Effective BiOCl Electrons Collector for Enhancing Photocarrier Separation of Bi2WO6/BiOCl Composite
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Photocatalysts
2.3. Characterization of Samples
2.4. Photocatalytic Activity Test
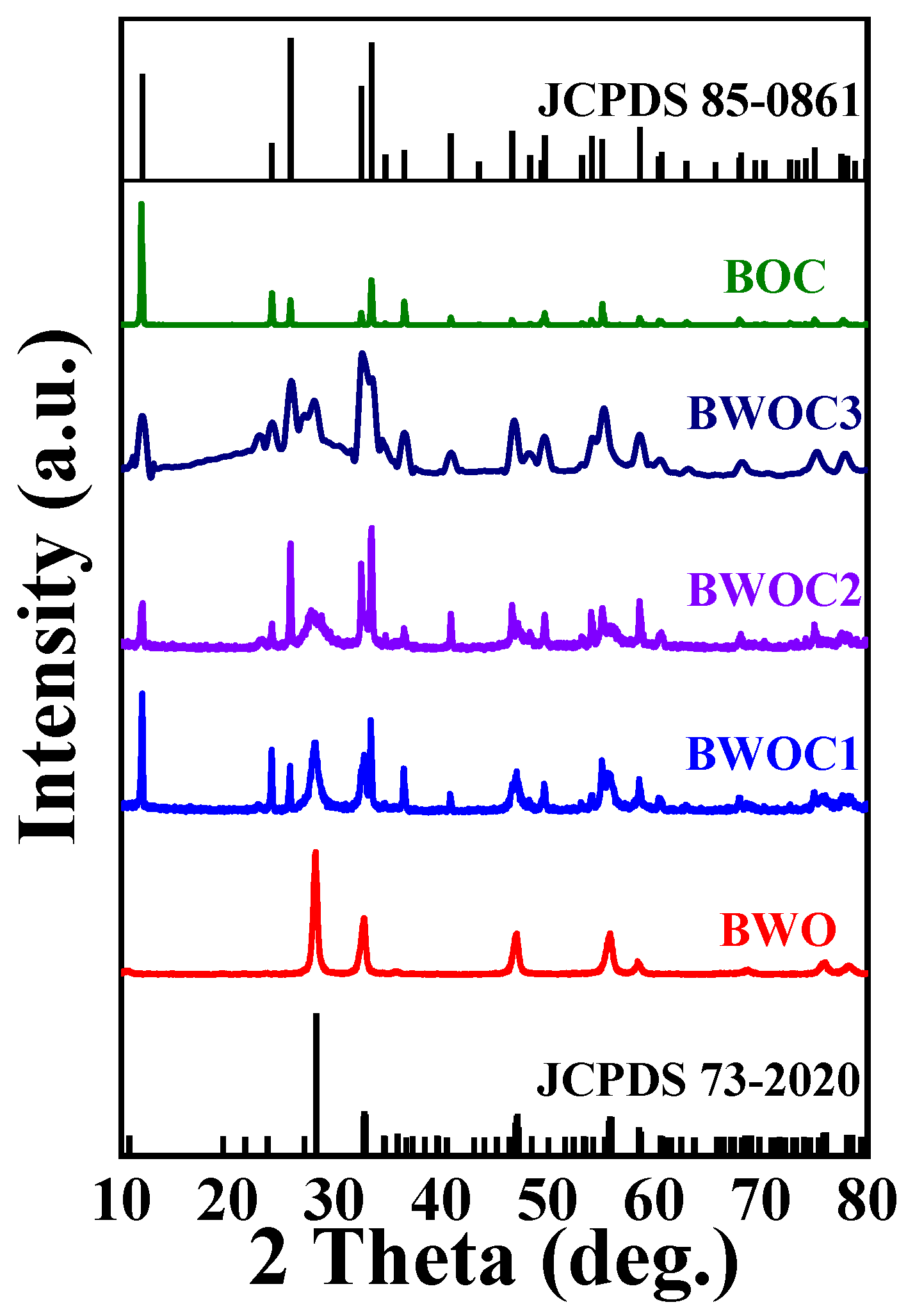
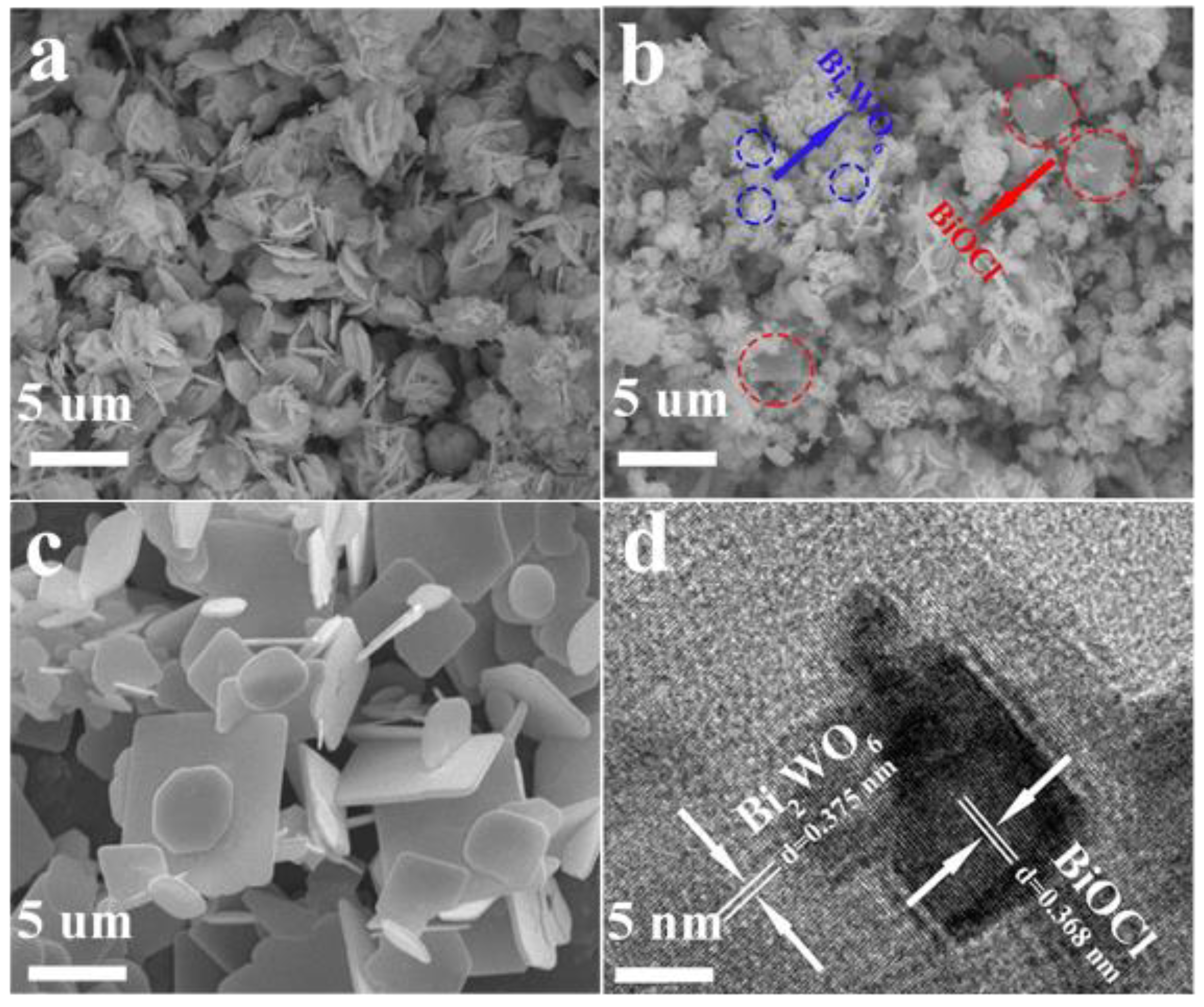
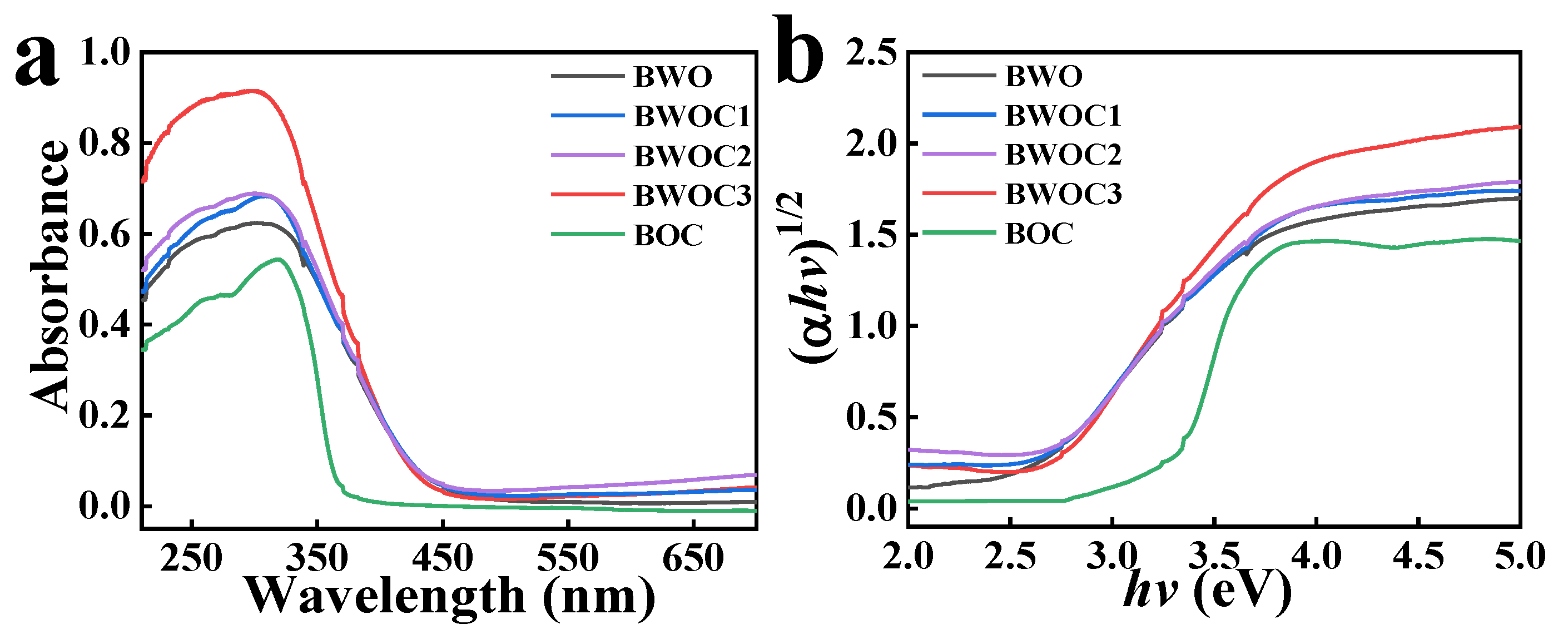
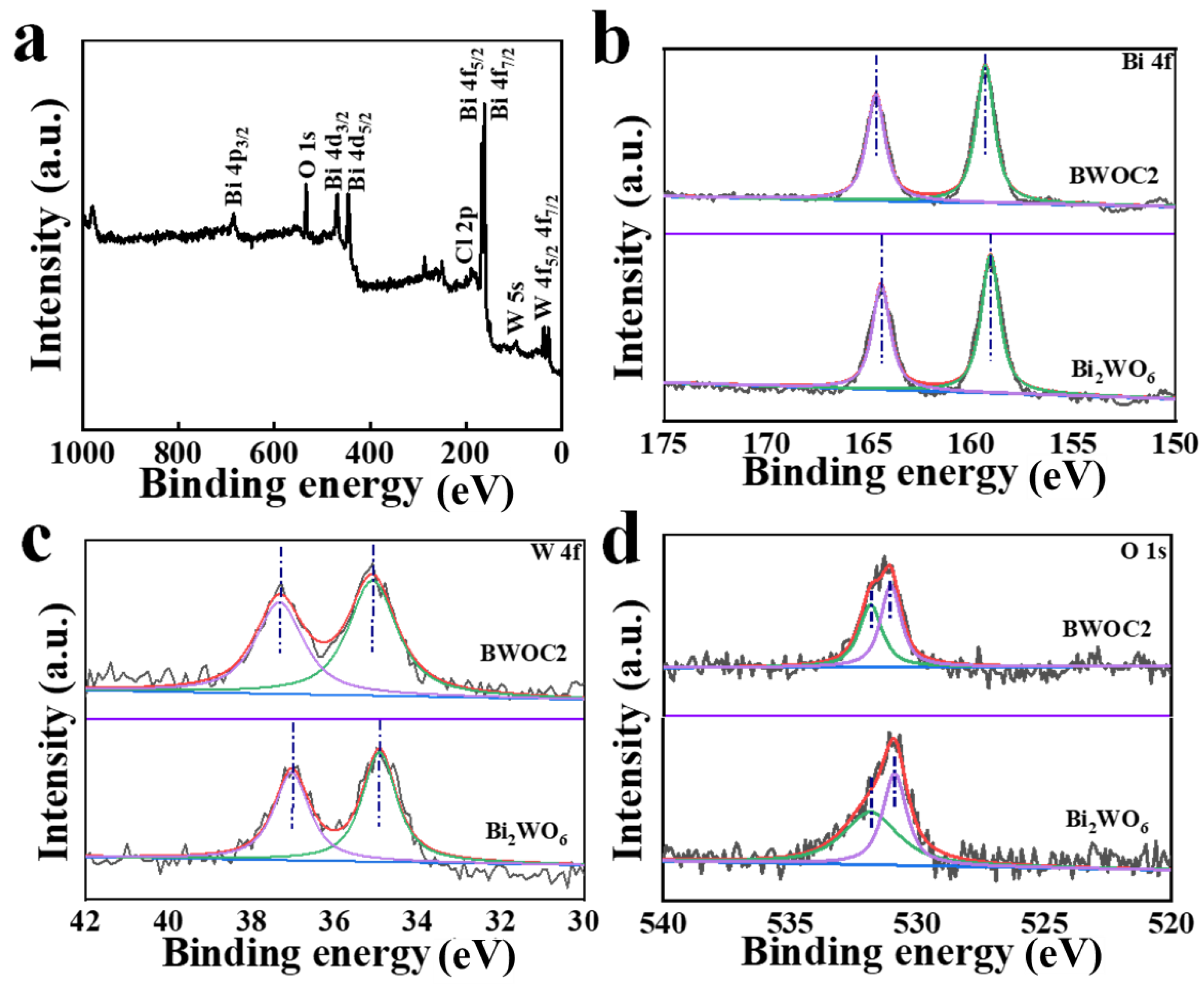
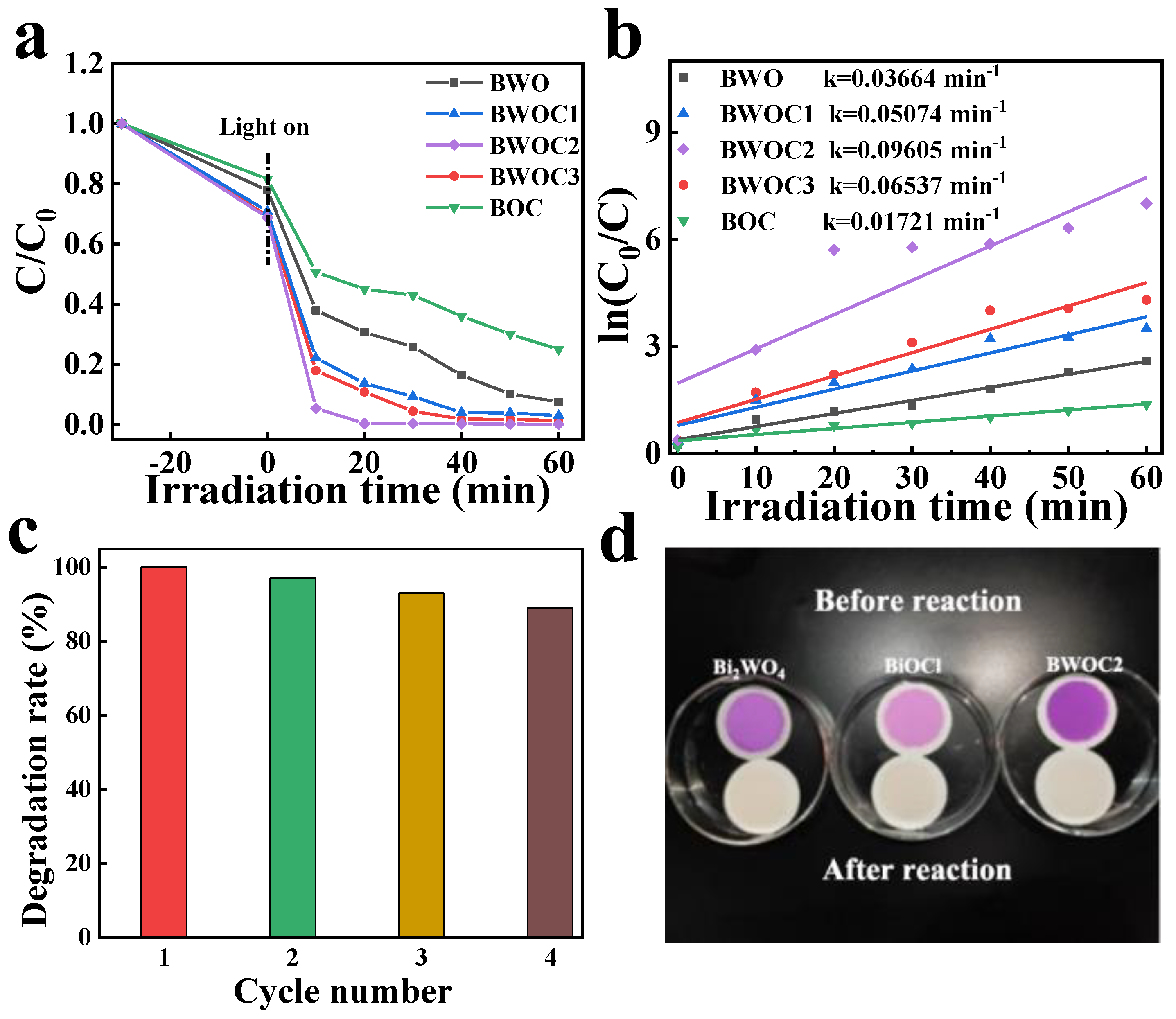
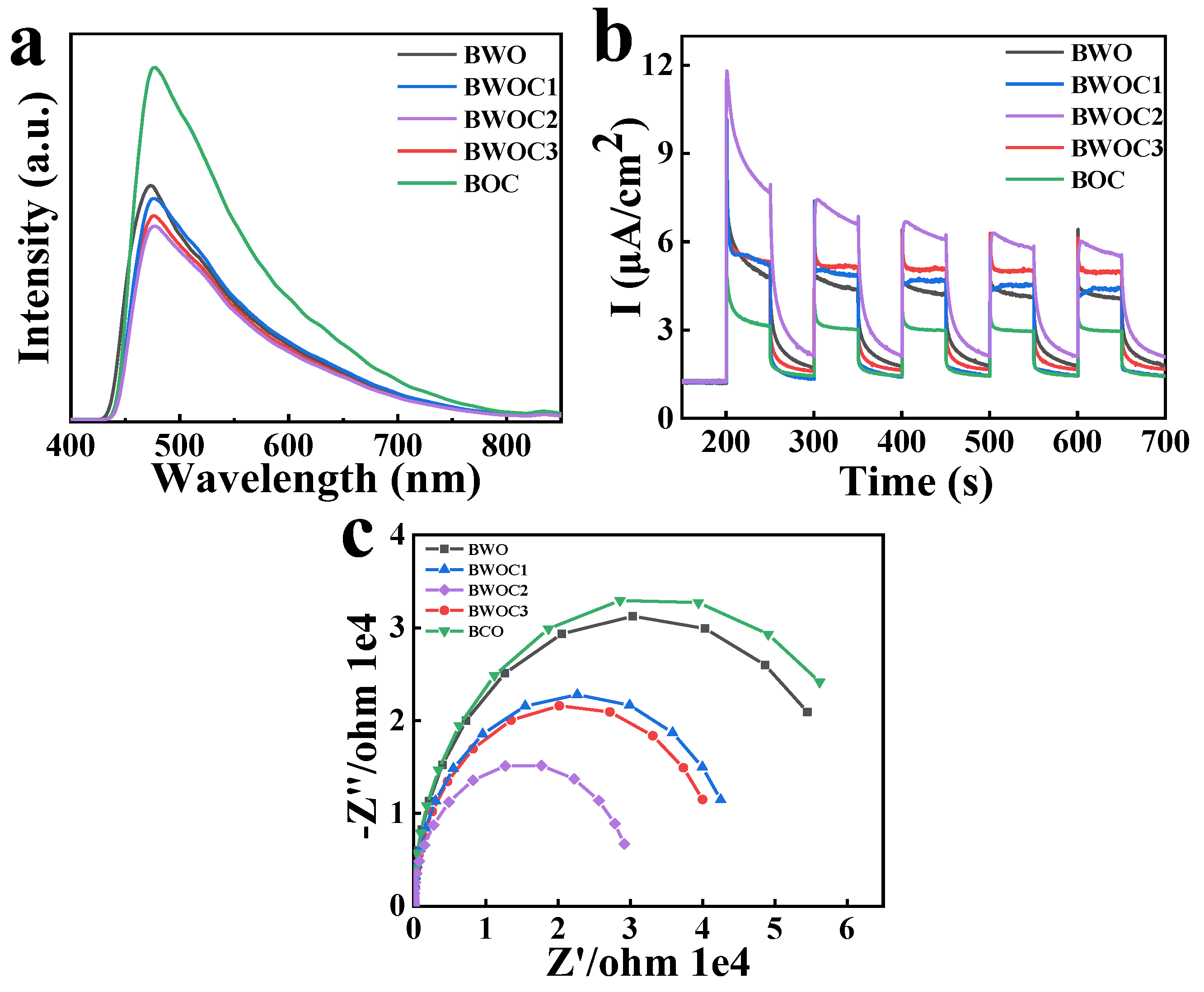
3. Results and Discussion
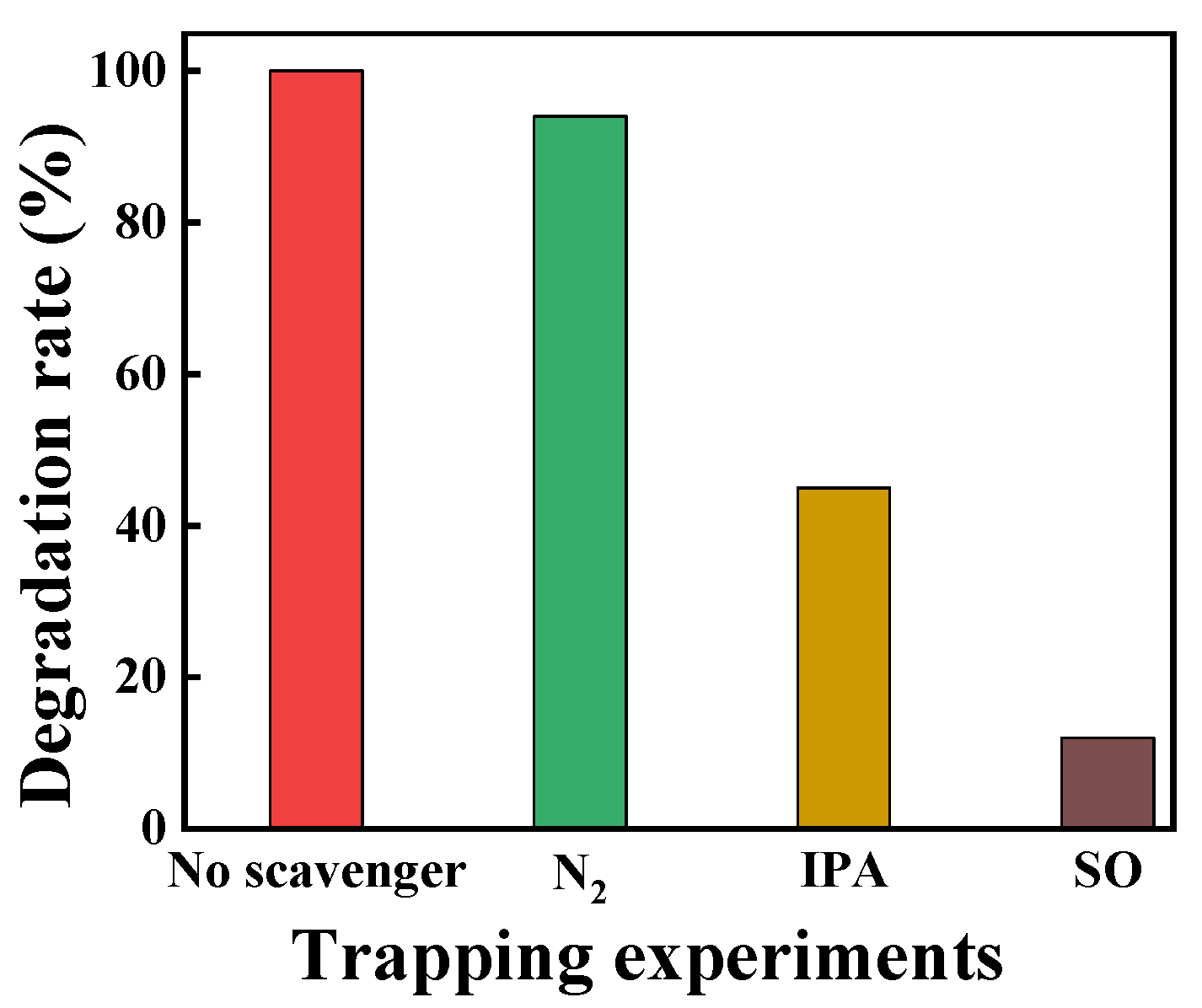
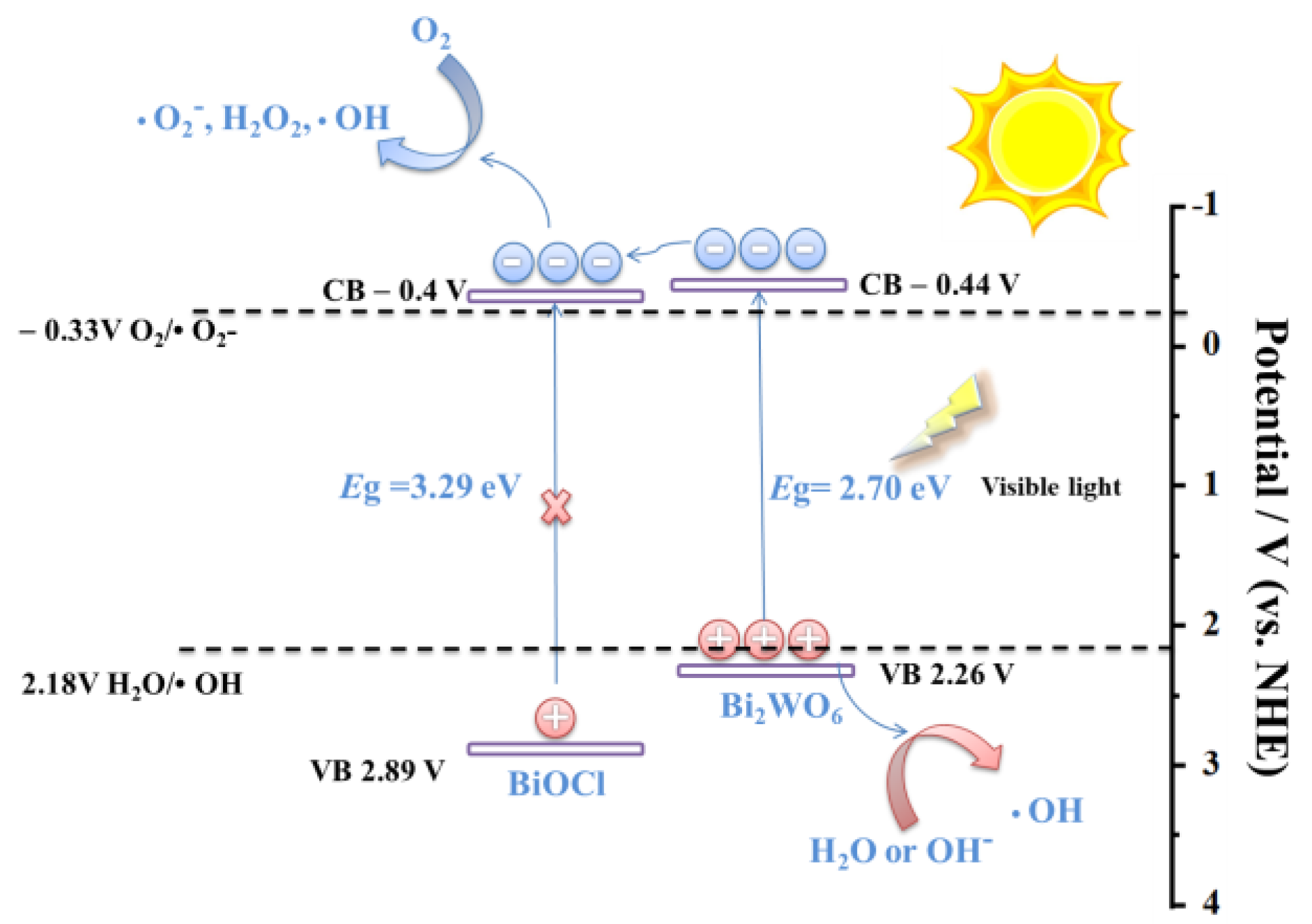
4. A Possible Mechanism for Photocatalytic Degradation of Rhodamine
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, B.; Zhang, Y.; Ran, J.; Jaroniec, M.; Qiao, S. Single-atom photocatalysts for emerging reactions. ACS Cent. Sci. 2021, 7, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Opoku, F.; Govender, K.K.; van Sittert, C.G.C.E.; Govender, P.P. Recent progress in the development of semiconductor—Based photocatalyst materials for applications in photocatalytic water splitting and degradation of pollutants. Adv. Sustain. Syst. 2017, 1, 1700006. [Google Scholar] [CrossRef]
- Spiridonov, V.V.; Liu, X.Y.; Zezin, S.B.; Panova, I.G.; Sybachin, A.V.; Yaroslavov, A.A. Hybrid nanocomposites of carboxymethyl cellulose cross-linked by in-situ formed Cu2O nanoparticles for photocatalytic applications. J. Organomet. Chem. 2020, 914, 121180. [Google Scholar] [CrossRef]
- Castro, K.A.D.F.; Rodrigues, J.M.M.; Faustino, M.; Tomé, J.P.C.; Cavaleiro, J.A.S.; Neves, M.d.P.M.S.; Simões, M.M.Q. Photocatalytic degradation of methyl orange mediated by a silica coated nanomagnet porphyrin hybrid. J. Organomet. Chem. 2021, 938, 121751. [Google Scholar] [CrossRef]
- Aquino, R.V.S.; Barbosa, A.A.; Ribeiro, L.B.; Oliveira, A.F.B.; Silva, J.P.; Azoubel, P.M.; Rocha, O.R.S. Degradation of leaf green food dye by heterogeneous photocatalysis with TiO2 over a polyethylene terephthalate plate. Chem. Pap. 2019, 73, 2501–2512. [Google Scholar] [CrossRef]
- Shen, R.; Ding, Y.; Li, S.; Zhang, P.; Xiang, Q.; Ng, Y.H.; Li, X. Constructing low-cost Ni3C/twin-crystal Zn0.5Cd0.5S heterojunction/homojunction nanohybrids for efficient photocatalytic H-2 evolution. Chin. J. Catal. 2021, 42, 25–36. [Google Scholar] [CrossRef]
- Wang, K.; Xing, Z.; Meng, D.; Zhang, S.; Li, Z.; Pan, K.; Zhou, W. Hollow MoSe2@Bi2S3/CdS Core-Shell Nanostructure as Dual Z-Scheme Heterojunctions with Enhanced Full Spectrum Photocatalytic-Photothermal Performance. Appl. Catal. B-Environ. 2021, 281, 119482. [Google Scholar] [CrossRef]
- He, F.; Meng, A.; Cheng, B.; Ho, W.; Yu, J. Enhanced photocatalytic H-2-production activity of WO3/TiO2 step-scheme heterojunction by graphene modification. Chin. J. Catal. 2020, 41, 9–20. [Google Scholar] [CrossRef]
- Liang, Z.; Shen, R.; Ng, Y.H.; Zhang, P.; Xiang, Q.; Li, X. A review on 2D MoS2 cocatalysts in photocatalytic H-2 production. J. Mater. Sci. Technol. 2020, 56, 89–121. [Google Scholar] [CrossRef]
- Shen, C.; Wen, X.; Fei, Z.; Liu, Z.; Mu, Q. Visible-light-driven activation of peroxymonosulfate for accelerating ciprofloxacin degradation using CeO2/Co3O4 p-n heterojunction photocatalysts. Chem. Eng. J. 2020, 391, 123612. [Google Scholar] [CrossRef]
- Liang, X.; Wang, P.; Gao, Y.; Huang, H.; Tong, F.; Zhang, Q.; Wang, Z.; Liu, Y.; Zheng, Z.; Dai, Y.; et al. Design and synthesis of porous M-ZnO/CeO2 microspheres as efficient plasmonic photocatalysts for nonpolar gaseous molecules oxidation: Insight into the role of oxygen vacancy defects and M = Ag, Au nanoparticles. Appl. Catal. B Environ. 2020, 260, 118151. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.; Xia, M.; An, H.; Bai, H.; Wei, J.; Yang, B.; Yang, G. Highly efficient charge transfer at 2D/2D layered P-La2Ti2O7/Bi2WO6 contact heterojunctions for upgraded visible-light-driven photocatalysis. Appl. Catal. B Environ. 2020, 261, 118244. [Google Scholar] [CrossRef]
- Jia, Z.; Li, T.; Zheng, Z.; Zhang, J.; Liu, J.; Li, R.; Wang, Y.; Zhang, X.; Wang, Y.; Fan, C. The BiOCl/diatomite composites for rapid photocatalytic degradation of ciprofloxacin: Efficiency, toxicity evaluation, mechanisms and pathways. Chem. Eng. J. 2020, 380, 122422. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Yang, S.; Lin, X.; Shi, W. Fabrication of a ternary heterostructure BiVO4 quantum dots/C-60/g-C3N4 photocatalyst with enhanced photocatalytic activity. J. Phys. Chem. Solids 2020, 136, 109164. [Google Scholar] [CrossRef]
- Shao, B.; Liu, X.; Liu, Z.; Zeng, G.; Liang, Q.; Liang, C.; Cheng, Y.; Zhang, W.; Liu, Y.; Gong, S. A novel double Z-scheme photocatalyst Ag3PO4/Bi2S3/Bi2O3 with enhanced visible-light photocatalytic performance for antibiotic degradation. Chem. Eng. J. 2019, 368, 730–745. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, J.; Chu, W.; Cahen, D.; He, T. Synergistic Effect of Charge Generation and Separation in Epitaxially Grown BiOCl/Bi2S3 Nano-Heterostructure. ACS Appl. Mater. Interfaces 2018, 10, 15304–15313. [Google Scholar] [CrossRef]
- Yang, W.; Ma, B.; Wang, W.; Wen, Y.; Zeng, D.; Shan, B. Enhanced photosensitized activity of a BiOCl-Bi2WO6 heterojunction by effective interfacial charge transfer. Phys. Chem. Chem. Phys. Pccp 2013, 15, 19387–19394. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Wang, Z.; Wei, X.; Liu, L.; Ren, Q.; Shi, H. p–n junction CuO/BiVO4 heterogeneous nanostructures: Synthesis and highly efficient visible-light photocatalytic performance. Dalton Trans. 2014, 43, 6735–6743. [Google Scholar] [CrossRef]
- Liu, S.; Tu, Y.; Dai, G. The effects of citrate ion on morphology and photocatalytic activity of flower-like Bi2O2CO3. Ceram. Int. 2014, 40, 2343–2348. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, M.; Chen, Q.G.; Fan, C.M.; Zhou, H.Y.; Xu, A.W. Novel one-dimensional Bi2O3–Bi2WO6 p–n hierarchical heterojunction with enhanced photocatalytic activity. J. Mater. Chem. A 2014, 2, 8517–8524. [Google Scholar] [CrossRef]
- He, Z.; Shi, Y.; Gao, C.; Wen, L.; Chen, J.; Song, S. BiOCl/BiVO4 p–n Heterojunction with Enhanced Photocatalytic Activity under Visible-Light Irradiation. J. Phys. Chem. C 2014, 118, 389–398. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Hu, J.S.; Pan, C.L.; Hou, C.M. Facile hydrothermal synthesis of novel Bi12TiO20-Bi2WO6 heterostructure photocatalyst with enhanced photocatalytic activity. Appl. Surf. Sci. 2015, 346, 33–40. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Fan, X.; Zhou, Y.; Wu, M.; Shi, J. Highly selective CO2 photoreduction to CO over g-C3N4/Bi2WO6 composites under visible light. J. Mater. Chem. A Mater. Energy Sustain. 2015, 3, 5189–5196. [Google Scholar] [CrossRef]
- Lupan, O.; Chow, L.; Ono, L.K.; Cuenya, B.R.; Chai, G.; Khallaf, H.; Schulte, A. Synthesis and Characterization of Ag- or Sb-Doped ZnO Nanorods by a Facile Hydrothermal Route. J. Phys. Chem. C 2010, 114, 12401–12408. [Google Scholar] [CrossRef]
- Li, Z.; Yin, L. Nitrogen-Doped MOF-Derived Micropores Carbon as Immobilizer for Small Sulfur Molecules as a Cathode for Lithium Sulfur Batteries with Excellent Electrochemical Performance. ACS Appl. Mater. Interfaces 2015, 7, 4029–4038. [Google Scholar] [CrossRef]
- Lin, L.; Huang, M.; Long, L.; Sun, Z.; Zheng, W.; Chen, D. Fabrication of a three-dimensional BiOBr/BiOI photocatalyst with enhanced visible light photocatalytic performance. Ceram. Int. 2014, 40, 11493–11501. [Google Scholar] [CrossRef]
- An, W.; Cui, W.; Liang, Y.; Hu, J.; Liu, L. Surface decoration of BiPO4 with BiOBr nanoflakes to build heterostructure photocatalysts with enhanced photocatalytic activity. Appl. Surf. Sci. 2015, 351, 1131–1139. [Google Scholar] [CrossRef]
- Chen, L.; Meng, D.; Wu, X.; Wang, A.; Wang, J.; Yu, M.; Liang, Y. Enhanced visible light photocatalytic performances of self-assembled hierarchically structured BiVO4/Bi2WO6 heterojunction composites with different morphologies. Rsc Adv. 2016, 6, 52300–52309. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Ji, M.; Wang, B.; Yin, S.; Zhang, Q.; Li, H. Advanced photocatalytic performance of graphene-like BN modified BiOBr flower-like materials for the removal of pollutants and mechanism insight. Appl. Catal. B Environ. 2016, 183, 254–262. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, Y.; Tian, G.; Fu, H.; Pan, K.; Zhou, J.; Yan, H. One-pot controlled synthesis of sea-urchin shaped Bi2S3/CdS hierarchical heterostructures with excellent visible light photocatalytic activity. Dalton Trans. 2014, 43, 12396–12404. [Google Scholar] [CrossRef]
- He, D.; Wang, L.; Xu, D.; Zhai, J.; Wang, D.; Xie, T. Investigation of Photocatalytic Activities over Bi2WO6/ZnWO4 Composite under UV Light and Its Photoinduced Charge Transfer Properties. ACS Appl. Mater. Interfaces 2011, 3, 3167–3171. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liang, B.; Zhang, M.; Long, Y.; Li, W. Enhanced photocatalytic properties of α-SnWO4 nanosheets modified by Ag nanoparticles. J. Colloid Interface Sci. 2017, 490, 46–52. [Google Scholar] [CrossRef]
- Chen, W.; Wu, S.C.; Xia, Z.J.; Huang, G.B.; Hu, J. Mesoporous g-C3N4 ultrathin nanosheets coupled with QDs self-decorated SnIn4S8 homojunctions towards highly efficient photocatalytic functional transformation. J. Alloys Compd. 2019, 809, 151859. [Google Scholar] [CrossRef]
- Cao, B.; Li, G.; Li, H. Hollow spherical RuO2@ TiO2@ Pt bifunctional photocatalyst for coupled H2 production and pollutant degradation. Appl. Catal. B Environ. 2016, 194, 42–49. [Google Scholar] [CrossRef]
- Cui, H.; Li, B.; Li, Z.; Li, X.; Xu, S. Z-scheme based CdS/CdWO4 heterojunction visible light photocatalyst for dye degradation and hydrogen evolution. Appl. Surf. Sci. J. Devoted Prop. Interfaces Relat. Synth. Behav. Mater. 2018, 455, 831–840. [Google Scholar] [CrossRef]
- Aisha, A.A.; Zoya, Z.; Shafik, A. Eco-friendly green synthesis of Ag@Fe bimetallic nanoparticles: Antioxidant, antimicrobial and photocatalytic degradation of bromothymol blue. J. Photochem. Photobiol. B Biol. 2018, 185, 143. [Google Scholar]
- Zhu, S.; Yang, C.; Li, F.; Li, T.; Zhang, M.; Cao, W. Improved photocatalytic Bi2WO6/BiOCl heterojunctions: One-step synthesis via an ionic-liquid assisted ultrasonic method and first-principles calculations. Mol. Catal. 2017, 435, 33–48. [Google Scholar] [CrossRef] [Green Version]
- Dong, F.; Li, Q.; Wing-Kei, H.O.; Wu, Z. The mechanism of enhanced visible light photocatalysis with micro-structurally optimized and graphene oxide coupled (BiO)2CO3. Chin. Sci. Bull. 2015, 60, 1915–1923. [Google Scholar]
- Ni, Z.; Shen, Y.; Xu, L.; Xiang, G.; Chen, M.; Shen, N.; Li, K.; Ni, K. Facile construction of 3D hierarchical flower-like Ag2WO4/Bi2WO6 Z-scheme heterojunction photocatalyst with enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2022, 576, 151868. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, B.; Ma, J.; Liu, L.; Luo, H.; Wang, W. The BiOBr/Bi/Bi2WO6 photocatalyst with SPR effect and Z-scheme heterojunction synergistically degraded RhB under visible light. Opt. Mater. 2021, 122, 111641. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, J.; Fang, N.; Fang, N.; Liang, J.; Shen, T.; Yuan, S. Investigation of photocatalytic performance of CuS/Bi2WO6 and degradation pathway of RhB in water. J. Water Supply Res. Technol.-Aqua 2020, 69, 145–159. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, X.; Liu, H.; Liu, Y.; Yu, T.; Sun, B. Synthesis of La-doped Bi2WO6 to study on the removal of RhB wastewater under visible light. J. Nanopart. Res. 2021, 23, 246. [Google Scholar] [CrossRef]
- Dumrongrojthanath, P.; Phuruangrat, A.; Doungarno, K.; Thongtem, T.; Patiphatpanya, P.; Thongtem, S. Microwave-hydrothermal synthesis of BiOBr/Bi2WO6 nanocomposites for enhanced photocatalytic performance. Ceram. Int. 2018, 44, S148–S151. [Google Scholar] [CrossRef]
- Lei, S.; Qin, C.; Tang, X.; Zhong, J.; Li, J.; Chen, J. Spiral carbon fibers modified Bi2WO6 with enhanced photocatalytic activity. J. Phys. Chem. Solids 2020, 141, 109430. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Wang, S.; Shu, M.; Wang, Y.; Cao, D. Effective BiOCl Electrons Collector for Enhancing Photocarrier Separation of Bi2WO6/BiOCl Composite. Chemistry 2022, 4, 765-775. https://doi.org/10.3390/chemistry4030054
Zheng Y, Wang S, Shu M, Wang Y, Cao D. Effective BiOCl Electrons Collector for Enhancing Photocarrier Separation of Bi2WO6/BiOCl Composite. Chemistry. 2022; 4(3):765-775. https://doi.org/10.3390/chemistry4030054
Chicago/Turabian StyleZheng, Yi, Siqi Wang, Min Shu, Yi Wang, and Dumeng Cao. 2022. "Effective BiOCl Electrons Collector for Enhancing Photocarrier Separation of Bi2WO6/BiOCl Composite" Chemistry 4, no. 3: 765-775. https://doi.org/10.3390/chemistry4030054
APA StyleZheng, Y., Wang, S., Shu, M., Wang, Y., & Cao, D. (2022). Effective BiOCl Electrons Collector for Enhancing Photocarrier Separation of Bi2WO6/BiOCl Composite. Chemistry, 4(3), 765-775. https://doi.org/10.3390/chemistry4030054





