Effects of Carboxyl Functionalized CNT on Electrochemical Behaviour of Polyluminol-CNT Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polyluminol-CNT Composites
2.3. Material Characterizations
2.4. Electrochemical Characterizations
2.5. First Principles Methods
3. Results and Discussion
3.1. First Principles Analysis
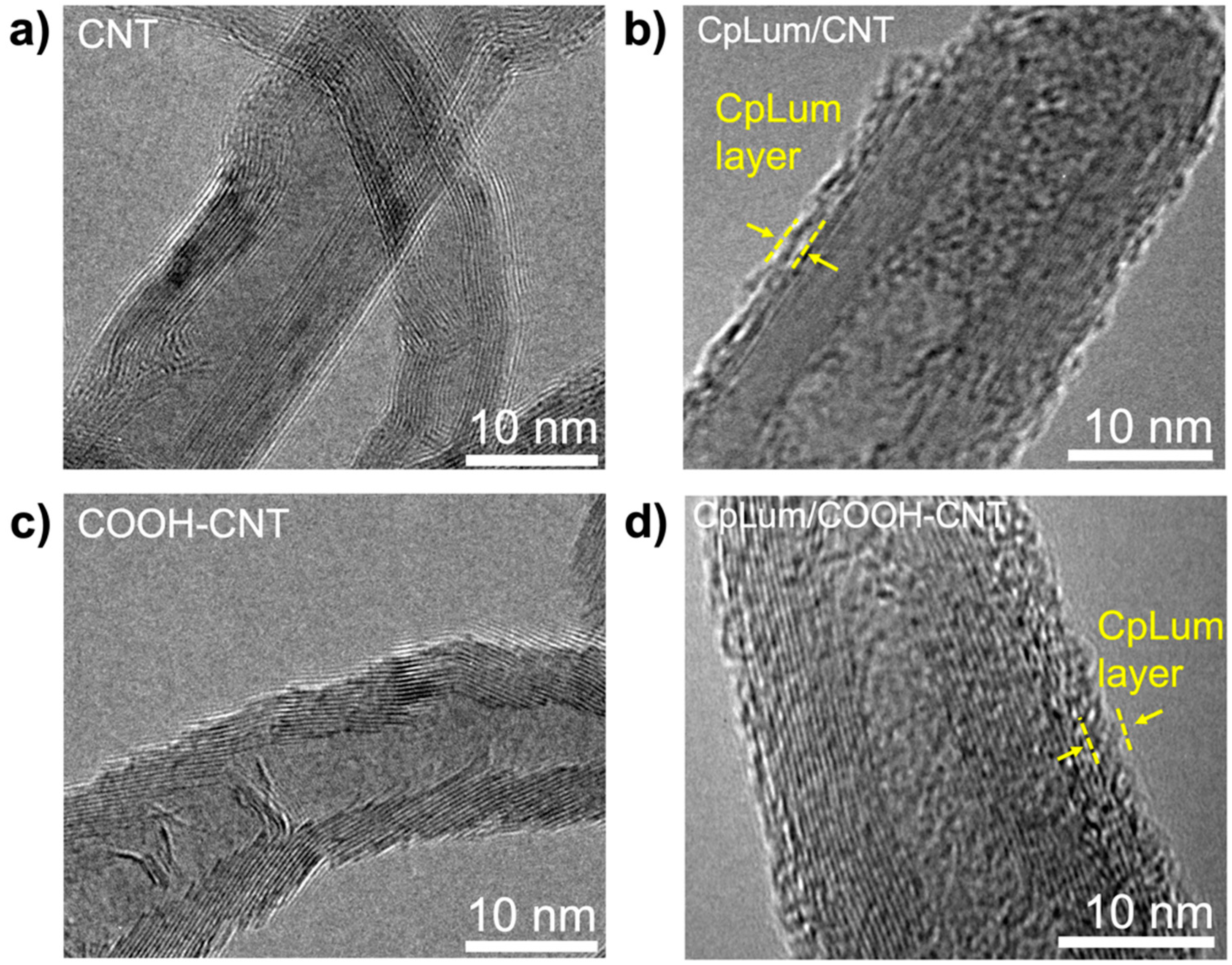
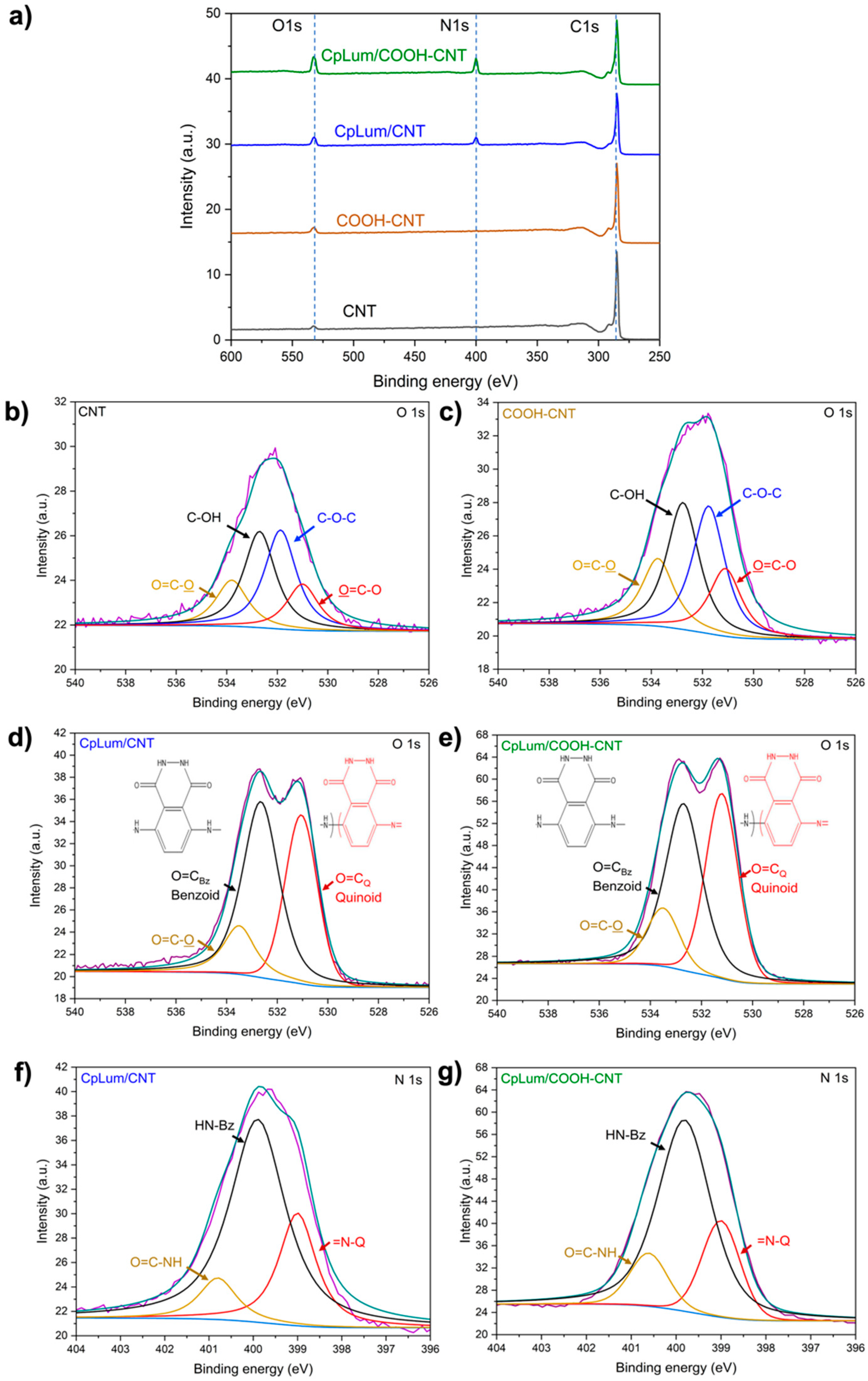
3.2. Material Surface Structure and Functionality Analysis
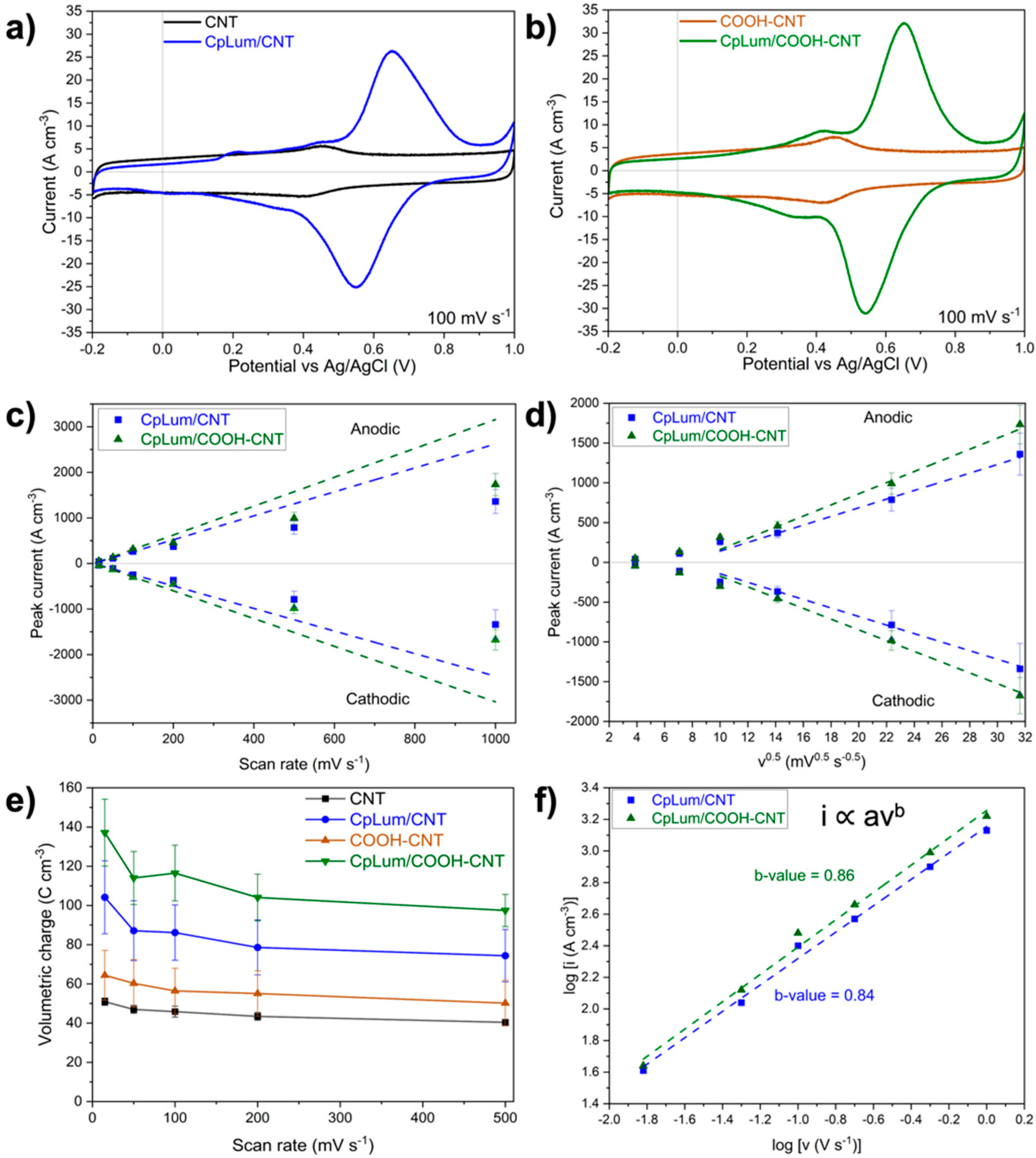
3.3. Electrochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- N’Diaye, J.; Bagchi, R.; Howe, J.; Lian, K. Redox Active Organic-Carbon Composites for Capacitive Electrodes: A Review. Sustain. Chem. 2021, 2, 407–440. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, D.; Song, X.; Du, W.; Zhao, X.; Zhang, D. Review on Carbon/Polyaniline Hybrids: Design and Synthesis for Supercapacitor. Molecules 2019, 24, 2263. [Google Scholar] [CrossRef]
- Gul, H.; Shah, A.-U.A.; Bilal, S. Fabrication of Eco-Friendly Solid-State Symmetric Ultracapacitor Device Based on Co-Doped PANI/GO Composite. Polymers 2019, 11, 1315. [Google Scholar] [CrossRef]
- Chang, H.-H.; Chang, C.-K.; Tsai, Y.-C.; Liao, C.-S. Electrochemically synthesized graphene/polypyrrole composites and their use in supercapacitor. Carbon 2012, 50, 2331–2336. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Y.; Guo, J.; Yan, X.; O’Connor, R.; Zhang, X.; Shen, N.Z.; Weeks, B.L.; Huang, X.; Wei, S.; et al. Electropolymerized Polypyrrole Nanocoatings on Carbon Paper for Electrochemical Energy Storage. ChemElectroChem 2014, 2, 119–126. [Google Scholar] [CrossRef]
- N’Diaye, J.; Chang, J.H.; Lian, K. The Capacitive Behavior of Polyluminol on Carbon Nanotubes Electrodes. ChemElectroChem 2019, 6, 5454–5461. [Google Scholar] [CrossRef]
- Geiselhart, C.M.; Barner-Kowollik, C.; Mutlu, H. Untapped toolbox of luminol based polymers. Polym. Chem. 2021, 12, 1732–1748. [Google Scholar] [CrossRef]
- Al-Ghaus, Z.; Akbarinejad, A.; Zhu, B.; Travas-Sejdic, J. Polyluminol-polyoxometalate hybrid hydrogels as flexible and soft supercapacitor electrodes. J. Mater. Chem. A 2021, 9, 20783–20793. [Google Scholar] [CrossRef]
- N’Diaye, J.; Lian, K. Investigation of the chemical structure and electrochemical activity of a chemically polymerized luminol. J. Electroanal. Chem. 2019, 839, 90–95. [Google Scholar] [CrossRef]
- Li, D.; He, J.; Ding, G.; Tang, Q.; Ying, Y.; He, J.; Zhong, C.; Liu, Y.; Feng, C.; Sun, Q.; et al. Borophene: Stretch-Driven Increase in Ultrahigh Thermal Conductance of Hydrogenated Borophene and Dimensionality Crossover in Phonon Transmission (Adv. Funct. Mater. 31/2018). Adv. Funct. Mater. 2018, 28, 1870213. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, S.H.; Kwon, S.; Cho, M.; Kim, K.H.; Han, T.H.; Lee, S.G. Tunable Electronic Properties of Nitrogen and Sulfur Doped Graphene: Density Functional Theory Approach. Nanomaterials 2019, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Liu, J.; Zhang, Y.; Wang, T. First-principles calculations of graphene-based polyaniline nano-hybrids for insight of electromagnetic properties and electronic structures. RSC Adv. 2016, 6, 73915–73923. [Google Scholar] [CrossRef]
- Lazar, P.; Karlický, F.; Jurečka, P.; Kocman, M.; Otyepková, E.; Šafářová, K.; Otyepka, M. Adsorption of Small Organic Molecules on Graphene. J. Am. Chem. Soc. 2013, 135, 6372–6377. [Google Scholar] [CrossRef] [PubMed]
- Mollenhauer, D.; Brieger, C.; Voloshina, E.; Paulus, B. Performance of Dispersion-Corrected DFT for the Weak Interaction between Aromatic Molecules and Extended Carbon-Based Systems. J. Phys. Chem. C 2015, 119, 1898–1904. [Google Scholar] [CrossRef]
- N’Diaye, J.; Elshazly, M.; Lian, K. Unraveling Synergistic Redox Interactions in Tetraphenylporphyrin–Polyluminol–Carbon Nanotube Composite for Capacitive Charge Storage. ACS Appl. Mater. Interfaces 2022, 14, 28359–28369. [Google Scholar] [CrossRef]
- Bogdanovskaya, V.; Vernigor, I.; Radina, M.; Sobolev, V.; Andreev, V.; Nikolskaya, N. Modified Carbon Nanotubes: Surface Properties and Activity in Oxygen Reduction Reaction. Catalysts 2021, 11, 1354. [Google Scholar] [CrossRef]
- Yang, H.; Yang, Y.; Liu, Y.; He, D.; Bai, J. Multi-scale study of CNT and CNT-COOH reinforced epoxy composites: Dispersion state, interfacial interaction vs. mechanical properties. Compos. Interfaces 2020, 28, 381–393. [Google Scholar] [CrossRef]
- Li, L.-X.; Li, F. The effect of carbonyl, carboxyl and hydroxyl groups on the capacitance of carbon nanotubes. New Carbon Mater. 2011, 26, 224–228. [Google Scholar] [CrossRef]
- Bernal, V.; Giraldo, L.; Moreno-Piraján, J.C. Physicochemical Properties of Activated Carbon: Their Effect on the Adsorption of Pharmaceutical Compounds and Adsorbate–Adsorbent Interactions. C 2018, 4, 62. [Google Scholar] [CrossRef]
- Wulandari, S.A.; Arifin; Widiyandari, H.; Subagio, A. Synthesis and characterization carboxyl functionalized Multi-Walled Carbon Nanotubes (MWCNT-COOH) and NH2 functionalized Multi-Walled Carbon Nanotubes (MWCNTNH2). J. Phys. Conf. Ser. 2018, 1025, 012005. [Google Scholar] [CrossRef]
- Supong, A.; Bhomick, P.C.; Karmaker, R.; Ezung, S.L.; Jamir, L.; Sinha, U.B.; Sinha, D. Experimental and theoretical insight into the adsorption of phenol and 2,4-dinitrophenol onto Tithonia diversifolia activated carbon. Appl. Surf. Sci. 2020, 529, 147046. [Google Scholar] [CrossRef]
- Vargas, A.M.M.; Cazetta, A.L.; Kunita, M.H.; Silva, T.L.; Almeida, V.C. Adsorption of methylene blue on activated carbon produced from flamboyant pods (Delonix regia): Study of adsorption isotherms and kinetic models. Chem. Eng. J. 2011, 168, 722–730. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Cheng, Y.; Jiang, S.P. Effect of Carbon Nanotubes on Direct Electron Transfer and Electrocatalytic Activity of Immobilized Glucose Oxidase. ACS Omega 2018, 3, 667–676. [Google Scholar] [CrossRef]
- David, T.; Mathad, J.K.; Padmavathi, T.; Vanaja, A. Part-A: Synthesis of polyaniline and carboxylic acid functionalized SWCNT composites for electromagnetic interference shielding coatings. Polymer 2014, 55, 5665–5672. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, Y.; Zheng, Y.; Dai, L.; Zhang, M.; Yang, J.; Chen, Y.; Chen, X.; Zhou, J. A Facile Synthesis of Polypyrrole/Carbon Nanotube Composites with Ultrathin, Uniform and Thickness-Tunable Polypyrrole Shells. Nanoscale Res. Lett. 2011, 6, 431. [Google Scholar] [CrossRef]
- Zhou, H.; Zhai, H.-J.; Zhi, X. Enhanced electrochemical performances of polypyrrole/carboxyl graphene/carbon nanotubes ternary composite for supercapacitors. Electrochim. Acta 2018, 290, 1–11. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Lopez-Ramon, M.V.; Carrasco-Marín, F. Changes in surface chemistry of activated carbons by wet oxidation. Carbon 2000, 38, 1995–2001. [Google Scholar] [CrossRef]
- Portet, C.; Chmiola, J.; Gogotsi, Y.; Park, S.; Lian, K. Electrochemical characterizations of carbon nanomaterials by the cavity microelectrode technique. Electrochim. Acta 2008, 53, 7675–7680. [Google Scholar] [CrossRef]
- Matsuda, Y.; Tahir-Kheli, J.; Goddard, I.W.A. Definitive Band Gaps for Single-Wall Carbon Nanotubes. J. Phys. Chem. Lett. 2010, 1, 2946–2950. [Google Scholar] [CrossRef]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Nardelli, M.B.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, R.; Gorni, T.; de Gironcoli, S. Nonlocal van der Waals density functional made simple and efficient. Phys. Rev. B 2013, 87, 041108. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter Mater. Phys. 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Bengtsson, L. Dipole correction for surface supercell calculations. Phys. Rev. B 1999, 59, 12301–12304. [Google Scholar] [CrossRef]
- Kokalj, A. XCrySDen—A new program for displaying crystalline structures and electron densities. J. Mol. Graph. Model. 1999, 17, 176–179. [Google Scholar] [CrossRef]
- Yu, M.; Trinkle, D.R. Accurate and efficient algorithm for Bader charge integration. J. Chem. Phys. 2011, 134, 064111. [Google Scholar] [CrossRef]
- Sanville, E.; Kenny, S.D.; Smith, R.; Henkelman, G. Improved grid-based algorithm for Bader charge allocation. J. Comput. Chem. 2007, 28, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2005, 36, 354–360. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef] [PubMed]
- McCreery, R.L. Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A. Chemical Functionalization of Carbon Nanotubes with Polymers: A Brief Overview. Macromol 2021, 1, 64–83. [Google Scholar] [CrossRef]
- Aarva, A.; Deringer, V.L.; Sainio, S.; Laurila, T.; Caro, M.A. Understanding X-Ray Spectroscopy of Carbonaceous Materials by Combining Experiments, Density Functional Theory, and Machine Learning. Part II: Quantitative Fitting of Spectra. Chem. Mater. 2019, 31, 9256–9267. [Google Scholar] [CrossRef]
- Cossaro, A.; Puppin, M.; Cvetko, D.; Kladnik, G.; Verdini, A.; Coreno, M.; de Simone, M.; Floreano, L.; Morgante, A. Tailoring SAM-on-SAM Formation. J. Phys. Chem. Lett. 2011, 2, 3124–3129. [Google Scholar] [CrossRef]
- Scotland, K.M.; Strong, O.K.; Parnis, J.M.; Vreugdenhil, A.J. DFT modeling of polyaniline: A computational investigation into the structure and band gap of polyaniline. Can. J. Chem. 2022, 100, 162–167. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, R.; Wang, Z.; Liu, H. Carboxyl-functionalized graphene oxide–polyaniline composite as a promising supercapacitor material. J. Mater. Chem. 2012, 22, 13619–13624. [Google Scholar] [CrossRef]
- Chaabani, A.; Ben Jabrallah, T.; Tahar, N.B. Electrochemical Oxidation of Ciprofloxacin on COOH-Functionalized Multi-Walled Carbon Nanotube–Coated Vitreous Carbon Electrode. Electrocatalysis 2022, 13, 402–413. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, A.J.; Dunn, B. Pseudocapacitive Contributions to Electrochemical Energy Storage in TiO2 (Anatase) Nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Ardizzone, S.; Fregonara, G.; Trasatti, S. “Inner” and “outer” active surface of ruo, electrodes. Electrochim. Acta 1990, 35, 263–267. [Google Scholar] [CrossRef]
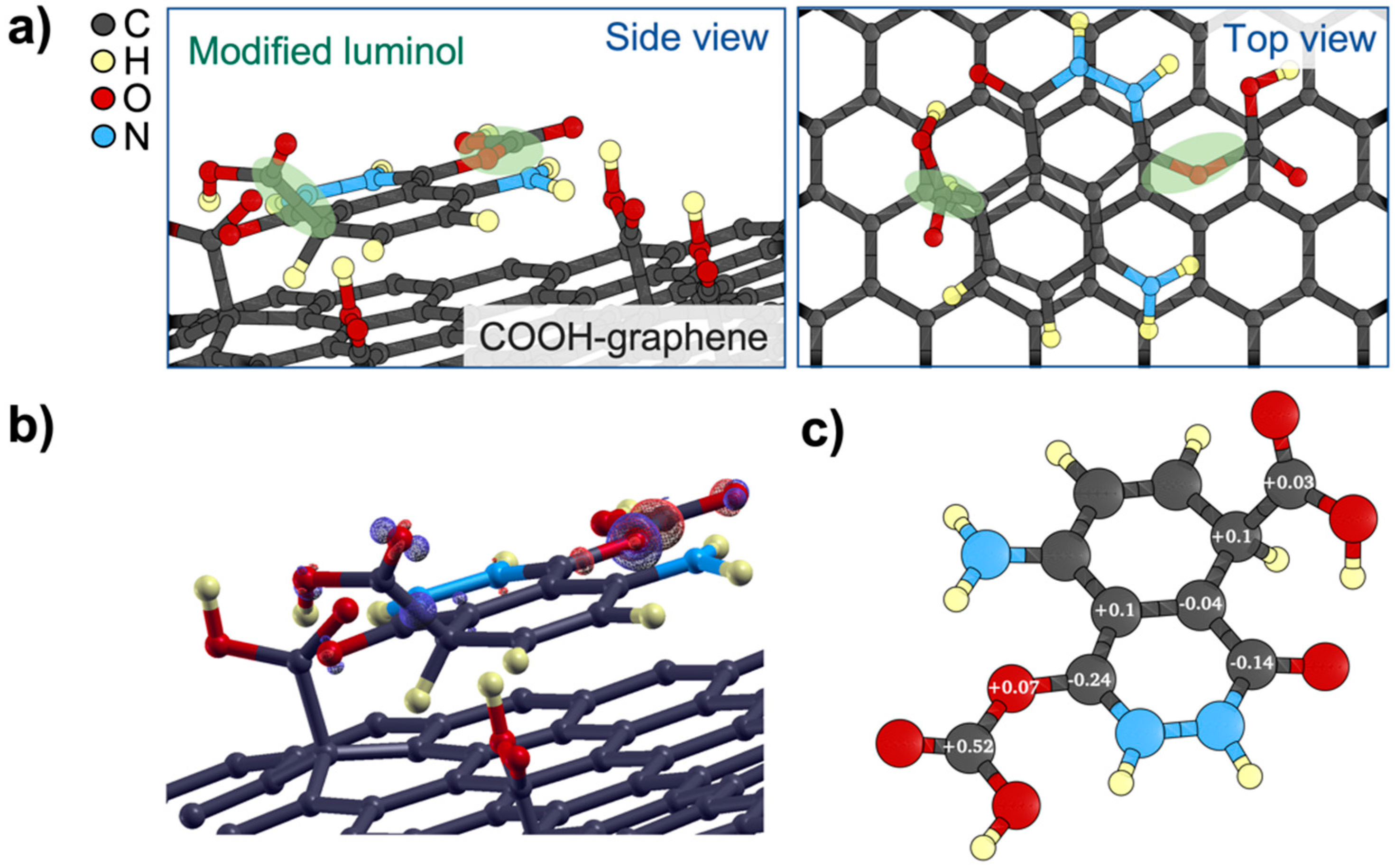


| Atop1 | Atop2 | Interstitial |
|---|---|---|
 | ||
| CNT (nm) | COOH-CNT (nm) | |
|---|---|---|
| Bare | 16.7 ± 3.3 | 16.5 ± 3.4 |
| CpLum-coated | 18.9 ± 3.2 | 18.9 ± 3.7 |
| Element | CNT [%] | CpLum/CNT [%] | COOH-CNT [%] | CpLum/COOH-CNT [%] |
|---|---|---|---|---|
| C1s | 98.1 ± 0.2 | 84.0 ± 4.7 | 93.2 ± 2.2 | 83.3 ± 4.9 |
| O1s | 1.9 ± 0.2 | 5.9 ± 1.8 | 6.8 ± 2.2 | 6.7 ± 2.1 |
| N1s | - | 10.1 ± 3.2 | - | 10.0 ± 2.8 |
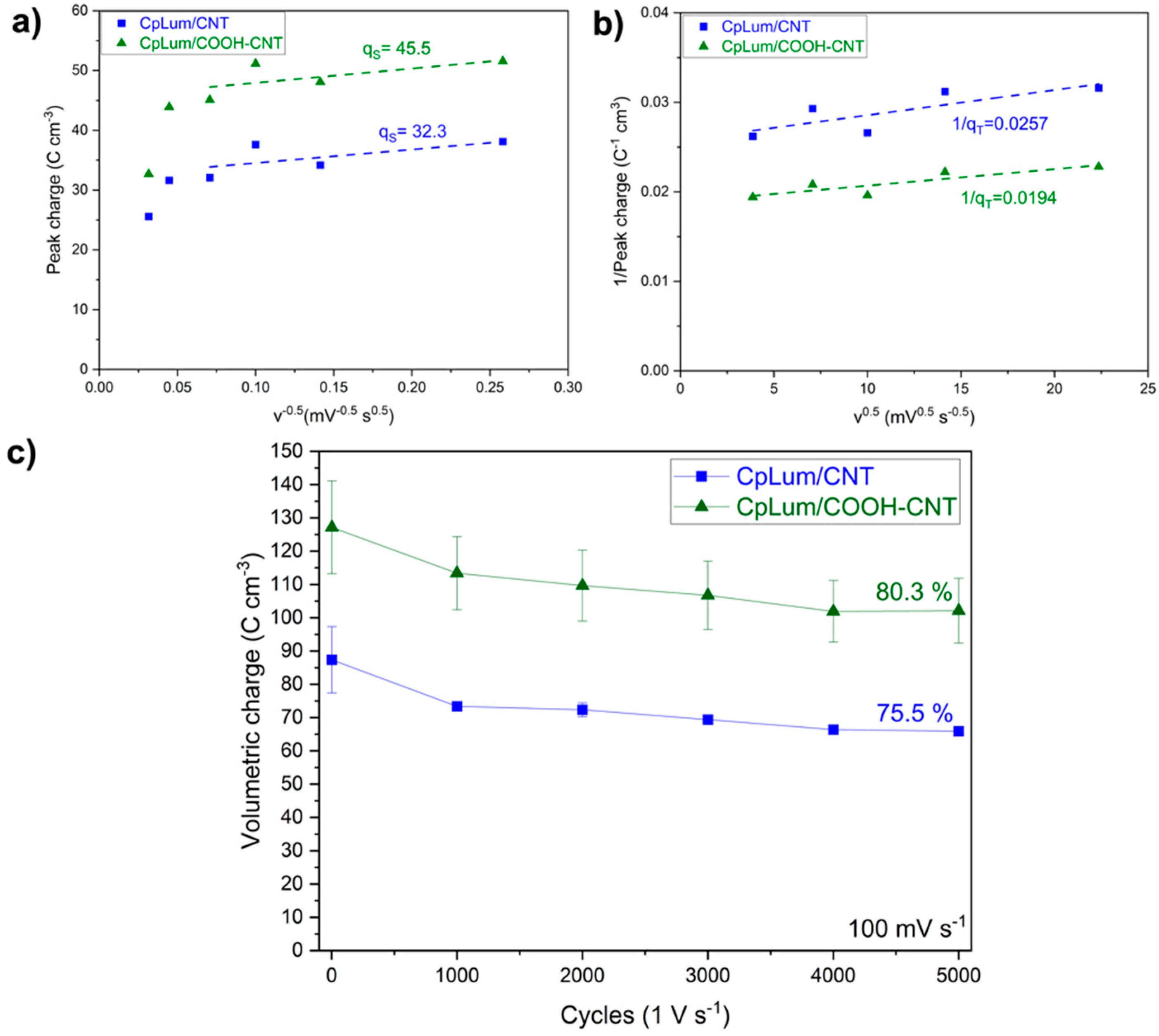
| Material | Total Peak Charge, qT (C cm−3) | Surface-Controlled Charge, qS (C cm−3) | Diffusion-Controlled Charge, qD (C cm−3) |
|---|---|---|---|
| CpLum/CNT | 38.8 | 32.3 | 6.6 |
| CpLum/COOH-CNT | 53.1 | 45.5 | 7.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagchi, R.; Elshazly, M.; N’Diaye, J.; Yu, D.; Howe, J.Y.; Lian, K. Effects of Carboxyl Functionalized CNT on Electrochemical Behaviour of Polyluminol-CNT Composites. Chemistry 2022, 4, 1561-1575. https://doi.org/10.3390/chemistry4040103
Bagchi R, Elshazly M, N’Diaye J, Yu D, Howe JY, Lian K. Effects of Carboxyl Functionalized CNT on Electrochemical Behaviour of Polyluminol-CNT Composites. Chemistry. 2022; 4(4):1561-1575. https://doi.org/10.3390/chemistry4040103
Chicago/Turabian StyleBagchi, Raunaq, Mohamed Elshazly, Jeanne N’Diaye, Dian Yu, Jane Y. Howe, and Keryn Lian. 2022. "Effects of Carboxyl Functionalized CNT on Electrochemical Behaviour of Polyluminol-CNT Composites" Chemistry 4, no. 4: 1561-1575. https://doi.org/10.3390/chemistry4040103
APA StyleBagchi, R., Elshazly, M., N’Diaye, J., Yu, D., Howe, J. Y., & Lian, K. (2022). Effects of Carboxyl Functionalized CNT on Electrochemical Behaviour of Polyluminol-CNT Composites. Chemistry, 4(4), 1561-1575. https://doi.org/10.3390/chemistry4040103






