Supramolecular Assemblies of Trinuclear Copper(II)-Pyrazolato Units: A Structural, Magnetic and EPR Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.2.1. X-Ray Crystallography
2.2.2. EPR Spectroscopy
2.2.3. Magnetic Measurements
2.3. Synthesis of Compounds (1) and (2)
2.3.1. Synthesis of [PPN]2{[Cu3(µ3-OH)(µ-4-Ph-pz)3Cl3]2[Cu (4-Ph-pzH)4]}Cl2} (1)
2.3.2. Synthesis of (PPN)[Cu3(µ3-OH)(µ-pz)3(µ1,1-N3)2(N3)] (2)
2.4. Theoretical Calculations
3. Results and Discussion
3.1. Synthesis
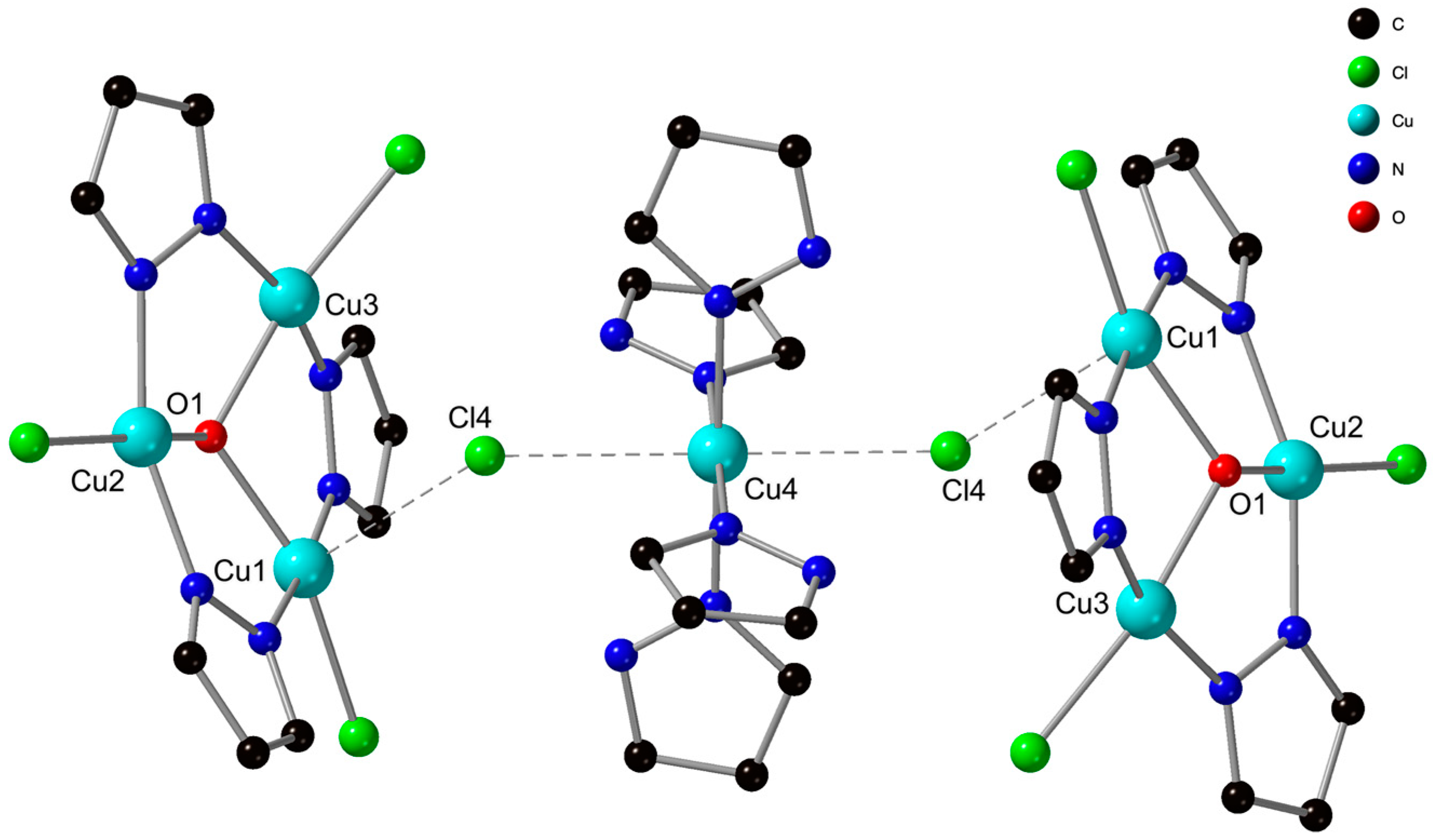
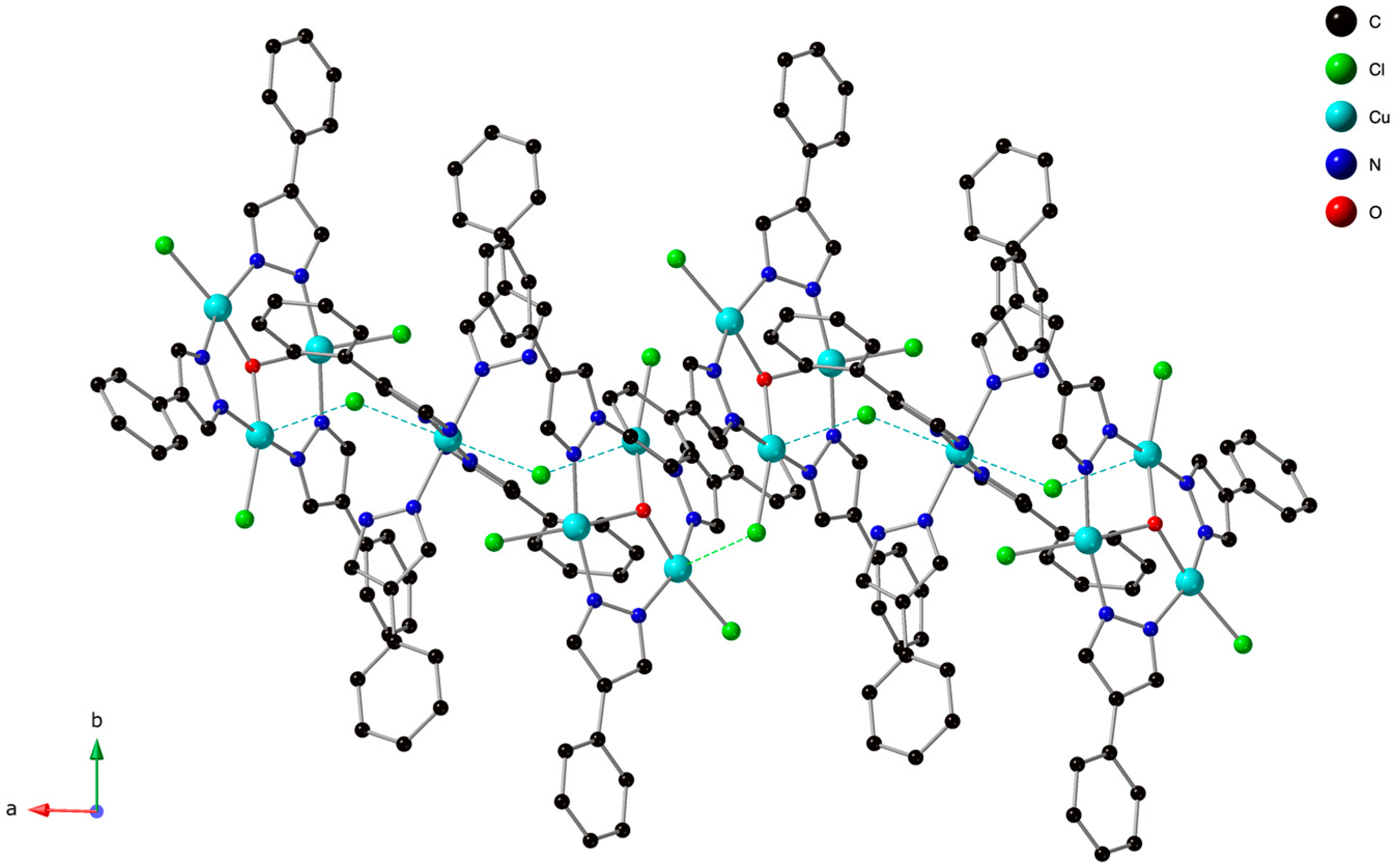
3.2. Crystal Structure Description of [PPN]2[{Cu3(µ3-OH)(µ-4-Ph-pz)3Cl3}2{Cu(4-Ph-pzH)4}]Cl2 (1)
3.3. Crystal Structure Description of (PPN)[Cu3(µ3-OH)(µ-pz)3(µ,κ1,1-N3)2(N3)] (2)
3.4. Infrared Spectra
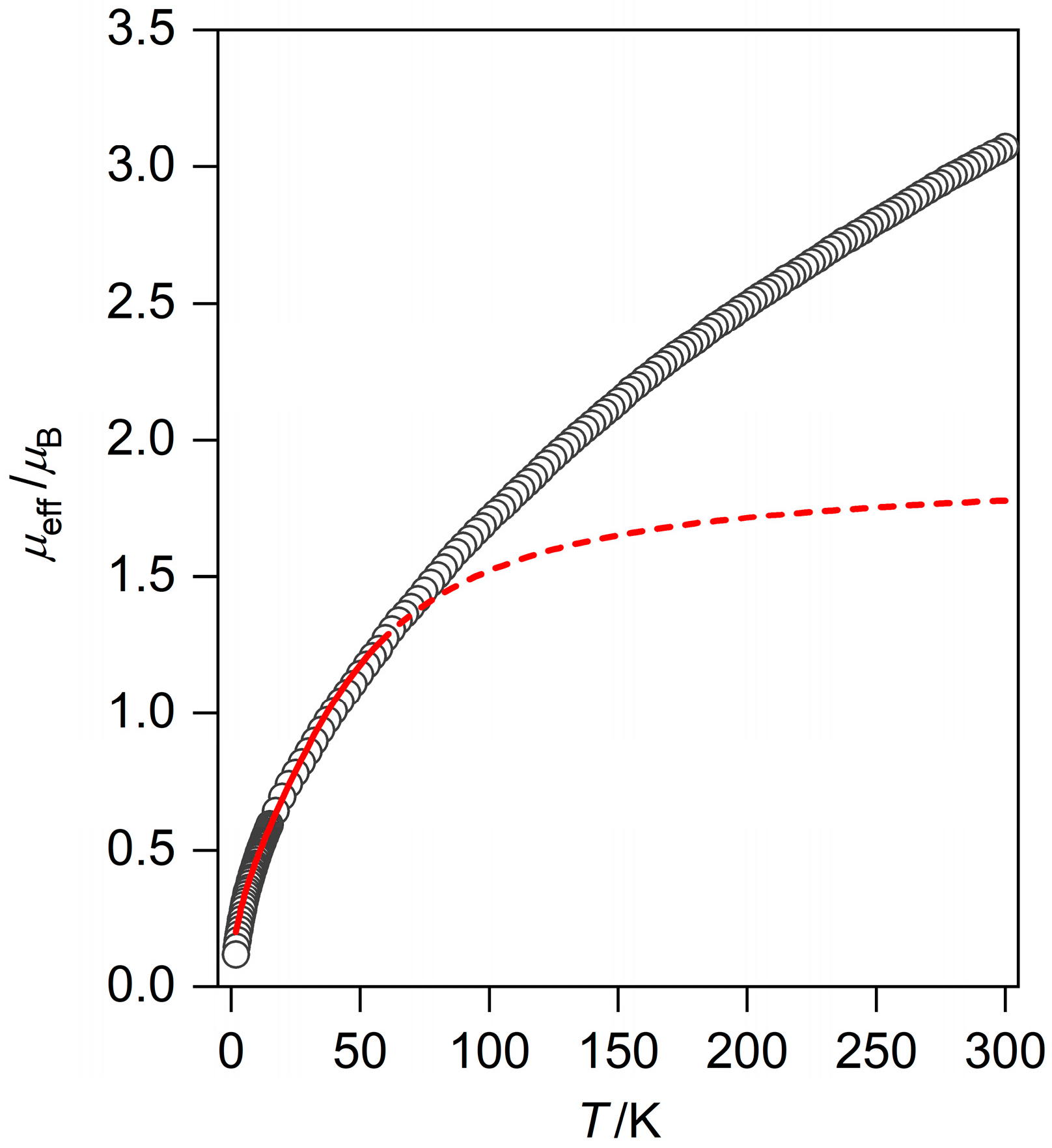
3.5. Magnetic Susceptibility of (1)
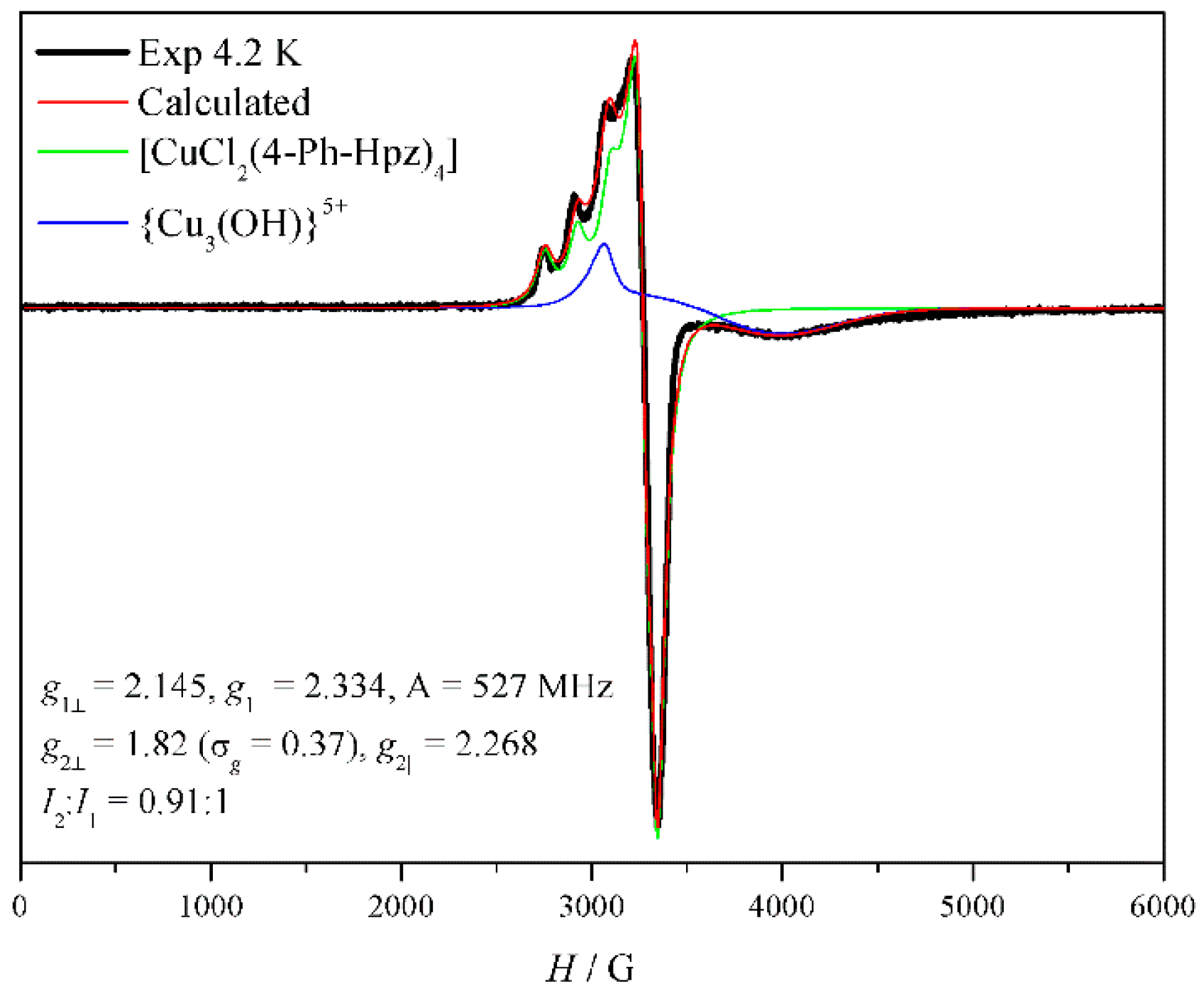
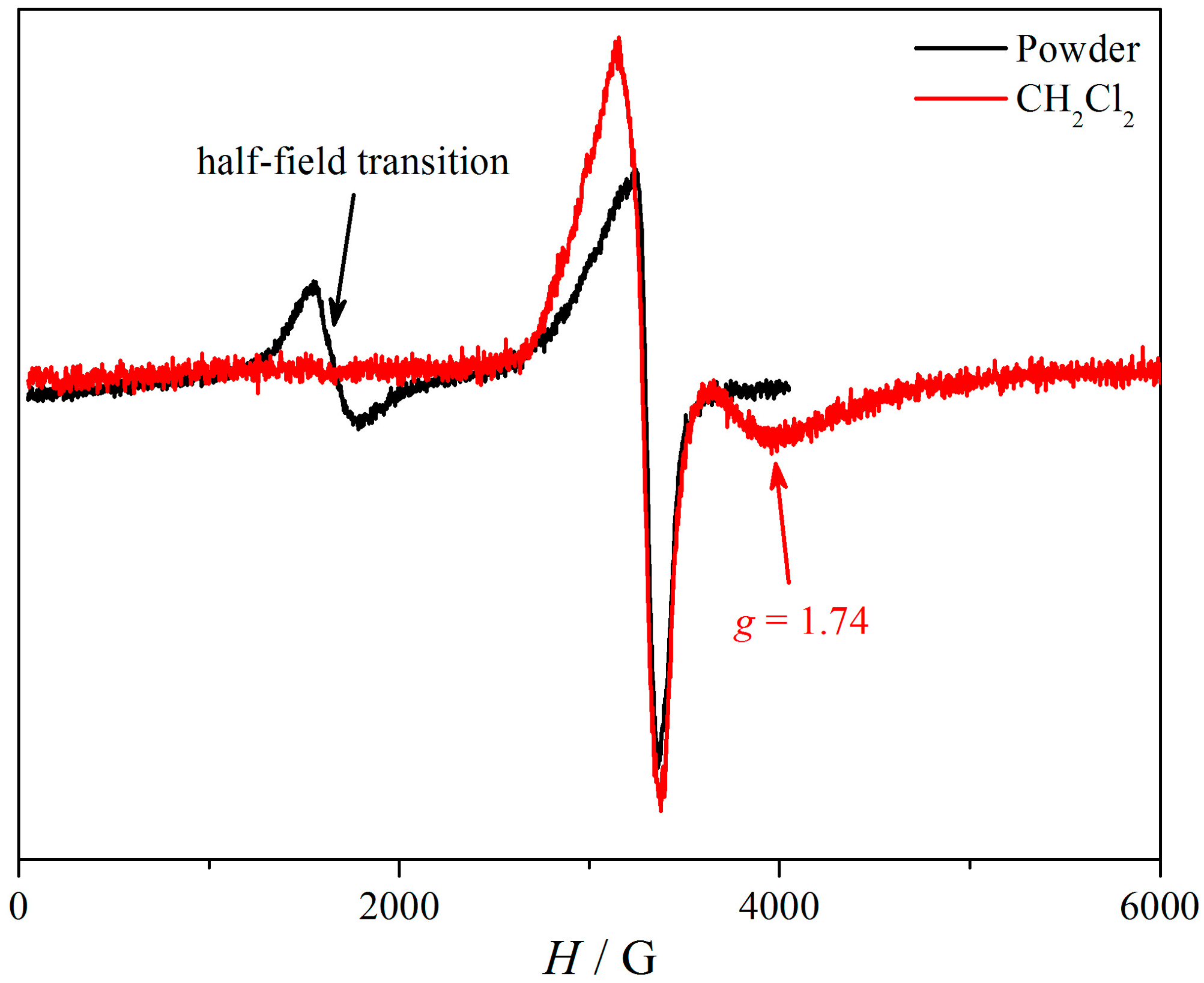
3.6. EPR Spectroscopy of (1)
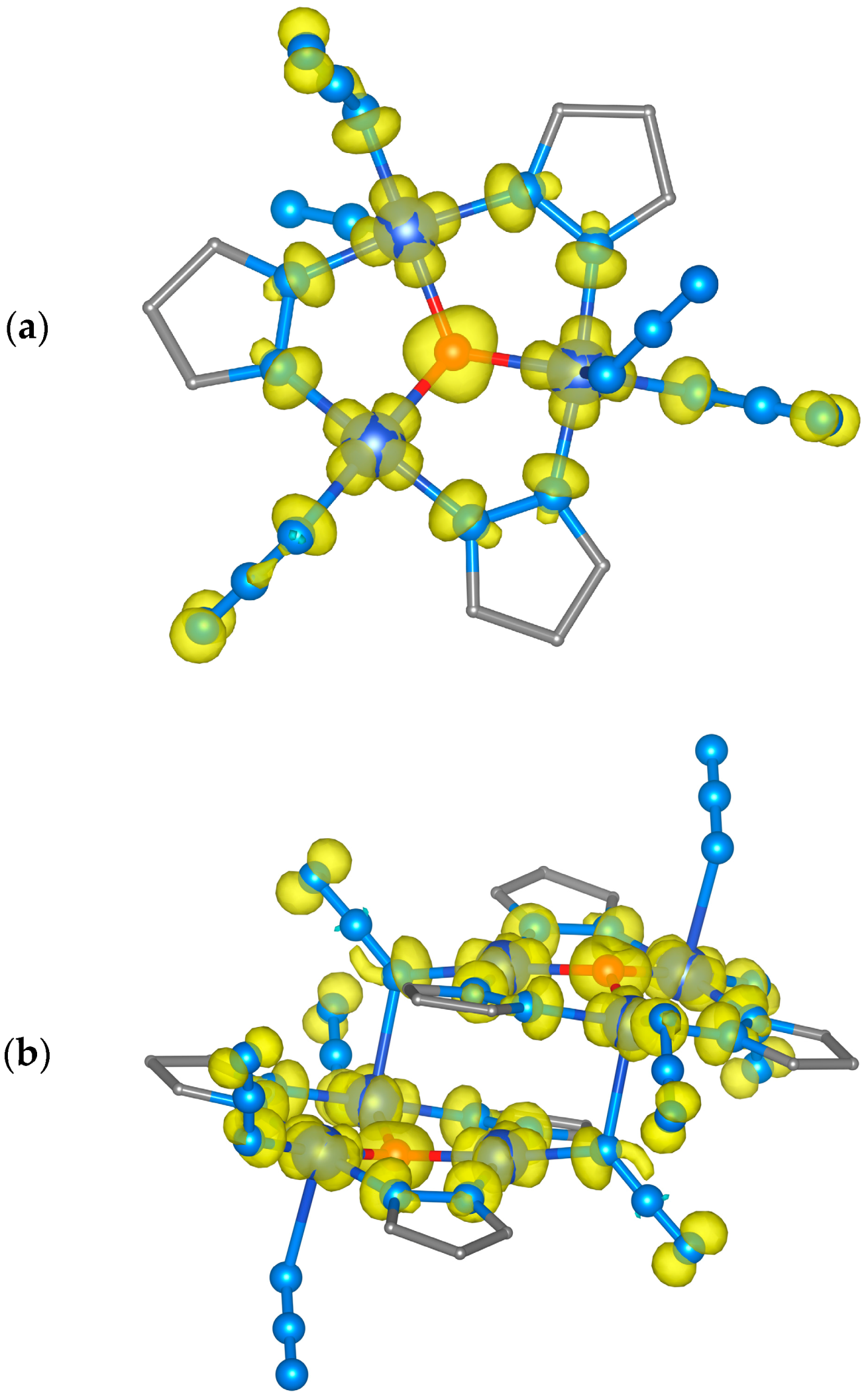
3.7. Theoretical DFT Calculations of (1)
3.8. Magnetic Susceptibility Studies of (2)
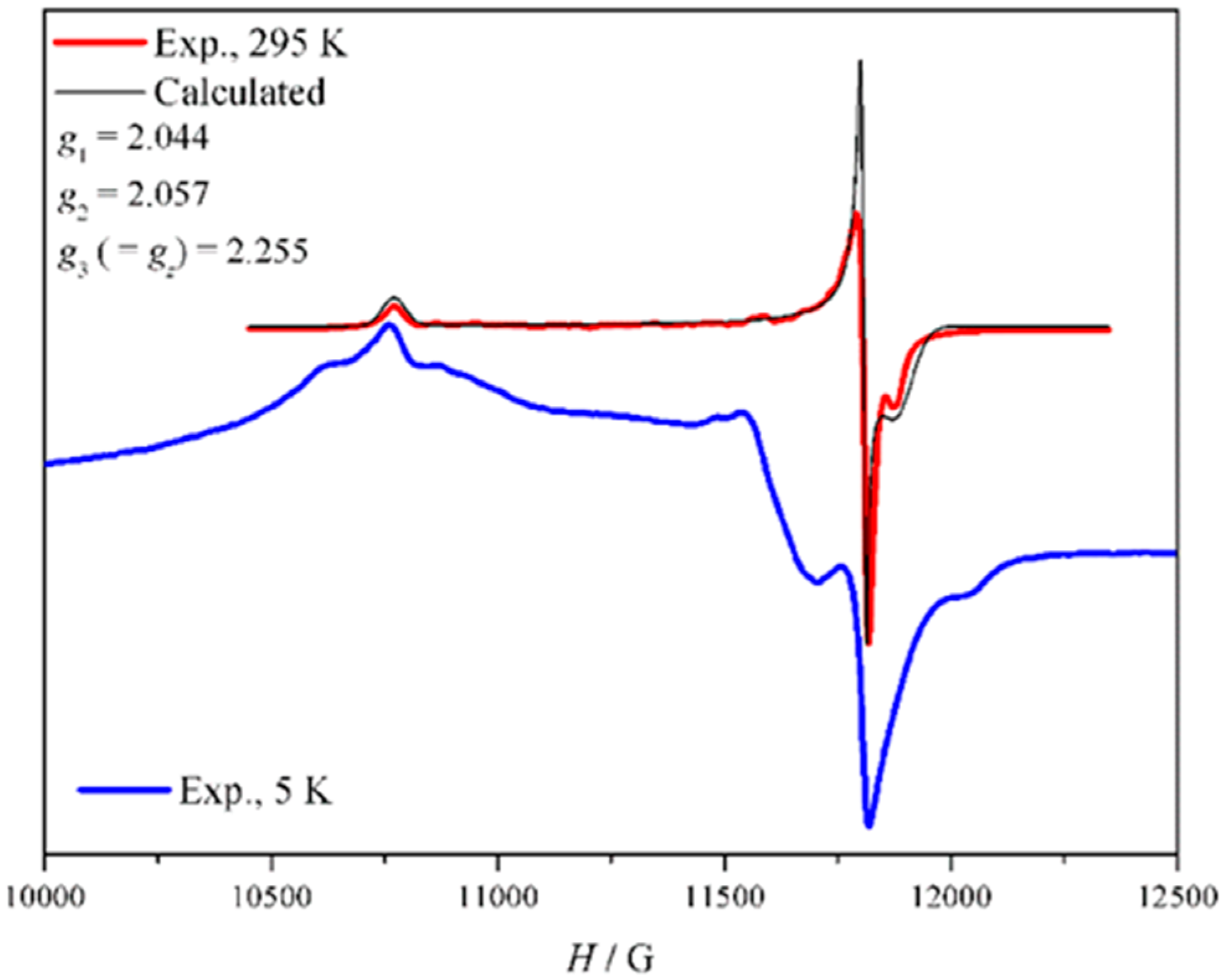
3.9. EPR Spectroscopy of (2)
3.10. Theoretical DFT Calculations of (2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pascual-Colino, J.; Beobide, G.; Castillo, O.; Lodewyckx, P.; Luque, A.; Pérez-Yáñez, S.; Román, P.; Velasco, L.F. Adenine nucleobase directed supramolecular architectures based on ferrimagnetic heptanuclear copper(II) entities and benzenecarboxylate anions. J. Inorg. Biochem. 2020, 202, 110865. [Google Scholar] [CrossRef]
- González, M.M.; Osiry, H.; Martínez, M.; Rodríguez-Hernández, J.; Lemus-Santana, A.A.; Reguera, E. Magnetic interaction in a 2D solid through hydrogen bonds and π-π stacking. J. Magn. Magn. Mater. 2019, 471, 70–76. [Google Scholar] [CrossRef]
- Pham, C.T.; Nguyen, T.H.; Matsumoto, K.; Nguyen, H.H. CuI/CuII Complexes with Dipicolinoylbis(N,N-diethylthiourea): Structures, Magnetism, and Guest Ion Exchange. Eur. J. Inorg. Chem. 2019, 2019, 4142–4146. [Google Scholar] [CrossRef]
- Chi, Y.-H.; Yu, L.; Shi, J.-M.; Zhang, Y.-Q.; Hu, T.-Q.; Zhang, G.-Q.; Shi, W.; Cheng, P. π–π Stacking and ferromagnetic coupling mechanism on a binuclear Cu(ii) complex. Dalton Trans. 2011, 40, 1453. [Google Scholar] [CrossRef] [PubMed]
- Ehlert, M.K.; Rettig, S.J.; Storr, A.; Thompson, R.C.; Trotter, J. Metal pyrazolate polymers. Part 1. Synthesis, structure, and magnetic properties of the [Cu(pz)2]x polymer. Can. J. Chem. 1989, 67, 1970–1974. [Google Scholar] [CrossRef]
- Ehlert, M.K.; Rettig, S.J.; Storr, A.; Thompson, R.C.; Trotter, J. Metal pyrazolate polymers. Part 2. Synthesis, structure, and magnetic properties of [Cu (4-Xpz) 2] x polymers (where X = Cl, Br, Me, H; pz = pyrazolate). Can. J. Chem. 1991, 69, 432–439. [Google Scholar] [CrossRef]
- Zueva, E.M.; Petrova, M.M.; Herchel, R.; Trávníček, Z.; Raptis, R.G.; Mathivathanan, L.; McGrady, J.E. Electronic structure and magnetic properties of a trigonal prismatic CuII6 cluster. Dalton Trans. 2009, 30, 5924–5932. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, S.; Lloret, F.; Bertomeu, I.; Alzuet, G.; Borrás, J.; García-Granda, S.; Liu-González, M.; Haasnoot, J.G. Cyclic Trinuclear and Chain of Cyclic Trinuclear Copper(II) Complexes Containing a Pyramidal Cu3O(H) Core. Crystal Structures and Magnetic Properties of [Cu3(μ3-OH)(aaat)3(H2O)3](NO3)2·H2O [aaat = 3-Acetylamino-5-amino-1,2,4-triazolate] and {[Cu3(μ3-OH)(aat)3(μ3-SO4)]·6H2O}n [aat = 3-Acetylamino-1,2,4-triazolate]: New Cases of Spin-Frustrated Systems. Inorg. Chem. 2002, 41, 5821–5830. [Google Scholar] [CrossRef]
- Ferrer, S.; Lloret, F.; Pardo, E.; Clemente-Juan, J.M.; Liu-González, M.; García-Granda, S. Antisymmetric Exchange in Triangular Tricopper(II) Complexes: Correlation among Structural, Magnetic, and Electron Paramagnetic Resonance Parameters. Inorg. Chem. 2012, 51, 985–1001. [Google Scholar] [CrossRef]
- Belinsky, M.I. Hyperfine Splittings in Spin-Frustrated Trinuclear Cu3 Clusters. Inorg. Chem. 2004, 43, 739–746. [Google Scholar] [CrossRef]
- Boča, R.; Dlháň, L.; Mezei, G.; Ortiz-Pérez, T.; Raptis, R.G.; Telser, J. Triangular, Ferromagnetically-Coupled CuII3−Pyrazolato Complexes as Possible Models of Particulate Methane Monooxygenase (pMMO). Inorg. Chem. 2003, 42, 5801–5803. [Google Scholar] [CrossRef] [PubMed]
- Angaridis, P.A.; Baran, P.; Boča, R.; Cervantes-Lee, F.; Haase, W.; Mezei, G.; Raptis, R.G.; Werner, R. Synthesis and Structural Characterization of Trinuclear CuII−Pyrazolato Complexes Containing μ3-OH, μ3-O, and μ3-Cl Ligands. Magnetic Susceptibility Study of [PPN]2[(μ3-O)Cu3(μ-pz)3Cl3]. Inorg. Chem. 2002, 41, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Mathivathanan, L.; Boudalis, A.K.; Turek, P.; Pissas, M.; Sanakis, Y.; Raptis, R.G. Interactions between H-bonded [CuII3(μ3-OH)] triangles; a combined magnetic susceptibility and EPR study. Phys. Chem. Chem. Phys. 2018, 20, 17234–17244. [Google Scholar] [CrossRef] [PubMed]
- Olguín, J.; Brooker, S. Synthesis of 3- and 5-formyl-4-phenyl-1H-pyrazoles: Promising head units for the generation of asymmetric imine ligands and mixed metal polynuclear complexes. New J. Chem. 2011, 35, 1242–1253. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 7th ed.; Elsevier/Butterworth-Heinemann: Amsterdam, The Netherlands; London, UK, 2013; ISBN 978-0-12-382161-4. [Google Scholar]
- APEX3; Bruker AXS Inc.: Madisson, WI, USA, 2017.
- SADABS; Bruker AXS Inc.: Madisson, WI, USA, 2001.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Thorn, A.; Dittrich, B.; Sheldrick, G.M. Enhanced rigid-bond restraints. Acta Crystallogr. A 2012, 68, 448–451. [Google Scholar] [CrossRef]
- Herchel, R.; Boča, R. Program Polymagnet; Slovak Technical University: Bratislava, Slovakia, 2006–2020. [Google Scholar]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, approximate and parallel Hartree–Fock and hybrid DFT calculations. A ‘chain-of-spheres’ algorithm for the Hartree–Fock exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Izsák, R.; Neese, F. An overlap fitted chain of spheres exchange method. J. Chem. Phys. 2011, 135, 144105. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Shi, K.; Mathivathanan, L.; Boudalis, A.K.; Turek, P.; Chakraborty, I.; Raptis, R.G. Nitrite Reduction by Trinuclear Copper Pyrazolate Complexes: An Example of a Catalytic, Synthetic Polynuclear NO Releasing System. Inorg. Chem. 2019. [Google Scholar] [CrossRef]
- Casarin, M.; Cingolani, A.; Di Nicola, C.; Falcomer, D.; Monari, M.; Pandolfo, L.; Pettinari, C. The Different Supramolecular Arrangements of the Triangular [Cu3 (μ3-OH)(μ-pz)3]2+ (pz = Pyrazolate) Secondary Building Units. Synthesis of a Coordination Polymer with Permanent Hexagonal Channels. Cryst. Growth Des. 2007, 7, 676–685. [Google Scholar] [CrossRef]
- Direm, A.; Tursun, M.; Parlak, C.; Benali-Cherif, N. Trans-dichlorotetrakis(1H-pyrazole-κN2)copper(II): Synthesis, crystal structure, hydrogen bonding graph-sets, vibrational and DFT studies. J. Mol. Struct. 2015, 1093, 208–218. [Google Scholar] [CrossRef]
- Sun, Y.-J.; Cheng, P.; Yan, S.-P.; Liao, D.-Z.; Jiang, Z.-H.; Shen, P.-W. Synthesis, crystal structure and properties of copper(II) complexes with different axial ligands and substituted pyrazoles. J. Mol. Struct. 2001, 597, 191–198. [Google Scholar] [CrossRef]
- Małecka, M.; Chęcińska, L. Di chloro tetrakis(3-phenyl pyrazole-κN2)copper(II). Acta Crystallogr. C 2003, 59, m115–m117. [Google Scholar] [CrossRef] [PubMed]
- Adak, P.; Das, C.; Ghosh, B.; Mondal, S.; Pakhira, B.; Sinn, E.; Blake, A.J.; O’Connor, A.E.; Chattopadhyay, S.K. Two pseudohalide-bridged Cu(II) complexes bearing the anthracene moiety: Synthesis, crystal structures and catecholase-like activity. Polyhedron 2016, 119, 39–48. [Google Scholar] [CrossRef]
- Ray, M.S.; Ghosh, A.; Bhattacharya, R.; Mukhopadhyay, G.; Drew, M.G.B.; Ribas, J. Different supramolecular hydrogen bond structures and significant changes in magnetic properties in dinuclear μ2-1,1-N3 copper(II) complexes with very similar tridentate Schiff base blocking ligands. Dalton Trans. 2004. [Google Scholar] [CrossRef] [PubMed]
- Boča, R.; Herchel, R. Antisymmetric exchange in polynuclear metal complexes. Coord. Chem. Rev. 2010, 254, 2973–3025. [Google Scholar] [CrossRef]
- Mathivathanan, L.; Al-Ameed, K.; Lazarou, K.; Trávníček, Z.; Sanakis, Y.; Herchel, R.; McGrady, J.E.; Raptis, R.G. A trigonal prismatic Cu6-pyrazolato complex containing a μ6-F ligand. Dalton Trans. 2015, 44, 20685–20691. [Google Scholar] [CrossRef]
- Moriya, T. Anisotropic Superexchange Interaction and Weak Ferromagnetism. Phys. Rev. 1960, 120, 91–98. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH: New York, NY, USA, 1993; ISBN 978-1-56081-566-2. [Google Scholar]
- Zheng, L.-L.; Leng, J.-D.; Zheng, S.-L.; Zhaxi, Y.-C.; Zhang, W.-X.; Tong, M.-L. Engineering delocalizing π electronic [CuII3(μ3-OH)(μ-pz)3]2+ species into organometallic frameworks by Ag-π coordination. CrystEngComm 2008, 10, 1467. [Google Scholar] [CrossRef]
- Hulsbergen, F.B.; ten Hoedt, R.W.M.; Verschoor, G.C.; Reedijk, J.; Spek, A.L. Synthesis, magnetic properties, and X-ray structure of catena-µ3-nitrato-O,O′,O″-[µ3-hydroxo-1-nitrato-1,2;1,3;2,3-tris(µ-pyrazolato-N,N′)-2,3-bis(pyrazole-N2)tricopper(II) monohydrate]. An unusual chain of trinuclear copper clusters. J. Chem. Soc. Dalton Trans. 1983. [Google Scholar] [CrossRef]
- Angaroni, M.; Ardizzoia, G.A.; Beringhelli, T.; Monica, G.L.; Gatteschi, D.; Masciocchi, N.; Moret, M. Oxidation reaction of [{Cu(Hpz)2Cl}2](Hpz = pyrazole): Synthesis of the trinuclear copper(II) hydroxo complexes [Cu3(OH)(pz)3(Hpz)2Cl2]·solv (solv = H2O or tetrahydrofuran). Formation, magnetic properties, and X-ray crystal structure of [Cu3(OH)(pz)3(py)2Cl2]·py (py = pyridine). J. Chem. Soc. Dalton Trans. 1990. [Google Scholar] [CrossRef]
- Ferrer, S.; Haasnoot, J.G.; Reedijk, J.; Müller, E.; Biagini Cingi, M.; Lanfranchi, M.; Manotti Lanfredi, A.M.; Ribas, J. Trinuclear N,N-Bridged Copper(II) Complexes Involving a Cu3OH Core: [Cu3(μ3-OH)L3A(H2O)2]A(H2O)x{L = 3-Acetylamino-1,2,4-triazolate; A = CF3SO3, NO3, ClO4; x = 0, 2} Synthesis, X-ray Structures, Spectroscopy, and Magnetic Properties. Inorg. Chem. 2000, 39, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Yi, L.; Cheng, P.; Liao, D.-Z.; Yan, S.-P. Synthesis and Characterization of a 3D Coordination Polymer Based on Trinuclear Triangular CuII as Secondary Building Units. Inorg. Chem. 2006, 45, 5799–5803. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, W.; Prosvirin, A.V.; Chieffo, V.; Dunbar, K.R.; Hudson, B.; Zubieta, J. Solid-State Coordination Chemistry of the Cu/Triazolate/X System (X = F−, Cl−, Br−, I−, OH−, and SO42−). Inorg. Chem. 2006, 45, 9346–9366. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; de Miranda, M.P.; McInnes, E.J.L.; Kilner, C.A.; Halcrow, M.A. Antisymmetric exchange in two tricopper(II) complexes containing a [Cu3(μ3-OMe)]5+ core. Dalton Trans. 2004. [Google Scholar] [CrossRef]
- Liu, J.-C.; Guo, G.-C.; Huang, J.-S.; You, X.-Z. Different Oxidation States of Copper(I, I/II, II) Thiocyanate Complexes Containing 1,2,4-Triazole as a Bridging Ligand: Syntheses, Crystal Structures, and Magnetic Properties of 2-D Polymer CuI(admtrz)SCN, Linear Trinuclear [CuI2CuII(admtrz)6(SCN)2](ClO4)2, and Triangular Trinuclear [CuII3(admtrz)4(SCN)3(μ3-OH)(H2O)](ClO4)2·H2O (admtrz = 4-Amino-3,5-dimethyl-1,2,4-triazole). Inorg. Chem. 2003, 42, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulou, A.N.; Margiolaki, I.; Psycharis, V.; Boudalis, A.K. Dynamic versus Static Character of the Magnetic Jahn–Teller Effect: Magnetostructural Studies of [Fe3O(O2CPh)6(py)3]ClO4 py. Inorg. Chem. 2017, 56, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Boudalis, A.K.; Rogez, G.; Turek, P. Determination of the Distributions of the Spin-Hamiltonian Parameters in Spin Triangles: A Combined Magnetic Susceptometry and Electron Paramagnetic Resonance Spectroscopic Study of the Highly Symmetric [Cr3O(PhCOO)6(py)3](ClO4)·0.5py. Inorg. Chem. 2018, 57, 13259–13269. [Google Scholar] [CrossRef]
- Ruiz, E.; Cano, J.; Alvarez, S.; Alemany, P. Broken symmetry approach to calculation of exchange coupling constants for homobinuclear and heterobinuclear transition metal complexes. J. Comput. Chem. 1999, 20, 1391–1400. [Google Scholar] [CrossRef]
- Ruiz, E.; Rodríguez-Fortea, A.; Cano, J.; Alvarez, S.; Alemany, P. About the calculation of exchange coupling constants in polynuclear transition metal complexes. J. Comput. Chem. 2003, 24, 982–989. [Google Scholar] [CrossRef]
- Neese, F. Prediction of molecular properties and molecular spectroscopy with density functional theory: From fundamental theory to exchange-coupling. Coord. Chem. Rev. 2009, 253, 526–563. [Google Scholar] [CrossRef]
- Hay, P.J.; Thibeault, J.C.; Hoffmann, R. Orbital interactions in metal dimer complexes. J. Am. Chem. Soc. 1975, 97, 4884–4899. [Google Scholar] [CrossRef]
- Ruiz, E.; Alemany, P.; Alvarez, S.; Cano, J. Toward the Prediction of Magnetic Coupling in Molecular Systems: Hydroxo- and Alkoxo-Bridged Cu(II) Binuclear Complexes. J. Am. Chem. Soc. 1997, 119, 1297–1303. [Google Scholar] [CrossRef]
- Johnston, D.C.; Kremer, R.K.; Troyer, M.; Wang, X.; Klümper, A.; Bud’ko, S.L.; Panchula, A.F.; Canfield, P.C. Thermodynamics of spin $S = 1/2$ antiferromagnetic uniform and alternating-exchange Heisenberg chains. Phys. Rev. B 2000, 61, 9558–9606. [Google Scholar] [CrossRef]
- Eaton, S.S.; More, K.M.; Sawant, B.M.; Eaton, G.R. Use of the ESR half-field transition to determine the interspin distance and the orientation of the interspin vector in systems with two unpaired electrons. J. Am. Chem. Soc. 1983, 105, 6560–6567. [Google Scholar] [CrossRef]
- Monroe, J.C.; Carvajal, M.A.; Deumal, M.; Landee, C.P.; Redemeyer, M.; Turnbull, M.M. Revisiting the Role of Hydrogen Bonding in the Strong Dimer Superexchange of a 2D Copper(II) Halide Honeycomb-Like Lattice: Structural and Magnetic Study. Inorg. Chem. 2020, 59, 6319–6331. [Google Scholar] [CrossRef] [PubMed]
- Matos, C.R.M.; Junior, H.C.S.; D’Amato, D.L.; de Souza, A.C.; Pinheiro, S.; Guedes, G.P.; Ferreira, G.B.; Alves, O.C.; de Almeida, F.B.; Garcia, F.; et al. Spin-frustration with two quasi-degenerated spin states of a copper(II) heptanuclear complex obtained from an amino acid ligand. Dalton Trans. 2020. [Google Scholar] [CrossRef]





| Compound a | Cu···Cu (Å) | −J, −zJ′/cm−1 | Ref. |
|---|---|---|---|
| [Cu3 (OH)(pz)3(Hpz)2(NO3)2]·H2O | 3.351 | 200, 0 | [45] |
| [Cu3(OH)(pz)3(py)2Cl2]·py | 3.112–3.321 | 140, 0 | [46] |
| [Cu3(OH)(aat)3(CF3SO3)(H2O)](CF3SO3) | 3.355 | 197.7, 0 | [47] |
| [Cu3(OH)(aat)3(NO3)(H2O)2](NO3)·(H2O)2 | 3.341 | 190.9, 0 | [47] |
| [Cu3(OH)(aat)3(ClO4)(H2O)2](ClO4) | 3.371 | 198.2, 0 | [47] |
| {[Cu3(O)(triazolate)3(OH)(H2O)6]}n | 3.388 | 112.6, 11.6 | [48] |
| [Cu3(triazolate)3(OH)][Cu2Br4] | 3.502 | 180, 68 | [49] |
| [Cu3Br(Hpz)2(pz)3(OCH3)]Br | 3.250–3.255 | 105, 0 | [50] |
| [Cu3(OH)(aaat)3(H2O)3](NO3)2·H2O | 3.347–3.393 | 195, 0 | [8] |
| {[Cu3(OH)(aat)3(SO4)]·6H2O}n | 3.337–3.364 | 185, 0 | [8] |
| [Cu3(admtrz)4(SCN)3(OH)(H2O)](ClO4)2·H2O | 3.254–3.318 | 120, 53 | [51] |
| [{Cu3(OH)(pz)3(Hpz)3}2SO4](NO3)2·MeCN·MeOH·1.5H2O | 3.182–3.354 | 180, 12.7 | [44] |
| [Ag(Hpz)2]2[{Ag2(Hpz)2(NO3)2}{Cu6(OH)2(pz)6(Hpz)6(SO4)}2](NO3)6·4H2O | 3.222–3.356 | 134, 10.5 | [44] |
| [{Ag(H2O)2}{Cu3(OH)(pz)3(Hpz)3(H2O)(ClO4)3}] | 3.302–3.372 | 158, 9.2 | [44] |
| [Et3NH][Cu3(OH)(pz)3(PhCOO)3] | 3.244–3.352 | 178, 57.5 | [13] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, K.; Mathivathanan, L.; Herchel, R.; Boudalis, A.K.; Raptis, R.G. Supramolecular Assemblies of Trinuclear Copper(II)-Pyrazolato Units: A Structural, Magnetic and EPR Study. Chemistry 2020, 2, 626-644. https://doi.org/10.3390/chemistry2030039
Shi K, Mathivathanan L, Herchel R, Boudalis AK, Raptis RG. Supramolecular Assemblies of Trinuclear Copper(II)-Pyrazolato Units: A Structural, Magnetic and EPR Study. Chemistry. 2020; 2(3):626-644. https://doi.org/10.3390/chemistry2030039
Chicago/Turabian StyleShi, Kaige, Logesh Mathivathanan, Radovan Herchel, Athanassios K. Boudalis, and Raphael G. Raptis. 2020. "Supramolecular Assemblies of Trinuclear Copper(II)-Pyrazolato Units: A Structural, Magnetic and EPR Study" Chemistry 2, no. 3: 626-644. https://doi.org/10.3390/chemistry2030039
APA StyleShi, K., Mathivathanan, L., Herchel, R., Boudalis, A. K., & Raptis, R. G. (2020). Supramolecular Assemblies of Trinuclear Copper(II)-Pyrazolato Units: A Structural, Magnetic and EPR Study. Chemistry, 2(3), 626-644. https://doi.org/10.3390/chemistry2030039





