1. Introduction
High performance polymers started to develop in the 1960s in order to meet the demands of the aerospace and electronics industries. They experienced fast growth, some of them being commercialized as thermally stable films, coatings, fibers, adhesives, foams, membranes, or moldings, with large use as components in the electronic and microelectronic industry, space vehicles, jet engines, nuclear reactors, the automotive industry, household items, etc. The evolving of modern industry requested advanced engineering polymers and plastics with various structures and properties tailored not only to meet high performance criteria in specific applications but also to captivate customers by providing new and unexpected advantages. Thus, some new properties as electronic, electro-optical, and photo-optical properties are desired to be maintained at high temperatures [
1,
2]. Aromatic polyimides display a great potential for this purpose, having excellent thermal, mechanical, dielectric, and optical properties, along with good chemical resistance and high dimensional stability [
3,
4]. These polymers can be obtained by various methods, the most commonly used being the polycondensation reaction of aromatic dianhydrides with aromatic diamines in polar aprotic solvents (
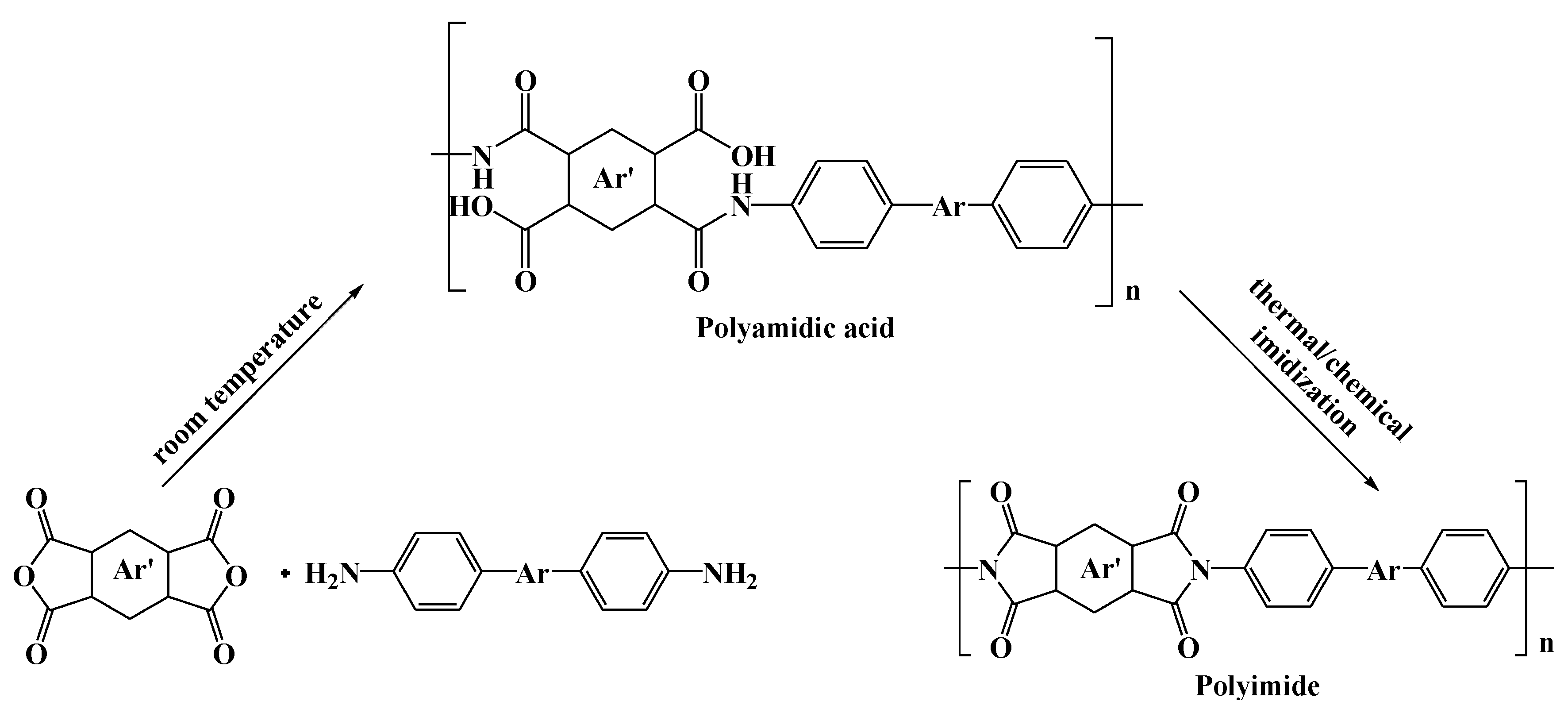
Scheme 1). Generally, the synthesis is carried out in two stages: in the first step, an intermediary polyamidic acid is obtained at room temperature, which is then subjected in the second step to a cyclodehydration process that can be performed thermally (in solution or film) or chemically (in solution). Although these polymers encountered difficult processing and are of high cost, they were intensively studied in the past for applications in various areas due to their high-end value, such as matrices for composites, films in (opto)electronic applications, structural adhesives and sealants, insulators for aircraft wire coatings, membranes for gas separation, etc. [
5]. More recently, polyimide materials started to be applied in flexible electronics due to their excellent mechanical strength, electrochemical stability, high glass transition temperature, and good flexibility [
6]. Polyimides are the most exploited materials for substrates and insulation in combination with metals for interconnection wires and electrode sites. Therefore, they became appropriate for use in medical devices and especially for encapsulation and insulation of active implants. In addition, polyimides have been used in sensors, biosensors, biochips, or as thin films for DNA or enzyme immobilization [
7]. With the great needs for high thermally-stable polymers able to perform well in advanced electrical, electronic and opto-electronic applications, such as high speed transportation and communication systems, high energy generation, storage and transmission, solar cells, smart windows, fuel cells, etc. [
8], polyimides will expand even more in the next years. However, this requires both new structural design and processing methods into exciting materials that implies the work of researchers from different fields as to integrate multiple functions in one material suitable to operate in the newest technologies.
In the pursuit to develop innovative polyimide materials endowed with versatile, multifunctional properties suitable for actual technologies, here we present our strategies to modify the classical polyimides chains to make them competitive in applications such as dielectrics, electron-transporting layers, flexible substrates, electrochromic devices, supercapacitors, energy storage, and others.
2. Polyimides for Dielectric/Substrate Materials
The dielectric constant (or relative permittivity) of polyimides typically ranges from 3 to 5 [
9]. However, for practical applications, there is need for polyimides with either lower dielectric constant (<3) for use as insulating and encapsulating material or interlayer dielectrics in the aerospace and microelectronic fields, or with high dielectric constant (>5) and breakdown strength suitable for use in film capacitors, electroactive actuators, and gate dielectrics. Each of these applications requires rational design of the chemical structures by adjusting the backbone of polyimide with specific groups, e.g., -CF
3 for dielectric constant decrease or -CN for dielectric constant increase.
In order to obtain polyimide materials endowed with low dielectric constants, the incorporation of various moieties with low polarity and polarizability is necessary [
10]. In this regard, one of our attempts involved the introduction of oxadiazole rings together with flexible ether linkages and bulky moieties such as fluorene, cyclohexane, isopropylidene, or hexafluoroisopropylidene in the polyimide chains (I,
Table 1) [
11]. Their synergism allowed us to obtain thin polyimide flexible and tough films with low dielectric constant, between 2.84 and 3.26, and low dielectric loss, below 0.012, in the frequency range 10–10
6 Hz (
Figure 1a). Moreover, each polyimide film showed high optical transparency in the visible domain and low cut-off wavelengths, with light transmittance beyond 66% at 450 nm and average transmittance in the visible region between 76 and 82% (
Figure 1b), demonstrating the potential for use of these films as flexible transparent substrates in plastic electronics.
Another approach in developing polyimides with low permittivity was focused on the combination of sulfone along with bulky hexafluoroisopropylidene (6F) units in the poly(amide-imide) polymer chains (II,
Table 1). According to the dielectric response, the obtained films were insulators, with dielectric constant between 2.4 and 3.9 at 1 Hz. However, only one polymer proved to have dielectric constant below 3 due to the
meta-catenation of the imide diacid segment. The films were slightly colored but highly transparent, with transmittance values around 80% beyond 500 nm [
12].
Modification of polyimide structures with fluorine atoms has been found in general to lower the dielectric constant [
13]. Thus, our work was focused on the polyimide loading with a high amount of fluorine through the incorporation of the 6F moiety in the dianhydride segment and trifluoromethyl groups on benzene rings (III,
Table 1). However, this strategy was detrimental to the polymers processing into thin films since only brittle free-standing films were obtained after optimization of the polyimides processing parameters [
14]. Still, to benefit of the useful properties induced by fluorine atoms in these polymers, it was necessary to blend them with polyimides having high film-forming ability and also containing fluorine atoms. Thus, blend films were developed, which displayed low relative permittivity at room temperature and 10–10
6 Hz, in the window 2.49–2.89 [
15].
Another challenge of our work was to develop miscible blends of a 6F-containing polyimide with a polyamide so that a series of hexafluoroisopropylidene-based polyimide-polyamide blends was synthesized (IV,
Table 1) [
16]. The dielectric constant was found to vary with the polyimide/polyamide ratio: the highest quantity of polyamide in the blend provided the highest dielectric constant (ε′ = 3.43), while at the lowest amount it induced only a reduced value (ε′ = 3.19).
The monomers incorporating bulky pendant groups are appropriate candidates for developing aromatic polyimides with good solubility and processability, mechanical properties, as well as low dielectric constant. When 4,4′- triphenylmethane (TPM) or Br-, F-, OH-substituted TPM was introduced into polyimide chains (V,
Table 1), a large variation of dielectric constant value was obtained. The effect of the substituent on the dielectric properties of polyimides was found beneficial, leading to the dielectric constant decrease [
17,
18]. This result was explained in terms of the lower interchain interactions and reduced polyimide chains packing into tight structures. The variation of the structural parameters in the dianhydride segments was also investigated for two TPM-based polyimide series. In the case of unsubtituted TPM units incorporated in polyimides, the lower values of dielectric constant were obtained when flexible 6F units were used (2.78 at 1 kHz and 24 °C) [
19]. In this case, the polarizability per structural unit and the permittivity decreased due to the increased free volume. By contrast, the polar carbonyl moiety promoted a significant increase of the dielectric constant (3.90 at 1 kHz and 24 °C). A less variation of the dielectric constant was observed when different bulky groups were introduced in flexible OH-substituted TPM-based polyimides. The most effective dielectric constant lowering was noticed in the case of fluorine-containing polyimide (3.04 at 100 Hz and room temperature) [
18]. However, the low dielectric constant, dielectric loss and DC conductivity over a wide range of temperatures demonstrated the good dielectric stability of the studied polymer films. In addition, when subjected to chemical resistance tests by using methyl ethyl ketone and an alkaline (NaOH) solution, these polymer films preserved the mechanical integrity, with no changes in appearance such as turning turbid (
Figure 2), which suggested their suitability for use as chemically resistant flexible substrates. Moreover, by painting one of these polyimide films with a conductive graphite paste, flexible electrodes were obtained, being appropriate for operating as working electrodes in cyclic voltammetry experiments.
With regard to the approaches to increase the dielectric constant of polyimides, several strategies have been applied so far, among which embedding polar groups (e.g., amide, CN), electron rich groups (e.g., bipyridine), and different organic fillers in polyimide matrices were the most often used [
20,
21]. In this context, we have developed jeffamine-containing polyimides (VI,
Table 1) that in addition were functionalized with polar nitrile groups. These polymers were exploited for dielectric applications in modern flexible/stretchable film capacitors [
22]. Due to the synergism between the selected structural units, amorphous semi-interpenetrated polymer networks structures with single
Tg resulted, which displayed one of the highest strain at break (516%) reported to date for this class of polymers. The polymers exhibited high dielectric constant values, between 5.1 and 19.2 at 1 MHz, depending on the grafting position of the nitrile moiety on the benzene ring in the diamine unit. These values were among the greatest known to date for the neat polyimides.
Table 1.
Structures of the polymers used in the studies reported in chapter 2.
Table 1.
Structures of the polymers used in the studies reported in chapter 2.
When CoCl
2/polyimide composites (VII,
Table 1) were developed, significant improvements of the dielectric constant were achieved [
23]. The dielectric constant varied with frequency and temperature for different loadings of CoCl
2. The highest amount of the salt introduced in the polyimide matrix led to the greatest dielectric constant, which reached approx. 8.5 at 20% CoCl
2 loading. The dielectric constant plotted versus the dielectric loss at various contents of CoCl
2 led to Cole–Cole plots (
Figure 3) whose profiles suggested the capacitive behavior of the composites even at a low salt content.
At the 20% CoCl2, the dielectric loss preponderantly increased in the low temperature regions with respect to the dielectric constant, when the Cole–Cole diagram profiles modified from arc to linear. At raised temperatures, these preserved linear, with a small exception in the case of the polyimide film with the highest salt loading. Such behavior was attributed to easier movement of the particle ions at high temperature, having at immediate effect the enhancement of the dielectric constant. As a consequence, an electrical conductivity as high as 2.57 × 10−7 S/cm was attained for PI-20% at 106 Hz.
3. n-Type Polyimide Materials
In recent years, great efforts were focused on the development of novel polymers with charge-transporting ability, either for holes or electrons, or even with ambipolar transport capability. While the polymers with electron donating properties (
p-type) registered fast development, the
n-type polymers able to transport electrons are less popular. One of the most frequently approached
n-type building block in polymers is the electron–withdrawing imide ring, which is the base for large number of donor–acceptor systems. Thus, aromatic polymers containing imide rings are redox-active due to the electrochemical reduction of imide at anion radical or dianion forms [
24,
25]. Six-membered imide rings (naphthaleneimide or peryleneimide) undergo easier reduction compared to their five-membered counterparts, thus emerging as an important class of
n-type units [
26,
27].
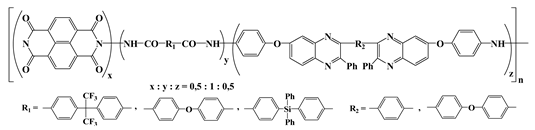
In our pursuit to contribute to the development of the
n-type polymer materials, our group has altered the polyimide chain structures with various functionalities. One example is the series of fluorinated copolyimides containing perylene, oxadiazole, and 6F moieties in the main chains (VIII,
Table 2) [
28]. Cyclic voltammetry studies showed that the polymers can be easily reduced (
n-doping) in the cathode region. In addition, they displayed low energy barrier between electron and hole injection from electrodes to polymer, thus being appropriate for use as active layers in electro-optical devices. When naphthalenediimide was incorporated together with 1,3,4-oxadiazole and flexible siloxane bridges in the main chains, electroactive polymers (IX,
Table 2) with ambipolar transport characteristics were obtained [
29]. Two or three oxidation peaks corresponding to the generation of radical cation or dication species were registered in the potential window between 0 and 2 V. When the potential was reversed to −2 V, one or two reduction peaks appeared, due to the formation of the radical anions of oxadiazole or/and naphthaleneimide units. Both the oxidation and reduction processes were found to be stable, with small shifts only of the cathodic peaks. The energy barrier for electron and hole injection into polymer was estimated in the range of 0.54–0.72 eV. With their good electron injection and transport properties these polymers are suitable as
n-type materials in optoelectronic applications.
Six-membered naphthalenediimide moiety was also incorporated in polyamides containing different flexible units by direct connection to amide group through the N–N bond (X,
Table 2) [
30]. An unusual supramolecular organization in spherical micellar structures was attained in thin polymer films prepared from these polymers, which favored the electrons transport. When a negative potential was applied to these films, electrochemical doping of polymer films occurred, making the films adherent to the electrode surface and electrically conductive. Thus, they exhibited good electron injection and transport properties and low energy barrier for electron injection. The introduction of additional
n-type quinoxaline units into this type of polymers (XI,
Table 2) improved the electron transport characteristics and the electrochemical stability of the polymer films [
31]. These were electrochemically active only in the cathodic region and displayed a CV wave with two reduction peaks attributed to the formation of radical anions localized on quinoxaline and naphthalenediimide. Although only the quinoxaline radical anions experienced oxidation when the current was reversed to 0 V, the overall reduction processes were find quite stable (
Figure 4a). In some cases, a large increase in the cathodic current occurred with the advancement of the scan numbers (
Figure 4b) as indicative of the electrochemical doping of these polymer films with negative (ClO4
−) ions, making the films adherent to the electrode surface and electrically conductive.
The effect of the diimide structure on the
n-type behavior of some five membered polyimides with donor-acceptor topology containing trifluoromethyl (CF
3)-substituted triphenylamine (TPA) as donor units (XIIa,
Table 2) has been investigated in [
32]. All polyimides displayed oxidation processes at onset potential between 1.11–1.17 V vs. SCE due to the generation of triphenylamine radical cations, and two independent reduction reactions at the phthalimide core, proving their ability for ambipolar transport characteristics. The benzophenontetracarboxylic dianhydride—derived polyimide underwent reduction at the lowest potential due to the easier reduction of benzophenone unit. Prototype devices were realized with this polymer in the configuration ITO/PEDOT:PSS/polyimide/Ag and preliminarily investigated with regard to the electrical characteristics. The polyimide films displayed an ohmic conduction in the lower range of applied voltage, whereas a non-ohmic conduction mechanism was observed in the higher range of applied voltage, which demonstrated a nonlinear Schottky behavior.
The same electron donating diamine was employed to build donor–acceptor systems with naphthalenediimide and perylenediimide dianhydrides (XIIb,
Table 2) [
33]. Due to the high electron-accepting properties induced by both CF
3 and naphthalene(di)imide or perylenediimide units, these polymers behaved as
n-type materials in
p−n heterojunction prototype diodes fabricated in the configuration ITO (or Al)/(PEDOT:PSS)/polyimide/eGaIn (
Figure 5a). These devices displayed asymmetrical and nonlinear
I−V characteristics as shown in
Figure 5b,c, with rectification ratio up to 63, shunt resistance up to 492 MΩ, low series resistance below 154 Ω, and current leakages up to 0.87 nA. The ideality factor was found lower than 5.48, which suggested the tunneling as the main phenomenon occurring in the diodes. The developed materials were recommended as alternatives to
n-type materials currently in use in advanced optoelectronic devices.
Grafting the strong 1,3,4-oxadiazole electron acceptor on the TPA unit in polyimides XIII (
Table 2) led to polymers which experienced difficult oxidation but easy reduction at low potentials, proving excellent ability for
n-doping and electron transport triggered by the oxadiazole ring [
34]. Electrical measurements on ITO/PEDOT:PSS/polyimide/eGaIn prototype devices demonstrated asymmetrical and nonlinear
I−V characteristics, with higher currents in the forward bias, typical for a diode. The electrical conduction was dependent on the conjugation strength of the tested polyimides, with satisfactory results in the case of PMDA-based polyimide. Due to the high electron-withdrawing effect exerted by the 1,3,4-oxadiazole unit, which modulated the HOMO−LUMO energy to low values, the investigated polymers can be acknowledged among the
n-type conducting materials for use in optoelectronic applications.
4. Electrochromic Polyimides
Chromic materials exhibit a reversible color change in response to an external stimulus such as temperature, light, current, etc. The color modification occurs due to the change in the absorption spectra of the materials across the UV–visible/near-infrared (NIR) region. Oxidation and reduction of certain compound when an electrical bias is applied can also lead to distinct photo-optical and color changes, a phenomenon known as electrochromism. The electrochromic materials must obey some criteria such as the ability for color change, short color switching time, large number of color switching cycles (stability), memory effect in open circuit conditions, or high color contrast. Among the variety of organic materials that exhibit electrochromism, propeller-shaped triphenylamine-based ones, including polymers, showed particular and appealing electrochromic features. Among them, high-performance polymers such as aromatic polyimides were scarcely studied as electrochromic materials. Due to their excellent thermal stability and film forming ability when adequately modified, these polymers are potential candidates in the electrochromism field. Along these lines, our work was concentrated on the development of novel six-membered polyimides (
Figure 6) with electrochromic behavior and their efficiency evaluation in prototype electrochromic devices [
35].
The electrochemical experiments evidenced both the hole and electron transport ability of these polymers, with the preponderant affinity for the electron transport (
n-doping). Since during the electrochemical oxidation of perylene-based polyimides the color switching between neutral and oxidized states was noticed, these polymers were investigated as active layers in electrochromic devices. The spectroelectrochemical measurements evidenced the evolution of some absorption bands upon potential increase, being associated with both polarons/bipolarons and radical cation formation (
Figure 7). The electrochromic devices changed the color between neutral and oxidized forms of the polymers. In the neutral state, the devices were yellow/light purple and turned into dark-green or violet at a voltage of 2.8–2.9 V. Both EC devices returned to the original colors when the potential was set back at 0.0 V.
The response time to coloring was found between 1.8 and 3.3 s, while the response time to bleaching was in the rage of 3.2–3.4 s (
Figure 8). The recovery of the realized electrochromic devices was approx. 90% after 30 cycles, denoting an excellent stability which was explained in terms of an extended polymer conjugation induced by the peryleneimide fragment.
The following attempt to develop electrochromic materials based on polyimides consisted in the introduction of diphenylamine in the side chains in the polyimides containing triphenylmethane [
36], which can undergo redox processes, as shown in
Figure 9.
The electrochromic behavior of the thin films prepared from these polymers was first evaluated in the three-electrode cell configuration, with them as working electrodes. When the potential was set at 1.15–1.25 V, their color turned to yellow-orange or pale-yellow due to the oxidation of the triphenylamine moiety. At higher potentials, the color of the films evolved from yellow-orange or pale-yellow to blue or light-blue due to the radical cations coupling. The highest electrochromic coloration efficiency, fastest color switching, and better stability was noticed in the case of PMDA-derived polyimide having the highest conjugated structure. Next, the polymers were tested in device configuration, as shown in
Figure 10.
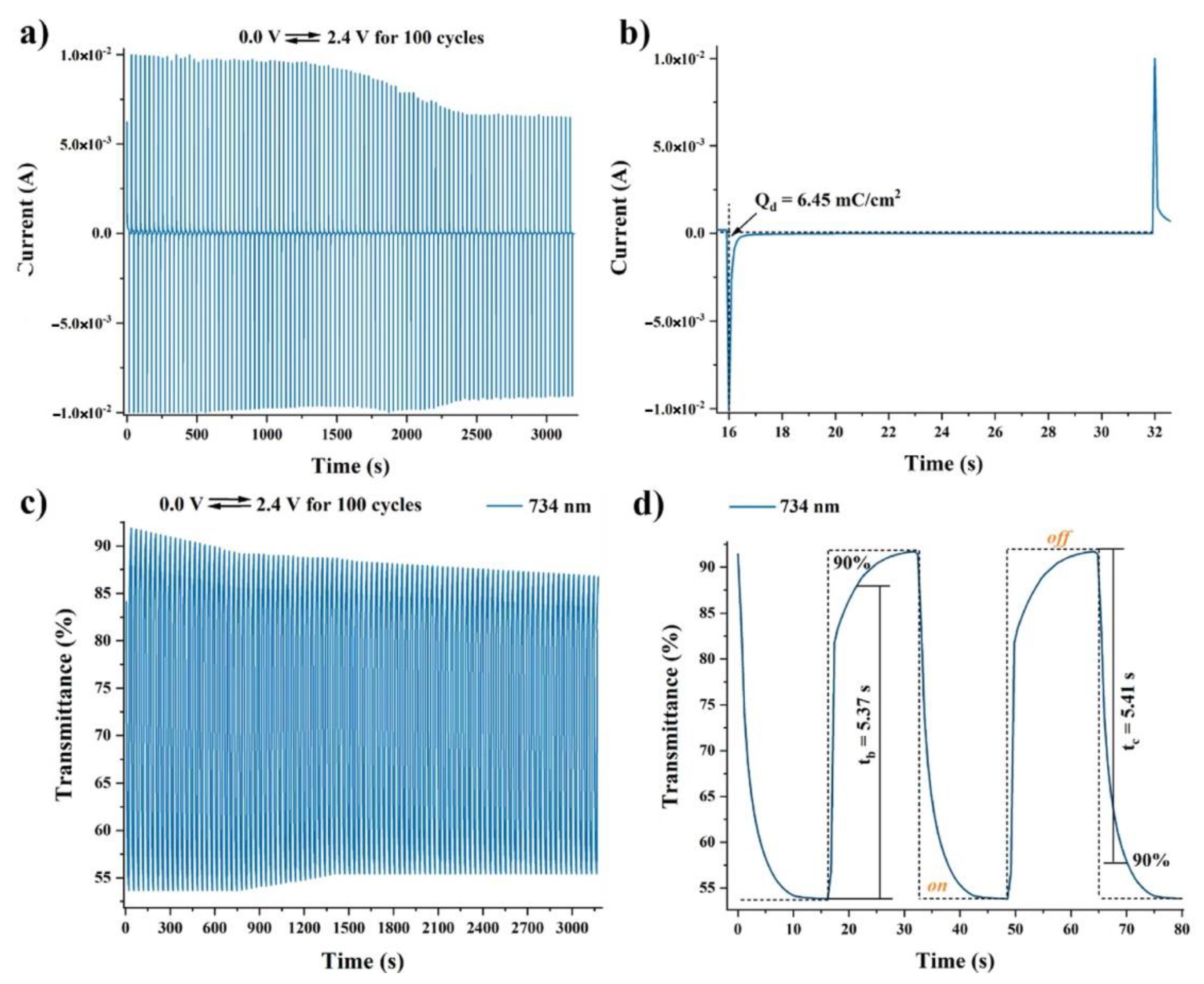
The applied potentials ranged from 0.0 up to maximum 2.4 V, with a 0.1 V sweep rate. The obtained spectroelectrochemical curves are presented in
Figure 11 for the electrochromic polymer of the series with the best performance (
P1).
The spectral changes determined by the radical cations formation led to the change of the devices’ color from transparent to orange or pale-yellow. At higher potentials, up to 2.4 V, a new absorption band evolved, which caused the modification of the device color from orange to blue or from pale-yellow to light-blue. In the case of
P1, the coloring and bleaching was obtained in 5.36 s and 5.41 s, respectively, while for the other polymers these parameters were almost 2 or 3 times higher. The optical contrast was 40% for
P1, 16% for
P2, and 11% for
P3, with a coloration efficiency in the range of 39–90 cm
2/C. The electrochromic stability estimated during 100 repeated cycles proved to be the best in the case of
P1 (about 85%), with a current drop up to 10% (
Figure 12).
5. Photochromic Polyimides
Azobenzene-containing compounds were largely studied as photochromic units to produce intelligent materials for optical applications such as data storage. The photochromic behaviour of azopolymers arises from the
trans-cis photo-isomerization of azobenzene moiety: this can be converted from
trans isomer to the
cis one by irradiation with UV light of 365 nm but also can be reversed from the
cis conformation to the
trans isomer either thermally or under irradiation with light of 420 nm [
37]. These processes are largely dependent on the polymer structure which incorporates the azobenzene units, which can be linear, dendritic, branched, crosslinked, etc, but also on the position (side chain or main chain) of the azobenzene fragment in the polymer [
38]. Most azopolymers with efficient photo-isomerisation behaviour display low thermal stability necessary for practical applications, fostering the research attention towards polyimides which are well-known for their high thermostability and other high performance properties. The research on photo-switchable polyimides was rather limited in the past, but rapidly expanded in recent years [
39,
40,
41], mainly due to the low isomerization efficiency of azobenzene induced by the rigid nature of polyimide chains. The incorporation of azobenzene into side chains of aromatic polyimides is a promising approach towards this aim, which may allow the azo chromophore alignment at elevated temperatures.
To expand the family of azobenzene polymers with appealing characteristics, our group have synthesized azopolyimides by incorporating some structural parameters, like azobenzene chromophore in the side chain and imide and flexible ether in the main chain (
Figure 13a) [
42]. Their UV-light irradiation both as solution and thin films led to the decrease of the UV-Vis absorption band from 353 nm due to the
trans-cis isomerisation of azobenzene. By further irradiation, the band decrease was maintained, but not so obvious. When the solution was stored in dark (for 18 h) the photo-induced
cis isomer started to relax to the
trans form and the characteristic absorption band reappeared. When the polymer solutions were stored in light, only partial relaxation of the
cis isomer up to equilibrium was noticed. These polymers showed direct light emission from the
trans isomer and indirect emission from the
cis-form. The investigation of the redox behavior of these polyimides modified with azo group, not assessed before, revealed that the reduction of the azobenzene moiety to the radical anion form is followed by protonation, while after the polymer oxidation in the solid state, chemical rearrangement or generation of new species may take place.
To increase the free movement of the azo group in polyimides, we further developed a series of polymers in which the highly photoresponsive azobenzene–naphthalene moiety was grafted on the triphenylmethane core of polyimides through a flexible spacer (
Figure 13b) [
43].
The detailed investigation of the
trans-cis isomerization mechanism evidenced that in solid state it occurs through a non-polarity assisted inversion, while in DMF the polarity-assisted rotation prevails. The
trans-cis conversion took place in a shorter time in solution (47 to 130 s), with a degree of conversion between 87 and 90.2%, being one the highest found for polyimides. In solid state, the polyimides photoresponse was preserved but after larger irradiation times (900 s), and with lower efficiency (68–75.7%) due to the rotational constrains of the azo chromophore in solid state (
Figure 14). Please note that these remarkable results were attained without any polymer dilution in solid state. The
cis-trans thermal relaxation was studied at 70 °C when almost full recovery of the
trans form was observed during 1200–2880 s both in solution and film. The stability of the
trans-cis and
cis–trans isomerizations were studied during 10 repeated cycles (
Figure 15). Accordingly, these were found very stable, up to 96.29% in solution and 92.68% in solid state.
One of these polyimides was further subjected to pulsed UV laser irradiation through phase masks at various fluencies and pulse numbers to generate surface relief gratings (SRGs), as shown in
Figure 16 [
44].
At 10 mJ/cm2 and 10 pulses of irradiation, the SRGs were not so obvious, and instead, at an energy density increase to 45 mJ/cm2, very nice and uniform SRGs were formed. At this high energy, but at superior pulses, the repetitive structures had better definition, increasing in height. Since at 100 pulses the SRG profiles appeared corrugated, it was concluded that a high energy and a small number of pulses are more appropriate to obtain well-defined SRGs. For understanding the formation mechanism of SRGs, molecular simulation and an advanced nanomechanical characterization were performed by AFM PinPoint mode, which were further correlated with the topographic images.
6. Polyimide Materials for Energy Storage Applications
The evolution of the power systems and electronic devices triggered the development of high-power and high-energy storage capacitors with rapid charging and discharging ability based on polymers. These have some advantages such as easy processing and low cost of production, low dielectric loss, and high breakdown strength, which makes them appropriate for use in hybrid electric vehicles, power transmission, microelectronic systems, etc. Nowadays, there is a high demand for dielectric capacitors able to operate in a high temperature range, and for this purpose the polyimides display a great potential also because of their high breakdown strength and thermal stability, low dielectric loss, and wide bandgap [
45]. Still, great effort has focused on polyimides to increase the permittivity of polyimides through the structural variation, which plays the key role in the dielectric behavior tuning of polyimides [
46]. To meet these demands, we exploited the synergism of nitrile and jeffamine polar groups for developing high dielectric constant neat polyimide films. Thus, a series of copolyimides containing both soft (polypropylene- and polyethylene-oxide) and hard (aromatic phenylene-based fragments substituted with CN groups) segments was synthesized (
Figure 17a) [
22]. As mentioned in
Section 2, these polyimides were processed in tough, flexible, and stretchable films with excellent adhesion to hydrophilic supports (
Figure 17b) that displayed high dielectric constants at 1 MHz, between 5.1 and 19.2.
According to the plots shown in
Figure 18b,c, the ε″(f) and σ(f) dependences display two different regimes: the resistive one at low frequencies due to the transport of electric charges and the capacitive one at high frequencies consistent with the dipolar relaxation. Thus, the dielectric behavior of these polymer films is significantly affected by the charge carrier’s conductivity. In addition, for these polymeric films, the presence of the Maxwell–Wagner–Sillars interfacial polarization process was highlighted, leading to high values of the DC conductivity. These were in the range of 10
−7–10
−6 S/cm at 10
4 Hz, being greater than those reported for classic polyimides or copolyimides whose σDC values ranged between 10
−13 and 10
−14 S/cm [
17].
The insulating ability of a material is determined by its dielectric breakdown strength which can be evaluated on the basis of the Weibull scale parameters. In this regard, it was found a significant difference between the three synthesized copolymers: at a probability of 63.2%, coPI-1 presented the highest value of the electrical breakdown resistance (220 V/µm), while for coPI-2 and coPI-3 the breakdown value was much lower (78 V/µm and 51 V/µm, respectively). Considering the permittivity values measured at 1 MHz and the dielectric breakdown strength, the energy storage density was obtained in the range of 0.221–1.092 J/cm3. The best performance was obtained with coPI-1, which can be regarded as a perspective dielectric stretchable material for flexible dielectric capacitors able to operate at high temperatures.
Electrochemical supercapacitors are other candidates for efficient energy storage devices that can meet the demands of advanced electronic systems. Since the electrode is the key component of the supercapacitor, which dictates the overall performance, huge research has been focused on the development of promising electrodes, and especially flexible electrodes [
47]. We brought contribution to this research field by developing unmodified multiwall-carbon nanotubes (MWCNTs)/polyimide composites at different MWCNTs loadings that were successfully tested as flexible electrodes in supercapacitors [
48]. The composite films were flexible and maintained their integrity during the rolling process, demonstrating good resistance to mechanical stress. According to the SEM images, a homogeneous dispersion of MWCNTs was attained, with nanotubes protuberating out of the polymer matrix (
Figure 19). The electrical conductivity varied from 1.14 × 10
−2 S/cm up to 1.11 S/cm, depending of the amount of MWCNTs loading. The electrical storage capacity of these composites was first tested in three electrode cell configuration by cyclic voltammetry (CV) and galvanostatic charge–discharge measurements, in both aqueous and organic electrolytes. The CV curves maintained a rectangular-like form with small distortion even at a high scan rate (500 mV/s), indicating a good energy storage capacity, especially for the composites with 5% and 10% MWCNTs.
The maximum specific gravimetric capacitance of 177 mF/g was obtained in the aqueous electrolyte for the composite with 10% MWCNTs. Due to the high volume of organic electrolyte ions, this parameter was approximately 20 times lower (9.02 mF/g) in the organic electrolyte. By galvanostatic charge–discharge (GCD) measurements, the maximum areal capacitance was found as 2.49 mF/cm2 in the aqueous electrolyte, and 0.17 mF/cm2 in the organic electrolyte, at 10 µA/cm2.
The most efficient composite film was tested in a simple, flexible symmetric supercapacitor, with tetrabutylammonium hexafluorophosphate/poly(methyl methacrylate) as gel electrolyte and filter paper as separator, without binder, current collector or any packaging material, but the electrical storage performance was low. In the pursuit to increase the energy storage efficiency, the composite film with 10% MWCNTs was coated with a graphite layer and tested in a supercapacitor having the structure presented in
Figure 20a. This supercapacitor had better performance, reaching 2.01 mF/cm
2 (or 84.6 mF/g) at 10 mV/s, as estimated from the CV curves (
Figure 20b). According to the GCD curves (
Figure 20c), the maximum areal capacitance of 0.183 mF/cm
2 was obtained at 3.67 µA/cm
2. At the same current density, the energy and power densities attained 14 nW × h × cm
−2 and 1.36 µW/cm
2, respectively. The obtained results can be considered as starting point for further development of high-temperature polyimide-based nanocomposites for use as active elements in flexible electronics.
7. Conclusions
In recent years, with the rapid development of versatile high-tech industries, there is a continuous demand for both academic and engineering knowledge on advanced polyimide-type materials. Due to the difficulties in polyimide processing and more exigent requirements, it was necessary to endow polyimides with new functions attractive for current technologies. To this aim, the generic objective of our research activities was to modify the structure of classic aromatic polyimides to render them both solubility in organic solvents and convenient processability by wet methods, as well as new functionalities, without altering the high performance properties characteristic of this class of polymers. Thus, the polyimides developed in ICMPP of the Romanian Academy demonstrated suitability for multiple applications, such as low k dielectrics or electrical insulators for microelectronics, high k dielectrics for film capacitors, electrode materials for supercapacitors, flexible substrates or n-type materials for optoelectronic devices, smart materials for electrochromic or photochromic applications, etc. Not less important and not addressed here, our focus was also directed towards developing gas-separation polyimide membranes and polyimide materials for sensors and biosensor applications. In some of these applications, the polyimide materials showed promising results, but several obstacles still exist and must be overcome. First of all is the high cost, which limits polyimide use in small quantities, more often for high-end value optoelectronic devices. Then, the integration of multiple functions in a single polyimide material is necessary to make it competitive for the fascinating electronic world of today, and finally, the technology for polyimide processing should be improved to get more attractive materials that can be used in harsh conditions such as elevated temperatures. We are confident that the research group from ICMPP will contribute in the future with valuable findings to the progress of all these fields.