Initial Coupling and Reaction Progression of Directly Deposited Biradical Graphene Nanoribbon Monomers on Iodine-Passivated Versus Pristine Ag(111)
Abstract
:1. Introduction
2. Materials and Methods
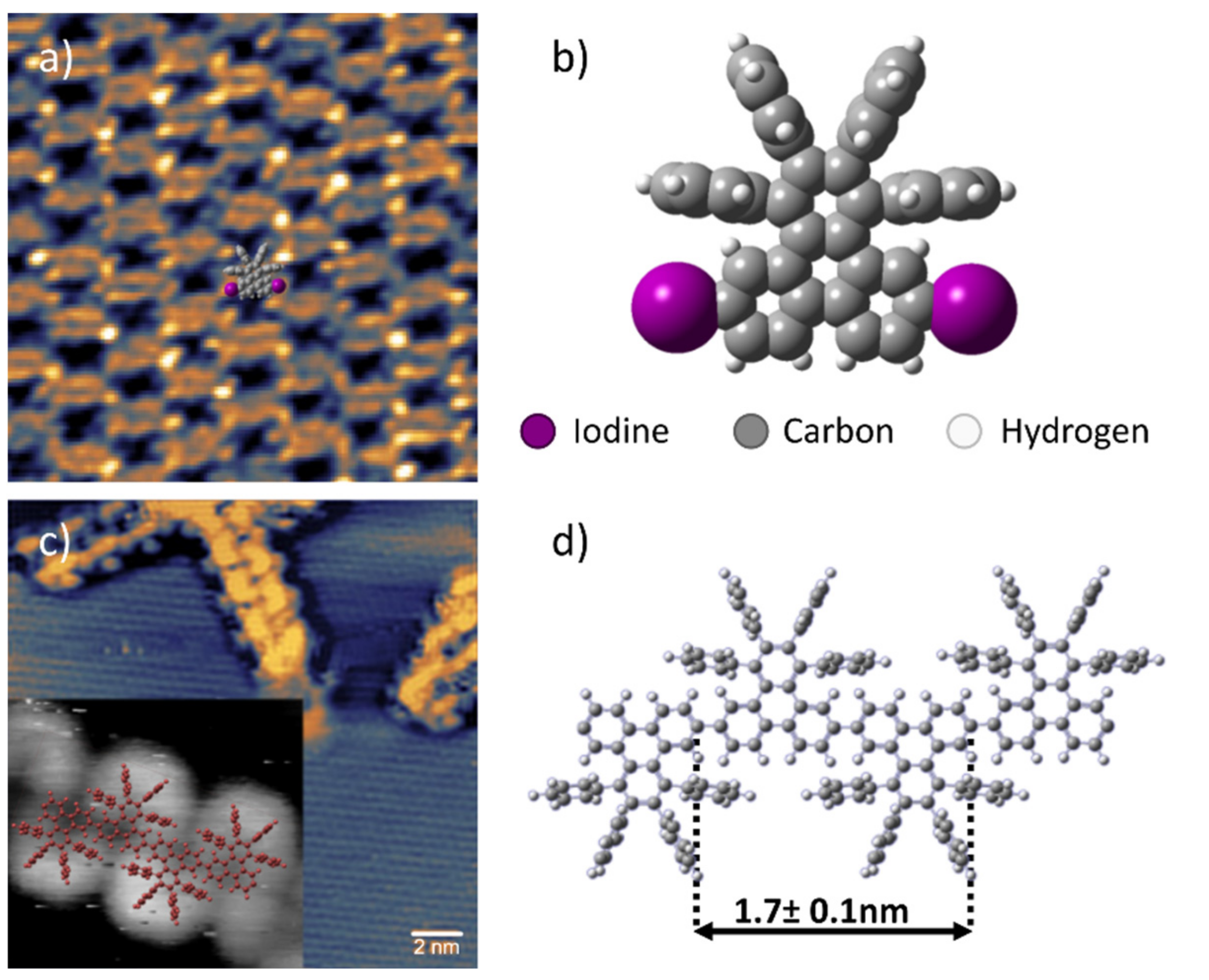
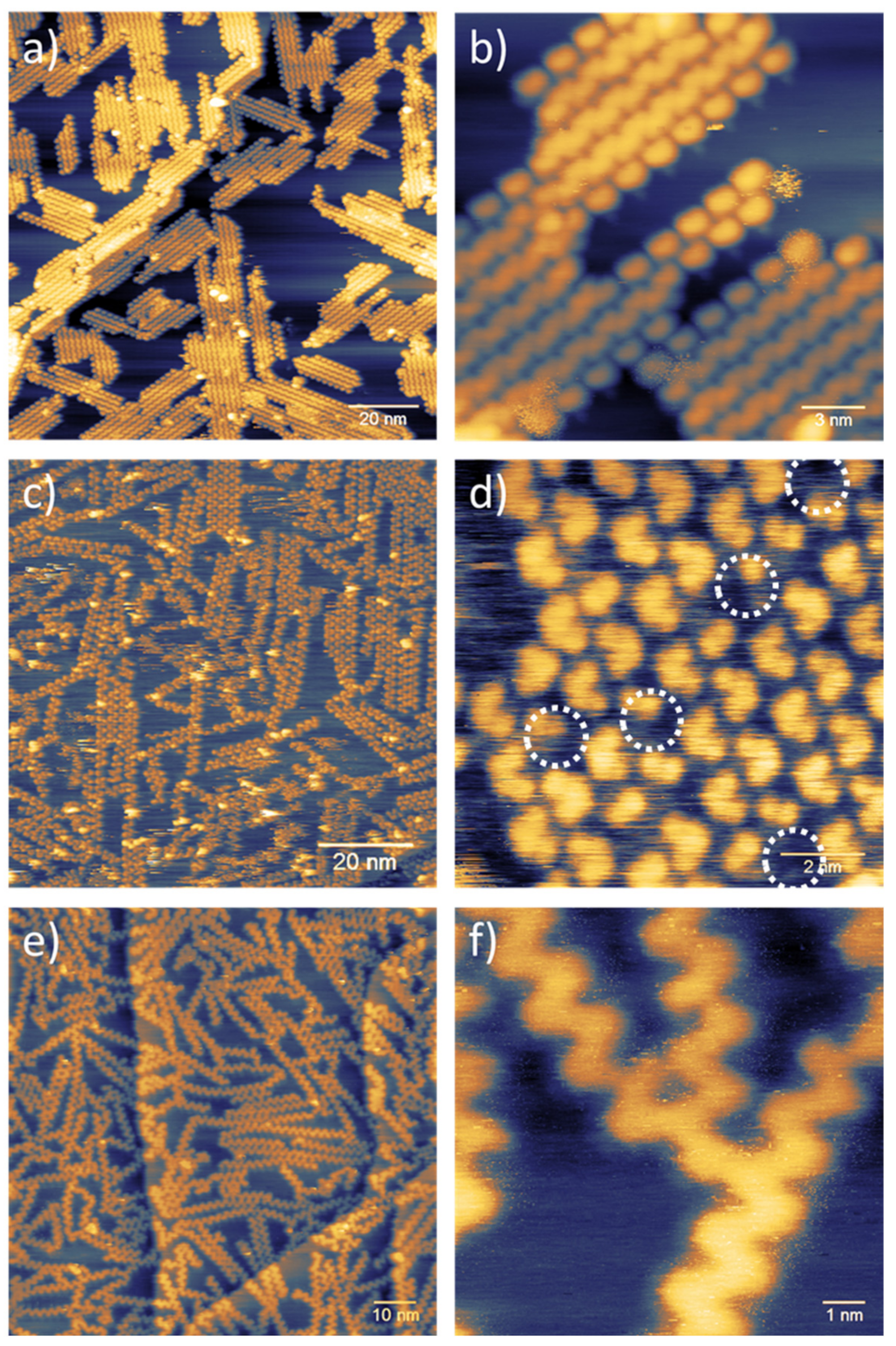
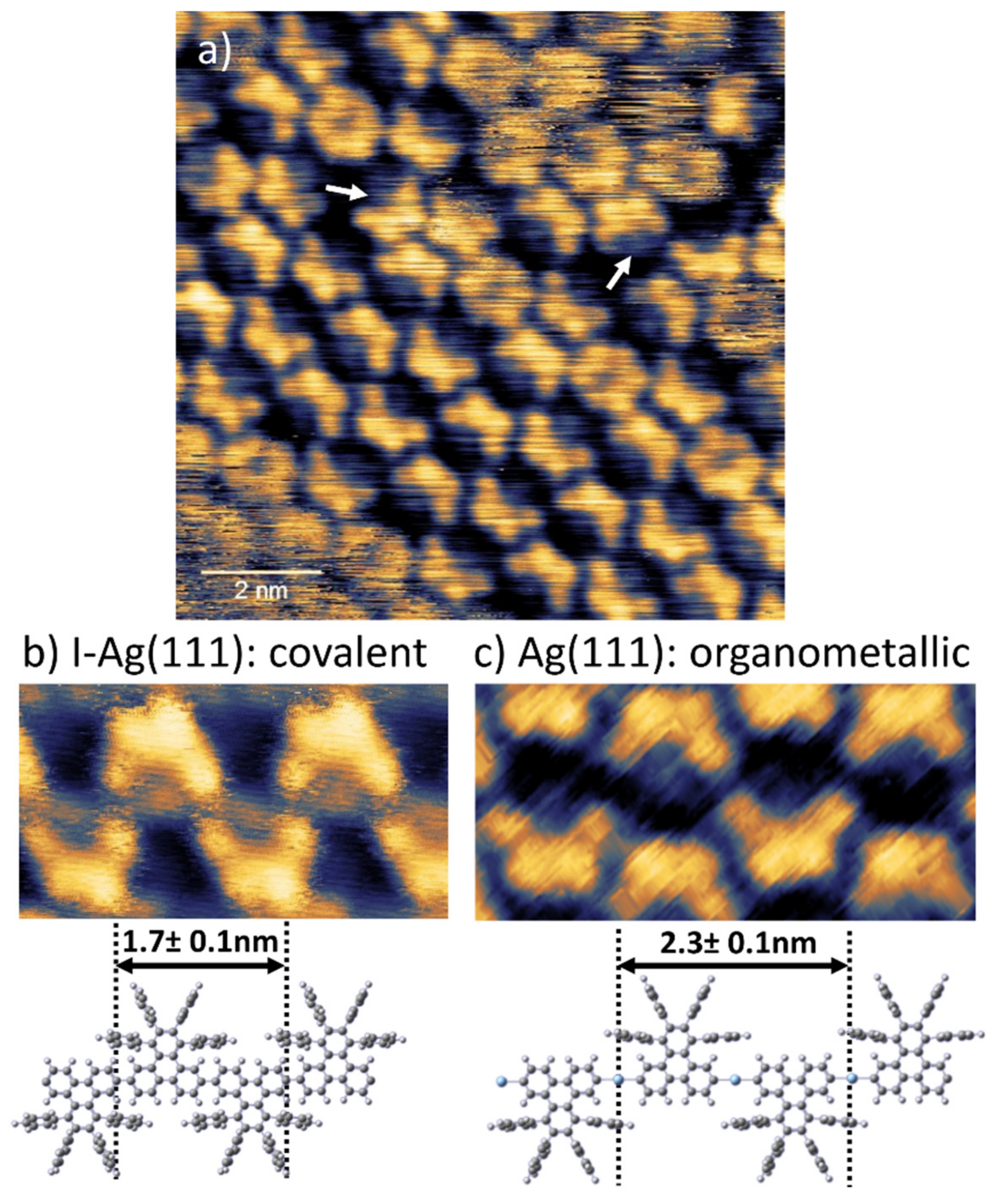
3. Results
4. Discussion and Summary
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photon. 2010, 4, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, W.; Kum, H.; Bae, S.-H.; Shim, J.; Kim, H.; Kong, L.; Meng, Y.; Wang, K.; Kim, C.; Kim, J. Path towards graphene commercialization from lab to market. Nat. Nano. 2019, 14, 927–938. [Google Scholar] [CrossRef]
- Schwierz, F. Graphene transistors. Nat. Nanotechnol. 2010, 5, 487–496. [Google Scholar] [CrossRef]
- Nakada, K.; Fujita, M.; Dresselhaus, G.; Dresselhaus, M.S. Edge state in graphene ribbons: Nanometer size effect and edge shape dependence. Phys. Rev. B 1996, 54, 17954–17961. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, X.; Zhang, L.; Lee, S.; Dai, H. Chemically Derived, Ultrasmooth Graphene Nanoribbon Semiconductors. Science 2008, 319, 1229–1232. [Google Scholar] [CrossRef]
- Han, M.Y.; Özyilmaz, B.; Zhang, Y.; Kim, P. Energy Band-Gap Engineering of Graphene Nanoribbons. Phys. Rev. Lett. 2007, 98, 206805. [Google Scholar] [CrossRef] [Green Version]
- Kosynkin, D.V.; Higginbotham, A.L.; Sinitskii, A.; Lomeda, J.R.; Dimiev, A.; Price, B.K.; Tour, J.M. Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 2009, 458, 872–876. [Google Scholar] [CrossRef] [Green Version]
- Son, Y.-W.; Cohen, M.L.; Louie, S.G. Energy Gaps in Graphene Nanoribbons. Phys. Rev. Lett. 2006, 97, 216803. [Google Scholar] [CrossRef] [Green Version]
- Barone, V.; Hod, O.; Scuseria, G.E. Electronic Structure and Stability of Semiconducting Graphene Nanoribbons. Nano Lett. 2006, 6, 2748–2754. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Guo, J. Effect of edge roughness in graphene nanoribbon transistors. Appl. Phys. Lett. 2007, 91, 073103. [Google Scholar] [CrossRef] [Green Version]
- Houtsma, R.S.K.; de la Rie, J.; Stöhr, M. Atomically precise graphene nanoribbons: Interplay of structural and electronic properties. Chem. Soc. Rev. 2021, 50, 6541–6568. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Ruffieux, P.; Jaafar, R.; Bieri, M.; Braun, T.; Blankenburg, S.; Muoth, M.; Seitsonen, A.P.; Saleh, M.; Feng, X. Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 2010, 466, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Ruffieux, P.; Wang, S.; Yang, B.; Sánchez-Sánchez, C.; Liu, J.; Dienel, T.; Talirz, L.; Shinde, P.; Pignedoli, C.A.; Passerone, D.; et al. On-surface synthesis of graphene nanoribbons with zigzag edge topology. Nature 2016, 531, 489–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talirz, L.; Ruffieux, P.; Fasel, R. On-Surface Synthesis of Atomically Precise Graphene Nanoribbons. Adv. Mater. 2016, 28, 6222–6231. [Google Scholar] [CrossRef]
- Lipton-Duffin, J.A.; Miwa, J.A.; Kondratenko, M.; Cicoira, F.; Sumpter, B.G.; Meunier, V.; Perepichka, D.F.; Rosei, F. Step-by-step growth of epitaxially aligned polythiophene by surface-confined reaction. Proc. Natl. Acad. Sci. USA 2010, 107, 11200–11204. [Google Scholar] [CrossRef] [Green Version]
- Blake, M.M.; Nanayakkara, S.U.; Claridge, S.A.; Fernández-Torres, L.C.; Sykes, E.C.H.; Weiss, P.S. Identifying Reactive Intermediates in the Ullmann Coupling Reaction by Scanning Tunneling Microscopy and Spectroscopy. J. Phys. Chem. A 2009, 113, 13167–13172. [Google Scholar] [CrossRef]
- Xi, M.; Bent, B.E. Mechanisms of the Ullmann coupling reaction in adsorbed monolayers. J. Am. Chem. Soc. 1993, 115, 7426–7433. [Google Scholar] [CrossRef]
- Grill, L.; Dyer, M.; Lafferentz, L.; Persson, M.; Peters, M.V.; Hecht, S. Nano-architectures by Covalent Assembly of Molecular Building Blocks. Nat. Nanotechnol. 2007, 2, 687. [Google Scholar] [CrossRef]
- Di Giovannantonio, M.; El Garah, M.; Lipton-Duffin, J.; Meunier, V.; Cardenas, L.; Fagot Revurat, Y.; Cossaro, A.; Verdini, A.; Perepichka, D.F.; Rosei, F.; et al. Insight into Organometallic Intermediate and Its Evolution to Covalent Bonding in Surface-Confined Ullmann Polymerization. ACS Nano 2013, 7, 8190–8198. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, J.; Strunskus, T.; Rastgoo-Lahrood, A.; Samanta, D.; Schmittel, M.; Lackinger, M. On-surface Ullmann polymerization via intermediate organometallic networks on Ag(111). Chem. Commun. 2014, 50, 7680–7682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritton, M.; Otte, K.; Björk, J.; Biswas, P.K.; Heckl, W.M.; Schmittel, M.; Lackinger, M. The influence of ortho-methyl substitution in organometallic self-assembly—A comparative study on Cu(111) vs. Ag(111). Chem. Commun. 2018, 54, 9745–9748. [Google Scholar] [CrossRef] [PubMed]
- Lischka, M.; Fritton, M.; Eichhorn, J.; Vyas, V.S.; Strunskus, T.; Lotsch, B.V.; Björk, J.; Heckl, W.M.; Lackinger, M. On-Surface Polymerization of 1,6-Dibromo-3,8-diiodpyrene—A Comparative Study on Au(111) Versus Ag(111) by STM, XPS, and NEXAFS. J. Phys. Chem. C 2018, 122, 5967–5977. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, C.; Brüller, S.; Sachdev, H.; Müllen, K.; Krieg, M.; Bettinger, H.F.; Nicolaï, A.; Meunier, V.; Talirz, L.; Fasel, R.; et al. On-Surface Synthesis of BN-Substituted Heteroaromatic Networks. ACS Nano 2015, 9, 9228–9235. [Google Scholar] [CrossRef]
- Massimi, L.; Ourdjini, O.; Lafferentz, L.; Koch, M.; Grill, L.; Cavaliere, E.; Gavioli, L.; Cardoso, C.; Prezzi, D.; Molinari, E.; et al. Surface-Assisted Reactions toward Formation of Graphene Nanoribbons on Au(110) Surface. J. Phys. Chem. C 2015, 119, 2427–2437. [Google Scholar] [CrossRef]
- Björk, J.; Stafström, S.; Hanke, F. Zipping Up: Cooperativity Drives the Synthesis of Graphene Nanoribbons. J. Am. Chem. Soc. 2011, 133, 14884–14887. [Google Scholar] [CrossRef]
- Lackinger, M. Surface-assisted Ullmann coupling. Chem. Commun. 2017, 53, 7872–7885. [Google Scholar] [CrossRef]
- Kolmer, M.; Steiner, A.K.; Izydorczyk, I.; Ko, W.; Engelund, M.; Szymonski, M.; Li, A.-P.; Amsharov, K. Rational synthesis of atomically precise graphene nanoribbons directly on metal oxide surfaces. Science 2020, 369, 571–575. [Google Scholar] [CrossRef]
- Llinas, J.P.; Fairbrother, A.; Borin Barin, G.; Shi, W.; Lee, K.; Wu, S.; Yong Choi, B.; Braganza, R.; Lear, J.; Kau, N.; et al. Short-channel field-effect transistors with 9-atom and 13-atom wide graphene nanoribbons. Nat. Commun. 2017, 8, 633. [Google Scholar] [CrossRef] [Green Version]
- Borin Barin, G.; Fairbrother, A.; Rotach, L.; Bayle, M.; Paillet, M.; Liang, L.; Meunier, V.; Hauert, R.; Dumslaff, T.; Narita, A.; et al. Surface-Synthesized Graphene Nanoribbons for Room Temperature Switching Devices: Substrate Transfer and ex Situ Characterization. ACS Appl. Nano Mater. 2019, 2, 2184–2192. [Google Scholar] [CrossRef] [Green Version]
- Galeotti, G.; Fritton, M.; Lackinger, M. Carbon-Carbon Coupling on Inert Surfaces by Deposition of En Route Generated Aryl Radicals. Angew. Chem. Int. Ed. 2020, 59, 22785–22789. [Google Scholar] [CrossRef] [PubMed]
- Lackinger, M. Synthesis on inert surfaces. Dalton T 2021, 50, 10020–10027. [Google Scholar] [CrossRef] [PubMed]
- Bronner, C.; Marangoni, T.; Rizzo, D.J.; Durr, R.A.; Jørgensen, J.H.; Fischer, F.R.; Crommie, M.F. Iodine versus Bromine Functionalization for Bottom-Up Graphene Nanoribbon Growth: Role of Diffusion. J. Phys. Chem. C 2017, 121, 18490–18495. [Google Scholar] [CrossRef] [Green Version]
- Thiessen, A.; Wettach, H.; Meerholz, K.; Neese, F.; Hoger, S.; Hertel, D. Control of electronic properties of triphenylene by substitution. Org. Electron. 2012, 13, 71–83. [Google Scholar] [CrossRef]
- Gutzler, R.; Heckl, W.M.; Lackinger, M. Combination of a Knudsen effusion cell with a quartz crystal microbalance: In situ measurement of molecular evaporation rates with a fully functional deposition source. Rev. Sci. Instrum. 2010, 81, 015108. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Richards, D.; Ertekin, E.; Grossman, J.C.; Strubbe, D.; Riley, J.; Guerrero, E. MIT Atomic-Scale Modeling Toolkit. 2021. Available online: https://nanohub.org/resources/ucb_compnano (accessed on 1 March 2022).
- Rastgoo-Lahrood, A.; Björk, J.; Lischka, M.; Eichhorn, J.; Kloft, S.; Fritton, M.; Strunskus, T.; Samanta, D.; Schmittel, M.; Heckl, W.M.; et al. Post-Synthetic Decoupling of On-Surface-Synthesized Covalent Nanostructures from Ag(111). Angew. Chem. Int. Ed. 2016, 55, 7650–7654. [Google Scholar] [CrossRef]
- Grossmann, L.; Duncan, D.A.; Jarvis, S.P.; Jones, R.G.; De, S.; Rosen, J.; Schmittel, M.; Heckl, W.M.; Björk, J.; Lackinger, M. Evolution of adsorption heights in the on-surface synthesis and decoupling of covalent organic networks on Ag(111) by normal-incidence X-ray standing wave. Nanoscale Horiz. 2022, 7, 51–62. [Google Scholar] [CrossRef]
- Gust, D. Restricted rotation in hexaarylbenzenes. J. Am. Chem. Soc. 1977, 99, 6980–6982. [Google Scholar] [CrossRef]
- Galeotti, G.; De Marchi, F.; Taerum, T.; Besteiro, L.; El Garah, M.; Lipton-Duffin, J.; Ebrahimi, M.; Perepichka, D.; Rosei, F. Surface-mediated Assembly, Polymerization and Degradation of Thiophene-based Monomers. Chem. Sci. 2019, 10, 5167–5175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berndt, W. Iodine Adsorption on Silver (111) Studied by LEED. Jpn. J. Appl. Phys. 1974, 13, 653. [Google Scholar] [CrossRef]
- Rastgoo-Lahrood, A.; Lischka, M.; Eichhorn, J.; Samanta, D.; Schmittel, M.; Heckl, W.M.; Lackinger, M. Reversible intercalation of iodine monolayers between on-surface synthesised covalent polyphenylene networks and Au(111). Nanoscale 2017, 9, 4995–5001. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; Vilas-Varela, M.; Kretz, B.; Garcia-Lekue, A.; Costache, M.V.; Paradinas, M.; Panighel, M.; Ceballos, G.; Valenzuela, S.O.; Peña, D.; et al. Bottom-up synthesis of multifunctional nanoporous graphene. Science 2018, 360, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Judd, C.J.; Junqueira, F.L.Q.; Haddow, S.L.; Champness, N.R.; Duncan, D.A.; Jones, R.G.; Saywell, A. Structural characterisation of molecular conformation and the incorporation of adatoms in an on-surface Ullmann-type reaction. Commun. Chem. 2020, 3, 166. [Google Scholar] [CrossRef]
- Zhang, Z.; Perepichka, D.F.; Khaliullin, R.Z. Adatoms in the Surface-Confined Ullmann Coupling of Phenyl Groups. J. Phys. Chem. Lett. 2021, 12, 11061–11069. [Google Scholar] [CrossRef]
- Fritton, M.; Duncan, D.A.; Deimel, P.S.; Rastgoo-Lahrood, A.; Allegretti, F.; Barth, J.V.; Heckl, W.M.; Björk, J.; Lackinger, M. The Role of Kinetics versus Thermodynamics in Surface-Assisted Ullmann Coupling on Gold and Silver Surfaces. J. Am. Chem. Soc. 2019, 141, 4824–48323. [Google Scholar] [CrossRef]
- Nacci, C.; Schied, M.; Civita, D.; Magnano, E.; Nappini, S.; Píš, I.; Grill, L. Thermal- vs Light-Induced On-Surface Polymerization. J. Phys. Chem. C 2021, 125, 22554–22561. [Google Scholar] [CrossRef]
- Song, W.; Martsinovich, N.; Heckl, W.M.; Lackinger, M. Thermodynamics of halogen bonded monolayer self-assembly at the liquid–solid interface. Chem. Commun. 2014, 50, 13465–13468. [Google Scholar] [CrossRef]
- Gutzler, R.; Fu, C.; Dadvand, A.; Hua, Y.; MacLeod, J.M.; Rosei, F.; Perepichka, D.F. Halogen Bonds in 2d Supramolecular Self-Assembly of Organic Semiconductors. Nanoscale 2012, 4, 5965. [Google Scholar] [CrossRef]
- Gatti, R.; MacLeod, J.M.; Lipton-Duffin, J.A.; Moiseev, A.G.; Perepichka, D.F.; Rosei, F. Substrate, Molecular Structure, and Solvent Effects in 2D Self-Assembly via Hydrogen and Halogen Bonding. J. Phys. Chem. C 2014, 118, 25505–25516. [Google Scholar] [CrossRef]
- Teyssandier, J.; Mali, K.S.; De Feyter, S. Halogen Bonding in Two-Dimensional Crystal Engineering. ChemistryOpen 2020, 9, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verzhbitskiy, I.A.; De Corato, M.; Ruini, A.; Molinari, E.; Narita, A.; Hu, Y.; Schwab, M.G.; Bruna, M.; Yoon, D.; Milana, S.; et al. Raman Fingerprints of Atomically Precise Graphene Nanoribbons. Nano Lett. 2016, 16, 3442–3447. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galeotti, G.; Fritton, M.; Lischka, M.; Obermann, S.; Ma, J.; Heckl, W.M.; Feng, X.; Lackinger, M. Initial Coupling and Reaction Progression of Directly Deposited Biradical Graphene Nanoribbon Monomers on Iodine-Passivated Versus Pristine Ag(111). Chemistry 2022, 4, 259-269. https://doi.org/10.3390/chemistry4020020
Galeotti G, Fritton M, Lischka M, Obermann S, Ma J, Heckl WM, Feng X, Lackinger M. Initial Coupling and Reaction Progression of Directly Deposited Biradical Graphene Nanoribbon Monomers on Iodine-Passivated Versus Pristine Ag(111). Chemistry. 2022; 4(2):259-269. https://doi.org/10.3390/chemistry4020020
Chicago/Turabian StyleGaleotti, Gianluca, Massimo Fritton, Matthias Lischka, Sebastian Obermann, Ji Ma, Wolfgang M. Heckl, Xinliang Feng, and Markus Lackinger. 2022. "Initial Coupling and Reaction Progression of Directly Deposited Biradical Graphene Nanoribbon Monomers on Iodine-Passivated Versus Pristine Ag(111)" Chemistry 4, no. 2: 259-269. https://doi.org/10.3390/chemistry4020020
APA StyleGaleotti, G., Fritton, M., Lischka, M., Obermann, S., Ma, J., Heckl, W. M., Feng, X., & Lackinger, M. (2022). Initial Coupling and Reaction Progression of Directly Deposited Biradical Graphene Nanoribbon Monomers on Iodine-Passivated Versus Pristine Ag(111). Chemistry, 4(2), 259-269. https://doi.org/10.3390/chemistry4020020






