Abstract
Rapid urban and industrial sectors generate massive amounts of wastewater, creating severe ecological disruption and harming living organisms. The number of harmful pollutants such as dyes, heavy metals, antibiotics, phenolic compounds, and volatile and several organic chemicals discharged into aquatic systems varies depending on the effluent composition of various sectors. MXene-based composites with unique characteristics were spotlighted as newly developed nanomaterials specifically for environmental-related applications. Therefore, this review broadly discusses the properties, basic principles of MXene, and synthesis routes for developing different MXene-based nanomaterials. The most current strategies on the energy and environmental applications of MXene-based nanomaterials, particularly in photocatalysis, adsorption, and water splitting, were deeply explored for the remediation of different pollutants and hydrogen (H2) evolution from wastewater. The detailed mechanism for H2 evolution and the remediation of industrial pollutants via photocatalysis and adsorption processes was elaborated. The multi-roles of MXene-based nanomaterials with their regeneration possibilities were emphasized. Several essential aspects, including the economic, toxicity and ecological power of MXene-based nanomaterials, were also discussed regarding their opportunity for industrialization. Finally, the perspectives and challenges behind newly developed MXene and MXene-based nanomaterials for environmental pollution were reviewed.
1. Introduction
Environmental pollutants such as toxic compounds and organic contaminants are discharged into environments alongside rapid industrialization developments and need to presently be at the forefront of global attention. Water resources are deemed contaminated when they contain toxicity and cause harmful effects on human health–the demand for sufficient clean water and energy is critical for the sustainability of the worldwide population growth. Water quality has worsened over the past few decades, mainly because of human activities and inappropriate use of natural water resources. According to the Natural Resources Defense Council (NRDC), approximately 1 billion of the world’s population is affected by water pollution by industrial effluents every year. In particular, people from low-income communities are significantly at risk because their homes are closest to the most polluting industries. Industries discharging effluents should obey the restrictions and regulations established by the Department of Environment.
Due to their high mobility, toxicity, and poor biodegradability, dye effluents from the textile, paper, and printing industries pose a serious risk to the environment and human health worldwide [1]. The colouring process, which uses a large amount of water and generates a large quantity of coloured wastewaters, is the main cause of high levels of dye pollution in wastewater effluent. Due to the discharge of more than 280,000 tonnes of textile manufacturing effluents annually worldwide, the textile industry is recognized as the primary source of aqueous waste and dye effluents [2]. The limits and regulations set forth by the Department of Environmental Protection must be followed by all discharged wastewater and effluents. According to Malaysia’s Fifth Schedule of Environmental Quality (Industrial Effluents) Regulation 2009 [3], the maximum permitted concentration of Color ADMI in wastewater is 100 for Standard A and 200 for Standard B. It has therefore become challenging to effectively remove such persistent organic pollutants from wastewater effluents in order to lower pollution.
Heavy metal ions are another major source of pollution. They are difficult to decompose or metabolize in organisms due to their high toxicity. Persistent organic pollutants are also causing the global environment to deteriorate. As a result, they are continuously condensed to exceed the allowable limit, endangering human health [4]. Organic pollution degradation strategies can purify oil, river water, wastewater, air, and other substances. Biodegradation, chemical oxidation, electrochemical conversion, or combustion can all be used to remove most organic pollutants. However, some low-level harmful organic pollutants are difficult to eliminate using the methods described above, and secondary pollution will occur.
In acknowledgement of safety requirements, researchers introduced numerous approaches to treating the wastewater excreted before the final release: biodegradation, photo/electrocatalysis, ozonation, adsorption, membrane separation, redox, and chemical precipitation. Nevertheless, considering the cost, technical challenges, and methods’ effectiveness, all the treatments mentioned should lace up with their drawbacks. In recent years, among the variety of removal techniques, photocatalytic degradation, also known as the advanced catalysis process, has proven essential advantages such as being environmentally friendly, reusability, and complete degradation. The history of engineering photocatalytic material can be traced back to 1972 when Fujishima and Honda applied the presence of TiO2 photocatalyst served as the starting point of photocatalytic reaction [5]. In conjunction with the expansion nanoparticle research area, nanomaterials show intriguing features such as a low removal capacity, high specific surface area, and unique environment flexibility [6].
The photocatalytic degradation process has been reported to degrade a broad range of organic matters such as dyes, phenols, and pharmaceutical waste including alkanolamines. Advanced photocatalytic nanomaterials are a promising technology to bridge the gap between global energy challenges and environmental remediation. At present, a significant amount of effort has also been put into developing highly efficient and robust photocatalysts. The photocatalysts were developed with carbon-based or two-dimensional (2D) nanomaterials such as carbon nanotubes, graphene oxides, metal-organic frameworks, and MXene. Remarkably, the research developed mentioned high-tech nanomaterials without needing expensive metal content. Among these photocatalysts, MXene has attracted significant interest in various areas, including environmental pollutants degradation, due to their unique and excellent physicochemical properties. Generally, MXene belongs to the family of transition metals and carbides and nitrides with an uneven distribution of functional groups. Nonetheless, graphene-based nanomaterials are the most well-known 2D material with a single atomic carbon layer, which leads to limited application, whereas MXene may reach 3, 5, or 7 atomic layers thick.
Therefore, MXene materials and their nanocomposite are advanced photocatalysts due to their high surface area, great strength, large interlayer spacing, nontoxicity, hydrophilicity, and environmental flexibility in recognition of the great potential of MXene that has grown to receive increased attention within a shorter period; this review chapter objective to provide a critical overview on the recent studies of MXene in environmental applications. The properties and fundamental principles of MXene were first studied to encourage a better understanding of the photocatalyst system. Then, we focused on several innovative MXene-based photocatalyst developments that mainly analyzed theoretical and experimental aspects, including TiO2-MXene, g-C3N4-MXene, and BiVO4-MXene. Afterwards, the research progress on the environmental pollution remediation of MXene/MXene-based photocatalyst is explained. The environmental application involved the removal of organic contaminants and concise hydrogen evolution reaction. As a final point, we present a summary of the current perspective and challenges to tackle future opportunities of MXene photocatalysts. The significant features of MXene-based nanomaterials in the current review were summarized in Figure 1.
Figure 1.
Significant features of MXene-based nanomaterials in the current review.
1.1. Background Study of MXene
In 2011, 2D-layered titanium carbide powder (Ti3C2Tx) was the first candidate of the MXene family introduced by Naguib et al. from Drexel University. As shown in Figure 2, MXene has a formula of Mn+1XnTx, which is fabricated by etching MAX phase (Mn+1AXn) in which M corresponds to transition metal, X denotes carbon/nitrogen, T indicates the functional groups such as hydroxyl, oxygen, or fluorine, A represents elements from the group, and n is an integer. MXene materials were initially investigated as a promising alternative for electrochemical energy storage in batteries and super capacitors due to their high electrical conductivity, structural stability, and hydrophilicity [7]. In 2011, 2D-layered titanium carbide powder (Ti3C2Tx) was the first candidate of the MXene family introduced by Naguib et al. from Drexel University [8]. MXene materials were broadly examined as vehicles for energy storage/delivery in devices such as Li/Na-ion batteries and supercapacitors, owing to their lamellar structures and exceptional electrical conductivity, in 2012 [9]. MXene was then used to remove various pollutants from the environment, including dye in 2014 [10], Cr (VI) in 2015 [11], U(VI) in 2016 [12], and N2 reduction in 2018 [13]. In recent developments, MXene has also been recommended for use as a negative electrode in acidic electrolytic solutions to establish higher concentration gradients and increase energy density [14]. Metallic MXenes have the potential to be tuned to match the semiconductor’s band edge by choosing specific compositions and managing their surface terminations, making them promising electrode materials for nanoelectronics devices.
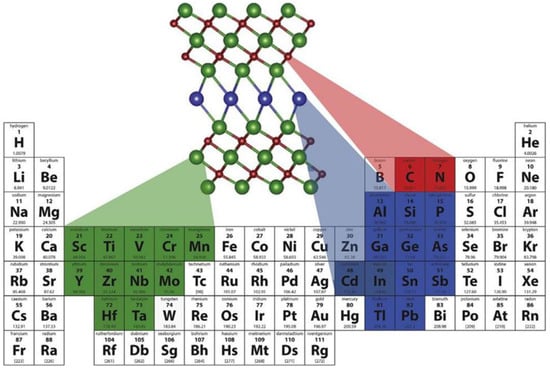
Figure 2.
MXene phases Mn+1AXn-producing elements (Reprinted with permission from Ref. [20]. 2019 Elsevier).
Furthermore, it was demonstrated that in situ Raman spectroscopy could be used to track how the O-termination in a Ti3C2Tx electrode protonated to form an OH-termination after being discharged in an H2SO4 solution [15]. Hantanasiridakul et al. also studied that this feature is much higher when compared to carbon-based components, which have a lower capacitance and narrowed potential gradient ranges of 1.0 V [15]. Due to its unique structural, physical, chemical, and functional properties, MXene is considered a designer nanomaterial for various applications in various industrial sectors [16]. Throughout the past several decades, the focus of research on this promising material has switched to the use of MXene in nanomembranes for water purification, nanostructures for energy conversion, and also multifunctional nanocomposites for photocatalytic applications, where it was used as a co-catalyst in order to create photocatalytic behavior [17]. MXene-semiconductor composites were also created to improve the performance of pure components by reducing agglomeration, increasing stability, and providing a conductive channel [18]. Furthermore, due to the rapid recombination of photoexcited charge carriers, single material photocatalysts are always insufficient, resulting in reduced quantum effectiveness and poor photocatalytic performance [19]. Thus, to improve the separation efficiency of photogenerated charge carriers, heterostructure photocatalysts were widely assembled. Finally, due to their unique 2D layered structure, MXene-based photocatalysts demonstrated a significant impact for environmental remediation applications.
1.2. Basic Principle of MXene
MXene belongs to transition metal, carbides, and nitrides with an uneven distribution of functional groups. MXene has a formula of Mn+1XnTx fabricated by etching MAX phase (Mn+1AXn) in which M corresponds to transition metal, X denotes carbon/nitrogen, T indicates the functional groups such as hydroxyl, oxygen or fluorine, A represents an element from the group, and n is an integer. Various functional groups of oxygen (-O), fluorine (F), and hydroxyl (-OH) were molded onto MXene surfaces. They were recognized using energy-dispersive X-ray spectroscopy (EDX) to detect the elemental compositions. The intralayer skeleton, interlayer, and surface terminating groups area make up the structure of MXene, Ti3C2. As confirmed by neutron diffraction, Ti is bonded to C via an ionic bond in the interlayer skeleton between the Van der Waals forces and hydrogen bonding between -O or F atoms on the surface [21]. MXene materials have gained significant attention for their wide variety of compositions, conductive properties, and unique morphology. The MXene compound possesses superior reflectivity in the ultraviolet (UV) light absorptions with the reported region from a wavelength between 300 and 500 nm, which was a crucial property for photocatalytic reactions with energy gaps of about 0.25–2.0 eV, as shown in Figure 3a,b [22].
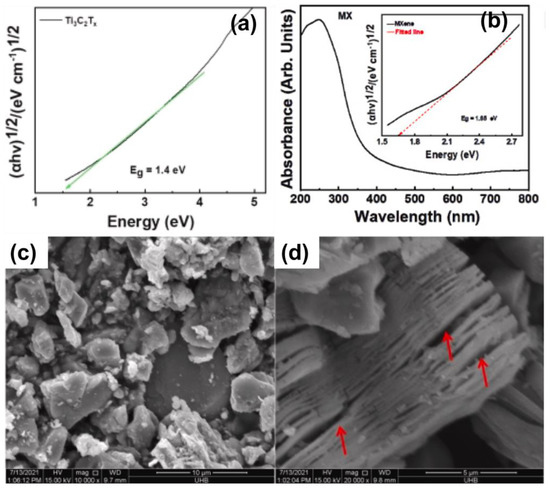
Figure 3.
UV-Visible Spectrum of MXene (a) (Reprinted with permission from Ref. [26]. 2022 Elsevier); (b) (Reprinted with permission from Ref. [27]. 2021 Elsevier) and SEM images of (c) Ti3AlC2 MAX, (d) MXene. (Reprinted with permission from Ref. [26]. 2022 Elsevier).
Moreover, the lattice layered structure of surface functionalized MXene allows for highly competent and robust photocatalysts with nitride and carbide bonding–this leads to an efficient generation of the reactive species responsible for pollutant degradation. In addition, MXene owns the hydrophilic nature incorporated with the active functional group on its surface to tackle ionic/molecular species, especially for environmental remediation. Furthermore, the morphology of the MXene flake’s structure was identified by some characterization methods, such as transmission electron microscopy (TEM) and scanning electron microscopy (SEM), which displayed accordion-like multilayer structures [23,24,25]. The most well-known 2D materials are graphene-based nanomaterials. Still, they only have a single atomic carbon layer which limits their application, whereas MXene may reach three, five, or seven atomic layers, as shown in Figure 3c,d. The inter-planar distance of MXene sheets was about 0.242 nm with the (1 0 3) plane of MXene.
2. Synthesis Routes of MXene
The introduction of MXene was first synthesized by etching an A layer from a MAX phase with the HF or HCl-LiF method. The enhancement of the hydrophilic compound due to the functionalization of MXene was the result of the etching method. The multilayered MXene is held weakly together by van der Waals bonds and hydrogen. Congruously, HCl and LiF solution’s etching method gives larger interlayer spacing than HF because of the expansion of hydrated Li-ion intercalation and chloride termination. Conversely, HCl–LiF is a lower fluorine content solution that creates a safer and easier method. However, the latter method’s concentration optimization of these two ingredients, LiF and HCl, improved the quality of the produced MXene [28]. Table 1 shows the summarization of the etching treatment using HF and HCl-LiF. It is observed that the reported etching durations are varied from 5 to 48 h to get a well-interspaced multilayer (M-MXene/Ti3C2Tx) or single-layer (S-MXene/Ti3C2Tx), depending on the etching agent and method used.

Table 1.
The recent development of etching treatment using HF and HCl-LiF.
Additionally, based on Khatun and Roy’s optimization analysis, 24 h of etching produces well-interspaced accordion-like multilayer MXene, and shorter etching times are insufficient to remove the ideal amount of Al. On the other hand, 48 h of etching causes pits to form and flakes to break, which weakens the MXene’s properties [29]. As a direct consequence, the developed MXene’s morphology and physicochemical characteristics vary depending on the etching duration.
Various fluoride-free etching methods have been developed to fabricate MXene to reduce its adverse effect on the environment, such as alkali-based etching, Lewis acid etching, and electrochemical etching (E-etching). There are a few reports on using alkaline etchants as fluorine-free synthetic methods. In the first work by Li et al. [39], an alkali-assisted hydrothermal method which inspired the Bayer process was used to prepare M-MXene. It was found that the Al from Ti3AlC2 was successfully removed at 270 ˚C in a 27.5 M NaOH solution for 12 h. Ideally, Al layers were attacked by OH; thus, Ti and Al were terminated with −OH and −O. As a result, this process yields a 92 wt. % multilayer Ti3C2Tx with an interlayer spacing of 1.2 nm. Granting this etching technique prevents the formation of −F terminal groups. Conversely, the high concentrations required of base and harsh reaction conditions are still dangerous and unsuitable for large-scale synthesis [40]. The Lewis acidic etching mechanism is proposed to etch MAX phases by direct redox coupling between the A element and the cation of Lewis acid molten salt [14].
Li et al. [41] reported an etching process of M-Ti3C2Tx prepared from Ti3SiC2 immersion in CuCl2 molten salt at 750 °C. The researchers describe that the exposed Si atoms were oxidised by Lewis acid Cu2+ resulting in volatile SiCl4 and associated with the reduction of Cu2+ to metallic Cu, then Clˉ anions reacting to form Ti3C2Cl2. Next, the Cu particles were removed by immersing the products in ammonium persulfate ((NH4)2S2O8), which also marks the O terminal surface groups. In addition, the E-etching technique approach was used for hydrolysis (to obtain MAX phase precursor with reduced lateral size) and a thermos-assisted electrochemical process (to remove the A-layer from the electrode) [42]. The etching process was performed in a two-electrode system with an anode and cathode where bulk Ti3AlC2 and chloride-containing electrolytes were chosen as apparent etching effects [43]. They suggested the electrolyte is composed of 1 M NH4Cl and 0.2 M tetramethylammonium hydroxide (TMA·OH) with a pH value above 9. Clˉ anions enabled rapid anodic Al etching, resulting in −OH termination on M-Ti3C2 surfaces, and the intercalation of ammonium species took place simultaneously. Herein, a fluoride-free, facile, and rapid method for MXene preparation was an advantage relative to E-etching.
Several other techniques, such as chemical vapour deposition and hydrothermal and salt templates, are exhibited as functional pathways for fluoride-free MXene preparation. Proper control towards surface functional groups, maximum specific surface distribution, and well-defined chemical stability towards synthesizing MXene should be the target to invent a feasible proposed approach and establish several environmental issues [44]. Although the superior properties of MXene were promising in photocatalysis applications, the performance of MXene is still hindered by its tendency to stack or aggregate, driven by van der Waals, which leads to a lower photocatalytic performance [45]. The high degree of electron-hole pair recombination rate is another factor that restricts the performance of MXene photocatalysts [25]. The photoluminescence (PL) test, which examined the energy released by the photocatalyst, was used to evaluate the separation of photogenerated charge carriers. It is important to note that the recombination of photogenerated charge carriers increases with PL intensity, which influences the overall performance of the photocatalytic system [46]. As more free electrons were available, the separation of photogenerated charge carriers improved and significantly increased the photocatalytic activity.
Heterojunctions were formerly thought to be one of the simplest and most effective ways to modify the internal electrical characteristic structure of MXene and increase its photocatalytic performance [47]. As a result, a new electric field within a space charge region will be developed, resulting in spatial photo charge carrier separation and migration. MXene-based photocatalysts are prepared by replacing the noble metal co-catalyst to enhance the physicochemical properties of the photocatalyst. Generally, the modification aims to improve the charge separation capacity of the photocatalysts [22]. The strategic view is that the best approach to maximize the use of MXene is to use it as a substrate, which facilitates hybridization with other nanomaterials and thus increases catalytic activity.
3. Environmental Applications
The presentation of MXene-based photocatalysts for environmental remediation and resource recovery has garnered maximum attention and has also been applied to developing advanced photocatalyst systems. The profound photocatalytic performance of the MXene-based photocatalyst is mainly governed by its evolution of particular surface termination groups (−OH, −O or −F) [48]. Generally, the pristine MXene (Ti3C2) is metallic, and the surface termination groups show semiconducting behaviors. Given the above unique features of the photocatalyst, researchers have attempted strategic experimental performances to reveal their role in an environmental application using the MXene hybridization structure. This section presents a detailed summary of recent prospective pollution control applications through MXene-based catalysts, including antibiotic removal, dye removal, and water splitting.
3.1. Photocatalysis Mechanism
The photocatalysis system mainly depends upon light energy or wavelengths and a photocatalyst. The semiconductors are generally utilized as photocatalysts, and their electronic structures could be helpful to serve as sensitizers for light irradiation [49]. In the degradation mechanism, the light source falls over the photocatalyst surface, and if this energy is equal to or greater than photocatalyst band gap energy, then electrons in the VB are disturbed and transferred towards the CB of the photocatalyst, leaving the holes in the VB. VB holes may oxidize donor molecules and generate hydroxyl radicals after a reaction with water molecules. On the other hand, electrons in CB produce superoxide radicals after a reaction with oxygen species. The produced holes and electrons may cause oxidation and reduction reaction processes that can be adsorbed over the photocatalyst surface to provide necessary outputs [50]. Similar photocatalysis mechanisms have been proposed in several studies. Vishal et al. proposed a photocatalysis mechanism using ZnO/Bi2WO6/M-MXene photocatalyst for ciprofloxacin under a visible-light system. Bi2WO6 and ZnO are activated under UV and visible-light systems, separating photogenerated electrons and holes. Photoelectrons from ZnO move towards the CB of Bi2WO6, and then these electrons are transferred into the CB of MXene. Firstly, MXene carried the photoelectrons towards active sites owing to its good metallic structure and produced H2O2 after reacting with adsorbed oxygen molecules. Furthermore, Bi2WO6 and ZnO also reacted with adsorbed molecules to produce H2O2 species, resulting in the generation of hydroxyl radicals. Secondly, holes in the VB of Bi2WO6 transferred towards ZnO VB and produced hydroxyl radicals after reacting with adsorbed water molecules that degrade ciprofloxacin pollutants [51]. In another study, TiO2/M-MXene photocatalyst was used to propose a photocatalysis mechanism for RhB. The photoelectrons in TiO2 move from VB towards CB, leaving holes in VB and producing photoelectrons and hole-pairs as shown in Figure 4 [52]. Similar photocatalysis mechanisms have also been investigated in other studies using TiO2/M-MXene for carbamazepine [53], CuFe2O4/M-MXene for sulfonamides [54], BLFMO-5/M-MXene hybrid for cango red [55], g-C3N4/S-MXene for RhB [56], CoO@TiO2/M-MXene, and Fe(Co)/M-MXene/ZSM-5 for phenol [57,58].
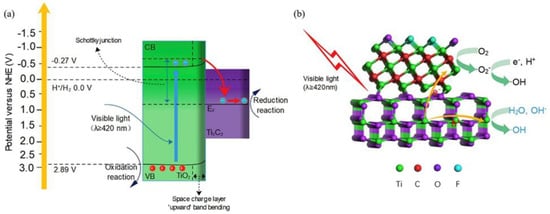
Figure 4.
(a) Charge transfer process in TiO2/M-MXene photocatalyst in visible-light system and (b) proposed photocatalytic degradation mechanism of RhB using TiO2/M-MXene. (Reprinted with permission from Ref. [52]. 2020 Elsevier).
3.2. Photocatalysis
The photocatalytic degradation of antibiotics and other different organic pollutants from wastewater has been considered the most effective method, owing to its simplicity, environment-friendliness, and short time-consuming nature [59,60,61]. In recent decades, pharmaceutical antibiotics have drawn increasing utilization in medical therapy and treatment worldwide. The annual consumption was reported to be 1 × 105 to 2 × 106 tons and was discharged to the aquatic environment [62]. Specifically, in 2019, the extensive consumption of antibiotics was accelerated due to the coronavirus disease 2019 (COVID-19) pandemic. Antibiotic pollutants are considered hazardous to human health and the environment to their water solubility, persistence, complex structure, pronounced chemical stability, and active metabolic residues [63].
Consequently, the development of antimicrobial resistance has appeared as a significant health problem for humans and other living creatures and the ecosystem disturbances in a natural water environment. The fast recombination of photo charge carriers’ efficiency and stability of a single photocatalyst minimizes its photo performance. Therefore, MXene assembled a co-catalyst to effectively minimize the photo charge carrier recombination effect and improve the photocatalytic power. Until now, several MXene and MXene-based photocatalysts have shown efficient photocatalytic performance for the remediation of different organic compounds from wastewater. Furthermore, previous studies have found that the combination of MXene and other co-catalysts provides abundant active sites and oxygen-containing functional groups that respond to the magnetic field. The magnetic field could boost the catalyst reactivity and enhance the degradation performance [64]. In literature, MXene-based photocatalysts have been widely tested for the effective degradation of antibiotics from wastewater. Cao et al. studied sulfamethazine photodegradation and compared the CuFe2O4, M-MXene, and CuFe2O4/M-MXene performances. The research suggested that CuFe2O4/M-MXene showed the best degradation performance with a reaction rate of 0.0128 min−1 compared to CuFe2O4 (k = 1.44 × 10−3 min−1) and M-MXene (k = 2.52 × 10−4 min−1) [54]. The mechanism of sulfamethazine degradation suggested by Cao et al. was •OH and H+ dominated the catalytic process, and dissolved oxygen generated •O2− to promote photocatalytic degradation. In another study in which sulfadimidine and tetracycline hydrochloride were degraded through the Ag2WO4/M-MXene composite photocatalyst and analyzed, it was demonstrated that the conductive properties of MXene can significantly enhance the photocatalytic power up to 88.6 and 62.9%, respectively. MXene may detect and transform into electrons, producing a stabilized Ag2WO4/M-MXene photocatalyst [65]. Sukidpaneenid et el. synthesized TiO2/M-MXene via a microwave-hydrothermal process in HCl/NaCl solutions, encouraging the development of fine TiO2 over the MXene parent structure. A TiO2/M-MXene photocatalyst was used to eliminate enrofloxacin antibiotic up to 93.4% in 4 h. The addition of NaCl played a substantial role during the synthesis of MXene-based photocatalysts by introducing sodium ions over the photocatalyst structure, improving photocatalytic power [66]. Sharma et al. reported the synthesis of a ZnO/Bi2WO6/M-MXene photocatalyst by a two-step electrostatic assembly process for the degradation of ciprofloxacin antibiotic up to 77% in 160 min under a natural sunlight system [51].
MXene-based photocatalysts have abundantly been employed to eliminate different types of dyes. The development of Z-scheme heterojunction photocatalysts could be an excellent photocatalytic agent. In this regard, Tu et al. prepared g-C3N4/S-MXene photocatalyst through a one-step H2O2 oxidation process. The development of Z-scheme heterojunction between MXene and g-C3N4 hybrid could have a solid power for a separate photogenerated recombination rate and is effective for the degradation of RhB and tetracycline up to 97.22 and 86.34%, respectively, in 60 min [56]. Literature showed that MXene has two-dimensional properties, good electrical performance, and surface hydrophilic functional groups, providing a great chance to build an excellent MXene hybrid. Sun et al. reported M-MXene-bridged-Ag/Ag3PO4 hybrid photocatalyst via an electrostatic self-assembly synthesis route. The synergetic impacts from MXene, Ag3PO4, and Ag effectively promote the photocatalytic activity for dye and Cr(IV) from wastewater [67]. Zhang et al. synthesized metal/M-MXene/ZSM-5 photocatalysts using a hydrothermal method to improve the visible-light absorption and catalytic power of ZSM-5 material. The incorporation of MXene and metals produced a narrow band gap energy, resulting in a superior photocatalytic activity for phenol up to 99% in just 25 min [57]. Therefore, an MXene-based catalyst is anticipated to be extended to a large variety of nanohybrids for the removal of different pollutants as demonstrated in Table 2.

Table 2.
Photocatalysis studies of different pollutants using MXene-based photocatalysts.
3.3. Adsorption Mechanism
In the adsorption mechanism, the adsorbate is strongly attributed to the surface of employed adsorbent until it has attained the equilibrium stage. The adsorption mechanism contains several significant steps such as (a) physical adsorption: settlement of adsorbate occurred over the surface of applied adsorbents, (b) complexation and precipitation: deposition of adsorbate occurred over the surface of the applied adsorbent, and (c) pore filling: condensation of adsorbate inside the pore of adsorbent. The adsorption mechanism has been defined in literature by employing different kinetic models. From the several kinetics equations, pseudo-first order, pseudo-second order, Elovich, and intraparticle diffusion kinetic model, equations were selected to understand the adsorption mechanism [78]. Ahsan et al. studied the adsorption mechanism of ciprofloxacin over sodium ion-based MXene adsorbent through different kinetic models. They revealed that the Elovich model provided the best understanding of the adsorption mechanism because of its higher R2 up to 0.99. Moreover, the chemisorption process is a rate-controlling step as experimental data obeying the Elovich equation [79]. Cai et al. selected pseudo-first-order and pseudo-second-order kinetic models to investigate the adsorption mechanism of MB and RhB dyes over phytic acid-based MXene composite and proposed that pseudo-second-order could be a more admirable model to explain the adsorption mechanism of both dyes providing a higher R2 up to 0.999 with a minimum difference between experimental and theoretical outcomes [80]. Similarly, other studies also proposed pseudo-second-order kinetics to understand the adsorption mechanism via MXene-based adsorbents [81,82,83,84].
The adsorption mechanism could be investigated more frequently through adsorption equilibrium studies by employing different isotherm models. The equilibrium relationship between adsorbate and adsorbent can be described using several isotherm equations that quantify the solute amount at room temperature. Langmuir, Freundlich, and Temkin isotherms have been the most commonly used models to produce isotherm profiles based on experimental results [61,85]. Yang et al. selected several isotherms to propose an interaction mechanism between Cr(IV) and S-MXene@IMIZ adsorbent. They revealed that the correlation coefficient R2 through Langmuir isotherm was higher up to 0.9949, which is closer to 1 than other isotherms. Therefore, Langmuir is a more suitable isotherm to explain the experimental data [86]. Moon et al. used ultrasound-assisted M-MXene adsorbent to propose the adsorption mechanism for MB dye through Langmuir and Freundlich isotherm equations. Their isotherm profiles described Freundlich isotherm as a more admirable model for explaining experimental data due to the higher R2 [87]. XPS analysis was also helpful in characterising the adsorption mechanism of 4-NP over hydrogel adsorbent. Their results revealed that several characteristic peaks, C1s, O1s and N1s, were significantly increased after the adsorption of 4-NP, indicating significant adsorption of 4-NP. The fitting peaks of C-O and C=O in O1s proposed that the adsorption mechanism mainly depends on hydrogen bonding between the adsorbent and the targeted pollutant.
Moreover, N-H could be a proper fitting group to improve the adsorption mechanism. Similarly, Dao et al. also explained the adsorption mechanism of tetracycline using alkalized M-MXene adsorbent, as shown in Figure 5. The weak electrostatic interaction is the main force responsible for the adsorption of tetracycline to an opposing charged adsorbent surface without metal use. The electrostatic interactions between negatively charged alkalized M-Mxene and positively charged metal ions become powerful by adding metal ions to the system. The surface modification of alkalized M-Mxene occurs through metal ions and produces a ternary complex of alkalized M-Mxene-metal ions-tetracycline to enhance the adsorption rate of tetracycline [88].
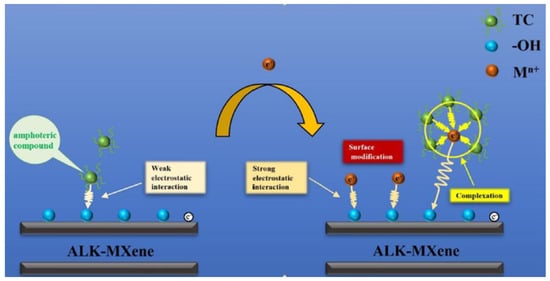
Figure 5.
Adsorption mechanism of tetracycline using alkalized M-MXene adsorbent. (Reprinted with permission from Ref. [88]. 2022 American Chemical Society).
3.4. Adsorption
The adsorption process provides reasonable compensations such as safe operations, recycling and remediation to the required concentration range. Moreover, the adsorption technique can provide an excellent removal rate for it to be commercially viable [76,86]. Due to the rapid development of industrialization, dye wastewater originating from textile, paper, and printing activities affected the ecological environment and human health. Dye pollutants are a family of organic compounds with high mobility, substantial toxicity, and poor biodegradability [1]. The aromatic molecular makeup of dyes were reported as cancer-causing and mutagenic, whereas the metal-containing dyes can cause severe renal system damage [10]. Many strategies were attempted to address this issue, primarily through adsorption, a potentially promising technique due to the easy operation and economical and energy saving [89]. Therefore, an MXene-based catalyst is a suitable choice to treat dye-polluted wastewater due to the surface functional group structures, effectively absorbing and activating the dye during adsorption activity. In past research, MXene and MXene-based materials have been widely employed to treat different pollutants through adsorption.
Enhancing the effectiveness of MXene, Wei et al. studied varied alkali functionalizations of MXene on dye adsorption performance. They reported that the alkali functionalization assists the expansion of the interlayer spacing of M-MXene and the surface functional group transformation, which enhances the adsorption capability and accelerates the removal rate for MB compared to pristine MXene [90]. The research proposed that NaOH-M-MXene obtained the fastest removal rate with higher methylene removal capacity (189 mg/g) compared to LiOH-M-MXene (121 mg/g), pristine-M-MXene (100 mg/g), and KOH-M-MXene (77 mg/g). The experimental data suggested most MXene-based catalysts’ interaction with dyes were monolayered in form and best described with Langmuir isotherm [91]. A M-MXene layer was composed of concentrated HF and pillared with terephthalate through a rapid direction method and showed the highest adsorption rate of 209 mg/g for MB owing to a higher surface area of 135.7 m2/g and greater interlayer spacing between free carboxylate groups of terephthalate and M-MXene sheets [92]. S-MXene@IMIZ composite was developed through the overlaying of polyimidazole chains over the MXene surface via a multicomponent process using a renewable chitosan reactant. The adsorption process showed that Cr(IV) was transformed into Cr(III) with a lower toxicity effect [86]. MXene nanosheets were incorporated with sodium ions through a fabrication process and used to treat ciprofloxacin antibiotics. Their adsorption results revealed that fast antibiotic and kinetics process removals are obtained through surface termination and spacing between sodium ion-based MXene layers [79]. In another research, tetracycline antibiotic was adsorbed from wastewater through hydroxyl-rich surface alkalized M-MXene adsorbent, prepared through an alkaline intercalation process. The possible influence of Cu(II), Co(II), Ni(II), Cd(II), and Cr(II) metal ions over the removal of antibiotics was investigated through alkalized M-MXene adsorbent. Their adsorption outcomes confirmed that metal ions showed a special removal rate, and the adsorption rate of antibiotics was successfully achieved up to 750% in the presence of Ni(II) metal ions. This highest adsorption happened at the hydroxyl site over alkalized M-MXene surface and showed improvement in surface complexation. Furthermore, increased antibiotic adsorption was also associated with the surface modification of the metal ions mechanism [88]. A summary of various MXene and MXene-based materials applications for different target pollutants removal is presented in Table 3.

Table 3.
Adsorption of different organic pollutants using MXene-based adsorbents.
3.5. Water Splitting Mechanism
The photocatalytic process is a chemical reaction that can be encouraged through photoirradiation under a suitable photocatalyst material. This kind of photocatalyst will have a solid ability to improve the chemical reaction without being used or converted into another phase. The basic working principle of the photocatalytic mechanism for H2 production via water splitting is simple, as shown in Figure 6. Initially, when light with energy more significant than the band gap of the photocatalyst is irradiated, the empty conduction band (CB) and the filled valence band (VB) are separated. The electrons in the VB are excited by the CB, and the electrons (e−) and hole pairs (h+) are generated. These e− and h+ reduce and oxidize the chemicals over the photocatalyst surface, respectively, unless they recombine to avoid clean chemical reactions. When the same amount of e− and h+ is used for a chemical reaction and recombination, the photocatalyst’s original structure (or chemical composition) does not change. Recently, several MXene-based photocatalysts have been proposed for photochemical reaction mechanisms to produce H2 under a visible-light activation system. Molybdenum@TiO2@M-MXene photocatalysts have effectively transformed and separated photoinduced electrons and hole pairs under a visible-light system, as shown in Figure 6. In the photocatalytic mechanism, a maximum number of photogenerated electrons inside the CB of TiO2 may quickly migrate towards the MXene side MoS2 impurity bandgap caused by molybdenum vacancies, resulting in an electron-rich environment over the surface planes of MoS2 and Mxene, via which H2 is produced through the reduction of water molecules [101]. In another study, ultra-small TiO2QDs were designed on S-MXene for a photocatalytic H2 mechanism via the water splitting process. In a proposed mechanism, photogenerated electrons excited from TiO2QDs transition from the CB to VB of TiO2QDs. Owing to its low interfacial resistance, e− rush from TiO2QDs towards S-MXene, facilitating an efficient charge separation effect. The metal-like properties of MXene make it easy to transfer photogenerated electrons to MXene. Consistent with this, the charge transfer path is significantly increased. This accelerates the separation of photogenerated charge carriers and promotes photocatalytic H2 formation [102]. A similar photocatalytic mechanism for H2 production via water splitting has been proposed in other research works using MXene-based photocatalysts [103,104,105,106,107].
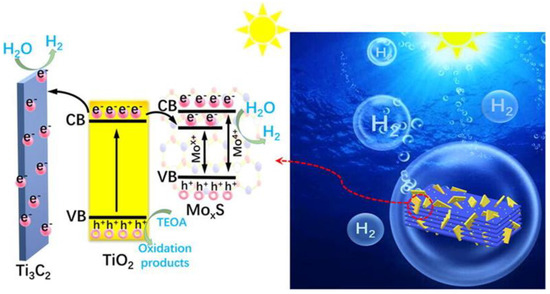
Figure 6.
A photocatalytic mechanism for H2 production using MoXS/MXene via water splitting. (Reprinted with permission from Ref. [101]. 2020 Elsevier).
3.6. Water Splitting
In addition to the versatile photocatalytic decomposition of pollutants, MXene-based catalysts significantly contribute to sustainable clean energy production. Environmental contamination and the energy crisis were global issues due to the rapid consumption of fossil fuels. Fossil fuel reserve depletion times for oil, coal, and gas are approximately 35, 107, and 37 years, respectively, and are computed by the modified formula from the Klass model [108]. Therefore, photocatalytic water-splitting technologies are an excellent way to replace fossil fuels such as hydrogen and oxygen. MXene has the potential as a promising candidate for hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) due to its unique features: (1) excellent metal conductivity, (2) higher hydrophilic functionalities, and (3) significant redox reactivity [25].
Wang et al. were one of the earlier researchers on MXene-based catalysts for hydrogen production. They reported that TiO2/M-Ti3C2Tx achieved the highest hydrogen evolution rates with 17.8 μmol g−1 h−1 compared to pure TiO2 (~5 μmol g−1 h−1) and pristine M-Ti3C2Tx (~1 μmol g−1 h−1) [109]. Ti3C2Tx in the studies was suggested to provide a 2D platform for intimate interactions with uniformly grown TiO2 nanoparticles and to promote the separation of photogenerated charge carriers. On the other hand, MXene, as a cocatalyst, helps to accelerate surface redox reactions and enhances charge transfer [110]. As the MXene-based catalysts hybrid technique has been developed and performed in water splitting, these new nanomaterials were proven excellent for HER and low-cost and efficient OER electrocatalysts. According to the literature, MXene–having −O termination–acted as an electron cocatalyst to enhance the water-splitting performance of semiconductors such as CdS, TiO2, and g-C3N4 [103,111].
On the other hand, many researchers believed that MXene with −OH surface adsorption could play a different role in the interfacial transfer of photogenerated electron hole-pairs [104]. Recently, the −OH terminated MXene system has been effectively utilized as a hole moderator for enhancing photo charge carrier and H2 efficiencies, such as Cu/TiO2@M-MXene [112] and PTO/TiO2@M-MXene [113]. In the same outline, Chang et al. have successfully developed anatase/rutile TiO2 over M-MXene via a hydrothermal process for efficient H2 generation of 4672 μmol/hrg, owing to optimum synthesis conditions that is 3.76 times greater than pure TiO2 [114]. In another research, M-MXene/ZXC1-XS have been successfully produced to attain excellent H2 generation. Their experimental results revealed the highest H2 evolution rate up to 14.17 mmol/hr owing to their collaborative influence of enhanced charge hole-pairs departure performance, oxidation potential, and enhancement of visible-light absorption capability [105]. Li et al. have developed a dynamic and stable MAPbl3/S-MXene photocatalyst system through the etching and exfoliation synthesis methods and received the highest H2 evaluation rate up to 3124 μmol/hrg6 times greater than that of pure MAPBI3 [106]. From the above discussions, it can be concluded that MXene is a universal cocatalyst that can improve the generation rate of H2 by providing different active sites. The application of MXene-based catalysts for water splitting is summarized in Table 4.

Table 4.
Summary of MXene-based catalysts for water splitting.
4. MXene-Based Nanomaterials
MXene-based photocatalysts are created by combining an MXene photocatalyst with another type of photocatalyst or co-catalyst [126]. Improved space charge transfer and separation can be achieved by combining MXene with semiconductors and selecting optimal CB and VB potentials. These nanostructures include 0D/2D, 1D/2D, 2D/2D heterostructures, and other combinations. The band alignment of an MXene-based photocatalyst with a different type of photocatalyst can be tuned to one of three different types. There are three varieties of heterostructure band alignment, as indicated in Figure 7: Type I (straddling gap), Type II (staggered gap), and Type III (broken gap) [127]. The CB and VB are combined in one layer on Type I, resulting in a straddling band alignment. As a result, when the linked photocatalyst absorbs photon energy equal to or greater than its band gap energy, electron-hole pairs in a single component within the heterostructure photocatalyst separate and migrate [128]. As a result, because the CB and VB of component B are at a lower and greater energy than component A, electrons and holes will be transported and accumulate on component A. Two separate CB and VB monolayers contribute to the Type II heterostructure, resulting in upward or downward band bending [70].
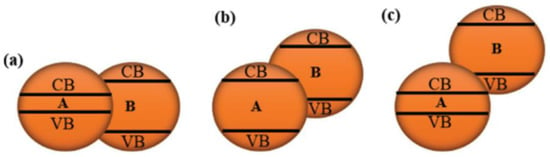
Figure 7.
Band alignment of heterostructures (a) Type I, (b) Type II, and (c) Type III. (Reprinted with permission from Ref. [127]. 2017 John Wiley and Sons).
Component B’s VB and CB potentials are higher than component A’s in Type II heterostructures due to the staggered band structure seen in Figure 7b [129]. Owing to the more negative CB position of B, photoexcited electrons (e−) can flow from B to A, whereas holes (h+) can travel oppositely from the more positive VB of A to B, resulting in overall efficient charge separation and improved photocatalytic activity [127]. On the other hand, the Type III heterostructure has no band gap or broken gap. Both the CB and VB of the coupled photocatalyst are not crosslinked in this situation, resulting in a gap between both band alignments in the heterostructure photocatalyst, which provides more substantial driving power for charge transfer [130]. Significant improvements in photocatalytic activity have been experimentally demonstrated using various MXene-based composites such as TiO2-MXene, g-C3N4-MXene, and BiVO4-MXene. The most widely used practice for synthesized photocatalyst composites is mechanical mixing, self-assembly, and in-situ decoration/oxidation [131]. Mechanical mixing offers electrostatic attraction in which negatively charged MXene is combined with a positively charged photocatalyst. At the same time, in situ decoration/oxidation provides a strong chemical bonding, which was essential in the particular design of applications. In general, the MXene-based heterostructure developed by coupling the MXene with other types of photocatalysts as a co-catalyst improves the electronic and optical properties of MXene [132].
4.1. MXene/TiO2 Composites
Titania, TiO2 was the most favourable and environmentally friendly promising photocatalyst, with a high photocatalytic activity that is closely related to its lattice structure. In this regard, this photocatalyst was capable of photoactivity reaction, a solid oxidising power, and high chemical/photo-stability. In the tetragonal unit cell of anatase, each titanium Ti atom is surrounded by six octahedra oxygen, O atoms. Although there are advantages such as a high carrier-forming capacity, toxicity, and good stability, TiO2 charge carriers tend to show rapid rearrangement and poor performance in visible light owing to their considerable band gap energy, 3.2 eV [133]. Anatase TiO2 also absorbs very little sunlight in the UV region (approximately 5%) owing to its considerable band gap energy [22]. Zhuang et al. produced a M-MXene/TiO2 composite using an electrostatic self-assembly technique and tested it for H2 evolution. During this preparation, Ti3AlC2 was etched by hydrofluoric acid (HF) 50 wt. % at room temperature for 2 h, as shown in Figure 8 [17]. MXene/TiO2 produced good photocatalytic power for H2 production owing to several factors, such as its enhancement in charge separation performance, reactive facet (101), and extension of light absorption [134]. The increased H2 evolution could be due to the heterogenous interface between MXene and TiO2 nanofibers [17]. MXene/TiO2 composites have also been tested for environmental applications owing to carbon and titanium compounds that greatly benefit environmental remediation. Siwanat et al. used the microwave hydrothermal method to prepare M-MXene/TiO2 composite in NaCl/HCl solutions and used it for the successful treatment of enrofloxacin from water. The addition of NaCl played a significant role in providing sodium ions, which intercalated over M-MXene/TiO2 composite to enhance the adsorption and photocatalysis performance of antibiotics from water. Furthermore, the maximum removal of antibiotics over M-MXene/TiO2 could be formed by hydroxyl radicals [66].
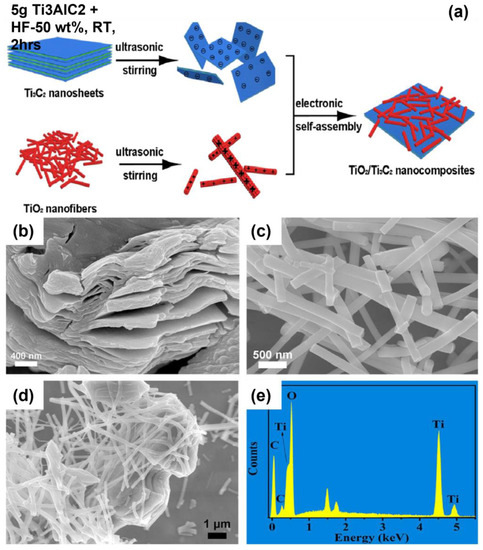
Figure 8.
(a) Schematic description for the synthesis of M-MXene/TiO2 composite SEM images of (b) TiO2, (c) MXene, and (d,e) MXene/TiO2 nanofibers. (Reprinted with permission from Ref. [17]. 2019 Elsevier).
Therefore, a number of strategies were conducted to inhibit the electro-hole pairs recombination, such as metal doping and coupling with other semiconductors, metal oxide, and carbon materials. Superior conductive MXene with known narrow bandgap energies would facilitate receiving electrons generated from other semiconductors [135]. The migration of generated electrons from TiO2 is favourable to increasing charge carriers’ separation efficiency and inhibiting electron-hole recombination. On the other hand, TiO2 nanoparticles act as spacers [136]. Table 5 shows TiO2/MXene composites with various synthesis techniques and their key findings.

Table 5.
Summary of TiO2-MXene-based photocatalyst synthesis techniques.
4.2. MXene/g-C3N4 Composites
Graphitic carbon nitride (GCN), g-C3N4, is a metal-free photocatalyst with a medium band-gap energy of 2.7 eV, [141]. The polymeric nature of g-C3N4 was structurally analogous to graphene, which was considered the most stable C3N4 type at ambient conditions. In addition, this photocatalyst is also highly resistant to thermal and chemical environments, making it a stable material. Furthermore, this nature allows for multiple excitations from a single photon’s absorption, thus effectively producing reactive species for pollutant degradation. Ideally, the compositions of g-C3N4, consisting only of carbon and nitrogen atoms were reported with a C/N molar ratio of 0.75 [142]. In addition, g-C3N4 has a surface termination as defects and nitrogen atoms for electron localization. It has a unique microstructure in which anchoring inorganic and organic functional groups act as active sites [143].
Conversely, g-C3N4 suffers from easy recombination of photo-generated electron-hole pairs, which limits its sorption capacity due to the low specific surface area and quantum efficiency [144]. Moreover, the non-utilization of visible light (450 nm) and low efficiency of charge separation and transfer of g-C3N4 itself restrict its further applications. To overcome the bulk individual g-C3N4, more light collectors and charge transfer tunnels need to implement in the photocatalyst composites to boost their photocatalytic activity [145]. Yujie et al. reported the preparation of S-MXene/g-C3N4 composite using an electrostatic attraction approach and tested it for H2 production, as shown in Figure 9a. The successful synthesis of S-MXene/g-C3N4 composite was confirmed through TEM analysis, as shown in Figure 9b,c. In characteristics, MXene produced a higher surface area of g-C3N4 and facilitated the density of active sites.
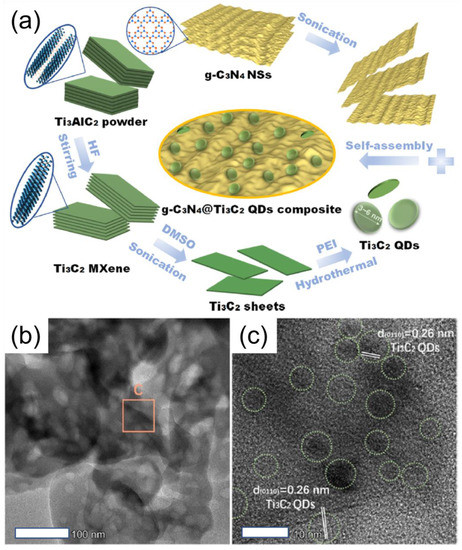
Figure 9.
(a) Schematic description for the preparation of Mxene/g-C3N4 composite using electrostatic attraction approach and (b,c) TEK images of Mxene/g-C3N4 composites. (Reprinted with permission from Ref. [148]. 2019 American Chemical Society).
Moreover, MXene showed good electronic conductivity, which could enhance the composite’s photo charge transfer performance [146]. In another research, Syahmi et al. prepared an M-MXene/g-C3N4 heterostructure photocatalyst with different loading of M-MXene from 1 to 12 wt. % using a wet impregnation process. Their photodegradation results revealed that 1 wt. % M-MXene/g-C3N4 provided the highest removal of MB under visible light owing to its excellent photo charge carrier between MXene and g-C3N4 and higher surface area properties [147]. It is believed that the combination of MXene and g-C3N4 could significantly improve the photocatalytic performance for H2 production and water remediation. Various reports have verified the advantages and excellence of g-C3N4-Mxene-based photocatalysts in the wide photocatalytic application, as summarized in Table 6. Generally, hybridization aims to improve photocatalyst-specific surface area and charge separation.

Table 6.
Summary of g-C3N4-Mxene based photocatalysts synthesis technique.
4.3. MXene/BiVO4 Composites
Bismuth vanadate (BiVO4) is a photocatalyst material with various intrinsic features such as a suitable band gap, proper band location, excellent stability, and a friendly environment. Photocatalytic properties of BiVO4 are strongly dependent on its crystal structure, which originates from the mineral pucherite in an orthorhombic structure [154]. Its improved photocatalytic performance was due to the better optical absorption achieved with a monoclinic scheelite structure with a band gap energy of 2.4 eV compared to a tetragonal structure with 2.9–3.1 eV [155]. This is the most significant photocatalyst for visible light water oxidation and photodegradation due to the narrow band gap, which can effectively oxidize water to release oxygen and generate active substances [156]. Primarily, BiVO4-based materials were extensively used for toxic pigments in the coating and plastic industry and have been shifted into photocatalytic applications due to their intrinsic crystal structure [157].
Nevertheless, the position of the conduction band of BiVO4 (ECB = 0.3 eV) in a more favourable spot severely limits the reduction capacity of BiVO4 and constraints in photocatalytic environmental remediation application [158]. Consequently, modest photocatalytic performance was observed in pristine BiVO4 due to the low electrical conductivity. On the other hand, slow water oxidation kinetics of BiVO4 occur due to the photogenerated holes on the photocatalyst surface, promoting the fast recombination of electron-hole pairs [127]. Herein, by modifying BiVO4 photocatalysts with MXene by in-situ engineered heterojunctions, the hybrid composite system can control the charge transfer process. Co-catalyst modification was also a primary fashion to accelerate surface redox kinetics. Therefore, the photo-generated holes migrated to the surface of MXene and generated an oxidising reaction. Yan et al. prepared M-MXene/BiVO4 composites through a spin coating of M-MXene over BiVO4 film surface to improve their kinetics for oxygen evolution purposes, as shown in Figure 10a, and TEM images are shown in Figure 10b,c. The thin coating of M-MXene acts as a co-catalyst to promote photo charge transfer carrier performance and reduce the photogenerated recombination rate to improve water oxidation performance [159]. The synthesis technique and critical findings of various BiVO4-Mxene-based photocatalysts are summarized in Table 7.
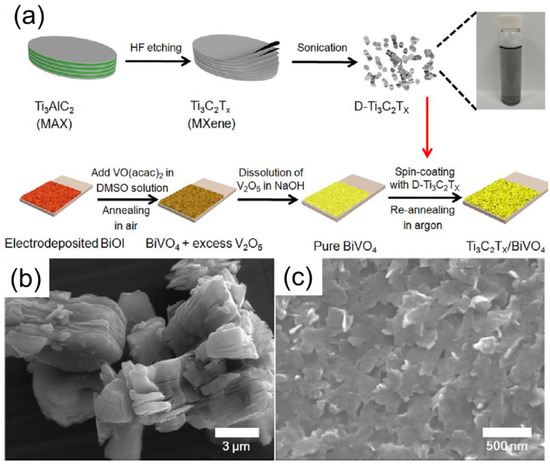
Figure 10.
(a) Schematic description for the synthesis of M-Mxene/BiVO4 composites and (b,c) TEM images of M-MXene/BiVO4. (Reprinted with permission from Ref. [159]. 2019 Elsevier).

Table 7.
Summary of BiVO4-MXene based photocatalysts synthesis technique.
5. Multi-Roles of MXene-Based Nanomaterials
5.1. Photocharge Separation and Transfer Role
In photocatalysis, improving the separation efficiency of photogenerated charge carriers has been a hot research topic. MXene, as a co-catalyst, could prevent photogenerated electrons and holes from recombination in the photocatalytic system. The Schottky junction might be experimentally established between semiconductor photocatalysts and MXene [163]. According to theoretical estimates, electrons could move from g-C3N4 to MXene in their composites due to the difference in their Fermi levels (EF) and the development of Schottky junctions. Furthermore, because MXene showed strong metallic conductivity and favourable H adsorption-free energy, H+ may be converted to H2, resulting in a significantly higher H2 production. Ag3PO4 has a high carrier recombination rate during the photocatalytic process and is readily converted to elemental silver. In light of this, a Schottky junction was built, and MXene was introduced as a co-catalyst to improve the photocatalytic performance and stability of Ag3PO4. Surprisingly, after eight cycles, the photocatalytic clearance rate of tetracycline hydrochloride solution (TC-H) over the Ag3PO4/MXene composite marginally decreased, indicating improved anti-photo corrosion efficacy and photocatalytic activity.
5.2. Photocatalytic Active Sites
The surface characteristics of materials are widely recognized to significantly impact their adsorption and photocatalytic activities. MXene and MXene-based materials have 2D heterostructures that increase the contact area with other 0D, 1D, and 2D materials and provide more surface reaction sites for photocatalysis applications. MXene etched using HF solution typically has a large number of −F terminations, but those etched with a mix of HCl and LiF have a large number of −O terminations. Ran et al. studied the influence of surface terminations on the photocatalytic property of MXene composites. They found that changing −F to −O/−OH enhanced the number of active sites, enhancing the photocatalytic H2 evolution [164]. Sun et al. also showed that MXene with −O terminations facilitated the separation of photogenerated charge carriers, resulting in a significant increase in H2 generation. Density functional theory (DFT) simulations revealed that MXene with −O terminations had a lower Gibbs free energy and improved H2 production rate than MXene with −F terminations. Although MXene with homogenous surface terminations has not been synthesized in the lab, the relative concentration of surface terminations can be controlled to improve photocatalytic stability [165].
6. Economic and Eco-Friendly Feasibility
MXene-based nanomaterials are attracting researchers’ interest for use in various disciplines due to their low cost of manufacturing and use and environmental friendliness. Figure 11 depicts MXene-based nanomaterials’ use to remove several contaminants. Different dyes such as methylene blue (MB), saffranine T (ST), neutral red (NR), antibiotics, heavy metals, and phenolic compounds are hazardous to the atmosphere and community. MXene-based nanomaterials’ active surface area, layered structure, and charged surface aid in removing hazardous substances from aqueous solutions. Several heavy metals, such as Pb(II), Cu(II), Cd (II), Cr(IV), and Hg(II), are inorganic and organic chemicals that are difficult to break down and accumulate in living things. MXene-based nanoparticles are used in this application because they include oxygen-containing groups and have a more extensive surface area for removing irons from effluent perfectly. Human respiratory infection and other health problems are caused by wastewater and certain volatile organic chemicals. MXene-based nanomaterials’ features, such as high adsorption energy and selectivity, help to remove toxic organic chemicals and hazardous water pollutants. Pu ions, uranium (II), and Neptune (Np) are radioactive constituents that are radiologically and chemically hazardous. They pollute the environment with nuclear waste. MXene-based nanomaterials are used to eliminate these radioactive materials because of their remarkable ability to resist higher radiation and good chemical compatibility. MXene-based nanomaterials also remove contaminants such as phosphates in the water, phenol, and highly charged cations, owing to their active surface morphology.
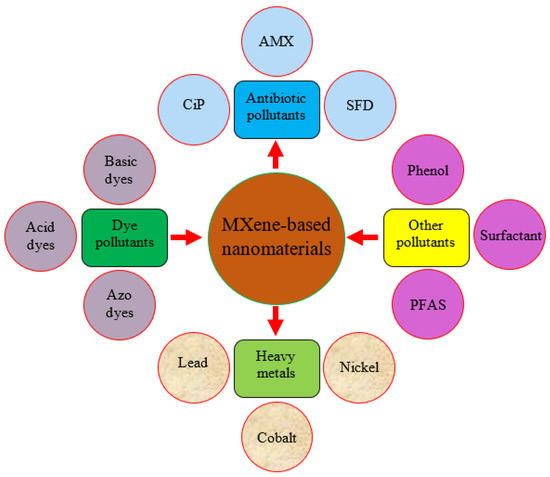
Figure 11.
Applications of MXene-based nanomaterials for the remediation of different pollutants from wastewater.
7. Regeneration Studies of MXene-Based Nanomaterials
When evaluating an adsorbent’s performance and cost-effectiveness, its ability to be recycled is a significant issue to consider. The adsorption capacities are highest in the early levels due to the accessibility of a greater surface area that gives free binding cores for the sorption of adsorbent materials. However, as the number of adsorption-regeneration rounds grows, pollutant removal and regeneration effectiveness decline. Several studies have investigated the regeneration studies of MXene-based nanomaterials, proving their extraordinary recyclability. Even more surprisingly, high-energy ball milling was discovered to resuscitate a few MXene-based adsorbents that absorb organic pollutants. Organic compounds do mineralize to graphitic and amorphous carbon during high-energy milling. This means that carbon-based components like carbides could be quickly revived using MC techniques. Vakili et al. evaluated the adsorptive characteristics of MXene sheets pillared by terephthalate. They also looked into whether or not the used adsorbent (after adsorption) could be reformatted into the MAX phase. After the colour had been absorbed, the wasted material was mostly titanium and carbon. A large percentage of this is due to the carbide MXene, but terephthalate pillars and methylene blue also play a role. As a result, the wasted adsorbent contained two of the three MAX phase components [92].
The used material was mixed with the appropriate amount of Al powder to resuscitate the MAX phase via MC manufacturing, and the MXene was then MC etched. The results show that the XRD pattern of the revived MXene was identical to that of the MXene produced from the MAX phase formed with graphite, demonstrating that T-MX can be regenerated indefinitely utilizing the MC process (passing through MC etching). As a result, the authors suggest that recyclable materials can be reused endlessly to eliminate organic contaminants from wastewater while ensuring their safe disposal. Furthermore, the recyclability of MXene for the removal of Cs ions was examined using 0.2 mol of HCl to desorb the particular ions. When the HCl solution was added, the number of H ions increased. The target contaminant and H ion adsorbed over MXene fought for exploitable surface sites, causing the pollutant to desorb from the MXene. In this experiment, the adsorbent removal percentage stayed over 90% after 5 days. The regenerations’ characterization remained essentially unchanged. However, when reincarnations increased, the removal efficiencies declined marginally, possibly due to a reduction in available functional sites on the adsorbent’s interface.
Furthermore, Shahzad et al. studied MXene by treating it with HNO3 or calcium nitrate solution (Ca(NO3)2) and regenerating the depleted DL-MXene by the treatment. On the other hand, the adsorbent was used to remove Cu(II) from the water, which was then recycled. The MXene adsorption rate after the initial regeneration set was 80%, but after the second and third studies, the adsorption rate dropped to around 47% and 33%, respectively, which is a considerable decrease compared to the results of the similar study [97]. Furthermore, Dong et al. investigated the regeneration capacities of cross-linked and un-cross-linked MXene-based materials, finding that cross-linked MXene (with a reusability potency of roughly 10 cycles) could achieve a greater adsorption rate than un-cross-linked MXene [166].
8. Conclusions and Future Challenges
MXene-based photocatalysts have emerged as promising candidates in developing advanced photocatalytic systems to address growing global concerns regarding clean water and energy sources, owing to their unique characteristics, composition, and tunable structural properties. The method of synthesizing MXene-based photocatalysts and their environmental remediation applications for uses such as antibiotic removal, adsorbents, photocatalysts for dye removal, and water splitting have been discussed extensively to better understand their characteristics. The photocatalytic performances shown by MXene-based photocatalysts were mainly governed by their fascinating features such as adjustable morphology and bandgap, high hydrophilicity, great thermal and chemical strength, and large specific surface area. Consequently, the broad application of MXene as a co-catalyst was explored, for instance in TiO2-MXene, g-C3N4-MXene, and BiVO4-MXene. Current research exertions have been dedicated to improving the biocompatibility, stability, and ecological power of MXene in the aquatic organism to boost its effectiveness in water remediation applications. Initial research established that MXene has higher a biocompatibility with no toxicity, even at higher concentrations. It is urgent to comprehensively examine possible threats associated with MXene materials.
Although much recent research and studies of photocatalytic performances were conducted on MXene-based photocatalysts, they have been widely utilized as adsorbents and photocatalysts for the successful remediation of different organic pollutants such as dyes and metal ions from wastewater. Still, this new promising nanomaterial is yet to be fully exploited and less studied compared to other two-dimensional and graphene nanomaterials. Additional improvements on the expansion of MXene-based photocatalysts were desired, such as (1) developing safe synthesis routes with low cost and high MXene yield, (2) introducing spacers to control the interlayer spacing of MXene-based photocatalysts which avoid the aggregation and stacking of MXene sheets, and (3) analyzing the photocatalytic performances of MXene-based photocatalysts using natural industrial wastewater and practical applications. It is incredibly significant to discover newly developed MXene nanomaterials, which could be more environmentally effective in wastewater-related applications. Subsequently, different MXene nanomaterials are already available and have been theoretically projected. More MXene nanomaterials can develop and become accessible for water remediation and other environmental applications. More research is required to cover the gap between newly proposed and experimentally possible MXene nanomaterials, with the hope of signifying the applicant structure with improved thermal, physical, chemical, and catalytic characteristics, which can be more appropriate for water purification and other environmental remediation. Experimental confirmation of modelling estimation is a significant way to facilitate the preparation of structures with improved electrocatalytic, photocatalytic, and other helpful characteristics. These additional efforts will open new routes for commercial applications for MXene.
Author Contributions
N.S., writing and original draft preparations, review and editing; M.Z., review and editing; M.F.R.S., conceptualization and review; S.S., conceptualization, comprehensive revision and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Higher Education (MOHE), grant number FRGS/1/2020/TK0/UTP/02/22 and Yayasan Universiti Teknologi PETRONAS (YUTP), grant number 015LC0-357.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sun, B.; Dong, X.; Li, H.; Shang, Y.; Zhang, Y.; Hu, F.; Gu, S.; Wu, Y.; Gao, T.; Zhou, G. Surface charge engineering for two-dimensional Ti2CTx MXene for highly efficient and selective removal of cationic dye from aqueous solution. Sep. Purif. Technol. 2021, 272, 118964. [Google Scholar] [CrossRef]
- Jin, X.C.; Liu, G.Q.; Xu, Z.H.; Tao, W.Y. Decolorization of a dye industry effluent by Aspergillus fumigatus XC6. Appl. Microbiol. Biotechnol. 2007, 74, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Department of Environment. Environmental Requirements: A Guide for Investors; Department of Environment: Putrajaya, Malaysia, 2010; pp. 1–77. [Google Scholar]
- Vunain, E.; Mishra, A.K.; Mamba, B.B. Dendrimers, mesoporous silicas and chitosan-based nanosorbents for the removal of heavy-metal ions: A review. Int. J. Biol. Macromol. 2016, 86, 570–586. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Q.; Zhang, B.; Tian, L.; Li, K.; Zhang, X. Recent advances in transition metal carbide electrocatalysts for oxygen evolution reaction. Catalysts 2020, 10, 1164. [Google Scholar] [CrossRef]
- Zhang, C.J.; Nicolosi, V. Graphene and MXene-based transparent conductive electrodes and supercapacitors. Energy Storage Mater. 2019, 16, 102–125. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Z.; Shen, P. Are MXenes Promising Anode Materials for Li Ion Batteries? Computational Studies on Electronic Properties and Li Storage Capability of Ti3C2 and Ti3C2X2 (X = F, OH) Monolayer. J. Am. Chem. Soc. 2012, 134, 16909–16916. [Google Scholar] [CrossRef]
- Mashtalir, O.; Cook, K.M.; Mochalin, V.N.; Crowe, M.; Barsoum, M.W.; Gogotsi, Y. Dye adsorption and decomposition on two- dimensional titanium carbide in aqueous media. J. Mater. Chem. A Mater. Energy Sustain. 2014, 2, 14334–14338. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, H.; Xiao, J.; Liu, D.; Liu, Z.; Qian, Y.; Xi, H. Adsorptive separation of methanol-acetone on isostructural series of metal-organic frameworks M-BTC (M = Ti, Fe, Cu, Co, Ru, Mo): A computational study of adsorption mechanisms and metal-substitution impacts. ACS Appl. Mater. Interfaces 2015, 7, 26930–26940. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, L.; Chen, K.; Zhang, Y.; Deng, Q.; Du, S.; Huang, Q.; Zheng, L.; Zhang, J.; Chai, Z.; et al. Loading Actinides in Multilayered Structures for Nuclear Waste Treatment: The First Case Study of Uranium Capture with Vanadium Carbide MXene. ACS Appl. Mater. Interfaces 2016, 8, 16396–16403. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, P. Catalyst: NH3 as an Energy Carrier. Chem 2017, 3, 709–712. [Google Scholar] [CrossRef]
- Kumar, J.A.; Prakash, P.; Krithiga, T.; Amarnath, D.J.; Premkumar, J.; Rajamohan, N.; Vasseghian, Y.; Saravanan, P.; Rajasimman, M. Methods of synthesis, characteristics, and environmental applications of MXene: A comprehensive review. Chemosphere 2022, 286, 131607. [Google Scholar] [CrossRef] [PubMed]
- Hantanasirisakul, K.; Gogotsi, Y. Electronic and Optical Properties of 2D Transition Metal Carbides and Nitrides (MXenes). Adv. Mater. 2018, 30, 1804779. [Google Scholar] [CrossRef] [PubMed]
- López-Grimau, V.; Gutiérrez, M.C. Decolourisation of simulated reactive dyebath effluents by electrochemical oxidation assisted by UV light. Chemosphere 2006, 62, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Liu, Y.; Meng, X. Fabrication of TiO2 nanofibers/MXene Ti3C2 nanocomposites for photocatalytic H2 evolution by electrostatic self-assembly. Appl. Surf. Sci. 2019, 496, 143647. [Google Scholar] [CrossRef]
- Jaffari, Z.H.; Abuabdou, S.M.A.; Ng, D.Q.; Bashir, M.J.K. Insight into two-dimensional MXenes for environmental applications: Recent progress, challenges, and prospects. FlatChem 2021, 28, 100256. [Google Scholar] [CrossRef]
- Prasad, C.; Yang, X.; Liu, Q.; Tang, H.; Rammohan, A.; Zulfiqar, S.; Zyryanov, G.V.; Shah, S. Recent advances in MXenes supported semiconductors based photocatalysts: Properties, synthesis and photocatalytic applications. J. Ind. Eng. Chem. 2020, 85, 1–33. [Google Scholar] [CrossRef]
- Ronchi, R.M.; Arantes, J.T.; Santos, S.F. Synthesis, structure, properties and applications of MXenes: Current status and perspectives. Ceram. Int. 2019, 45, 18167–18188. [Google Scholar] [CrossRef]
- Ibrahim, Y.; Meslam, M.; Eid, K.; Salah, B.; Abdullah, A.M.; Ozoemena, K.I.; Elzatahry, A.; Sharaf, M.A.; Sillanpää, M. A review of MXenes as emergent materials for dye removal from wastewater. Sep. Purif. Technol. 2022, 282, 120083. [Google Scholar] [CrossRef]
- Im, J.K.; Sohn, E.J.; Kim, S.; Jang, M.; Son, A.; Zoh, K.D.; Yoon, Y. Review of MXene-based nanocomposites for photocatalysis. Chemosphere 2021, 270, 129478. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, C.; Que, W. A novel two-dimensional accordion-like titanium carbide (MXene) for adsorption of Cr(VI) from aqueous solution. J. Adv. Dielectr. 2018, 8, 1850035. [Google Scholar] [CrossRef]
- Qu, J.; Teng, D.; Zhang, X.; Yang, Q.; Li, P.; Cao, Y. Preparation and regulation of two-dimensional Ti3C2Tx MXene for enhanced adsorption–photocatalytic degradation of organic dyes in wastewater. Ceram. Int. 2022, 48, 14451–14459. [Google Scholar] [CrossRef]
- Cao, S.; Shen, B.; Tong, T.; Fu, J.; Yu, J. 2D/2D Heterojunction of Ultrathin MXene/Bi2WO6 Nanosheets for Improved Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2018, 28, 1800136. [Google Scholar] [CrossRef]
- Alsafari, I.A. Synthesis of CuO/MXene nanocomposite to study its photocatalytic and antibacterial properties. Ceram. Int. 2022, 48, 10960–10968. [Google Scholar] [CrossRef]
- Alsafari, I.A.; Munir, S.; Zulfiqar, S.; Saif, M.S.; Warsi, M.F.; Shahid, M. Synthesis, characterization, photocatalytic and antibacterial properties of copper Ferrite/MXene (CuFe2O4/Ti3C2) nanohybrids. Ceram. Int. 2021, 47, 28874–28883. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Khatun, N.; Roy, S.C. Optimization of etching and sonication time to prepare monolayer Ti3C2Tx MXene flakes: A structural, vibrational, and optical spectroscopy study. Micro Nanostruct. 2022, 167, 207256. [Google Scholar] [CrossRef]
- Long, R.; Yu, Z.; Tan, Q.; Feng, X.; Zhu, X.; Li, X.; Wang, P. Ti3C2 MXene/NH2-MIL-88B(Fe): Research on the adsorption kinetics and photocatalytic performance of an efficient integrated photocatalytic adsorbent. Appl. Surf. Sci. 2021, 570, 151244. [Google Scholar] [CrossRef]
- Quyen, V.T.; Ha, L.T.T.; Thanh, D.M.; van Le, Q.; Viet, N.M.; Nham, N.T.; Thang, P.Q. Advanced synthesis of MXene-derived nanoflower-shaped TiO2@Ti3C2 heterojunction to enhance photocatalytic degradation of Rhodamine B. Environ. Technol. Innov. 2021, 21, 101286. [Google Scholar] [CrossRef]
- Thirumal, V.; Yuvakkumar, R.; Kumar, P.S.; Keerthana, S.P.; Ravi, G.; Velauthapillai, D.; Saravanakumar, B. Efficient photocatalytic degradation of hazardous pollutants by homemade kitchen blender novel technique via 2D-material of few-layer MXene nanosheets. Chemosphere 2021, 281, 130984. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hu, X.L.; Li, X.; Su, Z.M.; Zhou, E.L. Solvent induced two Cd-MOFs as luminescent sensors for picric acid, Fe3+ and Cr2O72−. J. Solid State Chem. 2021, 298, 122128. [Google Scholar] [CrossRef]
- Tahir, T.; Chaudhary, K.; Warsi, M.F.; Saif, M.S.; Alsafari, I.A.; Shakir, I.; Agboola, P.O.; Haider, S.; Zulfiqar, S. Synthesis of sponge like Gd3+ doped vanadium oxide/2D MXene composites for improved degradation of industrial effluents and pathogens. Ceram. Int. 2022, 48, 1969–1980. [Google Scholar] [CrossRef]
- My Tran, N.; Thanh Hoai Ta, Q.; Noh, J.S. Unusual synthesis of safflower-shaped TiO2/Ti3C2 heterostructures initiated from two-dimensional Ti3C2 MXene. Appl. Surf. Sci. 2021, 538, 148023. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Ling, Z.; Song, H.; Cai, Y.; Li, Z.; Zu, D.; Li, C. Ternary g-C3N4/TiO2/Ti3C2 MXene S-scheme heterojunction photocatalysts for NOx removal under visible light. Appl. Surf. Sci. 2021, 556, 149817. [Google Scholar] [CrossRef]
- Yu, M.; Liang, H.; Zhan, R.; Xu, L.; Niu, J. Sm-doped g-C3N4/Ti3C2 MXene heterojunction for visible-light photocatalytic degradation of ciprofloxacin. Chin. Chem. Lett. 2021, 32, 2155–2158. [Google Scholar] [CrossRef]
- Du, X.; Zhao, T.; Xiu, Z.; Xing, Z.; Li, Z.; Pan, K.; Yang, S.; Zhou, W. BiVO4@ZnIn2S4/Ti3C2 MXene quantum dots assembly all-solid-state direct Z-Scheme photocatalysts for efficient visible-light-driven overall water splitting. Appl. Mater. Today 2020, 20, 100719. [Google Scholar] [CrossRef]
- Li, T.; Yao, L.; Liu, Q.; Gu, J.; Luo, R.; Li, J.; Yan, X.; Wang, W.; Liu, P.; Chen, B.; et al. Fluorine-Free Synthesis of High-Purity Ti 3 C 2 T x (T=OH, O) via Alkali Treatment. Angew. Chem. 2018, 130, 6223–6227. [Google Scholar] [CrossRef]
- Xue, N.; Li, X.; Zhang, M.; Han, L.; Liu, Y.; Tao, X. Chemical-combined ball-milling synthesis of fluorine-free porous MXene for high-performance lithium ion batteries. ACS Appl. Energy Mater. 2020, 3, 10234–10241. [Google Scholar] [CrossRef]
- Li, Y.; Shao, H.; Lin, Z.; Lu, J.; Liu, L.; Duployer, B.; Persson, P.O.Å.; Eklund, P.; Hultman, L.; Li, M.; et al. A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nat. Mater. 2020, 19, 894–899. [Google Scholar] [CrossRef]
- Shi, L.; Wang, T.; Zhang, H.; Chang, K.; Meng, X.; Liu, H.; Ye, J. An Amine-Functionalized Iron(III) Metal–Organic Framework as Efficient Visible-Light Photocatalyst for Cr(VI) Reduction. Adv. Sci. 2015, 2, 1500006. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, P.; Wang, F.; Ricciardulli, A.G.; Lohe, M.R.; Blom, P.W.M.; Feng, X. Fluoride-Free Synthesis of Two-Dimensional Titanium Carbide (MXene) Using A Binary Aqueous System. Angew. Chem. 2018, 130, 15717–15721. [Google Scholar] [CrossRef]
- He, Y.; Zhang, M.; Shi, J.J.; Cen, Y.L.; Wu, M. Improvement of Visible-Light Photocatalytic Efficiency in a Novel InSe/Zr2CO2 Heterostructure for Overall Water Splitting. J. Phys. Chem. C 2019, 123, 12781–12790. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Xie, X.; Ren, C.E.; Makaryan, T.; Anasori, B.; Wang, G.; Gogotsi, Y. Hollow MXene Spheres and 3D Macroporous MXene Frameworks for Na-Ion Storage. Adv. Mater. 2017, 29, 1702410. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Wang, L.; Meng, X.; Shi, J.; Qi, C.; Zhang, Z.; Feng, L.; Li, C. 2D/2D BiOBr/Ti3C2 heterojunction with dual applications in both water detoxification and water splitting. J. Photochem. Photobiol. A Chem. 2020, 386, 112099. [Google Scholar] [CrossRef]
- Feng, X.; Yu, Z.; Sun, Y.; Long, R.; Shan, M.; Li, X.; Liu, Y.; Liu, J. Review MXenes as a new type of nanomaterial for environmental applications in the photocatalytic degradation of water pollutants. Ceram. Int. 2021, 47, 7321–7343. [Google Scholar] [CrossRef]
- Sreedhar, A.; Noh, J.S. Recent advances in partially and completely derived 2D Ti3C2 MXene based TiO2 nanocomposites towards photocatalytic applications: A review. Solar Energy 2021, 222, 48–73. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Sufian, S.; Mansor, N.; Rabat, N.E. Synthesis and characterization of TiO2-based nanostructures via fluorine-free solvothermal method for enhancing visible light photocatalytic activity: Experimental and theoretical approach. J. Photochem. Photobiol. A Chem. 2021, 404, 112834. [Google Scholar] [CrossRef]
- Saravanan, R.; Gracia, F.; Stephen, A. Nanocomposites for Visible Light-Induced Photocatalysis; Springer: Berlin/Heidelberg, Germany, 2017; pp. 19–40. [Google Scholar]
- Sharma, V.; Kumar, A.; Kumar, A.; Krishnan, V. Enhanced photocatalytic activity of two dimensional ternary nanocomposites of ZnO–Bi2WO6–Ti3C2 MXene under natural sunlight irradiation. Chemosphere 2022, 287, 132119. [Google Scholar] [CrossRef]
- Chen, L.; Ye, X.; Chen, S.; Ma, L.; Wang, Z.; Wang, Q.; Hua, N.; Xiao, X.; Cai, S.; Liu, X. Ti3C2 MXene nanosheet/TiO2 composites for efficient visible light photocatalytic activity. Ceram. Int. 2020, 46, 25895–25904. [Google Scholar] [CrossRef]
- Shahzad, A.; Rasool, K.; Nawaz, M.; Miran, W.; Jang, J.; Moztahida, M.; Mahmoud, K.A.; Lee, D.S. Heterostructural TiO2/Ti3C2Tx (MXene) for photocatalytic degradation of antiepileptic drug carbamazepine. Chem. Eng. J. 2018, 349, 748–755. [Google Scholar] [CrossRef]
- Cao, Y.; Fang, Y.; Lei, X.; Tan, B.; Hu, X.; Liu, B.; Chen, Q. Fabrication of novel CuFe2O4/MXene hierarchical heterostructures for enhanced photocatalytic degradation of sulfonamides under visible light. J. Hazard. Mater. 2020, 387, 122021. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.A.; Ali, S.I.; Amin, F.; Tariq, A.; Iqbal, M.Z.; Rizwan, S. La-and Mn-Codoped Bismuth Ferrite/Ti3C2 MXene Composites for Efficient Photocatalytic Degradation of Congo Red Dye. ACS Omega 2019, 4, 8661–8668. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Liu, Y.; Chen, M.; Zhou, Y.; Xie, Z.; Ma, L.; Li, L.; Yang, B. Carbon nitride coupled with Ti3C2-Mxene derived amorphous Ti-peroxo heterojunction for photocatalytic degradation of rhodamine B and tetracycline. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128448. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Chen, G.; Ji, F.; Shen, Y.; Peng, J.; Zhang, J. In situsynthesis of a hybrid Fe(Co)/MXene/ZSM-5 catalyst for phenol abatement. New J. Chem. 2021, 45, 16862–16871. [Google Scholar] [CrossRef]
- Ding, M.; Ao, W.; Xu, H.; Chen, W.; Tao, L.; Shen, Z.; Liu, H.; Lu, C.; Xie, Z. Facile construction of dual heterojunction CoO@TiO2/MXene hybrid with efficient and stable catalytic activity for phenol degradation with peroxymonosulfate under visible light irradiation. J. Hazard. Mater. 2021, 420, 126686. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Sufian, S.; Bahadar, A.; Lashari, N.; Rabat, N.E.; Mansor, N. Surface-fluorination of TiO2 photocatalysts for remediation of water pollution: A review. J. Clean. Prod. 2021, 317, 128354. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Sufian, S.; Rabat, N.E.; Mansor, N. Development of Photo-Fenton oxidation as green strategy for phenol degradation enhancement via DMF-controlled TiO2nanotubes under various oxidizing agents. J. Environ. Chem. Eng. 2021, 9, 104933. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Chowdhury, S.; Omar, A.A.; Siyal, A.A.; Sufian, S. Response surface methodology and artificial neural network for remediation of acid orange 7 using TiO2-P25: Optimization and modeling approach. Environ. Sci. Pollut. Res. 2020, 27, 34018–34036. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Q.; Xing, Y.; Liu, K.; Ling, W.; Yang, J.; Yang, Q.; Wu, T.; Zhang, J.; Pei, Z.; et al. Review of research on migration, distribution, biological effects, and analytical methods of microfibers in the environment. Sci. Total Environ. 2022, 855, 158922. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Li, R.; Geng, H.; Dong, C. Investigation of fine chalk dust particles’ chemical compositions and toxicities on alveolar macrophages in vitro. Chemosphere 2015, 120, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.; Sun, X. A Self-cleaning, catalytic titanium carbide (MXene) membrane for efficient tetracycline degradation through peroxymonosulfate activation: Performance evaluation and mechanism study. Sep. Purif. Technol. 2021, 279, 119796. [Google Scholar] [CrossRef]
- Fang, Y.; Cao, Y.; Chen, Q. Synthesis of an Ag2WO4/Ti3C2 Schottky composite by electrostatic traction and its photocatalytic activity. Ceram. Int. 2019, 45, 22298–22307. [Google Scholar] [CrossRef]
- Sukidpaneenid, S.; Chawengkijwanich, C.; Pokhum, C.; Isobe, T.; Opaprakasit, P.; Sreearunothai, P. Multi-function adsorbent-photocatalyst MXene-TiO2 composites for removal of enrofloxacin antibiotic from water. J. Environ. Sci. 2021, 124, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Tao, F.; Huang, Z.; Yan, W.; Zhang, Y.; Dong, X.; Wu, Y.; Zhou, G. Ti3C2 MXene-bridged Ag/Ag3PO4 hybrids toward enhanced visible-light-driven photocatalytic activity. Appl. Surf. Sci. 2021, 535, 147354. [Google Scholar] [CrossRef]
- Xu, D.; Ma, Y.; Wang, J.; Chen, W.; Tang, Y.; Li, X.; Li, L. Interfacial engineering of 2D/2D MXene heterostructures: Face-to-face contact for augmented photodegradation of amoxicillin. Chem. Eng. J. 2021, 426, 131246. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, N.; Tang, Z.R.; Anpo, M.; Xu, Y.J. Ti3C2Tx MXene as a Janus cocatalyst for concurrent promoted photoactivity and inhibited photocorrosion. Appl. Catal. B 2018, 237, 43–49. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Xiao, T.; Yuan, X.; Zeng, G.; Tu, W.; Wu, S.; Lee, H.Y.; Tan, Y.Z.; Chew, J.W. Formation of quasi-core-shell In2S3/anatase TiO2@metallic Ti3C2Tx hybrids with favorable charge transfer channels for excellent visible-light-photocatalytic performance. Appl. Catal. B 2018, 233, 213–225. [Google Scholar] [CrossRef]
- Ding, X.; Li, Y.; Li, C.; Wang, W.; Wang, L.; Feng, L.; Han, D. 2D visible-light-driven TiO2@Ti3C2/g-C3N4 ternary heterostructure for high photocatalytic activity. J. Mater. Sci. 2019, 54, 9385–9396. [Google Scholar] [CrossRef]
- Peng, C.; Wang, H.; Yu, H.; Peng, F. (111) TiO2-x/Ti3C2: Synergy of active facets, interfacial charge transfer and Ti3+ doping for enhance photocatalytic activity. Mater. Res. Bull. 2017, 89, 16–25. [Google Scholar] [CrossRef]
- Cheng, X.; Zu, L.; Jiang, Y.; Shi, D.; Cai, X.; Ni, Y.; Lin, S.; Qin, Y. A titanium-based photo-Fenton bifunctional catalyst of mp-MXene/TiO2-x nanodots for dramatic enhancement of catalytic efficiency in advanced oxidation processes. Chem. Commun. 2018, 54, 11622–11625. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Pan, Y.; Yin, M.; Xu, L.; Zhu, Y.; Pan, C. Facile synthesis of ternary Ti3C2−OH/ln2S3/CdS composite with efficient adsorption and photocatalytic performance towards organic dyes. J. Solid State Chem. 2019, 280, 120981. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, P.; Dai, L.; Bu, Z.; Li, X.; Yu, W.; Cao, Y.; Guan, J. Removal of pollutants via synergy of adsorption and photocatalysis over MXene-based nanocomposites. Chem. Eng. J. Adv. 2022, 10, 100285. [Google Scholar] [CrossRef]
- Li, K.; Lu, X.; Zhang, Y.; Liu, K.; Huang, Y.; Liu, H. Bi3TaO7/Ti3C2 heterojunctions for enhanced photocatalytic removal of water-borne contaminants. Environ. Res. 2020, 185, 109409. [Google Scholar] [CrossRef]
- Song, H.; Wang, Y.; Ling, Z.; Zu, D.; Li, Z.; Shen, Y.; Li, C. Enhanced photocatalytic degradation of perfluorooctanoic acid by Ti3C2 MXene-derived heterojunction photocatalyst: Application of intercalation strategy in DESs. Sci. Total Environ. 2020, 746, 141009. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Samsudin, M.F.R.; Sufian, S. Modelling and optimization of photocatalytic degradation of phenol via TiO2 nanoparticles: An insight into response surface methodology and artificial neural network. J. Photochem. Photobiol. A Chem. 2019, 384, 112039. [Google Scholar] [CrossRef]
- Ghani, A.A.; Shahzad, A.; Moztahida, M.; Tahir, K.; Jeon, H.; Kim, B.; Lee, D.S. Adsorption and electrochemical regeneration of intercalated Ti3C2Tx MXene for the removal of ciprofloxacin from wastewater. Chem. Eng. J. 2021, 421, 127780. [Google Scholar] [CrossRef]
- Cai, C.; Wang, R.; Liu, S.; Yan, X.; Zhang, L.; Wang, M.; Tong, Q.; Jiao, T. Synthesis of self-assembled phytic acid-MXene nanocomposites via a facile hydrothermal approach with elevated dye adsorption capacities. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124468. [Google Scholar] [CrossRef]
- Hao, C.; Li, G.; Wang, G.; Chen, W.; Wang, S. Preparation of acrylic acid modified alkalized MXene adsorbent and study on its dye adsorption performance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127730. [Google Scholar] [CrossRef]
- Jun, B.M.; Her, N.; Park, C.M.; Yoon, Y. Effective removal of Pb(ii) from synthetic wastewater using Ti3C2T: X MXene. Environ. Sci. 2020, 6, 173–180. [Google Scholar]
- Hu, X.; Chen, C.; Zhang, D.; Xue, Y. Kinetics, isotherm and chemical speciation analysis of Hg(Ⅱ) adsorption over oxygen-containing MXene adsorbent. Chemosphere 2021, 278, 130206. [Google Scholar] [CrossRef] [PubMed]
- Kadhom, M.; Kalash, K.; Al-Furaiji, M. Performance of 2D MXene as an adsorbent for malachite green removal. Chemosphere 2022, 290, 133256. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, M.; Lee, S.Y.; Mafize, A.A.; Kahar, N.A.M.A.; Johari, K.; Rabat, N.E. Efficient removal of PB(II) from aqueous solutions by using oil palm bio-waste/MWCNTs reinforced pva hydrogel composites: Kinetic, isotherm and thermodynamic modeling. Polymers 2020, 12, 430. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Hu, X.; Liang, J.; Huang, Q.; Dou, J.; Tian, J.; Deng, F.; Liu, M.; Zhang, X.; Wei, Y. Surface functionalization of MXene with chitosan through in-situ formation of polyimidazoles and its adsorption properties. J. Hazard. Mater. 2021, 419, 126220. [Google Scholar] [CrossRef] [PubMed]
- Jun, B.M.; Kim, S.; Rho, H.; Park, C.M.; Yoon, Y. Ultrasound-assisted Ti3C2Tx MXene adsorption of dyes: Removal performance and mechanism analyses via dynamic light scattering. Chemosphere 2020, 254, 126827. [Google Scholar] [CrossRef]
- Dao, X.; Hao, H.; Bi, J.; Sun, S.; Huang, X. Surface Complexation Enhanced Adsorption of Tetracycline by ALK-MXene. Ind Eng Chem Res 2022, 61, 6028–6036. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Wei, Z.; Peigen, Z.; Wubian, T.; Xia, Q.; Yamei, Z.; ZhengMing, S. Alkali treated Ti3C2Tx MXenes and their dye adsorption performance. Mater. Chem. Phys. 2018, 206, 270–276. [Google Scholar] [CrossRef]
- Lei, Y.; Cui, Y.; Huang, Q.; Dou, J.; Gan, D.; Deng, F.; Liu, M.; Li, X.; Zhang, X.; Wei, Y. Facile preparation of sulfonic groups functionalized Mxenes for efficient removal of methylene blue. Ceram. Int. 2019, 45, 17653–17661. [Google Scholar] [CrossRef]
- Vakili, M.; Cagnetta, G.; Huang, J.; Yu, G.; Yuan, J. Synthesis and regeneration of a MXene-based pollutant adsorbent by mechanochemical methods. Molecules 2019, 24, 2478. [Google Scholar] [CrossRef]
- Ren, J.; Zhu, Z.; Qiu, Y.; Yu, F.; Zhou, T.; Ma, J.; Zhao, J. Enhanced adsorption performance of alginate/MXene/CoFe2O4 for antibiotic and heavy metal under rotating magnetic field. Chemosphere 2021, 284, 131284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, J.; Zhang, Y.; Yu, Z.; Ji, R.; Zhou, X. Activation of peracetic acid with cobalt anchored on 2D sandwich-like MXenes (Co@MXenes) for organic contaminant degradation: High efficiency and contribution of acetylperoxyl radicals. Appl. Catal. B 2021, 297, 120475. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, M.; Huang, H.; Zhang, D.; Chen, J.; Mao, L.; Zhou, N.; Deng, F.; Zhang, X.; Wei, Y. A novel one-step strategy for preparation of Fe3O4-loaded Ti3C2 MXenes with high efficiency for removal organic dyes. Ceram. Int. 2020, 46, 11593–11601. [Google Scholar] [CrossRef]
- Peng, C.; Wei, P.; Chen, X.; Zhang, Y.; Zhu, F.; Cao, Y.; Wang, H.; Yu, H.; Peng, F. A hydrothermal etching route to synthesis of 2D MXene (Ti3C2, Nb2C): Enhanced exfoliation and improved adsorption performance. Ceram. Int. 2018, 44, 18886–18893. [Google Scholar] [CrossRef]
- Shahzad, A.; Nawaz, M.; Moztahida, M.; Jang, J.; Tahir, K.; Kim, J.; Lim, Y.; Vassiliadis, V.S.; Woo, S.H.; Lee, D.S. Ti3C2Tx MXene core-shell spheres for ultrahigh removal of mercuric ions. Chem. Eng. J. 2019, 368, 400–408. [Google Scholar] [CrossRef]
- Wang, L.; Song, H.; Yuan, L.; Li, Z.; Zhang, P.; Gibson, J.K.; Zheng, L.; Wang, H.; Chai, Z.; Shi, W. Effective Removal of Anionic Re(VII) by Surface-Modified Ti2CTx MXene Nanocomposites: Implications for Tc(VII) Sequestration. Environ. Sci. Technol. 2019, 53, 3739–3747. [Google Scholar] [CrossRef]
- Wang, Q.; Xiong, Y.; Xu, J.; Dong, F.; Xiong, Y. Oxidation-Resistant Cyclodextrin-Encapsulated-MXene/Poly (N-isopropylacrylamide) composite hydrogel as a thermosensitive adsorbent for phenols. Sep. Purif. Technol. 2022, 286, 120506. [Google Scholar] [CrossRef]
- Zhang, K.N.; Wang, C.Z.; Lü, Q.F.; Chen, M.H. Enzymatic hydrolysis lignin functionalized Ti3C2Tx nanosheets for effective removal of MB and Cu2+ ions. Int. J. Biol. Macromol. 2022, 209, 680–691. [Google Scholar] [CrossRef]
- Li, Y.; Ding, L.; Liang, Z.; Xue, Y.; Cui, H.; Tian, J. Synergetic effect of defects rich MoS2 and Ti3C2 MXene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2. Chem. Eng. J. 2020, 383, 123178. [Google Scholar] [CrossRef]
- Li, W.; Zhuang, C.; Li, Y.; Gao, C.; Jiang, W.; Sun, Z.; Qi, K. Anchoring ultra-small TiO2 quantum dots onto ultra-thin and large-sized Mxene nanosheets for highly efficient photocatalytic water splitting. Ceram. Int. 2021, 47, 21769–21776. [Google Scholar] [CrossRef]
- Huang, J.; Tao, J.; Liu, G.; Lu, L.; Tang, H.; Qiao, G. In situ construction of 1D CdS/2D Nb2CTx MXene Schottky heterojunction for enhanced photocatalytic hydrogen production activity. Appl. Surf. Sci. 2022, 573, 151491. [Google Scholar] [CrossRef]
- Peng, C.; Zhou, T.; Wei, P.; Ai, H.; Zhou, B.; Pan, H.; Xu, W.; Jia, J.; Zhang, K.; Wang, H.; et al. Regulation of the rutile/anatase TiO2 phase junction in-situ grown on −OH terminated Ti3C2Tx (MXene) towards remarkably enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 439, 135685. [Google Scholar] [CrossRef]
- Cao, B.; Wan, S.; Wang, Y.; Guo, H.; Ou, M.; Zhong, Q. Highly-efficient visible-light-driven photocatalytic H2 evolution integrated with microplastic degradation over MXene/ZnxCd1-xS photocatalyst. J. Colloid Interface Sci. 2022, 605, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lv, X.; Li, R.; Tao, X.; Zheng, Y. Stable and efficient Ti3C2 MXene/MAPbI3-HI system for visible-light-driven photocatalytic HI splitting. J. Power Sources 2022, 522, 231006. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zheng, T.; Sasaki, S.i.; Tamiaki, H.; Wang, X.F. Chlorophyll derivative sensitized monolayer Ti3C2Tx MXene nanosheets for photocatalytic hydrogen evolution. J. Photochem. Photobiol. A Chem. 2022, 427, 113792. [Google Scholar] [CrossRef]
- Abas, N.; Kalair, A.; Khan, N. Review of fossil fuels and future energy technologies. Futures 2015, 69, 31–49. [Google Scholar] [CrossRef]
- Wang, H.; Peng, R.; Hood, Z.D.; Naguib, M.; Adhikari, S.P.; Wu, Z. Titania Composites with 2 D Transition Metal Carbides as Photocatalysts for Hydrogen Production under Visible-Light Irradiation. ChemSusChem 2016, 9, 1490–1497. [Google Scholar] [CrossRef]
- Jin, C.; Rao, S.; Xie, J.; Sun, Z.; Gao, J.; Li, Y.; Li, B.; Liu, S.; Liu, L.; Liu, Q.; et al. Enhanced photocatalytic antibacterial performance by hierarchical TiO2/W18O49 Z-scheme heterojunction with Ti3C2Tx-MXene cocatalyst. Chem. Eng. J. 2022, 447, 137369. [Google Scholar] [CrossRef]
- Mun, S.J.; Park, S.J. Graphitic carbon nitride materials for photocatalytic hydrogen production via water splitting: A short review. Catalysts 2019, 9, 805. [Google Scholar] [CrossRef]
- Peng, C.; Xu, W.; Wei, P.; Liu, M.C.; Guo, L.; Wu, P.; Zhang, K.; Cao, Y.; Wang, H.; Yu, H.; et al. Manipulating photocatalytic pathway and activity of ternary Cu2O/(001)TiO2@Ti3C2Tx catalysts for H2 evolution: Effect of surface coverage. Int. J. Hydrogen. Energy 2019, 44, 29975–29985. [Google Scholar] [CrossRef]
- Yang, J.X.; Yu, W.B.; Li, C.F.; Dong, W.D.; Jiang, L.Q.; Zhou, N.; Zhuang, Z.P.; Liu, J.; Hu, Z.Y.; Zhao, H.; et al. PtO nanodots promoting Ti3C2 MXene in-situ converted Ti3C2/TiO2 composites for photocatalytic hydrogen production. Chem. Eng. J. 2021, 420, 129695. [Google Scholar] [CrossRef]
- Chang, H.; Li, X.; Shi, L.; Zhu, Y.R.; Yi, T.F. Towards high-performance electrocatalysts and photocatalysts: Design and construction of MXenes-based nanocomposites for water splitting. Chem. Eng. J. 2021, 421, 129944. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Xing, D.; Wang, J.; Zheng, L.; Wang, Z.; Wang, P.; Zheng, Z.; Cheng, H.; Dai, Y.; et al. 2D/2D heterostructure of ultrathin BiVO4/Ti3C2 nanosheets for photocatalytic overall Water splitting. Appl. Catal. B 2021, 285, 119855. [Google Scholar] [CrossRef]
- Li, Y.; Deng, X.; Tian, J.; Liang, Z.; Cui, H. Ti3C2 MXene-derived Ti3C2/TiO2 nanoflowers for noble-metal-free photocatalytic overall water splitting. Appl. Mater. Today 2018, 13, 217–227. [Google Scholar] [CrossRef]
- Su, T.; Hood, Z.D.; Naguib, M.; Bai, L.; Luo, S.; Rouleau, C.M.; Ivanov, I.N.; Ji, H.; Qin, Z.; Wu, Z. Monolayer Ti3C2Tx as an Effective Co-catalyst for Enhanced Photocatalytic Hydrogen Production over TiO2. ACS Appl. Energy Mater. 2019, 2, 4640–4651. [Google Scholar] [CrossRef]
- Yao, Z.; Sun, H.; Sui, H.; Liu, X. Construction of bpqds/Ti3C2@TiO2 composites with favorable charge transfer channels for enhanced photocatalytic activity under visible light irradiation. Nanomaterials 2020, 10, 452. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, Y.; Meng, X.; Gao, Y.; Dall’Agnese, Y.; Chen, G.; Dall’Agnese, C.; Wang, X.F. Eosin Y-sensitized partially oxidized Ti 3 C 2 MXene for photocatalytic hydrogen evolution. Catal. Sci. Technol. 2019, 9, 310–315. [Google Scholar] [CrossRef]
- Kong, X.; Gao, P.; Jiang, R.; Feng, J.; Yang, P.; Gai, S.; Chen, Y.; Chi, Q.; Xu, F.; Ye, W. Orderly layer-by-layered TiO2/carbon superstructures based on MXene’s defect engineeringfor efficient hydrogen evolution. Appl. Catal. A Gen 2020, 590, 117341. [Google Scholar] [CrossRef]
- Wang, J.; Shen, Y.; Liu, S.; Zhang, Y. Single 2D MXene precursor-derived TiO2 nanosheets with a uniform decoration of amorphous carbon for enhancing photocatalytic water splitting. Appl. Catal. B 2020, 270, 118885. [Google Scholar] [CrossRef]
- Han, X.; An, L.; Hu, Y.; Li, Y.; Hou, C.; Wang, H.; Zhang, Q. Ti3C2 MXene-derived carbon-doped TiO2 coupled with g-C3N4 as the visible-light photocatalysts for photocatalytic H2 generation. Appl. Catal. B 2020, 265, 118539. [Google Scholar] [CrossRef]
- Zhang, J.; Xing, C.; Shi, F. MoS2/Ti3C2 heterostructure for efficient visible-light photocatalytic hydrogen generation. Int. J. Hydrogen. Energy 2020, 45, 6291–6301. [Google Scholar] [CrossRef]
- Xiao, R.; Zhao, C.; Zou, Z.; Chen, Z.; Tian, L.; Xu, H.; Tang, H.; Liu, Q.; Lin, Z.; Yang, X. In situ fabrication of 1D CdS nanorod/2D Ti3C2 MXene nanosheet Schottky heterojunction toward enhanced photocatalytic hydrogen evolution. Appl. Catal. B 2020, 268, 118382. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, Y.; Teo, W.L.; Xie, A.; Guo, Y.; Dai, Y.; Zhou, W.; Jana, D.; Xian, Q.; Dong, W.; et al. Ultrathin ZnIn2S4 Nanosheets Anchored on Ti3C2Tx MXene for Photocatalytic H2 Evolution. Angew. Chem. 2020, 59, 11287–11292. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Opoku, F.; Govender, K.K.; Gertina, C.; Elizabeth, C.; Govender, P.P. Recent Progress in the Development of Semiconductor- Based Photocatalyst Materials for Applications in Photocatalytic Water Splitting and Degradation of Pollutants. Adv. Sustain. Syst. 2017, 1, 1700006. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, B.; Zhang, F.; Li, H.; Zheng, K.; Qiu, J.; Wu, L.; Yu, J.; Chen, X. Type-II AsP/Sc2CO2 van der Waals heterostructure: An excellent photocatalyst for overall water splitting. Int. J. Hydrogen. Energy 2021, 46, 32882–32892. [Google Scholar] [CrossRef]
- Meng, A.; Yu, J. Interface Science and Technology; Elsevier B.V.: Amsterdam, The Netherlands, 2020; pp. 161–191. [Google Scholar]
- Wang, Y.; Bayazit, M.K.; Moniz, S.J.A.; Ruan, Q.; Lau, C.C.; Martsinovich, N.; Tang, J. Linker-controlled polymeric photocatalyst for highly efficient hydrogen evolution from water. Energy Environ. Sci. 2017, 10, 1643–1651. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Z.; Yang, Q.; Tan, L.; Dong, L.; Dong, M. Ultrathin Ti3C2Tx (MXene) Nanosheet-Wrapped NiSe2 Octahedral Crystal for Enhanced Supercapacitor Performance and Synergetic Electrocatalytic Water Splitting. Nanomicro Lett. 2019, 11, 31. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y. Potential environmental applications of MXenes: A critical review. Chemosphere 2021, 271, 129578. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Zong, S.; Liu, J.; Huang, Z.; Liu, L.; Liu, J.; Zheng, J.; Fang, Y. Mxene-TiO2 composite with exposed {101} facets for the improved photocatalytic hydrogen evolution activity. J. Alloys Compd. 2022, 896, 163039. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, B.; Yan, B.; Li, G.; Nie, H.; Yang, K.; Zhang, C.; He, J. Few-layer Ti 3 C 2 T x (T = O, OH, or F) saturable absorber for a femtosecond bulk laser. Opt. Lett. 2018, 43, 3862. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zeng, M.; Zhou, J.; Chen, H.; Cong, G.; Liu, H.; Ji, M.; Zhu, C.; Xu, J. A one-pot synthesis of nitrogen doped porous MXene/TiO2 heterogeneous film for high-performance flexible energy storage. Chem. Eng. J. 2021, 426, 130765. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Han, L.; Li, H.; Wang, J.; Lu, T.; Pan, L. In-situ fabrication of few-layered MoS2 wrapped on TiO2-decorated MXene as anode material for durable lithium-ion storage. J. Colloid Interface Sci. 2021, 604, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, P.; Zhu, Y.; Zhu, X.; Zhang, Y.; Liu, M.; Liu, Y. In situ growth of TiO2 nanowires on Ti3C2 MXenes nanosheets as highly sensitive luminol electrochemiluminescent nanoplatform for glucose detection in fruits, sweat and serum samples. Biosens. Bioelectron. 2021, 194, 113600. [Google Scholar] [CrossRef]
- Wang, F.; Ma, X.; Zou, P.; Wang, G.; Xiong, Y.; Liu, Y.; Ren, F.; Xiong, X. Nitrogen-doped carbon decorated TiO2/Ti3C2Tx MXene composites as anode material for high-performance sodium-ion batteries. Surf. Coat. Technol. 2021, 422, 127568. [Google Scholar] [CrossRef]
- Yang, W.; Ma, G.; Fu, Y.; Peng, K.; Yang, H.; Zhan, X.; Yang, W.; Wang, L.; Hou, H. Rationally designed Ti3C2 MXene@TiO2/CuInS2 Schottky/S-scheme integrated heterojunction for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 429, 132381. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Fakhrul Ridhwan Samsudin, M.; Bacho, N.; Sufian, S. Nanocatalysts; IntechOpen: London, UK, 2019. [Google Scholar]
- Jo, W.K.; Lee, J.Y.; Selvam, N.C.S. Synthesis of MoS2 nanosheets loaded ZnO-g-C3N4 nanocomposites for enhanced photocatalytic applications. Chem. Eng. J. 2016, 289, 306–318. [Google Scholar] [CrossRef]
- Masih, D.; Ma, Y.; Rohani, S. Graphitic C3N4 based noble-metal-free photocatalyst systems: A review. Appl. Catal. B 2017, 206, 556–588. [Google Scholar] [CrossRef]
- Huang, K.; Li, C.; Wang, L.; Wang, W.; Meng, X. Layered Ti3C2 MXene and silver co-modified g-C3N4 with enhanced visible light-driven photocatalytic activity. Chem. Eng. J. 2021, 425, 131493. [Google Scholar] [CrossRef]
- Lu, Y.; He, J.; Luo, G. An improved synthesis of chitosan bead for Pb(II) adsorption. Chemical Engineering Journal 2013, 226, 271–278. [Google Scholar] [CrossRef]
- Nasri, M.S.I.; Samsudin, M.F.R.; Tahir, A.A.; Sufian, S. Effect of MXene Loaded on g-C3N4 Photocatalyst for the Photocatalytic Degradation of Methylene Blue. Energies 2022, 15, 955. [Google Scholar] [CrossRef]
- Li, Y.; Ding, L.; Guo, Y.; Liang, Z.; Cui, H.; Tian, J. Boosting the Photocatalytic Ability of g-C3N4 for Hydrogen Production by Ti3C2 MXene Quantum Dots. ACS Appl. Mater. Interfaces 2019, 11, 41440–41447. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, M.; Zhan, R.; Wang, X.; Peng, G.; Niu, J. Ti3C2 MXene-induced interface electron separation in g-C3N4/Ti3C2 MXene/MoSe2 Z-scheme heterojunction for enhancing visible light-irradiated enoxacin degradation. Sep. Purif. Technol. 2021, 275, 119194. [Google Scholar] [CrossRef]
- Liu, W.; Sun, M.; Ding, Z.; Gao, B.; Ding, W. Ti3C2 MXene embellished g-C3N4 nanosheets for improving photocatalytic redox capacity. J. Alloys Compd. 2021, 877, 160223. [Google Scholar] [CrossRef]
- Huang, K.; Li, C.; Zhang, X.; Wang, L.; Wang, W.; Meng, X. Self-assembly synthesis of phosphorus-doped tubular g-C3N4/Ti3C2 MXene Schottky junction for boosting photocatalytic hydrogen evolution. Green Energy Environ. 2021, 27, R713–R715. [Google Scholar] [CrossRef]
- Li, X.; Bai, Y.; Shi, X.; Huang, J.; Zhang, K.; Wang, R.; Ye, L. Mesoporous g-C3N4/MXene (Ti3C2Tx) heterojunction as a 2D electronic charge transfer for efficient photocatalytic CO2 reduction. Appl. Surf. Sci. 2021, 546, 149111. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, R.; Huang, J.; Jiang, Y.; Zhao, C.; Yang, X. In situ construction of protonated g-C3N4/Ti3C2 MXene Schottky heterojunctions for efficient photocatalytic hydrogen production. Chin. J. Catal. 2020, 42, 107–114. [Google Scholar] [CrossRef]
- Tan, H.L.; Amal, R.; Ng, Y.H. Exploring the Different Roles of Particle Size in Photoelectrochemical and Photocatalytic Water Oxidation on BiVO4. ACS Appl. Mater. Interfaces 2016, 8, 28607–28614. [Google Scholar] [CrossRef]
- Kudo, A.; Omori, K.; Kato, H. A novel aqueous process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Liu, C.; Fu, Y.; Zhao, J.; Wang, H.; Huang, H.; Liu, Y.; Dou, Y.; Shao, M.; Kang, Z. All-solid-state Z-scheme system of NiO/CDs/BiVO4 for visible light-driven efficient overall water splitting. Chem. Eng. J. 2019, 358, 134–142. [Google Scholar] [CrossRef]
- Yu, F.; Li, F.; Yao, T.; Du, J.; Liang, Y.; Wang, Y.; Han, H.; Sun, L. Fabrication and Kinetic Study of a Ferrihydrite-Modified BiVO4 Photoanode. ACS Catal. 2017, 7, 1868–1874. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Zhang, P.; Zhang, J.; Li, A.; Gong, J. Enhanced Surface Reaction Kinetics and Charge Separation of p-n Heterojunction Co3O4/BiVO4 Photoanodes. J. Am. Chem. Soc. 2015, 137, 8356–8359. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Fu, X.; Shang, Z.; Liu, J.; Luo, H. A BiVO4 film photoanode with re-annealing treatment and 2D thin Ti3C2Tx flakes decoration for enhanced photoelectrochemical water oxidation. Chem. Eng. J. 2019, 361, 853–861. [Google Scholar] [CrossRef]
- Soomro, R.A.; Jawaid, S.; Kalawar, N.H.; Tunesi, M.; Karakuş, S.; Kilislioğlu, A.; Willander, M. In-situ engineered MXene-TiO2/BiVO4 hybrid as an efficient photoelectrochemical platform for sensitive detection of soluble CD44 proteins. Biosens. Bioelectron. 2020, 166, 112439. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, H.; Wei, X.; Wu, Y.; Gu, W.; Hu, L.; Zhu, C. Efficient BiVO4 photoanode decorated with Ti3C2Tx MXene for enhanced photoelectrochemical sensing of Hg(II) ion. Anal. Chim. Acta 2020, 1119, 11–17. [Google Scholar] [CrossRef]
- Laureano-Anzaldo, C.M.; Robledo-Ortíz, J.R.; Manríquez-González, R. Zwitterionic cellulose as a promising sorbent for anionic and cationic dyes. Mater. Lett. 2021, 300, 130236. [Google Scholar] [CrossRef]
- Zhong, Q.; Li, Y.; Zhang, G. Two-dimensional MXene-based and MXene-derived photocatalysts: Recent developments and perspectives. Chem. Eng. J. 2021, 409, 128099. [Google Scholar] [CrossRef]
- Ran, J.; Gao, G.; Li, F.T.; Ma, T.Y.; Du, A.; Qiao, S.Z. Ti3C2 MXene co-catalyst on metal sulfide photo-absorbers for enhanced visible-light photocatalytic hydrogen production. Nat. Commun. 2017, 8, 13907. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, D.; Sun, Y.; Meng, X.; Gao, Y.; Dall’Agnese, Y.; Chen, G.; Wang, X.F. G-C3N4/Ti3C2Tx (MXenes) composite with oxidized surface groups for efficient photocatalytic hydrogen evolution. J. Mater. Chem. A Mater. 2018, 6, 9124–9131. [Google Scholar] [CrossRef]
- Dong, Y.; Sang, D.; He, C.; Sheng, X.; Lei, L. Mxene/alginate composites for lead and copper ion removal from aqueous solutions. RSC Adv. 2019, 9, 29015–29022. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).