Abstract
The integration of nanotheranostics into cancer treatment represents a transformative shift in oncology, combining precision diagnostics with targeted therapeutic interventions. This manuscript explores the advancements in nanotechnology-driven cancer therapies, highlighting the role of engineered nanoparticles, such as liposomes, dendrimers, polymeric micelles, and virus-like particles, in enhancing drug delivery, real-time imaging, and tumor-specific targeting. Additionally, emerging therapies, including immunotherapy, gene editing, and chromophore-assisted light inactivation (CALI), are discussed in the context of personalized medicine. The convergence of these strategies is poised to redefine cancer treatment paradigms, improving therapeutic efficacy while minimizing systemic toxicity. This review outlines the key challenges, current limitations, and future directions in nanotheranostic applications, emphasizing the need for interdisciplinary collaboration to optimize their clinical translation.
Keywords:
nanotheranostics; targeted nanomedicine; CALI therapy; cancer epitopes; liposome drug delivery; polymeric micelles; virus-like particles; nanoparticle imaging; photodynamic therapy; smart nanomaterials; biosensors in cancer; multimodal theranostics; nano-immunotherapy; biodegradable nanocarriers; microfluidic profiling 1. Introduction
Cancer treatment has undergone a profound transformation with the emergence of nanotheranostics [1] and other innovative therapies [2,3,4]. These advancements have enabled personalized, highly precise interventions that have significantly improved therapeutic outcomes. Researchers have emphasized the utility of nanomaterial-based agents, such as graphene and lipid nanoparticles, which facilitate targeted diagnosis and efficient treatment delivery, minimizing systemic toxicity and optimizing therapeutic responses [5].
The field of nanotheranostic integrates diagnostic and therapeutic modalities at the nanoscale, revolutionizing cancer detection, monitoring, and treatment strategies. Engineered nanoparticles are designed to deliver therapeutic agents selectively to tumor cells whilst simultaneously enabling real-time imaging and treatment monitoring. This dual functionality reduces the off-target effects and enhances precision, paving the way for adaptive and personalized treatment strategies [6,7,8].
Nanoparticles, including liposomes, dendrimers, aptamers, and other organic/inorganic nanocarriers, are widely employed for chemotherapeutic drug delivery, gene transfer, and immunotherapeutic applications (Figure 1). These nanocarriers can be functionalized with targeting ligands, such as antibodies or peptides, facilitating high specificity toward tumor-associated markers and sparing healthy tissues [9,10,11,12]. Additionally, many nanoparticles respond to tumor-specific stimuli, such as pH, temperature, or enzymatic activity, ensuring controlled drug release for enhanced efficacy [9,10,11,12].
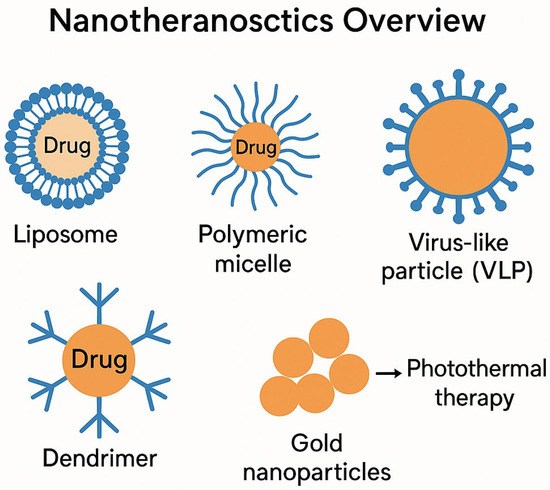
Figure 1.
Nanotheranostics overview: A schematic representation of nanomaterial-based approaches in cancer diagnostics and therapy, including liposomes, polymeric micelles, dendrimers, virus-like particles (VLPs), and gold nanoparticles. Each nanomaterial offers distinct advantages in drug delivery, imaging, immune modulation, and targeted therapy, enhancing precision in oncology treatments.
Beyond drug delivery, nanotheranostic platforms provide high-resolution imaging capabilities, utilizing modalities such as fluorescence, magnetic resonance imaging (MRI), and computed tomography (CT), enabling precise tumor localization and treatment tracking [13,14].
1.1. Overview of Cancer Treatment Challenges
Cancer treatment presents multifaceted challenges, spanning biological complexity, diagnostic hurdles, treatment resistance, economic constraints, and psychosocial impact [15,16,17]. A major obstacle is the heterogeneity and plasticity of cancer cells [18,19,20], which necessitates individualized therapeutic approaches. Cancer is not a singular disease, but comprises diverse types and subtypes, each with unique genetic and molecular characteristics. This variability complicates efforts to establish universal treatment protocols and necessitates targeted therapy strategies, which vary in their efficacy across patient populations [21,22]. Furthermore, tumor adaptability fosters resistance to traditional treatments, such as chemotherapy and radiation, undermining long-term disease control [23,24].
Early detection remains a critical challenge, as many cancers exhibit asymptomatic progression in their initial stages, leading to late-stage diagnoses that complicate interventions [25,26]. Additionally, unequal access to diagnostic technologies in low-resource settings further exacerbates the disparities in patient outcomes [27,28].
Treatment-associated toxicity is another pressing concern, with chemotherapy, radiation, and targeted therapies often inducing immune suppression, fatigue, and organ damage, diminishing quality of life and treatment adherence [29,30]. Furthermore, financial constraints pose additional barriers, as cutting-edge cancer therapies are frequently cost-prohibitive for patients and healthcare systems alike [31,32]. Addressing these limitations requires interdisciplinary collaboration to enhance treatment accessibility, affordability, and patient-centered care.
1.2. Nanotheranostics as a Promising Approach
Nanotheranostics represents a cutting-edge biomedical strategy that integrates diagnostic and therapeutic functions within a single nanoparticle-based platform [33,34]. Engineered nanomaterials, such as liposomes, polymeric nanoparticles, quantum dots, gold nanoparticles, and magnetic nanoparticles, are designed to deliver imaging agents, therapeutic payloads, and targeting ligands for selective tumor localization and drug administration [35,36,37,38].
This technology enables real-time monitoring of treatment responses, enhances drug targeting, and reduces systemic toxicity through biomarker-guided delivery and stimuli-responsive release mechanisms, triggered by tumor-specific factors, such as pH, enzymatic activity, or hypoxia [39,40,41,42]. Applications span multimodal imaging (MRI, CT, and fluorescence) and tailored interventions suited for personalized oncology [43,44,45,46].
Despite significant progress, nanotheranostics faces challenges in terms of biocompatibility, large-scale manufacturing, and regulatory approval. Nonetheless, innovations in material science and bioengineering continue to push these platforms toward routine clinical integration [45,46].
Building on these technological strides, the integration of nanotheranostics with immuno-oncology offers a compelling frontier for precision cancer therapy. Recent studies have illuminated the molecular and immunological underpinnings that govern therapeutic response, particularly in the context of immune checkpoint blockade. For instance, targeted next-generation sequencing has revealed specific genomic markers predictive of PD-1 inhibitor efficacy, underscoring the value of molecular profiling in patient stratification [47]. Tumor mutational burden (TMB) has emerged as a robust biomarker, with higher TMB correlating with improved responses to immunotherapy, likely due to increased neoantigen presentation and immune recognition [48,49].
Beyond genetic predictors, the tumor microenvironment and its interplay with the immune system play a pivotal role in modulating treatment outcomes. Genomic determinants have also been linked to radiation therapy responsiveness, suggesting that integrated approaches combining nanotheranostics with radiotherapy could enhance immunogenic cell death and therapeutic synergy [50]. The immune system’s dual role in tumor suppression and progression further complicates treatment paradigms yet offers opportunities for targeted modulation [51]. The cancer-immunity cycle, as conceptualized by Chen and Mellman, provides a framework for understanding how therapeutic interventions can amplify antitumor immunity at multiple stages [52].
T cell-based strategies continue to gain traction, with translational research highlighting their clinical relevance and potential for durable responses [53]. PD-1 blockade, for example, has been shown to reverse adaptive immune resistance, reinvigorating exhausted T cells and promoting tumor regression [54]. Combination therapies, such as nivolumab plus ipilimumab, have demonstrated enhanced efficacy in advanced melanoma, albeit with increased toxicity, reinforcing the need for precision-guided regimens [55]. Moreover, PD-L1 expression remains a key predictive biomarker, guiding treatment selection and informing prognosis [56].
Together, these findings underscore the importance of integrating molecular diagnostics, immune profiling, and nanotechnology to tailor cancer therapies. As nanotheranostics evolves, its convergence with immunotherapy promises to unlock new dimensions of personalized medicine, transforming cancer care through synergistic, biomarker-driven interventions.
2. Nanotechnology-Driven Cancer Solutions
2.1. Significance of Nanotheranostics
Clinically, nanotheranostics supports dynamic treatment monitoring and individualized therapy adjustment, especially critical in heterogeneous cancers with variable treatment responses [57,58]. Through targeted payload delivery and real-time imaging, these systems improve intervention timing and reduce adverse effects [59,60,61,62].
By customizing nanoparticle formulations to match tumor-specific molecular profiles, nanotheranostics enhance therapeutic accuracy and minimizes resistance [63,64]. These benefits extend beyond oncology to fields including cardiology, neurology, and infectious disease diagnostics [65,66,67,68].
2.2. Role of Nanoparticles in Enhancing Treatment Efficacy
Nanoparticles offer unique advantages in cancer therapy due to their high surface-area-to-volume ratio, tunable chemical properties, and ability to penetrate biological barriers [69,70,71]. Functionalized nanoparticles target tumor biomarkers with high selectivity, improving drug accumulation in malignant tissues while sparing healthy cells [72,73,74].
Advanced designs enable controlled drug release, maintaining therapeutic levels over time and minimizing systemic toxicity [75,76]. Metallic nanoparticles, such as gold and iron oxide, also serve as imaging contrast agents, improving tumor visualization via MRI and CT [77].
These innovative applications include photothermal therapy, where gold nanoparticles generate localized heat for tumor ablation [78,79], and gene delivery systems for transporting siRNA or CRISPR/Cas9 complexes to modulate oncogenic pathways [80,81]. As the research advances, nanoparticle-based modalities will continue to redefine the standards for cancer treatment efficacy and personalization [82].
3. Nanotechnology-Enabled Detection of Circulating Tumor Cells and Cancer Biomarkers
The detection and analysis of circulating tumor cells (CTCs) and cancer biomarkers (CBs) have emerged as pivotal components of precision oncology, offering real-time insights into tumor behavior, therapeutic responsiveness, and disease progression [83,84]. Leveraging nanotechnology, these indicators can now be captured and profiled with enhanced sensitivity and specificity, transforming early diagnosis and individualized treatment strategies.
CTCs are malignant cells shed from primary tumors into the bloodstream, serving as key mediators of metastasis [85,86]. Their molecular characterization reveals oncogenic mutations, resistance markers, and tumor heterogeneity—information critical for refining therapeutic decisions [87,88,89,90]. Nanotechnology facilitates their isolation via high-resolution microfluidic platforms and immunomagnetic separation techniques that exploit nanoscale functionalization to enhance detection sensitivity [91,92].
Cancer biomarkers, including proteins, DNA fragments, and metabolites, further complement liquid biopsy diagnostics by identifying the disease stage, predicting treatment outcomes, and guiding drug selection [93,94]. Nanoparticle-enabled biosensors and assay platforms have significantly improved biomarker recognition, enabling detection of key indicators, such as HER2 [95], PSA [96], and EGFR mutations [97], with increased precision.
The integration of nanotechnology into CTC and biomarker profiling has fostered non-invasive, real-time patient monitoring [98,99]. These systems amplify trace signals, reduce background interference, and facilitate multiplexed detection, streamlining cancer diagnostics across various tumor types.
3.1. Nanoscale Strategies for CTC Isolation and Monitoring
Nanotechnology enhances CTC detection by enabling highly sensitive liquid biopsy platforms that provide dynamic snapshots of tumor biology [100,101]. Unlike conventional imaging and biopsies, nanoscale filtration and antibody-coated magnetic nanoparticles can selectively isolate CTCs, supporting early detection and longitudinal monitoring [102].
CTC enumeration and profiling allow clinicians to track therapeutic effectiveness— declining counts signal treatment success, while increasing levels may reflect disease progression [103,104,105,106]. Nanostructured surfaces and molecular recognition elements also improve the capture efficiency, supporting downstream analyses of drug resistance mechanisms and metastatic potential [107,108,109,110]. Nevertheless, challenges such as CTC rarity and technological standardization persist, hampering further innovation in nanofluidic design and particle engineering [111,112].
3.2. Nanotechnology in Cancer Biomarker-Driven Therapy
Cancer biomarkers, when integrated with nanotechnology, enable personalized treatment strategies that target specific genetic and molecular abnormalities [113,114,115]. Nanocarriers conjugated with antibodies or aptamers selectively bind to biomarker-expressing cells, facilitating localized delivery of therapeutic agents and imaging probes [116,117].
For HER2-positive breast cancer, nanosystems delivering trastuzumab can enhance therapeutic precision [118,119]. Similarly, targeted nanoparticles can deliver TKIs to EGFR-mutant lung cancer cells, mitigating off-target effects [120,121]. Nanotechnology also plays a role in immunotherapy monitoring. Biosensors can detect PD-L1 expression, MSI, and TMB with high accuracy, informing checkpoint inhibitor eligibility and immune response profiling [122,123,124,125,126].
Advanced nanoparticle platforms further enable real-time tracking of circulating tumor DNA (ctDNA), empowering clinicians to adapt treatment plans dynamically [127,128,129,130]. Despite the current limitations regarding detection variability and cost, the ongoing developments in nanoproteomics and genomics are refining biomarker-guided interventions for optimal patient outcomes [131,132].
4. Integration of Cancer Treatment with Conventional Therapies
The integration of emerging cancer therapies with conventional treatments is reshaping oncology by combining innovation with established methodologies [133]. Traditional approaches, including surgery, chemotherapy, and radiotherapy, have long been the cornerstone of cancer care, offering proven efficacy for tumor reduction [134]. However, these techniques often pose challenges, including toxicity, resistance mechanisms, and treatment limitations [135]. Recent studies that exemplify this evolving landscape include the following:
- Nanocarrier-Assisted Drug Delivery in Immunotherapy: presented programmable nanocarriers that synergize with checkpoint blockades, improving selective tumor targeting and reducing systemic toxicity [136].
- Bioresponsive Platforms for Oral Peptide Delivery: introduced pH-sensitive nanoarchitectures that protect therapeutic peptides during gastrointestinal transit, offering a non-invasive alternative to injectable regimens and complementing traditional modalities [137].
By combining novel treatments, such as immunotherapy, gene editing, and targeted therapies, the medical field is enhancing precision and reducing toxicity [138]. Immunotherapy, through immune checkpoint inhibitors and CAR-T cell therapy, provides synergistic benefits when paired with conventional approaches [139]. For example, radiotherapy can enhance immune activation, making immunotherapeutic agents more effective [140].
Targeted therapies, including tyrosine kinase inhibitors (TKIs) and monoclonal antibodies, offer molecular-level precision, minimizing damage to healthy cells whilst improving tumor specificity [141,142]. Additionally, the fusion of chemotherapy and nanomedicine-based drug delivery systems optimizes treatment efficacy by reducing systemic toxicity [143].
Precision medicine further refines personalized treatment strategies, ensuring that therapies align with an individual’s tumor genetics and biomarker profile [144,145]. Notably, mutations such as BRCA1 in breast cancer or EGFR in lung cancer can guide specific treatment protocols, enhancing the therapeutic outcomes [146].
Despite its promise, integrated cancer treatment faces challenges, including clinical validation of combined approaches, multidisciplinary coordination, and financial constraints [147]. Continued research and policy adjustments are essential to ensuring broader access to advanced treatment combinations, ultimately improving long-term patient survival and quality of life [148].
4.1. Enhancement of Radiotherapy Through Nanotechnology
The integration of nanotechnology into radiotherapy has significantly improved cancer treatment by enabling precise tumor targeting, increasing therapeutic efficacy, and minimizing damage to surrounding healthy tissues [149]. Despite its effectiveness, radiotherapy poses challenges, such as radiation resistance, toxicity, and off-target effects, which nanotechnology helps address [150].
One key advancement is the development of radiosensitizers, which are nanoparticles designed to amplify radiation-induced damage in cancer cells [151]. Gold nanoparticles, for instance, have shown remarkable radiotherapy enhancement due to their high atomic number, which increases X-ray absorption and secondary electron generation, thereby intensifying tumor destruction while sparing adjacent tissues [152,153]. Similarly, hafnium oxide nanoparticles have demonstrated comparable radiosensitizing effects, thereby widening their clinical potential [154].
Nanotechnology also improves therapeutic agent delivery in combination with radiotherapy. Nanoparticles facilitate tumor-specific targeting, ensuring that radiation-enhancing agents accumulate preferentially within cancer cells, thereby improving efficacy whilst minimizing systemic toxicity [155].
Additionally, theranostic nanoparticles (which combine imaging and therapeutic functions) offer real-time tumor visualization, aiding in precise treatment adjustments [156,157]. Imaging agents, such as gadolinium and iron oxide nanoparticles, enhance MRI and CT scans, enabling accurate tumor localization and monitoring [158].
Another critical challenge in radiotherapy is tumor hypoxia, where oxygen-deficient regions exhibit radiation resistance [159]. Nanotechnology addresses this issue by introducing oxygen-carrying nanoparticles or reactive oxygen species (ROS)-generating nanoparticles, which reoxygenate the hypoxic tumor areas, thereby increasing radiation sensitivity and therapeutic efficacy [160,161].
These advances highlight nanotechnology’s crucial role in enhancing radiotherapy, improving tumor destruction, and reducing treatment-associated toxicity, ultimately leading to more effective cancer interventions [162].
4.2. Synergistic Effects of Nanoparticles with Chemotherapeutic Agents
Nanoparticles are revolutionizing chemotherapy by enhancing drug delivery, overcoming multidrug resistance (MDR), and improving therapeutic precision [163,164]. A major challenge in chemotherapy is the non-specific distribution of drugs, which often results in systemic toxicity and reduced efficacy. Nanoparticles circumvent this limitation by facilitating targeted drug delivery, ensuring higher drug concentrations at tumor sites whilst sparing healthy tissues [165,166].
Additionally, nanoparticles help combat MDR, a phenomenon in which cancer cells expel therapeutic agents via efflux pumps, such as P-glycoprotein, reducing drug effectiveness [167,168]. By bypassing these resistance mechanisms, nanoparticles improve intracellular drug retention, ensuring enhanced cancer cell destruction [169]. Moreover, nanoparticles can co-deliver chemotherapeutic drugs alongside MDR modulators, inhibiting efflux pathways and enhancing drug potency [170,171].
Nanoparticles also enable combination therapies, integrating chemotherapeutics with gene-silencing molecules (e.g., siRNA) [172] or immunomodulators [173,174] to target cancer through multiple mechanisms simultaneously. This multimodal approach disrupts tumor survival pathways, induces apoptosis, and strengthens immune responses [175].
Controlled drug release is another key advantage. Nanoparticles regulate therapeutic exposure, ensuring steady drug concentrations over extended periods, thereby reducing adverse effects and improving treatment adherence [176,177]. Additionally, stimuli-responsive nanoparticles, designed to release drugs upon exposure to pH, temperature, or enzymatic triggers, provide tumor-specific activation for optimal efficacy [178].
Together, these advances highlight nanoparticles’ vital role in modern chemotherapy, improving its efficacy, reducing toxicity, and overcoming resistance, ultimately leading to more effective cancer treatment strategies [179].
4.3. Role of Nanoparticles in Improving Photodynamic Therapy (PDT) Outcomes
Photodynamic therapy (PDT) is a minimally invasive cancer treatment that utilizes photosensitizers, light, and oxygen to generate ROS for tumor destruction [180]. Despite its potential, PDT faces limitations, including poor photosensitizer solubility, off-target effects, limited tissue penetration, and hypoxic tumor microenvironments [181,182]. Nanoparticles have emerged as key enhancers of PDT by improving their solubility, targeted delivery, tumor penetration, and controlled activation [183,184].
While this section focuses on PDT as a model system, the nanoparticle strategies discussed, such as enhanced solubility, targeted delivery, and tumor penetration, are not exclusive to PDT. These principles are equally applicable to other light-based therapies, like CALI, as well as conventional and emerging modalities, including chemotherapy, immunotherapy, and gene therapy. This underscores the versatility of nanotechnology for overcoming shared therapeutic barriers across diverse treatment platforms.
4.3.1. Improved Solubility and Bioavailability
Many photosensitizers suffer from poor aqueous solubility, limiting their therapeutic potential [185]. Nanoparticles, including liposomes, polymeric nanoparticles, and micelles, encapsulate hydrophobic photosensitizers, thereby enhancing their solubility, stability, and bioavailability, ensuring their efficient delivery to tumor sites [186].
4.3.2. Targeted Delivery
Nanoparticles can be functionalized with antibodies, peptides, or small molecules, enabling selective binding to tumor-specific receptors, thereby reducing off-target effects whilst enhancing photosensitizer accumulation in cancerous tissue [187,188].
4.3.3. Enhanced Tumor Penetration
The enhanced permeability and retention (EPR) effect allows for nanoparticles to accumulate preferentially in tumors due to their leaky vasculature [189]. Smaller nanoparticles penetrate deeper into tumor tissues, ensuring uniform photosensitizer distribution [190].
4.3.4. Overcoming Hypoxia
Hypoxic tumor microenvironments reduce ROS generation, weakening PDT’s effectiveness [191]. Oxygen-generating nanoparticles release oxygen in situ, alleviating hypoxia and enhancing therapeutic outcomes [192].
4.3.5. Controlled Release and Activation
Stimuli-responsive nanoparticles release photosensitizers in response to pH, enzymes, or light, ensuring localized therapy activation [193]. Additionally, upconversion nanoparticles convert near-infrared (NIR) light into visible light, enabling deeper tissue penetration [194].
4.3.6. Combination Therapies
Nanoparticles facilitate co-delivery of photosensitizers alongside chemotherapy, immunotherapy, or gene therapy, enhancing treatment efficacy and broadening the therapeutic potential [195].
These advancements underscore nanoparticles’ role in optimizing PDT, improving tumor targeting, therapeutic precision, and treatment efficacy [196].
4.4. Application of Chromophore-Assisted Light Inactivation (CALI) in Targeted Cancer Cell Therapy
Unlike the mechanistic breakdown used in Section 4.3 for PDT, CALI is presented here through a conceptual framework that highlights its therapeutic advantages and developmental limitations. This approach reflects CALI’s status as an emerging modality, where its clinical potential and translational challenges are best understood through a balanced evaluation of benefits and barriers. Accordingly, Section 4.4 is divided into two thematic subcategories: Section 4.4.1 discusses the therapeutic strengths of CALI, while Section 4.4.2 addresses the current limitations and prospects.
Chromophore-assisted light inactivation (CALI) is a novel therapeutic strategy that employs light-activated chromophores to selectively disrupt the biomolecules, proteins, or cellular structures associated with cancer cells [197,198]. This precision-driven method ensures localized cytotoxicity while sparing adjacent healthy tissues [199].
CALI operates through chromophore-conjugated molecules designed to bind to specific tumor-associated antigens or intracellular targets. Upon exposure to light, these chromophores generate reactive oxygen species (ROS) or other cytotoxic intermediates, triggering apoptosis or necrosis in the cancerous cells [200,201].
4.4.1. Therapeutic Advantages of CALI
- High Spatial Precision: Light-triggered activation ensures confined tissue damage, reducing collateral toxicity [202].
- Minimally Invasive Mechanism: Compared to conventional treatments like chemotherapy and radiotherapy, CALI offers localized action with fewer systemic effects [203].
- Molecular Versatility: Its targets range from oncogenic proteins to signaling nodes and metabolic pathways, enabling tailored therapeutic interventions [204,205].
- Combination Synergy: CALI can be integrated with photodynamic therapy (PDT) or nanoparticle-facilitated drug delivery systems to amplify its efficacy [206,207].
4.4.2. Limitations and Prospects
Despite its promise, CALI faces practical challenges, such as limited light penetration in deep tissues and issues surrounding chromophore stability and biocompatibility [208]. Emerging solutions include the use of near-infrared (NIR) light sources and nanoparticle-assisted chromophore delivery to enhance targeting and penetration [209,210].
5. Emerging Therapies in Cancer Treatment
A comprehensive overview of the molecular mechanisms underlying resistance to immune checkpoint inhibitors, highlighting the need for precision-guided interventions that can anticipate and overcome adaptive tumor responses has been reported [211]. Recent advances in cancer therapy have introduced highly innovative strategies aimed at targeting cancer cells with greater precision while minimizing damage to healthy tissues [212,213]. These emerging modalities, spanning immunotherapy, gene editing, epigenetic therapy, oncolytic virus therapy, therapeutic cancer vaccines, and stem cell therapy, are increasingly being revolutionized by nanotechnology. Nanomedicine platforms can enhance therapeutic efficacy by improving drug delivery, modulating immune responses, and overcoming biological barriers [214,215]. The following subsections detail how nanotechnology specifically contributes to each therapeutic domain.
5.1. Immunotherapy
Immunotherapy strengthens the body’s immune system to recognize and eliminate cancer cells. Established approaches include immune checkpoint inhibitors and CAR-T cell therapy [216,217]. Nanoparticles play a pivotal role as follows:
- Delivering immunomodulatory agents directly to immune cells.
- Enhancing CAR-T cell expansion through cytokine or gene payload delivery.
- Reducing systemic toxicity via tumor-targeted formulations.
5.1.1. Immune Checkpoint Inhibitors
PD-1/PD-L1 inhibitors (e.g., pembrolizumab, nivolumab) and CTLA-4 inhibitors (e.g., ipilimumab) have improved survival for melanoma and lung cancer [218,219,220,221]. Nanocarriers co-deliver checkpoint inhibitors with tumor antigens, enhancing immune activation and minimizing off-target effects.
5.1.2. CAR-T Cell Therapy
CAR-T therapy involves engineering T cells to target cancer antigens [222,223]. Nanoparticles facilitate this by delivering genetic constructs and stimulatory molecules, improving in vivo expansion and persistence.
Nanotechnology also supports biomarker-guided delivery systems to improve therapeutic precision and reduce immune-related adverse events [224,225].
5.2. Targeted Therapy
Targeted therapies inhibit molecular drivers of tumor growth [226]. Nanotechnology enhances these treatments by
- Improving the solubility and bioavailability of hydrophobic drugs.
- Enabling controlled release and tumor-specific accumulation.
- Facilitating combination strategies to overcome resistance.
5.2.1. Tyrosine Kinase Inhibitors (TKIs)
Drugs like imatinib (BCR-ABL) and gefitinib (EGFR) are effective in CML and lung cancer, while immune checkpoint pathways such as PD-L1 also play a critical role in tumor-induced immune suppression and therapeutic resistance [227,228,229]. Nanoformulations can improve the pharmacokinetics and enable sustained release, enhancing the therapeutic outcomes.
5.2.2. Monoclonal Antibodies
Trastuzumab targets HER2-positive breast cancer [230,231]. Nanoparticles conjugated with antibodies allow for dual targeting-receptor blockade plus cytotoxic payload delivery.
5.2.3. Small-Molecule Inhibitors
Vemurafenib targets BRAF mutations in melanoma [232]. Nanocarriers can enhance intracellular delivery and tumor penetration, addressing resistance mechanisms [233,234].
5.3. Gene Therapy
Gene therapy corrects cancer-driving mutations [235,236]. Nanotechnology is central to
- Protecting genetic material from enzymatic degradation.
- Facilitating cellular uptake and nuclear localization.
- Enabling non-viral delivery systems with reduced immunogenicity.
5.3.1. CRISPR-Cas9 Gene Editing
CRISPR-Cas9 enables precise DNA modifications, offering new avenues to investigate tumor mutational burden and mechanisms of immune evasion that influence cancer immunotherapy response [237,238,239]. Nanoparticles can deliver Cas9 and guide RNAs, improving the editing efficiency and reducing the off-target effects [240].
5.3.2. Tumor-Suppressor Gene Restoration
Restoring genes, like p53, promote tumor regression [241,242]. Nanocarriers can ensure localized delivery to tumor sites, enhancing the therapeutic specificity.
5.3.3. Immunogenomic Determinants of Checkpoint Inhibitor Response
Recent advances in immunotherapy have underscored the importance of immunogenomic profiling in predicting and enhancing responses to immune checkpoint blockade. Moreover, immunohistochemical quantification of PD-L1 expression on both tumor and immune cells provides a clinically validated biomarker to stratify patients for anti-PD-1/PD-L1 therapy and correlates with improved therapeutic outcomes [243]. Consequently, integrating immunogenomic biomarkers, including tumor mutational burden, neoantigen load, and immune gene signatures, enables precise patient stratification and improved selection for checkpoint inhibitor therapy [244]. However, the predictive value of PD-L1 expression remains nuanced; while elevated PD-L1 levels often correlate with improved responses, variability in detection methods and tumor heterogeneity complicate its utility as a standalone biomarker [245]. Tumor mutational burden (TMB) has emerged as a robust genomic indicator, with higher TMB linked to increased neoantigen load and enhanced immunogenicity, thereby improving the efficacy of checkpoint inhibitors across multiple cancer types [246].
5.4. Epigenetic Therapy
Epigenetic therapies modulate gene expression without altering DNA sequences [247,248]. Nanotechnology improves the following:
- Targeted delivery of epigenetic drugs.
- Drug stability and bioavailability.
- Synergistic effects through co-delivery platforms.
5.4.1. Histone Deacetylase (HDAC) Inhibitors
Checkpoint inhibitors can amplify antigen-specific T-cell activation and overcome tumor-induced immune suppression [249]. Building on this immunological foundation, vorinostat and panobinostat have been shown to reactivate silenced genes, offering promising avenues for epigenetic reprogramming in cancer therapy [250,251]. Nanoformulations of these agents further enhance tumor-specific accumulation and reduce systemic toxicity, improving therapeutic precision.
5.4.2. DNA Methyltransferase (DNMT) Inhibitors
Azacitidine and decitabine have restored gene expression in hematologic malignancies [252,253]. Nanoparticles can enable their co-delivery with other agents, amplifying their therapeutic synergy.
5.4.3. Modulating the Tumor Microenvironment for Enhanced Immunotherapy
The tumor microenvironment (TME) plays a pivotal role in shaping cancer progression and therapeutic response, particularly in the context of immunotherapy. Joyce and Fearon emphasized that the TME is not merely a passive backdrop but an active participant in tumor survival, immune evasion, and resistance to treatment, making it a compelling therapeutic target [254]. Notably, immunotherapeutic strategies can reprogram the TME to restore antitumor immunity, noting that the dynamic interplay between immune cells, stromal components, and signaling molecules determines the efficacy of checkpoint inhibitors [255]. Wei et al. further elucidated the fundamental mechanisms of immune checkpoint blockade, demonstrating how therapies targeting PD-1 and CTLA-4 pathways can overcome immunosuppressive barriers within the TME to elicit durable clinical responses [256]. Together, these insights underscore the necessity of integrating TME modulation into next-generation immunotherapeutic designs.
5.5. Oncolytic Virus Therapy
Oncolytic viruses selectively infect and destroy cancer cells [257,258]. Nanotechnology enhances this modality by the following:
- Improving viral stability and delivery.
- Facilitating deeper tissue penetration.
- Supporting combination therapies with immunomodulators.
5.5.1. Talimogene Laherparepvec (T-VEC)
T-VEC is FDA-approved for melanoma [259,260]. Nanoparticle carriers can improve its intratumoral delivery and immune activation.
5.5.2. Mechanisms of Action
- Direct Lysis: Viral replication leads to tumor destruction [261].
- Immune Activation: Antigen release recruits immune cells [262].
- Microenvironment Modulation: Enhances checkpoint inhibitor efficacy [263].
5.5.3. Tumor Microenvironment and Oncogenic Barriers to Immunotherapy
The tumor microenvironment (TME) plays a central role in shaping immune suppression and therapeutic resistance. Chen et al. highlighted how immunosuppressive cellular components within the TME, such as regulatory T cells, myeloid-derived suppressor cells, and tumor-associated macrophages, actively inhibit antitumor immune responses, thereby limiting the efficacy of immunotherapies [264].
Furthermore, it has been emphasized that the TME contributes to resistance against immune checkpoint blockade by fostering an immune-excluded phenotype and impairing T-cell infiltration [265]. In parallel, it has been demonstrated that tumor-intrinsic oncogenic pathways, including WNT/β-catenin signaling, can suppress immune recognition by preventing dendritic cell recruitment and CD8⁺ T cell priming, reinforcing immune escape mechanisms [266]. These insights underscore the need for therapeutic strategies that not only target immune checkpoints but also reprogram the TME and disrupt oncogenic signaling to restore immunogenicity.
5.6. Therapeutic Cancer Vaccines
Therapeutic vaccines stimulate immune recognition of cancer cells [267,268]. Nanoparticles can serve as
- Antigen carriers for cytotoxic T cell activation.
- Immune adjuvants to enhance the response durability.
- Platforms for personalized neoantigen delivery.
5.6.1. Mechanisms of Action
- Cytotoxic T Cell Activation: Nanocarriers deliver tumor antigens [269].
- Memory T Cell Generation: Sustains immune surveillance [270].
- Checkpoint Integration: Enhances response rates [271].
5.6.2. Examples of Therapeutic Cancer Vaccines
- Sipuleucel-T for prostate cancer [272].
- HPV-related vaccines for cervical and oropharyngeal cancers [273].
- KRAS vaccines for mutated tumors [274].
5.6.3. Mechanisms of Immune Escape and Resistance to Checkpoint Inhibitors
Despite the transformative impact of immune checkpoint inhibitors (ICIs), resistance remains a significant barrier to durable clinical success. It has been demonstrated that IL-10 can induce upregulation of TIM-3 on human T cells, leading to functional exhaustion and suppression of antitumor immunity [275]. This immunosuppressive signaling axis contributes to a hostile tumor microenvironment that undermines ICI efficacy. Paulson et al. further highlighted that therapy resistance in immunotherapy-treated cancers often arises from dynamic changes in antigen presentation, immune cell infiltration, and tumor-intrinsic adaptations that blunt immune recognition [276]. Complementing these findings, revealed that tumors can evade immune surveillance through transcriptional reprogramming and selective loss of checkpoint targets, reinforcing the complexity of resistance mechanisms and the need for combinatorial strategies to restore immune competence [277].
5.7. Stem Cell Therapy
Stem cell therapy regenerates damaged tissues and supports hematologic recovery [278,279,280]. Nanotechnology enhances the following:
- Stem cell tracking and imaging.
- Directed differentiation.
- Safe and efficient delivery to target tissues.
5.7.1. Applications for Cancer Treatment
- Hematopoietic Stem Cell Transplants: Restore blood cell production [281].
- Mesenchymal Stem Cells (MSCs): Exhibit anti-inflammatory and tumor-targeting properties [282].
- Induced Pluripotent Stem Cells (iPSCs): Enable patient-specific regenerative treatments [283].
5.7.2. Cellular and Metabolic Drivers of Immunotherapy Resistance
Overcoming resistance to cancer immunotherapy requires a nuanced understanding of the cellular and metabolic mechanisms that suppress immune function. It has been emphasized that immune cell exhaustion, marked by sustained expression of inhibitory receptors and diminished effector function can be pharmacologically modulated to restore antitumor immunity and improve therapeutic outcomes [284]. Furthermore, it has been documented how resistance to PD-1 blockade arises from multifactorial disruptions in antigen presentation, T-cell trafficking, and compensatory inhibitory pathways, necessitating combinatorial approaches to sustain immune activation [285]. Metabolic reprogramming within tumor cells also plays a critical role in immune escape, as Ho et al. demonstrated that altered glycolytic flux and nutrient competition can impair T-cell function and promote an immunosuppressive microenvironment [286]. Additionally, the role of tumor-associated macrophages (TAMs) in orchestrating immune suppression, angiogenesis, and therapy resistance, positioning TAMs as both biomarkers and therapeutic targets in the evolving landscape of immuno-oncology has been documented [287].
6. Immune Evasion and Therapeutic Resistance: Implications for Nanotheranostics
Cancer cells employ a diverse array of immune escape mechanisms that undermine the efficacy of immunotherapy, including downregulation of antigen presentation, secretion of immunosuppressive cytokines, and remodeling of the tumor microenvironment to exclude effector immune cells [288]. These adaptive responses not only facilitate tumor survival but also contribute to resistance against immune checkpoint inhibitors. Xiang et al. further emphasized that overcoming immunotherapy resistance requires multifaceted strategies, such as metabolic reprogramming, modulation of immune cell exhaustion, and rational combination therapies to restore immune competence and improve clinical outcomes [289]. While nanotheranostics offers a promising platform to deliver such interventions with precision, its clinical translation is hindered by limitations in long-term biocompatibility, regulatory standardization, and scalable manufacturing. Addressing these challenges will be critical to fully harness nanotheranostics as a vehicle for overcoming immune escape and resistance in next-generation cancer therapy.
6.1. Current Limitations in Nanotheranostics Applications
- Tumor Heterogeneity: Cancer cells exhibit diverse molecular profiles, complicating the design of universal nanotheranostic platforms. Tailoring nanoparticles to specific tumor subtypes remains a major hurdle [290].
- Biocompatibility and Long-Term Safety: Concerns persist regarding nanoparticle retention, clearance, and unforeseen toxicity. Longitudinal studies are essential to assess the chronic exposure risks and biodistribution [291].
- Manufacturing Scalability: Large-scale nanoparticle production demands standardized protocols to ensure reproducibility and batch-to-batch consistency for clinical applications [292].
- Regulatory Barriers: The approval process for nanomedicine lacks harmonized international guidelines, delaying clinical adoption and complicating global deployment [293].
- Economic Constraints: The high development costs for nanotheranostics, imaging technologies, and drug delivery systems limit accessibility, especially in low-resource settings [294].
Addressing these limitations will require interdisciplinary collaboration, regulatory reform, and cost-effective innovations to ensure the clinical integration of nanomedicine.
6.2. Future Research Opportunities and Potential Breakthroughs
Cancer research is advancing toward adaptive, programmable, and personalized nanomedicine platforms that will integrate nanotechnology with cutting-edge therapeutics to overcome biological barriers and improve patient outcomes [295,296].
Emerging Research Areas
- Programmable Adaptive Nanoparticles: Moving beyond static multifunctionality, next-generation nanoplatforms are being engineered to respond dynamically to tumor microenvironmental cues, such as pH, enzymatic activity, and hypoxia, to trigger site-specific drug release, imaging contrast enhancement, or immune activation in real time [297]. These platforms utilize modular design principles for customization across cancer types and therapeutic modalities.
- AI-Guided Nanomedicine Engineering: Artificial intelligence is increasingly being used to model nanoparticle–biological interactions, optimize surface functionalization, and simulate pharmacokinetics across diverse patient profiles [298]. Machine learning algorithms enable predictive design of nanocarriers with enhanced tumor penetration, reduced off-target effects, and tailored payload combinations, facilitating precision-guided nanotherapeutic development.
- Nanotheranostics-Enhanced Immunotherapy: Nanoparticles are being developed to deliver checkpoint inhibitors and tumor antigens directly to lymphoid tissues, enabling spatiotemporal control of immune activation [299]. Additionally, nanocarriers can modulate the tumor microenvironment to reverse immunosuppression, enhancing T cell infiltration and therapeutic durability.
- CRISPR-Cas9 and Nanomedicine Synergy: Non-viral nanoparticle carriers are being optimized for CRISPR-Cas9 delivery, enabling targeted genome editing with reduced immunogenicity and improved intracellular transport [300]. This synergy supports precise correction of oncogenic mutations and opens avenues for curative interventions in genetically driven cancers.
- Hybrid Imaging–Therapy Platforms: The integration of photoacoustic imaging with theranostic nanoparticles allows for real-time visualization of drug delivery and tumor response [301]. These dual-function platforms are advancing image-guided precision oncology by combining diagnostic and therapeutic capabilities in a single system.
With continued investment in research and technology, these innovations will redefine the boundaries of nanomedicine, enabling more effective, accessible, and patient-centered cancer therapies [302].
7. Discussion
The schematic diagram in Figure 1 illustrates the multifaceted role of nanotheranostics in cancer diagnostics and therapy, encompassing liposomes, polymeric micelles, dendrimers, virus-like particles (VLPs), and gold nanoparticles. These materials offer substantial advantages in targeted drug delivery, imaging, immune modulation, and multimodal therapy. The dendrimer molecule, with its branched polymeric structure, serves as a nanocarrier enabling photothermal therapy and gene delivery.
The immunotherapeutic potential of VLPs is especially relevant, as these engineered structures mimic viral capsids, efficiently delivering tumor-associated antigens. Gold nanorods, integral to photothermal therapy, enhance tumor imaging and ablation via laser-mediated heating. By combining therapeutic and diagnostic elements, nanotheranostics bridges molecular precision with enhanced treatment efficacy.
Figure 2 presents a mechanistic overview of chromophore-assisted light inactivation (CALI), where chromophores bind to tumor-associated proteins and initiate localized oxidative damage upon excitation. Light activation induces ROS generation, selectively disrupting cancer cells’ functioning while preserving the surrounding healthy tissues. This approach aligns with modern immune checkpoint therapies, as detailed in Figure 2, where molecular profiling of tumor epitopes enhances their immune-mediated destruction.
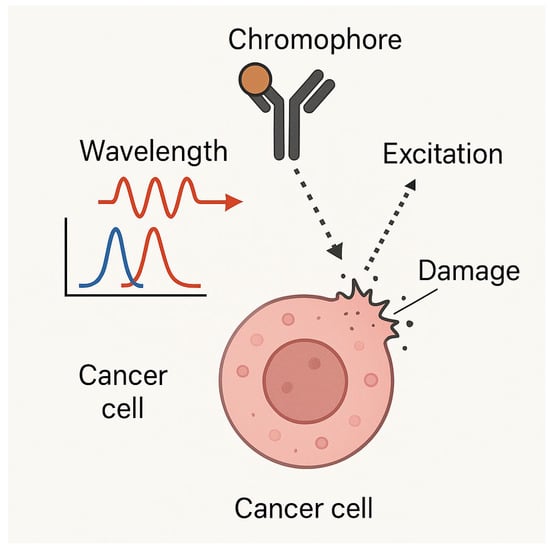
Figure 2.
Chromophore-assisted light inactivation (CALI) mechanism: A schematic representation of CALI-based targeted cancer cell destruction, showcasing chromophore-binding specificity, excitation–emission interactions, and localized damage induction. Light activation of chromophores leads to ROS generation, selectively inactivating key proteins and inducing apoptosis in cancer cells.
As highlighted in a recent report, the identification of actionable biomarkers has enabled the deployment of targeted treatments, including immune checkpoint inhibitors that modulate the PD-1/PD-L1 axis to restore antitumor immunity [303]. Specifically, these are agents such as nivolumab and pembrolizumab block PD-1 receptors on T cells, thereby reinvigorating immune responses against tumor cells; a mechanism that has demonstrated significant clinical efficacy in non-small cell lung cancer (NSCLC) [304,305,306]. These therapies not only exemplify the shift toward personalized oncology but also underscore the importance of molecular profiling in guiding treatment decisions [307].
These strategies complement CALI by amplifying cancer antigen exposure, improving tumor clearance through immune modulation. Immune checkpoint inhibitors such as atezolizumab, nivolumab, ipilimumab, durvalumab, and pembrolizumab have demonstrated significant efficacy across various cancer types by enhancing T cell-mediated antitumor responses. For instance, atezolizumab has shown superiority over chemotherapy in urothelial carcinoma [308], while the combination of nivolumab and ipilimumab has yielded promising outcomes in lung cancers with high tumor mutational burden [309]. Durvalumab, administered post-chemoradiotherapy, has improved survival in stage III NSCLC [310], and pembrolizumab has outperformed chemotherapy in PD-L1–positive NSCLC patients [311]. Nivolumab has also proven effective in previously treated NSCLC, offering a viable alternative to docetaxel [312]. Central to these therapies is PD-L1 expression, which serves as a predictive biomarker for response, guiding patient selection and optimizing therapeutic outcomes [313].
Liposomal drug delivery systems (Figure 3) have demonstrated structured encapsulation, targeted release, and prolonged drug circulation. The EPR effect enhances liposome accumulation in tumors, facilitating site-specific drug deployment whilst reducing systemic toxicity. Surface-functionalized liposomes, integrating targeting moieties, can improve cellular uptake in malignancies with known receptor overexpression. Liposomes thus serve as a key nanocarrier in chemotherapy and gene therapy applications.
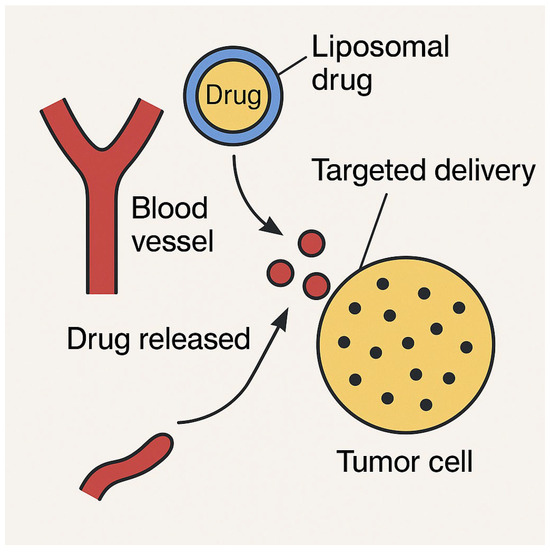
Figure 3.
Liposomal drug delivery: A schematic representation of liposomal drug delivery, illustrating the structural composition, encapsulation mechanism, and controlled release of therapeutic agents. This figure highlights the role of liposomes as nanocarriers, enhancing drug solubility, stability, and targeted delivery to cancer cells.
The tumor microenvironment (TME), depicted in Figure 4, presents the barriers that limit conventional drug penetration and efficacy. Hypoxic conditions, an acidic pH, and the extracellular matrix density can restrict therapeutic access to cancerous tissues. Smart nanoparticles, including pH-sensitive liposomes and dendrimers, exhibit environment-adaptive drug release, responding dynamically to tumoral conditions. Gold nanorods and graphene-based nanocomposites, essential for photothermal therapy, can overcome dense extracellular structures via heat-induced disruption, increasing the therapeutic precision.
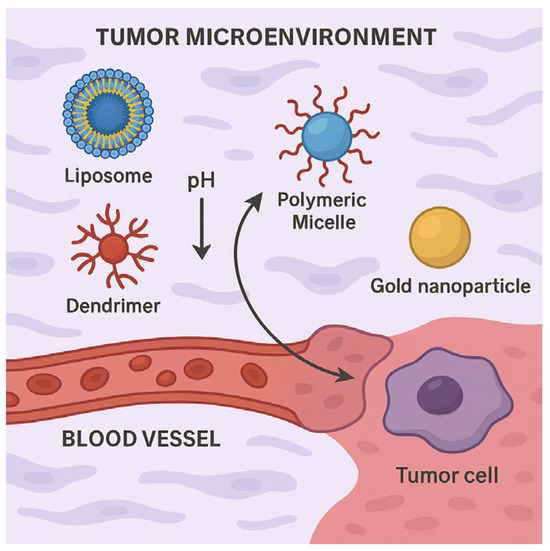
Figure 4.
Schematic representation of various nanocarriers (liposomes, polymeric micelles, dendrimers, and gold nanoparticle) interacting within tumor microenvironment via blood vessel, highlighting pH responsiveness and targeted delivery to tumor cell.
Figure 5 presents a pyramidal timeline capturing the major advancements in nanotheranostics, with each milestone contributing to the growing sophistication of cancer treatment strategies. This timeline chronicles the groundbreaking innovations, tracing the journey from the foundational theranostic concepts to modern multimodal applications in precision oncology. In 1988, the term theranostics emerged, heralding a paradigm shift by merging therapy and diagnostics into a unified approach to cancer management. This innovation enabled real-time monitoring of therapeutic responses, enhancing treatment adaptability and individualization. By 2005, gold nanoparticle-based imaging and photothermal therapy had revolutionized non-invasive cancer interventions. Gold nanoparticles, with their unique optical properties, facilitated tumor localization and selective ablation, marking a breakthrough in minimally invasive treatments. Moving forward, 2010 saw the expansion of polymeric micelle applications, significantly enhancing drug delivery efficiency. These nanocarriers improved their solubility and targeted release, reducing systemic toxicity and optimizing therapeutic precision in chemotherapy. A pivotal moment in 2015 was the introduction of virus-like particles (VLPs) into cancer immunotherapy. Engineered to mimic viral structures, VLPs stimulate robust immune responses, offering promising avenues for tumor antigen presentation and vaccine development. Their ability to activate adaptive immunity transformed the landscape of cancer immunotherapy. Finally, in 2024, an unprecedented leap in multimodal nanotheranostic systems enabled concurrent cancer imaging and therapy, marking a new frontier in personalized medicine. These integrated platforms now provide real-time monitoring, precision targeting, and dynamic adaptability, optimizing patient outcomes with minimized side effects. Through decades of continuous innovation, nanotheranostics has solidified itself as a foundational pillar in modern oncology, paving the way for next-generation therapies that will merge molecular precision with advanced therapeutic modalities.
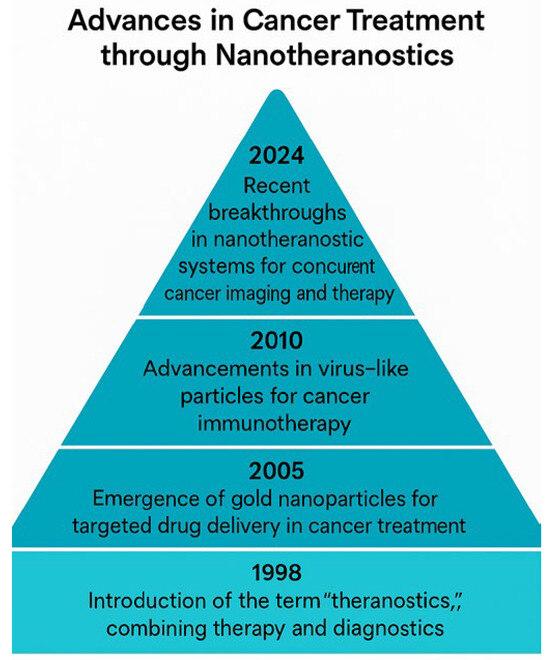
Figure 5.
Advances in cancer treatment through nanotheranostics: This pyramidal timeline illustrates the key milestones in nanotheranostics for cancer treatment, marking the significant advancements from 1998 to 2024. Each level of the pyramid corresponds to a breakthrough in a nanotechnology, showcasing the evolution of theranostics, gold nanoparticles, polymeric nanoparticles, virus-like particles, and advanced nanotheranostic systems for concurrent cancer imaging and therapy.
8. Regulatory and Manufacturing Considerations in Nanomedicine Development
The translation of nanomedicines from the bench to bedside demands a harmonized framework that addresses regulatory, manufacturing, and safety dimensions. This section synthesizes the current perspectives on FDA/EMA regulatory guidance, manufacturing scalability, quality control, and post-market surveillance, while proposing structural enhancements for scientific reporting.
8.1. Regulatory Frameworks: FDA and EMA Guidance
Nanomedicines are regulated under existing medicinal product frameworks, yet their unique physicochemical properties necessitate tailored guidance. The FDA has issued several documents addressing nanomaterial use in drug products, including guidance on liposome drug products and nanomaterial safety in cosmetics and biologics [314]. The EMA, through reflection papers and multidisciplinary guidelines, has emphasized the need for early scientific advice and case-by-case evaluation, particularly for nanosimilars and complex hybrid systems [315]. Despite these efforts, a unified regulatory definition of nanomedicines remains elusive, complicating cross-border harmonization [316].
8.2. Manufacturing Scalability and Batch Consistency
Scaling nanopharmaceutical production presents formidable challenges. Techniques such as microfluidics, supercritical fluid processing, and high-pressure homogenization offer promise, but often suffer from reproducibility issues [317]. Batch-to-batch variability in nanoparticle size, surface charge, and drug loading can significantly impact the pharmacokinetics and therapeutic efficacy. Quality-by-design (QbD) approaches and real-time analytics are increasingly being advocated to mitigate these risks [318,319].
8.3. GMP Requirements and Quality Control Measures
Good Manufacturing Practice (GMP) compliance for nanomedicines requires rigorous control of critical quality attributes (CQAs), including the particle size distribution, zeta potential, and encapsulation efficiency [320]. The PIC/S Guide to GMP PE009-16 outlines the updated expectations for investigational and commercial nanomedicines, emphasizing validated analytical methods and robust documentation [321]. However, conventional QC tools often lack sensitivity for nanoscale features, necessitating advanced characterization platforms, such as dynamic light scattering, electron microscopy, and nanoparticle tracking analysis [322].
8.4. Nanotoxicity: Mechanistic Insights and Risk Assessment
Nanotoxicity remains a central concern in regulatory evaluation. Mechanistic studies have revealed that nanoparticles may induce oxidative stress, membrane disruption, autophagy dysregulation, and inflammatory cascades [323]. Omics-based approaches and organ-on-chip models are emerging as powerful tools to predict the long-term toxicity and off-target effects [324]. Regulatory bodies are increasingly calling for chronic exposure data and mechanistic validation to support safety claims [325].
8.5. Environmental Impact and Lifecycle Assessments
The environmental risk assessment (ERA) of nanomedicines is underdeveloped but gaining traction. Studies have highlighted the need for lifecycle-based evaluations, encompassing nanoparticle release during manufacturing, usage, and disposal [326]. The OECD Working Party on Manufactured Nanomaterials and the EMA’s Horizon Scanning Reports have advocated for standardized ERA protocols, including environmental persistence, bioaccumulation, and ecotoxicity testing. Lifecycle assessments (LCAs) have further underscored the importance of sustainable nanomanufacturing practices [327].
8.6. Post-Market Surveillance and Pharmacovigilance
Post-marketing surveillance (PMS) is critical for capturing rare adverse events and long-term safety signals [328]. Regulatory agencies, such as the FDA, EMA, and TGA, mandate periodic safety update reports (PSURs), risk management plans (RMPs), and real-world evidence collection [329]. However, nanomedicine-specific PMS frameworks are still evolving. Integration of AI-driven signal detection and patient registries may enhance vigilance and responsiveness [330].
8.7. Enhanced Manuscript Organization and Terminology Standardization
To improve clarity and accessibility, we recommend restructuring review manuscripts to progress from fundamental principles (e.g., nanoparticle design, physicochemical properties) to clinical applications (e.g., oncology, infectious diseases). An executive summary should distill the key findings and recommendations for the stakeholders. Additionally, a glossary of standardized terminology, covering terms such as “PEGylation,” “zeta potential,” “nanosimilars,” and “QbD”, will aid interdisciplinary comprehension.
9. Conclusions
The integration of nanotheranostics and emerging therapies is redefining cancer treatment, offering precision-targeted interventions, reduced toxicity, and real-time therapeutic monitoring. Whilst technical, regulatory, and economic barriers remain, ongoing scientific advancements are accelerating their clinical translation. Future research directions (including AI-driven diagnostics, adaptive nanocarriers, and immunotherapy synergies) will shape the next generation of oncology. By fostering interdisciplinary collaboration, refining clinical accessibility, and enhancing treatment customization, nanotheranostics is set to revolutionize cancer care, paving the way for more personalized, effective, and minimally invasive therapies [331].
Author Contributions
Conceptualization, V.A. and I.E.C.; methodology, V.A.; software, V.A.; validation, V.A. and I.E.C.; formal analysis, V.A.; investigation, V.A. and I.E.C.; resources, V.A.; data curation, V.A.; writing—original draft preparation, V.A. and I.E.C.; writing—review and editing, V.A. and I.E.C.; visualization, V.A.; supervision, I.E.C.; project administration, V.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
During the preparation of this manuscript/study, the authors used Microsoft Copilot for the purposes of generating and compiling the references and images used in this review. The authors have reviewed and edited the references and take full responsibility for the content of this publication.
Conflicts of Interest
All authors declare no conflicts of interest.
References
- Chen, X. From quantum dots to cancer nanotheranostics. Nano Lett. 2016, 16, 5054–5063. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef] [PubMed]
- Lankoff, A.M.; Czerwińska, M.; Kruszewski, M. Advances in Nanotheranostic Systems for Concurrent Cancer Imaging and Therapy: An Overview of the Last 5 Years. Molecules 2024, 29, 5985. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Bukhari, S.I.; Imam, S.S.; Ahmad, M.Z.; Vuddanda, P.R.; Alshehri, S.; Mahdi, W.A.; Ahmad, J. Recent Progress in Lipid Nanoparticles for Cancer Theranostics: Opportunity and Challenges. Pharmaceutics 2021, 13, 840. [Google Scholar] [CrossRef]
- Salgueiro, M.J.; Zubillaga, M. Theranostic Nanoplatforms in Nuclear Medicine: Current Advances, Emerging Trends, and Perspectives for Personalized Oncology. J. Nanotheranostics 2025, 6, 27. [Google Scholar] [CrossRef]
- Gupta, D.; Roy, P.; Sharma, R.; Kasana, R.; Rathore, P.; Gupta, T.K. Recent nanotheranostic approaches in cancer research. Clin. Exp. Med. 2024, 24, 8. [Google Scholar] [CrossRef]
- Carrese, B.; Sanità, G.; Lamberti, A. Nanoparticles Design for Theranostic Approach in Cancer Disease. Cancers 2022, 14, 4654. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Simanek, E.E. Nonviral Vectors for Gene Delivery. Chem. Rev. 2009, 109, 259–302. [Google Scholar] [CrossRef]
- Fallatah, M.M.; Alradwan, I.; Alfayez, N.; Aodah, A.H.; Alkhrayef, M.; Majrashi, M.; Jamous, Y.F. Nanoparticles for Cancer Immunotherapy: Innovations and Challenges. Pharmaceuticals 2025, 18, 1086. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Mirshojaei, S.F.; Ahmadi, A.; Morales-Avila, E.; Ortiz-Reynoso, M.; Reyes-Perez, H. Radiolabelled Nanoparticles: Novel Classification of Radiopharmaceuticals for Molecular Imaging of Cancer. J. Drug Target. 2016, 24, 91–101. [Google Scholar] [CrossRef]
- Najdian, A.; Beiki, D.; Abbasi, M.; Gholamrezanezhad, A.; Ahmadzadehfar, H.; Amani, A.M.; Shafiee Ardestani, M.; Assadi, M. Exploring innovative strides in radiolabeled nanoparticle progress for multimodality cancer imaging and theranostic applications. Cancer Imaging 2024, 24, 127. [Google Scholar] [CrossRef]
- Bourgeois, A.; Horrill, T.; Mollison, A.; Stringer, E.; Lambert, L.K.; Stajduhar, K. Barriers to cancer treatment for people experiencing socioeconomic disadvantage in high-income countries: A scoping review. BMC Health Serv. Res. 2024, 24, 670. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Wang, C.-Y.; Chang, H.-H.; Chang, P.T.S.; Chang, C.-H.; Chu, T.Y.; Hsu, P.-C.; Kuo, C.-Y. Diagnostics and Therapy for Malignant Tumors. Biomedicines 2024, 12, 2659. [Google Scholar] [CrossRef] [PubMed]
- Iragorri, N.; de Oliveira, C.; Fitzgerald, N.; Essue, B. The Out-of-Pocket Cost Burden of Cancer Care—A Systematic Literature Review. Curr. Oncol. 2021, 28, 1216–1248. [Google Scholar] [CrossRef] [PubMed]
- Bhat, G.R.; Sethi, I.; Sadida, H.Q.; Rah, B.; Mir, R.; Algehainy, N.; Albalawi, I.A.; Masoodi, T.; Subbaraj, G.K.; Jamal, F.; et al. Cancer cell plasticity: From cellular, molecular, and genetic mechanisms to tumor heterogeneity and drug resistance. Cancer Metastasis Rev. 2024, 43, 197–228. [Google Scholar] [CrossRef] [PubMed]
- Proietto, M.; Crippa, M.; Damiani, C.; Pasquale, V.; Sacco, E.; Vanoni, M.; Gilardi, M. Tumor heterogeneity: Preclinical models, emerging technologies, and future applications. Front. Oncol. 2023, 13, 1164535. [Google Scholar] [CrossRef]
- Ghorbian, S. Cancer cell plasticity and therapeutic resistance: Mechanisms, crosstalk, and translational perspectives. Hereditas 2025, 162, 188. [Google Scholar] [CrossRef]
- Rituraj; Pal, R.S.; Wahlang, J.; Pal, Y.; Chaitanya, M.V.N.L.; Saxena, S. Precision oncology: Transforming cancer care through personalized medicine. Med. Oncol. 2025, 42, 246. [Google Scholar] [CrossRef]
- Wang, X. Highlights the recent important findings in cancer heterogeneity. Holist. Integr. Oncol. 2023, 2, 15. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer Drug Resistance: An Evolving Paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.V.; Lee, D.Y.; Li, B.; Quinlan, M.P.; Takahashi, F.; Maheswaran, S.; McDermott, U.; Azizian, N.; Zou, L.; Fischbach, M.A.; et al. A Chromatin-Mediated Reversible Drug-Tolerant State in Cancer Cell Subpopulations. Cell 2010, 141, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Terasawa, T.; Tadano, T.; Abe, K.; Sasaki, S.; Hosono, S.; Katayama, T.; Hoshi, K.; Nakayama, T.; Hamashima, C. Single-round performance of colorectal cancer screening programs: A network meta-analysis of randomized clinical trials. BMC Med. 2025, 23, 110. [Google Scholar] [CrossRef]
- Stefan, D.C.; Tang, S. Addressing cancer care in low- to middle-income countries: A call for sustainable innovations and impactful research. BMC Cancer 2023, 23, 756. [Google Scholar] [CrossRef]
- Ige, T.; Lewis, P.; Shelley, C.; Pistenmaa, D.; Coleman, C.N.; Aggarwal, A.; Dosanjh, M.; ICEC Survey Team. Understanding the challenges of delivering radiotherapy in low- and middle-income countries in Africa. J. Cancer Policy 2023, 35, 100372. [Google Scholar] [CrossRef]
- Du, R.; Wang, X.; Ma, L.; Larcher, L.M.; Tang, H.; Zhou, H.; Chen, C.; Wang, T. Adverse reactions of targeted therapy in cancer patients: A retrospective study of hospital medical data in China. BMC Cancer 2021, 21, 206. [Google Scholar] [CrossRef]
- Solanki, S.; Kumar, P.; Mumbrekar, K.D. Cancer therapy-induced cardiotoxicity: Mechanisms and mitigations. Heart Fail. Rev. 2025, 30, 1075–1092. [Google Scholar] [CrossRef]
- Zafar, S.Y.; Peppercorn, J.M.; Schrag, D.; Taylor, D.H., Jr.; Goetzinger, A.M.; Zhong, Y.; Abernethy, A.P. The Financial Toxicity of Cancer Treatment: A Pilot Study Assessing Out-of-Pocket Expenses and the Insured Cancer Patient’s Experience. Oncologist 2013, 18, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Kitaw, T.A.; Tilahun, B.D.; Zemariam, A.B.; Getie, A.; Bizuayehu, M.A.; Haile, R.N. The financial toxicity of cancer: Unveiling global burden and risk factors—A systematic review and meta-analysis. BMJ Glob. Health 2025, 10, e017133. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.-S. Nanotheranostics—Application and Further Development of Nanomedicine Strategies for Advanced Theranostics. Theranostics 2014, 4, 660–677. [Google Scholar] [CrossRef] [PubMed]
- Azimizonuzi, H.; Ghayourvahdat, A.; Ahmed, M.H.; Abdul Kareem, R.; Zrzor, A.J.; Mansoor, A.S.; Athab, Z.H.; Kalavi, S.A. A State-of-the-Art Review of the Recent Advances of Theranostic Liposome-Hybrid Nanoparticles in Cancer Treatment and Diagnosis. Cancer Cell Int. 2025, 25, 26. [Google Scholar] [CrossRef]
- Zhuang, L.; Lian, Y.; Zhu, T. Multifunctional gold nanoparticles: Bridging detection, diagnosis, and targeted therapy in cancer. Mol. Cancer 2025, 24, 228. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal Drug Delivery Systems: From Concept to Clinical Applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, In Vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef]
- Wu, P.; Ameen, T.; Zhang, H.R.; Wang, J.; Li, X.; Chen, C.; Liu, M.J.; Huang, R. Complementary black phosphorus tunneling field effect transistors. ACS Nano 2019, 13, 377–385. [Google Scholar] [CrossRef]
- Mi, P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef]
- Wu, J.; Du, K.; Bao, Y.; Xiong, M.; Chen, J.; Luo, Z.; Zhang, D.; Shi, Y. Docetaxel-loaded pH/ROS dual-responsive nanoparticles for the targeted treatment of gastric cancer. Cancer Nanotechnol. 2025, 16, 5. [Google Scholar] [CrossRef]
- Kapalatiya, H.; Madav, Y.; Tambe, V.S.; Wairkar, S. Enzyme-Responsive Smart Nanocarriers for Targeted Chemotherapy: An Overview. Drug Deliv. Transl. Res. 2022, 12, 1293–1305. [Google Scholar] [CrossRef] [PubMed]
- Vasylyshyn, T.; Huntošová, V.; Patsula, V.; Olejárová, S.; Slabý, C.; Jurašeková, Z.; Bánó, G.; Kubacková, J.; Šlouf, M.; Shapoval, O.; et al. Surface-engineered core–shell upconversion nanoparticles for effective hypericin delivery and multimodal imaging. Nanoscale 2025, 17, 10548–10562. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, M.; Muñoz-Unceta, N.; Matorras, A.; Jara, P.; Castro, C.; Cacho, D.; Caramelo, B.; Azueta, A.; Durán, I. Outcomes with atezolizumab in metastatic urothelial cancer: Real-world data from a single institution. Clin. Transl. Oncol. 2024, 26, 682–688. [Google Scholar] [CrossRef]
- Gupta, M.; Singh, S.P. Nanoparticles for multimodal imaging and theranostic applications in cancer diagnosis and treatment. J. Pharmacogn. Phytochem. 2024, 13, 236–243. [Google Scholar] [CrossRef]
- Gawne, P.J.; Ferreira, M.; Papaluca, M.; Grimm, J.; Decuzzi, P. New opportunities and old challenges in the clinical translation of nanotheranostics. Nat. Rev. Mater. 2023, 8, 1045–1062. [Google Scholar] [CrossRef]
- Miao, D.; Margolis, C.A.; Gao, W.; Voss, M.H.; Li, W.; Martini, D.J.; Norton, C.R.; Bossé, D.; Schadendorf, D.; Shah, S.P.; et al. Genomic Correlates of Response to Immune Checkpoint Blockade in Microsatellite Stable Solid Tumors. Nat. Genet. 2018, 50, 1270–1277. [Google Scholar] [CrossRef]
- Florou, V.; Floudas, C.S.; Maoz, A.; Naqash, A.R.; Norton, C.; Tan, A.C.; Sokol, E.S.; Frampton, G.; Soares, H.P.; Puri, S.; et al. Real-world pan-cancer landscape of frameshift mutations and their role in predicting responses to immune checkpoint inhibitors in cancers with low tumor mutational burden. J. Immunother. Cancer 2023, 11, e007440. [Google Scholar] [CrossRef]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J. Analysis of 100 000 Human Cancer Genomes Reveals the Landscape of Tumor Mutational Burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef]
- Bleaney, C.W.; Abdelaal, H.; Reardon, M.; Anandadas, C.; Hoskin, P.; Choudhury, A.; Forker, L. Clinical biomarkers of tumour radiosensitivity and predicting benefit from radiotherapy: A systematic review. Cancers 2024, 16, 1942. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Pio, R.; Ajona, D.; Ortiz-Espinosa, S.; Mantovani, A.; Lambris, J.D. Complementing the Cancer-Immunity Cycle. Front. Immunol. 2019, 10, 774. [Google Scholar] [CrossRef]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Koç, M.M.; Paksu, U.; Yetim, N.K.; Coşkun, B.; Özkan, E.H.; Erkovan, M. Nanoparticles in photothermal therapy-based medical and theranostic applications: An extensive review. Eur. Phys. J. Plus 2025, 140, 514. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Ji, D.-K.; Huang, Y.; Huang, W.; Dong, X.; Yao, D.; Wang, D. Bio-orthogonal click chemistry strategy for PD-L1-targeted imaging and pyroptosis-mediated chemo-immunotherapy of triple-negative breast cancer. J. Nanobiotechnol. 2024, 22, 461. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Li, R.; Liu, J.; Yang, L.; Zhang, Y.; Wang, Y.; Jiang, Y. Hybrid near-infrared-activated luminescent gold nanoparticle platform for efficient cancer therapy. Adv. Compos. Hybrid Mater. 2025, 8, 173. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, X.; Wu, L.; Wu, M.; James, T.D.; Zhang, R. Bioorthogonally activated probes for precise fluorescence imaging. Chem. Soc. Rev. 2025, 54, 1–36. [Google Scholar] [CrossRef]
- Ow, V.; Lin, Q.; Wong, J.H.M.; Sim, B.; Tan, Y.L.; Leow, Y.; Goh, R.; Loh, X.J. Understanding the interplay between pH and charges for theranostic nanomaterials. Nanoscale 2025, 17, 6960–6980. [Google Scholar] [CrossRef]
- Zhang, S.; Qin, S.; Xiao, Y.; Liu, Z.; Hu, X.; Huang, D.; Ye, X. Near-infrared luminescent materials: A review of their practical applications and prospective advancements. Dalton Trans. 2025, 54, 3538–3562. [Google Scholar] [CrossRef]
- Malla, P.; Wang, Y.-M.; Su, C.-H. New horizons for the therapeutic application of nanozymes in cancer treatment. J. Nanobiotechnol. 2025, 23, 130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Otsuka, A.; Nakamura, N.; Tatsumi, T.; Nakatsui, K.; Tsuzukiishi, T.; Sakanoue, T.; Shimazoe, K.; Ohta, S. 64Cu-chelated InP/ZnSe/ZnS QDs as PET/fluorescence dual-modal probe for tumor imaging. Sci. Technol. Adv. Mater. 2025, 26, 2463317. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, K.M.; Sharma, G.; Singh, A.P.; Siddiqui, J.A. Nanomedicine in Cancer Therapeutics: Current Perspectives from Bench to Bedside. Mol. Cancer 2025, 24, 169. [Google Scholar] [CrossRef] [PubMed]
- Maksymova, L.; Pilger, Y.A.; Nuhn, L.; Van Ginderachter, J.A. Nanobodies targeting the tumor microenvironment and their formulation as nanomedicines. Mol. Cancer 2025, 24, 65. [Google Scholar] [CrossRef]
- Nizam, I.; Aasaithambi, K.; Srinivasan, A.; Chidambaram, S.B.; Krishnamurthy, P.T.; Madhunapantula, S.R.; Thimmulappa, R.; Kuppusamy, G. Nanotheranostics in cardiovascular diseases: A novel tool. Int. J. Appl. Pharm. 2023, 15, 47521. [Google Scholar] [CrossRef]
- Huang, R.; Hu, Q.; Ko, C.-N.; Tang, F.K.; Xuan, S.; Wong, H.M.; Jin, L.; Li, X.; Leung, K.C.-F. Nano-based theranostic approaches for infection control: Current status and perspectives. Mater. Chem. Front. 2024, 8, 9–40. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, B.; Shen, Y.; Zhao, Y.; Fu, X.; Zhu, Y.; Gu, G.; Liu, C. Screening biomarkers for predicting the efficacy of immunotherapy in patients with PD-L1 overexpression. J. Cancer Res. Clin. Oncol. 2023, 149, 12965–12976. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, Y.; Li, Z.; Guo, L. Nanomedicine in cardiovascular and cerebrovascular diseases: Targeted nanozyme therapies and their clinical potential and current challenges. J. Nanobiotechnol. 2025, 23, 543. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Cosme Pecho, R.D.; Amin, A.H.; Akhavan-Sigari, R. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, M.J.; Kizhuveetil, U.; Johnson, A.; Nagarajan, R.; Muthuvijayan, V. Cancer nanomedicine: A review of nano-therapeutics and challenges ahead. RSC Adv. 2023, 13, 27863–27890. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Dash, P.; Das, P.; Nayak, B. Ligand-targeted polymeric nanoparticles for cancer chemotherapy. In Polymeric Nanoparticles for the Treatment of Solid Tumors; Springer: Cham, Switzerland, 2022; pp. 251–272. [Google Scholar] [CrossRef]
- Cheng, Y.; Morshed, R.A.; Auffinger, B.; Tobias, A.L.; Lesniak, M.S. Multifunctional Nanoparticles for Brain Tumor Imaging and Therapy. Adv. Drug Deliv. Rev. 2014, 66, 42–57. [Google Scholar] [CrossRef]
- Whitesides, G.M. The ‘Right’ Size in Nanobiotechnology. Nat. Biotechnol. 2003, 21, 1161–1165. [Google Scholar] [CrossRef]
- Unnikrishnan, G.; Joy, A.; Megha, M.; Kolanthai, E.; Senthilkumar, M. Exploration of inorganic nanoparticles for revolutionary drug delivery applications: A critical review. Discover Nano 2023, 18, 157. [Google Scholar] [CrossRef]
- Xie, D.; Sun, L.; Wu, M.; Li, Q. From detection to elimination: Iron-based nanomaterials driving tumor imaging and advanced therapies. Front. Oncol. 2025, 15, 1536779. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, M.; Xi, J.; Li, J.; Ma, R.; Ren, L.; Bai, Z.; Qi, K.; Li, X. Gold nanorods-based photothermal therapy: Interactions between biostructure, nanomaterial, and near-infrared irradiation. Nanoscale Res. Lett. 2022, 17, 68. [Google Scholar] [CrossRef]
- Choudhary, S.; Kaur, S.D.; Gandhi, H.; Pemmaraju, D.B.; Kapoor, D.N. An updated review on the potential of gold nanoparticles for cancer treatment and detection. Gold Bull. 2025, 58, 4. [Google Scholar] [CrossRef]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking Down Barriers: Advances in siRNA Delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef]
- Sun, W.; Ji, W.; Hall, J.M.; Hu, Q.; Wang, C.; Beisel, C.L.; Gu, Z. Self-Assembled DNA Nanoclews for the Efficient Delivery of CRISPR–Cas9. Angew. Chem. Int. Ed. 2015, 54, 12029–12033. [Google Scholar] [CrossRef]
- Appidi, T.; China, D.; Ștefan, G.-R.; Moreau, M.; Mao, S.; Velarde, E.; Toyang, N.; Lowe, H.; Rengan, A.K.; Ding, K.; et al. Engineered multifunctional nanoparticles for enhanced radiation therapy: Three-in-one approach for cancer treatment. Mol. Cancer 2025, 24, 68. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xia, W. Isolation of circulating tumor cells: Recent progress and future perspectives. Med-X 2024, 2, 28. [Google Scholar] [CrossRef]
- Yun, Y.; Kim, S.; Lee, S.-N.; Cho, H.-Y.; Choi, J.-W. Nanomaterial-based detection of circulating tumor cells and circulating cancer stem cells for cancer immunotherapy. Nano Convergence 2024, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Allard, W.J.; Matera, J.; Miller, M.C.; Repollet, M.; Connelly, M.C.; Rao, C.; Tibbe, A.G.; Uhr, J.W.; Terstappen, L.W.M.M. Tumor Cells Circulate in the Peripheral Blood of All Major Carcinomas but Not in Healthy Subjects or Patients with Benign Diseases. Clin. Cancer Res. 2004, 10, 6897–6904. [Google Scholar] [CrossRef]
- Yang, C.; Liu, C.; Zhang, Y.; Wang, H.; Zhao, J.; Li, X. Clinical applications of circulating tumor cells in metastasis and therapy. J. Hematol. Oncol. 2025, 18, 80. [Google Scholar] [CrossRef]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Circulating Breast Tumor Cells Exhibit Dynamic Changes in Epithelial and Mesenchymal Composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A.; et al. Isolation of Rare Circulating Tumor Cells in Cancer Patients by Microchip Technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef]
- Warkiani, M.E.; Guan, G.; Luan, K.B.; Lee, W.C.; Bhagat, A.A.S.; Chaudhuri, P.K.; Tan, D.S.W.; Lim, W.T.; Lee, S.C.; Chen, P.-C.; et al. Slanted Spiral Microfluidics for Ultra-fast, Label-free Isolation of Circulating Tumor Cells. Lab Chip 2014, 14, 128–137. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, X.; Guo, C.; Liu, Z.; Guo, X.; Tian, Z.; Wang, Z.; Yang, J.; Huang, X.; Ou, L. Greatly isolated heterogeneous circulating tumor cells using hybrid engineered cell membrane-camouflaged magnetic nanoparticles. J. Nanobiotechnol. 2024, 22, 231. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, J.; Li, Z.; Gou, Y. Microfluidic platform for circulating tumor cells isolation and detection. BIOCELL 2023, 47, 1439–1447. [Google Scholar] [CrossRef]
- Stevens, M.; Mentink, A.; Coumans, F.A.W.; Dathathri, E.; Isebia, K.T.; Kraan, J.; de Wit, R.; Martens, J.W.M.; Terstappen, L.W.M.M. Flow-based immunomagnetic enrichment of circulating tumor cells from diagnostic leukapheresis product. Mol. Oncol. 2023, 17, 13565. [Google Scholar] [CrossRef]
- Ashour, A.A.; Tayeb, F.J.; Felemban, M.F.; Shafie, A. Nanomaterial-based biosensors for cancer diagnosis: Trends and innovations (2022–2025). Microchim. Acta 2025, 192, 404. [Google Scholar] [CrossRef]
- Tan, W.; Zhu, Y.; Chen, S. Innovative approach to the detection of circulating tumor biomarkers: Multi-dimensional application of liposome technology. Lipids Health Dis. 2025, 24, 160. [Google Scholar] [CrossRef]
- Zhang, X.; Su, Y.; Ao, D.; Hong, Y.; Zou, M.; Xie, J.; Zhou, Q.; Wang, Y.; He, W.; Chen, Y. Label-free electrochemical immunosensor based on AuNPs/CCNTs/chitosan nanocomposites for carcinoembryonic antigen detection. J. Appl. Electrochem. 2025, 55, 1557–1569. [Google Scholar] [CrossRef]
- Lajoux, J.; Banguera-Ordoñez, Y.D.; Sena-Torralba, A.; Charbonnière, L.J.; Sy, M.; Goetz, J.; Maquieira, Á.; Morais, S. Breaking the picomolar barrier in lateral flow assays using Bright-Dtech-614 Europium nanoparticles. Microchem. J. 2025, 209, 112864. [Google Scholar] [CrossRef]
- Baruah, A.; Newar, R.; Das, S.; Kalita, N.; Nath, M.; Ghosh, P.; Chinnam, S.; Sarma, H.; Narayan, M. Biomedical applications of graphene-based nanomaterials: Recent progress, challenges, and prospects in highly sensitive biosensors. Discov. Nano 2024, 19, 103. [Google Scholar] [CrossRef] [PubMed]
- Agha, A.; Waheed, W.; Stiharu, I.; Nerguizian, V.; Destgeer, G.; Abu-Nada, E.; Alazzam, A. A review on microfluidic-assisted nanoparticle synthesis, and their applications using multiscale simulation methods. Discov. Nano 2023, 18, 18. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Z.; Yin, S.; Ma, X. Nanoplasmonic biosensors for precision medicine. Front. Chem. 2023, 11, 1209744. [Google Scholar] [CrossRef]
- Zhang, J.; Jian, X.; Bai, S.; Xu, G.; Du, M.; Guo, C.; Guan, Y. Impact of immunomagnetic nanoparticle size on capture efficiency, bioactivity, and proliferation ability of circulating tumor cells. J. Nanopart. Res. 2023, 25, 217. [Google Scholar] [CrossRef]
- Qi, X.; Lin, S.; Li, M. Atomic force microscopy combined with microfluidics for label-free sorting and automated nanomechanics of circulating tumor cells in liquid biopsy. Nanoscale 2025, 17, 8401–8415. [Google Scholar] [CrossRef]
- Tai, S.S.A.; Loo, H.L.; Bakhtiar, A.; Ho, P.C.-L.; Chuah, L.H. Antibody-conjugated polymer nanoparticles for brain cancer. Drug Deliv. Transl. Res. 2025, 15, 1–18. [Google Scholar] [CrossRef]
- Fabisiewicz, A.; Szostakowska-Rodzos, M.; Grzybowska, E.A. Improving the prognostic and predictive value of circulating tumor cell enumeration: Is longitudinal monitoring the answer? Int. J. Mol. Sci. 2024, 25, 10612. [Google Scholar] [CrossRef]
- Bashir, S.; Zia, M.A.; Shoukat, M.; Kaleem, I.; Bashir, S. Nanoparticles as a Novel Key Driver for the Isolation and Detection of Circulating Tumour Cells. Sci. Rep. 2024, 14, 67221. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.M.; et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, S. Nanomaterials in Tumor Immunotherapy: New Strategies and Challenges. Mol. Cancer 2023, 22, 94. [Google Scholar] [CrossRef] [PubMed]
- Stott, S.L.; Hsu, C.-H.; Tsukrov, D.I.; Yu, M.; Miyamoto, D.T.; Waltman, B.A.; Rothenberg, S.M.; Shah, A.M.; Smas, M.E.; Korir, G.K.; et al. Isolation of Circulating Tumor Cells Using a Microvortex-Generating Herringbone-Chip. Proc. Natl. Acad. Sci. USA 2010, 107, 18392–18397. [Google Scholar] [CrossRef]
- Ding, W.; Ye, W.; Liu, H.; Yang, J.; Chu, C.; Zhu, H.; Wang, J.; Zhou, L.; Zhao, M.; Liu, M. High-gradient microstructured hybrid microfluidic chip for rare tumor cell capture. Anal. Bioanal. Chem. 2025, 417, 2361–2374. [Google Scholar] [CrossRef]
- Rao, L.T.; Raz, A.; Patolsky, F. Biomarker analysis from complex biofluids by an on-chip chemically modified light-controlled vertical nanopillar array device. Nat. Protoc. 2024, 19, 1124–1146. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Y.; Lu, W.; Gong, T.; Ibáñez, E.; Cifuentes, A.; Lu, W. Microfluidic biosensors for biomarker detection in body fluids: A key approach for early cancer diagnosis. Biomark. Res. 2024, 12, 153. [Google Scholar] [CrossRef]
- Janjua, D.; Chaudhary, A.; Joshi, U.; Tripathi, T.; Kumar Jaggi, V.; Bharti, A.C. Redefining cancer care: Harnessing circulating tumor cells’ potential for improved diagnosis and prognosis. Cancer Cell Int. 2025, 25, 267. [Google Scholar] [CrossRef]
- Bednarz-Knoll, N.; Alix-Panabières, C.; Pantel, K. Plasticity of Disseminating Cancer Cells in Patients with Epithelial Malignancies. Cancer Metastasis Rev. 2011, 30, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Xi, M.; Li, J. Cancer metastasis: Molecular mechanisms and therapeutic interventions. Mol. Biomed. 2025, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Fasogbon, I.V.; Ondari, E.N.; Tusubira, D.; Kabuuka, T.; Abubakar, I.B.; Musyoka, A.M.; Aja, P.M. Advances and future directions of aptamer-functionalized nanoparticles for point-of-care disease diagnosis. Biol. Methods Protoc. 2025, 10, bpaf046. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Poria, R.; Kala, D.; Nagraik, R.; Dhir, Y.; Dhir, S.; Singh, B.; Kaushik, N.K.; Noorani, M.S.; Kumar, D.; et al. Recent advances in ctDNA detection using electrochemical biosensors for cancer. Discov. Oncol. 2024, 15, 517. [Google Scholar] [CrossRef]
- Narwade, M.; Shaikh, A.; Gajbhiye, K.R.; Kesharwani, P.; Gajbhiye, V. Advanced Cancer Targeting Using Aptamer Functionalized Nanocarriers for Site-Specific Cargo Delivery. Biomater. Res. 2023, 27, 42. [Google Scholar] [CrossRef]
- Yin, X.; Zhao, H.; He, Z.; Wang, X. Application of aptamer-functionalized nanomaterials in molecular imaging of tumors. Nanotechnol. Rev. 2023, 12, 107–136. [Google Scholar] [CrossRef]
- Lunawat, A.K.; Mukherjee, D.; Shivgotra, R.; Raikwar, S.; Awasthi, A.; Singh, A.; Singh, S.; Chandel, S.; Jain, S.K.; Thakur, S. Carboplatin Co Loaded 5 Fluorouracil Nanoparticles Conjugated with Trastuzumab for Targeted Therapy in HER2+ Heterogeneity Breast Cancer. AAPS PharmSciTech 2025, 26, 114. [Google Scholar] [CrossRef]
- Knetki-Wróblewska, M.; Dziadziuszko, R.; Jankowski, T.; Krawczyk, P.; Bryl, M.; Stencel, K.; Wrona, A.; Bandura, A.; Smok-Kalwat, J.; Rok-Knapińska, J.; et al. Pembrolizumab-combination therapy for NSCLC: Effectiveness and predictive factors in real-world practice. Front. Oncol. 2024, 14, 1341084. [Google Scholar] [CrossRef]
- Awad, M.M.; Forde, P.M.; Girard, N.; Spicer, J.; Wang, C.; Lu, S.; Mitsudomi, T.; Felip, E.; Broderick, S.R.; Swanson, S.J.; et al. Neoadjuvant nivolumab plus ipilimumab versus chemotherapy in resectable lung cancer: Exploratory analysis from CheckMate 816. J. Clin. Oncol. 2025, 43, 1453–1462. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Y.; Zheng, L.; Sun, X.; Zhao, Z.; Zheng, Y.; Tian, J. Efficacy and safety of consolidation durvalumab after chemoradiation therapy for stage III non-small-cell lung cancer: A systematic review and meta-analysis of real-world studies. Front. Pharmacol. 2023, 14, 1103927. [Google Scholar] [CrossRef]
- Liu, W.; Huo, G.; Chen, P. Clinical benefit of pembrolizumab in treatment of first-line non-small cell lung cancer: A systematic review and meta-analysis of clinical characteristics. BMC Cancer 2023, 23, 458. [Google Scholar] [CrossRef]
- Schenker, M.; Burotto, M.; Richardet, M.; Ciuleanu, T.; Gonçalves, A.; Steeghs, N.; Schoffski, P.; Ascierto, P.A.; Maio, M.; Lugowska, I.; et al. Randomized, open-label, phase 2 study of nivolumab plus ipilimumab or nivolumab monotherapy in patients with advanced or metastatic solid tumors of high tumor mutational burden. J. Immunother. Cancer 2024, 12, e008872. [Google Scholar] [CrossRef] [PubMed]
- Rosner, S.; Reuss, J.E.; Zahurak, M.; Zhang, J.; Zeng, Z.; Taube, J.; Anagnostou, V.; Smith, K.N.; Riemer, J.; Illei, P.B.; et al. Five-year clinical outcomes after neoadjuvant nivolumab in resectable non–small cell lung cancer. Clin. Cancer Res. 2023, 29, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Yajima, S.; Masuda, H. Immune checkpoint inhibitors and antibody-drug conjugates in urothelial carcinoma: Current landscape and future directions. Cancers 2025, 17, 1594. [Google Scholar] [CrossRef] [PubMed]
- Lieber, A.; Makai, A.; Orosz, Z.; Kardos, T.; Isaac, S.J.; Tornyi, I.; Bittner, N. The role of immunotherapy in early-stage and metastatic NSCLC. Pathol. Oncol. Res. 2024, 30, 1611713. [Google Scholar] [CrossRef]
- Stejskal, P.; Goodarzi, H.; Srovnal, J.; Hajdúch, M.; van ’t Veer, L.J.; Magbanua, M.J.M. Circulating tumor nucleic acids: Biology, release mechanisms, and clinical relevance. Mol. Cancer 2023, 22, 15. [Google Scholar] [CrossRef]
- Broche, J.; Kelemen, O.; Sekar, A.; Schütz, L.; Muyas, F.; Forschner, A.; Schroeder, C.; Ossowski, S. GeneBits: Ultra-sensitive tumour-informed ctDNA monitoring of treatment response and relapse in cancer patients. J. Transl. Med. 2025, 23, 964. Available online: https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-025-06993-3 (accessed on 2 June 2025). [CrossRef]
- Foster, B.; Pestano, G.; Kulisch, S.; Alexander, B.; Schroeder, C.; Taylor, C.; Kelemen, O.; Broche, J. ctDNA monitoring is providing a smarter way to track and treat cancer. Drug Discovery News. 2025. Available online: https://www.drugdiscoverynews.com/ctdna-monitoring-is-providing-a-smarter-way-to-track-and-treat-cancer-16443 (accessed on 2 June 2025).
- Bartolomucci, A.; Nobrega, M.; Ferrier, T.; Dickinson, K.; Kaorey, N.; Nadeau, A.; Castillo, A.; Burnier, J.V. Circulating tumor DNA to monitor treatment response in solid tumors and advance precision oncology. NPJ Precis. Oncol. 2025, 9, 876. [Google Scholar] [CrossRef]
- Kachhawaha, K.; Shah, R.; Singh, S.K. Clinical Sample Considerations in Proteomics-Based Biomarker Studies: Advancing Precision Medicine. In Protein Biomarkers: Discovery and Applications in Clinical Diagnostics; Singh, S.K., Chandra, P., Eds.; Springer: Singapore, 2025; pp. 21–43. [Google Scholar] [CrossRef]
- Abbasian, M.H.; Ardekani, A.M.; Sobhani, N.; Roudi, R. The Role of Genomics and Proteomics in Lung Cancer Early Detection and Treatment. Cancers 2022, 14, 5144. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Z.; Zhang, Y.; Zhang, C.; Xue, Q.; Zhang, G.; Tan, F. Recent advances in biomarkers for predicting the efficacy of immunotherapy in non-small cell lung cancer. Front. Immunol. 2025, 16, 1554871. [Google Scholar] [CrossRef]
- Zafar, A.; Khatoon, S.; Khan, M.J.; Abu, J.; Naeem, A. Advancements and Limitations in Traditional Anti-Cancer Therapies: A Comprehensive Review of Surgery, Chemotherapy, Radiation Therapy, and Hormonal Therapy. Discover Oncol. 2025, 16, 607. [Google Scholar] [CrossRef]
- Sulaiman, C.; George, B.P.; Balachandran, I.; Abrahamse, H. Cancer and traditional medicine: An integrative approach. Pharmaceuticals 2025, 18, 644. [Google Scholar] [CrossRef]
- Kiaie, S.H.; Salehi-Shadkami, H.; Sanaei, M.J.; Azizi, M.; Barough, M.S.; Nasr, M.S.; Sheibani, M. Nano-immunotherapy: Overcoming delivery challenge of immune checkpoint therapy. J. Nanobiotechnol. 2023, 21, 339. [Google Scholar] [CrossRef]
- Iriarte-Mesa, C.; Kleitz, F. Tailored mesoporous silica nanoparticles for overcoming gastrointestinal barriers: A perspective on advanced strategies for oral delivery. New J. Chem. 2025, 49, 10018–10034. [Google Scholar] [CrossRef]
- Park, H.; Yu, S.; Koo, T. Gene editing in cancer therapy: Overcoming drug resistance and enhancing precision medicine. Cancer Gene Ther. 2025, 32, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhou, J.; Tang, Y.; Huang, R.; Lu, C.; Qian, K.; Zhou, Q.; Zhang, J.; Yang, X.; Zhou, W.; et al. Advancements and challenges in CAR-T cell therapy for solid tumors: A comprehensive review of antigen targets, strategies, and future directions. Cancer Cell Int. 2025, 25, 313. [Google Scholar] [CrossRef]
- Xie, J.; Mao, Q.; Chen, J.; Shi, H.; Zhan, P.; Wang, H. Efficacy and safety of atezolizumab in the treatment of urothelial carcinoma: A systematic review and meta-analysis. World J. Surg. Oncol. 2025, 23, 133. [Google Scholar] [CrossRef]
- Kaur, R.; Bhardwaj, A.; Gupta, S. Cancer treatment therapies: Traditional to modern approaches to combat cancers. Mol. Biol. Rep. 2023, 50, 9663–9676. [Google Scholar] [CrossRef]
- Matsubara, J.; Mukai, K.; Kondo, T.; Yoshioka, M.; Kage, H.; Oda, K.; Kudo, R.; Ikeda, S.; Ebi, H.; Muro, K.; et al. First-line genomic profiling in previously untreated advanced solid tumors for identification of targeted therapy opportunities. JAMA Netw Open. 2023, 6, e2323336. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Ashley, E.A. Towards precision medicine. Nat. Rev. Genet. 2016, 17, 507–522. [Google Scholar] [CrossRef]
- Collins, F.S.; Varmus, H. A new initiative on precision medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef]
- Schettini, F.; Sirico, M.; Loddo, M.; Williams, G.H.; Hardisty, K.-M.; Scorer, P.; Thatcher, R.; Rivera, P.; Milani, M.; Strina, C. Next-generation sequencing-based evaluation of the actionable landscape of genomic alterations in solid tumors: The MOZART prospective observational study. Oncologist 2025, 30, oyae206. [Google Scholar] [CrossRef] [PubMed]
- Al-Lazikani, B.; Banerji, U.; Workman, P. Combinatorial drug therapy for cancer in the post-genomic era. Nat. Biotechnol. 2012, 30, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Zaim, R.; Redekop, W.K.; Uyl-de Groot, C.A. Cost-effectiveness of first-line nivolumab–ipilimumab combination therapy for advanced non-small-cell lung cancer: A systematic review. Front. Health Serv. 2023, 3, 1034256. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Hirst, D.G.; O’Sullivan, J.M. Gold nanoparticles as novel agents for cancer radiotherapy. Br. J. Radiol. 2012, 85, 101–113. [Google Scholar] [CrossRef]
- Song, X.; Sun, Z.; Li, L.; Zhou, L.; Yuan, S.; Yuan, S.; Sun, Z.; Li, L.; Zhou, L.; Yuan, S. Application of Nanomedicine in Radiotherapy Sensitization. Front. Oncol. 2023, 13, 1088878. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Jelveh, S.; Jalali, F.; van Prooijen, M.; Allen, C.; Bristow, R.G.; Hill, R.P.; Jaffray, D.A. Gold nanoparticles as radiation sensitizers for cancer therapy. Radiat. Res. 2010, 173, 719–728. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Slatkin, D.N.; Fazzari, M.J.; Smilowitz, H.M. Radiotherapy enhancement with gold nanoparticles. Nanomedicine 2008, 3, 703–712. [Google Scholar] [CrossRef]
- Loscertales, E.; López-Méndez, R.; Mateo, J.; Fraile, L.M.; Udías, J.M.; Espinosa, A.; España, S. Impact of gold nanoparticle size and coating on radiosensitization and generation of reactive oxygen species in cancer therapy. Nanoscale Adv. 2025, 7, 1204–1219. [Google Scholar] [CrossRef]
- Wang, J.; Pan, J.; Tang, Y.; Chen, J.; Fei, X.; Xue, W.; Liu, X. Advances of hafnium-based nanomaterials for cancer theranostics. Front. Chem. 2023, 11, 1283924. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Aime, S.; Hennink, W.E.; Storm, G.; Kiessling, F. Theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Hosny, S.; Mohamed, L.Z.; Ragab, M.S.; Alomoush, Q.K.; Abdalla, E.M.; Aly, S.A. Nanomaterials in biomedical applications: Opportunities and challenges—A review. Chem. Pap. 2025, 79, 2657–2678. [Google Scholar] [CrossRef]
- Brown, J.M.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef]
- Pi, F.; Deng, X.; Xue, Q.; Zheng, L.; Liu, H.; Yang, F.; Chen, T. Alleviating the hypoxic tumor microenvironment with MnO2-coated CeO2 nanoplatform for magnetic resonance imaging guided radiotherapy. J. Nanobiotechnol. 2023, 21, 90. [Google Scholar] [CrossRef]
- Mohanto, N.; Mondal, H.; Park, Y.-J.; Jee, J.-P. Therapeutic delivery of oxygen using artificial oxygen carriers demonstrates the possibility of treating a wide range of diseases. J. Nanobiotechnol. 2025, 23, 25. [Google Scholar] [CrossRef]
- Gerken, L.R.H.; Gerdes, M.E.; Pruschy, M.; Herrmann, I.K. Prospects of nanoparticle-based radioenhancement for radiotherapy. Mater. Horiz. 2023, 10, 4059–4082. [Google Scholar] [CrossRef]
- Farina, B.; Ramos Guerra, A.D.; Bermejo-Peláez, D.; Miras, C.P.; Peral, A.A.; Madueño, G.G.; Jaime, G.C.; Vilalta-Lacarra, A.; Pérez, J.R.; Muñoz-Barrutia, A.; et al. Integration of longitudinal deep-radiomics and clinical data improves the prediction of durable benefits to anti-PD-1/PD-L1 immunotherapy in advanced NSCLC patients. J. Transl. Med. 2023, 21, 174. [Google Scholar] [CrossRef]
- Rajbhandary, S.; Dhakal, H.; Shrestha, S. Tumor immune microenvironment (TIME) to enhance antitumor immunity. Eur. J. Med. Res. 2023, 28, 169. [Google Scholar] [CrossRef] [PubMed]
- Ly, P.-D.; Ly, K.-N.; Phan, H.-L.; Nguyen, H.H.T.; Duong, V.-A.; Nguyen, H.V. Recent advances in surface decoration of nanoparticles in drug delivery. Front. Nanotechnol. 2024, 6, 1456939. [Google Scholar] [CrossRef]
- Kumarasamy, R.V.; Natarajan, P.M.; Umapathy, V.R.; Roy, J.R.; Mironescu, M.; Palanisamy, C.P. Clinical applications and therapeutic potentials of advanced nanoparticles: A comprehensive review on completed human clinical trials. Front. Nanotechnol. 2024, 6, 1479993. [Google Scholar] [CrossRef]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in cancer therapy: Hierarchical targeting strategies and translational challenges. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef]
- Patel, H.; Li, J.; Bo, L.; Mehta, R.; Ashby, C.R.; Wang, S.; Cai, W.; Chen, Z.-S. Nanotechnology-based delivery systems to overcome drug resistance in cancer. Med. Rev. 2024, 4, 58. [Google Scholar] [CrossRef]
- Gajbhiye, K.R.; Salve, R.; Narwade, M.; Sheikh, A.; Kesharwani, P.; Gajbhiye, V. Lipid polymer hybrid nanoparticles: A custom-tailored next-generation approach for cancer therapeutics. Mol. Cancer 2023, 22, 160. [Google Scholar] [CrossRef]
- Ahmad, I.; Kushwaha, P.; Usmani, S.; Tiwari, A. Polymeric micelles: Revolutionizing cancer therapeutics for enhanced efficacy. BioNanoScience 2025, 15, 186. [Google Scholar] [CrossRef]
- Farhoudi, L.; Hosseinikhah, S.M.; Kazemi-Beydokhti, A.; Arabi, L.; Alavizadeh, S.H.; Moosavian, S.A.; Jaafari, M.R. pH-sensitive polymeric micelles enhance the co-delivery of doxorubicin and docetaxel: An emerging modality for treating breast cancer. Cancer Nanotechnol. 2024, 15, 37. [Google Scholar] [CrossRef]
- Wan, X.; Chen, C.; Zhan, J.; Ye, S.; Li, R.; Shen, M. Dendritic polylysine co-delivery of paclitaxel and siAXL enhances the sensitivity of triple-negative breast cancer chemotherapy. Front. Bioeng. Biotechnol. 2024, 12, 1415191. [Google Scholar] [CrossRef]
- Ramayanam, N.R.; Bukke, S.P.N.; Moka, M.K.; Dehingia, H.; Bordoloi, A.; Debbarma, R.; Kudumula, P.R.; Vuyyala, B.; Prasad, P.D.; Catherine, A. Advances in nanoparticle-based doxorubicin delivery: Precision strategies for targeted treatment of triple-negative breast cancer. Discover Nano 2025, 20, 111. [Google Scholar] [CrossRef]
- Hu, J.; Arvejeh, P.M.; Bone, S.; Hett, E.; Marincola, F.M.; Roh, K.-H. Nanocarriers for cutting-edge cancer immunotherapies. J. Transl. Med. 2025, 23, 447. [Google Scholar] [CrossRef]
- Sun, L.; Li, Z.; Lan, J.; Wu, Y.; Zhang, T.; Ding, Y. Better together: Nanoscale co-delivery systems of therapeutic agents for high-performance cancer therapy. Front. Pharmacol. 2024, 15, 1389922. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, L.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 2016, 7, 13193. [Google Scholar] [CrossRef] [PubMed]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Controlled Release 2001, 70, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Controlled Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Wei, X.; Song, M.; Li, W.; Huang, J.; Yang, G.; Wang, Y. Multifunctional Nanoplatforms Co-Delivering Combinatorial Dual-Drug for Eliminating Cancer Multidrug Resistance. Theranostics 2021, 11, 6334–6354. [Google Scholar] [CrossRef]
- Akpe, V.; Vernet, E.; Madu, C.; Obirai, J.C.; Brismar, H. Understanding the photochemical pathway of in vitro target delivery of aluminium phthalocyanine: A mechanistic approach using radical reaction chemistry. ChemPlusChem 2014, 79, 671–679. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Juarranz, A.; Jaen, P.; Sanz-Rodríguez, F.; Cuevas, J.; González, S. Photodynamic therapy of cancer. Basic principles and applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Lovell, J.F.; Jin, C.S.; Huynh, E.; Jin, H.; Kim, C.; Rubinstein, J.L.; Chan, W.C.W.; Cao, W.; Wang, L.V.; Zheng, G. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal imaging and therapeutic agents. Nat. Mater. 2011, 10, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Swain, A.; Jena, S.R.; Samanta, L. Smart nanocarriers for cancer: Harnessing exosomes and lipid systems in photodynamic and immunotherapy. Front. Immunol. 2025, 16, 1687953. [Google Scholar] [CrossRef]
- Thakur, S.; Godela, R.; Mandava, K.; Kolure, R. Advances in nanocarrier technology for drug encapsulation: A comprehensive overview. Discov. Mater. 2025, 5, 124. [Google Scholar] [CrossRef]
- Bechet, D.; Couleaud, P.; Frochot, C.; Viriot, M.L.; Guillemin, F.; Barberi-Heyob, M. Nanoparticles as vehicles for delivery of photodynamic therapy agents: An update. Nanomedicine 2008, 3, 830–844. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor microenvironment. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumors depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Qin, S.; Xu, Y.; Li, H.; Chen, H.; Yuan, Z. Recent advances in in situ oxygen-generating and oxygen-replenishing strategies for hypoxic-enhanced photodynamic therapy. Biomater. Sci. 2022, 10, 51–84. [Google Scholar] [CrossRef]
- Murugan, B.; Sagadevan, S.; Fatimah, I.; Oh, W.C.; Hossain, M.A.M.; Johan, M.R. Smart stimuli-responsive nanocarriers for cancer therapy–nanomedicine. Nanotechnol. Rev. 2021, 10, 1–17. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; La, H.; Zhu, R.; El-Banna, G.; Wei, Y.; Han, G. Upconverting NIR Photons for Bioimaging. Nanomaterials 2015, 5, 2148–2168. [Google Scholar] [CrossRef]
- Mousavi-Kiasary, S.M.; Senabreh, A.; Zandi, A.; Peña, R.; Cruz, F.; Adibi, A.; Hooshmand, N. Synergistic cancer therapies enhanced by nanoparticles: Advancing nanomedicine through multimodal strategies. Pharmaceutics 2025, 17, 682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, N.; Sun, H.; Fu, X.; Zhai, S.; Cui, J. Self-adjuvanting Photosensitizer Nanoparticles for Combination Photodynamic Immunotherapy. Biomater. Sci. 2021, 9, 5200–5210. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Na, J.; Liu, X.; Wu, P. Chromophore-Assisted Light Inactivation for Protein Degradation and Its Application in Biomedicine. Bioengineering 2024, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y.; Watanabe, W.; Matsunaga, S. Chromophore-assisted laser inactivation–towards a spatiotemporal–functional analysis of proteins, and the ablation of chromatin, organelle, and cell function. J. Cell Sci. 2014, 127, 1621–1629. [Google Scholar] [CrossRef]
- Serebrovskaya, E.O.; Lukyanov, K.A. Chromophore-Assisted Light Inactivation: A Powerful Tool to Study Protein Functions. In Singlet Oxygen: Applications in Biosciences and Nanosciences; Ogilby, P.R., Ed.; Royal Society of Chemistry: Cambridge, UK, 2016; pp. 185–203. [Google Scholar] [CrossRef]
- Shidara, H.; Jitsuki, S.; Takemoto, K. Chromophore-assisted light inactivation of target proteins for singularity biology. Biophys. Physicobiol. 2024, 21, e211009. [Google Scholar] [CrossRef]
- Bulina, M.E.; Chudakov, D.M.; Britanova, O.V.; Yanushevich, Y.G.; Staroverov, D.B.; Chepurnykh, T.V.; Merzlyak, E.M.; Shkrob, M.A.; Lukyanov, S.; Lukyanov, K.A. A genetically encoded photosensitizer. Nat. Biotechnol. 2006, 24, 95–99. [Google Scholar] [CrossRef]
- Du, W.; Shang, W.; Wen, W.; Deng, X.; Xie, D.; Zhang, Y.; Su, H.; Liu, H. Enhancing photodynamic therapy for cancer: A two-photon excited approach with a novel mitochondrial-targeted photosensitizer. Mater. Chem. Front. 2025, 9, 1234–1249. [Google Scholar] [CrossRef]
- Tour, O.; Meijer, R.M.; Zacharias, D.A.; Adams, S.R.; Tsien, R.Y. Genetically targeted chromophore-assisted light inactivation of proteins. Nat. Biotechnol. 2003, 21, 1505–1508. [Google Scholar] [CrossRef]
- Horstkotte, E.; Schröder, T.; Niewöhner, J.; Thiel, E.; Jay, D.G.; Henning, S.W. Toward Understanding the Mechanism of Chromophore-Assisted Laser Inactivation—Evidence for the Primary Photochemical Steps. Photochem. Photobiol. 2007, 83, 822–835. [Google Scholar] [CrossRef]
- Jay, D.G. Chromophore-Assisted Laser Inactivation. In Encyclopedia of Cancer, 2nd ed.; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1040–1044. [Google Scholar] [CrossRef]
- Kan, D.; Ding, R.; Yang, H.; Jia, Y.; Lei, K.; Wang, Z.; Zhang, W.; Yang, C.; Liu, Z.; Xie, F. Synergistic strategies in photodynamic combination therapy for cancer: Mechanisms, nanotechnology, and clinical translation. Front. Oncol. 2025, 15, 1607259. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, T.; Fujimoto, A.; Kawaguchi, H.; Kurosaki, T.; Kitamura, A. Stress granule dysfunction via chromophore-associated light inactivation. bioRxiv 2023. preprint. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, K.; Matsuda, T.; Sakai, N.; Fu, D.; Noda, M.; Uchiyama, S.; Kotera, I.; Arai, Y.; Horiuchi, M.; Fukui, K.; et al. SuperNova, a monomeric photosensitizing fluorescent protein for chromophore-assisted light inactivation. Sci. Rep. 2013, 3, 2629. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Qian, Y. Biodegradable fluorescent protein chromophore nanoparticles for hypoxic two-photon photodynamic therapy. Biomater. Sci. 2024, 12, 4567–4579. [Google Scholar] [CrossRef]
- Adeniyi, M.; Awosan, W. Nanoparticle-enhanced near-infrared fluorescence probes: A breakthrough in cancer imaging techniques. Int. J. Fundam. Med. Res. 2025, 1, 34883. [Google Scholar]
- Alsaafeen, B.H.; Ali, B.R.; Elkord, E. Resistance Mechanisms to Immune Checkpoint Inhibitors: Updated Insights. Mol. Cancer 2025, 24, 20. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, M.; Chen, R.; Ni, J.; Zhao, W.; Li, S.; Lu, X.; Jiao, H.; Cao, X. Innovative drugs promote precision cancer therapy. Clin. Cancer Bull. 2023, 2, 1. [Google Scholar] [CrossRef]
- Youssef, E.; Fletcher, B.; Palmer, D. Enhancing Precision in Cancer Treatment: The Role of Gene Therapy and Immune Modulation in Oncology. Front. Med. 2024, 11, 1527600. [Google Scholar] [CrossRef]
- Li, X.; Huang, Z.; Liao, Z.; Liu, A.; Huo, S. Transformable Nanodrugs for Overcoming the Biological Barriers in the Tumor Environment during Drug Delivery. Nanoscale 2023, 15, 8976–8992. [Google Scholar] [CrossRef]
- Labidi, S.; Meti, N.; Barua, R.; Li, M.; Riromar, J.; Jiang, D.M.; Fallah-Rad, N.; Sridhar, S.S.; Del Rincon, S.V.; Pezo, R.C.; et al. Clinical variables associated with immune checkpoint inhibitor outcomes in metastatic urothelial carcinoma: A multicentre retrospective cohort study. BMJ Open 2024, 14, e081480. [Google Scholar] [CrossRef]
- Kim, B. Designing healthcare for human use: Human factors and practical considerations for the translational process. Front. Health Serv. 2023, 3, 1188114. [Google Scholar] [CrossRef] [PubMed]
- Kishi, N.; Matsuo, Y.; Shintani, T.; Ogura, M.; Mitsuyoshi, T.; Araki, N.; Fujii, K.; Okumura, S.; Nakamatsu, K.; Kishi, T. Recurrence patterns and progression-free survival after chemoradiotherapy with or without consolidation durvalumab for stage III NSCLC. J. Radiat. Res. 2023, 64, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, K.; Mizugaki, H.; Ikezawa, Y.; Morita, R.; Yokoo, K.; Sumi, T.; Aso, M.; Kikuchi, H.; Nakamura, A.; Sekikawa, M.; et al. Real-world data of first-line treatment with pembrolizumab for NSCLC with high PD-L1 expression in elderly patients: A subgroup analysis of HOT/NJLCG2001. Jpn. J. Clin. Oncol. 2025, 55, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, J.; Zhang, L.; Cheng, S.; Yu, J. Network meta-analysis of first-line immune checkpoint inhibitor therapy in advanced non-squamous NSCLC patients with PD-L1 ≥50%. BMC Cancer 2023, 23, 791. [Google Scholar] [CrossRef]
- Richters, A.; Yildirim, H.; Booth, C.M.; Badillo, F.E.V.; Kiemeney, L.A.L.M.; Aben, K.K.H. Changes to primary end points in immune checkpoint inhibitor trials for urothelial, renal, and lung cancers: A systematic review. JAMA Oncol. 2023, 9, 1144–1147. [Google Scholar] [CrossRef]
- Zhang, W.; Dai, T.; Wang, D.; Zhu, Y.; Hua, W. Efficacy of neoadjuvant PD-1/PD-L1 inhibitor in resectable NSCLC: A meta-analysis based on randomized controlled trials. BMC Cancer 2024, 24, 1522. [Google Scholar] [CrossRef]
- Khan, T.H.; Muhammad, N.; Tarique, M.; Usmani, D.; Naz, H.; Sarode, A. The Role of Cancer-Specific Target Antigens in CAR T Cell Therapy in Hematological Malignancies. Curr. Tissue Microenviron. Rep. 2024, 5, 61–67. [Google Scholar] [CrossRef]
- Brentjens, R.J.; Davila, M.L.; Riviere, I.; Park, J.; Wang, X.; Cowell, L.G.; Bartido, S.; Stefanski, J.; Taylor, C.; Olszewska, M.; et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013, 5, 177ra38. [Google Scholar] [CrossRef]
- Ganpisetti, R.; Giridharan, S.; Vaskuri, G.S.S.J.; Narang, N.; Basim, P.; Dokmeci, M.R.; Ermis, M.; Rojekar, S.; Gholap, A.D.; Kommineni, N. Biological Nanocarriers in Cancer Therapy: Cutting Edge Innovations in Precision Drug Delivery. Biomolecules 2025, 15, 802. [Google Scholar] [CrossRef]
- Sabit, H.; Pawlik, T.M.; Radwan, F.; Abdel-Hakeem, M.; Abdel-Ghany, S.; Wadan, A.H.S.; Elzawahri, M.; El-Hashash, A.; Arneth, B. Precision nanomedicine: Navigating the tumor microenvironment for enhanced cancer immunotherapy and targeted drug delivery. Mol. Cancer 2025, 24, 160. [Google Scholar] [CrossRef]
- Dienstmann, R.; Rodon, J.; Barretina, J.; Tabernero, J. Genomic medicine frontier in human solid tumors: Prospects and challenges. J. Clin. Oncol. 2013, 31, 1874–1884. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Zhou, C.; Chen, Y.; Feng, X.; Tang, H. Real-world study of PD-1/L1 immune checkpoint inhibitors for advanced non-small cell lung cancer after resistance to EGFR-TKIs. Front. Oncol. 2023, 13, 1217872. [Google Scholar] [CrossRef] [PubMed]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus Docetaxel in Patients with Previously Treated Non-Small-Cell Lung Cancer (OAK): A Phase 3, Open-Label, Multicentre Randomised Controlled Trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.-Y. Exploring Treatment Options in Cancer: Tumor Treatment Strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Piccart-Gebhart, M.J.; Procter, M.; Leyland-Jones, B.; Goldhirsch, A.; Untch, M.; Smith, I.; Gianni, L.; Baselga, J.; Bell, R.; Jackisch, C.; et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 2005, 353, 1659–1672. [Google Scholar] [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef]
- Xie, J.; Mao, Q.Y.; Chen, J.H.; Shi, H.J.; Zhan, P.Q.; Wang, H.F. ASCO GU 2023: Final OS Analysis of Atezolizumab Monotherapy vs Chemotherapy in Untreated Locally Advanced or Metastatic Urothelial Carcinoma from the Phase 3 IMvigor130 Study. 2023. Available online: https://www.urotoday.com/conference-highlights/asco-gu-2023/asco-gu-2023-bladder-cancer/142544-asco-gu-2023-final-os-analysis-of-atezolizumab-monotherapy-vs-chemotherapy-in-untreated-locally-advanced-or-metastatic-urothelial-carcinoma-from-the-phase-3-imvigor130-study.html (accessed on 2 June 2025).
- Mounika, M.S.; Ganpisetti, R.; Bag, I.; Rana, R.; Sanjay, G.; Giribabu, K. 3D bioprinting models for novel breast cancer strategies. Res. J. Pharm. Technol. 2022, 15, 5576–5582. [Google Scholar] [CrossRef]
- Heo, Y.; Kim, W.-J.; Cho, Y.-J.; Jung, J.-W.; Kim, N.-S.; Choi, I.-Y. Advances in Cancer Genomics and Precision Oncology. Genes Genom. 2025, 47, 399–416. [Google Scholar] [CrossRef]
- Naldini, L. Gene therapy returns to centre stage. Nature 2015, 526, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Manguso, R.T.; Pope, H.W.; Zimmer, M.D.; Brown, F.D.; Yates, K.B.; Miller, B.C.; Collins, N.B.; Bi, K.; LaFleur, M.W.; Juneja, V.R.; et al. In vivo CRISPR screening identifies Pbrm1 as a suppressor of antitumor immunity. Nature 2017, 547, 413–418. [Google Scholar] [CrossRef]
- Mout, R.; Ray, M.; Yesilbag Tonga, G.; Lee, Y.W.; Tay, T.; Sasaki, K.; Rotello, V.M. Direct Cytosolic Delivery of CRISPR/Cas9-Ribonucleoprotein for Efficient Gene Editing. ACS Nano 2017, 11, 2452–2458. [Google Scholar] [CrossRef]
- Ventura, A.; Kirsch, D.G.; McLaughlin, M.E.; Tuveson, D.A.; Grimm, J.; Lintault, L.; Newman, J.; Reczek, E.E.; Weissleder, R.; Jacks, T. Restoration of p53 function leads to tumour regression in vivo. Nature 2007, 445, 661–665. [Google Scholar] [CrossRef]
- Xue, W.; Zender, L.; Miething, C.; Dickins, R.A.; Hernando, E.; Krizhanovsky, V.; Cordon-Cardo, C.; Lowe, S.W. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 2007, 445, 656–660. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.-S.; Hirsch, F.R. PD-L1 as a Biomarker of Response to Immune-Checkpoint Inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–361. [Google Scholar] [CrossRef]
- Wei, J.; Li, W.; Zhang, P.; Guo, F.; Liu, M. Current Trends in Sensitizing Immune Checkpoint Inhibitors for Cancer Treatment. Mol. Cancer 2024, 23, 279. [Google Scholar] [CrossRef]
- Qi, C.; Li, Y.; Zeng, H.; Wei, Q.; Tan, S.; Zhang, Y.; Li, W.; Tian, P. Current Status and Progress of PD-L1 Detection: Guiding Immunotherapy for Non-Small Cell Lung Cancer. Clin. Exp. Med. 2024, 24, 162. [Google Scholar] [CrossRef]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.A.; Stephens, P.J.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef]
- Egger, G.; Liang, G.; Aparicio, A.; Jones, P.A. Epigenetics in human disease and prospects for epigenetic therapy. Nature 2004, 429, 457–463. [Google Scholar] [CrossRef]
- Jones, P.A.; Baylin, S.B. The epigenomics of cancer. Cell 2007, 128, 683–692. [Google Scholar] [CrossRef]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef]
- Prince, H.M.; Bishton, M.J.; Harrison, S.J. Clinical studies of histone deacetylase inhibitors. Clin. Cancer Res. 2009, 15, 3958–3969. [Google Scholar] [CrossRef] [PubMed]
- West, A.C.; Johnstone, R.W. New and emerging HDAC inhibitors for cancer treatment. J. Clin. Investig. 2014, 124, 30–39. [Google Scholar] [CrossRef]
- Yang, X.; Lay, F.; Han, H.; Jones, P.A. Targeting DNA methylation for epigenetic therapy. Trends Pharmacol. Sci. 2010, 31, 536–546. [Google Scholar] [CrossRef]
- Suraweera, A.; O’Byrne, K.J.; Richard, D.J. Epigenetic Drugs in Cancer Therapy. Cancer Metastasis Rev. 2025, 44, 37. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Joyce, J.A.; Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef]
- Raza, A.; Mohsen, R.; Kanbour, A.; Zar Gul, A.R.; Philip, A.; Vijayakumar, S.; Hydrose, S.; Prabhu, K.S.; Al-Suwaidi, A.K.; Inchakalody, V.P.; et al. Serum immune mediators as novel predictors of response to anti-PD-1/PD-L1 therapy in NSCLC patients with high tissue-PD-L1 expression. Front. Immunol. 2023, 14, 1157100. [Google Scholar] [CrossRef]
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016, 107, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.J.; Peng, K.W.; Bell, J.C. Oncolytic Virotherapy. Nat. Biotechnol. 2012, 30, 658–670. [Google Scholar] [CrossRef]
- Andtbacka, R.H.I.; Collichio, F.A.; Harrington, K.J.; Middleton, M.R.; Downey, G.S.; Öhrling, K.; Kaufman, H.L.; Amatruda, T.; Senzer, N.; Chesney, J.; et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Bommareddy, P.K.; Shettigar, M.; Kaufman, H.L. Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 498–513. [Google Scholar] [CrossRef]
- Jiang, S.; Chai, H.; Tang, Q.; Shi, Z.; Zhou, L. Clinical Advances in Oncolytic Virus Therapy for Malignant Glioma: A Systematic Review. Discov. Oncol. 2023, 14, 183. [Google Scholar] [CrossRef]
- Guo, Z.S.; Liu, Z.; Kowalsky, S.P.; Feist, M.; Kalinski, P.; Bartlett, D.L. Oncolytic immunotherapy: Dying the right way is a key to eliciting potent antitumor immunity. Front. Oncol. 2014, 4, 74. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, L.; Xu, Y.; Liang, L.; Liu, L.; Chen, X.; Li, H.; Liu, H. The Progress and Prospects of Targeting the Adenosine Pathway in Cancer Immunotherapy. Biomark. Res. 2025, 13, 75. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.-X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef]
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015, 523, 231–235. [Google Scholar] [CrossRef]
- Luke, J.J.; Bao, R.; Sweis, R.F.; Spranger, S.; Gajewski, T.F. WNT/β-catenin pathway activation correlates with immune exclusion across human cancers. Cancer Res. 2019, 79, 5356–5362. [Google Scholar] [CrossRef]
- Melief, C.J.M.; van Hall, T.; Arens, R.; Ossendorp, F.; van der Burg, S.H. Therapeutic cancer vaccines. J. Clin. Investig. 2015, 125, 3401–3412. [Google Scholar] [CrossRef]
- Palucka, K.; Banchereau, J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer 2012, 12, 265–277. [Google Scholar] [CrossRef]
- Moon, J.J.; Suh, H.; Bershteyn, A.; Stephan, M.T.; Liu, H.; Ling, N.; Dryden, K.A.; Jones, H.; Zal, T.; Irvine, D.J. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat. Mater. 2011, 10, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu. Rev. Immunol. 2004, 22, 745–763. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.; Wang, X.; Han, M.; Guo, Y. Nanoparticle-Mediated Synergistic Chemoimmunotherapy for Cancer Treatment. Int. J. Nanomed. 2024, 19, 4533–4568. [Google Scholar] [CrossRef] [PubMed]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dolan, D.G.; Dreicer, R.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Jeong, M.; Jang, I. Comparative Effectiveness and Immunogenicity of Single-Dose and Multi-Dose Human Papillomavirus Vaccination: A Systematic Review. BMC Public Health 2025, 25, 2330. [Google Scholar] [CrossRef]
- Takeda, M.; Yoshida, S.; Inoue, T.; Sekido, Y.; Hata, T.; Hamabe, A.; Ogino, T.; Miyoshi, N.; Uemura, M.; Yamamoto, H.; et al. The Role of KRAS Mutations in Colorectal Cancer: Biological Insights, Clinical Implications, and Future Therapeutic Perspectives. Cancers 2025, 17, 428. [Google Scholar] [CrossRef]
- Hao, L.; Li, S.; Hu, X. New Insights into T-Cell Exhaustion in Liver Cancer: From Mechanism to Therapy. J. Cancer Res. Clin. Oncol. 2023, 149, 12543–12560. [Google Scholar] [CrossRef]
- Wang, B.; Han, Y.; Zhang, Y.; Zhao, Q.; Wei, J.; Xin, J. Overcoming Acquired Resistance to Cancer Immune Checkpoint Therapy: Potential Strategies Based on Molecular Mechanisms. Cell Biosci. 2023, 13, 120. [Google Scholar] [CrossRef]
- Benci, J.L.; Xu, B.; Qiu, Y.; Wu, T.D.; Dada, H.; Twyman-Saint Victor, C.; Cucolo, L.; Cordes, B.; Denize, T.; Jure-Kunkel, M.; et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell 2016, 167, 1540–1554.e12. [Google Scholar] [CrossRef]
- Thomas, E.D.; Lochte, H.L., Jr.; Lu, W.C.; Ferrebee, J.W. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N. Engl. J. Med. 1957, 257, 491–496. [Google Scholar] [CrossRef]
- Loren, A.W.; Mielcarek, M.; Bolaños-Meade, J.; Brammer, J.; Cowden, M.; Di Stasi, A.; El-Jawahri, A.; Elmariah, H.; Gundabolu, K.; Gutman, J.; et al. Hematopoietic Cell Transplantation, Version 3.2025, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2025, 23, e250047. [Google Scholar] [CrossRef]
- DeWolf, S.; Kuttiyara, J.; Vinci, P.; Fei, T.; Slingerland, J.; Katsamakis, Z.A.; Fein, J.A.; Gipson, B.; Lorenc, R.; Zinsmeyer, V.; et al. Bone Marrow and Blood Demonstrate Distinct Immune Reconstitution Patterns and Correlations with Relapse Post-Transplant. Blood Adv. 2025, 9, 15626. [Google Scholar] [CrossRef]
- Appelbaum, F.R. Hematopoietic-cell transplantation at 50. N. Engl. J. Med. 2007, 357, 1472–1475. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Sakuishi, K.; Apetoh, L.; Sullivan, J.M.; Blazar, B.R.; Kuchroo, V.K.; Anderson, A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore antitumor immunity. J. Exp. Med. 2010, 207, 2187–2194. [Google Scholar] [CrossRef]
- Moser, J.C.; Hu-Lieskovan, S. Mechanisms of resistance to PD-1 checkpoint blockade. Drugs 2020, 80, 459–465. [Google Scholar] [CrossRef]
- Ho, P.C.; Bihuniak, J.D.; Macintyre, A.N.; Staron, M.; Liu, X.; Amezquita, R.; Tsui, Y.C.; Cui, G.; Micevic, G.; Perales, J.C.; et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell 2015, 162, 1217–1228. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; To, K.K.W.; Zhu, S.; Wang, F.; Fu, L. Tumor-associated macrophages remodel the suppressive tumor immune microenvironment and targeted therapy for immunotherapy. J. Exp. Clin. Cancer Res. 2025, 44, 145. [Google Scholar] [CrossRef] [PubMed]
- Starzer, A.M.; Preusser, M.; Berghoff, A.S. Immune escape mechanisms and therapeutic approaches in cancer: The cancer-immunity cycle. Ther. Adv. Med. Oncol. 2022, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Xiong, W.; Xu, Y.; Zou, J.; Zeng, Y.; Liu, J.; Peng, Y.; Hu, C.; Wu, F. Immunometabolism: A New Dimension in Immunotherapy Resistance. Front. Med. 2023, 17, 585–616. [Google Scholar] [CrossRef]
- Swetha, K.L.; Roy, A. Tumor Heterogeneity and Nanoparticle-Mediated Tumor Targeting: The Importance of Delivery System Personalization. Drug Deliv. Transl. Res. 2018, 8, 1508–1526. [Google Scholar] [CrossRef]
- Khan, I.A.; Yu, T.; Yang, M.; Liu, J.; Chen, Z. A systematic review of toxicity, biodistribution, and biosafety in upconversion nanomaterials: Critical insights into toxicity mitigation strategies and future directions for safe applications. BME Front. 2025, 6, 0120. [Google Scholar] [CrossRef]
- de Souza Cardoso Delfino, C.; de Paula Pereira, M.C.; dos Santos Oliveira, M.; de Carvalho Favareto, I.; Valladão, V.S.; de Oliveira Mota, M.; Costa, M.V.B.; Sousa-Batista, A.J.; Balbino, T.A. Scaling nanopharmaceutical production for personalized medicine: Challenges and strategies. J. Nanopart. Res. 2025, 27, 108. [Google Scholar] [CrossRef]
- Foulkes, R.; Man, E.; Thind, J.; Yeung, S.; Joy, A.; Hoskins, C. The regulation of nanomaterials and nanomedicines for clinical application: Current and future perspectives. Biomater. Sci. 2020, 8, 4653–4664. [Google Scholar] [CrossRef]
- Joyce, P.; Allen, C.J.; Alonso, M.J.; Ashford, M.; Bradbury, M.S.; Germain, M.; Kavallaris, M.; Langer, R.; Lammers, T.; Peracchia, M.T.; et al. A Translational Framework to DELIVER Nanomedicines to the Clinic. Nat. Nanotechnol. 2024, 19, 1123–1138. [Google Scholar] [CrossRef]
- Mao, Y.; Xie, J.; Yang, F.; Luo, Y.; Du, J.; Xiang, H. Advances and Prospects of Precision Nanomedicine in Personalized Tumor Theranostics. Front. Cell Dev. Biol. 2024, 12, 1514399. [Google Scholar] [CrossRef]
- Ferrari, M. Cancer Nanotechnology: Opportunities and Challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Al Amili, M.; Guo, S. Tumor Microenvironment-Responsive Drug Delivery Based on Polymeric Micelles for Precision Cancer Therapy: Strategies and Prospects. Biomedicines 2024, 12, 417. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.-C.; Canchola, A.; Zhang, F.; Lin, Z. Machine Learning and Artificial Intelligence in Nanomedicine: Modeling Nanoparticle–Biological Interactions, Optimizing Surface Functionalization, and Predicting Pharmacokinetics across Patient Profiles. WIREs Nanomed. Nanobiotechnol. 2025, 17, e20027. [Google Scholar] [CrossRef]
- Qiu, J.; Wu, C. Smart Nanoparticle Delivery of Cancer Vaccines Enhances Tumor Immune Responses: A Review. Front. Nanotechnol. 2025, 7, 1564267. [Google Scholar] [CrossRef]
- Kim, M.; Hwang, Y.; Lim, S.; Jang, H.-K.; Kim, H.-O. Advances in Nanoparticles as Non-Viral Vectors for Efficient Delivery of CRISPR/Cas9. Pharmaceutics 2024, 16, 1197. [Google Scholar] [CrossRef]
- Tian, Y.; Carrillo-Malani, N.; Feng, K.; Miller, J.; Busch, T.M.; Sundaram, K.M.; Cheng, Z.; Amirshaghaghi, A.; Tsourkas, A. Theranostic Phthalocyanine and Naphthalocyanine Nanoparticles for Photoacoustic Imaging and Photothermal Therapy of Tumors. Nanotheranostics 2024, 8, 100–111. [Google Scholar] [CrossRef]
- Sorrentino, C.; Ciummo, S.L.; Di Carlo, E. Nanomedicine for Cancer Patient-Centered Care. MedComm 2024, 5, e767. [Google Scholar] [CrossRef]
- Chan, T.A.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.A.; Stenzinger, A.; Peters, S.; Curigliano, G.; Snyder, A.; Romero, P.; et al. Development of Tumor Mutation Burden as an Immunotherapy Biomarker: Utility for the Oncology Clinic. Ann. Oncol. 2019, 30, 44–56. [Google Scholar] [CrossRef]
- Papaporfyriou, A.; Bartziokas, K.; Apessos, I.; Mueller, J.; Leivaditis, V.; Koletsis, E.; Grapatsas, K. Comparative Efficacy and Safety of Neoadjuvant Immunotherapy with Nivolumab vs. Pembrolizumab in Resectable Non-Small Cell Lung Cancer: A Systematic Review. Curr. Oncol. 2024, 31, 6289–6299. [Google Scholar] [CrossRef]
- Liu, L.; Yan, Y.; Wang, Y.; Li, Z.; Yang, L.; Yu, K.; Zhao, Z. Comparative efficacy and safety of first-line PD-1/PD-L1 inhibitors in immunotherapy for non-small cell lung cancer: A network meta-analysis. Oncol. Lett. 2025, 29, 157. [Google Scholar] [CrossRef]
- Morgensztern, D.; Herbst, R.S. Nivolumab and Pembrolizumab for Non-Small Cell Lung Cancer. Clin. Cancer Res. 2016, 22, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.; Rizvi, N.A.; Chow, L.Q.M.; Borghaei, H.; Brahmer, J.R.; Harris, T.J.; Felip, E.; Cuffe, S.; Chen, Y.; Powney, R.; et al. Five-year overall survival with nivolumab in previously treated advanced NSCLC: Outcomes by PD-L1 expression. J. Clin. Oncol. 2021, 39, 723–733. [Google Scholar]
- Su, R.; Chen, Z.; Hong, D.; Jiang, S.; Yuan, Y.; Cai, X.; Hu, H.; Fu, C.; Huang, Z.; Wang, Z.; et al. Effectiveness and Safety of Immune Checkpoint Inhibitor Monotherapy in Advanced Upper Tract Urothelial Carcinoma: A Multicenter Real-World Study. Cancer Med. 2023, 12, 10587–10596. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Shiroyama, T.; Tamiya, M.; Minami, T.; Kinehara, Y.; Tamiya, A.; Suga, Y.; Kuge, T.; Mori, M.; Suzuki, H.; et al. Real-World Outcomes of Nivolumab Plus Ipilimumab and Pembrolizumab with Chemotherapy in Advanced NSCLC: A Multicenter Retrospective Comparative Study. Cancer Immunol. Immunother. 2024, 73, 4. [Google Scholar] [CrossRef]
- Facheris, G.; Cossali, G.; Imbrescia, J.; La Mattina, S.; Mataj, E.; Meli, N.; Volpi, G.; Triggiani, L.; Guerini, A.E.; Levi, G.; et al. Real-World Insights into the Impact of Durvalumab on Stage III Unresectable Non-Small Cell Lung Cancer—A Narrative Review. Cancers 2025, 17, 874. [Google Scholar] [CrossRef]
- Shalata, W.; Rabinovich, N.M.; Agbarya, A.; Yakobson, A.; Dudnik, Y.; Abu Jama, A.; Cohen, A.Y.; Shalata, S.; Abu Hamed, A.; Ber, T.I.; et al. Efficacy of pembrolizumab vs. nivolumab plus ipilimumab in metastatic NSCLC in relation to PD-L1 and TMB status. Cancers 2024, 16, 1825. [Google Scholar] [CrossRef]
- Shimizu, T.; Inoue, E.; Ohkuma, R.; Kobayashi, S.; Tsunoda, T.; Wada, S. Soluble PD-L1 changes in advanced NSCLC patients treated with PD-1 inhibitors: An individual patient data meta-analysis. Front. Immunol. 2023, 14, 1308381. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Ehmann, F.; Sakai-Kato, K.; Duncan, R.; Pérez de la Ossa, D.H.; Pita, R.; Vidal, J.M.; Kohli, A.; Tothfalusi, L.; Sanh, A.; Tinton, S.; et al. Next-Generation Nanomedicines and Nanosimilars: EU Regulators’ Initiatives Relating to The Development and Evaluation of Nanomedicines. Nanomedicine 2013, 8, 849–856. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Definition and Preferred Terms for Nanomaterials and Nanomedicines; WHO: Geneva, Switzerland, 2020; pp. 1–30. [Google Scholar]
- Marre, D.; Nystrom, A.M.; Zhang, H.; Fraser, D.; Miller, D.; Boyd, B.J.; Porter, C.J.H.; Prud’homme, R.K. Quality-by-Design Approach to the Development and Scale-Up of Nanomedicine Formulations. J. Control. Release 2019, 311, 55–68. [Google Scholar] [CrossRef]
- Sammasagi, S.S.; Sutar, K.P.; Hooli, S. Scale-Up and Quality Control Challenges in the Industrial Manufacturing of Nanoformulations: Current Trends and Future Perspectives. Int. J. Sci. Appl. Technol. 2025, 16, 2. [Google Scholar] [CrossRef]
- Aguiam, N.; Moura, L.I.F.; Oliveira, M.; Florindo, H.; Lopes, J.A. Process Analytics for the Manufacturing of Nanomedicines: Challenges and Opportunities. In Nanomedicine Manufacturing and Applications; Silva, A.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2025; Chapter 7; pp. 157–176. [Google Scholar] [CrossRef]
- Caputo, F.; Favre, G.; Borchard, G.; Calzolai, L.; Fisicaro, P.; Frejafon, E.; Günday-Türeli, N.; Koltsov, D.; Minelli, C.; Nelson, B.C.; et al. Toward an International Standardisation Roadmap for Nanomedicine. Drug Deliv. Transl. Res. 2024, 14, 2578–2588. [Google Scholar] [CrossRef] [PubMed]
- Havelikar, U.; Ghorpade, K.B.; Kumar, A.; Patel, A.; Singh, M.; Banjare, N.; Gupta, P.N. Comprehensive Insights into Mechanism of Nanotoxicity, Assessment Methods and Regulatory Challenges of Nanomedicines. Discover Nano 2024, 19, 165. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Multi-Organs-on-Chips: Towards Long-Term Biomedical Investigations. Molecules 2019, 24, 675. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tian, Y.; Yang, R.; Wang, H.; Allahou, L.W.; Chang, J.; Williams, G.; Knowles, J.C.; Poma, A. Nanotechnology in Healthcare: Safety and Environmental Risks. J. Nanobiotechnol. 2024, 22, 715. [Google Scholar] [CrossRef]
- Abu-Qdais, H.A.; Abu-Dalo, M.A.; Hajeer, Y.Y. Impacts of Nanosilver-Based Textile Products Using a Life Cycle Assessment. Sustainability 2021, 13, 3436. [Google Scholar] [CrossRef]
- Lai, R.W.S.; Yeung, K.W.Y.; Yung, M.M.N.; Leung, C.H.; Wong, C.S.; Chan, C.M.; Wong, M.H. Regulation of Engineered Nanomaterials: Current Challenges, Insights and Future Directions. Environ. Sci. Pollut. Res. 2018, 25, 3060–3077. [Google Scholar] [CrossRef]
- Cui, K.; Wang, L.; Huang, Y. Nanomaterial-Based Biosensors for Post-Marketing Biomarker Surveillance: Opportunities and Challenges. Front. Bioeng. Biotechnol. 2024, 12, 1414746. [Google Scholar] [CrossRef]
- Gomes, K.L.G.; da Silva, R.E.; da Silva, J.B.; Bosio, C.G.P.; Garbi Novaes, M.R.C. Post-Marketing Authorisation Safety and Efficacy Surveillance of Advanced Therapy Medicinal Products in Brazil, the European Union, the United States and Japan. Cytotherapy 2023, 25, 1113–1123. [Google Scholar] [CrossRef]
- Agrahari, V.; Choonara, Y.E.; Mosharraf, M.; Pillay, V. The Role of Artificial Intelligence and Machine Learning in Accelerating the Discovery and Development of Nanomedicine. Pharm. Res. 2024, 41, 2289–2297. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, F.D.; Monferrer, D.; Penon, O.; Rivera-Gil, P. Regulatory Pathways and Guidelines for Nanotechnology-Enabled Health Products: A Comparative Review of EU and US Frameworks. Front. Med. 2025, 12, 1544393. [Google Scholar] [CrossRef]
- Warner, J.; Prada Jardim, A.; Albera, C.; Smith, J.; Doe, R.; Li, Y. Artificial Intelligence-Driven Signal Detection in Pharmacovigilance: Leveraging Machine Learning and Patient Registries for Enhanced Drug Safety Surveillance. Pharm. Med. 2025, 39, 183–198. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).