Abstract
The recently discovered natural product (NP) (+)-floyocidin B with antimicrobial activity against Mycobacterium tuberculosis displays a hitherto unknown dihydroisoquinolinone scaffold in the class of the epoxyquinone NPs. The 4,5-regioselective functionalization of 2-chloropyridines was identified as a suitable strategy leading to the total syntheses of (+)-floyocidin B and analogs. In this paper, we present the long and winding evolution process to the final synthetic pathway, including model systems for route scouting and elucidation of side products, which enabled us to understand the unique reactivity of this unprecedented scaffold. A special focus was laid on method studies with different 2-chloropyridines, disclosing an unexpected effect of the 2-chloro substituent on the regioselectivity compared to 2-unsubstituted or carbon-substituted pyridines. Finally, a head-to-head comparison with the previously described synthesis of all four stereoisomers of the NP (−)-avicennone C revealed significant differences in the reactivity of these structurally closely related scaffolds.
1. Introduction
The class of the epoxyquinone natural products (NPs) [1] is known for its structural novelty and compounds with interesting biological activities. Antimicrobially active (+)-ambuic acid (1) [2] represents a prominent example, which inspired synthetic chemists to develop several total syntheses for this epoxyquinone (Figure 1) [3,4,5]. During a recent activity-guided screening campaign for new antitubercular NPs, further natural epoxyquinone congeners such as (+)-floyocidin A (3) and B (4) were discovered (Figure 1) [6]. Similarly to the structurally related NP (−)-avicennone C (2), for which the relative stereochemistry was misassigned based on nuclear magnetic resonance (NMR) data [7,8], structure elucidation of (+)-floyocidin B (4) was only possible by total synthesis [6]. Subsequently, the active-to-hit evaluation with (+)-floyocidin B (4) as a starting point resulted in synthetic analogs with even higher activities against Mycobacterium tuberculosis [6].
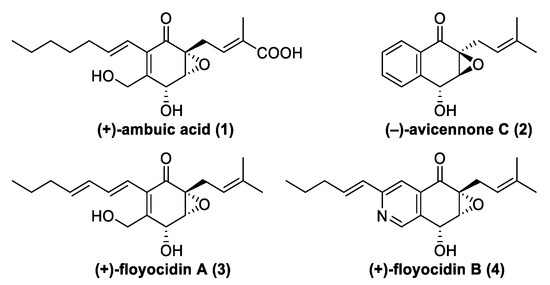
Figure 1.
Structures of NPs (+)-ambuic acid (1), (−)-avicennone C (2), and structurally related NPs (+)-floyocidin A (3) and (+)-floyocidin B (4).
Our previously reported syntheses of all four stereoisomers of the NPs (−)-avicennone C (2) [8] and the newly discovered (+)-floyocidin B (4) [6] for structure elucidation were the results of extensive route scouting [9]. Due to their structural similarity, the development of the total syntheses of 2 and 4 was performed simultaneously, and all gathered information about the chemical behavior was mutually adapted. Within this publication, we intend to present the full story of our route scouting as a case study with a special focus on the 4,5-regioselective functionalization of 2-chloropyridines, which may be of interest for further synthetic applications, e.g., in the field of medicinal chemistry.
For the total synthesis of (+)-floyocidin B (4) [6], chloro intermediate 5 was chosen as a key intermediate since it strategically allows not only for a late introduction of the pentenyl chain via Suzuki coupling, but also for a flexible decoration of the scaffold, which is advantageous for structure–activity relationship (SAR) investigations. Retrosynthetically, intermediate 5 was simplified to 2-chloro-4,5-substituted pyridines 6, which should be accessible from readily available 2-chloropyridines (Scheme 1). After an extensive literature search on regioselective pyridine functionalizations, we focused on three strategies considered to be promising for further investigations: (1) the 5-selective functionalization of 2-chloroisonicotinic acid (7) [10,11,12], (2) the 4-selective functionalization of 2-chloronicotinic acid (8) [13,14] or 2-chloro-5-bromopyridine (9) [15], and, finally, (3) the 5-selective halogen–metal exchange of the trihalogenated 2-chloro-4,5-dibromopyridine (10), which was unprecedented at the time when we started our investigations.
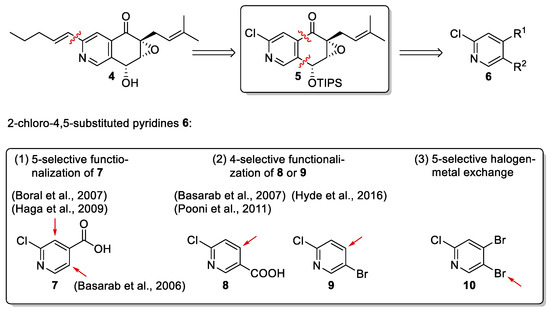
Scheme 1.
Retrosynthetic analysis of 2-chloro-4,5-substituted pyridines 6 [10,11,12,13,14,15].
2. Materials and Methods
Synthetic procedures and corresponding analytic data can be found in the Supplementary Materials.
3. Results and Discussion
Our first strategy to access key intermediate 2-chloro-4,5-substituted pyridine 6 was based on the work of Basarab et al. [10], who reported a selective functionalization of 2-chloroisonicotinic acid (7) in position 5 by directed ortho-lithiation using lithium tetramethylpiperidine (LiTMP), followed by trapping of the lithium species with DMF and conversion to the ethyl ester 11. However, we wanted to avoid the formation of a reactive aldehyde resulting from using DMF as the electrophile. Therefore, we chose benzaldhehyde as the test electrophile. The reaction resulted in lactone 12 as a single product, which was spontaneously formed from the initial addition product during aqueous work-up (Scheme 2). NMR analyses indicated functionalization at position 3, which is in accordance with the results reported by Boral et al. [11] and Haga et al. [12]. In order to evaluate the influence of the 2-chloro substituent on the regioselectivity of the metalation, 2-alkenyl-substituted isonicotinic acid 14 was used, which was obtained from methyl ester 13 by Suzuki cross-coupling and saponification. Lithiation of 14 and reaction with benzaldehyde exclusively gave lactone 15 with the desired regioselectivity (Scheme 2). To the best of our knowledge, no examples of functionalization of 2-alkyl-, 2-alkenyl-, and 2-aryl-substituted isonicotinic acids by lithiation are known in the literature. However, Johnston et al. published a directed ortho-arylation by palladium-catalyzed C–H activation of 2-alkyl-substituted isonicotinic acids with the same regioselectivity [16].
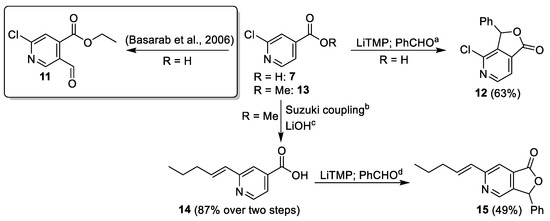
Scheme 2.
Functionalization of 2-substituted isonicotinic acids 7 and 14 [10]. Conditions: (a) n-BuLi, TMP, THF, −78 °C to −30 °C, 5 min; 7, −60 °C to −25 °C, 30 min; PhCHO, −78 °C, 2 h; (b) APhos Pd G3 (0.1 eq.), Cs2CO3, trans-1-penten-1-ylboronic acid pinacol ester, 1,4-dioxane/H2O 8:1, 100 °C; (c) LiOH, THF/H2O 10:1; (d) n-BuLi, TMP, THF, −78 °C to −30 °C, 5 min; 14, −60 °C to −25 °C, 30 min; PhCHO, −78 °C, 2 h.
Strategy two relied upon work on the lithiation of 2-chloronicotinic acid (8) and its di-isopropyl amide (18) with LiTMP, which is reported to proceed with excellent selectivity for the 4-position [13,14,17,18]. Additionally, Hyde et al. reported a lithiation of 2-chloro-5-bromopyridine (9) using LDA with the same regioselectivity [15]. However, a bromo- or iodo- in place of a carbonyl substituent would be advantageous for the execution of our envisaged cyclization strategies towards 4. Therefore, 9 served as the starting point for the synthesis of model compounds for cyclization. Trapping of lithiated 9 with Weinreb amides 21, 22, and 23 (Appendix A1) gave access to the putative cyclization precursors 24 (Appendix A2), 25, and 26 (Scheme 3).
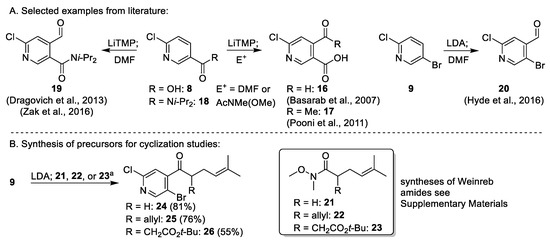
Scheme 3.
(A) Selected examples for regioselective lithiation of 2,5-substituted pyridines [13,14,15,17,18] and (B) synthesis of cyclization precursors 25 and 26. Conditions: (a) LDA, THF, −78 °C; 9, 1 h 10 min; 21, 22, or 23, 1 h 10 min.
Precursors 25 and 26 enabled us to evaluate potential cyclization strategies on simplified model systems. Those reactions served as pilots for our total syntheses of the NPs (−)-avicennone C [8] and (+)-floyocidin B [6]. Under standard Heck conditions, slow conversion of 25 into the exomethylene cylohexenone 27 and its aromatic isomer 28 was observed. Increased temperature led to full conversion of the starting material but yielded 28 as the major product, which pointed to the fact that for an improved synthesis strategy, a higher substituted cyclization precursor would be required to prevent aromatization via tautomerization and isomerization (Scheme 4). Guided by these findings, the palladium-catalyzed cyclization for the total synthesis of (−)-avicennone C (2) was developed [8]. The main differences lay in the presence of the aldehyde moiety instead of the terminal double bond and in a higher functionalized precursor preventing aromatization (Scheme 8) [8].
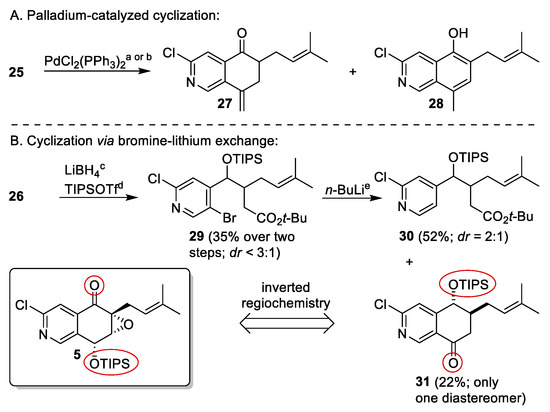
Scheme 4.
Model reactions for evaluation of cyclization strategies. Conditions: (a) PdCl2(PPh3)2 (0.05 eq.), NEt3, MeCN, reflux, 24 h; 4% for 27, 4% for 28, 56% reisolated 25; (b) Pd(PPh3)2Cl2 (0.05 eq.), NEt3, DMF, 120 °C, 16 h; traces for 27, 51% for 28; (c) LiBH4, THF; (d) TIPSOTf, 2,6-lutidine, CH2Cl2; (e) n-BuLi, THF, −100 °C, 25 min.
Our initial plan using 26 for a bromine–lithium exchange followed by spontaneous cyclization failed since we were unable to protect the ketone of 26 as a cyclic ketal (Appendix A3). In order to circumvent this issue, reduction to the corresponding alcohol and subsequent TIPS-protection was performed. The resulting diastereomeric mixture 29 was treated with n-BuLi at −100 °C yielding acyclic debrominated compound 30 as a diastereomeric mixture and cyclohexanone 31 as a single diastereomer. The outcome of this test reaction proved that careful control of the equivalents of n-BuLi employed led to a selective bromine–lithium exchange without nucleophilic attack of n-BuLi to the ester. The formation of debrominated compound 30 emphasized the requirement for either longer reaction times or higher temperatures for the cyclization, and the occurrence of 31 as a single diastereomer demonstrated a kinetic preference for the cyclization of one diastereomer. Optimization of the reaction conditions led to the alternative cyclization strategy in the total synthesis of (−)-avicennone C (2), in which the tert-butyl ester was replaced by a nitrile (Scheme 8) [8]. However, the positions of the ketone and protected alcohol function of 31, respectively, are inverted compared to the envisaged intermediate 5 of the synthesis of (+)-floyocidin B (4) (Scheme 4). Thus, we adapted these findings to our retrosynthetic analysis, in which position 5 of the 2-chloropyridine should be functionalized first and the ring closure should occur on position 4. These considerations culminated in strategy 3, which required the hitherto undisclosed trihalogenated 2-chloro-4,5-dibromopyridine (10) as a starting material.
In order to explore the regioselectivity of halogen–metal exchange reactions of trihalogenated pyridine 10, the metalation of 3,4-dibromopyridine (32) by bromine–magnesium exchange followed by quenching with an electrophile served as a model reaction for our approach. The reported regioselectivity with benzaldehyde [19,20] favoring regioisomer 34 was reproducible in our hands (Scheme 5, condition a). Encouraged by this result, we wanted to apply these conditions to the hitherto unknown 2-chloro analog 10 (Scheme 5), for which a synthetic access had to be developed. Halogenation of 2-chloro-5-bromopyridine (9) was not an option since trapping of the lithiated species with a bromination reagent such as NBS would result in an oxidative dimerization [21,22,23,24]. Finally, the Sandmeyer reaction of commercially available aminopyridine 35 yielded 10 in excellent yields using water-free reaction conditions (Scheme 5, condition b) [25], while under classical Sandmeyer conditions in aqueous medium, only moderate yields were observed due to low solubility of the starting material 35 and side-product formation (see Supplementary Materials).
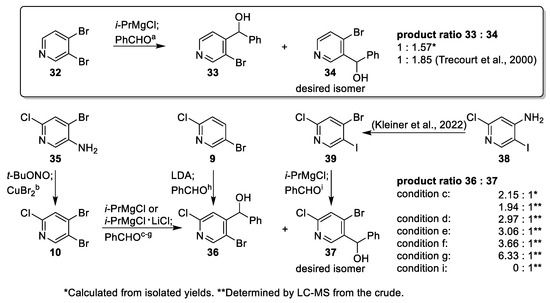
Scheme 5.
Model studies on the regioselectivity of halogen-magnesium exchange on different pyridines [6,20]. Conditions: (a) i-PrMgCl, THF, rt, 1 h; PhCHO, rt, overnight, 72% (sum of isomers), lit. [20]: 97%; (b) t-BuONO, CuBr2, MeCN, 0 °C to rt, 97%; (c) i-PrMgCl, THF, rt, 1 h; PhCHO, rt, overnight; 56% (sum of isomers); (d) i-PrMgCl, THF, 0 °C, 15 min; PhCHO, 0 °C to rt, overnight; (e) i-PrMgCl, THF, 0 °C, 50 min; PhCHO, 0 °C to rt, overnight; (f) i-PrMgCl, THF, −78 °C, 30 min; PhCHO, rt, overnight; (g) i-PrMgCl ∙ LiCl, THF, −78 °C, 30 min; PhCHO, rt, overnight; (h) LDA, THF, −78 °C, 1 h 10 min; PhCHO, −78 °C, 1 h, 49%; (i) i-PrMgCl, THF, −78 °C, 20 min; PhCHO, −78 °C, 1 h.
Prior to our regioselectivity optimization studies for the synthesis of the desired isomer 37 from 10, the undesired pure regioisomer 36 was prepared independently as an analytical reference compound starting from 9 [15] (Scheme 5, condition h) (Appendix A4). Our first attempt using 10 and i-PrMgCl at room temperature gave a selectivity of approximately 2:1 in favor of the undesired regioisomer 36 (Scheme 5, condition c) (Appendix A5). The observation that product ratios determined by LC-MS and calculated from isolated yields are compatible enabled us to evaluate the regioselectivities of the model reactions without isolation of the diastereomers. Lower reaction temperatures, different reaction times (Scheme 5, condition d–f), and the use of Knochel’s turbo Grignard reagent [26,27] (Scheme 5, condition g) did not improve the ratio toward the desired diastereomer 37. Analogously to the 2-chloroisonicotinic acids (Scheme 2), the 2-chloro substituent of pyridine 10 had a strong impact on the observed regioselectivities, which were inverted with respect to the 2-unsubstituted pyridine 32. Therefore, the desired selectivity of the halogen–magnesium exchange had to be achieved by a starting material with increased reactivity at the 5-position. Hence, we replaced 10 by the 5-iodopyridine 39 (Scheme 5, condition i), which was synthesized analogously to the bromo-analog 10 by Sandmeyer reaction [6].
With trihalogenated pyridine 39 in hand, we started our first generation synthesis of (+)-floyocidin B (Scheme 6). Indeed, halogen–magnesium exchange of 39 with i-PrMgCl occurred selectively at the iodinated 5-position and trapping of the magnesium organyl with aldehyde 40 led to regioselective formation of the two diastereomeric alcohols 41 and 42 in good yields, which were separated by flash chromatography [6]. TIPS-protection of the alcohol function and selective deprotection yielded primary alcohols 44 and 50 in only moderate yields due to considerable double-deprotection. In contrast, in the total synthesis of (−)-avicennone C (2), the deprotection of very similar compounds resulted in significantly higher selectivities (Scheme 8) [8]. Oxidation of primary alcohols 44 and 50 to aldehydes 46 and 52 with Dess–Martin periodinane (DMP) occurred smoothly. The intramolecular cyclization was performed under the palladium-catalyzed conditions described by Muratake et al. [28,29], which were applied for the first generation synthesis of (−)-avicennone C (2), too. In these reactions, side products were formed even under carefully monitored conditions for both diastereomers. The desired products 5 and 53 were isolated in low yields accompanied by secondary alcohols 47 and 54, which could easily be reoxidized with DMP (not shown). In the cyclization reaction of 46, the tetracyclic side product 48 was isolated from the reaction mixture, and its structure was elucidated by NMR analyses (Scheme 6).
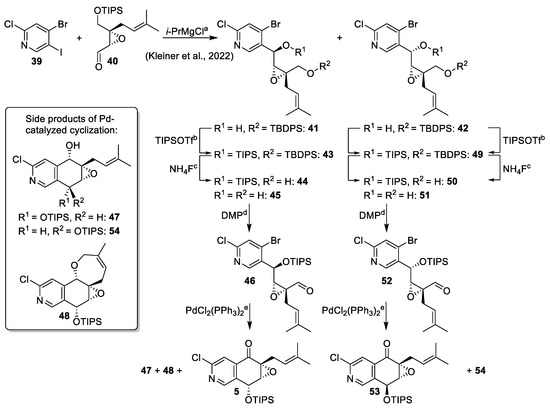
Scheme 6.
Synthesis of aldehydes 46 and 52 and palladium-catalyzed cyclization. Conditions: (a) i-PrMgCl, THF, −40 °C, 45 min; 40, −40 °C, 1.5 h; 49% for 41, 42% for 42 [6]; (b) TIPSOTf, 2,6-lutidine, CH2Cl2; 69% for 43, 90% for 49; (c) NH4F, MeOH/THF 12:1; 28% for 44, 53% for 45, 17% for 50, 61% for 51; (d) DMP, CH2Cl2; 92% for 46, 95% for 52; (e) PdCl2(PPh3)2 (0.1 eq.), Cs2CO3, toluene, reflux; 16% for 5, 15% for 47, 10% for 48, 42% for 53, 15% for 54.
The introduction of the pentenyl side chain was performed under Suzuki cross-coupling conditions, which had yielded model compound 14 in good yields (Scheme 2). However, only traces of the target molecule 55 were obtained (Scheme 7). Isolation and structure elucidation of the side products 56 and 57 identified the prenyl group and its reactivity in palladium-catalyzed reactions as the main cause for the low yield of 55. A similar tricyclic side product 58 was observed during the palladium-catalyzed reaction in the total synthesis of (−)-avicennone C (2) [8]. Careful monitoring led to an improved yield of 51% for 55 and less side-product formation. The total synthesis of (+)-floyocidin B (4) was completed after TIPS-deprotection (Scheme 7) [6].
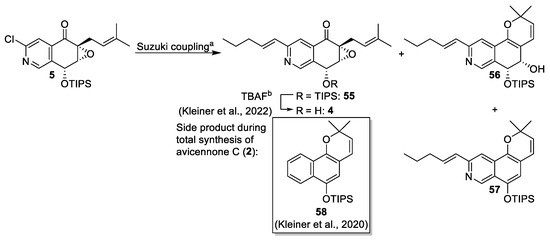
Scheme 7.
Suzuki reaction and investigation on cyclic side products [8]. Conditions: (a) APhos Pd G3 (0.1 eq.), Cs2CO3, trans-1-penten-1-ylboronic acid pinacol ester, 1,4-dioxane/H2O 8:1, 100 °C; 4% for 55, 27% for 56, 12% for 57; under carefully monitored conditions: 51% for 55; (b) TBAF, HOAc, THF; 60% [6].
The low-yielding reactions forced us to modify the strategy for the total synthesis of the other stereoisomers and the resynthesis of (+)-floyocidin B (4) to provide the required amounts for biological and physicochemical profiling. Significantly improved yields were achieved by a modified protection-deprotection strategy for the primary alcohols 44 and 50 utilizing acetate protection and selective acetyl migration (Scheme 8) [6]. A head-to-head comparison of the total syntheses of the avicennone C and the floyocidin B stereoisomers uncovered significant differences in the reactivity of these scaffolds, which only differ in the presence of the 2-chloropyridine in place of a phenyl moiety. While, in general, all reactions could be mutually adapted from one scaffold to the other, yields and side-product formation occurred differently. The discrepancy in yields of the two routes toward the primary alcohols 44/50 and 63/64 may be explained by the weak basicity of the pyridine, which favors yield-reducing double-deprotection in the shorter route and promotes acetyl migration in the four-step sequence.
The palladium-catalyzed intramolecular cyclization of aldehydes 46, 52, 65, and 66 gave only low (16% for 5) to moderate (42–48% for 53, 67, and 68) yields due to side-product formation and required an improvement (Scheme 8). A cyclization via bromine–lithium exchange of nitrile intermediates 59, 60, ent-69, and 70 led to yields > 80% in three of four cases. Surprisingly, the diastereomer 59 leading to the NP gave reproducibly low yields of the advanced intermediate 5, while the cyclizations for the other nitriles occurred smoothly. We can only hypothesize that either sterical or electronical properties of the linear precursors 46 and 59 with the same relative stereochemistry slow down the desired cyclization, which is responsible for the formation of different side products. For the material supply, this major drawback was partially compensated by a recycling strategy of the hydroxyl-epimer of (+)-floyocidin B (4) via Mitsunobu inversion and subsequent saponification [6].
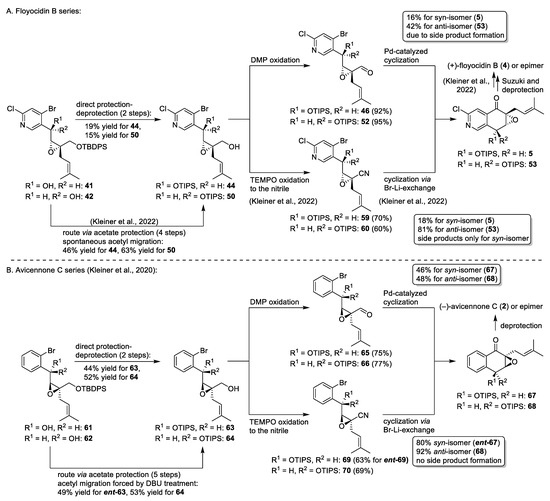
Scheme 8.
Head-to-head comparison of key steps of the total syntheses of (+)-floyocidin B (4) [6] and (−)-avicennone C (2) [8] and their epimers.
4. Conclusions
In conclusion, robust, scalable, and flexible synthetic routes to all possible stereoisomers of the epoxyquinone NPs (−)-avicennone C (2) and (+)-floyocidin B (4) have been developed. These total syntheses allowed for unambiguous determination of the absolute stereochemistry of the NPs to establish an access to a diverse variety of derivatives. Elucidation of occurring side products led to a profound understanding of the reactivity of the densely functionalized synthetic intermediates and enabled us to circumvent several pitfalls in the synthetic pathways. Solely, the reproducible low yields in the synthesis of advanced intermediate 5 via two different cyclization strategies remained a serious issue for the material supply, which was partially compensated by a recycling strategy of the hydroxy epimer of (+)-floyocidin B (4) via Mitsunobu inversion and subsequent saponification [6]. It is one of these painful examples, which many synthetic chemists may have experienced, in which diastereomers and even epimers can exhibit significantly different chemical reactivities.
Our observations on the regioselectivity of the functionalization of trihalogenated pyridines established 2-chloro-4,5-dibromopyridine and 4-bromo-2-chloro-5-iodopyridine as versatile starting materials for the preparation of orthogonally functionalized pyridine derivatives. Due to the role of pyridines as privileged scaffolds in medicinal chemistry our studies may be of general synthetic value beyond their importance for the SAR investigations on the (+)-floyocidin B scaffold.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry5010014/s1. The Supplementary Materials contain detailed procedures for synthesis of compounds and analytical data including 1H- and 13C-NMR spectra. They also include Scheme S1: Synthesis of the Weinreb amides 21, 22, and 23. Scheme S2: Sandmeyer reaction in aqueous medium. Scheme S3: Hypothesized mechanism of the formation of side product SI1 [30,31].
Author Contributions
Conceptualization, A.B., S.M.M.S. and C.P.; methodology, Y.K. and C.P.; investigation, Y.K.; data curation, Y.K.; writing—original draft preparation, Y.K. and C.P.; writing—review and editing, Y.K., A.B., S.M.M.S. and C.P.; supervision, A.B., P.H. and S.M.M.S.; project administration, P.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Hessen State Ministry of Higher Education, Research, and the Arts (HMWK) via generous grant for the LOEWE Research Center Insect Biotechnology and Bioresources. Sanofi and Evotec International contributed to the framework of Sanofi-Fraunhofer Natural Product Center of Excellence/Fraunhofer Evotec Natural Products Excellence Center.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Andreas Vilcinskas for general support, Till Schäberle, Michael Kurz, Cédric Couturier, Frédéric Jeannot, and Eric Bacqué for valuable discussions, the NMR department of the Justus Liebig University Giessen, and Christoph Hartwig for technical assistance.
Conflicts of Interest
P.H. and S.M.M.S. are or have been employed by Evotec International GmbH. A.B., P.H. and C.P. are or have been employed by Sanofi S.A. or one of its affiliates and are shareholders of Sanofi S.A.
Appendix A
1. Weinreb amides 21, 22, and 23 were prepared according to modified literature-know procedures from diethyl malonate in 4 and 5 steps, respectively. For details see Supplementary Materials (1.1.).
2. Ketone 24 could not be employed as a suitable precursor for further functionalization by alkylation at the α-position, because reduction of the ketone to the alcohol by LDA was identified as competing reaction. For details of synthesis of reduction product SI11 see Supplementary Materials (1.2.). Since the syntheses of 25 and 26 was possible using Weinreb amides 22 and 23, no attempts were made to achieve alkylation of 24 using different bases such as LiHMDS. Reduction of sterically hindered ketones using LDA is a known phenomenon. [32,33,34]
3. Several attempts for protection were performed under literature known conditions [35,36,37]. Forced reaction conditions only led to decomposition of ketone 26.
4. Comparison of the NOE correlations of 36 and 37 did not led to a fully convincing assignment of the regioisomers. Therefore 36 was synthesized by regioselective ortho-lithiation.
5. LC-MS analysis undercovered the formation of a side product SI1 during the reaction at room temperature. Its structure elucidation and proposal for the mechanism of its formation are provided in the Supplementary Materials.
References
- Mehta, G.; Sengupta, S. Progress in the total synthesis of epoxyquinone natural products: An update. Tetrahedron 2017, 73, 6223–6247. [Google Scholar] [CrossRef]
- Li, J.Y.; Harper, J.K.; Grant, D.M.; Tombe, B.O.; Bashyal, B.; Hess, W.M.; Strobel, G.A. Ambuic acid, a highly functionalized cyclohexenone with antifungal activity from Pestalotiopsis spp. and Monochaetia sp. Phytochemistry 2001, 56, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Johnson, R.P.; Porco, J.A. Total synthesis of the quinone epoxide dimer (+)-torreyanic acid: Application of a biomimetic oxidation/electrocyclization/Diels-Alder dimerization cascade. J. Am. Chem. Soc. 2003, 125, 5095–5106. [Google Scholar] [CrossRef] [PubMed]
- Mehta, G.; Pan, S.C. A total synthesis of the epoxyquinone based antifungal natural product (±)-ambuic acid. Tetrahedron Lett. 2005, 46, 3045–3048. [Google Scholar] [CrossRef]
- Jung, S.H.; Hwang, G.-S.; Lee, S.I.; Ryu, D.H. Total synthsis of (+)-ambuic acid: α-bromination with 1,2-dibromotetrachloroethane. J. Org. Chem. 2012, 77, 2513–2518. [Google Scholar] [CrossRef]
- Kleiner, Y.; Pöverlein, C.; Klädtke, J.; Kurz, M.; König, H.F.; Becker, J.; Mihajlovic, S.; Zubeil, F.; Marner, M.; Vilcinskas, A.; et al. The Discovery and Structure-Activity Evaluation of (+)-Floyocidin B and Synthetic Analogs. ChemMedChem 2022, 17, e202100644. [Google Scholar] [CrossRef]
- Han, L.; Huang, X.; Dahse, H.-M.; Moellmann, U.; Fu, H.; Grabley, S.; Sattler, I.; Lin, W. Unusual naphthoquinone derivatives from the twigs of Avicennia marina. J. Nat. Prod. 2007, 70, 923–927. [Google Scholar] [CrossRef]
- Kleiner, Y.; Hammann, P.; Becker, J.; Bauer, A.; Pöverlein, C.; Schuler, S.M.M. Total Synthesis and Structure Revision of (-)-Avicennone C. J. Org. Chem. 2020, 85, 13108–13120. [Google Scholar] [CrossRef]
- Kleiner, Y. Chemische und Biologische Evaluierung Neuartiger Epoxychinon-Naturstoffe und -Naturstoffhybride; Shaker Verlag: Aachen, Germany, 2021; ISBN 978-3-8440-8368-2. [Google Scholar]
- Basarab, G.; Dangel, B.; Fleming, P.R.; Gravestock, M.B.; Green, O.; Hauck, S.I.; Hill, P.; Hull, K.G.; Mullen, G.; Sherer, B.; et al. Antibacterial Piperidine Derivatives. WO2006GB00529 20060216, 16 February 2006. [Google Scholar]
- Boral, S.; Gao, X.; Wang, S.; Wurster, J.A.; Malone, T.C. Heteroaryl Dihydroindolones as Kinase Inhibitors. WO2007US64264 20070319, 19 March 2007. [Google Scholar]
- Haga, Y.; Sakamoto, T.; Shibata, T.; Nonoshita, K.; Ishikawa, M.; Suga, T.; Takahashi, H.; Takahashi, T.; Takahashi, H.; Ando, M.; et al. Discovery of trans-N-1-(2-fluorophenyl)-3-pyrazolyl-3-oxospiro6-azaisobenzofuran-1(3H),1’-cyclohexane-4’- carboxamide, a potent and orally active neuropeptide Y Y5 receptor antagonist. Bioorg. Med. Chem. 2009, 17, 6971–6982. [Google Scholar] [CrossRef]
- Basarab, G.S.; Bist, S.; Manchester, J.I.; Sherer, B. Chemical Compounds. US20070950105 20071204, 4 December 2007. [Google Scholar]
- Pooni, P.K.; Merchant, K.J.; Kerr, C.M.; Harrison, D. Benzazepine Derivatives for the Treatment of Central Nervous System Disorders. WO2011GB00016 20110107, 7 January 2011. [Google Scholar]
- Hyde, A.M.; Liu, Z.; Kosjek, B.; Tan, L.; Klapars, A.; Ashley, E.R.; Zhong, Y.-L.; Alvizo, O.; Agard, N.J.; Liu, G.; et al. Synthesis of the GPR40 Partial Agonist MK-8666 through a Kinetically Controlled Dynamic Enzymatic Ketone Reduction. Org. Lett. 2016, 18, 5888–5891. [Google Scholar] [CrossRef]
- Johnston, A.J.S.; Ling, K.B.; Sale, D.; Lebrasseur, N.; Larrosa, I. Direct ortho-Arylation of Pyridinecarboxylic Acids: Overcoming the Deactivating Effect of sp2-Nitrogen. Org. Lett. 2016, 18, 6094–6097. [Google Scholar] [CrossRef] [PubMed]
- Dragovich, P.S.; Bair, K.W.; Baumeister, T.; Ho, Y.-C.; Liederer, B.M.; Liu, X.; Liu, Y.; O’Brien, T.; Oeh, J.; Sampath, D.; et al. Identification of 2,3-dihydro-1H-pyrrolo [3,4-c]pyridine-derived ureas as potent inhibitors of human nicotinamide phosphoribosyltransferase (NAMPT). Bioorg. Med. Chem. Lett. 2013, 23, 4875–4885. [Google Scholar] [CrossRef]
- Zak, M.; Yuen, P.-W.; Liu, X.; Patel, S.; Sampath, D.; Oeh, J.; Liederer, B.M.; Wang, W.; O’Brien, T.; Xiao, Y.; et al. Minimizing CYP2C9 Inhibition of Exposed-Pyridine NAMPT (Nicotinamide Phosphoribosyltransferase) Inhibitors. J. Med. Chem. 2016, 59, 8345–8368. [Google Scholar] [CrossRef]
- Shi, L.; Chu, Y.; Knochel, P.; Mayr, H. Kinetics of bromine-magnesium exchange reactions in heteroaryl bromides. Org. Lett. 2009, 11, 3502–3505. [Google Scholar] [CrossRef]
- Trécourt, F.; Breton, G.; Bonnet, V.; Mongin, F.; Marsais, F.; Quéguiner, G. New Syntheses of Substituted Pyridines via Bromine–Magnesium Exchange. Tetrahedron 2000, 56, 1349–1360. [Google Scholar] [CrossRef]
- Abboud, M.; Mamane, V.; Aubert, E.; Lecomte, C.; Fort, Y. Synthesis of polyhalogenated 4,4’-bipyridines via a simple dimerization procedure. J. Org. Chem. 2010, 75, 3224–3231. [Google Scholar] [CrossRef] [PubMed]
- Mamane, V.; Peluso, P.; Aubert, E.; Cossu, S.; Pale, P. Chiral Hexahalogenated 4,4’-Bipyridines. J. Org. Chem. 2016, 81, 4576–4587. [Google Scholar] [CrossRef]
- Demangeat, C.; Saied, T.; Ramozzi, R.; Ingrosso, F.; Ruiz-Lopez, M.; Panossian, A.; Leroux, F.R.; Fort, Y.; Comoy, C. Transition-Metal-Free Approach for the Direct Arylation of Thiophene: Experimental and Theoretical Investigations towards the (Het)-Aryne Route. Eur. J. Org. Chem. 2019, 2019, 547–556. [Google Scholar] [CrossRef]
- Saied, T.; Demangeat, C.; Panossian, A.; Leroux, F.R.; Fort, Y.; Comoy, C. Transition-Metal-Free Heterobiaryl Synthesis via Aryne Coupling. Eur. J. Org. Chem. 2019, 2019, 5275–5284. [Google Scholar] [CrossRef]
- Honraedt, A.; Gallagher, T. Concise Entries to 4-Halo-2-pyridones and 3-Bromo-4-halo-2-pyridones. Synlett 2015, 27, 67–69. [Google Scholar] [CrossRef]
- Krasovskiy, A.; Knochel, P. A LiCl-mediated Br/Mg exchange reaction for the preparation of functionalized aryl- and heteroarylmagnesium compounds from organic bromides. Angew. Chem. Int. Ed. 2004, 43, 3333–3336. [Google Scholar] [CrossRef]
- Desaintjean, A.; Haupt, T.; Bole, L.J.; Judge, N.R.; Hevia, E.; Knochel, P. After our method studies had been completed, Knochel et al. published mixed Li/Mg organometallic species for the bromine-magnesium exchange on dibromopyridines, which led under certain conditions to different regioselectivities compared to i-PrMgCl*LiCl. Angew. Chem. Int. Ed. 2021, 60, 1513–1518. [Google Scholar] [CrossRef]
- Muratake, H.; Nakai, H. Intramolecular cyclization using palladium-catalyzed arylation toward formyl and nitro groups. Tetrahedron Lett. 1999, 40, 2355–2358. [Google Scholar] [CrossRef]
- Muratake, H.; Natsume, M.; Nakai, H. Palladium-catalyzed intramolecular α-arylation of aliphatic ketone, formyl, and nitro groups. Tetrahedron 2004, 60, 11783–11803. [Google Scholar] [CrossRef]
- Davies, J.; Angelini, L.; Alkhalifah, M.A.; Sanz, L.M.; Sheikh, N.S.; Leonori, D. Photoredox Synthesis of Arylhydroxylamines from Carboxylic Acids and Nitrosoarenes. Synthesis 2018, 50, 821–830. [Google Scholar]
- Sundalam, S.K.; Nilova, A.; Seidl, T.L.; Stuart, D.R. A Selective C− H deprotonation strategy to access functionalized arynes by using hypervalent iodine. Angew. Chem. Int. Ed. 2016, 55, 8431–8434. [Google Scholar] [CrossRef] [PubMed]
- Wittig, G.; Schmidt, H.-J.; Renner, H. Über Lithium-diäthylamid als Hydrid-Donator. Chem. Ber. 1962, 95, 2377–2383. [Google Scholar] [CrossRef]
- Kowalski, C.; Creary, X.; Rollin, A.J.; Burke, M.C. Reductions of .alpha.-substituted ketones by lithium diisopropylamide. J. Org. Chem. 1978, 43, 2601–2608. [Google Scholar] [CrossRef]
- Majewski, M.; Gleave, D.M. Reduction with lithium dialkylamides. J. Organomet. Chem. 1994, 470, 1–16. [Google Scholar] [CrossRef]
- Caserio, F.F., Jr.; Roberts, J.D. Small-ring Compounds. XXI. 3-Methylenecyclobutanone and Related Compounds1. J. Am. Chem. Soc. 1958, 80, 5837–5840. [Google Scholar] [CrossRef]
- LaMattina, J.L. The Synthesis of 2-Amino-4-(4-imidazolyl) pyridines. J. Heterocyclic Chem. 1983, 20, 533–538. [Google Scholar] [CrossRef]
- Henegar, K.E.; Ashford, S.W.; Baughman, T.A.; Sih, J.C.; Gu, R.-L. Practical Asymmetric Synthesis of (S)-4-Ethyl-7, 8-dihydro-4-hydroxy-1 H-pyrano [3, 4-f] indolizine-3, 6, 10 (4 H)-trione, a Key Intermediate for the Synthesis of Irinotecan and Other Camptothecin Analogs. J. Org. Chem. 1997, 62, 6588–6597. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).