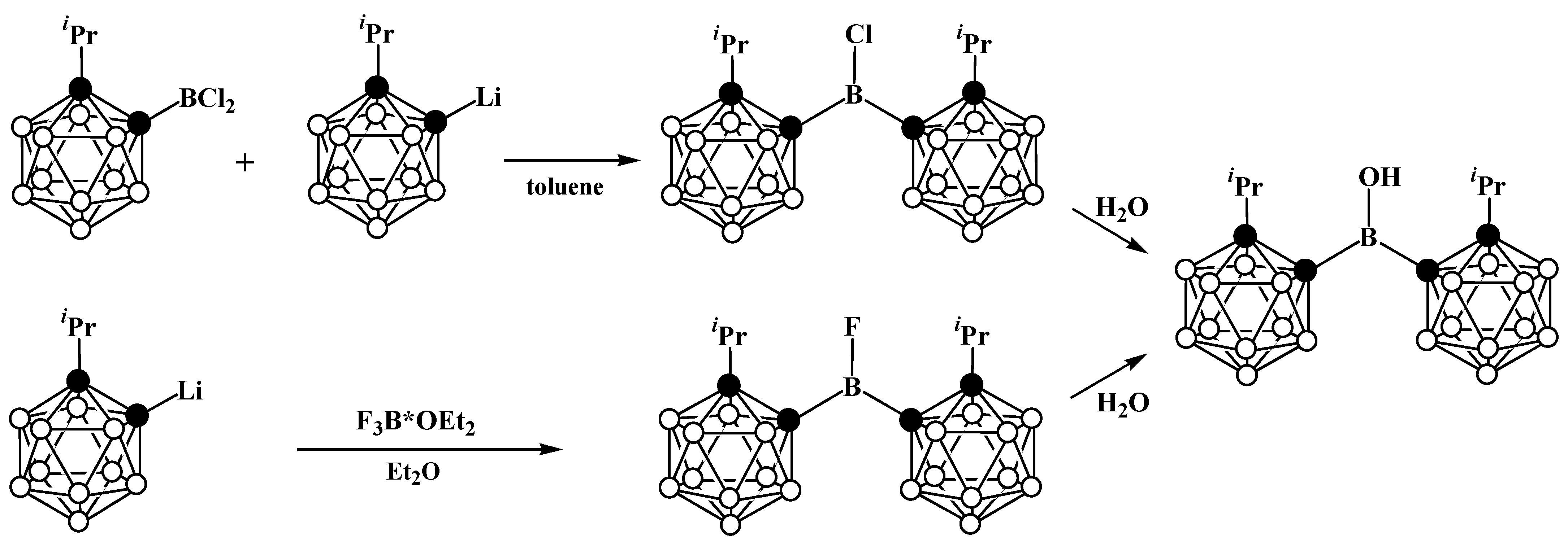
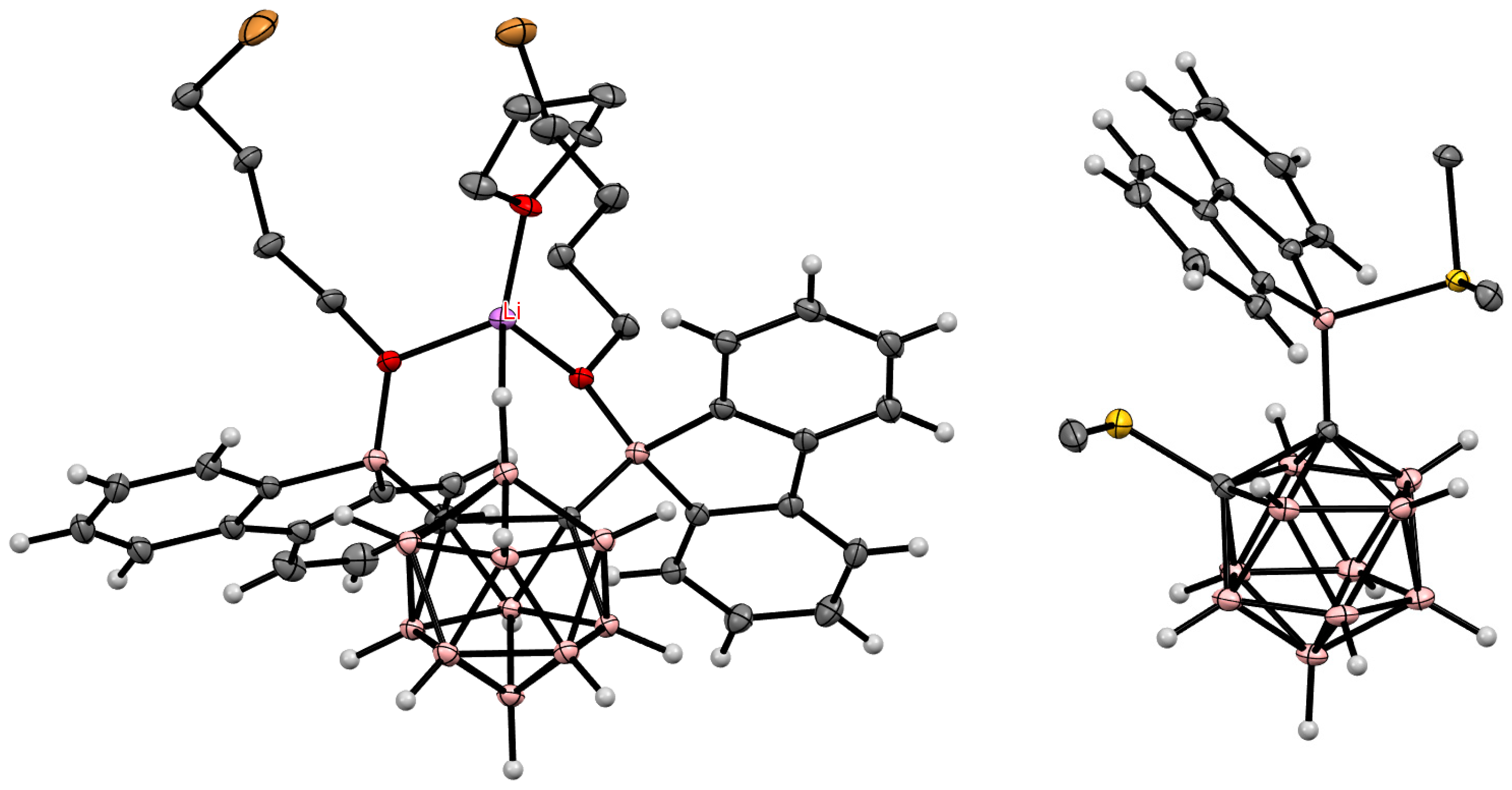
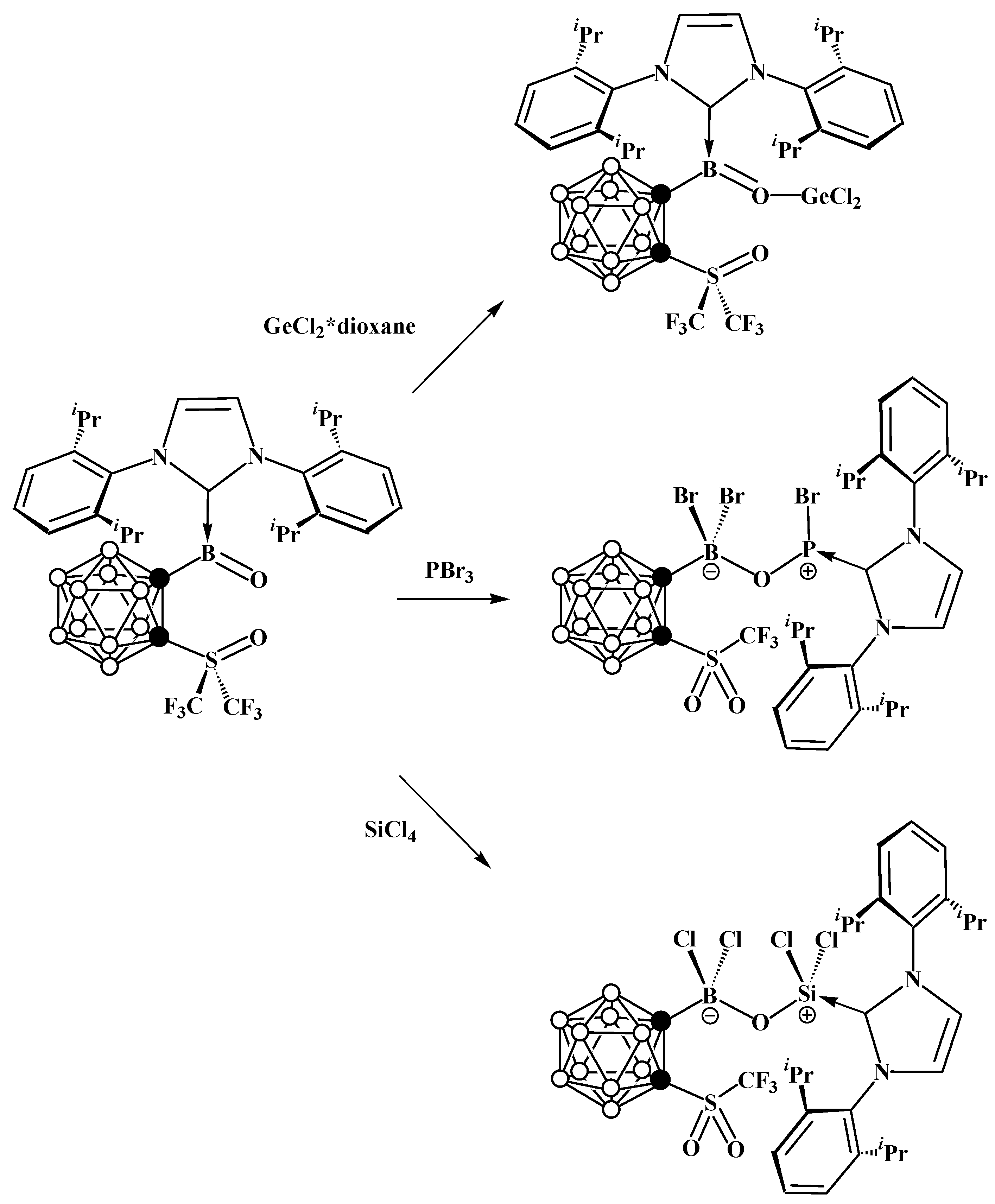
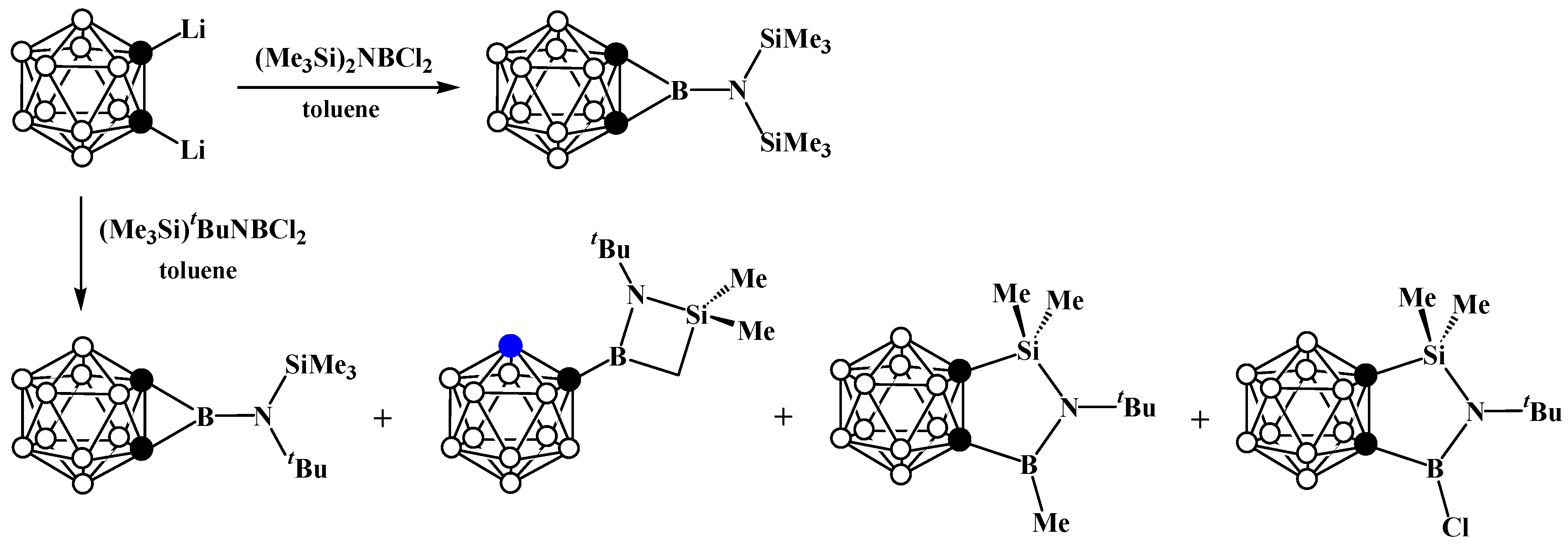
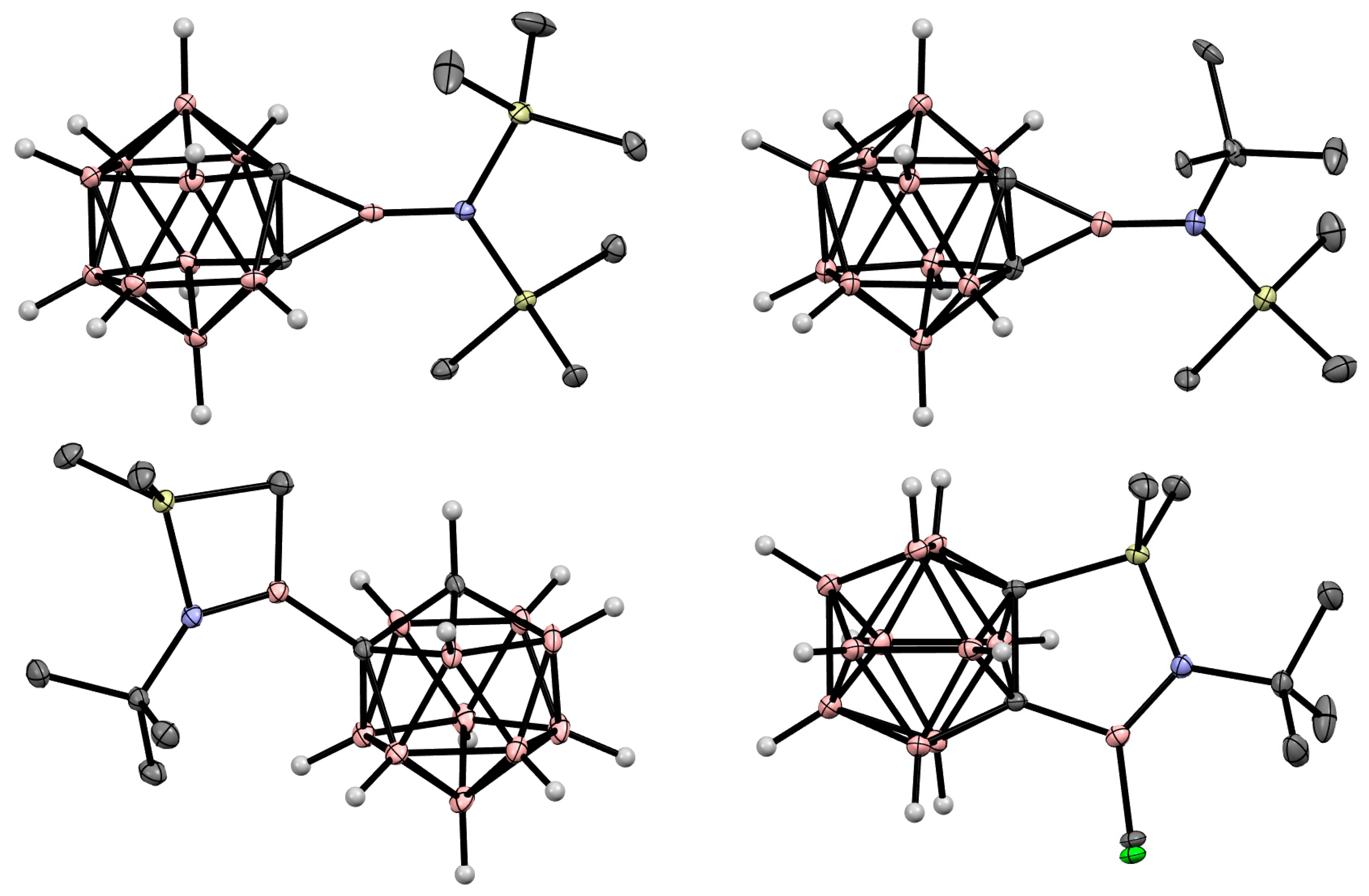
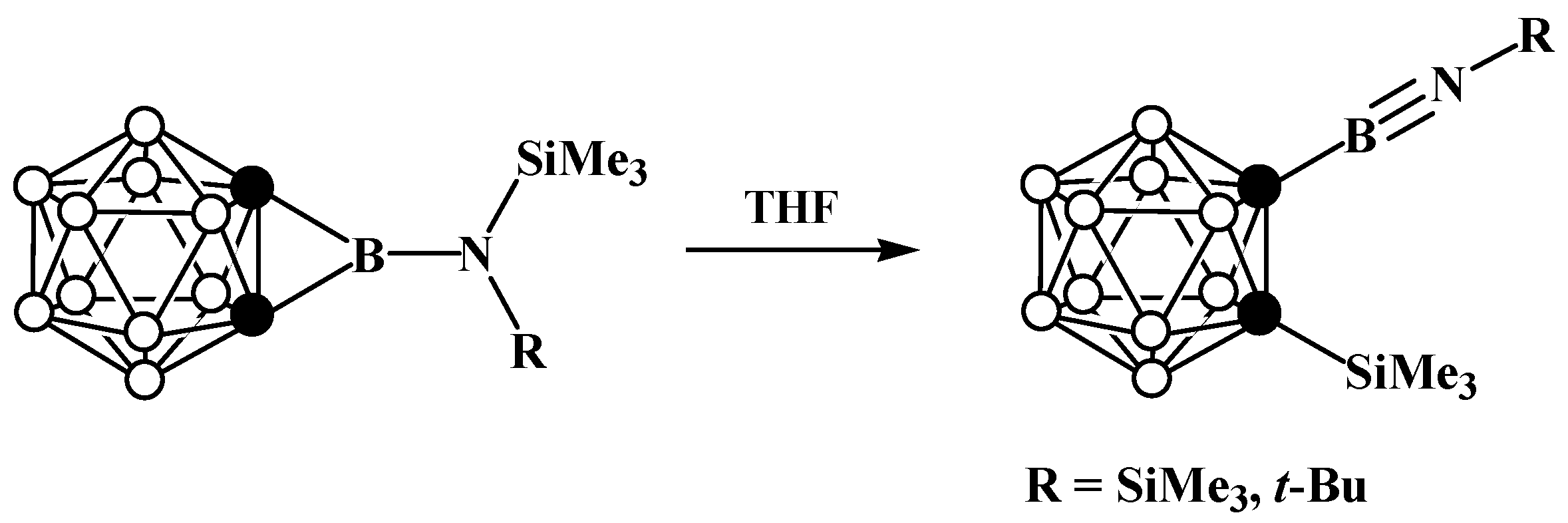
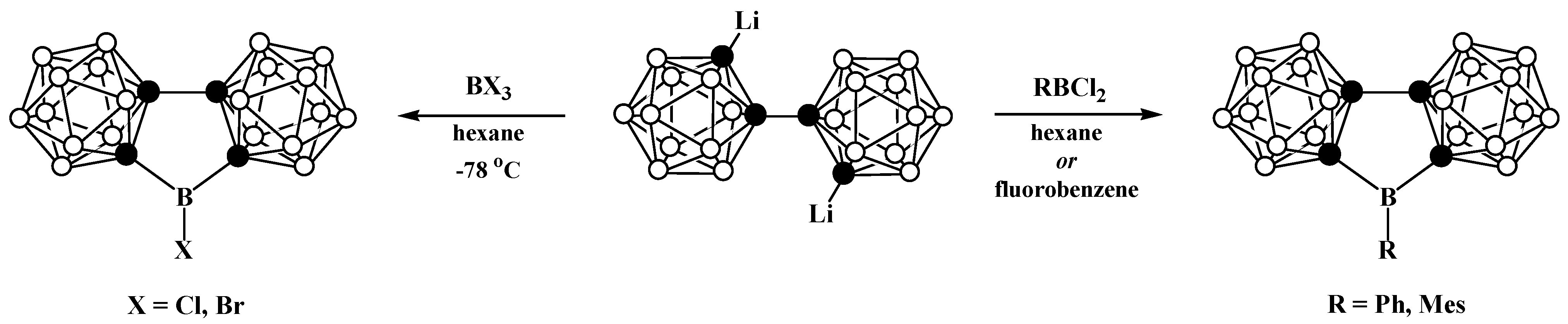
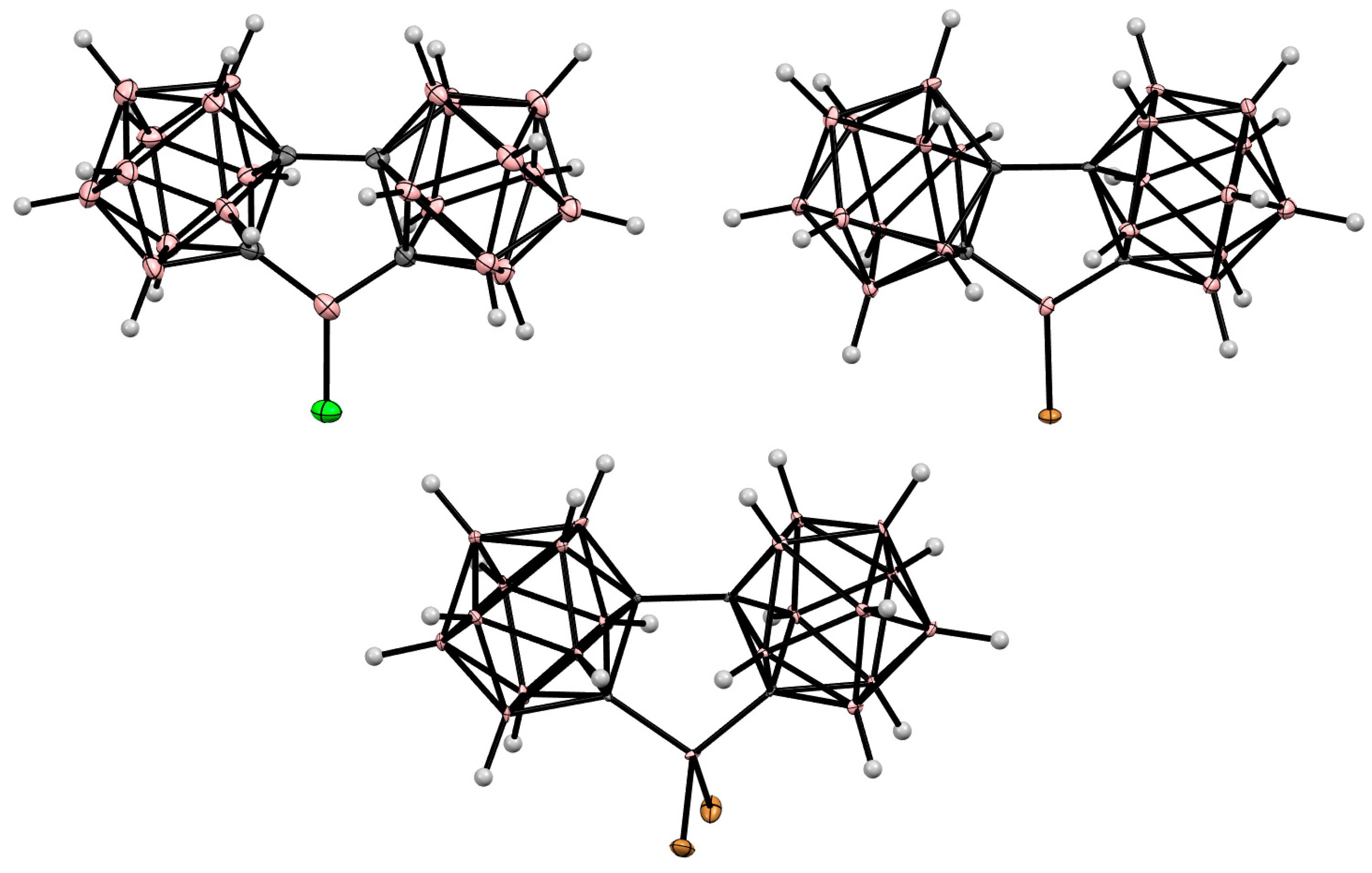
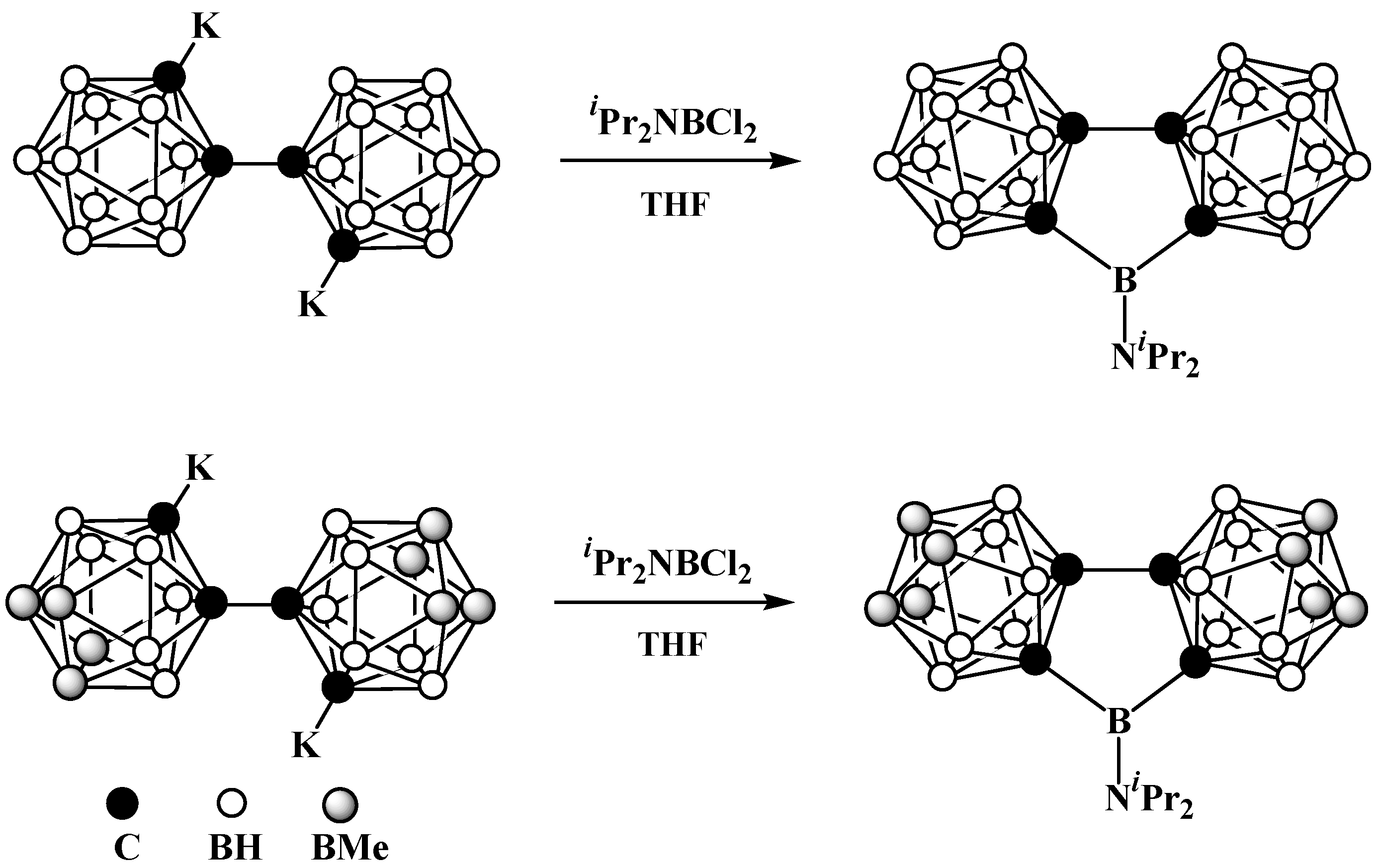
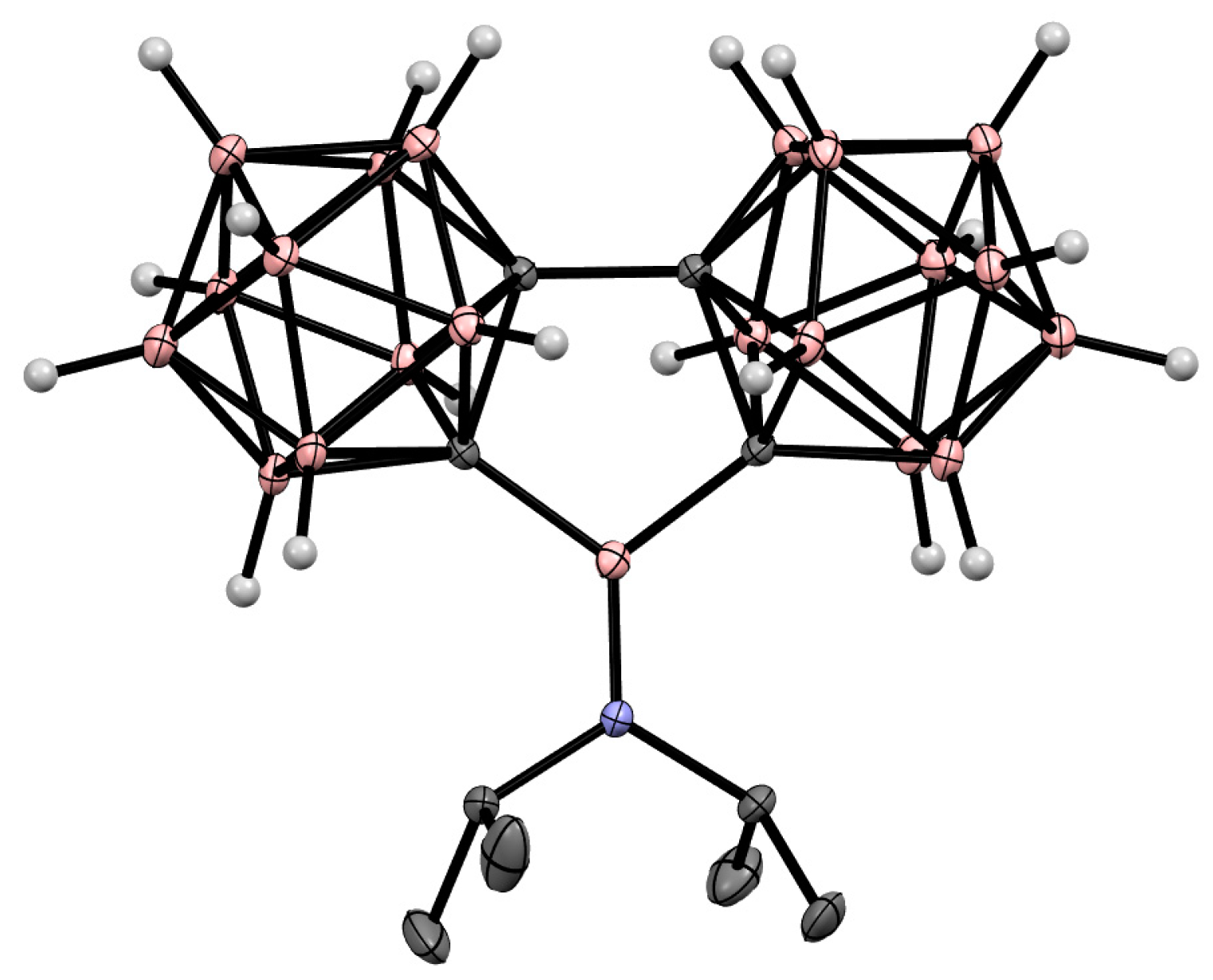
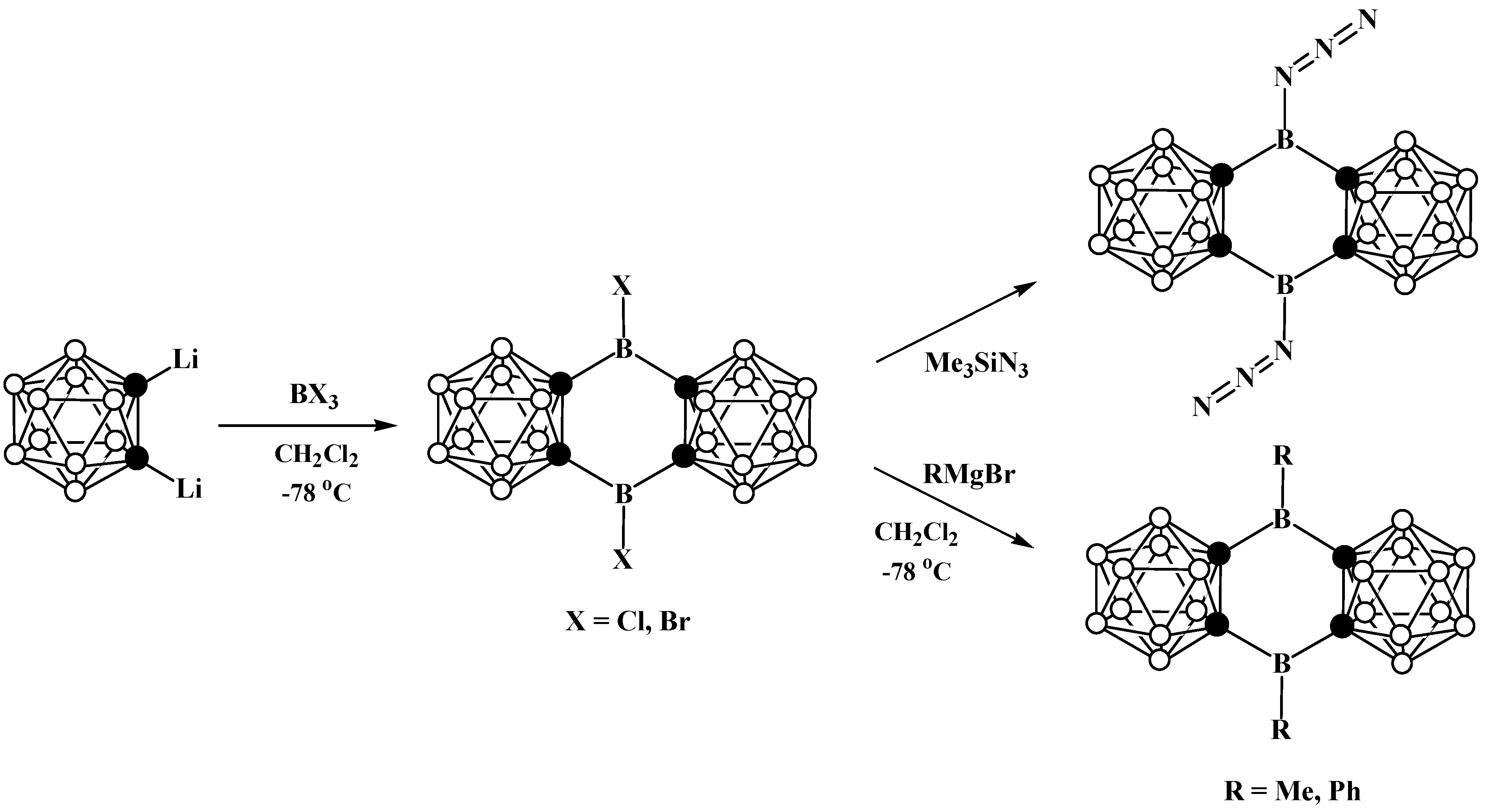
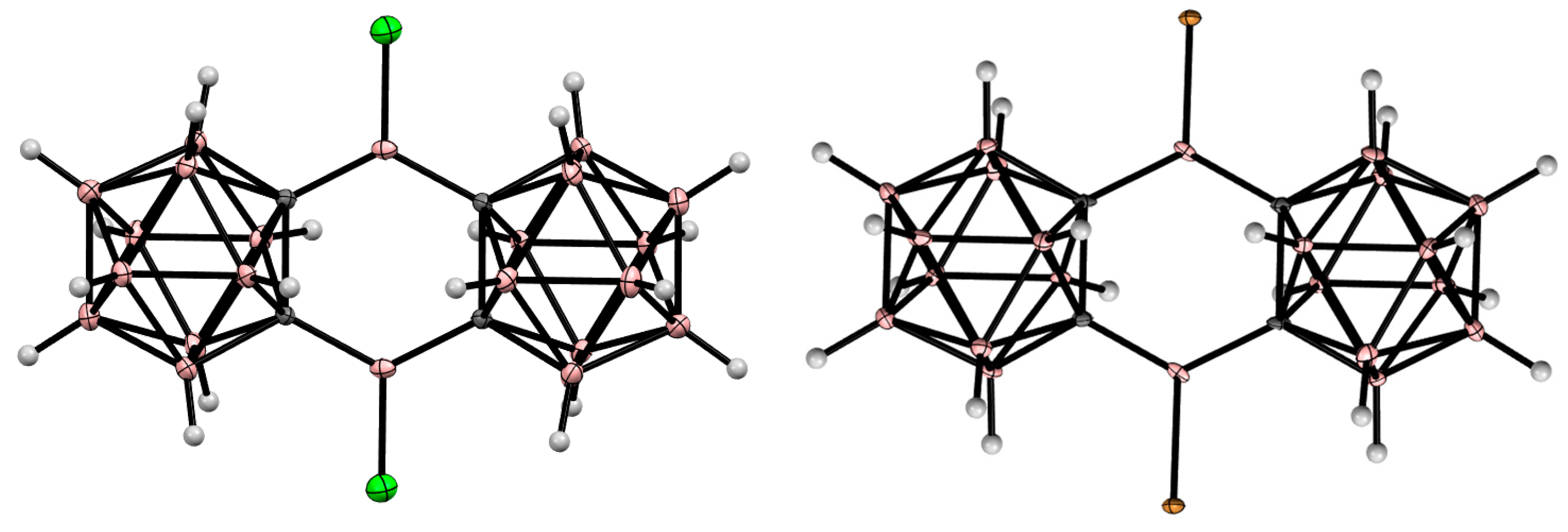
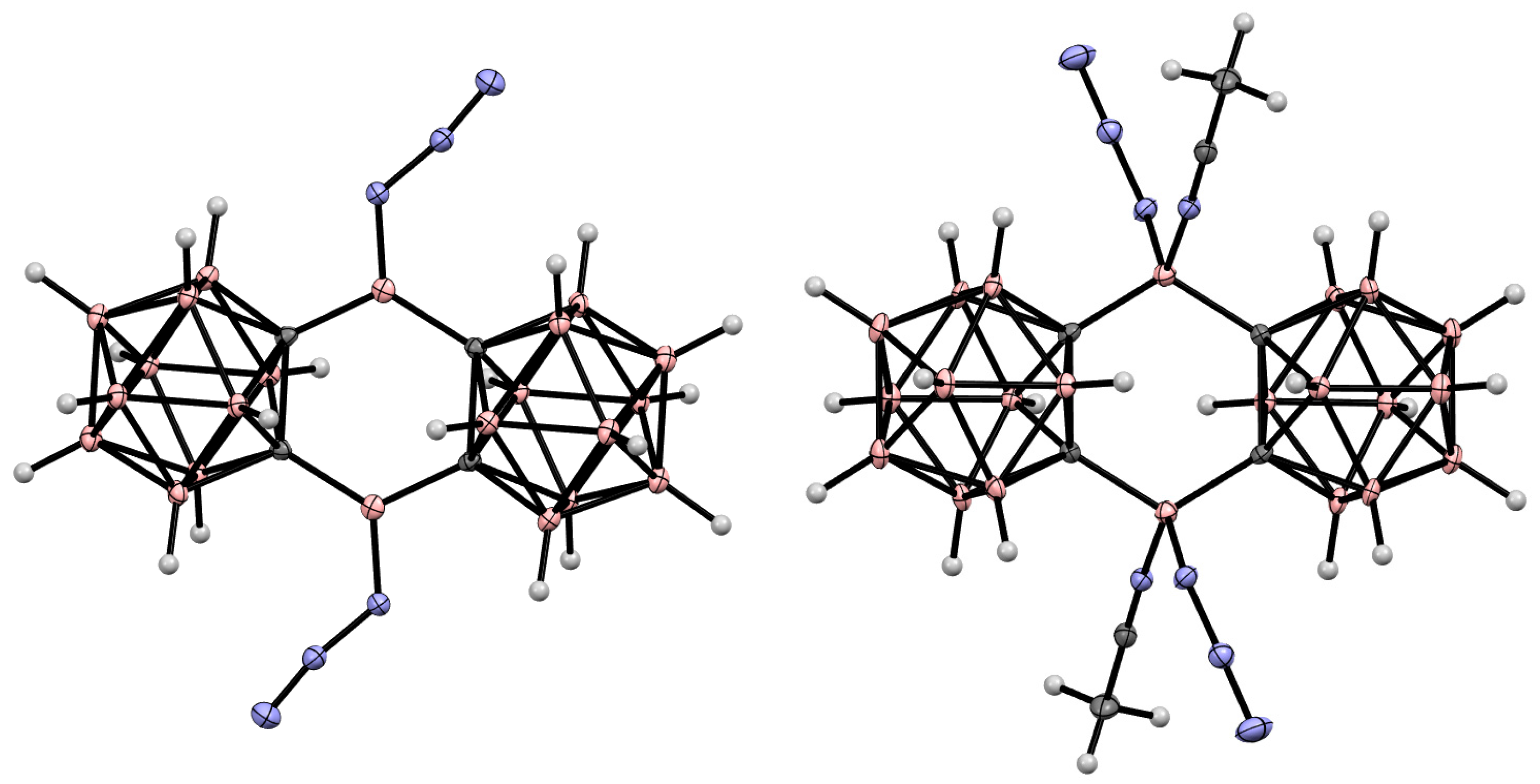
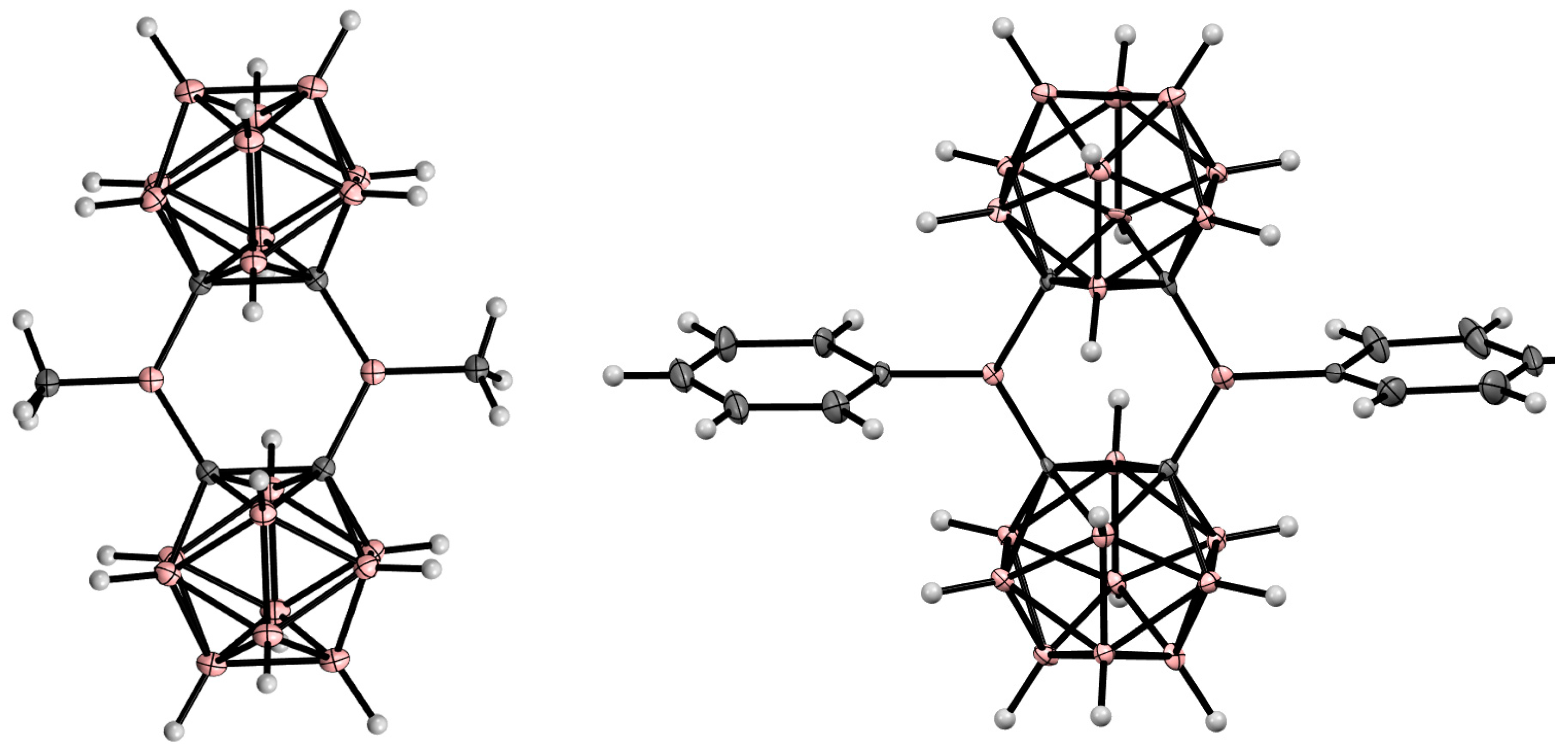
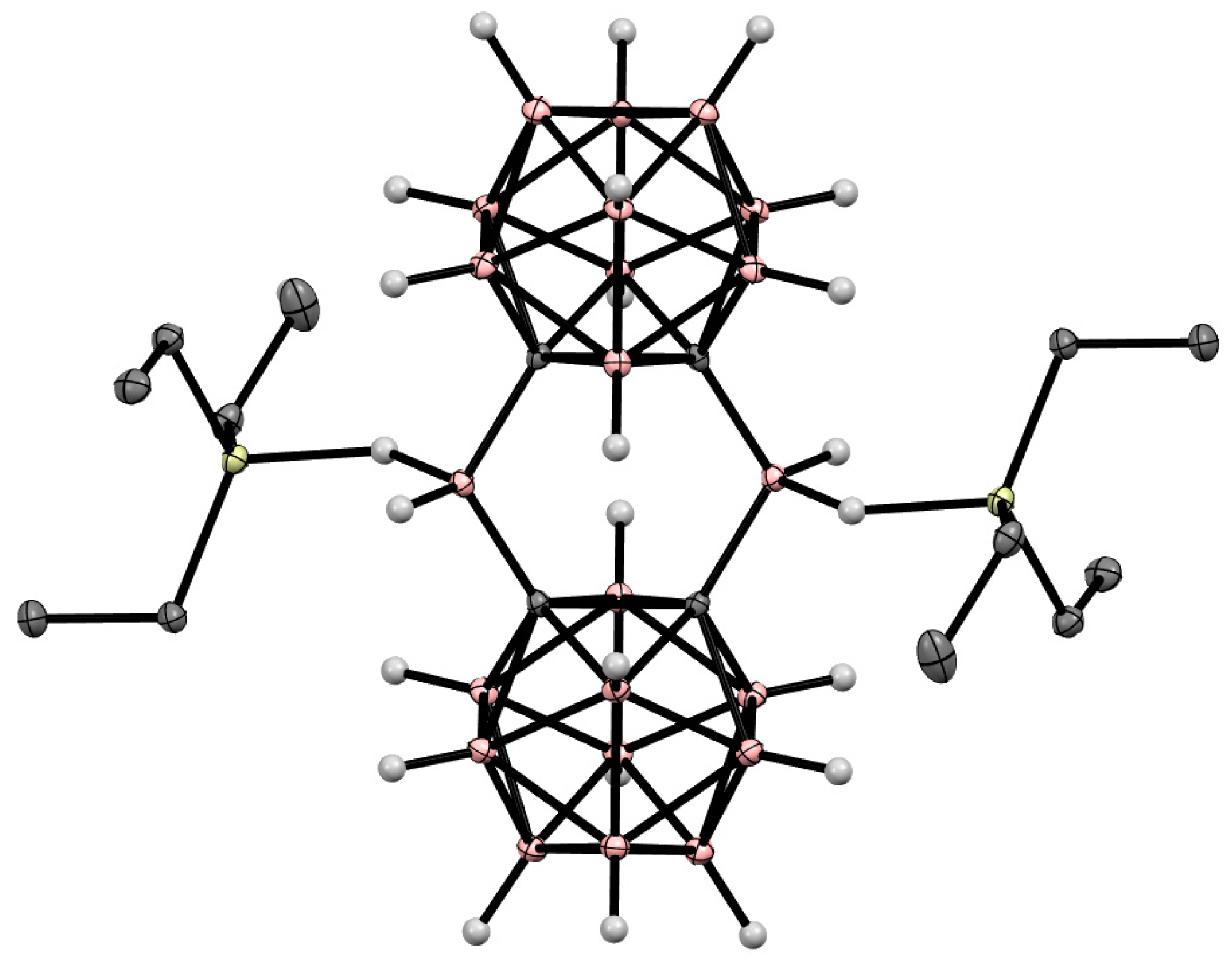
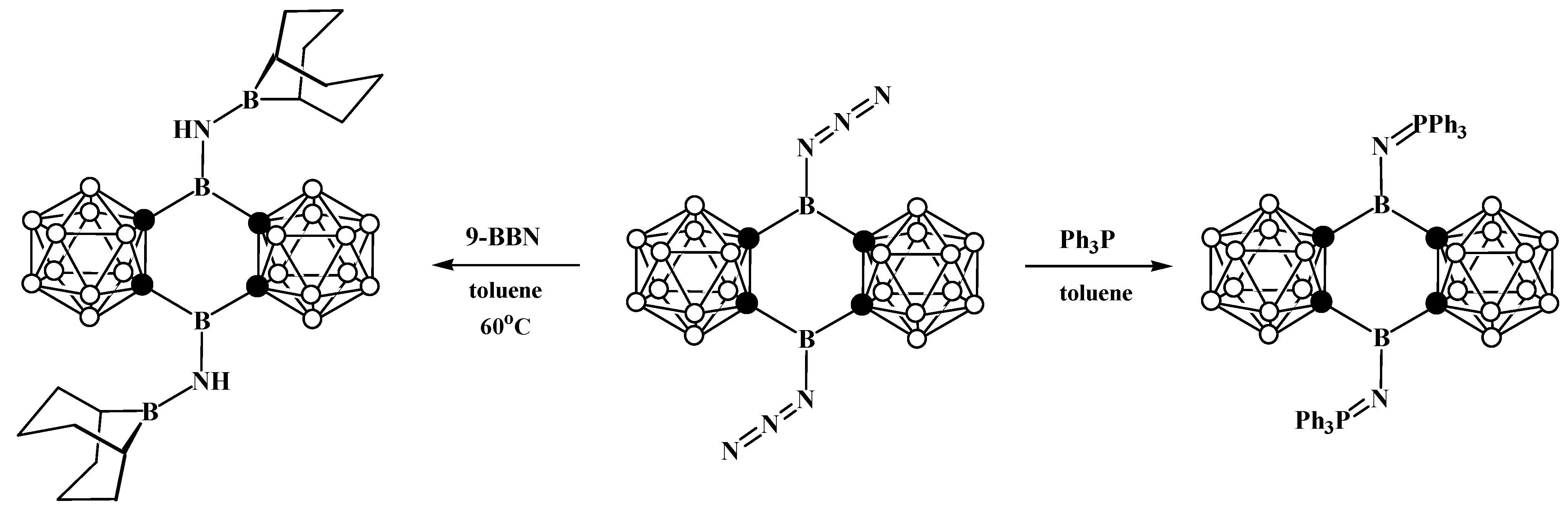
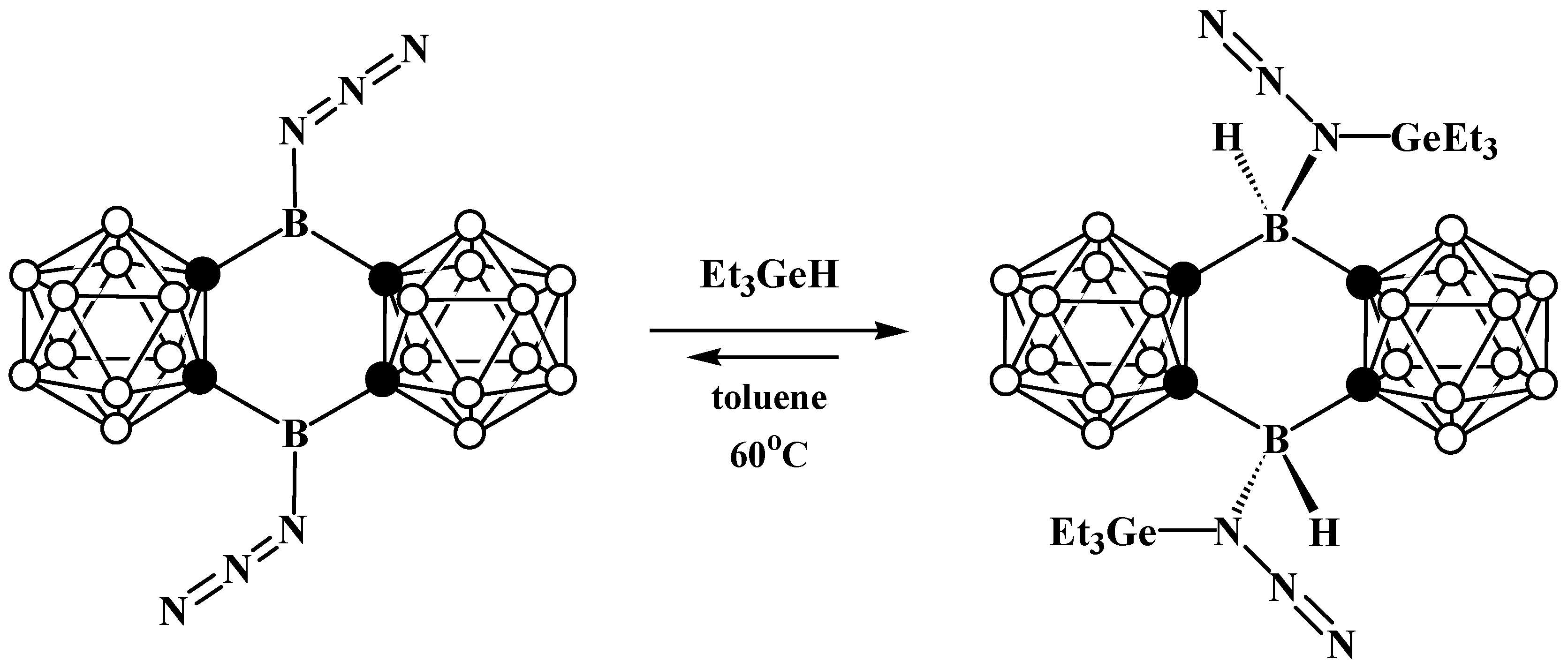
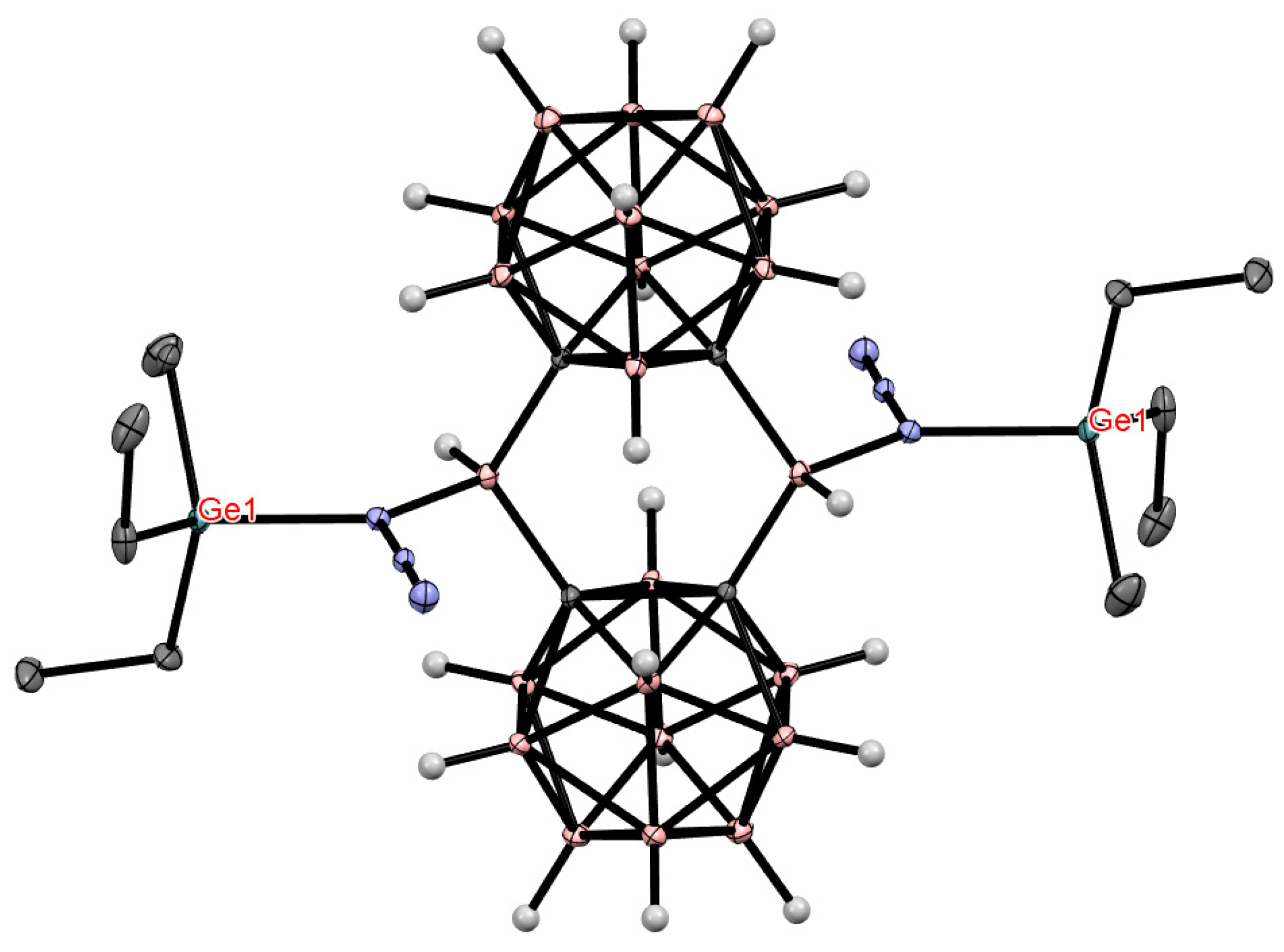
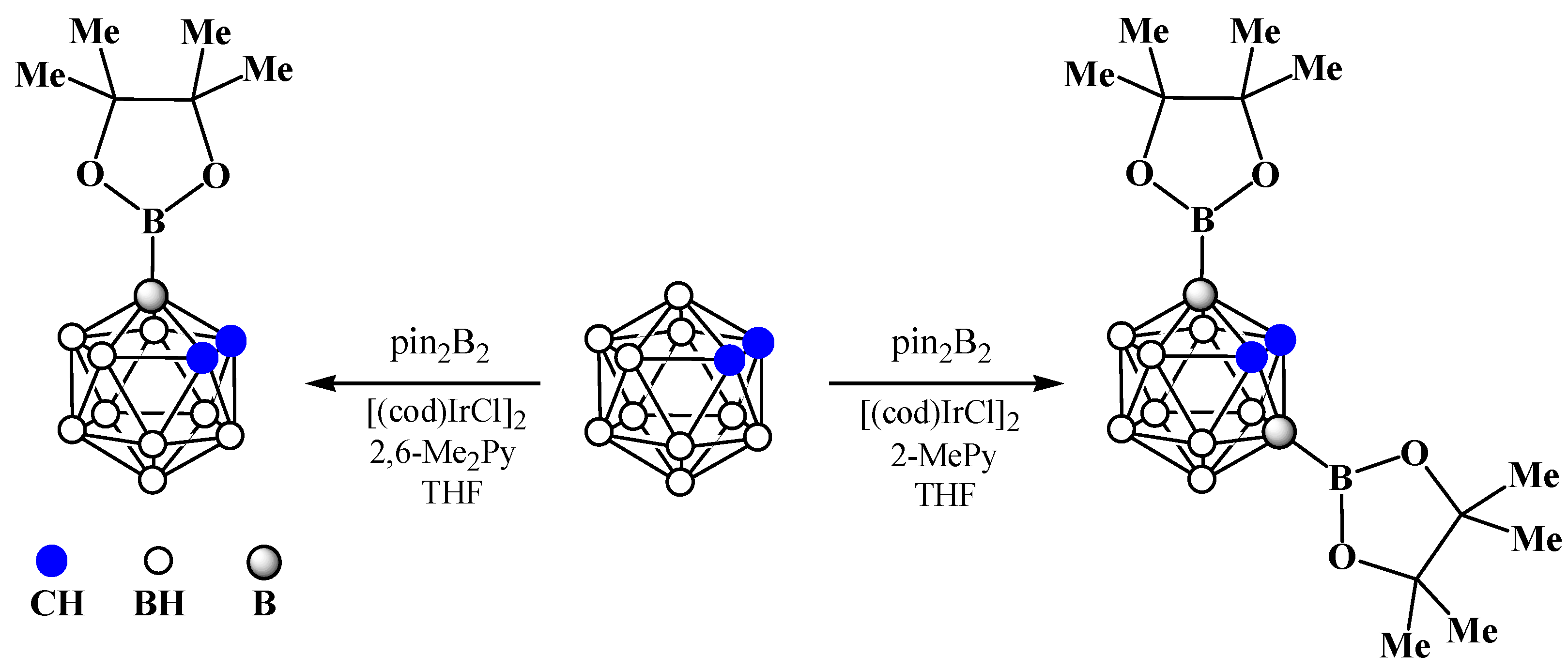
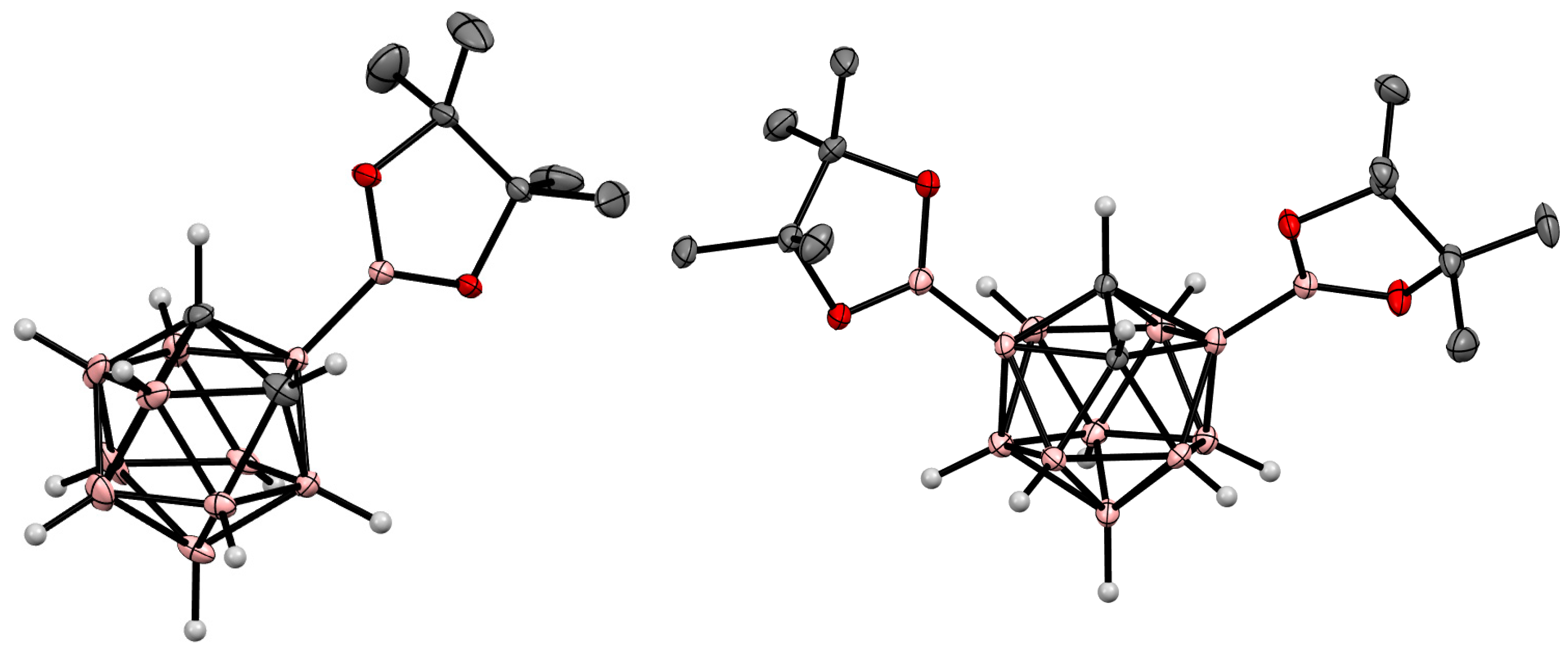
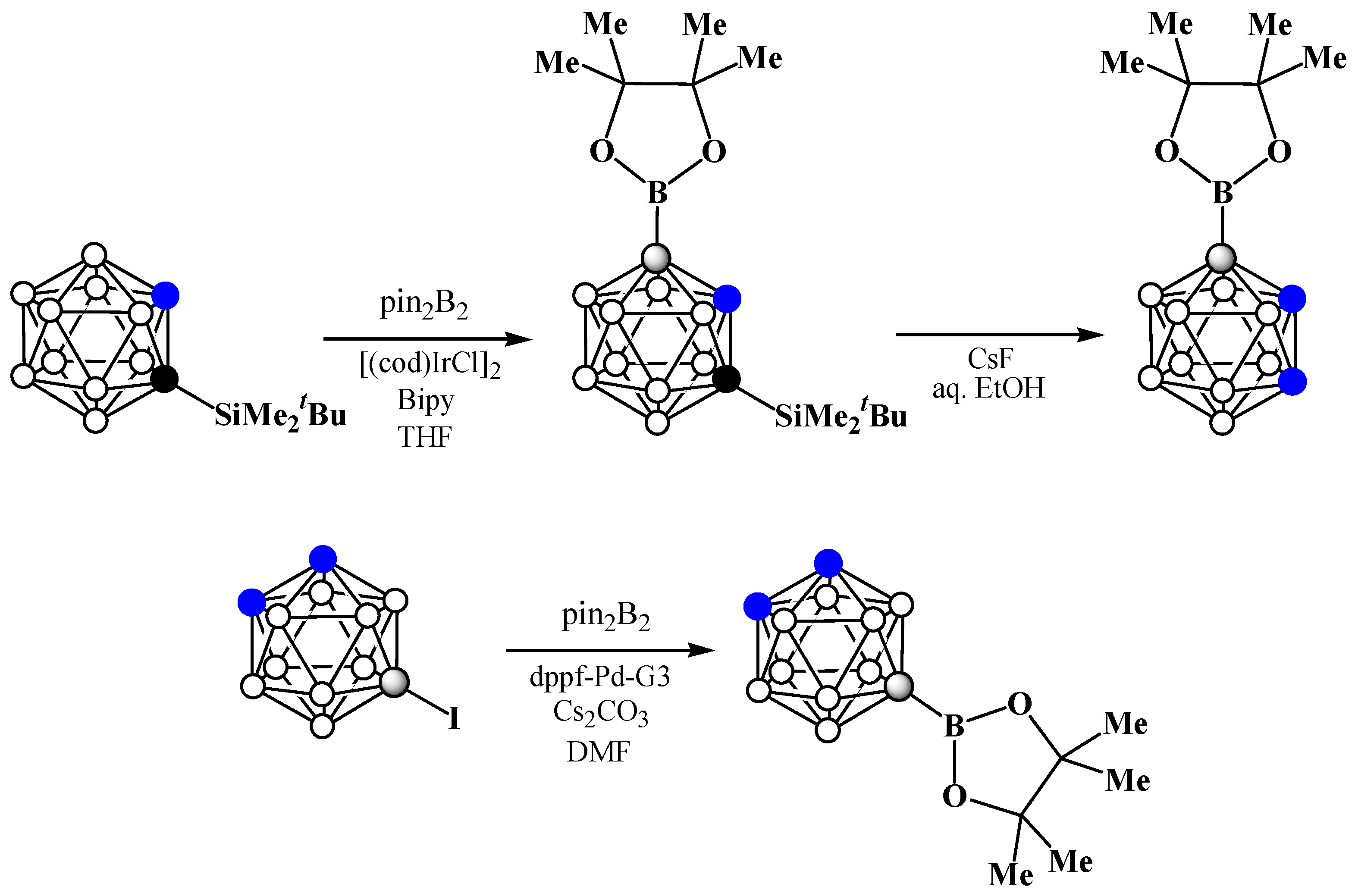
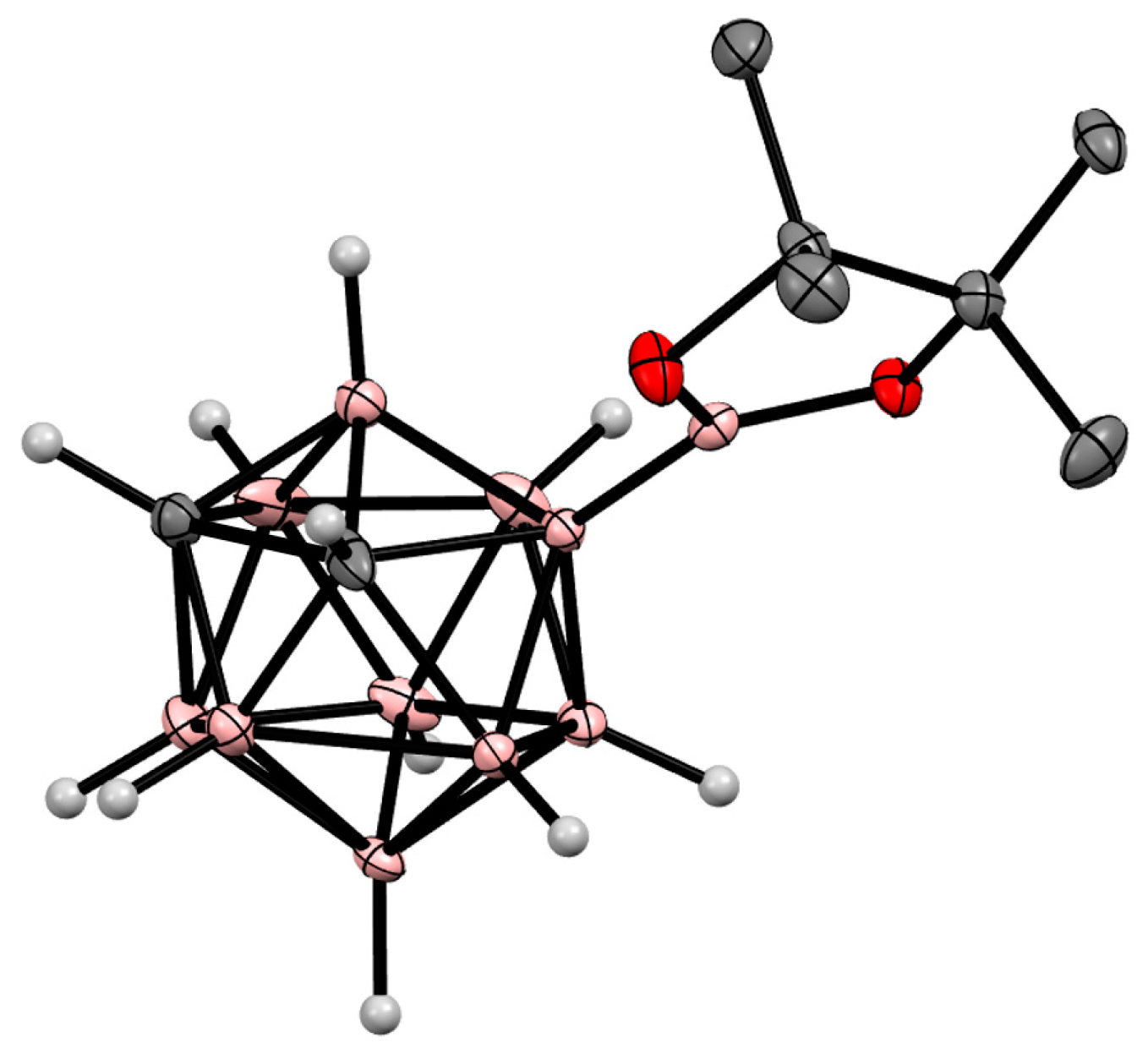
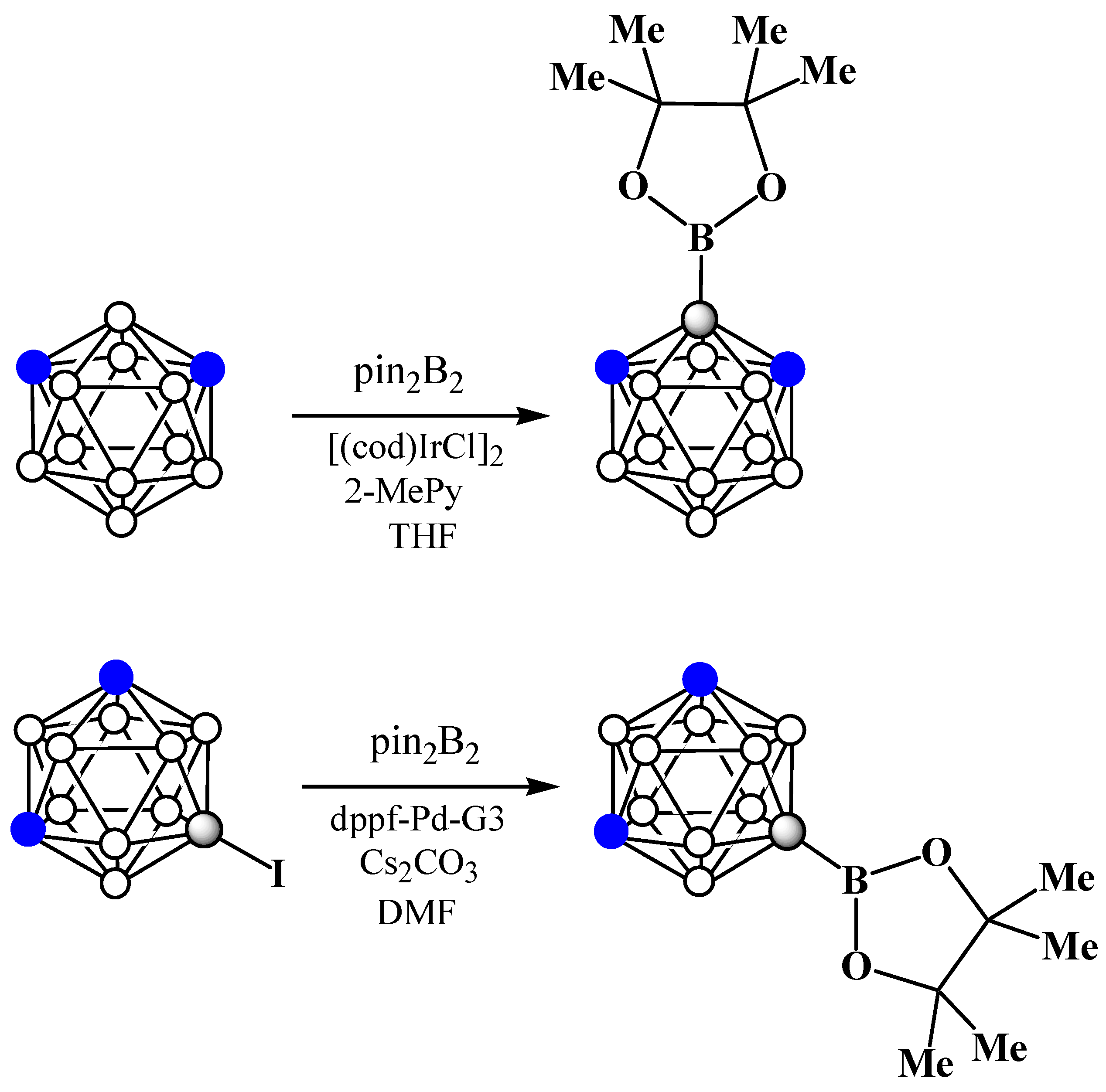
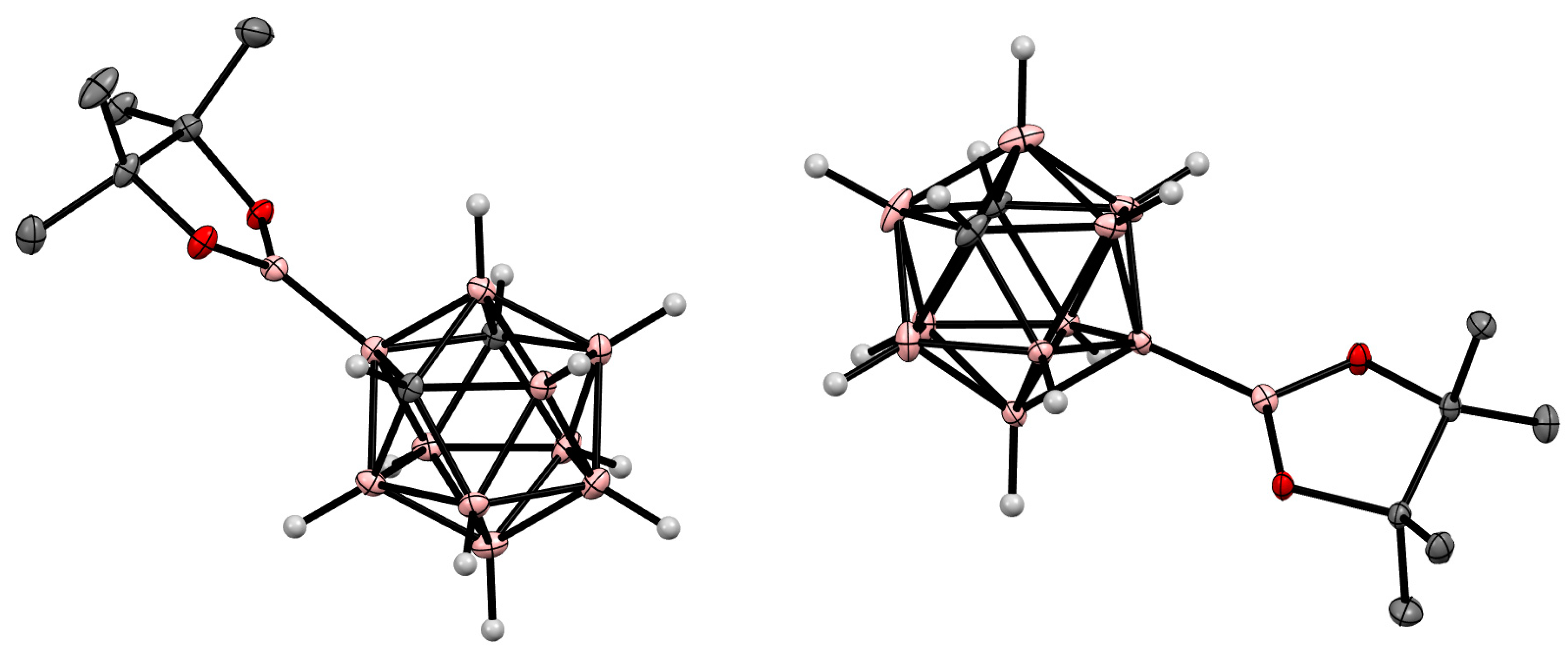
8. Carborane Derivatives with exo-Polyhedral Boron-Boron Bond
Funding
Data Availability Statement
Conflicts of Interest
References
- Heying, T.L.; Ager, J.W.; Clark, S.L.; Mangold, D.J.; Goldstein, H.L.; Hillman, M.; Polak, R.J.; Szymanski, J.W. A New Series of Organoboranes. I. Carboranes from the Reaction of Decaborane with Acetylenic Compounds. Inorg. Chem. 1963, 2, 1089–1092. [Google Scholar] [CrossRef]
- Heying, T.L.; Ager, J.W.; Clark, S.L.; Alexander, R.P.; Papetti, S.; Reid, J.A.; Trotz, S.I. A New Series of Organoboranes. III. Some Reactions of 1,2-Dicarbaclovododecaborane(12) and its Derivatives. Inorg. Chem. 1963, 2, 1097–1105. [Google Scholar] [CrossRef]
- Fein, M.M.; Bobinski, J.; Mayes, N.; Schwartz, N.; Cohen, M.S. Carboranes. I. The Preparation and Chemistry of 1-Isopropenylcarborane and its Derivatives (a New Family of Stable Clovoboranes). Inorg. Chem. 1963, 2, 1111–1115. [Google Scholar] [CrossRef]
- Zakharkin, L.I.; Stanko, V.I.; Brattsev, V.A.; Chapovskii, Y.A.; Okhlobystin, O.Y. Synthesis of a new class of organoboron compounds, B10C2H12(barene) and its derivatives. Russ. Chem. Bull. 1963, 12, 2074. [Google Scholar] [CrossRef]
- Zakharkin, L.I.; Stanko, V.I.; Klimova, A.I.; Chapovskii, Y.A. Metallation of B10C2H12(barene) and its derivatives by butyl lithium. Russ. Chem. Bull. 1963, 12, 2072. [Google Scholar] [CrossRef]
- Grimes, R.N. Carboranes, 3rd ed.; Academic Press: London, UK, 2016. [Google Scholar] [CrossRef]
- Sivaev, I.B. Polyhedral boranes and carboranes. In Comprehensive Organometallic Chemistry IV; Elsevier: Kidlington, UK, 2022; Volume 9, pp. 196–262. [Google Scholar] [CrossRef]
- Boone, J.L.; Brotherton, R.J.; Petterson, L.L. o-Carboranyl Derivatives of Boron Compounds. Inorg. Chem. 1965, 4, 910–912. [Google Scholar] [CrossRef]
- Mikhailov, B.M.; Shagova, E.A.; Potapova, T.V. Organoboron compounds. 211. Organoboron compounds of the carborane series. Russ. Chem. Bull. 1970, 19, 1923–1926. [Google Scholar] [CrossRef]
- Rai, D.N.; Venkatachelam, C.R.; Rudolph, R.W. The Preparation and Reaction Chemistry of Carboranyl Borane Adducts Derived from Trimethylamine Iodoborane. Synth. React. Inorg. Met. Nano Met. Chem. 1973, 3, 129–136. [Google Scholar] [CrossRef]
- Plešek, J.; Jelínek, T.; Heřmánek, S.; Stibr, B. Synthesis and reactions of 1,2-dicarba-closo-dodecarborane-1-yl tetrahydroborate(1-). Collect. Czechoslov. Chem. Commun. 1986, 51, 81–89. [Google Scholar] [CrossRef]
- Brown, D.A.; Colquhoun, H.M.; Daniels, J.A.; MacBride, J.A.H.; Stephenson, I.R.; Wade, K. Polymers and ceramics based on icosahedral carboranes. J. Mater. Chem. 1992, 2, 793–804. [Google Scholar] [CrossRef]
- Malan, C.; Morin, C. Synthesis of unsymmetrical C-disubstituted para-carboranes: Access to functionalized carboranyl-boronic acid and carboranol. Tetrahedron Lett. 1997, 38, 6599–6602. [Google Scholar] [CrossRef]
- Ohta, K.; Goto, T.; Yamazaki, H.; Pichierri, F.; Endo, Y. Facile and Efficient Synthesis of C-Hydroxycarboranes and C,C‘-Dihydroxycarboranes. Inorg. Chem. 2007, 46, 3966–3970. [Google Scholar] [CrossRef] [PubMed]
- Lyubimov, S.E.; Tyutyunov, A.A.; Vologzhanin, P.A.; Safronov, A.S.; Petrovskii, P.V.; Kalinin, V.N.; Gavrilov, K.N.; Davankov, V.A. Carborane-derived diphosphites: New ligands for Pd-catalyzed allylic amination. J. Organomet. Chem. 2008, 693, 3321–3323. [Google Scholar] [CrossRef]
- Lyubimov, S.E.; Davankov, V.A.; Gavrilov, K.N.; Grishina, T.B.; Rastorguev, E.A.; Tyutyunov, A.A.; Verbitskaya, T.A.; Kalinin, V.N.; Hey-Hawkins, E. Diamidophosphites with isomeric carborane fragments: A comparison of catalytic activity in asymmetric Pd-catalyzed allylic substitution reactions. Tetrahedron Lett. 2010, 51, 1682–1684. [Google Scholar] [CrossRef]
- Scholz, M.; Bensdorf, K.; Gust, R.; Hey-Hawkins, E. Asborin: The Carbaborane Analogue of Aspirin. Chemmedchem 2009, 4, 746–748. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Kaluđerović, G.N.; Kommera, H.; Paschke, R.; Will, J.; Sheldrick, W.S.; Hey-Hawkins, E. Carbaboranes as pharmacophores: Similarities and differences between aspirin and asborin. Eur. J. Med. Chem. 2011, 46, 1131–1139. [Google Scholar] [CrossRef]
- Scholz, M.; Blobaum, A.L.; Marnett, L.J.; Hey-Hawkins, E. Synthesis and evaluation of carbaborane derivatives of indomethacin as cyclooxygenase inhibitors. Bioorganic Med. Chem. 2011, 19, 3242–3248. [Google Scholar] [CrossRef]
- Ohta, K.; Ogawa, T.; Kaise, A.; Endo, Y. Enhanced estrogen receptor beta (ERβ) selectivity of fluorinated carborane-containing ER modulators. Bioorganic Med. Chem. Lett. 2013, 23, 6555–6558. [Google Scholar] [CrossRef]
- Kaise, A.; Ohta, K.; Endo, Y. Novel p-carborane-containing multitarget anticancer agents inspired by the metabolism of 17β-estradiol. Bioorganic Med. Chem. 2017, 25, 6371–6378. [Google Scholar] [CrossRef]
- Wrackmeyer, B.; Klimkina, E.V.; Milius, W. 1,3,2-Dioxaphospholanes with an Annelated 1,2-Dicarba- closo -dodecaborane(12) Unit: Formation and Dimerization. Eur. J. Inorg. Chem. 2013, 2014, 233–246. [Google Scholar] [CrossRef]
- Kazakov, G.S.; Stogniy, M.Y.; Sivaev, I.B.; Suponitsky, K.Y.; Godovikov, I.A.; Kirilin, A.D.; Bregadze, V.I. Synthesis of crown ethers with the incorporated cobalt bis(dicarbollide) fragment. J. Organomet. Chem. 2015, 798, 196–203. [Google Scholar] [CrossRef]
- Neumann, W.; Hiller, M.; Sárosi, M.B.; Lönnecke, P.; Hey-Hawkins, E. Reduction of hydroxy-functionalised carbaboranyl carboxylic acids and ketones by organolithium reagents. Dalton Trans. 2015, 44, 6638–6644. [Google Scholar] [CrossRef]
- Kuhnert, R.; Sárosi, M.-B.; George, S.; Lönnecke, P.; Hofmann, B.; Steinhilber, D.; Murganic, B.; Mijatovic, S.; Maksimovic-Ivanic, D.; Hey-Hawkins, E. CarbORev-5901: The First Carborane-Based Inhibitor of the 5-Lipoxygenase Pathway. Chemmedchem 2017, 12, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Stogniy, M.Y.; Erokhina, S.A.; Sivaev, I.B.; Bregadze, V.I. Synthesis of C-Methoxy- and C,C’-Dimethoxy-ortho-carboranes. J. Organomet. Chem. 2020, 927, 121523. [Google Scholar] [CrossRef]
- Svidlov, S.V.; Varzatskii, O.A.; Potapova, T.V.; Vologzhanina, A.V.; Bukalov, S.S.; Leites, L.A.; Voloshin, Y.Z.; Bubnov, Y.N. C-carboranylation of a quasi-aromatic iron(II) cage complex and its organic aromatic analog by the metal-catalyzed (promoted) cross-coupling reactions. Inorg. Chem. Commun. 2014, 43, 142–145. [Google Scholar] [CrossRef]
- Mukherjee, S.; Thilagar, P. Boron clusters in luminescent materials. Chem. Commun. 2016, 52, 1070–1093. [Google Scholar] [CrossRef] [PubMed]
- Núñez, R.; Tarrés, M.; Ferrer-Ugalde, A.; de Biani, F.F.; Teixidor, F. Electrochemistry and Photoluminescence of Icosahedral Carboranes, Boranes, Metallacarboranes, and Their Derivatives. Chem. Rev. 2016, 116, 14307–14378. [Google Scholar] [CrossRef]
- Ochi, J.; Tanaka, K.; Chujo, Y. Recent Progress in the Development of Solid-State Luminescent o -Carboranes with Stimuli Responsivity. Angew. Chem. Int. Ed. 2020, 132, 9925–9939. [Google Scholar] [CrossRef]
- Erdyakov, S.Y.; Voloshin, Y.Z.; Makarenko, I.G.; Lebed, E.G.; Potapova, T.V.; Ignatenko, A.V.; Vologzhanina, A.V.; Gurskii, M.E.; Bubnov, Y.N. New o-carboranyl-containing capping agents for d-metal tris-dioximates and first bis-C-carboranylboron-capped iron(II) clathrochelates: Synthesis and X-ray structure. Inorg. Chem. Commun. 2009, 12, 135–139. [Google Scholar] [CrossRef]
- Weber, L.; Kahlert, J.; Böhling, L.; Brockhinke, A.; Stammler, H.-G.; Neumann, B.; Harder, R.A.; Low, P.J.; Fox, M.A. Electrochemical and spectroelectrochemical studies of C-benzodiazaborolyl-ortho-carboranes. Dalton Trans. 2013, 42, 2266–2281. [Google Scholar] [CrossRef]
- Svidlov, S.V.; Voloshin, Y.Z.; Yurgina, N.S.; Potapova, T.V.; Belyy, A.Y.; Ananyev, I.; Bubnov, Y.N. Synthesis, structure, and reactivity of C-isopropyl-ortho-carborane organoboron derivatives. Russ. Chem. Bull. 2014, 63, 2343–2350. [Google Scholar] [CrossRef]
- Voloshin, Y.Z.; Erdyakov, S.Y.; Makarenko, I.G.; Lebed’, E.G.; Potapova, T.V.; Svidlov, S.V.; Starikova, Z.A.; Pol’Shin, E.V.; Gurskii, M.E.; Bubnov, Y.N. Macrocyclization of the semiclathrochelate o-carboranylboronate and n-butylboronate iron(II) oximehydrazonates: Synthesis and structure of clathrochelate products and unexpected allosteric effect of the apical substituent. Russ. Chem. Bull. 2007, 56, 1787–1794. [Google Scholar] [CrossRef]
- Mikhailov, B.M.; Shagova, E.A. 1-Isopropylcarboranyl-2-di-n-butylborane and some of its transformations. Russ. Chem. Bull. 1972, 21, 1188. [Google Scholar] [CrossRef]
- Mikhailov, B.M.; Shagova, E.A. Organoboron compounds. 357. The reaction of (1-isopropyl-o-carboran-2-yl)di-n-butylborane with aldehydes. Russ. Chem. Bull. 1979, 28, 549–552. [Google Scholar] [CrossRef]
- Mikhailov, B.M.; Shagova, E.A. Organoboron compounds. 360. Reaction of (1-isopropyl-o-carboran-2-yl)di-n-butylborane with aromatic nitriles. Russ. Chem. Bull. 1979, 28, 1250–1252. [Google Scholar] [CrossRef]
- Mikhailov, B.M.; Shagova, E.A. Organoboron compounds 356. The protolysis of (1-isopropyl-o-carboranyl-2)-di-n-butylborane. Russ. Chem. Bull. 1979, 28, 543–548. [Google Scholar] [CrossRef]
- Mikhailov, B.M.; Shagova, E.A. l,2-(1′-butyl-4′-methyl-2′, 3′-borocyclopentano)carborane. Russ. Chem. Bull. 1972, 21, 1407. [Google Scholar] [CrossRef]
- Mikhailov, B.M.; Shagova, E.A. Organoboron compounds. CCCIII. 1,2-(3′-boracyclopentano)- and 1,2-(3′-boracyclopenteno)-o-carborane. Z. Obshch. Khim. 1972, 42, 1052–1060. [Google Scholar]
- Spielvogel, B.F.; Rana, G.; Vyakaranam, K.; Grelck, K.; Dicke, K.E.; Dolash, B.D.; Li, S.-J.; Zheng, C.; Maguire, J.A.; Takagaki, M.; et al. A Novel Approach to the Syntheses of Functionalized, Water-Soluble Icosahedral Carboranyl Anions. Crystal Structure of Methyl N-[(Trimethylamineboryl)carbonyl]-L-tyrosinate: A Synthon for Novel Carboranylpeptides. Collect. Czechoslov. Chem. Commun. 2002, 67, 1095–1108. [Google Scholar] [CrossRef]
- Duda, K.; Himmelspach, A.; Landmann, J.; Kraus, F.; Finze, M. Synthesis of the fluorohydridoborate anions [BHF3]− and [1-HF2B-9,12-X2-closo-1,2-C2B10H9]− (X = H, I): Deboronation of 1,2- and 1,7-dicarba-closo-dodecaboranes with anhydrous [Me4N]F. Chem. Commun. 2016, 52, 13241–13244. [Google Scholar] [CrossRef]
- Dunks, G.B.; Wiersema, R.J.; Hawthorne, M.F. Chemical and structural studies of the B10C2H122− ions produced from icosahedral B10C2H12 carboranes. J. Am. Chem. Soc. 1973, 95, 3174–3179. [Google Scholar] [CrossRef]
- Kahlert, J.; Böhling, L.; Brockhinke, A.; Stammler, H.-G.; Neumann, B.; Rendina, L.M.; Low, P.J.; Weber, L.; Fox, M.A. Syntheses and reductions of C-dimesitylboryl-1,2-dicarba-closo-dodecaboranes. Dalton Trans. 2015, 44, 9766–9781. [Google Scholar] [CrossRef]
- Benton, A.; Watson, J.D.; Mansell, S.M.; Rosair, G.M.; Welch, A.J. The Lewis acidity of borylcarboranes. J. Organomet. Chem. 2020, 907, 121057. [Google Scholar] [CrossRef]
- Mikhailov, B.M.; Shagova, E.A. Bicarboranyl compounds of boron. Russ. Chem. Bull. 1972, 21, 1187. [Google Scholar] [CrossRef]
- Krebs, J.; Haehnel, M.; Krummenacher, I.; Friedrich, A.; Braunschweig, H.; Finze, M.; Ji, L.; Marder, T.B. Synthesis and structure of an o-carboranyl-substituted three-coordinate borane radical anion. Chem. Eur. J. 2021, 37, 1859–1867. [Google Scholar] [CrossRef]
- Akram, M.O.; Tidwell, J.R.; Dutton, J.L.; Martin, C.D. Tris(ortho-carboranyl)borane: An Isolable, Halogen-Free, Lewis Superacid. Angew. Chem. Int. Ed. 2022, 61, e202212073. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I. Lewis acidity of boron compounds. Coord. Chem. Rev. 2014, 270–271, 75–88. [Google Scholar] [CrossRef]
- Su, X.; Bartholome, T.A.; Tidwell, J.R.; Pujol, A.; Yruegas, S.; Martinez, J.J.; Martin, C.D. 9-Borafluorenes: Synthesis, Properties, and Reactivity. Chem. Rev. 2021, 121, 4147–4192. [Google Scholar] [CrossRef]
- He, J.; Rauch, F.; Finze, M.; Marder, T.B. (Hetero)arene-fused boroles: A broad spectrum of applications. Chem. Sci. 2021, 12, 128–147. [Google Scholar] [CrossRef]
- Bischof, T.; Guo, X.; Krummenacher, I.; Beßler, L.; Lin, Z.; Finze, M.; Braunschweig, H. Alkene insertion reactivity of a o-carboranyl-substituted 9-borafluorene. Chem. Sci. 2022, 13, 7492–7497. [Google Scholar] [CrossRef]
- Krebs, J.; Häfner, A.; Fuchs, S.; Guo, X.; Rauch, F.; Eichhorn, A.; Krummenacher, I.; Friedrich, A.; Ji, L.; Finze, M.; et al. Backbone-controlled LUMO energy induces intramolecular C–H activation in ortho-bis-9-borafluorene-substituted phenyl and o-carboranyl compounds leading to novel 9,10-diboraanthracene derivatives. Chem. Sci. 2022, 13, 14165–14178. [Google Scholar] [CrossRef]
- Nie, Y.; Miao, J.; Pritzkow, H.; Wadepohl, H.; Siebert, W. Weak intramolecular C–H···H–B and C–H···Cl interactions in C-aminoboryl-o-carboranes. J. Organomet. Chem. 2013, 747, 174–177. [Google Scholar] [CrossRef]
- Nie, Y.; Miao, J.; Wadepohl, H.; Pritzkow, H.; Oeser, T.; Siebert, W. Syntheses and Characterization ofo-Carboranes Containing Fusedexo-Polyhedral Di- and Triboraheterocycles. Z. Anorg. Allg. Chem. 2013, 639, 1188–1193. [Google Scholar] [CrossRef]
- Zi, G.; Li, H.-W.; Xie, Z. Synthesis, Structural Characterization, and Reactivity of Organolanthanide Complexes Derived from a New, Versatile Boron-Bridged Ligand, iPr2NB(C9H7)(C2B10H11). Organometallics 2002, 21, 1136–1145. [Google Scholar] [CrossRef]
- Zi, G.; Li, H.-W.; Xie, Z. Synthesis, Structural Characterization, and Catalytic Property of Group 4 Metal Carborane Compounds with a iPr2NB-Bridged Constrained-Geometry Ligand. Organometallics 2002, 21, 3850–3855. [Google Scholar] [CrossRef]
- Weber, L.; Eickhoff, D.; Marder, T.B.; Fox, M.A.; Low, P.J.; Dwyer, A.D.; Tozer, D.J.; Schwedler, S.; Brockhinke, A.; Stammler, H.-G.; et al. Experimental and theoretical studies on organic D-p-A systems containing three-coordinate boron moieties as both π-donor and π-acceptor. Chem. Eur. J. 2012, 18, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.; Böhling, L. The role of 2,3-dihydro-1-H-1,3,2-diazaboroles in luminescent molecules. Coord. Chem. Rev. 2015, 284, 236–275. [Google Scholar] [CrossRef]
- Weber, L.; Kahlert, J.; Brockhinke, R.; Böhling, L.; Brockhinke, A.; Stammler, H.; Neumann, B.; Harder, R.A.; Fox, M.A. Luminescence Properties of C -Diazaborolyl- ortho -Carboranes as Donor–Acceptor Systems. Chem. A Eur. J. 2012, 18, 8347–8357. [Google Scholar] [CrossRef]
- Weber, L.; Kahlert, J.; Brockhinke, R.; Böhling, L.; Halama, J.; Brockhinke, A.; Stammler, H.-G.; Neumann, B.; Nervi, C.; Harder, R.A.; et al. C,C′-Bis(benzodiazaborolyl)dicarba-closo-dodecaboranes: Synthesis, structures, photophysics and electrochemistry. Dalton Trans. 2013, 42, 10982–10996. [Google Scholar] [CrossRef]
- Benton, A.; Copeland, Z.; Mansell, S.M.; Rosair, G.M.; Welch, A.J. Exploiting the Electronic Tuneability of Carboranes as Supports for Frustrated Lewis Pairs. Molecules 2018, 23, 3099. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Lin, Z.; Xie, Z. The synthesis and structure of a carbene-stabilized iminocarboranyl-boron(i) compound. Chem. Commun. 2015, 51, 16817–16820. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Lin, Z.; Xie, Z. Synthesis and Structural Characterization of Carbene-Stabilized Carborane-Fused Azaborolyl Radical Cation and Dicarbollyl-Fused Azaborole. Organometallics 2016, 35, 2579–2582. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Xie, Z. Reversible Photothermal Isomerization of Carborane-Fused Azaborole to Borirane: Synthesis and Reactivity of Carbene-Stabilized Carborane-Fused Borirane. Angew. Chem. Int. Ed. 2017, 56, 9198–9201. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Yang, J.; Xie, Z. Synthesis, Structure, and Reactivity of Acid-Free Neutral Oxoborane. Angew. Chem. Int. Ed. 2021, 60, 19008–19012. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Yang, W.; Xiang, L.; Sun, W.; Ming, W.; Li, Y.; Lin, Z.; Ye, Q. Solution-Phase Synthesis of a Base-Free Benzoborirene and a Three-Dimensional Inorganic Analogue. J. Am. Chem. Soc. 2020, 142, 17243–17249. [Google Scholar] [CrossRef]
- Wang, J.; Jia, P.; Sun, W.; Wei, Y.; Lin, Z.; Ye, Q. Synthesis of Iminoboryl o-Carboranes by Lewis Base Promoted Aminoborirane-to-Iminoborane Isomerization. Inorg. Chem. 2022, 61, 8879–8886. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, J.; Yang, W.; Lin, Z.; Ye, Q. Boosting Ring Strain and Lewis Acidity of Borirane: Synthesis, Reactivity and Density Functional Theory Studies of an Uncoordinated Arylborirane Fused to o -Carborane. Chem. A Eur. J. 2023, 29, e202203265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, J.; Lin, Z.; Ye, Q. Synthesis, Characterization, and Properties of Three-Dimensional Analogues of 9-Borafluorenes. Inorg. Chem. 2022, 61, 18275–18284. [Google Scholar] [CrossRef] [PubMed]
- Yruegas, S.; Axtell, J.C.; Kirlikovali, K.O.; Spokoyny, A.M.; Martin, C.D. Synthesis of 9-borafluorene analogues featuring a three-dimensional 1,1′-bis(o-carborane) backbone. Chem. Commun. 2019, 55, 2892–2895. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Su, W.; Lin, Z.; Ye, Q. Synthesis, Characterization, and Density Functional Theory Studies of Three-Dimensional Inorganic Analogues of 9,10-Diboraanthracene—A New Class of Lewis Superacids. J. Am. Chem. Soc. 2021, 143, 8552–8558. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Wang, J.; Ye, Q. A Three-Dimensional Inorganic Analogue of 9,10-Diazido-9,10-Diboraanthracene: A Lewis Superacidic Azido Borane with Reactivity and Stability. Angew. Chem. Int. Ed. 2022, 61, 2202205506. [Google Scholar] [CrossRef]
- Entwistle, C.D.; Marder, T.B.; Smith, P.S.; Howard, J.A.; Fox, M.A.; Mason, S.A. Dimesitylborane monomer-dimer equilibrium in solution, and the solid-state structure of the dimer by single crystal neutron and X-ray diffraction. J. Organomet. Chem. 2003, 680, 165–172. [Google Scholar] [CrossRef]
- Cheng, R.; Qiu, Z.; Xie, Z. Iridium-catalysed regioselective borylation of carboranes via direct B–H activation. Nat. Commun. 2017, 8, 14827. [Google Scholar] [CrossRef] [PubMed]
- Shmal’Ko, A.V.; Anufriev, S.A.; Suponitsky, K.Y.; Sivaev, I.B. How to Protect ortho-Carborane from Decapitation—Practical Synthesis of 3,6-Dihalogen Derivatives 3,6-X2-1,2-C2B10H10 (X = Cl, Br, I). Inorganics 2022, 10, 207. [Google Scholar] [CrossRef]
- Mills, H.A.; Martin, J.L.; Rheingold, A.L.; Spokoyny, A.M. Oxidative Generation of Boron-Centered Radicals in Carboranes. J. Am. Chem. Soc. 2020, 142, 4586–4591. [Google Scholar] [CrossRef] [PubMed]
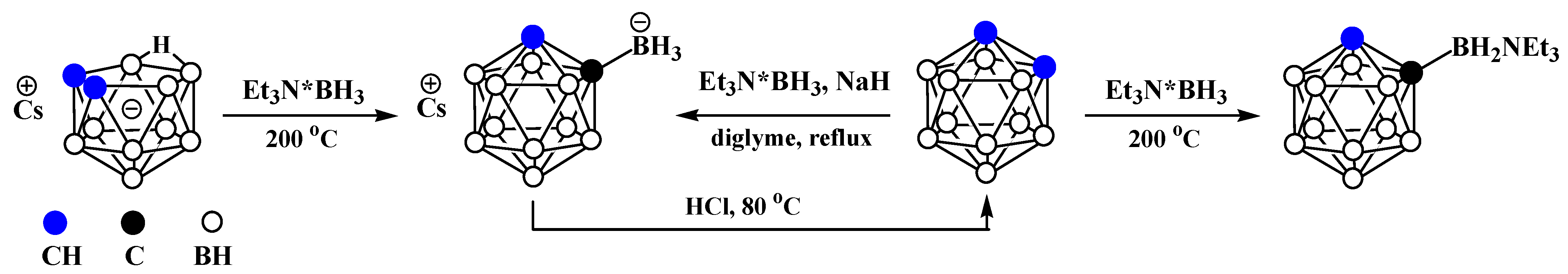
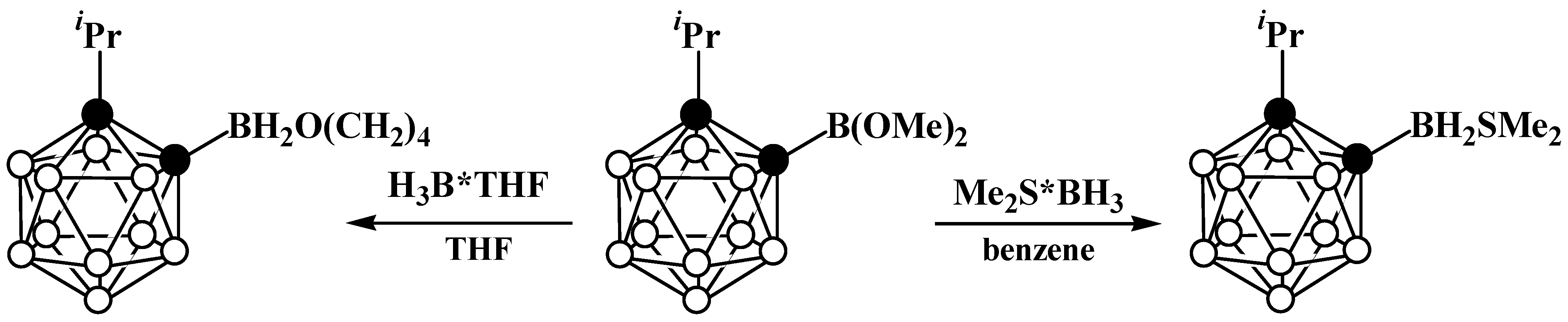
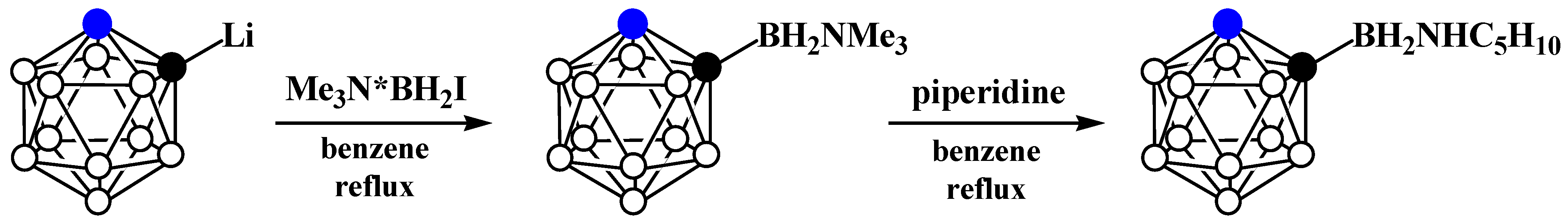
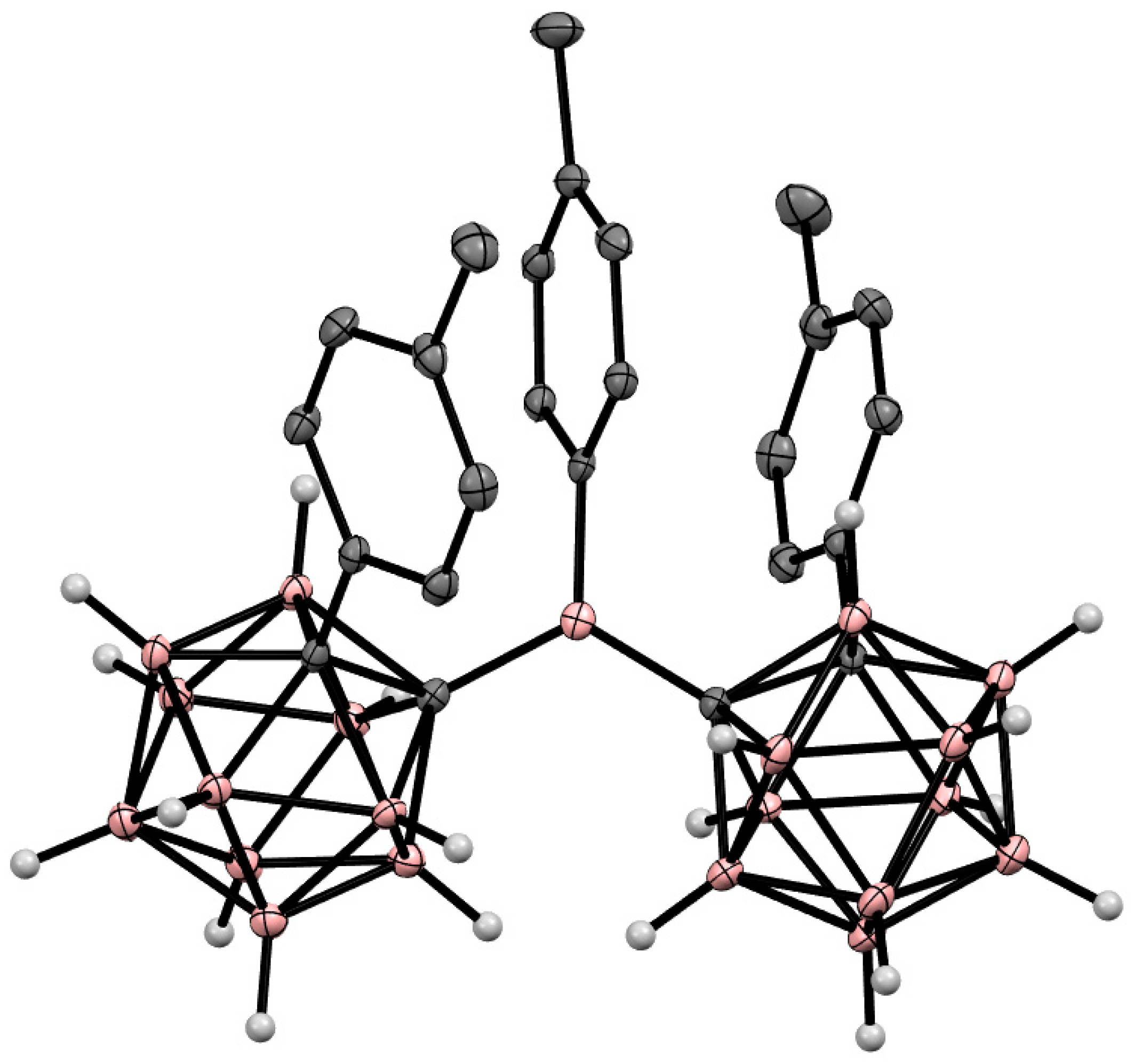
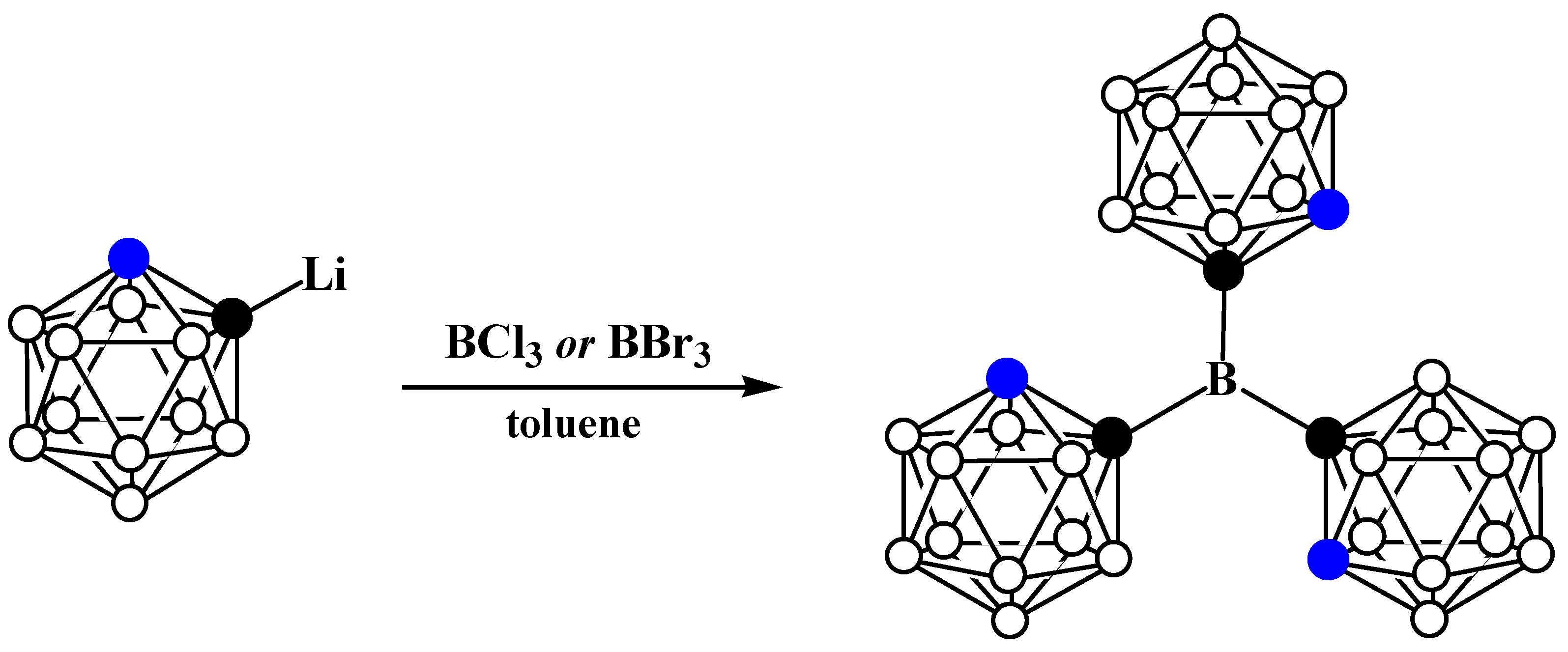
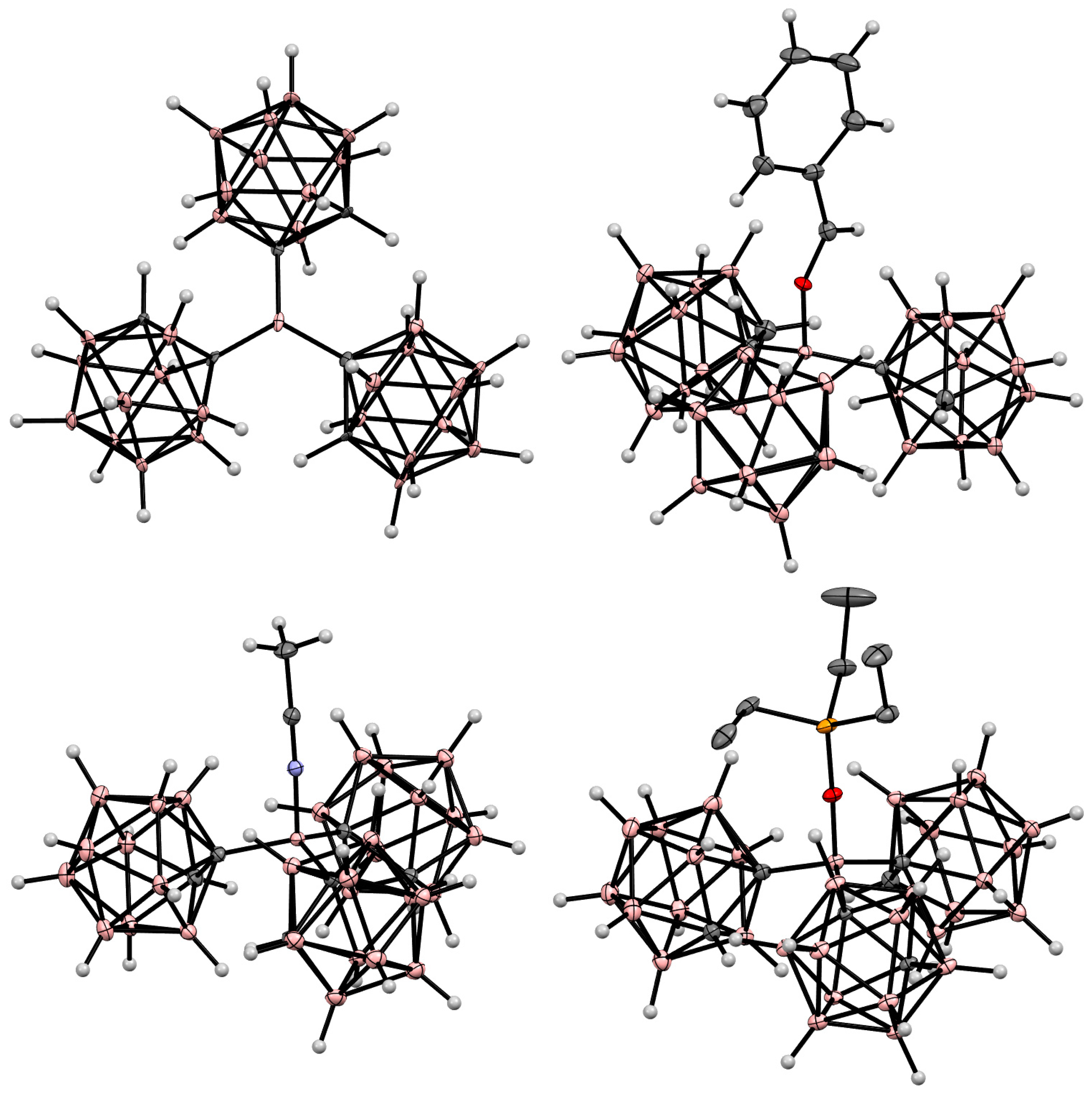
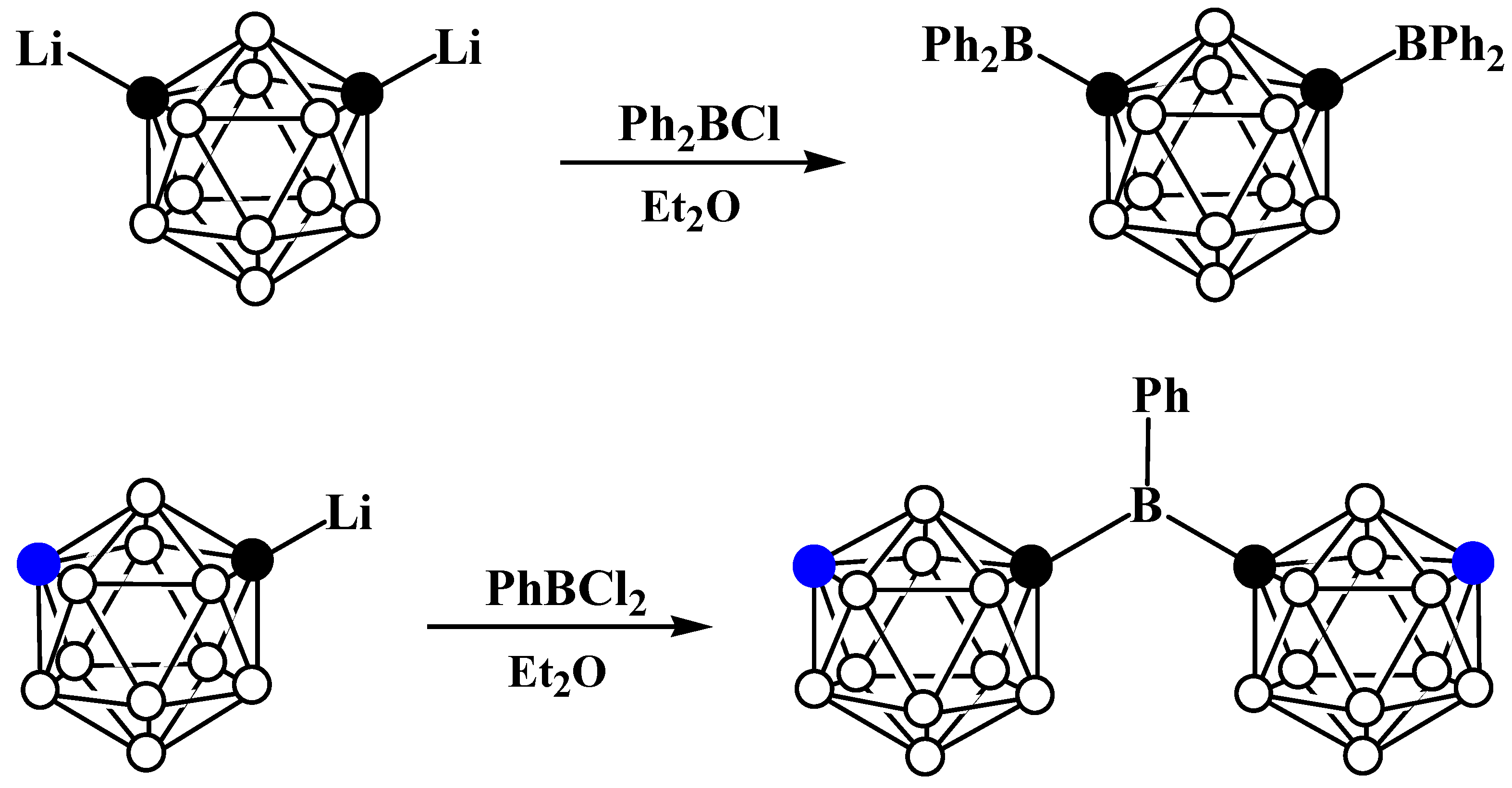





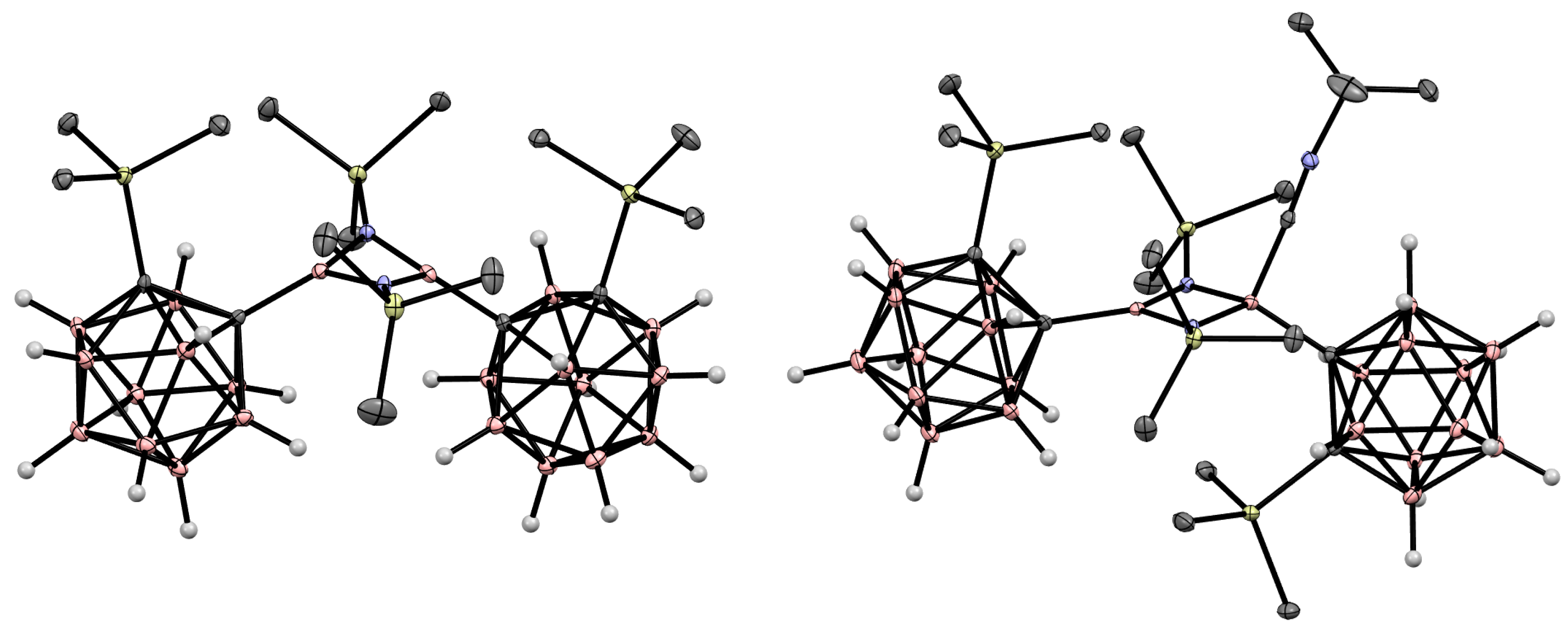

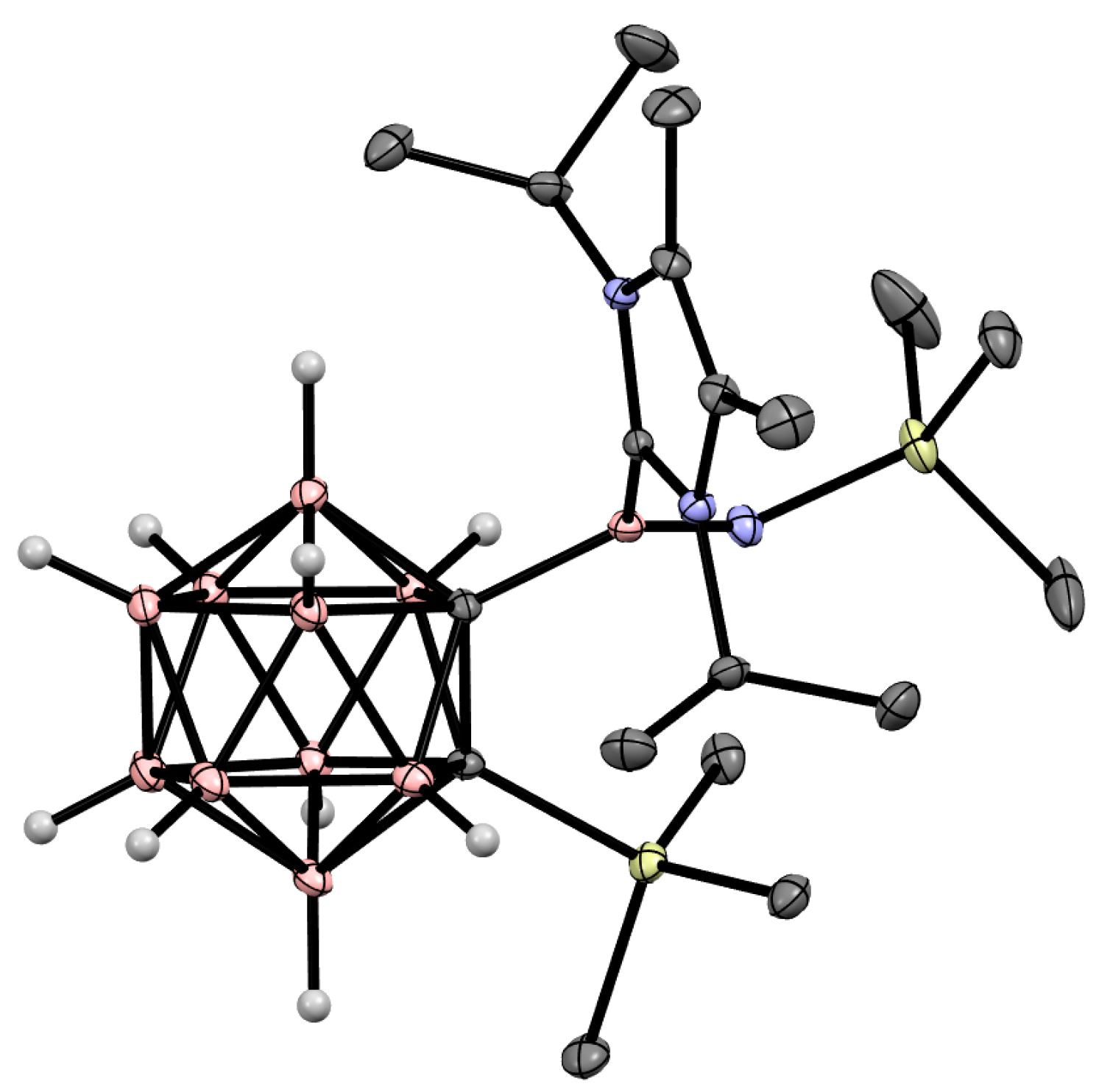
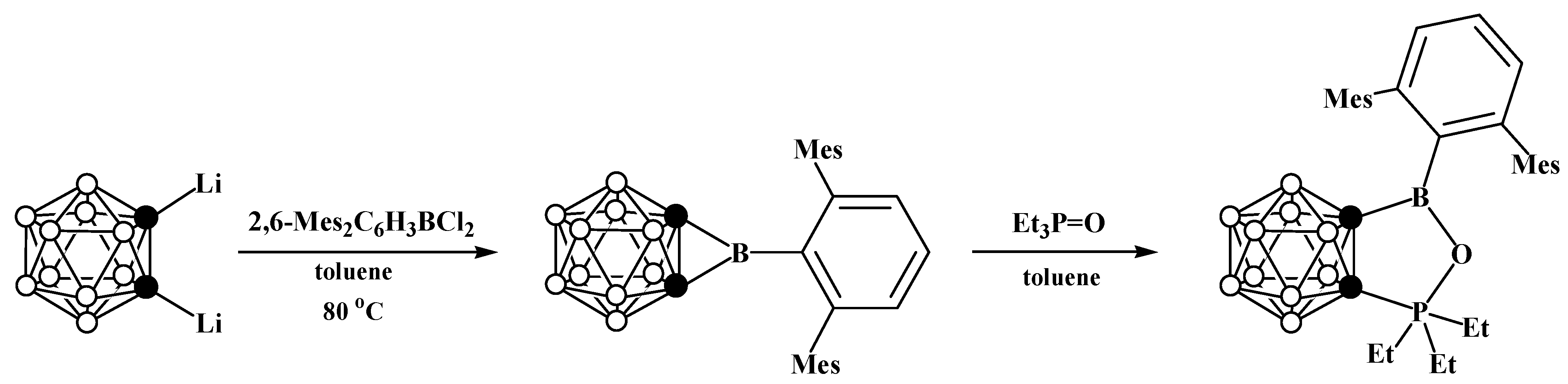
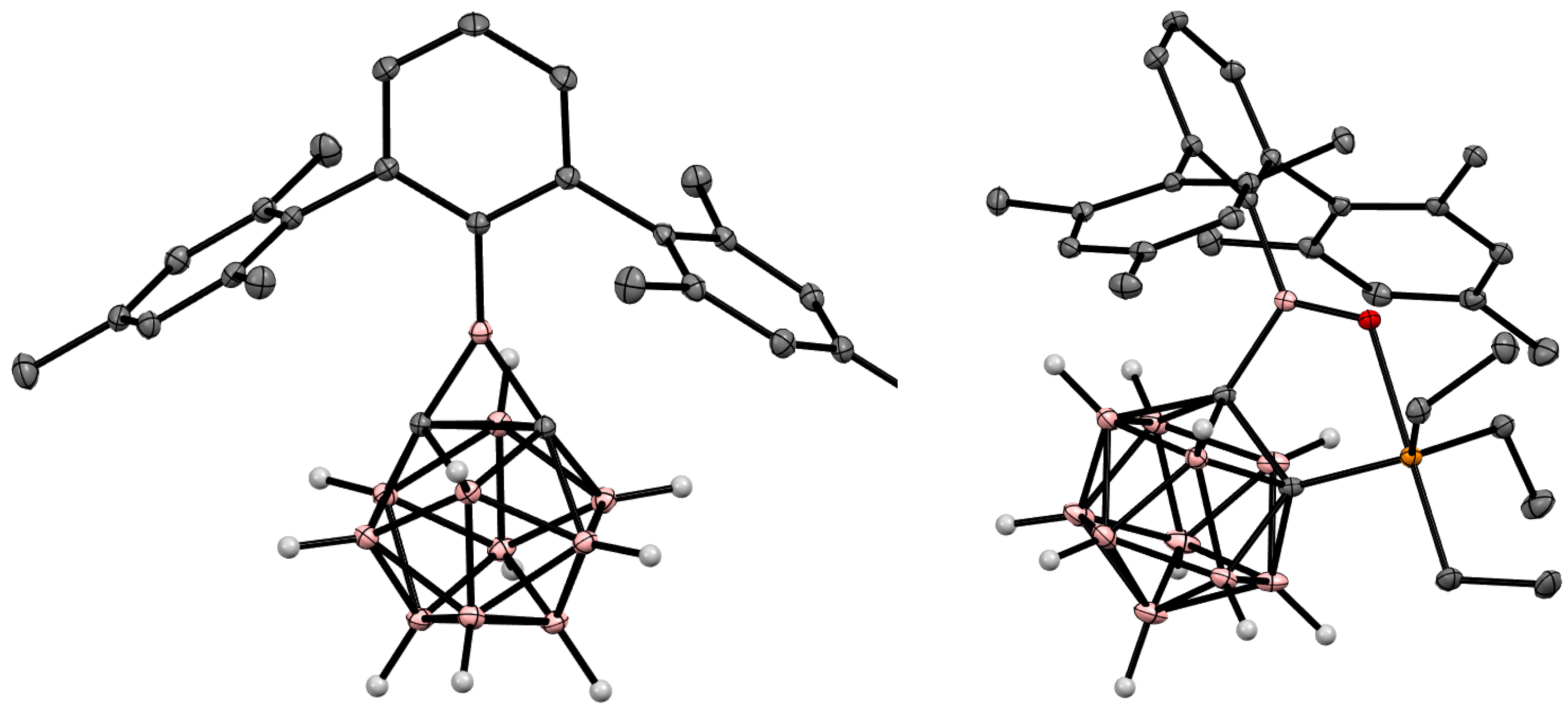

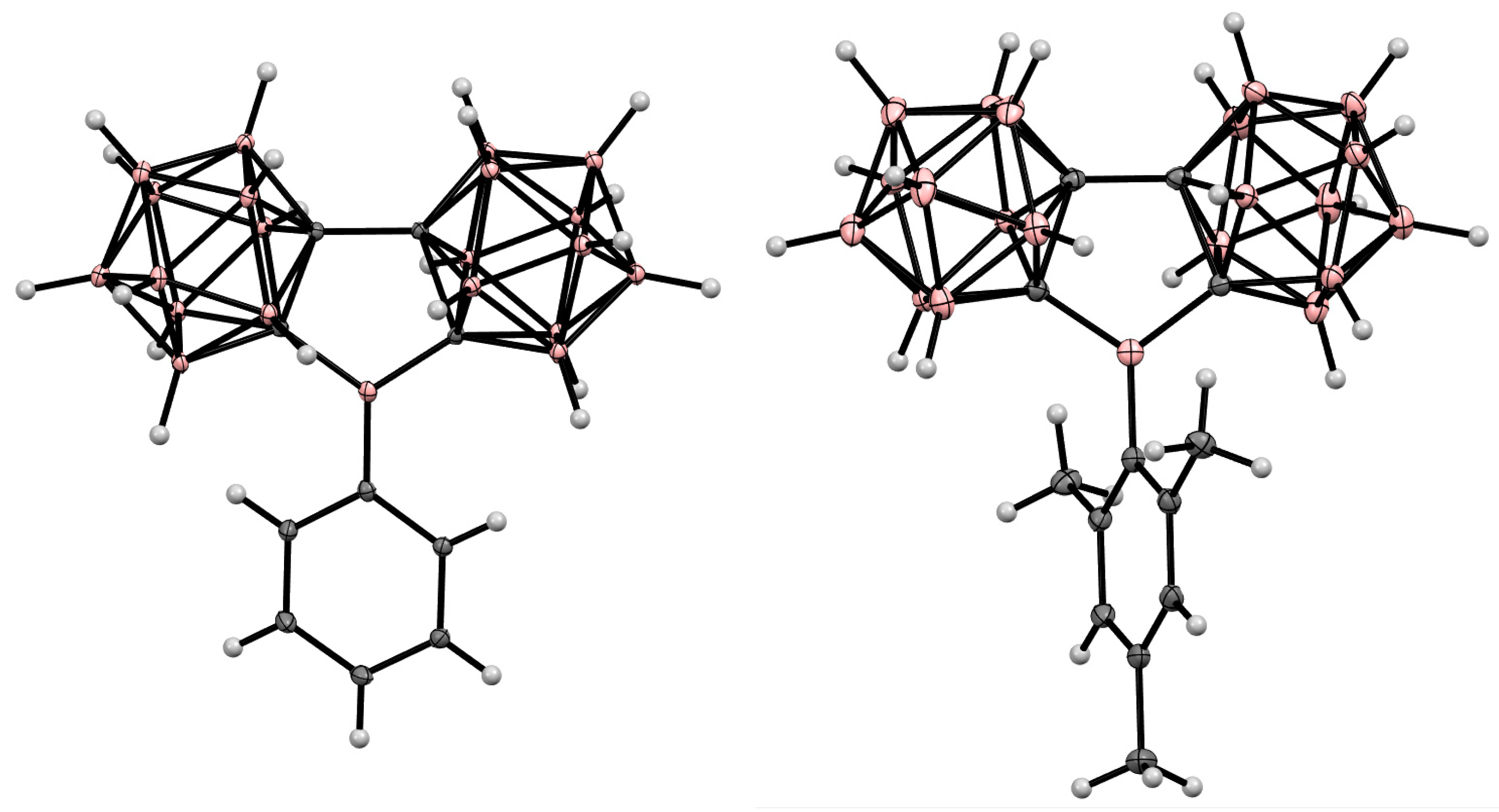
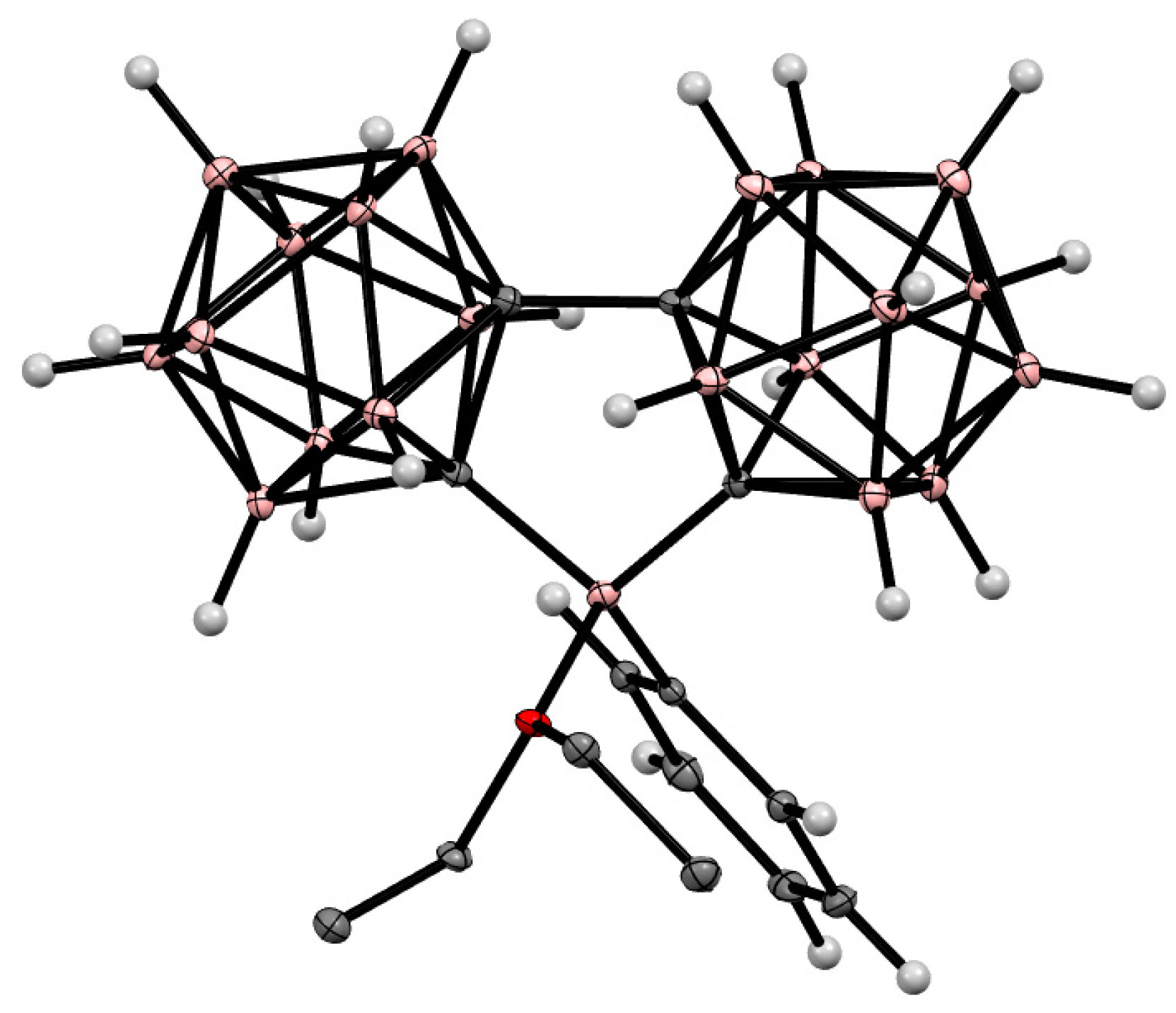
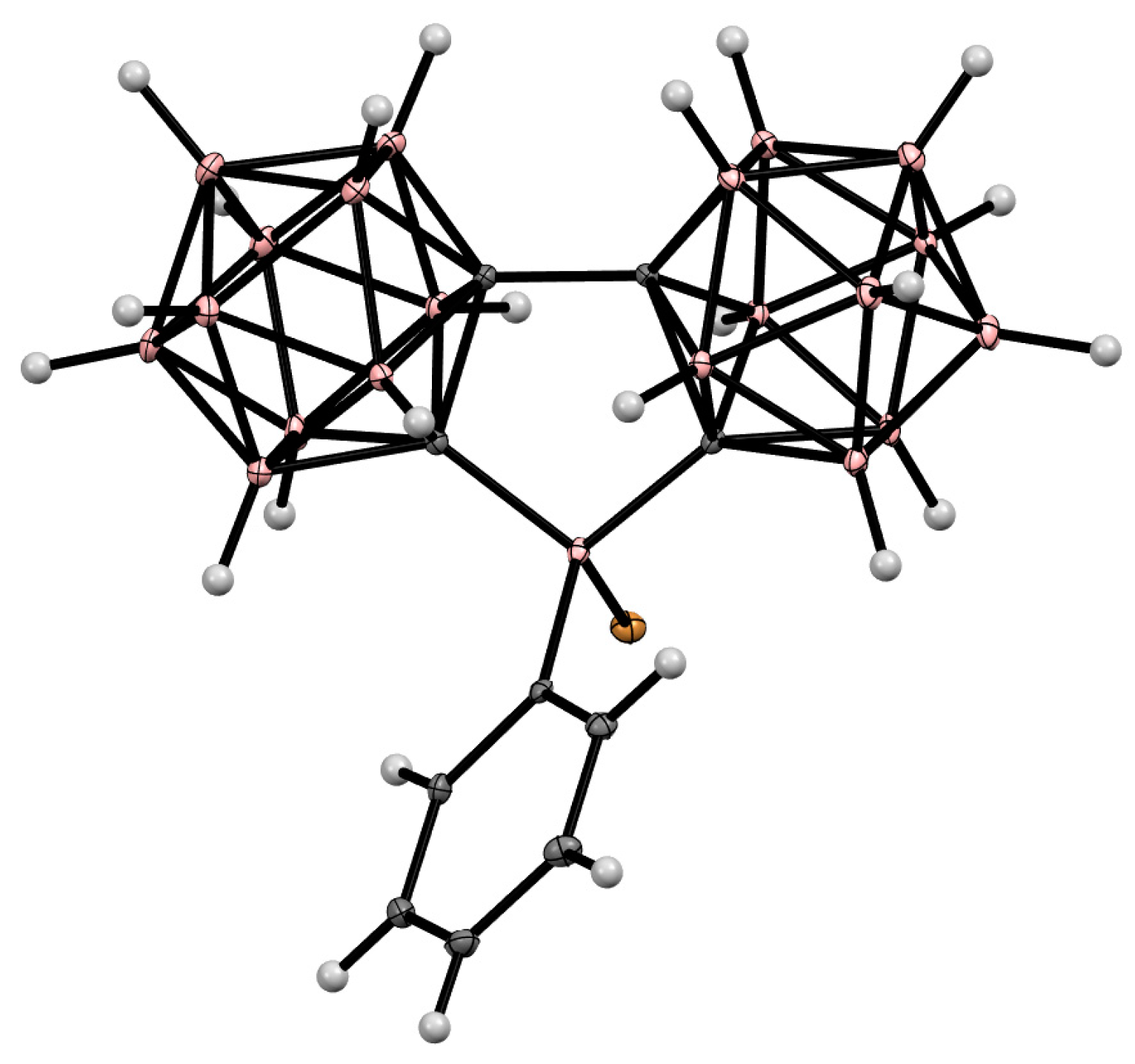
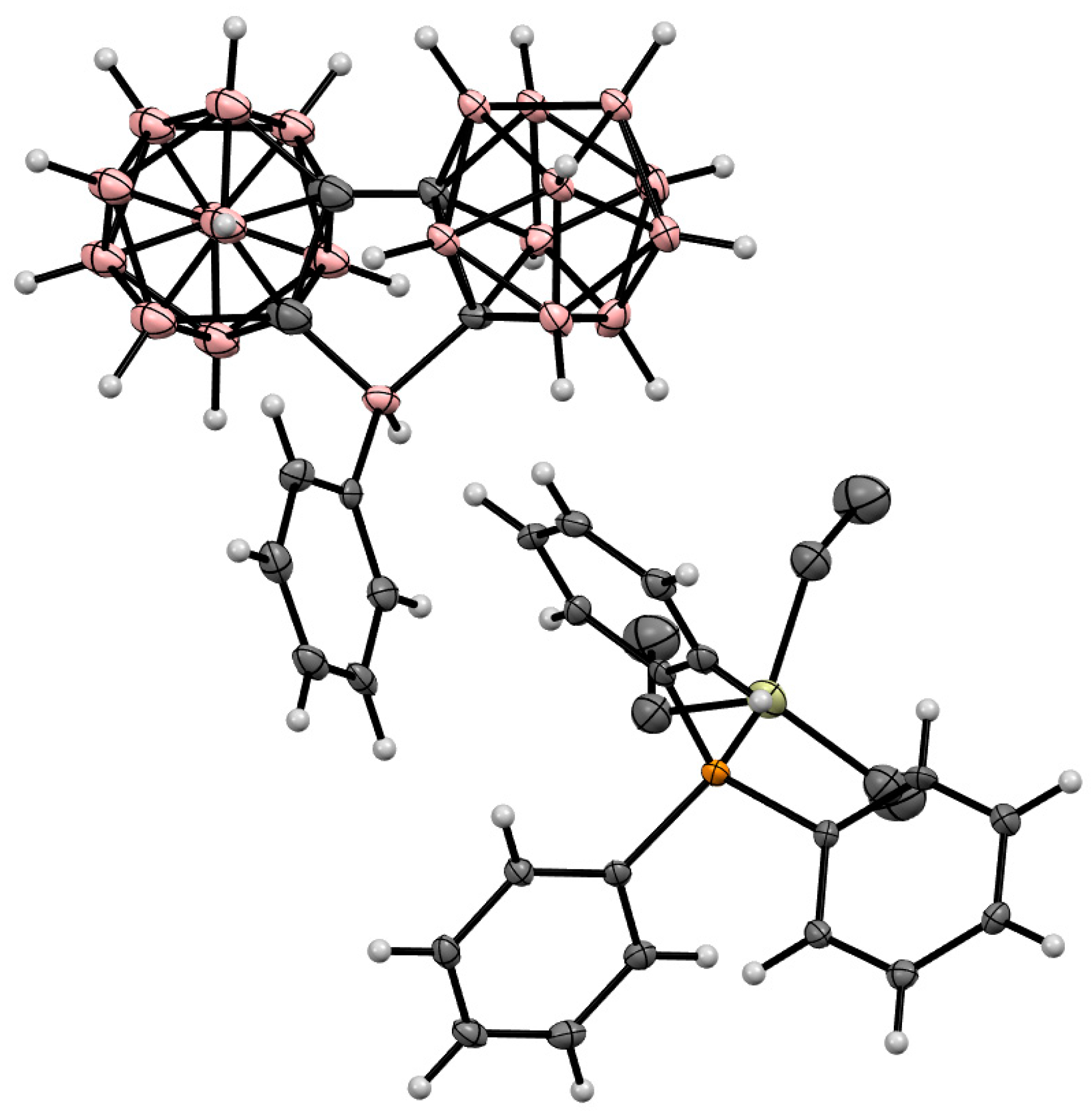
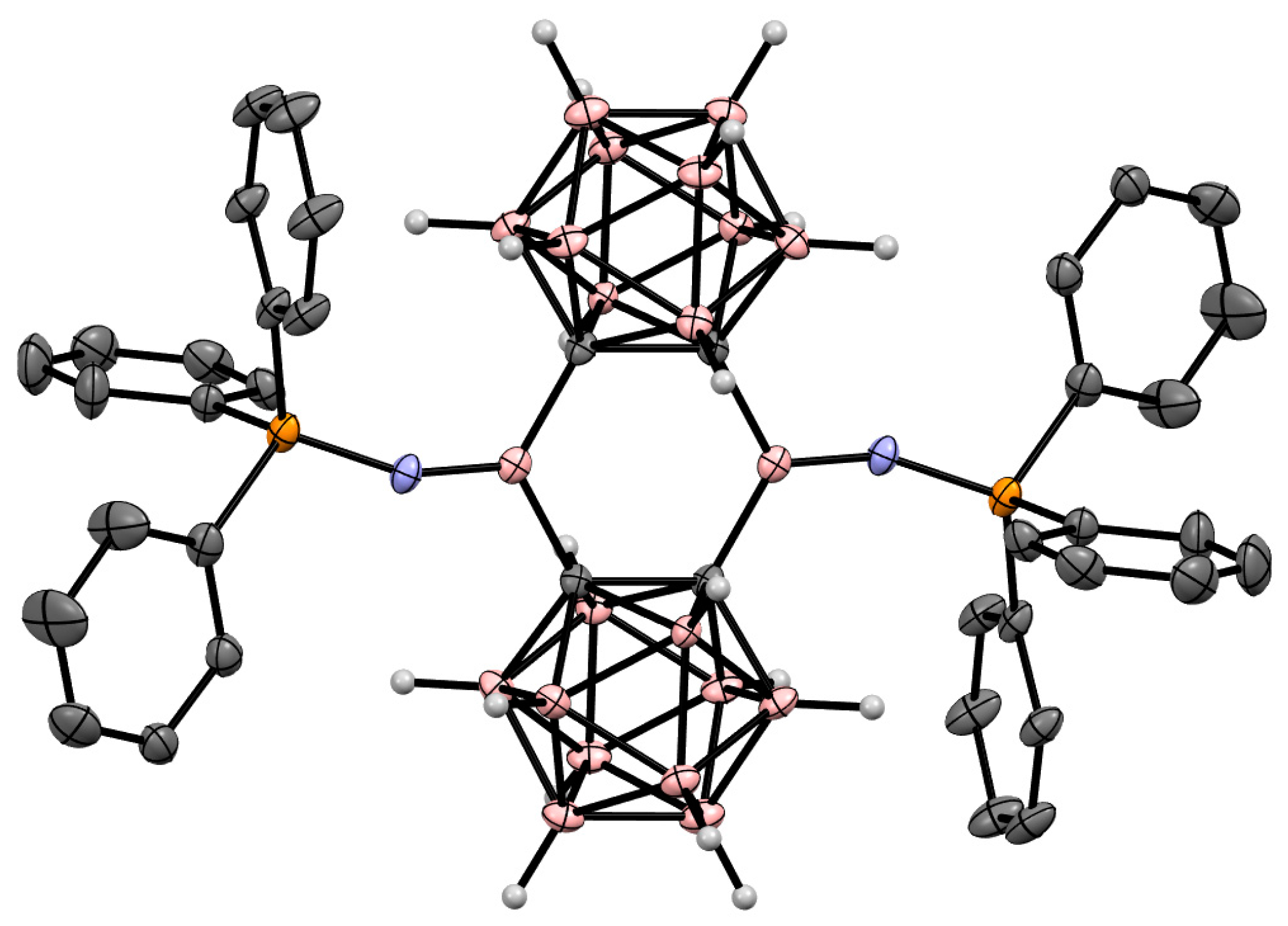
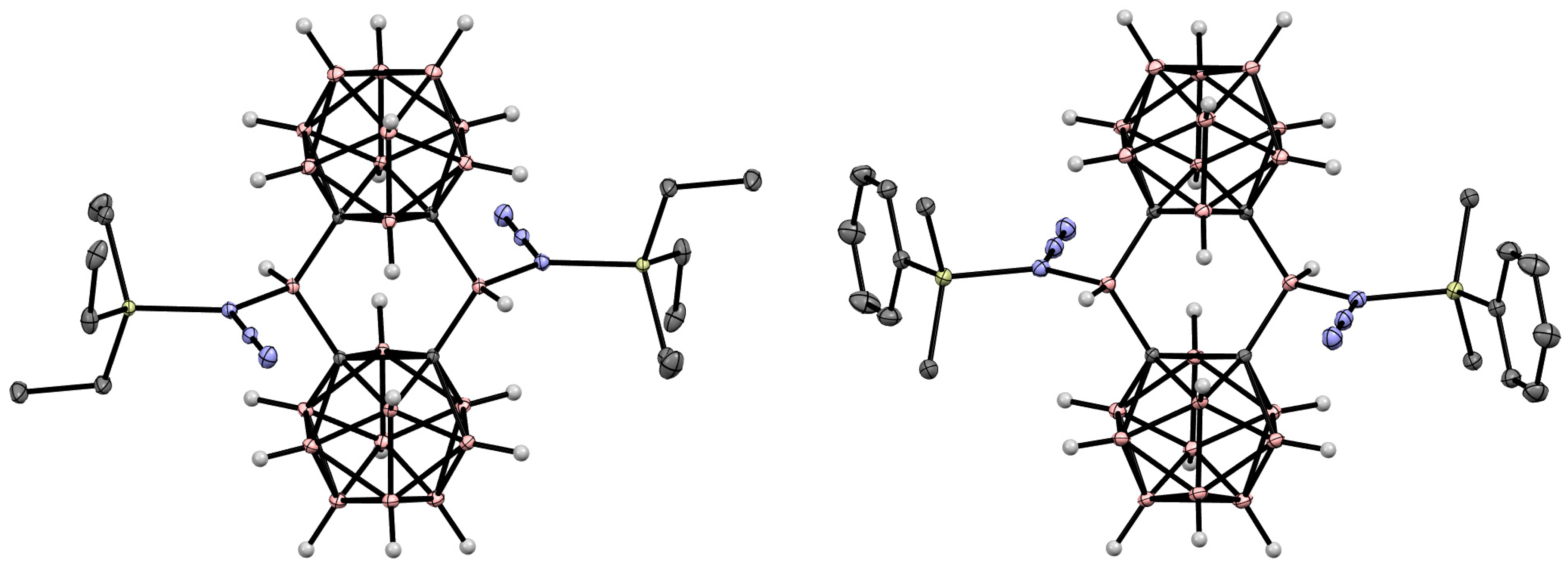
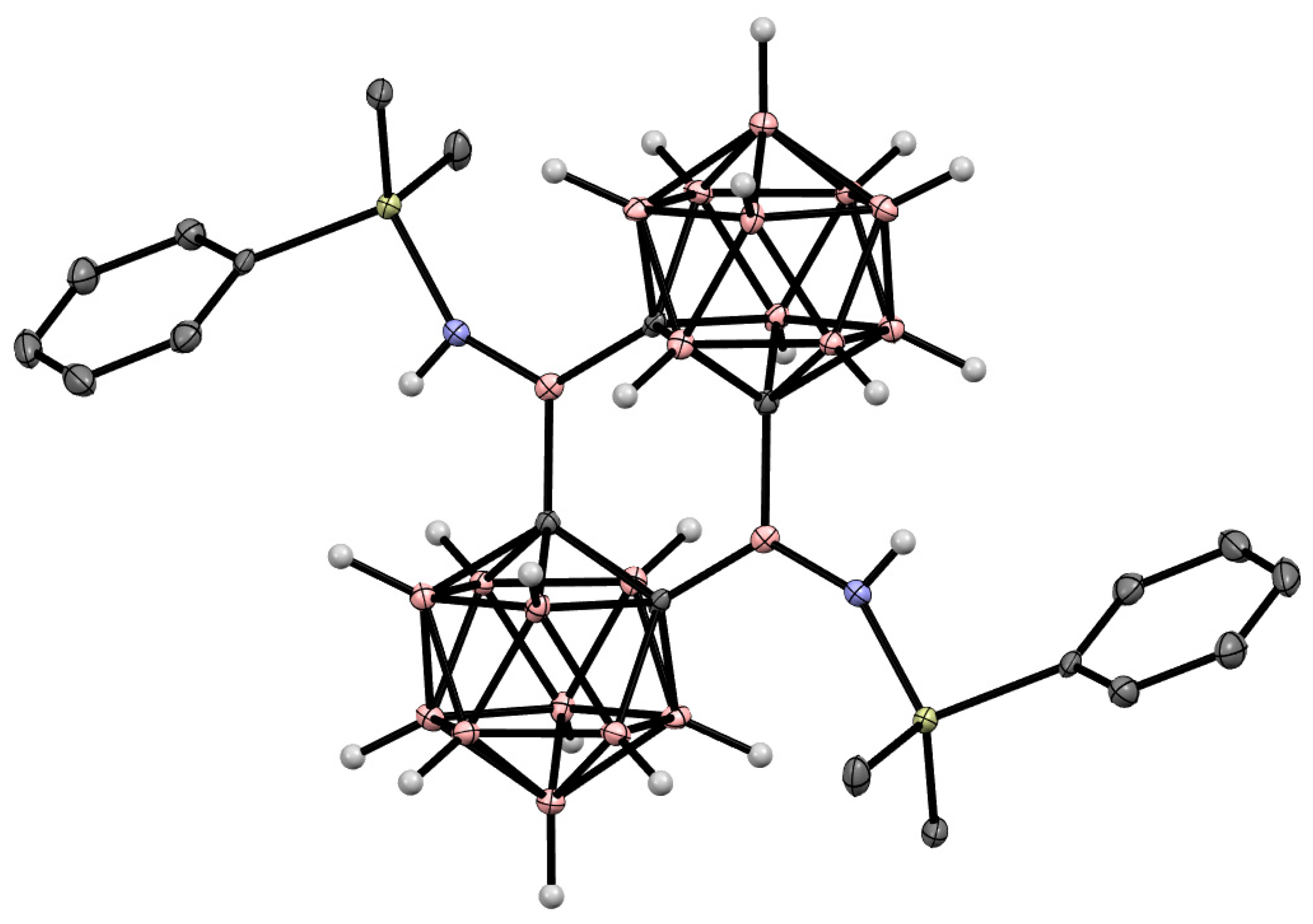
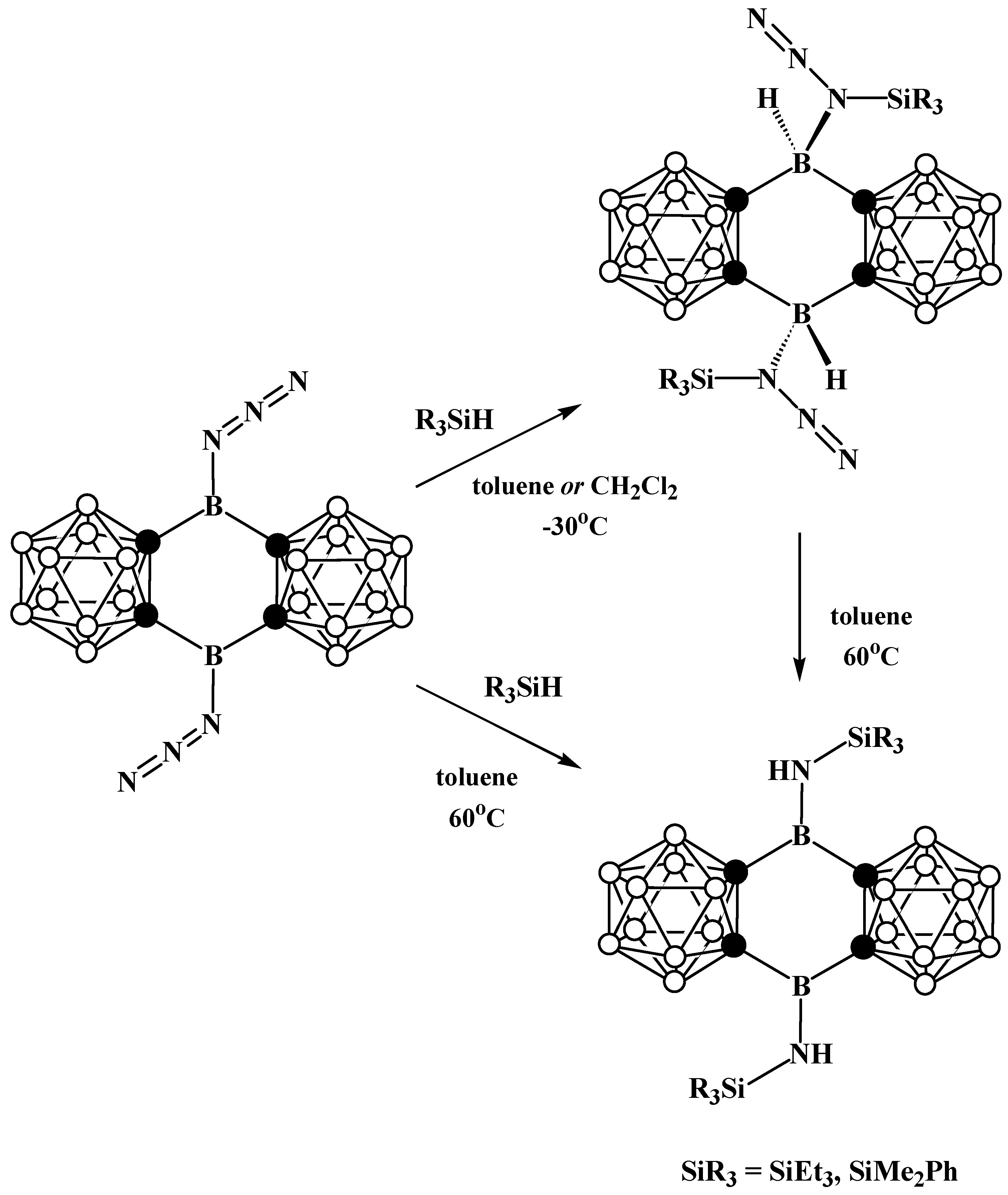
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivaev, I.B. Combining Two Types of Boron in One Molecule (To the 60th Anniversary of the First Synthesis of Carborane). Chemistry 2023, 5, 834-885. https://doi.org/10.3390/chemistry5020059
Sivaev IB. Combining Two Types of Boron in One Molecule (To the 60th Anniversary of the First Synthesis of Carborane). Chemistry. 2023; 5(2):834-885. https://doi.org/10.3390/chemistry5020059
Chicago/Turabian StyleSivaev, Igor B. 2023. "Combining Two Types of Boron in One Molecule (To the 60th Anniversary of the First Synthesis of Carborane)" Chemistry 5, no. 2: 834-885. https://doi.org/10.3390/chemistry5020059
APA StyleSivaev, I. B. (2023). Combining Two Types of Boron in One Molecule (To the 60th Anniversary of the First Synthesis of Carborane). Chemistry, 5(2), 834-885. https://doi.org/10.3390/chemistry5020059





