The Influence of Al Doping on the Optical Characteristics of ZnO Nanopowders Obtained by the Low-Cost Sol-Gel Method
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Temperature Effect on Crystallization of ZnO and Doping Effect on Crystallization of AZO Nanopowder
3.2. UV-VIS Spectroscopy Analysis
3.2.1. UV-Visible Characterization of Pure ZnO
3.2.2. UV-Visible Characterization of AZO
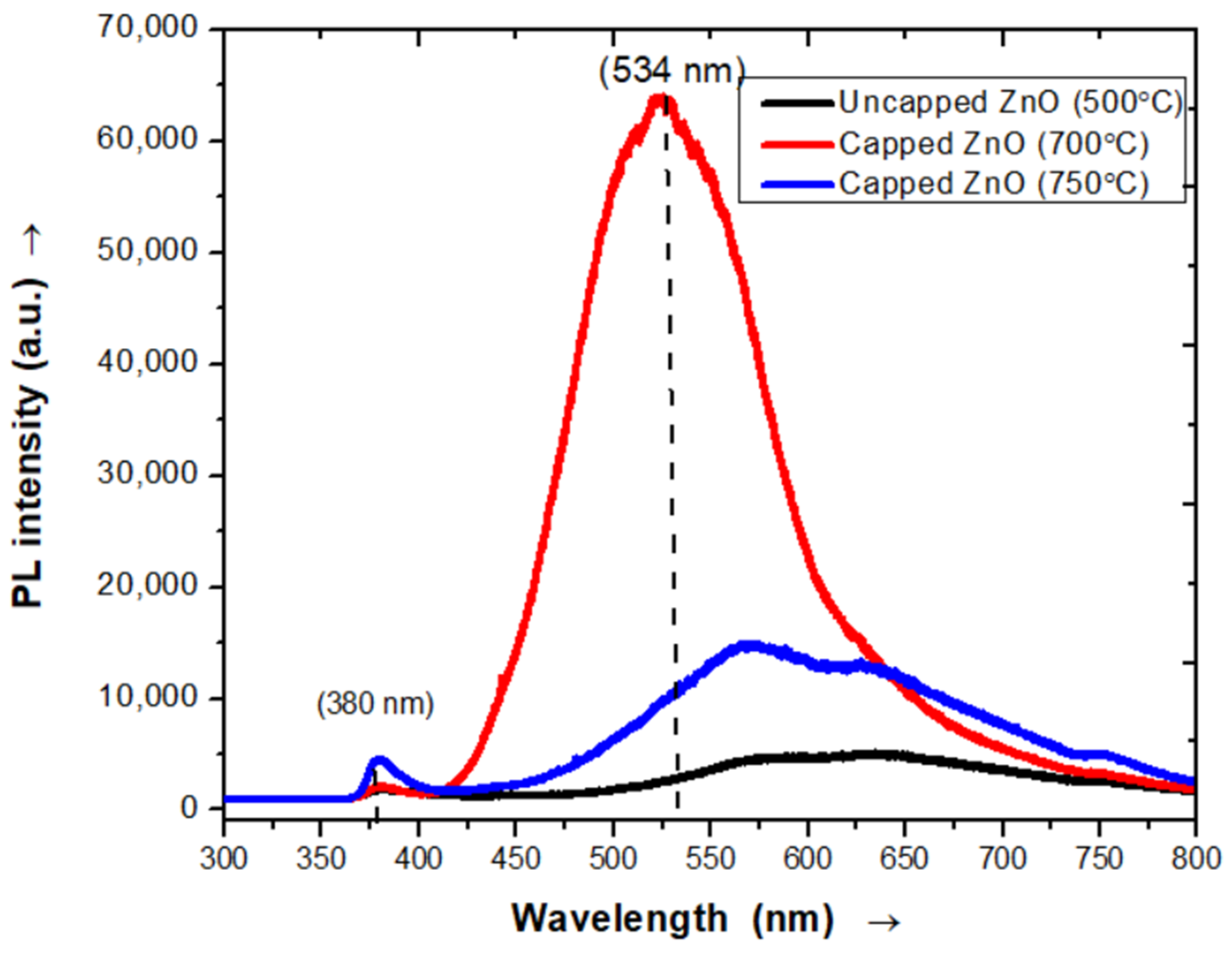
3.3. Photoluminescence Spectrum Analysis
3.3.1. Photoluminescence Spectra of Pure ZnO Nanopowder
3.3.2. Photoluminescence Spectra of AZO
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ZnO | Zinc oxide |
| AZO | Aluminum doped zinc oxide |
| XRD | X-ray diffraction |
| UV | Ultraviolet characterization |
| PL | Photoluminescence |
| NBE | Near band-edge |
| UV-VIS | Ultraviolet-visible |
References
- Shrisha, B.V.; Bhat, S.; Kushavah, D.; Gopalakrishna Naik, K. Hydrothermal growth and characterization of Al-doped ZnO nanorods. Mater. Today Proc. 2016, 3, 1693–1701. [Google Scholar] [CrossRef]
- Klingshirn, C.F.; Waag, A.; Hoffmann, A.; Geurts, J. Zinc oxide: From fundamental properties towards novel applications. SS Mater. 2010, 120, 233–266. [Google Scholar]
- Nurfani, E.; Lailani, A.; Kesuma, W.; Anrokhi, M.; Kadja, G.; Rozana, M. UV sensitivity enhancement in Fe-doped ZnO films grown by ultrafast spray pyrolysis. Opt. Mater. 2021, 112, 110768. [Google Scholar] [CrossRef]
- Shahedi, Z.; Jafari, M.R. Synthesis Al complex and investigating effect of doped ZnO nanoparticles in the electrical and optical efficiency of OLEDS. Appl. Phys. A 2017, 123, 98. [Google Scholar] [CrossRef]
- Varudkar, H.; Umadevi, G.; Nagaraju, P.; Dargad, J.; Mote, V. Fabrication of Al-doped ZnO nanoparticles and their application as a semiconductor-based gas sensor for the detection of ammonia. J. Mater. Sci. Mater. Electron. 2020, 31, 12579–12585. [Google Scholar] [CrossRef]
- Raghu, P.; Srinatha, N.; Naveen, C.; Mahesh, H.; Angadi, B. Investigation on the effect of Al concentration on the structural, optical and electrical properties of spin coated Al: ZnO thin films. J. Alloys Compd. 2017, 694, 68–75. [Google Scholar] [CrossRef]
- Li, Y.; Liao, C.; Tjong, S.C. Recent advances in zinc oxide nanostructures with antimicrobial activities. Int. J. Mol. Sci. 2020, 21, 8836. [Google Scholar] [CrossRef] [PubMed]
- Oprea, O.; Vasile, O.; Voicu, G.; Andronescu, E. The influence of the thermal treatment on luminescence properties of ZnO. Dig. J. Nanomater. Biostructures (DJNB) 2013, 8, 747–756. [Google Scholar]
- Chang, J.S.; Phuan, Y.W.; Chong, M.N.; Ocon, J.D. Exploration of a novel Type II 1D-ZnO nanorods/BiVO4 heterojunction photocatalyst for water depollution. J. Ind. Eng. Chem. 2020, 83, 303–314. [Google Scholar] [CrossRef]
- Chen, L.-C.; Tien, C.-H. The influence of hydrogen gas treatment on the characteristics of ZnO films. Open Crystallogr. J. 2009, 2, 11–14. [Google Scholar] [CrossRef]
- Tonto, P.; Mekasuwandumrong, O.; Phatanasri, S.; Pavarajarn, V.; Praserthdam, P. Preparation of ZnO nanorod by solvothermal reaction of zinc acetate in various alcohols. Ceram. Int. 2008, 34, 57–62. [Google Scholar] [CrossRef]
- Chang, J.S.; Strunk, J.; Chong, M.N.; Poh, P.E.; Ocon, J.D. Multi-dimensional zinc oxide (ZnO) nanoarchitectures as efficient photocatalysts: What is the fundamental factor that determines photoactivity in ZnO? J. Hazard. Mater. 2020, 381, 120958. [Google Scholar] [CrossRef]
- Al Asmar, R.; Atanas, J.; Ajaka, M.; Zaatar, Y.; Ferblantier, G.; Sauvajol, J.; Jabbour, J.; Juillaget, S.; Foucaran, A. Characterization and Raman investigations on high-quality ZnO thin films fabricated by reactive electron beam evaporation technique. J. Cryst. Growth 2005, 279, 394–402. [Google Scholar] [CrossRef]
- Oprea, O.; Vasile, O.; Voicu, G.; Craciun, L.; Andronescu, E. Photoluminescence, Magnetic Properties and Photocatalytic Activity of Gd 3+ Doped Zno Nanoparticles. Dig. J. Nanomater. Biostructures (DJNB) 2012, 7, 1757–1766. [Google Scholar]
- Singh, N.; Kaur, D.; M Mehra, R.; Kapoor, A. Effect of ageing in structural properties of ZnO nanoparticles with ph variation for application in solar cells. Open Renew. Energy J. 2012, 5, 15–18. [Google Scholar] [CrossRef]
- Wu, J.J.; Liu, S.C. Low-temperature growth of well-aligned ZnO nanorods by chemical vapor deposition. Adv. Mater. 2002, 14, 215–218. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Hydrothermal growth of ZnO nanostructures. Sci. Technol. Adv. Mater. 2009, 10, 013001. [Google Scholar] [CrossRef]
- Voicu, G.; Oprea, O.; Vasile, B.; Andronescu, E. Photoluminescence and photocatalytic activity of Mn-doped Zno nanoparticles. Dig. J. Nanomater. Biostructures (DJNB) 2013, 8, 667–675. [Google Scholar]
- Wahab, R.; Ansari, S.; Kim, Y.S.; Dar, M.; Shin, H.-S. Synthesis and characterization of hydrozincite and its conversion into zinc oxide nanoparticles. J. Alloys Compd. 2008, 461, 66–71. [Google Scholar] [CrossRef]
- Sharma, A.; Pathak, D.; Patil, D.S.; Dhiman, N.; Bhullar, V.; Mahajan, A. Electrospun PVP/TiO2 nanofibers for filtration and possible protection from various viruses like COVID-19. Technol. 2021, 9, 89. [Google Scholar] [CrossRef]
- Ghrib, T.; Massoudi, I.; Al-Otaibi, A.L.; Al-Malki, A.; Kharma, A.; Al-Hashem, E.; Al-Ghamdi, R.A.; Al-Zuraie, R.A. Effects of Terbium Doping on Structural, Optical and Photocatalytic Properties of ZnO Nanopowder Prepared by Solid-State Reaction. J. Inorg. Organomet. Polym. Mater. 2021, 31, 239–250. [Google Scholar] [CrossRef]
- He, H.; Yang, Q.; Wang, J.; Ye, Z. Layer-structured ZnO nanowire arrays with dominant surface-and acceptor-related emissions. Mater. Lett. 2011, 65, 1351–1354. [Google Scholar] [CrossRef]
- Liu, C.-P.; Wang, R.-C.; Kuo, C.-L.; Liang, Y.-H.; Chen, W.-Y. Recent patents on fabrication of nanowires. Recent Pat. Nanotechnol. 2007, 1, 11–20. [Google Scholar] [CrossRef]
- Huang, N.; Zhu, M.; Gao, L.; Gong, J.; Sun, C.; Jiang, X. A template-free sol–gel technique for controlled growth of ZnO nanorod arrays. Appl. Surf. Sci. 2011, 257, 6026–6033. [Google Scholar] [CrossRef]
- Sangpour, P.; Roozbehi, M.; Akhavan, O.; Moshfegh, A. ZnO nanowires from nanopillars: Influence of growth time. Curr. Nanosci. 2009, 5, 479–484. [Google Scholar] [CrossRef]
- Jia, G.; Wang, Y.; Yao, J. Fabrication and optical properties of well-aligned ZnO nanorods on sapphire prepared by chemical bath deposition. Dig. J. Nanomater. Biostructures 2012, 7, 261–267. [Google Scholar]
- Sharma, M.; Gayen, R.; Pal, A.; Kanjilal, D.; Chatterjee, R. Single phase formation of Fe-doped directional ZnO nanorod films: Study of cluster formation by complex impedance spectroscopy and removal of metal clustering by swift heavy ion irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020, 467, 73–79. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, P.; Late, D.; Singh, T.; More, M.; Joag, D.; Tiwari, R.; Hui, K.S.; Hui, K.; Srivastava, O. Optical and field emission properties in different nanostructures of ZnO. Dig. J. Nanomater. Bios 2012, 7, 795–806. [Google Scholar]
- Hashim, A.; Jaafar, M.; Ghazai, A.J.; Ahmed, N. Fabrication and characterization of tetraleg zinc oxide nanostruture using evaporation methode. Digest J. Nanomater. Biostructures 2012, 7, 487–491. [Google Scholar]
- Kou, H.; Zhang, X.; Du, Y.; Ye, W.; Lin, S.; Wang, C. Electrochemical synthesis of ZnO nanoflowers and nanosheets on porous Si as photoelectric materials. Appl. Surf. Sci. 2011, 257, 4643–4649. [Google Scholar] [CrossRef]
- Mikailzade, F.; Türkan, H.; Önal, F.; Karataş, Ö.; Kazan, S.; Zarbali, M.; Göktaş, A.; Tumbul, A. Structural, optical and magnetic characterization of nanorod-shaped polycrystalline Zn1−xMnxO films synthesized using sol–gel technique. Appl. Phys. A 2020, 126, 768. [Google Scholar] [CrossRef]
- Worku, A.K.; Ayele, D.W.; Habtu, N.G.; Melas, G.A.; Yemata, T.A.; Mekonnen, N.Y.; Teshager, M.A. Structural and thermal properties of pure and chromium doped zinc oxide nanoparticles. SN Appl. Sci. 2021, 3, 699. [Google Scholar] [CrossRef]
- Mouzaia, F.; Djouadi, D.; Chelouche, A.; Hammiche, L.; Touam, T. Particularities of pure and Al-doped ZnO nanostructures aerogels elaborated in supercritical isopropanol. Arab. J. Basic Appl. Sci. 2020, 27, 423–430. [Google Scholar] [CrossRef]
- Diguna, L.J.; Fitriani, A.D.; Liasari, B.R.; Timuda, G.E.; Widayatno, W.B.; Wismogroho, A.S.; Zeng, S.; Birowosuto, M.D.; Amal, M.I. Optical and Photodetection Properties of ZnO Nanoparticles Recovered from Zn Dross. Crystals 2021, 11, 6. [Google Scholar] [CrossRef]
- Mishra, P.N.; Pathak, D.; Mishra, P.K.; Kumar, V. Low-cost processing of pure and Al-doped capped ZnO nano powder for industry scale applications. Chalcogenide Lett. 2022, 19, 19–31. [Google Scholar] [CrossRef]
- Akhoondi, A.; Sharma, A.; Pathak, D.; Yusuf, M.; Demissie, T.B.; Guo, R.-t.; Ali, A. Hydrogen evolution via noble metals based photocatalysts: A review. Synth. Sinter. 2021, 1, 223–241. [Google Scholar] [CrossRef]
- T Chen, K.J.; Fang, T.H.; Hung, F.Y.; Ji, L.W.; Chang, S.J.; Young, S.J.; Hsiao, Y.J. The crystallization and physical properties of Al-doped ZnO nanoparticles. Appl. Surf. Sci. 2008, 254, 5791–5795. [Google Scholar] [CrossRef]
- Bedi, R.K.; Pathak, D.; Deepak; Kaur, D. Structural and optical properties of AgInSe2 films. Z. Kristallogr. Suppl. 2008, 27, 177–183. [Google Scholar] [CrossRef]
- Goh, E.; Xu, X.; McCormick, P. Effect of particle size on the UV absorbance of zinc oxide nanoparticles. Scr. Mater. 2014, 78, 49–52. [Google Scholar] [CrossRef]
- Umar, A.; Rahman, M.; Vaseem, M.; Hahn, Y.-B. Ultra-sensitive cholesterol biosensor based on low-temperature grown ZnO nanoparticles. Electrochem. Commun. 2009, 11, 118–121. [Google Scholar] [CrossRef]
- Zhao, L.; Lian, J.; Liu, Y.; Jiang, Q. Structural and optical properties of ZnO thin films deposited on quartz glass by pulsed laser deposition. Appl. Surf. Sci. 2006, 252, 8451–8455. [Google Scholar] [CrossRef]
- Hu, Y.; Lin, C.; Huang, J.-C.A. Dependences of the Al thickness and annealing temperature on the structural, optical and electrical properties in ZnO/Al multilayers. Thin Solid Film. 2006, 497, 130–134. [Google Scholar] [CrossRef]
- Filipponi, L.; Sutherland, D.; Center, I.N. Introduction to nanoscience and nanotechnologies. Nanoyou Teach. Train. Kit Nanosci. Nanotechnol. 2010, 188, 1–29. [Google Scholar]
- Rashid, A.R.A.; Hazwani, T.N.; Mukhtar, W.M.; Taib, N.A.M. Influence of annealing temperature on optical properties of Al doped ZnO nanoparticles via sol-gel methods. AIP Conf. Proc. 2018, 1972, 030006. [Google Scholar]
- Azizah, N.m.; Muhammady, S.; Purbayanto, M.A.K.; Nurfani, E.; Winata, T.; Sustini, E.; Widita, R.; Darma, Y. Influence of Al doping on the crystal structure, optical properties, and photodetecting performance of ZnO film. Prog. Nat. Sci. Mater. 2020, 30, 28–34. [Google Scholar] [CrossRef]
- Bao, D.; Yao, X.; Shinozaki, K.; Mizutani, N. Growth and electrical properties of Pb (Zr, Ti) O3 thin films by a chemical solution deposition method using zirconyl heptanoate as zirconium source. J. Cryst. Growth 2003, 259, 352–357. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Y.; Summers, C.J.; Wang, Z.L. Large-scale synthesis of six-nanometer-wide ZnO nanobelts. J. Phys. Chem. B 2004, 108, 8773–8777. [Google Scholar] [CrossRef]
- Huang, M.H.; Wu, Y.; Feick, H.; Tran, N.; Weber, E.; Yang, P. Catalytic growth of zinc oxide nanowires by vapor transport. Adv. Mater. 2001, 13, 113–116. [Google Scholar] [CrossRef]
- Williams, G.; Kamat, P.V. Graphene—Semiconductor nanocomposites: Excited—State interactions between ZnO nanoparticles and graphene oxide. Langmuir 2009, 25, 13869–13873. [Google Scholar] [CrossRef]
- Teke, A.; Özgür, Ü.; Doğan, S.; Gu, X.; Morkoç, H.; Nemeth, B.; Nause, J.; Everitt, H.O. Excitonic fine structure and recombination dynamics in single-crystalline ZnO. Phys. Rev. B 2004, 70, 195207. [Google Scholar] [CrossRef]
- Kumar, V.B.; Kumar, K.; Gedanken, A.; Paik, P. Facile synthesis of self-assembled spherical and mesoporous dandelion capsules of ZnO: Efficient carrier for DNA and anti-cancer drugs. J. Mater. Chem. B 2014, 2, 3956–3964. [Google Scholar] [CrossRef] [PubMed]
- Vanheusden, K.; Seager, C.; Warren, W.t.; Tallant, D.; Voigt, J. Correlation between photoluminescence and oxygen vacancies in ZnO phosphors. Appl. Phys. Lett. 1996, 68, 403–405. [Google Scholar] [CrossRef]
- Studenikin, S.; Golego, N.; Cocivera, M. Fabrication of green and orange photoluminescent, undoped ZnO films using spray pyrolysis. J. Appl. Phys. 1998, 84, 2287–2294. [Google Scholar] [CrossRef]
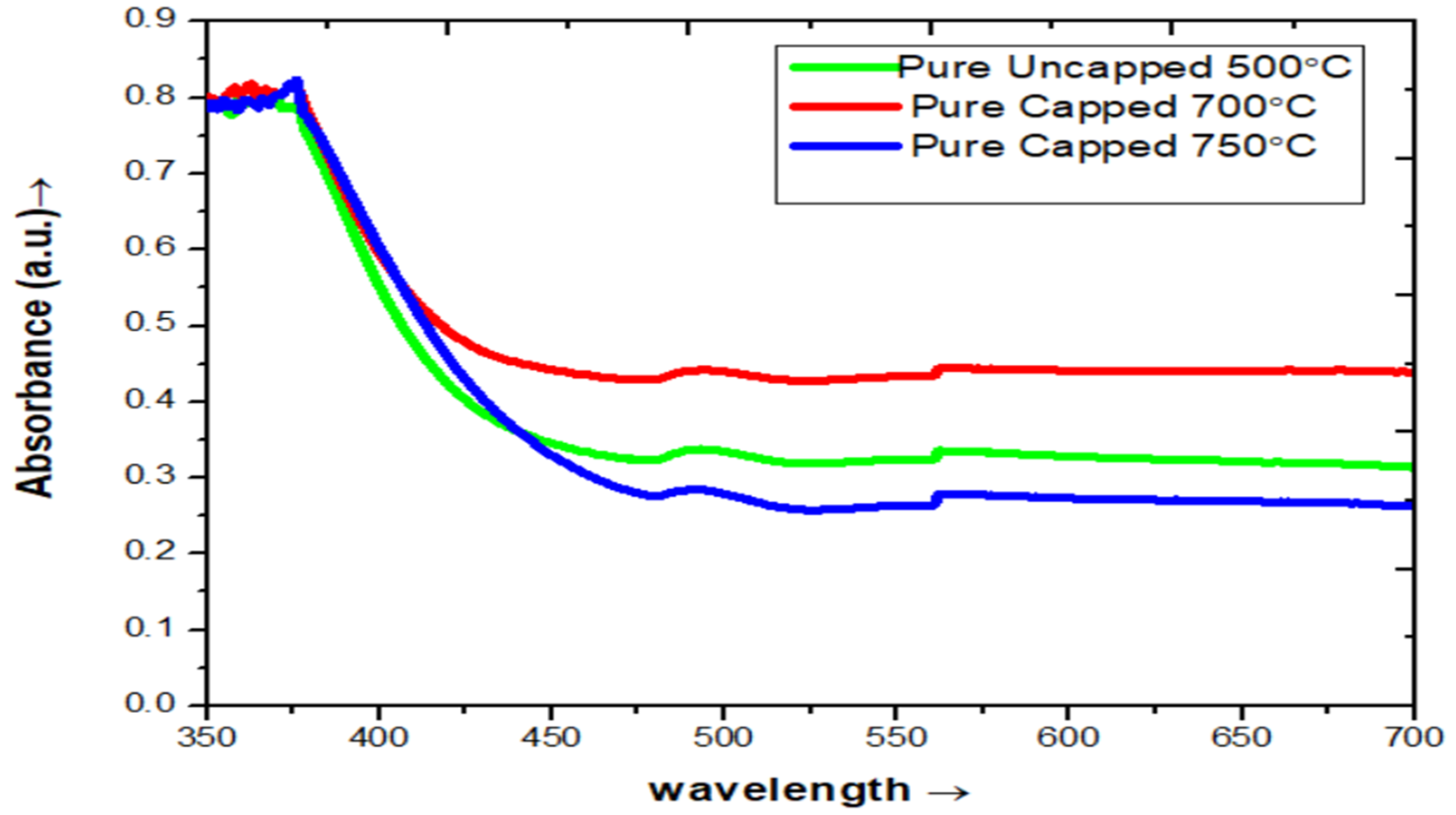
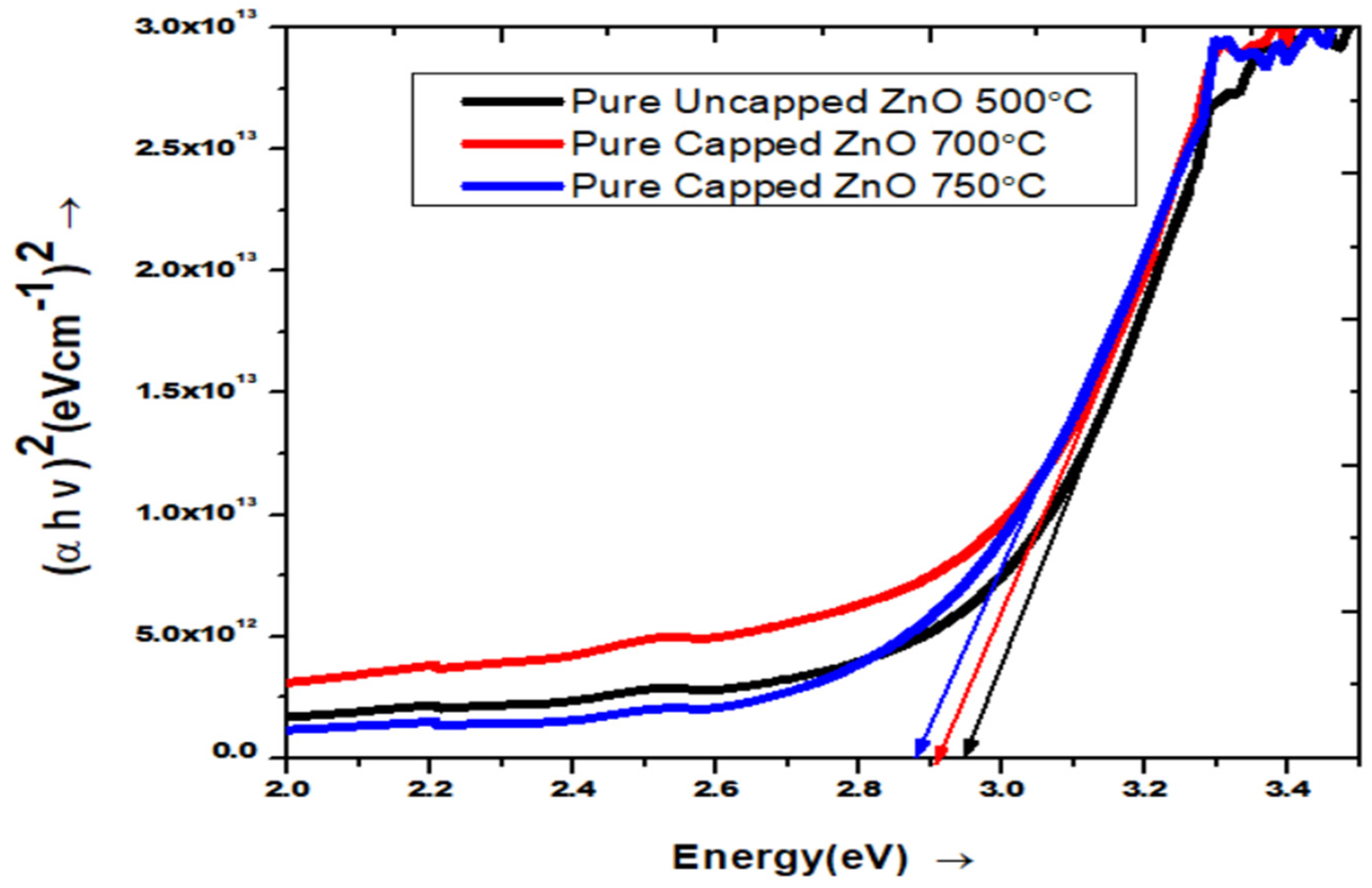

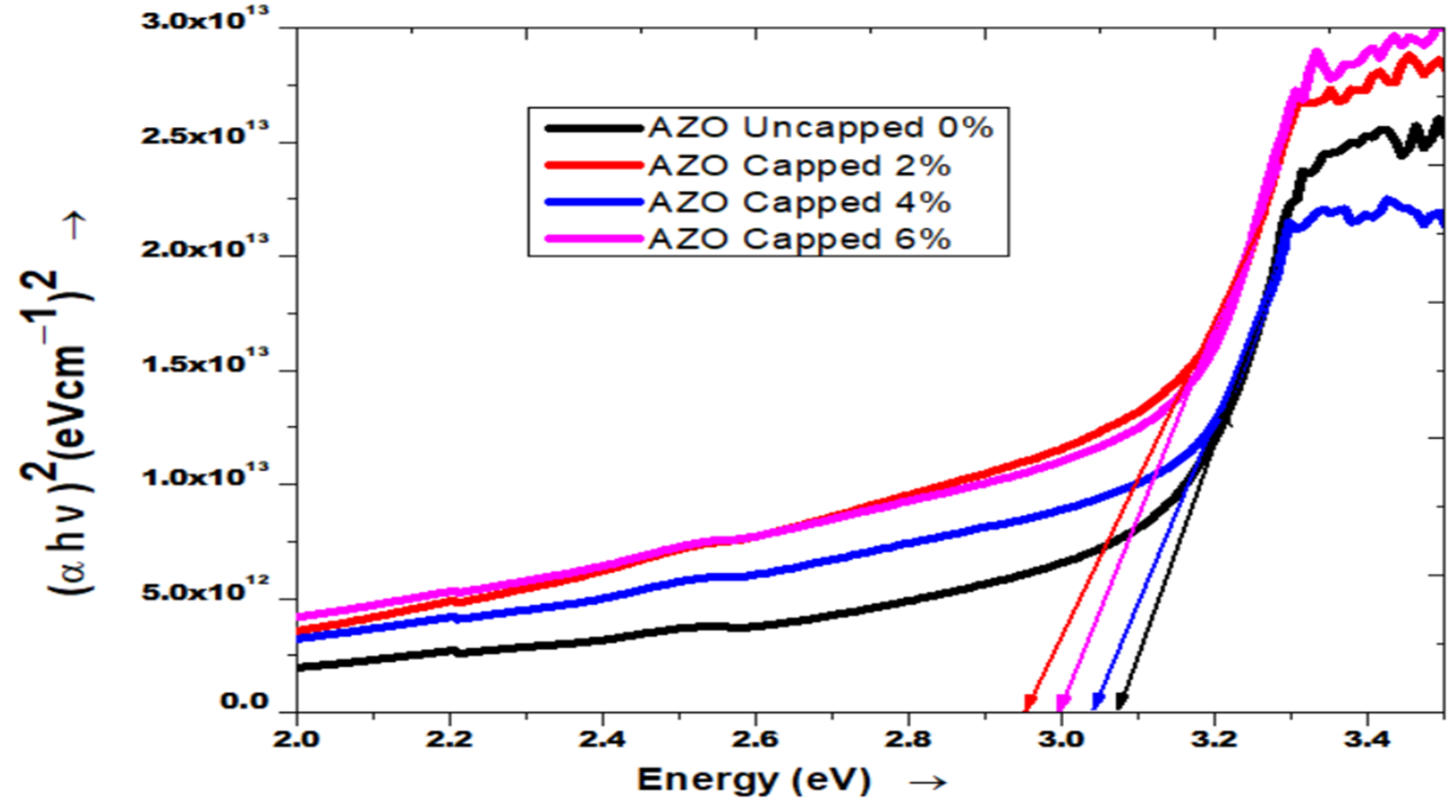

| Pure ZnO & Growth Temperature (°C) | Crystallite Size (nm) (From Scherrer Formula) | Band Gap (Eg) |
|---|---|---|
| Uncapped 500 | 14.19 | 2.97 |
| Capped 700 | 28.50 | 2.90 |
| Capped 750 | 34.17 | 2.84 |
| Al Content (%) in AZO Nanopowder at 500 °C | Crystallite Size (nm) (From Scherer Formula) | Band Gap (eV) |
|---|---|---|
| 0% (Uncapped) | 35.05 | 3.10 |
| 2% (capped) | 24.03 | 3.02 |
| 4% (capped) | 24.57 | 3.08 |
| 6% (capped) | 18.89 | 3.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, P.N.; Mishra, P.K.; Pathak, D. The Influence of Al Doping on the Optical Characteristics of ZnO Nanopowders Obtained by the Low-Cost Sol-Gel Method. Chemistry 2022, 4, 1136-1146. https://doi.org/10.3390/chemistry4040077
Mishra PN, Mishra PK, Pathak D. The Influence of Al Doping on the Optical Characteristics of ZnO Nanopowders Obtained by the Low-Cost Sol-Gel Method. Chemistry. 2022; 4(4):1136-1146. https://doi.org/10.3390/chemistry4040077
Chicago/Turabian StyleMishra, Pooja Nag, Pankaj Kumar Mishra, and Dinesh Pathak. 2022. "The Influence of Al Doping on the Optical Characteristics of ZnO Nanopowders Obtained by the Low-Cost Sol-Gel Method" Chemistry 4, no. 4: 1136-1146. https://doi.org/10.3390/chemistry4040077





