Recent Studies on the Antimicrobial Activity of Transition Metal Complexes of Groups 6–12
Abstract
1. Introduction
2. Group 6
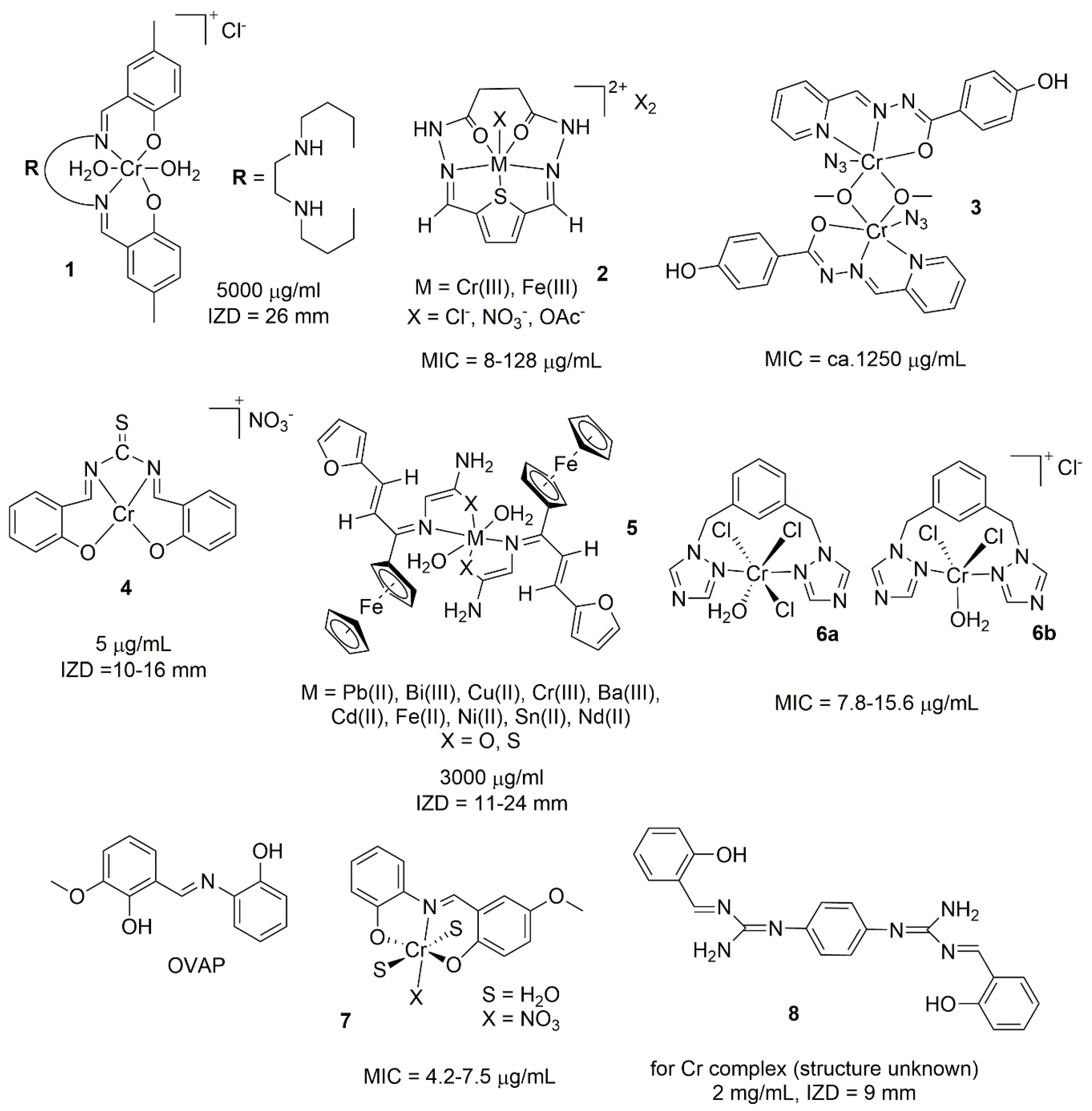
2.1. Chromium Complexes
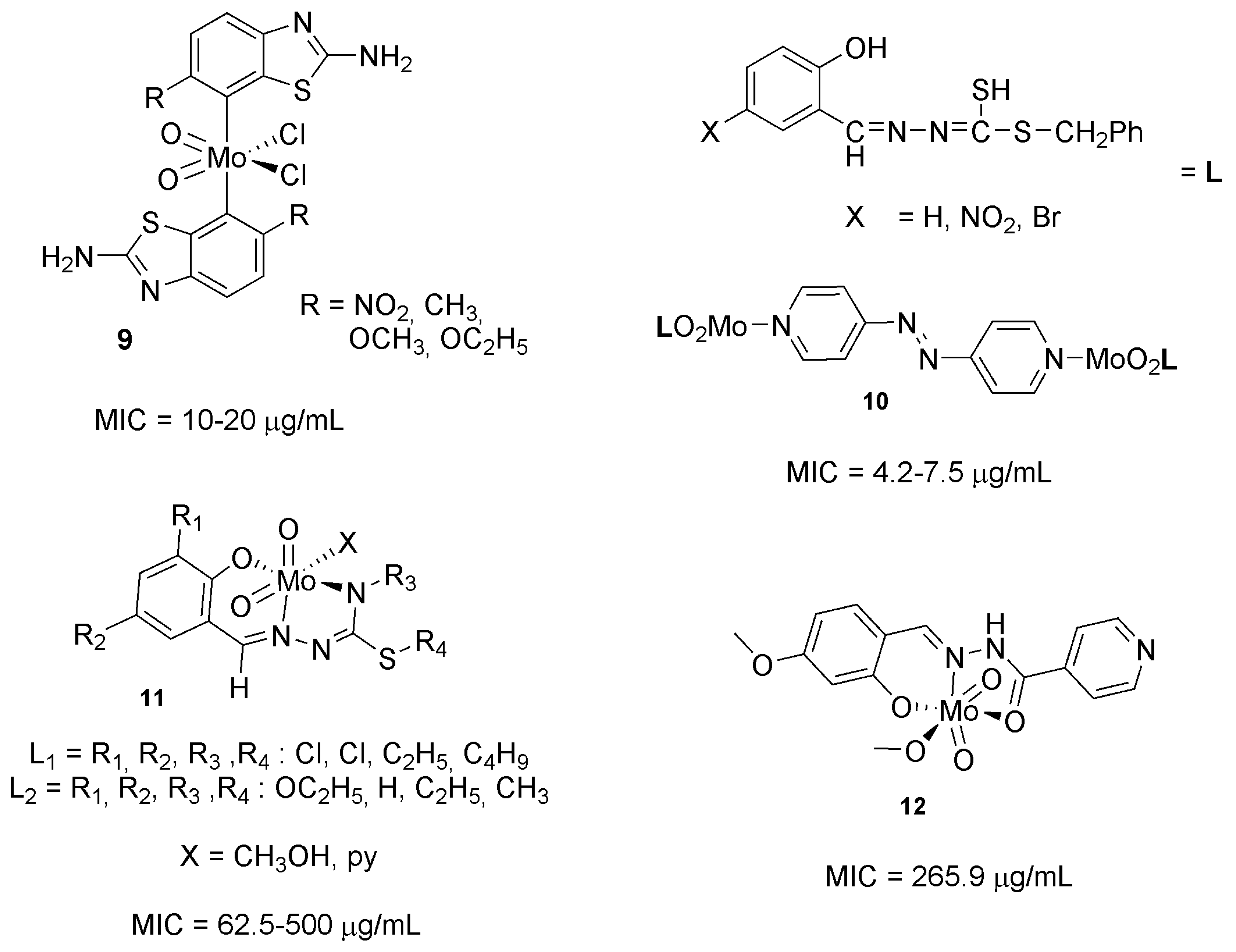
2.2. Molybdenum Complexes
3. Group 7
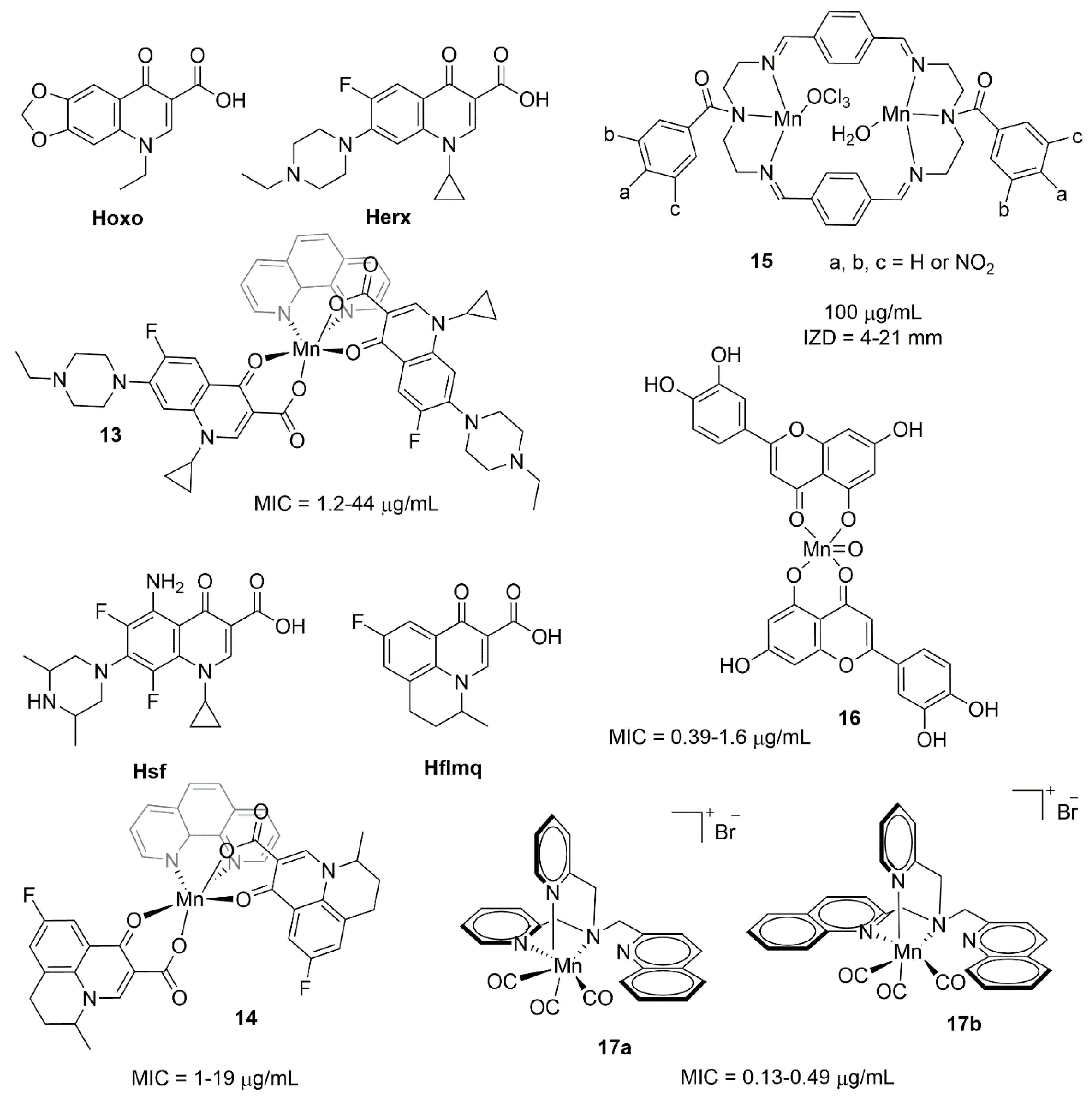
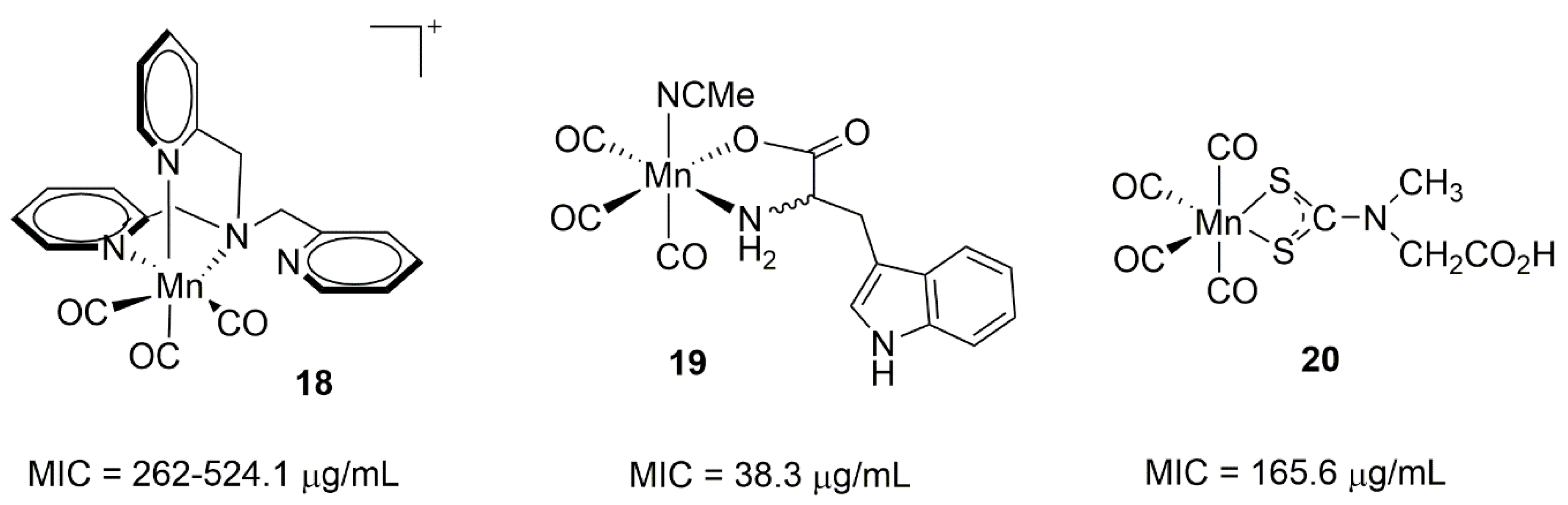
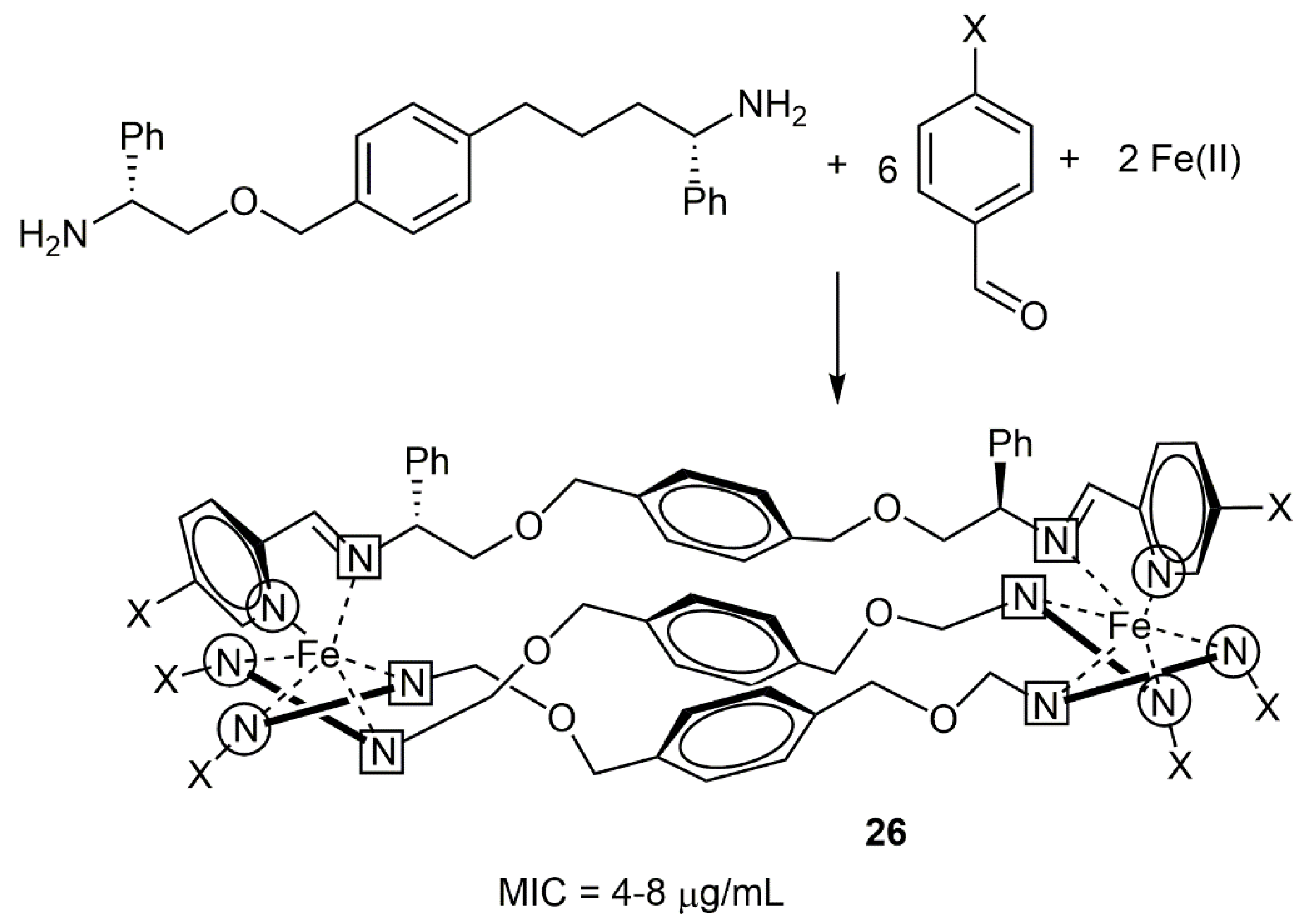
3.1. Manganese Complexes
3.2. Manganese Photoactivatable CO-Releasing Molecules (PhotoCORMs)
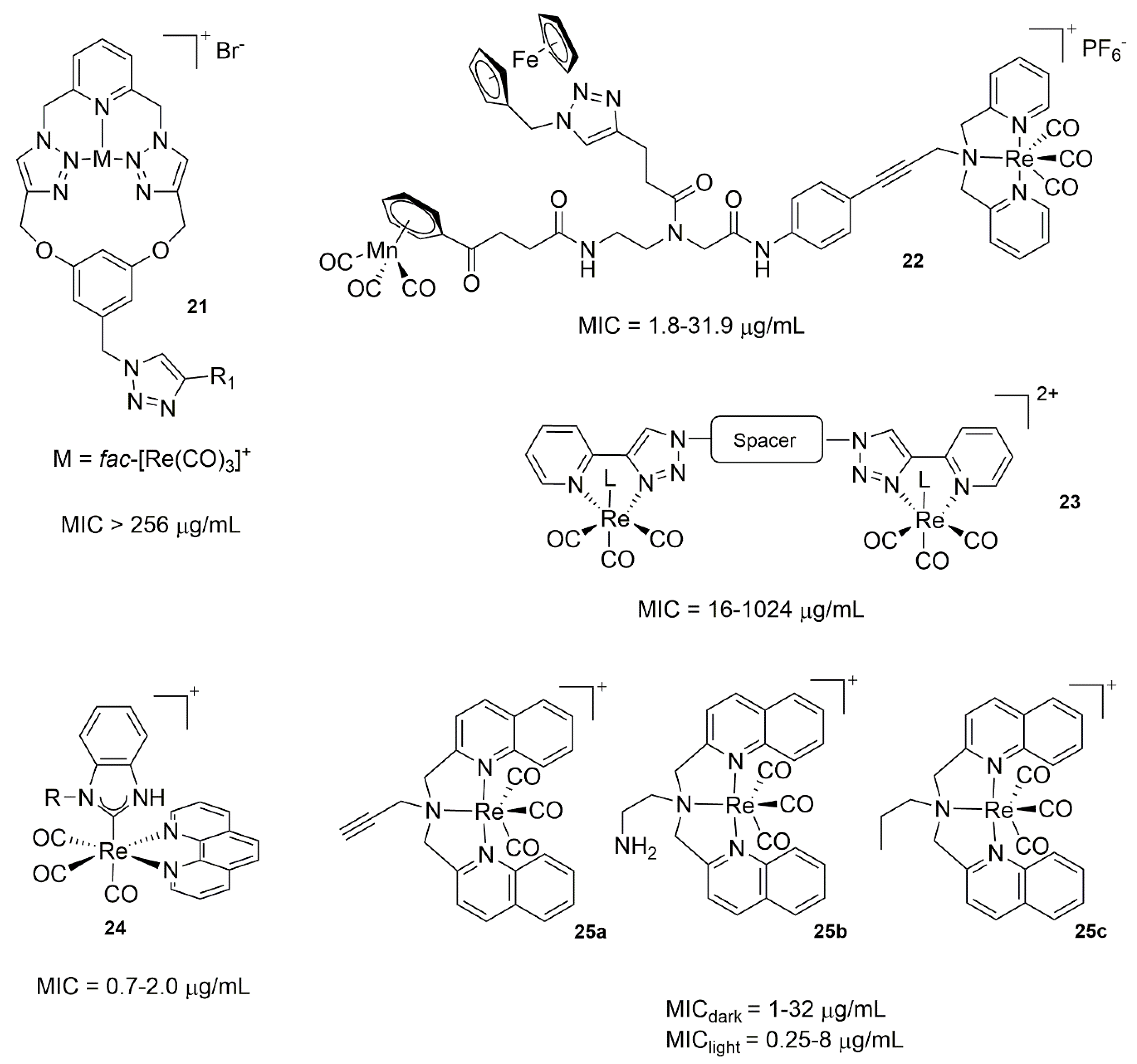
3.3. Rhenium Complexes
4. Group 8
4.1. Ruthenium Complexes
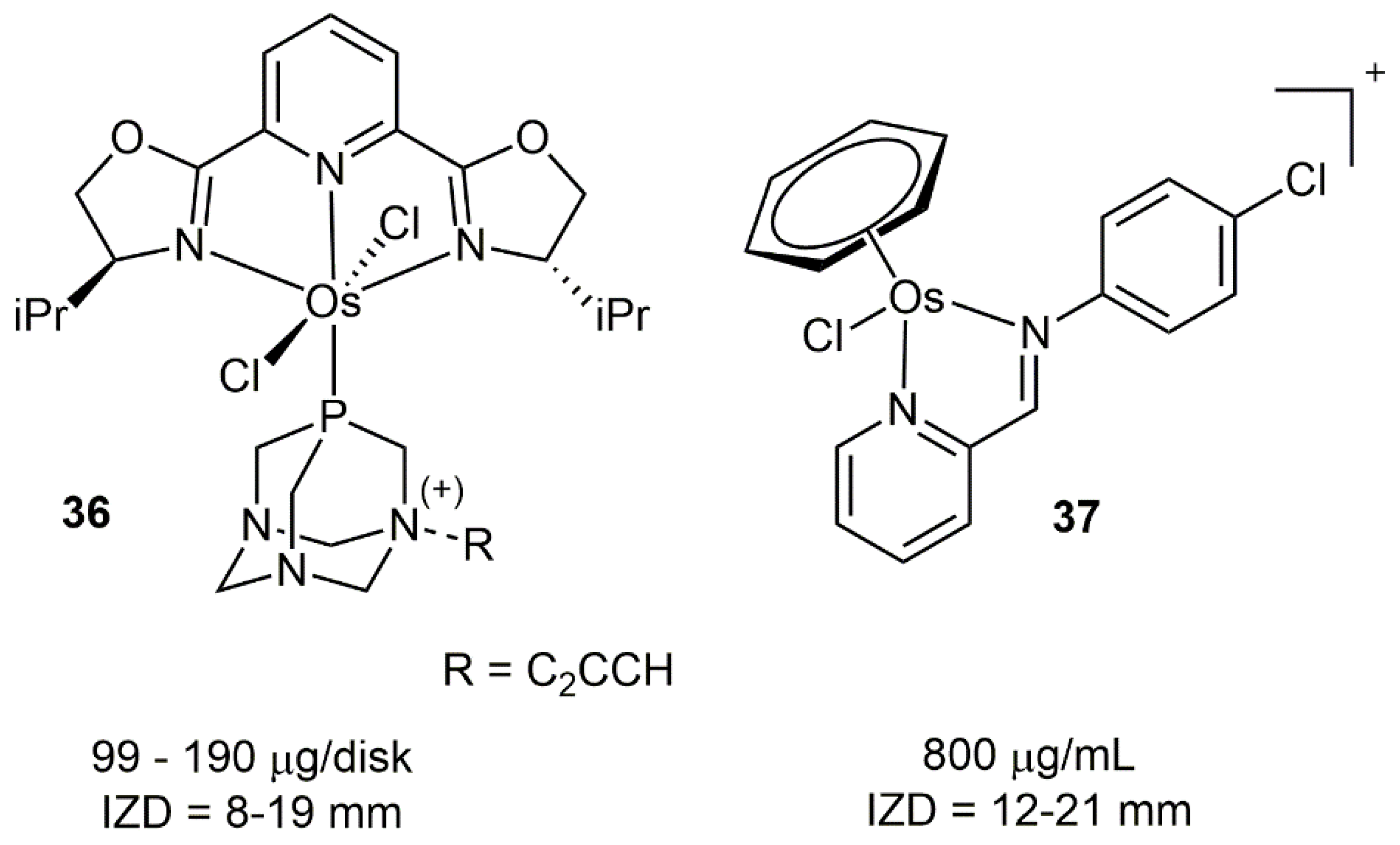
4.2. Osmium Complexes
5. Group 9
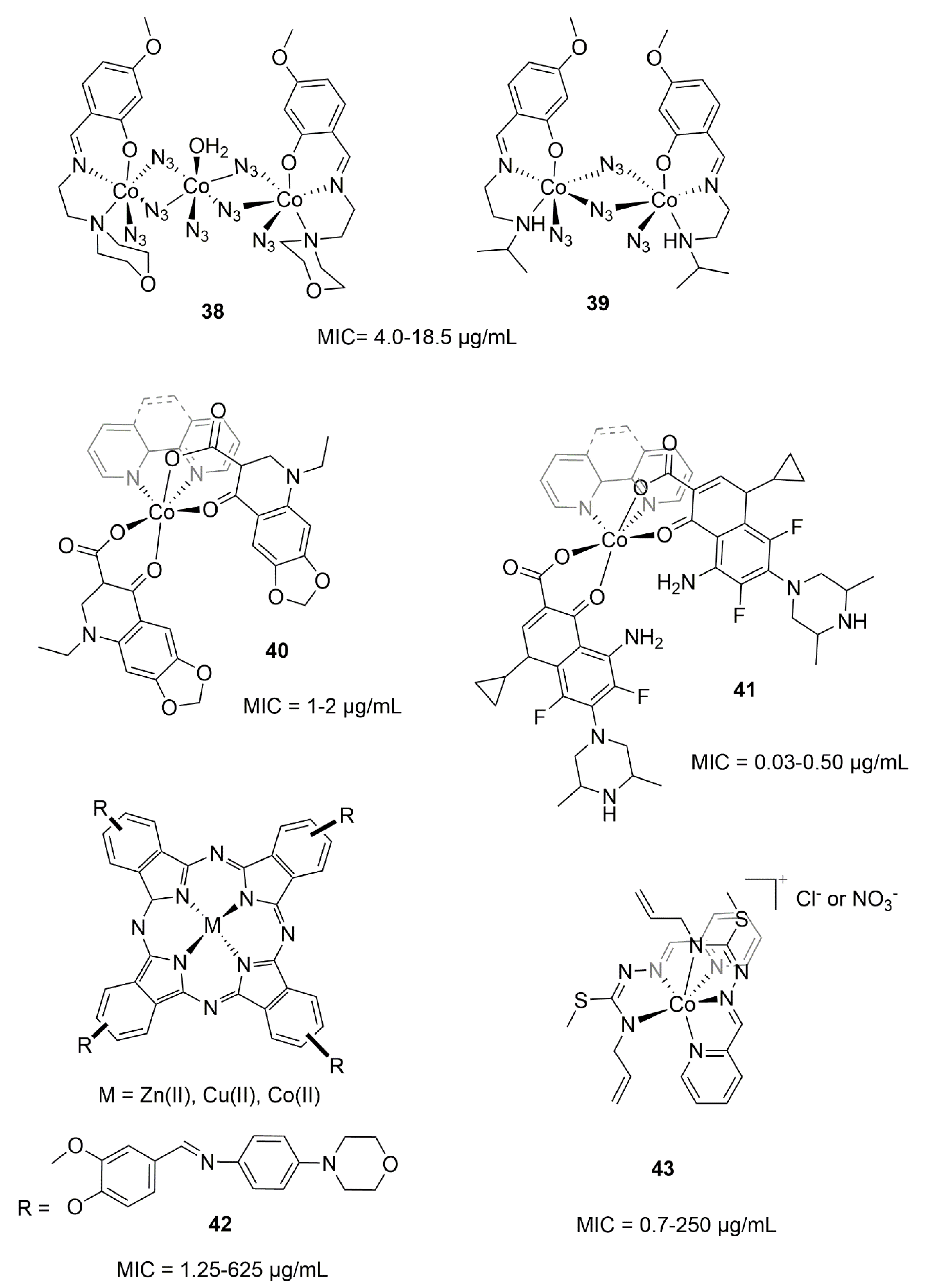
5.1. Cobalt Complexes
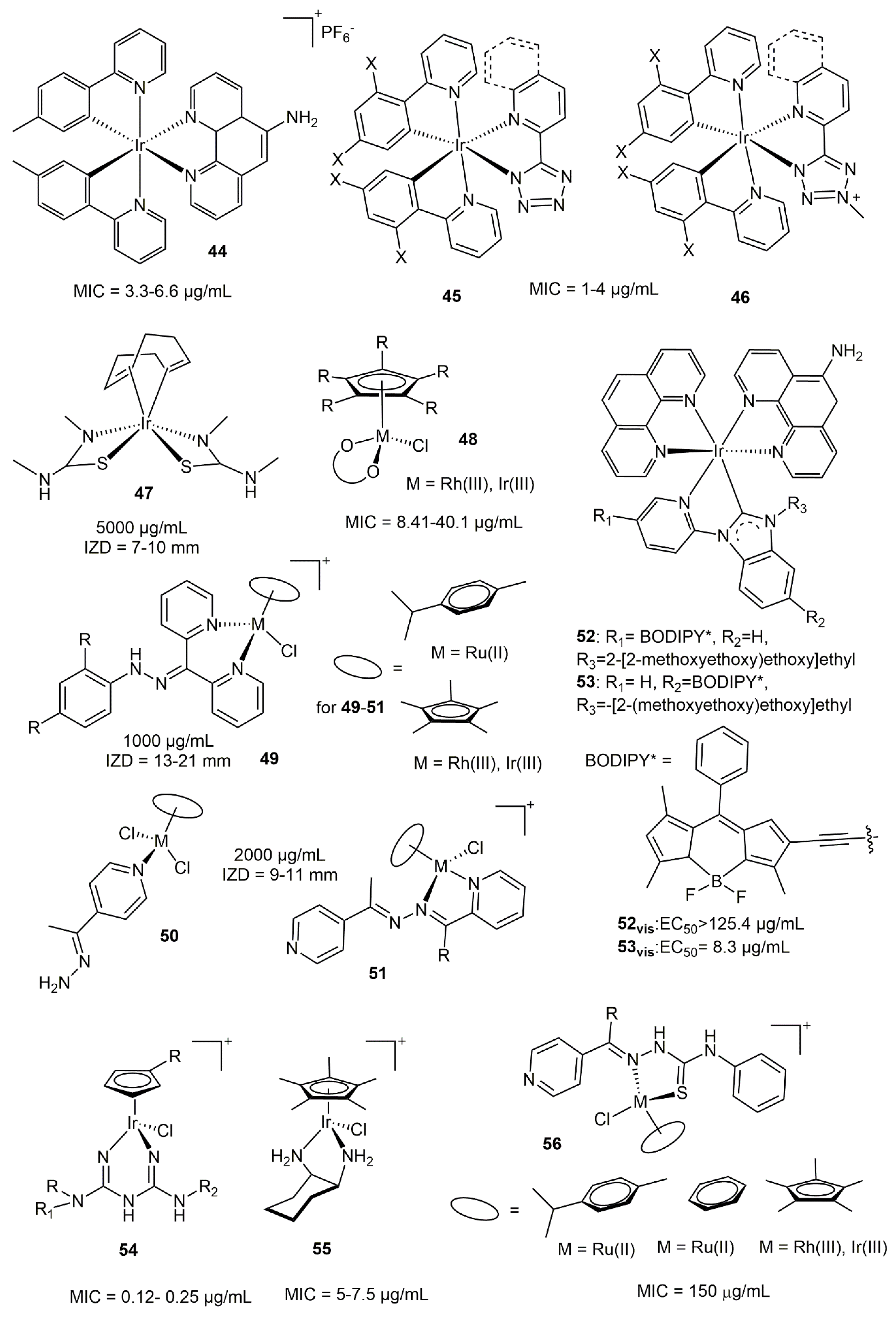
5.2. Rhodium and Iridium Complexes
6. Group 10
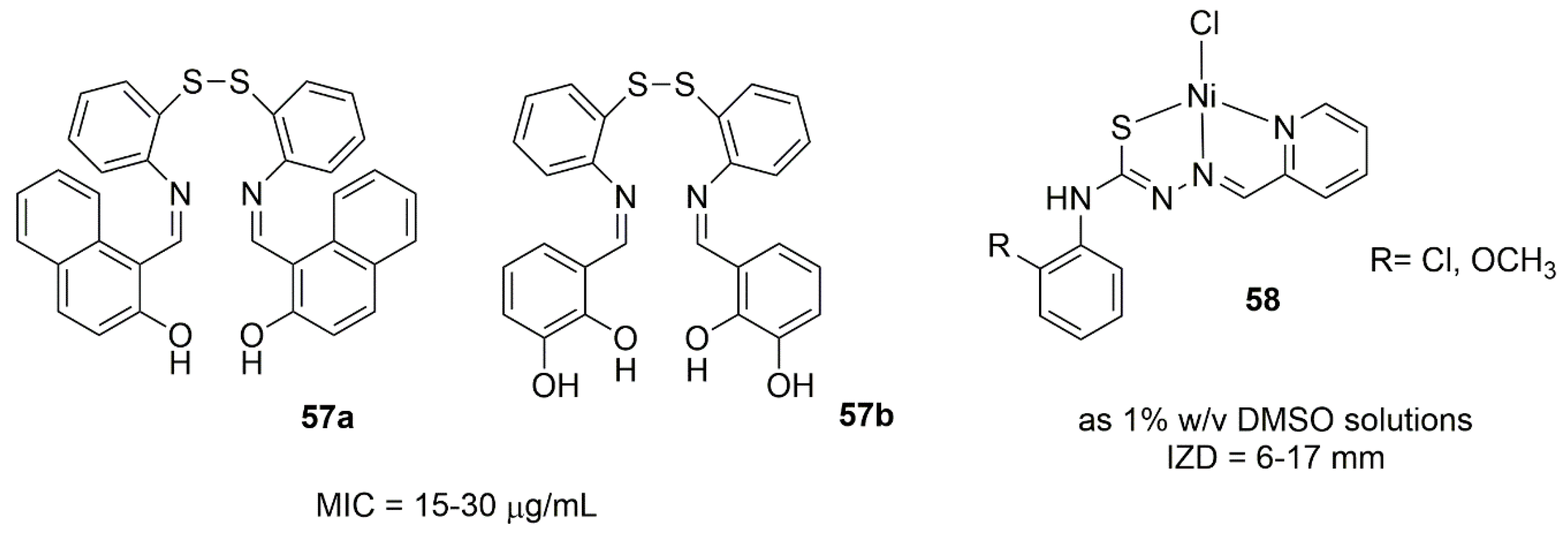
6.1. Nickel Complexes
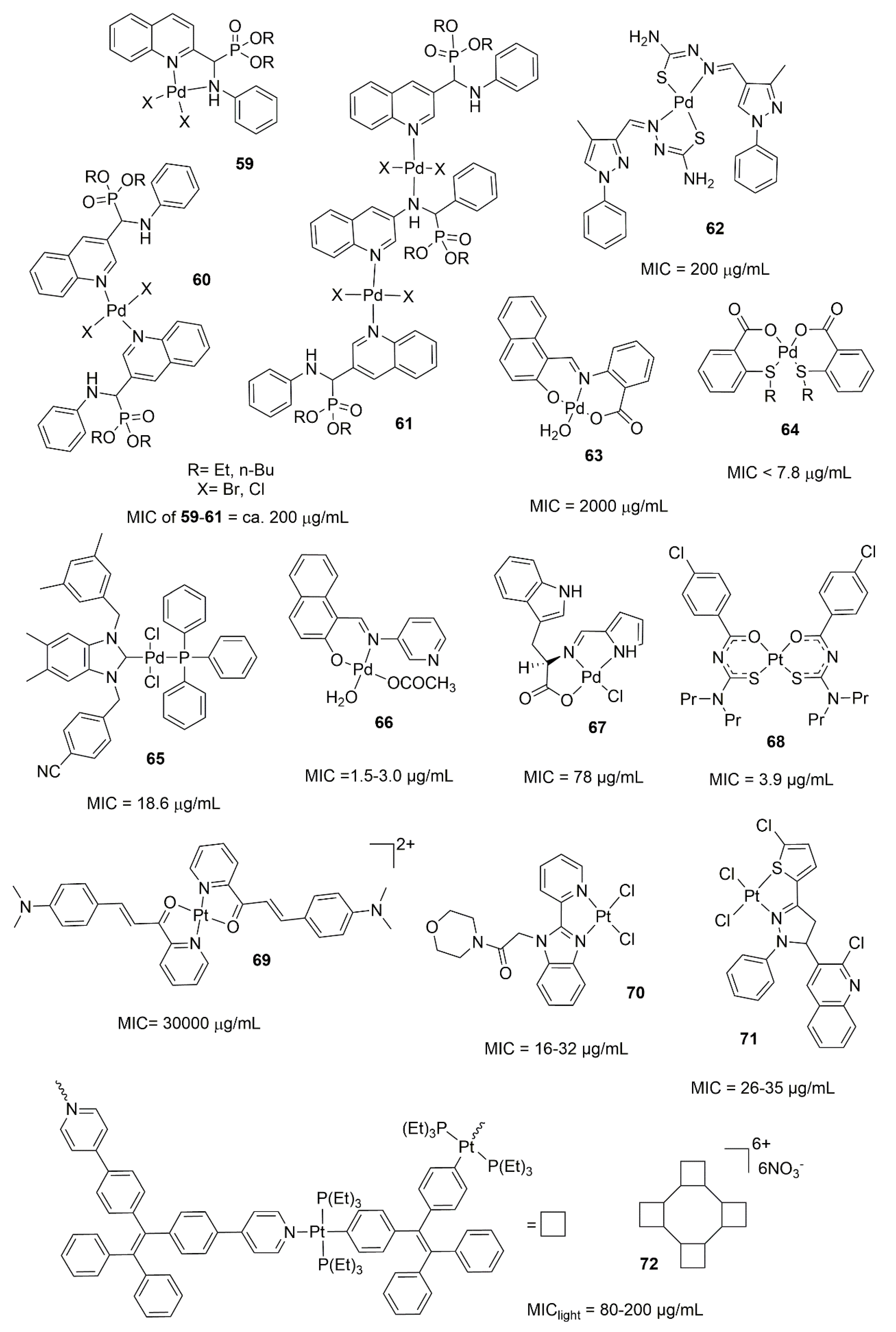
6.2. Palladium and Platinum Complexes
7. Group 11
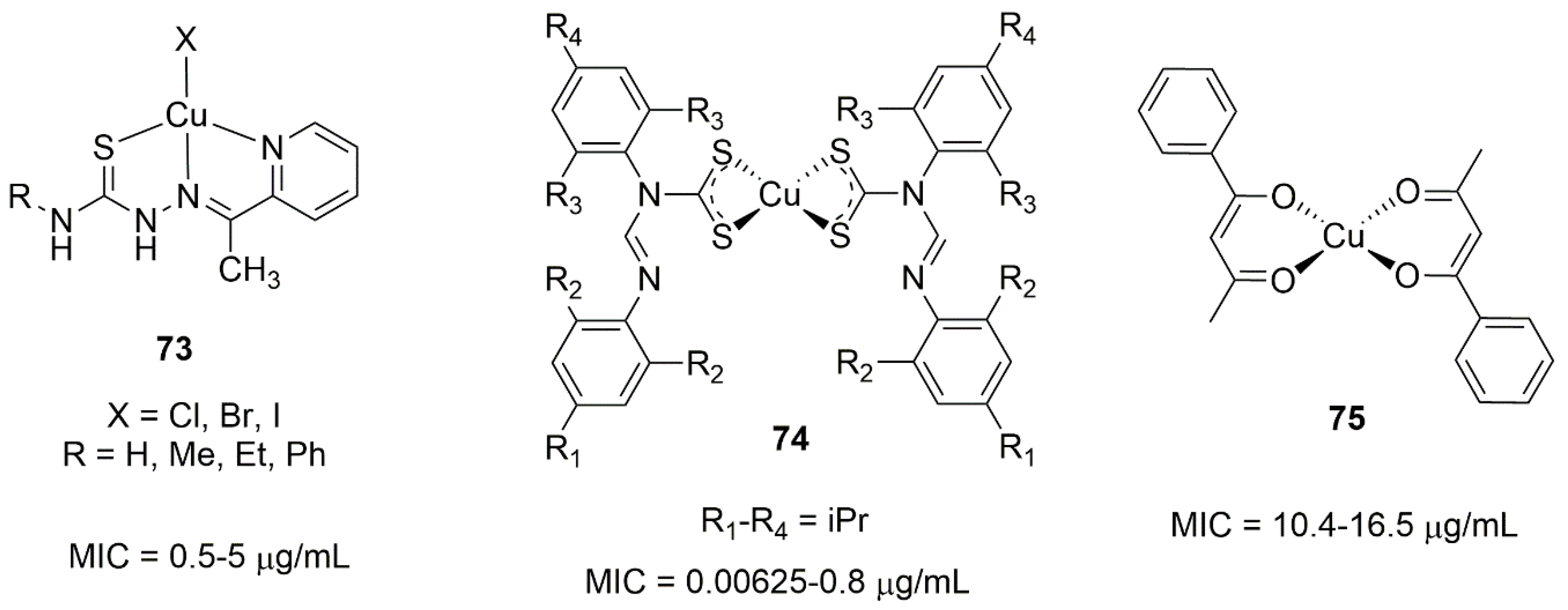
7.1. Copper Complexes
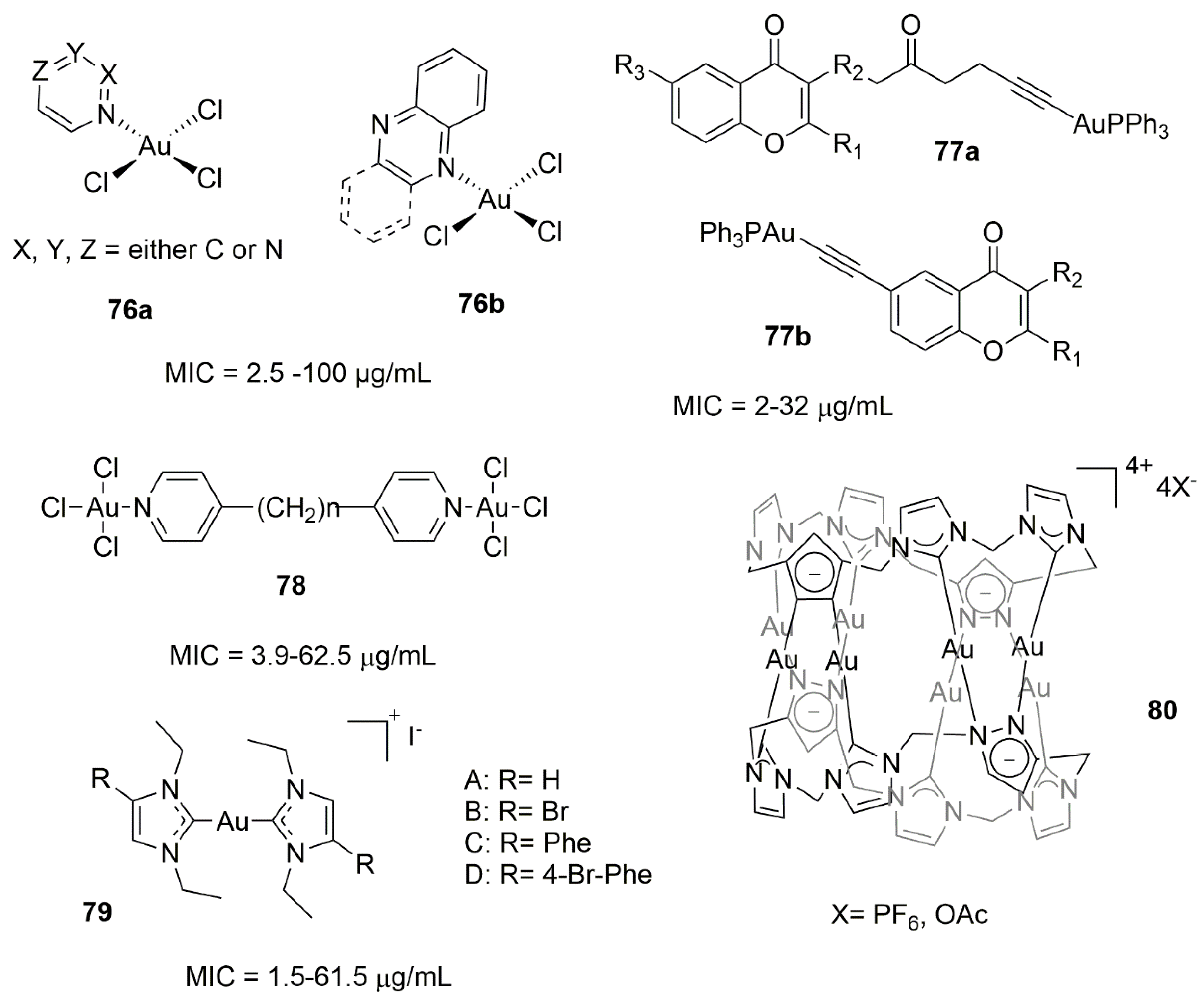
7.2. Gold Complexes
8. Group 12
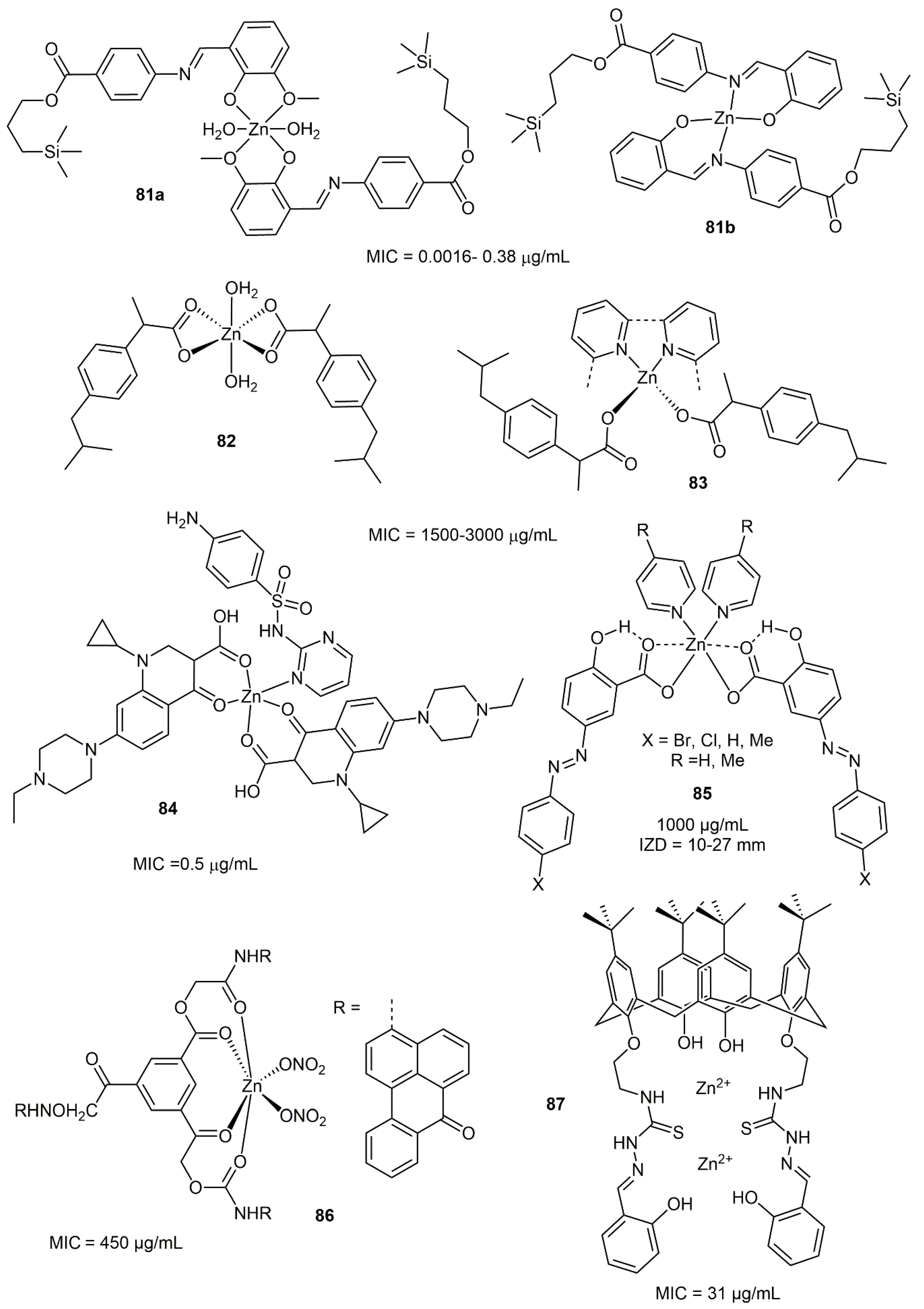
8.1. Zinc Complexes
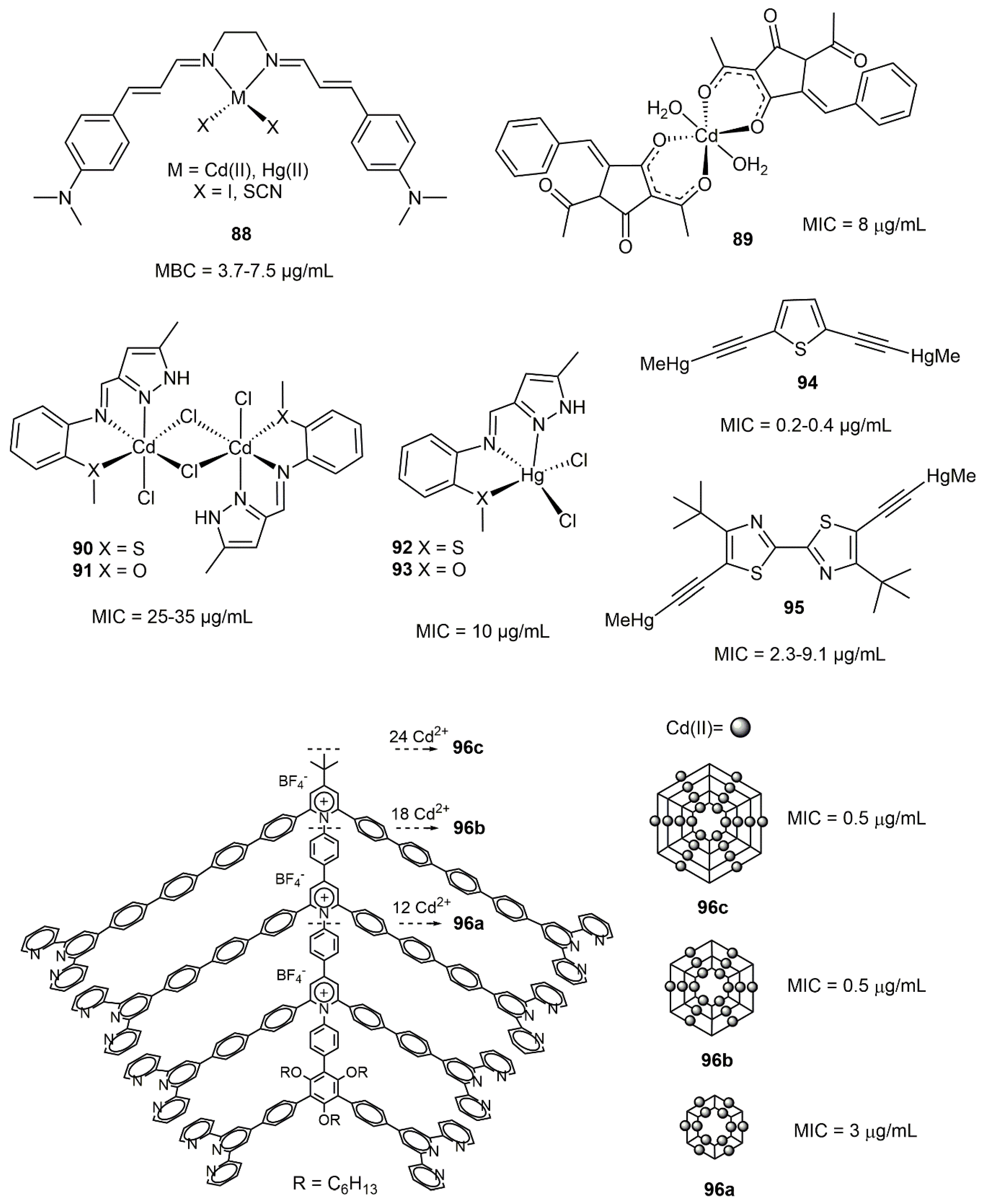
8.2. Cadmium and Mercury Complexes
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bush, K.; Courvalin, P.; Dantas, G.; Davies, J.; Eisenstein, B.; Huovinen, P.; Jacoby, G.A.; Kishony, R.; Kreiswirth, B.N.; Kutter, E.; et al. Tackling antibiotic resistance. Nat. Rev. Microbiol. 2011, 9, 894–896. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Bouley, R.; Ding, D.; Peng, Z.; Bastian, M.; Lastochkin, E.; Song, W.; Suckow, M.A.; Schroeder, V.A.; Wolter, W.R.; Mobashery, S.; et al. Structure-Activity Relationship for the 4(3H)-Quinazolinone Antibacterials. J. Med. Chem. 2016, 59, 5011–5021. [Google Scholar] [CrossRef]
- Ng, N.S.; Leverett, P.; Hibbs, D.E.; Yang, Q.; Bulanadi, J.C.; Wu, M.J.; Aldrich-Wright, J.R. The antimicrobial properties of some copper(II) and platinum(II) 1,10-phenanthroline complexes. Dalton Trans. 2013, 42, 3196–3209. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Z.; Chen, Y.; Xu, J.; Li, J.; Jiang, X. Synergy of non-antibiotic drugs and pyrimidinethiol on gold nanoparticles against superbugs. J. Am. Chem. Soc. 2013, 135, 12940–12943. [Google Scholar] [CrossRef]
- Xia, J.; Gao, J.; Kokudo, N.; Hasegawa, K.; Tang, W. Methicillin-resistant Staphylococcus aureus antibiotic resistance and virulence. Biosci. Trends 2013, 7, 113–121. [Google Scholar]
- Von Nussbaum, F.; Brands, M.; Hinzen, B.; Weigand, S.; Habich, D. Antibacterial natural products in medicinal chemistry-exodus or revival? Angew. Chem. Int. Ed. Engl. 2006, 45, 5072–5129. [Google Scholar] [CrossRef]
- Pawlowski, A.C.; Johnson, J.W.; Wright, G.D. Evolving medicinal chemistry strategies in antibiotic discovery. Curr. Opin. Biotech. 2016, 42, 108–117. [Google Scholar] [CrossRef]
- Talbot, G.H.; Jezek, A.; Murray, B.E.; Jones, R.N.; Ebright, R.H.; Nau, G.J.; Rodvold, K.A.; Newland, J.G.; Boucher, H.W.; Infectious Diseases Society of America. The Infectious Diseases Society of America’s 10 × ’20 Initiative (10 New Systemic Antibacterial Agents US Food and Drug Administration Approved by 2020): Is 20 × ’20 a Possibility? Arch. Clin. Infect. Dis. 2019, 69, 1–11. [Google Scholar] [CrossRef]
- Frei, A. Metal Complexes, an Untapped Source of Antibiotic Potential? Antibiotics 2020, 9, 90. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef]
- De Toledo, L.d.A.S.; Rosseto, H.C.; Bruschi, M.L. Iron oxide magnetic nanoparticles as antimicrobials for therapeutics. Pharm. Dev. Technol. 2018, 23, 316–323. [Google Scholar] [CrossRef]
- Dutta, P.; Wang, B. Zeolite-supported silver as antimicrobial agents. Coord. Chem. Rev. 2019, 383, 1–29. [Google Scholar] [CrossRef]
- Duval, R.E.; Gouyau, J.; Lamouroux, E. Limitations of Recent Studies Dealing with the Antibacterial Properties of Silver Nanoparticles: Fact and Opinion. Nanomaterials 2019, 9, 1775. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Mosleh, A.M.; Elghany, A.A.; Shams-Eldin, E.; Abu Serea, E.S.; Ali, S.A.; Shalan, A.E. Coated silver nanoparticles: Synthesis, cytotoxicity, and optical properties. RSC Adv. 2019, 9, 20118–20136. [Google Scholar] [CrossRef]
- Gunawan, C.; Marquis, C.P.; Amal, R.; Sotiriou, G.A.; Rice, S.A.; Harry, E.J. Widespread and Indiscriminate Nanosilver Use: Genuine Potential for Microbial Resistance. ACS Nano 2017, 11, 3438–3445. [Google Scholar] [CrossRef]
- Hashim, A.; Agool, I.R.; Kadhim, K.J. Modern Developments in Polymer Nanocomposites For Antibacterial and Antimicrobial Applications: A Review. J. Bionanosci. 2018, 12, 608–613. [Google Scholar] [CrossRef]
- Hussaini, S.Y.; Haque, R.A.; Razali, M.R. Recent progress in silver(I)-, gold(I)/(III)- and palladium(II)-N-heterocyclic carbene complexes: A review towards biological perspectives. J. Organomet. Chem. 2019, 882, 96–111. [Google Scholar] [CrossRef]
- Leitao, J.H.; Sousa, S.A.; Leite, S.A.; Carvalho, M. Silver Camphor Imine Complexes: Novel Antibacterial Compounds from Old Medicines. Antibiotics 2018, 7, 65. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Zoroddu, M.A. Medical Uses of Silver: History, Myths, and Scientific Evidence. J. Med. Chem. 2019, 62, 5923–5943. [Google Scholar] [CrossRef]
- Möhler, J.S.; Sim, W.; Blaskovich, M.A.T.; Cooper, M.A.; Ziora, Z.M. Silver bullets: A new lustre on an old antimicrobial agent. Biotech. Adv. 2018, 36, 1391–1411. [Google Scholar] [CrossRef] [PubMed]
- Rusu, A.; Hancu, G.; Cristina Munteanu, A.; Uivarosi, V. Development perspectives of silver complexes with antibacterial quinolones: Successful or not? J. Organomet. Chem. 2017, 839, 19–30. [Google Scholar] [CrossRef]
- Singh, R.; Shedbalkar, U.U.; Wadhwani, S.A.; Chopade, B.A. Bacteriagenic silver nanoparticles: Synthesis, mechanism, and applications. Appl. Microbiol. Biotechnol. 2015, 99, 4579–4593. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Patil, S.A.; Patil, S.A.; Patil, R.; Keri, R.S.; Budagumpi, S.; Balakrishna, G.R.; Tacke, M. N-heterocyclic carbene metal complexes as bio-organometallic antimicrobial and anticancer drugs. Future Med. Chem. 2015, 7, 1305–1333. [Google Scholar] [CrossRef]
- Li, F.; Collins, J.G.; Keene, F.R. Ruthenium complexes as antimicrobial agents. Chem. Soc. Rev. 2015, 44, 2529–2542. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G.; Metzler-Nolte, N. Small organometallic compounds as antibacterial agents. Dalton Trans. 2012, 41, 6350–6358. [Google Scholar] [CrossRef]
- Sierra, M.; Casarrubios, L.; De la Torre, M. Bio-Organometallic Derivatives of Antibacterial Drugs. Chemistry 2019, 25. [Google Scholar] [CrossRef]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; C, G.D.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef]
- Kumar, S.P.; Suresh, R.; Giribabu, K.; Manigandan, R.; Munusamy, S.; Muthamizh, S.; Narayanan, V. Synthesis and characterization of chromium(III) Schiff base complexes: Antimicrobial activity and its electrocatalytic sensing ability of catechol. Spectrochim. Acta A 2015, 139, 431–441. [Google Scholar] [CrossRef]
- Rathi, P.; Singh, D.P. Synthesis, antimicrobial, antioxidant and molecular docking studies of thiophene based macrocyclic Schiff base complexes. J. Mol. Struct. 2015, 1100, 208–214. [Google Scholar] [CrossRef]
- Shaabani, B.; Khandar, A.A.; Ramazani, N.; Fleck, M.; Mobaiyen, H.; Cunha-Silva, L. Chromium(III), manganese(II) and iron(III) complexes based on hydrazone Schiff-base and azide ligands: Synthesis, crystal structure and antimicrobial activity. J. Coord. Chem. 2017, 70, 696–708. [Google Scholar] [CrossRef]
- Kafi-Ahmadi, L.; Shirmohammadzadeh, L. Synthesis of Co(II) and Cr(III) salicylidenic Schiff base complexes derived from thiourea as precursors for nano-sized Co3O4 and Cr2O3 and their catalytic, antibacterial properties. J. Nanostruct. Chem. 2017, 7, 179–190. [Google Scholar] [CrossRef]
- Abdel Rahman, L.H.; Abu-Dief, A.M.; Hamdan, S.K.; Seleem, A.A. Nano Structure Iron(II) and Copper(II) Schiff-Base Complexes of a NNO Tridentate Ligand as New Antibiotic Agents. Spectral Thermal Behaviors and Dann Binding Ability. Int. J. Nanomater. Chem. 2015, 1, 65–77. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; El-Khatib, R.M.; Nassr, L.A.; Abu-Dief, A.M.; Ismael, M.; Seleem, A.A. Metal based pharmacologically active agents: Synthesis, structural characterization, molecular modeling, CT-DNA binding studies and in vitro antimicrobial screening of iron(II) bromosalicylidene amino acid chelates. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 366–378. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Nassr, L.A.E. Tailoring, physicochemical characterization, antibacterial and DNA binding mode studies of Cu(II) Schiff bases amino acid bioactive agents incorporating 5-bromo-2-hydroxybenzaldehyde. J. Iran. Chem. Soc. 2015, 12, 943–955. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Sheng, J.; Yin, D.-W.; Xin, H.; Yang, X.-M.; Qiao, Q.-Y.; Yang, Z.-J. Ferrocenyl chalcone-based Schiff bases and their metal complexes: Highly efficient, solvent-free synthesis, characterization, biological research. J. Organomet. Chem. 2018, 856, 27–33. [Google Scholar] [CrossRef]
- Murcia, R.A.; Leal, S.M.; Roa, M.V.; Nagles, E.; Munoz-Castro, A.; Hurtado, J.J. Development of Antibacterial and Antifungal Triazole Chromium(III) and Cobalt(II) Complexes: Synthesis and Biological Activity Evaluations. Molecules 2018, 23, 2013. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; El-Sagher, H.M.; Shehata, M.R. Fabrication, spectroscopic characterization, calf thymus DNA binding investigation, antioxidant and anticancer activities of some antibiotic azomethine Cu(II), Pd(II), Zn(II) and Cr(III) complexes. Appl. Organomet. Chem. 2019, 33, e4943. [Google Scholar] [CrossRef]
- El-Razek, S.E.A.; El-Gamasy, S.M.; Hassan, M.; Abdel-Aziz, M.S.; Nasr, S.M. Transition metal complexes of a multidentate Schiff base ligand containing guanidine moiety: Synthesis, characterization, anti-cancer effect, and anti-microbial activity. J. Mol. Struct. 2020, 1203, 127381. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; Newair, E.F.; Hamdan, S.K. Some new nano-sized Cr(III), Fe(II), Co(II), and Ni(II) complexes incorporating 2-((E)-(pyridine-2-ylimino)methyl)napthalen-1-ol ligand: Structural characterization, electrochemical, antioxidant, antimicrobial, antiviral assessment and DNA interaction. J. Photochem. Photobiol. 2016, 160, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Drzeżdżon, J.; Piotrowska-Kirschling, A.; Malinowski, J.; Kloska, A.; Gawdzik, B.; Chmurzyński, L.; Jacewicz, D. Antimicrobial, cytotoxic, and antioxidant activities and physicochemical characteristics of chromium(III) complexes with picolinate, dipicolinate, oxalate, 2,2′-bipyridine, and 4,4′-dimethoxy-2,2′-bipyridine as ligands in aqueous solutions. J. Mol. Liq. 2019, 282, 441–447. [Google Scholar] [CrossRef]
- Mahmoud, W.H.; Deghadi, R.G.; Mohamed, G.G. Metal complexes of novel Schiff base derived from iron sandwiched organometallic and 4-nitro-1,2-phenylenediamine: Synthesis, characterization, DFT studies, antimicrobial activities and molecular docking. Appl. Organomet. Chem. 2018, 32, e4289. [Google Scholar] [CrossRef]
- Pahontu, E.; Usataia, I.; Graur, V.; Chumakov, Y.; Petrenko, P.; Gudumac, V.; Gulea, A. Synthesis, characterization, crystal structure of novel Cu(II), Co(III), Fe(III) and Cr(III) complexes with 2-hydroxybenzaldehyde-4-allyl-S-methylisothiosemicarbazone: Antimicrobial, antioxidant and in vitro antiproliferative activity. Appl. Organomet. Chem. 2018, 32, e4544. [Google Scholar] [CrossRef]
- Srivastva, A.N.; Singh, N.P.; Shriwastaw, C.K. Physicochemical studies on bioactive Cr(III) coordination compounds with esters of hydrazine carboxylic acid as hetero donor ligands. Res. Chem. Intermed. 2017, 43, 5453–5465. [Google Scholar] [CrossRef]
- Saraswat, K.; Kant, R. Synthesis characterization and biological activity of some molybdenum(VI) complexes. Der Pharma. Chem. 2013, 5, 347–356. [Google Scholar]
- Wedd, A.G. Sulfido-Complexes of Molybdenum and Tungsten: Synthetic Aspects. In Studies in Inorganic Chemistry; Müller, A., Krebs, B., Eds.; Elsevier: Amsterdam, The Netherlands, 1984; Volume 5, pp. 181–193. [Google Scholar]
- Zhang, L.; Nelson, K.J.; Rajagopalan, K.V.; George, G.N. Structure of the Molybdenum Site of Escherichia coli Trimethylamine N-Oxide Reductase. Inorg. Chem. 2008, 47, 1074–1078. [Google Scholar] [CrossRef]
- Biswal, D.; Pramanik, N.R.; Chakrabarti, S.; Drew, M.G.B.; Mitra, P.; Acharya, K.; Biswas, S.; Mondal, T.K. Supramolecular frameworks of binuclear dioxomolybdenum(VI) complexes with ONS donor ligands using 4,4′-azopyridine as a pillar: Crystal structure, DFT calculations and biological study. N. J. Chem. 2015, 39, 8681–8694. [Google Scholar] [CrossRef]
- Çelen, Ş.; Eğlence-Bakır, S.; Şahin, M.; Deniz, I.; Celik, H.; Kizilcikli, I. Synthesis and characterization of new thiosemicarbazonato molybdenum(VI) complexes and their in vitro antimicrobial activities. J. Coord. Chem. 2019, 72, 1747–1758. [Google Scholar] [CrossRef]
- Sang, Y.-L.; Zhang, X.-H.; Lin, X.-S.; Liu, Y.-H.; Liu, X.-Y. Syntheses, crystal structures, and antibacterial activity of oxidovanadium(V) and dioxidomolybdenum(VI) complexes derived from N′-(2-hydroxy-4-methoxybenzylidene)isonicotinohydrazide. J. Coord. Chem. 2020, 73, 164–174. [Google Scholar] [CrossRef]
- Kirakci, K.; Zelenka, J.; Rumlová, M.; Cvačka, J.; Ruml, T.; Lang, K. Cationic octahedral molybdenum cluster complexes functionalized with mitochondria-targeting ligands: Photodynamic anticancer and antibacterial activities. Biomater. Sci. 2019, 7, 1386–1392. [Google Scholar] [CrossRef]
- Zampakou, M.; Akrivou, M.; Andreadou, E.G.; Raptopoulou, C.P.; Psycharis, V.; Pantazaki, A.A.; Psomas, G. Structure, antimicrobial activity, DNA- and albumin-binding of manganese(II) complexes with the quinolone antimicrobial agents oxolinic acid and enrofloxacin. J. Inorg. Biochem. 2013, 121, 88–99. [Google Scholar] [CrossRef]
- Barmpa, A.; Frousiou, O.; Kalogiannis, S.; Perdih, F.; Turel, I.; Psomas, G. Manganese(II) complexes of the quinolone family member flumequine: Structure, antimicrobial activity and affinity for albumins and calf-thymus DNA. Polyhedron 2018, 145, 166–175. [Google Scholar] [CrossRef]
- Arthi, P.; Shobana, S.; Srinivasan, P.; Prabhu, D.; Arulvasu, C.; Kalilur Rahiman, A. Dinuclear manganese(II) complexes of hexaazamacrocycles bearing N-benzoylated pendant separated by aromatic spacers: Antibacterial, DNA interaction, cytotoxic and molecular docking studies. J. Photoch. Photobio. B 2015, 153, 247–260. [Google Scholar] [CrossRef]
- Simpson, P.V.; Nagel, C.; Bruhn, H.; Schatzschneider, U. Antibacterial and Antiparasitic Activity of Manganese(I) Tricarbonyl Complexes with Ketoconazole, Miconazole, and Clotrimazole Ligands. Organometallics 2015, 34, 3809–3815. [Google Scholar] [CrossRef]
- Dong, H.; Yang, X.; He, J.; Cai, S.; Xiao, K.; Zhu, L. Enhanced antioxidant activity, antibacterial activity and hypoglycemic effect of luteolin by complexation with manganese(II) and its inhibition kinetics on xanthine oxidase. RSC Adv. 2017, 7, 53385–53395. [Google Scholar] [CrossRef]
- Güntzel, P.; Nagel, C.; Weigelt, J.; Betts, J.W.; Pattrick, C.A.; Southam, H.M.; La Ragione, R.M.; Poole, R.K.; Schatzschneider, U. Biological activity of manganese(I) tricarbonyl complexes on multidrug-resistant Gram-negative bacteria: From functional studies to in vivo activity in Galleria mellonella. Metallomics 2019, 11, 2033–2042. [Google Scholar] [CrossRef]
- Kottelat, E.; Chabert, V.; Crochet, A.; Fromm, K.M.; Zobi, F. Towards Cardiolite-Inspired Carbon Monoxide Releasing Molecules–Reactivity of d4, d5 Rhenium and d6 Manganese Carb-onyl Complexes with Isocyanide Ligands. Eur. J. Inorg. Chem. 2015, 2015, 5628–5638. [Google Scholar] [CrossRef]
- Devi, P.P.; Chipem, F.A.S.; Singh, C.B.; Lonibala, R.K. Complexation of 2-amino-3-(4-hydroxyphenyl)-N′-[(2-hydroxyphenyl) methylene] propane hydrazide with Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) ions: Structural characterization, DFT, DNA binding and antimicrobial studies. J. Mol. Struct. 2019, 1176, 7–18. [Google Scholar] [CrossRef]
- Jayakumar, S.; Mahendiran, D.; Rahiman, A.K. Theoretical, antimicrobial, antioxidant, in vitro cytotoxicity, and cyclin-dependent kinase 2 inhibitor studies of metal(II) complexes with bis(imidazol-1-yl)methane-based heteroscorpionate ligands. J. Coord. Chem. 2019, 72, 2015–2034. [Google Scholar] [CrossRef]
- Özgür, M.; Yılmaz, M.; Nishino, H.; Çinar Avar, E.; Dal, H.; Pekel, A.T.; Hökelek, T. Efficient syntheses and antimicrobial activities of new thiophene containing pyranone and quinolinone derivatives using manganese(III) acetate: The effect of thiophene on ring closure–opening reactions. N. J. Chem. 2019, 43, 5737–5751. [Google Scholar] [CrossRef]
- Pulina, N.A.; Kuznetsov, A.S.; Krasnova, A.I.; Novikova, V.V. Synthesis, Antimicrobial Activity, and Behavioral Response Effects of N-Substituted 4-Aryl-2-Hydroxy-4-Oxobut-2-Enoic Acid Hydrazides and Their Metal Complexes. Pharm. Chem. J. 2019, 53, 220–224. [Google Scholar] [CrossRef]
- Betts, J.; Nagel, C.; Schatzschneider, U.; Poole, R.; La Ragione, R.M. Antimicrobial activity of carbon monoxide-releasing molecule [Mn(CO)3(tpa-k3N)]Br versus multidrug-resistant isolates of Avian Pathogenic Escherichia coli and its synergy with colistin. PLoS ONE 2017, 12, e0186359. [Google Scholar] [CrossRef]
- Nagel, C.; McLean, S.; Poole, R.K.; Braunschweig, H.; Kramer, T.; Schatzschneider, U. Introducing [Mn(CO)3(tpa-k3N)]+ as a novel photoactivatable CO-releasing molecule with well-defined iCORM intermediates–synthesis, spectroscopy, and antibacterial activity. Dalton Trans. 2014, 43, 9986–9997. [Google Scholar] [CrossRef]
- Rana, N.; Jesse, H.E.; Tinajero-Trejo, M.; Butler, J.A.; Tarlit, J.D.; von Und Zur Muhlen, M.L.; Nagel, C.; Schatzschneider, U.; Poole, R.K. A manganese photosensitive tricarbonyl molecule [Mn(CO)3(tpa-k3N)]Br enhances antibiotic efficacy in a multi-drug-resistant Escherichia coli. Microbiology 2017, 163, 1477–1489. [Google Scholar] [CrossRef]
- Tinajero-Trejo, M.; Rana, N.; Nagel, C.; Jesse, H.E.; Smith, T.W.; Wareham, L.K.; Poole, R.K. Antimicrobial Activity of the Manganese Photoactivated Carbon Monoxide-Releasing Molecule [Mn(CO)3(tpa-k3N)]+ Against a Pathogenic Escherichia coli that Causes Urinary Infections. Antioxid. Redox. Sign. 2016, 24, 765–780. [Google Scholar] [CrossRef]
- Ward, J.S.; Lynam, J.M.; Moir, J.; Fairlamb, I.J.S. Visible-Light-Induced CO Release from a Therapeutically Viable Tryptophan-Derived Manganese(I) Carbonyl (TryptoCORM) Exhibiting Potent Inhibition against E. coli. Chem. Eur. J. 2014, 20, 15061–15068. [Google Scholar] [CrossRef]
- Ward, J.S.; Morgan, R.; Lynam, J.M.; Fairlamb, I.J.S.; Moir, J.W.B. Toxicity of tryptophan manganese(I) carbonyl (Trypto-CORM), against Neisseria gonorrhoeae. Med. Chem. Commun. 2017, 8, 346–352. [Google Scholar] [CrossRef]
- Crook, S.H.; Mann, B.E.; Meijer, A.J.H.M.; Adams, H.; Sawle, P.; Scapens, D.; Motterlini, R. [Mn(CO)4(S2CNMe(CH2CO2H))], a new water-soluble CO-releasing molecule. Dalton Trans. 2011, 40, 4230–4235. [Google Scholar] [CrossRef]
- Wareham, L.K.; McLean, S.; Begg, R.; Rana, N.; Ali, S.; Kendall, J.J.; Sanguinetti, G.; Mann, B.E.; Poole, R.K. The Broad-Spectrum Antimicrobial Potential of [Mn(CO)4(S2CNMe(CH2CO2H))], a Water-Soluble CO-Releasing Molecule (CORM-401): Intracellular Accumulation, Transcriptomic and Statistical Analyses, and Membrane Polarization. Antioxid. Redox Signal. 2018, 28, 1286–1308. [Google Scholar] [CrossRef]
- Noor, A.; Huff, G.S.; Kumar, S.V.; Lewis, J.E.M.; Paterson, B.M.; Schieber, C.; Donnelly, P.S.; Brooks, H.J.L.; Gordon, K.C.; Moratti, S.C.; et al. [Re(CO)3]+ Complexes of exo-Functionalized Tridentate “Click” Macrocycles: Synthesis, Stability, Photophysical Properties, Bioconjugation, and Antibacterial Activity. Organometallics 2014, 33, 7031–7043. [Google Scholar] [CrossRef]
- Patra, M.; Wenzel, M.; Prochnow, P.; Pierroz, V.; Gasser, G.; Bandow, J.E.; Metzler-Nolte, N. An organometallic structure-activity relationship study reveals the essential role of a Re(CO)3 moiety in the activity against gram-positive pathogens including MRSA. Chem. Sci. 2015, 6, 214–224. [Google Scholar] [CrossRef]
- Kumar, S.V.; Lo, W.K.C.; Brooks, H.J.L.; Hanton, L.R.; Crowley, J.D. Antimicrobial Properties of Mono- and Di-fac-rhenium Tricarbonyl 2-Pyridyl-1,2,3-triazole Complexes. Aust. J. Chem. 2016, 69, 489–498. [Google Scholar] [CrossRef]
- Siegmund, D.; Lorenz, N.; Gothe, Y.; Spies, C.; Geissler, B.; Prochnow, P.; Nuernberger, P.; Bandow, J.E.; Metzler-Nolte, N. Benzannulated Re(I)–NHC complexes: Synthesis, photophysical properties and antimicrobial activity. Dalton Trans. 2017, 46, 15269–15279. [Google Scholar] [CrossRef]
- Frei, A.; Amado, M.; Cooper, M.A.; Blaskovich, M.A.T. Light-Activated Rhenium Complexes with Dual Mode of Action against Bacteria. Chem. Eur. J. 2020, 26, 2852–2858. [Google Scholar] [CrossRef]
- Carreño, A.; Solís-Céspedes, E.; Zúñiga, C.; Nevermann, J.; Rivera-Zaldívar, M.M.; Gacitúa, M.; Ramírez-Osorio, A.; Páez-Hernández, D.; Arratia-Pérez, R.; Fuentes, J.A. Cyclic voltammetry, relativistic DFT calculations and biological test of cytotoxicity in walled-cell models of two classical rhenium(I) tricarbonyl complexes with 5-amine-1,10-phenanthroline. Chem. Phys. Lett. 2019, 715, 231–238. [Google Scholar] [CrossRef]
- Kydonaki, T.E.; Tsoukas, E.; Mendes, F.; Hatzidimitriou, A.G.; Paulo, A.; Papadopoulou, L.C.; Papagiannopoulou, D.; Psomas, G. Synthesis, characterization and biological evaluation of 99mTc/Re–tricarbonyl quinolone complexes. J. Inorg. Biochem. 2016, 160, 94–105. [Google Scholar] [CrossRef]
- Miller, R.G.; Vázquez-Hernández, M.; Prochnow, P.; Bandow, J.E.; Metzler-Nolte, N. A CuAAC Click Approach for the Introduction of Bidentate Metal Complexes to a Sulfanilamide-Derived Antibiotic Fragment. Inorg. Chem. 2019, 58, 9404–9413. [Google Scholar] [CrossRef]
- Pagoni, C.-C.; Xylouri, V.-S.; Kaiafas, G.C.; Lazou, M.; Bompola, G.; Tsoukas, E.; Papadopoulou, L.C.; Psomas, G.; Papagiannopoulou, D. Organometallic rhenium tricarbonyl–enrofloxacin and –levofloxacin complexes: Synthesis, albumin-binding, DNA-interaction and cell viability studies. J. Biol. Inorg. Chem. 2019, 24, 609–619. [Google Scholar] [CrossRef]
- Howson, S.E.; Bolhuis, A.; Brabec, V.; Clarkson, G.J.; Malina, J.; Rodger, A.; Scott, P. Optically pure, water-stable metallo-helical ‘flexicate’ assemblies with antibiotic activity. Nat. Chem. 2012, 4, 31–36. [Google Scholar] [CrossRef]
- Allardyce, C.S.; Dyson, P.J. Ruthenium in Medicine_ Current Clinical Uses and Future Prospects. Platin. Met. Rev. 2001, 45, 62–69. [Google Scholar]
- Atton, J.G.D.M.; Gillard, R.D. Equilibria in complexes of N-heterocyclic ligands. Part 33. Ruthenium(II) complex ions with chelating pyridyl-imidazoles. Transit. Met. Chem. 1981, 6, 351–355. [Google Scholar] [CrossRef]
- Bolhuis, A.; Hand, L.; Marshall, J.E.; Richards, A.D.; Rodger, A.; Aldrich-Wright, J. Antimicrobial activity of ruthenium-based intercalators. Eur. J. Pharm. Sci. 2011, 42, 313–317. [Google Scholar] [CrossRef]
- Gill, M.R.; Garcia-Lara, J.; Foster, S.J.; Smythe, C.; Battaglia, G.; Thomas, J.A. A ruthenium(II) polypyridyl complex for direct imaging of DNA structure in living cells. Nat. Chem. 2009, 1, 662–667. [Google Scholar] [CrossRef]
- Gill, M.R.; Thomas, J.A. Ruthenium(II) polypyridyl complexes and DNA—from structural probes to cellular imaging and therapeutics. Chem. Soc. Rev. 2012, 41, 3179–3192. [Google Scholar] [CrossRef]
- Keene, F.R.; Smith, J.A.; Collins, J.G. Metal complexes as structure-selective binding agents for nucleic acids. Coord. Chem. Rev. 2009, 253, 2021–2035. [Google Scholar] [CrossRef]
- Luedtke, N.W.; Hwang, J.S.; Nava, E.; Gut, D.; Kol, M.; Tor, Y. The DNA and RNA specificity of eilatin Ru(II) complexes as compared to eilatin and ethidium bromide. Nucleic Acids Res. 2003, 31, 5732–5740. [Google Scholar] [CrossRef]
- Matson, M.; Svensson, F.R.; Nordén, B.; Lincoln, P. Correlation Between Cellular Localization and Binding Preference to RNA, DNA, and Phospholipid Membrane for Luminescent Ruthenium(II) Complexes. J. Phys. Chem. B 2011, 115, 1706–1711. [Google Scholar] [CrossRef]
- Metcalfe, C.; Thomas, J.A. Kinetically inert transition metal complexes that reversibly bind to DNA. Chem. Soc. Rev. 2003, 32, 215–224. [Google Scholar] [CrossRef]
- Norden, B.; Lincoln, P.; Akerman, B.; Tuite, E. DNA interactions with substitution-inert transition metal ion complexes. Met. Ions Biol. Syst. 1996, 33, 177–252. [Google Scholar]
- Puckett, C.A.; Barton, J.K. Mechanism of Cellular Uptake of a Ruthenium Polypyridyl Complex. Biochemistry 2008, 47, 11711–11716. [Google Scholar] [CrossRef]
- Zeglis, B.M.; Pierre, V.C.; Barton, J.K. Metallo-intercalators and metallo-insertors. Chem. Commun. 2007, 44, 4565–4579. [Google Scholar] [CrossRef]
- Kumar, S.V.; Scottwell, S.Ø.; Waugh, E.; McAdam, C.J.; Hanton, L.R.; Brooks, H.J.L.; Crowley, J.D. Antimicrobial Properties of Tris(homoleptic) Ruthenium(II) 2-Pyridyl-1,2,3-triazole “Click” Complexes against Pathogenic Bacteria, Including Methicillin-Resistant Staphylococcus aureus (MRSA). Inorg. Chem. 2016, 55, 9767–9777. [Google Scholar] [CrossRef]
- Liao, G.; Ye, Z.; Liu, Y.; Fu, B.; Fu, C. Octahedral ruthenium(II) polypyridyl complexes as antimicrobial agents against mycobacterium. PeerJ. 2017, 5, e3252. [Google Scholar] [CrossRef]
- Li, X.; Heimann, K.; Li, F.; Warner, J.M.; Richard Keene, F.; Grant Collins, J. Dinuclear ruthenium(II) complexes containing one inert metal centre and one coordinatively-labile metal centre: Syntheses and biological activities. Dalton Trans. 2016, 45, 4017–4029. [Google Scholar] [CrossRef]
- Srivastava, P.; Shukla, M.; Kaul, G.; Chopra, S.; Patra, A.K. Rationally designed curcumin based ruthenium(II) antimicrobials effective against drug-resistant Staphylococcus aureus. Dalton Trans. 2019, 48, 11822–11828. [Google Scholar] [CrossRef]
- Kreofsky, N.W.; Dillenburg, M.D.; Villa, E.M.; Fletcher, J.T. Ru(II) coordination compounds of NN bidentate chelators with 1,2,3 triazole and isoquinoline subunits: Synthesis, spectroscopy and antimicrobial properties. Polyhedron 2020, 177, 114259. [Google Scholar] [CrossRef]
- Van Hilst, Q.V.C.; Vasdev, R.A.S.; Preston, D.; Findlay, J.A.; Scottwell, S.Ø.; Giles, G.I.; Brooks, H.J.L.; Crowley, J.D. Synthesis, Characterisation and Antimicrobial Studies of some 2,6-bis(1,2,3-Triazol-4-yl)Pyridine Ruthenium(II) “Click” Complexes. Asian J. Org. Chem. 2019, 8, 496–505. [Google Scholar] [CrossRef]
- Sun, B.; Sundaraneedi, M.K.; Southam, H.M.; Poole, R.K.; Musgrave, I.F.; Keene, F.R.; Collins, J.G. Synthesis and biological properties of tetranuclear ruthenium complexes containing the bis[4(4′-methyl-2,2′-bipyridyl)]-1,7-heptane ligand. Dalton Trans. 2019, 48, 14505–14515. [Google Scholar] [CrossRef]
- Menéndez-Pedregal, E.; Manteca, Á.; Sánchez, J.; Díez, J.; Gamasa, M.P.; Lastra, E. Antimicrobial and Antitumor Activity of Enantiopure Pybox–Osmium Complexes. Eur. J. Inorg. Chem. 2015, 2015, 1424–1432. [Google Scholar] [CrossRef]
- Gichumbi, J.M.; Friedrich, H.B.; Omondi, B.; Naicker, K.; Singh, M.; Chenia, H.Y. Synthesis, characterization, antiproliferative, and antimicrobial activity of osmium(II) half-sandwich complexes. J. Coord. Chem. 2018, 71, 342–354. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.-H. Syntheses, crystal structures and antibacterial activities of azido-bridged cobalt(III) complexes with Schiff bases. Transition Met. Chem. 2010, 35, 745–749. [Google Scholar] [CrossRef]
- Anjaneyulu, Y.; Rao, R.P. Preparation, Characterization and Antimicrobial Activity Studies on Some Ternary Complexes of Cu(II) with Acetylacetone and Various Salicylic Acids. Synth. React. Inorg. Met. Org. Chem. 1986, 16, 257–272. [Google Scholar] [CrossRef]
- Dharmaraj, N.; Viswanathamurthi, P.; Natarajan, K. Ruthenium(II) complexes containing bidentate Schiff bases and their antifungal activity. Transit. Met. Chem. 2001, 26, 105–109. [Google Scholar] [CrossRef]
- Irgi, E.P.; Geromichalos, G.D.; Balala, S.; Kljun, J.; Kalogiannis, S.; Papadopoulos, A.; Turel, I.; Psomas, G. Cobalt(II) complexes with the quinolone antimicrobial drug oxolinic acid: Structure and biological perspectives. RSC Adv. 2015, 5, 36353–36367. [Google Scholar] [CrossRef]
- Kouris, E.; Kalogiannis, S.; Perdih, F.; Turel, I.; Psomas, G. Cobalt(II) complexes of sparfloxacin: Characterization, structure, antimicrobial activity and interaction with DNA and albumins. J. Inorg. Biochem. 2016, 163, 18–27. [Google Scholar] [CrossRef]
- Turecka, K.; Chylewska, A.; Kawiak, A.; Waleron, K.F. Antifungal Activity and Mechanism of Action of the Co(III) Coordination Complexes With Diamine Chelate Ligands Against Reference and Clinical Strains of Candida spp. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Unluer, D.; Aktas Kamiloglu, A.; Direkel, S.; Bektas, E.; Kantekin, H.; Sancak, K. Synthesis and characterization of metallophthalocyanine with morpholine containing Schiff base and determination of their antimicrobial and antioxidant activities. J. Organomet. Chem. 2019, 900, 120936. [Google Scholar] [CrossRef]
- Balan, G.; Burduniuc, O.; Usataia, I.; Graur, V.; Chumakov, Y.; Petrenko, P.; Gudumac, V.; Gulea, A.; Pahontu, E. Novel 2-formylpyridine 4-allyl-S-methylisothiosemicarbazone and Zn(II), Cu(II), Ni(II) and Co(III) complexes: Synthesis, characterization, crystal structure, antioxidant, antimicrobial and antiproliferative activity. Appl. Organomet. Chem. 2020, 34, e5423. [Google Scholar] [CrossRef]
- Casanova, I.; Durán, M.L.; Viqueira, J.; Sousa-Pedrares, A.; Zani, F.; Real, J.A.; García-Vázquez, J.A. Metal complexes of a novel heterocyclic benzimidazole ligand formed by rearrangement-cyclization of the corresponding Schiff base. Electrosynthesis, structural characterization and antimicrobial activity. Dalton Trans. 2018, 47, 4325–4340. [Google Scholar] [CrossRef]
- Chandrasekhar, V.R.; Mookkandi Palsamy, K.; Lokesh, R.; Jegathalaprathaban, R.; Gurusamy, R. Biomolecular docking, antimicrobial and cytotoxic studies on new bidentate schiff base ligand derived metal(II) complexes. Appl. Organomet. Chem. 2019, 33, e4753. [Google Scholar] [CrossRef]
- Ebrahimipour, S.Y.; Machura, B.; Mohamadi, M.; Khaleghi, M. A novel cationic cobalt(III) Schiff base complex: Preparation, crystal structure, Hirshfeld surface analysis, antimicrobial activities and molecular docking. Microb. Pathog. 2017, 113, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Gałczyńska, K.; Ciepluch, K.; Madej, Ł.; Kurdziel, K.; Maciejewska, B.; Drulis-Kawa, Z.; Węgierek-Ciuk, A.; Lankoff, A.; Arabski, M. Selective cytotoxicity and antifungal properties of copper(II) and cobalt(II) complexes with imidazole-4-acetate anion or 1-allylimidazole. Sci. Rep. 2019, 9, 9777. [Google Scholar] [CrossRef] [PubMed]
- Orojloo, M.; Zolgharnein, P.; Solimannejad, M.; Amani, S. Synthesis and characterization of cobalt(II), nickel(II), copper(II) and zinc(II) complexes derived from two Schiff base ligands: Spectroscopic, thermal, magnetic moment, electrochemical and antimicrobial studies. Inorg. Chim. Acta 2017, 467, 227–237. [Google Scholar] [CrossRef]
- Palmucci, J.; Mahmudov, K.T.; Guedes da Silva, M.F.C.; Marchetti, F.; Pettinari, C.; Petrelli, D.; Vitali, L.A.; Quassinti, L.; Bramucci, M.; Lupidi, G.; et al. DNA and BSA binding, anticancer and antimicrobial properties of Co(II), Co(II/III), Cu(II) and Ag(I) complexes of arylhydrazones of barbituric acid. RSC Adv. 2016, 6, 4237–4249. [Google Scholar] [CrossRef]
- Sadhu, M.H.; Kumar, S.B.; Saini, J.K.; Purani, S.S.; Khanna, T.R. Mononuclear copper(II) and binuclear cobalt(II) complexes with halides and tetradentate nitrogen coordinate ligand: Synthesis, structures and bioactivities. Inorg. Chim. Acta 2017, 466, 219–227. [Google Scholar] [CrossRef]
- Saha, M.; Biswas, J.K.; Mondal, M.; Ghosh, D.; Mandal, S.; Cordes, D.B.; Slawin, A.M.Z.; Mandal, T.K.; Saha, N.C. Synthesis, characterization and antimicrobial activities of Co(III) and Ni(II) complexes with 5-methyl-3-formylpyrazole-N(4)-dihexylthiosemicarbazone (HMPzNHex2): X-ray crystallography and DFT calculations of [Co(MPzNHex2)2]ClO4·1.5H2O (I) and [Ni(HMPzNHex2)2]Cl2·2H2O (II). Inorg. Chim. Acta 2018, 483, 271–283. [Google Scholar] [CrossRef]
- Saha, S.; Sasmal, A.; Roy Choudhury, C.; Pilet, G.; Bauzá, A.; Frontera, A.; Chakraborty, S.; Mitra, S. Synthesis, crystal structure, antimicrobial screening and density functional theory calculation of nickel(II), cobalt(II) and zinc(II) mononuclear Schiff base complexes. Inorg. Chim. Acta 2015, 425, 211–220. [Google Scholar] [CrossRef]
- Vasdev, R.A.; Preston, D.; Scottwell, S.O.; Brooks, H.J.; Crowley, J.D.; Schramm, M.P. Oxidatively Locked [Co(2)L(3)]6+ Cylinders Derived from Bis(bidentate) 2-Pyridyl-1,2,3-triazole “Click” Ligands: Synthesis, Stability, and Antimicrobial Studies. Molecules 2016, 21, 1548. [Google Scholar] [CrossRef]
- Woźniczka, M.; Świątek, M.; Pająk, M.; Gądek-Sobczyńska, J.; Chmiela, M.; Gonciarz, W.; Lisiecki, P.; Pasternak, B.; Kufelnicki, A. Complexes in aqueous cobalt(II)–2-picolinehydroxamic acid system: Formation equilibria, DNA-binding ability, antimicrobial and cytotoxic properties. J. Inorg. Biochem. 2018, 187, 62–72. [Google Scholar] [CrossRef]
- Lu, L.; Liu, L.J.; Chao, W.C.; Zhong, H.J.; Wang, M.; Chen, X.P.; Lu, J.J.; Li, R.N.; Ma, D.L.; Leung, C.H. Identification of an iridium(III) complex with anti-bacterial and anti-cancer activity. Sci. Rep. 2015, 5, e14544. [Google Scholar] [CrossRef]
- Fiorini, V.; Zanoni, I.; Zacchini, S.; Costa, A.L.; Hochkoeppler, A.; Zanotti, V.; Ranieri, A.M.; Massi, M.; Stefan, A.; Stagni, S. Methylation of Ir(III)-tetrazolato complexes: An effective route to modulate the emission outputs and to switch to antimicrobial properties. Dalton Trans. 2017, 46, 12328–12338. [Google Scholar] [CrossRef]
- Kumar, S.; Purcell, W.; Conradie, J.; Bragg, R.R.; Langner, E.H.G. Synthesis, characterization, computational and antimicrobial activities of a novel iridium thiourea complex. New J. Chem. 2017, 41, 10919–10928. [Google Scholar] [CrossRef]
- DuChane, C.M.; Brown, L.C.; Dozier, V.S.; Merola, J.S. Synthesis, Characterization, and Antimicrobial Activity of Rh(III) and Ir(III) β-Diketonato Piano-Stool Compounds. Organometallics 2018, 37, 530–538. [Google Scholar] [CrossRef]
- Lapasam, A.; Dkhar, L.; Joshi, N.; Poluri, K.M.; Kollipara, M.R. Antimicrobial selectivity of ruthenium, rhodium, and iridium half sandwich complexes containing phenyl hydrazone Schiff base ligands towards B. thuringiensis and P. aeruginosa bacteria. Inorg. Chim. Acta 2019, 484, 255–263. [Google Scholar] [CrossRef]
- Lapasam, A.; Banothu, V.; Uma, A.; Kollipara, M. Synthesis, structural and antimicrobial studies of half-sandwich ruthenium, rhodium and iridium complexes containing nitrogen donor Schiff-base ligands. J. Mol. Struct. 2019, 1191. [Google Scholar] [CrossRef]
- Liu, B.; Monro, S.; Jabed, M.A.; Cameron, C.G.; Colón, K.L.; Xu, W.; Kilina, S.; McFarland, S.A.; Sun, W. Neutral iridium(III) complexes bearing BODIPY-substituted N-heterocyclic carbene (NHC) ligands: Synthesis, photophysics, in vitro theranostic photodynamic therapy, and antimicrobial activity. Photochem. Photobiol. Sci. 2019, 18, 2381–2396. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Moat, J.; McFeely, D.; Clarkson, G.; Hands-Portman, I.J.; Furner-Pardoe, J.P.; Harrison, F.; Dowson, C.G.; Sadler, P.J. Biguanide Iridium(III) Complexes with Potent Antimicrobial Activity. J. Med. Chem. 2018, 61, 7330–7344. [Google Scholar] [CrossRef] [PubMed]
- DuChane, C.M.; Karpin, G.W.; Ehrich, M.; Falkinham, J.O.; Merola, J.S. Iridium piano stool complexes with activity against S. aureus and MRSA: It is past time to truly think outside of the box. Med. Chem. Commun. 2019, 10, 1391–1398. [Google Scholar] [CrossRef]
- Lapasam, A.; Banothu, V.; Addepally, U.; Kollipara, M.R. Half-sandwich arene ruthenium, rhodium and iridium thiosemicarbazone complexes: Synthesis, characterization and biological evaluation. J. Chem. Sci. 2020, 132, 34. [Google Scholar] [CrossRef]
- Raj, P.; Singh, A.; Singh, A.; Singh, N. Syntheses and Photophysical Properties of Schiff Base Ni(II) Complexes: Application for Sustainable Antibacterial Activity and Cytotoxicity. ACS Sustain. Chem. Eng. 2017, 5, 6070–6080. [Google Scholar] [CrossRef]
- Ibrahim, A.B.M.; Farh, M.K.; El-Gyar, S.A.; El-Gahami, M.A.; Fouad, D.M.; Silva, F.; Santos, I.C.; Paulo, A. Synthesis, structural studies and antimicrobial activities of manganese, nickel and copper complexes of two new tridentate 2-formylpyridine thiosemicarbazone ligands. Inorg. Chem. Commun. 2018, 96, 194–201. [Google Scholar] [CrossRef]
- Juribašić, M.; Molčanov, K.; Kojić-Prodić, B.; Bellotto, L.; Kralj, M.; Zani, F.; Tušek-Božić, L. Palladium(II) complexes of quinolinylaminophosphonates: Synthesis, structural characterization, antitumor and antimicrobial activity. J. Inorg. Biochem. 2011, 105, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, L.V.; Burgos, A.E.; Brandão, P.F.B. Synthesis, Characterization, and Antimicrobial Activity of the Ligand 3-Methylpyrazole- 4-Carboxaldehyde Thiosemicarbazone and Its Pd(II) Complex. Phosphorus Sulfur Relat. Elem. 2014, 189, 52–59. [Google Scholar] [CrossRef]
- Radić, G.P.; Glođović, V.V.; Radojević, I.D.; Stefanović, O.D.; Čomić, L.R.; Ratković, Z.R.; Valkonen, A.; Rissanen, K.; Trifunović, S.R. Synthesis, characterization and antimicrobial activity of palladium(II) complexes with some alkyl derivates of thiosalicylic acids: Crystal structure of the bis(S-benzyl-thiosalicylate)–palladium(II) complex, [Pd(S-bz-thiosal)2]. Polyhedron 2012, 31, 69–76. [Google Scholar] [CrossRef]
- Boubakri, L.; Mansour, L.; Harrath, A.H.; Özdemir, I.; Yaşar, S.; Hamdi, N. N-Heterocyclic carbene-Pd(II)-PPh3 complexes as a new highly efficient catalyst system for the Sonogashira cross-coupling reaction: Synthesis, characterization and biological activities. J. Coord. Chem. 2018, 71, 183–199. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Abdel-Rahman, L.H.; Abdel-Mawgoud, A.A.H. A robust in vitro Anticancer, Antioxidant and Antimicrobial Agents Based on New Metal-Azomethine Chelates Incorporating Ag(I), Pd(II) and VO(II) Cations: Probing the Aspects of DNA Interaction. Appl. Organomet. Chem. 2020, 34, e5373. [Google Scholar] [CrossRef]
- Nyawade, E.A.; Onani, M.O.; Meyer, S.; Dube, P. Synthesis, characterization and antibacterial activity studies of new 2-pyrral-L-amino acid Schiff base palladium (II) complexes. Chem. Pap. 2020, 1–11. [Google Scholar] [CrossRef]
- Solmaz, U.; Gumus, I.; Binzet, G.; Celik, O.; Balci, G.K.; Dogen, A.; Arslan, H. Synthesis, characterization, crystal structure, and antimicrobial studies of novel thiourea derivative ligands and their platinum complexes. J. Coord. Chem. 2018, 71, 200–218. [Google Scholar] [CrossRef]
- Gaber, M.; El-Ghamry, H.A.; Mansour, M.A. Pd(II) and Pt(II) chalcone complexes. Synthesis, spectral characterization, molecular modeling, biomolecular docking, antimicrobial and antitumor activities. J. Photochem. Photobiol. 2018, 354, 163–174. [Google Scholar] [CrossRef]
- Mansour, A.M. Antifungal activity, DNA and lysozyme binding affinity of Pd(II) and Pt(II) complexes bearing N,N-pyridylbenzimidazole ligand. J. Coord. Chem. 2018, 71, 3381–3391. [Google Scholar] [CrossRef]
- Lunagariya, M.V.; Thakor, K.P.; Varma, R.R.; Waghela, B.N.; Pathak, C.; Patel, M.N. Synthesis, characterization and biological application of 5-quinoline 1,3,5-trisubstituted pyrazole based platinum(II) complexes. Med. Chem. Commun. 2018, 9, 282–298. [Google Scholar] [CrossRef]
- Gao, S.; Yan, X.; Xie, G.; Zhu, M.; Ju, X.; Stang, P.J.; Tian, Y.; Niu, Z. Membrane intercalation-enhanced photodynamic inactivation of bacteria by a metallacycle and TAT-decorated virus coat protein. Proc. Natl. Acad. Sci. USA 2019, 116, 23437–23443. [Google Scholar] [CrossRef]
- Alam, M.N.; Yu, J.Q.; Beale, P.; Turner, P.; Proschogo, N.; Huq, F. Crystal Structure, Antitumour and Antibacterial Activity of Imidazo[1, 2–α]pyridine Ligand Containing Palladium Complexes. ChemistrySelect 2020, 5, 668–673. [Google Scholar] [CrossRef]
- Basto, A.P.; Muller, J.; Rubbiani, R.; Stibal, D.; Giannini, F.; Suss-Fink, G.; Balmer, V.; Hemphill, A.; Gasser, G.; Furrer, J. Characterization of the Activities of Dinuclear Thiolato-Bridged Arene Ruthenium Complexes against Toxoplasma gondii. Antimicrob. Agents Chemother. 2017, 61, e01031-17. [Google Scholar] [CrossRef] [PubMed]
- Bugarčić, Z.M.; Divac, V.M.; Kostić, M.D.; Janković, N.Ž.; Heinemann, F.W.; Radulović, N.S.; Stojanović-Radić, Z.Z. Synthesis, crystal and solution structures and antimicrobial screening of palladium(II) complexes with 2-(phenylselanylmethyl)oxolane and 2-(phenylselanylmethyl)oxane as ligands. J. Inorg. Biochem. 2015, 143, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Choo, K.B.; Mah, W.L.; Lee, S.M.; Lee, W.L.; Cheow, Y.L. Palladium complexes of bidentate pyridine N-heterocyclic carbenes: Optical resolution, antimicrobial and cytotoxicity studies. Appl. Organomet. Chem. 2018, 32, e4377. [Google Scholar] [CrossRef]
- Farkasová, V.; Drweesh, S.A.; Lüköová, A.; Sabolová, D.; Radojević, I.D.; Čomić, L.R.; Vasić, S.M.; Paulíková, H.; Fečko, S.; Balašková, T.; et al. Low-dimensional compounds containing bioactive ligands. Part VIII: DNA interaction, antimicrobial and antitumor activities of ionic 5,7-dihalo-8-quinolinolato palladium(II) complexes with K+ and Cs+ cations. J. Inorg. Biochem. 2017, 167, 80–88. [Google Scholar] [CrossRef]
- Gorle, A.K.; Li, X.; Primrose, S.; Li, F.; Feterl, M.; Kinobe, R.T.; Heimann, K.; Warner, J.M.; Keene, F.R.; Collins, J.G. Oligonuclear polypyridylruthenium(II) complexes: Selectivity between bacteria and eukaryotic cells. J. Antimicrob. Chemother. 2016, 71, 1547–1555. [Google Scholar] [CrossRef]
- Laurent, Q.; Batchelor, L.K.; Dyson, P.J. Applying a Trojan Horse Strategy to Ruthenium Complexes in the Pursuit of Novel Antibacterial Agents. Organometallics 2018, 37, 915–923. [Google Scholar] [CrossRef]
- Li, F.; Feterl, M.; Mulyana, Y.; Warner, J.M.; Collins, J.G.; Keene, F.R. In vitro susceptibility and cellular uptake for a new class of antimicrobial agents: Dinuclear ruthenium(II) complexes. J. Antimicrob. Chemother. 2012, 67, 2686–2695. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, B.; Kell, R.E.M.; Southam, H.M.; Butler, J.A.; Li, X.; Poole, R.K.; Keene, F.R.; Collins, J.G. The Antimicrobial Activity of Mononuclear Ruthenium(II) Complexes Containing the dppz Ligand. ChemPlusChem 2018, 83, 643–650. [Google Scholar] [CrossRef]
- Onar, G.; Gürses, C.; Karataş, M.O.; Balcıoğlu, S.; Akbay, N.; Özdemir, N.; Ateş, B.; Alıcı, B. Palladium(II) and ruthenium(II) complexes of benzotriazole functionalized N-heterocyclic carbenes: Cytotoxicity, antimicrobial, and DNA interaction studies. J. Organomet. Chem. 2019, 886, 48–56. [Google Scholar] [CrossRef]
- Pahontu, E.; Paraschivescu, C.; Ilies, D.C.; Poirier, D.; Oprean, C.; Paunescu, V.; Gulea, A.; Rosu, T.; Bratu, O. Synthesis and Characterization of Novel Cu(II), Pd(II) and Pt(II) Complexes with 8-Ethyl-2-hydroxytricyclo(7.3.1.0(2,7))tridecan-13-one-thiosemicarbazone: Antimicrobial and in Vitro Antiproliferative Activity. Molecules 2016, 21, 674. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.C.; Saha Roy, A.; Banerjee, S.; Banerjee, A.; Das Saha, K.; Bhadra, R.; Pramanik, K.; Ghosh, P. Palladium(II) and platinum(II) complexes of glyoxalbis(N-aryl)osazone: Molecular and electronic structures, anti-microbial activities and DNA-binding study. New J. Chem. 2019, 43, 9891–9901. [Google Scholar] [CrossRef]
- Satheesh, C.E.; Raghavendra Kumar, P.; Sharma, P.; Lingaraju, K.; Palakshamurthy, B.S.; Raja Naika, H. Synthesis, characterisation and antimicrobial activity of new palladium and nickel complexes containing Schiff bases. Inorg. Chim. Acta 2016, 442, 1–9. [Google Scholar] [CrossRef]
- Southam, H.M.; Butler, J.A.; Chapman, J.A.; Poole, R.K. Chapter One—The Microbiology of Ruthenium Complexes. Adv. Microb. Physiol. 2017, 71, 1–96. [Google Scholar]
- Turel, I.; Kljun, J.; Perdih, F.; Morozova, E.; Bakulev, V.; Kasyanenko, N.; Byl, J.A.; Osheroff, N. First ruthenium organometallic complex of antibacterial agent ofloxacin. Crystal structure and interactions with DNA. Inorg. Chem. 2010, 49, 10750–10752. [Google Scholar] [CrossRef]
- Yang, Y.; Liao, G.; Fu, C. Recent Advances on Octahedral Polypyridyl Ruthenium(II) Complexes as Antimicrobial Agents. Polymers 2018, 10, 650. [Google Scholar] [CrossRef]
- Jević, V.V.; Radić, G.P.; Stefanović, O.D.; Radojević, I.D.; Vasić, S.; Čomić, L.R.; Đinović, V.M.; Trifunović, S.R. Part XXIII. Synthesis and characterization of platinum(IV) complexes with O,O′-dialkyl esters of (S,S)-ethylenediamine-N,N′-di-2-(3-methyl)butanoic acid and bromido ligands. Antimicrobial, antibiofilm and antioxidant screening. Inorg. Chim. Acta 2016, 442, 105–110. [Google Scholar] [CrossRef]
- Dkhar, L.; Banothu, V.; Kaminsky, W.; Kollipara, M.R. Synthesis of half sandwich platinum group metal complexes containing pyridyl benzothiazole hydrazones: Study of bonding modes and antimicrobial activity. J. Organomet. Chem. 2020, 914, 121225. [Google Scholar] [CrossRef]
- Sankarganesh, M.; Vijay Solomon, R.; Dhaveethu Raja, J. Platinum complex with pyrimidine- and morpholine-based ligand: Synthesis, spectroscopic, DFT, TDDFT, catalytic reduction, in vitro anticancer, antioxidant, antimicrobial, DNA binding and molecular modeling studies. J. Biomol. Struct. Dyn. 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Choo, K.B.; Lee, S.M.; Lee, W.L.; Cheow, Y.L. Synthesis, characterization, in vitro antimicrobial and anticancer studies of new platinum N-heterocyclic carbene (NHC) complexes and unexpected nickel complexes. J. Organomet. Chem. 2019, 898, 120868. [Google Scholar] [CrossRef]
- Musa, T.M.; Al-jibouri, M.N.; Al-bayati, R.I.H. Synthesis, Characterization and Antimicrobial Study of nickel(II), palladium(II), platinum(II), rhodium(III), cadmium(II) and zirconium(IV) complexes with (E)-1-(benzo[d]thiazol-2-yl)-4-(hydroxy(2-hydroxyphenyl)methylene)-3-methyl-1H-pyrazol-5(4H)-one. J. Phys. Conf. Ser. 2018, 1032, 012057. [Google Scholar] [CrossRef]
- Bobinihi, F.F.; Onwudiwe, D.C.; Ekennia, A.C.; Okpareke, O.C.; Arderne, C.; Lane, J.R. Group 10 metal complexes of dithiocarbamates derived from primary anilines: Synthesis, characterization, computational and antimicrobial studies. Polyhedron 2019, 158, 296–310. [Google Scholar] [CrossRef]
- Stojković, D.L.; Jevtić, V.V.; Radić, G.P.; Đukić, M.B.; Jelić, R.M.; Zarić, M.M.; Anđelković, M.V.; Mišić, M.S.; Baskić, D.D.; Trifunović, S.R. Stereospecific ligands and their complexes. XXIV. Synthesis, characterization and some biological properties of Pd(II) and Pt(II) complexes with R2-S,S-eddtyr. New J. Chem. 2018, 42, 3924–3935. [Google Scholar] [CrossRef]
- Ahmad, S.; Seerat ur, R.; Rüffer, T.; Khalid, T.; Isab, A.A.; Al-Arfaj, A.R.; Saleem, M.; Khan, I.U.; Choudhary, M.A. Crystal structure and antimicrobial activity of a transplatin adduct of N,N′-dimethylthiourea, trans-[Pt(NH3)2(dmtu)2]Cl2. Monatsh. Chem. 2017, 148, 669–674. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Ekennia, A.C.; Mogwase, B.M.S.; Olubiyi, O.O.; Hosten, E. Palladium(II) and platinum(II) complexes of N-butyl-N-phenyldithiocarbamate: Synthesis, characterization, biological activities and molecular docking studies. Inorg. Chim. Acta 2016, 450, 69–80. [Google Scholar] [CrossRef]
- Kaushal, M.; Lobana, T.S.; Nim, L.; Bala, R.; Arora, D.S.; Garcia-Santos, I.; Duff, C.E.; Jasinski, J.P. Synthesis of 2-acetylpyridine-N-substituted thiosemicarbazonates of copper(II) with high antimicrobial activity against methicillin resistant S. aureus, K. pneumoniae 1 and C. albicans. New J. Chem. 2019, 43, 11727–11742. [Google Scholar] [CrossRef]
- Oladipo, S.D.; Omondi, B.; Mocktar, C. Synthesis and structural studies of nickel(II)- and copper(II)-N,N′-diarylformamidine dithiocarbamate complexes as antimicrobial and antioxidant agents. Polyhedron 2019, 170, 712–722. [Google Scholar] [CrossRef]
- Krishnegowda, H.M.; Karthik, C.S.; Marichannegowda, M.H.; Kumara, K.; Kudigana, P.J.; Lingappa, M.; Mallu, P.; Neratur, L.K. Synthesis and structural studies of 1-phenyl-1,3-butanedione copper(II) complexes as an excellent antimicrobial agent against methicillin-resistant Staphylococcus aureus. Inorg. Chim. Acta 2019, 484, 227–236. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Gold compounds in medicine: Potential anti-tumour agents. Gold Bull. 2003, 36, 117–124. [Google Scholar] [CrossRef]
- Glišić, B.Đ.; Djuran, M.I. Gold complexes as antimicrobial agents: An overview of different biological activities in relation to the oxidation state of the gold ion and the ligand structure. Dalton Trans. 2014, 43, 5950–5969. [Google Scholar] [CrossRef] [PubMed]
- Savić, N.D.; Milivojevic, D.R.; Glišić, B.Đ.; Ilic-Tomic, T.; Veselinovic, J.; Pavic, A.; Vasiljevic, B.; Nikodinovic-Runic, J.; Djuran, M.I. A comparative antimicrobial and toxicological study of gold(III) and silver(I) complexes with aromatic nitrogen-containing heterocycles: Synergistic activity and improved selectivity index of Au(III)/Ag(I) complexes mixture. RSC Adv. 2016, 6, 13193–13206. [Google Scholar] [CrossRef][Green Version]
- Hikisz, P.; Szczupak, L.; Koceva-Chyla, A.; Gu Spiel, A.; Oehninger, L.; Ott, I.; Therrien, B.; Solecka, J.; Kowalski, K. Anticancer and Antibacterial Activity Studies of Gold(I)-Alkynyl Chromones. Molecules 2015, 20, 19699–19718. [Google Scholar] [CrossRef]
- Schmidt, C.; Karge, B.; Misgeld, R.; Prokop, A.; Brönstrup, M.; Ott, I. Biscarbene gold(I) complexes: Structure–activity-relationships regarding antibacterial effects, cytotoxicity, TrxR inhibition and cellular bioavailability. Med. Chem. Commun. 2017, 8, 1681–1689. [Google Scholar] [CrossRef]
- Pöthig, A.; Ahmed, S.; Winther-Larsen, H.C.; Guan, S.; Altmann, P.J.; Kudermann, J.; Santos Andresen, A.M.; Gjøen, T.; Høgmoen Åstrand, O.A. Antimicrobial Activity and Cytotoxicity of Ag(I) and Au(I) Pillarplexes. Front. Chem. 2018, 6, 584. [Google Scholar] [CrossRef]
- Crichton, R. Biological Inorganic Chemistry, 3rd ed.; Elsevier: London, UK, 2019; pp. 339–362. ISBN 978-0-12-811741-5. [Google Scholar]
- Cuevas, L.; Koyanagi, A. Zinc and infection: A review. Ann. Trop. Paediatr. 2005, 25, 149–160. [Google Scholar] [CrossRef]
- Harrison, P.M.; Hoare, R.J. Metals in Biochemistry; Chapman and Hall: London, UK; New York, NY, USA, 1980. [Google Scholar]
- Osredkar, J. Copper and Zinc, Biological Role and Significance of Copper/Zinc Imbalance. J. Clin. Toxicol. 2011, 3, 495. [Google Scholar] [CrossRef]
- Zelenak, V.; Gyoryova, K.; Mlynarcik, D. Antibacterial and Antifungal Activity of Zinc(II) Carboxylates with/without N-Donor Organic LIigands. Met. Based Drugs 2002, 8, 269–274. [Google Scholar] [CrossRef]
- Zaltariov, M.-F.; Cazacu, M.; Avadanei, M.; Shova, S.; Balan, M.; Vornicu, N.; Vlad, A.; Dobrov, A.; Varganici, C.-D. Synthesis, characterization and antimicrobial activity of new Cu(II) and Zn(II) complexes with Schiff bases derived from trimethylsilyl-propyl-p-aminobenzoate. Polyhedron 2015, 100, 121–131. [Google Scholar] [CrossRef]
- Abu Ali, H.; Omar, S.N.; Darawsheh, M.D.; Fares, H. Synthesis, characterization and antimicrobial activity of zinc(II) ibuprofen complexes with nitrogen-based ligands. J. Coord. Chem. 2016, 69, 1110–1122. [Google Scholar] [CrossRef]
- Boughougal, A.; Cherchali, F.Z.; Messai, A.; Attik, N.; Decoret, D.; Hologne, M.; Sanglar, C.; Pilet, G.; Tommasino, J.B.; Luneau, D. New model of metalloantibiotic: Synthesis, structure and biological activity of a zinc(II) mononuclear complex carrying two enrofloxacin and sulfadiazine antibiotics. N. J. Chem. 2018, 42, 15346–15352. [Google Scholar] [CrossRef]
- Basu Baul, T.; Nongsiej, K.; Ka-Ot, A.; Joshi, S.R.; León, I.; Höpfl, H. Tweaking the affinity of aryl-substituted diazosalicylato- and pyridine ligands towards Zn(II) and its neighbors in the periodic system of the elements, Cu(II) and Cd(II), and their antimicrobial activity. Appl. Organomet. Chem. 2019, 33, e4905. [Google Scholar] [CrossRef]
- Staneva, D.; Vasileva-Tonkova, E.; Grabchev, I. A New Bioactive Complex between Zn(II) and a Fluorescent Symmetrical Benzanthrone Tripod for an Antibacterial Textile. Materials 2019, 12, 3473. [Google Scholar] [CrossRef]
- Bahojb Noruzi, E.; Shaabani, B.; Geremia, S.; Hickey, N.; Nitti, P.; Kafil, H.S. Synthesis, Crystal Structure, and Biological Activity of a Multidentate Calix[4]arene Ligand Doubly Functionalized by 2-Hydroxybenzeledene-Thiosemicarbazone. Molecules 2020, 25, 370. [Google Scholar] [CrossRef]
- Montazerozohori, M.; Nasr-Esfahani, M.; Hoseinpour, M.; Naghiha, A.; Zahedi, S. Synthesis of some new antibacterial active cadmium and mercury complexes of 4-(3-(2-(4-(dimethyl aminophenyl allylidene aminopropyl-imino)prop-1-ethyl)-N,N-dimethyl benzene amine. Chem. Spec. Bioavailab. 2014, 26, 240–248. [Google Scholar] [CrossRef]
- Agertt, V.A.; Bonez, P.C.; Rossi, G.G.; Flores, V.d.C.; Siqueira, F.d.S.; Mizdal, C.R.; Marques, L.L.; de Oliveira, G.N.M.; de Campos, M.M.A. Identification of antimicrobial activity among new sulfonamide metal complexes for combating rapidly growing mycobacteria. BioMetals 2016, 29, 807–816. [Google Scholar] [CrossRef]
- Matiadis, D.; Tsironis, D.; Stefanou, V.; Elliott, A.G.; Kordatos, K.; Zahariou, G.; Ioannidis, N.; McKee, V.; Panagiotopoulou, A.; Igglessi-Markopoulou, O.; et al. Synthesis, characterization and antimicrobial activity of N-acetyl-3-acetyl-5-benzylidene tetramic acid-metal complexes. X-ray analysis and identification of the Cd(II) complex as a potent antifungal agent. J. Inorg. Biochem. 2019, 194, 65–73. [Google Scholar] [CrossRef]
- Mandal, S.; Mondal, M.; Biswas, J.K.; Cordes, D.B.; Slawin, A.M.Z.; Butcher, R.J.; Saha, M.; Chandra Saha, N. Synthesis, characterization and antimicrobial activity of some nickel, cadmium and mercury complexes of 5-methyl pyrazole-3yl-N-(2′-methylthiophenyl) methyleneimine, (MPzOATA) ligand. J. Mol. Struct. 2018, 1152, 189–198. [Google Scholar] [CrossRef]
- Mandal, S.; Das, M.; Das, P.; Samanta, A.; Butcher, R.J.; Saha, M.; Alswaidan, I.A.; Rhyman, L.; Ramasami, P.; Saha, N.C. Synthesis, characterization, DFT and antimicrobial studies of transition metal ion complexes of a new schiff base ligand, 5-methylpyrazole-3yl-N-(2′-hydroxyphenylamine)methyleneimine, (MPzOAP). J. Mol. Struct. 2019, 1178, 100–111. [Google Scholar] [CrossRef]
- Lam, P.L.; Lu, G.L.; Choi, K.H.; Lin, Z.; Kok, S.H.L.; Lee, K.K.H.; Lam, K.H.; Li, H.; Gambari, R.; Bian, Z.X.; et al. Antimicrobial and toxicological evaluations of binuclear mercury(II) bis(alkynyl) complexes containing oligothiophenes and bithiazoles. RSC Adv. 2016, 6, 16736–16744. [Google Scholar] [CrossRef]
- Wang, H.; Qian, X.; Wang, K.; Su, M.; Haoyang, W.-W.; Jiang, X.; Brzozowski, R.; Wang, M.; Gao, X.; Li, Y.; et al. Supramolecular Kandinsky circles with high antibacterial activity. Nat. Commun. 2018, 9, 1815. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasiri Sovari, S.; Zobi, F. Recent Studies on the Antimicrobial Activity of Transition Metal Complexes of Groups 6–12. Chemistry 2020, 2, 418-452. https://doi.org/10.3390/chemistry2020026
Nasiri Sovari S, Zobi F. Recent Studies on the Antimicrobial Activity of Transition Metal Complexes of Groups 6–12. Chemistry. 2020; 2(2):418-452. https://doi.org/10.3390/chemistry2020026
Chicago/Turabian StyleNasiri Sovari, Sara, and Fabio Zobi. 2020. "Recent Studies on the Antimicrobial Activity of Transition Metal Complexes of Groups 6–12" Chemistry 2, no. 2: 418-452. https://doi.org/10.3390/chemistry2020026
APA StyleNasiri Sovari, S., & Zobi, F. (2020). Recent Studies on the Antimicrobial Activity of Transition Metal Complexes of Groups 6–12. Chemistry, 2(2), 418-452. https://doi.org/10.3390/chemistry2020026





