Abstract
Lanthanide (LnIII) ions were successfully chelated and sensitized with a tripodal ligand. The absolute LnIII-centered emission efficiencies were ~3% for both the europium(III) (EuIII) and terbium (TbIII) complexes and up to 54% for the cerium(III) (CeIII) complex. The differences in emission quantum yields for the early lanthanides (CeIII) and the mid lanthanides (EuIII and TbIII) were attributed to their d–f and f–f nature, respectively. Despite the low quantum yield of the EuIII complex, the combination of the residual ligand fluorescence and the red EuIII emission resulted in a bluish-white material with the Commission Internationale de l’Eclairage (CIE) coordinates (0.258, 0.242). Thus, metal complexes of the ligand could be used in the generation of single-component white-light-emitting materials.
Keywords:
lanthanides; luminescence; tripodal; sensitization; cerium; lifetimes; wLEDs; complexes; white light; ligand 1. Introduction
The unique properties of trivalent lanthanide, LnIII, ions have resulted in their applications in imaging, sensing, and telecommunications [1,2,3,4,5]. The emission spectra of LnIII ions are sharp as the 4f orbitals of LnIII ions are shielded from the external environment by the filled 5s and 5p orbitals [4]. These emission spectra arise from f–f transitions; thus, they are parity forbidden by the Laporte rule [4]. Consequently, LnIII ions have long emission lifetimes which makes them ideal probes for imaging of biological tissue, as their long emission lifetimes can be discriminated from the autofluorescence of biological tissue [4]. Another consequence of the parity-forbidden nature of their emission is that LnIII ions have low molar absorptivities (ε = 1–10 M−1cm−1); thus, their direct excitation is inefficient [4,6]. Therefore, LnIII ions are often coordinated to organic ligands with well-matched triplet, 3T, excited energy levels that upon excitation can transfer the energy from their excited energy levels (singlet, 1S, and triplet, 3T) to the excited emissive energy levels of the coordinated LnIII ions [4,7,8,9,10,11,12]. As a result, extensive research has been devoted to the design and synthesis of organic ligands to sensitize the luminescence of LnIII ions [11,13,14,15,16].
However, most efforts are focused on monodentate ligands and often require several synthetic steps that are tedious and reagent consuming. Polybenzimidazole ligands are a less explored class of chelating ligands for sensitizing LnIII ions due to their limited solubility [15,17,18,19,20,21,22,23,24,25]. This class of ligands provides an opportunity for easy functionalization to yield versatile ligands with improved solubility. Therefore, there remains a need for multifunctional ligands with well-matched 3T excited states for LnIII sensitization that can be synthesized in a few steps and are soluble in a variety of solvents. Efficient sensitization of LnIII ions can be achieved by using ligands with high molar extinction coefficients and significant spectral overlap between the emission of the ligand and the absorption of the LnIII ion [9]. In this work, the efficiency of energy transfer from a triethyl 2,2′,2″(2,2′,2″nitrilotris(methylene)tris(1H-benzimidazole-2,1-diyl)) triethanoate (BimOEt3) ligand to LnIII ions was investigated using LnIII-complexes of the tripodal ligand. The tripodal BimOEt3 ligand was chosen due to its ease of synthesis, high molar extinction coefficient, and high solubility in protic solvents [20,21]. The photophysical properties, along with the emission lifetimes and quantum yields of the three molecular complexes, are discussed.
2. Results and Discussion
2.1. Synthesis and Characterization of BimOEt3
The BimOEt3 ligand was synthesized following literature reports [20,21,26,27] and the successful synthesis was confirmed by a combination of 1H and 13C NMR spectroscopy (Figures S1–S3). Investigation of the photophysics of BimOEt3 in ethanol via UV–Vis absorption and fluorescence spectroscopy showed three absorption bands (253, 277, and 285 nm) and emission maxima at 322 nm that were attributed to singlet 1π→1π* and 1π*→1π transitions of the benzimidazole groups, respectively (Figure 1) [28]. The emission spectrum of BimOEt3 ranges from 300 to 500 nm with a maximum at 322 nm when excited at 280 nm (Figure 1). The measured absolute fluorescence quantum yield, φL–Ln, and the lifetime, τ, of BimOEt3 (ethanol, 298 K) were 8.1 ± 2.0% and 1.1 ± 0.1 ns, respectively.
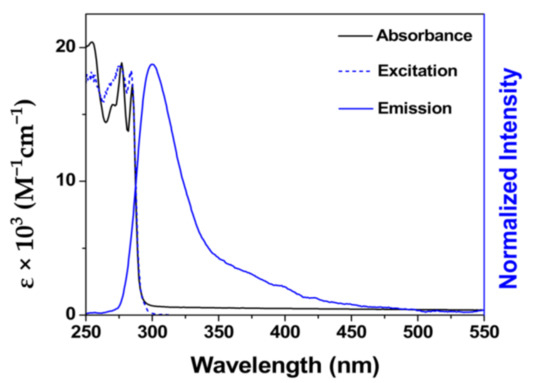
Figure 1.
UV–Vis absorption, excitation, and emission spectra of 1.0 × 10−4 M BimOEt3 in ethanol at 298 K.
2.2. Photophysical Properties of the LnIII-BimOEt3 Complex
LnIII complexes were prepared by mixing 1:2 LnIII-to-BimOEt3 stoichiometric amounts in ethanol/acetonitrile solutions due to the tetradentate nature of BimOEt3 and the photophysics of the resulting complexes investigated (details in Supporting Information). The coordination of the metal ions was determined through infrared spectroscopy. The infrared spectra showed that the carbonyl C=O stretch shifted from 1736 cm−1 to 1745 cm−1 following complexation of EuIII and TbIII ions by the ligand (Figure 2) [1,2,3,4,13,15,19,29,30]. In contrast, the C=O stretch of the CeIII complex remained at 1736 cm−1. This was initially surprising and prompted further investigation. Thus, as a proof-of-concept, single crystals of the CeIII complex were grown by slow diffusion in an ethanol/acetonitrile/ether solvent mixture and analyzed via single-crystal X-ray diffraction. The solution to the structure revealed that the CeIII ions were coordinated to the ligand via the central nitrogen and the imidazole nitrogen atoms of BimOEt3 (Figure S4). This finding suggested that BimOEt3 can selectively bind early (CeIII) and mid (EuIII and TbIII) LnIII ions via its nitrogen and carbonyl groups, respectively. However, further studies by a combination of 1H and 13C NMR spectral analysis of the metal complexes indicated that the EuIII complex is unstable in solution since the proton resonances of the EuIII complex are not shifted compared to the free ligand (Figures S1–S3). In contrast, the yttrium(III) (YIII) and CeIII complexes were stable in solution as suggested by the shifts in the proton resonances of the central methylene groups. The three absorption transitions of BimOEt3 remain at 253, 276, and 284 nm but with subtle changes in their intensities (Figure 1 and Figure S5).
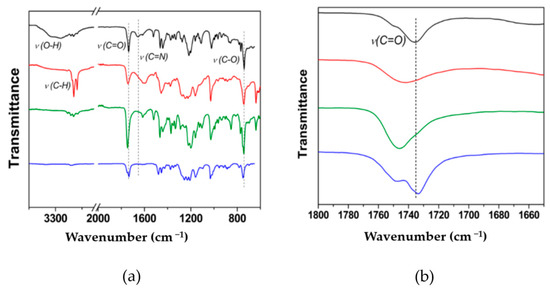
Figure 2.
Infrared spectra of BimOEt3 (black trace), EuIII complex (red trace), TbIII complex (green trace), and CeIII complex (blue trace) with assignment of relevant vibrational frequencies shown in (a) full and (b) selected carbonyl stretching region.
Low-temperature measurements using the analogous GdIII-BimOEt3 complex indicated that the excited singlet, 1S, and triplet, 3T, energy levels of BimOEt3 were 27,800 and 24,500 cm−1, respectively (Table 1 and Figure 6a,b). Thus, the excited 3T energy level of BimOEt3 is well positioned to transfer energy to the 5D0 and 5D4 emitting levels of EuIII and TbIII ions, respectively [9,31,32]. The excitation of the EuIII and TbIII complexes at 280 nm resulted in typical red 5D0→7FJ (0–4) and green 5D4→7FJ (6–3) transitions of EuIII and TbIII, respectively (Figure 3a,b). However, a broad emission band in the range 350–600 nm was observed in both complexes but was more intense in the EuIII complex. This residual ligand emission suggests inefficient energy transfer from the ligand to the metal ions. The measured absolute emission quantum yields for both the EuIII and TbIII complexes were 3% following excitation at 280 nm (Table 1). However, directly exciting the EuIII and TbIII complexes at 394 and 484 nm, respectively, resulted in intrinsic LnIII emission efficiencies of 6% and 4%, respectively. The low efficiencies of the EuIII and TbIII complexes are comparable to values (1–13%) reported for analogous polybenzimidazole-type ligands and are attributed to non-radiative deactivation caused by the rotational freedom via the ester groups of the tripodal ligand [28,33]. Evidence of fluorescence quenching by the ester groups was observed following functionalization of the BimH3 ligand to BimOEt3 (Scheme S1). Using the measured excited energy levels of BimOEt3, the sensitization mechanism of the LnIII ions by the ligand can be described by the Jablonski diagram below (Figure 4).

Table 1.
The excited singlet (1S) and triplet (3T) energy levels, emission quantum yields (φL–Ln), intrinsic quantum yields (φLn–Ln), and lifetimes (τ) of the 1:2 lanthanide (LnIII)-to-ligand complexes in air-saturated ethanol at 298 K.
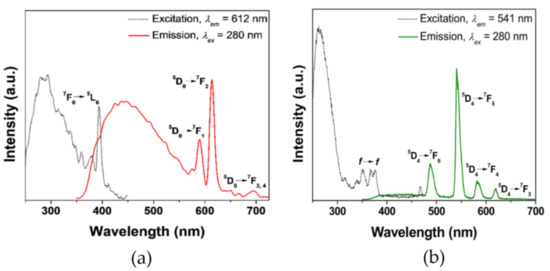
Figure 3.
Excitation (dashed traces) and emission (solid traces) of 1.0 × 10−5 M (a) EuIII and (b) TbIII complexes of BimOEt3 in ethanol at 298 K.
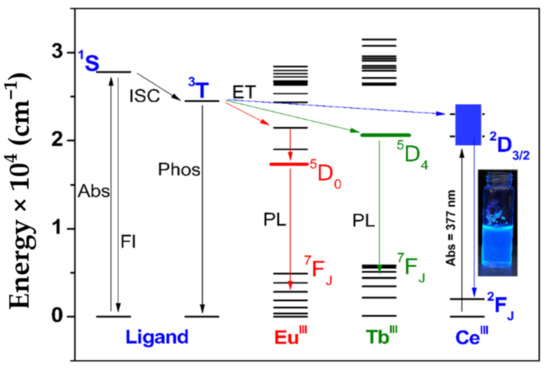
Figure 4.
Energy level diagram showing the sensitization mechanism of EuIII, TbIII, and CeIII ions by BimOEt3. The nonradiative deactivation pathways are removed for clarity. In the diagram, Abs is absorbance, Fl is fluorescence, PL is photoluminescence, ISC is intersystem crossing, and ET is energy transfer. Energy transfer from the singlet, 1S, to the excited levels of the LnIII ions is omitted for clarity. The inset shows the intense blue emission of the CeIII complex in ethanol under a handheld 365-nm UV lamp irradiation.
Despite the low emission quantum yields, a bluish-white-emitting material was obtained through a combination of the residual blue ligand fluorescence and weak-red emission of the EuIII complex with the CIE coordinates (0.258, 0.242). This suggests that single-component white-light-emitting devices (wLEDs) could be generated using metal complexes of BimOEt3 (Figure 5) [10,34,35,36].
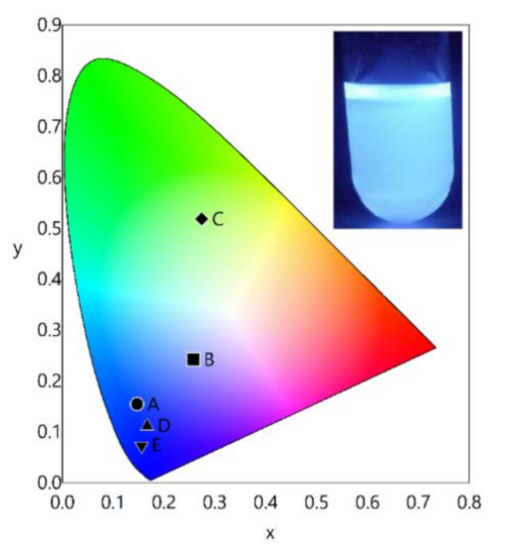
Figure 5.
CIE coordinate diagram of (A) BimOEt3 and its (B) EuIII complex, (C) TbIII complex, (D) CeIII complex at 280 nm excitation, and (E) CeIII complex at 377 nm excitation. The insert shows the intense bluish-white emission of the EuIII-BimOEt3 complex in ethanol under 365-nm UV irradiation.
In contrast to the EuIII and TbIII complexes, excitation of the CeIII complex at 280 nm resulted in two broad emission bands that are attributed to the 1π*→1π transitions of the ligand and the 2D3/2→2FJ (J = 5/2–7/2) transitions of the CeIII ion (Figure 6a,b). In addition, another transition at 489 nm was observed in the CeIII emission spectrum and can be attributed to ligand perturbation of the CeIII ion [37]. Further analysis of the 2D3/2→2FJ (J = 5/2–7/2) transitions of the CeIII emission at 77 K revealed two distinct bands at 434 nm (23,041 cm−1) and 472 nm (21,186 cm−1) with a resulting energy gap of ~1900 cm−1, which is in agreement with previous reports (Figure 6b) [19,21,37].
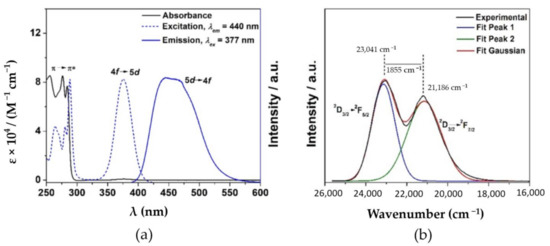
Figure 6.
(a) UV–Vis absorption, excitation, and emission spectra of the CeIII complex in ethanol and its (b) fluorescence in 2-methyl-THF at 77 K with excitation at 377 nm.
The emission lifetimes of the EuIII and TbIII complexes were fit to bi-exponential function. The observed lifetimes do not arise from the ligand states by comparison to the GdIII data and, therefore, are assigned as arising from the presence of two LnIII coordination environments in solution (Table 1 and Figure S7a,b). This was assigned to contributions arising from the lack of stability of the EuIII and TbIII complexes in solution.
In contrast, the measured emission lifetime of the CeIII complex was fit to a mono-exponential function following excitation at 280 or 377 nm (Table 1 and Figure S8a,b). In contrast to the EuIII and TbIII emission lifetimes, the CeIII emission lifetime was short lived (55.0 ± 0.2 ns), consistent with a lifetime expected for a parity allowed 5d–4f transition (Table 1) [21]. Excitation of the CeIII complex at 280 and 377 nm results in emission quantum yields of 17.6 ± 2.0% and 53.6 ± 1.3%, respectively. These values are similar to reports by Zheng et al. [21] and Harada et al. [19] for analogous CeIII-polybenzimidazole complexes. Using the measured emission quantum yields, the efficiency of energy transfer, , from BimOEt3 to CeIII was determined to be 33% using the equation , where the subscripts L−Ln and the Ln−Ln denote the overall emission and the 5d–4f CeIII emission quantum yields, respectively. These results are in agreement with reports by Harada et al. [19] and Zheng et al. [21].
3. Materials and Methods
3.1. Materials
All the chemicals used were reagent grade and used as received.
3.2. General Procedures
The infrared spectra were acquired on a PerkinElmer spectrum 100 FT-IR spectrometer (PerkinElmer, Shelton, CT, USA) in the range 650–4000 cm−1 with a 4.0 cm−1 resolution and 8 scans per sample. The spectra were corrected for H2O and CO2 vibrations before data acquisition. The UV–Vis absorption spectra were acquired using a Cary Varian spectrophotometer operating at a medium scan speed in the range 800–200 nm. The emission spectra were acquired on a Horiba Fluoromax-4 spectrofluorimeter (Horiba Scientific, Piscataway, NJ, USA) equipped with a 150 W CW Ozone free xenon arc lamp, Czerny–Turner monochromators with excitation grating blazed at 330 nm (1200 groove/mm), and emission grating blazed at 500 nm (1200 grooves/mm). All spectra were corrected for the instrument response function and the intensity of the lamp. The emission lifetimes of the samples were acquired by exciting the samples with an Nd:YAG laser that was focused through a variable neutral density filter (Edinburg F-B01 laser mount) and a 2-mm diameter iris (Newport ID-1.0). The emission lifetimes of the CeIII complex were acquired using a TCSPC system equipped with a 370-nm NanoLED.
3.3. Measurement of Ligand Excited States
The excited singlet (1S) and triplet (3T) energy levels of BimOEt3 (Florida State University, Tallahassee, FL, USA) were determined by measuring the emission spectra of the analogous gadolinium (GdIII) complexes at 77 K [31]. 2-Methyltetrahydrofuran solutions (Alfa Aesar, Tewksbury, MA, USA) of the complexes were excited at 295 nm and the emission spectra collected in the range 300–700 nm. The emission spectra were deconvoluted into their Franck–Condon progression and the highest energy peak acquired at either the zero delay or gated emission taken as the excited 1S and 3T energy levels, respectively. The delay time for the fluorescence spectrum was acquired at 0 ms delay while the phosphorescence spectrum was acquired at 0.5 ms delay.
3.4. X-ray Crystallography
The slow-diffusion of a 1:1 ethanol/acetonitrile (Florida State University, Tallahassee, FL, USA) mixture in diethyl ether vapor yielded X-ray-quality single-crystals of BimOEt3 after a week. A crystal was mounted on a glass fiber of a Rigaku XtaLAB Synergy-DW dual wavelength X-ray diffractometer (CuKα = 1.54184 Å) at 298 K (Rigaku, Houston, TX, USA). Data reduction was performed using empirical absorption correction based on “multi-scan”. The structures were solved by the intrinsic phasing and least-square refinements using ShelXT and ShelXL (2014/2, Bruker AXS, Madison, WI, USA) of the Olex2 package [38,39,40]. The best crystal was selected for the data collection; however, the crystal still had quite a few type A and B alerts in the Cifcheck file due to a highly disordered structure caused by unstable solvent (trifluoromethane-sulfonate) in the unit cell.
4. Conclusions
The versatility of a tripodal ligand was demonstrated with successful sensitization of LnIII ions in molecular complexes, leading to an intense blue emission with a quantum yield of 54% for the CeIII complex. However, the measured emission quantum yields of the mid-lanthanide complexes (EuIII and TbIII) were 3%. Despite the low quantum yield of the EuIII complex, the combination of the residual ligand fluorescence and the red EuIII emission resulted in a bluish-white-emitting material with the CIE coordinates (0.258, 0.242). Thus, metal complexes of the ligand could be used in the generation of single-component-white-light-emitting materials. Although with improved solubility in polar solvents, the functionalization with ester groups leads to a rapid quenching of the ligand fluorescence. The coordination nature of the ligand to the metal ions was revealed through a combination of infrared and NMR spectroscopy, as well as single-crystal X-ray diffraction analysis of the metal complexes of BimOEt3. The results serve as a starting point for further studies to better understand the stability of tripodal polybenzimidazole ligands for lanthanide separation/extraction and imaging applications.
Supplementary Materials
The following are available online at https://www.mdpi.com/2624-8549/3/1/11/s1, Schemes S1 and S2: synthesis details; Figures S1–S3: NMR spectra; Figure S4: single-crystal X-ray data; Figure S5: UV–Vis absorption spectra; Figure S6: ligand excited singlet and triplet energy levels; Figure S7; EuIII and TbIII emission decay curves; Figure S8: CeIII emission decay curve. Crystallographic data for BimOEt3 have been deposited with the Cambridge Crystallographic Data Centre, CCDC No. 2054591.
Author Contributions
Conceptualization, R.A.T. and G.F.S.; methodology, R.A.T.; validation, R.A.T., R.E.O., and X.L.; formal analysis, R.A.T.; investigation, R.A.T. and R.E.O.; resources, G.F.S.; data curation, R.A.T.; writing—original draft preparation, R.A.T.; writing—review and editing, R.A.T., R.E.O., and X.L.; supervision, G.F.S.; project administration, R.A.T. and G.F.S.; funding acquisition, G.F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Foundation (NSF CHE-1608364) “SusChEM: Understanding Microwave Interactions to Control Magnetic Nanocrystal Growth from a Single Source Precursor”. Time resolved studies were collected on a transient absorption instrument supported by the National Science Foundation under Grant Number CHE-1531629. This work made use of the Rigaku Synergy-S single-crystal X-ray diffractometer which was acquired through the NSF MRI program (award CHE-1828362).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and in the Supplementary Material.
Acknowledgments
The authors acknowledge Carl Conti for assistance with 1H NMR data collection.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bünzli, J.-C.G. Lanthanide Luminescence for Biomedical Analyses and Imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Eliseeva, S.V. Lanthanide NIR luminescence for telecommunications, bioanalyses and solar energy conversion. J. Rare Earths 2010, 28, 824–842. [Google Scholar] [CrossRef]
- Eliseeva, S.; Bünzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Eliseeva, S.V. Intriguing aspects of lanthanide luminescence. Chem. Sci. 2013, 4, 1939–1949. [Google Scholar] [CrossRef]
- Abbett, R.L.; Tigaa, R.A.; Sonawane, S.L.; Strouse, G.F.; Schlenoff, J.B. Hydrophobic Versus Hydrophilic Polyelectrolyte Multilayers for Emissive Europium Films. ACS Appl. Polym. Mater. 2021. [Google Scholar] [CrossRef]
- Hardy, D.A.; Tigaa, R.A.; McBride, J.R.; Ortega, R.E.; Strouse, G.F. Structure–function correlation: Engineering high quantum yields in down-shifting nanophosphors. J. Am. Chem. Soc. 2019. [Google Scholar] [CrossRef]
- de Bettencourt-Dias, A. Small Molecule Luminescent Lanthanide Ion Complexes–Photophysical Characterization and Recent Developments. Curr. Org. Chem. 2007, 11, 1460–1480. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Hardy, D.A.; Tigaa, R.A.; Ortega, R.E.; McBride, J.R.; Strouse, G.F. Breaking Latva’s Rule by Energy Hopping in a Tb(III): ZnAl2O4 Nanospinel. J. Phys. Chem. C 2019. [Google Scholar] [CrossRef]
- Tigaa, R.A.; Aerken, X.; Fuchs, A.; de Bettencourt-Dias, A. Sensitization of LnIII (Ln = Eu, Tb, Tm) Ion Luminescence by Functionalized Polycarbonate-Based Materials and White Light Generation. Eur. J. Inorg. Chem. 2017. [Google Scholar] [CrossRef]
- Tigaa, R.A.; Lucas, G.J.; de Bettencourt-Dias, A. ZnS Nanoparticles Sensitize Luminescence of Capping-Ligand-Bound Lanthanide Ions. Inorg. Chem. 2017, 56, 3260–3268. [Google Scholar] [CrossRef]
- Guino-o, M.A.; Bustrom, B.; Tigaa, R.A.; de Bettencourt-Dias, A. Microwave-assisted synthesis of ternary lanthanide(2-thenoyltrifluoroacetone)3(triphenylphosphine oxide)2 complexes. Inorg. Chim. Acta 2017, 464, 23–30. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293, 19–47. [Google Scholar] [CrossRef]
- Chauvin, A.-S.; Gumy, F.; Imbert, D.; Bünzli, J.-C.G. Europium and Terbium tris(Dipicolinates) as Secondary Standards for Quantum Yield Determination. Spectrosc. Lett. 2004, 37, 517–532. [Google Scholar] [CrossRef]
- Shavaleev, N.M.; Eliseeva, S.V.; Scopelliti, R.; Bünzli, J.-C.G. Tridentate Benzimidazole-Pyridine-Tetrazolates as Sensitizers of Europium Luminescence. Inorg. Chem. 2014, 53, 5171–5178. [Google Scholar] [CrossRef]
- Tigaa, R.A.; de Bettencourt-Dias, A. Synthesis and Characterization of Two Tritylthio-Derivatives: 1-Bromo-3-Tritylthiopropane and 2-(Tritylthio)-Ethanethiol. J. Chem. Cryst. 2017, 47, 233–240. [Google Scholar] [CrossRef]
- Liu, L.; Feng, H.N.; Fu, G.-R.; Li, B.-N.; Lv, X.-Q.; Wong, W.-K.; Jones, R.A. Efficient NIR (near-infrared) luminescent ZnLn-grafted (Ln = Nd, Yb or Er) PNBE (Poly(norbornene)). J. Lumin. 2017, 186, 23–29. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, H.; Liu, L.; Yu, C.; Lv, X.; Zhu, X.; Wong, W.-K.; Jones, R.A.; Pan, R.A.; Pan, M.; et al. The first example of Tb3-containing metallopolymer-type hybrid materials with efficient and high color-purity green luminescence. Dalton Trans. 2015, 44, 6229–6241. [Google Scholar] [CrossRef]
- Harada, T.; Hasegawa, R.; Nishiyama, K. Efficient 4f–5d Emission Processes of Ce3+ Complexes with Benzimidazole-based Tetradentate Ligands. Chem. Lett. 2014, 43, 1496–1498. [Google Scholar] [CrossRef]
- Rodionov, V.O.; Presolski, S.I.; Gardinier, S.; Lim, Y.-H.; Finn, M.G. Benzimidazole and Related Ligands for Cu-Catalyzed Azide−Alkyne Cycloaddition. J. Am. Chem. Soc. 2007, 129, 12696–12704. [Google Scholar] [CrossRef]
- Zheng, X.-L.; Liu, Y.; Pan, M.; Lv, X.-Q.; Zhang, J.-Y.; Zhao, C.-Y.; Tong, Y.-X.; Su, C.Y. Bright Blue-Emitting Ce3+ Complexes with Encapsulating Polybenzimidazole Tripodal Ligands as Potential Electroluminescent Devices. Angew. Chem. Int. Ed. 2007, 46, 7399–7403. [Google Scholar] [CrossRef]
- Renaud, F.; Piguet, C.; Bernardinelli, G.; Bünzli, J.-C.G.; Hopfgartner, G. In Search for Mononuclear Helical Lanthanide Building Blocks with Predetermined Properties: Lanthanide Complexes with Diethyl Pyridine-2, 6-Dicarboxylate. Chem. Eur. J. 2006, 3, 1660–1667. [Google Scholar] [CrossRef]
- Wietzke, R.; Mazzanti, M. Strong intramolecular π–π interactions favor the formation of 2:1 (L:M) lanthanide complexes of tris(2-benzimidazolylmethyl)amine. Chem. Commun. 1999, 209–210. [Google Scholar] [CrossRef]
- de Bettencourt-Dias, A.; Tigaa, R.A. Sensitization of near-infrared LnIII [Ln = Yb or Nd] ions using water-soluble, band gap tuneable 3-MPA-capped CdS nanoparticles. J. Mater. Chem. C 2018, 6, 2814–2821. [Google Scholar] [CrossRef]
- Tigaa, R.A.; Monteiro, J.H.S.K.; Silva-Hernandez, S.; de Bettencourt-Dias, A. LnIII-centered emission sensitized through fluorescent carbon dots. J. Lumin. 2017, 192, 1273–1277. [Google Scholar] [CrossRef]
- Kunkely, H.; Vogler, A. Optical metal-to-ligand charge transfer in tris(pyrazine-2-carboxylato)cerium(III): Absorption and emission. J. Photochem. Photobiol. A 2002, 151, 45–47. [Google Scholar] [CrossRef]
- Baschieri, A.; Mazzanti, A.; Stagni, S.; Sambri, L. Triple Click to Tripodal Triazole-Based Ligands–Synthesis and Characterization of Blue-Emitting Ce3+ Complexes-Baschieri-2013-European Journal of Inorganic Chemistry-Wiley Online Library. Eur. J. Inorg. Chem. 2013, 2432–2439. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, M.; Yang, Q.-Y.; Fu, L.; Li, K.; Wei, S.-C.; Su, C.-Y. sDual-Emission from a Single-Phase Eu–Ag Metal–Organic Framework: An Alternative Way to Get White-Light Phosphor. Chem. Mater. 2012, 24, 1954–1960. [Google Scholar] [CrossRef]
- Shavaleev, N.M.; Eliseeva, S.V.; Scopelliti, R.; Bünzli, J.-C.G. Influence of Symmetry on the Luminescence and Radiative Lifetime of Nine-Coordinate Europium Complexes. Inorg. Chem. 2015. [Google Scholar] [CrossRef]
- Chauvin, A.-S.; Gumy, F.; Imbert, D.; Bünzli, J.-C.G. Europium and Terbium tris(Dipicolinates) as Secondary Standards for Quantum Yield Determination (Erratum). Spectrosc. Lett. 2007, 40, 193. [Google Scholar] [CrossRef]
- Crosby, G.A.; Whan, R.E.; Alire, R.M. Intramolecular Energy Transfer in Rare Earth Chelates. Role of the Triplet State. J. Chem. Phys. 1961, 34, 743–748. [Google Scholar] [CrossRef]
- Latva, M.; Takalo, H.; Mukkala, V.-M.; Matachescu, C.; Rodríguez-Ubis, J.C.; Kankare, J. Correlation between the lowest triplet state energy level of the ligand and lanthanide(III) luminescence quantum yield. J. Lumin. 1997, 75, 149–169. [Google Scholar] [CrossRef]
- Pan, M.; Zheng, X.-L.; Liu, Y.; Liu, W.-S.; Su, C.-Y. Structural and photoluminescent studies of lanthanide complexes with tripodal triRNTB (N-substituted tris(benzimidazol-2-ylmethyl)amine): Ligand substituent, anionic and secondary ligand effects. Dalton Trans. 2009, 12, 2157–2169. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Su, P.; Zhang, Z.; Fu, G.; Li, B.; Lv, X. Red to white polymer light-emitting diode (PLED) based on Eu3+-Zn2+-Gd3+-containing metallopolymer. J. Mater. Chem. C 2017, 5, 4780–4787. [Google Scholar] [CrossRef]
- Liu, L.; Fu, G.; Li, B.; Lu, X.; Wong, W.-K.; Jones, R.A. Single-component Eu3+-Tb3+-Gd3+-grafted polymer with ultra-high color rendering index white-light emission. RSC Adv. 2017, 7, 6762–6771. [Google Scholar] [CrossRef]
- Zhang, Z.; He, Y.-N.; LIu, L.; Lv, X.-Q.; Zhu, X.J.; Wong, W.-K.; Pan, M.; Su, C.-Y. Pure white-light and colour-tuning of Eu3+-Gd3+-containing metallopolymer. Chem. Commun. 2016, 52, 3713–3716. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.R.; Kudinov, K.; Tyagi, P.; Hill, C.K.; Bradforth, S.E.; Nadea, L. Photoluminescence of cerium fluoride and cerium-doped lanthanum fluoride nanoparticles and investigation of energy transfer to photosensitizer molecules. Phys. Chem. Chem. Phys. 2014, 16, 12441–12453. [Google Scholar] [CrossRef] [PubMed]
- University of Göttingen. SADABS 2.10; University of Göttingen: Göttingen, Germany, 2003. [Google Scholar]
- SHELXTL. Version 6.12, Structure Determination Software Suite; Bruker AXS: Madison, WI, USA, 2001. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).