Polymer/Enzyme Composite Materials—Versatile Catalysts with Multiple Applications
Abstract
1. Introduction
2. Enzymes—Structure, Activity, and Characteristics
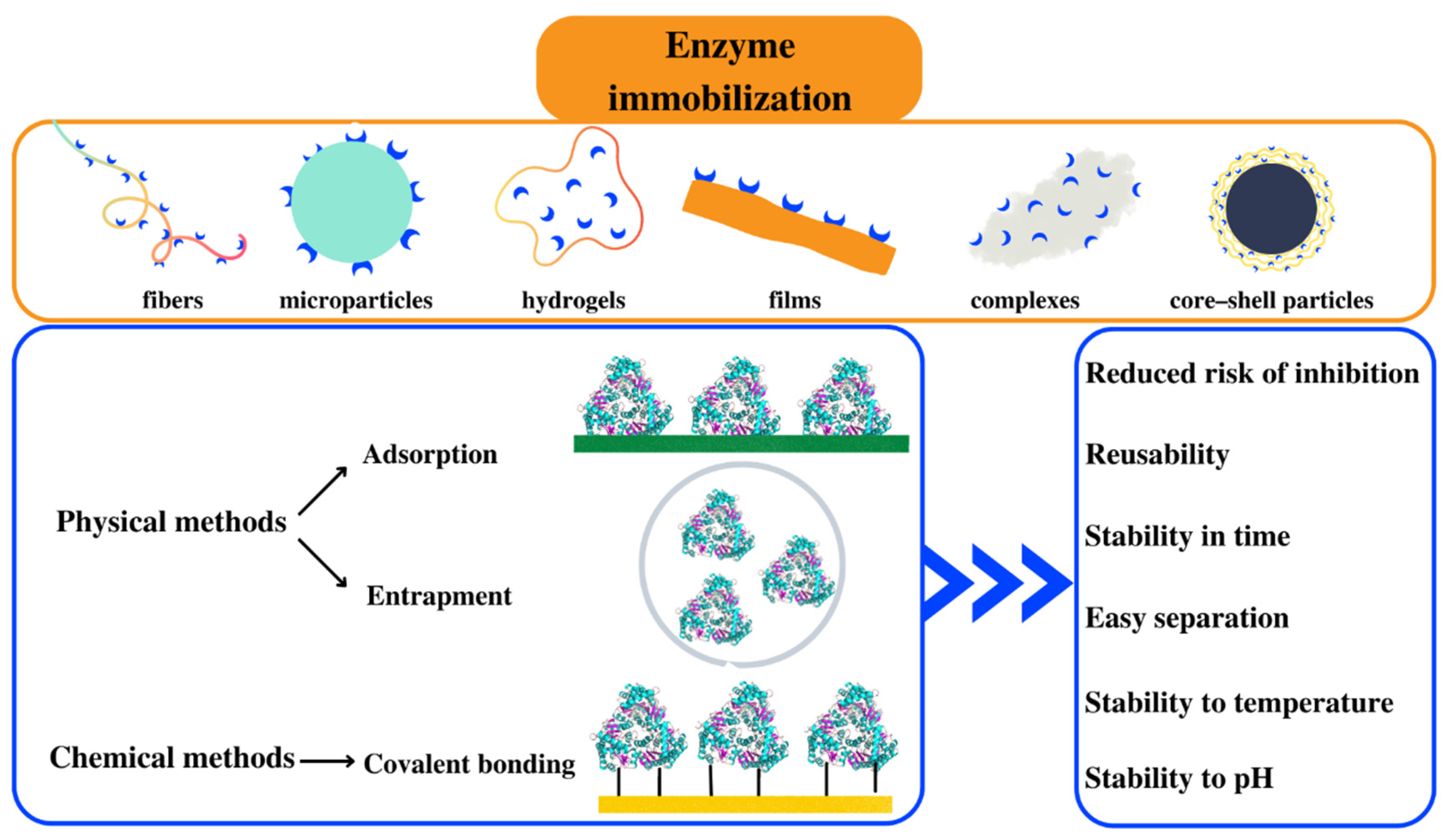
3. Methods of Enzyme Immobilization on Polymeric Supports
3.1. Immobilization by Adsorption
3.2. Immobilization by Entrapment
3.3. Immobilization by Covalent Bonding
4. Polymer/Enzyme Composite Materials
4.1. Polymeric Fibers
4.2. Polymeric Hydrogels
4.3. Polymeric Micro- and Nanoparticles
4.4. Core–Shell Particles
4.4.1. Single-Layer Core–Shell Composite Materials
4.4.2. Multilayer Core–Shell Composite Materials
4.5. Thin Polymeric Films
4.6. Polymer/Enzyme Complexes
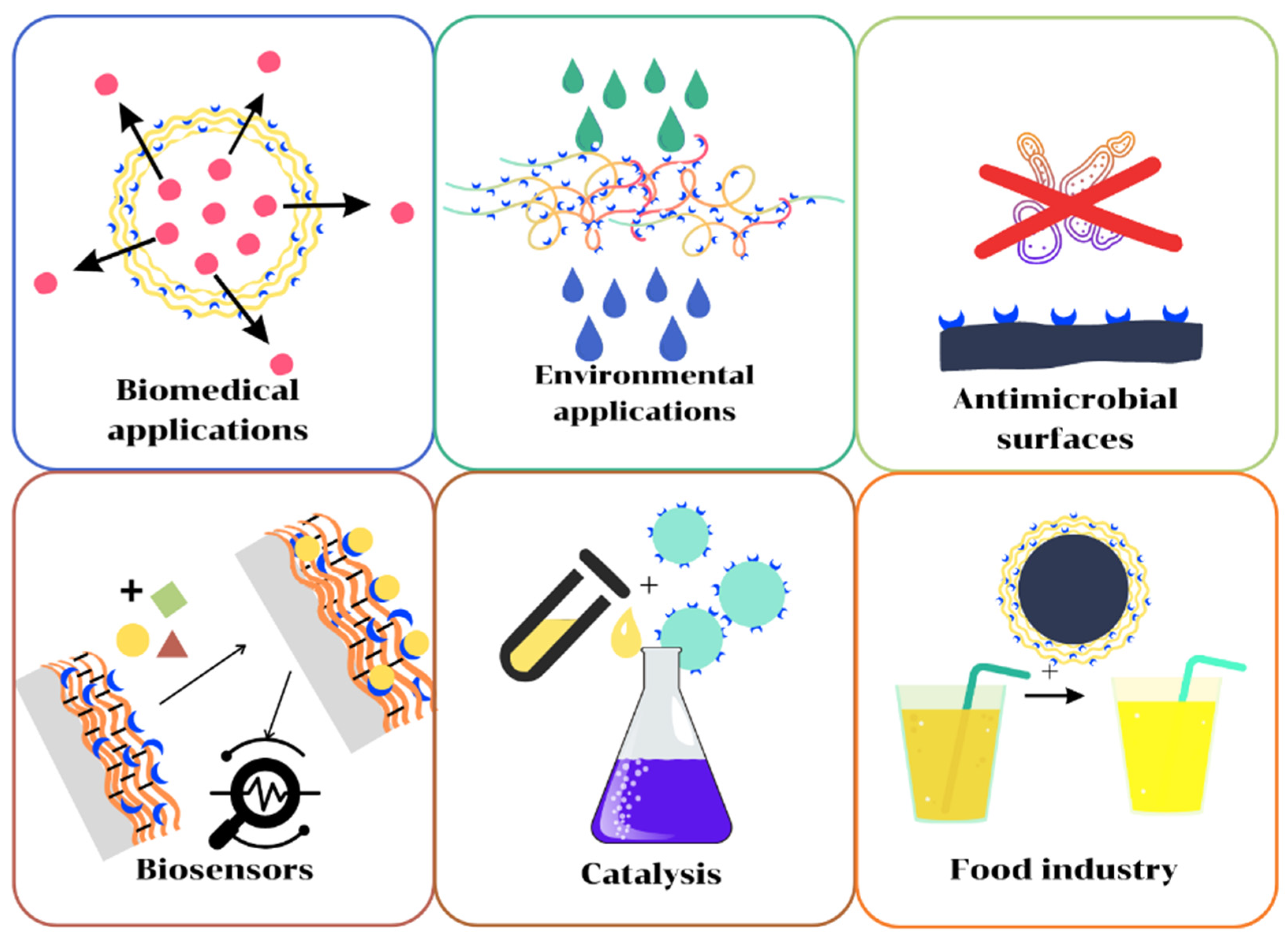
5. Applications of Polymer/Enzyme Composite Materials
5.1. Applications in Environmental Protection
5.2. Catalysts for Chemical Industry
5.3. Biosensors
5.4. Antimicrobial Applications
5.5. Biomedical Applications
5.6. Applications for Food Industry
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Li, Y.; Wang, X.; Sun, J. Layer-by-layer assembly for rapid fabrication of thick polymeric films. Chem. Soc. Rev. 2012, 41, 5998–6009. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; Ismail, A.E.; Zoica Dinu, C. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Bilal, M.; Dourado, C.; Mehmood, T.; Nadeem, F.; Tabassam, Q.; Fernando, L.; Ferreira, R. Immobilized lipases-based nano-biocatalytic systems—A versatile platform with incredible biotechnological potential. Int. J. Biol. Macromol. 2021, 175, 108–122. [Google Scholar] [CrossRef]
- Aggarwal, S.; Chakravarty, A.; Ikram, S. A comprehensive review on incredible renewable carriers as promising platforms for enzyme immobilization & thereof strategies. Int. J. Biol. Macromol. 2021, 167, 962–986. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, K.; Su, Z.; Bj, S. Tailor-made novel electrospun polystyrene/poly (D,L-lactide-co-glycolide) for oxidoreductases immobilization: Improvement of catalytic properties under extreme reaction conditions. Bioorg. Chem. 2021, 114, 105036. [Google Scholar] [CrossRef]
- BRENDA Enzyme Database. Available online: www.brenda-enzymes.org (accessed on 4 June 2022).
- Copeland, R.A. Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis, 2nd ed.; Wiley-VCH: New York, NY, USA, 2000. [Google Scholar]
- Nelson, J.M.; Griffin, E.G. Adsorption of invertase. J. Am. Chem. Soc. 1916, 38, 1109–1115. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilization in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Tran, D.N.; Balkus, K.J. Enzyme immobilization via electrospinning. Top. Catal. 2012, 55, 1057–1069. [Google Scholar] [CrossRef]
- Zahirinejad, S.; Hemmati, R.; Homaei, A.; Dinari, A.; Hosseinkhani, S.; Mohammadi, S.; Vianello, F. Nano-organic supports for enzyme immobilization: Scopes and perspectives. Colloids Surf. B 2021, 204, 111774. [Google Scholar] [CrossRef]
- Jin, W.; Wang, Z.; Peng, D.; Shen, W.; Zhu, Z.; Cheng, S.; Li, B.; Huang, Q. Effect of linear charge density of polysaccharides on interactions with α-amylase: Self-Assembling behavior and application in enzyme immobilization. Food Chem. 2020, 331, 127320. [Google Scholar] [CrossRef]
- Briones, A.V.; Sato, T. Encapsulation of glucose oxidase (GOD) in polyelectrolyte complexes of chitosan–carrageenan. React. Funct. Polym. 2010, 70, 19–27. [Google Scholar] [CrossRef]
- Meridor, D.; Gedanken, A. Preparation of enzyme nanoparticles and studying the catalytic activity of the immobilized nanoparticles on polyethylene films. Ultrason. Sonochem. 2013, 20, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dong, J.; Bhattacharjee, S.; Wijeratne, S.; Bruening, M.L.; Baker, G.L. Increased protein sorption in poly(acrylic acid)-containing films through incorporation of comb-like polymers and film adsorption at low pH and high ionic strength. Langmuir 2013, 29, 2946–2954. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Ming, H.; Li, L.; Li, M.; Gao, J.; Han, T.; Wang, Y. Fabrication of an in-situ co-immobilized enzyme in mesoporous silica for synthesizing GSH with ATP regeneration. Mol. Catal. 2020, 486, 110870. [Google Scholar] [CrossRef]
- Gopinath, S.; Sugunan, S. Enzymes immobilized on montmorillonite K10: Effect of adsorption and grafting on the surface properties and the enzyme activity. Appl. Clay Sci. 2010, 35, 67–75. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, W.; Cai, Y. Enzyme-enhanced adsorption of laccase immobilized graphene oxide for micro-pollutant removal. Sep. Purif. Technol. 2022, 294, 121178. [Google Scholar] [CrossRef]
- Liu, D.; Chen, J.; Shi, Y. Advances on methods and easy separated support materials for enzymes immobilization. Trends Anal. Chem. 2018, 102, 332–342. [Google Scholar] [CrossRef]
- Cao, L. Carrier-Bound Immobilized Enzymes: Principles, Application and Design; Wiley-VCH: New York, NY, USA, 2005. [Google Scholar]
- Tacias-Pascacio, V.G.; García-Parra, E.; Vela-Gutiérrez, G.; Virgen-Ortiz, J.J.; Berenguer-Murcia, Á.; Alcántara, A.R.; Fernandez-Lafuente, R. Genipin as an emergent tool in the design of biocatalysts: Mechanism of reaction and applications. Catalysts 2019, 9, 1035. [Google Scholar] [CrossRef]
- Ashkan, Z.; Hemmati, R.; Homaei, A.; Dinari, A.; Jamlidoost, M.; Tashakor, A. Immobilization of enzymes on nanoinorganic support materials: An update. Int. J. Biol. Macromol. 2021, 168, 708–721. [Google Scholar] [CrossRef]
- Zhang, S.; Bilal, M.; Zdarta, J.; Cui, J.; Kumar, A.; Franco, M.; Fernando, L.; Ferreira, R.; Iqbal, H.M.N. Biopolymers and nanostructured materials to develop pectinases-based immobilized nano-biocatalytic systems for biotechnological applications. Food Res. Int. 2021, 140, 109979. [Google Scholar] [CrossRef]
- Soares, A.M.B.F.; Gonçalves, L.M.O.; Ferreira, R.D.S.; de Souza, J.M.; Fangueiro, R.; Alves, M.M.M.; Carvalho, F.A.A.; Mendes, A.N.; Cantanhêde, W. Immobilization of papain enzyme on a hybrid support containing zinc oxide nanoparticles and chitosan for clinical applications. Carbohydr. Polym. 2020, 243, 116498. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Electrospun fibers and their application in drug controlled release, biological dressings, tissue repair, and enzyme immobilization. RSC Adv. 2019, 9, 25712–25729. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Q.; Huang, F.; Wei, Q. Chapter 26—Electrospun Nanofibers for Enzyme Immobilization. In Electrospinning: Nanofabrication and Applications; Ding, B., Wang, X., Yu, J., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 765–781. [Google Scholar] [CrossRef]
- Shinde, P.; Musameh, M.; Gao, Y.; Robinson, A.J.; Louis, I. Immobilization and stabilization of alcohol dehydrogenase on polyvinyl alcohol fibre. Biotechnol. Rep. 2018, 19, e00260. [Google Scholar] [CrossRef] [PubMed]
- Porto, M.D.A.; dos Santos, J.P.; Hackbart, H.; Bruni, G.P.; Fonseca, L.M.; da Rosa Zavareze, E.; Dias, A.R.G. Immobilization of α-amylase in ultrafine polyvinyl alcohol (PVA) fibers via electrospinning and their stability on different substrates. Int. J. Biol. Macromol. 2019, 126, 834–841. [Google Scholar] [CrossRef]
- Hosseini, A.; Ramezani, S.; Tabibiazar, M.; Mohammadi, M. Immobilization of α-amylase in ethylcellulose electrospun fibers using emulsion-electrospinning method. Carbohydr. Polym. 2021, 278, 118919. [Google Scholar] [CrossRef]
- Saallah, S.; Naim, M.N.; Lenggoro, I.W.; Noriznan, M.; Fitrah, N.; Bakar, A.; Gen, M. Immobilisation of cyclodextrin glucanotransferase into polyvinyl alcohol (PVA) nanofibres via electrospinning. Biotechnol. Rep. 2016, 10, 44–48. [Google Scholar] [CrossRef]
- Temocin, Z.; Inal, M.; Gokgoz, M.; Yigitoglu, M. Immobilization of horseradish peroxidase on electrospun poly(vinyl alcohol)–polyacrylamide blend nanofiber membrane and its use in the conversion. Polym. Bull. 2018, 75, 1843–1865. [Google Scholar] [CrossRef]
- Jankowska, K.; Zdarta, J.; Grzywaczyk, A.; Pinelo, M.; Jesionowski, T. Horseradish peroxidase immobilized onto electrospun fibres and its application in decolourisation of dyes from model sea water. Process Biochem. 2021, 102, 10–21. [Google Scholar] [CrossRef]
- Sarathi, M.; Doraiswamy, N.; Pennathur, G. Enhanced stability of immobilized keratinolytic protease on electrospun nanofibers. Prep. Biochem. Biotechnol. 2019, 49, 695–703. [Google Scholar] [CrossRef]
- Jhuang, J.; Lin, S.; Chen, L.; Lou, S.; Chen, S.; Chen, H. Development of immobilized laccase-based time temperature indicator by electrospinning zein fiber. Food Packag. Shelf Life 2020, 23, 100436. [Google Scholar] [CrossRef]
- Jankowska, K.; Zdarta, J.; Grzywaczyk, A.; Kije, E.; Biadasz, A. Electrospun poly(methyl methacrylate)/polyaniline fibers as a support for laccase immobilisation and use in dye decolourisation. Environ. Res. 2020, 184, 109332. [Google Scholar] [CrossRef]
- Jhuang, J.; Lou, S.; Lin, S.; Hsin, S.; Chen, L.; Chen, H. Immobilizing laccase on electrospun chitosan fiber to prepare time-temperature indicator for food quality monitoring. Innov. Food Sci. Emerg. Technol. 2020, 63, 102370. [Google Scholar] [CrossRef]
- Bayazidi, P.; Almasi, H.; Asl, A.K. Immobilization of lysozyme on bacterial cellulose nanofibers: Characteristics, antimicrobial activity and morphological properties. Int. J. Biol. Macromol. 2017, 107, 2544–2551. [Google Scholar] [CrossRef]
- Moreno-Cortez, I.E.; Romero-García, J.; González-González, V.; Garcia-Gutierrez, D.I.; Garza-Navarro, M.A.; Cruz-Silva, R. Encapsulation and immobilization of papain in electrospun nanofibrous membranes of PVA cross-linked with glutaraldehyde vapor. Mater. Sci. Eng. C 2015, 52, 306–314. [Google Scholar] [CrossRef]
- Labus, K.; Wolanin, K.; Radosinski, L. Comparative study on enzyme immobilization using natural hydrogel matrices—experimental studies. Catalysts 2020, 10, 489. [Google Scholar] [CrossRef]
- Jafary, F.; Panjehpour, M.; Varshosaz, J.; Yaghmaei, P. Stability improvement of immobilized alkaline phosphatase using chitosan. Braz. J. Chem. Eng. 2016, 33, 243–250. [Google Scholar] [CrossRef]
- Manzo, R.M.; Ceruti, R.J.; Bonazza, H.L.; Adriano, W.S.; Sihufe, G.A.; Mammarella, E.J. Immobilization of carboxypeptidase A into modified chitosan matrixes by covalent attachment. Appl. Biochem. Biotechnol. 2018, 185, 1029–1043. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, M.N.; Hu, H.; Wang, W.; Zhang, X. Enhanced bio-catalytic performance and dye degradation potential of chitosan-encapsulated horseradish peroxidase in a packed bed reactor system. Sci. Total Environ. 2017, 575, 1352–1360. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Iqbal, H.M.N.; Hu, H.; Wang, W.; Zhang, X. Horseradish peroxidase immobilization by copolymerization into cross-linked polyacrylamide gel and its dye degradation and detoxification potential. Int. J. Biol. Macromol. 2018, 113, 983–990. [Google Scholar] [CrossRef]
- Morales Urrea, D.A.; Fernández Gimenez, A.V.; Rodriguez, Y.E.; Contreras, E.M. Immobilization of horseradish peroxidase in Ca-alginate beads: Evaluation of the enzyme leakage on the overall removal of an azo-dye and mathematical modeling. Process Saf. Environ. Prot. 2021, 156, 134–143. [Google Scholar] [CrossRef]
- Vassiliadi, E.; Xenakis, A.; Zoumpanioti, M. Chitosan hydrogels: A new and simple matrix for lipase catalysed biosyntheses. Mol. Catal. 2018, 445, 206–212. [Google Scholar] [CrossRef]
- Asgher, M.; Noreen, S.; Bilal, M. Enhancement of catalytic, reusability, and long-term stability features of Trametes versicolor IBL-04 laccase immobilized on different polymers. Int. J. Biol. Macromol. 2017, 95, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Popović, N.; Stanišić, M.; Ilić Đurđić, K.; Prodanović, O.; Polović, N.; Prodanović, R. Dopamine-modified pectin for a Streptomyces cyaneus laccase induced microbeads formation, immobilization, and textile dyes decolorization. Environ. Technol. Innov. 2021, 22, 101399. [Google Scholar] [CrossRef]
- Bonazza, H.L.; Manzo, R.M.; Santos, J.C.S.; Mammarella, E.J. Operational and thermal stability analysis of Thermomyces lanuginosus lipase covalently immobilized onto modified chitosan supports. Appl. Biochem. Biotechnol. 2017, 184, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Helal, S.E.; Abdelhady, H.M.; Abou-Taleb, K.A.; Hassan, M.G.; Amer, M.M. Lipase from Rhizopus oryzae R1: In-depth characterization, immobilization, and evaluation in biodiesel production. J. Genet. Eng. Biotechnol. 2021, 19. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.L.; da Silva, O.S.; Converti, A.; Porto, T.S. Thermodynamic and kinetic studies on pectinase extracted from Aspergillus aculeatus: Free and immobilized enzyme entrapped in alginate beads. Int. J. Biol. Macromol. 2018, 115, 1088–1093. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, F.; Zhou, B.; Li, J.; Li, B.; Liang, H. Immobilization of pectinases into calcium alginate microspheres for fruit juice application. Food Hydrocoll. 2019, 89, 691–699. [Google Scholar] [CrossRef]
- Rehman, H.U.; Aman, A.; Nawaz, M.A.; Karim, A.; Ghani, M.; Baloch, A.H.; Qader, S.A.U. Immobilization of pectin depolymerising polygalacturonase using different polymers. Int. J. Biol. Macromol. 2016, 82, 127–133. [Google Scholar] [CrossRef]
- Awad, G.E.A.; Ghanem, A.F.; Abdel, W.A.; Wahba, M.I. Functionalized κ -carrageenan/hyperbranched poly(amidoamine) for protease immobilization: Thermodynamics and stability studies. Int. J. Biol. Macromol. 2020, 148, 1140–1155. [Google Scholar] [CrossRef]
- Dhiman, S.; Srivastava, B.; Singh, G.; Khatri, M.; Arya, S.K. Immobilization of mannase on sodium alginate-grafted-β-cyclodextrin: An easy and cost effective approach for the improvement of enzyme properties. Int. J. Biol. Macromol. 2019, 156, 1347–1358. [Google Scholar] [CrossRef]
- Ghiorghita, C.A.; Bucatariu, F.; Dragan, E.S. Influence of cross-linking in loading/release applications of polyelectrolyte multilayer assemblies. A review. Mater. Sci. Eng. C 2019, 105, 110050. [Google Scholar] [CrossRef]
- Bucatariu, F.; Petrila, L.-M.; Teodosiu, C.; Mihai, M. Versatile nanostructured SiO2/cross-linked polyelectrolyte composites for emerging pollutants removal from aqueous media. C. R. Chim. 2021, 25, 95–108. [Google Scholar] [CrossRef]
- Işık, M. High Stability of immobilized acetylcholinesterase on chitosan beads. ChemistrySelect 2020, 5, 4623–4627. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Li, X.; Lee, B.S.; Jung, S.; Lee, M. Enhancing the thermo-stability and anti-biofilm activity of alginate lyase by immobilization on low molecular weight chitosan nanoparticles. Int. J. Mol. Sci. 2019, 20, 4565. [Google Scholar] [CrossRef]
- Leonida, M.; Belbekhouche, S.; Adams, F.; Kiran, U.; Choudhary, D.; Kumar, I. Enzyme nanovehicles: Histaminase and catalase delivered in nanoparticulate chitosan. Int. J. Pharm. 2019, 557, 145–153. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Lau, Y.S.; Yang, K. Entrapment of cross-linked cellulase colloids in alginate beads for hydrolysis of cellulose. Colloids Surf. B 2016, 145, 862–869. [Google Scholar] [CrossRef]
- Rakmai, J.; Cheirsilp, B.; Prasertsan, P. Enhanced thermal stability of cyclodextrin glycosyltransferase in alginate–gelatin mixed gel beads and the application for β-cyclodextrin production. Biocatal. Agric. Biotechnol. 2015, 4, 717–726. [Google Scholar] [CrossRef]
- Li, J.; Feng, T.; Han, L.; Zhang, M.; Jiang, T. Fabricating biopolymer-inorganic hybrid microspheres for enzyme immobilization: Connect membrane emulsification with biomimetic mineralization. Particuology 2021, 64, 171–177. [Google Scholar] [CrossRef]
- Melo, M.N.; Pereira, F.M.; Rocha, M.A.; Ribeiro, J.G.; Diz, F.M.; Monteiro, W.F.; Ligabue, R.A.; Severino, P.; Fricks, A.T. Immobilization and characterization of horseradish peroxidase into chitosan and chitosan/PEG nanoparticles: A comparative study. Process Biochem. 2020, 98, 160–171. [Google Scholar] [CrossRef]
- Singh, R.S.; Singh, R.P.; Kennedy, J.F. Immobilization of yeast inulinase on chitosan beads for the hydrolysis of inulin in a batch system. Int. J. Biol. Macromol. 2016, 95, 87–93. [Google Scholar] [CrossRef]
- Sun, H.; Wei, Y.; Zheng, X.; Jiang, X. Preparation of uniform polyurea microspheres at high yield by precipitation polymerization and their use for laccase immobilization. Polymer 2021, 216, 123432. [Google Scholar] [CrossRef]
- Traffano Schiffo, M.V.V.; Aguirre Calvo, T.R.; Castro-Giraldez, M.; Fito, P.J.; Santagapita, P.R. Alginate beads containing lactase: Stability and microstructure. Biomacromolecules 2017, 18, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, L.I.R.; Possebom, G.; Alvez Valandro, E.; Cansian, R.L.; Paroul, N.; de Oliveira, D.; Treichel, H. Application of home-made lipase in the production of geranyl propionate by esterification of geraniol and propionic acid in solvent-free system. Biocatal. Agric. Biotechnol. 2015, 4, 44–48. [Google Scholar] [CrossRef]
- Sanchez, D.A.; Tonetto, G.M.; Ferreira, M.L. Screening of lipases with unusual high activity in the sn-2 esterification of 1,3-dicaprin under mild operating conditions. J. Agric. Food Chem. 2017, 65, 5010–5017. [Google Scholar] [CrossRef] [PubMed]
- Akil, E.; Pereira, A.S.; El-Bacha, T.; Amaral, F.F.; Torres, A.G. Efficient production of bioactive structured lipids by fast acidolysis catalyzed by Yarrowia lipolytica lipase, free and immobilized in chitosan-alginate beads, in solvent-free medium. Int. J. Biol. Macromol. 2020, 163, 910–918. [Google Scholar] [CrossRef]
- Ozaltin, K.; Postnikov, P.S.; Trusova, M.E.; Sedlarik, V.; Di, A. Polysaccharides based microspheres for multiple encapsulations and simultaneous release of proteases. Int. J. Biol. Macromol. 2019, 132, 24–31. [Google Scholar] [CrossRef]
- Petrila, L.-M.; Bucatariu, F.; Mihai, M.; Teodosiu, C. Polyelectrolyte multilayers: An overview on fabrication, properties, and biomedical and environmental applications. Materials 2021, 14, 4152. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Iqbal, H.M.N. Magnetic nanoparticles as versatile carriers for enzymes immobilization: A review. Int. J. Biol. Macromol. 2018, 120, 2530–2544. [Google Scholar] [CrossRef]
- Bedade, D.K.; Sutar, Y.B.; Singhal, R.S. Chitosan coated calcium alginate beads for covalent immobilization of acrylamidase: Process parameters and removal of acrylamide from coffee. Food Chem. 2019, 275, 95–104. [Google Scholar] [CrossRef]
- Raghu, S.; Pennathur, G. Enhancing the stability of a carboxylesterase by entrapment in chitosan coated alginate beads. Turk. J. Biol. 2018, 42, 307–318. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, W. Preparation of chitosan-based nanoparticles for enzyme immobilization. Int. J. Biol. Macromol. 2019, 126, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Tizchang, S.; Khiabani, M.S.; Mokarram, R.R.; Hamishehkar, H. Bacterial cellulose nano crystal as hydrocolloid matrix in immobilized β-galactosidase onto silicon dioxide nanoparticles. LWT 2020, 123, 109091. [Google Scholar] [CrossRef]
- Mehandia, S.; Ahmad, S.; Sharma, S.C.; Arya, S.K. Decolorization and detoxification of textile effluent by immobilized laccase-ACS into chitosan-clay composite beads using a packed bed reactor system: An ecofriendly approach. J. Water Process Eng. 2022, 47, 102662. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Lima, P.J.M.; Pinheiro, B.B.; Freire, T.M.; Dutra, L.M.U.; Fechine, P.B.A.; Goncalves, L.R.B.; de Souza, M.C.M.; dos Santos, J.C.S.; Fernandez-Lafuente, R. Immobilization of lipase A from Candida antarctica onto chitosan-coated magnetic nanoparticles. Int. J. Mol. Sci. 2019, 20, 4018. [Google Scholar] [CrossRef]
- Hou, C.; Wang, Y.; Zhu, H.; Wei, H. Construction of enzyme immobilization system through metal-polyphenol assisted Fe3O4 chitosan hybrid microcapsules. Chem. Eng. J. 2016, 283, 397–403. [Google Scholar] [CrossRef]
- Sikora, A.; Chełminiak-Dudkiewicz, D.; Siódmiak, T.; Tarczykowska, A.; Dariusz, W.; Ziegler-Borowska, M.; Piotr, M. Enantioselective acetylation of (R,S)-atenolol: The use of Candida rugosa lipases immobilized onto magnetic chitosan nanoparticles in enzyme-catalyzed biotransformation. J. Molec. Catal. B Enzym. 2016, 134, 43–50. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Wang, L.; Wang, S. Stability and catalytic properties of lipase immobilized on chitosan encapsulated magnetic nanoparticles cross-linked with genipin and glutaraldehyde. J. Chem. Technol. Biotechnol. 2015, 76, 991–996. [Google Scholar] [CrossRef]
- Todea, A.; Hiseni, A.; Otten, L.G.; Arends, I.W.C.E.; Peter, F.; Carmen, G.; Boeriu, C.G. Increase of stability of oleate hydratase by appropriate immobilization technique and conditions. J. Mol. Catal. B Enzym. 2015, 119, 40–47. [Google Scholar] [CrossRef]
- Magro, L.D.; de Moura, K.S.; Backes, B.E.; de Menezes, E.W.; Benvenutti, E.V.; Nicolodi, S.; Klein, M.P.; Fernandez-Lafuente, R.; Rodrigues, R.C. Immobilization of pectinase on chitosan-magnetic particles: Influence of particle preparation protocol on enzyme properties for fruit juice clarification. Biotechnol. Rep. 2019, 24, e00373. [Google Scholar] [CrossRef]
- De Villiers, M.M.; Otto, D.P.; Strydom, S.J.; Lvov, Y.M. Introduction to nanocoatings produced by layer-by-layer (LbL) self-assembly. Adv. Drug Deliv. Rev. 2011, 63, 701–715. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Tang, N.; Cheng, P.; Xiang, J.; Du, W.; Wang, X. Bioinspired stability improvement of layer-by-layer microcapsules using a bioadhesive for enzyme encapsulation. React. Funct. Polym. 2016, 99, 73–79. [Google Scholar] [CrossRef]
- Sakr, O.S.; Borchard, G. Encapsulation of enzymes in layer-by-layer (LbL) structures: Latest advances and applications. Biomacromolecules 2013, 14, 2117–2135. [Google Scholar] [CrossRef] [PubMed]
- Barsan, M.M.; David, M.; Florescu, M.; Țugulea, L.; Brett, C.M.A. A new self-assembled layer-by-layer glucose biosensor based on chitosan biopolymer entrapped enzyme with nitrogen doped graphene. Bioelectrochemistry 2014, 99, 46–52. [Google Scholar] [CrossRef]
- Perazzini, R.; Saladino, R.; Guazzaroni, M.; Crestini, C. A novel and efficient oxidative functionalization of lignin by layer-by-layer immobilised horseradish peroxidase. Bioorg. Med. Chem. 2011, 19, 440–447. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Wang, X.S.; Qin, X.; Chen, Q.; Anzai, J. Enzyme microcapsules with substrate selective permeability constructed via layer-by-layer polyelectrolyte self-assembly. Mater. Sci. Eng. C 2012, 32, 569–573. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Rios, N.S.; Mendez-Sanchez, C.; Lokha, Y.; Carballares, D.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Coimmobilization of different lipases: Simple layer by layer enzyme spatial ordering. Int. J. Biol. Macromol. 2020, 145, 856–864. [Google Scholar] [CrossRef]
- Ghiorghita, C.A.; Bucatariu, F.; Dragan, E.S. Poly(N,N-dimethylamino) ethyl methacrylate/sodium alginate multilayers and their interaction with proteins/enzymes. Int. J. Biol. Macromol. 2018, 107, 1584–1590. [Google Scholar] [CrossRef]
- Bucatariu, F.; Ghiorghita, C.A.; Simon, F.; Bellmann, C.; Dragan, E.S. Poly(ethyleneimine) cross-linked multilayers deposited onto solid surfaces and enzyme immobilization as a function of the film properties. Appl. Surf. Sci. 2013, 280, 812–819. [Google Scholar] [CrossRef]
- Bucatariu, F.; Ghiorghita, C.A.; Cocarta, A.I.; Dragan, E.S. Cross-linked multilayer-dye films deposited onto silica surfaces with high affinity for pepsin. Appl. Surf. Sci. 2016, 390, 320–327. [Google Scholar] [CrossRef]
- Amorosi, C.; Michel, M.; Avérous, L.; Toniazzo, V.; Ruch, D.; Ball, V. Plasma polymer films as an alternative to (PSS-PAH)n or (PSS-PDADMAC)n films to retain active enzymes in exponentially growing polyelectrolyte multilayers. Colloids Surf. B 2012, 97, 124–131. [Google Scholar] [CrossRef]
- Benucci, I.; Liburdi, K.; Cacciotti, I.; Lombardelli, C.; Zappino, M.; Nanni, F.; Esti, M. Chitosan/clay nanocomposite films as supports for enzyme immobilization: An innovative green approach for winemaking applications. Food Hydrocoll. 2018, 74, 124–131. [Google Scholar] [CrossRef]
- He, L.; Lan, W.; Cen, L.; Chen, S.; Liu, S.; Liu, Y.; Ao, X.; Yang, Y. Improving catalase stability by its immobilization on grass carp (Ctenopharyngodon idella) scale collagen self-assembly films. Mater. Sci. Eng. C 2019, 105, 110024. [Google Scholar] [CrossRef] [PubMed]
- Hosu, O.; Lettieri, M.; Papara, N.; Ravalli, A.; Sandulescu, R.; Cristea, C.; Marrazza, G. Colorimetric multienzymatic smart sensors for hydrogen peroxide, glucose and catechol screening analysis. Talanta 2019, 204, 525–532. [Google Scholar] [CrossRef]
- Homma, T.; Kondo, M.; Kuwahara, T.; Shimomura, M. Polyaniline/poly(acrylic acid) composite film: A promising material for enzyme-aided electrochemical sensors. Eur. Polym. J. 2015, 62, 139–144. [Google Scholar] [CrossRef]
- Zhu, Y.; Qiu, F.; Rong, J.; Zhang, T.; Mao, K.; Yang, D. Covalent laccase immobilization on the surface of poly(vinylidene fluoride) polymer membrane for enhanced biocatalytic removal of dyes pollutants from aqueous environment. Colloids Surf. B 2020, 191, 111025. [Google Scholar] [CrossRef]
- Li, S.; Zhao, S.; Hou, Y.; Chen, G.; Chen, Y.; Zhang, Z. Polylactic acid (PLA) modified by polyethylene glycol (PEG) for the immobilization of lipase. Appl. Biochem. Biotechnol. 2020, 190, 982–996. [Google Scholar] [CrossRef]
- Benbow, N.L.; Sebben, D.A.; Karpiniec, S.; Stringer, D.; Krasowska, M.; Beattie, D.A. Lysozyme uptake into pharmaceutical grade fucoidan/chitosan polyelectrolyte multilayers under physiological conditions. J. Colloid Interface Sci. 2020, 565, 555–566. [Google Scholar] [CrossRef]
- Visan, A.; Cristescu, R.; Stefan, N.; Miroiu, M.; Nita, C.; Socol, M.; Florica, C.; Rasoga, O.; Zgura, I.; Sima, L.E.; et al. Antimicrobial polycaprolactone/polyethylene glycol embedded lysozyme coatings of Ti implants for osteoblast functional properties in tissue engineering. Appl. Surf. Sci. 2017, 417, 234–243. [Google Scholar] [CrossRef]
- Fu, J.; Schlenoff, J.B. Driving forces for oppositely charged polyion association in aqueous solutions: Enthalpic, entropic, but not electrostatic. J. Am. Chem. Soc. 2015, 138, 980–990. [Google Scholar] [CrossRef]
- Salehi, A.; Desai, P.S.; Li, J.; Steele, C.A.; Larson, R.G. Relationship between polyelectrolyte bulk complexation and kinetics of their layer-by-layer assembly. Macromolecules 2014, 48, 400–409. [Google Scholar] [CrossRef]
- Moschakis, T.; Biliaderis, C.G. Biopolymer-based coacervates: Structures, functionality and applications in food products. Curr. Opin. Colloid Interface Sci. 2017, 28, 96–109. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, L.; Li, Y.; Zhang, X.; Xu, S.; Yang, G.; Delair, T. Chitosan-based colloidal polyelectrolyte complexes for drug delivery: A review. Carbohydr. Polym. 2020, 238, 116126. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.L.; Li, Y.; Priftis, D.; Leon, L.; Tirrell, M. The effect of salt on the complex coacervation of vinyl polyelectrolytes. Polymers 2014, 6, 1756–1772. [Google Scholar] [CrossRef]
- Karim, Z.; Adnan, R.; Husain, Q. β-cyclodextrine chitosan complex as the immobilization matrix for horseradish peroxidase and its application for the removal of azo dyes from textile effluent. Int. Biodeterior. Biodegrad. 2012, 72, 10–17. [Google Scholar] [CrossRef]
- Zheng, J.; Gao, Q.; Ge, G.; Wu, J.; Tang, C.; Zhao, M. Dynamic equilibrium of β-conglycinin/lysozyme heteroprotein complex coacervates. Food Hydrocoll. 2022, 124, 107339. [Google Scholar] [CrossRef]
- Xu, W.; Jin, W.; Zhang, C.; Li, Z.; Lin, L.; Huang, Q.; Ye, S.; Li, B. Curcumin loaded and protective system based on complex of κ-carrageenan and lysozyme. Food Res. Int. 2014, 59, 61–66. [Google Scholar] [CrossRef]
- Antonov, Y.A.; Moldenaers, P.; Cardinaels, R. Complexation of lysozyme with sodium caseinate and micellar casein in aqueous buffered solutions. Food Hydrocoll. 2017, 62, 102–118. [Google Scholar] [CrossRef]
- Antonov, Y.A.; Zhuravleva, I.L.; Cardinaels, R.; Moldenaers, P. Macromolecular complexes of lysozyme with kappa carrageenan. Food Hydrocoll. 2018, 74, 227–238. [Google Scholar] [CrossRef]
- Kubelbeck, S.; Mikhael, J.; Schoof, S.; Andrieu-Brunsen, A.; Baier, G. Immobilization of α-amylase in polyelectrolyte complexes. J. Appl. Polym. Sci. 2017, 15, 45036. [Google Scholar] [CrossRef]
- Souza, C.J.F.; Garcia-Rojas, E.E.; Souza, C.S.F.; Vriesmann, L.C.; Vicente, J.; De Carvalho, M.G.; Petkowicz, C.L.O.; Favaro-Trindade, C.S. Immobilization of β-galactosidase by complexation: Effect of interaction on the properties of the enzyme. Int. J. Biol. Macromol. 2019, 122, 594–602. [Google Scholar] [CrossRef]
- Souza, C.J.F.; Garcia-Rojas, E.E.; Favaro-Trindade, C.S. Lactase (β -galactosidase) immobilization by complex formation: Impact of biopolymers on enzyme activity. Food Hydrocoll. 2018, 83, 88–96. [Google Scholar] [CrossRef]
- Bucatariu, F.; Teodosiu, C.; Morosanu, I.; Fighir, D.; Ciobanu, R.; Petrila, L.-M.; Mihai, M. An overview on composite sorbents based on polyelectrolytes used in advanced wastewater treatment. Polymers 2021, 13, 3963. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Pinelo, M. Developments in support materials for immobilization of oxidoreductases: A comprehensive review. Adv. Colloid Interface Sci. 2018, 258, 1–20. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M. Sandal reactive dyes decolorization and cytotoxicity reduction using manganese peroxidase immobilized onto polyvinyl alcohol–alginate beads. Chem. Cent. J. 2015, 9, 47. [Google Scholar] [CrossRef]
- Aricov, L.; Leonties, A.R.; Gîfu, I.C.; Preda, D.; Raducan, A.; Anghel, D.-F. Enhancement of laccase immobilization onto wet chitosan microspheres using an iterative protocol and its potential to remove micropollutants. J. Environ. Manag. 2020, 276, 111326. [Google Scholar] [CrossRef]
- Maryskova, M.; Schaabova, M.; Tomankova, H.; Novotny, V.; Rysova, M. Wastewater treatment by novel polyamide/polyethylenimine nanofibers with immobilized laccase. Water 2020, 12, 588. [Google Scholar] [CrossRef]
- Leontieș, A.R.; Răducan, A.; Culiță, D.C.; Alexandrescu, E.; Moroșan, A.; Mihaiescu, D.E.; Aricov, L. Laccase immobilized on chitosan-polyacrylic acid microspheres as highly efficient biocatalyst for naphthol green B and indigo carmine degradation. Chem. Eng. J. 2022, 439, 135654. [Google Scholar] [CrossRef]
- Vera, M.; Nyanhongo, G.S.; Guebitz, G.M. Polymeric microspheres as support to co-immobilized Agaricus bisporus and Trametes versicolor laccases and their application in diazinon degradation. Arab. J. Chem. 2019, 13, 4218–4227. [Google Scholar] [CrossRef]
- Lassouane, F.; Aït-Amar, H.; Amrani, S.; Rodriguez-Couto, S. A promising laccase immobilization approach for Bisphenol A removal from aqueous solutions. Bioresour. Technol. 2018, 271, 360–367. [Google Scholar] [CrossRef]
- Pereira Cipolatti, E.; Valério, A.; Henriques, R.O.; Pinto, M.C.C.; Fernandez Lorente, G.; Manoel, E.A.; Guisan, J.M.; Ninow, J.L.; de Oliveira, D.; Costa Pessela, B. Production of new nanobiocatalysts via immobilization of lipase B from C. antarctica on polyurethane nanosupports for application on food and pharmaceutical industries. Int. J. Biol. Macromol. 2020, 165, 2957–2963. [Google Scholar] [CrossRef]
- Pan, J.; Ou, Z.; Tang, L.; Shi, H. Enhancement of catalytic activity of lipase-immobilized Fe3O4 -chitosan microsphere for enantioselective acetylation of racemic 1-phenylethylamine. Korean J. Chem. Eng. 2019, 36, 729–739. [Google Scholar] [CrossRef]
- Melo, A.D.Q.; Silva, F.F.M.; dos Santos, J.C.S.; Fernandez-Lafuente, R.; Lemos, T.L.G.; Dias Filho, F.A. Synthesis of benzyl acetate catalyzed by lipase immobilized in nontoxic chitosan-polyphosphate beads. Molecules 2017, 22, 2165. [Google Scholar] [CrossRef]
- Tudorache, M.; Gheorghe, A.; Negoi, A.; Enache, M.; Maria, G.-M.; Parvulescu, V. Bifunctional carbohydrate biopolymers entrapped lipase as catalyst for the two consecutive conversions of α-pinene to oxy-derivative. Carbohydr. Polym. 2016, 152, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Enzyme immobilized nanomaterials as electrochemical biosensors for detection of biomolecules. Enzyme Microb. Technol. 2022, 156, 110006. [Google Scholar] [CrossRef] [PubMed]
- Tutunaru, O.; Mihailescu, C.M.; Savin, M.; Tincu, B.C.; Stoian, M.C.; Muscalu, G.S.; Firtat, B.; Dinulescu, S.; Craciun, G.; Moldovan, C.A.; et al. Acetylcholinesterase entrapment onto carboxyl-modified single-walled carbon nanotubes and poly(3,4-ethylenedioxythiophene) nanocomposite film, electrosynthesis characterization, and sensor application for dichlorvos detection in apple juice. Microchem. J. 2021, 169, 106573. [Google Scholar] [CrossRef]
- Cano-Raya, C.; Dencheva, N.V.; Braz, J.F.; Malfois, M.; Denchev, Z.Z. Optical biosensor for catechol determination based on laccase-immobilized anionic polyamide 6 microparticles. J. Appl. Polym. Sci. 2020, 137, e49131. [Google Scholar] [CrossRef]
- Aldea, A.; Leote, R.J.B.; Matei, E.; Evanghelidis, A.; Enculescu, I.; Diculescu, V.C. Gold coated electrospun polymeric fibers as new electrode platform for glucose oxidase immobilization. Microchem. J. 2021, 165, 106108. [Google Scholar] [CrossRef]
- Yuan, M.; Dai, F.; Li, D.; Fan, Y.; Xiang, W.; Tao, F.; Cheng, Y.; Deng, H. Lysozyme/collagen multilayers layer-by-layer deposited nanofibers with enhanced biocompatibility and antibacterial activity. Mater. Sci. Eng. C 2020, 112, 110868. [Google Scholar] [CrossRef]
- Baidamshina, D.R.; Koroleva, V.A.; Olshannikova, S.S.; Trizna, E.Y.; Bogachev, M.I.; Artyukhov, V.G.; Holyavka, M.G.; Kayumov, A.R. Biochemical properties and anti-biofilm activity of chitosan-immobilized papain. Mar. Drugs 2021, 19, 197. [Google Scholar] [CrossRef]
- Filho Moreira, R.N.F.; Vasconcelos, N.F.; Andrade, F.K.; de Freitas Rosa, M.; Silveira Vieira, R. Papain immobilized on alginate membrane for wound dressing application. Colloids Surf. B 2020, 194, 111222. [Google Scholar] [CrossRef]
- Teske, M.; Kießlich, T.; Fischer, J.; Bahl, H.; Wulf, K. Immobilizing hydrolytic active papain on biodegradable PLLA for biofilm inhibition in cardiovascular applications. Curr. Dir. Biomed. Eng. 2020, 6, 6–9. [Google Scholar] [CrossRef]
- Sklyarenko, A.V.; El, M.A.; Kurochkina, V.B.; Yarotsky, S.V. Enzymatic synthesis of β-lactam acids (Review). Appl. Biochem. Microbiol. 2015, 51, 627–640. [Google Scholar] [CrossRef]
- Mohammadi, M.; Habibi, Z.; Gandomkar, S.; Yousefi, M. A novel approach for bioconjugation of Rhizomucor miehei lipase (RML) onto amine-functionalized supports; Application for enantioselective resolution of rac-ibuprofen. Int. J. Biol. Macromol. 2018, 117, 523–531. [Google Scholar] [CrossRef]
- Gilani, S.L.; Najafpour, G.D.; Heydarzadeh, H.D.; Moghadamnia, A. Enantioselective synthesis of (S)-naproxen using immobilized lipase on chitosan beads. Chirality 2017, 29, 304–314. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Iqbal, H.M.N.; Hu, H. Gelatin-immobilized manganese peroxidase with novel catalytic characteristics and its industrial exploitation for fruit juice clarification purposes. Catal. Lett. 2016, 146, 2221–2228. [Google Scholar] [CrossRef]
- Irshad, M.; Murtza, A.; Zafar, M.; Bhatti, K.H.; Rehman, A.; Anwar, Z. Chitosan-immobilized pectinolytics with novel catalytic features and fruit juice clarification potentialities. Int. J. Biol. Macromol. 2017, 104, 242–250. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Cacciotti, I.; Esti, M. Papain covalently immobilized on chitosan–clay nanocomposite films: Application in synthetic and real white wine. Nanomaterials 2020, 10, 1622. [Google Scholar] [CrossRef]
- Fernández-Pacheco, P.; García-Béjar, B.; Briones Pérez, A.; Arévalo-Villena, M. Free and immobilised β-glucosidases in oenology: Biotechnological characterisation and its effect on enhancement of wine aroma. Front. Microbiol. 2021, 12, 723815. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, C.; Zou, Y.; Li, Y.; Zhang, H. Immobilization of lysozyme on layer-by-layer self-assembled electrospun films: Characterization and antibacterial activity in milk. Food Hydrocoll. 2021, 113, 106468. [Google Scholar] [CrossRef]


| Enzyme | Polymer | Immobilization Method | Observations | Reference |
|---|---|---|---|---|
| Alcohol dehydrogenase | polystyrene poly(D,L-lactide-co-glycolide) | Covalent bonding | Stable for 7 catalytic cycles, maintaining ~20% of the enzymatic activity | [5] |
| Poly(vinyl alcohol) (PVA) | Covalent bonding | Stable for 8 cycles of reaction, maintaining 60% of the initial enzymatic activity | [27] | |
| α-Amylase | PVA | Entrapment | stable at 80 °C, maintaining 80% of the enzymatic activity at pH = 8 | [28] |
| Ethyl cellulose | entrapment | stable for 15 catalytic cycles, maintaining 50% of the enzymatic activity | [29] | |
| Cyclodextrin— glucan transferase | PVA | Covalent bonding | A 31% increase in the enzymatic activity compared with the control sample | [30] |
| Horseradish peroxidase | PVA PAM | Entrapment | Stable for 25 catalytic cycles, maintaining 54% of the enzymatic activity | [31] |
| Polyamide | Adsorption covalent bonding | Capable of degrading 70% of the targeted dyes (Reactive Black 5 and malachite green) | [32] | |
| Keratinolytic protease | PVA | Covalent bonding | 88% efficiency in the degradation of chicken feathers | [33] |
| Laccase | Zein | Covalent bonding | Used as a time–temperature indicator for food quality control | [34] |
| Poly(methyl methacrylate) (PMMA) Polyaniline | Adsorption Covalent bonding | Stable for 10 catalytic cycles maintaining 80% of the enzymatic activity | [35] | |
| CHI PVA | Covalent bonding | Used as a time-temperature indicator for food samples | [36] | |
| Polystyrene Poly(D,L-lactide-co-glycolide) | Covalent bonding | Stable for 7 catalytic cycles, maintaining ~20% of the enzymatic activity | [5] | |
| Lysozyme | CHI | Covalent bonding | Stable for 9 reaction cycles, 70% of the enzyme activity is maintained | [37] |
| Adsorption | ||||
| Papain | PVA | Covalent bonding | Stable after 14 days of storage, maintaining 40% of the initial activity | [38] |
| Enzyme | Polymer | Immobilization Method | Observations | Reference |
|---|---|---|---|---|
| Alkaline phosphatase | CHI | Covalent bonding | 71% immobilization yield | [40] |
| Carboxypeptidase A | CHI | Covalent bonding | 86% immobilization yield | [41] |
| Horseradish peroxidase | CHI | Entrapment | ~93% immobilization yield | [42] |
| PAM | Entrapment | able to catalyze the degradation of 90% of the dye sample tested | [43] | |
| ALGCa | Entrapment | Successfully used for the oxidation of azo-dye Orange II | [44] | |
| Laccase | CHI | Entrapment | Stable for 12 catalytic cycles, maintaining 90% of the enzymatic activity | [45] |
| PAM | Entrapment | ~73% maximum immobilization yield | [46] | |
| Agar-agar | Entrapment | ~80% maximum immobilization yield | [46] | |
| Gelatin | Entrapment | ~64% maximum immobilization yield | [46] | |
| Pectin | Entrapment | Removed ~60% of azo dye Amido Black 10B after 10 catalytic cycles | [47] | |
| Lipase | CHI | Covalent bonding | Best results registered for the use of glycidol + ethylenediamine as cross-linking agents | [48] |
| CHI | Adsorption Covalent bonding | 99% immobilization yield for the covalent bonding | [49] |
| Enzyme | Polymer | Immobilization Method | Observations | Reference |
|---|---|---|---|---|
| Acetylcholinesterase | CHI | Covalent bonding | 81% enzymatic activity after 60 days of storage | [57] |
| Alginate lyase | CHI | Covalent bonding | Antibacterial activity against P. aeruginosa | [58] |
| Catalase Diamine oxidase | CHI | Covalent bonding | 100% enzymatic activity after storage for 5 months at −20 °C | [59] |
| Cellulase | ALGNa | Covalent bonding | 67% enzymatic activity after 10 reaction cycles | [60] |
| Cyclodextrin glycosyltransferase | ALGNa gelatin | Entrapment | Can be used for the synthesis of β-cyclodextrin (highest yield of 8.6 g/L) | [61] |
| Glycerol dehydrogenase | ALGNa gelatin | Entrapment | Enzymatic activity was halved after 21 days of storage | [62] |
| Horseradish peroxidase | CHI PEG | Entrapment | Immobilization yield of 65.8% for CHI and 51.7% for CHI/PEG | [63] |
| Inulinase | CHI | Covalent bonding | Suitable to hydrolyze inulin (84.5% at 125 rpm after 4 h) | [64] |
| Laccase | Polyurea | Covalent bonding | Able to degrade Congo red and RBBR dyes | [65] |
| Lactase | ALGNa Guar gum | Covalent bonding | ALG/guar gum/ trehalose exhibiting the highest stability at storage, freezing and freeze/thaw cycles | [66] |
| Lipase | ALGCa | Covalent bonding | 50% enzymatic activity after 4 cycles of reaction | [67] |
| CHI | Adsorption | 33% yield in the synthesis of 1,3-dicaproyl-2-palmitoyl glycerol | [68] | |
| CHI ALG | Entrapment | 100% enzymatic activity after 5 reaction cycles | [69] |
| Enzyme | Polymer | Immobilization Method | Observations | Reference |
|---|---|---|---|---|
| Acrylamidase | ALG CHI | Covalent bonding | Optimal enzymatic activity at pH = 8.5 and 65 °C | [73] |
| Carboxylesterase | ALG CHI | Entrapment | 80% enzymatic activity after storage at 4 °C for 40 days | [74] |
| Glucoamylase | CHI | Covalent bonding | 80% enzymatic activity after 10 cycles of reaction | [75] |
| β-galactosidase | Bacterial cellulose | Covalent bonding | 80% enzymatic activity after 12 cycles of use | [76] |
| Laccase | CHI | Entrapment | 78% decolorization of textile effluent samples | [77] |
| CHI | Covalent bonding adsorption | 84% immobilization yield, 78% decolorization of textile effluent sample | [78] | |
| CHI | Entrapment | More than 70% enzymatic activity after 10 cycles of use | [79] | |
| Lipase | CHI | Covalent bonding | 67% enantioselectivity in the acetylation of racemic atenolol | [80] |
| CHI | Covalent bonding | 95% enzymatic activity after 7 days of storage at 25 °C | [81] | |
| Oleate hydratase | CHI | Covalent bonding | 75% enzymatic activity after 5 cycles of reaction | [82] |
| Enzyme | Polymer | Immobilization Method | Observations | Reference |
|---|---|---|---|---|
| Glucosidase | Polystyrene sulfonate (PSS) Polyallylamine hydrochloride (PAH) | Entrapment | 60% enzymatic activity after 15 reaction cycles | [86] |
| CHI PSS | Adsorption | Used as a biosensor, exhibited high sensitivity and operational stability | [87] | |
| Horseradish peroxidase | PSS PAH | Covalent bonding | 90% enzymatic activity after 10 cycles of reaction | [88] |
| PAH PSS | Entrapment | 90% enzymatic activity after 30 days of storage at 4 °C | [89] | |
| Lipase | PEI | Covalent bonding | The enzymatic activity of the multilayer embedding different types of lipases was around 190 U/g | [90] |
| Lysozyme | PDMAEM ALGNa | Adsorption | The immobilization yield was around 5.5% | [91] |
| PEI | Covalent bonding | The immobilization of lysozyme did not strongly influenced the surface charge of the material | [92] | |
| Pepsin | PEI PMMA | Adsorption | The amount of pepsin immobilized was around 200 mg/g | [93] |
| PEI | Covalent bonding | The amount of pepsin immobilized was around 200 mg/g | [92] | |
| PDMAEMA ALGNa | Adsorption | The amount of pepsin immobilized was around 200 mg/g | [91] |
| Enzyme | Polymer | Immobilization Method | Observations | Reference |
|---|---|---|---|---|
| Alkaline phosphatase | PSS PAH | Adsorption | The manufactured films were able to prevent the enzyme leaching | [94] |
| PSS Poly(diallyldimethylammonium chloride) (PDADMAC) | Adsorption | [94] | ||
| Bromelain | CHI | Covalent bonding | The films exhibited improved mechanical characteristics | [95] |
| Catalase | Collagen | Covalent bonding | ~50% enzymatic activity after 22 cycles of use | [96] |
| Glucose oxidase | Poly(aniline-co-anthranilic acid) | Adsorption | The film was used as a sensor for glucose with a limit of detection of 14 ± 2 μM | [97] |
| Glucosidase | Polyaniline PAA | Covalent bonding | The film was used as a glucose sensor | [98] |
| Horseradish peroxidase | Poly(aniline-co-anthranilic acid) | Adsorption | The film was used as a sensor for glucose with a limit of detection of 14 ± 2 μM | [97] |
| Laccase | Poly(vinylidene fluoride) | Covalent bonding | The composite film was able to retain 97.1% of Congo red | [99] |
| Lipase | Polylactic acid Polyethylene glycol (PEG) | Covalent bonding | 70% enzymatic activity after 30 days of storage | [100] |
| Lysozyme | CHI | Entrapment | The amount of enzyme immobilized was dependent on the immobilization time | [101] |
| PAH PAA Poly(2-hydroxyethyl methacrylate)-g-poly(acrylic acid) | Adsorption | The immobilization of the enzyme leads to a 400% increase in the film thickness | [15] | |
| Polycaprolactone PEG | Entrapment | The film exhibited antimicrobial activity against Escherichia coli, Bacillus subtilis, Enterococcus faecalis, and Staphylococcus aureus strains | [102] |
| Enzyme | Polymer | Immobilization Method | Observations | Reference |
|---|---|---|---|---|
| Glucose oxidase | CHI carrageenan | Adsorption | 80.2% enzymatic activity in pH = 1.2 solution, 73.3% in chitosanase solution and 66.4% in pepsin solution | [13] |
| Horseradish peroxidase | CHI β-cyclodextrin | Adsorption | Able to completely degrade textile dyes after 15 days of operation | [108] |
| Lysozyme | β-conglycinin | Adsorption | The amount of enzyme immobilized was strongly influenced by the presence of NaCl | [109] |
| κ-carrageenan | Adsorption | The microparticles were able to entrap 70% curcumin | [110] | |
| Sodium caseinate | Adsorption | Stable complexes at pH~11 | [111] | |
| κ-carrageenan | Adsorption | Stable complexes at pH~11 | [112] | |
| A-amylase | λ-carrageenan | Adsorption | 70% of enzyme activity after exposure to pH = 3 for 1 h | [12] |
| Pectin | Adsorption | Unstable at pH < 3 | [12] | |
| PEI PAA | Adsorption | The enzymatic activity after immobilization was around 80% | [113] | |
| β-galactosidase | ALGNa | Adsorption | Maximum enzymatic activity at pH = 5 | [114] |
| ALGNa Ɩ-carrageenan | Adsorption | Maximum enzymatic activity at pH = 7 | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrila, L.-M.; Grădinaru, V.R.; Bucatariu, F.; Mihai, M. Polymer/Enzyme Composite Materials—Versatile Catalysts with Multiple Applications. Chemistry 2022, 4, 1312-1338. https://doi.org/10.3390/chemistry4040087
Petrila L-M, Grădinaru VR, Bucatariu F, Mihai M. Polymer/Enzyme Composite Materials—Versatile Catalysts with Multiple Applications. Chemistry. 2022; 4(4):1312-1338. https://doi.org/10.3390/chemistry4040087
Chicago/Turabian StylePetrila, Larisa-Maria, Vasile Robert Grădinaru, Florin Bucatariu, and Marcela Mihai. 2022. "Polymer/Enzyme Composite Materials—Versatile Catalysts with Multiple Applications" Chemistry 4, no. 4: 1312-1338. https://doi.org/10.3390/chemistry4040087
APA StylePetrila, L.-M., Grădinaru, V. R., Bucatariu, F., & Mihai, M. (2022). Polymer/Enzyme Composite Materials—Versatile Catalysts with Multiple Applications. Chemistry, 4(4), 1312-1338. https://doi.org/10.3390/chemistry4040087










