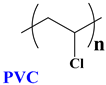
Synthesized and Designed New Modified Poly(vinyl chloride) Structures to Enhance Their Photo-Resistance Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
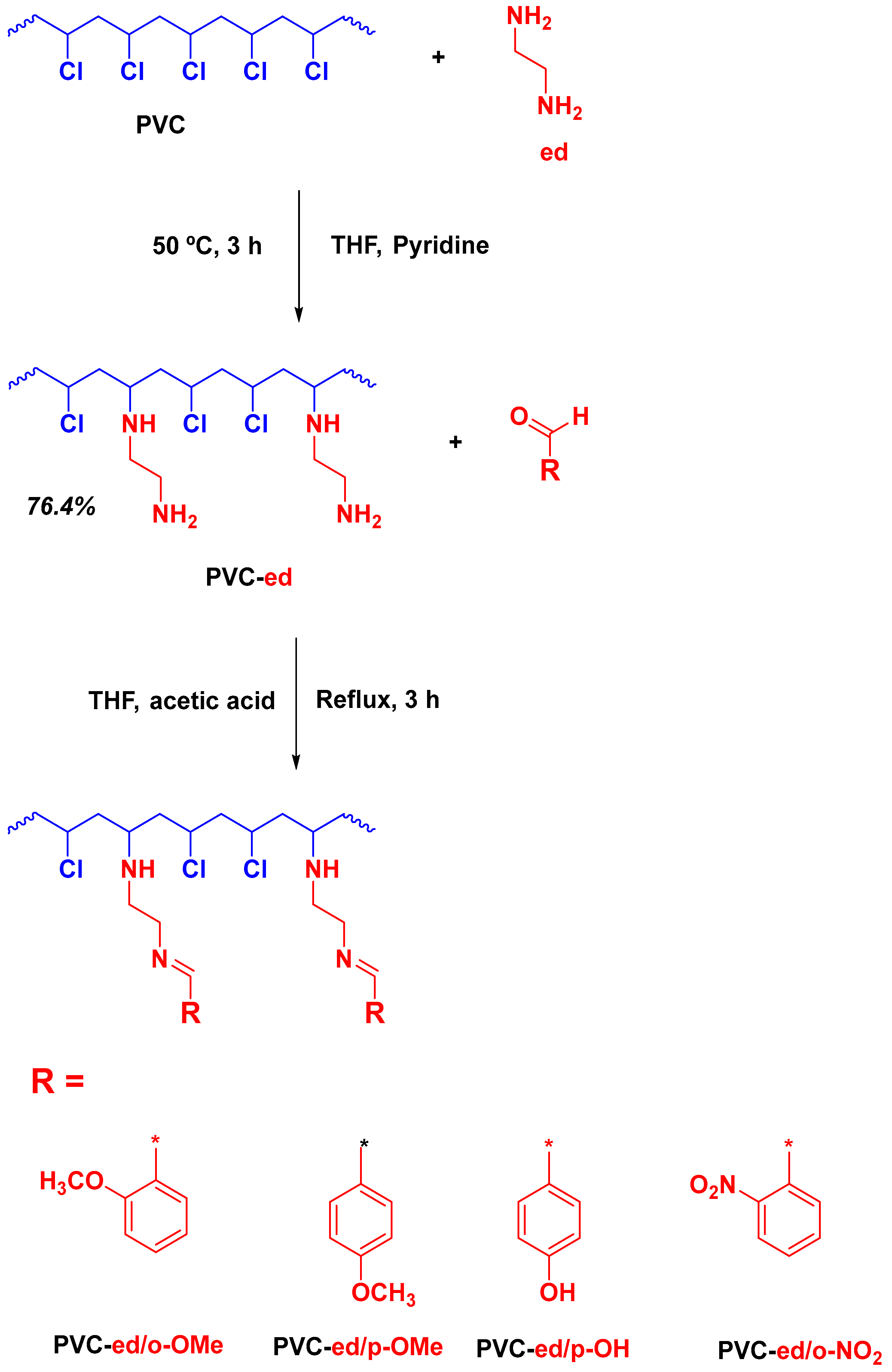
2.3. Synthesis
2.3.1. Synthesis of Modified PVC Based on Ethylene Diamine (PVC-ed)
2.3.2. Synthesis of PVC-ed Schiff-Bases (General Procedure)
2.4. Preparation of Poly(vinyl chloride) (PVC) Film (Blank)
2.5. Doping PVC Film with 0.04 Blend Modified Mixed of PVC-ed/R
2.6. Photodegradation Measuring Methods
2.6.1. Irradiation of PVC Films by UV Light-Accelerated UV Weathering
2.6.2. By FTIR Spectrophotometry
2.6.3. By Weight Loss
2.6.4. By Morphological Study
3. Results and Discussions
3.1. Synthesis of Modified Aromatic Schiff bases PVCs
3.2. Characterization of Synthesized Modified PVC Polymers
3.2.1. Utilizing Fourier Transform Infrared Spectroscopy (FTIR)
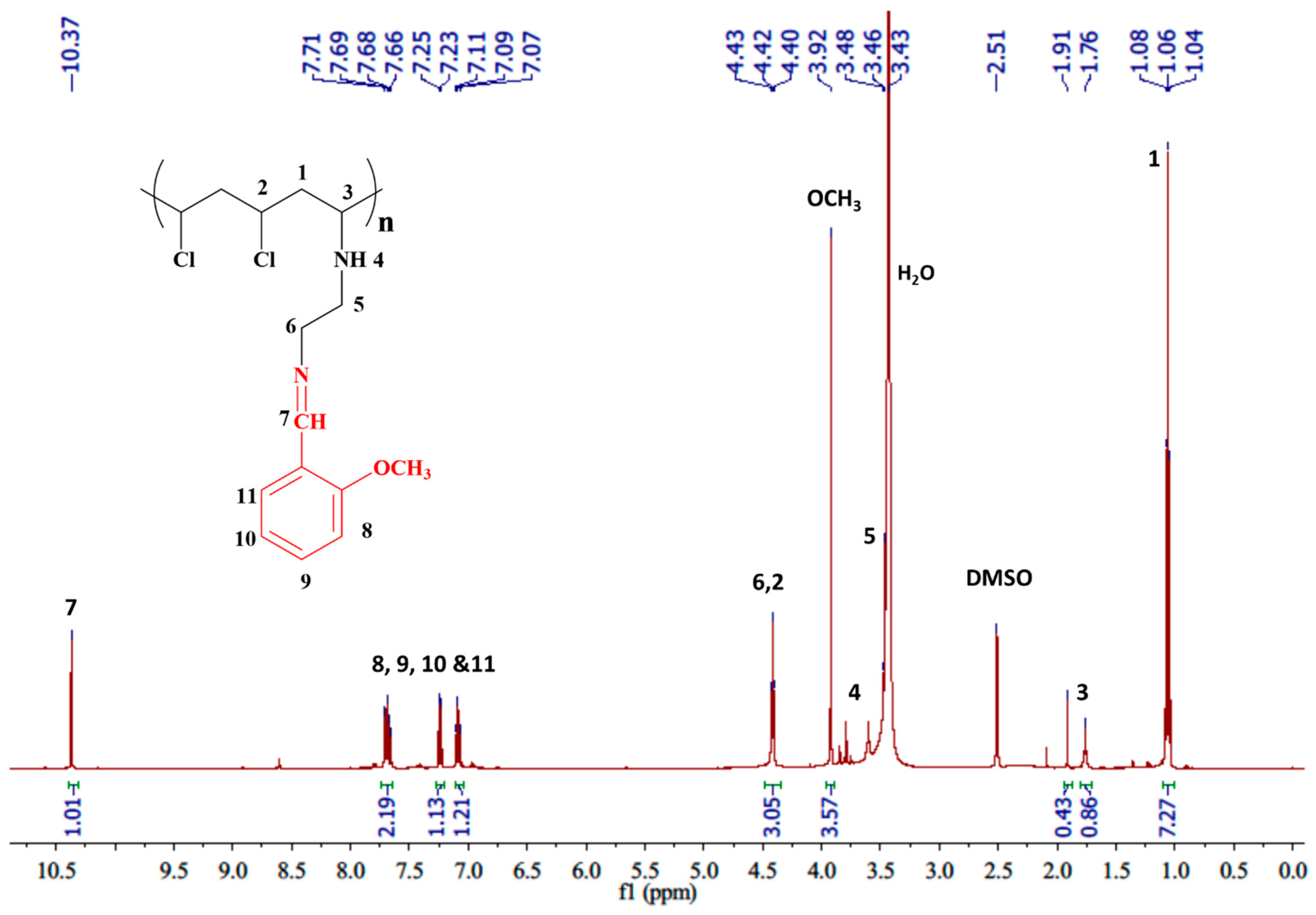
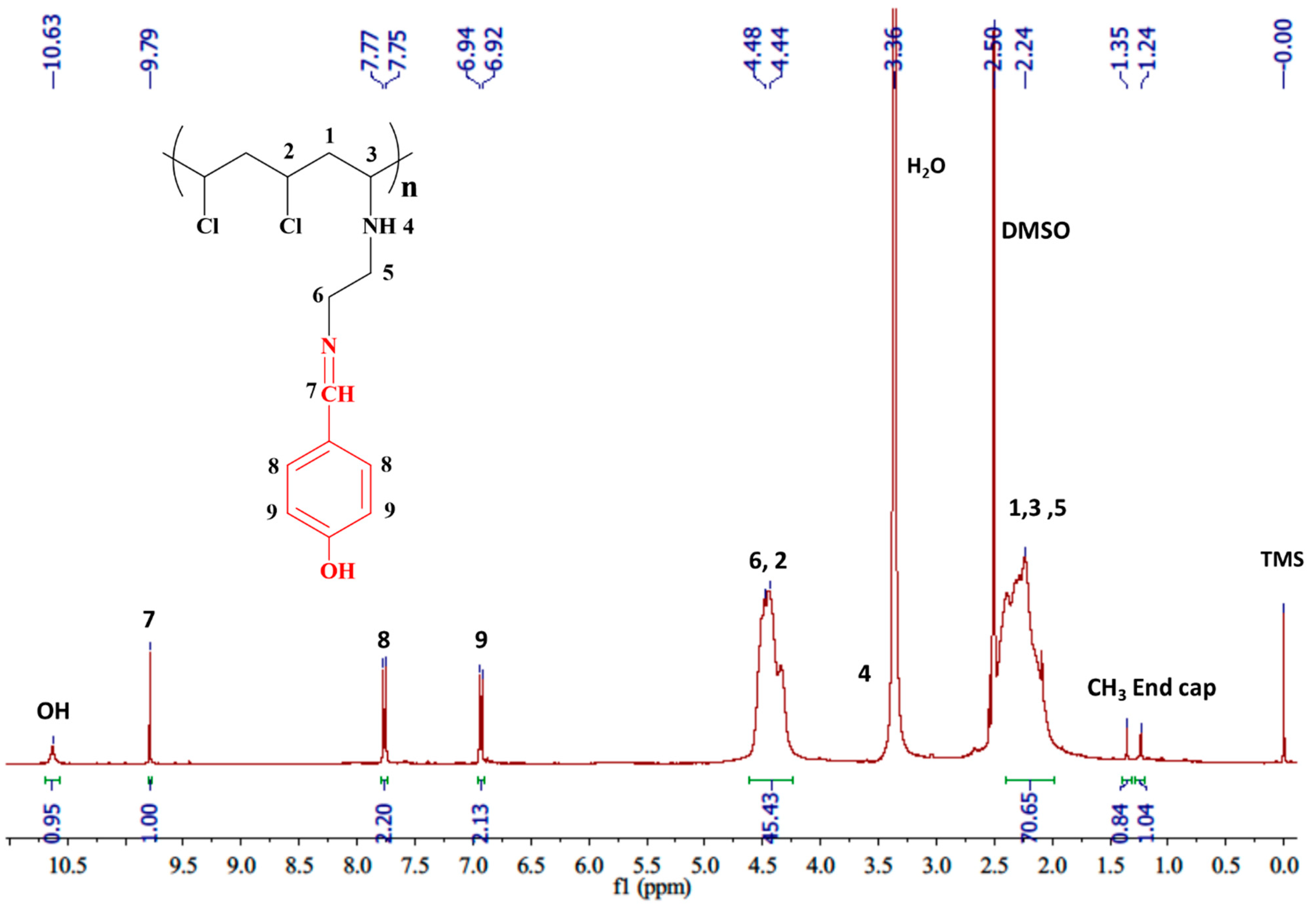
3.2.2. Utilizing Proton Nuclear Magnetic Resonance Spectrometer (1H-NMR)
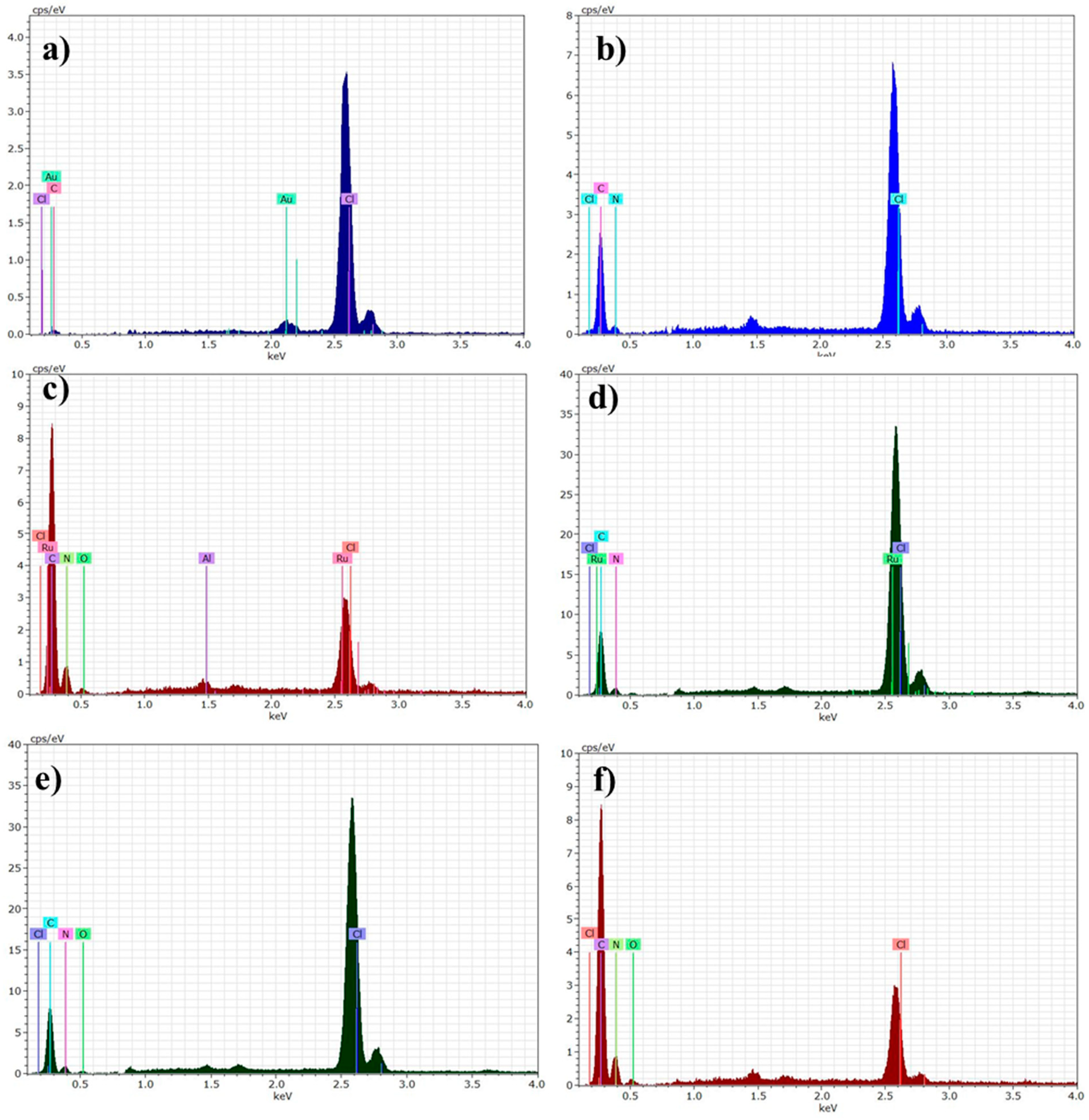
3.2.3. Energy Dispersive X-ray (EXD) of PVC Films
3.3. Synthesized Modified Polymers as Photo-stabilizers of PVC Films
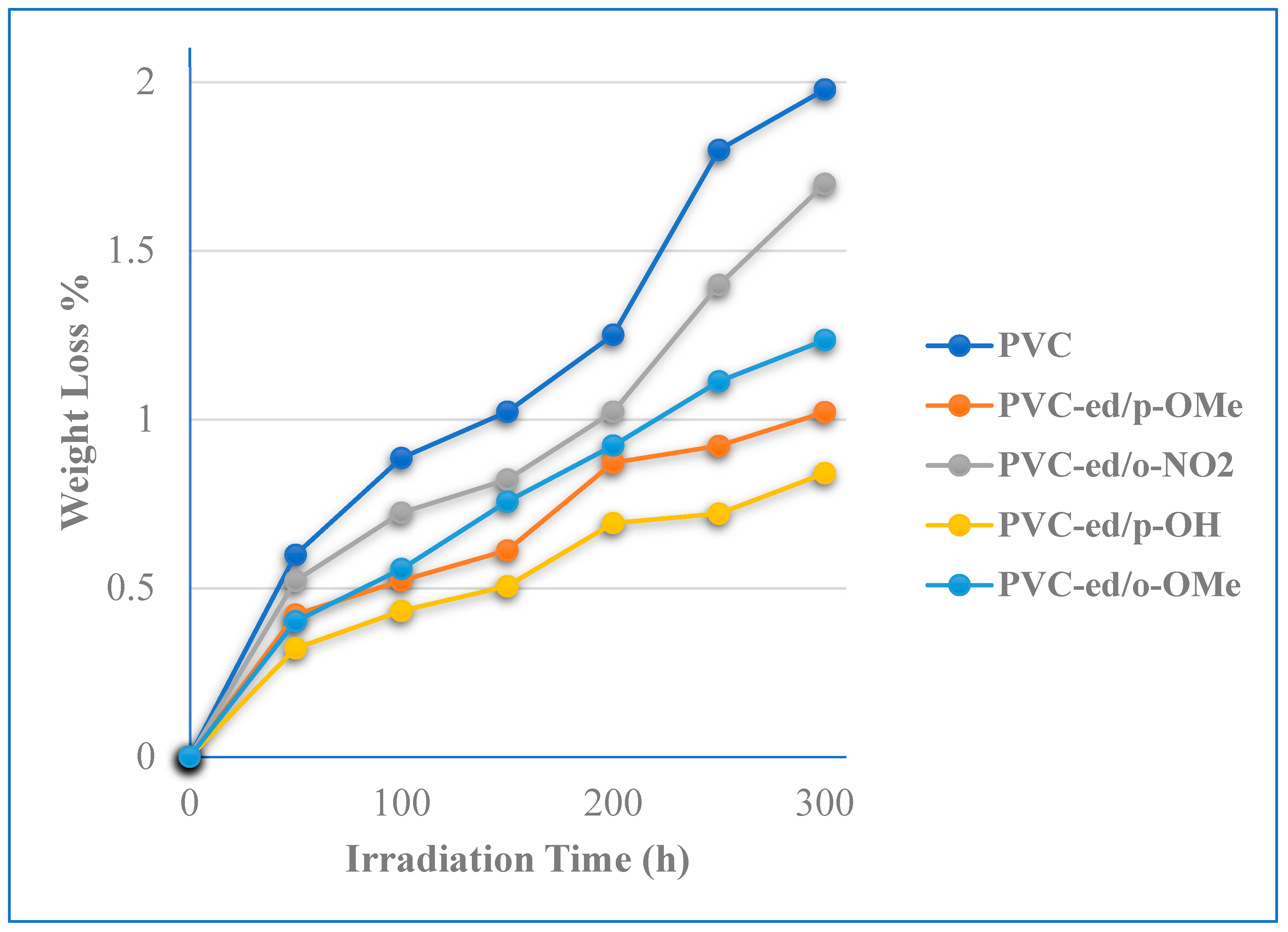
3.3.1. Evaluation of Stabilizing Efficiency of PVC Films by Weight Loss
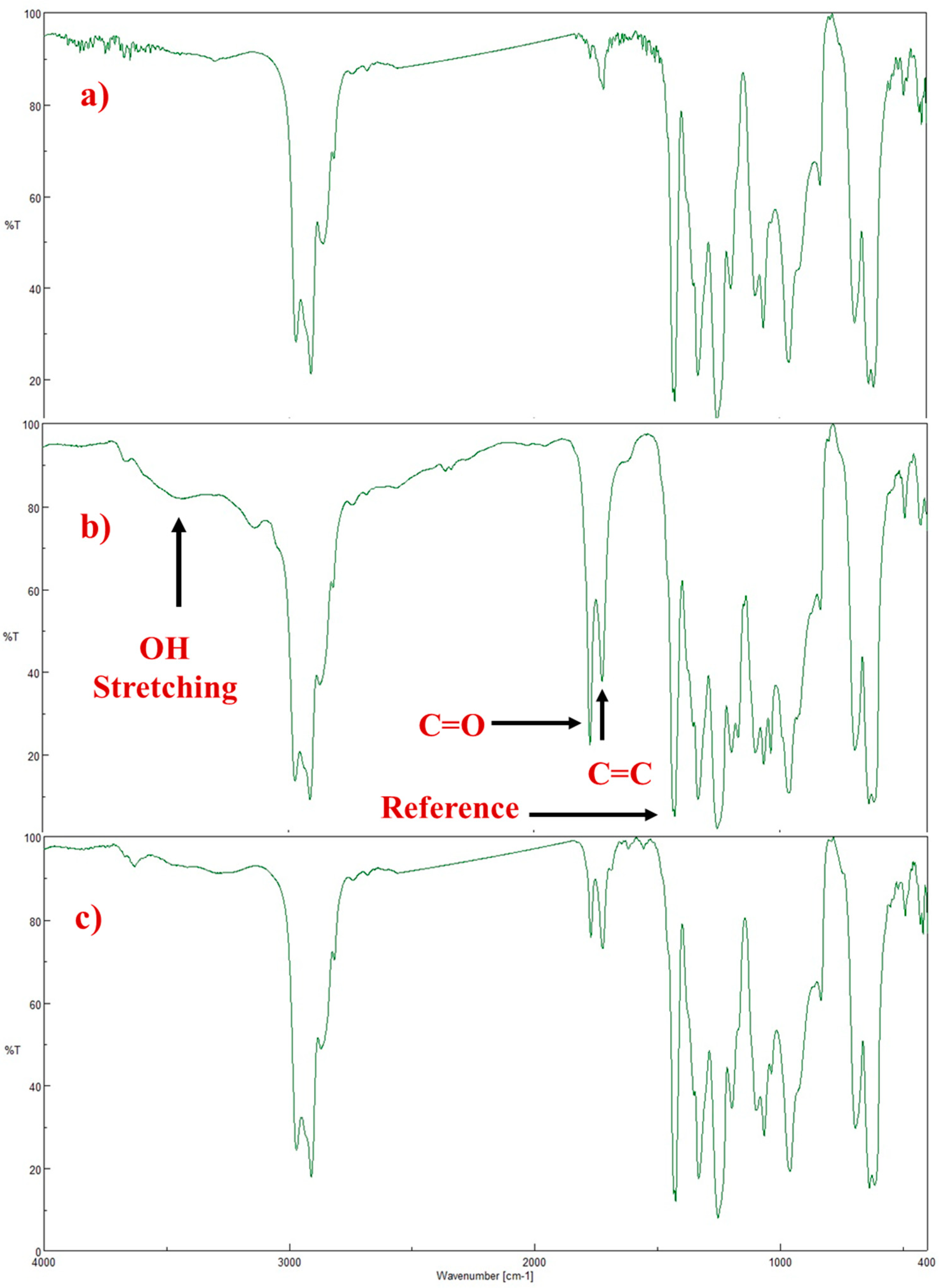
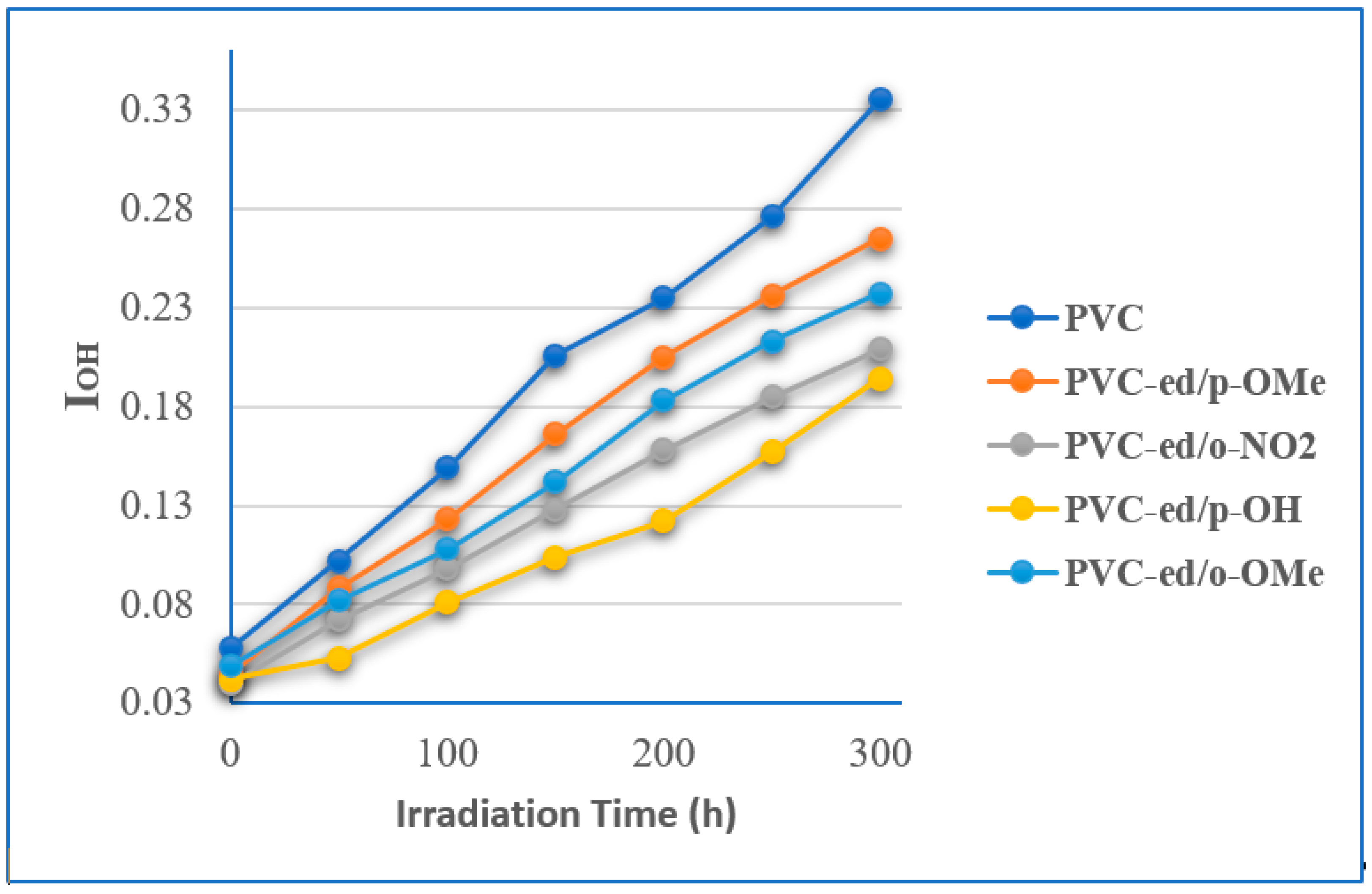
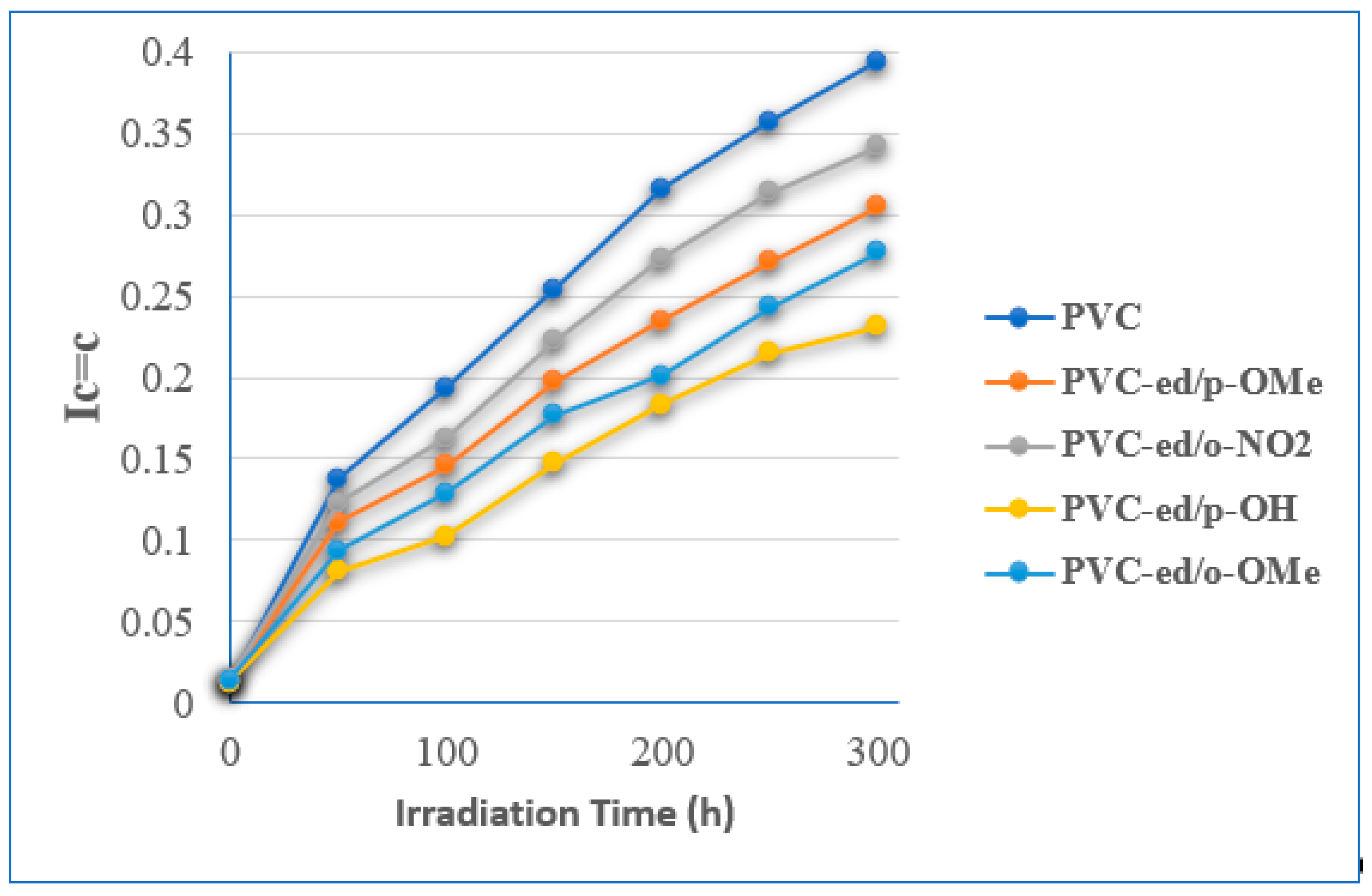
3.3.2. FTIR Spectroscopy to Estimate the Stability of PVCs Films
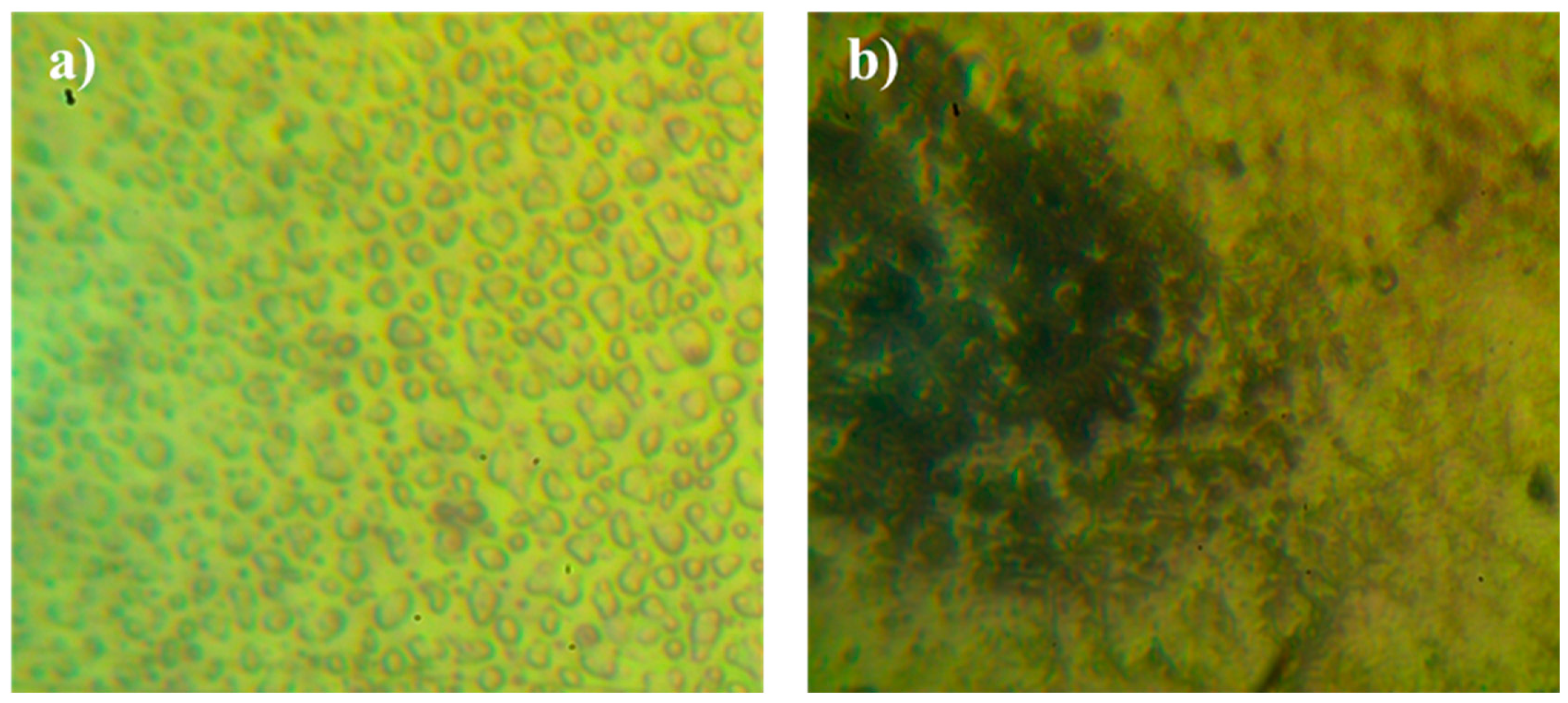
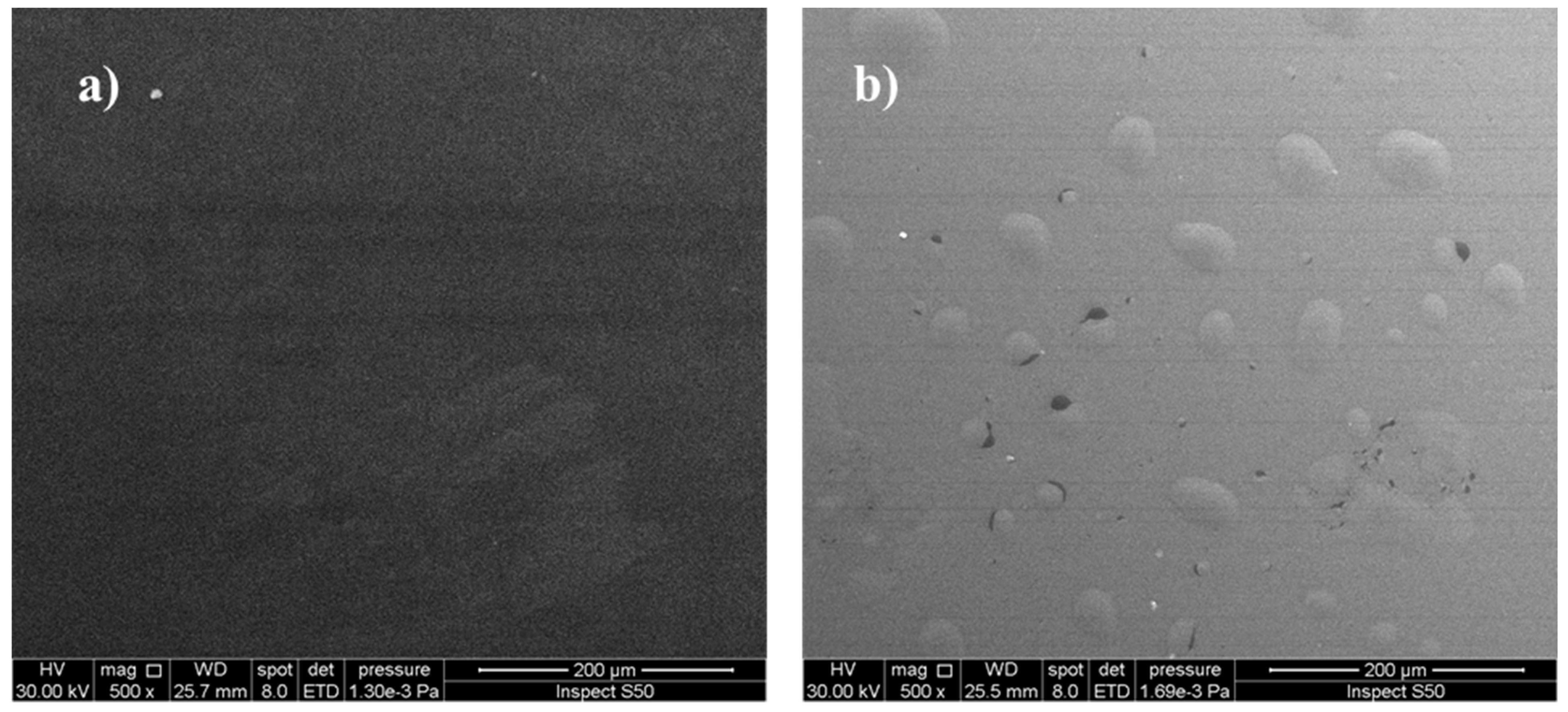
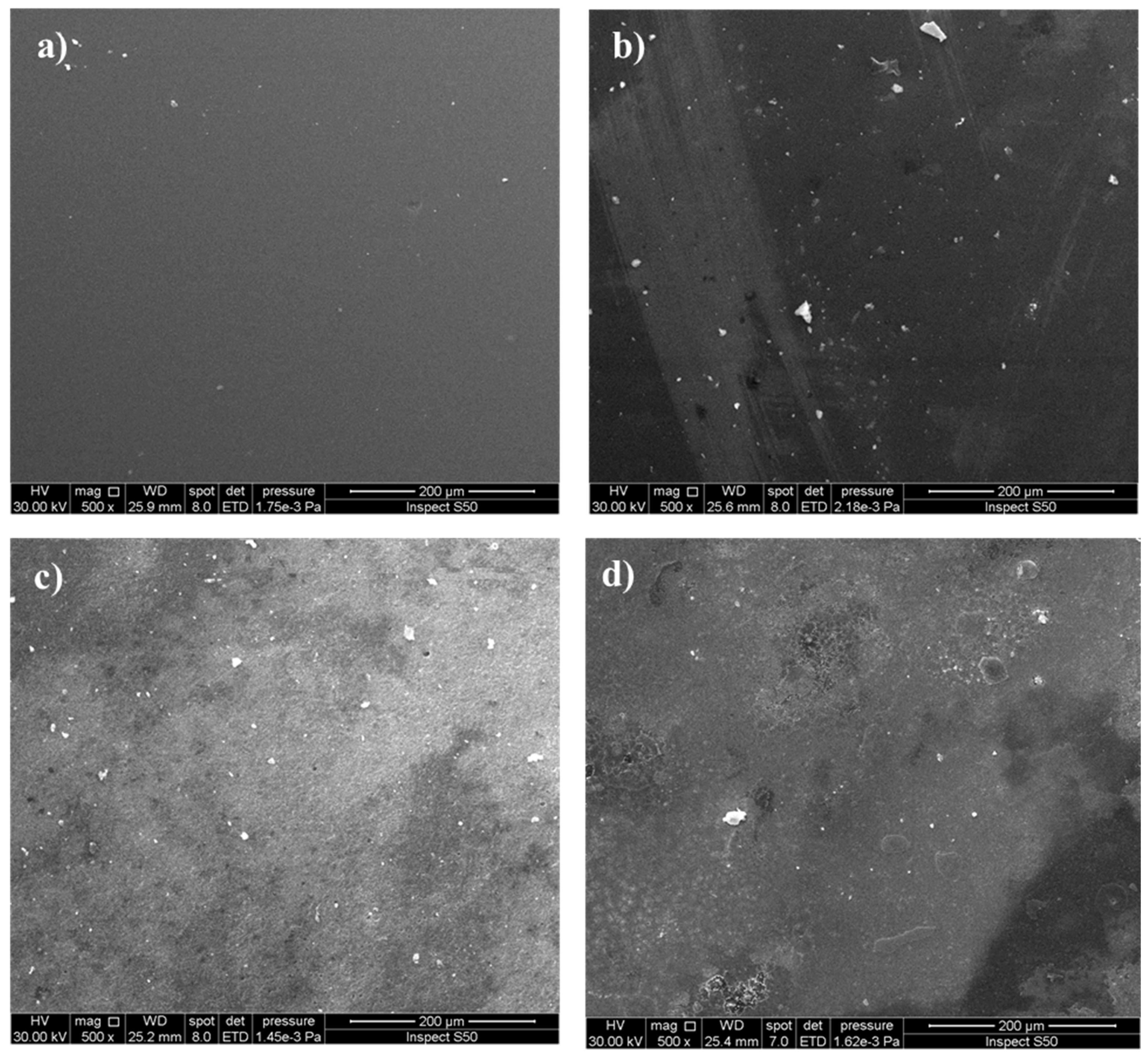
3.3.3. Analysis the Surface Morphology of PVC Films
Microscopic Images to Study the Morphology of PVC Films
Scanning Electron Microscopy (SEM) to Study the Morphology of PVC Films
Atomic Force Microscope (AFM) to Exhibit PVC Photostability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Martínez, G.; Millán, J.L. Stereoselective nucleophilic substitution of poly(vinyl chloride) with potassium 4-acetamidothiophenolate. J. Polym. Sci. Part A: Polym. Chem. 2004, 42, 1857–1867. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Yassin, A.A.; Khalil, K.D.; Sabaa, M.W. Organic thermal stabilizers for rigid poly(vinyl chloride) I. Barbituric and thiobarbituric acids. Polym. Degrad. Stab. 2000, 70, 5–10. [Google Scholar] [CrossRef]
- Khalaf, M.; Fadhil, Z.; Al-Mashhadani, M.H.; Abdallh, M.; Bufaroosha, M.; Majeed, A.; Salih, N.; Yousif, E. PVC Films Per-formance Stabilized By Dibutyltin (IV) Complex For Sustainable Environment. J. Phys. Conf. Ser. 2020, 1664, 012072. [Google Scholar] [CrossRef]
- Dong, W.; Ruan, X.; Ni, Z.; Chen, M. Influence of soy protein isolate on the thermal stability of poly(vinyl chloride) in the presence or absence of calcium and zinc stearates. Polym. Degrad. Stab. 2013, 98, 96–101. [Google Scholar] [CrossRef]
- Fråne, A.; Miliute-Plepiene, J.; Almasi, A.M.; Westöö, A.-K. PVC Waste Treatment in the Nordic Countries; Nordic Council of Ministers: Copenhagen, Denmark, 2019. [Google Scholar] [CrossRef]
- Erythropel, H.C.; Shipley, S.; Börmann, A.; Nicell, J.; Maric, M.; Leask, R.L. Designing green plasticizers: Influence of molecule geometry and alkyl chain length on the plasticizing effectiveness of diester plasticizers in PVC blends. Polymer 2016, 89, 18–27. [Google Scholar] [CrossRef]
- Li, D.; Xie, L.; Fu, M.; Zhang, J.; Indrawirawan, S.; Zhang, Y.; Tang, S. Synergistic effects of lanthanum-pentaerythritol alkoxide with zinc stearates and with beta-diketone on the thermal stability of poly(vinyl chloride). Polym. Degrad. Stab. 2015, 114, 52–59. [Google Scholar] [CrossRef]
- Fu, M.; Li, D.; Liu, H.; Ai, H.; Zhang, Y.; Zhang, L. Synergistic effects of zinc-mannitol alkoxide with calcium/zinc stearates and with β-diketone on thermal stability of rigid poly(vinyl chloride). J. Polym. Res. 2016, 23, 13. [Google Scholar] [CrossRef]
- Ahmed, A.; Abdallh, M.; Al-Mashhadani, M.H.; Ahmed, D.S.; Bufaroosha, M.; Jawad, A.H.; Yousif, E. Environmental Stability of Poly(Vinyl Chloride) Modified by Schiff’s Base under Exposure to UV. Biointerface Res. Appl. Chem. 2021, 11, 13465–13473. [Google Scholar]
- Sabaa, M.W.; Oraby, E.H.; Abdel Naby, A.S.; Mohammed, R.R. Anthraquinone derivatives as organic stabilizers for rigid poly(vinyl chloride) against photo-degradation. Eur. Polym. J. 2005, 41, 2530–2543. [Google Scholar] [CrossRef]
- Fadhil, Z.; Zageer, D.S.; Faris, A.H.; Al-Mashhadani, M.H.; Ahmed, A.; Hashim, H.; Yousif, E. Extracted lignin from oil palm empty fruit bunch as natural eco-friendly poly (vinyl chloride) photo-stabilizer. Mater. Sci. Energy Technol. 2022, 5, 15–21. [Google Scholar] [CrossRef]
- Bufaroosha, M.; Salih, N.; Hadi, A.G.; Ahmed, D.S.; Al-Mashhadani, M.H.; Yousif, E. The Effect of UV Aging on the Structure of PVC in the Presence of Organotin(IV) Compounds. Al-Nahrain J. Sci. 2020, 23, 57–61. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Al-mashhadani, M.H.; Hashim, H.; Ahmed, D.S.; Yousif, E. Morphological, color impact and spectro-scopic studies of new schiff base derived from 1, 2, 4-triazole ring. Prog. Color Colorants Coat. (PCCC) 2021, 14, 27–34. [Google Scholar]
- Arkls, E.; Balkose, D. Thermal stabilization of poly (vinyl chloride) by organotin compound. Polym. Degrad. Stab. 2005, 88, 46–51. [Google Scholar]
- Al-Tikrity, E.T.; Yaseen, A.A.; Yousif, E.; Ahmed, D.S.; Al-Mashhadani, M.H. Impact on Poly (Vinyl chloride) of tri-methoprim schiff bases as stabilizers. Polym. Polym. Compos. 2022, 30, 09673911221094020. [Google Scholar]
- Alhaydary, E.; Yousif, E.; Al-Mashhadani, M.H.; Ahmed, D.S.; Jawad, A.H.; Bufaroosha, M.; Ahmed, A.A. Sulfameth-oxazole as a ligand to synthesize di-and tri-alkyltin (IV) complexes and using as excellent photo-stabilizers for PVC. J. Polym. Res. 2021, 28, 1–19. [Google Scholar] [CrossRef]
- Kalouskova, R.; Novotna, M.; Vymazal, Z. Investigation of thermal stabilization of poly(vinyl chloride) by lead stearate and its combination with synthetic hydrotalcite. Polym. Degrad. Stab. 2004, 85, 903–909. [Google Scholar] [CrossRef]
- Watheq, B.; Yousif, E.; Al-Mashhadani, M.H.; Mohammed, A.; Ahmed, D.S.; Kadhom, M.; Jawad, A.H. A Surface Mor-phological Study, Poly(Vinyl Chloride) Photo-Stabilizers Utilizing Ibuprofen Tin Complexes against Ultraviolet Radiation. Surfaces 2020, 3, 579–593. [Google Scholar] [CrossRef]
- Pan, Y.-T.; Wang, D.-Y. Fabrication of low-fire-hazard flexible poly (vinyl chloride) via reutilization of heavy metal biosorbents. J. Hazard. Mater. 2017, 339, 143–153. [Google Scholar] [CrossRef]
- Silva, T.F.; Soares, B.G.; Ferreira, S.C.; Livi, S. Silylated montmorillonite as nanofillers for plasticized PVC nanocomposites: Effect of the plasticizer. Appl. Clay Sci. 2014, 99, 93–99. [Google Scholar] [CrossRef]
- Krishnan, G.R.; Sreeraj, M.K.; Sreekumar, K. Modification of poly(vinyl chloride) with pendant metal complex for catalytic applications. Comptes Rendus. Chim. 2013, 16, 736–741. [Google Scholar] [CrossRef]
- Elashmawi, I.; Alatawi, N.S.; Elsayed, N.H. Preparation and characterization of polymer nanocomposites based on PVDF/PVC doped with graphene nanoparticles. Results Phys. 2017, 7, 636–640. [Google Scholar] [CrossRef]
- Wirawan, R.; Sapuan, S.; Yunus, R.; Abdan, K.; Sapuan, M.S. Properties of sugarcane bagasse/ poly(vinyl chloride) composites after various treatments. J. Compos. Mater. 2011, 45, 1667–1674. [Google Scholar] [CrossRef]
- Mahdi, S.A.; Ahmed, A.A.; Yousif, E.; Al-Mashhadani, M.H.; Hashim, H.; Jawad, A.H. New organic PVC photo-stabilizers derived from synthesised novel coumarine moieties. Mater. Sci. Energy Technol. 2022, 5, 278–293. [Google Scholar] [CrossRef]
- Lu, J.Z.; Wu, Q. Surface and interfacial characterization of wood-PVC composite: Imaging morphology and wetting behavior. Wood Fiber Sci. 2007, 37, 95–111. [Google Scholar]
- Abdrahman, M.F.; Zainudin, E. Properties of Kenaf Filled Unplasticized Polyvinyl Chloride Composites. Compos. Sci. Technol. 2011, 471–472, 507–512. [Google Scholar] [CrossRef]
- Ahmed, A.; Al-Mashhadani, M.H.; Ahmed, D.S.; Ahmed, A.A.; Yousif, E.; Yusop, R.M. Preparation of Polymeric films containing Schiff base as UV-Absorber with Good Resistance against UV-Photoaging. Biointerface Res. Appl. Chem. 2021, 11, 12743–12749. [Google Scholar]
- Moulay, S. Chemical modification of poly(vinyl chloride)-still on the run. Prog. Polym. Sci. 2010, 35, 303–331. [Google Scholar] [CrossRef]
- Moulay, S.; Zeffouni, Z. Pyridination of poly(vinyl chloride) via a homolytic pathway. Chin. J. Polym. Sci. 2007, 25, 297–302. [Google Scholar] [CrossRef]
- Saleh, T.; Yousif, E.; Al-Tikrity, E.; Bufaroosha, M.; Husain, A.; Al-Mashhadani, M.H. Modification of PVC with captopril and complexation reaction for preparing photostability and thermal stability of PVC. Mater. Sci. Energy Technol. 2022, 5, 311–323. [Google Scholar] [CrossRef]





| Modified PVC | 1H NMR (400 MHz: DMSO-d6, δ, ppm, J in Hz) |
|---|---|
 | 4.43 (m, 1H, Cl–CH), 2.33 (m, 2H, CH2) 2.09 (s, end cap CH3), 1.23 (s, end cap CH3) |
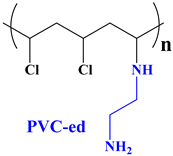 | 4.43 (m, 1H, Cl–CH), 3.05–3.90 (br. m, overlap with H2O peak, 3H, NH, NH2), 2.85 (s, 4H, 2N–CH2), 2.55–2.01 (br. m, 3H, N–CH, CH2), 1.35 (s, CH3 End cap), 1.23 (s, CH3 End cap). |
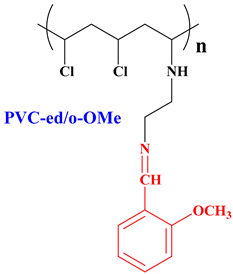 | 10.37 (s, 1H, N=CH), 7.69 (m, 2H, Ar), 7.24 (m, 1H, Ar), 7.09 (m, 1H, Ar), 4.42 (m, 3H, =N–CH2, Cl–CH), 3.92 (s, 3H, OCH3), 3.26–3.90 (br. m, overlap with H2O peak, 3H, NH, N–CH2), 1.76 (m, 1H, N–CH), 1.06 (m, 2H, CH2) |
 | 10.63 (s, exch 1H, OH), 9.79 (s, 1H, N=CH), 7.76 (d, J = 8.0 Hz, 2H, Ar), 6.93 (d, J = 8.0 Hz, 2H, Ar), 4.60–4.15 (br. m, 3H, =N–CH2, Cl–CH), 3.36 (m, overlap with H2O peak, 1H, NH), 2.5–1.9 (br. m, overlap with DMSO peak, 5H, NH, CH2, N–CH, CH2), 1.35 (s, CH3 End cap), 1.24 (s, CH3 End cap) |
 | 10.12 (s, 1H, N=CH), 7.71 (m, 1H, Ar), 7.44 (m, 1H, Ar), 7.24 (m, 2H, Ar), 3.79–3.70 (m, 3H, =N–CH2, Cl–CH), 3.35–3.20 (m, 4H, overlap with H2O peak, NH, OCH3), 2.52–2.39 (m, overlap with DMSO peak, 3H, N–CH2, N–CH), 1.79 (m, 2H, CH2) |
 | 10.24 (s, 1H, N=CH), 8.58 (m, 1H, Ar), 8.15 (m, 1H, Ar), 7.93 (m, 2H, Ar), 3.97–3.90 (m, 3H, =N–CH2, Cl–CH), 3.45–3.30 (m, 1H, overlap with H2O peak, NH), 2.55–2.48 (m, overlap with DMSO peak, 3H, N–CH2, N–CH), 2.09 (m, 2H, CH2). |
| Films | Roughness Average (nm) | Root Mean Square (Sq) (nm) |
|---|---|---|
| Blank PVC before irradiation | 1.91 | 2.52 |
| Blank PVC after irradiation | 5.25 | 7.05 |
| PVC-ed/p-OH | 1.92 | 2.5 |
| PVC-ed/o-OMe | 2.05 | 2.92 |
| PVC-ed/p-OMe | 4.52 | 5.88 |
| PVC-ed/o-NO2 | 3.09 | 4.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, A.A.; Al-Mashhadani, M.H.; Al-Dahhan, W.H.; Hashim, H.; Yousif, E. Synthesized and Designed New Modified Poly(vinyl chloride) Structures to Enhance Their Photo-Resistance Characteristics. Chemistry 2022, 4, 1101-1122. https://doi.org/10.3390/chemistry4040075
Hasan AA, Al-Mashhadani MH, Al-Dahhan WH, Hashim H, Yousif E. Synthesized and Designed New Modified Poly(vinyl chloride) Structures to Enhance Their Photo-Resistance Characteristics. Chemistry. 2022; 4(4):1101-1122. https://doi.org/10.3390/chemistry4040075
Chicago/Turabian StyleHasan, Amer Adnan, Mohammed H. Al-Mashhadani, Wedad H. Al-Dahhan, Hassan Hashim, and Emad Yousif. 2022. "Synthesized and Designed New Modified Poly(vinyl chloride) Structures to Enhance Their Photo-Resistance Characteristics" Chemistry 4, no. 4: 1101-1122. https://doi.org/10.3390/chemistry4040075
APA StyleHasan, A. A., Al-Mashhadani, M. H., Al-Dahhan, W. H., Hashim, H., & Yousif, E. (2022). Synthesized and Designed New Modified Poly(vinyl chloride) Structures to Enhance Their Photo-Resistance Characteristics. Chemistry, 4(4), 1101-1122. https://doi.org/10.3390/chemistry4040075








