Amino Acids and Peptides as Versatile Ligands in the Synthesis of Antiproliferative Gold Complexes †
Abstract
:1. Introduction
2. Gold(I) Complexes Containing Amino Acids and Peptides Moieties
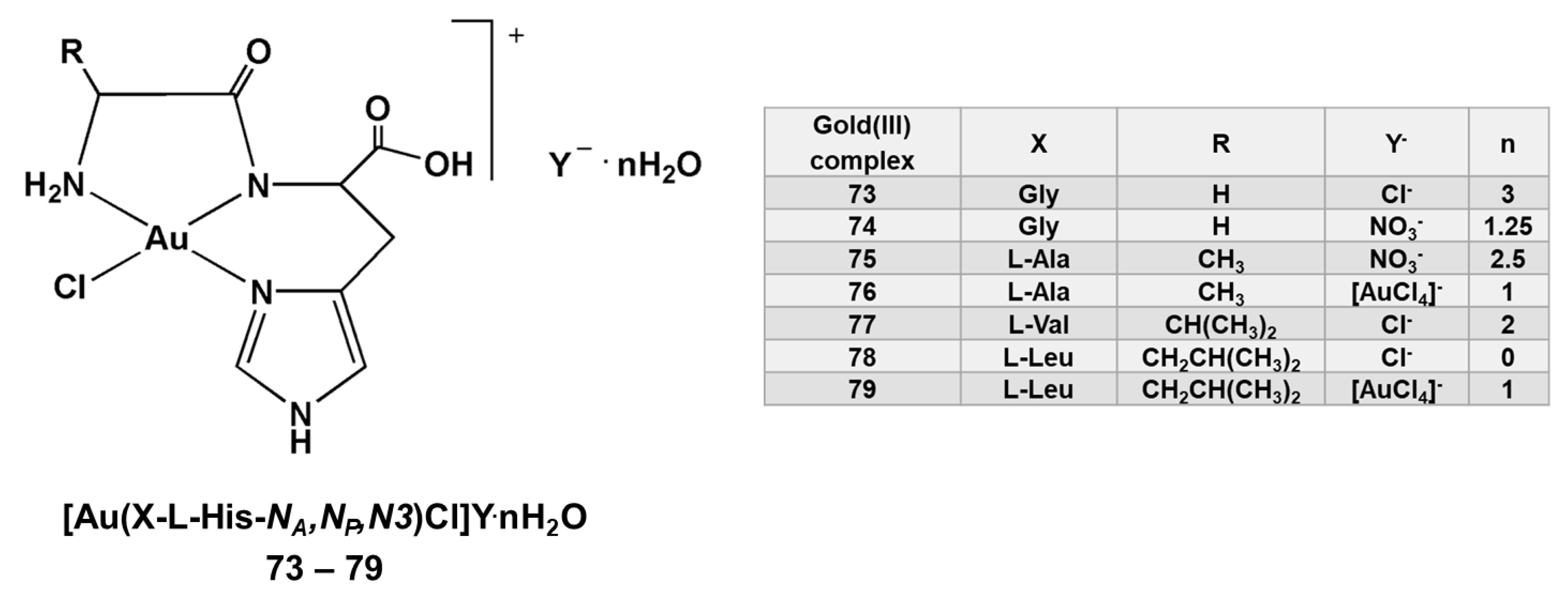
3. Gold(III) Complexes Containing Amino Acids and Peptides Moieties
4. Conclusions
Funding
Conflicts of Interest
References
- Huaizhi, Z.; Yuantao, N. China’s ancient gold drugs. Gold Bull. 2001, 34, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Benedek, T.G. The history of gold therapy for tuberculosis. J. Hist. Med. Allied Sci. 2004, 59, 50–89. [Google Scholar] [CrossRef] [PubMed]
- Fricker, S.P. Medical uses of gold compounds: Past, present and future. Gold Bull. 1996, 29, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Ott, I. On the medicinal chemistry of gold complexes as anticancer drugs. Coord. Chem. Rev. 2009, 253, 1670–1681. [Google Scholar] [CrossRef]
- Nobili, S.; Mini, E.; Landini, I.; Gabbiani, C.; Casini, A.; Messori, L. Gold compounds as anticancer agents: Chemistry, cellular pharmacology, and preclinical studies. Med. Res. Rev. 2010, 30, 550–580. [Google Scholar] [CrossRef] [PubMed]
- Berners-Price, S.J.; Filipovska, A. Gold compounds as therapeutic agents for human diseases. Metallomics 2011, 3, 863–873. [Google Scholar] [CrossRef]
- Bertrand, B.; Casini, A. A golden future in medicinal inorganic chemistry: The promise of anticancer gold organometallic compounds. Dalton Trans. 2014, 43, 4209–4219. [Google Scholar] [CrossRef]
- Nardon, C.; Boscutti, G.; Fregona, D. Beyond platinums: Gold complexes as anticancer agents. Anticancer Res. 2014, 34, 487–492. [Google Scholar]
- Lima, J.C.; Rodríguez, L. Phosphine-Gold(I) compounds as anticancer agents: General description and mechanisms of action. Anti-Cancer Agents Med. Chem. 2011, 11, 921–928. [Google Scholar] [CrossRef]
- Milacic, V.; Fregona, D.; Dou, Q.P. Gold complexes as prospective metal-based anticancer drugs. Histol. Histopathol. 2008, 23, 101–108. [Google Scholar]
- Kostova, I. Gold coordination complexes as anticancer agents. Anti-Cancer Agents Med. Chem. 2006, 6, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.I.; Ooi, K.K.; Tiekink, E.R.T. Gold-based medicine: A paradigm shift in anti-cancer therapy? Molecules 2018, 23, 1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora, M.; Gimeno, M.C.; Visbal, R. Recent advances in Gold–NHC complexes with biological properties. Chem. Soc. Rev. 2019, 48, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Glišić, B.Đ.; Djuran, M.I. Gold complexes as antimicrobial agents: An overview of different biological activities in relation to the oxidation state of the gold ion and the ligand structure. Dalton Trans. 2014, 43, 5950–5969. [Google Scholar] [CrossRef]
- Navarro, M.; Gabbiani, C.; Messori, L.; Gambino, D. Metal-based drugs for malaria, trypanosomiasis and leishmaniasis: Recent achievements and perspectives. Drug Discov. Today 2010, 15, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Kastin, A.J. (Ed.) Handbook of Biologically Active Peptides; Elsevier: Amsterdam, The Netherland, 2006. [Google Scholar]
- Peacock, A.F.A.; Bullen, G.A.; Gethings, L.A.; Williams, J.P.; Kriel, F.H.; Coates, J. Gold-phosphine binding to De Novo designed coiled coil peptides. J. Inorg. Biochem. 2012, 117, 298–305. [Google Scholar] [CrossRef]
- Astruc, D. Why is ferrocene so exceptional? Eur. J. Inorg. Chem. 2017, 2017, 6–29. [Google Scholar] [CrossRef]
- Gimeno, M.C.; Goitia, H.; Laguna, A.; Luque, M.E.; Villacampa, M.D.; Sepúlveda, C.; Meireles, M. Conjugates of ferrocene with biological compounds. Coordination to gold complexes and antitumoral properties. J. Inorg. Biochem. 2011, 105, 1373–1382. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Bernal, J.; Villacampa, M.D.; Cativiela, C.; Laguna, A.; Gimeno, M.C. Synthesis of new gold(I) thiolates containing amino acid moieties with potential biological interest. Inorg. Chem. 2013, 52, 6473–6480. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Gracia-Fleta, L.; Marzo, I.; Cativiela, C.; Laguna, A.; Gimeno, M.C. Gold(I) thiolates containing amino acid moieties. Cytotoxicity and structure–activity relationship studies. Dalton Trans. 2014, 43, 17054–17066. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.; Marzo, I.; Cativiela, C.; Laguna, A.; Gimeno, M.C. Highly cytotoxic bioconjugated gold(I) complexes with cysteine—containing dipeptides. Chem. Eur. J. 2015, 21, 11088–11095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, A.; Cativiela, C.; Laguna, A.; Gimeno, M.C. Bioactive gold(I) complexes with 4-mercaptoproline derivatives. Dalton Trans. 2016, 45, 13483–13490. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.; Gimeno, M.C.; Marzo, I.; Metzler-Nolte, N. Synthesis, characterization, and cytotoxic activity of AuI N,S-heterocyclic carbenes derived from peptides containing L-thiazolylalanine. Eur. J. Inorg. Chem. 2014, 2014, 2512–2519. [Google Scholar] [CrossRef]
- Lemke, J.; Pinto, A.; Niehoff, P.; Vasylyeva, V.; Metzler-Nolte, N. Synthesis, structural characterisation and anti-proliferative activity of NHC gold amino acid and peptide conjugates. Dalton Trans. 2009, 7063–7070. [Google Scholar] [CrossRef]
- Kouodom, M.N.; Ronconi, L.; Celegato, M.; Nardon, C.; Marchiò, L.; Dou, Q.P.; Aldinucci, D.; Formaggio, F.; Fregona, D. Toward the selective delivery of chemotherapeutics into tumor cells by targeting peptide transporters: Tailored gold-based anticancer peptidomimetics. J. Med. Chem. 2012, 55, 2212–2226. [Google Scholar] [CrossRef]
- Brown, K.L.; Hancock, R.E.W. Cationic host defense (antimicrobial) peptides. Curr. Opin. Immunol. 2006, 18, 24–30. [Google Scholar] [CrossRef]
- Johnstone, S.A.; Gelmon, K.; Mayer, L.D.; Hancock, R.E.; Bally, M.B. In vitro characterization of the anticancer activity of membrane-active cationic peptides. I. Peptide-mediated cytotoxicity and peptide-enhanced cytotoxic activity of doxorubicin against wild-type and p-glycoprotein over-expressing tumor cell lines. Anti-Cancer Drug Des. 2000, 15, 151–160. [Google Scholar]
- Toniolo, C.; Brückner, H. Peptaibiotics; Wiley-VCD: Weinheim, Germany; Wiley-VHCA: Zürich, Switzerland, 2009. [Google Scholar]
- Kouodom, M.N.; Boscutti, G.; Celegato, M.; Crisma, M.; Sitran, S.; Aldinucci, D.; Formaggio, F.; Ronconi, L.; Fregona, D. Rational design of gold(III)-dithiocarbamato peptidomimetics for the targeted anticancer chemotherapy. J. Inorg. Biochem. 2012, 117, 248–260. [Google Scholar] [CrossRef]
- Carotti, S.; Marcon, G.; Marussich, M.; Mazzei, T.; Messori, L.; Mini, E.; Orioli, P. Cytotoxicity and DNA binding properties of a chloro glycylhistidinate gold(III) complex (GHAu). Chem. Biol. Interact. 2000, 125, 29–38. [Google Scholar] [CrossRef]
- Glišić, B.Đ.; Stanić, Z.D.; Rajković, S.; Kojić, V.; Bogdanović, G.; Djuran, M.I. Solution study under physiological conditions and cytotoxic activity of the gold(III) complexes with L-histidine-containing peptides. J. Serb. Chem. Soc. 2013, 78, 1911–1924. [Google Scholar] [CrossRef]
- Warżajtis, B.; Glišić, B.Đ.; Savić, N.D.; Pavic, A.; Vojnovic, S.; Veselinović, A.; Nikodinovic-Runic, J.; Rychlewska, U.; Djuran, M.I. Mononuclear gold(III) complexes with L-histidine-containing dipeptides: Tuning the structural and biological properties by variation of the N-terminal amino acid and counter anion. Dalton Trans. 2017, 46, 2594–2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radulović, N.S.; Stojanović, N.M.; Glišić, B.Đ.; Randjelović, P.J.; Stojanović-Radić, Z.Z.; Mitić, K.V.; Nikolić, M.G.; Djuran, M.I. Water-soluble gold(III) complexes with N-donor ligands as potential immunomodulatory and antibiofilm agents. Polyhedron 2018, 141, 164–180. [Google Scholar] [CrossRef]
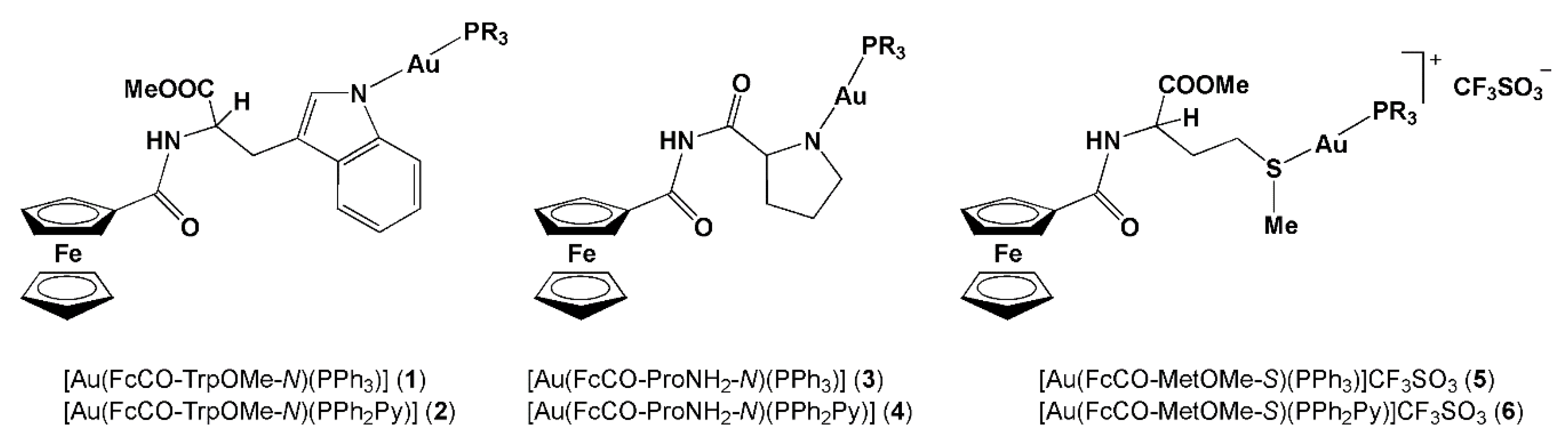

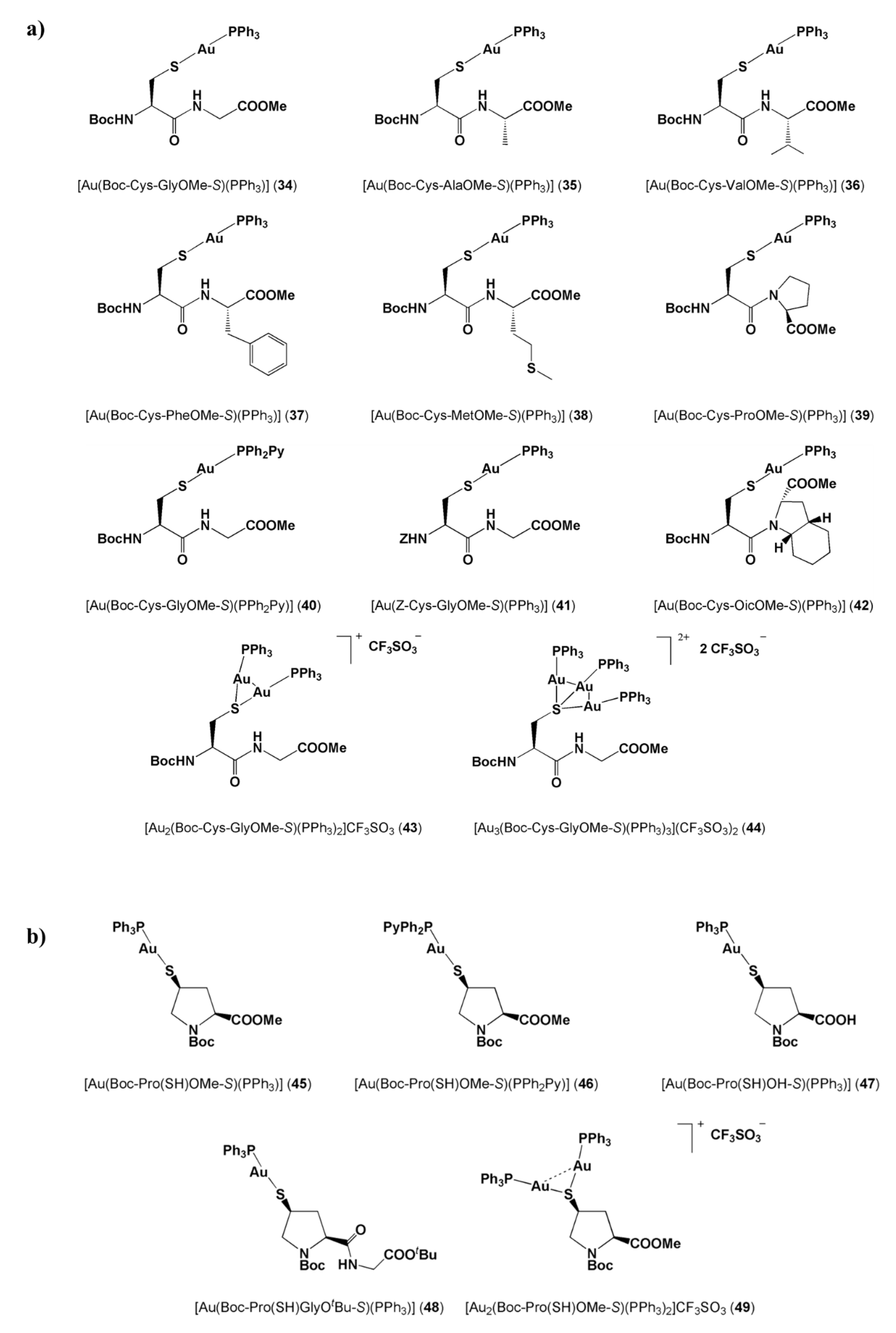

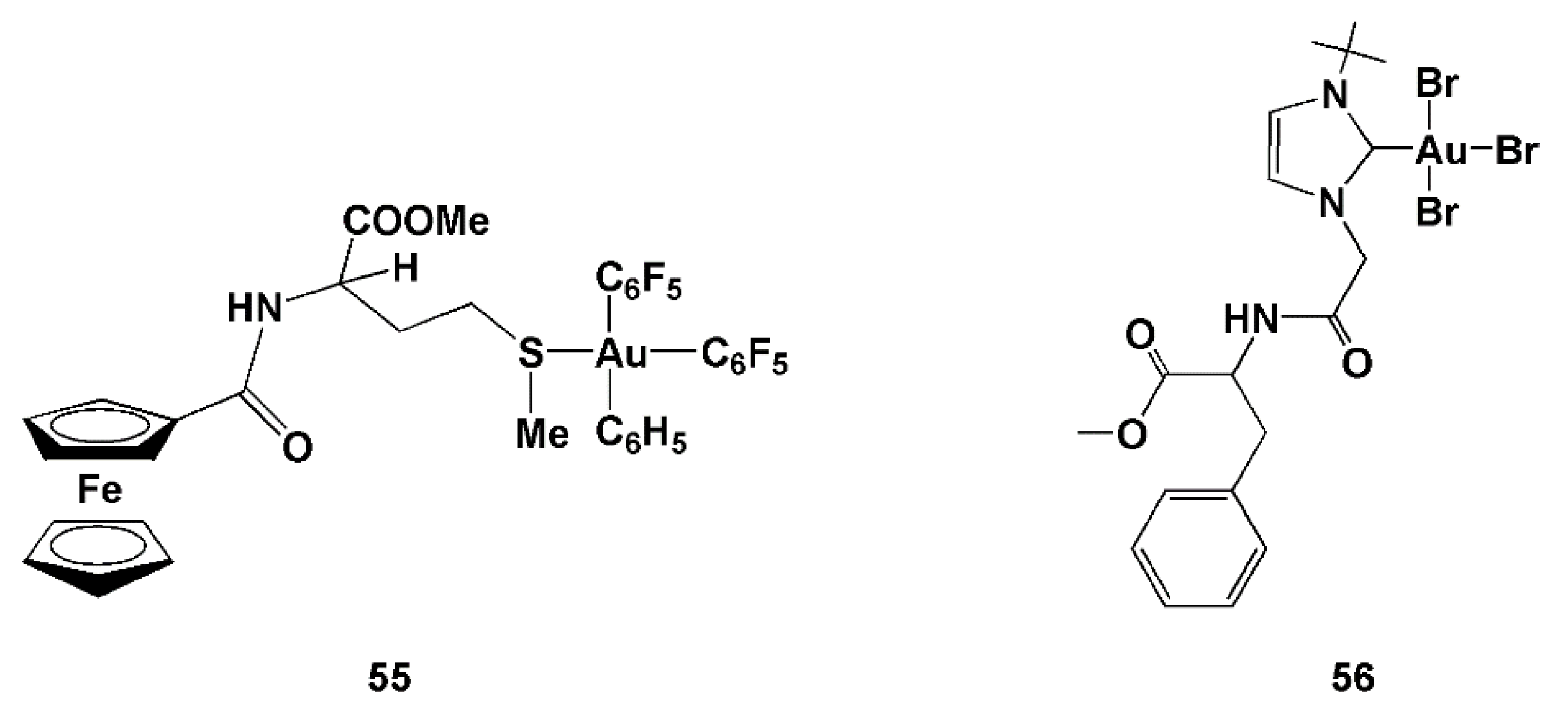
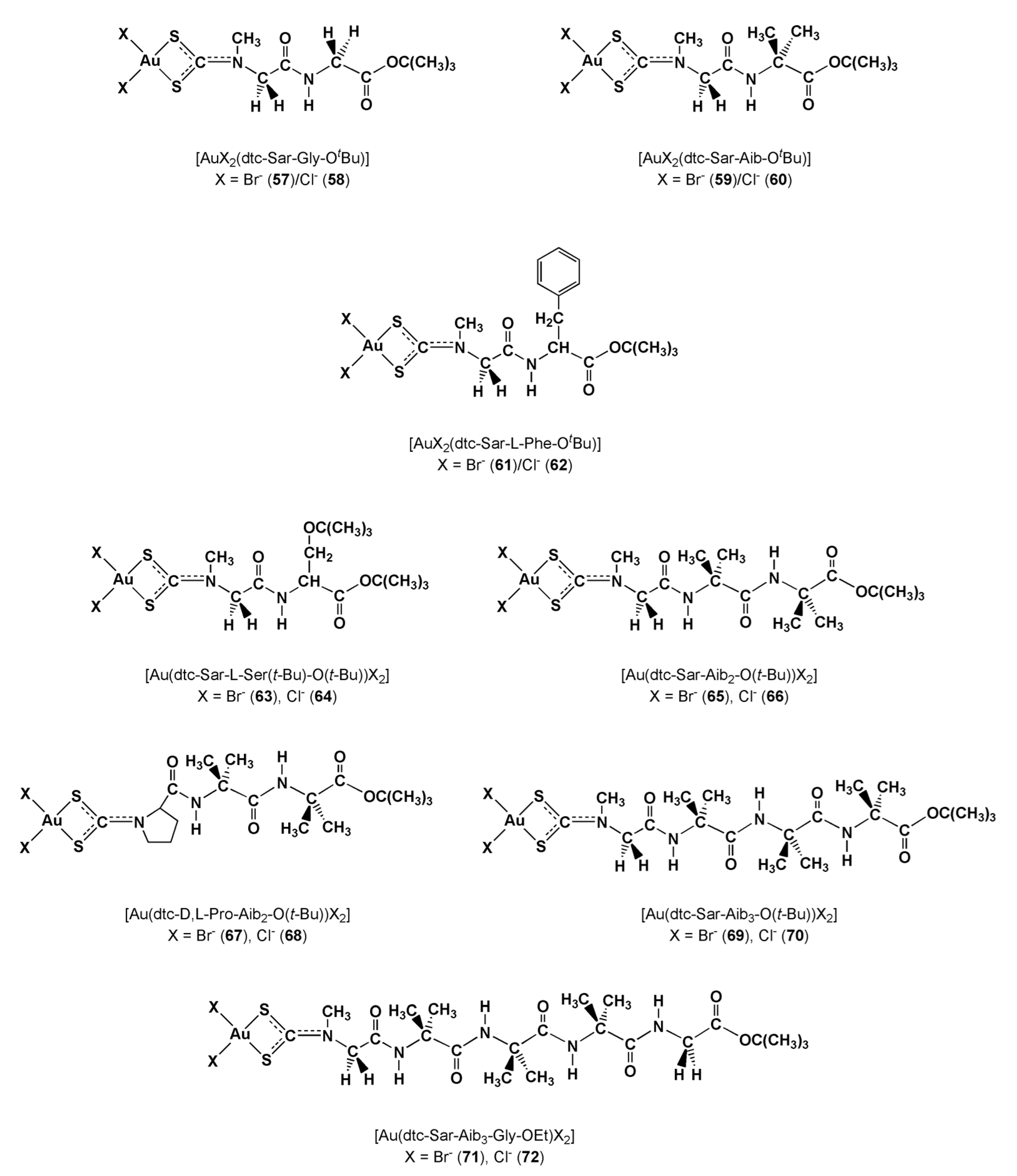

| Cell Line a | HeLa | MCF-7 | N1E-115 | |
|---|---|---|---|---|
| Complex | ||||
| 1 | 32 ± 1.8 | 15 ± 1.2 | 27 ± 2.2 | |
| 2 | 22 ± 1.9 | 45 ± 1.7 | 26 ± 1.6 | |
| 3 | 28 ± 1.6 | 52 ± 1.2 | 54 ± 2.3 | |
| 4 | 29 ± 1.3 | 32 ± 2.5 | ˂10 ± 2.2 | |
| 5 | 18 ± 1.4 | 15 ± 1.8 | 29 ± 1.7 | |
| Cell Line a | A549 | MiaPaca2 | Jurkat | 293T | R69 | |
|---|---|---|---|---|---|---|
| Complex | ||||||
| [Au(SPyCOOH)(PPh3)] | 15.5 ± 0.92 | 9.2 ± 0.28 | 4.6 ± 0.08 | 4.6 ± 0.13 | 16 ± 0.64 | |
| [Au(SPyCOOH)(PR3)], R = methyl ester of amino acid | ||||||
| 7 | 11.5 ± 0.55 | 9.7 ± 0.22 | 3.8 ± 0.07 | 4.2 ± 0.08 | 8.6 ± 0.33 | |
| 8 | 13.7 ± 0.71 | 11.0 ± 0.20 | 4.0 ± 0.07 | 2.7 ± 0.07 | 2.2 ± 0.08 | |
| 9 | 10.9 ± 0.40 | 10.2 ± 0.25 | 3.3 ± 0.05 | 10.7 ± 0.31 | 19.0 ± 0.60 | |
| 10 | 8.9 ± 0.36 | 12.3 ± 0.37 | 4.0 ± 0.08 | 5.5 ± 0.16 | 14.0 ± 0.59 | |
| 11 | 8.2 ± 0.41 | 12.8 ± 0.32 | 4.1 ± 0.06 | 3.7 ± 0.07 | 9.6 ± 0.36 | |
| 12 | 7.4 ± 0.34 | 9.4 ± 0.19 | 2.4 ± 0.04 | 10.0 ± 0.19 | 4.0 ± 0.13 | |
| [Au(SPyCOOH)(PR3)], R = amino acid | ||||||
| 13 | 14.7 ± 0.88 | 8.2 ± 0.13 | 7.6 ± 0.11 | 35.2 ± 0.53 | 25.9 ± 1.04 | |
| 14 | 7.7 ± 0.22 | 10.7 ± 0.16 | 3.7 ± 0.06 | 12.8 ± 0.27 | 6.1 ± 0.18 | |
| 15 | 14.7 ± 0.20 | 12.3 ± 0.32 | 4.3 ± 0.08 | 11.3 ± 0.29 | 6.5 ± 0.25 | |
| 16 | 15.9 ± 0.50 | 11.5 ± 0.29 | 6.7 ± 0.13 | 65.5 ± 1.12 | 33.1 ± 1.13 | |
| 17 | 14.1 ± 0.55 | 14.5 ± 0.26 | 3.6 ± 0.06 | 49.3 ± 1.53 | 28.4 ± 1.16 | |
| 18 | 14.3 ± 0.61 | 11.6 ± 0.20 | 7.5 ± 0.07 | 3.0 ± 0.08 | 4.0 ± 0.11 | |
| [Au(SPyCOOH)(PR3)], R = amide derivative of amino acid | ||||||
| 19 | 28.3 ± 1.02 | 27.2 ± 0.65 | 3.9 ± 0.06 | 14.4 ± 0.42 | 4.9 ± 0.16 | |
| 20 | 19.1 ± 0.67 | 8.1 ± 0.23 | 3.9 ± 0.07 | 8.1 ± 0.28 | 1.4 ± 0.04 | |
| 21 | 14.4 ± 0.60 | 12.5 ± 0.32 | 3.8 ± 0.06 | 7.6 ± 0.26 | 5.2 ± 0.15 | |
| 22 | 18.8 ± 0.71 | 14.1 ± 0.29 | 3.7 ± 0.06 | 14.6 ± 0.57 | 6.8 ± 0.23 | |
| 23 | 19.4 ± 0.62 | 15.2 ± 0.33 | 5.3 ± 0.11 | 13.6 ± 0.40 | 3.0 ± 0.09 | |
| 24 | 30.5 ± 0.82 | 19.2 ± 0.36 | 7.7 ± 0.15 | 5.8 ± 0.16 | 4.0 ± 0.16 | |
| Complexes obtained by structural modifications of [Au(SPyCOR)(PPh3)] | ||||||
| 25 | 15.7 ± 0.66 | 17.4 ± 0.48 | 3.8 ± 0.04 | 12.0 ± 0.19 | 3.2 ± 0.13 | |
| 26 | 8.3 ± 0.39 | 13.1 ± 0.26 | 3.4 ± 0.06 | 3.5 ± 0.11 | 2.8 ± 0.08 | |
| 27 | 32.5 ± 1.24 | 29.3 ± 0.70 | 36.5 ± 0.77 | >25 | 10.4 ± 0.35 | |
| 28 | 18.7 ± 0.64 | 22.5 ± 0.67 | 8.6 ± 0.14 | 17.9 ± 0.45 | 15.4 ± 0.60 | |
| 29 | 33.5 ± 1.31 | >50 | NT | 8.3 ± 0.19 | 1.9 ± 0.05 | |
| 30 | 16.5 ± 0.92 | 17.1 ± 0.39 | 4.2 ± 0.05 | 7.7 ± 0.24 | 3.0 ± 0.08 | |
| 31 | 18.3 ± 0.75 | 15.1 ± 0.27 | 3.6 ± 0.06 | >25 | 1.2 ± 0.02 | |
| 32 | >50 | >50 | NT | >25 | 10.7 ± 0.29 | |
| 33 | 4.1 ± 0.11 | 1.2 ± 0.04 | 0.9 ± 0.07 | 4.5 ± 0.14 | 0.8 ± 0.02 | |
| Cisplatin | 105 ± 0.90 | 71 ± 0.80 | 7.4 ± 0.10 | 14.0 ± 0.20 | 65.0 ± 0.92 | |
| Cell Line | A549 | MiaPaca2 | Jurkat | |
|---|---|---|---|---|
| Complex | ||||
| Complexes with cysteine-containing dipeptides | ||||
| 34 | 1.5 ± 0.2 | 2.0 ± 0.2 | 0.9 ± 0.1 | |
| 35 | 1.9 ± 0.1 | 1.9 ± 0.1 | 1.6 ± 0.1 | |
| 36 | 2.3 ± 0.1 | 3.0 ± 0.1 | 2.2 ± 0.1 | |
| 37 | 15.6 ± 0.11 | 5.4 ± 0.1 | 0.4 ± 0.1 | |
| 38 | 4.8 ± 0.1 | 1.8 ± 0.1 | 1.7 ± 0.1 | |
| 39 | 3.0 ± 0.1 | 0.7 ± 0.1 | 0.5 ± 0.1 | |
| 40 | 5.0 ± 0.2 | 0.5 ± 0.1 | 0.8 ± 0.1 | |
| 41 | 2.7 ± 0.1 | 1.5 ± 0.1 | 1.1 ± 0.1 | |
| 42 | 2.1 ± 0.1 | 1.2 ± 0.1 | 1.5 ± 0.1 | |
| 43 | 1.8 ± 0.1 | 0.1 ± 0.1 | 0.6 ± 0.1 | |
| 44 | 3.5 ± 0.1 | 1.5 ± 0.1 | 0.8 ± 0.1 | |
| Complexes with 4-mercaptoproline | ||||
| 45 | 1.8 ± 0.15 | 3.0 ± 0.19 | 0.8 ± 0.08 | |
| 46 | 3.8 ± 0.37 | 6.1 ± 0.54 | 3.5 ± 0.32 | |
| 47 | >25 | >25 | 9.3 ± 0.65 | |
| 48 | 3.5 ± 0.29 | 2.3 ± 0.22 | 0.6 ± 0.08 | |
| 49 | 1.9 ± 0.16 | 1.8 ± 0.17 | 0.5 ± 0.07 | |
| Complexes with N,S-heterocyclic carbenes | ||||
| 50 | 0.4 ± 0.01 | 16.6 ± 0.2 | 6.2 ± 0.1 | |
| 51 | >25 | 24.8 ± 0.1 | ca. 25 | |
| Cisplatin | 105 ± 0.90 | 71 ± 0.80 | 7.4 ± 0.10 | |
| Cell Line | HeLa | HepG2 | HT-29 | ||||
|---|---|---|---|---|---|---|---|
| Complex | CV a | Resazurin | CV | Resazurin | CV | Resazurin | |
| 52 | 45.5 ± 4.8 | 52.7 ± 5.0 | 61.4 ± 7.4 | 71.3 ± 5.9 | 63.8 ± 6.7 | 58.9 ± 4.3 | |
| 53 | 3.4 ± 1.3 | 8.3 ± 1.4 | 15.2 ± 1.7 | 20.4 ± 0.9 | 10.5 ± 1.9 | 16.9 ± 1.7 | |
| 54 | 17.3 ± 3 | 29.4 ± 1.8 | 28.1 ± 4.5 | 30.0 ± 2.6 | 26.8 ± 2.1 | 34.6 ± 1.8 | |
| Cell Line a | PC3 | DU145 | 2008 | C13 | L540 | |
|---|---|---|---|---|---|---|
| Complex | ||||||
| [AuX2(dtc-Sar-AA-OtBu)] AA = Gly, X = Br− (57)/Cl− (58); Aib, X = Br− (59)/Cl− (60); L-Phe, X = Br− (61)/Cl− (62) | ||||||
| 57 | 1.3 ± 0.1 | 4.5 ± 0.9 | 18.0 ± 1.6 | 11.5 ± 1.2 | 2.1 ± 0.2 | |
| 58 | 1.6 ± 0.1 | 2.5 ± 0.3 | 13.2 ± 1.1 | 15.9 ± 1.3 | 3.4 ± 0.2 | |
| 59 | 0.8 ± 0.1 | 1.4 ± 0.1 | 4.5 ± 0.2 | 3.7 ± 0.3 | 1.5 ± 0.2 | |
| 60 | 1.1 ± 0.1 | 2.2 ± 0.1 | 4.7 ± 0.2 | 5.1 ± 0.4 | 1.7 ± 0.3 | |
| 61 | 16.8 ± 1.7 | 13.2 ± 1.2 | 41.2 ± 4.0 | 17.2 ± 1.8 | 16.4 ± 1.5 | |
| 62 | 16.5 ± 1.6 | 15.3 ± 1.5 | 43.4 ± 3.8 | 21.5 ± 1.5 | 7.3 ± 0.5 | |
| [AuX2(dtc-Sar-L-Ser(tBu)-OtBu))] X = Br− (63)/Cl− (64) | ||||||
| 63 | 5.2 ± 0.5 | NT | 17.5 ± 1.6 | 17.0 ± 1.6 | 3.4 ± 0.2 | |
| 64 | 6.5 ± 0.7 | NT | 15.2 ± 1.5 | 16.1 ± 1.3 | 1.4 ± 0.1 | |
| [AuX2(dtc-AA-Aib2-OtBu)] AA = Sar, X = Br− (65)/Cl− (66); D,L-Pro, X = Br− (67)/Cl− (68) | ||||||
| 65 | 5.8 ± 0.6 | NT | 12.0 ± 1.1 | 15.0 ± 1.3 | 3.8 ± 0.4 | |
| 66 | 5.8 ± 0.5 | NT | 14.2 ± 1.5 | 15.4 ± 1.4 | 4.1 ± 0.5 | |
| 67 | 6.3 ± 0.7 | NT | 16.3 ± 1.4 | 11.5 ± 1.1 | 2.2 ± 0.1 | |
| 68 | 3.0 ± 0.2 | NT | 8.2 ± 0.7 | 7.8 ± 0.5 | 2.2 ± 0.2 | |
| [AuX2(dtc-Sar-Aib3-OtBu)] X = Br− (69)/Cl− (70) | ||||||
| 69 | 16.0 ± 1.7 | NT | 42.8 ± 4.1 | 17.5 ± 1.6 | 3.9 ± 0.4 | |
| 70 | 16.1 ± 0.6 | NT | 43.5 ± 1.2 | 11.5 ± 1.2 | 3.5 ± 0.2 | |
| [AuX2(dtc-Sar-Aib3-Gly-OEt)] X = Br− (71)/Cl− (72) | ||||||
| 71 | 15.0 ± 1.4 | NT | 29.2 ± 3.0 | 22.9 ± 1.9 | 5.4 ± 0.5 | |
| 72 | 20.0 ± 1.9 | NT | 27.8 ± 2.3 | 35.5 ± 3.9 | 5.0 ± 0.8 | |
| Cisplatin | 3.3 ± 0.3 | 4.5 ± 0.1 | 19.4 ± 1.2 | 117.2 ± 9.1 | 2.5 ± 0.1 | |
| Cell Line a | MRC-5 | MCF-7 | HT-29 | HL-60 | Raji | HeLa | A549 | |
|---|---|---|---|---|---|---|---|---|
| Complex | ||||||||
| 73 | >200 | 150 | 150 | |||||
| 74 | >200 | 19.68 ± 0.23 | 14.70 ± 1.36 | 11.93 ± 1.02 | 3.30 ± 0.02 | >200 | >200 | |
| 75 | >200 | >100 | >100 | >100 | >100 | 170 | 170 | |
| 76 | 150b | 75 | 30 | |||||
| 77 | >200 | >200 | >200 | |||||
| 78 | 170 | 55 | 115 | |||||
| 79 | 100 | 65 | 75 | |||||
| Cisplatin | 0.48 ± 0.02 | 1.56 ± 0.26 | 18.60 ± 2.32 | 10.31 ± 2.54 | 2.25 ± 0.10 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrejević, T.P.; Glišić, B.Đ.; Djuran, M.I. Amino Acids and Peptides as Versatile Ligands in the Synthesis of Antiproliferative Gold Complexes. Chemistry 2020, 2, 203-218. https://doi.org/10.3390/chemistry2020013
Andrejević TP, Glišić BĐ, Djuran MI. Amino Acids and Peptides as Versatile Ligands in the Synthesis of Antiproliferative Gold Complexes. Chemistry. 2020; 2(2):203-218. https://doi.org/10.3390/chemistry2020013
Chicago/Turabian StyleAndrejević, Tina P., Biljana Đ. Glišić, and Miloš I. Djuran. 2020. "Amino Acids and Peptides as Versatile Ligands in the Synthesis of Antiproliferative Gold Complexes" Chemistry 2, no. 2: 203-218. https://doi.org/10.3390/chemistry2020013
APA StyleAndrejević, T. P., Glišić, B. Đ., & Djuran, M. I. (2020). Amino Acids and Peptides as Versatile Ligands in the Synthesis of Antiproliferative Gold Complexes. Chemistry, 2(2), 203-218. https://doi.org/10.3390/chemistry2020013







