Coinage Metal Complexes Containing Perfluorinated Carboxylates
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Silver(I) Carboxylates
2.1.1. [Ag(O2CCF3)(η2-MeC6H5)]n (1)
2.1.2. [Ag2(O2CCF2CF3)2(η2-MeC6H5)]n (2)
2.1.3. [Ag{O2C(CF2)2CF3}]n (3)
2.1.4. [Ag(O2CC6F5)(MeC6H5)]n (4)
2.1.5. [Ag(O3SCF3)]n (5)
2.2. Preparation of the Gold(I) Carboxylates
2.2.1. [(Ph3P)Au(O2CCF3)] (1a)
2.2.2. [(Ph3P)Au(O2CCF2CF3)] (2a)
2.2.3. [(Ph3P)Au{O2C(CF2)2CF3}] (3a)
2.2.4. [(Ph3P)Au(O2CC6F5)}] (4a)
2.2.5. [(dppb)(AuO2CCF3)2] (5a)
2.2.6. [(dppb)(AuO2CC6F5)2] (6a)
2.2.7. [(Xantphos)(AuO2CCF2CF3)2] (7a)
2.2.8. [(Xantphos){AuO2C(CF2)2CF3}2] (8a)
2.3. Preparation of the Bimetallic Silver(I)-Gold(I) Carboxylates
2.3.1. [(CF3CO2)2AgAu(PPh3)]2 (9a)
2.3.2. [(CF3CF2CO2)2AgAu(PPh3)]2 (10a)
2.3.3. [{CF3(CF2)2CO2}2AgAu(PPh3)]2 (11a)
2.3.4. [(CF3CO2)2AgAu(dppb)]2 (12a)
2.3.5. [(C6F5CO2)2AgAu(dppb)]2 (13a)
2.3.6. [{CF3(CF2)2CO2}2AgAu(Xantphos)]2 (14a)
2.4. Preparation of the Perfluorinated Copper Complexes
2.4.1. [Cu2(O2CCF3)4(dioxane)]n (16)
2.4.2. [Cu(O2CCF3)(PPh3)3] (17)
2.4.3. [Cu(O2CCF2CF3)(PPh3)3]·MeOH (18)
2.4.4. [Cu{O2C(CF2)2CF3}(PPh3)3] (19)
2.4.5. [Cu(O2CC6F5)(PPh3)3] (20)
2.4.6. [Cu(O2CCF2CF3)(PPh3)2] (21)
2.5. X-ray Crystallography
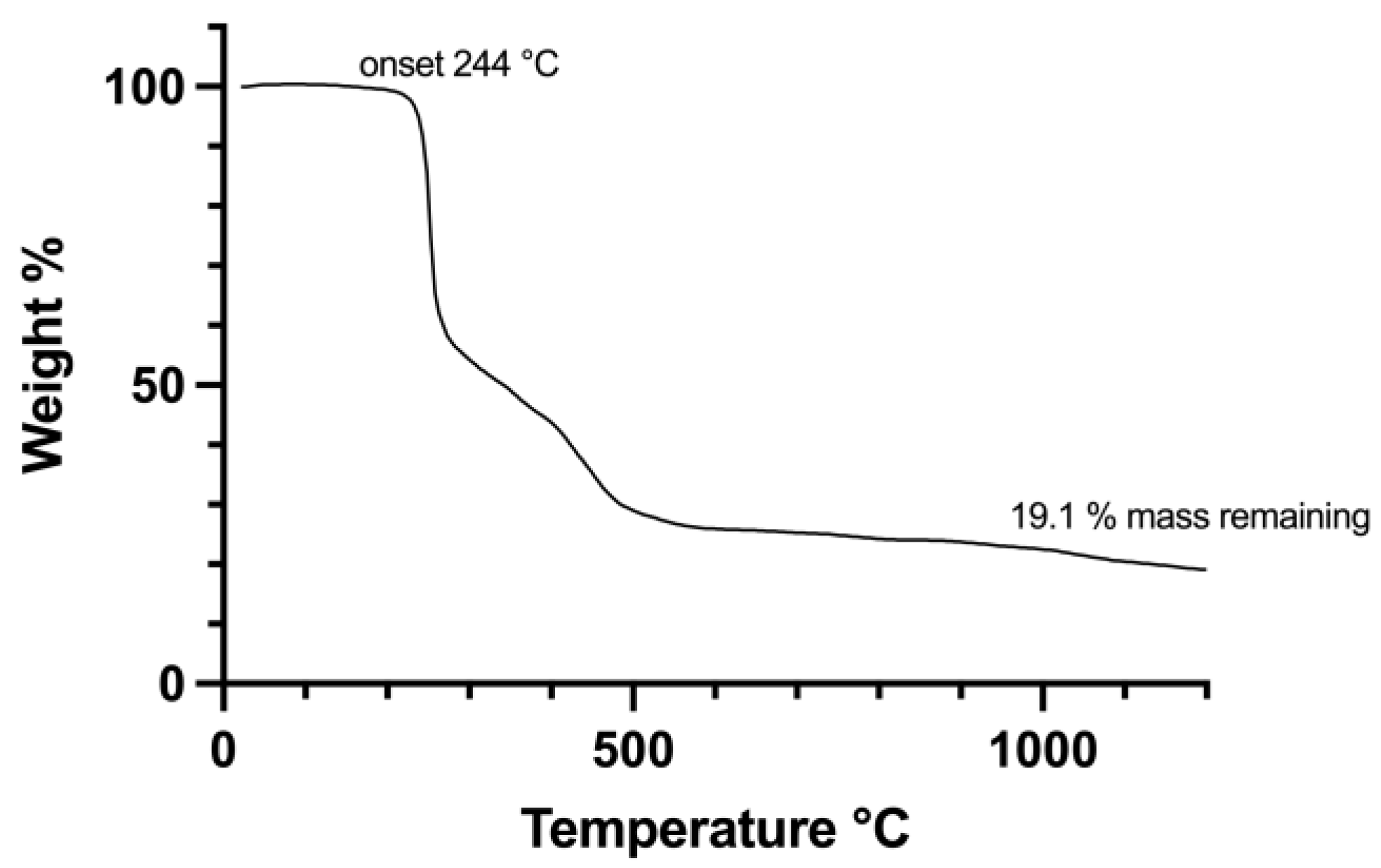
3. Results and Discussion
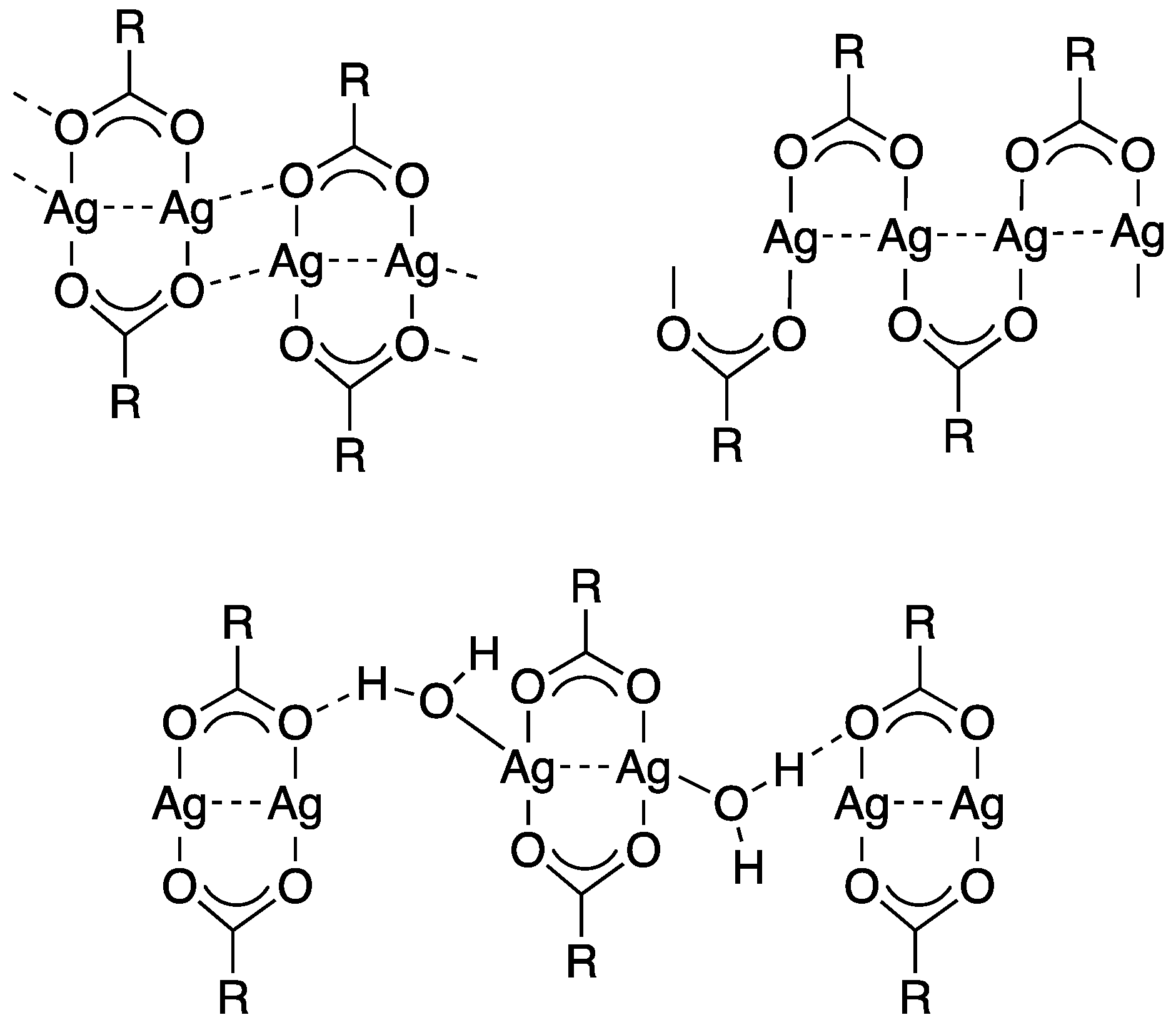
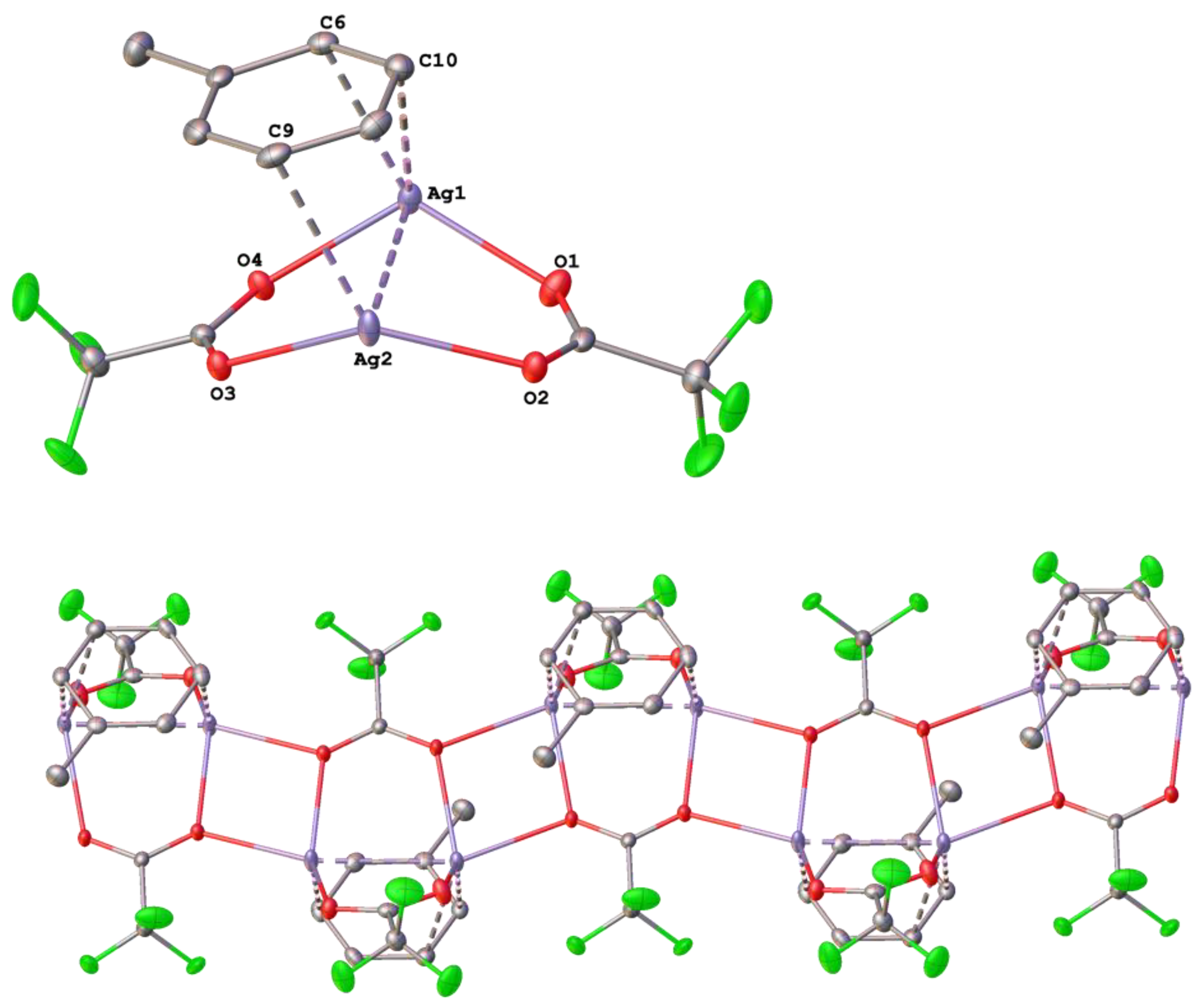
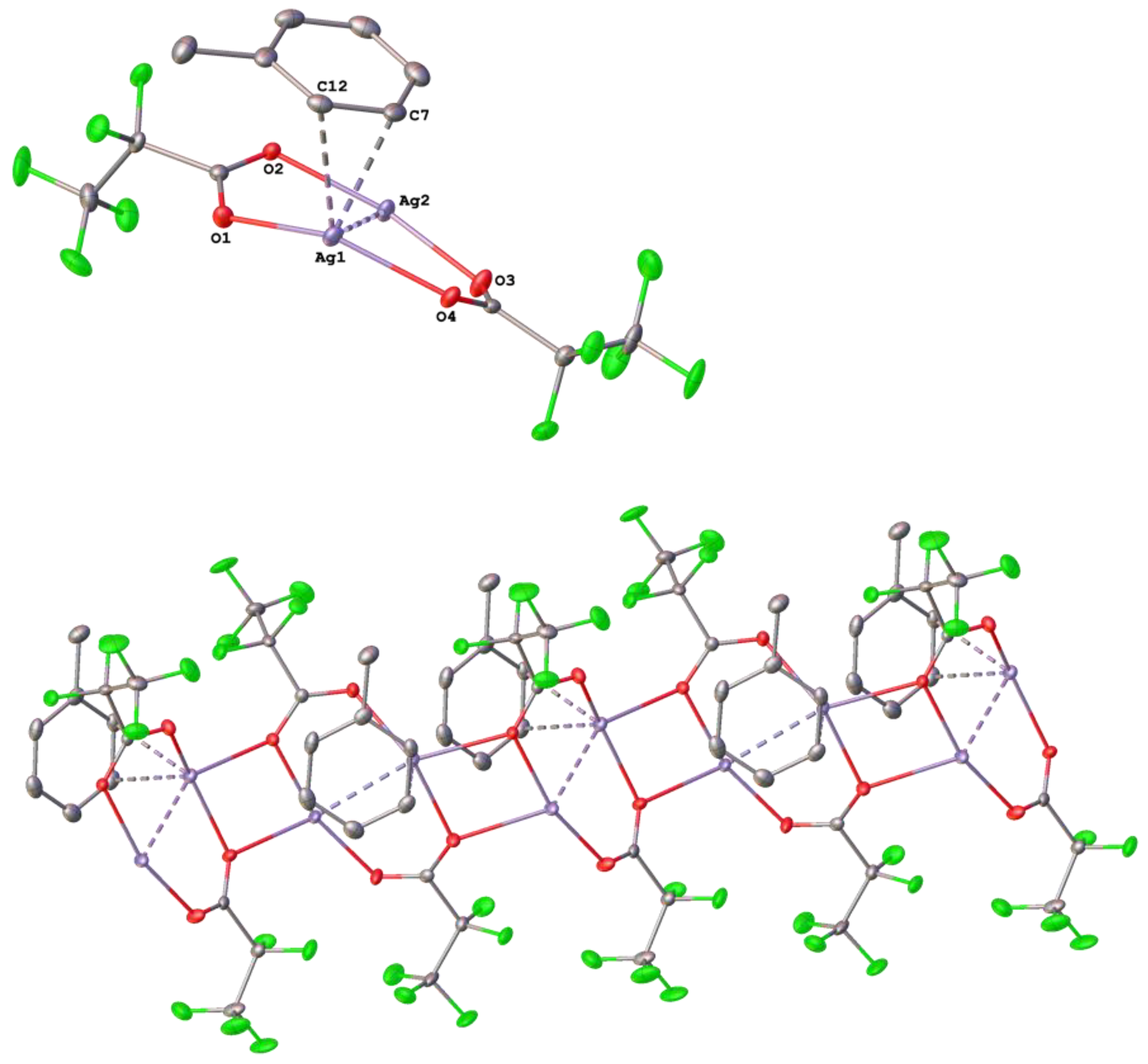
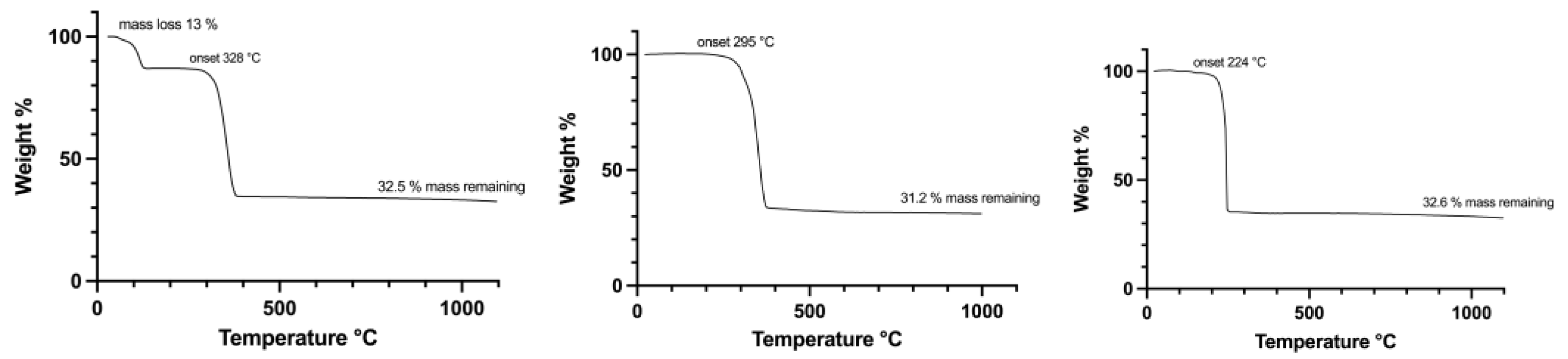
3.1. Silver(I) Complexes with Perfluorinated Carboxylato Ligands
3.2. Gold(I) Complexes with Perfluorinated Carboxylato Ligands
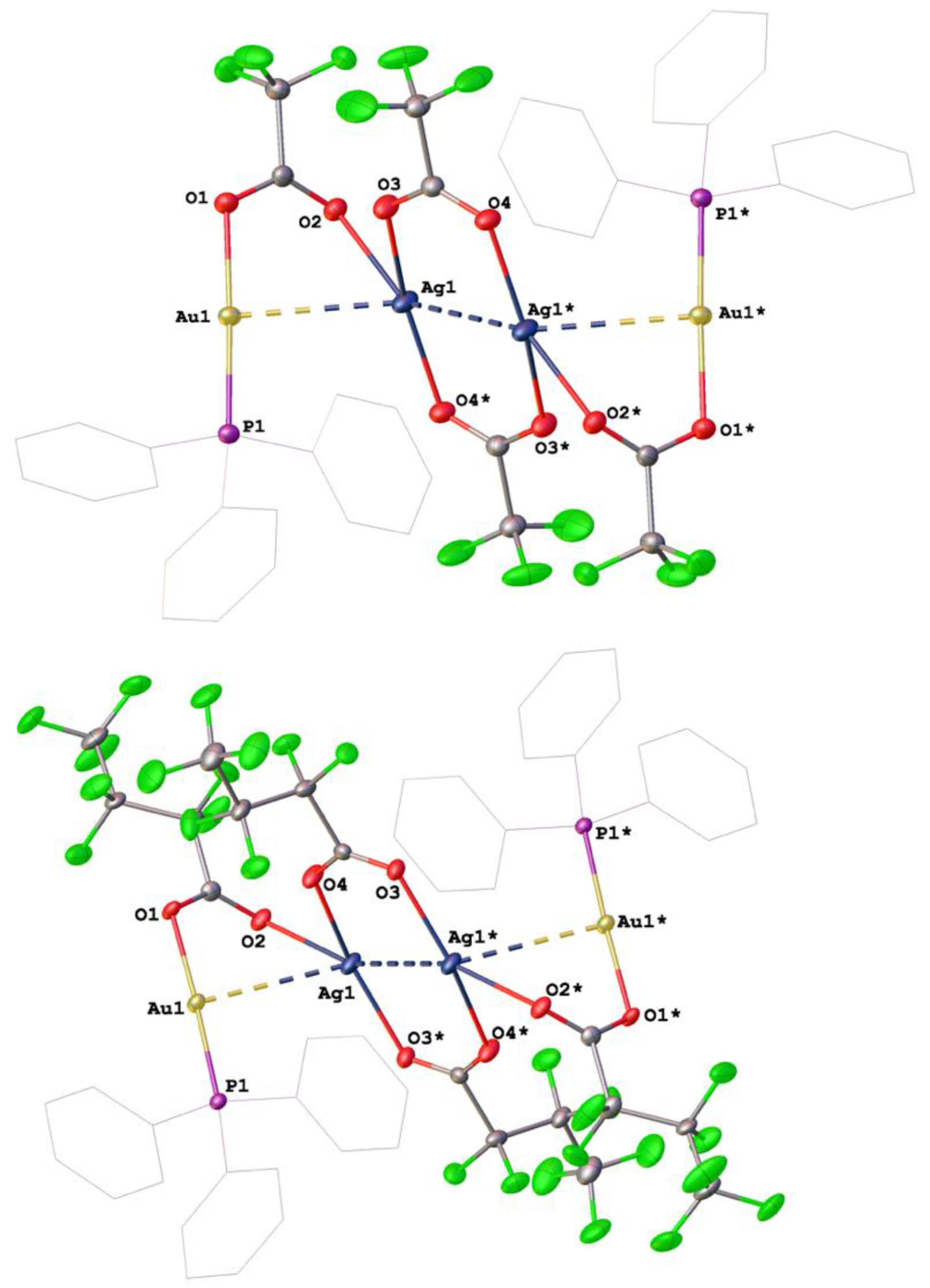
3.3. Bimetallic Silver/Gold Complexes with Perfluorinated Carboxylato Ligands
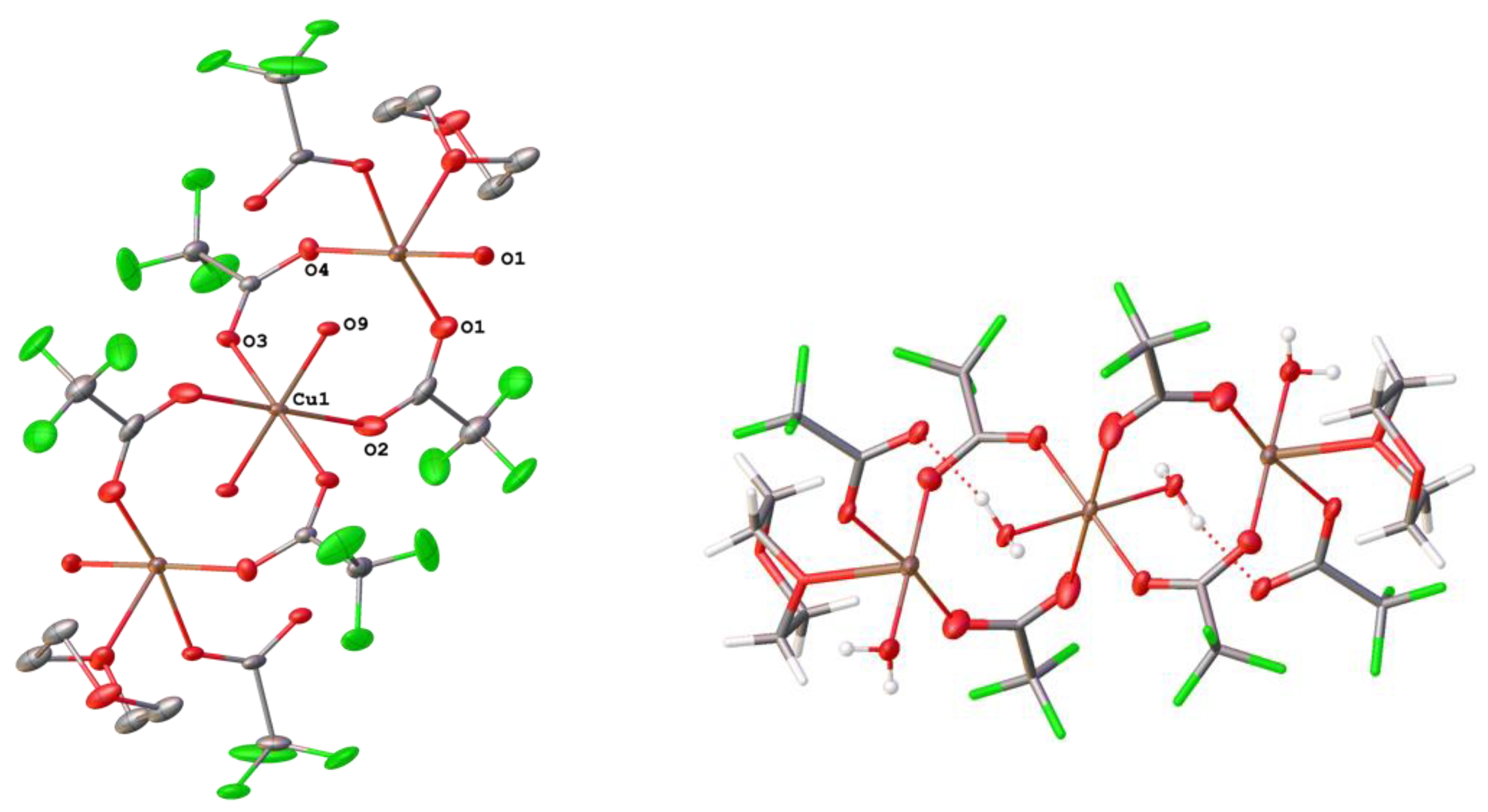
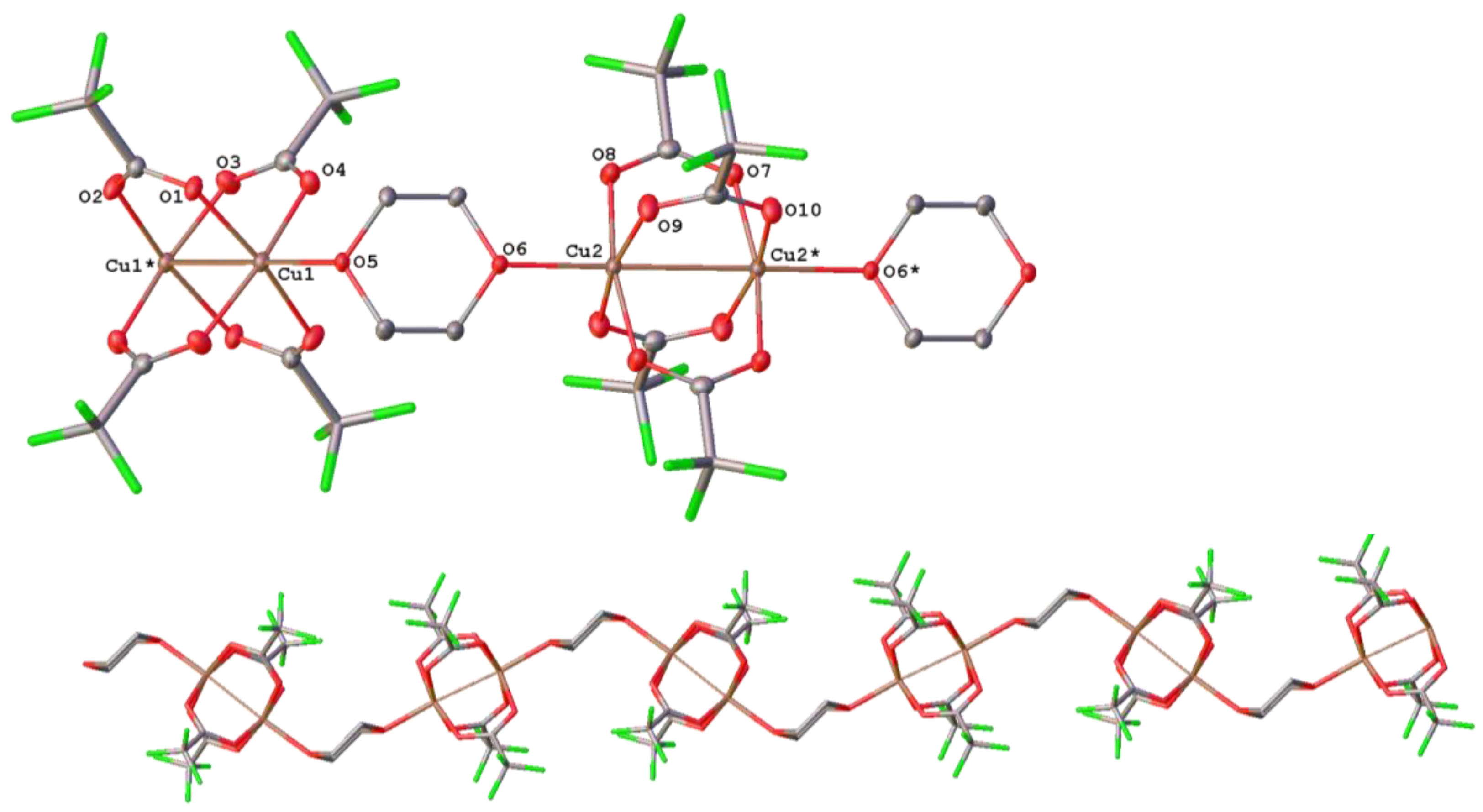
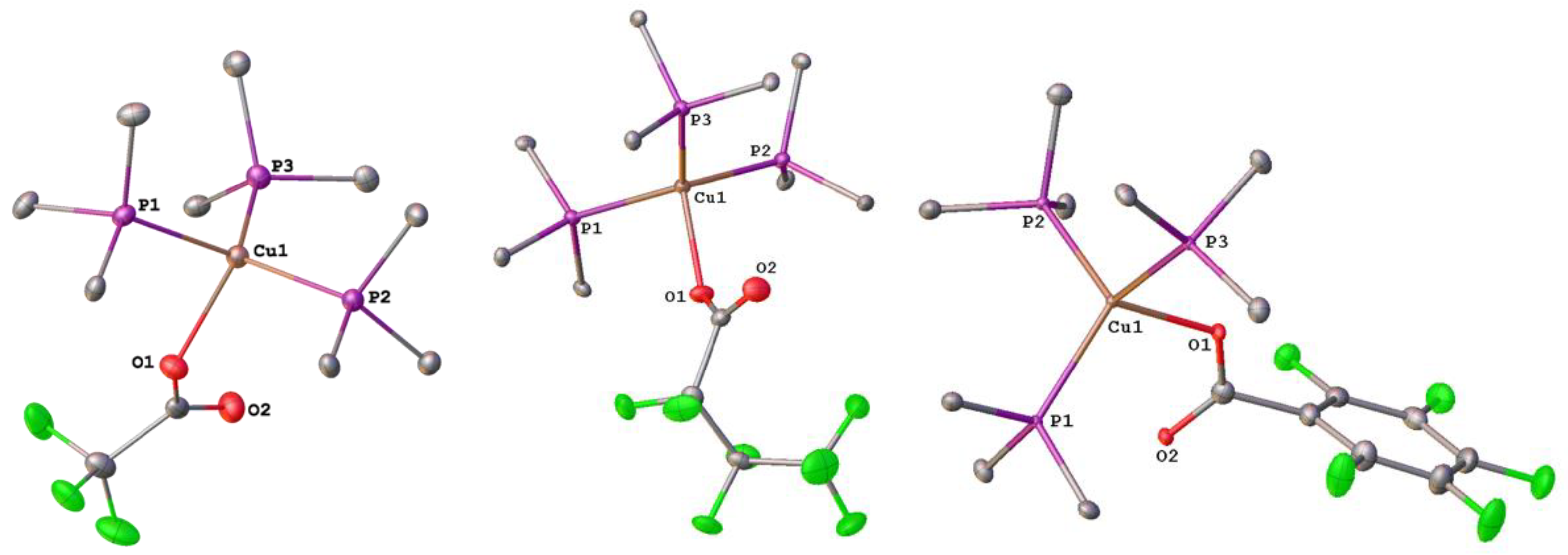
3.4. Copper Complexes with Perfluorinated Carboxylato Ligands
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hauptschein, M.; Grosse, A.V. Perfluoroalkyl halides prepared from silver perfluoro-fatty acid salts. I. Perfluoroalkyl iodides. J. Am. Chem. Soc. 1951, 73, 2461–2463. [Google Scholar] [CrossRef]
- Adams, S.K.; Edwards, D.A.; Richards, R. Silver(I) carboxylates. I. Mass spectra and low frequency infrared spectra. Inorg. Chim. Acta 1975, 12, 163–166. [Google Scholar]
- Edwards, D.A.; Harker, R.M.; Mahon, M.F.; Molloy, K.C. Aerosol-assisted chemical vapour deposition (AACVD) of silver films from triorganophosphine adducts of silver carboxylates, including the structure of [Ag(O2CC3F7)(PPh3)2]. Inorg. Chim. Acta 2002, 328, 134–146. [Google Scholar] [CrossRef]
- Römbke, P.; Schier, A.; Schmidbaur, H.; Cronje, S.; Raubenheimer, H. Mono- and bimetallic gold(I) and silver(I) pentafluoropropionates and related compounds. Inorg. Chim. Acta 2004, 357, 235–242. [Google Scholar]
- Torroba, J.; Aynsley, J.; Tuzimotoa, P.A.; Bruce, D.W. One-pot synthesis of anhydrous silver carboxylates from silver(I) fluoride. RSC Adv. 2012, 2, 12866–12869. [Google Scholar]
- Blakeslee, A.E.; Hoard, J.L. The structure of silver perfluorobutyrate. J. Am. Chem. Soc. 1956, 78, 3029–3033. [Google Scholar] [CrossRef]
- Griffin, R.G.; Ellett, J.D.; Mehring, M.; Bullitt, J.G.; Waugh, J.S. Single crystal study of the 19F shielding tensors of a trifluoromethyl group. J. Chem. Phys. 1972, 57, 2147–2155. [Google Scholar]
- Weigand, H.; Tyrra, W.; Naumann, D. The structure of silverpentafluorobenzoate monohydrate, AgCO2C6F5·H2O. Z. Anorg. Allg. Chem. 2008, 634, 2125–2126. [Google Scholar] [CrossRef]
- Lamann, R.; Hülsen, M.; Dolg, M.; Ruschewitz, U. Syntheses, crystal structures and thermal behavior of five new complexes containing 2,4,6-trifluorobenzoate as ligand. Z. Anorg. Allg. Chem. 2012, 638, 1424–1431. [Google Scholar] [CrossRef]
- Swarts, F. Sur quelques trifluoroacétates. Bull. Soc. Chim. Belg. 1939, 48, 176–191. [Google Scholar]
- Bruce, M.I.; Nicholson, B.K.; Shawkataly, O.B. Synthesis of gold-containing mixed metal cluster compleces. Inorg. Synth. 1989, 26, 324. [Google Scholar]
- Römbke, P.; Schier, A.; Schmidbaur, H. Gold(I) carboxylates and fluorocarboxylates. Z. Naturforsch. 2002, 57b, 605–609. [Google Scholar] [CrossRef]
- Edwards, D.A.; White, J.W. Copper(II) pentafluorobenzoates: Preparations, magnetic and spectroscopic features, and reation with triphenylphosphine. J. Inorg. Nucl. Chem. 1978, 40, 1335–1339. [Google Scholar] [CrossRef]
- Handa, M.; Ishitobi, Y.; Yakuwa, T.; Yoshioka, D.; Ishida, H.; Mikuriya, M.; Hiromitsu, I.; Tanaka, H.; Ikeue, T. A polymer complex [Cu(O2CC6F5)2(pyz)]n formed from copper(II) pentafluorobenzoate and pyrazine. Bull. Chem. Soc. Jpn. 2009, 82, 1277–1279. [Google Scholar] [CrossRef]
- Krupoder, S.A.; Danilovich, V.S.; Miller, A.O.; Furin, G.G. Synthesis and properties of the volatile polyfluoroaryl and polyfluorocarboxy derivatives of metals and metalloids - Prospective materials for microelectronics. Russ. J. Org. Chem. 1994, 30, 1243–1251. [Google Scholar]
- Kabsch, W. XDS. Acta Cryst. 2010, D66, 125–132. [Google Scholar]
- CrysAlisPro 41.123a, 40.53; Rigaku Oxford Diffraction Ltd.: Oxford, UK, 2022.
- DATCOL, Bruker AXS: Billerica, MA, USA, 2006.
- Sheldrick, G.M. SADABS 2.03; Universiät Göttingen: Göttingen, Germany, 2002. [Google Scholar]
- APEX3, Crystallography Software Suite, Bruker AXS: Billerica, MA, USA, 2017.
- Sheldrick, G.M. SHELXT - Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97, Program for Crystal Structure Refinement. University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Szlyk, E.; Lakomska, I.; Grodzicki, A. Thermal and spectroscopic studies of the Ag(I) salts with fluorinated carboxylic and sulfonic acid residues. Thermochim. Acta 1993, 223, 207–212. [Google Scholar] [CrossRef]
- Taylor, I.F., Jr.; Hall, E.A.; Amma, E.L. Metal ion-aromatic complexes. VII. Crystal and molecular structure of bis(m-xylene)silver perchlorate. J. Am. Chem. Soc. 1969, 91, 5745–5749. [Google Scholar] [CrossRef]
- Karpova, E.V.; Boltalin, A.I.; Korenev, Y.M.; Troyanov, S.I. Silver(I) mono- and trifluoroacetates: Thermal stability and crystal structure. Russ. J. Coord. Chem. 1999, 25, 65–68. [Google Scholar]
- Schmidbaur, H.; Schier, A. Argentophilic interactions. Angew. Chem., Int. Ed. 2015, 54, 746–784. [Google Scholar]
- Szczesny, R.; Szlyk, E. Thermal decomposition of some silver(I) carboxylates under nitrogen atmosphere. J. Therm. Anal. Calorim. 2013, 111, 1325–1330. [Google Scholar]
- Szlyk, E.; Lakomska, I.; Grodzicki, A. Studies of Au(I) complexes with triphenylphosphine and perfluorinated carboxylates. Polish J. Chem. 1995, 69, 1103–1108. [Google Scholar]
- Deák, A.; Tunyogi, T.; Tárkányi, G.; Király, P.; Pálinkás, G. Self-assembly of gold(i) with diphosphine and bitopic nitrogen donor linkers in the presence of trifluoroacetate anion: Formation of coordination polymerversus discrete macrocycle. CrystEngComm 2007, 9, 640–643. [Google Scholar]
- Appel, M.; Schloter, K.; Heidrich, J.; Beck, W. Metallorganische Lewis-Säuren: XXVII. Metallorganische Verbindungen mit Anionen der Fluorsulfonsäure, sowie von perfluorierten Sulfon- und Carbonsäuren. J. Organomet. Chem. 1987, 322, 77–88. [Google Scholar] [CrossRef]
- Tunyogi, T.; Deák, A. [m-4,5-Bis(diphenylphosphino)-9,9-di-methylxanthene]bis[(trifluoroacetato)-gold(I)] and its dichloromethane 0.58-solvate. Acta Cryst. 2010, C66, m133–m136. [Google Scholar]
- Schmidbaur, H.; Schier, A. A briefing on aurophilicity. Chem. Soc. Rev. 2008, 37, 1931–1951. [Google Scholar]
- Bartlett, P.N.; Cheng, F.; Cook, D.A.; Hector, A.L.; Levason, W.; Reid, G.; Zhang, W. Synthesis and structure of [{C7F15CO2}2AgAu(PPh3)]2 and its use in electrodeposition of gold–silver alloys. Inorg. Chim. Acta 2010, 363, 1048–1051. [Google Scholar] [CrossRef]
- Fernández, E.J.; López-de-Luzuriaga, J.M.; Monge, M.; Rodriguez, M.A.; Crespo, O.; Gimeno, M.C.; Laguna, A.; Jones, P.G. Heteropolynuclear complexes with the ligand Ph2PCH2SPh: Theoretical evidence for metallophilic Au-M interactions. Chem. Eur. J. 2000, 6, 636–644. [Google Scholar] [CrossRef]
- Wheaton, C.A.; Jennings, M.C.; Puddephatt, R.J. Complexes of gold(I) with a chiral diphosphine ligand: A polymer with Au-Ag and Ag-Ag metallophilic bonds. Z. Naturforsch. 2009, 64b, 1469–1477. [Google Scholar] [CrossRef]
- Catterick, J.; Thornton, P. Structures and physical properties of polynuclear carboxylates. Adv. Inorg. Chem. Radiochem. 1977, 20, 291–362. [Google Scholar]
- Larionov, S.V.; Glinskaya, L.A.; Klevtsova, R.F.; Lvov, P.E.; Ikorskii, V.N. Preparation, crystal od molecular structure, and magnetic properties of an adduct of copper(II) pentafluorobenzoate with 1,4-dioxane. Russ. J. Inorg. Chem. 1991, 36, 1413–1415. [Google Scholar]
- Karpova, E.V.; Boltalin, A.I.; Korenev, Y.M.; Troyanov, S.I. Synthesis and X-ray diffraction analysis of copper(II) fluoroacetates Cu(CF3COO)2 and Cu(CHF2COO)2 0.5H2O containing polymeric chains in their structures. Russ. J. Coord. Chem. 2000, 26, 361–366. [Google Scholar]
- Zhang, J.; Hubert-Pfalzgraf, L.G.; Luneau, D. Synthesis, characterization and molecular structures of Cu(II) and Ba(II) fluorinated carboxylate complexes. Polyhedron 2005, 24, 1185–1195. [Google Scholar] [CrossRef]
- Moreland, J.A.; Doedens, R.J. Synthesis, crystal structure, and magnetic properties of a dimeric quinoline adduct of copper(II) trifluoroacetate. J. Am. Chem. Soc. 1975, 97, 508–513. [Google Scholar] [CrossRef]
- Karpova, E.V.; Boltalin, A.I.; Zakharov, M.A.; Sorokina, N.I.; Korenev, Y.M.; Troyanov, S.I. Synthesis and crystal structure of copper(II) trifluoroacetates, Cu2(CF3COO)4 · 2 CH3CN and Cu(CF3COO)2(H2O)4. Z. Anorg. Allg. Chem. 1998, 624, 741–744. [Google Scholar] [CrossRef]
- Vives, G.; Mason, S.A.; Prince, P.D.; Junk, P.C.; Steed, J.W. Intramolecular versus intermolecular hydrogen bonding of coordinated acetate to organic acids: A neutron, X-ray, and database study. Cryst. Growth Des. 2003, 3, 699–704. [Google Scholar] [CrossRef]
- Nefedov, S.E.; Kushan, E.V.; Yakovleva, M.A.; Chikhichin, D.G.; Kotseruba, V.A.; Levchenko, O.A.; Kamalov, G.L. Binuclear complexes with the “Chinese Lantern” geometry as intermediates in the liquid-phase oxidation of dibenzyl ether with atmospheric oxygen in the presence of copper(II) carboxylates. Russ. J. Coord. Chem. 2012, 38, 224–231. [Google Scholar] [CrossRef]
- Gahlot, S.; Purohit, B.; Jeanneau, E.; Mishra, S. Coinage metal complexes with di-tertiary-butyl sulfide as precursors with ultra-low decomposition temperature. Chem. Eur. J. 2021, 27, 10826–10832. [Google Scholar] [CrossRef]
- Motreff, A.; Correa da Costa, R.; Allouchi, H.; Duttine, M.; Mathonière, C.; Duboc, C.; Vincent, J.M. Dramatic solid-state humidity-induced modification of the magnetic coupling in a dimeric fluorous copper(II)−carboxylate complex. Inorg. Chem. 2009, 48, 5623–5625. [Google Scholar] [CrossRef] [PubMed]
- Han, L.J.; Kong, Y.J.; Huang, M.M. Magnetic properties and crystal structures of two copper coordination compounds with pentafluorobenzoate ligand. Inorg. Chim. Acta 2021, 514, 120019. [Google Scholar]
- Kani, Y.; Tsuchimoto, M.; Ohba, S.; Tokii, T. catena-Poly[[tetrakis(m-2,2-dimethyl-propionato-O:O´)dicopper(II)]-m-dioxane-O:O´] and catena-poly-[[tetrakis(m-3,3-dimethylbutyrato-O:O´)dicopper(II)]-m-dioxane-O,O´]. Acta Cryst. 2000, C56, e80–e81. [Google Scholar]
- Borel, M.M.; Leclaire, A. Etude des Propionates Métalliques. V. Détermination de la Structure du Bis-propionato Cuivre(II) 0,5 Dioxanne. Acta Cryst. 1976, B32, 1275–1278. [Google Scholar] [CrossRef]
- Smart, P.; Mínguez Espallargas, G.; Brammer, L. Competition between coordination network and halogen bond network formation: Towards halogen-bond functionalised network materials using copper-iodobenzoate units. CrystEngComm 2008, 10, 1335–1344. [Google Scholar] [CrossRef]
- Boniak, L.; Borel, M.M.; Busnot, F.; Leclaire, A. Structure de [Cu(C6H5COO)2(C4H8O2)0.5)]2 2 C4H8O2. Mise en évidence d’une conformation "twist" du dioxane. Rev. Chim. Miner. 1979, 16, 501–508. [Google Scholar]
- Hammond, B.; Jardine, F.H.; Vohra, A.G. Carboxylatocopper(I) complexes. J. Inorg. Nucl. Chem. 1971, 33, 1017–1024. [Google Scholar] [CrossRef]
- Hart, R.D.; Healy, P.C.; Hope, G.A.; Turner, D.W.; White, A.H. Electrochemical synthesis and structural characterization of bis(triphenylphosphine)copper(I) fluoroacetates. J. Chem. Soc. Dalton Trans. 1994, 773–779. [Google Scholar] [CrossRef]
- Vykoukal, V.; Halasta, V.; Babiak, M.; Bursik, J.; Pinkas, J. Morphology control in AgCu nanoalloy synthesis by molecular Cu(I) precursors. Inorg. Chem. 2019, 58, 15246–15254. [Google Scholar]
- Devereux, M.; McCann, M.; Cronin, J.F.; Cardin, C.; Convery, M.; Quillet, V. Synthesis and X-ray crystal structure of the copper(I) dicarboxylic acid complex [Cu(η1-bdoaH)(PPh3)3] (bdoaH2 = benzene-1,2- dioxyacetic acid). Polyhedron 1994, 13, 2359–2366. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Holtcamp, M.W.; Khandelwal, B.; Klausmeyer, K.K.; Reibenspies, J.H. A more intimate examination of the role of copper(I) in the decarboxylation of derivatives of malonic acid. Comparisons with zinc(II) analogs. Inorg. Chem. 1995, 34, 2389–2398. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Holtcamp, M.W.; Khandelwal, B.; Reibenspies, J.H. Intramolecular and intermolecular hydrogen bonding in triphenylphosphine derivatives of copper(I) carboxylates, (Ph3P)2CuO2C(CH2)nCOOH. Role of copper(I) in the decarboxylation of malonic acid and its derivatives. Inorg. Chem. 1994, 33, 531–537. [Google Scholar] [CrossRef]
- Mauro, A.E.; Porta, C.C.; De Simone, C.A.; Zukerman-Schpector, J.; Catellano, E.E. Synthesis and structural studies of (3,5-dinitrobenzoate)bis(triphenylphosphine)copper(I). Polyhedron 1993, 12, 1141–1143. [Google Scholar] [CrossRef]
- Szlyk, E.; Szymanska, I. Studies of new volatile copper(I) complexes with triphenylphosphite and perfluorinated carboxylates. Polyhedron 1999, 18, 2941–2948. [Google Scholar] [CrossRef]








| 1 | 2 | 2a | 4a | 6a | 7a | 9a | 11a | |
|---|---|---|---|---|---|---|---|---|
| CCDC code | 2167914 | 2167908 | 2167925 | 2167926 | 2167923 | 2167921 | 2167924 | 2167915 |
| Empirical formula | C11H8F6O4Ag2 | C13H8F10O4Ag | C21H15F5O2PAu | C25H15F5O2PAu | C44H24F10O4P2Au2 | C45H32F10O5P2Au2 | C44H30F12O8P2Ag2Au2 | C52H30F28O8P2Ag2Au2 |
| Formula weight | 533.91 | 633.93 | 622.27 | 670.31 | 1262.50 | 1298.58 | 1586.29 | 1986.37 |
| Crystal system | Triclinic | Monoclinic | Monoclinic | Monoclinic | Triclinic | Triclinic | Triclinic | Triclinic |
| Space group | P-1 | P21/c | P21/n | P21/n | P-1 | P-1 | P-1 | P-1 |
| Unit cell dimensions | a = 9.1704(5) Å b = 9.6753(6) Å c = 10.1292(6) Å α = 61.546(6)° β = 64.823(5)° γ = 85.495(5)° | a = 12.1816(9) Å b = 13.4270(12) Å c = 10.6126(9) Å α = 90.0° β = 94.470(7)° γ = 90.0° | a = 11.8549(10) Å b = 11.7981(10) Å c = 14.7984(13) Å α = 90.0° β = 104.747(3)° γ = 90.0° | a = 13.2968(6) Å b = 6.6460(5) Å c = 25.3473(14) Å α = 90.0° β = 97.943(6)° γ = 90.0° | a = 11.9016(13) Å b = 13.5895(7) Å c = 14.8220(7) Å α = 65.906(6)° β = 83.007(5)° γ = 89.395(6)° | a = 10.243(3) Å b = 13.205(7) Å c = 17.993(7) Å α = 70.86(3)° β = 87.40(3)° γ = 71.03(4)° | a = 7.9232(5) Å b = 11.8065(9) Å c = 13.2352(12) Å α = 85.362(5)° β = 73.848(8)° γ = 73.859(7)° | a = 10.3296(5) Å b = 11.8286(6) Å c = 13.5824(7) Å α = 75.056(4)° β = 75.850(4)° γ = 70.362(5)° |
| Volume Å3 | 706.34(8) | 1730.5(2) | 2001.6(3) | 2218.5(2) | 2170.1(3) | 2169.3(17) | 1142.33(16) | 1487.29(14) |
| Z | 2 | 4 | 4 | 4 | 2 | 2 | 1 | 1 |
| ρcalc g/cm3 | 2.510 | 2.433 | 2.065 | 2.007 | 1.932 | 1.988 | 2.306 | 2.218 |
| μ mm−1 | 2.857 | 2.385 | 7.491 | 6.767 | 6.911 | 6.918 | 7.418 | 5.759 |
| F(000) | 508.0 | 1208.0 | 1184.0 | 1280.0 | 1196.0 | 1240.0 | 748.0 | 940.0 |
| Crystal size | 0.02 × 0.05 × 0.10 | 0.02 × 0.05 × 0.11 | 0.022 × 0.057 × 0.059 | 0.011 × 0.025 × 0.045 | 0.08 × 0.09 × 0.12 | 0.04 × 0.08 × 0.08 | 0.035 × 0.04 × 0.16 | 0.03 × 0.05 × 0.07 |
| 2θ range for data collection | 4.97 to 64.904° | 5.768 to 58.862° | 3.946 to 67.612° | 4.697 to 72.105° | 5.31 to 74.062° | 5.48 to 62.874° | 5.554 to 72.200° | 4.834 to 58.974° |
| Reflections collected | 7127 | 9243 | 74,095 | 53,830 | 104,934 | 52,648 | 52,950 | 13,672 |
| Independent reflections | 7127 | 4023 | 8037 | 10,522 | 22,074 | 14,289 | 8942 | 6916 |
| Data/restraints/parameters | 7127/0/210 | 4023/0/263 | 8037/0/271 | 10,522/0/307 | 22,074/0/559 | 14,289/0/589 | 8942/0/316 | 6916/0/424 |
| Goodness-of-fit on F2 | 0.942 | 1.044 | 1.056 | 1.019 | 1.027 | 1.024 | 1.015 | 1.044 |
| Final R indices [I > 2σ(I)] | R1 = 0.0296 wR2 = 0.0657 | R1 = 0.0226 wR2 = 0.0431 | R1 = 0.0164 wR2 = 0.0353 | R1 = 0.0304 wR2 = 0.0481 | R1 = 0.0306 wR2 = 0.0639 | R1 = 0.0699 wR2 = 0.1600 | R1 = 0.0274 wR2 = 0.0511 | R1 = 0.0300 wR2 = 0.0448 |
| Final R indices [all data] | R1 = 0.0429 wR2 = 0.0684 | R1 = 0.0286 wR2 = 0.0451 | R1 = 0.0221 wR2 = 0.0371 | R1 = 0.0589 wR2 = 0.0534 | R1 = 0.0472 wR2 = 0.0681 | R1 = 0.1181 wR2 = 0.1861 | R1 = 0.0429 wR2 = 0.0544 | R1 = 0.0435 wR2 = 0.0491 |
| Largest difference peak/hole e/Å3 | 1.84/−1.06 | 0.60/−0.53 | 1.4/−0.6 | 1.0/−1.3 | 2.8/−2.7 | 3.8/−3.9 | 0.9/−1.9 | 1.00/−1.29 |
| 14a | 15 | 16 | 17 | 19 | 20 | 21 | ||
| CCDC code | 2167922 | 2167916 | 2167909 | 2167913 | 2167912 | 2167910 | 2167911 | |
| Empirical formula | C55H32F28O9P2Ag2Au2 | C24H32F18O22Cu | C12H8F12O10Cu2 | C56H45F3O2P3Cu | C58H45F7O2P3Cu | C63H53F5O4P3Cu | C39H30F5O2P2Cu | |
| Formula weight | 2042.42 | 1205.11 | 667.26 | 963.37 | 1063.39 | 1125.50 | 751.11 | |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Triclinic | Triclinic | Triclinic | Triclinic | |
| Space group | P21/c | P21/n | P21/n | P-1 | P-1 | P-1 | P-1 | |
| Unit cell dimensions | a = 20.778(3) Å b = 15.689(3) Å c = 18.248(3) Å α = 90.0° β = 97.595(14)° γ = 90.0° | a = 12.7528(7) Å b = 10.3794(5) Å c = 18.5367(14) Å α = 90.0° β = 108.717(7)° γ = 90.0° | a = 8.7675(2) Å b = 14.5136(4) Å c = 16.7461(4) Å α = 90.0° β = 98.800(2)° γ = 90.0° | a = 12.9761(3) Å b = 17.9813(4) Å c = 21.5160(6) Å α = 82.311(2)° β = 83.547(2)° γ = 88.1828(19)° | a = 11.3935(4) Å b = 12.2780(4) Å c = 18.7548(6) Å α = 87.683(3)° β = 78.047(3)° γ = 72.226(3)° | a = 13.0559(3) Å b = 13.1731(4) Å c = 18.7364(4) Å α = 95.532(2)° β = 105.660(2)° γ = 114.835(3)° | a = 12.1576(8) Å b = 12.9136(10) Å c = 13.1932(10) Å α = 89.326(6)° β = 66.955(7)° γ = 63.060(7)° | |
| Volume Å3 | 5896.1(16) | 2323.9(3) | 2105.82(9) | 4942.9(2) | 2443.47(15) | 2733.51(14) | 1664.2(2) | |
| Z | 4 | 2 | 4 | 4 | 2 | 2 | 2 | |
| ρcalc g/cm3 | 2.299 | 1.722 | 2.105 | 1.295 | 1.445 | 1.367 | 1.499 | |
| μ mm−1 | 5.816 | 1.504 | 2.174 | 0.591 | 0.616 | 0.553 | 0.816 | |
| F(000) | 3872.0 | 1202.0 | 1304.0 | 1992.0 | 1092.0 | 1164.0 | 768.0 | |
| Crystal size | 0.04 × 0.05 × 0.12 | 0.05 × 0.07 × 0.09 | 0.02 × 0.07 × 0.08 | 0.06 × 0.07 × 0.12 | 0.07 × 0.09 × 0.09 | 0.06 × 0.09 × 0.11 | 0.04 × 0.06 × 0.08 | |
| 2θ range for data collection | 5.2 to 52.744° | 4.666 to 58.686° | 4.922 to 59.016° | 4.978 to 59.088° | 4.81 to 58.838° | 4.652 to 59.004° | 5.458 to 58.654° | |
| Reflections collected | 80,334 | 13,733 | 11,030 | 52,923 | 23,872 | 27,958 | 15,883 | |
| Independent reflections | 12,054 | 5405 | 4885 | 23,105 | 11,311 | 12,725 | 7621 | |
| Data/restraints/parameters | 12,054/36/912 | 5405/0/298 | 4885/24/344 | 23,105/0/1171 | 11,311/0/640 | 12,725/0/689 | 7621/0/442 | |
| Goodness-of-fit on F2 | 1.052 | 1.050 | 1.042 | 1.023 | 1.026 | 1.028 | 1.039 | |
| Final R indices [I > 2σ(I)] | R1 = 0.0370 wR2 = 0.0746 | R1 = 0.0921 wR2 = 0.2197 | R1 = 0.0317 wR2 = 0.0709 | R1 = 0.0470 wR2 = 0.1073 | R1 = 0.0329 wR2 = 0.0722 | R1 = 0.0387 wR2 = 0.0956 | R1 = 0.0273 wR2 = 0.0648 | |
| Final R indices [all data] | R1 = 0.0569 wR2 = 0.0831 | R1 = 0.1190 wR2 = 0.2417 | R1 = 0.0420 wR2 = 0.0757 | R1 = 0.0619 wR2 = 0.1155 | R1 = 0.0417 wR2 = 0.0768 | R1 = 0.0478 wR2 = 0.1011 | R1 = 0.0321 wR2 = 0.0671 | |
| Largest difference peak/hole e/Å3 | 1.93/−1.66 | 3.43/−1.94 | 0.49/−0.69 | 2.79/−1.62 | 0.42/−0.47 | 2.02/−0.62 | 0.46/−0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piani, R.; Beele, B.B.; Rust, J.; Lehmann, C.W.; Mohr, F. Coinage Metal Complexes Containing Perfluorinated Carboxylates. Chemistry 2023, 5, 813-833. https://doi.org/10.3390/chemistry5020058
Piani R, Beele BB, Rust J, Lehmann CW, Mohr F. Coinage Metal Complexes Containing Perfluorinated Carboxylates. Chemistry. 2023; 5(2):813-833. https://doi.org/10.3390/chemistry5020058
Chicago/Turabian StylePiani, Robin, Björn B. Beele, Jörg Rust, Christian W. Lehmann, and Fabian Mohr. 2023. "Coinage Metal Complexes Containing Perfluorinated Carboxylates" Chemistry 5, no. 2: 813-833. https://doi.org/10.3390/chemistry5020058
APA StylePiani, R., Beele, B. B., Rust, J., Lehmann, C. W., & Mohr, F. (2023). Coinage Metal Complexes Containing Perfluorinated Carboxylates. Chemistry, 5(2), 813-833. https://doi.org/10.3390/chemistry5020058








