Self-Assembly and Gelation Study of Dipeptide Isomers with Norvaline and Phenylalanine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and General Methods
2.2. Dipeptide Synthesis and Purification
2.3. Circular Dichroism
2.4. Self-Assembly Tests
2.5. Transmission Electron Microscopy (TEM)
2.6. Raman Microspectroscopy
2.7. Single-Crystal X-ray Diffraction (XRD)
3. Results and Discussion
3.1. Dipeptide Preparation and Spectroscopic Characterization
3.2. Self-Assembly Tests
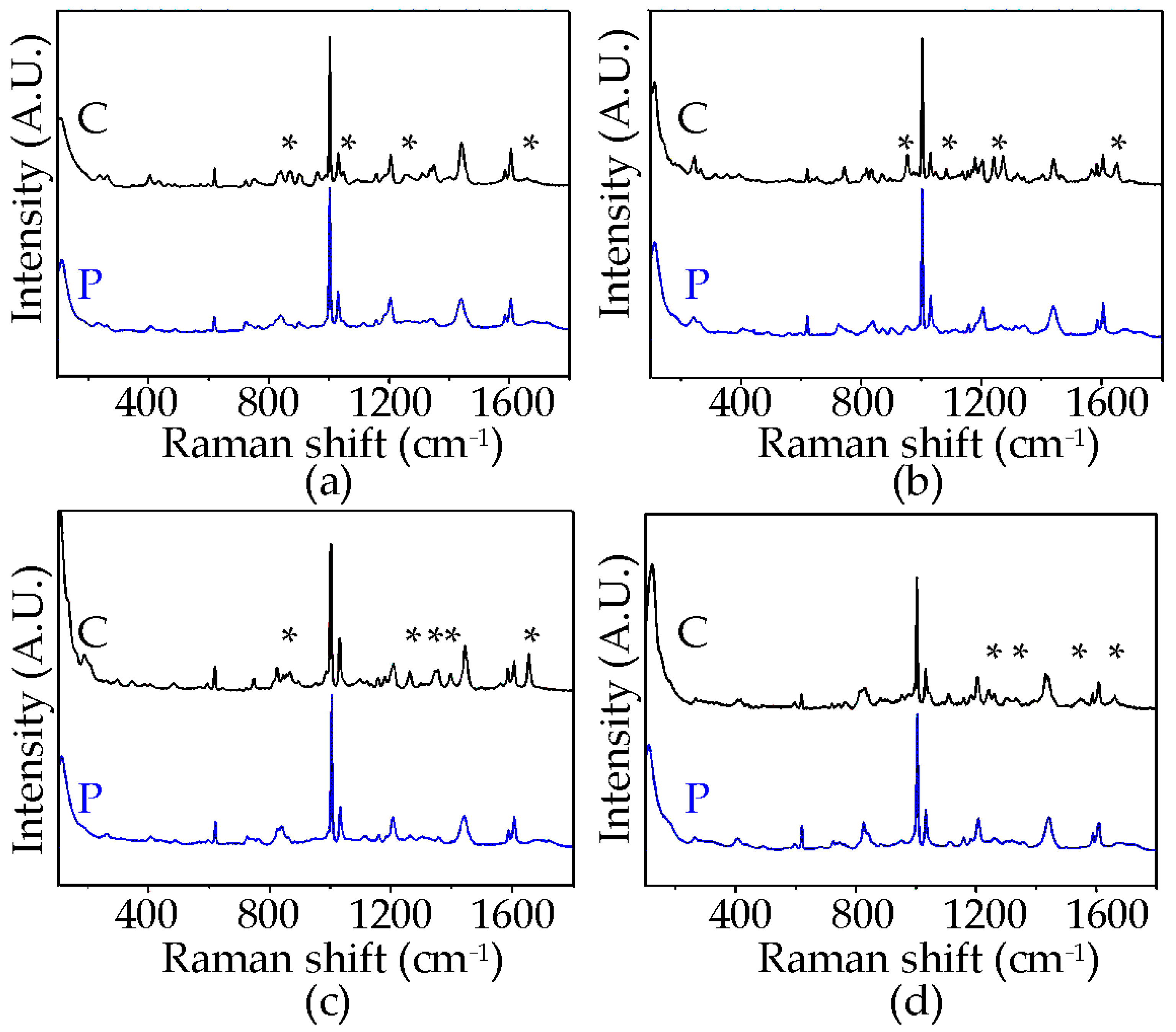
3.3. Raman Spectroscopy
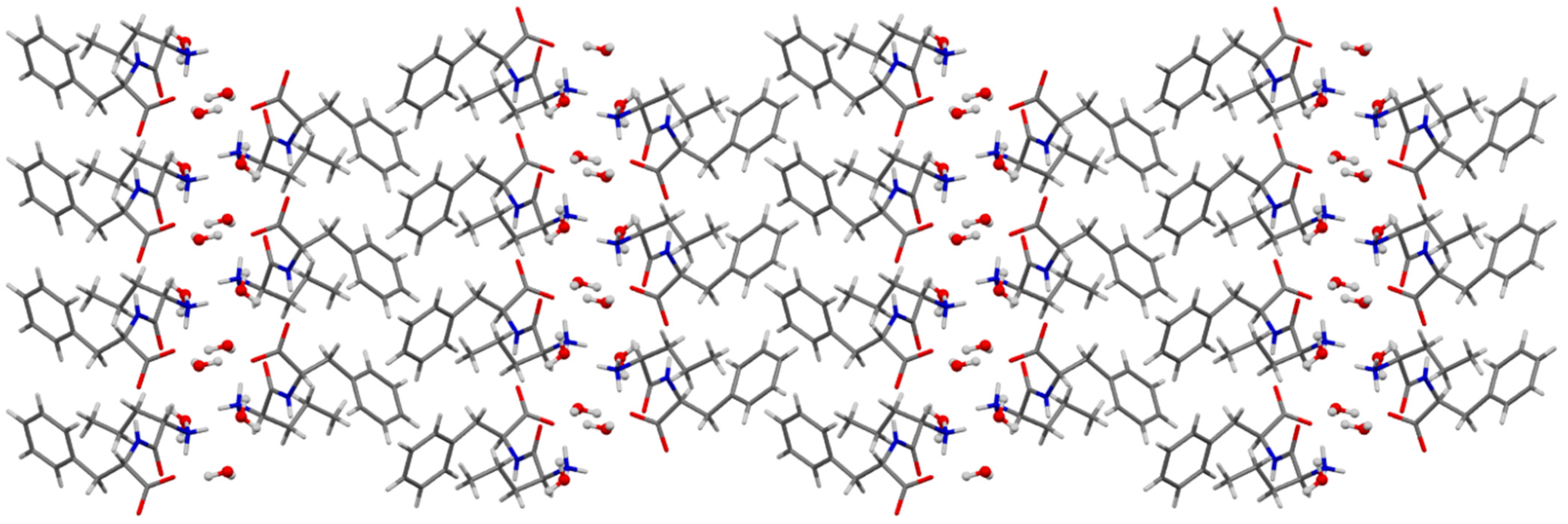
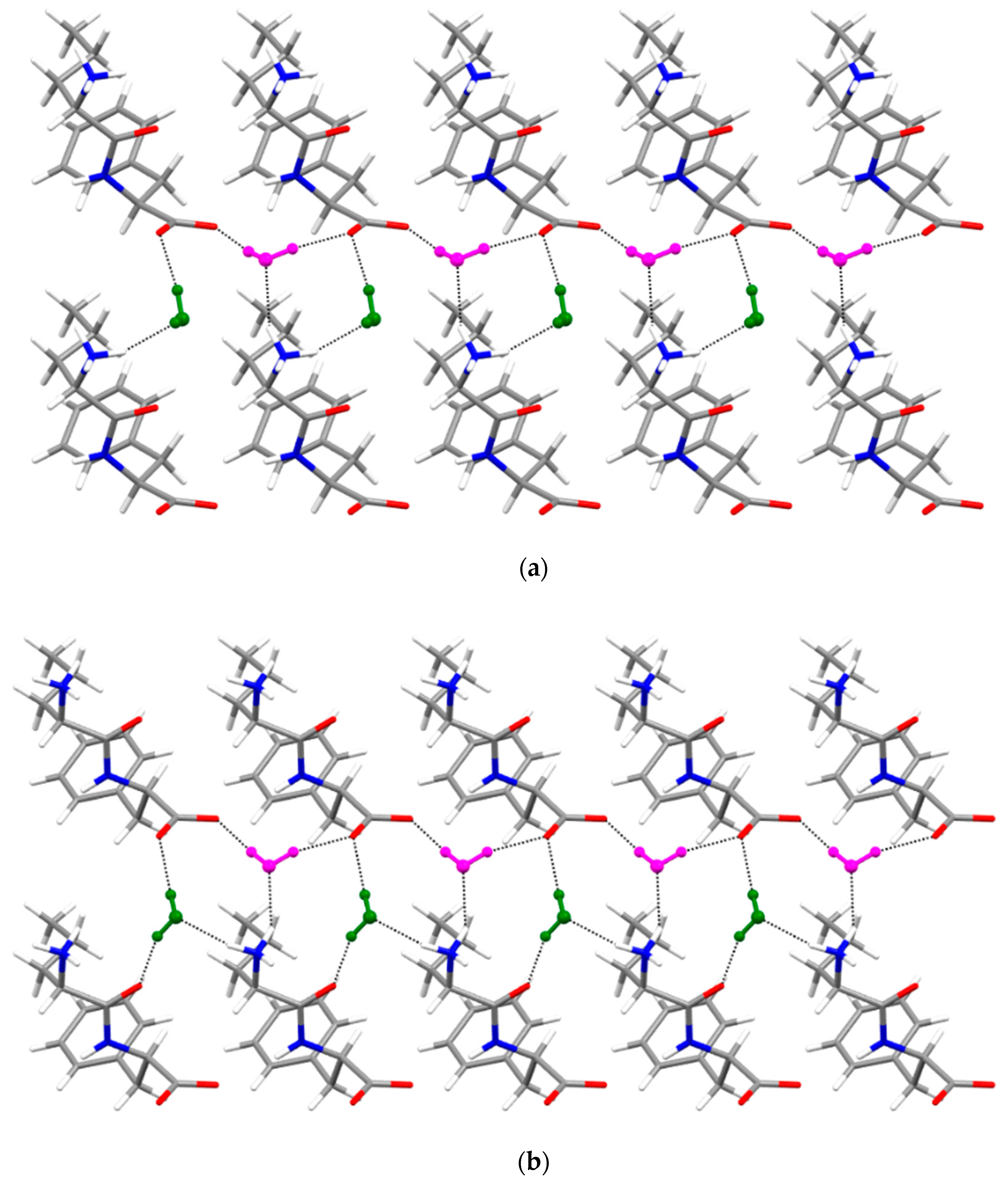
3.4. Single-Crystal X-ray Diffraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, D.J. Dipeptide and tripeptide conjugates as low-molecular-weight hydrogelators. Macromol. Biosci. 2011, 11, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tao, K.; Ji, W.; Makam, P.; Rencus-Lazar, S.; Gazit, E. Self-assembly of cyclic dipeptides: Platforms for functional materials. Prot. Pept. Lett. 2020, 27, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Rissanou, A.N.; Simatos, G.; Siachouli, P.; Harmandaris, V.; Mitraki, A. Self-assembly of alanine-isoleucine and isoleucine-isoleucine dipeptides through atomistic simulations and experiments. J. Phys. Chem. B 2020, 124, 7102–7114. [Google Scholar] [CrossRef] [PubMed]
- Balachandra, C.; Padhi, D.; Govindaraju, T. Cyclic dipeptide: A privileged molecular scaffold to derive structural diversity and functional utility. ChemMedChem 2021, 16, 2558–2587. [Google Scholar] [CrossRef] [PubMed]
- Scarel, M.; Marchesan, S. Diketopiperazine gels: New horizons from the self-assembly of cyclic dipeptides. Molecules 2021, 26, 3376. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef]
- Brahmachari, S.; Arnon, Z.A.; Frydman-Marom, A.; Gazit, E.; Adler-Abramovich, L. Diphenylalanine as a reductionist model for the mechanistic characterization of β-amyloid modulators. ACS Nano 2017, 11, 5960–5969. [Google Scholar] [CrossRef]
- Safaryan, S.; Slabov, V.; Kopyl, S.; Romanyuk, K.; Bdikin, I.; Vasilev, S.; Zelenovskiy, P.; Shur, V.Y.; Uslamin, E.A.; Pidko, E.A.; et al. Diphenylalanine-based microribbons for piezoelectric applications via inkjet printing. ACS Appl. Mater. Interfaces 2018, 10, 10543–10551. [Google Scholar] [CrossRef]
- Zelenovskiy, P.S.; Domingues, E.M.; Slabov, V.; Kopyl, S.; Ugolkov, V.L.; Figueiredo, F.M.L.; Kholkin, A.L. Efficient water self-diffusion in diphenylalanine peptide nanotubes. ACS Appl. Mater. Interfaces 2020, 12, 27485–27492. [Google Scholar] [CrossRef]
- Erdoğan, H. Cation-based approach to morphological diversity of diphenylalanine dipeptide structures. Soft Matter 2021, 17, 5221–5230. [Google Scholar] [CrossRef]
- Diaferia, C.; Roviello, V.; Morelli, G.; Accardo, A. Self-assembly of pegylated diphenylalanines into photoluminescent fibrillary aggregates. Chemphyschem 2019, 20, 2774–2782. [Google Scholar] [CrossRef] [PubMed]
- Rajbhandary, A.; Nilsson, B.L. Investigating the effects of peptoid substitutions in self-assembly of fmoc-diphenylalanine derivatives. Biopolymers 2017, 108, e22994. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.D.; Wojciechowski, J.P.; Robinson, A.B.; Heu, C.; Garvey, C.J.; Ratcliffe, J.; Waddington, L.J.; Gardiner, J.; Thordarson, P. Controlling self-assembly of diphenylalanine peptides at high ph using heterocyclic capping groups. Sci. Rep. 2017, 7, 43947. [Google Scholar] [CrossRef] [PubMed]
- Karikis, K.; Butkiewicz, A.; Folias, F.; Charalambidis, G.; Kokotidou, C.; Charisiadis, A.; Nikolaou, V.; Nikoloudakis, E.; Frelek, J.; Mitraki, A.; et al. Self-assembly of (boron-dipyrromethane)-diphenylalanine conjugates forming chiral supramolecular materials. Nanoscale 2018, 10, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Dudukovic, N.A.; Hudson, B.C.; Paravastu, A.K.; Zukoski, C.F. Self-assembly pathways and polymorphism in peptide-based nanostructures. Nanoscale 2018, 10, 1508–1516. [Google Scholar] [CrossRef]
- Diaferia, C.; Avitabile, C.; Leone, M.; Gallo, E.; Saviano, M.; Accardo, A.; Romanelli, A. Diphenylalanine motif drives self-assembling in hybrid pna-peptide conjugates. Chem. Eur. J. 2021, 27, 14307–14316. [Google Scholar] [CrossRef]
- Garcia, A.M.; Melchionna, M.; Bellotto, O.; Kralj, S.; Semeraro, S.; Parisi, E.; Iglesias, D.; D’Andrea, P.; De Zorzi, R.; Vargiu, A.V.; et al. Nanoscale assembly of functional peptides with divergent programming elements. ACS Nano 2021, 15, 3015–3025. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Xing, R.; Yan, X. Covalently assembled dipeptide nanoparticles with adjustable fluorescence emission for multicolor bioimaging. ChemBioChem 2019, 20, 555–560. [Google Scholar] [CrossRef]
- Yuran, S.; Razvag, Y.; Das, P.; Reches, M. Self-assembly of azide containing dipeptides. J. Pept. Sci. 2014, 20, 479–486. [Google Scholar] [CrossRef]
- Kralj, S.; Bellotto, O.; Parisi, E.; Garcia, A.M.; Iglesias, D.; Semeraro, S.; Deganutti, C.; D’Andrea, P.; Vargiu, A.V.; Geremia, S.; et al. Heterochirality and halogenation control phe-phe hierarchical assembly. ACS Nano 2020, 14, 16951–16961. [Google Scholar] [CrossRef]
- Görbitz, C.H. Nanotube formation by hydrophobic dipeptides. Chem. Eur. J. 2001, 7, 5153–5159. [Google Scholar] [CrossRef]
- Görbitz, C.H. The structure of nanotubes formed by diphenylalanine, the core recognition motif of alzheimer’s beta-amyloid polypeptide. Chem. Commun. 2006, 2332–2334. [Google Scholar] [CrossRef] [PubMed]
- Görbitz, C.H. Microporous organic materials from hydrophobic dipeptides. Chem. Eur. J. 2007, 13, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Görbitz, C. L-isoleucyl-L-phenylalanine dihydrate. Acta Cryst. C 2004, 60, o371–o373. [Google Scholar] [CrossRef]
- Görbitz, C. L-phenylalanyl- L-isoleucine 0.88-hydrate. Acta Cryst. C 2004, 60, o810–o812. [Google Scholar] [CrossRef]
- de Groot, N.S.; Parella, T.; Aviles, F.X.; Vendrell, J.; Ventura, S. Ile-phe dipeptide self-assembly: Clues to amyloid formation. Biophys. J. 2007, 92, 1732–1741. [Google Scholar] [CrossRef]
- Bellotto, O.; Kralj, S.; Melchionna, M.; Pengo, P.; Kisovec, M.; Podobnik, M.; De Zorzi, R.; Marchesan, S. Self-assembly of unprotected dipeptides into hydrogels: Water-channels make the difference. ChemBioChem 2022, 23, e202100518. [Google Scholar] [CrossRef]
- Kurbasic, M.; Semeraro, S.; Garcia, A.M.; Kralj, S.; Parisi, E.; Deganutti, C.; De Zorzi, R.; Marchesan, S. Microwave-assisted cyclization of unprotected dipeptides in water to 2,5-piperazinediones and self-assembly study of products and reagents. Synthesis 2019, 51, 2829–2838. [Google Scholar] [CrossRef]
- Görbitz, C. β turns, water cage formation and hydrogen bonding in the structures of L-valyl-L-phenylalanine. Acta Cryst. B 2002, 58, 512–518. [Google Scholar] [CrossRef]
- Görbitz, C. An NH3+…Phenyl interaction in L-phenylalanyl- L-valine. Acta Cryst. C 2000, 56, 1496–1498. [Google Scholar] [CrossRef]
- Bellotto, O.; Pierri, G.; Rozhin, P.; Polentarutti, M.; Kralj, S.; D’Andrea, P.; Tedesco, C.; Marchesan, S. Dipeptide self-assembly into water-channels and gel biomaterial. Org. Biomol. Chem. 2022, 20, 6211–6218. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Carreño, C.; Becerra, A.; Lazcano, A. Norvaline and norleucine may have been more abundant protein components during early stages of cell evolution. Orig. Life Evol. Biosph. 2013, 43, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Pokrovskiy, M.V.; Korokin, M.V.; Tsepeleva, S.A.; Pokrovskaya, T.G.; Gureev, V.V.; Konovalova, E.A.; Gudyrev, O.S.; Kochkarov, V.I.; Korokina, L.V.; Dudina, E.N.; et al. Arginase inhibitor in the pharmacological correction of endothelial dysfunction. Int. J. Hypertens. 2011, 2011, 515047. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Konovalova, E.A.; Chernomortseva, E.S. The study of the endothelial protective properties of the L-norvaline combination with mexidol in the simulation of L-NAME-induced NO deficiency. Res. Result Pharmacol. 2019, 5, 41–46. [Google Scholar] [CrossRef][Green Version]
- Bolt, H.L.; Williams, C.E.J.; Brooks, R.V.; Zuckermann, R.N.; Cobb, S.L.; Bromley, E.H.C. Log D versus HPLC derived hydrophobicity: The development of predictive tools to aid in the rational design of bioactive peptoids. Pept. Sci. 2017, 108, e23014. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.M.; Iglesias, D.; Parisi, E.; Styan, K.E.; Waddington, L.J.; Deganutti, C.; De Zorzi, R.; Grassi, M.; Melchionna, M.; Vargiu, A.V.; et al. Chirality effects on peptide self-assembly unraveled from molecules to materials. Chem 2018, 4, 1862–1876. [Google Scholar] [CrossRef]
- Amdursky, N.; Stevens, M.M. Circular dichroism of amino acids: Following the structural formation of phenylalanine. ChemPhysChem 2015, 16, 2768–2774. [Google Scholar] [CrossRef]
- Mazzier, D.; Mosconi, D.; Marafon, G.; Reheman, A.; Toniolo, C.; Moretto, A. Light-driven topochemical polymerization under organogel conditions of a symmetrical dipeptide-diacetylene system. J. Pept. Sci. 2017, 23, 155–161. [Google Scholar] [CrossRef]
- Profit, A.A.; Vedad, J.; Desamero, R.Z. Peptide conjugates of benzene carboxylic acids as agonists and antagonists of amylin aggregation. Bioconjug. Chem. 2017, 28, 666–677. [Google Scholar] [CrossRef]
- Ronen, M.; Kalanoor, B.S.; Oren, Z.; Ron, I.; Tischler, Y.R.; Gerber, D. Characterization of peptides self-assembly by low frequency Raman spectroscopy. RSC Adv. 2018, 8, 16161–16170. [Google Scholar] [CrossRef]
- Levine, M.S.; Ghosh, M.; Hesser, M.; Hennessy, N.; DiGuiseppi, D.M.; Adler-Abramovich, L.; Schweitzer-Stenner, R. Formation of peptide-based oligomers in dimethylsulfoxide: Identifying the precursor of fibril formation. Soft Matter 2020, 16, 7860–7868. [Google Scholar] [CrossRef] [PubMed]
- Scarel, E.; Bellotto, O.; Rozhin, P.; Kralj, S.; Tortora, M.; Vargiu, A.V.; De Zorzi, R.; Rossi, B.; Marchesan, S. Single-atom substitution enables supramolecular diversity from dipeptide building blocks. Soft Matter 2022, 18, 2129–2136. [Google Scholar] [CrossRef]
- Hernández, B.; Pflüger, F.; Kruglik, S.G.; Ghomi, M. Characteristic Raman lines of phenylalanine analyzed by a multiconformational approach. J. Raman Spectrosc. 2013, 44, 827–833. [Google Scholar] [CrossRef]
- Fischer, W.B.; Eysel, H.H. Polarized raman spectra and intensities of aromatic amino acids phenylalanine, tyrosine and tryptophan. Spectrochim. Acta A Mol. Spectrosc. 1992, 48, 725–732. [Google Scholar] [CrossRef]
- Thakuria, R.; Nath, N.K.; Saha, B.K. The Nature and Applications of π–π Interactions: A Perspective. Cryst. Growth Des. 2019, 19, 523–528. [Google Scholar] [CrossRef]
- Grabowski, S.J.; Lipkowski, P. Characteristics of X-H···π Interactions: Ab Initio and QTAIM Studies. J. Phys. Chem. A 2011, 115, 4765–4773. [Google Scholar] [CrossRef]
- Picci, G.; Marchesan, S.; Caltagirone, C. Ion channels and transporters as therapeutic agents: From biomolecules to supramolecular medicinal chemistry. Biomedicines 2022, 10, 885. [Google Scholar] [CrossRef]
- Roy, A.; Talukdar, P. Recent advances in bioactive artificial ionophores. ChemBioChem 2021, 22, 2925–2940. [Google Scholar] [CrossRef]
- Wu, X.; Howe, E.N.W.; Gale, P.A. Supramolecular transmembrane anion transport: New assays and insights. Acc. Chem. Res. 2018, 51, 1870–1879. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, J.H.; Chen, X.X.; Ren, H.L.; Feng, X.L.; Wang, J.L.; Xiao, J.H. Combination of L-arginine and L-norvaline protects against pulmonary fibrosis progression induced by bleomycin in mice. Biomed. Pharmacother. 2019, 113, 108768. [Google Scholar] [CrossRef]
- Javrushyan, H.; Nadiryan, E.; Grigoryan, A.; Avtandilyan, N.; Maloyan, A. Antihyperglycemic activity of L-norvaline and L-arginine in high-fat diet and streptozotocin-treated male rats. Exp. Mol. Pathol. 2022, 126, 104763. [Google Scholar] [CrossRef] [PubMed]
- Polis, B.; Gurevich, V.; Assa, M.; Samson, A.O. Norvaline restores the bbb integrity in a mouse model of Alzheimer’s disease. Int. J. Mol. Sci. 2019, 20, 4616. [Google Scholar] [CrossRef]
- Polis, B.; Srikanth, K.D.; Gurevich, V.; Gil-Henn, H.; Samson, A.O. L-norvaline, a new therapeutic agent against Alzheimer’s disease. Neural Regen. Res. 2019, 14, 1562–1572. [Google Scholar] [CrossRef] [PubMed]
- Gilinsky, M.A.; Polityko, Y.K.; Markel, A.L.; Latysheva, T.V.; Samson, A.O.; Polis, B.; Naumenko, S.E. Norvaline reduces blood pressure and induces diuresis in rats with inherited stress-induced arterial hypertension. BioMed. Res. Int. 2020, 2020, 4935386. [Google Scholar] [CrossRef] [PubMed]
- Battye, T.G.G.; Kontogiannis, L.; Johnson, O.; Powell, H.R.; Leslie, A.G.W. iMOSFLM: A New Graphical Interface for Diffraction-Image Processing with MOSFLM. Acta Crystallogr. Sect. D 2011, 67, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.R. Scaling and assessment of data quality. Acta Crystallogr. Sect. D 2006, 62, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.R.; Murshudov, G.N. How good are my data and what is the resolution? Acta Crystallogr. Sect. D Biol. Crystallogr. 2013, 69, 1204–1214. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
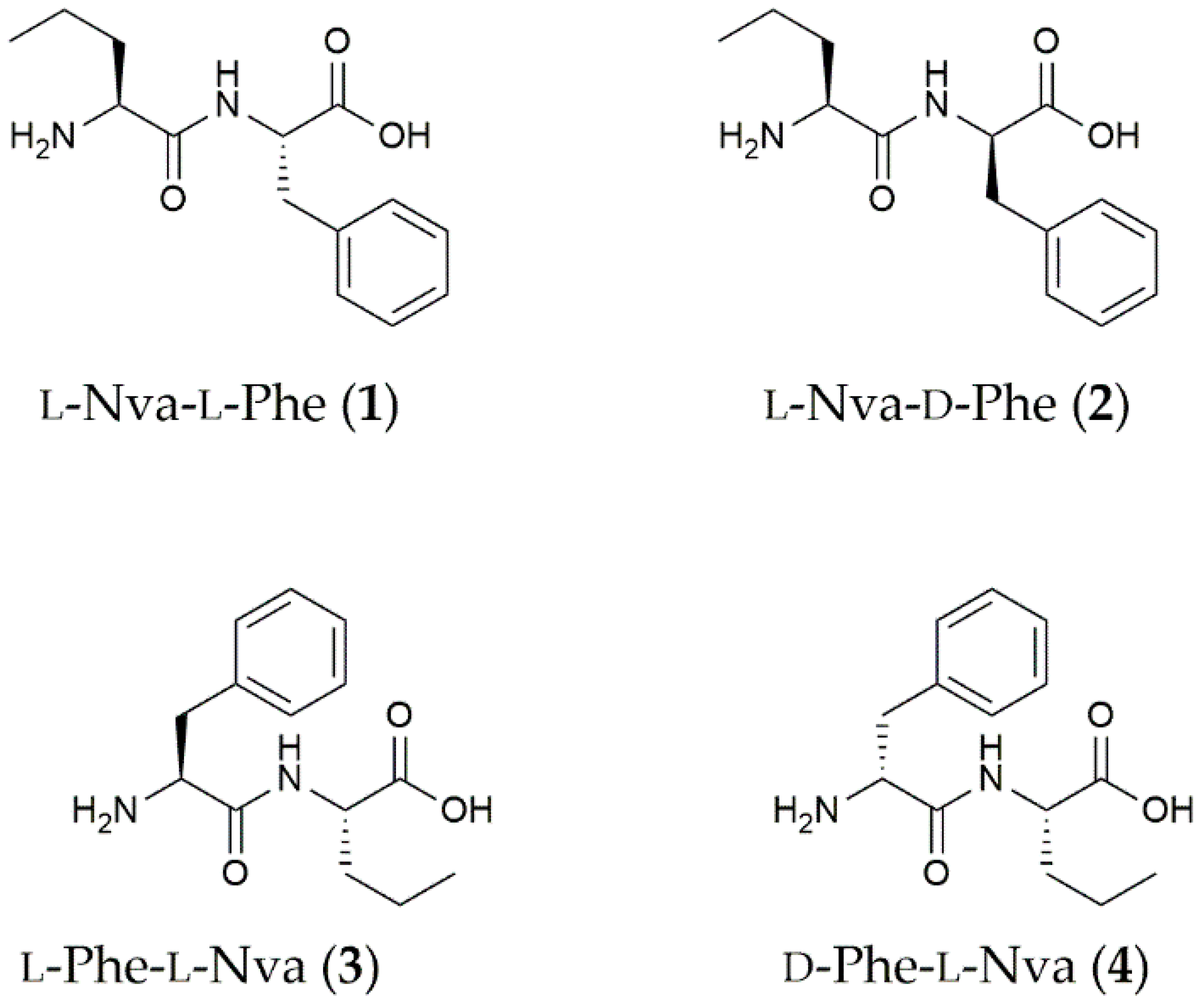


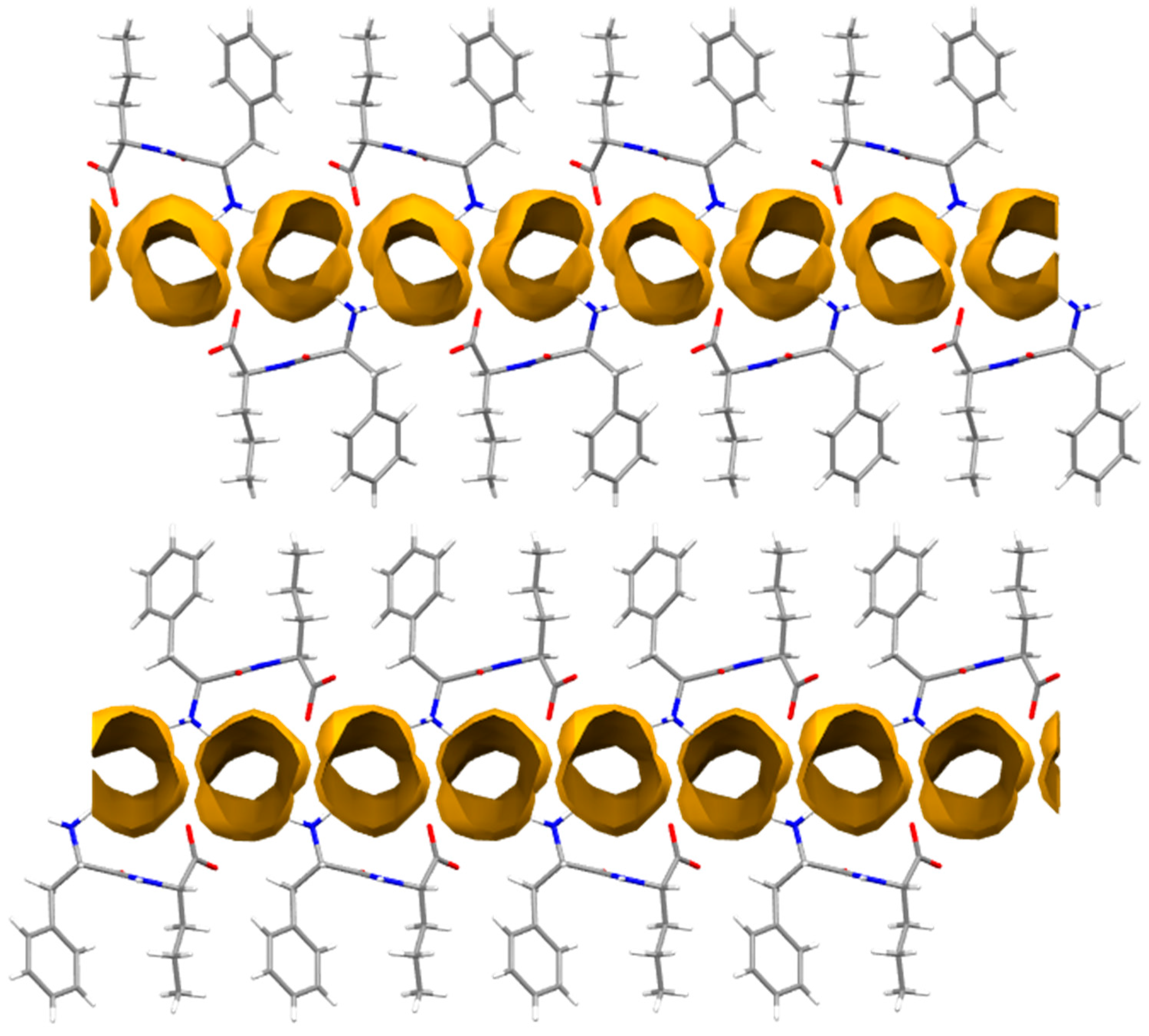

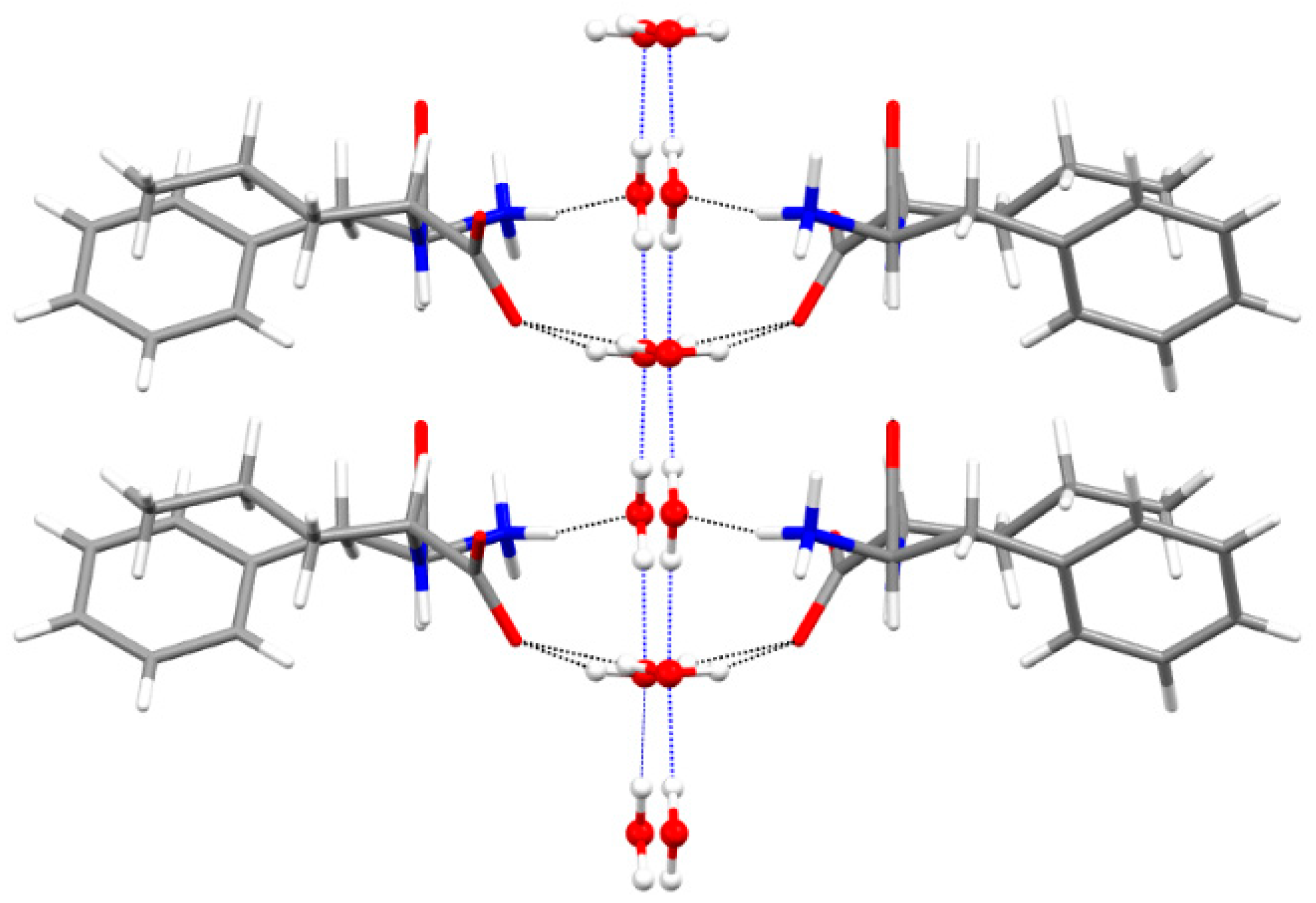
| Solvent | L-Nva-L-Phe (1) | L-Nva-D-Phe (2) | L-Phe-L-Nva (3) | D-Phe-L-Nva (4) |
|---|---|---|---|---|
| PBS buffer | Crystal | Crystal | Crystal | Crystal |
| MeOH | Crystal | Crystal | Crystal | Crystal |
| EtOH | Crystal | Crystal | Crystal | Crystal |
| iPrOH | Crystal | Crystal | Crystal | Crystal |
| MeCN | Crystal | Crystal | Crystal | Gel 1 |
| Acetone | Precipitate | Precipitate | Precipitate | Precipitate |
| Argan oil | Precipitate | Precipitate | Precipitate | Precipitate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarel, E.; Pierri, G.; Rozhin, P.; Adorinni, S.; Polentarutti, M.; Tedesco, C.; Marchesan, S. Self-Assembly and Gelation Study of Dipeptide Isomers with Norvaline and Phenylalanine. Chemistry 2022, 4, 1417-1428. https://doi.org/10.3390/chemistry4040093
Scarel E, Pierri G, Rozhin P, Adorinni S, Polentarutti M, Tedesco C, Marchesan S. Self-Assembly and Gelation Study of Dipeptide Isomers with Norvaline and Phenylalanine. Chemistry. 2022; 4(4):1417-1428. https://doi.org/10.3390/chemistry4040093
Chicago/Turabian StyleScarel, Erica, Giovanni Pierri, Petr Rozhin, Simone Adorinni, Maurizio Polentarutti, Consiglia Tedesco, and Silvia Marchesan. 2022. "Self-Assembly and Gelation Study of Dipeptide Isomers with Norvaline and Phenylalanine" Chemistry 4, no. 4: 1417-1428. https://doi.org/10.3390/chemistry4040093
APA StyleScarel, E., Pierri, G., Rozhin, P., Adorinni, S., Polentarutti, M., Tedesco, C., & Marchesan, S. (2022). Self-Assembly and Gelation Study of Dipeptide Isomers with Norvaline and Phenylalanine. Chemistry, 4(4), 1417-1428. https://doi.org/10.3390/chemistry4040093






